Abstract
While radiation nephropathy is a major problem associated with radiotherapy, the exact mechanisms underlying its pathogenesis and the mediators involved in kidney deterioration remain to be elucidated. In view of the finding that senescence is typically increased post-irradiation, the present study examined whether ionizing radiation may cause kidney injury by enhancing premature senescence. The present study explored the relevance of the aging suppressor, Klotho, which has anti-aging activity and is highly expressed in murine renal cells/kidney tissues, under irradiation conditions. Firstly, the effects of radiation on mouse inner medullary collecting duct-3 (mIMCD-3) cells and kidney tissues of mice were assessed. Subsequently, the mRNA expression levels of Klotho, TNF-α and ADAM metallopeptidase domain (ADAM)9/10/17 were analyzed by reverse transcription-quantitative PCR following exposure to radiation. In addition, the levels of these proteins were measured by western blotting or ELISA. The results revealed that irradiation of mIMCD-3 cells clearly triggered cellular senescence. Notably, Klotho gene expression was considerably decreased in radiation-exposed mIMCD-3 cells and in the kidney tissues of irradiated BALB/c mice, and the corresponding translated protein was consistently expressed following radiation exposure. Moreover, expression of TNF-α, a negative regulator of Klotho, was significantly increased, whereas ADAM9/10/17, an ectodomain shedding enzyme of Klotho, was decreased in irradiated mIMCD-3 cells and in the kidney tissues of BALB/c mice. Collectively, these data suggested that TNF-α-mediated inhibition of Klotho expression and blockage of soluble Klotho formation via decreased ADAM expression following irradiation may contribute to the development of renal dysfunction through acceleration of radiation-induced cellular senescence.
Keywords: radiation, senescence, renal epithelial cells, Klotho, TNF-α, ADAM metallopeptidase domain
Introduction
Exposure to radiation causes damage to healthy organs. The side effects of radiotherapy, including recurrence, secondary cancer and normal tissue injury, are among the clinical problems faced by patients with cancer. Notably, partial or total body exposure to 5–10 Gy irradiation in a single fraction is not lethal but can lead to nephropathy (1). Accidental radiation exposure has been suggested to induce chronic kidney disease (CKD) as a potential late-onset effect; however, the underlying factors have yet to be investigated in detail. Radiation-induced nephropathy is often observed in patients with gastrointestinal or gynecological cancer, lymphoma and sarcoma of the upper abdomen (2). A previous study on renal failure-associated mortality in atomic bomb survivors suggested that radiation-induced renal dysfunction was a contributory factor to increased circulatory disease (3). Assessment of renal function should be included in the long-term follow-up plan for patients with substantial clinical or accidental radiation exposure to the kidney region, since CKD has a significant role in human morbidity and mortality. The precise mechanisms underlying pathogenesis and/or mediators involved in radiation-induced nephropathy are currently under investigation.
Generally, exposure to ionizing radiation (IR) induces direct injury to normal tissues and promotes generation of reactive oxygen species (ROS) that cause damage to macromolecules (4). Formation of excessive ROS has been implicated in the aging process and pathogenesis of various human diseases (5). Cellular senescence is a physiological process through which cells lose the ability to divide and proliferate, contributing to various physiological and pathological processes of biological aging. Typically, cellular senescence encompasses irreversible growth arrest, normal tissue repair and tumor suppression, and is induced in response to IR (6,7). Senescent cells secrete proinflammatory factors, a condition known as senescence-associated secretory phenotype (SASP) (5). Several molecules of SASP are associated with radiation-induced normal tissue injury, including TNF-α, IL-1, IL-6, TGF-β, epidermal growth factor and vascular endothelial growth factor (8). In particular, the inflammatory cytokine TNF-α has been shown to be rapidly and consistently expressed in irradiated and adjacent tissue, and to be involved in the acute phase reaction (8). Deficiency of TNF-α in a lung injury model has been reported to effectively prevent symptoms of radiation pneumonitis (9). While TNF-α is implicated in radiation mucositis, enteritis and dermatitis, its specific involvement in radiation-induced kidney injury has not been fully elucidated (8).
Klotho, an aging suppressor gene (10), is expressed in several tissues and is inhibited by TNF-α (11). The highest levels of Klotho are detected in the kidney and brain. The protein is additionally expressed in parathyroid glands and the heart, but with lower abundance (12). Emerging evidence has indicated that deficiency of Klotho is an early biomarker for CKD and acute kidney injury (10). Two existing forms of Klotho with distinct functions have been identified to date; specifically, a transmembrane and a soluble secreted form. The extracellular domain of Klotho is cleaved by ADAM metallopeptidase domain (ADAM)9/10/17 to generate a soluble secreted form (10). A previous study has revealed that deficiency in transmembrane and soluble forms of Klotho in patients with CKD contributed to the pathogenesis of secondary hyperparathyroidism, vascular calcification, left ventricular hypertrophy and worsening of kidney injury (13). Klotho expression and cleavage processes by ADAM9/10/17 have been widely examined in a range of pathophysiological conditions; however, the precise roles of these molecules in CKD remain to be established (14,15).
The present study hypothesized that TNF-α may exert an inhibitory effect on the expression of Klotho, which in turn may promote radiation-induced aging of renal epithelial cells. The aim of the present study was to demonstrate that IR induces senescence in mouse internal medulla collection duct-3 (mIMCD-3) cells. Therefore, the mRNA and protein expression levels of TNF-α and Klotho were detected following exposure to radiation. In addition, changes to the expression of the ectodomain shedding enzyme of Klotho, ADAM9/10/17, were investigated. These data intended to comprehensively suggest that radiation-induced renal dysfunction may be associated with inhibition of Klotho activity and promotion of cellular senescence. Finally, the present study aimed to provide insight into the pathogenic mechanisms responsible for radiation-induced kidney injury.
Materials and methods
Cell culture
The mIMCD-3 (CRL-2123™) cell line was obtained from the American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium:F-12 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (GenDEPOT), 100 U/ml penicillin and 100 µg/ml streptomycin. Cells were grown in a humidified atmosphere containing 95% air and 5% CO2 at 37°C.
In vitro and in vivo irradiation
For γ-irradiation, cells were exposed to a total of 6 Gy for 2 min (dose rate, 3 Gy/min) with a 137Cs γ-ray source (Gamma-Service Medical GmbH). BALB/c and C57BL/6 mice were purchased from Koatech Co., Ltd. Mice were housed at 22±2°C and 50±10% humidity under a 12-h light/dark cycle. Five animals were housed per cage with free access to drinking water and standard mouse chow. For irradiation, 8-week-old (male; weight, 20–25 g) mice were placed in well-ventilated custom jigs, allowing for precise delivery of radiation using the X-Rad 320 system (Precision Inc.; 260 kV, 10 mA) to a total of 6 Gy for 3 min (dose rate, 2 Gy/min). Sham-irradiated mice were similarly placed in custom jigs and positioned in the X-Rad 320 system without X-ray exposure. A UNIDOSE® universal dosimeter (PTW-Freiburg) was used to measure the dose rate (16). The control and IR-treated mouse groups (n=3) were euthanized 1 and 3 days after irradiation by exposure to CO2 (30% flow rate of gradual-fill CO2). Death was confirmed by monitoring cardiac and respiratory arrest. Subsequently, kidneys were removed, frozen in liquid nitrogen and stored at −70°C. For in vivo studies, all animal experiments were conducted in accordance with the Guidelines for the Use and Care of Laboratory Animals (16) and the study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Korea Institute of Radiological and Medical Sciences (KIRAMS; IACUC approval no. 2016-0065; Seoul, South Korea).
Cell counting and proliferation assays
Cells were seeded onto 6-well plates (2×104/well), incubated for 24 h, and subjected to 6 Gy radiation. Cell numbers were counted 24, 48 and 72 h after radiation and cells were suspended in fresh cell culture medium. Viable cells were visualized using trypan blue exclusion (17) and counted with an Automated Cell Counter (Bio-Rad Laboratories, Inc.). Cell proliferation was assessed using the WST assay (Cyto X cell viability assay kit; LPS Solution) according to the manufacturer's instructions.
Fluorescence-activated cell sorting analysis
Cell cycle analysis was determined using a BD Accuri™ C6 Plus flow cytometer (BD Biosciences). Briefly, cells were seeded onto 6-well plates (2×104/well), incubated for 24 h, and subjected to 6 Gy irradiation. After 72 h, cells were harvested by centrifugation at 200 × g for 5 min at room temperature, washed with PBS, fixed overnight at 4°C with ice-cold 75% ethanol, and incubated with RNase A (10 µg/ml) and propidium iodide (PI; 50 µg/ml in 0.1% sodium citrate with 0.1% NP-40) for 15 min at room temperature in the dark. Cells in the G1, S and G2/M phases were identified based on fluorescence intensity and cell cycle distribution was analyzed using BD Accuri™ C6 Plus software version 1.0.23.1 (BD Biosciences).
Western blotting and ELISA
Total cell lysates were prepared in RIPA lysis buffer (GenDEPOT) containing a freshly added protease inhibitor cocktail (GenDEPOT). The protein concentration was then measured using the Bradford assay (Bio-Rad Laboratories, Inc.) Protein extracts (15 µg) were then mixed with SDS sample buffer, boiled for 5 min and separated by SDS-PAGE on 12% (w/v) gels. Subsequently, proteins were transferred to nitrocellulose membranes (EMD Millipore). Blots were blocked in 5% BSA solution (BSA in 1X TBS-0.1% Tween-20; GenDEPOT) at room temperature for 1 h. Blots were incubated overnight at 4°C with primary antibodies (1:1,000 dilution) against heterochromatin protein 1γ (HP1γ; cat. no. ab56978), ADAM9 (cat. no. ab186833), ADAM10 (cat. no. ab1997) ADAM17 (cat. no. ab2051) (all Abcam), sirtuin 1 (SIRT1; cat. no. sc-15404), β-actin (cat. no. sc-47778) (both Santa Cruz Biotechnology, Inc.) and V5 (cat. no. R-960-25; Invitrogen; Thermo Fisher Scientific, Inc.) followed by incubation with HRP-conjugated goat anti-rabbit (cat. no. SA002-500) and goat anti-mouse (cat. no. SA001-500) secondary antibodies (1:5,000 dilution; both GenDEPOT) for 30 min at room temperature. Protein bands were detected with Western Lightning Plus ECL (PerkinElmer) and semi-quantification of protein expression was visualized using an Amersham Imager 600 (GE Healthcare).
Klotho and TNF-α levels were measured in cell lysates using the Mouse TNF-α ELISA Ready-Set-Go (cat. no. 88-7324; Invitrogen; Thermo Fisher Scientific, Inc.) and Mouse Klotho ELISA (cat. no. LS-F6578; LSBio) kits. Experiments were performed according to the manufacturer's instructions without further optimization and absorbance was read at 450 nm using the Multiskan FC microplate photometer (Thermo Fisher Scientific, Inc.).
Immunocytochemistry
mIMCD-3 cells were seeded on 8-well cell culture slides (4×103/well) (SPL Life Sciences), incubated for 24 h, and subjected to 6 Gy irradiation. After 72 h, slides were rinsed with PBS and fixed in 4% paraformaldehyde overnight at 4°C, followed by permeabilization in 0.1% Triton X-100 for 10 min at room temperature and rinsing with PBS. Cells were blocked in 5% BSA solution (BSA in 1X TBS-0.1% Tween-20) (GenDEPOT) at room temperature for 1 h. Subsequently, cells were incubated overnight at 4°C with primary antibodies (1:100 dilution) against HP1γ and SIRT1, followed by incubation with the following secondary antibodies (1:200 dilution): Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (cat. no. A11008) and Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (cat. no. A11001) (Invitrogen; Thermo Fisher Scientific, Inc.) for 2 h at room temperature in the dark. Specimens were then treated with 2 µg/ml PI (Sigma-Aldrich; Merck KGaA) in PBS for 5 min at room temperature, mounted on glass slides, and observed under a Zeiss LSM 710 confocal microscope (Carl Zeiss AG).
Reverse transcription-PCR (RT-PCR) and RT-quantitative (q)PCR
Total RNA was isolated from cells and tissues using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and quantified via formaldehyde-agarose gel electrophoresis. Single-stranded cDNA was synthesized from RNA (4 µg) using 0.27 µg oligo dT and amfiRivert reverse transcriptase (cat. no. R5600-200; GenDEPOT) according to the manufacturer's instructions. The desired cDNA fragments were amplified via RT-qPCR using the following primers: Klotho, forward 5′-TGTGAATGAGGCTCTGAAAGC-3′, reverse 5′-GAGCGATCACTAAGTGAATACG-3′; TNF-α, forward 5′-CCACCACGCTCTTCTGTCTAC-3′, reverse 5′-AGGGTCTGGGCCATAGAACT-3′; ADAM9, forward 5′-GGATATGGAGGAAGCGTGGA-3′, reverse 5′-GCAACAAGGGGGACGATTAG-3′; ADAM10, forward 5′-AGCAACATCTGGGGACAAAC-3′, reverse 5′-TGGCCAGATTCAACAAAACA-3′; ADAM17, forward 5′-GTACGTCGATGCAGAGCAAA-3′, reverse 5′-GAAATCCCAAAATCGCTCAA-3′; and GAPDH, forward 5′-AAGGGCTCATGACCACAGTC-3′ and reverse 5′-TTCAGCTCTGGGATGACCTT-3′. To determine mRNA expression, reactions were conducted in a total volume of 20 µl containing 1 µl cDNA, 5X Hot FIREPol® EvaGreen® qPCR Supermix (Solis BioDyne) and the relevant primers. The Mic qPCR cycler for real-time PCR (Bio Molecular Systems) was used to assess mRNA expression. Expression data were normalized to the geometric mean of the housekeeping gene, GAPDH, to control the variability in expression levels and analyzed using the 2−ΔΔCq method (18).
Transfection
A total of 1 day prior to transfection, cells were seeded onto 60-mm dishes (4×105/well). For pcDNA3.1/V5-His-TOPO vector plasmid (cat. no. K480001, Invitrogen; Thermo Fisher Scientific, Inc) and Klotho expression plasmid (V5-klotho; cat. no. 17712; Addgene, Inc.) DNA transfection, 8 µg DNA was added to 0.5 ml Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.), followed by the addition of 0.5 ml Opti-MEM containing 20 µl Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). The mixture was incubated at room temperature for 5 min and was then added to cells. After incubation for 6 h at 37°C, the medium was changed, followed by incubation of cells for 24 h and exposure to 6 Gy radiation. The empty pcDNA3.1/V5-His-TOPO vector was used as the negative control.
Statistical analysis
Data are presented as the mean ± SD of at least three experiments. Statistical comparisons among the groups were analyzed with unpaired Student's t-test, or one-way ANOVA and Tukey's post hoc test using SPSS software version 23 (IBM Corp.). P<0.05 was considered to indicate a statistically significant difference.
Results
Radiation induces senescence of kidney cells
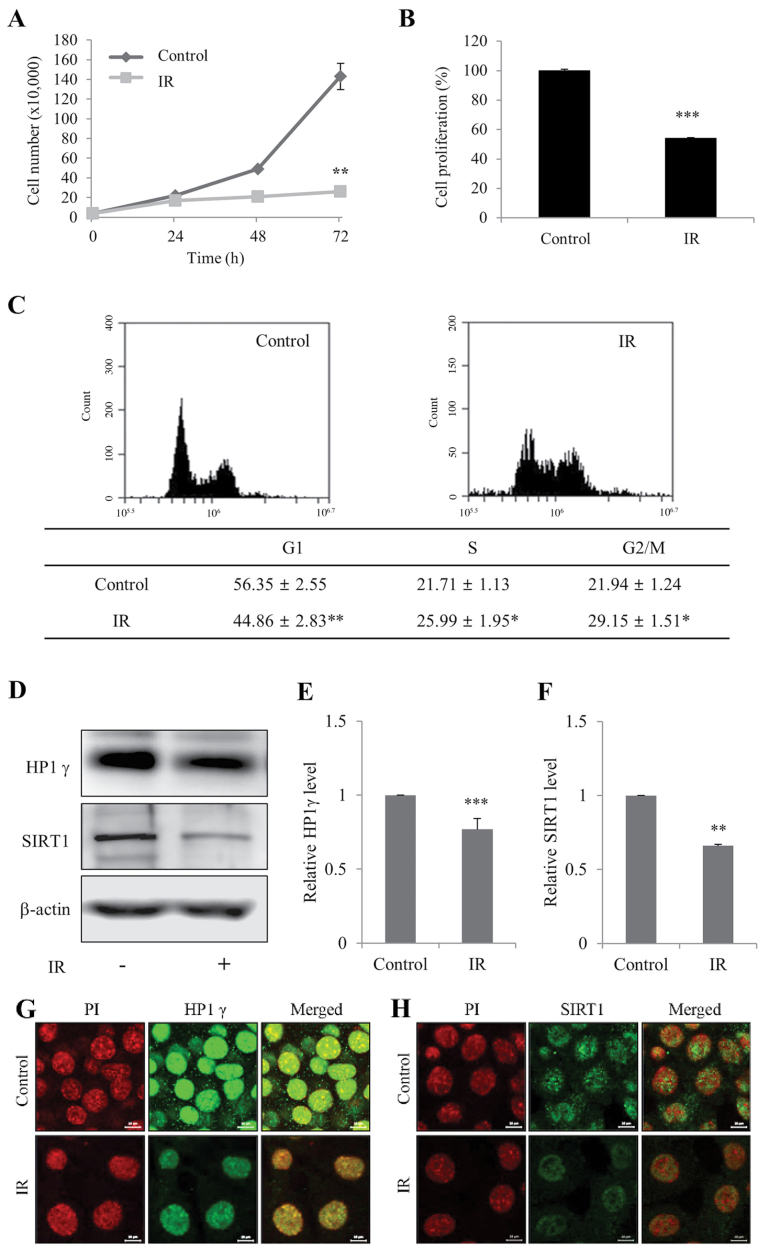
To determine whether IR induces cellular senescence in mIMCD-3 cells, cell viability and senescence-associated markers, HP1γ and SIRT1 (19), were initially measured. Cell viability and the proportion of cells in G1 phase of the cell cycle were significantly decreased in mIMCD-3 cells in response to irradiation (Fig. 1A-C). In addition, the expression levels of HP1γ and SIRT1 levels were significantly decreased in irradiated mIMCD-3 cells (Fig. 1D-F), as determined via western blotting. Immunofluorescence findings confirmed the IR-induced decrease in the expression levels of HP1γ and SIRT1 (Fig. 1G and H). These results indicated that irradiation may suppress viability and promote senescence of kidney cells.
Figure 1.
Radiation induces senescence of mIMCD-3 cells. (A) Control and IR-treated viable mIMCD-3 cells were visualized using Trypan blue exclusion and counted. (B) Control and IR-treated cell proliferation wase determined using the WST assay. Data are presented as a percentage of control cell growth. (C) Control and IR-treated mIMCD-3 cells were stained with PI and subjected to flow cytometry. (D) Protein expression levels of HP1γ and SIRT1 were determined via western blotting, with β-actin as the loading control. Semi-quantification of (E) HP1γ and (F) SIRT1 protein expression levels in cell lysates relative to the control group. Immunofluorescence analysis of (G) HP1γ and (H) SIRT1 using a fluorescein isothiocyanate-conjugated secondary antibody and PI nuclear staining followed by confocal microscopy (scale bar, 10 µm). mIMCD-3 cells were treated with or without 6 Gy radiation and cultured for 72 h. Data are expressed as the mean ± SD (n=4), *P<0.05, **P<0.01, ***P<0.001 vs. Control. mIMCD-3, mouse inner medullary collecting duct-3; IR, ionizing radiation; HP1γ, heterochromatin protein 1γ; SIRT1, sirtuin 1; PI, propidium iodide.
Radiation enhances TNF-α expression in kidney cells and tissues of mice
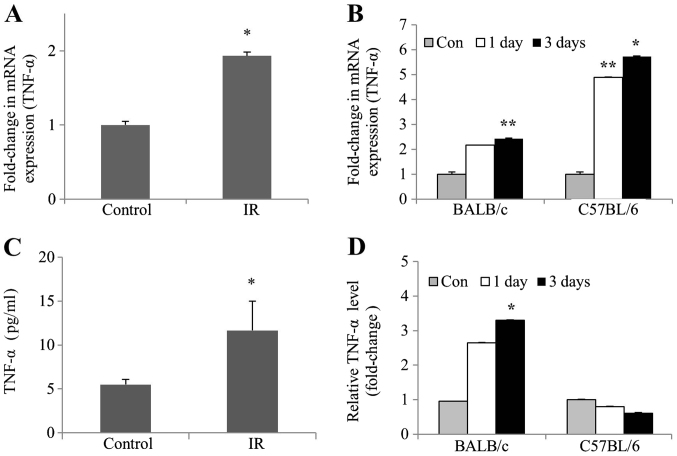
To determine whether TNF-α levels were affected by IR in vivo and in vitro, TNF-α mRNA expression and protein levels were examined in irradiated mIMCD-3 cells, as well as in the kidney tissues of BALB/c and C57BL/6 mice. RT-qPCR experiments revealed a significant increase in the mRNA expression levels of TNF-α, both in irradiated mIMCD-3 and C57BL/6 mice. However, the mRNA expression levels of TNF-α in BALB/c mice were only slightly increased (Fig. 2A and B). Consistent with this finding, ELISA experiments revealed a subsequent increase in TNF-α protein levels. Furthermore, TNF-α protein levels were significantly increased in irradiated BALB/c mice but only slightly decreased in irradiated C57BL mice (Fig. 2C and D). These collective data clearly demonstrated that the protein and mRNA expression levels of TNF-α were increased in irradiated kidney cells and BALB/c mice tissues.
Figure 2.
Upregulation of TNF-α mRNA expression and protein levels in irradiated mIMCD-3 cells, and renal tissues of BALB/c and C57BL6 mice. (A) mIMCD-3 cells, and (B) BALB/c and C57BL/6 mice were treated with or without 6 Gy radiation. After 24 and/or 72 h, reverse transcription-quantitative PCR was applied to assess TNF-α mRNA expression using GAPDH as the loading or internal control. ELISA was conducted to measure (C) protein levels of TNF-α (pg/ml) in mIMCD-3 cells, and (D) fold-change values of TNF-α levels in BALB/c and C57BL/6 mice relative to the control group. Data are presented as the mean ± SD (n=4). *P<0.05, **P<0.01 vs. Control. mIMCD-3, mouse inner medullary collecting duct-3; IR, ionizing radiation.
mRNA expression levels of Klotho are decreased in irradiated kidney cells and tissues of mice
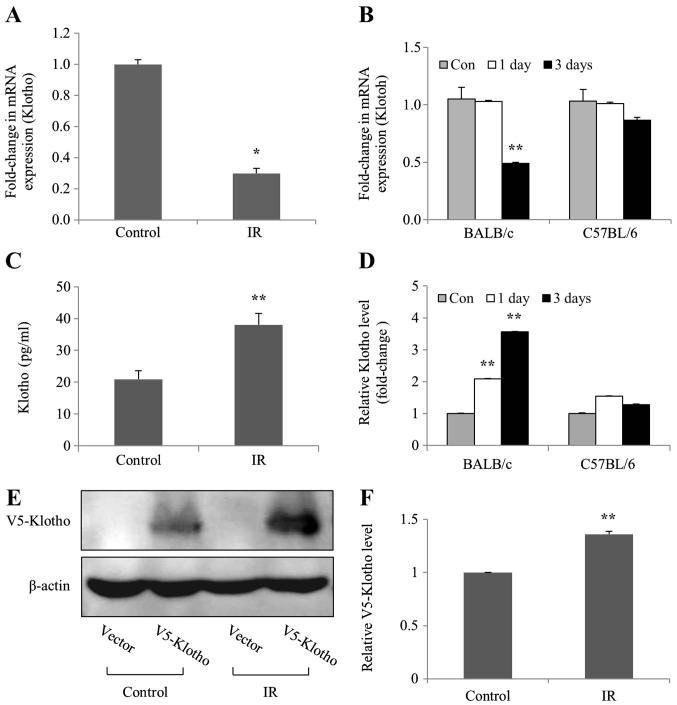
To investigate whether levels of the aging suppressor Klotho were affected by IR in vivo and in vitro, the mRNA expression and protein levels of Klotho were examined in irradiated mIMCD-3 cells, and in the kidney tissues of BALB/c and C57BL/6 mice. The mRNA expression levels of Klotho were markedly decreased in irradiated mIMCD-3 cells, and in BALB/c mice (Fig. 3A and B), as determined by RT-qPCR. By contrast, ELISA detected considerably increased Klotho protein levels in mIMCD-3 cells and BALB/c mice following radiation; however, Klotho protein levels were slightly increased on days 1 and 3 compared with the control in C57BL/6 mice (Fig. 3C and D). Transfection experiments were performed to confirm the action site (membrane-anchored or soluble secreted) of the Klotho protein expressed in the cells. Consistent with ELISA findings, the results of western blot analysis revealed increased levels of exogenous V5-tagged Klotho (membrane-anchored) protein in irradiated mIMCD-3 cells (Fig. 3E and F). Notably, these findings suggested that the mRNA expression levels of Klotho were decreased, whereas the expression levels of the corresponding protein were increased in irradiated mIMCD-3 cells and BALB/c mice.
Figure 3.
mRNA expression levels of Klotho are inhibited in mIMCD-3 cells, and renal tissues of BALB/c and C57BL6 mice after radiation exposure. (A) mIMCD-3 cells, and (B) BALB/c and C57BL/6 mice were treated with or without 6 Gy radiation. After 24 and/or 72 h, reverse transcription-quantitative PCR was applied to assess the mRNA expression levels of Klotho using GAPDH as the loading or internal control. ELISA was conducted to measure (C) Klotho protein levels (pg/ml) in mIMCD-3 cells, and (D) fold-change values of Klotho levels in BALB/c and C57BL/6 mice relative to the control group. (E) Following transient transfection with V5-tagged Klotho expression plasmid, mIMCD-3 cells were exposed to 6 Gy radiation and protein expression levels of Anti-V5 in cell lysates were determined via western blotting using Klotho-specific antibodies 24 h after irradiation. Variations in protein loading among samples were controlled by monitoring the β-actin level. (F) Semi-quantification of V5-tagged Klotho protein expression levels in IR-irradiated cell lysates relative to V5-tagged Klotho protein expression levels in control group. Data are presented as the mean ± SD (n=4). *P<0.05, **P<0.01 vs. Control. mIMCD-3, mouse inner medullary collecting duct-3; IR, ionizing radiation.
ADAM9/10/17 levels are decreased in irradiated kidney cells and tissues of mice
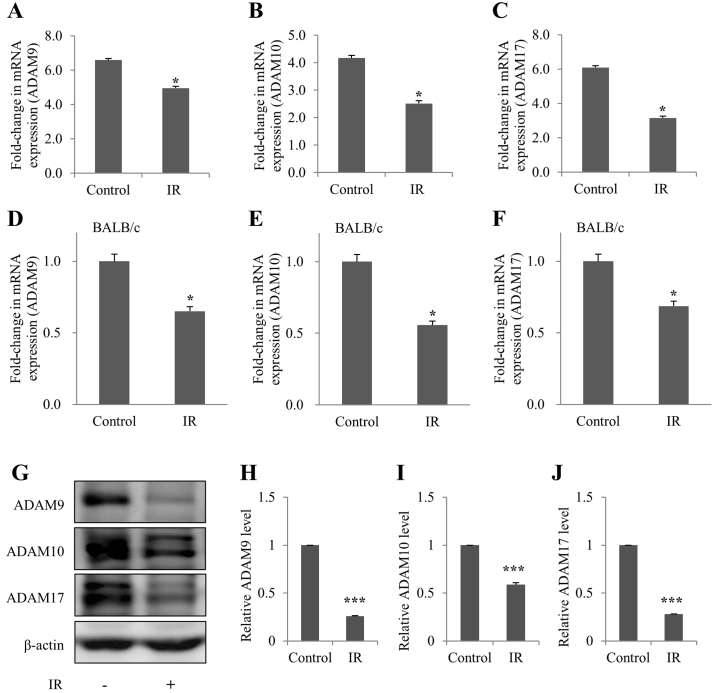
The mRNA expression levels of ADAM9/10/17 were subsequently detected in irradiated mIMCD-3 cells, and in the kidney tissues of BALB/c mice, in order to determine whether ADAM9/10/17, an ectodomain shedding enzyme of Klotho, was affected by IR in vivo and in vitro. As determined by RT-qPCR, the mRNA expression levels of ADAM9/10/17 were decreased in irradiated mIMCD-3 cells and BALB/c mice (Fig. 4A-F). In addition, ADAM9/10/17 protein expression was decreased in irradiated mIMCD-3 cells (Fig. 4G-J), as determined via western blot analysis.
Figure 4.
Downregulation of ADAM9/10/17 mRNA expression levels in irradiated mIMCD-3 cells and renal tissues of BALB/c mice. (A-C) mIMCD-3 cells and (D-F) BALB/c mice were treated with or without 6 Gy radiation. After 72 h, reverse transcription-quantitative PCR was applied to assess (A and D) ADAM9, (B and E) ADAM10 and (C and F) ADAM17 mRNA expression, using GAPDH as the loading or internal control. (G) Protein expression levels of ADAM9/10/17 were determined via western blotting using β-actin as the loading control. Semi-quantification of (H) ADAM9, (I) ADAM10 and (J) ADAM17 levels in cell lysates relative to the control groups. Data are presented as the mean ± SD (n=4). *P<0.05, ***P<0.001 vs. Control. mIMCD-3, mouse inner medullary collecting duct-3; IR, ionizing radiation.
Discussion
There is growing awareness that radiation induces injury in the normal tissue of organs that are late to respond to radiation, such as the brain, kidneys and lungs, involving complex and dynamic responses between multiple cell types that lead not only to targeted cell death, but also to acute and chronic alterations in cell function (20). The pathogenesis of radiation-induced renal dysfunction is multifaceted and is associated with the sequential interaction of all cell types, including vascular, tubular and glomerular cells, in the kidney. In particular, renal tubular cells and the surrounding interstitium are notably radiosensitive (1,21–23). Data from the present study revealed that irradiation induced cellular senescence in mIMCD-3 mouse kidney cells. Among the genes associated with cellular senescence, the mRNA expression levels of the aging suppressor, Klotho (24), were decreased and its negative regulator, TNF-α (11,25), were increased in irradiated kidney cells and mouse kidney tissues.
Cellular senescence is a common response of normal cells to radiation, which contributes to tissue injury (26). A previous study demonstrated the increased activity of senescence pathways in multiple kidney diseases (27). In addition, a reduction in the number of renal senescent cells via replacement with non-senescent cells has been reported to decrease aging-related injury (28). In the present study, the expression levels of HP1γ, a commonly used marker of senescence, were decreased in mIMCD-3 cells. Additionally, irradiation induced downregulation of SIRT1 protein, supporting the concept that radiation exposure may promote cellular senescence of mIMCD-3 kidney cells.
Senescent cells secrete various proinflammatory cytokines associated with normal tissue injury following irradiation (8). In previous studies, Klotho suppressed TNF-α-induced expression of adhesion molecules in endothelial cells (24) and proinflammatory cytokines reduced Klotho expression in the kidney (29), supporting the association of Klotho with inflammation in kidney cells. Klotho is an aging suppressor protein that is abundant in kidney tissue and Klotho-knockout mice have been shown to develop a syndrome similar to premature aging (30,31). The present study focused on determining the relevance of radiation-induced cellular senescence and the involvement of Klotho. Despite recent focus on the role of Klotho in the kidney, its precise functions with regards to pathophysiological implications remain to be established.
Klotho is a transmembrane protein whose extracellular domain is cleaved by the ectodomain shedding enzyme, ADAM9/10/17, to generate large amounts of soluble protein into the blood, urine and cerebrospinal fluid. The Klotho protein exists as both membrane-anchored and soluble secreted forms (30,32) with potentially distinct functions. Membrane Klotho forms a complex with fibroblast growth factor (FGF) receptors (FGFRs) and functions as a coreceptor for FGF23, a bone-derived hormone that controls mineral homeostasis. Soluble Klotho functions as an endocrine factor with important roles in numerous processes, including antisenescence, energy metabolism, inhibition of Wnt signaling, antioxidation, and modulation of ion transport independently of FGF23 and FGFRs (33–35). In particular, the present study showed that while Klotho gene transcription was downregulated, the corresponding translated protein was consistently expressed after radiation exposure. In addition, it was confirmed that the location of the expressed Klotho protein was not truncated and was anchored on the cell membrane with increased levels of the exogenous V5-tagged Klotho (membrane-anchored) protein detected after mIMCD-3 cells radiation exposure. ADAMs are membrane-anchored cell surface and secreted proteins containing disintegrin and metalloproteinase domains that are critical for regulation of cell phenotypes via effects on cell adhesion, migration, proteolysis, and modulation of cell signaling and biological responses (36). In the present study, the expression of the ectodomain shedding enzyme of Klotho, ADAM9/10/17, was reduced following exposure of the kidney to radiation. However, BALB/c mice are sensitive to radiation and C57BL/6 mice are resistant (37,38); therefore, this study evaluated ADAMs expression in BALB/c mice. Differences in radiation-induced genomic instability between these mouse strains necessitate further study of the expression levels of ADAMs that respond differently to radiation-sensitive or resistant mouse strains. Notably, a decline in blood Klotho levels has previously been detected in patients with aging-related disorders, such as cardiovascular disease, cancer, hypertension and kidney disease (39). The present findings suggested that a reduction in Klotho gene expression, along with disruption of physiological cleavage of Klotho via inhibition of ADAM, may contribute to radiation-induced renal dysfunction via the acceleration of senescence.
Commonly, damage of renal tubules is the ultimate pathway leading to end-stage kidney disease. Accumulating studies have suggested that the pathogenesis of radiation-induced nephropathy is multifaceted and involves the unpredictable interactions of all cell types of the kidney, as well as a number of various inflammatory elements in the kidney (21,22,40,41). Critically, some types of soluble Klotho may exist in the blood, urine and other body fluids, where it performs a multitude of functions in renal inflammatory response. However these pleiotropic roles are poorly understood (8,41–43). Moreover, the differences between BALB/c and C57BL/6 mice with regards to changes in TNF-α and ADAM9/10/17 expression caused by irradiation may be assessed in future research. The differences in mouse strains with regards to inflammation and tubular cell responses during damage may provide compelling potential targets for additional studies of responsiveness to the development of radiation-induced nephropathy (44–47).
In conclusion, to the best of our knowledge, the present study is the first to indicate that radiation-induced renal dysfunction may be associated with TNF-α-mediated inhibition of Klotho gene expression, which could accelerate cellular senescence of the kidney. Based on the current data, it may be suggested that kidney dysfunction due to irradiation is caused by decreased production of soluble Klotho via reduced expression of ADAM9/10/17. These novel findings provide insights into the pathogenic mechanisms underlying radiation-induced kidney injury, which should facilitate the development of treatment strategies and support the efficacy of Klotho as a biomarker. Therefore, Klotho may not only be useful as a diagnostic and/or prognostic marker, but could present a promising target to prevent and retard radiation-induced kidney injury. Further investigation of the functional involvement of Klotho-mediated renal tubular injury is imperative and will be the main point of further study, along with overcoming limitations, such as the use of a single cell line, a limited number of animals, pathological mechanism studies, and experimental animal model selection based on mouse strain difference. Therefore, future studies are focusing on identifying the mechanisms of action of ADAMs, and the various forms of Klotho that perform numerous functions, in order to understand the mechanisms underlying translational modification in irradiated kidney cells/tissues and serum.
Acknowledgements
Not applicable.
Funding
This research was supported by a grant from the Korea Institute of Radiological and Medical Sciences (KIRAMS), funded by the Ministry of Science, ICT and Future Planning, Republic of Korea (grant no. 50531-2020).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
EJK, ML and DYK designed the study, performed most of the experiments, analyzed the data, and wrote the manuscript. EJK and ML analyzed the data and provided advice on the project. EJK supervised the project and provided financial support. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The experimental protocol was conducted under the Guidelines for the Use and Care of Laboratory Animals and the study was approved by the IACUC of the Korea Institute of Radiological and Medical Sciences (KIRAMS; IACUC approval no. 2016-0065; Seoul, South Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cohen EP, Robbins ME. Radiation nephropathy. Seminars in nephrology Elsevier. 2003:486–499. doi: 10.1016/S0270-9295(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 2.Baradaran-Ghahfarokhi M. Radiation-induced kidney injury. J Renal Inj Prev. 2012;1:49–50. doi: 10.12861/jrip.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Little MP. A review of non-cancer effects, especially circulatory and ocular diseases. Radiat Environ Biophys. 2013;52:435–449. doi: 10.1007/s00411-013-0484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei J, Wang B, Wang H, Meng L, Zhao Q, Li X, Xin Y, Jiang X. Radiation-induced normal tissue damage: oxidative stress and epigenetic mechanisms. Oxid Med Cell Longev. 2019;2019:3010342. doi: 10.1155/2019/3010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salminen A, Kauppinen A, Kaarniranta K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP) Cell Signal. 2012;24:835–845. doi: 10.1016/j.cellsig.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM. Cellular senescence in renal ageing and disease. Nat Rev Nephrol. 2017;13:77–89. doi: 10.1038/nrneph.2016.183. [DOI] [PubMed] [Google Scholar]
- 7.Malaquin N, Tu V, Rodier F. Cellular Senescence. Springer; 2019. Assessing Functional Roles of the Senescence-Associated Secretory Phenotype (SASP) pp. 45–55. [DOI] [PubMed] [Google Scholar]
- 8.Citrin DE, Mitchell JB. Mechanisms of normal tissue injury from irradiation. Semin Radiat Oncol. 2017;27:316–324. doi: 10.1016/j.semradonc.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill RP, Zaidi A, Mahmood J, Jelveh S. Investigations into the role of inflammation in normal tissue response to irradiation. Radiother Oncol. 2011;101:73–79. doi: 10.1016/j.radonc.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, Yamanari T, Kikumoto Y, Uchida HA, Kitamura S, et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8:e56695. doi: 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thurston RD, Larmonier CB, Majewski PM, Ramalingam R, Midura-Kiela M, Laubitz D, Vandewalle A, Besselsen DG, Mühlbauer M, Jobin C, et al. Tumor necrosis factor and interferon-γ down-regulate Klotho in mice with colitis. Gastroenterology. 2010;138:1384–1394, 1394.e1-2. doi: 10.1053/j.gastro.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu MC, Kuro-o M, Moe OW. Klotho and kidney disease. J Nephrol. 2010;23(Suppl 16):S136–S144. [PMC free article] [PubMed] [Google Scholar]
- 13.Komaba H, Lanske B. Vitamin D in Chronic Kidney Disease. Springer Cham; 2016. Vitamin D and Klotho in Chronic Kidney Disease; pp. 179–194. [DOI] [Google Scholar]
- 14.Erin N, Türker S, Elpek Ö, Yildirim B. ADAM proteases involved in inflammation are differentially altered in patients with gastritis or ulcer. Exp Ther Med. 2018;15:1999–2005. doi: 10.3892/etm.2017.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Loon EP, Pulskens WP, van der Hagen EA, Lavrijsen M, Vervloet MG, van Goor H, Bindels RJ, Hoenderop JG. Shedding of klotho by ADAMs in the kidney. Am J Physiol Renal Physiol. 2015;309:F359–F368. doi: 10.1152/ajprenal.00240.2014. [DOI] [PubMed] [Google Scholar]
- 16.Kim EJ, Lee M, Kim DY, Kim KI, Yi JY. Mechanisms of energy metabolism in skeletal muscle mitochondria following radiation exposure. Cells. 2019;8:950. doi: 10.3390/cells8090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McRobb LS, McKay MJ, Gamble JR, Grace M, Moutrie V, Santos ED, Lee VS, Zhao Z, Molloy MP, Stoodley MA. Ionizing radiation reduces ADAM10 expression in brain microvascular endothelial cells undergoing stress-induced senescence. Aging (Albany NY) 2017;9:1248–1268. doi: 10.18632/aging.101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Slizhov P, Dolinina T, Pleskach N, Vasilishina AA, Zherebtsov SV, Bulatnikova MA, Mikhelson VM, Spivak IM. Markers of aging in cells of patients with cockayne syndrome. General and individual differences. Cell Tissue Biol. 2018;12:296–306. doi: 10.1134/S1990519X18040090. [DOI] [Google Scholar]
- 20.Zhao W, Chuang EY, Mishra M, Awwad R, Bisht K, Sun L, Nguyen P, Pennington JD, Wang TJ, Bradbury CM, et al. Distinct effects of ionizing radiation on in vivo murine kidney and brain normal tissue gene expression. Clin Cancer Res. 2006;12:3823–3830. doi: 10.1158/1078-0432.CCR-05-2418. [DOI] [PubMed] [Google Scholar]
- 21.Glatstein E, Fajardo LF, Brown JM. Radiation injury in the mouse kidney - I. Sequential light microscopic study. Int J Radiat Oncol Biol Phys. 1977;2:933–943. doi: 10.1016/0360-3016(77)90191-2. [DOI] [PubMed] [Google Scholar]
- 22.Williams MV. The cellular basis of renal injury by radiation. Br J Cancer Suppl. 1986;7:257–264. [PMC free article] [PubMed] [Google Scholar]
- 23.Jaggi JS, Seshan SV, McDevitt MR, Sgouros G, Hyjek E, Scheinberg DA. Mitigation of radiation nephropathy after internal alpha-particle irradiation of kidneys. Int J Radiat Oncol Biol Phys. 2006;64:1503–1512. doi: 10.1016/j.ijrobp.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 24.Maekawa Y, Ishikawa K, Yasuda O, Oguro R, Hanasaki H, Kida I, Takemura Y, Ohishi M, Katsuya T, Rakugi H. Klotho suppresses TNF-α-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35:341–346. doi: 10.1007/s12020-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 25.Navarro-González JF, Sánchez-Niño MD, Donate-Correa J, Martín-Núñez E, Ferri C, Pérez-Delgado N, Górriz JL, Martínez-Castelao A, Ortiz A, Mora-Fernández C. Effects of pentoxifylline on soluble Klotho concentrations and renal tubular cell expression in diabetic kidney disease. Diabetes Care. 2018;41:1817–1820. doi: 10.2337/dc18-0078. [DOI] [PubMed] [Google Scholar]
- 26.Hong EH, Lee SJ, Kim JS, Lee KH, Um HD, Kim JH, Kim SJ, Kim JI, Hwang SG. Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase. J Biol Chem. 2010;285:1283–1295. doi: 10.1074/jbc.M109.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muñoz-Espín D, Serrano M. Cellular senescence: From physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Fogo AB. Cell senescence in the aging kidney. J Am Soc Nephrol. 2010;21:1436–1439. doi: 10.1681/ASN.2010020205. [DOI] [PubMed] [Google Scholar]
- 29.Moreno JA, Izquierdo MC, Sanchez-Niño MD, Suárez-Alvarez B, Lopez-Larrea C, Jakubowski A, Blanco J, Ramirez R, Selgas R, Ruiz-Ortega M, et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol. 2011;22:1315–1325. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.John GB, Cheng C-Y, Kuro-o M. Role of Klotho in aging, phosphate metabolism, and CKD. Am J Kidney Dis. 2011;58:127–134. doi: 10.1053/j.ajkd.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y, Sun Z. Molecular basis of Klotho: From gene to function in aging. Endocr Rev. 2015;36:174–193. doi: 10.1210/er.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavik I, Jaeger P, Ebner L, Wagner CA, Petzold K, Spichtig D, Poster D, Wüthrich RP, Russmann S, Serra AL. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: A sequence suggested from a cross-sectional study. Nephrol Dial Transplant. 2013;28:352–359. doi: 10.1093/ndt/gfs460. [DOI] [PubMed] [Google Scholar]
- 33.Lu X, Hu MC. Klotho/FGF23 axis in chronic kidney disease and cardiovascular disease. Kidney Dis. 2017;3:15–23. doi: 10.1159/000452880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuro-O M. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019;15:27–44. doi: 10.1038/s41581-018-0078-3. [DOI] [PubMed] [Google Scholar]
- 35.Hu MC, Kuro-o M, Moe OW. Klotho and chronic kidney disease. Contrib Nephrol. 2013;180:47–63. doi: 10.1159/000346778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palau V, Pascual J, Soler MJ, Riera M. Role of ADAM17 in kidney disease. Am J Physiol Renal Physiol. 2019;317:F333–F342. doi: 10.1152/ajprenal.00625.2018. [DOI] [PubMed] [Google Scholar]
- 37.Ponnaiya B, Cornforth MN, Ullrich RL. Radiation-induced chromosomal instability in BALB/c and C57BL/6 mice: The difference is as clear as black and white. Radiat Res. 1997;147:121–125. doi: 10.2307/3579411. [DOI] [PubMed] [Google Scholar]
- 38.Hamasaki K, Imai K, Hayashi T, Nakachi K, Kusunoki Y. Radiation sensitivity and genomic instability in the hematopoietic system: Frequencies of micronucleated reticulocytes in whole-body X-irradiated BALB/c and C57BL/6 mice. Cancer Sci. 2007;98:1840–1844. doi: 10.1111/j.1349-7006.2007.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JH, Hwang KH, Park KS, Kong ID, Cha SK. Biological role of anti-aging protein Klotho. J Lifestyle Med. 2015;5:1–6. doi: 10.15280/jlm.2015.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kal HB, van Kempen-Harteveld ML. Renal dysfunction after total body irradiation: dose-effect relationship. Int J Radiat Oncol Biol Phys. 2006;65:1228–1232. doi: 10.1016/j.ijrobp.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron, Exp Nephrol. 2005;101:e67–e74. doi: 10.1159/000086500. [DOI] [PubMed] [Google Scholar]
- 42.Kala J. Radiation-induced kidney injury. J Onco-Nephrology. 2019;3:160–167. doi: 10.1177/2399369319865271. [DOI] [Google Scholar]
- 43.Fujiu K, Manabe I, Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest. 2011;121:3425–3441. doi: 10.1172/JCI57582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puri TS, Shakaib MI, Chang A, Mathew L, Olayinka O, Minto AW, Sarav M, Hack BK, Quigg RJ. Chronic kidney disease induced in mice by reversible unilateral ureteral obstruction is dependent on genetic background. Am J Physiol Renal Physiol. 2010;298:F1024–F1032. doi: 10.1152/ajprenal.00384.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Kooten C, Daha MR, van Es LA. Tubular epithelial cells: A critical cell type in the regulation of renal inflammatory processes. Exp Nephrol. 1999;7:429–437. doi: 10.1159/000020622. [DOI] [PubMed] [Google Scholar]
- 46.Jevnikar AM, Brennan DC, Singer GG, Heng JE, Maslinski W, Wuthrich RP, Glimcher LH, Kelley VE. Stimulated kidney tubular epithelial cells express membrane associated and secreted TNF α. Kidney Int. 1991;40:203–211. doi: 10.1038/ki.1991.201. [DOI] [PubMed] [Google Scholar]
- 47.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.