Abstract
Objectives
To study evidence for construct validity, the aim was to describe the outcome from the recently developed Diabetes Questionnaire, assess the associations of that outcome with clinical variables and generic health-related quality of life, and study the sensitivity to differences between clinically relevant groups of glycaemic control in adults with type 1 and type 2 diabetes in a nation-wide setting.
Design
Cross-sectional survey.
Setting
Swedish diabetes care clinics connected to the National Diabetes Register (NDR).
Participants
Among 2479 adults with type 1 diabetes and 2469 with type 2 diabetes selected at random from the NDR, 1373 (55.4%) with type 1 and 1353 (54.8%) with type 2 diabetes chose to participate.
Outcome measures
The Diabetes Questionnaire, the generic 36-item Short Form version 2 (SF-36v2) health survey and clinical variables.
Results
Related to the prespecified assumptions, supporting evidence for construct validity for the Diabetes Questionnaire was found. Supporting divergent validity, the statistically significant correlations with the clinical variables were few and weak. In relation to the SF-36v2 and in support of convergent validity, the strongest correlations were seen in the Diabetes Questionnaire scales General Well-being and Mood and Energy. In those scales, machine learning analyses showed that about 40%–45% of the variance was explained by the SF-36v2 results and clinical variables. In multiple regression analyses among three groups with differing levels of glycated haemoglobin adjusted for demographics, other risk factors, and diabetes complications, the high-risk group had, in support of sensitivity to clinically relevant groups, statistically significant lower scores than the well-controlled group in most Diabetes Questionnaire scales.
Conclusions
This nation-wide study shows that the Diabetes Questionnaire captures some generic health-related quality-of-life dimensions, in addition to adding diabetes-specific information not covered by the SF-36v2 and clinical variables. The Diabetes Questionnaire is also sensitive to differences between clinically relevant groups of glycaemic control.
Keywords: general diabetes, clinical audit, quality in health care
Strengths and limitations of this study.
The cross-sectional study used a large, heterogeneous nationwide sample of adults with type 1 diabetes and adults with type 2 diabetes selected at random.
Respondents were representative of the 2015 population in the Swedish National Diabetes Register.
The Diabetes Questionnaire scales scores were related to relevant clinical variables and a well-known and often recommended measure of generic health-related quality of life.
The analyses were limited to the respondents and might reflect a group with greater motivation for participation.
The questionnaires were only offered in Swedish.
Introduction
Everyday life with diabetes as an adult is a complex challenge. Diabetes makes individuals responsible for self-management to avoid serious short-term and long-term complications, while balancing self-perceived health and well-being in the present as well as in the future.1–6 To support skills for self-management is a central task of diabetes care, and the individual patient’s prerequisites, wishes and available evidence must be taken into account.1 4–6 An important step for the Swedish National Diabetes Register (NDR) has therefore been to broaden healthcare provider perspectives and enable a systematic collection of adults’ perspectives of living with diabetes and their experiences of whether they are offered adequate support from diabetes care.7–10 The newly developed Diabetes Questionnaire is intended to support meetings with individuals and provide a means for quality improvement at the local, regional and national levels.7–9
The Diabetes Questionnaire was developed from interviews with adults with type 1 or type 2 diabetes that identified a broad range of aspects important to the target group, such as well-being, impact on daily life, capabilities to manage diabetes and support from diabetes care.9 In line with Sen’s capability approach,11 12 the Diabetes Questionnaire focuses on the individual’s opportunities, prerequisites and possible barriers to live a good life with diabetes.7–9 Supporting evidence for content validity, face validity and ease of items understandability and answerability has been presented.8 9 In addition, supporting evidence for test–retest reliability and that the scales can be used for comparison between men and women, between different age groups, and, for most scales, between type 1 and type 2 diabetes have been provided.7 8 Furthermore, the scales can detect differences between clinically relevant subgroups, such as diabetes type, diabetes treatment, age group and gender.7 We have also begun to study the associations with clinical variables by showing low individual-level correlations with glycated haemoglobin (HbA1c), systolic blood pressure (SBP) and low-density lipoprotein (LDL) cholesterol.7
This study adds to previous work and reports on an extended analysis of the evidence for construct validity. Construct validity concerns the confidence that a questionnaire captures the construct it was intended to measure.13 It is a measurement property that involves a complex process using a variety of techniques studying differences between relevant groups and prespecified assumptions of logical relationships to scores of a range of other measures and patient characteristics.13 14 The assumptions can postulate which aspects are expected to be related to each other, presenting evidence for convergent validity, and which aspects are expected to be relatively unrelated, supporting evidence for divergent, also known as discriminant, validity.13 For this work, we chose to focus on differences between subgroups of glycaemic control as measured by HbA1c and the relations to clinical variables relevant for diabetes care and an often-recommended generic measure of health-related quality of life, the 36-item Short Form version 2 (SF-36v2) health survey. To study evidence for construct validity, the aim was to describe the outcome from the Diabetes Questionnaire, to assess the associations of that outcome with clinical variables and generic health-related quality of life, and to study the sensitivity to differences between clinically relevant groups of glycaemic control in adults with type 1 and type 2 diabetes in a nationwide setting.
Methods
Sample and data-collection
In this cross-sectional survey, 2479 adults with type 1 diabetes and 2469 with type 2 diabetes were selected at random without replacement from the Swedish NDR. Eligibility criteria were being alive, 18–80 years of age, and recorded in the NDR during the period from 30 September 2014 to 1 October 2015 with at least one recorded test of HbA1c level during the previous 12 months. With these criteria, 29 245 adults with type 1 diabetes at hospital outpatient clinics and 208 852 adults with type 2 diabetes at primary healthcare centres were eligible for recruitment. In the data collection phase, we aimed at a sample size allowing for subgroup analyses.
The Diabetes Questionnaire, the SF-36v2 survey, and a prepaid return envelope were sent by email in October 2015 to survey selectees and again to non-respondents after 30 days.7 15 Both questionnaires were answered by 1373 (55.4%) individuals with type 1 diabetes and 1353 (54.8%) with type 2 diabetes.15 With small differences in response rate depending on the questionnaires in question, the sample has been described as previously focusing on the scale development of the Diabetes Questionnaire7 and separate analyses of the SF-36v2 data.15 Age, sex and clinical variables (diabetes type defined by clinical diagnosis, diabetes duration, HbA1c level, cardiovascular risk factors, complications, physical activity level and receipt of medical treatment) recorded because of their relevance to high-quality diabetes care were collected from the NDR.
Diabetes Questionnaire
The Diabetes Questionnaire is a 33-item self-reporting questionnaire having a total of 12 scales divided into two main parts.7 8 Part 1 has 22 items on eight scales and acts as a patient-reported outcome measure (PROM). These scales are General Wellbeing (GenW), Mood and Energy (MoE), Free of Worries about blood sugar (FreW), Capabilities to Manage your Diabetes (ManD), Diet and Exercise (DiEx), Not Limited by Diabetes (NLD), Not Limited by Blood Sugar (NLBS) and Support from Others (SuO). Part 2 is an 11-item patient-reported experience measure (PREM) with four scales. Those scales are Support from Diabetes Care (SuDC), Access to Diabetes Care (AcDC), Continuity in Diabetes Care (CoDC) and Medical Devices and Medical Treatment (MDMT). All scales are scored from 0 to 100, with higher scores representing the more desirable outcome. The scales ManD, NLBS and MDMT are specific to diabetes type.7
SF-36v2 survey
The SF-36v2 survey is a self-reporting questionnaire for generic health-related quality of life with support for its validity and reliability in overall populations, such as people with diabetes.3 16–20 We used the self-administered standard form in Swedish and software from QualityMetric. The eight domains produced are physical functioning (PF); role-physical, that is role limitations due to physical health problems; bodily pain; general health (GH); vitality (VT); social functioning (SF); role-emotional, that is role limitations due to mental health problems; and mental health (MH). The domains are scored from 0 to 100. Higher scores indicate a better general health-related quality of life.16 17
Prespecified assumptions
As the Diabetes Questionnaire is intended to measure patient perspectives on how they feel, how their diabetes treatment is going and their experiences of support from diabetes care, the prespecified assumptions for correlations with clinical variables and the SF-36v2 were as follows:
Based on clinical experience, it was proposed that, in support of divergent validity, a small number of negative and weak correlations would be found between the Diabetes Questionnaire scales and the clinical variables, mostly related to the HbA1c level. There would be no correlations with SBP and LDL cholesterol.
Based on examinations of the content in the two questionnaires, it was proposed that in support of convergent validity, the Diabetes Questionnaire PROM scales GenW, MoE, FreW, ManD, DiEx, NLD and NLBS would have more and stronger correlations to the SF-36v2 domains, as compared with the PROM scale SuO and the PREM scales (SuDC, AcDC, CoDC and MDMT). Observed correlations would be positive, with the strongest in GenW and MoE. In support of divergent validity strong correlations were not expected across the other scales. Correlations ≥0.60 were considered as very strong, 0.50 to <0.60 as strong, 0.40 to <0.50 as moderate and <0.40 as weak.
Statistical analysis
The data for participants with type 1 and type 2 diabetes were analysed separately. The descriptive statistics for each variable are based on non-missing observations. The continuous variables are given as means and SD for normal distributions and as medians and IQRs for skewed distributions. The categorical variables are presented as numbers and percentages. The generation of scale scores from the Diabetes Questionnaire is described in detail elsewhere.7 The SF-36v2 domain scores were generated using the manual and licensed software from QualityMetric.17
In relation to the prespecified assumptions, Spearman’s rank correlation was used to study the monoton associations between the Diabetes Questionnaire scale scores and the clinical variables age, diabetes duration, HbA1c level, body mass index (BMI), LDL cholesterol and SBP, as well as between the scores from the Diabetes Questionnaire scales and the SF-36v2 domains. To broaden the analysis, machine learning using random forests was conducted to investigate non-linear associations between the Diabetes Questionnaire scales and the SF-36v2 domains together with clinical variables (age, sex, diabetes duration, HbA1c level, BMI, LDL cholesterol and SBP). Random forest is a general tree-based regression and classification method that uses bootstrapping to create a large number of regressions of classification trees that are combined to produce a model prediction.21 The use of a large number of trees allows the model to depict non-linear associations without the need to prespecify these in a model, while at the same time guarding against overfit.21 First, the variance in all Diabetes Questionnaire scales was examined in relation to the SF-36v2 domains and the clinical variables together. Next, the variable importance of the SF-36v2 domains and the clinical variables as predictors of the PROM scales GenW and MoE were examined. We also examined the percent variance in HbA1c explained by another clinical variable, the Diabetes Questionnaire scales, and the SF-36v2 domains together. The results are given as percent of the total variance. Each model contained 1000 trees.
To study the sensitivity of the Diabetes Questionnaire scales to clinically relevant groups of glycaemic control, group-level associations between the Diabetes Questionnaire scales and glycaemic control as measured by HbA1c, unadjusted and adjusted multiple regression analyses were conducted in the same manner as previously described for the SF-36v2 data.15 HbA1c was considered as a categorical variable divided into three clinically relevant groups corresponding to differing levels of glycaemic control and consequently differing levels of the risk of diabetes complications according to international and Swedish treatment guidelines.4 22 The three groups were well-controlled (<52 mmol/mol), sub-optimal (52–69 mmol/mol) and high-risk (≥70 mmol/mol). For the three HbA1c groups, the least square mean estimates and 95% CIs were calculated for each scale. The scale observations were modelled with a linear model with fixed effects for the HbA1c group (exposure), age, sex, diabetes duration, BMI, SBP, LDL-cholesterol, micro-albuminuria and macro-albuminuria, estimated glomerular filtration rate, retinopathy, smoking status, physical activity level, previous coronary heart disease, previous stroke, and receipt of antihypertensive and lipid lowering treatments. Missing data were imputed 10 times, using multiple chained equations. The analyses were performed separately for each imputed data set, and the results were subsequently combined using Rubin’s rules. The results are presented as least square mean estimates with 95% CIs.
The extent of missing data was 0% for age and sex, 7.2% for clinical variables (range 0%–36.5%), 1.7% for the SF-36v2 domains (range 0%–3.3% for individual dimensions) and 4.8% for the Diabetes Questionnaire scales (range 0.3%–34.7% for individual scales). For the Diabetes Questionnaire, the higher extent of missing data is likely related to having ‘not applicable’ as a response alternative in some scales, which at this stage was treated as missing data. For scales without ‘not applicable’ as a response alternative, the range for missing data was 0.3%–2.8%.
The standardised mean difference was used to examine the data balance between the HbA1c groups and the deviation from the means in the clinical and demographic data. A significance level of 5% was used throughout; no allowance was made for multiplicity of statistical tests. The analyses were conducted using SAS V.9.4 and R V.3.4.4.
Patient and public involvement statement
The Diabetes Questionnaire was based on qualitative interviews with adults living with diabetes.8 9 Adults with diabetes and representatives from patient organisations participated in expert reviews during the development and initial testing.8 Adults with diabetes were involved in the pretesting phase by participating in cognitive interviews and being consulted to comment on questionnaire revisions.8 The analyses presented here as the previous scale development and evaluation of reliability and validity relied on the contributions from those adults with diabetes who responded to the questionnaires.7 8 The Swedish Diabetes Foundation, the national patient organisation, has expressed their support for the project.
Results
Among respondents with type 1 diabetes, 50.3% were men. The averages of key statistics were 48.6 years for age, 24.7 years for diabetes duration, and 62 mmol/mol for HbA1c level. Among respondents with type 2 diabetes, 60.8% were men. Corresponding averages were 66.6 years for age, 9.4 years for diabetes duration, and 53 mmol/mol for HbA1c level (table 1). The crude means and SD for the Diabetes Questionnaire scales are given in online supplemental table S1. The clinical characteristics of non-respondents are given in online supplemental table S2.
Table 1.
Clinical and demographic characteristics of the respondents separated by diabetes type and glycated haemoglobin (HbA1c) level
| Variable | Type 1 diabetes | Type 2 diabetes | ||||||||
| All | HbA1c <52 mmol/mol | HbA1c 52–69 mmol/mol | HbA1c ≥70 mmol/mol | Standardised mean difference, SMD | All | HbA1c <52 mmol/mol | HbA1c 52–69 mmol/mol | HbA1c ≥70 mmol/mol | Standardised mean difference, SMD | |
| Number (%) | 1373 | 284 (20.7%) | 781 (56.9%) | 308 (22.4%) | 1353 | 725 (53.6%) | 503 (37.2%) | 125(9.2%) | ||
| Men, n (%) | 690 (50.3) | 152 (53.5) | 391 (50.1) | 147 (47.7) | 0.077 | 822 (60.8) | 444 (61.2) | 302 (60.0) | 76 (60.8) | 0.016 |
| Age, years (SD) | 48.6 (16.4) | 46.9 (17.0) | 49.6 (16.1) | 47.8 (16.3) | 0.113 | 66.6 (9.1) | 66.5 (9.1) | 66.9 (9.0) | 65.5 (9.7) | 0.103 |
| Diabetes duration, years (IQR) | 22.0 (12.0–36.0) | 19.0 (7.0–32.0) | 23.0 (13.0–37.0) | 24.0 (13.0–37.0) | 0.150 | 8.0 (4.0–14.0) | 6.0 (3.0–11.0) | 10.0 (6.0–16.0) | 13.0 (6.0–17.0) | 0.443 |
| HbA1c mmol/mol (SD) | 62 (12.7) | 53 (12.5) | ||||||||
| BMI, kg/m2 (SD) | 26.0 (4.2) | 25.2 (3.8) | 26.0 (4.2) | 26.7 (4.6) | 0.239 | 29.9 (5.3) | 29.3 (5.2) | 30.3 (5.4) | 32.0 (5.5) | 0.332 |
| Systolic blood pressure, mm Hg (SD) | 127.0 (14.0) | 124.8 (14.0) | 127.5 (13.8) | 127.8 (14.2) | 0.145 | 134.3 (14.3) | 134.0 (14.4) | 134.5 (13.7) | 135.1 (16.5) | 0.046 |
| Antihypertensive medication, n (%) | 589 (44.7) | 99 (36.9) | 341 (45.3) | 149 (50.2) | 0.179 | 1070 (80.1) | 572 (79.6) | 404 (81.9) | 94 (76.4) | 0.091 |
| LDL-cholesterol, mmol/L (SD) | 2.4 (0.8) | 2.5 (0.8) | 2.4 (0.8) | 2.5 (0.8) | 0.077 | 2.5 (0.9) | 2.5 (0.9) | 2.4 (0.9) | 2.5 (1.0) | 0.026 |
| Lipid-lowering medication, n (%) | 642 (48.4) | 94 (34.6) | 378 (49.8) | 170 (57.6) | 0.315 | 900 (68.1) | 472 (66.6) | 344 (70.1) | 84 (69.4) | 0.050 |
| Micro-albuminuria, n (%) | 132 (10.3) | 12 (4.6) | 70 (9.5) | 50 (17.6) | 0.285 | 194 (18.0) | 80 (13.9) | 83 (20.1) | 31 (34.1) | 0.323 |
| Macro-albuminuria, n (%) | 31 (2.6) | 5 (2.1) | 12 (1.8) | 14 (5.2) | 0.126 | 52 (5.0) | 27 (4.8) | 20 (5.1) | 5 (6.1) | 0.037 |
| Estimated glomerular filtration rate, mL/min (SD) | 90.0 (23.5) | 90.6 (20.7) | 89.1 (22.6) | 91.6 (27.7) | 0.071 | 82.3 (23.5) | 82.5 (22.3) | 81.9 (24.0) | 83.4 (27.9) | 0.038 |
| Retinopathy, n (%) | 875 (65.9) | 137 (50.6) | 520 (68.2) | 218 (74.1) | 0.333 | 327 (29.4) | 128 (21.7) | 153 (36.3) | 46 (47.0) | 0.366 |
| Coronary heart disease, n (%) | 83 (6.3) | 9 (3.3) | 53 (7.0) | 21 (7.1) | 0.113 | 279 (22.4) | 136 (20.2) | 111 (24.0) | 32 (28.6) | 0.130 |
| Stroke, n (%) | 48 (3.6) | 5 (1.9) | 32 (4.2) | 11 (3.7) | 0.093 | 96 (7.8) | 48 (7.2) | 40 (8.9) | 8 (7.1) | 0.043 |
| Smoker, n (%) | 135 (10.1) | 14 (5.1) | 78 (10.2) | 43 (14.4) | 0.214 | 162 (12.9) | 79 (11.7) | 58 (12.3) | 25 (23.1) | 0.203 |
| Physical activity, daily, n (%) | 359 (27.6) | 90 (33.5) | 203 (27.2) | 66 (23.2) | 0.334 | 426 (34.9) | 251 (38.7) | 157 (33.9) | 18 (16.7) | 0.410 |
| Diabetes treatment | 0.136 | 0.813 | ||||||||
| Diet alone, n (%) | 195 (14.4) | 172 (23.7) | 19 (3.8) | 4 (3.3) | ||||||
| Oral hypoglycaemic agent alone, n (%) | 718 (53.1) | 419 (57.8) | 261 (52.0) | 38 (30.9) | ||||||
| Insulin alone, n (%) | 1335 (97.2) | 271 (95.4) | 764 (97.8) | 300 (97.4) | 130 (9.6) | 46 (6.3) | 63 (12.5) | 21 (17.1) | ||
| Insulin and oral agent, n (%) | 32 (2.3) | 9 (3.2) | 15 (1.9) | 8 (2.6) | 266 (19.7) | 76 (10.5) | 140 (27.9) | 50 (40.7) | ||
| Insulin pump users, n (%) | 356 (26.2) | 66 (23.8) | 221 (28.5) | 69 (22.5) | 0.091 | 2 (0.5) | 1 (0.9) | 1 (0.5) | 0 (0.0) | 0.093 |
BMI, body mass index; LDL, low-density lipoprotein.
bmjopen-2020-038966supp001.pdf (693.7KB, pdf)
The descriptive statistics are presented as the means and SD for normally distributed continuous variables, the median and IQR for skewed distributions, or number and percentages for categorical variables.
Monoton correlations related to the proposed assumptions between the Diabetes Questionnaire scale scores and the clinical variables
In line with the assumptions and in support for divergent validity, there were few statistically significant monoton correlations between the Diabetes Questionnaire scales and the clinical variables. Observed correlations were weak, and most were negative. The results are shown as heat maps in online supplemental figures S1, S2 with details provided in online supplemental tables S3 and S4.
As assumed, the HbA1c level was the variable with most statistically significant correlations across the Diabetes Questionnaire scales. Statistically significant but weak correlations between having a lower and better HbA1c level and higher and better scores were seen in several Diabetes Questionnaire scales. For participants with type 1 diabetes, significant weak negative correlations (−0.12 to −0.25) were seen in the five Diabetes Questionnaire PROM scales GenW, FreW, ManD, DiEx and NLBS. The strongest correlations were seen in ManD and DiEx. Among participants with type 2 diabetes, statistically significant but weak negative correlations (−0.13 to −0.24) were seen in the seven Diabetes Questionnaire PROM scales GenW, MoE, FreW, ManD, DiEx, NLD and NLBS and in the two PREM scales SuDC and AcDC. The strongest correlations were seen in MoE, FreW and ManD, with generally stronger correlations in the PROM scales than in the PREM scales online supplemental figure S1, S2 and tables S3, S4.
For age, statistically significant positive correlations showed that a higher age was weakly associated with higher and better scores in several Diabetes Questionnaire scales. For participants with type 1 diabetes, statistically significant weak positive correlations (0.11–0.19) were seen in the four PROM scales MoE, FreW, ManD and DiEx, and in the two PREM scales AcDC and MDMT. The highest correlations were seen in MoE, FreW and MDMT. Among participants with type 2 diabetes, statistically significant weak positive correlations (0.12–0.16) were seen in the six PROM scales GenW, MoE, FreW, ManD and DiEx. The highest correlations were seen in MoE, FreW and DiEx. For LDL cholesterol and SBP, the results came up to the expectations of no statistically significant correlations. However, for participants with type 1 diabetes, a statistically significant negative correlation showed that a lower SBP was weakly associated with better scores in MoE. A lower BMI showed statistically significant weak negative correlations with higher scores in DiEx in both diabetes types as with GenW and MoE in type 2 diabetes. For diabetes duration, statistically significant positive correlations showed that a longer duration was weakly associated with higher scores in FreW and ManD for participants with type 1 diabetes. For those with type 2 diabetes, statistically significant negative correlations showed that a longer duration was associated with lower scores in FreW and NLBS online supplemental figure S1, S2 and tables S3, S4.
Monoton correlations related to the proposed assumptions between scores in the Diabetes Questionnaire scales and the SF-36v2 domains
In line with the assumptions and in support for convergent validity, the statistically significant monoton correlations between the Diabetes Questionnaire scales and the SF-36v2 domains were stronger in seven of the PROM scales as compared with the PROM scale SuO and the PREM scales. As expected, the observed statistically significant correlations were all positive, showing an association between higher scores in both questionnaires. The results are shown in figures 1 and 2 and online supplemental tables S5 and S6.
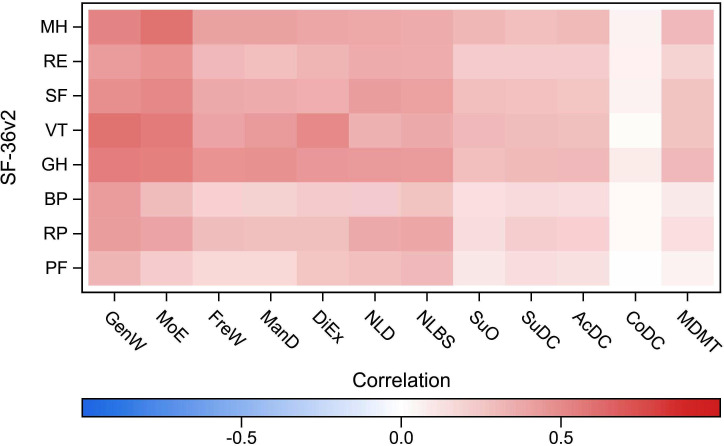
Figure 1.
Spearman’s rank correlation between the Diabetes Questionnaire scales and the SF-36v2 domains in type 1 diabetes. Diabetes Questionnaire scales: AcDC, Access to Diabetes Care; CoDC, Continuity in Diabetes Care; DiEx, Diet and Exercise; FreW, Free of Worries about blood sugar; GenW, General Wellbeing; ManD, Capabilities to Manage your Diabetes; MDMT, Medical Devices and Medical Treatment; MoE, Mood and Energy; NLBS, Not Limited by Blood Sugar; NLD, Not Limited by Diabetes; SuDC, Support from Diabetes Care; SuO, Support from Others. SF-36v2 domains: BP, bodily pain; GH, general health; MH, mental health; PF, physical functioning; RE, role-emotional; RP, role-physical; SF, social functioning; VT, vitality. SF-36v2, 36-item Short Form version 2.
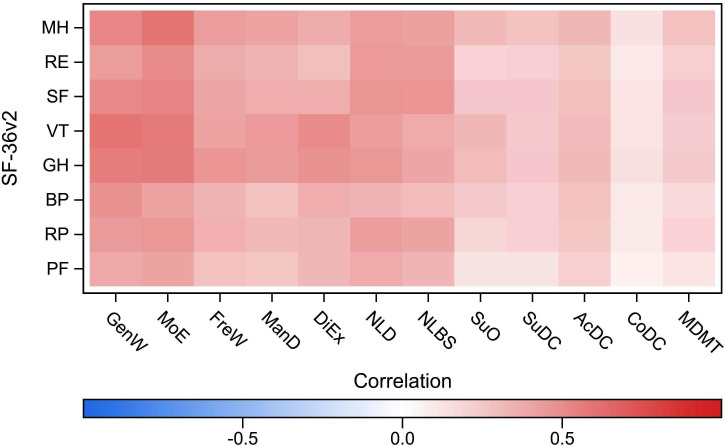
Figure 2.
Spearman’s rank correlation between the Diabetes Questionnaire scales and the SF-36v2 domains in type 2 diabetes. Diabetes Questionnaire scales: AcDC, Access to Diabetes Care; CoDC, Continuity in Diabetes Care; DiEx, Diet and Exercise; FreW, Free of Worries about blood sugar; GenW, General Wellbeing; ManD, Capabilities to Manage your Diabetes; MDMT, Medical Devices and Medical Treatment; MoE, Mood and Energy; NLBS, Not Limited by Blood Sugar; NLD, Not Limited by Diabetes; SuDC, Support from Diabetes Care; SuO, Support from Others. SF-36v2 domains: BP, bodily pain; GH, general health; MH, mental health; PF, physical functioning; RE, role-emotional; RP, role-physical; SF, social functioning; VT, vitality. SF-36v2, 36-item Short Form version 2.
As assumed, the strongest correlations were seen in the Diabetes Questionnaire PROM scales GenW and MoE. Statistically significant positive correlations showed that higher scores in GenW and MoE were strongly associated with higher scores in about half of the SF-36v2 domains. In GenW, statistically significant positive correlations were seen with the SF-36v2 domains PF, GH, VT and MH. The correlations were very strong with VT (0.60), strong with GH and MH (0.51–0.56) and weak with PF. Among those with type 2 diabetes, there were also statistically significant strong positive correlations between GenW and SF (0.51). In MoE, statistically significant positive correlations were seen with the SF-36v2 domains GH, VT, SF and MH. The correlations were very strong with MH (0.60) and strong with GH, VT and SF (0.51–0.58). Among those with type 2 diabetes, statistically significant strong positive correlations were also seen between MoE and RF (0.51). For both diabetes types, statistically significant strong positive correlations were also seen between the PROM scale DiEx and the VT domain (0.51). Statistically significant moderate positive correlations were also seen between the PROM scales and SF-36v2 domains. In NLD and NLBS, statistically significant moderate positive correlations were more common in type 2 diabetes than in type 1 diabetes. In support for divergent validity, the PROM scale SuO and the PREM scales, statistically significant correlations were weak (0.11–0.32) or absent (figures 1 and 2, online supplemental tables S5 and S6.
Non-linear associations to clinical variables and SF-36v2 domains together
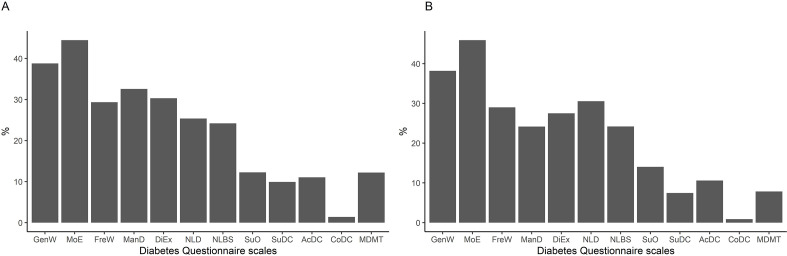
The results from the machine learning analysis are shown in figure 3 and online supplemental figure S3. Similar results were seen for type 1 and type 2 diabetes. Among the PROM scales, the variance was explained by the SF-36v2 domains together with the clinical variables to almost 40% in GenW and to around 45% in MoE. In FreW, ManD, DiEx, NLD and NLBS, the variance was explained to about 25%–30% and in SuO to about 10%. Among the PREM scales, SuDC, AcDC and MDMT were explained to about 10% or below. In CoDC, almost no variance was explained (figure 3). As predictors of the Diabetes Questionnaire PROM scales GenW and MoE, the variables with the highest importance were the SF-36v2 domains GH, VT and MH. LDL cholesterol and SBP had low variable importance (online supplemental figure S3). The per cent variance in HbA1c explained by other clinical variables, the SF-36v2 domains, and the Diabetes Questionnaire scales together was low, around 5% in type 1 diabetes and around 10% in type 2 diabetes. Consequently, the importance of the other clinical variables, the SF-36v2 domains, and the Diabetes Questionnaire scales as predictors of HbA1c was not examined.
Figure 3.
Per cent variance in the diabetes questionnaire scales explained by the SF-36v2 domains and clinical variables in type 1 (A) and type 2 diabetes (B). AcDC, Access to Diabetes Care; CoDC, Continuity in Diabetes Care; DiEx, Diet and Exercise; FreW, Free of Worries about blood sugar; GenW, General Wellbeing; ManD, Capabilities to Manage your Diabetes; MDMT: Medical Devices and Medical Treatment; MoE, Mood and Energy; NLBS, Not Limited by Blood Sugar; NLD, Not Limited by Diabetes; SF-36v2, 36-item Short Form version 2; SuDC: Support from Diabetes Care; SuO, Support from Others.
Sensitivity of the Diabetes Questionnaire scales to clinically relevant groups of glycaemic control
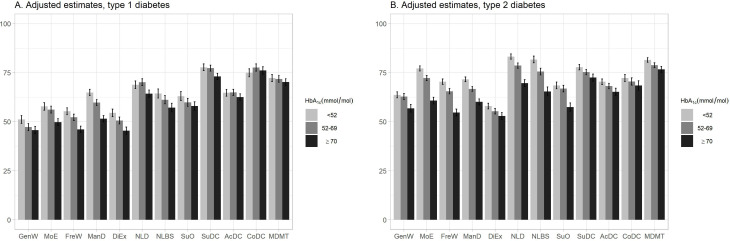
The results from the adjusted regression analyses of the Diabetes Questionnaire scales and the HbA1c groups are presented separately for participants with type 1 and type 2 diabetes in figure 4. The least square mean estimates and CIs from the unadjusted and adjusted analyses are given detail in online supplemental table S7.
Figure 4.
Adjusted least square mean estimates with 95% CIs for the Diabetes Questionnaire scales in type 1 diabetes (A) and type 2 diabetes (B) separated by glycated haemoglobin (HbA1c) level. Adjusted for age, sex, diabetes duration, body mass index, systolic blood pressure, LDL cholesterol level, micro-albuminuria and macro-albuminuria, estimated glomerular filtration rate, retinopathy, smoking status, physical activity level, receipt of antihypertensive and lipid lowering treatments, previous coronary heart disease and previous stroke. AcDC, Access to Diabetes Care; CoDC, Continuity in Diabetes Care; DiEx, Diet and Exercise; FreW, Free of Worries about blood sugar; GenW, General Wellbeing; LDL, low-density lipoprotein; ManD, Capabilities to Manage your Diabetes; MDMT, Medical Devices and Medical Treatment; MoE, Mood and Energy; NLBS, Not Limited by Blood Sugar; NLD, Not Limited by Diabetes; SuDC, Support from Diabetes Care; SuO, Support from Others.
Among those with type 1 diabetes, the adjusted analysis of the HbA1c groups showed significantly lower scores for the high-risk group than the well-controlled group in the eight PROM scales GenW, MoE, FreW, ManD, DiEx, NLD, NLBS and SuO as in the PREM scale SuDC. The largest between-group differences were seen in the PROM scales ManD and DiEx, where the well-controlled group had the significantly highest means, followed by the suboptimal group and the high-risk group. Among those with type 2 diabetes, the adjusted analysis showed that the high-risk group had significantly lower scores than the well-controlled group in all scales but CoDC. In the five PROM scales MoE, FreW, ManD, NLD and NLBS, the well-controlled group had the significantly highest means, followed by the suboptimal and high-risk groups. The largest between-group differences were seen in MoE, FreW, NLD and NLBS (figure 4, online supplemental table S7).
Discussion
From a nationwide setting with a large sample of adults with type 1 and type 2 diabetes selected at random, we present the outcome from the Diabetes Questionnaire. To study construct validity, we assess convergent and divergent associations of that outcome with clinical variables and generic health-related quality of life, as measured by the SF-36v2 and assess the sensitivity to differences between clinically relevant groups of glycaemic control. We found supporting evidence for construct validity in both type 1 and type 2 diabetes. As expected, and in support for divergent validity, there were few statistically significant correlations with the clinical variables. The observed correlations were weak, and most were negative. Also as expected, and in support for convergent validity, the correlations with the SF-36v2 domains were positive; the strongest correlations were found in the Diabetes Questionnaire PROM scales GenW and MoE. Furthermore, either weak or no correlations were seen in the PREM scales, supporting divergent validity. In machine learning analyses, the SF-36v2 domains and the clinical variables together explained the variance in the PROM scales GenW and MoE to about 40%–45%. In the other scales, the variance explained was low. In regression analyses among three groups with differing levels of HbA1c adjusted for demographics, other risk factors, and diabetes complications, the high-risk group had, in support of sensitivity to clinically relevant groups of glycaemic control, statistically significantly lower scores than the well-controlled group in most Diabetes Questionnaire scales for participants with type 1 diabetes and in almost all scales for those with type 2 diabetes. Statistically significant differences between all three groups of glycaemic control were seen in two scales for type 1 diabetes and in five scales for type 2 diabetes.
Findings and implications
Evaluating the measurement qualities of a questionnaire is a complex and cumulative effort.13 14 In this study, we continue the evaluation of the Diabetes Questionnaire by addressing its construct validity. The results in relation to divergent validity show supporting evidence that the Diabetes Questionnaire targets different concepts than the clinical variables for diabetes care traditionally covered by the NDR. Thus, the central aspects covered by the Diabetes Questionnaire including patient perspectives on how they feel, how their diabetes treatment is going, or their experiences of support from diabetes care cannot be measured by HbA1c or other tested clinical variables. Nor can the clinical variables be estimated through the Diabetes Questionnaire. We need the combination. There is a growing emphasis that the perspectives of those living with diabetes should be part of clinical meetings and be given priority among outcomes in diabetes care assessments.1 5 6 23–25 Supplementing decision-making by adding the patient’s perspective is suggested to increase the focus on these aspects in clinical meetings2 26 and to enhance the quality of care.26–28 In Sweden, the Patient Act strengthens the patient’s position and possibilities for shared decision-making and states that the individual patient’s prerequisites and wishes should be taken into account.29 There is also a growing movement towards person-centred care aiming for partnership that is centred on the patient’s experience and individual prerequisites, resources and barriers. An important basis is the patient’s story.30 We hope that the Diabetes Questionnaire can support the patient story if used in the clinical meetings together with the clinical variables.
The Diabetes Questionnaire is unique in being developed to support clinical meetings with individuals and to be used as a means for quality improvement through longitudinal assessment at a local, regional and national levels within the frame of a nationwide healthcare quality register.7–9 Many other questionnaires for diabetes were developed to target a specific aspect within intervention studies.3 18 19 The Diabetes Questionnaire has a broad approach with aspects identified as important to adults with diabetes.8 9 The Diabetes Questionnaire is also developed using the vocabulary and phrasing of people with diabetes,8 unlike many other questionnaires that often use academic or professional jargon. In this study, we found supporting evidence that the Diabetes Questionnaire is sensitive to statistically significant differences between clinically relevant subgroups with differing levels of glycaemic control. The Diabetes Questionnaire was also in support of convergent validity found to capture some aspects of generic health-related quality of life, while also in support of divergent validity adding aspects that are not covered by the often-recommended SF-36v2. For routine use within clinical diabetes care, the Diabetes Questionnaire is likely more relevant than the generic SF-36v2. A limitation of the Diabetes Questionnaire is, however, that it is currently only available in Swedish. Consequently, there is limited opportunity for international comparisons. The opportunities and barriers related to clinical use of the Diabetes Questionnaire are currently being studied from the perspectives of professionals and adults with diabetes.
Strengths and weaknesses
Among the strengths of this study are the large and heterogeneous sample of adults with type 1 and type 2 diabetes selected at random from the nation-wide NDR. The respondents were representative of the 2015 population in the NDR (data on file). The results can be considered representative of the Swedish adult population with diabetes related to the coverage rate of about 90% in 2015 when around 40 000 adults with type 1 diabetes and 347 000 with type 2 diabetes were registered in the NDR. Through the NDR, we had access to clinical variables relevant for diabetes care and background data for the non-respondents. Another strength is the use of a well-known measure of health-related quality of life. As there is a lack of agreed-upon benchmarks for how strong positive correlations between questionnaires addressing subjective aspects should be to support convergent construct validity,31 32 this study based the division of the correlation strength on reports that such correlations generally are low,31 33 often within the range 0.20–0.4033 or 0.40–0.60.31 A correlation of 0.60 has been suggested to be extremely strong, as the random error of measurement of the two questionnaires impede perfect correlations.31 As the Diabetes Questionnaire and the SF-36v2 do not measure the exact same construct, there were no prerequisites for broad strong correlations.14 31 33
Our study also has limitations. The analyses were limited to the respondents and might reflect a group that is more motivated to participate. Another limitation is that the questionnaires were only offered in Swedish, potentially resulting in a higher proportion of foreign-born individuals among the non-responders than among the respondents. Furthermore, the cross-sectional design means that it is not possible to make causal conclusions.
Future perspectives
The evaluation of construct validity is a work of putting the pieces together.13 14 Consequently, more studies are needed to relate the Diabetes Questionnaire to different concepts and measures. An important task for diabetes care is to identify suitable interventions that adequately can support individuals with diabetes. The Diabetes Questionnaire can be an important contribution to identify the need and focus for targeted interventions, especially for adults with low scores. In future studies, it is important to evaluate the potential of using scores from the Diabetes Questionnaire scales as the primary selection base or in combination with, for example, HbA1c levels or BMI. It is also essential to evaluate whether the Diabetes Questionnaire scales are responsive to actual changes and can be used as an evaluative tool adding patient perspectives to both nursing and medical interventions, longitudinal assessments and quality improvement. The NDR is established as a clinical and a national assessment tool in Swedish diabetes care.4 34–36 By now, the Diabetes Questionnaire is digitally and freely available for use by all clinics in Sweden connected to the NDR. The Diabetes Questionnaire is also included as the basis for developmental quality indicators in the Swedish national guidelines for diabetes care.4 In the future, the Diabetes Questionnaire can be among the established quality indicators bringing patient perspectives to the fore for diabetes care.
Conclusion
This nation-wide study shows that the Diabetes Questionnaire captures some generic health-related quality of life dimensions as well as adds diabetes-specific information not covered by the SF-36v2 and clinical variables. The Diabetes Questionnaire is also sensitive to differences between clinically relevant groups of glycaemic control.
Supplementary Material
Acknowledgments
The authors wish to thank Ebba Linder for facilitating data collection, Mervete Miftaraj for data management and Ann-Marie Svensson for intellectual advice and support during the processes of design and ethical approval. Parts of the presented data have been published in an abstract and were presented at the Annual Meeting of the European Association for the Study of Diabetes (EASD) in 2019 and within a doctoral thesis at Gothenburg University, Sweden, in 2019.
Footnotes
Contributors: MSE made substantial contributions to the design of the work, applying for ethical approval and funding, interpreting the data and drafting and revising the manuscript (major contributor). JL and U-BJ supervised and made substantial contributions to the design of the work, applied for funding, made intellectual contributions in the interpretation of the data, critically revised the manuscript for important intellectual content, and contributed experience and knowledge from diabetes care and research in diabetes and health-related quality of life. SB made substantial contributions to the design of the work, made intellectual contributions in the interpretation of the data and critically revised the manuscript for important intellectual content. BP made substantial contributions to the design of the work; performed the selection of the random sample; made intellectual contributions in the interpretation of the data; critically revised the manuscript for important intellectual content, and contributed statistical advice, experience and knowledge in the research of generic health-related quality of life and patient-reported outcome. SF made substantial contributions to the design of the work, contributed substantial statistical advice, was the major contributor in analysing the data, made substantial intellectual contributions in the interpretation of the data, and critically revised the manuscript for important intellectual content. SG supervised and made substantial contributions to the design of the work; applied for ethical approval and funding; made intellectual contributions in interpretation of the data; critically revised the manuscript for important intellectual content, and contributed medical experience and knowledge from diabetes care, diabetes research, and research using healthcare quality registers. KE-O supervised and made substantial contributions to the design of the work; applied for ethical approval and funding; generated the SF-36v2 data; interpreted the data; critically revised the manuscript for important intellectual content, and contributed medical experience and knowledge from diabetes care, diabetes research and research using healthcare quality registers. All authors read and approved the final manuscript as well as consented to be on the author list.
Funding: This work was supported by Dalarna University, Falun; Uppsala University, Uppsala; the Sahlgrenska University Hospital, Gothenburg; the Swedish Diabetes Foundation; and the Sophiahemmet Foundation, Stockholm. Unrestricted grants were provided by Astra Zeneca, MSD, Novo Nordisk and the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (Nos. ALFGBG-725311 and ALFGBG-698991). None of the funding providers have influenced the design of the study; the collection, analysis or interpretation of data; the writing of the manuscript, or any publication decision at any stage.
Competing interests: KE-O reports grants from the ALF agreement (ALFGBG 698991), during the conduct of the study; personal fees from Abbott, personal fees from Lilly, personal fees from Novo Nordisk, personal fees from Bayer, outside the submitted work; SG reports grants from the ALF-agreement (ALFGBG 725311), during the conduct of the study; grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Eli Lilly, grants and personal fees from Merck Sharp & Dohme, grants and personal fees from Novo Nordisk, grants and personal fees from Sanofi, outside the submitted work; the other authors declare that they have nothing to disclose.
Patient consent for publication: Not required.
Ethics approval: The study conforms to the Declaration of Helsinki and was approved by the Regional Ethical Review Board in Gothenburg, Sweden (No. 029-15, T600-15). Participants gave their informed consent. The letter to the participants contained information about the study’s purpose, the voluntary nature of their participation and their right to end participation. The letter also disclosed information about the NDR, methods of handling personal data, confidentiality measures and contact details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The data that support the findings of this study are not publicly available. The study presented here has been subject to review by an ethical board and approved for publication related to the specific aim of our research project. With reference to the European General Data Protection Regulation, the data are personal and therefore confidential.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Young-Hyman D, de Groot M, Hill-Briggs F, et al. Psychosocial care for people with diabetes: a position statement of the American diabetes association. Diabetes Care 2016;39:2126–40. 10.2337/dc16-2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polonsky WH. Emotional and quality-of-life aspects of diabetes management. Curr Diab Rep 2002;2:153–9. 10.1007/s11892-002-0075-5 [DOI] [PubMed] [Google Scholar]
- 3.Speight J, Reaney MD, Barnard KD. Not all roads lead to Rome-a review of quality of life measurement in adults with diabetes. Diabet Med 2009;26:315–27. 10.1111/j.1464-5491.2009.02682.x [DOI] [PubMed] [Google Scholar]
- 4.National Board of Health and Welfare (Socialstyrelsen) National guidelines for diabetes care. [In Swedish]. Nationella riktlinjer för diabetesvård: Stöd för styrning och ledning, 2018. Available: http://www.socialstyrelsen.se/ [Accessed 16 Oct 2019].
- 5.American Diabetes Association 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43:S37–47. 10.2337/dc20-S004 [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43:S48–65. 10.2337/dc20-S005 [DOI] [PubMed] [Google Scholar]
- 7.Borg S, Eeg-Olofsson K, Palaszewski B, et al. Patient-Reported outcome and experience measures for diabetes: development of scale models, differences between patient groups and relationships with cardiovascular and diabetes complication risk factors, in a combined registry and survey study in Sweden. BMJ Open 2019;9:e025033. 10.1136/bmjopen-2018-025033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svedbo Engström M, Leksell J, Johansson U-B, et al. A disease-specific questionnaire for measuring patient-reported outcomes and experiences in the Swedish national diabetes register: development and evaluation of content validity, face validity, and test-retest reliability. Patient Educ Couns 2018;101:139–46. 10.1016/j.pec.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 9.Svedbo Engström M, Leksell J, Johansson U-B, et al. What is important for you? A qualitative interview study of living with diabetes and experiences of diabetes care to establish a basis for a tailored patient-reported outcome measure for the Swedish national diabetes register. BMJ Open 2016;6:e010249. 10.1136/bmjopen-2015-010249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borg S, Palaszewski B, Gerdtham U-G, et al. Patient-Reported outcome measures and risk factors in a quality registry: a basis for more patient-centered diabetes care in Sweden. Int J Environ Res Public Health 2014;11:12223–46. 10.3390/ijerph111212223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robeyns I. Sen's capability approach and general inequality: selecting relevant capabilities. Fem Econ 2003;9:61–92. 10.1080/1354570022000078024 [DOI] [Google Scholar]
- 12.Sen AK, Nussbaum MC. The quality of life. Oxford: Clarendon Press, 1993. [Google Scholar]
- 13.Fayers PM, Machin D. Quality of life: the assessment, analysis, and reporting of patient-reported outcomes. Third ed Chichester, West Sussex, UK; Hoboken, NJ: John Wiley & Sons Inc, 2016. [Google Scholar]
- 14.Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol 2010;63:737–45. 10.1016/j.jclinepi.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 15.Svedbo Engström M, Leksell J, Johansson U-B, et al. Health-Related quality of life and glycaemic control among adults with type 1 and type 2 diabetes – a nationwide cross-sectional study. Health Qual Life Outcomes 2019;17:1–11. 10.1186/s12955-019-1212-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware JE. Sf-36 health survey update. Spine 2000;25:3130–9. 10.1097/00007632-200012150-00008 [DOI] [PubMed] [Google Scholar]
- 17.Maruish ME. User’s manual for the SF-36v2 Health Survey. 3rd ed Lincoln, RI: QualityMetric Incorporated, 2011. [Google Scholar]
- 18.Fitzpatrick R, Bowling A, Gibbons E, et al. A structured review of patient-reported measures in relation to selected chronic conditions, perceptions of quality of care and carer impact national centre for health outcomes development (Oxford site): unit of health-care epidemiology, department of public health, University of Oxford, 2006. Available: http://phi.uhce.ox.ac.uk/
- 19.Gibbons E, Fitzpatrick R, Patient Reported Outcome Measurement Group . A structured review of patient-reported outcome measures (PROMs) for diabetes. University of Oxford, 2009. [Google Scholar]
- 20.Norris SL, McNally TK, Zhang X, et al. Published norms underestimate the health-related quality of life among persons with type 2 diabetes. J Clin Epidemiol 2011;64:358–65. 10.1016/j.jclinepi.2010.04.016 [DOI] [PubMed] [Google Scholar]
- 21.Breiman L. Random forests. Mach Learn 2001;45:5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- 22.American Diabetes Association 6. Glycemic Targets: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43:S66–76. 10.2337/dc20-S006 [DOI] [PubMed] [Google Scholar]
- 23.Jones A, Vallis M, Pouwer F. If it does not significantly change HbA1c levels why should we waste time on it? a plea for the prioritization of psychological well-being in people with diabetes. Diabet Med 2015;32:155–63. 10.1111/dme.12620 [DOI] [PubMed] [Google Scholar]
- 24.Glasgow RE, Peeples M, Skovlund SE. Where is the patient in diabetes performance measures?: the case for including patient-centered and self-management measures. Diabetes Care 2008;31:1046–50. 10.2337/dc07-1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.IDF Clinical Guidelines Task Force Global guideline for type 2 diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med 2006;23:579–93. 10.1111/j.1464-5491.2006.01918.x [DOI] [PubMed] [Google Scholar]
- 26.Kotronoulas G, Kearney N, Maguire R, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 2014;32:1480–501. 10.1200/JCO.2013.53.5948 [DOI] [PubMed] [Google Scholar]
- 27.Reay N. How to measure patient experience and outcomes to demonstrate quality in care. Nurs Times 2010;106:12–14. [PubMed] [Google Scholar]
- 28.Snyder CF, Aaronson NK, Choucair AK, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res 2012;21:1305–14. 10.1007/s11136-011-0054-x [DOI] [PubMed] [Google Scholar]
- 29.Socialdepartementet Patient Act, (SFS 2014:821). [In Swedish]. Patientlag (SFS 2014:821. Stockholm, Sweden. [Google Scholar]
- 30.Ekman I, Swedberg K, Taft C, et al. Person-centered care--ready for prime time. Eur J Cardiovasc Nurs 2011;10:248–51. 10.1016/j.ejcnurse.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 31.McDowell I. Measuring health: a guide to rating scales and questionnaires. 3rd ed Oxford; New York: Oxford University Press, 2006. [Google Scholar]
- 32.Post MW. What to Do With "Moderate" Reliability and Validity Coefficients? Arch Phys Med Rehabil 2016;97:1051–2. 10.1016/j.apmr.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 33.Polit DF, Beck CT. Nursing research: generating and assessing evidence for nursing practice. 10th ed Philadelphia: Wolters Kluwer Health/Lippincott Williams and Wilkins, 2016. [Google Scholar]
- 34.Eliasson B, Gudbjörnsdottir S. Diabetes care--improvement through measurement. Diabetes Res Clin Pract 2014;106 Suppl 2:S291–4. 10.1016/S0168-8227(14)70732-6 [DOI] [PubMed] [Google Scholar]
- 35.Gudbjörnsdottir S, Cederholm J, Nilsson PM, et al. The National diabetes register in Sweden: an implementation of the St. Vincent Declaration for quality improvement in diabetes care. Diabetes Care 2003;26:1270–6. 10.2337/diacare.26.4.1270 [DOI] [PubMed] [Google Scholar]
- 36.Svensson AM, Gudbjörnsdottir S, Samuelsson P, et al. 20 years of successful improvements. Gothenburg, Sweden, 2016. Available: www.ndr.nu
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038966supp001.pdf (693.7KB, pdf)