Graphical abstract
Keywords: SARS-COV-2, Pathophysiology, Immune response, Diagnostics, Vaccine development.
Abstract
Coronaviruses are a group of enveloped RNA viruses that are diversely found in humans and now declared a global pandemic by the World Health Organization in March 2020. The population's susceptibility to these highly pathogenic coronaviruses has contributed to large outbreaks, evolved into public health events, and rapidly transmitted globally. Thus, there is an urgent need to develop effective therapies and vaccines against this disease. In the primary stage of severe acute respiratory syndrome coronavirus (SARS-COV-2) infection, the signs and symptoms are nonspecific, and many more cases have been observed than initially expected. Genome sequencing is performed regularly to identify genetic changes to SARS-COV-2, and vaccine development is focused on manufacture, production, and based on specific problems, and very few are available on recent developments in the prevention of outbreaks. The aim of this review article to explore recent updates on SARS-COV-2 in the context of pathogenesis during disease progression, and innate acquired mechanisms of defense, This includes advances in diagnostics, susceptibility, and severity of host-virus genome interactions, modes of transmission, active compounds being used in pre-clinical and clinical trials for the treatment of patients, vaccine developments, and the effectiveness of SARS-COV-2 prevention and control measures. We have summarized the importance of pathophysiology immune response, Diagnostics, vaccine development currently approaches explored for SARS-COV-2.
1. Introduction
The highly contagious and pathogenic novel SARS-CoV-2, causative agent ongoing COVID-19 pandemic, has spread rapidly and posed a health threat of unprecedented magnitude on the global population. This group includes more than 100 viruses commonly found in various animal species, including bats, pangolins, horses, cats, cattle, camels, goats, and humans [1], [2], [3]. Coronaviruses are classified in order Nidovirales, family Coronaviridae, and positive-sense, single-stranded RNA (ssRNA) viruses with a large genome size of approximately 30 kb. There are seven types of coronavirus found in humans, belonging to “alpha and beta” groups, which frequently mutate and show cross-species transmission. Like the other two highly pathogenic coronaviruses SARS-CoV and MERS-CoV, SARS-CoV-2 also caused severe respiratory illness and even death. The first human coronavirus (alpha-HCoV-229E) was identified in the 1960 s and caused an upper respiratory tract infection, like the common cold [4], [5]. Most people are infected with one or more types of viruses. Rarely, coronaviruses infect animals and become new zoonoses, which can infect people and spread in the human population [5]. A new alpha human coronavirus was discovered in 2003, which was associated with pneumonia, rhinorrhea, and laryngotracheobronchitis in children, particularly in immune-compromised individuals. The beta coronaviruses (type 2) are categorized into lineages A, B, C, and D. One member of lineage A (HCoV-OC-43) was discovered in 1967, and a second (HCoV-HKU) was identified in 2005, associated with diarrhea, acute rhinitis, and infection of the lower respiratory tract. From lineage B, SARS-CoV was identified in 2002–03 and its mode of transmission was bat to civet and then, via civet, to humans [2], [3], [4], [5]. A second highly transmissible virus from lineage B was discovered in December 2019 in Wuhan, China, and now known as SARS-CoV-2. Another coronavirus of interest belongs to lineage C and caused an outbreak of Middle East Respiratory Syndrome (MERS) in 2012, from camels to humans [6].
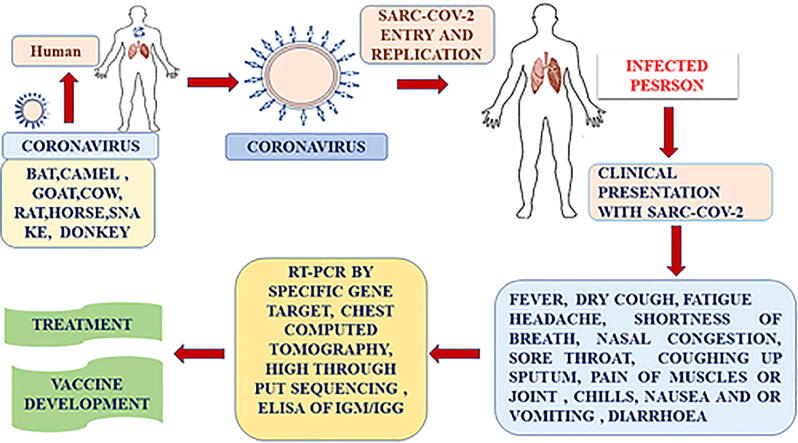
In 2019 in Wuhan, China there were several unusual cases of pneumonia, presenting with a dry cough, dyspnea, fever, and lung tissue damage [3]. The source of many of these cases was recorded as being the Wuhan wild animal and seafood market [7]. On 12th January 2020, China shared the genetic sequence of the infectious agent. China reported virus originated from wild bats and was similar to SARS, hence the infectious agent became known as SARS-CoV-2 at the end of January [8] and declared an emergency a pandemic (global outbreak) of disease [1], [9]. SARS-CoV-2 is highly transmissible and 15,296,926 cases and 628,903 deaths from SARS-CoV-2 were recorded in over 200 counties on 24th July 2020. We compared these figures with other coronavirus outbreaks, such as SARS in 2003, where the number of infected persons was approximately 8000, with a 9.5% case fatality rate. MERS in 2012 had approximately 2,500 cases, with a mortality rate of approximately 35% [10], [11]. The virus targets the respiratory system and its transmission is by contact, droplets, and fomites from another infected person who may be symptomatic or asymptomatic [12]. The incubation period is around 2 to 14 days [9]. The main symptoms are dry cough, fever, sore throat, and shortness of breath, leading to pneumonia and acute respiratory distress (ARDS), which may require intensive care as shown in Fig. 1 [13]. Overall, the mortality rate is approximately 3%, and increases with age, over 60. The mortality rate is also higher in people with diabetes, heart disease, and kidney disease [14]. In COVID-19 patients, a characteristic feature is a lymphocytopenia and CT chest scans show ground glass-like features, indicative of viral pneumonia [15], [16]. Diagnosis of SARS-CoV-2 is made with real-time PCR, by identifying the RNA load via nasal swab (NS) and throat swab (TS), and/or by X-ray and CT scans [15]. The main treatments are supportive, such as antivirals, antimalarials, steroids, and antibiotics [17]. At present, no approved treatments or vaccines are available against SARS-CoV-2 as shown in Fig. 1. However, randomized multicentric clinical trials are under way to look for treatment and vaccine options.
Fig. 1.
Flow Diagram of transmission, diagnosis, clinical presentation, and treatment for COVOD-19.
1.1. Pathogenesis during the progression of SARS-COV-2 infection
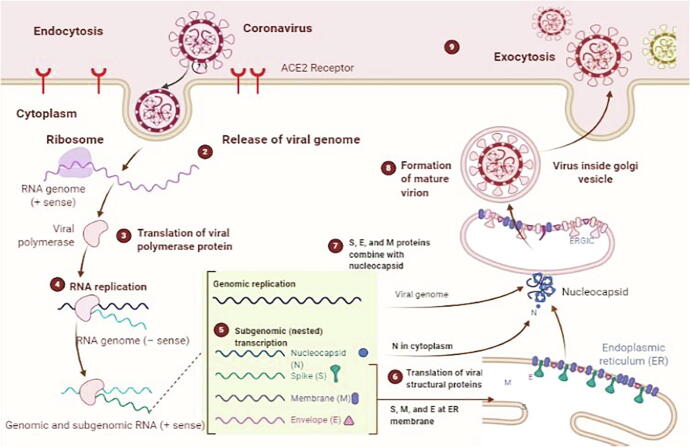
MERS and SARS share the same mechanism as SARS-CoV-2. Spike protein (S2) binds to the Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1) receptor protein, which is expressed in epithelial cells [18]. The virus enters the host cells via receptor-mediated endocytosis and is uncoated, releasing RNA genome into the cytoplasm see human SARS-COV-2 life cycle as shown in Fig. 2 [15]. Spike protein also has another function; binds to ACE2, causing down-regulation, which leads, eventually, to lung injury [19]. The liver produces an inactive form of angiotensin, which circulates in the blood in response to the enzyme renin released by the kidney, and which converts angiotensin to angiotensin-I [13], [14]. This circulates in blood in an inactive form and converted to angiotensin-II by an angiotensin-converting enzyme (ACE or ACE1) [20]. This enzyme maintains blood pressure and mediates tissue injury via a series of non-hemodynamic effects [21]. ACE2 would normally cleave angiotensin-II, as part of a negative feedback loop. tTherefore, if ACE2 is downregulated, this would increase blood pressure in SARS-COV-2 patients. Furthermore, angiotensin-II can bind to receptors in the lungs, called type 1a angiotensin-II receptors (AGTR1A), which increases the permeability of the lungs. Here, a problem arises when the downregulation of ACE2 simultaneously upregulates ACE1, due to negative feedback [22], [23], [24], and this leads to excessive angiotensin-II. This binds to the AGTR1A receptors, which then causes excessive vascular pulmonary activity, the pathology observed in lung tissue associated with pulmonary destruction [22], [23], [24].
Fig. 2.
Life Cycle of Highly Pathogenic Human Coronaviruses. the figure was created using BioRender (https://biorender.com/).
1.2. Innate and acquired mechanisms of defense against SARS-CoV-2
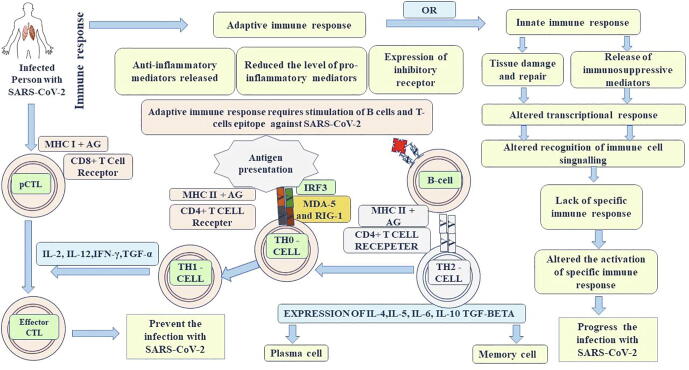
The entry of immune cells into the lungs to fight SARS-CoV-2 leads to ARDS [25], [26] and it's associated with the upregulation of pro-inflammatory cytokines and chemokines shown in Fig. 3 [27]. In SARS-CoV-2 infected patients, IL-1β, IL-16, CXC chemokines, and ligand 2 (CCL2) levels are high [28]. Individuals recovred from SARS-CoV-2 have shown an early expression of interferons IFN-α, IFN-γ, CXCL-10, and CCL2 [29]. IFN-α is an antiviral protein released by infected cells and serves as a signal to produce other anti-viral proteins, so that nearby host cells are more protected. It activates other immune cells, such as macrophages [29]. IFN-γ is a part of the immune system that destroys particular cells that are infected with SARS-CoV-2 [30]. Chemo attractants such as CXCL-10 and CCL2 are released to guide other cells to destroy infected cells [31], [32]. The adaptive immune response is known to be vital to survival, severe SARS-CoV-2 infection is related to a failure to switch from an innate immune response to an adaptive immune response. Infected people lose their memory cells, effector cells, or enough antibodies to reduce the viral load, unbalancing the host-pathogen interaction [33]. NF-kB an important pro-inflammatory cytokine that regulates some genes encoding other cytokines and chemokines as shown in Fig. 3 [34] that are released by cells and which trigger an immune response against the infection. Nucleocapsid proteins (N-proteins) of SARS-CoV-2 bind to viral RNA, as part of the assembly process, and this may be prevented by host proteins such as MDAS and RIG-1 [35]. These proteins detect viral RNA and trigger cell destruction [36] The EB protein of SARS-CoV-2 inhibited by MAD-5 and RIG-1. If MAD-5 and RIG-1 proteins can function, they activate the mitochondrial surface protein MAVS, and this protein activates E3 ubiquitin ligases, leading to the production of NF-kB, which enters the nucleus and activates pro-inflammatory cytokines [37]. The E3 ubiquitin ligases also activate IRF-3 and IRF-7 transcription factors, which enter the nucleus and upregulate interferon α, γ, and activate immune cells (Fig. 3) [3], [38]. However, the M−protein of SARS-CoV-2 inhibits the phosphorylation of IRF-3 and IRF-7 [36]. Therefore, these transcription factors cannot go to the nucleus and cannot up-regulate the interferon at transcription level [3]. The PLPro protein of SARS-CoV-2 is capable of inhibiting the activation of NF-kB, therefore no response in the nucleus and upregulation of pro-inflammatory proteins. In some cases, the production of sufficient cytokines decreases the number of interferons [35], [39], [40], [41].
Fig. 3.
Immune response the SARS-Co-V2 infection.
1.3. Advances in the diagnosis of SARS-COV-2
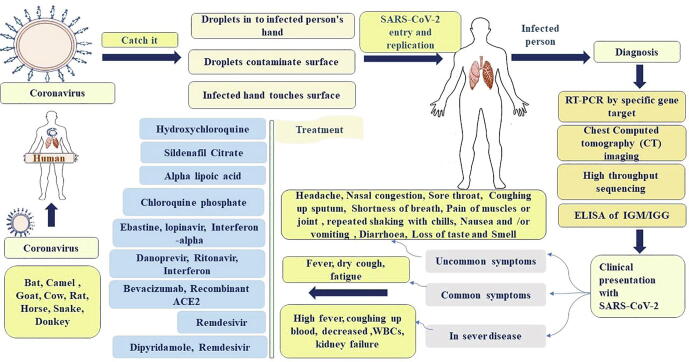
The gold standard for diagnosis is RT-PCR, and the Centre for Disease Control and Prevention (CDC) has developed an RT-PCR kit based on a specific target sequence as shown in Fig. 1 [42], [43]. Various countries have developed their RT-PCR kits, such as Germany (targeting RdRP, E, and N genes), China (targeting ORF1ab and N), Hong Kong (targeting nsp14 and N genes), Japan (targeting multiple proteins), and Thailand (targeting the N gene). Various diagnostic assays are being developed to detect the virus. Some kits are only able to detect one strain of SARS-CoV-2, and some are sensitive to any SARS agent. The specificity of RT-PCR depends on the expertise of individuals, and samples must be taken from the lower or upper respiratory tract. Moreover, single negative samples do not rule out MERS-CoV infection [15], [17]
RT-PCR can often miss the identification together and has some time less sensitivity senstive for acute infection [42]. Tested sample, approximately 3% of those who have negative PCR results have SARS-CoV-2. There could be reasons, for this, perhaps early at the pandemic not enough virus samples for detection and the diagnostic kits were not used correctly [44]. The RT-PCR test may improve over time and suggested, meanwhile, that chest CT is used to identify patients for further testing, which will help to inform isolation and treatment as shown in Fig. 1 [45], [46]. CT scan is also good technology to screening for testing but due to poor access to laboratory testing, or in a situation where centralized public health workers are overstretched [47]. In patients with SARS-CoV-2 symptoms, such as fever, cough, and shortness of breath, clinicians often carry out chest X-rays, which are relatively cheap and easy.
The most common abnormal finding that of ground-glass opacities, portions of the lungs show up on X-ray images as a hazy shade of gray, like frosted glass, instead of being black [48], [49]. It is important to know that chest X-rays are not very sensitive to SARS-CoV-2. Patients who are highly suspected of having SARS-COV-2 viral pneumonia should probably receive chest CT, even if their chest X-ray is negative [27], [50]. Chest CT provides a more detailed view, with the most common finding, again, being ground-glass opacities scattered throughout the lungs [51], [52]. These represent tiny air sacs or alveoli that are filled with fluid and that turn a shade of grey on the CT scan. In severe and advanced infections, more fluid accumulates in the lobes of the lungs, and the appearance progresses to a solid, white consolidation and finally a crazy-paving pattern, with swelling of the interstitial space along the walls of the lung lobules [53], [54]. A chest X-ray is informative but not very sensitive or specific to SARS-CoV-2; therefore, individuals should undergo RT-PCR [54], [55]. Many countries have been isolating patients with classic CT findings until laboratory tests help to identify the cause of disease. It is also important to evaluate each patient, based on the severity of the disease [55]. Individuals can be asymptomatic or have a very mild form of the disease; the sensitivity of a chest CT falls to about 50% in the first 48 h of symptoms or with individuals with the mild disease [51]. ELISA was used to detect antibodies to MERS-CoV for earlier detection, which is based on the N and S proteins of MERS-Co-V. Micro-neutralization is used to detect specific antibodies in the serum sample, this test is considered more sensitive and specific for the analysis of neutralizing antibodies. The presence of antibodies indicates that the immune response has developed in the infected person, but serology tests are recommended for observation purposes only, not for a diagnosis.
1.4. Case classification and clinical complications with SARS-CoV-2 infection
The new CDC and WHO list of possible SARS-CoV-2 symptoms includes fever, cough, difficulty in breathing, repeated shaking with chills, muscle pain, headache, sore throat, and loss of taste and smell as shown in Fig. 1. In a few cases, patients experience extreme fatigue, diarrhea, and fever 37.8 °C or above [3], [9], [10], [11], [13], [56]. Symptoms usually occur 2–14 days after exposure to the virus, but may also be observed for up to 28 days. Not all individuals with SARS-CoV-2 show all symptoms and these range from mild to severe [56]. The test result showed viral nucleic acid declared as a positive SARS-CoV-2 test [14], [15], [16]. Suspected cases are those with symptoms who have been exposed to infected cases [13]. Clinically diagnosed cases are those having symptoms, prior exposure, and the presence of a lung imaging feature consisting of coronavirus pneumonia [34], [46]. Asymptomatic cases are free from symptoms but test positive for viral nucleic acid. SARS-CoV-2 infection is classified into three categories mild, severe, and critical. Mild cases have either no sign of pneumonia or mild pneumonia [27]. Severe cases show problems of oxygenation, breathing, and characteristic radiological findings on a CT-Scan [57], [58]. In severe cases, breathing problems are classified as dyspnea, and patients have a respiratory frequency (RF) > 30/Min [12], [14], [55], [58]. Severity also classified as an oxygen saturation < 93% and PF ratio < 300 [55]. The illness is considered to be severe if lung infiltration or pneumonia occupying more than 50% of their lung field and is seen on a chest radiograph or CT-scan [55], [59]. In critical cases, acute respiratory distress syndrome (ARDS) is a major clinical complication of SARS-CoV-2 infection [60]. This syndrome is characterized by hypoxic respiratory failure and bilateral infiltration due to diffuse alveolar damage [61]. This damage can cause respiratory failure, which is associated with V/Q mismatch and pulmonary shunting [62], [63]. Large immune-mediated cytokine cascades cause lung alveolar damage, patients are unable to successfully ventilate, and develop respiratory failure [64]. Septic shock causes vasodilation and leads to multiple organ dysfunction, especially in the kidney. Some SARS-CoV-2 patients develop renal failure, in addition to respiratory failure, and may require life support [65]. Patients who develop these complications with SARS-COV-2 have much higher mortality rates [66]. The overall case fatality rate in the SARS-CoV-2 pandemic near about 3.5%, but in elderly patients, this rises to 14%. Fatality is higher for patients with cardiovascular disease, diabetes , chronic respiratory disease , and cancer [67], [68]. Some clinicians have reported the presence of blood clots in young adults (aged < 50 years), which causes serious problems such as heart attack and stroke [69].
1.5. Susceptibility to mutation and severity of host-virus genome interactions
SARS-CoV-2 has mutated into at least 30 different genetic variants. Of these, 19 are novel strains, including six mutations in the spike protein. Different strains have affected different parts of the world. This may lead to potential difficulties in finding a universal method of diagnosis or treatment. Strains from 11 randomly chosen SARS-CoV-2 patients from Hangzhou were isolated and analyzed by deep sequencing [70], [71], [72]. Researchers said that the virus has acquired mutations that are capable of substantially changing its pathogenicity. Some of the most pathogenic strains of the virus are more pathogenic than normal strains [73], [74]. One mutated strain of SARS-CoV-2 has been more lethal across Europe and New York than in other parts of the United States and other countries [75]. The diversity of viral strains under investigation leads to a consideration of the impact of these mutations on the development of vaccines and drugs. Mutations in the virus genome might cause the disease to be more virulent, more severe, or more transmissible [18], [76]. Microbiologists from the Peking School of Life Sciences in Beijing, and the Institute Pasteur of Shanghai, have studied the genome of 103 samples of SARC-CoV-2 [18]. They analyzed the evolutionary relationship between the virus strains and their sequenced genomes. They identified two lineages the milder or less infectious S type and the highly infectious, more virulent, and more severe L type [77]. The genetic difference between S and L lineages is in the receptor-binding domain. The L type is more prevalent (70%) in current cases [78], [79]. The L type was prevalent in the early stages of the outbreak in Wuhan. Later on, the S type became prevalent in China because of lockdown control [80]. The Chinese lockdown successfully closed down the spread by isolating people, and this approach seemed to reduce the number of cases of L type SARS-CoV-2 in Wuhan after January 2020 [79], [80]. If the Chinese lockdown halted the L type, the subsequent pandemic may be of the S type, which is believed to be less virulent [79], [80], [81]. There are also the possibility of infection with both viral types in an individual with SARS-CoV-2. One patient in the US has tested positive for both the S and L types, so it appears that both lineages are persisting in the pandemic [81], [82].
2. Drugs used in pre-clinical and clinical therapy
There is no specific treatment currently available drugs for SARS-CoV-2 [70], [71], [77]. Some drugs are under investigation and being tested as a potential treatment of SARS-COV-2, as shown in Table 1, Table 2. The antiviral agent famvir has shown efficacy against SARS-CoV-2, and clinical trials have been conducted in 70 patients in Shenzhen, Guangdong province [72], [83], [84], [85], [86], [87]. Another drug option is remdesevir, an adenosine analog, which is incorporated into the nascent viral RNA chain and results in premature termination. However, the drug is still in a clinical trial [88], a randomized control trial involving 1063 patients, funded by the US, improved recovery from advanced SARS-CoV-2. Preliminary results indicate that patients treated with remdesevir showed a 31% improvement in recovery time over placebo [88]. Chloroquine widely used as an anti-malarial and as a treatment for autoimmune disease, and also reported as having anti-viral activity, blocking virus infection by increasing the endosomal pH required for virus-cell fusion as well as interference with glycosylation of the cellular receptor of SARS-CoV-2 [89]. In addition to its anti-viral activity, chloroquine has immune-modulating activity, which may synergistically enhance anti-viral effects in vivo. Meanwhile, a clinician from South Korea has reported the use of an anti-HIV drug combination, lopinavir, and ritonavir, to successfully treat SARS-CoV-2 shown in Fig. 1 [90], [91]. Researchers in China have reported full recovery from SARS-CoV-2 their blood plasma for possible use as a new treatment of the virus [87], [92], an approach is known as hyperimmune globin therapy. Corticosteroids should be avoided because of their potential in prolonging viral replication, as previously observed in MERS-CoV patients. These are the treatment options currently available, but all are under trial, as part of an effort to test more than 60 types of potential therapeutic drugs, as shown in Table 2.
Table 1.
List of potential therapeutic option for SARS-COV-2.
| S.N. | Drug | Mechanism | Risk | Remark | References |
|---|---|---|---|---|---|
| 1 | Lopinavir | Lopinavir bind to membrane protein and enzyme for coronavirus replication | Cardiac arrhythmias. Carefulness in patients with liver diseases | Preclinical | [16], [93] |
| 2 | Remdesivir | Inhibit the activity of RNA-dependent RNA polymerases; excise nucleotide analog inhibitors. | Nausea and vomiting | Preclinical | [88] |
| 3 | Chloroquine (Antimalarial) | Viral DNA and RNA polymerase, viral protein, glycosylation, ACE2 cellular receptor inhibition, immunomodulation of cytokine release. | Cardiac arrhythmias, retinal damage, especially, G6PD deficiency, Caution in diabetics | Chloroquine has activity against pneumonia patients with SARS-CoV-2 infection; however, specific data are not available. | [89] |
| 4 | Antimalarial (Hydroxychloroquine) | In vitro activity against SARS-CoV-2, inhibition of viral enzymes or processes such as viral DNA and RNA polymerase; 5ACE2 inhibition. | Diabetics Significant drug interactions | Hydroxychloroquine has activity against SARS-CoV-2. | [93] |
| 5 | Azithromycin | Reducing chemotaxis of neutrophils (PMNs) to the lungs by inhibiting cytokines (i.e., IL-8), inhibition | Risk of cardiac arrhythmias | Reduce the excessive cytokine production associated with respiratory viral infections | [93] |
| 6 | Azithromycin Macrolide Antibacterial | Downregulate inflammatory responses | Blocking the activation of nuclear transcription factors. | Reduce the excessive cytokine production associated with respiratory viral infections | [93] |
| 7 | Tocilizumab | IL-6 Receptor-Inhibiting Monoclonal Antibody | GI perforation, hepatotoxicity | Tocilizumab inhibits IL-6-mediated receptors | [94] |
| 8 | SARS-COV-2 Convalescent Plasma | Contain antibodies to SARS-CoV-2 | SARS-CoV-2 neutralizing antibody titers may be conducted (optimally greater than 1:320) | FDA approval required | [95] |
Table 2.
List of probable drug targets against SARS-CoV-2.
| Targeted viral components | Mechanism of action | Status | References |
|---|---|---|---|
| Viral spike glycoprotein S | |||
| S1 | Monoclonal antibodies against RBD inhibits virus-host cell binding. | Under progress | [97] |
| S2 | HR2P and P1 peptides inhibit fusion of S with a host cell receptor | Under progress | [98] |
| Oligosaccharides (S) | Griffithsin binds to oligosaccharides on S and inhibits virus-host cell binding | Under progress | [99] |
| S | siRNA based dsRNA inhibits the replication of SARS-CoV-2. | Under progress | [100] |
| Viral envelope, membrane, nucleocapsid, and accessory proteins | |||
| E | siRNA based dsRNA inhibits the replication of SARS-CoV-2. | Under progress | [101] |
| Hexamethylene amiloride ion act as ion channel inhibitors for SARS-CoV-2. | Under progress | [102] | |
| M | siRNA based dsRNA, inhibits the replication of SARS-CoV-2. | Under progress | [103] |
| N | siRNA, PJ34, Monoclonal antibodies | Under progress | [104] |
| Accessory proteins | siRNA – dsRNA act as ORF protein inhibitors, SARS-CoV-2. | Under progress | [17] |
| Viral enzymes-based inhibiters | |||
| PLpro | GRL0617, Inhibitors of PLpro activity (Optimal activty) | Under progress | [105] |
| 3CLpro | Lopinavir, Inhibitors of 3CLpro activity; Broad spectrum effective in SARS-CoV-2. patients in non-randomized trials | Under progress | [10] |
| RdRp | Ribavirin, inhibits viral RNA synthesis found effective SARS-CoV-2.; dose need to optimize | Under progress | [106] |
| BCX4430 | Based on analogue to adenosine and inhibit viral RNA polymerase activity; Broad spectrum | Under progress | [107] |
| RdRp | Analogues of acyclovir inhibit RdRp, effective against MERS–CoV and HCoV-NL63 • Further investigations required. | Under progress | [108] |
| Helicase | 5-Hydroxychromone derivatives disable the function of helicase | Under progress | [109] |
| SSYA10-001 and ADKs, disable the function of helicase Broad spectrum, effective against SARS-CoV-2. | Under progress | [110] | |
| Angiotensin converting enzyme | Benazepril (Lotensin), perindopril (Aceon), quinapril (Accupril), trandolapril (Mavik) Angiotensin -converting enzyme inhibitors and angiotensin-II receptor Blocker | Under progress | [111] |
3. Vaccine development for SARS-CoV-2: hope against hope
Scientists are designing vaccines at various laboratories and institutions around the world as shown in Table 2. Vaccines are made using a highly conserved part of the viral genome, and more than 90 vaccines are under progress against SARS-CoV-2. Some technologies are being used that have never been used before for developing licensed vaccines. Other research groups have begun clinical trials on humans, while others are testing in animal models [96]. Potential vaccines needed to be work for all strains and any mutated variants, and likely to be very challenging to find a single vaccine that works for all [88]. The aim of vaccine development to expose the body to the antigen, without causing the disease. However, antigens will still cause an immune response that can block or kill the virus, if persons become infected later.
One challenge that the antibody response against S and L is slightly different [112], and L type vaccine induces slightly different antibodies, it is unclear if this will confer cross-immunity to S type and vice versa [83]. In the context of the current waves of infection, unclear whether becoming co-infected with both L and S types at the same time leads to more serious illness [113]. SARS-CoV-2 are RNA-based viruses with a 3.4 kbp genome and a couple of changes in the genome of this virus [96], [113]. Mutations have been observed in S2 receptors, which are proteins responsible for the infection of host cells. Unfortunately, these viruses are more transmissible and provided challenges to vaccine development, and also need more study in this field [10].
There are several types of SARS-CoV-2 vaccine currently under trial as shown in Table 3, based on genome. Seven research groups are working on inactivated vaccines of SARS-CoV-2 [164]. Twenty-five teams are developing viral vector vaccines, where the virus is genetically altered to induce the body to produce viral proteins. Some viral vector vaccines can destroy replicates inside the host cell, while others prevent replication due to the inactivation of viral genes. Almost 20 groups use the genetic sequences for SARS-CoV-2 proteins, which induce the immune response. Once these genetic instructions (RNA/DNA) are inserted into human cells, the host cells produce viral proteins. The viral spike protein is the main target of RNA/DNA vaccines. Another type of vaccine involves injecting SARS-CoV-2 proteins directly into the body. They can also use small fragments of protein (peptides) or the isolated outer-shell protein of SARS-CoV-2, such proteins can induce an immune response without damaging cells. Germany and the United Kingdom have approved their first two SARS-CoV-2 vaccines. The Paul Ehrlich Institute (PEI) approved for the first clinical trial of RNA-based BNT162b1 vaccine, developed with the use of a lipid nanoparticle, non-viral gene delivery system. The cell then transcribes and translates this genetic information into proteins, which generates an immune response. A team of research scientists working on three other, similar, mRNA vaccines. Similarly, Oxford also launching a trial of the chadOx1 nCoV-19 vaccine, which is based on chimpanzee adenovirus, which includes the spike or S protein on the surface of SARS-CoV-2. The mRNA vaccine candidate developed by Imperial College is being tested in an animal model.
Table 3.
List of SARS-CoV-2 Vaccine under clinical trial.
| S.N. | Vaccine development | Product category of vaccine | Type of candidate vaccine description | Current status- | Developer | References |
|---|---|---|---|---|---|---|
| 1 | INO-4800 | DNA based | DNA plasmid delivered by electroporation targeted for S1-RBD-protein | Phase-II and III | novio Pharmaceuticals, CEPI, Korea National Institute of Health, International Vaccine Institute (IVI); Thermo Fisher Scientific; Beijing Advaccine Biotechnology; Richter-Helm BioLogic | [114] |
| 2 | BNT162 (a1, b1, b2, c2) | RNA Based | S1-RBD-protein targeted | Phase-II and III | (BioNTech, Fosun Pharma,Pfizer); Rentschler ; Biopharma ; Fosun Pharma | [115] |
| 3 | SARS-CoV-2/aAPC | lentiviral vector | lentiviral vector, pathogen-specific artificial antigen-presenting dendritic cells targeted spike (S) glycoprotein | Phase I | (Shenzhen Geno-Immune Medical Institute) | [116] |
| 4 | LV-SMENP-DC | lentiviral vector | lentiviral minigene vaccine, dendritic cells modified with lentiviral vector and based on multiple viral antigens | Phase I | (Shenzhen Geno-Immune Medical Institute) | [117] |
| 5 | bacTRL-Spike | DNA based | DNA; bacTRL-targeted on Spike protein | Phase I | (Symvivo Corporation, University of British Columbia, Dalhousie University) | [118] |
| 6 | Ad5-nCoV | Non-replicating viral vector | Recombinant adenovirus type 5 vector targeted on Spike protein | Phase-III | (CanSino Biologics,Institute of Biotechnology of the Academy of Military Medical Sciences); Beijing Institute of Biotechnology; Canada's National Research Council; Petrovax | [43] |
| 7 | ChAdOx1 nCoV-19 | Adenoviral vector | ChAdOx1 is a replication-deficient simian adenoviral vector derived from isolate Y25 targeted on Spike protein | Phase-III | United Kingdom | [119] |
| 8 | mRNA-1273 | RNA Vaccine | lipid nanoparticle dispersion containing messenger RNA targeted on Spike protein | Phase I | (Moderna, US National Institute of Allergy and Infectious Diseases) | [120] |
| 9 | Ad5-nCoV | Spike protein | recombinant adenovirus type 5 vector | Phase I | (CanSino Biologics, Institute of Biotechnology of the Academy of Military Medical Sciences) | [121] |
| 10 | Ad5-nCoV | Non-Replicating | Non-Replicating Viral Vector 2nd Gen E2b- Ad5 Spike, RBD | Phase 2 ChiCTR2000031781 Phase 1 ChiCTR2000030906 | Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China; CanSino Biological Inc./Beijing Institute of Biotechnology | [122] |
| 11 | DNA plasmid vaccine Electroporation device | DNA | DNA plasmid. Vaccine encoding spike (S) protein delivered by Electroporation | Phase 1 NCT04336410 | Inovio Pharmaceuticals | [115] |
| 12 | d TBD | Inactivated | Live Attenuated Virus Deoptimized | Preclinical | Osaka University/ BIKEN/ NIBIOHN | [123] |
| 13 | Dendritic cell-based vaccine | Non-replicating | Non-replicating viral vector; dendritic cell-based vaccine | Pre-clinical | Vaxart / Emergent BioSolutions | [124] |
| 14 | Parainfluenza virus 5 | Non-replicating viral vector | parainfluenza virus 5 (PIV5)-based vaccine expressing the spike protein | Pre-clinical | Centro Nacional Biotecnologia (CNB-CSIC), Spain | 130) |
| 15 | Dendritic cell-based vaccine | Virus-like particle | Virus-like particle-based Dendritic Cell (DC)- targeting vaccine | Pre-clinical | University of Manitoba | [125] |
| 16 | GX-19 | DNA | DNA Vaccine expressing SARC-CoV-2 S-protein | Pre-Clinical | Genexine Consortium | [126] |
| 17 | DNA with electroporation | DNA Vaccine | Adenovirus-vectored vaccine (ChAdOx1 nCoV-19) expressing the SARS-CoV-2 spike protein | Pre-Clinical | Karolinska Institute / Cobra Biologics (OPENCORONA Project) | [127] |
| 18 | Recombinant SARS CoV-2 glycoprotein nanoparticle vaccine | Protein Subunit | Recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M | Phase-I | Novavax | [128] |
| 19 | Recombinant deactivated rabies virus containing S1 | Protein Subunit | Non-replicating viral vector | Pre-clinical | Bharat Biotech/ Thomas Jefferson University (INDIA) | [129] |
| 20 | Adenovirus Type 5 Vector | Replicating viral vector | Ad5-nCoV is a recombinant adenovirus type-5 vector (Ad5) vaccine | Pre-clinical | Zydus Cadila (INDIA) | [130] |
| 21 | ChAdOx1-S | Non-Replicating Viral Vector | The immune response generated against the spike protein | Phase-III | University of Oxford/AstraZeneca | [131] |
| 22 | LNPencapsulated mRNA | RNA | Lipid nanoparticle-encapsulated mRNA (mRNA-LNP) encoding the receptor-binding domain (RBD) | Phase-I | Moderna/NIAID | [132] |
| 23 | Plasmid DNA | DNA | Microneedle array for delivery spike protein based | Pre-Clinical | Immunomic Therapeutics, Inc./EpiVax, Inc./PharmaJet | [133] |
| 24 | NasoVAX | Non-Replicating Viral Vector | adenovirus-based NasoVAX expressing SARS2-CoV spike protein | Pre-Clinical | Altimmune | [134] |
| 25 | Deoptimized live attenuated vaccines | Live Attenuated Virus | Codon deoptimized live attenuated vaccines | Pre-Clinical | Codagenix/Serum Institute of India | [135] |
| 26 | Inactivated + alum | Inactivated viral protein | As viral subunit protein Inactivated | Phase -II | Sinovac | [136] |
| 27 | 3 LNP-mRNAs | RNA based | mRNA vaccines encode antigens | Phase-III | BioNTech/Fosun Pharma/Pfizer | [137] |
| 28 | mRNA | RNA | a vaccine targeting the Spike protein receptor-binding domain | Pre-Clinical | Curevac | [138] |
| 29 | Plant-derived VLP | Virus-like particle | QVLP vaccine induced a substantial and sustained increase of hemagglutinin-specific polyfunctional CD4 T cells, | Phase I | Medicago Inc. | [139] |
| 30 | VLP | VLP | The target antigen on the surface of the virus like particle | Pre-Clinical | ARTES Biotechnology | [140] |
| 31 | Codon deoptimized live attenuated virus | Live attenuated virus | live-attenuated vaccine, it has the potential to stimulate a robust T cell and antibody immune response | Pre-Clinical | Codagenix / Serum Institute of India (INDIA) | [141] |
| 32 | Codon deoptimized live attenuated virus | Live attenuated virus | Live Attenuated Covid-19 vaccine based on codon de-optimized technology. | Pre-Clinical | Indian Immunologicals Ltd/ Griffith University (INDIA) | [142] |
| 33 | VLA2001, Inactivated | Inactivated | Inactivated + CpG 1018, Spike protein receptor-binding domain | Pre-clinical | Valneva/ Dynavax | [143] |
| 34 | Ad5 vector | Non-Replicating Viral Vector | Ad5 adjuvanted Oral Vaccine platform | Pre-Clinical | Vaxart | [144] |
| 35 | protein-based adjuvanted | Protein Subunit | Native like Trimeric subunit Spike Protein vaccine | Pre-Clinical | Clover Biopharmaceuticals Inc./GSK/Dynavax | [145] |
| 36 | Microneedle arrays S1 subunit | Protein Subunit | MNA-MERS-S1 subunit vaccines | Pre-Clinical | Univ. of Pittsburgh | [146] |
| 37 | OMV-based vaccine | Protein subunit | Receptor-binding domain (RBD) in the N-terminal surface subunit (S1), and then employs its C-terminal transmembrane subunit (S2) to fuse with the host cell membrane | Pre-Clinical | BiOMViS Srl/Univ. of Trento | [147] |
| 39 | S protein | Protein Subunit | Protein Subunit S protein (baculovirus production), Ii-Key peptide targeted for S protein | Pre-Clinical | Sanofi Pasteur/GSK | [148] |
| 40 | gp-96 backbone | Protein Subunit | Activates CD8 T cells, antigen presenting | Pre-Clinical | Heat Biologics/Univ. Of Miami | [149] |
| 41 | Peptide vaccine | Protein Subunit | Synthetic Long Peptide Vaccine candidate for S and M proteins | Pre-Clinical | FBRI SRC VB VECTOR,Rospotrebnadzor, Koltsovo | [150] |
| 42 | SARS-COV-2 XWG-03 truncated S | Protein subunit | COVID-19 XWG-03 truncated S (spike) proteins. | Pre-Clinical | Innovax/Xiamen Univ./GSK | [151] |
| 43 | OMV-based vaccine | Protein subunit | Outer Membrane Vesicle (OMV)-subunit | Pre-Clinical | Quadram Institute Biosciences | [152] |
| 44 | Measles Vector | Replicating Viral Vector | Non-Replicating Viral Vector Oral Vaccine platform. (S, N targets). | Pre-Clinical | Institute Pasteur/Themis/Univ. of Pittsburg Center for Vaccine Research/Merck | [153] |
| 45 | Replication competent VSV chimeric virus technology (VSVΔG) | Replicating Viral Vector | Replication competent VSV chimeric virus technology (VSVΔG) delivering the SARS-CoV-2 Spike (S) glycoprotein. | Pre-Clinical | IAVI/Merck | [154] |
| 46 | M2SR | Replicating Viral Vector | M2-deficient single replication (M2SR) influenza vector | Pre-Clinical | UW-Madison/FluGen/Bharat Biotech | [155] |
| 47 | Ii-Key peptide | Protein Subunit | The peptide sequence includes at least one cell-permeable peptide (CPP) domain | Pre-Clinical | Generex/EpiVax | [156] |
| 48 | Molecular clamp stabilized Spike protein | Protein Subunit | Molecular clamp stabilized Spike protein iis a COVID-19 protein-based vaccine | Pre-Clinical | University of Queensland/GSK/Dynavax | [157] |
| 49 | Oral Ad5 S | Non-Replicating Viral Vector | Adenovirus serotype 5 (Ad5)-based vaccine vectors | Pre-Clinical | Stabilitech Biopharma Ltd | [158] |
| 50 | Spike-based | Protein subunit | Protein Subunit RBD-based. | Pre-Clinical | University of Alberta | [159] |
| 51 | Measles Virus | RNA | Live attenuated measles virus (S, N targets) is a COVID-19 viral-vector vaccine (S, N targets) | Pre-Clinical | DZIF - German Center for Infection Research | [160] |
| 52 | Live viral vectored vaccine based on attenuated influenza virus backbone (intranasal) | Replicating Viral Vector | Live viral vectored vaccine based on attenuated influenza virus backbone (intranasal) | Pre-Clinical | BiOCAD and IEM | [161] |
| 53 | Ad26.COV2-S | Non-Replicating Viral Vector | Ad26 (alone or with MVA boost) | Pre-Clinical | Janssen Pharmaceutical Companies | [162] |
| 54 | MVA-S encoded | Non-replicating viral vector | Non-replicating viral vector MVA-S encoded SARS-CoV2; MVA-S encoded | Pre-clinical | DZIF – German Center for Infection Research | [163] |
4. Neutralizing monoclonal antibodies against SARS-CoV-2
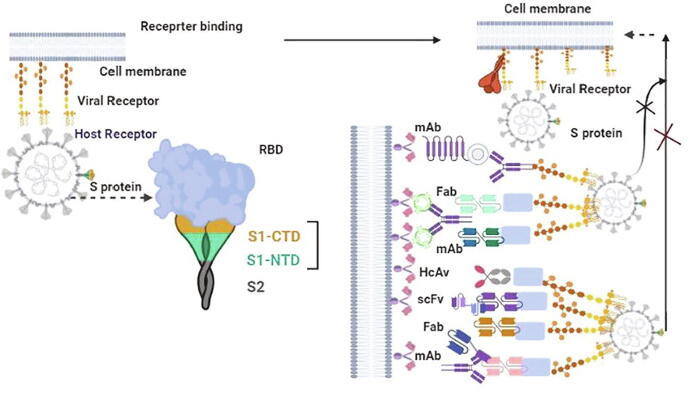
Neutralizing antibodies (Nabs) of coronaviruses tend to target the turmeric spike (S) protein (S1 and S2) and mediate the entry into a host cell. Which is actively involved in the attachment of cell and fusion of the cell membrane via the formation of a six-helix bundle (6-HB) fusion core [164]. The S protein has AA (Amino Acid) that are 77.5% identical and bind to ACE2 on human cells through the S1B domain. S1-RBD and S2 are identified as potential targets for Nabs. To identify antibodies that could potentially neutralize SARS-CoV2. SARS-CoV-2 patents generate immunoglobulin F (IgG) types of antibodies, which is binding to SARS-CoV-2 elements after the onset of disease and blocking SARS-CoV-2 entry of host cells, called neutralizing antibody (NAbs) [164], [165]. Possible targets of nAbs on the S protein of SARS-CoV-2as shown in Fig. 4. Monoclonal antibody (mAb), antigen-binding fragment (Fab), single-chain variable region fragment (scFv), or single-domain antibody binds to the RBD, S1 subunit (non-RBD, including NTD), or S2 of the viral S protein, blocking binding between the RBD and the respective receptor (for RBD-targeting nAbs), interfering with the conformational change of S (for S1-targeting nAbs), or hindering S2- mediated membrane fusion (for S2-targeting nAbs), leading to the inhibition of infection with SARS-CoV-2 in the host cells. Several human monoclonal antibodies (mAbs) have been developed from memory B cells [166], [167]. A researcher from the field developed 51 cell lines from the humanized mice and evaluate the efficacy and 47D11 were found active and targeted against the S1B receptor binding domain of SARS-CoV-2. Pseudo typed lentiviral-based neutralization assay was performed to identify the efficacy of Nabs. The potential of pseudo-virus-neutralizing efficacy suggested that the neutralizing capacity of SARS-CoV-2-specific depends on the magnitude and binding affinity of antibodies [165]. Representative SARS-CoV RBD and MERS-CoV RBD-Targeting nAbs are mentioned in table 4.
Fig. 4.
Potential targets of nAbs against SARS-CoV-2. This figure was created by using BioRender (https://biorender.com/).
Table 4.
Representative SARS-CoV RBD- and MERS-CoV RBD-Targeting nAbs.
| S.N. | Antibody discovery Neutralizing monoclonal antibodies | Palatiform | Epitope clusters | binding affinities | Protective efficacy | IC50 | References |
|---|---|---|---|---|---|---|---|
| 1 | 3F11 | Humanized phage display library | sdAb (a single-domain antibody) llama Animal immunization and sequencing RBD PsV (pseudovirus)neutralization | Blocking RBD–ACE2 | Protect mice against the challenge of SARS | IC50 = 0.14 μg/ml. | [165] |
| 2 | ab1 | B cells of convalescent patients; Single-cell sequencing | Neutralize live SARS-CoV (strain Urbani) infection RBD in the S1. | Blocking RBD–ACE2 | Full protection of mice: 0.3 mg of IgG1 ab1. | Reporter Gene neutralization assay: 200 ng/ml; LV neutralization: ND100 < 400 ng/ml | [167] |
| 3 | CB6 | B cells of convalescent patients | Neutralize live SARS-CoV (strain Urbani) infection RBD in the S1. | Blocking RBD–ACE2 | Protection of rhesus macaques: 50 mg/kg. | PsV neutralization: ND50 = 0.036 μg/ml; LV neutralization: ND50 = 0.036 μg/ml; | [168] |
| 4 | P2C-1F11 | The plasma of convalescing patients | SARS-CoV (strain Urbani) infection RBD | Blocking RBD–ACE2. | Protection of mice. | PsV neutralization: IC50 = 0.03 μg/ml. | [169] |
| 5 | rRBD-15 | A synthetic human Fab antibody library | SARS-CoV (strain Urbani) infection RBD | Blocking RBD–ACE2. | Protection of mice. | PsV neutralization: IC50 = 12.2 nM. | [170] |
| 6 | CC12.1 | B cells of convalescent patients | SARS-CoV infection RBD | Blocking RBD–ACE2. | Full protection of Syrian hamsters: Antibody serum concentration of ~ 22 μg/ml. | PsV neutralization: IC50 = 0.019 μg/ml. | [171] |
| 7 | COVA1-18 | B cells of convalescent patients | SARS-CoV infection RBD | Blocking RBD–ACE2. | Protection of Syrian hamsters: Antibody serum concentration. | PsV neutralization: IC50 = 8 ng/ml | [172] |
| 8 | BD-368–2 | B cells of convalescent patients; Single cell sequencing | Neutralize live SARS-CoV (strain Urbani) infection RBD in the S1. | Blocking RBD–ACE2 RBD in the S1 domain with high affinity. | B cells of convalescent patients; Single cell sequencing | PsV neutralization: IC50 = 1.2 ng/ml; LV neutralization: IC50 = 15 ng/ml | [166] |
| 9 | B38 | Peripheral blood of SARS-CoV-2-infected patients | Neutralize live SARS-CoV (strain Urbani) infection RBD | Blocking RBD–ACE2. | Protection of mice: Lung viral load reduced by 32.8% compared with PBS control | LV (live virus) neutralization: IC50 = 0.177 μg/ml; | [173] |
| 10 | H4 | Peripheral blood of SARS-CoV-2-infected patients | Neutralize live SARS-CoV (strain Urbani) infection RBD | Blocking RBD–ACE2. | Protection of mice: Lung viral load reduced by 26% compared with PBS control. | LV neutralization: IC50 = 0.896 μg/ml; | [173] |
| 11 | 7B11 | Animal immunization; hybridoma technology | Neutralize live SARS-CoV (strain Urbani) infection RBD | Blocking RBD–ACE2. | Protection of mice, Lung viral load reduced by 26% compared with PBS control. | PsV neutralization: IC50 = 10 μg/ml. | [174] |
| 12 | CR3022 | Gene cloning; Protein expression | SARS-CoV infection RBD. | Blocking RBD–ACE2. | LV neutralization: IC50 = ~ 0.114 μg/ml. | [175] | |
| 13 | 4A8 | Peripheral blood of convalescent patients | NTD | PsV neutralization: EC50 = 49 μg/ml | [176] | ||
| 14 | VHH-72-Fc | Animal immunization and sequencing | SARS-CoV (strain Urbani) infection RBD | Blocking RBD–ACE2. | Protection of mice. | PsV neutralization: IC50 ~ 0.2 μg/ml. | [177] |
| 15 | 311mab–31B5 | B cells of convalescent patients | SARS-CoV (strain Urbani) infection RBD | Blocking RBD–ACE2. | Protection of mice. | PsV neutralization: IC50 = 33.8 ng/ml; PsV neutralization: IC50 = 69.8 ng/ml. | [173] |
| 16 | H014 | Animal immunization and phage display | SARS-CoV infection RBD. | Blocking RBD–ACE2. | Protection of mice: Lung viral load reduced by about 10 ~ 100 folds compared with PBS control. | PsV neutralization: IC50 = 3 nM; | [178] |
| 17 | COV2-2196 | Peripheral blood of convalescent patients | SARS-CoV infection RBD. | Blocking RBD–ACE2. | Protection of hamsters: Viral RNA copy numbers and infectious virus titers in lung tissues were reduced by 4 logs or more compared with PBS control. | PsV neutralization: IC50 = 0.7 ng/ml | [179] |
| 18 | COVA2-15 | A synthetic human Fab antibody library | SARS-CoV infection RBD. | Blocking RBD–ACE2. | Protection of mice. | LV neutralization: IC50 = 7 ng/ml. | [172] |
| 19 | REGN10989 | Transgenic mice; Peripheral blood of SARS-CoV-2-infected patients; Next Generation Sequencing | SARS-CoV infection RBD. | Blocking RBD–ACE2. | Protection of mice. | PsV neutralization: IC50 = 7.23 pM. | [180] |
4.1. Effectiveness of SARS-CoV-2 prevention and control procedures
According to the WHO and CDC, hand washing is the most effective procedure to prevent infection. Asymptomatic spreaders may be present in the community, feeling quite well but still able to pass on the virus [45], [72]. It is critical to understand that the asymptomatic spreading of the virus has been shown and that it can be transmitted before symptoms or detection [27]. There are three important concepts: identity, isolation, and information. Once a patient identified, they need to take actions regarding personal hygiene and prevention, such as limiting contact with others as much as possible, staying at home, using available PPE (N-Mask or FFP2-standard masks set by the European Union, gloves, and goggles), and avoid touching the eyes, nose, mouth, and wash hands. Used PPE should be discarded properly to prevent SARS-CoV-2 infection [62]. Hand hygiene should be maintained, such as washing hands for 30 s with an effective soap or hand sanitizer with at least 70% alcohol [181]. High-touch surfaces that may be contaminated with SARS-CoV-2 can also be cleaned, using EPA-approved disinfectants. There is no any disinfectant currently approved by the EPA specifically for SARS-CoV-2, but several disinfectants are available [182]. Current observations suggest that people of all ages are generally susceptible to this new infectious disease. However, those who are in close contact with patients with symptomatic and asymptomatic SARS-CoV-2, including health care workers and other patients in a hospital, are at higher risk of infection [182]. As of February 14, 2020, a total of 1716 health care workers in China were infected with SARS-CoV-2, comprising 3.8% of all patients nationally, 6 of whom have died [182], [183]. A team of research scientists at the Aix-Marseille University, in France, found that some SARS-CoV-2 strains can replicate at 60 °C, and the virus are not completely eliminated until the temperature rises to 90–100 °C.
Peoples with chronic disease may suffer from increased stress due to the coronavirus pandemic because SARS-CoV-2 disease poses higher health risks for them. Stress during the pandemic, including fear, concerns about health and loved ones, changes in routine eating patterns, and difficulty in concentrating, can lead to a worsening of existing health problems, increased use of tobacco, alcohol, and other drugs. Peoples with pre-existing mental health conditions should be aware of worsening caused by the pandemic. Stress can be reduced by taking care of close friends and family, and by helping others to cope, making the community stronger. It will be better to avoid stressful activities that cause anxiety, to keep updated with accurate information, to meditate regularly, try to eat healthy, balanced meals, exercise regularly, sleep more often, and develop an understanding of the actual risk.
5. Conclusion
SARS-CoV-2 continues to increase its death toll as more people become infected. More than 215 countries are reporting new cases, and many struggle to limit their spread. SARS-CoV-2 has origins in mutations to coronavirus strains in animals, such as bats and pangolin. Poorly treated animals are stressed and are responsible for the emergence of new diseases. Wet markets where animals are in close contact with humans are the perfect breeding grounds for new diseases. Coronavirus not only causes respiratory problems, but may also damage the heart, kidneys, liver, and other organs. Half of the SARS-CoV-2 patients have blood or protein in their urine samples, an indication of early kidney damage. SARS-CoV-2 may also cause inflammation of the heart muscles and can cause disturbances in the heart rhythm, ultimately leading to cardiac arrest. The human gastrointestinal tract contains 100 times more ACE2 receptors than the lungs. Research shows that the virus could use these receptors to gain entry into other organs of the body and this might lead to multi-organ failure. The current scenario of the SARS-CoV-2 pandemic proves that the world was not prepared for a disaster on such a scale. In the event of another pandemic in the future, do you believe the world will have learned lessons to prepare for what comes next, such as funding medical research, building better health care systems and infrastructure, and tracking potentially lethal viruses. Researchers are actively working on the characterization of new emerging strains, drug discovery, and the development of SARS-CoV-2 infections. Already, some excellent tools and techniques have been developed for use in the fight against SARS-CoV-2. There are 92 vaccines under development, 45 antibodies, 12 cell-based and, 5 RNA-based therapies, and 52 other therapeutic options are currently being explored to fight the new SARS-CoV-2. Various clinical trials are underway to investigate potential therapies against the SARS-COV-2 pandemic, but no effective treatment is effective available, to date. We hope this work will contribute to protecting and treating people at risk of developing a vaccine for severe SARS-CoV-2 infections.
6. Author’s contribution
D.D.S and D.K.Y. conceived and designed the project and collected data from the literature and write the manuscript. I.H. and E.H.C, support editing the Manuscript. All authors contributed to the interpretation and discussion of the results. All authors read and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
D. D. S. thankful to Amity University Rajasthan, Jaipur, India. D.K.Y. thankful to Gachon Institute of Pharmaceutical Science and the Department of Pharmacy, College of Pharmacy, Gachon University of Medicine and Science, Incheon, Korea for providing the computational modeling facility. We are thankful to Chandni Kumari for support to Manuscript reference and editing. D.K.Y. was thankful to the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology, who supported this study (No. 2017R1C1B2003380). This research was also partially supported by the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (NRF-2016K1A4A3914113).
Contributor Information
Eun-Ha Choi, Email: choipdp@gmail.com.
Dharmendra Kumar Yadav, Email: dharmendra30oct@gmail.com.
References
- 1.V.M. Cucinotta D, WHO Declares COVID-19 a Pandemic, Acta Bio Med 91(1) (2020) 157-160. [DOI] [PMC free article] [PubMed]
- 2.Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci. 2020;16(10):1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1) doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon L.L.M., Peiris M. Emergence of a novel human coronavirus threatening human health. Nat Med. 2020;26(3):317–319. doi: 10.1038/s41591-020-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omrani A.S., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV): animal to human interaction. Pathogens Global Health. 2016;109(8):354–362. doi: 10.1080/20477724.2015.1122852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang S., Du L., Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerg Microbes Infect. 2020;9(1):275–277. doi: 10.1080/22221751.2020.1723441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackenzie J.S., Smith D.W. COVID-19: a novel zoonotic disease caused by a coronavirus from China: what we know and what we don't. Microbiol Australia. 2020;41(1) doi: 10.1071/MA20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 10.Yang X., Yu Y., Xu J., Shu H., Xia J.A., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15) doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raoult D., Zumla A., Locatelli F., Ippolito G., Kroemer G. Coronavirus infections: epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020;4(4):66–75. doi: 10.15698/cst2020.04.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan G. A novel coronavirus capable of lethal human infections: an emerging picture. Virol J. 2013;10(1) doi: 10.1186/1743-422X-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30(8):4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jean S.-S., Lee P.-I., Hsueh P.-R. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim C.H. SARS-CoV-2 Evolutionary Adaptation toward Host Entry and Recognition of Receptor O-Acetyl Sialylation in Virus-Host Interaction. Int J Mol Sci. 2020;21(12):4549. doi: 10.3390/ijms21124549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge X.-Y., Li J.-L., Yang X.-L., Chmura A.A., Zhu G., Epstein J.H. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1) doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demiselle J., Fage N., Radermacher P., Asfar P. Vasopressin and its analogues in shock states: a review. Ann Intensive Care. 2020;10(1) doi: 10.1186/s13613-020-0628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6(1) doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakodkar P., Kaka N., Baig M.N. A comprehensive literature review on the clinical presentation, and management of the pandemic Coronavirus Disease 2019 (COVID-19) Cureus. 2020 doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rico-Mesa J.S., White A., Anderson A.S. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr Cardiol Rep. 2020;22(5) doi: 10.1007/s11886-020-01291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell B, Moss C, George G, Santaolalla A, Cope A, Papa S, Van Hemelrijck M, Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence, ecancermedicalscience 14 (2020). [DOI] [PMC free article] [PubMed]
- 26.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng H., Xiong R., He R., Lin W., Hao B., Zhang L. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J Infection. 2020;81(1):e33–e39. doi: 10.1016/j.jinf.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torres T., Puig L. Managing cutaneous immune-mediated diseases during the COVID-19 pandemic. Am J Clin Dermatol. 2020;21(3):307–311. doi: 10.1007/s40257-020-00514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Alwis R., Chen S., Gan E.S., Ooi E.E. Impact of immune enhancement on Covid-19 polyclonal hyperimmune globulin therapy and vaccine development. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choreño-Parra J.A., Thirunavukkarasu S., Zúñiga J., Khader S.A. The protective and pathogenic roles of CXCL17 in human health and disease: potential in respiratory medicine. Cytokine Growth Factor Rev. 2020;53:53–62. doi: 10.1016/j.cytogfr.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. GeroScience. 2020;42(2):505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizzo P., Vieceli Dalla Sega F., Fortini F., Marracino L., Rapezzi C., Ferrari R. COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Basic Res Cardiol. 2020;115(3) doi: 10.1007/s00395-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerging Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30(4):343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choy E.H., De Benedetti F., Takeuchi T., Hashizume M., John M.R., Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16(6):335–345. doi: 10.1038/s41584-020-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saghazadeh A., Rezaei N. Immune-epidemiological parameters of the novel coronavirus – a perspective. Expert Rev Clin Immunol. 2020;16(5):465–470. doi: 10.1080/1744666X.2020.1750954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felsenstein S., Herbert J.A., McNamara P.S., Hedrich C.M. COVID-19: immunology and treatment options. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerging Microbes Infect. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zolfaghari Emameh R., Nosrati H., Taheri R.A. Combination of biodata mining and computational modelling in identification and characterization of ORF1ab polyprotein of SARS-CoV-2 isolated from oronasopharynx of an Iranian patient. Biol Procedures Online. 2020;22(1) doi: 10.1186/s12575-020-00121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.C.T.N. NCT04334980, Evaluating the Safety, Tolerability and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19, ClicinalTrials.gov (2020).
- 44.Haveri A., Smura T., Kuivanen S., Österlund P., Hepojoki J., Ikonen N. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Eurosurveillance. 2020;25(11) doi: 10.2807/1560-7917.ES.2020.25.11.2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19. Pediatric Infectious Dis J. 2020;39(5):355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu Q., Guan H., Sun Z., Huang L., Chen C., Ai T. Early CT features and temporal lung changes in COVID-19 pneumonia in Wuhan, China. Eur J Radiol. 2020;128 doi: 10.1016/j.ejrad.2020.109017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schweitzer W., Ruder T., Baumeister R., Bolliger S., Thali M., Meixner E. Implications for forensic death investigations from first Swiss post-mortem CT in a case of non-hospital treatment with COVID-19. Forensic Imaging. 2020;21 [Google Scholar]
- 48.Butt C., Gill J., Chun D., Babu B.A. Deep learning system to screen coronavirus disease 2019 pneumonia. Appl Intelligence. 2020:1–7. doi: 10.1007/s10489-020-01714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z., Ding L., Chen G., Zhao C., Luo X., Li X. Clinical Time Features and Chest Imaging of 85 Patients With COVID-19 in Zhuhai, China. Front Med. 2020;7 doi: 10.3389/fmed.2020.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eastman R.T., Roth J.S., Brimacombe K.R., Simeonov A., Shen M., Patnaik S. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6(5):672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan L., Li D., Xue H., Zhang L., Liu Z., Zhang B. Progress and prospect on imaging diagnosis of COVID-19. Chin J Acad Radiol. 2020;3(1):4–13. doi: 10.1007/s42058-020-00031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the Treatment of Covid-19 — preliminary report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ucar F., Korkmaz D. COVIDiagnosis-Net: deep Bayes-SqueezeNet based diagnosis of the coronavirus disease 2019 (COVID-19) from X-ray images. Med Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bao C., Liu X., Zhang H., Li Y., Liu J. Coronavirus Disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am College Radiol. 2020;17(6):701–709. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amanati A., Karimi A., Fahimzad A., Shamshiri A.R., Fallah F., Mahdavi A. Incidence of ventilator-associated pneumonia in critically Ill children undergoing mechanical ventilation in pediatric intensive care unit. Children. 2017;4(7) doi: 10.3390/children4070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prevention C.F.D.C.a. Coronavirus (COVID-19),:2020. https://www.cdc.gov/cdc-info/index.html
- 57.Addie D.D., le Poder S., Burr P., Decaro N., Graham E., Hofmann-Lehmann R. Utility of feline coronavirus antibody tests. J Feline Med Surg. 2014;17(2):152–162. doi: 10.1177/1098612X14538873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhat R., Hamid A., Kunin J.R., Saboo S.S., Batra K., Baruah D. Chest imaging in patients hospitalized With COVID-19 infection - a case series. Curr Probl Diagn Radiol. 2020;49(4):294–301. doi: 10.1067/j.cpradiol.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bor C., Demirag K., Okcu O., Cankayali I., Uyar M. Ventilator-associated pneumonia in critically Ill patients with intensive antibiotic usage. Pakistan J Med Sci. 1969;31(6) doi: 10.12669/pjms.316.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mantuani D., Frazee B., Fahimi J., Nagdev A. Point-of-care multi-organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. Western J Emergency Med. 2016;17(1):46–53. doi: 10.5811/westjem.2015.11.28525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tersalvi G., Vicenzi M., Calabretta D., Biasco L., Pedrazzini G., Winterton D. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Cardiac Fail. 2020;26(6):470–475. doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lai C.-C., Liu Y.H., Wang C.-Y., Wang Y.-H., Hsueh S.-C., Yen M.-Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J Microbiol Immunol Infect. 2020;53(3):404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El Zowalaty M.E., Järhult J.D. From SARS to COVID-19: a previously unknown SARS- related coronavirus (SARS-CoV-2) of pandemic potential infecting humans – Call for a One Health approach. One Health. 2020;9 doi: 10.1016/j.onehlt.2020.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lomoro P., Verde F., Zerboni F., Simonetti I., Borghi C., Fachinetti C. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7 doi: 10.1016/j.ejro.2020.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395(10230):1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abduljalil J.M., Abduljalil B.M. Epidemiology, genome, and clinical features of the pandemic SARS-CoV-2: a recent view. New Microb New Infections. 2020;35 doi: 10.1016/j.nmni.2020.100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh D.D., Han I., Choi E.-H., Yadav D.K. Recent advances in pathophysiology, drug development and future perspectives of SARS-CoV-2. Front Cell Dev Biol. 2020;8(580202) doi: 10.3389/fcell.2020.580202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar S., Zhi K., Mukherji A., Gerth K. Repurposing antiviral protease inhibitors using extracellular vesicles for potential therapy of COVID-19. Viruses. 2020;12(5) doi: 10.3390/v12050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee P.-I., Hu Y.-L., Chen P.-Y., Huang Y.-C., Hsueh P.-R. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53(3):371–372. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu F.-C., Li Y.-H., Guan X.-H., Hou L.-H., Wang W.-J., Li J.-X. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dietz L., Horve P.F., Coil D.A., Fretz M., Eisen J.A., Van Den Wymelenberg K., Gilbert J.A. 2019 Novel Coronavirus (COVID-19) pandemic: built environment considerations to reduce transmission. mSystems. 2020;5(2) doi: 10.1128/mSystems.00245-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pachetti M., Marini B., Benedetti F., Giudici F., Mauro E., Storici P. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J Transl Med. 2020;18(1):179. doi: 10.1186/s12967-020-02344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lythgoe M.P., Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol Sci. 2020;41(6):363–382. doi: 10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kolifarhood G., Aghaali M., Mozafar Saadati H., Taherpour N., Rahimi S., Izadi N. Epidemiological and Clinical Aspects of COVID-19; a Narrative Review. Arch Acad Emerg Med. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 80.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rello J., Storti E., Belliato M., Serrano R. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.01028-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saber-Ayad M., Saleh M.A., Abu-Gharbieh E. The rationale for potential pharmacotherapy of COVID-19. Pharmaceuticals. 2020;13(5) doi: 10.3390/ph13050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chow N., Fleming-Dutra K., Gierke R., Hall A., Hughes M., Pilishvili T. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 — United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tobaiqy M., Qashqary M., Al-Dahery S., Mujallad A., Hershan A.A., Kamal M.A. Therapeutic management of patients with COVID-19: a systematic review. Infection Prevention Practice. 2020;2(3):100061. doi: 10.1016/j.infpip.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen L., Niu J., Wang C., Huang B., Wang W., Zhu N. High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J Virol. 2019;93(12):e00023–19. doi: 10.1128/JVI.00023-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar Y, Singh H, Patel C.N. In silico prediction of potential inhibitors for the main protease of SARS-CoV-2 using molecular docking and dynamics simulation based drug-repurposing. J Infect Public Health. 2020;13(9):1210–1223. doi: 10.1016/j.jiph.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pawelec G., Weng N.-P. Can an effective SARS-CoV-2 vaccine be developed for the older population? Immunity Ageing. 2020;17(1) doi: 10.1186/s12979-020-00180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.N.I.O. Health, NIH clinical trial shows Remdesivir accelerates recovery from advanced COVID-19, 2020.
- 90.Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo G., Ye L., Pan K., Chen Y., Xing D., Yan K. New Insights of Emerging SARS-CoV-2: epidemiology, etiology, clinical features, clinical treatment, and prevention. Front Cell Dev Biol. 2020;8:410. doi: 10.3389/fcell.2020.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID-19 treatment. Revista Panamericana de Salud Pública. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.C.T.N. NCT04310228, Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019, ClicinalTrials.gov (2020).
- 95.Drug U.F.A. Recommendations for Investigational COVID-19 Convalescent Plasma. fda.gov. 2020 [Google Scholar]
- 96.Gibson W.J., Nafee T., Travis R., Yee M., Kerneis M., Ohman M. Machine learning versus traditional risk stratification methods in acute coronary syndrome: a pooled randomized clinical trial analysis. J Thromb Thrombolysis. 2019;49(1):1–9. doi: 10.1007/s11239-019-01940-8. [DOI] [PubMed] [Google Scholar]
- 97.Tang X.C., Agnihothram S.S., Jiao Y., Stanhope J., Graham R.L., Peterson E.C. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc Natl Acad Sci. 2014;111(19):E2018–E2026. doi: 10.1073/pnas.1402074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Du L., Zhao G., Yang Y., Qiu H., Wang L., Kou Z. A conformation-dependent neutralizing monoclonal antibody specifically targeting receptor-binding domain in Middle East respiratory syndrome coronavirus spike protein. J Virol. 2014;88(12):7045–7053. doi: 10.1128/JVI.00433-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barton C., Kouokam J.C., Lasnik A.B., Foreman O., Cambon A., Brock G. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob Agents Chemother. 2014;58(1):120–127. doi: 10.1128/AAC.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O'Keefe B.R., Giomarelli B., Barnard D.L., Shenoy S.R., Chan P.K.S., McMahon J.B. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family coronaviridae. J Virol. 2010;84(5):2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barnard D.L., Kumaki Y. Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy. Future Virol. 2011;6(5):615–631. doi: 10.2217/fvl.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baric R.S., Pervushin K., Tan E., Parthasarathy K., Lin X., Jiang F.L. Structure and inhibition of the SARS coronavirus envelope protein ion channel. PLoS Pathog. 2009;5(7):e1000511. doi: 10.1371/journal.ppat.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He M.L., Zheng B.J., Chen Y., Wong K.L., Huang J.D., Lin M.C. Development of interfering RNA agents to inhibit SARS-associated coronavirus infection and replication. Hong Kong Med J. 2009;15(3 Suppl 4):28–31. [PubMed] [Google Scholar]
- 104.Liao H.-I., Olson C.A., Hwang S., Deng H., Wong E., Baric R.S. mRNA display design of fibronectin-based intrabodies that detect and inhibit severe acute respiratory syndrome coronavirus nucleocapsid protein. J Biol Chem. 2009;284(26):17512–17520. doi: 10.1074/jbc.M901547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Báez-Santos Y.M., St S.E., John A.D. Mesecar. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mandal R.S., Ta A., Sinha R., Theeya N., Ghosh A., Tasneem M. Ribavirin suppresses bacterial virulence by targeting LysR-type transcriptional regulators. Sci Rep. 2016;6(1):39454. doi: 10.1038/srep39454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taylor R., Kotian P., Warren T., Panchal R., Bavari S., Julander J. BCX4430 – a broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J Infection Public Health. 2016;9(3):220–226. doi: 10.1016/j.jiph.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pruijssers A.J., Denison M.R. Nucleoside analogues for the treatment of coronavirus infections. Curr Opin Virol. 2019;35:57–62. doi: 10.1016/j.coviro.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Adedeji A.O., Singh K., Calcaterra N.E., DeDiego M.L., Enjuanes L., Weiss S. Severe acute respiratory syndrome coronavirus replication inhibitor that interferes with the nucleic acid unwinding of the viral helicase. Antimicrob Agents Chemother. 2012;56(9):4718–4728. doi: 10.1128/AAC.00957-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Adedeji A.O., Singh K., Kassim A., Coleman C.M., Elliott R., Weiss S.R. Evaluation of SSYA10-001 as a replication inhibitor of severe acute respiratory syndrome, mouse hepatitis, and middle east respiratory syndrome coronaviruses. Antimicrob Agents Chemother. 2014;58(8):4894–4898. doi: 10.1128/AAC.02994-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen Y.J., Li L.J., Tang W.L., Song J.Y., Qiu R., Li Q. First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension. Cochrane Database Syst Rev. 2018:CD008170. doi: 10.1002/14651858.CD008170.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hotez P.J., Corry D.B., Bottazzi M.E. COVID-19 vaccine design: the Janus face of immune enhancement. Nat Rev Immunol. 2020;20(6):347–348. doi: 10.1038/s41577-020-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ojha R., Gupta N., Naik B., Singh S., Verma V.K., Prusty D. High throughput and comprehensive approach to develop multiepitope vaccine against minacious COVID-19. Eur J Pharm Sci. 2020;151:105375. doi: 10.1016/j.ejps.2020.105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.C.T.N. NCT04346277, Compassionate Use Open-Label Anti-CD14 Treatment in Patients With SARS-CoV-2 (COVID-19), ClinicalTrials.gov (2020).
- 115. NCT04336410 C.T.N., Safety Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers. ClinicalTrials.gov. 2020 [Google Scholar]
- 116. NCT04368728 C.T.N. Study to Describe the Safety, Tolerability, Immunogenicity, and Potential Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Adults. ClinicalTrials.gov. 2020 [Google Scholar]
- 117. NCT04299724 C.T.N. Safety and Immunity of Covid-19 aAPC Vaccine. ClinicalTrials.gov. 2020 [Google Scholar]
- 118. NCT04276896 C.T.N. Immunity and Safety of Covid-19 Synthetic Minigene Vaccin. ClinicalTrials.gov. 2020 [Google Scholar]
- 119. NCT04341389 C.T.N. A Phase II Clinical Trial to Evaluate the Recombinant Novel Coronavirus Vaccine (Adenovirus Vector) ClinicalTrials.gov. 2020 [Google Scholar]
- 120.TrialSiteNews . University of Oxford Commences Clinical Trial for Vaccine Candidate (ChAdOx1 nCoV-19) Targeting COVID-19, Clinal trials. Covid-19, University of Oxford, vaccine; 2020. [Google Scholar]
- 121. NCT04283461 C.T.N. Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis SARS CoV-2 Infection. ClinicalTrials.gov. 2020 [Google Scholar]
- 122.ChiCTR2000031781, A randomized, double-blinded, placebo-controlled phase II clinical trial for Recombinant Novel Coronavirus (2019-nCOV) Vaccine (Adenovirus Vector), Chinese clinical trial registry (2020).
- 123.Innovation N.I.O.B. Laboratory of Vaccine Materials, nibiohn.go.jp. 2020 [Google Scholar]
- 124.B. B, Vaxart Announces Additional Positive Pre-Clinical Data for its Oral COVID-19 Vaccine Program, in: I. Vaxart (Ed.) 2020.
- 125.C. RUTKOWSKI, UM alumnus on front lines of COVID-19 research in China, news.umanitoba.ca (2020).
- 126.L. Han-soo, Genexine, Binex to develop COVID-19 vaccine, koreabiomed.com (2020).
- 127.Smith T.R.F., Patel A., Ramos S., Elwood D., Zhu X., Yan J. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11(1):2601. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.C.T. Arena, Covid-19: China to launch clinical trials for coronavirus vaccine, clinicaltrialsarena.com (2020).
- 129.Hamilton E, UW–Madison, FluGen, Bharat Biotech to develop CoroFlu, a coronavirus vaccine, warf.org (2020).
- 130.Zydus Cadila launches a fast tracked programme to develop vaccine for the novel coronavirus, 2019-nCoV (COVID-19); 2020.
- 131.I. TV, Oxford University’s COVID-19 vaccine moves to next stage of human trial, indiatvnews.com (2020).
- 132.Novakowski S., Jiang K., Prakash G., Kastrup C. Delivery of mRNA to platelets using lipid nanoparticles. Sci Rep. 2019;9(1):552. doi: 10.1038/s41598-018-36910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.P. Technology, Covid-19: Entos and Takis to work on separate vaccines, pharmaceutical-technology.com (2020).
- 134.COVID-19 Vaccine Candidates assaygenie.com 2020.
- 135.C.T. Arena, Serum Institute of India brings Covid-19 vaccine into animal testing, clinicaltrialsarena.com (2020).
- 136.C.t.n. NCT01273233, Safety of an Inactivated Enterovirus Type 71 Vaccine in Healthy Adults, clinicaltrials.gov (2013).
- 137.P. Inc, BioNTech and Pfizer announce regulatory approval from German authority Paul-Ehrlich-Institut to commence first clinical trial of COVID-19 vaccine candidates, in: B. Media (Ed.) 2020.
- 138.CureVac, CureVaćs Optimized mRNA Platform Provides Positive Pre-Clinical Results at Low Dose for Coronavirus Vaccine Candidate, in: curevac.com (Ed.) 2020.
- 139.Xu Ruodan, Shi Mingfei, Li Jing, Song Ping. Construction of SARS-CoV-2 Virus-Like Particles by Mammalian Expression System.Front Bioeng. Biotechnol. 2020;8:862. doi: 10.3389/fbioe.2020.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qian C, Liu X, Xu Q. Recent Progress on the Versatility of Virus-Like Particles. Vaccines (Basel) 2020;8(1):139. doi: 10.3390/vaccines8010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Syomin B.V., Ilyin Y.V. Virus-Like Particles as an Instrument of Vaccine Production. Molecular Biology. 2019;53:323–334. doi: 10.1134/S0026893319030154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jeyanathan M., Afkhami S., Smaill F. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Valneva, Valneva and Dynavax Announce Collaboration to Advance Vaccine Development for COVID-19, in: valneva.com (Ed.) 2020.
- 144.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dynavax, Dynavax and Clover Biopharmaceuticals Announce Research Collaboration to Evaluate Coronavirus (COVID-19) Vaccine Candidate with CpG 1018 Adjuvant, in: D.T. Corporation (Ed.) 2020.
- 146.Kim E, Erdos G, Huang S, Kenniston T.W., Balmert S.C., Carey C.D. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020;55:102743. doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Francesca M., Rossi O., Francesca N., Francesca M. Francesca Micoli. OMV Vaccines and the Role of TLR Agonists in Immune Response. Int J Mol Sci. 2020;21(12):4416. doi: 10.3390/ijms21124416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009 Mar;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Saha R.P., Sharma AR, Singh M.K., Samanta S, Bhakta S, Mandal S. Repurposing Drugs, Ongoing Vaccine, and New Therapeutic Development Initiatives Against COVID-19. Front Pharmacol. 2020;11:1258. doi: 10.3389/fphar.2020.01258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kalita P., Padhi A.K., Zhang K.Y.J., Tripathi T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb Pathog. 2020;145 doi: 10.1016/j.micpath.2020.104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.P. Technology, GSK partners with Innovax Biotech on Covid-19 vaccine, 2020.
- 152.Rabaan A.A., Al-Ahmed S.H., Sah R., Al-Tawfiq J.A., Al-Qaaneh A.M., Al-Jamea L.H., Woodman A., Al-Qahtani M., Haque S., Harapan H., Bonilla-Aldana D.K., Kumar P., Dhama K., Rodriguez-Morales A.J.. Recent advances in vaccine and immunotherapy for COVID-19. Human vaccines & immunotherapeutics 2020; 1–12. Advance online publication. 10.1080/21645515.2020.1825896. [DOI] [PMC free article] [PubMed]
- 153.Ng WH, Liu X, Mahalingam S. Development of vaccines for SARS-CoV-2. F1000Res. 2020;9:F1000 Faculty Rev-991. 10.12688/f1000research.25998.1 [DOI]
- 154.Iavi, Iavi and merck collaborate to develop vaccine against sars cov-2, in: iavi.org (Ed.) 2020.
- 155.Eiden J, Volckaert B, Rudenko O. Single Intranasal (IN) Dose of M2SR (M2-Deficient Single Replication) Live Influenza Vaccine Protects Adults Against Subsequent Challenge with a Substantially Drifted H3N2 Strain. Open Forum Infect Dis. 2019;6(Suppl 2):S967–S968. doi: 10.1093/ofid/ofz360.2425. [DOI] [Google Scholar]
- 156.GlobeNewswire, Generex Signs Contract with EpiVax to Develop Ii Key Peptide Vaccines to Address the Coronavirus Pandemic, 2020.
- 157.Young P.R. Disease X ver1.0: COVID-19. Microbiology Australia 41, 2020;109-112. 10.1071/MA20028 [DOI]
- 158.C. R, Adenoviral vectors are the new COVID-19 vaccine front-runners. Can they overcome their checkered past?, 2020.
- 159.N. J, University of Alberta researchers in race against time to create COVID-19 vaccine, ualberta.ac (2020).
- 160.Dzif, The DZIF focuses on SARS-CoV-2 research, dzif.de (2020).
- 161.B. O, Candidats a vaccí contra la Covid-19, segons l'Organització Mundial de la Salut 2020.
- 162.TrialSiteNews, CanSino Biological, Moderna, and INOVIO Lead COVID-19 Vaccine Race; 42 Other Candidates in Pre-Clinical Stage, 2020.
- 163.Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 Vaccines at Pandemic Speed. The New England Journal of Medicine. 2020:1–5. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 164.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chi X., Liu X., Wang C., Zhang X., Li X., Hou J. Humanized single domain antibodies neutralize SARS-CoV-2 by targeting the spike receptor binding domain. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-18387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cao Y., Su B., Guo X., Sun W., Deng Y., Bao L. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020;182(1):73–84.e16. doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li W., Drelich A., Martinez D.R., Gralinski L., Chen C., Sun Z. Potent neutralization of SARS-CoV-2 in vitro and in an animal model by a human monoclonal antibody. BioRxiv. 2020 [Google Scholar]
- 168.Shi R., Shan C., Duan X., Chen Z., Liu P., Song J. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584(7819):120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- 169.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584(7819):115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 170.Liu J., Zhang S., Zhang T., Xiao H., Du J., Zeng S. Isolation of a human monoclonal antibody specific for the receptor binding domain of SARS-CoV-2 using a competitive phage biopanning strategy. Antibody Therapeutics. 2020;3(2):95–100. doi: 10.1093/abt/tbaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W.-T. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020 doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brouwer P.J.M., Caniels T.G., van der Straten K., Snitselaar J.L., Aldon Y., Bangaru S. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369(6504):643–650. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen X., Li R., Pan Z., Qian C., Yang Y., You R. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647–649. doi: 10.1038/s41423-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tai W., Zhang X., He Y., Jiang S., Du L. Identification of SARS-CoV RBD-targeting monoclonal antibodies with cross-reactive or neutralizing activity against SARS-CoV-2. Antiviral Res. 2020;179 doi: 10.1016/j.antiviral.2020.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Huo J., Zhao Y., Ren J., Zhou D., Duyvesteyn H.M.E., Ginn H.M. Neutralization of SARS-CoV-2 by destruction of the prefusion spike. Cell Host Microbe. 2020;28(3):445–454.e6. doi: 10.1016/j.chom.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chu H., Chan J.F.-W., Yuen T.T.-T., Shuai H., Yuan S., Wang Y. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1(1):e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wrapp D., De Vlieger D., Corbett K.S., Torres G.M., Wang N., Van Breedam W. Structural basis for potent neutralization of betacoronaviruses by single-domain camelid antibodies. Cell. 2020;181(5):1004–1015.e15. doi: 10.1016/j.cell.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lv Z., Deng Y.-Q., Ye Q., Cao L., Sun C.-Y., Fan C. Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody. Science. 2020;369(6510):1505–1509. doi: 10.1126/science.abc5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zost S.J., Gilchuk P., Case J.B., Binshtein E., Chen R.E., Nkolola J.P. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584(7821):443–449. doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020 doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccines Immunother. 2020;16(6):1232–1238. doi: 10.1080/21645515.2020.1735227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang J., Wang Z. Strengths, weaknesses, opportunities and threats (SWOT) analysis of china’s prevention and control strategy for the COVID-19 epidemic. Int J Environ Res Public Health. 2020;17(7) doi: 10.3390/ijerph17072235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xu C., Jin J., Song J., Yang Y., Yao M., Zhang Y. Application of refined management in prevention and control of the coronavirus disease 2019 epidemic in non-isolated areas of a general hospital. Int J Nursing Sci. 2020;7(2):143–147. doi: 10.1016/j.ijnss.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]