Abstract
Adjuvant nutritional treatment is a commonly overlooked topic when treating lethal viral diseases as COVID-19 pandemic. We recently introduced TaibUVID nutritional supplements (nigella sativa, chamomile and natural honey) as adjuvants for COVID-19 contacts, patients and public prophylaxis. TaibUVID Forte adds costus, senna and fennel to TaibUVID. Meta-analyses and systematic reviews confirmed evidence-based therapeutic benefits of TaibUVID components in treating many human diseases e.g. diabetes mellitus and hypertension, common co-morbidities in COVID-19 patients. Double-blind clinical trials for treating COVID-19 patients with TaibUVID supplements were inapplicable. In this retrospective study in Egypt, COVID-19 patients and contacts knew TaibUVID via social media and voluntarily used them. 65% of COVID-19 patients (n = 13) received both pharmacological treatments and adjuvant TaibUVID nutritional supplements. 35% (n = 7) received TaibUVID only. Lymphopenia rapidly improved to lymphocytosis upon regular TaibUVID intake. TaibUVID nutritional supplements helped COVID-19 contacts’ prophylaxis. 70% of COVID-19 contacts (n = 14) (on regular TaibUVID intake) did not get SARS-COV2 infection. 30% (n = 6) were not using TaibUVID regularly and got mild flu-like symptoms and upon using both TaibUVID and pharmacological treatments, all improved and got negative nasopharyngeal swabs PCR. COVID-19 contacts were mainly physicians (40%, n = 8) (dealing with COVID-19 patients daily) and members of physicians’ families (45%). Main presentations reported by COVID-19 patients (n = 20) were cough (90%), fever (55%), anosmia (45%), taste loss (45%), sore throat (45%), respiratory difficulty (45%) and malaise (35%). TaibUVID inhalation therapy (nigella sativa/anthemis/costus solution nebulization) was used by 65% of COVID-19 patients (n = 13) and alleviated respiratory manifestations e.g. cough and respiratory difficulty and was life-saving in some cases. 70% of COVID-19 patients (n = 14) improved in 1-4 days, 25% (n = 5) improved in 5-10 days while 5% improved in more than 10 days. TaibUVID nutritional supplements were tolerable and significantly satisfactory (P<0.01). 81.25% of COVID-19 patients (n = 13) did not report side effects. 18.25% (n = 3) reported mild diarrhea, sweating and hyperglycemia (not confirmed to be due to TaibUVID supplements). 31.25% of patients (n = 5) were satisfied by 100% with TaibUVID nutritional supplements. 37.5% (n = 6) of patients were satisfied by 75%. In conclusion, TaibUVID nutritional supplements are recommended for public prophylaxis (to decrease emergence of new cases) and treatment in COVID-19 pandemic. Clinical trials and further investigations are recommended.
Keywords: COVID-19, TaibUVID, Nigella sativa, chamomile, natural honey, nasopharyngeal swab PCR, contacts, cases
Introduction
COVID-19 (Wuhan-2019-nCoV) is a global pandemic according to World Health Organization with higher contagiousness and infectivity than both the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) [1]. COVID-19 pandemic is continuously expanding to affect more people worldwide despite strict WHO measures. In some countries, hospitals are overcrowded while many major hospitals turned to be mere COVID-19 centers. The concept of home treatment for contacts and mild COVID-19 cases should be widely employed in the near future due to the lack of beds in hospitals having increased patients’ numbers and the mild to moderate nature of many COVID-19 patients who may have respiratory symptoms with normal initial chest X-ray (Figure 1A). Home treatment for COVID-19 may relieve the burdens over health authorities. Among the best world success systems for managing COVID-19 pandemic is the Saudi management. They professionally provided a high standard of medical services for all people (citizens and non-citizens equally) for free and set strict legislations to combat pandemic spread. That was fruitful in decreasing mortality to below international standards.
Figure 1.
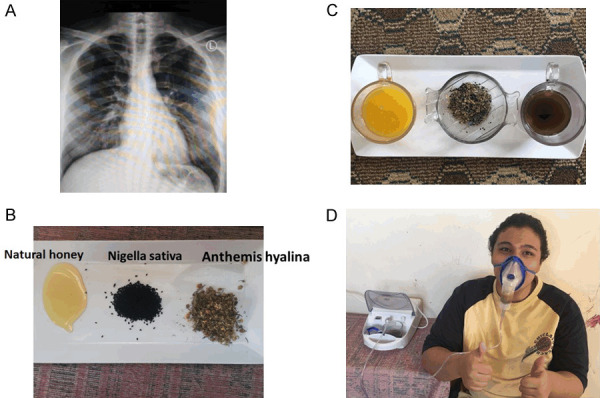
Components of TaibUVID nutritional therapy are natural products as nutritional supplements for COVID-19 patients. A. COVID-19 patient may present with respiratory symptoms while chest X-ray is within normal features. B. TaibUVID nutritional supplements include: Honey: 15 ml (one large tablespoon) of natural honey/dose. Anthemis hyalina (Chamomile): 1 gram/dose. Nigella sativa: 2 grams/dose or 5 ml nigella sativa oil/dose. Orange juice: one cup of natural orange juice/dose. C. TaibUVID inhalation therapy is a promising novel modality that was effective for treating COVID-19 patients in this study. D. TaibUVID inhalation therapy is a simple respiratory treatment that was welcomed and tolerated in this study. Self-administration of TaibUVID inhalation therapy (nigella/Anthemis/costus decoction) dramatically relieves profuse cough and respiratory difficulty. Both nigella sativa and anthemis hyalina were reported to decrease coronavirus replication to 5% and zero%, respectively. Both exert tissue-protective antioxidant effects. TaibUVID Plus is TaibUVID + nigella sativa/anthemis hyalina/costus decoction solution. Decoction nebulization should be a new addition to emergency departments and inpatient departments in hospitals that may help rapid recovery and shorter hospital stay. Simply, nigella sativa seeds (6-15 grams) are added to anthemis hyalina (1-2 grams) and 500 ml water then heating for 5 minutes and leaving few minutes to cool up ± adding 1 ml clove oil. Applying this decoction via a nebulizer dramatically alleviated continuous cough and respiratory difficulty and was life-saving in many COVID-19 patients. Fortunately, all TaibUVID components are available as home remedies for COVID-19 contacts and cases. If there is no nebulizer at home, simply heat the decoction inside a pot while partly covering the pot and inhaling the vapor.
Natural products may carry a lot of hope in combating lethal COVID-19 infection. Natural products as nigella sativa and honey induce lymphocytosis that may counteract the hematological changes (e.g. lymphopenia) induced by COVID-19 infection [2,3]. Meta-analyses and systematic reviews confirmed potent evidence-based therapeutic benefits of nigella sativa in treating many human diseases that may be found as co-morbidities worsening COVID-19 outcomes. Nigella sativa improved the laboratory parameters of hyperglycemia, diabetes control and insulin resistance. Nigella sativa exerts cardio-protective effects in cardiac patients [4] and decreases body weight and body mass index in adults [5]. Nigella sativa is also an evidence-based remedy for decreasing inflammation, inhibiting oxidative stress, and modulating the immune system in patients having rheumatoid arthritis [6]. Importantly, nigella sativa is an evidence-based remedy in the regulation of immune reactions implicated in various infectious and non-infectious conditions including different types of allergy, autoimmunity, and cancer [7]. Both nigella sativa and anthemis hyalina (chamomile) significantly and maximally decreased the replication of coronaviruses [8]. Based on that, nigella sativa may be promising for treating COVID-19 co-morbidities.
With the difficulties in providing effective and safe specific pharmacological treatments (or potent prophylaxis) to combat COVID-19 pandemic that afflicted millions of people around the world, natural products with evidence-based medicinal properties may be a life-saving, cheap and safe option. Natural honey is an evidence-based remedy in causing tissue repair and tissue-protective effects where honey is quite effective in the prophylaxis and treatment of radiation- and chemoradiation-induced oral mucositis [9]. Moreover, honey dressings proved effective in promoting better wound healing than silver sulfadiazine for burns [10]. Chamomile is also an evidence-based remedy for many therapeutic and nontherapeutic purposes [11]. Unfortunately, many physicians may discourage using natural products for preventive and therapeutic purposes based on the fact that medical schools (unlike pharmacy schools) are not teaching a knowledge about medicinal plants in their medical curriculum. Graduated physicians grow their experience with no knowledge about the therapeutic benefits of available medicinal plants or natural products and their life-saving roles in catastrophic pandemics as COVID-19. Previously, we already discussed the importance of including prophetic medicine and integrative medicine remedies in medical curriculum of modern medical schools [12,13].
Recently, we introduced TaibUVID nutritional supplements (derived from prophetic medicine) as suggested medicinal adjuvants that can be entirely prepared and given at home (or at hospital kitchens) even in poor societies. As previously reported, TaibUVID is a suggested medicinal nutritional supplement including nigella sativa (2 grams/dose), anthemis hyalina (chamomile 1 gram/dose) and natural products e.g. natural honey (15 ml/dose) that are better to be followed by drinking a cup of fresh orange juice with no chemical additions or formulations [14]. TaibUVID, TaibUVID Plus and TaibUVID Forte that adds senna, fennel and costus to TaibUVID (Figure 1B-D; Tables 1, 2 and 3) include cheap, available, commonly used and publicly acceptable food items that may carry a lot of therapeutic benefits to COVID-19 contacts and patients. Regular TaibUVID doses are suggested to be prepared and administered at home (particularly in poor societies) or hospital kitchens to combat COVID-19 pandemic and to alleviate the heavy burdens on hospitals [14]. Media and social media campaigns and orientation talks may play a role in expanding the nutritional awareness regarding TaibUVID widespread use aiming at rapid COVID-19 eradication. Lack of one or more of TaibUVID components should never delay starting nutritional therapy with any currently available TaibUVID components. Available components of TaibUVID nutritional treatment should be started orally and by inhalation as soon as possible according to the suggested nutritional protocol (Table 3). Fortunately, TaibUVID met a lot of public success among Egyptian patients who started preparing it at home and used it (Tables 4, 5 and 6).
Table 1.
TaibUVID inhalation therapy preparation for COVID-19 patients
| TaibUVID inhalation therapy preparation |
|---|
| N.B. TaibUVID Plus = TaibUVID + TaibUVID inhalation therapy. |
| Nigella sativa seeds (10-15 grams) are added to anthemis hyalina (2-4 gram) + costus (2-5 gram) and 500 ml water then heating for 5 minutes and leaving few minutes to cool up ± adding 1 ml clove oil (Figure 1B-D). Applying this decoction via a nebulizer dramatically alleviated the continuous cough and respiratory difficulty and was life-saving in many COVID-19 patients. If there is no nebulizer at home, simply heat the decoction inside a pot while covering the pot and inhale the vapor. |
Table 2.
Expected improvements after TaibUVID nutritional supplements
| Expected improvements after TaibUVID nutritional supplements |
|---|
| • May help rapid reversion from positive to negative nasal swab PCR. |
| • May decrease COVID-19 morbidity and mortality. |
| • May increase tissue-protective effects till rapid cure. |
| • May increase patients’ immunity (increased WBCs, CD4 lymphocytes, CD8 lymphocytes and interferon-gamma). |
| • May combat cytokine storm in COVID-19 pandemic positive patients. |
| • May combat oxidative stress-induced tissue damage and may alleviate related markers (causing increased antioxidants, decreased malondialdehyde, increased glutathione peroxidase, increased catalase and increased total antioxidant capacity). |
Table 3.
Updated TaibUVID adjuvants protocol (as adjuvants to pharmacological protocols) for prophylaxis and treatment
| TaibUVID and TaibUVID Forte preparations |
|---|
| A. Large TaibUVID mix preparation (about 30 doses) (more practical and simple): |
| Each dose = one large spoonful = 2 grams nigella sativa + 1 gram chamomile + 10 ml natural honey |
| One large TaibUVID mix equals: |
| • 2 large tablespoons of Chamomile powder (about 30 grams). |
| • 4 large tablespoons of Nigella sativa powder (= about 60 grams). |
| Mix the powder well. |
| • Add 500 grams natural honey and mix well. |
| - One dose of TaibUVID equals one large table spoonful (or 1.5 large disposable plastic spoonful). |
| - It is recommended to put TaibUVID dose in a cup of warm water to dissolve while squeezing a fresh lemon or orange with it. Give to patient. |
| B. Large TaibUVID Forte mix preparation (about 30 doses): |
| Each dose = one large spoonful = 2 grams nigella sativa + 1 gram chamomile + 10 ml natural honey + 1 gram fennel + 0.5 gram costus + 0.5 gram senna |
| N.B. senna is a laxative containing emodin that blocks coronavirus spike protein. |
| One TaibUVID Forte stock mix equals: |
| • 2 large tablespoons of Chamomile powder (about 30 grams). |
| • 2 large tablespoons of fennel powder (about 30 grams). |
| • 1 large tablespoon of costus powder (about 15 grams). |
| • 1 large tablespoon of senna powder (about 15 grams). |
| • 4 large tablespoon of nigella sativa powder (about 60 grams). |
| Mix the powder well. |
| • Add 500 grams natural honey and mix well. |
| - One dose of TaibUVID equals one large table spoonful (or 1.5 large disposable plastic spoonful). |
| - It is recommended to put TaibUVID Forte dose in a cup of warm water to dissolve while squeezing a fresh lemon or orange with it. Give to patient. |
| N.B. Senna induces diarrhea. If diarrhea is disliked, senna dose can be reduced or omitted. Senna should be given for at least first 3 days. Emodin in senna blocks SARS-COV-2 virus spike protein. This decreases infectivity and cellular adhesion. |
| Updated nutritional supplements protocol |
| 1. For public prophylaxis: One TaibUVID dose (once daily till eradicating COVID-19 pandemic). |
| 2. For prophylaxis of medical personnels, nurses and patient’s contacts: Small nasal cotton pieces + Face mask + Face shield………. |
| - TaibUVID Forte nutritional supplement (twice daily till eradicating COVID-19 pandemic). |
| 3. For mild or asymptomatic COVID-19 cases: TaibUVID nutritional supplement (three times daily for 7 days or till getting -ve nasal swab PCR). Then once daily for prophylaxis till COVID-19 eradication. |
| 4. For moderate COVID-19 cases (with respiratory symptoms e.g. cough, difficult breathing, acute wheezing,….): TaibUVID Forte nutritional supplement (5 times daily for 7 days). If symptoms persist or deteriorate, One TaibUVID Forte dose for another 7 days. |
| - Give TaibUVID inhalation therapy a via nebulizer (5 times daily for 7 days). |
| 5. For severe COVID-19 cases with respiratory symptoms e.g. pneumonia, continuous cough, difficult breathing, acute wheezing,….): TaibUVID Forte (5 times daily for 7-14 days) and TaibUVID inhalation therapy (5 times daily for 7-14 days). |
| 6. - Give inhalation therapy via nebulizer (5 times daily for 7-14 days). |
| 7. For ventilated patients or comatose patients: TaibUVID Forte supplements should be given by ICU physicians: |
| - TaibUVID Forte (5 times daily till cure-God willing-mixed in an electric mixer with 400 ml water) is given via a nasogastric tube + inhalation therapy (via ventilator setting adjusted by ICU physicians). |
Table 4.
Complete blood count of patient #1 at time of COVID-19 diagnosis (before starting TaibUVID) and few days after starting TaibUVID nutritional supplement
| At time of COVID-19 diagnosis (before starting TaibUVID nutritional supplement) | 11 days later (after starting TaibUVID nutritional supplement) | |
|---|---|---|
| White blood cells | 4.09 (4-11 × 103/µL) | 5 (4-11 × 103/µL) |
| Red blood cells | 4.88 (3.5-5.8 × 106/µL) | 5.28 (3.5-5.8 × 106/µL) |
| Hemoglobin | 13.3 (13.5-17.5 g/dL) | 14.9 (13.5-17.5 g/dL) |
| HCT | 40.26 (30-50%) | 44.1 (30-50%) |
| MCV | 83 (80-97 fL) | 83.5 (80-97 fL) |
| MCH | 27.2 (26-33.5 pg) | 28.2 (26-33.5 pg) |
| MCHC | 33 (31.5-36 g/dL) | 33.8 (31.5-36 g/dL) |
| Platelets | 201 (150-400 × 103/µL) | 226 (150-400 × 103/µL) |
| Neutrophils | 77% (43-76%) | 47% (43-76%) |
| Lymphocytes | 17.7% (20-40%) | 44% (20-40%) |
| Monocytes | 5.3% (2-10%) | 7% (2-10%) |
| Eosinophils | 0.2% (1-6%) | 2% (1-6%) |
| Basophils | 0.5% (0-1%) | 0% (0-1%) |
TaibUVID-induced lymphocytosis is evident.
Table 5.
COVID-19 positive patients who received TaibUVID nutritional treatments. N.B. PP is pharmacological protocol
| Patient number | Job | Main symptoms and disease course | TaibUVID components given to patient | Time taken for nasal swab PCR to be negative | Comments |
|---|---|---|---|---|---|
| #1 | Teacher | Continuous cough, malaise, anosmia, taste loss, high fever | TaibUVID + inhalation therapy + PP | 4 days | - Patient felt good improvement 4 days after TaibUVID nutritional therapy |
| #2 | Teacher | Continuous cough, respiratory difficulty, fever, malaise | TaibUVID + PP | At time of next nasal swab (5 days after TaibUVID treatment) | - Patient felt good improvement 2 days after TaibUVID nutritional therapy |
| - Oxygen saturation decreased | - The next day his voice was comfortable with no cough during a telephone call | ||||
| - His voice was interrupted by cough during a telephone call | - He added clove oil inhalation | ||||
| #3 | Physician | - Moderate cough, mild respiratory difficulty | TaibUVID + inhalation therapy | At time of next nasal swab (4 days after initiating TaibUVID treatment) | - Patient felt moderate improvement 1 day after TaibUVID nutritional therapy |
| - 2 successive +ve nasal swabs PCR before starting TaibUVID | - The next day his voice was comfortable during a telephone call | ||||
| #4 | Physician | Moderate cough, mild respiratory difficulty | TaibUVID + inhalation therapy + PP | At time of next nasal swab (4 days after initiating TaibUVID treatment) | - Patient felt marked improvement 1 day after TaibUVID nutritional therapy |
| - The next day his voice was comfortable during a telephone call | |||||
| #5 | Physician’s wife | Moderate cough, mild respiratory difficulty | TaibUVID + inhalation therapy | At time of next nasal swab (4 days after initiating TaibUVID treatment) | - Patient felt marked improvement 1 day after TaibUVID nutritional therapy |
| - 2 successive +ve nasal swabs PCR before starting TaibUVID | - She is wife of patient #2 | ||||
| #6 | Physician | Moderate cough, moderate respiratory difficulty | TaibUVID + inhalation therapy | At time of next nasal swab (4 days after initiating TaibUVID treatment) | - Patient felt marked improvement 1 day after TaibUVID nutritional therapy |
| - 2 successive +ve nasal swabs PCR before starting TaibUVID | |||||
| #7 | Physician | Moderate cough, moderate respiratory difficulty | TaibUVID + inhalation therapy | At time of next nasal swab (2 days after initiating TaibUVID treatment) | - Cough and respiration difficulty markedly improved after nigella sativa decoction therapy (5 times per day) and TaibUVID (5 times per day) |
| #8 | Physician | Mild cough | TaibUVID | At time of next nasal swab (3 days after initiating TaibUVID treatment) | - He has diabetes mellitus and started TaibUVID (once daily) for 2 weeks before exposure to infection |
| - He got infection after dealing with a positive COVID-19 patient. He had mild cough only | |||||
| - A dramatic improvement occurred upon increasing TaibUVID to 5 doses/day | |||||
| - Nasal swab PCR became negative soon | |||||
| - Some of his colleagues died with COVID-19 infection | |||||
| #9 | Teacher | Difficult breathing and moderate cough | TaibUVID + inhalation therapy | At time of next nasal swab (1 day after initiating TaibUVID treatment) | Nigella sativa decoction inhalation dramatically and immediately relieved cough and difficult inspiration |
| #10 | Teacher | malaise, anosmia, taste loss | TaibUVID + inhalation therapy | At time of next nasal swab (1 day after initiating TaibUVID treatment) | Patient felt good improvement 1 day after TaibUVID nutritional therapy |
| #11 | Physician | Fever | TaibUVID + PP inhalation therapy | 9 days | |
| #12 | Engineer | Fever, cough, respiratory difficulty | TaibUVID Forte (without senna) + Inhalation therapy + PP | 8 days | Fever subsided |
| Cough improved | |||||
| #13 | Physician | Fever, cough, malaise, anosmia, taste loss, sore throat | TaibUVID | 3 days | |
| #14 | Physician | Mild cough, sore throat | TaibUVID + PP | 2 days | |
| #15 | Physician’s wife | Mild cough, sore throat | TaibUVID + PP | 2 days | |
| #16 | Physician | Fever, cough, sore throat, anosmia, taste loss | TaibUVID Forte + inhalation therapy + PP | 10 days | |
| #17 | Physician’s wife | Fever, cough, sore throat, anosmia, taste loss | TaibUVID Forte + PP | 10 days | |
| #18 | Professor | Fever, cough, sore throat, anosmia, taste loss, hypoxemia, malaise | TaibUVID + PP | 20 days | He received oxygen at hospital |
| #19 | house wife | Fever, cough, sore throat, anosmia, respiratory difficulty, taste loss | TaibUVID + PP | 7 days | |
| #20 | Physician | Fever, cough, sore throat, anosmia, taste loss + lympopenia | TaibUVID Forte + inhalation therapy + PP | 7 days |
Table 6.
COVID-19 contacts (physicians and non-physicians receiving TaibUVID nutritional therapy for prophylaxis)
| Patient number | job | TaibUVID components given to patient | Comments |
|---|---|---|---|
| #1 | Physician at emergency department with high flow of COVID-19 cases | TaibUVID | His nasal swab PCR was negative with no symptoms. A large number of his colleagues got COVID-19 infection. |
| #2 | Physician at emergency department with high flow of COVID-19 cases | TaibUVID | His nasal swab PCR is negative with no symptoms. A large number of his colleges got COVID-19 infection. |
| #3 | Physician at emergency department with high flow of COVID-19 cases | TaibUVID | His nasal swab PCR is negative with no symptoms. He got flu-like symptoms for 2 days (no nasopharyngeal swab was done) |
| ?????? COVID-19 positive | |||
| #4 | University professor and physician (he was a contact of a positive case) | TaibUVID | His nasal swab PCR is negative with no symptoms |
| #5 | Physician (she was a contact of a positive case) | TaibUVID | Her nasal swab PCR is negative with no symptoms. She got mild flu-like symptoms for 2 days (no nasopharyngeal swab was done) |
| ?????? COVID-19 positive | |||
| #6 | Physician’s house wife (she was a contact of her husband, a positive case) | TaibUVID | Her nasal swab PCR is negative with no symptoms. She got mild flu-like symptoms for 2 days (no nasopharyngeal swab was done) |
| ?????? COVID-19 positive | |||
| #7 | Teacher. Husband of a positive case | TaibUVID | His nasal swab PCR is negative with no symptoms |
| #8 | Son of a positive case | TaibUVID | His nasal swab PCR is still negative with no symptoms |
| #9 | Daughter of a positive case | TaibUVID | Her nasal swab PCR is still negative with no symptoms |
| #10 | Physician in a fever hospital | TaibUVID | He did not catch infection till now |
| #11 | Physician’s Wife | TaibUVID | She is healthy (no symptoms till now) |
| #12-17 | A family contacting COVID-19 case | TaibUVID | They were contacts of a positive case. (no symptoms till now) |
| #18 | Physician at a COVID-19 isolation hospital | TaibUVID | He was not taking TaibUVID regularly. He caught infection |
| #19 | Physician at a COVID-19 isolation hospital | TaibUVID | He was not taking TaibUVID regularly. He caught infection. His case was severe. |
| #20 | Physician’s wife | TaibUVID | She was not taking TaibUVID regularly. She caught infection |
Concurrent simultaneous TaibUVID administration may potentiate the efforts in isolating positive cases. For us, all patients should stick to the medical advices given by physicians and should also stick to the standard approved pharmacological protocols for treating COVID-19 cases. TaibUVID is a suggested adjuvant nutritional supplement that may be beneficial in raising immunity and exerting tissue protection. Potential TaibUVID-induced therapeutic benefits may minimize the efforts exerted in treating COVID-19 emergencies (Figure 3). TaibUVID components are safe natural products that carry promising evident antiviral, tissue-protective and immune potentiating effects. TaibUVID Plus includes both oral TaibUVID plus TaibUVID inhalation therapy (inhalation of nigella sativa/anthemis/costus decoction nebulization). TaibUVID inhalation therapy is a novel local inhalation treatment to relieve respiratory manifestations in COVID-19. TaibUVID inhalation therapy (nigella/anthemis/costus decoction nebulization) is suggested to be a new addition to the pharmacy of emergency departments and inpatient departments in hospitals as a life-saving treatment for COVID-19-induced continuous cough and respiratory difficulty. TaibUVID Plus components (particularly nigella sativa/anthemis ± costus decoction nebulization) is better to be available at inpatient departments at hospitals. Decoction nebulization is suggested to help gaining rapid recovery and minimizing inpatient admission period [14].
Figure 3.
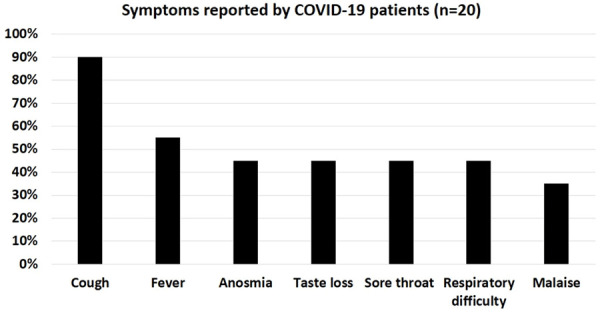
Main symptoms presented by COVID-19 patients. COVID-19 patients in this study (n = 20) presented with cough (90%), fever (55%), anosmia (45%), taste loss (45%), sore throat (45%), respiratory difficulty (45%) and malaise (35%).
Patients and methods
Ethics committee approval
Ethics Committee Approval for performing the study was obtained from Center of Scientific Foundation for Experimental Studies and Research, Ismailia, Egypt.
Double-blind comparative clinical trial was not applicable
Recently, the prevention and treatment protocols using TaibUVID nutritional supplements were suggested as adjuvants for preventing and treating COVID-19 contacts and patients [14]. Some updating modifications were added to the treatment protocol (Table 3) to manage any possible delay in improvement. We planned to perform a clinical trial using two study groups (standard COVID-19 protocol vs. standard COVID-19 protocol and TaibUVID nutritional supplement). Unfortunately, that was not applicable. All components of TaibUVID nutritional supplements are natural and available in food stores and are not harmful. However, suggested clinical trials were not applicable.
Social media helped data collection
Upon publishing our recent article as a suggested adjuvant preventive and treatment protocol for managing COVID-19 patients and cases using the nutritional supplements (TaibUVID, TaibUVID Plus [14] and TaibUVID Forte), that was publicly acceptable among COVID-19 contacts and patients in Egypt who are using social media. Fortunately, all components of the suggested nutritional supplements are available everywhere. At their homes, patients themselves, contacts and their families prepared TaibUVID doses and TaibUVID (nigella sativa/anthemis ± costus) decoction inhalation solution and administered it. Applying this decoction via a nebulizer dramatically alleviated the continuous cough and respiratory difficulty. One patient who developed a sudden respiratory difficulty got an immediate relief after administering nigella/anthemis decoction inhalation. He reported that it was life-saving for him (Table 5).
A small questionnaire was given to estimate patients’ opinions regarding safety of TaibUVID nutritional supplements and satisfaction of patients with them. Due to the pandemic nature of COVID-19 infection and the extensive public concerns expressed in social media, there was an extensive public interest in natural products particularly suggested TaibUVID supplements. Many physicians and non-physicians were interested in TaibUVID and used social media (facebook, youtube and whatsApp) to cite it and shared that knowledge with their friends. TaibUVID nutritional supplements received a public acceptance and reputation particularly in Upper Egypt (in the south in Sohag governorate) where people prepared TaibUVID by themselves at home. COVID-19 patients and contacts added TaibUVID to their regular diets. Many emergency department physicians and their families started to use it. However, still a lot of physicians are not interested in natural products as preventive or therapeutic treatments and did not take TaibUVID prophylactic doses to prevent hospital risks of infection. All physicians took suitable other preventive measures (masks, gowns, face shields…). Members of a single family (8 persons) were contacts of a COVID-19 positive case and entered a quarantine for 14 days (taking TaibUVID nutritional supplement). Two nasopharyngeal swab PCR tests were done for all family members and were negative (Table 6).
Retrospective data collection and analysis
COVID-19 patients and contacts reported their own experiences with COVID-19 infection, pharmacological treatments and TaibUVID nutritional supplements (oral and inhalation therapy). Key points regarding disease progress and improvement were tabulated (Figures 4, 5). Symptomatology and related health issues were graphed and presented (Figures 1, 2 and 3). Patients gave agreements and consents to participate in the study.
Figure 4.
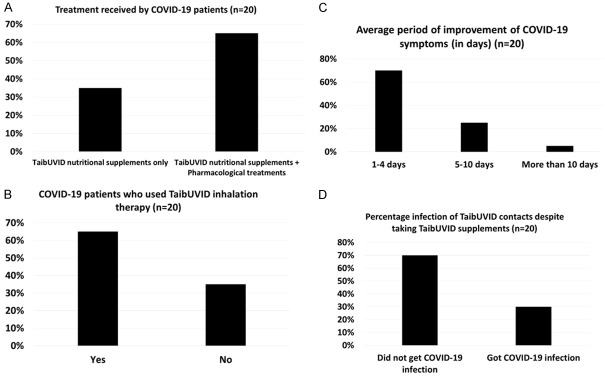
Treatments received by COVID-19 patients. A. 65% of COVID-19 patients received pharmacological treatments and TaibUVID nutritional supplements. 35% of COVID-19 patients received TaibUVID nutritional supplements only. B. 65% of COVID-19 patients received TaibUVID inhalation therapy. C. 70% of COVID-19 patients improved in 1-4 days, 25% improved in 5-10 days while 5% improved in more than 10 days. D. 70% of COVID-19 contacts (on regular TaibUVID supplements intake) did not catch COVID-19 infection. 30% of COVID-19 contacts (on non-regular TaibUVID supplements intake) caught COVID-19 infection. Symptoms of 2 out of those 6 contacts rapidly disappeared (without performing PCR) upon starting regular TaibUVID intake. 5 out of those 6 contacts (25%) got dramatic rapid disappearance of symptoms upon regular TaibUVID intake. One case (5%) was sever and improved in hospital (using pharmacological treatments and TaibUVID nutritional supplements).
Figure 5.
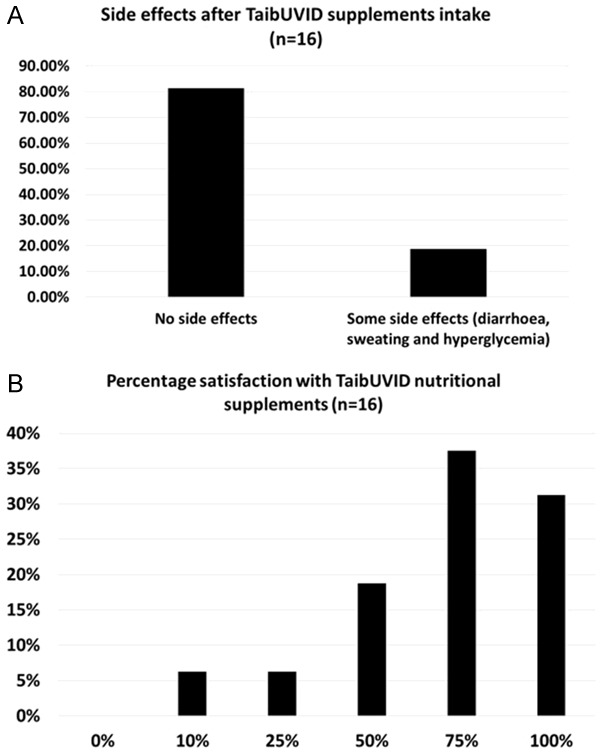
TaibUVID nutritional supplements were tolerable and satisfactory to COVID-19 patients. (A) 81.25% of COVID-19 patients (n = 16) did not report any side effects with TaibUVID nutritional supplements. 18.25% of COVID-19 patients reported mild side effects as diarrhea, sweating and hyperglycemia that are not confirmed to be due to TaibUVID supplements intake. (B) COVID-19 patients’ satisfaction with TaibUVID supplements. 31.25% of patients were satisfied 100% with TaibUVID nutritional supplements. 37.5% of patients were satisfied 75% with TaibUVID nutritional supplements (B). Student’s t test revealed that COVID-19 patients expressed a significantly high satisfaction percent with TaibUVID nutritional supplement (70.87%±6.86) (p<0.01).
Figure 2.
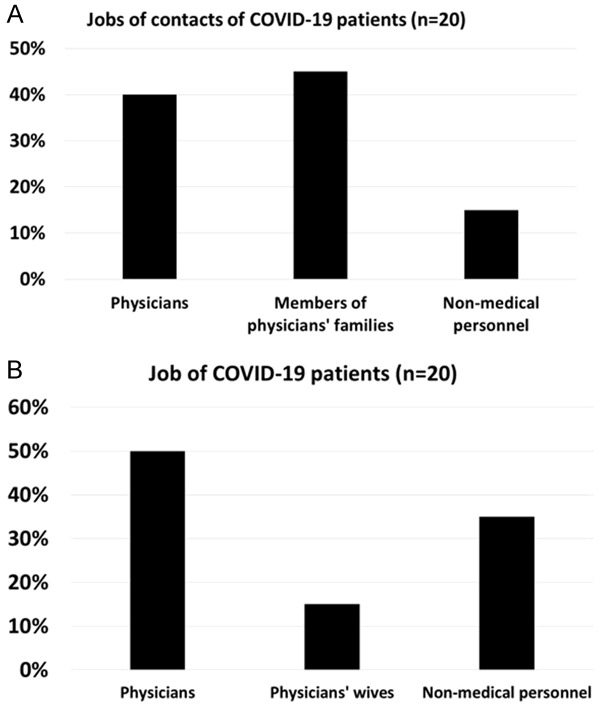
Occupational risks for COVID-19 infection. A. COVID-19 infection risks are higher in certain occupations. In this study, 40% of COVID-19 contacts at risk of infection (n = 20) were physicians (40%), physicians’ families (45%) and then non-medical personnel (15%). B. Percentages of COVID-19 patients included in this study were affected by jobs nature. Physicians were 50%, Physicians’ wives were 15% and non-medical personnel were 35%.
Statistical analysis
SPSS version 16 was used to calculate and analyze the descriptive statistics (frequencies) to determine the statistics regarding patients’ symptomatology, duration till improvement and TaibUVID-induced contacts prophylaxis. Descriptive statistics (frequencies) were also used to validate patients’ and contacts’ evaluation of given nutritional supplements. One sample t test was used with significance values set at *p<0.05, **p<0.01 and ***p<0.001.
Results
TaibUVID helps improving hematological parameters in COVID-19 patients
Some COVID-19 patients may have leukopenia and/or lymphopenia (reflects immunological defects) that may be related to COVID-19 infection (Table 4). Upon treatment with pharmacological protocols and TaibUVID nutritional supplements for few days, leukopenia and lymphopenia were corrected to normal levels and even lymphocytosis (Table 4). White blood cells count increased from 4090 cells/cc to 5000 cells/cc (normal range: 4000-11000/cc) over 11 days while lymphocytes percentage increased from 17.7% to 44% i.e. lymphocytosis (normal range: 20-40%) (TaibUVID effect) (Table 4).
TaibUVID nutritional supplements helped COVID-19 contacts’ prophylaxis
COVID-19 patients may present with respiratory symptoms (cough, anosmia, taste loss…) (Tables 5, 6) while chest X-ray is normal (Figure 1A). Vast majority (85%, n = 17) of COVID-19 contacts in this study were physicians’ families (45%, n = 9) and physicians (40%, n = 8). Non-medical personnel were a minority (15%, n = 3) (Figure 2A). Majority of COVID-19 patients were physicians (50%, n = 10), physicians’ family members (15%, n = 3) while the remainder (35%, n = 7) were non-medical personnel (Figure 2B).
Main symptomatology presentation in COVID-19 patients
Main presentations reported by COVID-19 patients (n = 20) were cough (90%, n = 18), fever (55%, n = 11), anosmia (45%, n = 9), taste loss (45%, n = 9), sore throat (45%, n = 9), respiratory difficulty (45%, n = 9) and malaise (35%, n = 7) (Figure 3).
TaibUVID nutritional supplements and pharmacological treatments
100% of COVID-19 contacts (n = 20) (Table 6) and 35% of COVID-19 patients (n = 7) (Figure 4A) received TaibUVID supplements only. 65% of COVID-19 patients (n = 13) received both pharmacological treatments and TaibUVID nutritional supplements (Figure 4A). 65% of COVID-19 patients (n = 13) received adjuvant TaibUVID inhalation therapy (Figure 4A).
Assessment of TaibUVID effects on COVID-19 contacts and patients
Upon regular TaibUVID intake, many COVID-19 contacts (70%, n = 14) did not get COVID-19 infection or have positive nasopharyngeal swabs PCR till now (about 4 successive months). Among those were many emergency department physicians who are extensively dealing with COVID-19 patients (Table 5) daily while some other colleagues (not taking TaibUVID) caught infection and acquired symptoms. All physicians were taking necessary health precautions. In addition, members of a single family (8 persons) who were contacts of a COVID-19 positive case and entered a quarantine for 14 days (taking TaibUVID nutritional supplement as a prophylaxis) were also in a good health with negative two successive nasopharyngeal swab PCR tests separated by 6 days (Table 6). 6 contacts (4 physicians and 2 wives) (30%, n = 6) who non-regularly used TaibUVID nutritional supplements got mild flu-like symptoms (????? COVID-19 infection) (Figure 4D). 4 out of 6 those COVID-19 contacts had confirmed COVID-19 infection. Symptoms of 2 out of 6 contacts rapidly disappeared (without performing PCR) upon TaibUVID intake. 5 out of 6 (25%) got dramatic rapid disappearance of symptoms upon regular TaibUVID intake. One case (5%) was severe and improved in the hospital (using pharmacological treatments and TaibUVID nutritional supplements) (Figure 5).
Time course and progress of TaibUVID-induced improvements
Majority of COVID-19 patients (65%, n = 13) received TaibUVID supplements as an adjuvant treatment to pharmacological protocols while 35% of patients (n = 7) received TaibUVID supplements alone (Figure 4A). TaibUVID inhalation therapy (nigella sativa/anthemis/costus decoction nebulization using vapor inhalation via a nebulizer) is a novel local treatment that was used and effectively alleviated the respiratory manifestations in 65% of COVID-19 patients (n = 13) (Figure 4B). That dramatically alleviated COVID-19-induced continuous cough and respiratory difficulty and was life-saving in some cases (Table 5). Since introducing TaibUVID and TaibUVID Plus nutritional therapies for COVID-19 pandemic, many Egyptian patients (n = 20) with confirmed positive COVID-19 PCR nasopharyngeal swabs decided to voluntarily administer home-made TaibUVID nutritional doses (3-5 times/day). In many COVID-19 patients (70%, n = 14), 25% of patients (n = 5) took 5-10 days to get recovery while only 5% (a single patient) needed more than 10 days to recover (Figure 4C). 70% of healthy contacts (n = 14) who are keeping using regular oral TaibUVID intake did not catch COVID-19 infection (Figure 4D).
Patients’ satisfaction with TaibUVID nutritional supplement
A small questionnaire was distributed to validate patients’ satisfaction with TaibUVID nutritional supplements. 16 out of 20 patients (80% response rate) answered the questionnaire. 13 patients (81.25%) did not report any side effects after TaibUVID administration. 3 patients (18.75%) reported diarrohea (n = 1, 6.25%), sweating (n = 1, 6.25%) and hyperglycemia (n = 1, 6.25%) (Figure 5A). 5 patients (31.25%) were satisfied by 100% with TaibUVID nutritional supplements. 6 patients (37.5%) were satisfied by 75% with TaibUVID nutritional supplements (Figure 5B). Mean ± SEM of one sample test (against test value 50) revealed that COVID-19 patients expressed a significantly high satisfaction percent with TaibUVID nutritional supplement (70.87%±6.89) (p<0.01).
Discussion
TaibUVID nutritional supplements include TaibUVID, TaibUVID Plus and TaibUVID Forte having natural, safe, available and cheap components (Tables 1, 2 and 3). TaibUVID components are nigella sativa, anthemis hyalina (chamomile) and natural honey. TaibUVID Forte includes TaibUVID in addition to three medicinal plants (costus, fennel and senna). Therapeutic rationale beyond choosing these natural products at scheduled doses is explained and discussed herein. Nigella sativa, natural honey and chamomile were reported (in many systematic reviews and meta-analyses studies) to treat many different human diseases [4-7,9-11] that may be seen as co-morbidities in COVID-19 patients. COVID-19-induced morbidity and mortality are governed by many factors e.g. patients’ immunity and co-morbidities e.g. diabetes mellitus, hypertension, and hyperlipidemia. In a previous report, we confirmed safety and effectiveness of TaibUVID nutritional supplements in treating COVID-19 patients having variable severities of illness [15]. Nigella sativa is quite safe, effective, cheap, available and tolerable for treating human patients having diabetes mellitus, uncontrolled diabetes mellitus with uncontrolled hyperlipidemia [16]. Nigella sativa effectively treated patients having insulin resistance syndrome [17]. Cardiac patients and asthmatic patients infected with COVID-19 pandemic are a vulnerable group. Nigella sativa exerted evident improvements of cardiac dysfunction [18] uncontrolled hypertension [19] and asthma [20,21]. COVID-19 may deteriorate patient’s conditions having chronic illnesses and associated co-morbidities. Nigella sativa treatment improved the overall outcomes in patients having rheumatoid arthritis [22], allergic rhinitis [23], helicobacter pylori infection [23] and psoriasis [24]. All those categories of patients (if acquire COVID-19 infection) will benefit from nigella sativa treatment for treating both COVID-19 infection and associated co-morbidities.
Nigella sativa is the first and major component of TaibUVID nutritional therapy. Nigella sativa was reported to be safe at high doses (20 grams/kg) in broiler chicken [25]. Nigella sativa enhances immune stimulation (increases natural killer cells counts and activities, increases bone marrow cells, increases white blood cells counts and increases interferon-gamma serum levels) i.e. nigella sativa may benefit enhancing immunity in COVID-19 subjects. Nigella sativa protected the lung tissues and other body tissues, exerted strong broad spectrum antiviral effects and treated many diseases e.g. diabetes and hypertension (that may be COVID-19 co-morbidities). Nigella sativa is a quite potentiator of pharmacological treatments. In allergic lung conditions, nigella sativa protected the lung tissues and lessened the pathological tissue damage induced by ovalbumin sensitization [26,27]. Nigella sativa can be looked at as a perfect and complete pharmacy. Nigella sativa contains a plenty of tissue-protective and antiviral active ingredients (causing tissue-protection and antiviral effects) e.g. thymoquinone, p-cymene, cis-carveol, carvacrol, thymol, α-phellandrene, α-pinene, β-pinene, trans-anethole, α-longipinene, longifolene [27], alpha-terpinene, gamma-terpinene, p-cymene, terpinen-4-ol, alpha-terpineol, thymol, citral, 1,8-cineole [26] and α-hederin [28,29]. Thymoquinone is the most enormous ingredient and is famous for its tissue-protective and antiviral effects e.g. thymoquinone effectively suppressed Ebstein Barr virus-infected B cells [26]. Both α-hederin and thymoquinone (of nigella sativa) have anti-inflammatory and bronchodilatory effects [28]. Such active ingredients evidently exerted dose-dependent antiviral effects against herpes virus particles thereby inactivating viral infection [30]. In addition, alpha-pinene and beta-myrcene (in nigella sativa) were reported to exert evident antiviral activity against herpes simplex virus-1 [29]. Moreover, alpha-pinene-derived pharmacological agents were reported to exert potent anti-viral activity against both thymidine kinase +ve and thymidine kinase -ve varicella zoster virus [31].
Natural honey exerts potent antiviral effects. Honey was effective in treating many viral conditions e.g. recurrent attacks of herpetic lesions (labial and genital) [32] and influenza virus infections [33]. Honey was also reported to treat herpes simplex gingivostomatitis in children [32]. All those effects may benefit COVID-19 patients.
Anthemis hyalina (chamomile) was reported to decrease coronaviruses replication to zero levels i.e. complete 100% inhibition of replication [8]. Anthemis contains a lot of the antiviral ingredients present in nigella sativa [34]. This may explain its potent antiviral effects. A high safe dose of chamomile (20 grams/day) was reported to be used in diabetic patients [35]. Clove was also reported to exert potent antiviral effects [36,37].
Senna (cassia angustifolia) is a natural laxative that contains emodin. Emodin was reported to block the spike protein of coronaviruses that may help SARS-COV-2 infectivity [38]. This may benefit COVID-19 patients and decrease coronaviruses infectivity. Senna is not carcinogenic even after daily administration for 2 years at a dosage of 300 mg/kg/day in rats [39]. However, being a laxative, senna may induce diarrhea for those using TaibUVID Forte preparation. This has a lot of therapeutic benefits to counteract constipation-induced health harms. If senna-induced diarrhea is disliked, senna may better be used for few days then omitted from TaibUVID Forte recipe.
Costus (saussurea lappa) ingredients (costunolide and dehydrocostus lactone) exert potent antiviral effects e.g. suppressed hepatitis B surface antigen (HBsAg) and hepatitis B envelop antigen (HBeAg) [40]. Costus is well-known to exert potent anti-inflammatory effects, inhibit gram-positive and gram-negative bacteria and inhibit platelet aggregation [41,42]. This may counteract COVID-19 pathogenesis and complications. Costus was reported to be safe at a high dose (1.5 g/kg/d) in experimental animals where it decreased insulin resistance and triglycerides [43]. Costus is well-known to be antimutagenic with a high margin of safety with no toxicity or teratogenicity [44].
Fennel (foeniculum vulgare) exerts strong antiviral effects against Herpes simplex virus type-1 and parainfluenza viruses. Fennel exerts strong bronchodilatory activity and antithrombotic activity against platelet aggregation induced by ADP, arachidonic acid, and collagen. Fennel exerts antipyretic activity against hyperpyrexia. Fennel stimulates the ciliary motility of the respiratory apparatus (expectorant effect) and enhances the excretion of foreign particles [45]. This may benefit COVID-19 patients. Fennel extract is safe at high doses e.g. at 3 grams/kg (orally) where fennel did not cause any type of toxicity [45].
Social media enriched public knowledge regarding therapeutic benefits of TaibUVID nutritional supplements. We also report here the updated protocol for TaibUVID prophylaxis and treatment for contacts and cases of variable severity (Tables 1, 2 and 3). Our data carries a promising report for preventive and therapeutic effects exerted by TaibUVID adjuvant nutritional supplements given to COVID-19 patients and contacts. We report also that members of a single family (8 persons) were contacts of a COVID-19 positive case and entered a quarantine for 14 days (taking TaibUVID nutritional supplement with no other drugs). They kept negative nasopharyngeal swab PCR tests (Table 6). Upon regular TaibUVID intake (once daily), majority of COVID-19 contacts (14 out of 20) did not get positive nasopharyngeal swabs PCR (Table 6 and Figure 4D). This was true also for many other family members upon regular intake of prophylactic doses of TaibUVID (data not shown). That was true also for many other emergency department physicians who are dealing with COVID-19 patients extensively and on a daily basis (data not shown). However, we should keep in mind that acquiring COVID-19 infection depends on other factors e.g. virus load, exposure time, contacts’ immunity, performing protective measures and others. Physicians and nurses receive the highest infection risk and may transmit that to their families. Our data confirmed that certain jobs may increase the possibility of COVID-19 infection. Physicians and their families carry the heaviest burden being the majority of potential contacts (85%, n = 17) who were facing the highest risks of COVID-19 infection (Figure 2A). Our data revealed that majority of COVID-19 patients were physicians (50%, n = 10), physicians’ family members (15%, n = 3) while the remainder (35%, n = 7) were non-medical personnel (Figure 2B). That may be explained in light of the high viral load and longer duration of exposure (occupational risks).
TaibUVID nutritional supplements will not prevent COVID-19 virus entry into human body but are likely to minimize its harmful effects via enhancing antiviral immunity, exerting antiviral effects and conferring tissue-protective effects. TaibUVID inhalation therapy are likely to exert direct antiviral effects at the airways and the respiratory system. Collectively, COVID-19 contacts on regular TaibUVID intake are likely to be in a better immunological and general condition to deal with COVID-19 pandemic than ordinary people provided that other factors (e.g. virus load and protective measures) are the same.
We report here that some COVID-19 patients may present with leukopenia and/or lymphopenia (reflecting immunological defects) that may be related to COVID-19 infection (Table 4). Upon treatment with pharmacological protocols and TaibUVID nutritional supplements for few days, leukopenia and lymphopenia were corrected to normal levels and even lymphocytosis (Table 4). White blood cells count increased from 4090 cells/cc to 5000 cells/cc (normal range: 4000-11000/cc) over 11 days while lymphocytes percentage increased from 17.7% to 44% (normal range: 20-40%) (TaibUVID effect) (Table 4). We report also that COVID-19 patients presented with cough (90%, n = 18), fever (55%, n = 11), anosmia (45%, n = 9), taste loss (45%, n = 9), sore throat (45%, n = 9), respiratory difficulty (45%, n = 9) and malaise (35%, n = 7) (Figure 3).
We report that many patients having confirmed symptomatic COVID-19 infection and their families voluntarily prepared TaibUVID nutritional supplements at home as previously explained [13]. Majority of COVID-19 patients (65%, n = 13) in this study received TaibUVID supplements as adjuvants to pharmacological treatments (Figure 4A). Other patients who developed some respiratory symptoms (moderate to severe cough and respiratory difficulty) received oral TaibUVID and TaibUVID inhalation therapy (of nigella sativa/anthemis vapor). More interestingly, we report also that the majority (70%, n = 14) of COVID-19 patients having positive nasopharyngeal swab PCR tests reverted to negative PCR within few days (1-4 days) that was shorter than the average of those not using TaibUVID (1-2 weeks) (Figure 4C). 95% of COVID-19 patients (n = 19) took less than 10 days to revert to negative nasopharyngeal swab PCR while only one patient (5%) needed more than 10 days to revert to negative nasopharyngeal swab PCR (Figure 4C). 25% of the contacts (n = 5) got mild symptoms e.g. mild cough and diarrhea for 1-2 days (without doing nasopharyngeal swab PCR) and they received TaibUVID supplements only. They were considered potential COVID-19 patients. One patient (5%) developed intensive cough and got positive nasopharyngeal swab PCR and received both pharmacological treatment and TaibUVID Forte supplements (oral and inhalation therapy). He got improved within few days (Figure 4D). Being a physician, the patient used interrupted TaibUVID prophylaxis and was exposed to COVID-19 patients in his hospital. High virus load with decreased TaibUVID intake might be responsible for that. In previous reports, inhalation therapy of nigella sativa and chamomile were successful in treating asthmatic patients [46] while nigella sativa solution inhalation improved the liver functions in other hepatic patients [47].
Regarding the safety issues beyond TaibUVID nutritional supplements, majority of patients (81.25%, n = 13) in this study confirmed that they did not face any side effects during use of TaibUVID nutritional supplements (Figure 5A).The minor side effects reported by 3 patients (18.75%) (out of 16 patients) who answered the questionnaire were sweating, hyperglycemia and diarrhea (Figure 5A). Sweating is unlikely to be due to the nutritional supplements and is mostly due to COVID-19 infection itself. Diarrhoea is a common manifestation in the course of febrile viral illnesses and is also unlikely to result from TaibUVID nutritional supplements. Hyperglycemia in diabetic subjects is a common finding and is mostly related to diabetes control (dietary and pharmacological). However, although natural honey is always accused to cause hyperglycemia, natural honey is reported to cause hypoglycemia and exert anti-diabetic effects [48]. Hyperglycemia reported as a side effects in this study might be due to infection itself or the consumption of an artificial honey (non-natural honey) that is made of excess glucose and honey flavor.
TaibUVID nutritional supplements gained satisfaction of 87.5% of patients (n = 13) participating in the questionnaire (n = 16) (P<0.01) (Figure 5B). 5 patients (31.25% of) were satisfied 100% with TaibUVID nutritional supplements. 6 patients (37.5%) were satisfied by 75% with TaibUVID nutritional supplements (Figure 5B). It may be argued that improvements of patients in this study occurred spontaneously. We do not think so as rapid reversion to negative nasopharyngeal swab PCR did not occur in all patients. In addition, TaibUVID effect (increased lymphocytes percentage with TaibUVID intake) did not occur spontaneously but it is a reported hematological effect of both natural honey and nigella sativa. It may be argued that patients’ improvements are attributed to pharmacological protocols only. We do not think so. 35% of patients (n = 7) in this study improved upon using TaibUVID supplements without taking any medications. In addition, pharmacological protocols are not reported to increase bone marrow cellularity or improve lymphopenia towards lymphocytosis. On the contrary, TaibUVID components-induced antioxidant (tissue-protective) effects may alleviate side effects induced by pharmacological protocols.
Our data strongly recommends using TaibUVID and TaibUVID Forte for public prophylaxis to decrease the emergence of new COVID-19 cases and for treating patients in the hope of accelerating recovery and alleviating pharmacological side effects.
Conclusion
All TaibUVID components are available and can be prepared as home (or hospital) nutritional supplements for prophylaxis of COVID-19 contacts and adjuvant treatment of COVID-19 cases. Being cheap natural products with potent antiviral effects, TaibUVID and TaibUVID Forte supplements carry a lot of hope as adjuvants or (sole treatments in mild cases) for rapidly eradicating COVID-19 pandemic. TaibUVID components are not harmful or interfere with current pharmacological treatments to COVID-19 patients. TaibUVID advantages include easy home preparation, self-intake, contacts eradication, patients’ relief and potentiating isolation and treatment efforts exerted by health authorities. TaibUVID inhalation therapy should be a new addition to emergency departments and inpatient departments in hospitals to help rapid recovery and short hospital stay. TaibUVID supplements improved lymphopenia within few days. Fortunately, all TaibUVID components are available everywhere for welfare of COVID-19 contacts and cases. Physicians and medical personnel face the highest risk of COVID-19 infection. Majority of patients (70%) taking TaibUVID improved in less than 4 days. TaibUVID supplements helps public prophylaxis against COVID-19 infection (70% or more). Negiligible side effects were reported by some patients. There is a big patients’ satisfaction (87.5%) with TaibUVID nutritional supplements.
Acknowledgements
The authors declare that they did not receive any financial support from any source for this research article.
Disclosure of conflict of interest
None.
References
- 1.Yan Y, Shin WI, Pang YX, Meng Y, Lai J, You C, Zhao H, Lester E, Wu T, Pang CH. The first 75 days of novel coronavirus (SARS-CoV-2) outbreak: recent advances, prevention, and treatment. Int J Environ Res Public Health. 2020;17:2323. doi: 10.3390/ijerph17072323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abuharfeil N, Al-Oran R, Abo-Shehada M. The effect of bee honey on the proliferative activity of human B-and T-lymphocytes and the activity of phagocytes. Food Agr Immunol. 1999;11:169–177. [Google Scholar]
- 3.El-Shanshory M, Hablas NM, Aboonq MS, Fakhreldin AR, Attia M, Arafa W, Mariah RA, Baghdadi H, Ayat M, Zolaly M, Nabo MMH, Almaramhy HH, El-Sawy SA, Zidan M, Elshazley M, Alharbi R, Mostafa S, Abou-El-Naga M, El Sayed SM. Nigella sativa improves anemia, enhances immunity and relieves iron overload-induced oxidative stress as a novel promising treatment in children having beta-thalassemia major. J Herb Med. 2019;16:100245. [Google Scholar]
- 4.Hamdan A, Haji Idrus R, Mokhtar MH. Effects of nigella sativa on type-2 diabetes mellitus: a systematic review. Int J Environ Res Public Health. 2019;16:4911. doi: 10.3390/ijerph16244911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mousavi SM, Sheikhi A, Varkaneh HK, Zarezadeh M, Rahmani J, Milajerdi A. Effect of Nigella sativa supplementation on obesity indices: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2018;38:48–57. doi: 10.1016/j.ctim.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Khabbazi A, Javadivala Z, Seyedsadjadi N, Malek Mahdavi A. A systematic review of the potential effects of nigella sativa on rheumatoid arthritis. Planta Med. 2020;86:457–469. doi: 10.1055/a-1143-8521. [DOI] [PubMed] [Google Scholar]
- 7.Majdalawieh AF, Fayyad MW. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review. Int Immunopharmacol. 2015;28:295–304. doi: 10.1016/j.intimp.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Ulasli M, Gurses SA, Bayraktar R, Yumrutas O, Oztuzcu S, Igci M, Igci YZ, Cakmak EA, Arslan A. The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Mol Biol Rep. 2014;41:1703–1711. doi: 10.1007/s11033-014-3019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Münstedt K, Momm F, Hübner J. Honey in the management of side effects of radiotherapy- or radio/chemotherapy-induced oral mucositis. A systematic review. Complement Ther Clin Pract. 2019;34:145–152. doi: 10.1016/j.ctcp.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Aziz Z, Hassan BA. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50–57. doi: 10.1016/j.burns.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Miraj S, Alesaeidi S. A systematic review study of therapeutic effects of Matricaria recuitta chamomile (chamomile) Electron Physician. 2016;8:3024. doi: 10.19082/3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamouda O, Sweilam M, Abdellah A, Aboonq MS, Abdel-Halim OB, El Sayed SM, Hamouda AO. Outcome and future perspectives of pioneering integrative medicine education in Taibah university: ten years’ experience in saudi Arabia medical schools (a medical education article) Am J Educ Res. 2019:69–75. [Google Scholar]
- 13.Hamouda O, Sweilam M, Abdellah A, El Sayed SM. Evaluation of pioneering introduction of integrative and prophetic medicine education in an Arabic medical school (Taibah University, Saudi Arabia): 10 years’ experience. J Int Med Res. 2019;47:2157–2165. doi: 10.1177/0300060519831174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Sayed SM, Almaramhy HH, Aljehani YT, Okashah AM, El-Anzi ME, AlHarbi MB, El-Tahlawi R, Nabo MMH, Aboonq MS, Hamouda O, Alhadramy O. The evidence-based TaibUVID nutritional treatment for minimizing covid-19 fatalities and morbidity and eradicating COVID-19 pandemic: a novel approach for better outcomes (A Treatment Protocol) Am J Public Health Res. 2020;8:54–60. [Google Scholar]
- 15.El Sayed SM, Bahashwan SA, Aboonq MS, Hussam Baghdadi H, Elshazley M, Okashah AM, El Rashedy AG, Abou El-Magd RM, Aljehani YT, El-Tahlawi R, Nabo MMN, Hamouda O, El-Murr A, Soliman TM, Mahmoud HS, Abu-Elnaga M, Hassan MM. IJMDC. 2020;4:1375–1389. [Google Scholar]
- 16.Bamosa AO, Kaatabi H, Lebdaa FM, Elq A, Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. 2010;54:344–354. [PubMed] [Google Scholar]
- 17.Najmi A, Nasiruddin M, Khan RA, Haque SF. Effect of Nigella sativa oil on various clinical and biochemical parameters of insulin resistance syndrome. Int J Diabetes Dev Ctries. 2008;28:11–4. doi: 10.4103/0973-3930.41980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bamosa A, Kaatabi H, Badar A, Al-Khadra A, Al Elq A, Abou-Hozaifa B, Lebda F, Al-Almaie S. Nigella sativa: a potential natural protective agent against cardiac dysfunction in patients with type 2 diabetes mellitus. J Family Community Med. 2015;22:88–95. doi: 10.4103/2230-8229.155380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leong XF, Rais Mustafa M, Jaarin K. Nigella sativa and its protective role in oxidative stress and hypertension. Evid Based Complement Alternat Med. 2013;2013:120732. doi: 10.1155/2013/120732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boskabady MH, Javan H, Sajady M, Rakhshandeh H. The possible prophylactic effect of Nigella sativa seed extract in asthmatic patients. Fundam Clin Pharmacol. 2007;21:559–566. doi: 10.1111/j.1472-8206.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 21.Boskabady M, Mohsenpoor N, Takaloo L. Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine. 2010;17:707–713. doi: 10.1016/j.phymed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Gheita TA, Kenawy SA. Effectiveness of Nigella sativa oil in the management of rheumatoid arthritis patients: a placebo controlled study. Phytother Res. 2012;26:1246–1248. doi: 10.1002/ptr.3679. [DOI] [PubMed] [Google Scholar]
- 23.Nikakhlagh S, Rahim F, Aryani FH, Syahpoush A, Brougerdnya MG, Saki N. Herbal treatment of allergic rhinitis: the use of Nigella sativa. Am J Otolaryngol. 2011;32:402–407. doi: 10.1016/j.amjoto.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Dwarampudi LP, Palaniswamy D, Nithyanantham M, Raghu PS. Antipsoriatic activity and cytotoxicity of ethanolic extract of Nigella sativa seeds. Pharmacogn Mag. 2012;8:268–272. doi: 10.4103/0973-1296.103650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar P, Patra AK, Mandal GP, Samanta I, Pradhan S. Effect of black cumin seeds on growth performance, nutrient utilization, immunity, gut health and nitrogen excretion in broiler chickens. J Sci Food Agric. 2017;97:3742–3751. doi: 10.1002/jsfa.8237. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, Damanhouri ZA, Anwar F. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarwar A, Latif Z. GC-MS characterisation and antibacterial activity evaluation of Nigella sativa oil against diverse strains of Salmonella. Nat Prod Res. 2015;29:447–451. doi: 10.1080/14786419.2014.947493. [DOI] [PubMed] [Google Scholar]
- 28.Saadat S, Mohammadi M, Fallahi M, Aslani M. The protective effect of α-hederin, the active constituent of Nigella sativa, on tracheal responsiveness and lung inflammation in ovalbumin-sensitized guinea pigs. J Physiol Sci. 2015;65:285–292. doi: 10.1007/s12576-015-0367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loizzo MR, Saab A, Tundis R, Statti GA, Lampronti I, Menichini F, Gambari R, Cinatl J, Doerr HW. Phytochemical analysis and in vitro evaluation of the biological activity against herpes simplex virus type 1 (HSV-1) of Cedrus libani A. Rich. Phytomedicine. 2008;15:79–83. doi: 10.1016/j.phymed.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Astani A, Reichling J, Schnitzler P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother Res. 2010;24:673–9. doi: 10.1002/ptr.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.López C, Balo C, Blanco JM, Fernández F, De Clercq E, Balzarini J. A cyclobutane carbonucleoside with marked selectivity against TK+ and TK- varicella zoster virus. Nucleosides Nucleotides Nucleic Acids. 2001;20:1133–1135. doi: 10.1081/NCN-100002505. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Naby Awad OG, Hamad AH. Honey can help in herpes simplex gingivostomatitis in children: prospective randomized double blind placebo controlled clinical trial. Am J Otolaryngol. 2018;39:759–763. doi: 10.1016/j.amjoto.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe K, Rahmasari R, Matsunaga A, Haruyama T, Kobayashi N. Anti-influenza viral effects of honey in vitro: potent high activity of manuka honey. Arch Med Res. 2014;45:359–365. doi: 10.1016/j.arcmed.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Rustaiyan A, Masoudi M, Danaii E, Taherkhani M, Aghajani Z. Composition of the essential oils of Anthemis hyalina DC., Achillea nobilis L. and Cichorium intybus L. Three asteraceae herbs growing wild in Iran. J Essent Oil Bear Pl. 2012;24:472–480. [Google Scholar]
- 35.Kaseb F, Yazdanpanah Z, Biregani AN, Yazdi NB, Yazdanpanah Z. The effect of chamomile (Matricaria recutita L.) infusion on blood glucose, lipid profile and kidney function in type 2 diabetic patients: a randomized clinical trial. J Nut Int Med. 2018;20:110–118. [Google Scholar]
- 36.Aboubakr HA, Nauertz A, Luong NT, Agrawal S, El-Sohaimy SA, Youssef MM, Goyal SM. In vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus. J Food Prot. 2016;79:1001–1012. doi: 10.4315/0362-028X.JFP-15-593. [DOI] [PubMed] [Google Scholar]
- 37.Cortés-Rojas DF, de Souza CR, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90–96. doi: 10.1016/S2221-1691(14)60215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho TY, Wu SL, Chen JC, Li CC, Hsiang CY. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell J, Mengs U, McPherson S, Zijlstra J, Dettmar P, Gregson R, Tigner J. An oral carcinogenicity and toxicity study of senna (Tinnevelly senna fruits) in the rat. Arch Toxicol. 2006;80:34–44. doi: 10.1007/s00204-005-0021-9. [DOI] [PubMed] [Google Scholar]
- 40.Chen HC, Chou CK, Lee SD, Wang JC, Yeh SF. Active compounds from Saussurea lappa Clarks that suppress hepatitis B virus surface antigen gene expression in human hepatoma cells. Antiviral Res. 1995;27:99–109. doi: 10.1016/0166-3542(94)00083-k. [DOI] [PubMed] [Google Scholar]
- 41.Hasson SS, Al-Balushi MS, Alharthy K, Al-Busaidi JZ, Aldaihani MS, Othman MS, Said EA, Habal O, Sallam TA, Aljabri AA, Ahmedidris M. Evaluation of anti-resistant activity of Auklandia (Saussurea lappa) root against some human pathogens. Asian Pac J Trop Biomed. 2013;3:557–562. doi: 10.1016/S2221-1691(13)60113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu HH, Lee JS, Lee KH, Kim KY, You YO. Saussurea lappa inhibits the growth, acid production, adhesion, and water-insoluble glucan synthesis of Streptococcus mutans. J Ethnopharmacol. 2007;111:413–417. doi: 10.1016/j.jep.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Subasinghe H, Hettihewa L, Gunawardena S, Liyanage T. Methanol and water extracts of Costus speciosus (j. könig) sm. leaves reverse the high-fat-diet induced peripheral insulin resistance in experimental Wistar rats. Int Res J Pharm. 2014;5:44–49. [Google Scholar]
- 44.Marzook EA, El-Bayoumy AS, Marzook FA. Preclinical evaluation of carnosine and costus as hematological protective agents against gamma radiation. J Rad Res Appl Sci. 2019;12:304–310. [Google Scholar]
- 45.Badgujar SB, Patel VV, Bandivdekar AH. Foeniculum vulgare Mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res Int. 2014;2014:1–32. doi: 10.1155/2014/842674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Jawad FH, Al-Razzuqi RA, Hashim HM, Ismael AH. Broncho-relaxant activity of Nigella sativa versus anthemisnobilis in chronic bronchial asthma; a comparative study of efficacy. IOSR J Pharmac. 2012;2:81–83. [Google Scholar]
- 47.Mostafa EF, Ibrahim AM, Hamed EF. Effect of intrapulmonary inhalation of honey, nigella sativa and curcumin on liver function in patients with chronic liver disease. Afro-Egypt J Infect Endem Dis. 2012;2:148–154. [Google Scholar]
- 48.Bobiş O, Dezmirean DS, Moise AR. Honey and diabetes: the importance of natural simple sugars in diet for preventing and treating different type of diabetes. Oxid Med Cell Longev. 2018;2018:4757893. doi: 10.1155/2018/4757893. [DOI] [PMC free article] [PubMed] [Google Scholar]