Abstract
Liver damage involves oxidative stress and a progression from chronic hepatitis to hepatocellular carcinoma (HCC). The increased incidence of liver disease in Egypt and other countries in the last decade, coupled with poor prognosis, justify the critical need to introduce alternative chemopreventive agents that may protect against liver damage. The aim of this study was to evaluate the efficacy of exopolysaccharide-peptide (PSP) complex extracted from Pleurotus ostreatus as a hepatoprotective agent against diethylnitrosamine (DEN)/carbon tetrachloride (CCL4)-induced hepatocellular damage in rats. The levels of liver injury markers (ALT, AST and ALP) were substantially increased following DEN/CCl4 treatment. DEN/CCl4 - induced oxidative stress was confirmed by elevated levels of lipid peroxidation and decreased levels of superoxide dismutase, glutathione-S-transferase, and reduced glutathione. PSP reversed these alterations in the liver and serum, and provided protection evidenced by reversal of histopathological changes in the liver. The present study demonstrated that PSP extract from P. ostreatus exhibited hepatoprotective and antioxidant effects against DEN/CCl4-induced hepatocellular damage in rats. Given the high prevalence of HCV-related liver damage in Egypt, our results suggest further clinical evaluation of P. ostreatus extracts and their potential hepatoprotective effects in patients with liver disease.
Keywords: Pleurotus ostreatus, Liver, Antioxidants, Diethylnitrosamine
Highlights
-
•
Polysaccharo-peptide complex from Pleurotus ostreatus in Egypt is cytotoxic in the liver cancer cell line HepG2
-
•
Polysaccharo-peptide complex protects against chemically induced liver damage in rats
-
•
Polysaccharo-peptide complex activates the antioxidant system in the liver
-
•
Polysaccharo-peptide complex reverses the chemically induced hematotoxicity
1. Introduction
Medicinal mushrooms have been shown to stimulate immune function, contribute to glucose homoeostasis and to modulate detoxification, as well as exert anti-inflammatory, and anticancer activities (reviewed in Ref. [1]). They are rich sources of antioxidants such as thiamine (vitamin B1), riboflavin (vitamin B2), nicotinic acid (vitamin B3), biotin and ascorbic acid (vitamin C) [2], as well as carotenoids, polyphenolic compounds and flavonoids, which prevent free radical damage and reduce the risk of chronic diseases [3]. The genus Pleurotus comprises about 40 species, that are commonly referred to as ‘‘oyster mushrooms”. Pleurotus ostreatus extracts have been shown to exhibit hypolipidemic [3,4], immunostimulant, antiproliferative and antitumor activities [5,6], as well as anti-inflammatory, antioxidant [[7], [8], [9]], and antifungal activities [10].
Extracts from P. ostreatus, such as polysaccharide-peptide complex (PSP), possess hepatoprotective properties against liver damage caused by toxic chemicals. Aqueous extracts contain high concentrations of cysteine, methionine and aspartic acid. They improve the antioxidant status and were shown to revert hepatic damage [7,9]. Treatment of rats with polysaccharides from the P. ostreatus mycelium are reported to protect against carbon tetrachloride (CCl4)-induced hepatic damage [11].
Liver damage involves oxidative stress and generally a progression from steatosis to chronic hepatitis, fibrosis, cirrhosis and hepatocellular carcinoma (HCC) [12]. Egypt has one of the highest global burdens of hepatitis C virus (HCV) infection, with an estimated 10% in people between 15 and 59 years of age, and thousands die every year of the consequences of HCV, such as liver cirrhosis and HCC [13].
The antioxidant enzymes catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (Gpx) act in concert to protect cellular components from damage by free radicals [14,15]. The reduction of their activity is associated with the accumulation of oxidants, leading to loss of integrity and function of cell membranes, contributing to cell injury. GSH is an important non-enzymatic antioxidant which promotes the detoxification of several toxic metabolites. The depletion of GSH promotes generation of reactive oxygen species (ROS) and oxidative stress with a cascade of effects thereby affecting functional as well as structural integrity of cell and organelle membranes [16]. The GSTs are a multigene family of isozymes that catalyse the conjugation of GSH to a variety of electrophilic compounds, and thereby exert a critical role in cellular protection against ROS [17].
Liver injury results in an increase in serum concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Both aminotransferases are highly concentrated in the liver. Alkaline phosphatase (ALP), another indicator of liver damage, is mainly derived from the liver and bones in healthy adults [18]. An increase in serum ALP levels is frequently associated with a variety of diseases, such as extrahepatic bile obstruction, intrahepatic cholestasis, infiltrative liver disease and hepatitis [19].
The increased incidence of liver disease in Egypt in the last decade, coupled with the poor prognosis, justify the critical need to introduce alternative chemopreventive agents that may protect against liver damage. We have previously shown that β-glucans extracted in our labs from the inedible mushroom Schizophylum commune reduced the incidence of HCC in dimyethyl-benz [a]anthracene (DMBA)-treated mice and this effect was coupled with an increase in apoptosis [20]. Using a similar approach, the aims of the present study were (1) to extract crude polysaccharo peptide complex (PSP) from P. ostreatus, and (2) to study its potential hepatoprotective and antioxidant effects in an experimental model of DEN/CCl4-induced hepatotoxicity in rats.
2. Materials and methods
2.1. Preparation of the mushroom extract by fermentation
Pure mycelia of Pleurotus ostreatus were obtained from the Agricultural Research Center (Giza, Egypt). The culture was maintained on potato-dextrose agar (PDA) and sub-cultured every month. The mushroom extract was prepared according to [21] (supplementary data). Total protein and total sugar contents were determined by Lowry method [22] using BSA as standard and by phenol sulfuric acid method [23] using glucose as standard, respectively.
2.2. Spectroscopy and nuclear magnetic resonance
Ultraviolet and infrared absorptions were determined as described in [20]. The 1H and 13C nuclear magnetic resonance (NMR) spectroscopy was performed according to the method described by [24]. NMR spectra were obtained using a 500 MHz JEOL spectrometer (Japan).
2.3. Animals
Male albino Wistar rats were purchased from the National Research Center for biological products (Cairo, Egypt). Animal care and the experimental protocols were in accordance with the guidelines of the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). Five animals were housed per cage and kept on standard diet (mouse chow) and water ad libitum, 22 °C room temperature (RT), 50 ± 10% humidity and 12-h light/dark cycle. The food was withdrawn 18–24 h before the experiment.
2.4. Induction of hepatotoxicity and experimental animal groups
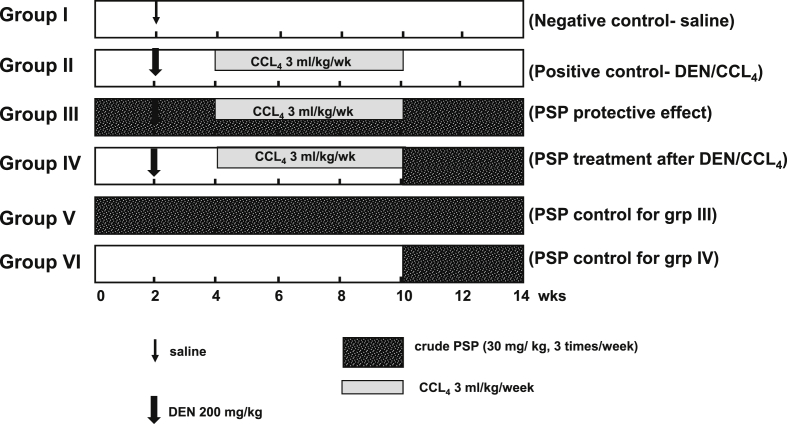
Forty-Eight male albino Wistar rats weighing 100–150 g were randomly divided into six groups of eight rats each (Fig. 1). Group I received only saline solution intraperitoneal (i.p). Groups II, III and IV each received one dose of 200 mg/kg body weight of DEN. After 2 weeks of DEN administration, hepatotoxicity was promoted through subcutaneous (Sc) injections of CCl4 (3 ml/kg/week) for 6 weeks [25]. Group II served as positive (toxin) control. In order to study whether early administration of PSP would have a protective effect against DEN/CCl4 hepatoxicity, Group III received PSP extract at a dose of 30 mg/kg body weight, 3 times/week, i.p for 2 weeks before DEN administration and for the remaining 12 weeks of the experiment. The PSP dose was selected based on a preliminary acute toxicity test (Table S1). Group IV received 30 mg/kg body weight of PSP, 3 times/week, i.p for the last 4 weeks of the experiment after DEN/CCl4 treatment. Group V: Served as a control group for group III and received the same PSP dose for 14 weeks. Group VI: Served as a control group for group IV and received the same PSP dose for the last 4 weeks of the experiment.
Fig. 1.
Experimental groups: Group 1 was treated with saline, group II with DEN/CCL4 at the doses shown, group III received PSP at the dose shown for 14 weeks, group IV received the same dose of PSP for 4 weeks after administration of DEN/CCL4. Groups V and VI served as PSP controls for groups III and IV, respectively.
2.5. Cell culture and cell viability
HepG2 cell line (DSMZ, Braunschweig, Germany) was cultured in RPMI 1640 (Gibco, Paisley, UK) with 10% fetal bovine serum, penicillin (100 units/ml), streptomycin (50 μg/ml) (Hyclone, Logan, UT) at 37 °C in a humidified atmosphere containing 5% CO2 and confirmed authentic using Short Tandem Repeat kit AmpFSTR identifier (Applied Biosystems, Foster City, CA). HepG2 cells were plated in 96-well plates at 104 cells/well and treated after 24 h with serial dilutions of the crude partially soluble PSP in DMSO. Cell viability was assessed after 48 h PSP exposure using Sulforhodamine B (SRB) stain.
2.6. Tissue sampling and biochemical assays
Animals were subjected to mild ether anesthesia following overnight fast, blood was collected from the abdominal aorta and the serum was separated by centrifugation and stored at −80 °C for evaluation of the following parameters using commercially available kits (Biosystem S.A., Spain): ALT, AST and ALP. Whole blood was collected in EDTA-coated tubes and used for the determination of WBCs, RBCs and Hb. Cells were analyzed using a flow cytometry-based Advia 2120 Hematology system. The liver was washed twice with ice-cold saline. A portion of the liver was homogenized (10%, w/v) in ice-cold sodium potassium phosphate buffer (0.01 M, pH 7.4) containing 1.15% KCl. The homogenate was centrifuged at 10,000×g for 20 min at 4 °C, and the supernatant was used for the determination of: LPO [26]; SOD [27]; GSH [28]; and GST activity [29].
2.7. Histopathology
Liver sections were cut immediately, fixed in 10% neutral buffered formalin, processed, and embedded in paraffin. Sections (4–5 μm) were prepared and mounted on glass slides and stained with hematoxylin and eosin (H&E) for histopathological evaluation.
2.8. Statistical analyses
Data were expressed as mean ± SD for 8 rats per group. Comparison between the means of various treatment groups was analyzed using least significant difference (LSD) test. A value of p < 0.05 was considered significant. Statistical analysis was performed using the SPSS statistical package version 16.00 (SPSS Inc, Chicago, Illinois, USA).
3. Results
3.1. Extraction and structural analysis of the exopolysaccharide-peptide (PSP) complex from Pleurotus ostreatus
Ethanol precipitation of PSP and its elemental analysis, as well as results from IR and 13C NMR are shown in the supplementary data (Figs. S1–7). The partially soluble PSP had a total carbohydrate content of 88.85% and 5.3% of soluble protein.
3.2. PSP extract is cytotoxic to HepG2 liver cancer cell line
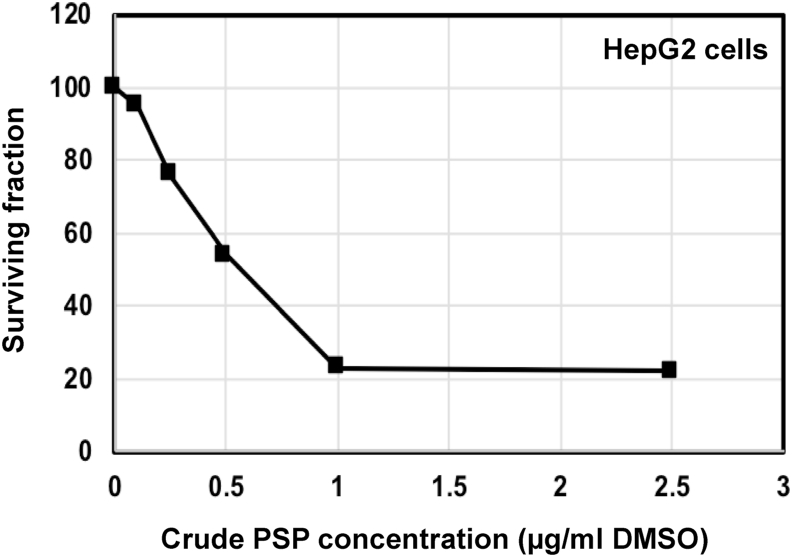
The viability of the human liver cancer cell line (HepG2) was measured after the addition of increasing concentrations of PSP (0, 1, 2.5, 5 and 10 μg/ml DMSO) for 48 h (Fig. 2). The IC50 was inferred from the graph to be 0.5 μg/mL.
Fig. 2.
Cytotoxicity of PSP extract from P. ostreatus in HepG2 cell line.
3.3. Effect of PSP extract on serum biomarkers of liver damage
Administration of DEN/CCL4 induced liver damage but no liver tumors were observed. Hepatotoxicity was confirmed by histopathology. Treatment with DEN/CCL4 induced a significant 6-fold increase in serum ALT levels compared to control indicating liver damage (Group II). Administration of PSP to groups III and IV significantly reduced serum ALT levels almost back to normal. A similar pattern of results was obtained for other serum biomarkers; a significant 2- and 1.5-fold increase in AST and ALP levels, respectively, was observed in group II animals, compared to all other experimental groups. This effect was reversed upon treatment with PSP extract (Group III, IV). There was no significant difference in ALT, AST or ALP levels between groups III, IV, V and VI compared to negative control (Group I) or between groups III and V, or IV and VI, respectively (Table 1).
Table 1.
PSP extract reverses the hepatotoxic effect of DEN/CCl4 on ALT, AST and ALP levels.
| Experimental Groups |
Group I |
Group II |
Group III |
Group IV |
Group V |
Group VI |
|---|---|---|---|---|---|---|
| ALT (U/L) (mean ± SD) |
39.57 ± 2.60 | 243.20 ± 84.73 | 45.83 ± 2.63 | 34.90 ± 4.68 | 36.15 ± 3.80 | 30.09 ± 3.74 |
| F (p) | 35.254* (<0.001) | |||||
| P1 | <0.001* | 0.757 | 0.818 | 0.866 | 0.640 | |
| P2 | <0.001* | <0.001* | <0.001* | <0.001* | ||
| P3 | 0.633 | |||||
| P4 | 0.812 | |||||
|
AST (U/L) (mean ± SD) |
158.67 ± 18.75 | 338.67 ± 103.56 | 190.17 ± 21.85 | 139.00 ± 19.96 | 177.17 ± 29.46 | 145.50 ± 11.24 |
| F (p) | 15.469* (<0.001) | |||||
| p1 | <0.001* | 0.249 | 0.469 | 0.496 | 0.627 | |
| p2 | <0.001* | <0.001* | <0.001* | <0.001* | ||
| p3 | 0.631 | |||||
| p4 | 0.810 | |||||
|
ALP (U/L) (mean ± SD) |
168.33 ± 48.14 | 268.83 ± 26.41 | 186.17 ± 62.37 | 198.00 ± 20.79 | 192.50 ± 19.09 | 179.83 ± 57.65 |
| F (p) | 4.174* (0.005) | |||||
| p1 | <0.001* | 0.477 | 0.240 | 0.337 | 0.646 | |
| p2 | 0.002* | 0.008* | 0.004* | 0.001* | ||
| p3 | 0.800 | |||||
| p4 | 0.469 | |||||
F: F test (ANOVA), p1: p value of LSD test between negative control and other groups, p2: p value of LSD test between group II and group III, IV, V and VI, p3: p value of LSD test between group III and group V, p4: p value of LSD test between group IV and group VI, *: Statistically significant at p ≤ 0.05.
3.4. Effect of PSP extract on oxidative stress biomarkers
Treatment of rats with DEN/CCL4 resulted in a 1.4-fold significant increase in the level of hepatic lipid peroxidation (LPO) compared to group I. Administration of PSP to groups III and IV resulted in a significant decrease in hepatic LPO levels by 1.6- and 1.5-fold, respectively, compared to group II rats.
A highly significant decrease in hepatic LPO was shown in group V that received only PSP for the 14 weeks of the experiment (control group for group III) compared to groups I, II and III. However, the level of hepatic LPO did not show any significant difference in rats receiving PSP only for 4 weeks (Group VI) when compared to either group IV or to group I rats (Table 2).
Table 2.
PSP extract reduces hepatic lipid peroxidation in rats treated with DEN/CCl4.
| Experimental Groups |
Group I |
Group II |
Group III |
Group IV |
Group V |
Group VI |
|---|---|---|---|---|---|---|
| LPO (nmol/g liver) (mean ± SD) |
153.02 ± 27.58 | 217.03 ± 18.81 | 134.28 ± 20.72 | 147.19 ± 18.04 | 105.39 ± 20.38 | 144.43 ± 8.25 |
| F (p) | 20.746* (<0.001) | |||||
| p1 | <0.001* | <0.112 | 0.614 | <0.001* | <0.458 | |
| p2 | <0.001* | <0.001* | <0.001* | <0.001* | ||
| p3 | 0.002* | |||||
| p4 | 0.268 | |||||
F: F test (ANOVA), p1: p value of LSD test between negative control and other groups, p2: p value of LSD test between group II and group III, IV, V and VI, p3: p value of LSD test between group III and group V, p4: p value of LSD test between group IV and group VI, *: Statistically significant at p ≤ 0.05.
Administration of DEN/CCl4 (Group II) resulted in a significant decrease in both serum and hepatic SOD, as well as in hepatic GST activity compared to their corresponding values in the negative control group I rats. Administration of PSP reversed this effect in groups III and IV (Table 3).
Table 3.
Effect of PSP extract on serum and hepatic superoxide dismutase (SOD), hepatic glutathione -S- transferase (GST) activities, and GSH concentration.
| Experimental Groups |
Group I |
Group II |
Group III |
Group IV |
Group V |
Group VI |
|---|---|---|---|---|---|---|
| Serum SOD (mean ± SD) | 7.72 ± 2.33 | 5.84 ± 0.83 | 6.62 ± 0.99 | 6.84 ± 0.98 | 10.23 ± 1.54 | 8.47 ± 1.09 |
| F (p) | 7.619* (<0.001) | |||||
| p1 | 0.026* | 0.180 | 0.285 | 0.004* | 0.357 | |
| p2 | 0.342 | 0.222 | <0.001* | 0.003* | ||
| p3 | <0.001* | |||||
| p4 | 0.052 | |||||
| Hepatic SOD (mean ± SD) | 1.16 ± 0.07 | 0.68 ± 0.05 | 0.81 ± 0.04 | 0.89 ± 0.13 | 0.93 ± 0.15 | 1.00 ± 0.16 |
| F (p) | 13.241* (<0.001) | |||||
| p1 | <0.001* | <0.001* | <0.001* | 0.001* | 0.017* | |
| p2 | 0.045* | 0.003* | <0.001* | <0.001* | ||
| p3 | 0.077 | |||||
| p4 | 0.089 | |||||
| Hepatic GST (mean ± SD) | 2.76 ± 0.71 | 1.44 ± 0.30 | 2.34 ± 0.68 | 2.24 ± 0.42 | 2.86 ± 0.64 | 3.05 ± 0.36 |
| F (p) | 6.890* (<0.001) | |||||
| p1 | <0.001* | 0.183 | 0.107 | 0.757 | 0.372 | |
| p2 | 0.008* | 0.016* | <0.001* | <0.001* | ||
| p3 | 0.104 | |||||
| p4 | 0.016* | |||||
|
GSH (mg/g liver) (mean ± SD) |
115.36 ± 51.18 | 43.01 ± 11.89 | 100.58 ± 22.26 | 83.38 ± 11.20 | 109.85 ± 40.46 | 89.55 ± 31.43 |
| F (p) | 4.077* (0.006) | |||||
| p1 | <0.001* | 0.425 | 0.090 | 0.765 | 0.168 | |
| p2 | 0.013* | 0.035* | 0.001* | 0.016 | ||
| p3 | 0.616 | |||||
| p4 | 0.738 | |||||
F: F test (ANOVA).
p1: p value of LSD test between negative control and other groups.
p2: p value of LSD test between group II and group III, IV, V and VI.
p3: p value of LSD test between group III and group V.
p4: p value of LSD test between group IV and group VI.
*: Statistically significant at p ≤ 0.05.
The activity of both serum SOD and hepatic GST in groups III and IV, which received PSP either before or after the hepatotoxins, did not show any significant change compared to the corresponding values in group I rats. Serum SOD in group V rats that served as control for group III and received PSP for 14 weeks, showed highly significant increase compared to rats of both group I and group III (Table 3).
DEN/CCl4 treated group (Group II) showed a significant 2.7-fold decrease in the level of hepatic GSH compared to the hepatic GSH in the control group I. Administration of PSP in groups III and IV reversed this effect resulting in hepatic GSH concentrations approaching that in the control group I (Table 3).
3.5. PSP extract increases WBCs, RBCs and haemoglobin concentration in rats treated with DEN/CCl4
DEN/CCl4 caused a significant decrease in WBCs and RBCs count as well as in the hemoglobin (Hb) levels in group II rats compared to all other groups (Table 4). Treatment with PSP significantly alleviated this hematotoxicity, bringing RBCs count and Hb levels to levels approximating those of control group I rats.
Table 4.
PSP extract increases white (WBCs), red (RBCs) cells’ counts and hemoglobin (Hb) concentration in rats treated with DEN/CCl4.
| Experimental Groups | Group I | Group II | Group III | Group IV | Group V | Group VI | |
|---|---|---|---|---|---|---|---|
| WBCs count (#*103/L) (mean ± SD) |
11.40 ± 3.54 | 6.70 ± 1.14 | 16.65 ± 7.23 | 11.73 ± 2.63 | 16.24 ± 3.00 | 15.98 ± 3.17 | |
| F (p) | 5.165* (0.003) | ||||||
| p1 | 0.076 | 0.062 | 0.904 | 0.069 | 0.084 | ||
| p2 | 0.001* | 0.060 | 0.001* | 0.001* | |||
| RBCs count (#*106/L) (mean ± SD) |
7.78 ± 0.22 | 4.88 ± 0.95 | 7.45 ± 0.42 | 7.63 ± 0.59 | 7.51 ± 0.33 | 7.75 ± 0.55 | |
| F (p) | 18.600* (<0.001) | ||||||
| p1 | <0.001* | 0.424 | 0.696 | 0.485 | 0.932 | ||
| p2 | <0.001* | <0.001* | <0.001* | <0.001* | |||
| Hb (g/dl) (mean ± SD) |
12.78 ± 1.44 | 7.92 ± 1.63 | 10.93 ± 0.63 | 11.32 ± 0.83 | 11.98 ± 0.81 | 12.25 ± 0.64 | |
| F (p) | 12.151* (<0.001) | ||||||
| p1 | <0.001* | 0.025* | 0.058 | 0.286 | 0.501 | ||
| p2 | <0.001* | <0.001* | <0.001* | <0.001* | |||
F: F test (ANOVA).
p1: p value of LSD test between negative control and other groups.
p2: p value of LSD test between group II and group III, IV, V and VI.
*: Statistically significant at p ≤ 0.05.
3.6. Histopathology
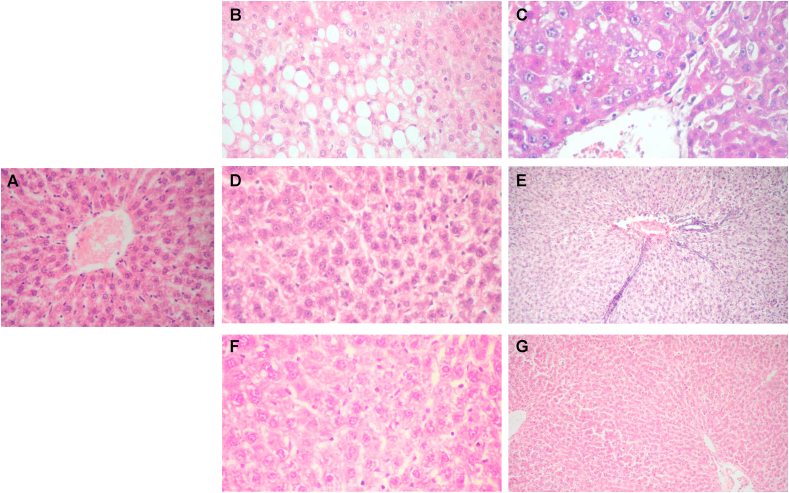
Liver sections from control rats (Group I) showed normal arrangement of hepatocytes (Fig. 3A). Rats treated with DEN/CCL4 (Group II) showed mild portal mononuclear inflammatory infiltrate, fibrosis, lytic necrosis, frequent apoptotic bodies and distended portal veins. Steatosis manifest by marked accumulation of lipids (Fig. 3B), as well as dysplastic hepatocytes with karyomegaly and prominent macronucleoli (Fig. 3C) were also evident. Group III rats showed more or less normal liver architecture (Fig. 3D), however, rats post-treated with PSP (Group IV), showed minimal portal inflammatory infiltrate (Fig. 3E). Groups V and VI treated only with PSP showed minimal changes (Fig. 3F and G).
Fig. 3.
H&E stained sections of liver from a negative control rat (Group I) showing intact normal hepatic architecture (X100) (A). DEN/CCL4 -treated rats (Group II) showed mild portal mononuclear inflammatory infiltrate and marked macrosteatotic changes of the hepatocytes (X400) (B), markedly dysplastic hepatocytes with karyomegaly and prominent macronucleoli (X400) (C). Group III rats demonstrated almost normal hepatic architecture (X400) (D), while group IV rats showed minimal portal inflammatory infiltrate (X100) (E). Administration of PSP for 14 weeks (Group V) (X400) (F), or for the last 4 weeks of the experiment (Group VI) showed almost normal morphology (X100) (G).
4. Discussion
Despite significant advances in surgical treatments and locoregional therapies, prognosis is still poor for patients with liver disease, and for those with recurrent hepatocellular carcinoma (HCC). Therefore, chemoprevention, and lifestyle changes are important as a supportive approach for the protection from liver disease. Most cases of HCC in humans develop in the setting of chronic liver disease, characterized by inflammation, cirrhosis, fibrosis and steatosis [30]. In the present study we used the DEN/CCl4-model to mimic liver damage. This disease model can be used for studying fibrogenesis and HCC [31]. The goal of this study was to characterize the in vivo putative hepatoprotective and antioxidant effects of P. ostreatus rather than it's anticancer activity. The present study demonstrated that the exopolysaccharide peptide complex (PSP), isolated from P. ostreatus exhibited hepato-, haemato-protective, and antioxidant activities in DEN/CCl4-treated rats.
Furthermore, PSP extract was cytotoxic in the human liver cancer cell line HepG2. These results are consistent with those of [32] in human colorectal and leukaemia cell lines, with [33] against gastric cancer in vitro and in vivo, and with [34] in HepG2, HCT 116 and HeLa cell lines. Additionally, previous reports demonstrated that extracts from P. ostreatus suppressed proliferation of breast cancer (MCF-7, MDA-MB-231) and colon cancer (HT-29, HCT-116) cells, without affecting proliferation of epithelial mammary MCF-10A and normal colon FHC cells [5]. In a similar study [35], showed that the normal liver cell line WRL-68 was more resistant than the liver cancer cell lines (Huh7, HepG2, SMMC-7721 and Hep3B) to the cytotoxic effects of a polysaccharide-protein (PSP) complex isolated from Pleurotus pulmonarius. Protein extracts from P. ostreatus were found to induce apoptosis in SW480 cells through ROS production, GSH depletion and mitochondrial dysfunction [32].
DEN and CCl4 generate reactive oxygen species (ROS), which cause cellular injury [15]. In the present study, DEN/CCl4 increased serum ALT, AST and ALP levels concomitant with histopathological modifications in the liver reflecting the induction of acute hepatotoxicity and hepatic structural damage [25,36]. PSP reduced ALT, AST and ALP activities suggesting a potential reversal of liver injury, accompanied with an improved histomorphology of the liver. This is consistent with previous work by Refs. [7,36] on P. ostreatus extracts, by Ref. [37] on silymarin, and by Ref. [38] on silymarin and Anji white tea polyphenols.
Bioactivation of DEN and CCl4 by cytochrome P450 system in the liver yields highly reactive free radicals that can react with proteins or lipids, or abstract a hydrogen atom from an unsaturated lipid, thus initiating lipid peroxidation [36,37]. Free radical scavenging enzymes such as SOD, CAT, GPx and GST are the first line of defence against oxidative injury. The inhibition of antioxidant system may cause the accumulation of H2O2 or products of its decomposition [17]. In the present study, the exposure to DEN/CCl4 resulted in increased levels of hepatic lipid peroxides and depleted hepatic SOD, GST activity as well as reduced GSH concentration. PSP extract-treated animals showed a significant reduction in the levels of hepatic oxidation markers with concomitant amelioration in the hepatic antioxidant defence system, which could be related to its ability to scavenge ROS, thus preventing further damage to membrane lipids. Among the antioxidant compounds in the oyster mushroom are polyphenols, which can act as hydrogen donors, to neutralize ROS and inhibit the formation of O2- and OH-, the main inducers of lipid peroxidation [39]. Our results are in agreement with reports by Refs. [14] showing the antioxidant effects of a PSP complex extracted from P. abalonus-fruiting bodies on senescence-accelerated mice. Similar results were reported by Ref. [40] using Paraquat as liver damage inducer.
In the present study, the decrease in haematological parameters (WBCs, RBCs and Hb), in DEN/CCl4-treated rats, is in agreement with [41]. The destruction of RBCs reflects the abnormality in hepatocellular functions and potential changes in the membrane cholesterol and phospholipid ratio. One of potential mechanism of DEN/CCl4 -induced hemolysis is oxidative stress induction as reflected in this study by increased lipid peroxidation. This effect is similar to the effect of cadmium-induced haematological toxicity [42]. The administration of PSP caused an amelioration of the haematological parameters (WBCs; RBCs count & Hb level) probably through lowering lipid peroxidation levels in cell membranes; leading to decreased haemolysis of RBCs and/or due to the prevention of free radicals induced damage. Similar results were reported by Ref. [43]. The increase in WBCs counts in the present study may be due to an activation of immune response [44] as reported by Ref. [45] that polysaccharides from P ostreatus induced NK-cell mediated cytotoxicity against lung and breast cancer cells mediated by NKG2D and IFNγ upregulation.
The present study has some limitations in the fact that the active component in the polysaccharide-peptide complex (PSP) extract from P. ostreatus has not been identified. Extracts contain several compounds, and future research should focus on isolation and characterization of the active therapeutic compound in the PSP extract. Additionally, although our goal, in the present study, was to investigate the hepatoprotective effects of PSP against liver injury, it would have been relevant to investigate it's antineoplastic effects on HCC, as well. Future preclinical studies should be directed towards studying the interaction of PSP with conventional chemo- and radiotherapy, as well as with targeted therapy in liver and other types of malignancies.
5. Conclusion
In conclusion, this study confirms that extracts from locally available oyster mushroom (P. ostreatus) have hepatoprotective, and antioxidant effects against liver damage induced in rats by DEN/CCL4. Hence, it is recommended to invest in the commercial cultivation of oyster mushroom in Egypt for nutritional and medicinal purposes. Since Egypt is one of the countries with high prevalence of hepatitis C infection, our results suggest further clinical evaluation of P. ostreatus and its potential hepatoprotective effects in patients with liver disease.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Nihad M. Abdel-Monem: Conceptualization, Project administration, Supervision, Validation, Writing - review & editing. Mohammad A. El-Saadani: Conceptualization, Project administration, Supervision, Validation, Writing - review & editing. Ayman S. Daba: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing - review & editing. Samar R. Saleh: Data curation, Formal analysis, Investigation, Methodology, Resources, Writing - original draft, Roles/Writing - original draft, Writing - review & editing. Eiman Aleem: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing - original draft, Roles/Writing - original draft, Writing - review & editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100852.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Aleem E. β-Glucans and their applications in cancer therapy: focus on human studies. Anticancer Agents Med Chem. 2013;13:709–719. doi: 10.2174/1871520611313050005. [DOI] [PubMed] [Google Scholar]
- 2.Mallavadhani U.V., Sudhakar A.V.S., Satyanarayana K.V.S., Mahapatra A., Li W., vanBreemen R.B. Chemical and analytical screening of some edible mushrooms. Food Chem. 2006;95:58–64. [Google Scholar]
- 3.Alam N., Yoon K.N., Lee T.S., Lee U.Y. Hypolipidemic activities of dietary Pleurotus ostreatus in hypercholesterolemic rats. MYCOBIOLOGY. 2011;39:45–51. doi: 10.4489/MYCO.2011.39.1.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobek P., Ozdin L., Galbavy S. Dose- and time-dependent hypocholesterolemic effect of oyster mushroom (Pleurotus ostreatus) in rats. Nutrition. 1998;14:282–286. doi: 10.1016/s0899-9007(97)00471-1. [DOI] [PubMed] [Google Scholar]
- 5.Jedinak A., Sliva D. Pleurotus ostreatus inhibits proliferation of human breast and colon cancer cells through p53-dependent as well as p53-independent pathway. Int. J. Oncol. 2008;33:1307–1313. [PMC free article] [PubMed] [Google Scholar]
- 6.Tong H., Xia F., Feng K., Sun G., Gao X., Sun L., Jiang R., Tian D., Sun X. Structural characterization and in vitro antitumor activity of a novel polysaccharide isolated from the fruiting bodies of Pleurotus ostreatus. Bioresour. Technol. 2009;100:1682–1686. doi: 10.1016/j.biortech.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Jayakumar T., Ramesh E., Geraldine P. Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl(4)-induced liver injury in rats. Food Chem. Toxicol. 2006;44:1989–1996. doi: 10.1016/j.fct.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Jayakumar T., Sakthivel M., Thomas P.A., Geraldine P. Pleurotus ostreatus, an oyster mushroom, decreases the oxidative stress induced by carbon tetrachloride in rat kidneys, heart and brain. Chem. Biol. Interact. 2008;176:108–120. doi: 10.1016/j.cbi.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Jayakumar T., Thomas P.A., Geraldine P. Protective effect of an extract of the oyster mushroom, Pleurotus ostreatus, on antioxidants of major organs of aged rats. Exp. Gerontol. 2007;42:183–191. doi: 10.1016/j.exger.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Chu K.T., Xia L., Ng T.B. Pleurostrin, an antifungal peptide from the oyster mushroom. Peptides. 2005;26:2098–2103. doi: 10.1016/j.peptides.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Zhu B., Li Y., Hu T., Zhang Y. The hepatoprotective effect of polysaccharides from Pleurotus ostreatus on carbon tetrachloride-induced acute liver injury rats. Int. J. Biol. Macromol. 2019;131:1–9. doi: 10.1016/j.ijbiomac.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 12.Kodavanti P.R., Joshi U.M., Young R.A., Meydrech E.F., Mehendale H.M. Protection of hepatotoxic and lethal effects of CCl4 by partial hepatectomy. Toxicol. Pathol. 1989;17:494–505. doi: 10.1177/019262338901700304. [DOI] [PubMed] [Google Scholar]
- 13.Population M.o.H.a. In: Plan of Action for the Prevention, Care & Treatment of Viral Hepatitis. Population H.a., editor. 2018. pp. 1–61.http://www.emro.who.int/images/stories/egypt/VH_Plan_of_Action_FINAL_PRINT1.pdf Egypt 2014-2018. Egypt. [Google Scholar]
- 14.Li L., Ng T.B., Song M., Yuan F., Liu Z.K., Wang C.L., Jiang Y., Fu M., Liu F. A polysaccharide-peptide complex from abalone mushroom (Pleurotus abalonus) fruiting bodies increases activities and gene expression of antioxidant enzymes and reduces lipid peroxidation in senescence-accelerated mice. Appl. Microbiol. Biotechnol. 2007;75:863–869. doi: 10.1007/s00253-007-0865-4. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q., Kong B., Li G., Liu N., Xia X. Hepatoprotective and antioxidant effects of porcine plasma protein hydrolysates on carbon tetrachloride-induced liver damage in rats. Food Chem. Toxicol. 2011;49:1316–1321. doi: 10.1016/j.fct.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Sreepriya M., Bali G. Effects of administration of Embelin and Curcumin on lipid peroxidation, hepatic glutathione antioxidant defense and hematopoietic system during N-nitrosodiethylamine/Phenobarbital-induced hepatocarcinogenesis in Wistar rats. Mol. Cell. Biochem. 2006;284:49–55. doi: 10.1007/s11010-005-9012-7. [DOI] [PubMed] [Google Scholar]
- 17.Pari L., Suresh A. Effect of grape (Vitis vinifera L.) leaf extract on alcohol induced oxidative stress in rats. Food Chem. Toxicol. 2008;46:1627–1634. doi: 10.1016/j.fct.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Sj F.L.M.P.M. Liver Function tests and the objective evaluation of the patient with liver disease. In: Zakim D a.B.T., editor. Hepatology: A Textbook of Liver Disease. WB Saunders; Philadelphia, USA: 1996. pp. 791–833. [Google Scholar]
- 19.Wiwanitkit V. High serum alkaline phosphatase levels, a study in 181 Thai adult hospitalized patients. BMC Fam. Pract. 2001;2:2. doi: 10.1186/1471-2296-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansour A., Daba A., Baddour N., El-Saadani M., Aleem E. Schizophyllan inhibits the development of mammary and hepatic carcinomas induced by 7,12 dimethylbenz(alpha)anthracene and decreases cell proliferation: comparison with tamoxifen. J. Canc. Res. Clin. Oncol. 2012;138:1579–1596. doi: 10.1007/s00432-012-1224-0. [DOI] [PubMed] [Google Scholar]
- 21.Sarangi I., Ghosh D., Bhutia S.K., Mallick S.K., Maiti T.K. Anti-tumor and immunomodulating effects of Pleurotus ostreatus mycelia-derived proteoglycans. Int. Immunopharm. 2006;6:1287–1297. doi: 10.1016/j.intimp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Dubois M.G., Hamilton K.A., J K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- 24.Abraham P R.J.L. Heyden; London: 1978. Proton and Carbon-13 NMR Spectroscopy:an Integrated Approach. [Google Scholar]
- 25.Shaarawy S.M., Tohamy A.A., Elgendy S.M., Elmageed Z.Y., Bahnasy A., Mohamed M.S., Kandil E., Matrougui K. Protective effects of garlic and silymarin on NDEA-induced rats hepatotoxicity. Int. J. Biol. Sci. 2009;5:549–557. doi: 10.7150/ijbs.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 27.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 28.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 29.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 30.Shankaraiah R.C., Gramantieri L., Fornari F., Sabbioni S., Callegari E., Negrini M. Animal models of hepatocellular carcinoma prevention. Cancers. 2019:11. doi: 10.3390/cancers11111792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uehara T., Pogribny I.P., Rusyn I. The DEN and CCl4 -induced mouse model of fibrosis and inflammation-associated hepatocellular carcinoma. Curr Protoc Pharmacol. 2014;66:14 30 11–10. doi: 10.1002/0471141755.ph1430s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J.Y., Chen C.H., Chang W.H., Chung K.T., Liu Y.W., Lu F.J., Chen C.H. Anti-cancer effects of protein extracts from calvatia lilacina, Pleurotus ostreatus and volvariella volvacea. Evid Based Complement Alternat Med. 2011;2011:982368. doi: 10.1093/ecam/neq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao X.Y., Liu J.L., Yang W., Hou X., Li Q.J. Antitumor activity of polysaccharide extracted from Pleurotus ostreatus mycelia against gastric cancer in vitro and in vivo. Mol. Med. Rep. 2015;12:2383–2389. doi: 10.3892/mmr.2015.3648. [DOI] [PubMed] [Google Scholar]
- 34.Younis A.S., Wu J., F S., El Shikh H., Hassan F., Elaasser M. Effectiveness of different solvents extracts from edible mushrooms in inhibiting the growth of tumor cells. Cancer Biology. 2014;4:1–15. [Google Scholar]
- 35.Xu W., Huang J.J., Cheung P.C. Extract of Pleurotus pulmonarius suppresses liver cancer development and progression through inhibition of VEGF-induced PI3K/AKT signaling pathway. PloS One. 2012;7 doi: 10.1371/journal.pone.0034406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nada S.A., Omara E.A., Abdel-Salam O.M., Zahran H.G. Mushroom insoluble polysaccharides prevent carbon tetrachloride-induced hepatotoxicity in rat. Food Chem. Toxicol. 2010;48:3184–3188. doi: 10.1016/j.fct.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Pradeep K., Mohan C.V., Gobianand K., Karthikeyan S. Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur. J. Pharmacol. 2007;560:110–116. doi: 10.1016/j.ejphar.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Wang R., Yang Z., Zhang J., Mu J., Zhou X., Zhao X. Liver injury induced by carbon tetrachloride in mice is prevented by the antioxidant capacity of Anji white tea polyphenols. Antioxidants (Basel) 2019 Mar;8(3):64. doi: 10.3390/antiox8030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez C. Reactive oxygen species and antioxidant properties from mushrooms. Synth Syst Biotechnol. 2017;2:13–22. doi: 10.1016/j.synbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutyarso E.S. Taurine and oyster mushroom (Pleurotus ostreatus) prevents oxidative damage in liver of mice induced by Paraquat. Biomedical and Pharmacology Journal. 2017;10:1993–2000. [Google Scholar]
- 41.El-Sheikh N.M., Khalil F.A. L-arginine and L-glutamine as immunonutrients and modulating agents for oxidative stress and toxicity induced by sodium nitrite in rats. Food Chem. Toxicol. 2011;49:758–762. doi: 10.1016/j.fct.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 42.Andjelkovic M., Buha Djordjevic A., Antonijevic E., Antonijevic B., Stanic M., Kotur-Stevuljevic J., Spasojevic-Kalimanovska V., Jovanovic M., Boricic N., Wallace D., Bulat Z. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int. J. Environ. Res. Publ. Health. 2019;16 doi: 10.3390/ijerph16020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ubhenin A.N., C C., Dingwoke E.J. Protective effects of Pleurotus ostreatus in ameliorating carbon tetrachloride (ccl4) induced liver injury in Wistar rats. Med. Plant Res. 2019;13:104–111. [Google Scholar]
- 44.Oluyemi K.A., Omotuyi I.O., Jimoh O.R., Adesanya O.A., Saalu C.L., Josiah S.J. Erythropoietic and anti-obesity effects of Garcinia cambogia (bitter kola) in Wistar rats. Biotechnol. Appl. Biochem. 2007;46:69–72. doi: 10.1042/BA20060105. [DOI] [PubMed] [Google Scholar]
- 45.El-Deeb N.M., El-Adawi H.I., El-Wahab A.E.A., Haddad A.M., El Enshasy H.A., He Y.W., Davis K.R. Modulation of NKG2D, KIR2DL and cytokine production by Pleurotus ostreatus glucan enhances natural killer cell cytotoxicity toward cancer cells. Front Cell Dev Biol. 2019;7:165. doi: 10.3389/fcell.2019.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.