Abstract
Background
It remains unknown whether individuals who regularly use preventive medications receive the same benefit from healthy lifestyle as those who do not use medications. We aimed to examine the associations of healthy lifestyle with mortality according to use of major preventive medications, including aspirin, antihypertensives, and lipid‐lowering medications.
Methods and Results
Among 79 043 women in the Nurses' Health Study (1988–2014) and 39 544 men in the Health Professionals Follow‐up Study (1986–2014), we defined a healthy lifestyle score based on body mass index, smoking, physical activity, diet, and alcohol intake. We estimated multivariable hazard ratios (HRs) and population‐attributable risks of death from any cause, cardiovascular disease, cancer, and other causes in relation to healthy lifestyle according to medication use. We documented 35 195 deaths. A similar association of healthy lifestyle score with lower all‐cause mortality was observed among medication users (HR, 0.82 per unit increment; 95% CI, 0.81–0.82) and nonusers (HR, 0.81; 95% CI, 0.79–0.83) (P interaction=0.54). The fraction of premature deaths that might be prevented by adherence to the 5 healthy lifestyle factors among medication users and nonusers was 38% (95% CI, 32%–42%) and 40% (95% CI, 29%–50%) for all‐cause mortality, 37% (95% CI, 27%–46%) and 45% (95% CI, 18%–66%) for cardiovascular disease mortality, and 38% (95% CI, 28%–46%) and 33% (95% CI, 14%–49%) for cancer mortality, respectively.
Conclusions
Adherence to a healthy lifestyle confers substantial benefit for prevention of premature death among both regular users and nonusers of preventive medications. Adherence to a healthy lifestyle remains important even among individuals regularly using preventive medications.
Keywords: lifestyle, mortality, nutrition, preventive medication, primary prevention
Subject Categories: Lifestyle, Epidemiology, Obesity, Primary Prevention, Diet and Nutrition
Nonstandard Abbreviations and Acronyms
- AHEI
Alternate Healthy Eating Index
- BMI
body mass index
- CVD
cardiovascular disease
- HR
hazard ratio
- HPFS
Health Professionals Follow‐up Study
- NHS
Nurses' Health Study
- PAR
population‐attributable risk
Clinical Perspective
What Is New?
In this cohort study with a follow‐up of 26 years, we found a similar benefit of healthy lifestyle among regular users and nonusers of major preventive medications.
What Are the Clinical Implications?
The findings indicate that adhering to a healthy lifestyle is always beneficial for health, regardless of the use of preventive medications.
Cardiovascular disease (CVD) and cancer are the 2 leading causes of death, accounting for almost a half (CVD, 23%; cancer, 22%) of all deaths in the United States in 2017. 1 Major preventive medications, including aspirin, antihypertensives, and lipid‐lowering medications, are among the most commonly used medications in US adults. 2 , 3 , 4 , 5 During 2012 to 2018, ≈47% of adults aged 45 to 75 years reported current use of aspirin for primary prevention of CVD, 2 62% of hypertensive patients used antihypertensives, 3 and 28% of those 40 years or older used statins, with the prevalence increasing to 48% among those 75 years or older. 4 While these medications have an established benefit for reducing CVD incidence in high‐risk populations and recurrent events in patients with CVD history, 6 , 7 , 8 their benefit for primary prevention in an average‐risk population remains controversial. 9 , 10 , 11 Moreover, although some evidence indicates potential of these medications in cancer prevention, 12 the benefit is limited to certain cancer type (ie, aspirin for colorectal cancer 13 ) and remains inconclusive for others. 14 , 15 , 16
On the other hand, heathy lifestyle practices, including no smoking, being physically active, eating a healthy diet, limiting alcohol drinking, and maintaining a healthy body weight, have been associated with a substantial reduction in incidence and mortality of CVD 17 and cancer. 18 A recent report revealed that >60% of all‐cause premature deaths can be potentially prevented by adherence to healthy lifestyle, 19 indicating an important role of lifestyle modification in the prevention of premature death. Mechanistically, a healthy lifestyle has been shown to act on similar pathways as preventive medications, such as control of blood pressure, reduction of inflammation, and improvement of lipid profiles. 20 , 21 , 22 However, it remains unknown whether individuals who regularly use preventive medications would still benefit from healthy lifestyle practices as those not using the medications. Addressing this question has great clinical implications as a result of the ever‐increasing prevalence of preventive medication use 5 and related concerns about the “health certificate effect” (medication users may consider being certified healthy and thus have less incentive to make lifestyle modification). 23
Therefore, we prospectively assessed the association of healthy lifestyles with all‐cause and cause‐specific mortality among users and nonusers of common preventive medications within 2 large cohorts in the United States, including NHS (Nurses' Health Study) and HPFS (Health Professionals Follow‐up Study). We also estimated the population‐attributable risk (PAR) associated with healthy lifestyles according to medication use.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Brigham and Women's/Harvard Cohorts at https://sites.google.com/channing.harvard.edu/cohortdocs/getting-started/collaborations-consortia?authuser=2.
Study Population
NHS and HPFS are 2 ongoing US cohorts that enrolled 121 700 registered female nurses aged 30 to 55 years in 1976 and 51 529 male health professionals aged 40 to 75 years in 1986, respectively. Similar follow‐up procedures have been applied in the 2 cohorts, with over 90% of follow‐up. 24 , 25 Briefly, follow‐up questionnaires were administered at baseline enrollment and every 2 years thereafter to collect updated lifestyle and medical information. Diet was assessed using validated food frequency questionnaires every 4 years. 26 , 27 In the current study, we defined baseline as the year of 1988 for NHS and 1986 for HPFS, when we started to assess the use of major preventive medications, including aspirin, antihypertensives, and lipid‐lowering medications. Informed consent was obtained from all participants. The study protocol was approved by the institutional review boards of the Brigham and Women's Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Among participants who returned baseline questionnaires (NHS, 1988; HPFS, 1986), we excluded participants who had a history of CVD (including myocardial infarction and stroke), cancer (except nonmelanoma skin cancer), or diabetes mellitus; reported implausible energy intakes (<500 or >3500 kcal/d for women; <800 or >4200 kcal/d for men); with a body mass index (BMI) of <18.5 kg/m2; or with missing data on lifestyle exposures or medication use. After these exclusions, 79 043 women and 39 544 men were included in the analysis (Figure S1).
Assessment of Lifestyle Factors
We included 5 lifestyle factors: BMI, cigarette smoking, physical activity, diet, and alcohol intake. Height, body weight, cigarette smoking, and physical activity were self‐reported through biennial questionnaires. Cigarette smoking was evaluated based on both pack‐years and the current status reported biennially. 28 Physical activity was assessed by the total hours per week of moderate to vigorous intensity activity (including brisk walking) that requires the expenditure of at least 3 metabolic equivalents per hour. Alcohol use and diet were self‐reported every 4 years by food frequency questionnaires. 26 , 27 Diet quality was assessed with the Alternate Healthy Eating Index (AHEI) 2010 score, 29 which was designed to target food choices and macronutrient sources associated with reduced chronic disease risk. Supplemental information of the lifestyle and covariates assessment is provided in Data S1.
For each of the 5 lifestyle factors, we defined a binary criterion, by which the participants received a score of 1 if they met the criterion and 0 otherwise. The healthy lifestyle comprised a BMI ≥18.5 and <27.5 kg/m2, never smoking, physical activity for ≥30 minutes per day of moderate to vigorous intensity activity, an AHEI score in the upper 40th percentile within each cohort, and light to moderate alcohol intake (>0 and <1 drink [14 g alcohol] per day for women and <2 drinks per day for men). An overall healthy lifestyle score (range, 0–5) was then calculated by summing the 5 scores, with a higher score indicating a healthier lifestyle.
Assessment of Medication Use
We considered aspirin, antihypertensives, and lipid‐lowering medications as common preventive medications assessed on the biennial questionnaires. In NHS, we assessed the use of standard‐dose (325 mg) aspirin since 1980, baby aspirin (81 mg) since 2000, and antihypertensives and lipid‐lowering medications since 1988. Regular aspirin use was defined as use of ≥2 tablets of standard aspirin per week. For the years when baby aspirin was separately assessed, the dose of baby aspirin was divided by 4 and combined with standard aspirin. Regular use of antihypertensives and lipid‐lowering medications was defined as use ≥2 times per week. In HPFS, all medications were assessed since 1986 and the same definitions as in NHS were used to define medication users. Detailed assessment methods are provided in the Data S1.
For each medication, participants were considered as nonusers up until the first time they reported regular use and as users thereafter for the remainder of the follow‐up. Duration of regular medication use (in years) was also calculated as a time‐varying variable by accumulating the follow‐up time during which regular use was reported.
Ascertainment of Deaths
Deaths were identified from state vital statistics records, the National Death Index, next of kin, and the postal system. Using these methods, we were able to ascertain >96% of the deaths in each cohort. 30 , 31 The primary cause of death was identified from medical records (65% in NHS and 59% in HPFS), death certificates (26% in NHS and 29% in HPFS), or next‐of‐kin or other contact person (9% in NHS and 12% in HPFS). For the current analysis, we assessed all‐cause and cause‐specific deaths caused by CVD (International Classification of Diseases, Eighth Revision [ICD‐8], codes 390–458), cancer (codes 140–207), and other causes.
Statistical Analysis
Data were analyzed from October 2, 2018, to August 22, 2019. Details about statistical analysis are provided in Data S1. Both medication use and lifestyle factors were analyzed as time‐varying variables. To capture long‐term exposures and reduce random within‐person variation, we calculated cumulative average levels of physical activity, AHEI score, and alcohol intake from our repeated questionnaires. For BMI, to minimize reverse causality resulting from weight loss caused by preexisting diseases, we used the maximum BMI reported throughout the follow‐up. 32 When data on the lifestyle factors were missing at a given questionnaire cycle, the last nonmissing observation was carried forward. We stopped updating lifestyle information when a participant reported a diagnosis of cancer (except nonmelanoma skin cancer), diabetes mellitus, stroke, coronary heart disease, or angina, because these conditions may lead to lifestyle changes. 33 In analysis of mortality attributable to other causes than CVD and cancer, we also stopped updating lifestyle information when Parkinson disease or chronic obstructive pulmonary disease was reported.
Participants contributed person‐time from return of the baseline questionnaire (1988 for NHS and 1986 for HPFS) until the date of death, loss to follow‐up, or end of the follow‐up period (June 30, 2014, for NHS and January 31, 2014, for HPFS), whichever came first. We used time varying–, age‐, period‐, and cohort‐stratified Cox proportional hazards regression models to estimate the hazard ratios (HRs) and 95% CIs for mortality associated with individual lifestyle factors and healthy lifestyle score. All analyses were performed separately according to medication use. We assessed the interaction between medication use and lifestyle using Wald test for the product term of the 2 variables. In the multivariable analyses, we performed mutual adjustment for the lifestyle factors and also adjusted for several potential confounding factors, including ethnicity; current multivitamin use; family history of diabetes mellitus, cancer, and myocardial infarction; and menopausal status and hormone use (women only).
We calculated the hypothetical PARs 34 and 95% CIs 35 to estimate the proportion of premature deaths that could have been avoided if all participants in each cohort had adhered to the healthy lifestyle practices, assuming a causal relationship between lifestyle and mortality. To calculate the PARs, we used the multivariable pooled logistic regression models to calculate the relative risk of mortality by comparing participants who met the healthy criteria for all of the 5 lifestyle factors to the remaining participants. 36
The analyses were first conducted in each cohort separately. Because no appreciable difference was detected by cohort (Table S1), we conducted pooled analysis using cohort‐stratified Cox proportional hazards regression model. As secondary analyses, we further stratified by tertiles of duration of medication use (1–4, 5–12, and ≥13 years among medication users) and by group of medications (aspirin, antihypertensives, and lipid‐lowering medications). To assess whether the influence of healthy lifestyle differs by age, we examined the associations separately among participants younger than 70 and those 70 years and older. We also stratified the analysis by the major indications for use of the medications of interest, including hypertension, hyperlipidemia, myocardial infarction, stroke, angina, and diabetes mellitus.
Results
In the 2 cohorts of 118 587 participants with a median of 26 years (2 818 177 person‐years) of follow‐up, we documented 35 195 deaths, of which 8259 were caused by CVD, 11 369 by cancer, and 15 567 by other causes. Table 1 shows the characteristics of participants according to medication use. Overall, 72% and 70% of all person‐years in NHS and HPFS, respectively, were reported as medication users. Compared with nonusers, medication users were older, more likely to have a history of hypertension and hyperlipidemia, and a lower healthy lifestyle score.
Table 1.
Age‐Standardized Characteristics* of Study Participants According to Regular Use of Common Preventive Medications † in NHS and HPFS
| NHS (n=79 043) | HPFS (n=39 544) | |||
|---|---|---|---|---|
| Medication Users | Medication Nonusers | Medication Users | Medication Nonusers | |
| Person‐y, No. (% within cohort) | 1 335 136 (72) | 529 607 (28) | 670 478 (70) | 282 956 (30) |
| Age, y | 67.4 | 59.5 | 66.2 | 58.4 |
| White, % | 98 | 97 | 95 | 93 |
| Current multivitamin use, % | 50 | 36 | 50 | 33 |
| History of hypertension, % | 58 | 18 | 48 | 14 |
| History of hyperlipidemia, % | 62 | 40 | 51 | 27 |
| Family history of diabetes mellitus, % | 29 | 25 | 21 | 18 |
| Family history of myocardial infarction, % | 26 | 20 | 34 | 27 |
| Family history of cancer, % | 14 | 14 | 36 | 33 |
| Regular use of aspirin, % | 79 | … | 86 | … |
| Regular use of antihypertensives, % | 59 | … | 47 | … |
| Regular use of lipid‐lowering medications, % | 31 | … | 29 | … |
| BMI, kg/m2 | 28.1 | 26.4 | 27.0 | 26.2 |
| Current smoker, % | 12 | 15 | 7 | 8 |
| Pack‐y of smoking‡ | 23.4 | 24.7 | 21.4 | 20.7 |
| Physical activity, min/d | 16.9 | 19.1 | 29.5 | 29.7 |
| AHEI score | 47.1 | 46.5 | 47.7 | 47.9 |
| Alcohol intake, g/d | 5.8 | 5.6 | 11.8 | 9.7 |
| Healthy lifestyle score, %§ | ||||
| 0 | 5 | 4 | 4 | 3 |
| 1 | 23 | 19 | 17 | 15 |
| 2 | 34 | 34 | 30 | 28 |
| 3 | 25 | 29 | 29 | 30 |
| 4 | 10 | 13 | 17 | 19 |
| 5 | 2 | 3 | 5 | 6 |
| Mean | 2.2 | 2.4 | 2.5 | 2.6 |
HPFS indicates Health Professionals' Follow‐up Study; and NHS, Nurses' Health Study.
Updated information throughout follow‐up was used to calculate the means for continuous variables and percentage for categorical variables. All variables are age‐standardized except age and person‐years.
The medications included aspirin, antihypertensives, and lipid‐lowering medications; regular medication use was defined as use of ≥2 tablets per week or ≥2 times per week.
Among ever‐smokers only.
Healthy lifestyle score (range, 0–5) was defined as the number of the 5 healthy lifestyle factors: healthy body weight (body mass index [BMI], ≥18.5 and <27.5 kg/m2), never smoking, light to moderate alcohol intake (>0 and <1 drink [14 g alcohol] per day for women, and >0 and <2 drink [28 g alcohol] per day for men), physically active (≥30 min/d of moderate to vigorous intensity activity), and high‐quality diet (upper 40% of Alternate Healthy Eating Index [AHEI] score).
All of the 5 individual lifestyle factors were significantly associated with all‐cause mortality, and the associations were similar between medication users and nonusers (all P interaction >0.25) (Figure 1). When the 5 factors were analyzed collectively as a healthy lifestyle score, each unit increment in the score was associated with an HR for all‐cause mortality of 0.82 (95% CI, 0.81–0.82) among medication users and 0.81 (95 CI, 0.79–0.83) among medication nonusers (P interaction=0.54) (Figure 2). Compared with individuals with a healthy lifestyle score of 0, those with a score of 5 had an HR for all‐cause mortality of 0.38 (95% CI, 0.35–0.42) and 0.33 (95 CI, 0.27–0.40) among medication users and nonusers, respectively.
Figure 1.
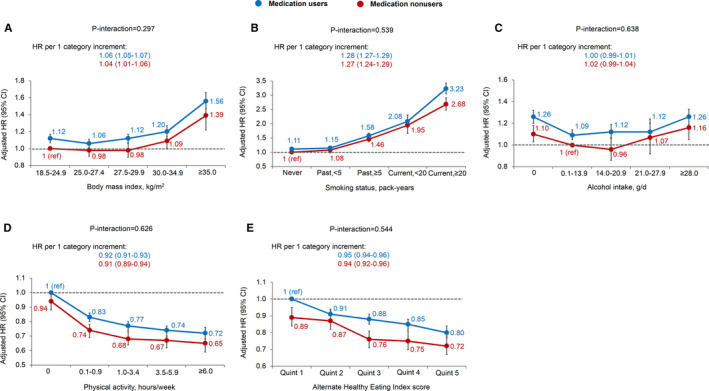
Associations of body mass index (A), smoking (B), alcohol intake (C), physical activity (D), and the Alternate Healthy Eating Index score (E) with all‐cause mortality according to regular use of common preventive medications. Common preventive medications included aspirin, antihypertensives, and lipid‐lowering medications; regular medication use was defined as use ≥2 tablets per week or ≥2 times per week. Multivariable Cox proportional hazards regression was used to calculate the hazard ratios (HRs) and 95% CIs while adjusting for age, calendar period, ethnicity, current multivitamin use, family history of diabetes mellitus, myocardial infarction, or cancer; menopausal status and hormone use (women only); and the other 4 of the 5 lifestyle factors. Error bars indicate 95% CIs.
Figure 2.
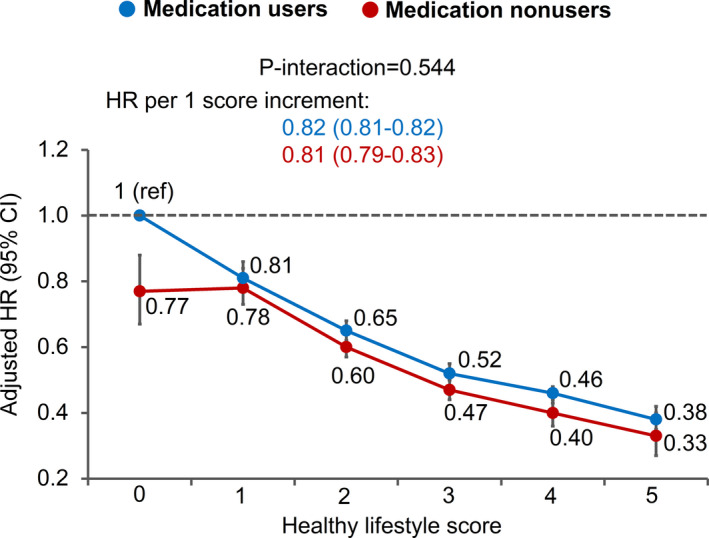
Association of healthy lifestyle score with all‐cause mortality according to regular use of common preventive medications. Healthy lifestyle score (range, 0–5) was defined as the number of the 5 healthy lifestyle factors: healthy body weight (body mass index, ≥18.5 and <27.5 kg/m2), never smoking, light to moderate alcohol intake (>0 and <1 drink [14 g alcohol] per day for women, >0 and <2 drink [28 g alcohol] per day for men), physical active (≥30 min/d of moderate to vigorous intensity activity), and high‐quality diet (upper 40% of Alternate Healthy Eating Index score). Common preventive medications included aspirin, antihypertensives, and lipid‐lowering medications; regular medication use was defined as use ≥2 tablets per week or ≥2 times per week. Multivariable Cox proportional hazards regression was used to calculate the hazard ratios (HRs) and 95% CIs while adjusting for age, calendar period, ethnicity, current multivitamin use, family history of diabetes mellitus, myocardial infarction, or cancer; and menopausal status and hormone use (women only). Error bars indicate 95% CIs.
For cause‐specific mortality, healthy lifestyle score was inversely associated with mortality of CVD, cancer, and other causes in both medication users and nonusers (Figures S2 through S4). The association for CVD mortality appeared to be stronger in medication nonusers (HR, 0.74; 95 CI, 0.69–0.78) than users (HR, 0.81; 95% CI, 0.79–0.82) (P interaction=0.01), whereas similar associations were found between the 2 groups for cancer mortality (users: HR, 0.83 [95% CI, 0.81–0.84]; nonusers: HR, 0.84 [95 CI, 0.81–0.87]; P interaction=0.49) and other mortality (users: HR, 0.82 [95% CI, 0.81–0.83]; nonusers: HR, 0.82 [95 CI, 0.78–0.85]; P interaction=0.94).
We then calculated the PAR of mortality associated with healthy lifestyle practice (Table 2). For all‐cause mortality, similar PARs were observed among medication users and nonusers for each of the lifestyle factors. The combined PAR for all of the 5 healthy lifestyle factors was 38% (95% CI, 32%–42%) among medication users and 40% (95% CI, 29%–50%) among nonusers. For CVD mortality, the PAR was relatively lower among medication users (37%; 95% CI, 27%–46%) than nonusers (45%; 95% CI, 18%–66%). No appreciable difference between the 2 groups was found in the PAR for cancer mortality (users: 38% [95% CI, 28%–46%]; nonusers: 33% [95% CI, 14%–49%]) or for other mortality (users: 33% [95% CI, 25%–41%]; nonusers: 38% [95% CI, 20%–54%]).
Table 2.
Prevalence of Individual and Combined Healthy Lifestyle Factors and Corresponding PAR Estimates for All‐Cause and Cause‐Specific Mortality According to Regular Use of Common Preventive Medications*
| Lifestyle | Medication Users | Medication Nonusers | ||
|---|---|---|---|---|
| Prevalence, % | PAR (95% CI), %† | Prevalence, % | PAR (95% CI), %† | |
| All‐cause mortality | ||||
| Never smoking | 44 | 23 (22–24) | 47 | 24 (21–27) |
| Physical activity of ≥30 min/d‡ | 24 | 11 (9–13) | 27 | 10 (5–15) |
| Diet quality of upper 40%§ | 40 | 6 (4–7) | 38 | 8 (4–12) |
| Light to moderate alcohol intake‖ | 65 | 5 (4–6) | 63 | 4 (2–6) |
| BMI <27.5 kg/m2 | 58 | 3 (2–4) | 71 | 1 (−1 to 3) |
| All of the 5 healthy lifestyle factors | 3 | 38 (32–42) | 4 | 40 (29–50) |
| CVD mortality | ||||
| Never smoking | 44 | 16 (14–19) | 47 | 24 (17–31) |
| Physical activity of ≥30 min/d‡ | 24 | 13 (8–17) | 27 | 5 (−7 to 17) |
| Diet quality of upper 40%§ | 40 | 1 (−2 to 4) | 38 | 13 (4–22) |
| Light to moderate alcohol intake‖ | 65 | 8 (6–9) | 63 | 4 (−2 to 10) |
| BMI <27.5 kg/m2 | 58 | 7 (5–9) | 71 | 12 (7–17) |
| All of the 5 healthy lifestyle factors | 3 | 37 (27–46) | 4 | 45 (18–66) |
| Cancer mortality | ||||
| Never smoking | 44 | 29 (27–31) | 47 | 26 (21–30) |
| Physical activity of ≥30 min/d‡ | 24 | 5 (1–9) | 27 | 6 (−2 to 13) |
| Diet quality of upper 40%§ | 40 | 4 (2–7) | 38 | 5 (−1 to 10) |
| Light to moderate alcohol intake‖ | 65 | 4 (2–5) | 63 | 0 (−5 to 5) |
| BMI <27.5 kg/m2 | 58 | 2 (0–4) | 71 | 1 (−2 to 4) |
| All of the 5 healthy lifestyle factors | 3 | 38 (28–46) | 4 | 33 (14–49) |
| Other mortality | ||||
| Never smoking | 44 | 20 (18–22) | 47 | 19 (14–24) |
| Physical activity of ≥30 min/d‡ | 24 | 10 (6–13) | 27 | 14 (7–22) |
| Diet quality of upper 40%§ | 40 | 8 (6–10) | 38 | 8 (2–13) |
| Light to moderate alcohol intake‖ | 65 | 5 (4–7) | 63 | 8 (5–12) |
| BMI <27.5 kg/m2 | 58 | 1 (0–3) | 71 | −8 (−14 to −1) |
| All of the 5 healthy lifestyle factors | 3 | 33 (25–41) | 4 | 38 (20–54) |
BMI indicates body mass index; and CVD, cardiovascular disease.
The medications included aspirin, antihypertensives, and lipid‐lowering medications; regular medication use was defined as use ≥2 tablets per week or ≥2 times per week.
Population‐attributable risks (PARs) and 95% CIs were calculated while adjusting for age, calendar period, ethnicity, current multivitamin use, family history of diabetes mellitus, myocardial infarction, or cancer; menopausal status and hormone use (women only); and the other 4 of the 5 lifestyle factors (except “all of the 5 healthy lifestyle factors”).
Physical activity was of moderate to vigorous intensity requiring the expenditure of ≥3 metabolic equivalents per hour.
Diet quality was based on the Alternate Healthy Eating Index score.
Light to moderate alcohol intake was defined as >0 and <1 drink (14 g alcohol) per day for women, and >0 and <2 drink (28 g alcohol) per day for men.
We also assessed the PAR among medication users by duration of use and medication groups (Figure 3). A lower PAR was observed among medication users with a duration of ≥13 years than those with 1 to 4 and 5 to 12 years for all‐cause mortality (31% versus 36% and 43%), CVD mortality (23% versus 43% and 46%), and other mortality (26% versus 40% and 34%). No substantial difference in the PAR was found among users of aspirin, antihypertensives, and lipid‐lowering medications, with the estimates ranging from 30% to 38% for all‐cause mortality, 24% to 38% for CVD mortality, 36% to 40% for cancer mortality, and 28% to 33% for other mortality. When stratified by age, the PAR was higher among medication users younger than 70 years (51%; 95% CI, 40%–61%) than users 70 years and older (35%; 95% CI, 29%–40%) (Table S2). When stratified by the common indications for use of these medications, we observed similar PARs among medication users who did (36%; 95% CI, 30%–42%) and did not (31%; 95% CI, 18%–43%) have the preexisting conditions (Table S3).
Figure 3.
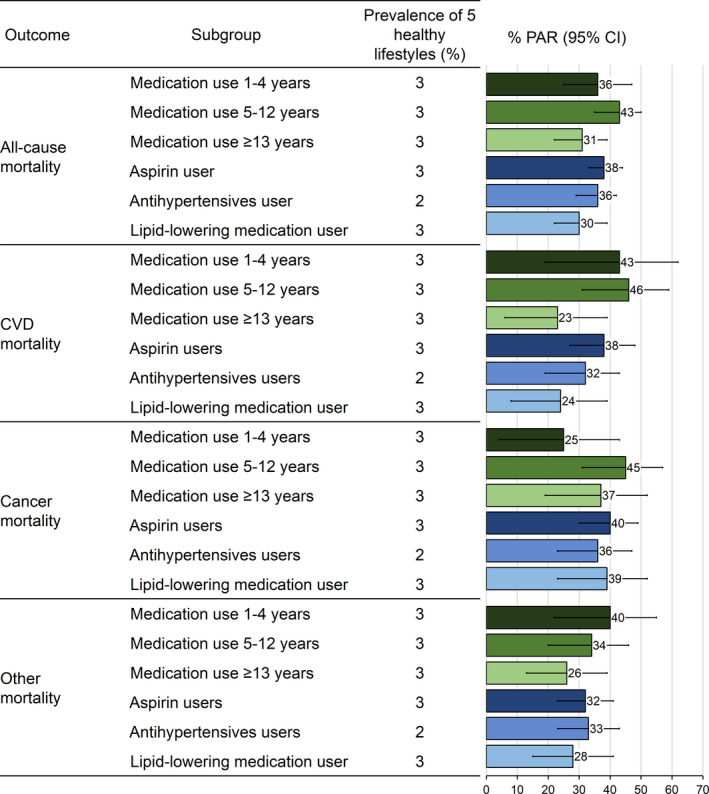
Prevalence and population‐attributable risk (PAR) (95% CI) estimates of the 5 healthy lifestyle factors for all‐cause and cause‐specific mortality among medication users by use duration and medication type. The 5 healthy lifestyles included healthy body weight (body mass index, ≥18.5 and <27.5 kg/m2), never smoking, light to moderate alcohol intake (>0 and <1 drink [14 g alcohol] per day for women, >0 and <2 drink [28 g alcohol] per day for men), physical active (≥30 min/d of moderate to vigorous intensity activity), and high‐quality diet (upper 40% of Alternate Healthy Eating Index score). Regular medication use was defined as use ≥2 tablets per week or ≥2 times per week. PARs and 95% CIs were calculated while adjusting for age, calendar period, ethnicity, current multivitamin use, family history of diabetes mellitus, myocardial infarction, or cancer; and menopausal status and hormone use (women only). Error bars indicate 95% CIs. CVD indicates cardiovascular disease.
Discussion
In 2 large prospective cohorts, we found that ≈40% of total premature deaths in both medication users and nonusers can potentially be prevented by adoption of a healthy lifestyle characterized by never smoking, being physical active, eating a high‐quality diet, consuming light to moderate alcohol, and maintaining a BMI ≥18.5 and <27.5 kg/m2. Similar estimates according to medication use were found for cancer mortality, whereas the estimate for CVD mortality tended to be higher in medication nonusers (45%) than users (37%). These findings indicate that healthy lifestyle may confer substantial benefit in preventing premature death, regardless of preventive medication use. Adherence to a healthy lifestyle remains important even among individuals regularly using preventive medications.
Common preventive medications, including aspirin, antihypertensives, and lipid‐lowering medications, have established benefit mainly in reducing recurrent CVD 6 , 7 , 8 and certain cancers (eg, aspirin for colorectal cancer 13 ). However, a considerable increase in use of these medications for primary prevention of CVD has been documented. 2 , 4 , 37 A recent study showed that a polypill containing anticoagulant aspirin, a cholesterol‐lowering statin, and 2 antihypertensives reduced the risk of major cardiovascular events. 38 According to the 2017 National Health Interview Survey, 29 million people 40 years and older were taking an aspirin a day despite having no known CVD. 37 On the other hand, given the perceived challenges in lifestyle modification, 39 individuals who regularly take preventive medication may consider being certified healthy and thus have less incentive to adopt or sustain healthy lifestyle practices (health certificate effect), posing challenges for implementation of primary prevention among presumably high‐risk populations. 23 , 40 Moreover, there is little evidence regarding whether medication use modifies the health benefit of healthy lifestyle practices. Stamatakis et al 40 reported that physical activity protected against premature death to a similar extent among medication users and nonusers. Chiuve et al 41 found that a substantial fraction of incident CVD is potentially preventable in both users and nonusers of lipid‐lowering and antihypertensive medications. However, because the benefit of these medications is predominantly established for CVD and largely unclear for cancer and other diseases, it remains an open question how overall health is influenced by healthy lifestyles among users and nonusers of medications.
The 5 factors examined in the current study are among the most prevalent lifestyle factors that have been shown to confer a substantial benefit in reducing mortality. Smoking has been the primary cause for nearly 80% of deaths from CVD and chronic obstruction pulmonary disease and at least 30% of cancer deaths. 42 Heavy alcohol consumption has been significantly associated with increased risk of all‐cause death by 11% and cancer death by 27%. 43 Also, risk of all‐cause mortality has been increased by 20% to 40% among overweight people and by 2 to 3 times among those with obesity. 44 In contrast, physical activity and diet quality in accordance with recommendations has a considerable benefit in reducing mortality of all causes, CVD, and cancer. 45 , 46 The potential pathways through which healthy lifestyle reduces mortality risk include reduced chronic inflammation, 20 enhanced artery endothelial function, 21 and improved lipid profiles and insulin sensitivity. 20 , 22 Although the benefit of a healthy lifestyle among high‐risk or diseased individuals has been reported, 47 , 48 , 49 , 50 it remains to be determined whether a healthy lifestyle confers a similar benefit among regular users and nonusers of preventive medications. In the current study, we found a similar association between a healthy lifestyle and mortality. Our findings reinforce the indispensable role of lifestyle modification in preventing premature death, irrespective of medication use.
Our findings have important clinical and public health implications. First, although pharmacological therapy and a healthy lifestyle share certain mechanisms, among individuals regularly using preventive medications, ≈40% of premature death cases are still potentially preventable through healthy lifestyle, and this benefit appeared not to vary by preexisting indications of the medication use. Our finding provides supporting evidence for combining lifestyle modification (“lifestyle therapy” 51 ) and pharmacological therapy to conquer the increasing prevalence of chronic diseases. Second, we observed a different association of healthy lifestyle between medication users and nonusers only with CVD mortality, but not with cancer mortality or other mortality. This finding is in line with the primary benefit of these medications against CVD and suggests that lifestyle factors may reduce mortality attributable to causes other than CVD through mechanisms independent of those by the preventive medications.
Study Strengths and Limitations
Our study has several strengths, including the large sample size, long‐term follow‐up of up to 28 years, detailed and repeated assessment of medication use, and rigorous adjustment for potential confounding factors. Several limitations should also be noted. First, we applied a less stringent threshold to define healthy lifestyle (eg, the upper limit for BMI was set at 27.5 rather than 25, as commonly used to define normal body weight) to preserve statistical power because even with the current threshold, only 3% to 4% of participants met the healthy criteria for all of the 5 lifestyle factors. Therefore, the PARs for healthy lifestyles may have been underestimated. Second, our medication, lifestyle, and anthropometric (eg, height and weight) data were all based on self‐report and thus subject to measurement error. However, the accuracy and stability of these self‐reported data have been well documented in our cohorts, 52 , 53 , 54 as well as other study populations. 55 Third, our study participants were all health professionals and predominantly white, thereby limiting our ability to stratify the analyses by race and reducing the generalizability of our findings. However, it is unlikely that the biological effect of lifestyle would have a substantial difference between health professionals and the general population. Moreover, given the higher health consciousness and better lifestyle profiles of health professionals, we may have underestimated the benefit of lifestyles on the population scale.
Conclusions
A healthy lifestyle may confer similar substantial benefit in preventing premature death in regular users and nonusers of preventive medications. Adherence to a healthy lifestyle is still important even among individuals regularly using preventive medications.
Sources of Funding
This work was supported by the American Cancer Society Mentored Research Scholar Grant (MRSG‐17‐220‐01 ‐ NEC to Song); by the US National Institutes of Health grants (P01 CA87969 to M. J. Stampfer; U01 CA186107 to M. J. Stampfer; P01 CA55075, to W. C. Willett; UM1 CA167552 to W. C. Willett; K24 DK098311, R01 CA137178, R01 CA202704, R01 CA176726, to Chan; K99 CA215314 and R00 CA215314 to Song). Dr Chan is a Stuart and Suzanne Steele MGH Research Scholar. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article.
Disclosures
Chan previously served as a consultant for Bayer Healthcare and Pfizer Inc. for work unrelated to the topic of this article. This study was not funded by Bayer Healthcare or Pfizer Inc. The remaining authors have no disclosures to report.
Supporting information
Acknowledgments
The authors thank the participants and staff of NHS and HPFS for their continued contributions, as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming. The authors assume full responsibility for analyses and interpretation of these data.
(J Am Heart Assoc. 2020;9:e016692 DOI: 10.1161/JAHA.119.016692
For Sources of Funding and Disclosures, see page 9.
References
- 1. Murphy SL, Xu J, Kochanek KD, Arias E. Mortality in the United States, 2017. NCHS Data Brief. 2018;1–8. [PubMed] [Google Scholar]
- 2. Williams CD, Chan AT, Elman MR, Kristensen AH, Miser WF, Pignone MP, Stafford RS, McGregor JC. Aspirin use among adults in the U.S.: results of a national survey. Am J Prev Med. 2015;501–508. [DOI] [PubMed] [Google Scholar]
- 3. Fang J, Gillespie C, Ayala C, Loustalot F. Prevalence of self‐reported hypertension and antihypertensive medication use among adults aged >/=18 years—United States, 2011–2015. MMWR Morb Mortal Wkly Rep. 2018;219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gu Q, Paulose‐Ram R, Burt VL, Kit BK. Prescription cholesterol‐lowering medication use in adults aged 40 and over: United States, 2003–2012. NCHS Data Brief. 2014;1–8. [PubMed] [Google Scholar]
- 5. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;1818–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tramacere I, Boncoraglio GB, Banzi R, Del Giovane C, Kwag KH, Squizzato A, Moja L. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: a systematic review and network meta‐analysis. BMC Med. 2019;67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rothwell PM, Algra A, Chen Z, Diener HC, Norrving B, Mehta Z. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time‐course analysis of randomised trials. Lancet. 2016;365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson AM, Hu T, Eshelbrenner CL, Reynolds K, He J, Bazzano LA. Antihypertensive treatment and secondary prevention of cardiovascular disease events among persons without hypertension: a meta‐analysis. JAMA. 2011;913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, Howard G, Pearson TA, Rothwell PM, Ruilope LM, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2018;1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hippisley‐Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fretheim A, Odgaard‐Jensen J, Brors O, Madsen S, Njolstad I, Norheim OF, Svilaas A, Kristiansen IS, Thurmer H, Flottorp S. Comparative effectiveness of antihypertensive medication for primary prevention of cardiovascular disease: systematic review and multiple treatments meta‐analysis. BMC Med. 2012;33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsao AS, Kim ES, Hong WK. Chemoprevention of cancer. CA Cancer J Clin. 2004;150–180. [DOI] [PubMed] [Google Scholar]
- 13. Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long‐term effect of aspirin on colorectal cancer incidence and mortality: 20‐year follow‐up of five randomised trials. Lancet. 2010;1741–1750. [DOI] [PubMed] [Google Scholar]
- 14. Cao Y, Nishihara R, Wu K, Wang M, Ogino S, Willett WC, Spiegelman D, Fuchs CS, Giovannucci EL, Chan AT. Population‐wide impact of long‐term use of aspirin and the risk for cancer. JAMA Oncol. 2016;762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bowles EJA, Yu O, Ziebell R, Chen L, Boudreau DM, Ritzwoller DP, Hubbard RA, Boggs JM, Burnett‐Hartman AN, Sterrett A, et al. Cardiovascular medication use and risks of colon cancer recurrences and additional cancer events: a cohort study. BMC Cancer. 2019;270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta‐analysis. JAMA. 2006;74–80. [DOI] [PubMed] [Google Scholar]
- 17. Colpani V, Baena CP, Jaspers L, van Dijk GM, Farajzadegan Z, Dhana K, Tielemans MJ, Voortman T, Freak‐Poli R, Veloso GGV, et al. Lifestyle factors, cardiovascular disease and all‐cause mortality in middle‐aged and elderly women: a systematic review and meta‐analysis. Eur J Epidemiol. 2018;831–845. [DOI] [PubMed] [Google Scholar]
- 18. Song M, Giovannucci E. Preventable incidence and mortality of carcinoma associated with lifestyle factors among white adults in the United States. JAMA Oncol. 2016;1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Pan A, Wang DD, Liu X, Dhana K, Franco OH, Kaptoge S, Di Angelantonio E, Stampfer M, Willett WC, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. 2018;345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;2169–2180. [DOI] [PubMed] [Google Scholar]
- 21. Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;454–460. [DOI] [PubMed] [Google Scholar]
- 22. Aucott L, Gray D, Rothnie H, Thapa M, Waweru C. Effects of lifestyle interventions and long‐term weight loss on lipid outcomes—a systematic review. Obes Rev. 2011;e412–e425. [DOI] [PubMed] [Google Scholar]
- 23. Deutekom M, Vansenne F, McCaffery K, Essink‐Bot ML, Stronks K, Bossuyt PM. The effects of screening on health behaviour: a summary of the results of randomized controlled trials. J Public Health (Oxf). 2011;71–79. [DOI] [PubMed] [Google Scholar]
- 24. Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20‐year contribution to the understanding of health among women. J Womens Health. 1997;49–62. [DOI] [PubMed] [Google Scholar]
- 25. Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;464–468. [DOI] [PubMed] [Google Scholar]
- 26. van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;51–65. [DOI] [PubMed] [Google Scholar]
- 28. Sarna L, Bialous SA, Cooley ME, Jun HJ, Feskanich D. Impact of smoking and smoking cessation on health‐related quality of life in women in the Nurses' Health Study. Qual Life Res. 2008;1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, Hennekens CH. Test of the national death index. Am J Epidemiol. 1984;837–839. [DOI] [PubMed] [Google Scholar]
- 31. Rich‐Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;1016–1019. [DOI] [PubMed] [Google Scholar]
- 32. Yu E, Ley SH, Manson JE, Willett W, Satija A, Hu FB, Stokes A. Weight history and all‐cause and cause‐specific mortality in three prospective cohort studies. Ann Intern Med. 2017;613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, Giovannucci EL. Association of animal and plant protein intake with all‐cause and cause‐specific mortality. JAMA Intern Med. 2016;1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case‐control data. Am J Epidemiol. 1985;904–914. [DOI] [PubMed] [Google Scholar]
- 35. Platz EA, Willett WC, Colditz GA, Rimm EB, Spiegelman D, Giovannucci E. Proportion of colon cancer risk that might be preventable in a cohort of middle‐aged US men. Cancer Causes Control. 2000;579–588. [DOI] [PubMed] [Google Scholar]
- 36. Wacholder S, Benichou J, Heineman EF, Hartge P, Hoover RN. Attributable risk: advantages of a broad definition of exposure. Am J Epidemiol. 1994;303–309. [DOI] [PubMed] [Google Scholar]
- 37. O'Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: results from the 2017 National Health Interview Survey. Ann Intern Med. 2019;596–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roshandel G, Khoshnia M, Poustchi H, Hemming K, Kamangar F, Gharavi A, Ostovaneh M, Nateghi A, Majed M, Navabakhsh B. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases: a pragmatic cluster randomized controlled trial. Lancet. 2019;672–683. [DOI] [PubMed] [Google Scholar]
- 39. Tunstall‐Pedoe H. The decline in coronary heart disease; did it fall or was it pushed? BMJ. 2012;d7809. [DOI] [PubMed] [Google Scholar]
- 40. Stamatakis E, Hamer M, Primatesta P. Cardiovascular medication, physical activity and mortality: cross‐sectional population study with ongoing mortality follow‐up. Heart. 2009;448–453. [DOI] [PubMed] [Google Scholar]
- 41. Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid‐lowering and antihypertensive medications. Circulation. 2006;160–167. [DOI] [PubMed] [Google Scholar]
- 42. Smoking‐attributable mortality, years of potential life lost, and productivity losses–United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;1226–1228. [PubMed] [Google Scholar]
- 43. Xi B, Veeranki SP, Zhao M, Ma C, Yan Y, Mi J. Relationship of alcohol consumption to all‐cause, cardiovascular, and cancer‐related mortality in U.S. adults. J Am Coll Cardiol. 2017;913–922. [DOI] [PubMed] [Google Scholar]
- 44. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard‐Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;763–778. [DOI] [PubMed] [Google Scholar]
- 45. Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, Campbell PT, Freedman M, Weiderpass E, Adami HO, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose‐response relationship. JAMA Intern Med. 2015;959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sotos‐Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, Willett WC, Rimm EB, Hu FB. Association of changes in diet quality with total and cause‐specific mortality. N Engl J Med. 2017;143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fang J, Wylie‐Rosett J, Alderman MH. Exercise and cardiovascular outcomes by hypertensive status: NHANES I epidemiological follow‐up study, 1971–1992. Am J Hypertens. 2005;751–758. [DOI] [PubMed] [Google Scholar]
- 48. Parikh A, Lipsitz SR, Natarajan S. Association between a dash‐like diet and mortality in adults with hypertension: findings from a population‐based follow‐up study. Am J Hypertens. 2009;409–416. [DOI] [PubMed] [Google Scholar]
- 49. Tuomilehto J, Schwarz P, Lindstrom J. Long‐term benefits from lifestyle interventions for type 2 diabetes prevention: time to expand the efforts. Diabetes Care. 2011;e003192 DOI: 10.1161/JAHA.116.003192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rebholz CM, Anderson CA, Grams ME, Bazzano LA, Crews DC, Chang AR, Coresh J, Appel LJ. Relationship of the American Heart Association's Impact Goals (Life's Simple 7) with risk of chronic kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) Cohort Study. J Am Heart Assoc. 2016;5:e003192 DOI: 10.1161/JAHA.116.003192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lianov L, Johnson M. Physician competencies for prescribing lifestyle medicine. JAMA. 2010;202–203. [DOI] [PubMed] [Google Scholar]
- 52. Rimm EB, Stampfer MJ, Colditz GA, Chute CG,Litin LB, Willett WC. Validity of self‐reported waist and hip circumferences in men and women. Epidemiology. 1990;466–473. [DOI] [PubMed] [Google Scholar]
- 53. Chasan‐Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self‐administered physical activity questionnaire for male health professionals. Epidemiology. 1996;81–86. [DOI] [PubMed] [Google Scholar]
- 54. Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;570–572. [PubMed] [Google Scholar]
- 55. Pitkala KH, Strandberg TE, Tilvis RS. Interest in healthy lifestyle and adherence to medications: impact on mortality among elderly cardiovascular patients in the DEBATE Study. Patient Educ Couns. 2007;44–49. [DOI] [PubMed] [Google Scholar]
- 56. Hu FB, Sigal RJ, Rich‐Edwards JW, Colditz GA, Solomon CG, Willett WC, Speizer FE, Manson JE. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;1433–1439. [DOI] [PubMed] [Google Scholar]
- 57. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;531–540. [DOI] [PubMed] [Google Scholar]
- 58. Giovannucci E, Stampfer MJ, Colditz GA, Manson JE, Rosner BA, Longnecker MP, Speizer FE, Willett WC. Recall and selection bias in reporting past alcohol consumption among breast cancer cases. Cancer Causes Control. 1993;441–448. [DOI] [PubMed] [Google Scholar]
- 59. Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self‐administered questionnaire. Am J Epidemiol. 1991;810–817. [DOI] [PubMed] [Google Scholar]
- 60. Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age‐related macular degeneration in women. JAMA. 1996;1141–1146. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


