Abstract
Context:
Lipodystrophies are heterogeneous, genetic or acquired disorders characterized by selective loss of body fat and predisposition to insulin resistance. The extent of fat loss determines the severity of associated metabolic complications such as diabetes mellitus, hypertriglyceridemia, and hepatic steatosis.
Evidence Acquisition and Synthesis:
Both original and review articles were found via PubMed search reporting on clinical features and management of various types of lipodystrophies and were integrated with the author's knowledge of the field.
Conclusion:
The autosomal recessive congenital generalized lipodystrophy and autosomal dominant familial partial lipodystrophy (FPL) are the two most common types of genetic lipodystrophies. Mutations in AGPAT2, BSCL2, CAV1, and PTRF have been reported in congenital generalized lipodystrophy and in LMNA, PPARG, AKT2, and PLIN1 in FPL. CIDEC is the disease gene for autosomal recessive, FPL and LMNA and ZMPSTE24 for autosomal recessive, mandibuloacral dysplasia-associated lipodystrophy. Recently, an autosomal recessive autoinflammatory lipodystrophy syndrome was reported to be due to PSMB8 mutation. Molecular genetic bases of many rare forms of genetic lipodystrophies remain to be elucidated. The most prevalent subtype of acquired lipodystrophy currently occurs with prolonged duration of protease inhibitor-containing, highly-active antiretroviral therapy in HIV-infected patients. The acquired generalized and partial lipodystrophies are mainly autoimmune in origin and display complement abnormalities. Localized lipodystrophies occur due to drug or vaccine injections, pressure, panniculitis, and other unknown reasons. The current management includes cosmetic surgery and early identification and treatment of metabolic and other complications with diet, exercise, hypoglycemic drugs, and lipid-lowering agents.
Lipodystrophies are heterogeneous disorders characterized by selective loss of body fat (1, 2). Some investigators use the term “lipoatrophy” for these disorders. Although many patients develop lipodystrophy due to genetic defects, others develop it due to various acquired conditions. These disorders have been reported in the medical literature for more than 100 yr. Acquired partial lipodystrophy (APL) was the first one to be reported approximately 125 yr ago (3), followed by the acquired generalized variety (AGL) (4), and only about 56 yr ago (5), the phenotype of the first genetic variety, congenital generalized lipodystrophy (CGL), was reported. Interestingly, a new lipodystrophy syndrome was recognized in HIV-infected patients treated with highly active antiretroviral therapy (HAART) including HIV-1 protease inhibitors (PI) [abbreviated as LD-HIV (lipodystrophy in HIV-infected patients)] about 13 yr ago (6), which actually has become the most prevalent type currently among all lipodystrophies.
There is considerable heterogeneity related to the pattern and extent of fat loss among various types of lipodystrophies. In some patients, fat is lost from small, discrete areas (localized variety); in others from the limbs (partial variety); whereas some have fat loss from nearly the entire body (generalized variety). Although localized lipodystrophies mainly have cosmetic implications only, the partial and generalized lipodystrophies in addition predispose the patients to insulin resistance and its associated complications such as diabetes mellitus, hypertriglyceridemia, hepatic steatosis, polycystic ovaries, and acanthosis nigricans. The extent of fat loss determines the severity of metabolic and other complications (1, 2, 7).
In the last decade or so, considerable progress has been made in elucidating the molecular genetic basis of many types of inherited lipodystrophies, and to date 11 genetic loci have been discovered. A few novel genetic syndromes of lipodystrophies have also been reported, but their molecular basis remains to be elucidated. Some progress has also been made in understanding the pathogenesis of LD-HIV. This recent progress will be reviewed here.
Genetic Lipodystrophies (Table 1)
Table 1.
Classification, clinical features, and molecular basis of genetic lipodystrophies
| Type | Subtype (gene) | Key clinical features | Molecular basis/other comments |
|---|---|---|---|
| Autosomal recessive | |||
| Congenital generalized lipodystrophy (CGL)a | CGL1 (AGPAT2) | Lack of metabolically active adipose tissue since birth | AGPATs are key enzymes required for triglyceride and phospholipids biosynthesis. AGPATs acylate lysophosphatidic acid to form phosphatidic acid. AGPAT2 is highly expressed in adipose tissue. |
| CGL2 (BSCL2) | Lack of both metabolically active and mechanical adipose tissue since birth, mild mental retardation, cardiomyopathy | BSCL2 encodes seipin, which may play a role in fusion of small lipid droplets and in adipocyte differentiation. | |
| CGL3 (CAV1) | Single patient with extreme lack of body fat, short stature, and vitamin D resistance | Caveolin 1 is an integral component of caveolae, present in abundance on adipocyte membranes. Caveolin 1 binds fatty acids and translocates them to lipid droplets. | |
| CGL4 (PTRF) | Extreme lack of body fat, congenital myopathy, pyloric stenosis, and cardiomyopathy | PTRF (also known as cavin) is involved in biogenesis of caveolae and regulates expression of caveolins 1 and 3. | |
| Mandibuloacral dysplasia (MAD)a | Type A (LMNA) | Skeletal anomalies, loss of sc fat from the extremities and trunk | Lamins A and C are nuclear lamina proteins and LMNA mutations may disrupt nuclear function resulting in premature cell death in many tissues. |
| Type B (ZMPSTE24) | Skeletal anomalies, more generalized loss of fat, premature renal failure, progeroid features | ZMPSTE24 is required for posttranslational processing of carboxy-terminal residues of prelamin A to form lamin A. Accumulation of farnesylated prelamin A may disrupt nuclear function in several tissues. | |
| Autoinflammatory syndromes | JMP (PSMB8) | Joint contractures, Muscle atrophy, Microcytic anemia and Panniculitis-induced lipodystrophy | PSMB8 encodes β5i, a catalytic subunit of the immunoproteasomes. Immunoproteasome-mediated proteolysis generates immunogenic epitopes presented by MHC class I molecules. |
| CANDLE (Unknown) | Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated Temperature | New syndrome reported in six patients. | |
| Familial partial lipodystrophy (FPL) | (CIDEC) | Single patient with loss of sc fat from limbs, multilocular, small lipid droplets in adipocytes | CIDEC is a lipid droplet associated protein that inhibits lipolysis and promotes formation of unilocular lipid droplet in adipocytes. |
| SHORT syndrome | (Unknown) | Short stature, Hyperextensibility or inguinal hernia, Ocular depression, Rieger anomaly and Teething delay | Variable loss of sc fat is observed. |
| MDP syndrome | (Unknown) | Mandibular hypoplasia, Deafness, Progeroid features, undescended testes and male hypogonadism | Patients usually present with generalized loss of sc fat. |
| Neonatal progeroid syndrome | (Unknown) | Generalized loss of body fat and muscle mass, and progeroid appearance at birth | Molecular basis is unknown. |
| Autosomal dominant | |||
| Familial partial lipodystrophy (FPL)a | FPLD1, Kobberling (unknown) | Loss of sc fat from the extremities | Phenotype not well characterized. |
| FPLD2, Dunnigan (LMNA) | Loss of sc fat from the extremities and trunk (sparing the face and neck) at puberty | Lamins A and C are nuclear lamina proteins and specific mutations may disrupt nuclear function resulting in premature death of adipocytes. | |
| FPLD3 (PPARG) | Loss of sc fat from the extremities, especially from distal regions | PPARγ is a critical transcription factor required for adipogenesis. Dominant negative PPARγ mutations may inhibit adipocyte differentiation. | |
| FPLD4 (AKT2) | Single family reported with loss of sc fat from the extremities | AKT2, also known as protein kinase B, is involved in adipocyte differentiation and downstream insulin receptor signaling. | |
| FPLD5 (PLIN1) | Loss of sc fat from the extremities with small adipocytes and increased fibrosis of adipose tissue | Perilipin 1 is an integral component of lipid droplet membranes and is essential for lipid storage and hormone regulated lipolysis. | |
| Atypical progeroid syndrome | (LMNA) | Variable loss of sc fat, progeroid features | Different heterozygous mostly de novo mutations in LMNA cause nuclear dysfunction. |
| Hutchinson-Gilford progeria | (LMNA) | Generalized loss of sc fat, progeroid features | Specific de novo LMNA mutations induce abnormal splicing and accumulation of truncated farnesylated prelamin A. |
| SHORT syndrome | Unknown | See above | Variable loss of sc fat. |
PLIN1, Perilipin; MHC, major histocompatibility complex.
Additional rare types for which the genetic basis is not known are not included.
The genetic lipodystrophies have been reported in about 1000 patients, and their estimated prevalence in the general population (based on the assumption that only one fourth of the patients may be reported) could be less than one in a million. Recent progress in the characterization of the phenotypes of various types of genetic lipodystrophies and elucidation of the molecular defect in many of them has led to increased recognition of these syndromes. Affected females are recognized easily and thus are reported more often than males.
CGL, Berardinelli-Seip syndrome
This autosomal recessive disorder was reported initially by Berardinelli (5) from Brazil and Seip (8) from Scandinavia. Since then, about 300 patients have been reported in the literature, including clusters of patients from Lebanon and Brazil from consanguineous families (9–12). Patients with CGL are recognized at birth or soon thereafter due to a near-total lack of body fat and prominent muscularity that causes a severe and striking phenotype (Fig. 1A). The infants may have hepatosplenomegaly and umbilical prominence or hernia. Only a few patients develop diabetes during infancy, but most often diabetes appears during the teenage years or later. Later during childhood, acanthosis nigricans develops on extensive areas of the skin. Children usually have a voracious appetite and accelerated growth. Female patients develop hirsutism, may have clitoromegaly, and have irregular menstrual periods or oligoamenorrhea. Rarely, premature pubarche and menarche have been seen. Many of the patients have polycystic ovaries, and successful pregnancy is extremely rare. Fertility, however, is normal in affected men. Other uncommon manifestations include hypertrophic cardiomyopathy, mild mental retardation, and focal lytic lesions in the appendicular bones after puberty (9, 10). Diabetes, hyperlipidemia, and hepatic steatosis pose a therapeutic challenge, and patients can develop diabetic nephropathy and retinopathy, recurrent attacks of acute pancreatitis, and occasionally cirrhosis, which are the causes of morbidity and mortality.
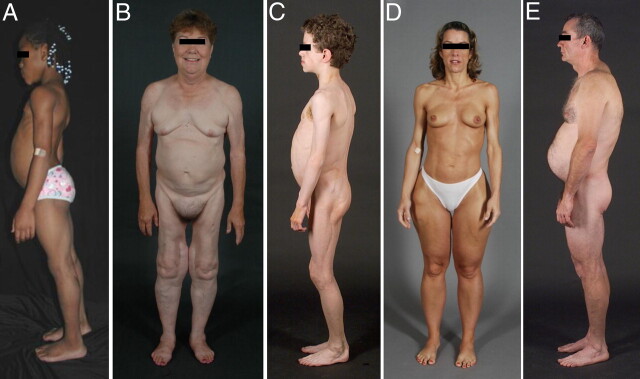
Fig. 1.
Clinical features of patients with various types of lipodystrophies. A, Lateral view of an 8-yr-old African-American female with CGL (also known as Berardinelli-Seip congenital lipodystrophy), type 1 due to homozygous c.377insT (p.Pro128AlafsX19) mutation in AGPAT2. The patient had generalized loss of sc fat with mild acanthosis nigricans in the axillae and neck. She had umbilical prominence and acromegaloid features (enlarged mandible, hands, and feet). B, Anterior view of a 65-yr-old Caucasian female with FPL of the Dunnigan variety due to heterozygous p.Arg482Gln mutation in LMNA. She had marked loss of sc fat from the limbs and anterior truncal region. The breasts were atrophic. She had increased sc fat deposits in the face, anterior neck, suprapubic and vulvar region, and medial parts of the knees. C, Lateral view of an 8-yr-old German boy with AGL. He started experiencing generalized loss of sc fat at age 3 with marked acanthosis nigricans in the neck, axillae, and groin. He developed Crohn's disease at age 11, requiring hemicolectomy at age 13. D, Anterior view of a 39-yr-old Caucasian female with APL (Barraquer-Simons syndrome). She had marked loss of sc fat from the face, neck, upper extremities, chest, and abdomen but had increased sc fat deposition in the lower extremities. E, Lateral view of a 39-yr-old Caucasian male infected with HIV with PI-containing HAART-induced lipodystrophy. He had marked loss of sc fat from the face and limbs but had increased sc fat deposition in the neck region, anteriorly and posteriorly showing buffalo hump. Abdomen was protuberant due to excess intraabdominal fat. He had been on PI-containing antiretroviral therapy for more than 7 yr. [Panel A was reproduced from V. Simha and A. Garg: Lipodystrophy: lessons in lipid and energy metabolism. Curr Opin Lipidol 17:162–169, 2006 (94), with permission. © Lippincott Williams & Williams.]
Genome-wide linkage analysis with positional cloning strategy led to the identification of two genes for CGL: 1-acylglycerol-3-phosphate O-acyltransferase 2 (AGPAT2) on chromosome 9q34 (13, 14) and Berardinelli-Seip congenital lipodystrophy 2 (BSCL2) on chromosome 11q13 (15). Since then, the candidate gene approach led to the identification of two other genes, caveolin 1 (CAV1) (16) and polymerase I and transcript release factor (PTRF) (17). CGL due to AGPAT2 (CGL1) and BSCL2 (CGL2) mutations are the most common varieties and have been reported in patients of various ethnicities. Although most of the patients of African origin have CGL1, those from Lebanon have CGL2 due to founder mutations. Only one Brazilian girl has been reported with homozygous CAV1 mutation (CGL3) (16), but so far 21 patients with PTRF mutations (CGL4) have been reported (17–19). The Brazilian girl with CGL3 also had short stature and presumed vitamin D resistance (16). Those with PTRF mutations also suffered from congenital myopathy, percussion-induced myoedema, pyloric stenosis, atlantoaxial instability, cardiac rhythm disturbances including prolonged QT interval and exercise-induced ventricular tachycardia, and sudden death (17–19).
AGPATs are critical enzymes involved in the biosynthesis of triglyceride and phospholipids from glycerol-3-phosphate. They catalyze acylation of fatty acids at the sn-2 position of glycerol moiety and convert lysophosphatidic acid to phosphatidic acid (Fig. 2). Each of the 11 known AGPAT isoforms is encoded by a distinct gene and has distinct tissue expression and biochemical properties (20, 21). AGPAT2 is highly expressed in the adipose tissue, and its deficiency may cause lipodystrophy by limiting triglyceride or phospholipid biosynthesis there (22).
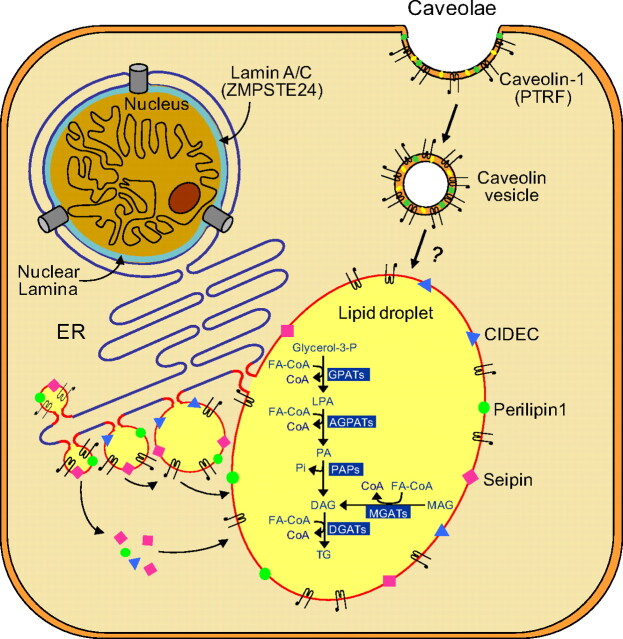
Fig. 2.
Lipid droplet formation in adipocytes. Lipid droplets (LD) are organelles that store triglycerides (TG) intracellularly. They form as budding vesicles at the endoplasmic reticulum (ER) that fuse in adipocytes to form one large LD. Many proteins, such as CIDEC (shown in blue triangles), seipin (pink squares), and perilipin 1 (green circles) are present on the LD membrane. CIDEC and seipin may be involved in fusion of LDs to form a larger LD, whereas perilipin 1 is essential for lipid storage and hormone-mediated lipolysis. Caveolae are formed from lipid rafts on the cell surface, which include cholesterol (yellow symbols), glycosphingolipids (green symbols), and caveolin-1 (black hairpin-like symbols). Endocytosis of caveolae forms caveolin vesicles that may directly merge with lipid droplets and thus translocating fatty acids to LDs. PTRF controls expression of caveolin 1 and 3 (data not shown). The classical and alternative pathways involved in the biosynthesis of TG are shown inside the lipid droplet. In the adipose tissue, TG synthesis requires glycerol-3-phosphate as the initial substrate (classical pathway), whereas in the small intestine, synthesis of TG can occur via an alternative pathway using monoacylglycerol (MAG) as the initial substrate. Acylation of glycerol-3-phosphate using fatty acyl coenzyme A (FA-CoA) at the sn-1 position is catalyzed by glycerol-3-phosphate acyltransferases (GPATs), resulting in the formation of 1-acylglycerol-3-phosphate or lysophosphatidic acid (LPA). LPA is then acylated at the sn-2 position by AGPATs to yield phosphatidic acid (PA). Removal of phosphate group from PA by PA phosphatases (PAP) produces diacylglycerol (DAG). Further acylation of DAG at the sn-3 position by diacylglycerol acyltransferases (DGATs) finally produces TG. In the alternative pathway, MAG is acylated to DAG by monoacylglycerol acyltransferases (MGATs) which is then further converted to TG. Lamin A/C are integral components of nuclear lamina (shown in blue color) and interact with nuclear membrane proteins as well as chromatin. Zinc metalloproteinase (ZMPSTE24) is critical for posttranslational processing of prelamin A to its mature form, lamin A. [Modified from A. Garg and A. K. Agarwal: Caveolin-1, a new locus for human lipodystrophy. J Clin Endocrinol Metab 93:1183–1185, 2008 (26), with permission. © The Endocrine Society. And from A. Garg and A. K. Agarwal: Lipodystrophies: disorders of adipose tissue biology. Biochem Biophys Acta 1791:507–513, 2009 (95), with permission. © Elsevier.]
The BSCL2-encoded protein, seipin, appears to play a role in lipid droplet formation and may also be involved in adipocyte differentiation (Figs. 2 and 3) (23–25). Caveolin 1 is an integral component of caveolae, which are specialized microdomains seen in abundance on adipocyte membranes (26). Caveolin 1 binds fatty acids and translocates them to lipid droplets (Fig. 2). PTRF (also known as cavin) is involved in biogenesis of caveolae and regulates expression of caveolins 1 and 3 (17).
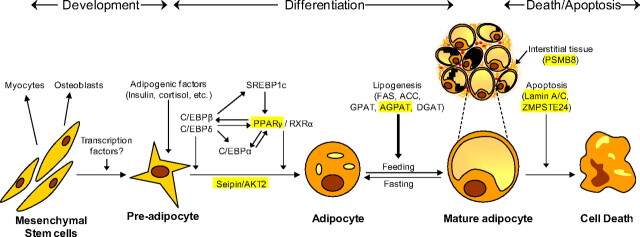
Fig. 3.
Pathways involved in the development, differentiation, and death of adipocytes. The pluripotent mesenchymal stem cells can form preadipocytes, myocytes, or osteoblasts depending upon the various cues. In response to various signals from hormones such as insulin and steroids and induction of adipogenic transcription factors, a series of changes are initiated in preadipocytes that lead to their differentiation to adipocytes. The transcription factors, CCAAT (cytidine-cytidine-adenosine-adenosine-thymidine)-enhancer-binding proteins (C/EBP) β/δ are the first to be up-regulated and then stimulate other transcription factors such as PPARγ, C/EBPα, and sterol regulatory element-binding protein (SREBP) 1c. Some other genes such as preadipocyte factor 1 (Pref1), a known adipogenesis inhibitor, are down-regulated. Mature adipocytes are activated, resulting in the overexpression of lipogenic genes like fatty acid synthase (FAS), acetyl coenzyme A carboxylase (ACC), GPAT, AGPAT, and diacylglycerol acyltransferases (DGAT) for biosynthesis of triglycerides and phospholipids. The size of the lipid droplets is reduced upon fasting and increases with increased substrate availability. Available data suggest that the BSCL2-encoded protein, seipin, and AKT2 may be involved in adipocyte differentiation, whereas the AGPAT2 affects triglyceride synthesis. Clinical evidence from lipodystrophy patients harboring LMNA or ZMPSTE24 mutations suggests that nuclear dysfunction may accelerate apoptosis/death of mature adipocytes. Interstitial tissue may also play an important role in adipocyte survival. Mutations in PSMB8, which encodes β5i, a catalytic subunit of the immunoproteasomes, may induce autoinflammatory syndrome, resulting in infiltration of lymphocytes in adipose tissue (panniculitis) and death of nearby adipocytes. [Modified from A. K. Agarwal and A. Garg: Genetic disorders of adipose tissue development, differentiation and death. Annu Rev Genomics Hum Genet 7:175–199, 2006 (96), with permission. © Annual Reviews.]
Patients with BSCL2 mutations have the most severe variety of CGL and are born without any body fat. They lack both the “mechanical” adipose tissue located in the retroorbital, palm, sole, and in periarticular regions as well as the “metabolically active” adipose tissue located in the sc, intraabdominal, intrathoracic, and other areas (27). In comparison, those with AGPAT2, CAV1, and PTRF mutations do not have any loss of mechanical fat (16, 18, 27, 28). In addition, those with CAV1 and PTRF mutations have preserved bone marrow fat (16, 18, 28), which is absent in patients with CGL1 and CGL2. There are a few patients with CGL who do not have mutations in the four known genes and may lead us to yet more novel CGL genes.
Mandibuloacral dysplasia (MAD)-associated lipodystrophy
MAD is characterized by skeletal abnormalities such as mandibular and clavicular hypoplasia and acroosteolysis (29, 30). Patients also develop progeroid manifestations such as cutaneous atrophy with prominent superficial vasculature and mottled hyperpigmentation, thin beaked nose, and hair loss. Other clinical features include delayed dentition and closure of cranial sutures, crowded teeth, joint stiffness, and two types of lipodystrophy: partial (type A) or generalized (type B) (31). Metabolic complications are mild, but diabetes, glucose intolerance, insulin resistance, mild hypertriglyceridemia, and low levels of high-density lipoprotein cholesterol have been reported in some patients with MAD (31, 32).
Patients with MAD harbor mutations in lamin A/C (LMNA) or zinc metalloproteinase (ZMPSTE24) (33, 34). ZMPSTE24 is involved in posttranslational proteolytic processing of prelamin A to mature lamin A. This process involves farnesylation of carboxy-terminal cysteine in the CAAX motif of prelamin A, followed by proteolytic cleavage of AAX tripeptide by ZMPSTE24, carboxymethylation of cysteine residue, and finally another proteolytic removal of 15 additional carboxy-terminal residues by ZMPSTE24. Thus, ZMPSTE24 deficiency can result in accumulation of toxic farnesylated prelamin A in cells. Although MAD due to LMNA mutations has been reported in approximately 30 patients, only eight MAD patients are known with ZMPSTE24 mutations. Interestingly, several patients were reported from Italy, all of them harboring a founder LMNA mutation (33).
There is some phenotypic heterogeneity between the two subtypes of MAD; those with ZMPSTE24 mutations are premature at birth, show clinical manifestations earlier in life, and are predisposed to developing focal segmental glomerulosclerosis and calcified skin nodules (35, 36).
Autoinflammatory syndromes
Joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced (JMP) lipodystrophy syndrome
We recently reported a distinct autosomal recessive, autoinflammatory syndrome in three patients belonging to two pedigrees from Portugal and Mexico who presented with JMP syndrome during childhood (37). Three other patients with very similar features had been previously reported from Japan (38, 39). Other reported features include intermittent fever, hypergammaglobulinemia, elevated erythrocyte sedimentation rate, hepatosplenomegaly, and calcification of basal ganglia.
We further conducted genome-wide linkage analysis in the two pedigrees and looked for regions of extended homozygosity in the affected patients, which identified the locus on chromosome 6. Sequencing of candidate genes involved in immune response in this region led us to a homozygous, missense, loss of function mutation in proteasome subunit, β-type, 8 (PSMB8) gene in both the pedigrees (40). PSMB8 encodes the β5i subunit of the immunoproteasome (41). Immunoproteasome-mediated proteolysis generates immunogenic epitopes presented by major histocompatibility complex class I molecules. Mutation in PSMB8 may trigger autoinflammatory response resulting in infiltration of adipose tissue with lymphocytes and other immune cells and loss of nearby adipocytes.
Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome
Two groups have recently reported a total of five patients who presented with onset of recurrent fever and annular violaceous plaques during infancy and resulting in loss of sc fat from the face and upper limbs later during childhood (42, 43). They also had violaceous swelling of the eyelids, hepatosplenomegaly, arthralgias, hypochromic anemia, increased erythrocyte sedimentation rate, and basal ganglia calcifications. The mode of inheritance seems to be autosomal recessive, but the molecular basis remains unknown.
Familial partial lipodystrophy (FPL)
FPL is characterized by the onset of fat loss from the limbs and other regions of the body usually during childhood, puberty, or later. Affected patients have normal body fat distribution during infancy and early childhood. Many regions of the body, such as the face, neck, and intraabdominal region are spared, and patients accumulate excess body fat in the nonlipodystrophic regions (Fig. 1B) (44–46). Diagnosis of affected men is difficult because many normal men have a muscular physique. Diabetes and metabolic complications usually occur during adulthood, and women are more severely affected than men (47). Acanthosis nigricans is usually mild and is present in the neck, axillae, and groins. Most affected women reproduce normally, but approximately one fourth of women have irregular periods and hirsutism suggestive of polycystic ovarian syndrome (47). Some patients also develop mild to moderate myopathy, cardiomyopathy, and conduction system abnormalities indicative of a multisystem dystrophy (48, 49).
Genome-wide linkage analysis with positional cloning strategy led to the identification of the first gene for FPL, Dunnigan variety, i.e. LMNA, on chromosome 1q21–22 (50–53), an integral component of nuclear lamina. Thereafter, the candidate gene approach has led to the identification of four other genes: peroxisome proliferator-activated receptor γ (PPARG; (54–56), a key transcription factor involved in adipocyte differentiation; v-AKT murine thymoma oncogene homolog 2 (AKT2) (57), involved in downstream insulin signaling; and cell death-inducing DNA fragmentation factor a-like effector c (CIDEC) (58) and perilipin 1 (PLIN1) (59), both involved in lipid droplet formation (60). FPL, Dunnigan due to LMNA mutations is the most common variety, with more than 300 patients reported (2), whereas only approximately 30 patients have been reported to harbor PPARG mutations (54–56), four patients from one family with AKT2 mutation (57, 61), and six patients from three families with PLIN1 mutations (59). A single patient with autosomal recessive FPL was found to harbor a homozygous missense mutation in CIDEC (58). The histopathology of the sc adipose tissue of the patient revealed multilocular, small lipid droplets in adipocytes (58, 62). Adipocyte loss in patients with LMNA mutations may be due to disruption of nuclear envelope function and integrity resulting in premature cell death (63). Patients with PPARG and AKT2 mutations may develop lipodystrophy due to defective adipocyte differentiation. Patients with PLIN1 mutations reportedly had smaller adipocytes with increased infiltration of macrophages and fibrosis in the adipose tissue (59).
The phenotypic heterogeneity between various types of FPL has not been clearly delineated. It appears that most patients with classical FPL, Dunnigan variety [not those with atypical variety (64)] have more severe loss of sc fat from the extremities compared with those with PLIN1 and PPARG mutations (54, 59). Patients with PPARG mutations have more severe lipodystrophy in the distal regions of extremities (calves and forearms) compared with proximal regions (thighs and arms) (54). Increased sc fat accumulation has been reported in the face and neck region in FPL due to LMNA, PPARG, and PLIN1 mutations (46, 59, 65). Phenotypic data on patients with AKT2 and CIDEC mutations are limited, and thus further in-depth studies of body fat distribution in additional patients with these subtypes are required. There are many patients with FPL phenotype who do not have mutations in the five known genes, and thus novel FPL genes remain to be discovered.
Other rare genetic syndromes
Heterozygous LMNA mutations can also cause variable amounts of fat loss with partial or generalized lipodystrophies in patients with atypical progeroid syndrome (66) and Hutchinson-Gilford progeria syndrome (67). We recently reported a novel autosomal recessive syndrome with mandibular hypoplasia, deafness, progeroid features (MDP)-associated lipodystrophy (68). All males with MDP had undescended testes and hypogonadism. One adult female showed lack of breast development. Another rare syndrome presents with short stature, hyperextensibility or inguinal hernia, ocular depression, Rieger anomaly, and teething delay (SHORT) –associated lipodystrophy and follows both autosomal recessive and dominant inheritance patterns (69, 70). Finally, the autosomal recessive, neonatal progeroid (Weidemann-Rautenstrauch) syndrome presents with generalized loss of body fat and muscle mass and progeroid appearance at birth (71). The molecular basis of these syndromes remains unknown.
Acquired Lipodystrophies (Table 2)
Table 2.
Classification, clinical features, and pathogenetic basis of acquired lipodystrophies
| Type | Subtype | Clinical features | Pathogenetic basis/other comments |
|---|---|---|---|
| Lipodystrophy in HIV-infected patients | PI-induced NRTI-induced |
Loss of sc fat from the face and extremities and excess fat deposition in the neck and abdomen | PI may inhibit ZMPSTE24 and/or cause dysregulation of transcription factors involved in adipogenesis. |
| NRTI may inhibit mitochondrial polymerase-γ and cause mitochondrial toxicity. | |||
| Acquired partial lipodystrophy | Autoimmune MPGN-associated Idiopathic |
Loss of sc fat from the face, neck, upper limbs, and trunk, sparing the lower abdomen and lower limbs | Low serum complement 3 levels and presence of an autoantibody, complement 3 nephritic factor, in most of the patients suggest autoimmune-mediated loss of adipose tissue. |
| Acquired generalized lipodystrophy | Autoimmune Panniculitis-associated Idiopathic |
Generalized loss of fat associated with tender sc nodules, autoimmune or other diseases | Panniculitis preceding the loss of sc fat and association of autoimmune diseases suggest immune-mediated loss of adipose tissue. Other mechanisms may also be involved. |
| Localized lipodystrophy | Drug-induced Panniculitis-induced Pressure-induced Centrifugal Idiopathic |
Loss of sc fat from small areas of the body | Multiple mechanisms including local drug-induced, immune-mediated, or pressure-induced atrophy of adipose tissue. Other unknown mechanisms may also be involved. |
MPGN, Membranoproliferative glomerulonephritis.
Despite recognition of acquired lipodystrophies for more than a century, progress in understanding underlying pathogenetic mechanisms has been slow. APL has been reported in approximately 250 cases of various ethnicities with a male-to-female ratio of 1:4 (72), and AGL in approximately 100 cases, mostly Caucasians with a male-to-female ratio of 1:3 (73). LD-HIV is estimated to be affecting more than 100,000 patients in the United States and many more in other countries (74–76).
AGL—Lawrence syndrome
The onset of loss of sc fat in patients with AGL occurs usually during childhood (73). The pattern and extent of fat loss is quite variable (Fig. 1C). Most of the patients have generalized loss of fat, but in a few of them, some areas of the body such as intraabdominal and bone marrow fat are spared. AGL patients are highly likely to develop severe hepatic steatosis and fibrosis, diabetes, and hypertriglyceridemia, which pose a therapeutic challenge.
The pathogenesis of fat loss in patients with AGL may be variable, and the exact mechanisms remain unknown. In approximately one fourth, panniculitis (which presents clinically as sc inflammatory nodules) precedes loss of fat. Panniculitis initially results in localized lipodystrophy that can spread to become generalized. Another one fourth of cases have associated autoimmune diseases such as juvenile dermatomyositis. In the remaining patients with idiopathic variety, multiple unknown mechanisms are likely involved (73). The panniculitis-associated AGL usually presents with less severe fat loss and metabolic complications than the other types (73). Some patients with AGL have been reported to have chronic hepatitis with autoimmune features and low serum complement 4 levels, suggesting involvement of the classical complement pathway in the pathogenesis of fat loss (77).
APL—Barraquer-Simons syndrome
The onset of APL in most patients occurs before the age of 15 yr. Fat loss occurs gradually in a symmetric fashion, first affecting the face and then spreading downward. Most patients lose fat from the face, neck, upper extremities, and trunk, and sc fat from the lower abdomen and legs is spared (Fig. 1D). In fact, many accumulate excess sc fat in the hips and legs. Metabolic complications are usually not seen. However, approximately one fifth of the patients develop membranoproliferative glomerulonephritis, and later on some of them develop drusen (72). There is strong evidence to suggest that fat loss involves autoimmune-mediated destruction of adipocytes because more than 80% of the patients have low serum levels of complement 3 and a circulating autoantibody called complement 3-nephritic factor that blocks degradation of the enzyme C3 convertase (72). Although exact pathogenesis of fat loss is not known, it could be due to C3-nephritic factor-induced lysis of adipocytes expressing factor D (78). Rare variants in LMNB2 were reported in five patients with APL, but two of the four variants were also found in normal controls (79). The reported loss of sc fat from the thighs, legs, and gluteal region, and the presence of diabetes in three patients and type IV or V hyperlipoproteinemia in four patients is quite atypical for patients with APL (79).
HAART-induced lipodystrophy in HIV-infected patients (LD-HIV)
LD-HIV usually appears after receiving PI-containing HAART for 2 yr or more (Fig. 1E). Most patients gradually lose sc fat from the arms, legs, and face. Fat loss from the face can be so severe as to result in emaciated appearance. Some areas of the body are spared, and some patients accumulate excess fat there manifesting as buffalo hump, double chin, and increased waist circumference (74). The fat loss worsens with ongoing HAART therapy and does not reverse on discontinuation of PI. Many patients develop hypertriglyceridemia, but only a few develop diabetes mellitus.
Both PIs and nucleoside reverse transcriptase inhibitors (NRTIs) are implicated. PIs may cause lipodystrophy by inhibiting ZMPSTE24, resulting in accumulation of toxic farnesylated prelamin A (80). Other mechanisms, such as altered expression of key transcription factors involved in lipogenesis and adipocyte differentiation, and sterol regulatory element binding protein 1c and PPARγ, may also be involved (81). PIs may induce insulin resistance by inhibiting glucose transporter 4 expression (82). NRTIs (especially zidovudine and stavudine) have been proposed to induce fat loss by inhibiting mitochondrial polymerase-γ and causing mitochondrial toxicity (83, 84). Because PIs or NRTIs are usually given together as part of the HAART, the individual effects of these drugs on the phenotype remain unclear. It is likely that, whereas PI induce peripheral lipodystrophy and metabolic abnormalities similar to that seen in patients with FPL, Dunnigan or those with MAD, type B, NRTIs may be responsible for fat accumulation in the neck region (buffalo hump) as seen in patients with multiple symmetric lipomatosis due to mitochondrial DNA mutations (85).
Localized lipodystrophy
Localized lipodystrophies present with focal loss of sc fat, usually causing one or more dimples. In some patients, large contiguous or anatomically distinct areas on any region of the body may be involved. Localized loss of sc fat usually occurs due to sc injection of various drugs, panniculitis, pressure, and other mechanisms (1).
Diagnosis and Prognosis
Although the phenotype of CGL is so striking that the diagnosis should be apparent at birth, the diagnosis can be delayed in children with APL, atypical progeroid syndrome, and AGL for several years until they see a specialist. Lipodystrophies should be considered in differential diagnosis of patients presenting with early diabetes, severe hypertriglyceridemia, hepatic steatosis, hepatosplenomegaly, acanthosis nigricans, and polycystic ovarian syndrome. A thorough physical examination of “lean” patients with these metabolic complications to look for evidence of fat loss should clinch the diagnosis. It is especially important to examine the extremities and hips for signs of fat loss and muscular prominence. Some patients may present with excess sc fat deposition in various anatomic regions and may resemble patients with Cushing's syndrome and truncal obesity. In those suspected to have genetic lipodystrophies, an in-depth pedigree analysis should be conducted to understand the mode of inheritance. Parents should be probed for any consanguinity. Careful examination of the male first-degree relatives can sometimes reveal previously undiagnosed FPL.
The diagnosis of various types of lipodystrophies is mainly clinical. Laboratory tests can only provide additional supportive evidence. All patients except those with localized lipodystrophy should be tested for glucose intolerance, serum lipids, liver function, and hyperuricemia. Measurement of serum leptin does not help diagnostically but may predict response to investigational metreleptin replacement therapy. Analysis of serum complement 3 and 4, and complement 3 nephritic factor and urinalysis for proteinuria should be conducted in patients with APL and AGL. Skeletal surveys may reveal lytic bone lesions in appendicular skeleton in CGL patients and various skeletal defects in MAD patients. A deep skin biopsy clinches the diagnosis of panniculitis or neutrophilic dermatosis. Electrocardiography, Holter monitoring, echocardiography, and stress test should be conducted for patients suspected of having cardiomyopathy or coronary heart disease.
Beyond a physical examination, skinfold thickness measurement, dual-energy x-ray absorptiometry, and a whole-body T-1 weighted magnetic resonance imaging can provide information on the pattern of fat loss. Genetic testing, including prenatal diagnosis, is available for AGPAT2, BSCL2, LMNA, ZMPSTE24, and PPARG in clinical laboratories, and some research laboratories including our own perform genetic testing of all known genes.
Patients with lipodystrophies should be differentiated from those with anorexia nervosa, cachexia, starvation, diencephalic syndrome, Cushing's syndrome, generalized and truncal obesity, multiple symmetric lipomatosis, and other rare progeroid syndromes and disorders affecting growth and development.
Patients with generalized lipodystrophies are predisposed to developing acute pancreatitis, cirrhosis, end-stage diabetic renal disease requiring renal transplantation, and blindness due to diabetic retinopathy. Many patients with FPL die of coronary heart disease or cardiomyopathy and rhythm disturbances (48, 49, 86). Some patients with MAD die during childhood of unknown reasons, and some others die during early adulthood due to complications of renal failure due to focal segmental glomerulosclerosis (35, 36). Patients with APL who develop membranoproliferative glomerulonephritis may eventually succumb to renal failure (72). Sudden death has been reported during childhood in CGL, type 4, likely due to arrhythmias (19). Patients with LD-HIV are predisposed to developing coronary heart disease (76).
Treatment
Treatment of lipodystrophies is quite challenging. Proper counseling of the parents as far as pathogenesis and expected course of the type of lipodystrophy is critical for allaying stress and psychological sequelae in children affected with lipodystrophies. Parents should provide support to affected children to help them adjust among their friends and classmates. Because reversal of the lost adipose tissue is not possible, cosmetic surgery to improve appearance and management of metabolic complications are the only therapeutic options. Autologous adipose tissue transplantation or implantation of dermal fillers can improve facial appearance. Unwanted excess adipose tissue from the chin, buffalo hump, and vulvar region can be surgically excised or removed by liposuction. CGL patients can undergo reconstructive facial surgery including mandibular resection.
For lack of clinical trial evidence, all patients are advised to consume high-carbohydrate, low-fat diets. These diets can reduce chylomicronemia but may raise very low-density lipoprotein triglycerides. Increased physical activity, especially aerobic training, can lower insulin resistance. Patients with FPL with concomitant cardiomyopathy, however, may avoid strenuous exercise (87). Lytic bone lesions in patients with CGL usually do not pose increased risk of fractures.
No controlled clinical trials have been conducted to help guide drug therapy for metabolic complications. For severe hypertriglyceridemia, fibrates and fish oil should be used and may be combined with statins in some patients. Metformin should be the first-line therapy for diabetes. There is no hard evidence to show that thiazolidinediones can improve fat deposition in lipodystrophic regions (88). Instead, in patients with partial lipodystrophies, thiazolidinediones can potentially increase unwanted fat deposition in nonlipodystrophic regions. Whether thiazolidinediones should be the therapy of choice in FPL patients with PPARG mutations is not clear (89). For many patients with generalized lipodystrophy, insulin therapy is needed. If the daily dose of insulin exceeds 200 U, U-500 insulin should be used. Some patients may have difficulty injecting insulin because they may not have sc fat in the abdomen or thighs. Whether vitamin E supplementation is helpful in reducing hepatic steatosis in patients with lipodystrophy is not clear. Although, sc metreleptin replacement therapy can dramatically improve diabetes control, hepatic steatosis, and hypertriglyceridemia in severely hypoleptinemic patients with generalized lipodystrophy (90, 91), its effects in patients with FPL so far have been equivocal (92). Metreleptin therapy remains investigational and is not yet approved by the U.S. Food and Drug Administration. For patients with LD-HIV, switching PI with other antiretroviral agents may improve dyslipidemia and insulin resistance; however, lipodystrophy may not improve. Recently, a GH-releasing factor analog, tesamorelin, was approved for reducing excess visceral fat in LD-HIV patients (93). However, it does not improve fat loss.
Acknowledgments
Disclosure Summary: The author is coholder of a patent for use of leptin for treating human lipoatrophy and a method of determining predisposition to said treatment but receives no financial compensation. He receives research funds from and is a consultant to Amylin Pharmaceutical Inc.
Abbreviations:
- AGL
Acquired generalized lipodystrophy
- AGPAT
1-acylglycerol-3-phosphate O-acyltransferase
- AKT2
v-AKT murine thymoma oncogene homolog 2
- APL
acquired partial lipodystrophy
- BSCL2
Berardinelli-Seip congenital lipodystrophy 2
- CAV1
caveolin 1
- CGL
congenital generalized lipodystrophy
- CIDEC
cell death-inducing DNA fragmentation factor a-like effector c
- FPL
familial partial lipodystrophy
- HAART
highly active antiretroviral therapy
- LD-HIV
lipodystrophy in HIV-infected patients
- LMNA
lamin A/C
- MAD
mandibuloacral dysplasia
- NRTI
nucleoside reverse transcriptase inhibitor
- PPARG or PPARγ
peroxisome proliferator-activated receptor γ
- PSMB8
proteasome subunit, β-type, 8
- PTRF
polymerase I and transcript release factor
- ZMPSTE24
zinc metalloproteinase.
References
- 1. Garg A. 2000. Lipodystrophies. Am J Med 108:143–152 [DOI] [PubMed] [Google Scholar]
- 2. Garg A. 2004. Acquired and inherited lipodystrophies. N Engl J Med 350:1220–1234 [DOI] [PubMed] [Google Scholar]
- 3. Mitchell SW. 1885. Singular case of absence of adipose matter in the upper half of the body. Am J Med Sci 90:105–106 [Google Scholar]
- 4. Ziegler LH. 1928. Lipodystrophies: report of seven cases. Brain 51:145–167 [Google Scholar]
- 5. Berardinelli W. 1954. An undiagnosed endocrinometabolic syndrome: report of 2 cases. J Clin Endocrinol Metab 14:193–204 [DOI] [PubMed] [Google Scholar]
- 6. Carr A , Samaras K , Burton S , Law M , Freund J , Chisholm DJ , Cooper DA 1998. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 12:F51–F58 [DOI] [PubMed] [Google Scholar]
- 7. Garg A , Misra A 2004. Lipodystrophies: rare disorders causing metabolic syndrome. Endocrinol Metab Clin North Am 33:305–331 [DOI] [PubMed] [Google Scholar]
- 8. Seip M. 1959. Lipodystrophy and gigantism with associated endocrine manifestations: a new diencephalic syndrome? Acta Paediatrica 48:555–574 [PubMed] [Google Scholar]
- 9. Van Maldergem L , Magré J , Khallouf TE , Gedde-Dahl T , Delépine M , Trygstad O , Seemanova E , Stephenson T , Albott CS , Bonnici F , Panz VR , Medina JL , Bogalho P , Huet F , Savasta S , Verloes A , Robert JJ , Loret H , De Kerdanet M , Tubiana-Rufi N , Mégarbané A , Maassen J , Polak M , Lacombe D , Kahn CR , Silveira EL , D'Abronzo FH , Grigorescu F , Lathrop M , Capeau J , O'Rahilly S 2002. Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J Med Genet 39:722–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agarwal AK , Simha V , Oral EA , Moran SA , Gorden P , O'Rahilly S , Zaidi Z , Gurakan F , Arslanian SA , Klar A , Ricker A , White NH , Bindl L , Herbst K , Kennel K , Patel SB , Al-Gazali L , Garg A 2003. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab 88:4840–4847 [DOI] [PubMed] [Google Scholar]
- 11. Pardini VC , Victória IM , Rocha SM , Andrade DG , Rocha AM , Pieroni FB , Milagres G , Purisch S , Velho G 1998. Leptin levels, β-cell function, and insulin sensitivity in families with congenital and acquired generalized lipoatrophic diabetes. J Clin Endocrinol Metab 83:503–508 [DOI] [PubMed] [Google Scholar]
- 12. Magré J , Delépine M , Van Maldergem L , Robert JJ , Maassen JA , Meier M , Panz VR , Kim CA , Tubiana-Rufi N , Czernichow P , Seemanova E , Buchanan CR , Lacombe D , Vigouroux C , Lascols O , Kahn CR , Capeau J , Lathrop M 2003. Prevalence of mutations in AGPAT2 among human lipodystrophies. Diabetes 52:1573–1578 [DOI] [PubMed] [Google Scholar]
- 13. Garg A , Wilson R , Barnes R , Arioglu E , Zaidi Z , Gurakan F , Kocak N , O'Rahilly S , Taylor SI , Patel SB , Bowcock AM 1999. A gene for congenital generalized lipodystrophy maps to human chromosome 9q34. J Clin Endocrinol Metab 84:3390–3394 [DOI] [PubMed] [Google Scholar]
- 14. Agarwal AK , Arioglu E , De Almeida S , Akkoc N , Taylor SI , Bowcock AM , Barnes RI , Garg A 2002. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet 31:21–23 [DOI] [PubMed] [Google Scholar]
- 15. Magré J , Delépine M , Khallouf E , Gedde-Dahl T , Van Maldergem L , Sobel E , Papp J , Meier M , Mégarbané A , Bachy A , Verloes A , d'Abronzo FH , Seemanova E , Assan R , Baudic N , Bourut C , Czernichow P , Huet F , Grigorescu F , de Kerdanet M , Lacombe D , Labrune P , Lanza M , Loret H , Matsuda F , Navarro J , Nivelon-Chevalier A , Polak M , Robert JJ , Tric P , Tubiana-Rufi N , Vigouroux C , Weissenbach J , Savasta S , Maassen JA , Trygstad O , Bogalho P , Freitas P , Medina JL , Bonnicci F , Joffe BI , Loyson G , Panz VR , Raal FJ , O'Rahilly S , Stephenson T , Kahn CR , Lathrop M , Capeau J 2001. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet 28:365–370 [DOI] [PubMed] [Google Scholar]
- 16. Kim CA , Delépine M , Boutet E , El Mourabit H , Le Lay S , Meier M , Nemani M , Bridel E , Leite CC , Bertola DR , Semple RK , O'Rahilly S , Dugail I , Capeau J , Lathrop M , Magré J 2008. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab 93:1129–1134 [DOI] [PubMed] [Google Scholar]
- 17. Hayashi YK , Matsuda C , Ogawa M , Goto K , Tominaga K , Mitsuhashi S , Park YE , Nonaka I , Hino-Fukuyo N , Haginoya K , Sugano H , Nishino I 2009. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest 119:2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shastry S , Delgado MR , Dirik E , Turkmen M , Agarwal AK , Garg A 2010. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am J Med Genet A 152A:2245–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajab A , Straub V , McCann LJ , Seelow D , Varon R , Barresi R , Schulze A , Lucke B , Lützkendorf S , Karbasiyan M , Bachmann S , Spuler S , Schuelke M 2010. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet 6:e1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takeuchi K , Reue K 2009. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab 296:E1195–E1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agarwal AK , Garg A 2010. Enzymatic activity of the human 1-acylglycerol-3-phosphate-O-acyltransferase isoform 11: upregulated in breast and cervical cancers. J Lipid Res 51:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agarwal AK , Garg A 2003. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol Metab 14:214–221 [DOI] [PubMed] [Google Scholar]
- 23. Szymanski KM , Binns D , Bartz R , Grishin NV , Li WP , Agarwal AK , Garg A , Anderson RG , Goodman JM 2007. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci USA 104:20890–20895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fei W , Shui G , Gaeta B , Du X , Kuerschner L , Li P , Brown AJ , Wenk MR , Parton RG , Yang H 2008. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J Cell Biol 180:473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Payne VA , Grimsey N , Tuthill A , Virtue S , Gray SL , Dalla Nora E , Semple RK , O'Rahilly S , Rochford JJ 2008. The human lipodystrophy gene BSCL2/seipin may be essential for normal adipocyte differentiation. Diabetes 57:2055–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garg A , Agarwal AK 2008. Caveolin-1: a new locus for human lipodystrophy. J Clin Endocrinol Metab 93:1183–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simha V , Garg A 2003. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy due to mutations in the AGPAT2 or Seipin genes. J Clin Endocrinol Metab 88:5433–5437 [DOI] [PubMed] [Google Scholar]
- 28. Simha V , Agarwal AK , Aronin PA , Iannaccone ST , Garg A 2008. Novel subtype of congenital generalized lipodystrophy associated with muscular weakness and cervical spine instability. Am J Med Genet A 146A:2318–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sensenbrenner JA , Fiorelli G 1971. New syndrome manifested by mandibular hypoplasia, acroosteolysis, stiff joints and cutaneous atrophy (mandibuloacral dysplasia) in two unrelated boys. In: , Bergsma D ed. The clinical delineation of birth defects: orofacial structures. Baltimore, MD: Williams, Wilkins; 293–297 [PubMed] [Google Scholar]
- 30. Young LW , Radebaugh JF , Rubin P , Sensenbrenner JA , Fiorelli G , McKusick VA 1971. New syndrome manifested by mandibular hypoplasia, acroosteolysis, stiff joints and cutaneous atrophy (mandibuloacral dysplasia) in two unrelated boys. Birth Defects 7:291–297 [PubMed] [Google Scholar]
- 31. Simha V , Agarwal AK , Oral EA , Fryns JP , Garg A 2003. Genetic and phenotypic heterogeneity in patients with mandibuloacral dysplasia-associated lipodystrophy. J Clin Endocrinol Metab 88:2821–2824 [DOI] [PubMed] [Google Scholar]
- 32. Freidenberg GR , Cutler DL , Jones MC , Hall B , Mier RJ , Culler F , Jones KL , Lozzio C , Kaufmann S 1992. Severe insulin resistance and diabetes mellitus in mandibuloacral dysplasia. Am J Dis Child 146:93–99 [DOI] [PubMed] [Google Scholar]
- 33. Novelli G , Muchir A , Sangiuolo F , Helbling-Leclerc A , D'Apice MR , Massart C , Capon F , Sbraccia P , Federici M , Lauro R , Tudisco C , Pallotta R , Scarano G , Dallapiccola B , Merlini L , Bonne G 2002. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am J Hum Genet 71:426–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agarwal AK , Fryns JP , Auchus RJ , Garg A 2003. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet 12:1995–2001 [DOI] [PubMed] [Google Scholar]
- 35. Agarwal AK , Zhou XJ , Hall RK , Nicholls K , Bankier A , Van Esch H , Fryns JP , Garg A 2006. Focal segmental glomerulosclerosis in patients with mandibuloacral dysplasia due to zinc metalloproteinase deficiency. J Investig Med 54:208–213 [DOI] [PubMed] [Google Scholar]
- 36. Ahmad Z , Zackai E , Medne L , Garg A 2010. Early onset mandibuloacral dysplasia due to compound heterozygous mutations in ZMPSTE24. Am J Med Genet A 152A:2703–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garg A , Hernandez MD , Sousa AB , Subramanyam L , Martínez de Villarreal L , dos Santos HG , Barboza O 2010. An autosomal recessive syndrome of joint contractures, muscular atrophy, microcytic anemia, and panniculitis-associated lipodystrophy. J Clin Endocrinol Metab 95:E58–E63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Horikoshi A , Iwabuchi S , Iizuka Y , Hagiwara T , Amaki I 1980. [A case of partial lipodystrophy with erythema, dactylic deformities, calcification of the basal ganglia, immunological disorders and low IQ level (author's translation)]. Rinsho Shinkeigaku 20:173–180 [PubMed] [Google Scholar]
- 39. Tanaka M , Miyatani N , Yamada S , Miyashita K , Toyoshima I , Sakuma K , Tanaka K , Yuasa T , Miyatake T , Tsubaki T 1993. Hereditary lipo-muscular atrophy with joint contracture, skin eruptions and hyper-γ-globulinemia: a new syndrome. Intern Med 32:42–45 [DOI] [PubMed] [Google Scholar]
- 40. Agarwal AK , Xing C , DeMartino GN , Mizrachi D , Hernandez MD , Sousa AB , Martínez de Villarreal L , dos Santos HG , Garg A 2010. PSMB8 encoding the β5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet 87:866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rivett AJ , Hearn AR 2004. Proteasome function in antigen presentation: immunoproteasome complexes, peptide production, and interactions with viral proteins. Curr Protein Pept Sci 5:153–161 [DOI] [PubMed] [Google Scholar]
- 42. Torrelo A , Patel S , Colmenero I , Gurbindo D , Lendínez F , Hernández A , López-Robledillo JC , Dadban A , Requena L , Paller AS 2010. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome. J Am Acad Dermatol 62:489–495 [DOI] [PubMed] [Google Scholar]
- 43. Ramot Y , Czarnowicki T , Maly A , Navon-Elkan P , Zlotogorski A 9 June 2010. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature syndrome: a case report. Pediatr Dermatol 10.1111/j.1525-1470.2010.01163.x [DOI] [PubMed] [Google Scholar]
- 44. Dunnigan MG , Cochrane MA , Kelly A , Scott JW 1974. Familial lipoatrophic diabetes with dominant transmission. A new syndrome. Q J Med 43:33–48 [PubMed] [Google Scholar]
- 45. Köbberling J , Dunnigan MG 1986. Familial partial lipodystrophy: two types of an X linked dominant syndrome, lethal in the hemizygous state. J Med Genet 23:120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garg A , Peshock RM , Fleckenstein JL 1999. Adipose tissue distribution in patients with familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab 84:170–174 [DOI] [PubMed] [Google Scholar]
- 47. Garg A. 2000. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab 85:1776–1782 [DOI] [PubMed] [Google Scholar]
- 48. Garg A , Speckman RA , Bowcock AM 2002. Multisystem dystrophy syndrome due to novel missense mutations in the amino-terminal head and α-helical rod domains of the lamin A/C gene. Am J Med 112:549–555 [DOI] [PubMed] [Google Scholar]
- 49. Subramanyam L , Simha V , Garg A 2010. Overlapping syndrome with familial partial lipodystrophy, Dunnigan variety and cardiomyopathy due to amino-terminal heterozygous missense lamin A/C mutations. Clin Genet 78:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peters JM , Barnes R , Bennett L , Gitomer WM , Bowcock AM , Garg A 1998. Localization of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q21–22. Nat Genet 18:292–295 [DOI] [PubMed] [Google Scholar]
- 51. Cao H , Hegele RA 2000. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet 9:109–112 [DOI] [PubMed] [Google Scholar]
- 52. Shackleton S , Lloyd DJ , Jackson SN , Evans R , Niermeijer MF , Singh BM , Schmidt H , Brabant G , Kumar S , Durrington PN , Gregory S , O'Rahilly S , Trembath RC 2000. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet 24:153–156 [DOI] [PubMed] [Google Scholar]
- 53. Speckman RA , Garg A , Du F , Bennett L , Veile R , Arioglu E , Taylor SI , Lovett M , Bowcock AM 2000. Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C-terminal domain of lamin A/C. Am J Hum Genet 66:1192–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Agarwal AK , Garg A 2002. A novel heterozygous mutation in peroxisome proliferator-activated receptor-γ gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab 87:408–411 [DOI] [PubMed] [Google Scholar]
- 55. Hegele RA , Cao H , Frankowski C , Mathews ST , Leff T 2002. PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes 51:3586–3590 [DOI] [PubMed] [Google Scholar]
- 56. Semple RK , Chatterjee VK , O'Rahilly S 2006. PPAR γ and human metabolic disease. J Clin Invest 116:581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. George S , Rochford JJ , Wolfrum C , Gray SL , Schinner S , Wilson JC , Soos MA , Murgatroyd PR , Williams RM , Acerini CL , Dunger DB , Barford D , Umpleby AM , Wareham NJ , Davies HA , Schafer AJ , Stoffel M , O'Rahilly S , Barroso I 2004. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 304:1325–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rubio-Cabezas O , Puri V , Murano I , Saudek V , Semple RK , Dash S , Hyden CS , Bottomley W , Vigouroux C , Magré J , Raymond-Barker P , Murgatroyd PR , Chawla A , Skepper JN , Chatterjee VK , Suliman S , Patch AM , Agarwal AK , Garg A , Barroso I , Cinti S , Czech MP , Argente J , O'Rahilly S , Savage DB 2009. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med 1:280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gandotra S , Le Dour C , Bottomley W , Cervera P , Giral P , Reznik Y , Charpentier G , Auclair M , Delépine M , Barroso I , Semple RK , Lathrop M , Lascols O , Capeau J , O'Rahilly S , Magré J , Savage DB , Vigouroux C 2011. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med 364:740–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Olofsson SO , Boström P , Andersson L , Rutberg M , Levin M , Perman J , Borén J 2008. Triglyceride containing lipid droplets and lipid droplet-associated proteins. Curr Opin Lipidol 19:441–447 [DOI] [PubMed] [Google Scholar]
- 61. Semple RK , Sleigh A , Murgatroyd PR , Adams CA , Bluck L , Jackson S , Vottero A , Kanabar D , Charlton-Menys V , Durrington P , Soos MA , Carpenter TA , Lomas DJ , Cochran EK , Gorden P , O'Rahilly S , Savage DB 2009. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest 119:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nishino N , Tamori Y , Tateya S , Kawaguchi T , Shibakusa T , Mizunoya W , Inoue K , Kitazawa R , Kitazawa S , Matsuki Y , Hiramatsu R , Masubuchi S , Omachi A , Kimura K , Saito M , Amo T , Ohta S , Yamaguchi T , Osumi T , Cheng J , Fujimoto T , Nakao H , Nakao K , Aiba A , Okamura H , Fushiki T , Kasuga M 2008. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest 118:2808–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Caron M , Auclair M , Donadille B , Béréziat V , Guerci B , Laville M , Narbonne H , Bodemer C , Lascols O , Capeau J , Vigouroux C 2007. Human lipodystrophies linked to mutations in A-type lamins and to HIV protease inhibitor therapy are both associated with prelamin A accumulation, oxidative stress and premature cellular senescence. Cell Death Differ 14:1759–1767 [DOI] [PubMed] [Google Scholar]
- 64. Garg A , Vinaitheerthan M , Weatherall PT , Bowcock AM 2001. Phenotypic heterogeneity in patients with familial partial lipodystrophy (Dunnigan variety) related to the site of mis-sense mutations in lamin A/C (LMNA) gene. J Clin Endocrinol Metab 86:59–65 [DOI] [PubMed] [Google Scholar]
- 65. Al-Shali K , Cao H , Knoers N , Hermus AR , Tack CJ , Hegele RA 2004. A single-base mutation in the peroxisome proliferator-activated receptor γ4 promoter associated with altered in vitro expression and partial lipodystrophy. J Clin Endocrinol Metab 89:5655–5660 [DOI] [PubMed] [Google Scholar]
- 66. Garg A , Subramanyam L , Agarwal AK , Simha V , Levine B , D'Apice MR , Novelli G , Crow Y 2009. Atypical progeroid syndrome due to heterozygous missense LMNA mutations. J Clin Endocrinol Metab 94:4971–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Merideth MA , Gordon LB , Clauss S , Sachdev V , Smith AC , Perry MB , Brewer CC , Zalewski C , Kim HJ , Solomon B , Brooks BP , Gerber LH , Turner ML , Domingo DL , Hart TC , Graf J , Reynolds JC , Gropman A , Yanovski JA , Gerhard-Herman M , Collins FS , Nabel EG , Cannon RO , Gahl WA , Introne WJ 2008. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med 358:592–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shastry S , Simha V , Godbole K , Sbraccia P , Melancon S , Yajnik CS , Novelli G , Kroiss M , Garg A 2010. A novel syndrome of mandibular hypoplasia, deafness, and progeroid features associated with lipodystrophy, undescended testes, and male hypogonadism. J Clin Endocrinol Metab 95:E192–E197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sensenbrenner JA , Hussels IE , Levin LS 1975. A low birthweight syndrome? Rieger syndrome. Birth Defects 11:423–426 [PubMed] [Google Scholar]
- 70. Gorlin RJ , Cervenka J , Moller K , Horrobin M , Witkop J 1975. Rieger anomaly and growth retardation (the S-H-O-R-T syndrome). Birth Defects 11:46–48 [PubMed] [Google Scholar]
- 71. O'Neill B , Simha V , Kotha V , Garg A 2007. Body fat distribution and metabolic variables in patients with neonatal progeroid syndrome. Am J Med Genet A 143A:1421–1430 [DOI] [PubMed] [Google Scholar]
- 72. Misra A , Peethambaram A , Garg A 2004. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine (Baltimore) 83:18–34 [DOI] [PubMed] [Google Scholar]
- 73. Misra A , Garg A 2003. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine 82:129–146 [DOI] [PubMed] [Google Scholar]
- 74. Chen D , Misra A , Garg A 2002. Lipodystrophy in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab 87:4845–4856 [DOI] [PubMed] [Google Scholar]
- 75. Carr A. 2000. HIV protease inhibitor-related lipodystrophy syndrome. Clin Infect Dis 30(Suppl 2):S135–S142 [DOI] [PubMed] [Google Scholar]
- 76. Grinspoon S , Carr A 2005. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 352:48–62 [DOI] [PubMed] [Google Scholar]
- 77. Savage DB , Semple RK , Clatworthy MR , Lyons PA , Morgan BP , Cochran EK , Gorden P , Raymond-Barker P , Murgatroyd PR , Adams C , Scobie I , Mufti GJ , Alexander GJ , Thiru S , Murano I , Cinti S , Chaudhry AN , Smith KG , O'Rahilly S 2009. Complement abnormalities in acquired lipodystrophy revisited. J Clin Endocrinol Metab 94:10–16 [DOI] [PubMed] [Google Scholar]
- 78. Mathieson PW , Würzner R , Oliveria DB , Lachmann PJ , Peters DK 1993. Complement-mediated adipocyte lysis by nephritic factor sera. J Exp Med 177:1827–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hegele RA , Cao H , Liu DM , Costain GA , Charlton-Menys V , Rodger NW , Durrington PN 2006. Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy. Am J Hum Genet 79:383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hudon SE , Coffinier C , Michaelis S , Fong LG , Young SG , Hrycyna CA 2008. HIV-protease inhibitors block the enzymatic activity of purified Ste24p. Biochem Biophys Res Commun 374:365–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bastard JP , Caron M , Vidal H , Jan V , Auclair M , Vigouroux C , Luboinski J , Laville M , Maachi M , Girard PM , Rozenbaum W , Levan P , Capeau J 2002. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet 359:1026–1031 [DOI] [PubMed] [Google Scholar]
- 82. Murata H , Hruz PW , Mueckler M 2000. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem 275:20251–20254 [DOI] [PubMed] [Google Scholar]
- 83. Carr A , Miller J , Law M , Cooper DA 2000. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS 14:F25–F32 [DOI] [PubMed] [Google Scholar]
- 84. Lee H , Hanes J , Johnson KA 2003. Toxicity of nucleoside analogues used to treat AIDS and the selectivity of the mitochondrial DNA polymerase. Biochemistry 42:14711–14719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Klopstock T , Naumann M , Seibel P , Shalke B , Reiners K , Reichmann H 1997. Mitochondrial DNA mutations in multiple symmetric lipomatosis. Mol Cell Biochem 174:271–275 [PubMed] [Google Scholar]
- 86. Hegele RA. 2000. Familial partial lipodystrophy: a monogenic form of the insulin resistance syndrome. Mol Genet Metab 71:539–544 [DOI] [PubMed] [Google Scholar]
- 87. Pasotti M , Klersy C , Pilotto A , Marziliano N , Rapezzi C , Serio A , Mannarino S , Gambarin F , Favalli V , Grasso M , Agozzino M , Campana C , Gavazzi A , Febo O , Marini M , Landolina M , Mortara A , Piccolo G , Viganò M , Tavazzi L , Arbustini E 2008. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol 52:1250–1260 [DOI] [PubMed] [Google Scholar]
- 88. Simha V , Rao S , Garg A 2008. Prolonged thiazolidinedione therapy does not reverse fat loss in patients with familial partial lipodystrophy, Dunnigan variety. Diabetes Obes Metab 10:1275–1276 [DOI] [PubMed] [Google Scholar]
- 89. Tan GD , Savage DB , Fielding BA , Collins J , Hodson L , Humphreys SM , O'Rahilly S , Chatterjee K , Frayn KN , Karpe F 2008. Fatty acid metabolism in patients with PPARγ mutations. J Clin Endocrinol Metab 93:4462–4470 [DOI] [PubMed] [Google Scholar]
- 90. Oral EA , Simha V , Ruiz E , Andewelt A , Premkumar A , Snell P , Wagner AJ , DePaoli AM , Reitman ML , Taylor SI , Gorden P , Garg A 2002. Leptin-replacement therapy for lipodystrophy. N Engl J Med 346:570–578 [DOI] [PubMed] [Google Scholar]
- 91. Simha V , Szczepaniak LS , Wagner AJ , DePaoli AM , Garg A 2003. Effect of leptin replacement on intrahepatic and intramyocellular lipid content in patients with generalized lipodystrophy. Diabetes Care 26:30–35 [DOI] [PubMed] [Google Scholar]
- 92. Park JY , Javor ED , Cochran EK , DePaoli AM , Gorden P 2007. Long-term efficacy of leptin replacement in patients with Dunnigan-type familial partial lipodystrophy. Metabolism 56:508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Falutz J , Mamputu JC , Potvin D , Moyle G , Soulban G , Loughrey H , Marsolais C , Turner R , Grinspoon S 2010. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J Clin Endocrinol Metab 95:4291–4304 [DOI] [PubMed] [Google Scholar]
- 94. Simha V , Garg A 2006. Lipodystrophy: lessons in lipid and energy metabolism. Curr Opin Lipidol 17:162–169 [DOI] [PubMed] [Google Scholar]
- 95. Garg A , Agarwal AK 2009. Lipodystrophies: disorders of adipose tissue biology. Biochem Biophys Acta 1791:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Agarwal AK , Garg A 2006. Genetic disorders of adipose tissue development, differentiation and death. Annu Rev Genomics Hum Genet 7:175–199 [DOI] [PubMed] [Google Scholar]