Abstract
Background
The widespread nature of coronavirus disease 2019 (COVID-19) has been unprecedented. We sought to analyze its global impact with a survey on colorectal cancer care during the pandemic.
Methods
The impact of coronavirus disease 2019 on preoperative assessment, elective surgery, and postoperative management of colorectal cancer patients was explored by a 35-item survey, which was distributed worldwide to members of surgical societies with an interest in colorectal cancer care. Respondents were divided into 2 comparator groups: (1) “delay” group: colorectal cancer care affected by the pandemic and (2) “no delay” group: unaltered colorectal cancer practice.
Results
A total of 1,051 respondents from 84 countries completed the survey. No substantial differences in demographics were found between the delay (745, 70.9%) and no delay (306, 29.1%) groups. Suspension of multidisciplinary team meetings, staff members quarantined or relocated to coronavirus disease 2019 units, units fully dedicated to coronavirus disease 2019 care, and personal protective equipment not readily available were factors significantly associated to delays in endoscopy, radiology, surgery, histopathology, and prolonged chemoradiation therapy-to-surgery intervals. In the delay group, 48.9% of respondents reported a change in the initial surgical plan, and 26.3% reported a shift from elective to urgent operations. Recovery of colorectal cancer care was associated with the status of the outbreak. Practicing in coronavirus disease-free units, no change in operative slots and staff members not relocated to coronavirus disease 2019 units were statistically associated with unaltered colorectal cancer care in the no delay group, while the geographic distribution was not.
Conclusion
Global changes in diagnostic and therapeutic colorectal cancer practices were evident. Changes were associated with differences in health care delivery systems, hospital’s preparedness, resource availability, and local coronavirus disease 2019 prevalence rather than geographic factors. Strategic planning is required to optimize colorectal cancer care.
Introduction
The widespread nature and impact of the coronavirus disease 2019 (COVID-19) pandemic has been unprecedented.1 The global transmission of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been rapid because of the high infectivity and a relatively high rate of asymptomatic carriers in a highly mobile and interconnected world.2 As of August 20, 2020, the World Health Organization confirmed 22,256,220 cases of COVID-19 globally, including 782,456 deaths.3
A lack of preparedness and a lack of appreciation of the gravity of the pandemic have led to significant strains on health care systems around the world. In the first half of 2020, most nations’ health care resources were overwhelmed by COVID-19, and many hospitals essentially became coronavirus accepting hospitals during the emergency phase.4 , 5 The impact of COVID-19 on global oncological care has been profound.6, 7, 8 COVIDSurg Collaborative estimated that 28,404,603 elective operations were cancelled or postponed worldwide during the 12 weeks of peak disruption, with 38% being for cancer.4 Colorectal cancer (CRC) is the third leading cause of cancer related deaths globally. The pandemic has led to major disruptions and delays in CRC practice, which may adversely affect survival outcomes for several years to come.8
The primary aim of our survey was to analyze the global impact of the COVID-19 outbreak on both diagnosis and treatment of CRC. The secondary aim was to explore which factors were associated with changes in CRC care or with unaffected practice.
Methods
Surgical divisions treating CRC across the world were eligible to participate, including those in countries that did not have COVID-19 outbreaks during the study period. Only 1 collaborator per surgical division was eligible to take part, although multiple divisions from the same hospital could participate in the survey. To obtain a representative sample of participants, national and international surgical societies with an interest in CRC care from 6 geographic regions were asked to endorse the study and disseminate the survey by e-mail to their members. The societies had no role in study design, data collection, analysis, and interpretation, or in the writing of the report. To overcome the temporal bias of distribution, the link to the online survey was made available for 3 weeks, from May 20 to June 10, 2020. A newsletter with a reminder was sent every week. Informed consent was obtained by voluntary participation, and no compensation was offered. The study was registered at ClinicalTrials.gov (NCT 04488549).
Survey
A 35-item survey on DElayed COloRectal cancer care during COVID-19 pandemic (DECOR-19) (Appendix 2) was designed by the steering committees formed by the principal investigators. Meetings were conducted via teleconference to define the appropriateness, feasibility, and preliminary validity of the questions to include. Further validation of the survey was achieved by pilot testing on 10 surgery residents to ensure adequate sentence construction and correct interpretation of the questions. We elected not to delay the survey process by performing a formal full validation to glean insights from the results in an expeditious manner in this critical period.
(1) The platform “Online surveys” (formerly BOS – Bristol Online Survey), developed by the University of Bristol in accordance with the Consolidated Criteria for Reporting Qualitative Research and the Checklist for Reporting Results of Internet E-Surveys9 (Appendix 3), was used. Proprietary survey software and local servers were used to ensure data protection. The fully deidentified dataset was kept on password protected computers. Responses were single or multiple choice, numeric, and open text. All questions were set as mandatory fields with real-time validation and automated skip logic to prevent missing data and avoid illogical or incompatible responses. No randomization of items was used. Quantitative data were automatically collected by the software and exported to a tabulated format. Estimated mean time to complete the survey was 10 to 15 minutes. The survey was structured in the following 4 sections: Demographics and personal practice (Q.1–Q.13): including the respondent’s gender, country, hospital level, total number of hospital beds, specialty-specific (Q.8: general surgery/colorectal surgery) division, annual volume of CRC surgery and laparoscopic CRC resections in the division, and average long-course chemoradiation therapy (CRT)-to-surgery interval for rectal cancer.
After demographics, respondents were asked if they experienced any delay in CRC care (Q.14). There were 2 comparator groups: (1) “no delay”: respondents were redirected to a single question (Q.35) investigating the reasons of unaffected practice and (2) “delay”: respondents continued the survey with the following sections:
(2) Hospital response to COVID-19 emergency (Q.15–Q.22): to capture the current status of CRC care, exploring the overall changes in term of resource allocation. The section included hospital’s preparedness to COVID-19, ready availability of external facilities for CRC surgery, presence of cancer care coordinator, personal protective equipment (PPE) availability, status of elective CRC surgery, elective CRC patients needing urgent surgery, CRC patients status, and staff members status.
(3) Delay in CRC care (Q.23–Q.33): investigating any delays across the various fields of practice (ie, endoscopy, radiology, surgery, radiotherapy, oncology, histopathology, multidisciplinary team [MDT] meetings) and the relative reasons, any change in the original management plan and types of complication determining a shift from elective to emergency surgery.
(4) Recovery of CRC care (Q.34): assessing the recovery of CRC practice at the date of the survey completion (fully recovered, improved, persistently limited).
Statistical analysis
Continuous variables were summarized by means and standard deviations and categorical variables by proportions. Comparisons of categorical variables across groups were made by Pearson’s χ2 tests. A series of hierarchical binary and ordinal logistic regression models were performed to assess the association between respondents’ preferences and their characteristics, with geographical area as a random effect. The Brant test was performed to assess the proportional odds assumption in the ordinal logistic model. Uni and multivariable hierarchical logistic models were fitted to explore the association between delay and a predefined set of covariates (demographics, hospital characteristics, and respondents’ personal practice in CRC care). To assess the factors associated with the recovery of practice, first, the time interval in days between the date of achievement of the 100th COVID-19 positive case in the respondent’s country and the date of recovery (fully recovered or improved) or the date of persistently limited CRC care was calculated and then was fitted a 0-inflated negative binomial regression.10 Adjustments to the P values for multiple testing were not performed, and statistical significance was assessed using alpha = 0.05. All analyses were performed using Stata 16 (StataCorp LLC, College Station, TX).
Results
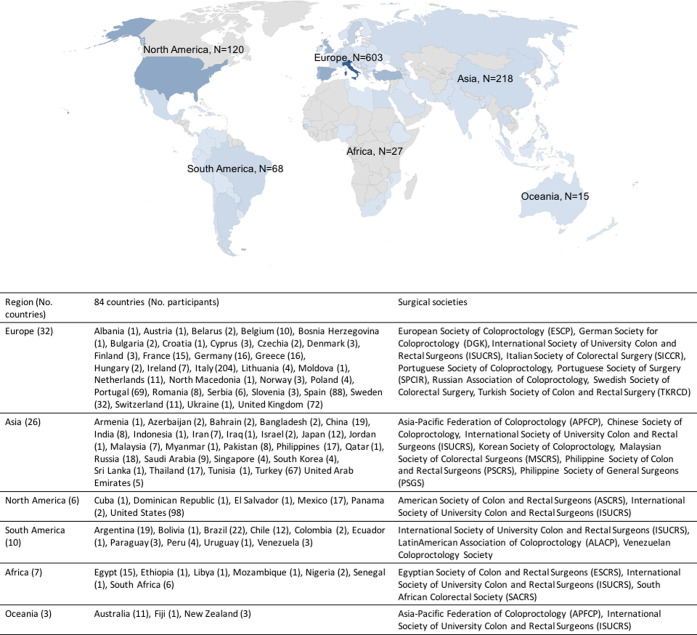
Twenty national and international surgical societies from 6 geographic regions endorsed the study and disseminated the survey to their members in the time frame (May 20–June 10, 2020) (Fig 1 ).
Fig 1.
Geographic distribution with country of origin of respondents (N = 1,051).
Demographics and personal practice
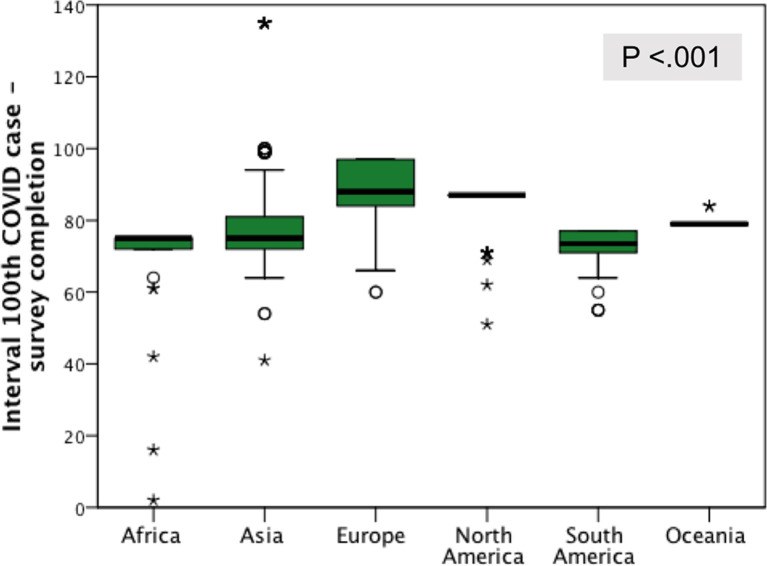
A total of 1,051 respondents, representing 1,051 colorectal or surgical divisions, from 84 countries (Fig 1) completed the survey and were included in the final analysis: Europe 603 respondents (57.4%), Asia 218 (20.7%), North America 120 (11.4%), South America 68 (6.5%), Africa 27 (2.6%), and Oceania 15 (1.4%). The mean interval between the achievement of the 100th COVID-19 case in each respondent’s country and the date of survey completion was higher for respondents from North America (70 days) and Europe (64 days) (Fig 2 ). Mean time spent to complete the survey was 10.8 (standard deviation, 3.8) minutes.
Fig 2.
Geographic distribution of respondents by the interval (d) between the date of achievement of the 100th COVID-19 case in their own country and the date of survey completion.
Respondents were mostly men (78.7%), practicing in general surgery divisions (76.3%) and academic hospitals (61.1%) with mid to high bed volume (>250 beds, 89.2%) (Table I ). A large majority of divisions (78.8%) performed >50 colon cancer surgeries per year, with 31.5% reporting this case volume for rectal cancer. Thirty-five percent of respondents reported regular use of laparoscopy in >75% of cases in CRC surgery (Table I). Most respondents (70.7%) indicated 8 to 12 weeks as the optimal long-course CRT-to-surgery interval in rectal cancer. Demographics and personal practice were consistent across the geographical regions, and the only difference in this proportional distribution was found in the annual number of surgeries for rectal cancer, which were more frequent in Asia (Table I).
Table I.
Demographics across 6 geographical regions (N = 1,051 global respondents)
|
N = 1,051 (100%) |
Asia 218 (20.7) |
Europe 603 (57.4) |
N America 120 (11.4) |
S America 68 (6.5) |
Africa 27 (2.6) |
Oceania 15 (1.4) |
|
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Males | 827 (78.7) | 183 (83.9) | 459 (76.1) | 93 (77.5) | 56 (82.4) | 24 (88.9) | 12 (80.0) |
| Females | 224 (21.3) | 35 (16.1) | 144 (23.9) | 27 (22.5) | 12 (17.6) | 3 (11.1) | 3 (20.0) |
| Type of hospital | |||||||
| Academic | 642 (61.1) | 165 (75.7) | 337 (55.9) | 57 (47.5) | 52 (76.5) | 23 (85.2) | 8 (53.3) |
| Nonacademic teaching | 312 (29.7) | 34 (15.6) | 213 (35.3) | 46 (38.3) | 14 (20.6) | 0.0 (0) | 5 (33.4) |
| Nonteaching | 97 (9.2) | 19 (8.7) | 53 (8.8) | 17 (14.2) | 2 (2.9) | 4 (14.8) | 2 (13.3) |
| Number of beds | |||||||
| ≤250 | 113 (10.8) | 19 (8.7) | 55 (9.5) | 17 (14.2) | 12 (17.6) | 8 (29.6) | 2 (13.3) |
| 251–750 | 535 (50.9) | 80 (36.7) | 322 (53.3) | 74 (61.7) | 47 (69.2) | 4 (14.8) | 8 (53.3) |
| 751–1,250 | 242 (23.0) | 66 (30.3) | 136 (21.8) | 22 (18.3) | 6 (8.8) | 8 (29.6) | 4 (26.7) |
| >1,250 | 161 (15.3) | 53 (24.3) | 90 (15.4) | 7 (5.8) | 3 (4.4) | 7 (26.0) | 1 (6.7) |
| Type of division | |||||||
| Colorectal | 248 (23.7) | 60 (27.5) | 111 (18.4) | 42 (35.0) | 19 (27.9) | 11 (40.7) | 5 (33.4) |
| General surgery | 803 (76.3) | 158 (72.5) | 492 (81.6) | 78 (65.0) | 49 (72.1) | 16 (59.3) | 10 (66.6) |
| Annual number of colon cancer surgery | |||||||
| ≤50 | 223 (21.2) | 52 (23.8) | 111 (18.4) | 26 (21.7) | 19 (27.9) | 13 (48.2) | 2 (13.3) |
| 51–150 | 516 (49.1) | 81 (37.2) | 334 (55.4) | 51 (42.5) | 34 (50.0) | 9 (33.3) | 7 (46.7) |
| >150 | 312 (29.7) | 85 (39.0) | 158 (26.2) | 43 (35.8) | 15 (22.1) | 5 (18.5) | 6 (40.0) |
| Laparoscopy (%) | |||||||
| <25 | 221 (21.0) | 78 (35.8) | 89 (14.8) | 18 (15.0) | 22 (32.4) | 14 (51.9) | 0 (0) |
| 25–50 | 172 (16.4) | 45 (20.6) | 86 (14.3) | 17 (14.2) | 17 (25.0) | 4 (14.8) | 3 (20.0) |
| 50–75 | 286 (27.2) | 37 (17.0) | 194 (32.2) | 32 (26.6) | 7 (10.2) | 7 (25.9) | 9 (60.0) |
| >75 | 372 (35.4) | 58 (26.6) | 234 (38.8) | 53 (44.2) | 22 (32.4) | 2 (7.4) | 3 (20.0) |
| Annual number of rectal cancer surgery | |||||||
| ≤50 | 720 (68.5) | 106 (48.6) | 455 (75.5) | 75 (62.5) | 51 (75.0) | 23 (85.2) | 10 (66.6) |
| 51–150 | 252 (24.0) | 73 (33.5) | 125 (20.7) | 36 (30.0) | 12 (17.6) | 2 (7.4) | 4 (26.7) |
| >150 | 79 (7.5) | 39 (17.9) | 23 (3.8) | 9 (7.5) | 5 (7.4) | 2 (7.4) | 1 (6.7) |
| Laparoscopy (%) | |||||||
| <25 | 277 (26.4) | 81 (37.2) | 123 (20.4) | 21 (17.5) | 32 (47.1) | 15 (57.6) | 5 (33.4) |
| 25–50 | 171 (16.3) | 42 (19.3) | 92 (15.3) | 26 (21.7) | 6 (8.8) | 2 (7.4) | 3 (20.0) |
| 50–75 | 239 (22.7) | 30 (13.8) | 166 (27.5) | 25 (20.8) | 7 (10.2) | 7 (25.9) | 4 (26.6) |
| >75 | 364 (34.6) | 65 (29.7) | 222 (36.8) | 48 (40.0) | 23 (33.9) | 3 (11.1) | 3 (20.0) |
| Long-course CRT-surgery interval | |||||||
| ≤8 | 271 (25.8) | 62 (28.4) | 172 (28.5) | 19 (15.7) | 6 (8.8) | 11 (40.7) | 1 (6.7) |
| 8–12 | 743 (70.7) | 152 (69.7) | 407 (67.5) | 100 (83.4) | 54 (79.4) | 16 (59.3) | 14 (93.3) |
| >12 | 37 (3.5) | 4 (1.9) | 24 (4.0) | 1 (0.9) | 8 (11.8) | 0.0 (0) | 0.0 (0) |
Overall, 745 respondents (70.9%) experienced some delays in CRC care (delay group) and 306 respondents (29.1%) did not (no delay group). These 2 groups were substantially homogeneous for all demographics and personal practices (Table II ). The geographical distribution between the 2 groups was also similar and proportionally consistent with the overall population of 1,051 respondents.
Table II.
Characteristics of the 2 groups of delay and no delay CRC practice (N = 1,051 global respondents)
| Delay n = 745 (70.9%) |
No delay n = 306 (29.1%) |
P | |
|---|---|---|---|
| Geographical region | |||
| Europe | 422 (56.6) | 181 (59.2) | .141 |
| Asia | 158 (21.3) | 60 (19.6) | |
| North America | 82 (11.0) | 38 (12.4) | |
| South America | 57 (7.7) | 11 (3.6) | |
| Africa | 16 (2.1) | 11 (3.6) | |
| Oceania | 10 (1.3) | 5 (1.6) | |
| Peak reached | |||
| Yes | 617 (82.8) | 253 (82.7) | .957 |
| No | 128 (17.2) | 53 (17.3) | |
| Sex | |||
| Males | 586 (78.7) | 241 (78.8) | .971 |
| Females | 159 (21.3) | 65 (21.2) | |
| Type of hospital | |||
| Academic | 482 (64.7) | 160 (52.3) | .001 |
| Nonacademic teaching | 199 (26.7) | 113 (36.9) | |
| Nonteaching | 64 (8.6) | 33 (10.8) | |
| Number of beds | |||
| ≤250 | 74 (9.9) | 39 (12.7) | .156 |
| 251–750 | 370 (49.7) | 165 (53.9) | |
| 751–1,250 | 180 (24.2) | 62 (20.3) | |
| >1,250 | 121 (16.2) | 40 (13.1) | |
| Type of division | |||
| Colorectal | 182 (24.4) | 66 (21.6) | .362 |
| General surgery | 563 (75.6) | 240 (78.4) | |
| Annual number of colon cancer surgery | |||
| ≤50 | 150 (20.1) | 73 (23.8) | .001 |
| 51–150 | 348 (46.7) | 167 (54.6) | |
| >150 | 247 (33.2) | 66 (21.6) | |
| Laparoscopy (%) | |||
| <25 | 168 (22.6) | 53 (17.3) | .136 |
| 25–50 | 125 (16.7) | 47 (15.3) | |
| 50–75 | 202(27.1) | 84 (27.5) | |
| >75 | 250 (33.6) | 122 (39.9) | |
| Annual number of rectal cancer surgery | |||
| ≤50 | 494 (66.3) | 226 (73.9) | .054 |
| 51–150 | 190 (25.5) | 62 (20.3) | |
| >150 | 61 (8.2) | 18 (5.9) | |
| Laparoscopy (%) | |||
| <25 | 205 (27.5) | 72 (23.5) | .114 |
| 25–50 | 119(16.0) | 52 (17.0) | |
| 50–75 | 178(23.9) | 61 (19.9) | |
| >75 | 243 (32.6) | 121 (39.6) | |
| Long-course CRT- surgery interval |
|||
| <8 | 494 (66.3) | 226 (73.8) | .413 |
| 8–12 | 190 (25.5) | 62 (20.3) | |
| >12 | 61 (8.2) | 18 (5.9) |
Hospital response to COVID-19 emergency
Among 745 respondents in the delay group, 694 (93.2%) reported that their hospitals had participated in the emergency by either providing fully dedicated support (16.8%) or partially dedicating (76.4%) clinical activities to the management of SARS-CoV-2 patients (Table III ): (1) 97.3% (725 respondents) reported that elective surgery was affected by COVID-19: 376 (50.5%) respondents reported that surgical capacity was reduced (>50% according to 186 respondents), and 349 (46.8%) stated that elective surgery was temporarily suspended (≥5 weeks according to 296 respondents); (2) 85.6% (638 respondents) reported that PPE was readily available; (3) 64.3% (479 respondents) reported that their hospitals relocated resources to COVID-19 free external facilities for elective CRC surgery; (4) 52.1% (388 respondents) reported that staff members were diagnosed with SARS-CoV-2 and were quarantined; and (5) 45.4% (338 respondents) reported that staff members were relocated from surgical divisions to COVID-19 units (>40% of staff in 94 divisions). The geographical distribution of the respondents did not significantly impact hospitals’ organization.
Table III.
Characteristics of respondents reporting delays in CRC care across 6 geographical regions (n = 745 reporting delayed care)
|
n = 745 (100%) |
Asia 158 (21.2) |
Europe 422 (56.6) |
N America 82 (11.0) |
S America 57 (7.8) |
Africa 16 (2.1) |
Oceania 10 (1.3) |
|
|---|---|---|---|---|---|---|---|
| Hospital involvement in COVID-19 care | |||||||
| Fully dedicated | 125 (16.8) | 38 (24.1) | 68 (16.1) | 13 (15.9) | 4 (7.0) | 2 (12.5) | 0.0 (0) |
| Partially dedicated | 569 (76.4) | 102 (64.5) | 326 (77.3) | 68 (82.9) | 53 (93.0) | 11 (68.7) | 9 (90.0) |
| Not involved | 51 (6.8) | 18 (11.4) | 28 (6.6) | 1 (1.2) | 0.0 (0) | 3 (18.8) | 1 (10.0) |
| Readily available | |||||||
| External facilities for CRC surgery | 479 (64.3) | 103 (65.2) | 291 (69.0) | 26 (31.7) | 42 (73.7) | 10 (62.5) | 7 (70.0) |
| Cancer care coordinator | 420 (56.4) | 80 (50.6) | 238 (56.4) | 57 (69.5) | 32 (56.1) | 7 (43.7) | 6 (60.0) |
| Personal protective equipment | 638 (85.6) | 146 (92.4) | 357 (84.6) | 70 (85.4) | 44 (77.2) | 12 (75.0) | 9 (90.0) |
| Status of elective CRC surgery | |||||||
| Temporarily put on hold | 349 (46.8) | 73 (46.2) | 182 (43.1) | 60 (73.2) | 21 (36.8) | 8 (50.0) | 5 (50.0) |
| ≤4 weeks | 53 (15.2) | 8 (11.0) | 29 (15.9) | 7 (11.7) | 5 (23.8) | 4 (50.0) | 0.0 (0) |
| 5–8 weeks | 170 (48.7) | 37 (50.7) | 89 (48.9) | 33 (55.0) | 5 (23.8) | 3 (37.5) | 3 (60.0) |
| >8 weeks | 126 (36.1) | 28 (38.3) | 64 (35.2) | 20 (33.3) | 11 (52.4) | 1 (12.5) | 2 (40.0) |
| Temporarily reduced | 376 (50.5) | 83 (52.5) | 222 (52.6) | 22 (26.8) | 36 (63.2) | 8 (50.0) | 5 (50.0) |
| ≤50% | 190 (50.5) | 45 (54.2) | 114 (51.4) | 9 (50.9) | 17 (47.2) | 2 (25.0) | 3 (60.0) |
| >50% | 186 (49.5) | 38 (45.8) | 108 (48.6) | 13 (59.1) | 19 (52.8) | 6 (75.0) | 2 (40.0) |
| Unaffected | 20 (2.7) | 2 (1.3) | 18 (4.3) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Elective CRC patients | |||||||
| Needing urgent surgery | 196 (26.3) | 37 (23.4) | 122 (28.9) | 17 (20.7) | 15 (26.3) | 3 (18.8) | 2 (20.0) |
| Initial CRC care plan | |||||||
| Changed | 365 (49.0) | 86 (54.4) | 206 (48.8) | 31 (37.8) | 30 (52.6) | 9 (56.3) | 3 (30.0) |
| CRC patients | |||||||
| Refusing surgery | 300 (40.3) | 70 (44.3) | 154 (36.5) | 46 (56.1) | 25 (49.3) | 3 (18.8) | 2 (20.0) |
| Being COVID-19+ on surgery | 145 (19.5) | 19 (12.0) | 109 (25.8) | 9 (11.0) | 7 (12.3) | 0.0 (0) | 1 (10.0) |
| Becoming COVID-19+ postoperative | 198 (26.6) | 28 (17.7) | 146 (34.6) | 8 (9.8) | 13 (22.8) | 2 (12.5) | 1 (10.0) |
| Staff members | |||||||
| Quarantined | 388 (52.1) | 74 (46.8) | 245 (58.1) | 40 (48.8) | 23 (40.4) | 4 (25.0) | 2 (20.0) |
| <10% | 179 (46.1) | 40 (54.1) | 104 (42.5) | 27 (67.5) | 7 (30.4) | 0.0 (0) | 1 (50.0) |
| 10–20% | 153 (39.5) | 28 (37.8) | 100 (40.8) | 11 (27.5) | 10 (43.5) | 3 (75.0) | 1 (50.0) |
| >20% | 56 (14.4) | 6 (8.1) | 41 (16.7) | 2 (5.0) | 6 (26.1) | 1 (25.0) | 0.0 (0) |
| Relocated to COVID units | 338 (45.4) | 66 (41.8) | 215 (50.9) | 39 (47.6) | 13 (22.8) | 3 (18.8) | 2 (20.0) |
| <20% | 190 (56.2) | 49 (74.2) | 109 (50.7) | 23 (59.0) | 7 (53.8) | 1 (33.3) | 1 (50.0) |
| 20–40% | 54 (16.0) | 6 (9.1) | 33 (15.3) | 8 (20.5) | 4 (30.8) | 2 (66.7) | 1 (50.0) |
| >40% | 94 (27.8) | 11 (16.7) | 73 (34.0) | 8 (20.5) | 2 (15.4) | 0.0 (0) | 0.0 (0) |
| MDT meetings | |||||||
| Suspended | 364 (48.9) | 102 (64.6) | 192 (45.5) | 31 (37.8) | 31 (54.4) | 7 (43.8) | 1 (10.0) |
COVID-19 significantly affected CRC care. Among the 745 respondents of the delay group (Table III) (1) 48.9% (365 respondents) reported a change of the initial surgical plan; (2) 48.5% (364 respondents) stated that MDT meetings were suspended; (3) 40.3% (300 respondents) replied that CRC patients refused surgery during the COVID-19 emergency phase; (4) 26.6% (198 respondents) reported that they had patients who developed COVID-19 postoperatively; (5) 26.3% (196 respondents) reported that CRC patients originally planned for elective operations needed urgent surgery; and (6) 26.2% (195 respondents) performed CRC surgery in COVID-19 patients.
Delay in CRC care
The multivariable hierarchical logistic regression model (Table IV ) showed a 38% lower risk (odds ratio [OR] = 0.62, 95% confidence interval [CI] 0.45–0.85; P = .003) of delay among respondents from nonacademic teaching versus academic hospitals and a 72% higher risk (OR = 1.72, 95% CI 1.07–2.76; P = .026) among those reporting high versus low case volume of colon cancer surgeries (Table IV).
Table IV.
Multivariable hierarchical logistic regression model exploring the association between delay and a preselected covariate set in CRC care (N = 1,051 global respondents)
| Adjusted OR |
95% Lower |
CI Upper |
P | |
|---|---|---|---|---|
| Sex | ||||
| Female (reference) | ||||
| Males | 1.01 | 0.73 | 1.41 | .948 |
| Type of hospital | ||||
| Academic (reference) | ||||
| Nonacademic teaching | 0.62 | 0.45 | 0.85 | .003 |
| Nonteaching | 0.72 | 0.44 | 1.19 | .203 |
| Number of beds | ||||
| <250 (reference) | ||||
| 251–750 | 1.06 | 0.67 | 1.70 | .797 |
| 751–1,250 | 1.11 | 0.64 | 1.92 | .708 |
| >1,250 | 1.03 | 0.56 | 1.91 | .922 |
| Type of division | ||||
| Colorectal (reference) | ||||
| General surgery | 1.02 | 0.73 | 1.44 | .903 |
| Colon cancer surgeries per y | ||||
| ≤50 (reference) | ||||
| 51–150 | 0.98 | 0.68 | 1.40 | .905 |
| >150 | 1.72 | 1.07 | 2.76 | .026 |
| Rectal cancer surgeries per y | ||||
| ≤50 (reference) | ||||
| 51–150 | 0.95 | 0.64 | 1.41 | .790 |
| >150 | 0.87 | 0.45 | 1.67 | .680 |
Overall, in the delay group (745 respondents), the original surgical management plan was changed according to 365 (48.9%) respondents, and the original protocol of neoadjuvant therapy was changed according to 157 (21.1%) respondents. Changes were more likely to occur among respondents reporting staff members quarantined (OR 1.38, 95% CI 1.01–1.90; P = .045) or relocated to COVID-19 units (OR 1.55, 95% CI 1.13–2.13; P = .006).
Endoscopy and radiology
Endoscopic procedures for CRC were the most affected diagnostic techniques by the COVID-19 emergency (73.7% [549/745] of respondents). The delay in radiology was reported by 45% (335/745) of respondents (Table V ). The multivariable hierarchical logistic regression model (Table VI ) demonstrated the following effects on the risk of delay in endoscopy: (1) 82% higher risk (OR = 1.82, 95% CI 1.26–2.62; P = .001) in divisions where staff members were relocated to COVID-19 units; (2) 58% higher risk (OR = 1.58, 95% CI 1.10–2.27; P = .013) in divisions where staff members were quarantined; (3) 64% lower risk (OR = 0.36, 95% CI 0.15–0.84; P = .017) in high-volume hospitals (versus low-volume hospitals); and (4) 42% lower risk (OR = 0.58, 95% CI 0.36–0.99; P = .045) in divisions partially dedicated to SARS-CoV-2 (versus fully dedicated).
Table V.
Delays in CRC care across 6 geographical regions (n = 745 reporting delayed care)
|
n = 745 (100%) |
Asia 158 (21.2) |
Europe 422 (56.6) |
N America 82 (11.0) |
S America 57 (7.7) |
Africa 16 (2.1) |
Oceania 10 (1.3) |
P | |
|---|---|---|---|---|---|---|---|---|
| Endoscopy | 549 (73.7) | 109 (69.0) | 310 (73.5) | 64 (78.0) | 47 (82.5) | 12 (75.0) | 7 (70.0) | .421 |
| Radiology | 335 (45.0) | 69 (43.7) | 199 (47.2) | 32 (39.0) | 21 (36.8) | 10 (62.5) | 4 (40.0) | .336 |
| Neoadjuvant CRT | 196 (26.3) | 47 (29.7) | 106 (25.1) | 16 (19.5) | 22 (38.6) | 2 (12.5) | 3 (30.0) | .097 |
| Prolonged CRT interval | 324 (43.5) | 83 (52.5) | 175 (41.5) | 26 (31.7) | 31 (54.4) | 7 (43.8) | 2 (20.0) | .008 |
| Surgery | 434 (58.3) | 90 (57.0) | 257 (60.9) | 43 (52.4) | 34 (59.6) | 7 (43.8) | 3 (30.0) | .208 |
| Histopathology | 131 (17.6) | 43 (27.2) | 55 (13.0) | 9 (11.0) | 19 (33.3) | 4 (25.0) | 1(10.0) | <.001 |
Table VI.
Multivariable hierarchical logistic regression model assessing delays in CRC care (n = 745 reporting delayed care)
| Endoscopy (n = 549) |
Radiology (n = 335) |
Surgery (n = 434) |
Histopathology (n = 131) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| Type of hospital | ||||||||||||
| Academic (reference) | ||||||||||||
| Nonacademic teaching | 0.90 | 0.58–1.39 | .638 | 0.70 | 0.48–1.02 | .065 | 0.95 | 0.65–1.39 | .808 | 0.63 | 0.37–1.07 | .089 |
| Nonteaching | 0.64 | 0.33–1.23 | .179 | 0.57 | 0.30–1.08 | .083 | 0.91 | 0.50–1.67 | .758 | 0.23 | 0.08–0.62 | .004 |
| Number of beds | ||||||||||||
| ≤250 (reference) | ||||||||||||
| 251–750 | 0.75 | 0.38–1.46 | .398 | 0.86 | 0.48–1.56 | .627 | 1.11 | 0.61–1.98 | .737 | 0.52 | 0.26–1.05 | .068 |
| 751–1,250 | 0.76 | 0.35–1.65 | .485 | 0.91 | 0.46–1.79 | .785 | 1.30 | 0.66–2.54 | .452 | 0.36 | 0.15–0.83 | .017 |
| >1,250 | 0.36 | 0.15–0.84 | .017 | 0.54 | 0.26–1.15 | .110 | 1.00 | 0.47–2.11 | .996 | 0.54 | 0.22–1.35 | .186 |
| Type of division | ||||||||||||
| Colorectal (reference) | ||||||||||||
| General surgery | 0.83 | 0.55–1.30 | .413 | 0.78 | 0.53–1.15 | .208 | 0.67 | 0.45–0.99 | .045 | 1.49 | 0.87–2.54 | .149 |
| Colon cancer surgery per y | ||||||||||||
| ≤50 (reference) | ||||||||||||
| 51–150 | 1.51 | 0.94–2.42 | .087 | 1.13 | 0.73–1.75 | .572 | 1.42 | 0.92–2.18 | .114 | 0.78 | 0.45–1.33 | .356 |
| >150 | 1.77 | 0.97–3.22 | .061 | 0.96 | 0.57–1.64 | .888 | 1.51 | 0.89–2.56 | .126 | 0.73 | 0.36–1.44 | .360 |
| Rectal cancer surgery per y | ||||||||||||
| ≤50 (reference) | ||||||||||||
| 51–150 | 1.12 | 0.68–1.87 | .652 | 1.56 | 1.01–2.40 | .045 | 0.75 | 0.49–1.16 | .200 | 0.70 | 0.38–1.29 | .247 |
| >150 | 1.17 | 0.52–2.64 | .705 | 1.86 | 0.93–3.71 | .079 | 1.34 | 0.65–2.74 | .426 | 1.30 | 0.55–3.06 | .547 |
| Hospital response to COVID-19 | ||||||||||||
| Fully dedicated (reference) | ||||||||||||
| Partially dedicated | 0.58 | 0.36–0.99 | .045 | 0.80 | 0.52–1.22 | .295 | 0.49 | 0.36–0.77 | .002 | 0.71 | 0.41–1.22 | .211 |
| Not involved | 1.04 | 0.43–2.51 | .934 | 0.87 | 0.42–1.79 | .698 | 0.62 | 0.29–1.31 | .208 | 1.77 | 0.75–4.14 | .190 |
| External facilities for CRC surgery | 0.68 | 0.46–1.00 | .052 | 0.89 | 0.63–1.24 | .477 | 0.98 | 0.70–1.38 | .918 | 0.87 | 0.56–1.37 | .556 |
| Cancer care coordinator | 1.03 | 0.72–1.47 | .874 | 0.92 | 0.67–1.26 | .605 | 1.07 | 0.78–1.47 | .681 | 1.47 | 0.96–2.26 | .077 |
| PPE readily available | 0.70 | 0.41–1.21 | .206 | 0.52 | 0.24–0.81 | .003 | 0.59 | 0.37–0.93 | .023 | 0.44 | 0.26–0.75 | .002 |
| Staff members quarantined | 1.58 | 1.10–2.27 | .013 | 0.79 | 0.57–1.10 | .148 | 1.34 | 0.97–1.84 | .074 | 0.99 | 0.64–1.53 | .971 |
| Staff members relocated | 1.82 | 1.26–2.62 | .001 | 1.69 | 1.23–2.33 | .001 | 1.34 | 0.97–1.85 | .075 | 1.05 | 0.68–1.63 | .826 |
| MDT meetings suspended | 0.81 | 0.57–1.16 | .250 | 1.39 | 1.01–1.90 | .042 | 1.40 | 1.02–1.92 | .039 | 2.06 | 1.23–2.26 | .001 |
The multivariable hierarchical logistic regression model (Table VII ) demonstrated the following effects on the risk of delay in radiology: (1) 69% higher risk (OR = 1.69, 95% CI 1.23–2.33; P = .001) in divisions where staff members were relocated to COVID-19 units; (2) 56% higher risk (OR = 1.56, 95% CI 1.01–2.40; P = .045) in divisions with medium volume of annual rectal cancer surgeries (versus low volume); (3) 39% higher risk (OR = 1.39, 95% CI 1.01–1.90; P = .042) in divisions where MDT meetings were suspended; (4) 48% lower risk (OR = 0.52, 95% CI 0.24–0.81’ P = .003) when PPE was readily available.
Table VII.
Multivariable ordinal logistic regression model assessing the recovery of CRC care (n = 745 reporting delayed care)
| Fully recovered versus improved versus persistently limited |
||||
|---|---|---|---|---|
| Adjusted OR |
95% Lower |
CI Upper |
P | |
| Sex | ||||
| Female (reference) | ||||
| Male | 0.91 | 0.64 | 1.30 | .611 |
| Type of hospital | ||||
| Academic (reference) | ||||
| Nonacademic teaching | 0.81 | 0.57 | 1.16 | .257 |
| Nonteaching | 0.65 | 0.37 | 1.15 | .140 |
| Number of beds | ||||
| <250 (reference) | ||||
| 251–750 | 0.59 | 0.34 | 1.04 | .066 |
| 751–1,250 | 0.62 | 0.33 | 1.17 | .138 |
| >1,250 | 0.58 | 0.29 | 1.18 | .135 |
| Type of division | ||||
| Colorectal (reference) | ||||
| General surgery | 0.89 | 0.62 | 1.27 | .514 |
| Colon cancer surgeries per y | ||||
| ≤50 (reference) | ||||
| 51–150 | 1.06 | 0.70 | 1.59 | .797 |
| >150 | 1.72 | 0.61 | 1.64 | .990 |
| Rectal cancer surgeries per y | ||||
| ≤50 (reference) | ||||
| 51–150 | 0.65 | 0.43 | 0.97 | .036 |
| >150 | 0.97 | 0.50 | 1.87 | .926 |
| Hospital response to COVID-19 | ||||
| Fully dedicated (reference) | ||||
| Partially dedicated | 1.05 | 0.70 | 1.59 | .798 |
| Not involved | 0.75 | 0.38 | 1.50 | .418 |
| External facilities for CRC surgery | 0.81 | 0.59 | 1.11 | .183 |
| Cancer care coordinator | 0.76 | 0.57 | 1.03 | .073 |
| PPE readily available | 0.81 | 0.54 | 1.22 | .318 |
| Staff members quarantined | 1.66 | 1.22 | 2.45 | .001 |
| Staff members relocated | 1.09 | 0.81 | 1.47 | .572 |
| MDT meetings suspended | 0.77 | 0.57 | 1.04 | .086 |
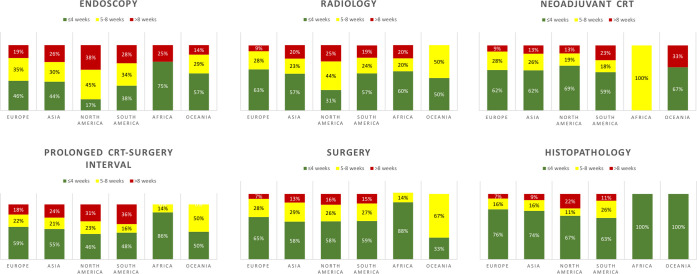
Delays in diagnostics for CRC beyond 4 weeks were more prevalent in North America, with 53 out of 64 respondents (83%) reporting delay in endoscopic procedures and 22 out of 32 respondents (69%) reporting delay in radiological investigations (Fig 3 ).
Fig 3.
Delay (weeks) in colorectal cancer care across the various fields of practice (745 respondents).
Surgery
Colorectal cancer surgery was delayed in 58.3% (434/745) of divisions. For the majority of respondents (90.1% [391/434]), the delay was 5 to 8 weeks beyond normal wait time, exceeding 8 weeks for 43 respondents (9.9%) (Table V, Fig 3). The multivariable hierarchical logistic regression model (Table VI) demonstrated the following effects on the risk of delay in surgery: (1) 40% higher risk (OR = 1.40, 95% CI 1.02–1.92; P = .039) in divisions where MDT meetings were suspended; (2) 51% lower risk (OR = 0.49, 95% CI 0.36–0.77; P = .002) in divisions partially dedicated to COVID-19 (versus fully dedicated); (3) 41% lower risk (OR = 0.59, 95% CI 0.37–0.93; P = .023) when PPE was readily available; and (4) 33% lower risk (OR = 0.67, 95% CI 0.45–0.99; P = .045) among respondents from general surgery divisions (versus colorectal divisions).
Overall, 48.9% (365/745) of respondents changed the original surgical plan (multiple alternatives): from laparoscopic to open (37.3%, 136/365); from colorectal resections to CRT (28.2%, 103/365); from transanal minimally invasive surgery/transanal endoscopic microsurgery to neoadjuvant radiotherapy (19%, 69/365); from colorectal resections to stenting (10%, 37/365); from robotic to open (8.0%, 29/365); from robotic to laparoscopic (6.0%, 22/365); and from transanal minimally invasive surgery/transanal endoscopic microsurgery to abdominal surgery (4.2%, 15/365). The reported reasons for changes in surgical plans (multiple alternatives) were shortage of theater slots (52%, 190/365 respondents), shortage of staff members and personnel (30%, 111/365), disease progression (28%, 103/365), and concerns over aerosolization in laparoscopic/robotic surgery (18%, 64/365).
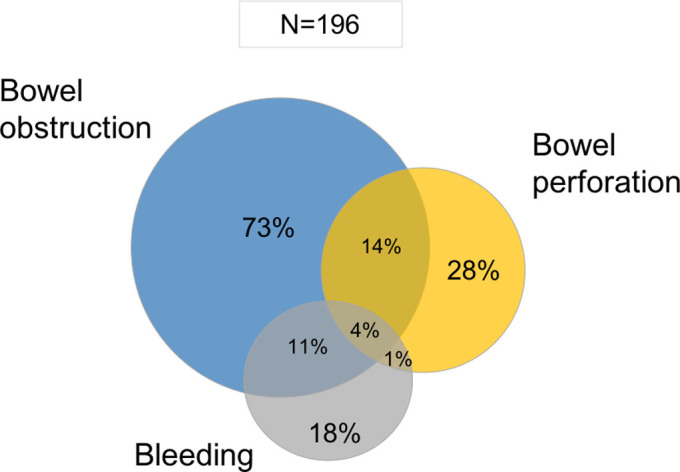
Overall, 26.3% (196/745) of respondents reported that CRC patients scheduled for elective surgery needed urgent surgery owing to (multiple alternatives) bowel obstruction (73%), bowel perforation (28%), or bleeding (18%) (Supplementary Fig 1).
Neoadjuvant CRT
One hundred and ninety-six of 745 respondents (26.3%) reported that neoadjuvant CRT was postponed for rectal cancer patients (Table V, Fig 3). The delay was ≤4 weeks for 61.7% (121/196) of respondents and ≥5 weeks for 38.3% (75/196) of respondents.
Overall, 21.1% (157/745) of respondents changed the original oncological plan for rectal cancer patients from neoadjuvant CRT and surgery to surgery only (86%, 135/157) and from long-course CRT to short-course CRT (13%, 22/157).
In addition, 43.5% (324/745) of respondents also reported that the long-course neoadjuvant CRT-to-surgery interval for rectal cancer patients was prolonged beyond the optimal 8 to 12 weeks interval (43.2% [140/324] of respondents ≥5 weeks) (Table V). A factor statistically associated to this delay was the suspension of MDT meetings (OR 1.64, 95% CI 1.20–2.24; P = .002).
Histopathology
Histopathological assessment was affected for 17.6% (131/745) of respondents. The delay was more prevalent in South America (19/57, 33.3%; P < .001) (Table V, Fig 3). The multivariable hierarchical logistic regression model (Table VI) demonstrated the following effects on the risk of delay in histopathology: (1) 77% lower risk (OR = 0.63, 95% CI 0.08–0.62; P = .004) in nonteaching hospitals (versus academic hospitals); (2) 66% lower risk (OR = 0.44, 95% CI 0.26–0.75; P = .002) when PPE was readily available; (3) 64% lower risk (OR = 0.36, 95% CI 0.15–0.83; P = .017) in mid- to high-bed-volume hospitals (versus low bed volume); and (4) 206% higher risk in divisions where MDT meetings were suspended (OR = 2.06, 95% CI 1.23–2.26; P = .001).
Recovery of CRC care
Recovery of CRC care at the date of the survey completion (May 20–June 10, 2020) (Appendices 4–5) mirrors the status of the outbreak throughout the geographical regions. Overall, CRC care was “improved but not fully recovered” to pre-COVID status for 56.4% (420/745) of respondents. The highest prevalence was in Europe (65.9%, 278/422) and North America (58.5%, 48/82). At the time of the survey, in these 2 regions were nations both at the peak and at the transition phase of the emergency. CRC care status was “persistently limited” for 26% (194/745) of respondents. The highest prevalence was in Africa (75%, 12/16) and South America (72%, 41/57), 2 regions where most nations were at the initial phase of the emergency at the time of the study. A “fully recovered” CRC practice was reported by 17.6% (131/745) of respondents. The highest prevalence was in Asia (25.3%, 40/158), where some nations were at the end of the emergency phase at the time of the survey. These data are consistent with the 0-inflated negative binomial regression model (Table VII) exploring the interval (days) between the date of achievement of the 100th COVID-19 case and the date of recovery of CRC care. Persistently limited practice was significantly associated with a shorter interval (mean interval ratio 0.41 [95% CI 0.35–0.47]; P < .001) compared with fully recovered practice.
The multivariable hierarchical logistic regression model (Table VII) demonstrated the following effects on recovery of CRC practice: 66% higher risk of persistently limited practice (OR = 1.66, 95% CI 1.22–2.45; P = .001) in divisions where staff members were quarantined and 35% lower risk of persistently limited practice (OR = 0.65, 95% CI 0.43–0.97; P = .036) in divisions with medium volume of annual rectal cancer surgeries (versus low volume).
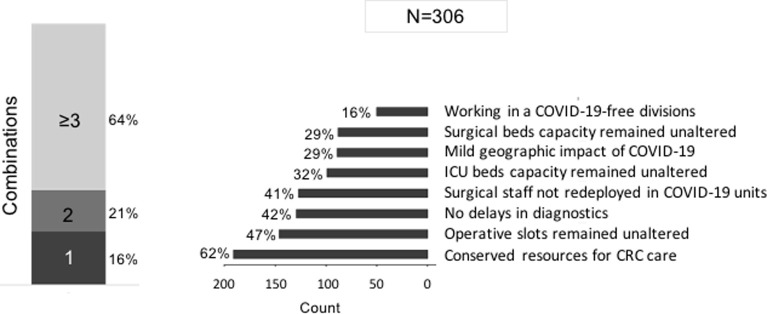
Analysis of the no delay group
The no delay group included 29% (306/1,051) of respondents. The reasons reported for unaltered CRC practice were (more than 1 factor could be reported) (1) preservation of resources for CRC care (62%, 190/306); (2) no changes in operative slots (47%, 144/306); (3) no delay in diagnostics (42%, 129/306); (4) surgical staff not redeployed from surgical divisions to COVID-19 units (41%, 125/306); (5) no change in intensive care unit bed capacity for CRC patients (32%, 98/306); and (6) no change in surgical bed capacity for CRC patients (29%, 89/306). A combination of ≥3 of factors was reported by 64% (196/306) of respondents (Fig 4 ).
Fig 4.
Reported reasons of no delay in colorectal cancer care (306 respondents).
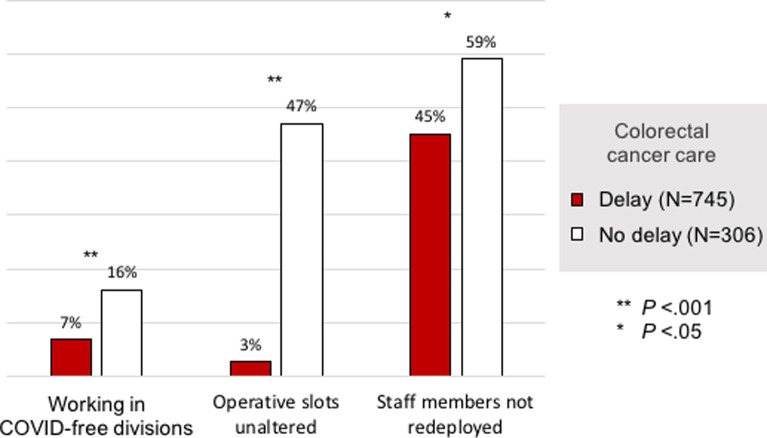
Three main statistically significant reasons for unaltered CRC care comparing the no delay to the delay group were identified: (1) practicing in COVID-free divisions (16% vs 7%; P < .001); (2) no change in operative slots (47% vs 3%; P < .001); and (3) staff members not redeployed from surgical divisions to COVID-19 units (59% vs 45%; P = .037) (Supplementary Fig 2).
Discussion
COVID-19 introduced a global challenge for the management of CRC. In our survey, changes in both diagnostic and therapeutic practices were reported by 71% (745/1,051: delay group) of respondents. Endoscopic and radiologic procedures were highly affected by the COVID-19 emergency. Elective CRC surgery was impacted for almost all respondents (97.3%), with planned procedures being temporarily suspended (46.8%) or capacity reduced (50.5%). Our results are consistent with an earlier survey on the global impact of COVID-19 in CRC patients completed by 289 surgeons in April 2020 during the emergency phase.11 This study showed that outpatients services, cancer screening, diagnostics and treatment were all transiently suspended. Another study on elective oncological surgery in Italy during the COVID-19 emergency phase demonstrated that 70% of surgical divisions had a reduction of hospital beds with an associated 76% reduction of surgical activity owing to the relocation of resources.12 Evidence is limited regarding the effect of diagnostic or surgical delays on CRC specific outcomes.12, 13 Maringe et al8 estimated a 17% increase in the number of deaths of CRC patients up to year 5 as a result of diagnostic delays owing to the COVID-19 pandemic in United Kingdom. In a retrospective cohort study, Lee et al14 reported that the diagnosis-to-treatment interval (DTI) for all CRC, regardless of cancer staging, should not exceed 30 days. In another cohort study, Kucejko et al15 reported that the ideal timing of definitive resection in colon cancer is between 3 and 6 weeks after initial diagnosis to achieve a modest but significant improvement in overall survival. The COVID-19 pandemic has increased the DTI for CRC. Turaga and Girotra16 reported that CRC surgery can be safely delayed beyond the normal wait time up to 4 weeks without having a significant impact on patient survival or cancer progression. However, in our survey, 58.3% of respondents in the delay group reported that COVID-19 prolonged DTI to ≥5 weeks beyond normal wait time. Moreover, 43.5% of respondents reported a prolonged long-course CRT-to-surgery interval for rectal cancer patients to ≥5 weeks beyond the optimal 8 to 12 weeks interval. Indeed, according to Turaga and Girotra,16 this delay is less likely to cause harm. They reported that a postponement period of 6 weeks beyond the optimal long-course CRT-to-surgery interval for rectal cancer patients may be considered safe. Nevertheless, it remains unclear whether a prolonged time interval to surgery beyond the current recommended interval of 8 to 12 weeks results in increased morbidity or better pathological response.17, 18, 19, 20
COVID-19 also increased the risk of urgent surgery or changed the decision-making process.21 , 22 In this survey, 26.3% (196/745) of respondents reported that CRC patients scheduled for elective surgery needed urgent surgery. Moreover, 49% (365/475) of responders changed the original surgical plan, and 21% (157/745) changed the original oncologic plan. Reasons for the changes were shortage of theater slots, shortage of staff members and personnel, disease progression, and concerns over aerosolization in laparoscopic/robotic surgery. Regarding this last factor, however, a number of societies (Society of American Gastrointestinal and Endoscopic Surgeons, European Association of Endoscopic Surgery, and Australian College of Surgeons) reported that laparoscopy may be appropriate.13 The closed cavity in laparoscopy enables smoke control and airborne particles in the abdomen can be safely eliminated through filtered evacuation systems.23, 24, 25, 26 Although there is no compelling evidence that laparoscopy increases the risk of airborne transmission, appropriate safety measures are recommended. All members of the surgical team should wear appropriate PPE,14 and the pneumoperitoneum should be slowly released in a controlled manner to minimize the spread of airborne particles.26 , 27 Despite these guidelines, 45.2% of respondents in the current study changed their surgical approach from laparoscopic/robotic to open.
The COVID-19 pandemic changed the functioning and organization of hospitals around the world. During the surge, restrictive measures were adopted to reduce COVID-19 exposure and to preserve human and material resources.28, 29, 30, 31 In our survey, we found that delays in CRC care were associated with differences in health care delivery systems, hospital preparedness, resource availability, and local COVID-19 prevalence, while the geographical distribution of the respondents did not impact significantly. Important factors included hospitals dedicating their services to COVID-19 care, quarantine and/or redeployment of staff, MDT meetings suspension, and the lack of readily available PPE (Table VIII ). These factors mirror the statistically significant reason for unaffected CRC practice in the no delay group: practicing in COVID-free divisions, no change in number of operative slots, and staff members not redeployed from surgical divisions to COVID-19 units.
Table VIII.
Reasons of delay in CRC care (n = 745 reporting delayed care)
| Delays |
Prolonged CRT-surgery interval | Change of original plan | ||||
|---|---|---|---|---|---|---|
| Endoscopy | Radiology | Surgery | Histopathology | |||
| Academic hospitals | x | |||||
| Colorectal divisions | x | |||||
| High-volume hospitals | x | x | ||||
| Medium volume of rectal cancer surgery | x | |||||
| Units fully dedicated to COVID-19 care | x | x | ||||
| PPE not readily available | x | x | x | |||
| Staff members quarantined | x | x | ||||
| Staff members redeployed to COVID-19 units | x | x | x | |||
| MDT meetings suspended | x | x | x | x | ||
The recovery of health care systems is a complex process owing to the impact of cancelled and postponed operations. Recommended principles for rescheduling have been outlined by the American College of Surgeons, American Hospital Association, American Society of Anesthesiologists, and the Association of Perioperative Nurses.32 The American College of Surgeons also provided principles for the safe resumption of elective surgery organized in 2 parts: core facility checklist items (general facility policies, structures and processes, outcomes reporting) and surgery-specific checklist items (policies, structures and processes, outcomes reporting) including measures to protect the patient and protocols in place for safe protection of medical first-line teams.32 In our survey, recovery of CRC care was associated with the stage of the virus outbreak at the time of study completion. Independent of the geographical region, the likelihood of reduced CRC practice was 66% higher among respondents reporting quarantined staff members (P = .001) and 35% lower among those working in divisions with medium volume of rectal cancer surgeries (compared with low volume; P = .036).
Our results indicate that cancer pathways need to swiftly be re-established and maintained at a near normal throughput, with attention to the backlog of patients, in order to reduce the impact of the COVID-19 pandemic.33 Hospitals need to assume standard-of-care when the benefits exceed COVID-related mortality.34 , 35 However, Caricato et al36 reported that oncological programs proposed in Italy to guarantee elective surgical activity were only successful in 19% of the regions. In the current study, we identified a crucial role of MDT meetings in CRC care. Meeting suspension was associated with delays in radiology, surgery, and histopathology and prolonged the CRT-to-surgery interval (Table VIII).
In our survey, the relative homogeneity of delays seemed to reflect the lack of any absolute relation to either the geographical location or the status of the outbreak. Specifically, even within geographical regions at the same time points, some hospitals had delays while others did not. Thus, delays or lack thereof appeared to be more owing to an individual hospital’s organization and preparedness. The plans implemented at hospitals at which no delays were experienced could be shared as “best practices” so that other facilities could avoid delays during future virus surges. Conversely, the geographical distribution was important if we consider the recovery of CRC care, because the status of the outbreak was associated with the recovery of standard clinical activities in those hospitals who were most affected by the COVID-19 emergency.
Our study has several limitations inherent in surveys, including voluntary participation and recall and selections bias. The respondents included a preponderance of male general surgeons from large academic centers in Europe, Asia, and North America (Fig 1). Therefore, data from all global regions are not equally distributed or robust. This geographic distribution mirrored the areas of highest prevalence of COVID-19 at the time of survey distribution (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200530-covid-19-sitrep-131.pdf?sfvrsn=d31ba4b3_2) (Fig 2). It is therefore reasonable to assume that surgeons from countries with low COVID-19 case prevalence were less motivated to take part.
Another limitation is the lack of a formal full validation process of the survey, which was elected to obtain results in an expeditious manner in this critical period. The impact of subsequent surges is also unknown, as is the long-term effect of the delays on diagnosis and/or treatment. Despite these limitations, our data provide important insights regarding the impact of COVID-19 pandemic in CRC care.
In conclusion, during the COVID-19 pandemic, global changes in both diagnostic and therapeutic CRC practices were evident. This problem cannot be solved by sharing best practices as the inability to render CRC care was directly related to hospital preparedness and availability of resources rather than to geographical factors. Future surges may again challenge human and material resources. Therefore, a solution to this disparity could potentially be addressed by sharing resources and/or transfer of patients among institutions. The implementation of such practices may nevertheless be challenging because of differences in health care systems.
Conflict of interest/Disclosure
GAS, UG, SMR, JWNM, AM, GLDT, GG, and CT have no relevant financial disclosures. SDW receives consulting fees from Karl Storz, Medtronic, Intuitive, Takeda and Stryker and royalties for intellectual property license from Karl Storz, Medtronic, and Intuitive.
Financial/Support
None.
Approved for Distribution
This Survey was approved for distribution by the following International Societies: American Society of Colon and Rectal Surgeons (ASCRS), Asia-Pacific Federation of Coloproctology (APFCP), Chinese Society of Coloproctology, Egyptian Society of Colon and Rectal Surgeons (ESCRS), European Society of Coloproctology (ESCP), German Society for Coloproctology (DGK), International Society of University Colon and Rectal Surgeons (ISUCRS), Italian Society of Colorectal Surgery (SICCR), Korean Society of Coloproctology, Latin American Association of Coloproctology (ALACP), Malaysian Society of Colorectal Surgeons (MSCRS), Philippine Society of Colon and Rectal Surgeons (PSCRS), Philippine Society of General Surgeons (PSGS), Portuguese Society of Coloproctology, Portuguese Society of Surgery (SPCIR), Russian Association of Coloproctology, South African Colorectal Society (SACRS), Swedish Society of Colorectal Surgery, Turkish Society of Colon, and Rectal Surgery (TKRCD) and Venezuelan Coloproctology Society. The Societies had no role in study design, data collection, analysis, and interpretation, or in the writing of this report. The views expressed are those of the authors and not necessarily those of the Societies.
Footnotes
Giulio A. Santoro and Ugo Grossi contributed equally to this article.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.surg.2020.11.008.
Supplementary materials
Supplementary Fig 1.
Types for complication determining a shift from elective to emergency surgery.
Supplementary Fig 2.
Comparison between delay and no delay groups in colorectal cancer care (1,051 respondents).
References
- 1.Lango M.N. How did we get here? A short history of COVID-19 and other coronavirus-related epidemics. Head Neck. 2020;42:1535–1538. doi: 10.1002/hed.26275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai Y., Yao L., Wei T., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Coronavirus disease (COVID-19) pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 4.COVIDSurg Collaborative Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020 doi: 10.1002/bjs.11746. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sud A., Jones M.E., Broggio J., et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31:1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nunoo-Mensah J.W., Rizk M., Caushaj P.F., et al. and the ISUCRS COVID-19 Participating Investigator Group COVID-19 and the global impact on colorectal practice and surgery. Clin Colorectal Cancer. 2020;19:178–190 e1. doi: 10.1016/j.clcc.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuderer N.M., Choueiri T.K., Shah D.P., et al. and the COVID-19 and Cancer Consortium Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet E-Surveys (CHERRIES) J Med Internet Res. 2004;6:e34. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Worldometer COVID-19 Coronavirus pandemic. 2020. https://www.worldometers.info/coronavirus/
- 11.Bagaria S.P., Heckman M.G., Diehl N.N., Parker A., Wasif N. Delay to colectomy and survival for patients diagnosed with colon cancer. J Invest Surg. 2019;32:350–357. doi: 10.1080/08941939.2017.1421732. [DOI] [PubMed] [Google Scholar]
- 12.Torzilli G., Vigano L., Galvanin J., et al. A snapshot of elective oncological surgery in Italy during COVID-19 emergency: pearls, pitfalls, and perspectives. Ann Surg. 2020;272:e112–e117. doi: 10.1097/SLA.0000000000004081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hangaard Hansen C., Gögenur M., Tvilling Madsen M., Gögenur I. The effect of time from diagnosis to surgery on oncological outcomes in patients undergoing surgery for colon cancer: a systematic review. Eur J Surg Oncol. 2018;44:1479–1485. doi: 10.1016/j.ejso.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y.H., Kung P.T., Wang Y.H., Kuo W.Y., Kao S.L., Tsai W.C. Effect of length of time from diagnosis to treatment on colorectal cancer survival: a population-based study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kucejko R.J., Holleran T.J., Stein D.E., Poggio J.L. How soon should patients with colon cancer undergo definitive resection? Dis Colon Rectum. 2020;63:172–182. doi: 10.1097/DCR.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 16.Turaga K.K., Girotra S. Are we harming cancer patients by delaying their cancer surgery during the COVID-19 pandemic? Ann Surg. 2020 doi: 10.1097/SLA.0000000000003967. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sloothaak D.A.M., Geijsen D.E., van Leersum N.J., et al. and the Dutch Surgical Colorectal Audit. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg. 2013;100:933–939. doi: 10.1002/bjs.9112. [DOI] [PubMed] [Google Scholar]
- 18.Habr-Gama A., São Julião G.P., Fernandez L.M., et al. Achieving a complete clinical response after neoadjuvant chemoradiation that does not require surgical resection: it may take longer than you think! Dis Colon Rectum. 2019;62:802–808. doi: 10.1097/DCR.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 19.Lichthardt S., Wagner J., Löb S., et al. Pathological complete response due to a prolonged time interval between preoperative chemoradiation and surgery in locally advanced rectal cancer: analysis from the German StuDoQ|Rectal carcinoma registry. BMC Cancer. 2020;20:49. doi: 10.1186/s12885-020-6538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lefèvre J.H., Mineur L., Cachanado M., et al. and the French Research Group of Rectal Cancer Surgery (GRECCAR) Does a longer waiting period after neoadjuvant radio-chemotherapy improve the oncological prognosis of rectal cancer?: three years' follow-up results of the Greccar-6 randomized multicenter trial. Ann Surg. 2019;270:747–754. doi: 10.1097/SLA.0000000000003530. [DOI] [PubMed] [Google Scholar]
- 21.Zanus G., Romano M., Santoro G.A., Rossi S., Grossi U. Impact of COVID-19 on urgent surgical activity. Br J Surg. 2020 doi: 10.1002/bjs.11856. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng M.H., Boni L., Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: Lessons learned in China and Italy. Ann Surg. 2020;272:e5–e6. doi: 10.1097/SLA.0000000000003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mintz Y., Arezzo A., Boni L., et al. and the Technology Committee of the European Association for Endoscopic Surgery A low-cost, safe, and effective method for smoke evacuation in laparoscopic surgery for suspected coronavirus patients. Ann Surg. 2020;272:e7–e8. doi: 10.1097/SLA.0000000000003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mintz Y., Arezzo A., Boni L., et al. The risk of COVID-19 transmission by laparoscopic smoke may be lower than for laparotomy: a narrative review. Surg Endosc. 2020;34:3298–3305. doi: 10.1007/s00464-020-07652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies C.G., Khan M.N., Ghauri A.S., Ranaboldo C.J. Blood and body fluid splashes during surgery--the need for eye protection and masks. Ann R Coll Surg Engl. 2007;89:770–772. doi: 10.1308/003588407X209301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianco F., Incollingo P., Grossi U., Gallo G. Preventing transmission among operating room staff during COVID-19 pandemic: the role of the Aerosol Box and other personal protective equipment. Updates Surg. 2020;72:907–910. doi: 10.1007/s13304-020-00818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallo G., La Torre M., Pietroletti R., et al. Italian society of colorectal surgery recommendations for good clinical practice in colorectal surgery during the novel coronavirus pandemic. Tech Coloproctol. 2020;24:501–505. doi: 10.1007/s10151-020-02209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spinelli A., Pellino G. COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg. 2020;107:785–787. doi: 10.1002/bjs.11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brindle M.E., Gawande A. Managing COVID-19 in surgical systems. Ann Surg. 2020;272:e1–e2. doi: 10.1097/SLA.0000000000003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Englehardt R.K., Nowak B.M., Seger M.V., Duperier F.D. Contamination resulting from aerosolized fluid during laparoscopic surgery. JSLS. 2014;18 doi: 10.4293/JSLS.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hojaij F.C., Chinelatto L.A., Boog G.H.P., Kasmirski J.A., Lopes J.V.Z., Sacramento F.M. Surgical practice in the current COVID-19 pandemic: A rapid systematic review. Clinics (Sao Paulo) 2020;75:e1923. doi: 10.6061/clinics/2020/e1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American College of Surgeons (ACS) Post-COVID-19 readiness checklist for resuming surgery. 2020. https://www.facs.org/covid-19/checklist
- 33.Nunoo-Mensah J.W., Giordano P., Chung-Faye G. COVID-19: An opportunity to reimagine colorectal cancer diagnostic testing—A new paradigm shift. Clin Col Can. 2020;19:227–230. doi: 10.1016/j.clcc.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coccolini F., Perrone G., Chiarugi M., et al. Surgery in COVID-19 patients: operational directives. World J Emerg Surg. 2020;15:25. doi: 10.1186/s13017-020-00307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Saverio S., Pata F., Gallo G., et al. Coronavirus pandemic and Colorectal surgery: practical advice based on the Italian experience. Colorectal Dis. 2020;22 doi: 10.1111/codi.15056. 625-364. [DOI] [PubMed] [Google Scholar]
- 36.Caricato M., Baiocchi G.L., Crafa F., et al. and the Italian Colorectal Anastomotic Leakage (iCral) study group Colorectal surgery in Italy during the Covid19 outbreak: a survey from the iCral study group. Updates Surg. 2020;72:249–257. doi: 10.1007/s13304-020-00760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.