Key Points
Question
What is the association between the implementation of a teledermatology triage system at Zuckerberg San Francisco General Hospital and the organizational expenses for the provision of outpatient dermatology care?
Findings
In this cost minimization analysis incorporating personnel and teledermatology technology costs of 2098 patients referred to the dermatology department, teledermatology saved $140 per newly referred dermatology patient compared with a conventional dermatology care model.
Meaning
This study suggests that using teledermatology to triage and manage dermatology patients within a capitated health care system may be associated with significant cost savings.
Abstract
Importance
Teledermatology (TD) enables remote triage and management of dermatology patients. Previous analyses of TD systems have demonstrated improved access to care but an inconsistent fiscal impact.
Objective
To compare the organizationwide cost of managing newly referred dermatology patients within a TD triage system vs a conventional dermatology care model at the Zuckerberg San Francisco General Hospital and Trauma Center (hereafter referred to as the ZSFG) in California.
Design, Setting, and Participants
A retrospective cost minimization analysis was conducted of 2098 patients referred to the dermatology department at the ZSFG between June 1 and December 31, 2017.
Intervention
Implementation of the TD triage system in January 2015.
Main Outcomes and Measures
The main outcome was mean cost to the health care organization to manage newly referred dermatology patients with or without TD triage. To estimate costs, decision-tree models were constructed to characterize possible care paths with TD triage and within a conventional dermatology care model. Costs associated with primary care visits, dermatology visits, and TD visits were then applied to the decision-tree models to estimate the mean cost of managing patients following each care path for 6 months. The mean cost for each visit type incorporated personnel costs, with the mean cost per TD consultation also incorporating software implementation and maintenance costs. Finally, ZSFG patient data were applied within the models to evaluate branch probabilities, enabling calculation of mean cost per patient within each model.
Results
The analysis captured 2098 patients (1154 men [55.0%]; mean [SD] age, 53.4 [16.8] years), with 1099 (52.4%) having Medi-Cal insurance and 879 (41.9%) identifying as non-White. In the decision-tree model with TD triage, the mean (SD) cost per patient to the health care organization was $559.84 ($319.29). In the decision-tree model for conventional dermatology care, the mean (SD) cost per patient was $699.96 ($390.24). Therefore, the TD model demonstrated a statistically significant mean (SE) cost savings of $140.12 ($11.01) per patient. Given an annual dermatology referral volume of 3150 patients, the analysis estimates an annual savings of $441 378.
Conclusions and Relevance
Implementation of a TD triage system within the dermatology department at the ZSFG was associated with cost savings, suggesting that managed health care settings may experience significant cost savings from using TD to triage and manage patients.
This cost minimization analysis compares the organization-wide cost of managing newly referred dermatology patients within a teledermatology triage system vs a conventional dermatology care model.
Introduction
Teledermatology (TD) enables remote evaluation and management of patients with dermatologic disease. Studies have found that TD improves patient access to care1,2,3,4,5 and clinical efficiency3,4,6,7,8,9 without forgoing diagnostic accuracy7,10,11 or negatively affecting patient experience.7,10,12,13 Despite the documented health and logistical benefits of TD, to our knowledge, literature on the cost-effectiveness of TD is scarce and has produced mixed results overall.9,10,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30 Among studies evaluating TD within managed health care systems that would benefit most from improved cost-effectiveness, some have found TD to produce cost savings,15,17 others have found TD produces cost savings only when societal costs are included,16,23 and one has found TD to be more costly.21 Furthermore, only 2 studies incorporated the costs of managing dermatology patients after the initial consultation,16,23 and those studies excluded primary care follow-up costs associated with the management of the patient’s skin disease. We sought to address these gaps through a cost minimization analysis of the TD system at Zuckerberg San Francisco General Hospital and Trauma Center (hereafter referred to as the ZSFG) in California.
Methods
Study Setting
The dermatology clinic at the ZSFG is the primary dermatology referral site for the San Francisco Health Network, supported by the San Francisco Department of Public Health. The ZSFG provides subspecialty care services to approximately 150 000 San Francisco residents per year primarily through managed care payment agreements, provides care to a diverse patient population, and acts as the public safety-net hospital for the city’s most vulnerable populations. This study was reviewed by the University of California, San Francisco (UCSF) Human Research Protection Program Institutional Review Board and granted exempt certification on April 19, 2018, because it involved the collection of existing data, documents, and records that were recorded in a deidentified manner. By granting exempt certification, the UCSF Institutional Review Board also waived the requirement of patient consent because the data were deidentified.
TD Program
The ZSFG store-and-forward TD program was introduced in January 2015 and is described in depth in a previous publication.6 In short, for all nonemergency patient referrals, the referring clinicians are required to upload patient photographs and a brief history through a web-based telemedicine platform (Medweb, version 7.0.11). An attending dermatologist and 3 to 4 dermatology residents convene weekly to review TD cases and determine which patients require an in-person appointment at the ZSFG dermatology clinic. When a dermatology visit is not recommended, the referring clinician is expected to coordinate the dermatologist’s recommended workup and treatment plan. When patients require a dermatology visit, the TD platform sends electronic communications to dermatology clinic staff to schedule appointments.
Study Design
We conducted a retrospective cost minimization analysis of dermatology care delivery at the ZSFG. Decision-tree modeling was performed to estimate the mean cost to manage newly referred dermatology patients for 6 months within a TD triage care model and the conventional dermatology care model without TD.
Patient Population
The analysis captured all adult patients who received consultations through the ZSFG’s TD triage system between June 1 and December 31, 2017. We selected a period after December 2016 to account for the 2-year phased rollout of the TD program and selected the 7-month interval of June to December 2017 because of data availability. No sample size calculation was performed because data were collected on all patients within the study period.
Cost Data
Within the decision-tree model, cost contributors included primary care provider (PCP) visits, dermatology clinic visits, and TD consultations. Cost calculations included personnel costs within each of these settings and technological costs for TD consultations. Personnel costs accounted for attending physicians, residents, nurse practitioners, medical assistants, registered nurses, front office staff, and temporary workers. Mean personnel salaries and number of full-time equivalents within each setting were provided by respective clinic administrators, including salary and fringe benefits. Fringe benefits were provided by the UCSF Finance and Administration Department and were consistent across clinical sites using the 2017 UCPath composite benefit rates set by UCSF.31 Technological costs accounted for software license installation, software support, maintenance services, equipment, and training for the ZSFG and referring clinic users, as informed by the public software license agreement between San Francisco County and Nexsys Electronics Inc (DBA Medweb). Cost estimates excluded costs for rent, utilities, and clinic supplies because of data unavailability.
Patients attended PCP visits at 1 of 14 referring clinics. The cost per visit at the Richard H. Fine People’s Clinic (ie, the 1M clinic) at the ZSFG was used as the estimated cost for PCP visits because the 1M clinic contributes the largest proportion of total TD consultations, both the 1M clinic and the dermatology clinic are hospital based and use trainees, and calculating the cost for PCP visits at each of the unique referring clinics would be unfeasible. To compute the mean cost per 1M clinic visit, dermatology clinic visit, and TD consultation, costs within each setting for 12 months, 7 months, and 7 months, respectively, were divided by the number of patients managed within each setting during the same period. These periods were selected because of data availability. The number of 1M clinic visits was provided by clinic administrators, the number of dermatology visits was provided by managers at the ZSFG’s Specialty Care and Diagnostics Department, and the number of TD consultations was obtained by generating a report within Medweb.
Decision Tree and Mean Patient Cost
Two decision-tree models were created to represent patient flow within a TD triage model and a traditional dermatology care model. Branch probabilities were evaluated through medical record review or through reports generated by Medweb. Dermatology clinic and PCP clinic visits were included only if the patient attended the visit, if the visit occurred within 6 months of the patient’s TD consultation, and if the patient’s dermatologic symptom was included in the “History of Present Illness” and “Assessment and Plan” sections of the associated visit note. We chose a 6-month management period because a shorter follow-up interval may not capture follow-up visits, whereas a longer period might skew results in favor of sicker patients with more complex problems requiring frequent dermatology visits. Previous TD cost studies analyzed 4 to 9 months of follow-up.16,23
In the TD triage model, all patients began with a PCP visit and TD consultation. The model then branched to represent patients who were or were not triaged to a dermatology clinic visit. Patients triaged to not receive a dermatology visit reached a final chance node with 2 branches based on whether they attended any relevant PCP visits. Patients triaged to a dermatology clinic visit reached a chance node with 2 potential branches based on whether they attended or did not attend their dermatology appointment. Patients who did not attend their dermatology clinic appointment reached a final chance node with 2 branches based on whether they attended any relevant PCP visits.
For the conventional care model, branch probabilities and follow-up visits were extrapolated from the TD triage model. All patients began with a PCP visit and were subsequently referred to the dermatology clinic. The model then branched to represent patients who attended their dermatology appointment and patients who did not attend their dermatology appointment. Patients who did not attend their dermatology appointment reached a chance node with 2 branches based on whether they attended additional PCP visits.
To evaluate the association between TD triage and health care organization costs, we subtracted the mean patient cost within the TD model from the mean patient cost within the conventional care model. We then multiplied the difference by the total number of TD consultations in 2017 to estimate the annual fiscal impact.
Statistical and Sensitivity Analysis
The mean per-patient cost of managing newly referred dermatology patients was compared between the models by conducting a 2-tailed z test. We also performed sensitivity analyses by independently varying the cost of TD visits, the cost of dermatology clinic visits, the cost of PCP visits, and the proportion of patients triaged through TD to receive a dermatology clinic visit to evaluate the values needed to equilibrate the mean cost between the 2 models. The statistical and sensitivity analyses were performed using Microsoft Excel, version 16.32 (Microsoft Corp), and P < .05 was deemed statistically significant.
Results
Characteristics of the Study Population
The analysis captured 2098 patients from the San Francisco Health Network system (Table). The sample represented the diverse and socioeconomically disadvantaged patient population at the ZSFG, with 676 patients (32.2%) speaking a primary language other than English, 879 patients (41.9%) identifying as non-White, and 1099 patients (52.4%) having Medi-Cal. The top 3 diagnostic categories within our sample were benign growth (671 [32.0%]), infection (304 [14.5%]), and eczematous dermatitis (261 [12.4%]).
Table. Demographic Characteristics of Study Populations.
| Characteristic | Study sample (N = 2098) |
|---|---|
| Female, No. (%) | 944 (45.0) |
| Race/ethnicity, No. (%) | |
| Non-Hispanic White | 665 (31.7) |
| Hispanic White | 554 (26.4) |
| Asian | 476 (22.7) |
| Black or African American | 202 (9.6) |
| Other | 201 (9.6) |
| Age, mean (SD), y | 53.4 (16.8) |
| Health care coverage, No. (%) | |
| Medi-Cal | 1099 (52.4) |
| Medicare | 468 (22.3) |
| Healthy San Francisco | 218 (10.4) |
| Other coverage | 275 (13.1) |
| Uninsured | 38 (1.8) |
| Primary language, No. (%) | |
| English | 1422 (67.8) |
| Spanish | 365 (17.4) |
| Cantonese | 180 (8.6) |
| Other | 131 (6.2) |
| Primary diagnosis, No. (%) | |
| Benign growth (including seborrheic keratosis) | 671 (32.0) |
| Neoplasm of uncertain significance | 136 (6.5) |
| Lichen simplex chronicus or prurigo nodularis | 103 (4.9) |
| Eczematous dermatitis | 261 (12.4) |
| Psoriasis | 117 (5.6) |
| Acne or rosacea | 110 (5.2) |
| Infection | 304 (14.5) |
| Hair or nail condition | 93 (4.4) |
| Other | 303 (14.4) |
Mean Cost per Patient Visit
Total costs for each visit type were summed over the corresponding time period and divided by the total number of patient visits. The resulting mean costs were $272.80 per primary care patient visit, $324.90 per dermatology clinic visit, and $44.25 per TD consult (eTable in the Supplement).
Mean Cost per Patient Within Decision-Tree Models and Cost Savings Associated With TD
The mean (SD) cost per patient within the TD triage model was $559.84 ($319.29) (Figure 1). In contrast, the estimated mean (SD) cost per patient within the conventional care model was $699.96 ($390.24) (Figure 2). The mean (SE) per-patient cost savings associated with TD implementation was $140.12 ($11.01), which represents a statistically significant difference (P < .001). Given an annual dermatology referral volume of 3150 patients, the analysis estimates an annual savings of $441 378.
Figure 1. Teledermatology (TD) Decision Tree.
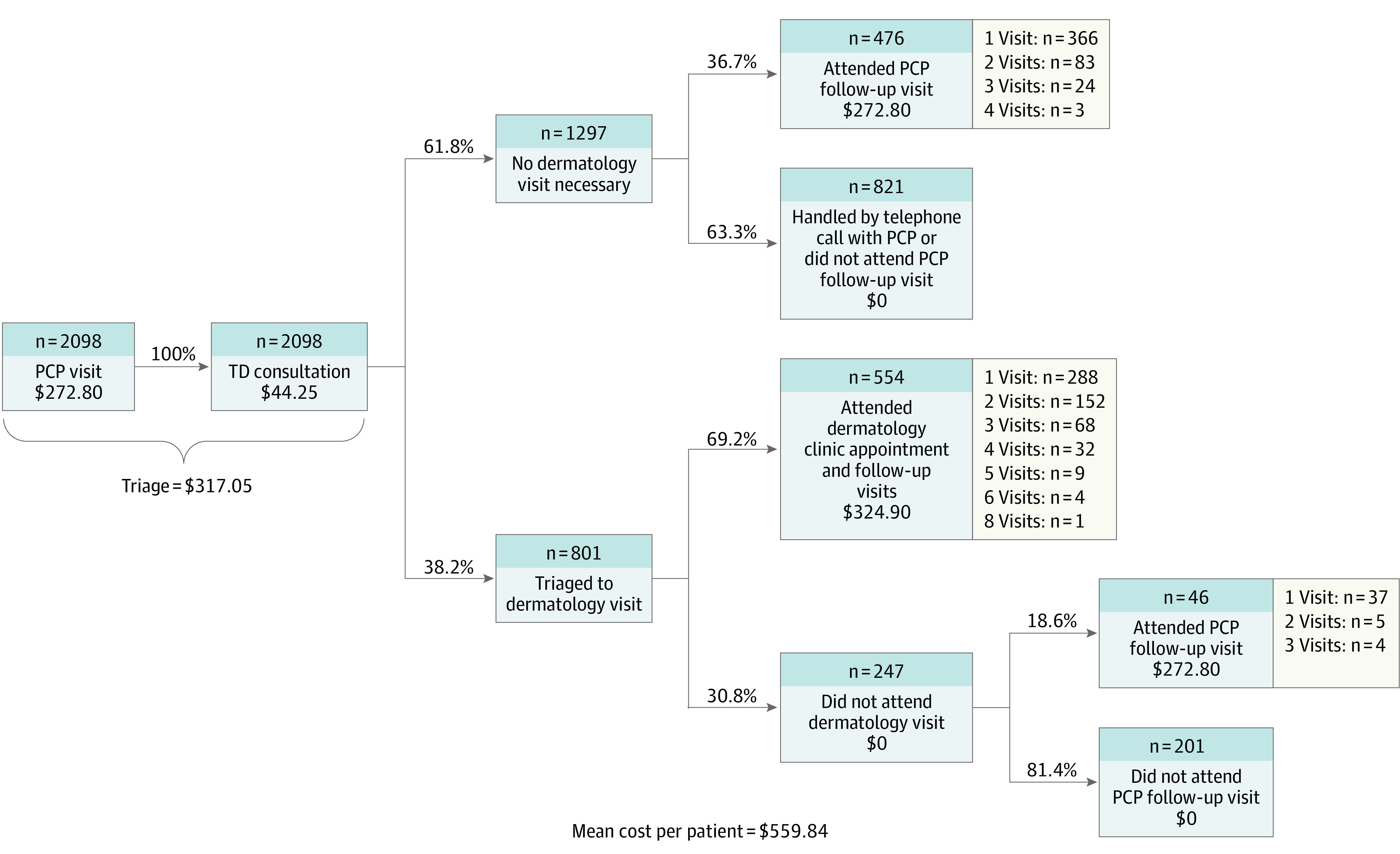
PCP indicates primary care physician.
Figure 2. Conventional Care Decision Tree.
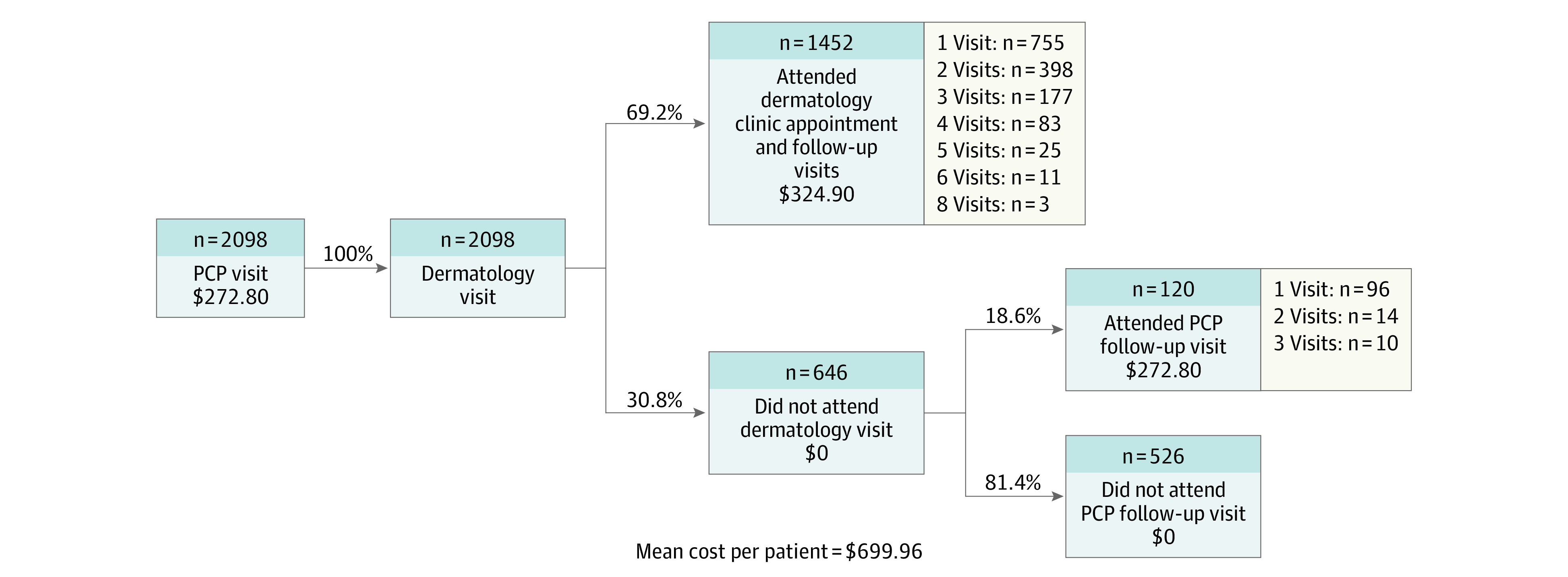
PCP indicates primary care physician.
Sensitivity Analysis
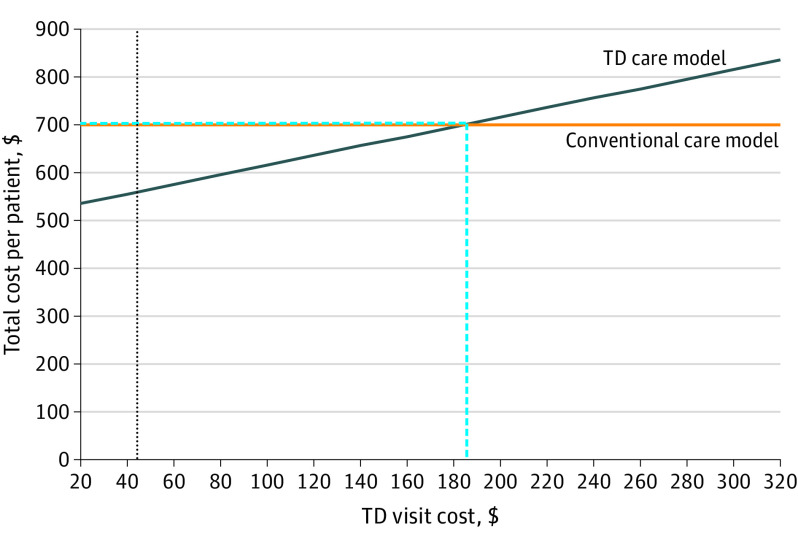
The 2 models produced the same per-patient cost of $699.96 when TD consultation costs were increased to $184.23, which represents a 316% increase from our value of $44.25 (Figure 3). The 2 models produced the same per-patient cost of $473.72 when dermatology visit costs were decreased to $144.35, representing a 56% relative decrease from our value of $324.90 (eFigure 1 in the Supplement). The 2 models produced the same per-patient cost of $1305.70 when PCP visit costs were increased to $837.08, representing a 207% relative increase from our value of $272.80 (eFigure 2 in the Supplement). Last, the 2 models produced the same per-patient cost of $699.96 when TD triage rates to a dermatology appointment increased to 85.3%, which represents a 123% increase from our value of 38.2% (eFigure 3 in the Supplement).
Figure 3. Sensitivity Analysis of Teledermatology (TD) Visit Cost.
The cost per TD consultation evaluation in our model was $44.25. Through our sensitivity analysis, we determined that if the TD consultation evaluation had cost $184.23 (the threshold value), then the per-patient cost to deliver dermatology care for 6 months to new patients would have equilibrated at $699.96. The vertical dashed blue line indicates the threshold value for TD consultation evaluation cost needed for the per-patient overall cost of the TD triage system and conventional care model to be equivalent. The horizontal dashed blue line indicates the per-patient overall cost that each system equilibrates to at the threshold value for TD consultation evaluation cost. The dotted black vertical line indicates the true calculated study value for TD consultation evaluation cost.
Discussion
We conducted a retrospective cost minimization analysis using decision-tree modeling to analyze cost differences of managing newly referred dermatology patients for 6 months with or without a TD triage system. Implementation of a TD triage system was associated with a statistically significant cost savings of $140.12 per patient relative to the conventional care model when factoring in personnel and TD technological costs. Sensitivity analysis demonstrated that TD consultations would need to be more than 4 times more expensive for the TD triage and conventional care models to become cost neutral. The novel aspects of our study include decision-tree cost modeling, which, to our knowledge, has previously only been used in 1 non–US-based cost analysis of TD,22 and the use of true costs rather than estimates or national mean values.
Factors associated with increased cost savings in past analyses include high patient volume being triaged through TD26,27 and increased percentage of dermatology clinic visits avoided.21 Our study meets both criteria, with an annual TD consultation volume greater than all but 1 previously described TD systems17 and the percentage of dermatology clinic visits avoided toward the upper range demonstrated in previous studies.3,4,7,8,9,10
A promising aspect of our findings is that TD produced cost savings even though the analysis included TD implementation costs. Many costs associated with the implementation of TD, including the purchase of equipment and the training of personnel, tend to be front loaded and have been suggested as the reason for the increased relative cost of TD in previous analyses.19,24 Therefore, the cost savings of the ZSFG’s TD system should be lowest at the outset and should steadily increase over time.
For several reasons, our analyses may underestimate TD’s fiscal benefits when applied to other health care systems. First, TD is primarily associated with reduced health care costs through avoidance of relatively expensive dermatology visits, so systems with lower rates of unattended appointments have more to gain compared with our system. Second, our analysis excluded societal costs. Past studies have consistently demonstrated that TD reduces societal costs by enabling patients to attend fewer in-person appointments and by providing more timely dermatology diagnosis and treatment.4,16,17,23,27,32 Third, the ZSFG system relatively underpays dermatologists and overpays PCPs compared with national mean values,33 leading to relatively more expensive PCP visits and inexpensive dermatology visits. These findings likely dampened the cost savings resulting from avoided dermatology visits within the TD model. Fourth, past studies have reported that TD is associated with improvements in PCPs’ knowledge of dermatologic diseases,34,35 suggesting that TD may lead to reduced dermatology referrals over time as PCPs become more comfortable managing simple dermatologic problems.
Limitations
Our study has several limitations. First, we included only personnel costs and direct TD costs without accounting for other fixed costs such as rent, supplies, and utilities. We excluded these costs because patient visits and review of TD cases took place within space on the ZSFG campus, which is owned and operated by the city and county of San Francisco. The spaces are similar in capital improvements, no rent is paid, and utilities are not metered separately, with nonpersonnel overhead integrated into the greater hospital expenses.
Second, we did not account for revenue generated from billing. The ZSFG is a federally qualified health center that does not bill fee for service and cares primarily for patients with government insurance (Medi-Cal and Medicare). Health care models that collect significant revenue from fee-for-service billing may have different results, limiting generalizability. Another limitation in generalizability may stem from differences in electronic record systems and the costs of implementing and integrating TD software.
Third, we extrapolated rates of unattended appointments, PCP follow-up, and dermatology follow-up from our study population to the conventional care model because of lack of data. Although we presume that rates of unattended appointments and PCP follow-up within a conventional care model would have been similar to those found within the TD model, it is possible that conventional care would yield an increased relative proportion of simple problems that could be managed with a single dermatology clinic visit. Therefore, the extrapolated data may overestimate dermatology follow-ups in the conventional care model, thus overvaluing the cost of the conventional model.
Fourth, nonattendance at appointments within our health care system incurred no direct cost. We attributed no cost to missed appointments because the dermatology clinic at the ZSFG intentionally overbooks appointment slots with the assumption that some patients will not attend. This assumption may not be applicable to fee-for-service care models that would lose potential revenue.
Fifth, we lacked data analyzing clinical outcomes among the ZSFG dermatology patients before and after TD implementation. Therefore, we operated under the assumption that the clinical outcomes would be equivalent in both models based on previous studies demonstrating that TD produced equivalent23,36,37,38,39,40 or improved41 clinical outcomes among dermatology patients.
Sixth, within the conventional care model, we assumed that all patients attending a PCP visit for a dermatologic symptom would be referred to a dermatologist. A previous analysis found that PCPs would have independently managed 60% of the consultations they sent via TD if operating within a traditional care model.15 The study results have not been validated in other systems, and we were unable to conduct a similar analysis without introducing potential recall bias.
Conclusions
Implementation of TD at the ZSFG was associated with a significant reduction in the mean cost of managing patients referred to the dermatology department. Therefore, TD has the potential to produce cost savings when applied in closed health care systems. Areas of future investigation include analyzing TD’s impact on clinician workload, clinical outcomes, and emergency department visits.
eTable. Cost Breakdown per Patient Visit
eFigure 1. Sensitivity Analysis of Dermatology Visit Cost
eFigure 2. Sensitivity Analysis of PCP Visit Cost
eFigure 3. Sensitivity Analysis of TD Triage Rates to Dermatology Visit
References
- 1.Bezalel S, Fabri P, Park HS. Implementation of store-and-forward teledermatology and its associated effect on patient access in a Veterans Affairs dermatology clinic. JAMA Dermatol. 2015;151(5):556-557. doi: 10.1001/jamadermatol.2014.5272 [DOI] [PubMed] [Google Scholar]
- 2.Carter ZA, Goldman S, Anderson K, et al. Creation of an internal teledermatology store-and-forward system in an existing electronic health record: a pilot study in a safety-net public health and hospital system. JAMA Dermatol. 2017;153(7):644-650. doi: 10.1001/jamadermatol.2017.0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chansky PB, Simpson CL, Lipoff JB. Implementation of a teletriage system improves access to dermatologic care in an underserved clinic: a retrospective review. J Investig Dermatol. 2017;137(5)(suppl_1):S59. doi: 10.1016/j.jid.2017.02.359 [DOI] [PubMed] [Google Scholar]
- 4.McGoey ST, Oakley A, Rademaker M. Waikato teledermatology: a pilot project for improving access in New Zealand. J Telemed Telecare. 2015;21(7):414-419. doi: 10.1177/1357633X15583216 [DOI] [PubMed] [Google Scholar]
- 5.Whited JD, Hall RP, Foy ME, et al. Teledermatology’s impact on time to intervention among referrals to a dermatology consult service. Telemed J E Health. 2002;8(3):313-321. doi: 10.1089/15305620260353207 [DOI] [PubMed] [Google Scholar]
- 6.Zakaria A, Maurer T, Su G, Amerson E. Impact of teledermatology on the accessibility and efficiency of dermatology care in an urban safety-net hospital: a pre-post analysis. J Am Acad Dermatol. 2019;81(6):1446-1452. doi: 10.1016/j.jaad.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 7.Whited JD. Teledermatology. Med Clin North Am. 2015;99(6):1365-1379, xiv. doi: 10.1016/j.mcna.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 8.Leavitt ER, Kessler S, Pun S, et al. Teledermatology as a tool to improve access to care for medically underserved populations: a retrospective descriptive study. J Am Acad Dermatol. 2016;75(6):1259-1261. doi: 10.1016/j.jaad.2016.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landow SM, Mateus A, Korgavkar K, Nightingale D, Weinstock MA. Teledermatology: key factors associated with reducing face-to-face dermatology visits. J Am Acad Dermatol. 2014;71(3):570-576. doi: 10.1016/j.jaad.2014.02.021 [DOI] [PubMed] [Google Scholar]
- 10.Campagna M, Naka F, Lu J. Teledermatology: an updated overview of clinical applications and reimbursement policies. Int J Womens Dermatol. 2017;3(3):176-179. doi: 10.1016/j.ijwd.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osei-tutu A, Shih T, Rosen A, et al. Mobile teledermatology in Ghana: sending and answering consults via mobile platform. J Am Acad Dermatol. 2013;69(2):e90-e91. doi: 10.1016/j.jaad.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 12.Ford JA, Pereira A. Does teledermatology reduces secondary care referrals and is it acceptable to patients and doctors?: a service evaluation. J Eval Clin Pract. 2015;21(4):710-716. doi: 10.1111/jep.12373 [DOI] [PubMed] [Google Scholar]
- 13.Weinstock MA, Nguyen FQ, Risica PM. Patient and referring provider satisfaction with teledermatology. J Am Acad Dermatol. 2002;47(1):68-72. doi: 10.1067/mjd.2002.119666 [DOI] [PubMed] [Google Scholar]
- 14.Armstrong AW, Dorer DJ, Lugn NE, Kvedar JC. Economic evaluation of interactive teledermatology compared with conventional care. Telemed J E Health. 2007;13(2):91-99. doi: 10.1089/tmj.2006.0035 [DOI] [PubMed] [Google Scholar]
- 15.Yang X, Barbieri JS, Kovarik CL. Cost analysis of a store-and-forward teledermatology consult system in Philadelphia. J Am Acad Dermatol. 2019;81(3):758-764. doi: 10.1016/j.jaad.2018.09.036 [DOI] [PubMed] [Google Scholar]
- 16.Datta SK, Warshaw EM, Edison KE, et al. Cost and utility analysis of a store-and-forward teledermatology referral system: a randomized clinical trial. JAMA Dermatol. 2015;151(12):1323-1329. doi: 10.1001/jamadermatol.2015.2362 [DOI] [PubMed] [Google Scholar]
- 17.Vidal-Alaball J, Garcia Domingo JL, Garcia Cuyàs F, et al. A cost savings analysis of asynchronous teledermatology compared to face-to-face dermatology in Catalonia. BMC Health Serv Res. 2018;18(1):650. doi: 10.1186/s12913-018-3464-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livingstone J, Solomon J. An assessment of the cost-effectiveness, safety of referral and patient satisfaction of a general practice teledermatology service. London J Prim Care (Abingdon). 2015;7(2):31-35. doi: 10.1080/17571472.2015.11493433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wootton R, Bloomer SE, Corbett R, et al. Multicentre randomised control trial comparing real time teledermatology with conventional outpatient dermatological care: societal cost-benefit analysis. BMJ. 2000;320(7244):1252-1256. doi: 10.1136/bmj.320.7244.1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whited JD. Economic analysis of telemedicine and the teledermatology paradigm. Telemed J E Health. 2010;16(2):223-228. doi: 10.1089/tmj.2009.0100 [DOI] [PubMed] [Google Scholar]
- 21.Whited JD, Datta S, Hall RP, et al. An economic analysis of a store and forward teledermatology consult system. Telemed J E Health. 2003;9(4):351-360. doi: 10.1089/153056203772744671 [DOI] [PubMed] [Google Scholar]
- 22.Eminović N, Dijkgraaf MG, Berghout RM, Prins AH, Bindels PJ, de Keizer NF. A cost minimisation analysis in teledermatology: model-based approach. BMC Health Serv Res. 2010;10(1):251. doi: 10.1186/1472-6963-10-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pak HS, Datta SK, Triplett CA, Lindquist JH, Grambow SC, Whited JD. Cost minimization analysis of a store-and-forward teledermatology consult system. Telemed J E Health. 2009;15(2):160-165. doi: 10.1089/tmj.2008.0083 [DOI] [PubMed] [Google Scholar]
- 24.Reid DS, Weaver LE, Sargeant JM, et al. Telemedicine in Nova Scotia: report of a pilot study. Telemed J. 1998;4(3):249-258. doi: 10.1089/tmj.1.1998.4.249 [DOI] [PubMed] [Google Scholar]
- 25.Snoswell C, Finnane A, Janda M, Soyer HP, Whitty JA. Cost-effectiveness of store-and-forward teledermatology: a systematic review. JAMA Dermatol. 2016;152(6):702-708. doi: 10.1001/jamadermatol.2016.0525 [DOI] [PubMed] [Google Scholar]
- 26.Lamminen H, Lamminen J, Ruohonen K, Uusitalo H. A cost study of teleconsultation for primary-care ophthalmology and dermatology. J Telemed Telecare. 2001;7(3):167-173. doi: 10.1258/1357633011936336 [DOI] [PubMed] [Google Scholar]
- 27.Loane MA, Oakley A, Rademaker M, et al. A cost-minimization analysis of the societal costs of realtime teledermatology compared with conventional care: results from a randomized controlled trial in New Zealand. J Telemed Telecare. 2001;7(4):233-238. doi: 10.1258/1357633011936453 [DOI] [PubMed] [Google Scholar]
- 28.Lee JJ, English JC III. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19(2):253-260. doi: 10.1007/s40257-017-0317-6 [DOI] [PubMed] [Google Scholar]
- 29.de la Torre-Díez I, López-Coronado M, Vaca C, Aguado JS, de Castro C. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: a systematic review. Telemed J E Health. 2015;21(2):81-85. doi: 10.1089/tmj.2014.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang RH, Barbieri JS, Nguyen HP, et al. ; Group for Research of Policy Dynamics in Dermatology . Clinical effectiveness and cost-effectiveness of teledermatology: where are we now, and what are the barriers to adoption? J Am Acad Dermatol. 2020;83(1):299-307. doi: 10.1016/j.jaad.2020.01.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UCSF Office of Budget and Resource Management. Rates. Updated June 29, 2020. Accessed October 16, 2020. https://brm.ucsf.edu/cbr/rates
- 32.Byamba K, Syed-Abdul S, García-Romero M, et al. Mobile teledermatology for a prompter and more efficient dermatological care in rural Mongolia. Br J Dermatol. 2015;173(1):265-267. doi: 10.1111/bjd.13607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medscape. Medscape physician compensation report 2017. Accessed March 18, 2020. https://www.medscape.com/sites/public/physician-comp/2017
- 34.Barbieri JS, Nelson CA, Bream KD, Kovarik CL. Primary care providers’ perceptions of mobile store-and-forward teledermatology. Dermatol Online J. 2015;21(8):13030/qt2jt0h05w. [PubMed] [Google Scholar]
- 35.Mohan GC, Molina GE, Stavert R. Store and forward teledermatology improves dermatology knowledge among referring primary care providers: a survey-based cohort study. J Am Acad Dermatol. 2018;79(5):960-961. doi: 10.1016/j.jaad.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 36.Pak H, Triplett CA, Lindquist JH, Grambow SC, Whited JD. Store-and-forward teledermatology results in similar clinical outcomes to conventional clinic-based care. J Telemed Telecare. 2007;13(1):26-30. doi: 10.1258/135763307779701185 [DOI] [PubMed] [Google Scholar]
- 37.Whited JD, Warshaw EM, Kapur K, et al. Clinical course outcomes for store and forward teledermatology versus conventional consultation: a randomized trial. J Telemed Telecare. 2013;19(4):197-204. doi: 10.1177/1357633x13487116 [DOI] [PubMed] [Google Scholar]
- 38.Frühauf J, Kröck S, Quehenberger F, et al. Mobile teledermatology helping patients control high-need acne: a randomized controlled trial. J Eur Acad Dermatol Venereol. 2015;29(5):919-924. doi: 10.1111/jdv.12723 [DOI] [PubMed] [Google Scholar]
- 39.Marcin JP, Nesbitt TS, Cole SL, et al. Changes in diagnosis, treatment, and clinical improvement among patients receiving telemedicine consultations. Telemed J E Health. 2005;11(1):36-43. doi: 10.1089/tmj.2005.11.36 [DOI] [PubMed] [Google Scholar]
- 40.Lamel S, Chambers CJ, Ratnarathorn M, Armstrong AW. Impact of live interactive teledermatology on diagnosis, disease management, and clinical outcomes. Arch Dermatol. 2012;148(1):61-65. doi: 10.1001/archdermatol.2011.1157 [DOI] [PubMed] [Google Scholar]
- 41.Ferrándiz L, Ruiz-de-Casas A, Martin-Gutierrez FJ, et al. Effect of teledermatology on the prognosis of patients with cutaneous melanoma. Arch Dermatol. 2012;148(9):1025-1028. doi: 10.1001/archdermatol.2012.778 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Cost Breakdown per Patient Visit
eFigure 1. Sensitivity Analysis of Dermatology Visit Cost
eFigure 2. Sensitivity Analysis of PCP Visit Cost
eFigure 3. Sensitivity Analysis of TD Triage Rates to Dermatology Visit