Abstract
The heterogeneous and anisotropic articular cartilage is generally studied as a layered structure of ‘zones’ with unique composition and architecture, which is difficult to recapitulate using current approaches. We here present a novel hybrid bioprinting strategy to generate zonally-stratified cartilage. Scaffold-free tissue strands (TSs) were made of human adipose-derived stem cells (ADSCs) or pre-differentiated ADSCs. Cartilage TSs with pre-differentiated ADSCs exhibited improved mechanical properties and up-regulated expression of cartilage-specific markers at both transcription and protein levels as compared to TSs with ADSCs being differentiated in the form of strands and TSs of non-transfected ADSCs. Using the novel hybrid approach integrating a new aspiration-assisted and extrusion-based bioprinting techniques, we demonstrated the bioprinting of zonally-stratified cartilage with vertically aligned TSs at the bottom zone and horizontally-aligned TSs at the superficial zone, in which collagen fibers were aligned with designated orientation in each zone imitating the anatomical regions and matrix orientation of native articular cartilage. In addition, mechanical testing study revealed a compression modulus of ~1.1 MPa, which is similar to that of human articular cartilage. The prominent findings highlighted the potential of this novel bioprinting approach for building biologically-, mechanically, and histologically-relevant cartilage for tissue engineering purposes.
Keywords: scaffold-free bioprinting, biofabrication, zonally-stratified articular cartilage, adipose-derived stem cells
1. Introduction
Damage to the articular cartilage of the knee is a common and debilitating injury. Over 60% of patients who underwent arthroscopy have been found to have Grade III or IV injury.[1] These focal injuries themselves are frequently symptomatic, functionally limiting and require further surgical treatment. Furthermore, they often progress to further cartilage degeneration and can result in posttraumatic osteoarthritis (OA).[2] Articular cartilage repair is highly challenging as articular cartilage has limited intrinsic repair capacity.[3] Current clinical treatments include microfracture, auto or allo osteochondral grafting and cell-based techniques such as autologous chondrocyte implantation (ACI). All of these techniques, to date, have significant shortcomings. Microfracture is not effective for large defects as it results in the formation of fibrocartilage that is biomechanically inferior.[4, 5] Autografting is widely used; however, donor-site morbidity and complications limit its availability and clinical use.[4, 5] ACI results in peri-operative improvement but ~40% of all surgeries must be revised due to graft hypertrophy and delamination, and the resulting repair tissue has poor histologic organization.[6] Allografts are limited by the donor supply and carry the risk of disease transmission. While the state-of-the-art techniques have promising initial clinical results, their long-term efficacy is limited by the formation of fibrous tissue, apoptosis, mechanical failure, and further cartilage degeneration.[7–9] Therefore, novel bioprinting approaches are essential to overcome the limitations of abovementioned approaches.
The structure and composition of healthy articular cartilage varies along the chondral axis; therefore, many of the limitations of current techniques can be attributed to the lack of proper spatial control of biological signals for guiding cell differentiation, hyaline cartilage formation, and integration with the underlying bone.[10–12] The heterogeneous and anisotropic cartilage is generally studied as a layered structure of ‘zones’ that possess mechanical properties reflecting each zone’s composition and architecture,[13] which is difficult to recapitulate using current approaches. Scaffold-based approaches for supporting tissue growth and maturation have been well explored in cartilage regeneration;[9] however, cells in scaffolds have limited interactions and signaling as they are confined in the scaffold matrix, which is vitally important for differentiation of cells as well as mechanotransductive signaling between cells in order to regenerate articular cartilage with anisotropic structural, biological and mechanical characteristics.[9, 14]
Three-dimensional (3D) bioprinting has the ability to engineer cartilage with control on zonal cellular and extracellular matrix (ECM) arrangement;[15–17] however, such a bioprinting mechanism has not been feasible thus far. To address these challenges, we present a novel bioprinitng approach to engineer chondral tissue grafts integrating scaffold-free cartilage tissue strands (TSs) and zonally-stratified bioprinting arrangement to collectively modulate the heterotypic reconstruction of zone-specific chondral tissues. We aim to fabricate human articular cartilage using chondrocytes differentiated from human adipose-derived stem cells (ADSCs). ‘Cartilage TSs’, a scaffold-free biomaterial, is a bioink made of densely packed cells and their endogenous ECM. Cartilage TSs are scalable in length [18] and bioprintable [19], and possess appealing properties such as mechanical stability, rapid self-assembly through tissue fusion and higher cell densities promoting rapid chondrogenic maturation. Here, this study demonstrates a number of novel aspects, as compared to our previous work which proposed TS fabrication as a novel process.[19] First of all, bovine chondrocytes were used in our previous work to make TSs, whereas the current study used human primary ADSCs as a cell source. Hence, this study offers a close-looped practical strategy for regenerative medicine, which uses autologous stem cells from surgical waste (fat tissue in this case) to fabricate tissue substitutes with clinically-amenable scale and biologically-relevant microstructure. Secondly, this study revealed that cells in TSs grew and spread along the strand axis depositing the collagen matrix longitudinally and facilitating an anisotropic orientation. Cellular and collagen orientation was carefully investigated in the current study leading to better understanding of cellular organization within TSs. Thirdly and most importantly, we presented a novel hybrid biofabrication technique, which has not been demonstrated before. Deposition of TSs in horizontal and vertical orientation was investigated via the utilization of extrusion-based bioprinting and AAB techniques, in order to generate zonally-stratified structure, which is a great advancement beyond what was accomplished in our previous work.
2. Results
2.1. Formation and evaluation of TSs
In this study, three groups of TSs were fabricated and compared (Figure 1 and Table 1). ADSCs in Group 1 were pre-differentiated to chondrocytes in a monolayer culture, followed by making TSs using chondrocytes. In Group 2, TSs were fabricated using ADSCs, which were the differentiated towards chondrocytes in a 3D strand form. TSs in Group 3 were made of ADSCs without any differentiation and used as a control group. In all groups, ADSCs or fully-differentiated chondrocytes were injected into alginate capsules in order to generate TSs (Figure 2a). After microinjection, densely packed cells (with density of ~1.6×105 cells/mm) filled the capsules with an inner diameter of ~800 μm (Figure 2b) and began to aggregate due to their close interaction, which follows a three-step model involving initial cell-cell contact, cadherin accumulation, and aggregate compaction.[20–22] The diameters of TSs in Group 1 decreased more rapidly than those in Groups 2 and 3 showing considerable contraction during aggregation. During the extended culture period (3 weeks), TSs in all groups maintained their integrity inside the capsules without apparent morphological changes (Figure 2a). In terms of diameters, TSs in Group 1 compacted until Day 4 reaching a minimum diameter of ~500 μm due to cell aggregation, and then increased in diameter gradually to ~600 μm after 21 days of culture which could be attributed to the abundant ECM deposition during chondrogenic differentiation. The diameter change for TSs in Group 2 exhibited a similar trend as compared to that for Group 1. The diameter of TSs in Group 3 was relatively constant as compared to those of other two groups, although TSs in Group 3 contracted slightly until Day 4.
Figure 1.

Schematic overview of fabrication of scaffold-free zonally-stratified articular cartilage: human ADSCs were expanded and differentiated under chondrogenic induction followed by centrifuging them to obtain dense pellet. Cells were then microinjected into alginate capsules to facilitate cell aggregation followed by de-crosslinking of alginate capsules to obtain TSs. Zonally-stratified cartilage was comprised of the vertically aligned bottom zone, which was fabricated using an aspiration-assisted bioprinting (AAB) technique with the assistance of a pin array and the superficial zone, which was bioprinted using an extrusion-based bioprinting technique. After tissue fusion, the superficial zone was transferred onto the bottom zone and assembled via tissue fusion.
Table 1.
Conditions of cell culture in different groups
| Cell type | Chondrogenic media during TS formation | Culture time | |
|---|---|---|---|
| Group 1 | Pre-differentiated ADSC (in chondrogenic media for 21 days) | √ | 21 days |
| Group 2 | ADSC | √ | 21 days |
| Group 3 | ADSC | × | 21 days |
Figure 2.
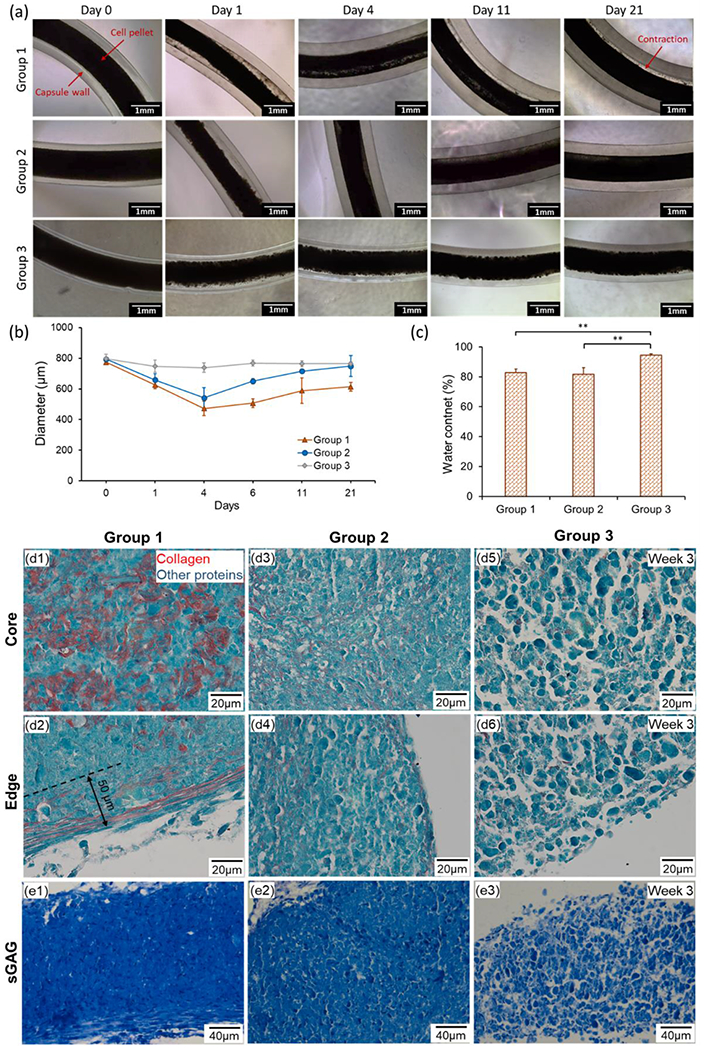
(a) Morphology and (b) diameter change of TSs in alginate capsules cultured for 3 weeks. (c) Water content of different groups of TSs (n=3, **p < 0.01). (d) Images of histological sections stained for Picrosirius Red/Fast Green demonstrating collagen content at the core and edges of TSs of different groups after the 3-week culture. (e) sGAG content stained by Toluidine Blue in different groups after the 3-week culture.
Viability of cell in monolayer culture prior to microinjection process was 95 ± 2%. The normalized cell viability after microinjection process with respect to that before injection was determined to be 0.95 ± 0.11, demonstrating that the microinjection process did not result in a significant decrease in cell viability (p=0.663, W-value: 12.00). Water content of TSs was also investigated after de-crosslinking of capsules. TSs in Groups 1 and 2 demonstrated similar water content (~80%) while the control group showed higher water content at around 95%.
In addition, cartilage-specific ECM production in different groups was histologically assessed. Results of Picrosirius Red/Fast Green staining exhibited remarkable production of collagen in the core of TSs in Group 1 (Figure 2d1). At the edge, thick collagen fibers continuously organized along the TSs with a depth of ~50 μm (Figure 2d2). In regards to Group 2, thinner and discrete collagen fibrils were stained across the entire strand domain as compared to Group 1 (Figures 2d3–d4). Minimal amount of collagen was observed in Group 3; however, collagen was not organized in the form of fibrils (Figures 2d5–d6). Moreover, denser ECM organization was observed in Group 1, both at the center and edges, and Group 3 exhibited the least dense morphology. The Toluidine Blue staining was stronger in Group 1 compared to other groups indicating that ECM in Group 1 contained a higher amount of sulfated glycosaminoglycan (sGAG, Figure 2e).
In order to demonstrate the expression of other cartilage-specific proteins including COL-II and Aggrecan, immunofluorescent imaging was performed. COL-II is the predominant type of collagen in cartilage, which forms a cohesive network and largely contributes to the tensile stiffness and strength of cartilage.[23] Aggrecan is the major proteoglycan in articular cartilage, which provides a hydrated gel structure that endows cartilage with load-bearing properties.[24] Fluorescent images revealed that both COL-II and Aggrecan was strongly positive for Group 1, both in sagittal and cross-sectional views (Figures 3a and d). In Group 2, although COL-II and Aggrecan were observed, signal was weaker than that of Group 1, indicating lower expression of both proteins (Figures 3b and e). In Group 3, COL-II and Aggrecan staining was weak (Figures 3c and f), which were consistent with Picrosirius Red staining. In addition, TSs in all groups showed circular cross-sections indicating the cylindrical morphology of TSs.
Figure 3.
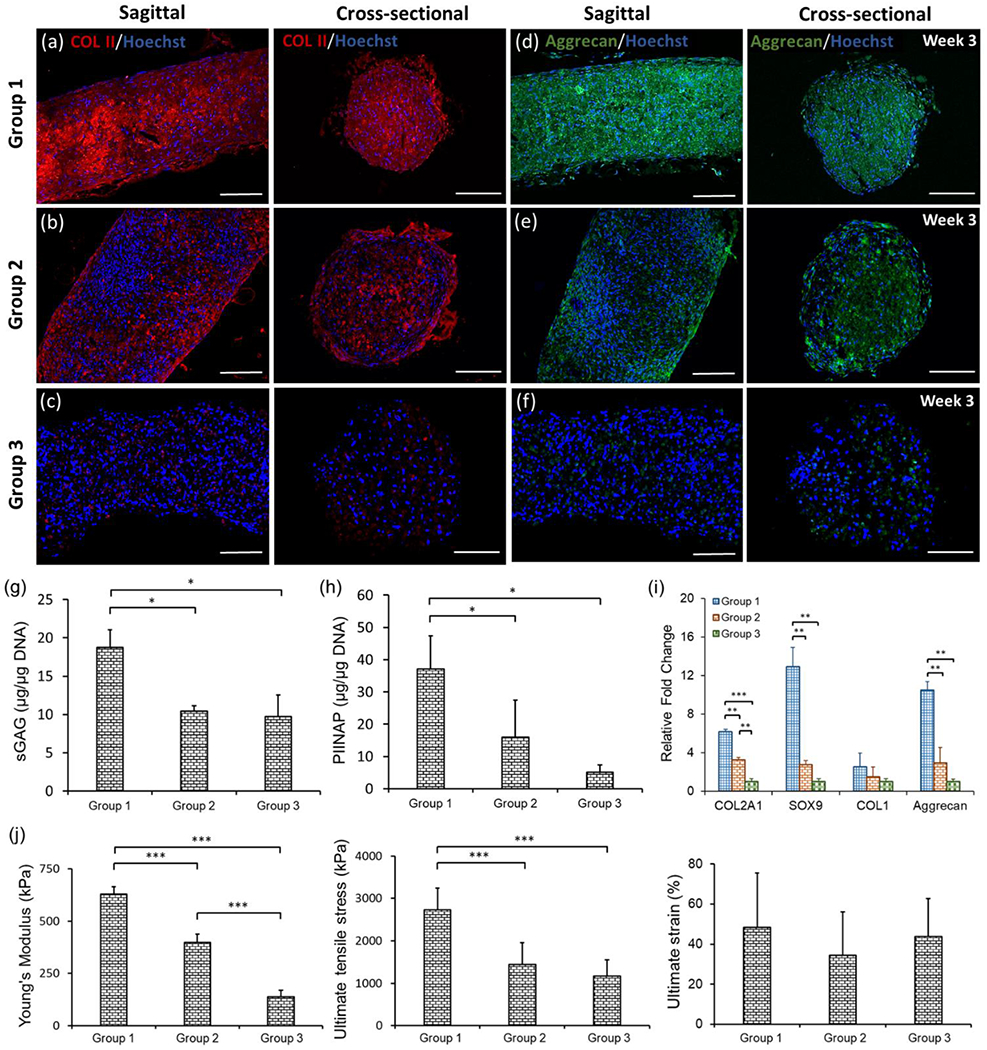
Expression of cartilage-specific markers, quantification of protein and gene expression, and mechanical properties in different groups of TSs. (a-c) COL-II and (d-f) Aggrecan immunostaining in the sagittal and cross-sectional views of TSs in different groups after the 3-week culture. (g) sGAG content measurement by DMMB assay, which was normalized to DNA amount (n=3, *p < 0.05). (h) COL-II synthesis from TSs with normalization to DNA amount (n=3, *p < 0.05). (i) Real-time PCR analysis of TSs demonstrating expression of cartilage-specific genes including COL2A1, Sox9, COL1 and Aggrecan in different groups (n=3, **p < 0.01, and ***p < 0.001). (j) Mechanical analysis of three types of TSs over the 3-week incubation period, including Young’s modulus, ultimate tensile stress and failure strain (n=3, ***p < 0.001).
After three weeks of culture, sGAG content in TSs and Type IIA collagen N-propeptide (PIIANP) released into the culture media were quantified. In the 1,9-dimethylmethylene blue (DMMB) assay, Group 1 presented sGAG content of 18.7 μg/μg DNA, which was significantly higher compared to other two groups (Figure 3g). Meanwhile, Groups 2 and 3 exhibited similar sGAG expression, which were 10.5 and 9.7 μg/μg DNA, respectively. The results of PIIANP quantitative analysis confirmed upregulated COL-II expression in Group 1 at 37.2 μg/μg DNA as compared to 16.1 μg/μg DNA in Group 2 and 5.1 μg/μg DNA in Group 3 (Figure 3h). Although Group 2 exhibited higher COL-II production than Group 3, no significant differences were present. The functionality of TSs was further validated by a gene expression study. The results of gene expression analysis revealed significantly higher expression of cartilage-specific markers in Group 1 than the other two groups (Figure 3i). Effectively, COL2A1 gene expression for Group 1 demonstrated ~2- and 6-fold increase compared to that for Groups 2 and 3, respectively. Meanwhile, Sox9 expression for Group1 showed ~5- and 13-fold increase compared to that for Groups 2 and 3, and Aggrecan expression for Group 1 exhibited ~4- and 11-fold increase compared to that for Groups 2 and 3, respectively. COL1 gene expression in Group 1 was slightly higher than those in Groups 2 and 3 without statistical significance; however, COL1 expression for Group 1 was weaker compared to the expression of COL2A1, Sox9 and Aggrecan.
Mechanical testing was performed after three weeks of culture for all groups. Quantitatively, Group 1 exhibited much higher mechanical properties, where Young’s modulus was ~2- and 5-fold higher than that for Groups 2 and 3, respectively (Figure 3j). The ultimate tensile stress for Group 1 showed ~2-fold increase as compared to that for both Groups 2 and 3 (p < 0.05), which indicated the impact of chondrogenesis on mechanical enhancement of TSs. No significant difference was observed for the ultimate strain among all groups.
2.2. Evaluation of mechanical properties and cellular orientation in pre-differentiated cartilage TSs
Since TSs in Group 1 exhibited superior biological and mechanical properties, they were considered more suitable for construction of the zonally-stratified cartilage. Therefore, TSs in Group 1 were further investigated for this purpose. Mechanical properties of TSs with pre-differentiated chondrocytes exhibited higher values upon further culture (Figure 4a), which was evidenced by the ~3-fold increase in Young’s modulus (2.1 MPa) and ~2-fold increase in ultimate tensile stress (5.6 MPa, p < 0.05) at week 6. In addition, the ultimate strain showed a slight increase without statistical significance (p > 0.05).
Figure 4.
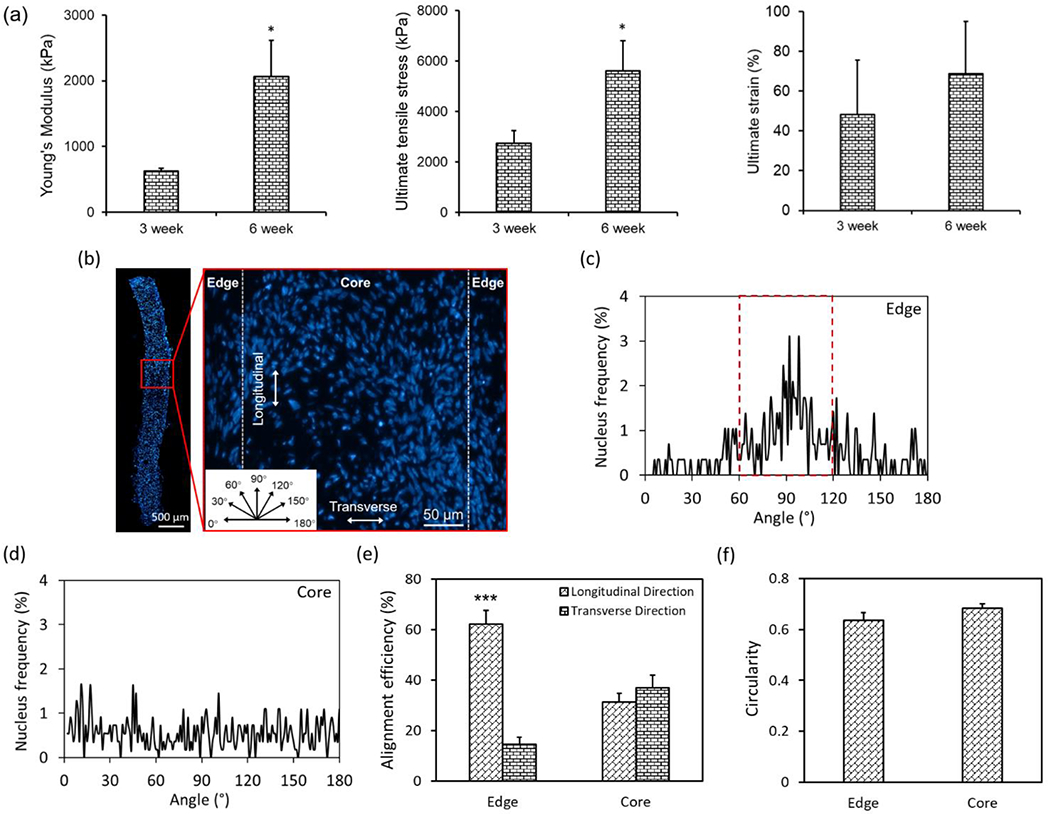
Quantification of mechanical properties and cellular alignment and orientation within TSs in Group 1. (a) Comparison of mechanical properties of TSs in Group 1 after 3 and 6 weeks of culture (n=3, *p < 0.05). (b) A fluorescent image showing nuclei distribution throughout the histological section of a TS in Group 1 after 3 weeks of culture, which was divided into two regions (edge and core) based on the distance to edges (insert: angular orientation, which was used to define the nucleus angle). (c-d) Analysis of nucleus angle frequency at two regions. (e) Quantitative analysis of nucleus alignment efficiency and (f) circularity (n=4; ***p < 0.001).
As aligned collagenous morphology was observed, particularly along the edges of TSs in Group 1 (Figure 2d2), the alignment of chondrocytes was further investigated by dividing the histological images of TSs into two regions (i.e., edge and core) according to the distance from edges (Figure 4b). Analysis of nucleus orientation provided detailed information (e.g., peak distribution) in different regions. In the edge regions, the nucleus angle distribution exhibited a single dominating peak along the longitudinal direction of TSs (Figure 4c). In contrast, there was no dominant peak observed in the core region (Figure 4d). In terms of the quantitative nucleus alignment efficiency, more than 60% of nuclei oriented along the longitudinal direction of TSs (60-120°, Figure 4e), with ~15% of those orienting along the transverse direction in the edge regions (0-30° or 150-180°). As expected, the alignment frequency of cells in the core region was ~30% indicating no preferential orientation. In addition, there was no significant difference observed for cell circularity in both regions, which were around 0.6 indicating the ellipse shape of nuclei (Figure 4f).
2.3. Bioprinting of zonally-stratified cartilage
The capability of fabricating zonally-stratified cartilage using cartilage TSs (Group 1) was evidenced by a novel hybrid approach. Using the multi-arm bioprinter (MABP) and a customized print head (Figures 5a1–a2) as explained in our previous work,[19] TSs were successfully extruded. Bioprinted TSs aligned in parallel along the movement direction of the nozzle (Figure 5a3), and attached each other during the bioprinting process. Cell viability before and after extrusion was determined to be 91 ± 1% and 91 ± 3% (n=3, p=1.000, W-value: 11.00, Figure 5a4), respectively, demonstrating that the extrusion process did not result in any significant changes in cell viability. TSs fused during 1-week culture and integrated into a single patch of tissue (Figure 5c1). It should be noted that TSs made of pre-differentiated chondrocytes were mechanically strong enough to endure the external forces experienced during their transfer and extrusion.
Figure 5.
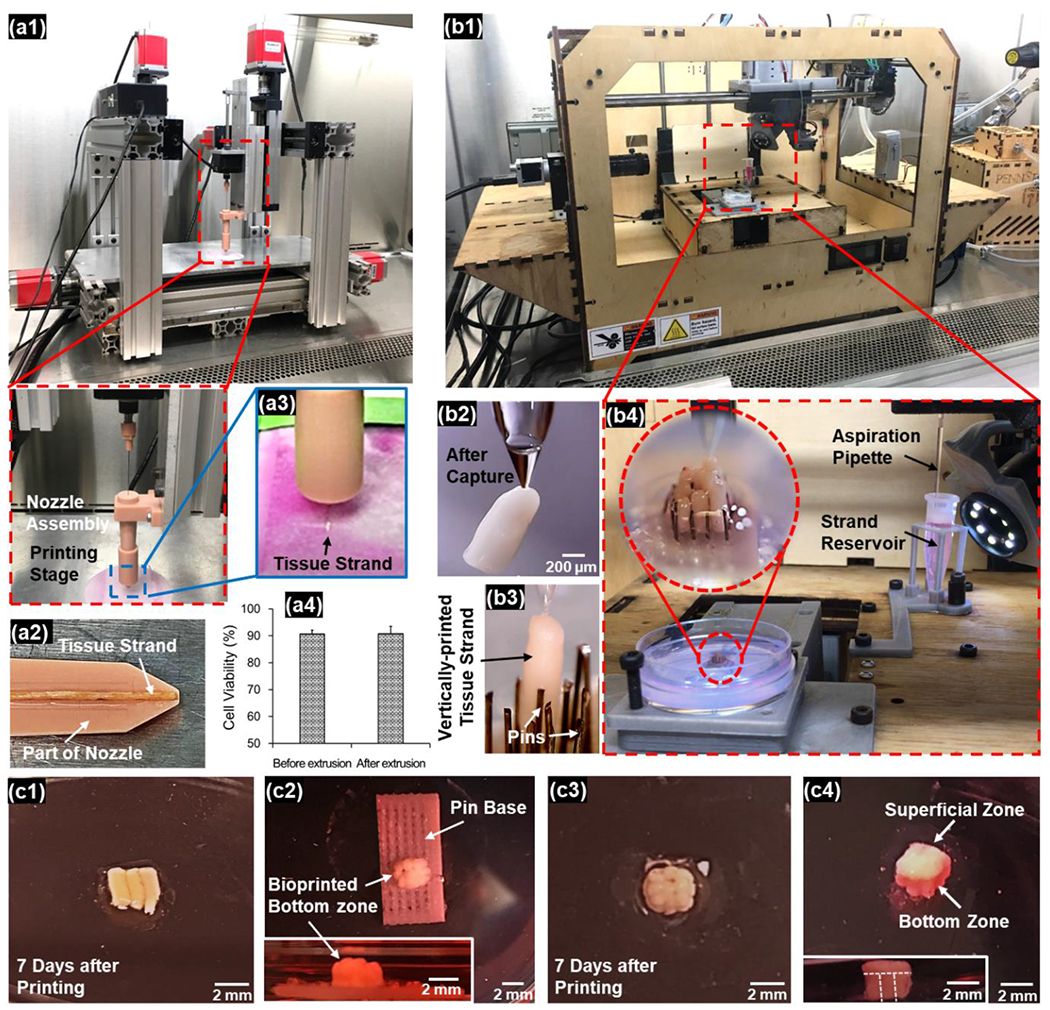
Hybrid bioprinting of scaffold-free zonally-stratified articular cartilage: (a) bioprinting of the superficial zone using the MABP (a1) with a detachable nozzle assembly (a2). Loaded TSs were extruded with the movement of nozzle (a3). Extrusion process did not result in any significant changes in cell viability (a4). (b) Bioprinting of the bottom zone using the AAB technique (b1). The pick-and-place procedure (b2) was performed to place the TSs into the pin array repetitively (b3) to obtain a well-defined 3×3 arrangement of TSs (b4). (c) The bioprinted superficial zone (c1) was immobilized by alginate hydrogel and cultured for a week to facilitate tissue fusion. The bioprinted bottom zone was also immobilized by alginate hydrogel, followed by pins being removed (c2). The construct was then detached from the pin base (c3) and cultured for a week to facilitate tissue fusion. The bioprinted superficial zone was transferred onto the bottom zone and cultured for another week to generate the whole cartilage with two distinct zones (c4).
The bottom zone, on the other hand, was bioprinted in a vertical arrangement using a novel aspiration-assisted bioprinting (AAB) technique described in our recent work[25] (Figure 5b1) with the assistance of a pin array. Bioprinting was performed in a pick-and-place fashion of individual TSs (Figures 5b2–b3). The customized glass nozzle with a diameter of ~80 μm and vacuum pressure of ~300 mmHg was used to capture the TS from the cell media. The TS was then lifted and transferred to the pin array. With the assistance of a charge-coupled device (CCD) camera mounted aside the nozzle, the position of TSs relative to the pin array was monitored in real time, which supported the accurate placement of TSs into the designated slots on the pin array. The pin array assisted to adjust the position of TSs to make them slide into each slot vertically with the advancement of the pipette tip at the speed of 10 mm/s in downwards direction. After a TS was placed at the designated position, aspiration was terminated and the TS was detached from the pipette tip due to gravity. After bioprinting, the bottom zone showed vertically placed TSs with a well-defined 3×3 array arrangement, with close strand-to-strand proximity enabling tissue adhesion and potential fusion for structural integrity (Figure 5b4). There was no significant damage observed on TSs when it was aspirated by the vacuum pressure indicating their structural integrity. TSs did not fall during aspiration and transfer processes demonstrating the sufficiency of the applied 300 mmHg aspiration pressure. Since the transfer of TSs from the reservoir to the pin array (which was hydrated with culture media) was completed within seconds, no significant dehydration was observed on TSs.
After the removal of pins and the base (Figure 5c2), constructs maintained their structural integrity (Figure 5c3). With a week of additional culture, the fused superficial zone was assembled to the bottom zone, followed by another week of culture for complete fusion of both zones. The final tissue demonstrated zonally-stratified arrangement apparently with sufficient integrity indicating the success of the presented bioprinting approach (Figure 5c4).
2.4. Evaluation of zonally-stratified articular cartilage
The mechanical test exhibited that the compressive modulus of printed bioprinted cartilage was 1.09 ± 0.08 MPa (Figure 6a), which is comparable with respect to the native human articular cartilage (e.g., 0.24-1 MPa).[26, 27] In order to visualize the cellular and matrix organization in different zones, both superficial and bottom zones of the bioprinted tissues were sectioned and stained (Figure 6b). The cross-sectional image of the superficial zone with hematoxylin and eosin (H&E) staining revealed well-fused TSs (Figure 6c). Nuclei staining was conducted and cell alignment was quantified by dividing strands to “edge” and “core” regions as explained before. The analysis of alignment efficiency exhibited that cells in the edge regions had significantly higher alignment efficiency in the longitudinal direction as compared to those along the transverse direction (44% vs. 22%, p < 0.05, Figures 6d1–d2), while cells in the core regions did not exhibit a dominant orientation. These findings suggested that the cellular alignment observed at the boundaries was maintained even after TSs fused. Additionally, no significant difference in cell circularity was observed between edge and core regions, and the ellipse shape of nuclei was maintained (Figure 6d3). The cross-sectional image of the bottom zone with H&E staining displayed a 3×3 matrix, with TSs fusing to the neighboring ones and generating a single patch of tissue (Figure 6e).
Figure 6.
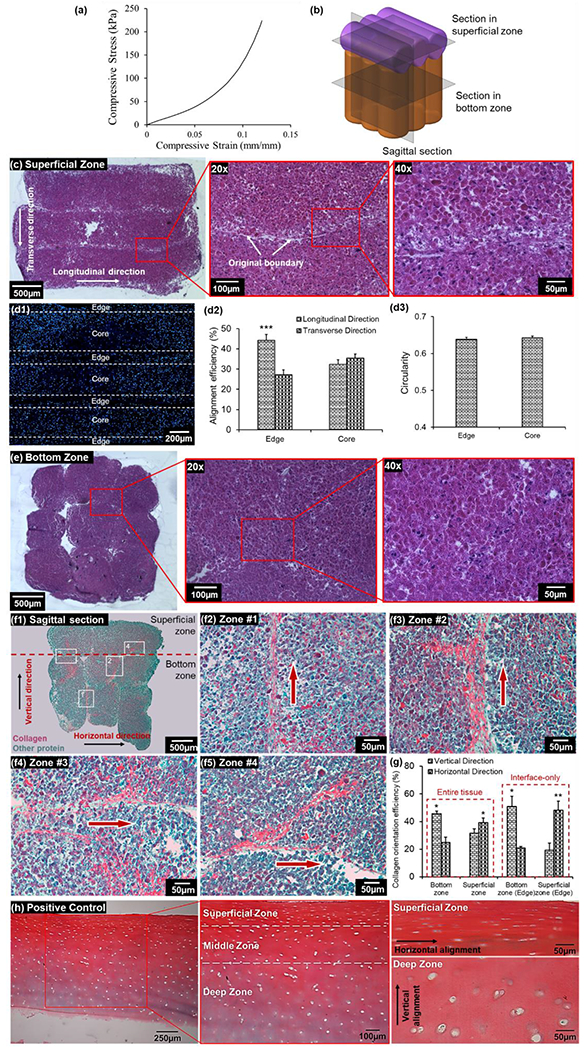
Characterization of fabricated zonally-stratified cartilage. (a) Stress-strain curves of the compression test on the bioprinted cartilage. (b) A schematic diagram showing positions of transverse and sagittal sectioning in the bioprinted articular cartilage. (c) H&E staining of the superficial zone with different magnifications demonstrating the fusion of TSs and cellular morphology. (d) A fluorescent image of nuclei on a section of the superficial zone, which was divided into two zones (edge and core) on each TS domain (d1), and the analysis of nuclei alignment (d2) and elongation (d3) of these regions (n=3, ***p < 0.001). (e) H&E straining of the bottom zone with different magnifications demonstrating the fusion of TSs and chondrocyte morphology. (f) Sagittal sections of bioprinted cartilage with Picrosirius Red/Fast Green straining demonstrating the stratified arrangement and collagen organization in (f1) whole cartilage and (f2-f5) at the boundary regions, which were labelled in (f1). (g) Quantitative measurement of collagen alignment efficiency at the superficial and bottom zones of the entire tissue, as well as at the interfaces of TSs (n=3, *p < 0.05 and **p < 0.01). (h) Picrosirius Red/Fast Green straining on a section of human cartilage as a positive control showing the zonally-stratified arrangement of collagen.
In addition to transverse sections, sagittal sectioning was also conducted to investigate the collagen arrangement inside the whole cartilage. The sagittal section of the tissue showed that the superficial and bottom zones were well integrated, with horizontally-oriented TSs at the superficial zone and vertically-oriented ones at the bottom zone, which recapitulated the stratified anatomy of native cartilage (Figure 6f). Picrosirius Red staining facilitated visualization of the collagen matrix within the tissue and the collagen orientation, particularly at the boundaries of fused TSs, were highlighted (Figure 6f2–f5). In general, collagen fibers were aligned along the periphery of TSs. Collagen fibers at the bottom zone were observed to be aligned vertically at the boundaries of TSs, and those at the superficial zone were aligned horizontally. This observation of collagen alignment was consistent with that in individual TSs (Figure 2d2), which indicated that the tissue fusion did not significantly impact the collagen orientation of individual TSs. The collagen alignment at the superficial and bottom zones was holistically quantified. Results showed that the amount of vertically-aligned collagen fibers (45%) were significantly higher than horizontally-aligned collagen fibers (24%) at the bottom zone (p < 0.05, Figure 6g). As expected, the opposite result was observed at the superficial zone, which was 31% and 39% for vertical and horizontal fibers, respectively (p < 0.05). In addition, the collagen alignment at TS boundaries was also quantified. Due to the exclusion of randomly-oriented collagen, greater differences between vertically- and horizontally- aligned fibers were observed, where vertical and horizontal collagen fibers were 51% and 21% at the bottom zone (p < 0.05, Figure 6g), and 19% and 48% at the superficial zone (p < 0.05), respectively.
3. Discussions
In this study, we presented a new hybrid bioprinting approach to build zonally-stratified articular cartilage in order to mimic the anatomy of native cartilage using chondrocytes differentiated from human ADSCs. The results demonstrated that TSs in Group 1 had greater ECM deposition (i.e., collagen and sGAG) as compared to the other groups. The quantitative measurement of proteins (i.e., sGAG and COL-II) and gene expression (i.e., COL2A1, SOX9 and Aggrecan) were consistent with the staining results, in which Group 1 showed superior outcomes. In Group 1, the expression level of these hyaline cartilage-specific genes was stronger than that for COL1 indicating that Group 1 strands better imitated the composition of hyaline cartilage instead of fibrocartilage. TSs in Groups 1 and 2 exhibited increased diameter after four days of TS formation till Day 21 (Figure 2b), which could be attributed to the abundant ECM deposition during chondrogenic differentiation. Thereafter, the TS diameter became constant, which could be due to the compaction of TS. Water contents of TSs in Groups 1 and 2 were in the range of that of human cartilage (i.e., 60 to 85% by wet weight [23]), demonstrating the biological relevance of the fabricated TSs made of chondrocytes. Mechanical testing results also indicated that TSs in Group 1 possessed superior mechanical properties, including Young’s modulus and ultimate tensile stress, compared to other groups, which could be attributed to substantial collagen fiber formation within these TSs. Collagen is responsible for maintaining the structural integrity and mechanical function of tissues in most multicellular organisms.[28, 29] Young’s modulus and ultimate tensile stress of TSs in Group 1 at Week 6 were in the same order of magnitude as those in native human cartilage (1-15 MPa and 7-13 MPa, respectively).[30, 31]
Cells in Group 1 were fully differentiated in 2D culture prior to forming TSs; however, cells in Group 2 were differentiated while the cells were compacted into strands. Although it has been reported that 3D culture (e.g., hydrogel encapsulation and tissue spheroid) induced more reliable chondrogenic differentiation of stem cells than conventional monolayer culture,[32–34] TSs in Group 2 exhibited lower chondrogenic functionality, which might be due to their thick nature, limiting the diffusion of chondrogenic differentiation media into the depth of TSs, and hence slowed down the chondrogenesis of ADSCs.[22] In addition, since the differentiated chondrocytes in Group 1 aggregated in the form of TSs, it could be assumed that the 3D environment prevented the chondrocytes from dedifferentiation and preserved their chondrogenic phenotype.[35] Due to these appealing properties of TSs made of pre-differentiated chondrocytes, Group 1 was preferred for bioprinting experiments. It should be noted the used diameter of TSs is satisfactory to maintain the viability of chondrocytes because of their anaerobic nature.[22]
Cellular alignment at different depth of TSs demonstrated that cells within ~50 μm distance from edges were highly oriented along the longitudinal direction of TSs (Figure 4e). At the boundaries of TSs, cell movement subjected to the wall of alginate capsules. As a result, cells could be guided by the inner surface of the capsule wall, and tended to align along the longitudinal direction of capsules. Arrangement of cells, located at the core of TSs, revealed a random angle distribution. The oriented cells and collagen fibers were mostly observed at a depth of ~50 μm from the edge of TSs. Hence, the overall proportion of cell (or collagen) heterogeneity is supposed to increase with decreased TS diameter, which could be obtained using alginate capsules with a smaller size. As shown in Figure 6g, despite we obtained anisotropy in the entire tissue, the collagen orientation efficiency was higher when only the interface regions were considered. However, this proof-of-concept study has shown a promising attempt to generate zonally-stratified tissues using a scaffold-free approach. The anisotropy in tissues could be further improved by decreasing the diameter of TSs or stimulating them mechanically in a dynamic culture.[36] Cell orientation was considered to affect the alignment of deposited collagen matrix.[37, 38] The mechanism for production of aligned collagen fibers in this study was probably attributed to cell contractility and motility.[39, 40] Cells aligned in the longitudinal direction of TSs and applied contraction forces along the long axes of cells, which aligned collagen fibers in the same direction.[37, 41, 42] The superior mechanical properties in TSs of Group 1 could be attributed to the greater collagen production and its alignment with respect to the other groups.[43]
Articular cartilage exhibits great heterogeneity and complex microarchitecture, which is comprised of three anatomic zones, namely the superficial, middle and deep zones.[44] The thin superficial zone occupies approximately 10-20% of articular cartilage thickness, where the collagen fibers are aligned parallel to the articular surface. The middle zone and deep zone composed 70-90% of the total volume, and collagen fibrils are arranged perpendicular to the articular surface, particularly at the deep zone (Figure 6h). Although the middle zone was absent in our current design, the cartilage construct in this study had a top layer with ~20% thickness (i.e., 0.6 mm) and bottom layer with ~80% thickness (i.e., 2.8 mm), which largely mimicked the anatomy of native cartilage. The compressive modulus of bioprinted cartilage was comparable to the native human articular cartilage, and it could be expected that the mechanical properties of bioprinted cartilage would be further improved if they were cultivated for a longer period of time.
Generally speaking, as distinguished from scaffold-based approaches, scaffold-free constructs possessed higher cell density and ECM formation, which is also free of issues such as degradation and digestion of the polymer matrix. In addition, immobilization of cells and limited cell-cell interactions in hydrogel-based approaches slows down the tissue maturation process. Regarding the 3D bioprinting of zonally-stratified articular cartilage, Ren et al. provided an approach to imitate the zonal structure using a cell density gradient of 3:2:1 in different region of bioprinted constructs.[45] Most recently, Daly et al. inkjet bioprinted mesenchymal stromal cells (MSCs) and chondrocytes into polycaprolactone (PCL) microchambers, which functioned as a support structure guiding the formation of cell aggregates and leading to the development of stratified cartilage tissue with a depth-dependent collagen fiber architecture.[46] In their study, parallel collagen orientation presented in the superficial zone due to the cell migration on the top of the microchamber walls, and the vertical collagen orientation in the depth of scaffold was enhanced resulting from the dynamic culture.[46] However, the presence of PCL microchambers might induce issues such as slow degradation and voids after degradation, which restricts the tissue growth. In our study, taking the advantage of the cellular and collagen alignment within individual TSs, the fabricated cartilage was able to mimic stratified architecture of the native cartilage by assembling the TSs in different orientations through bioprinting (Figure 6h). Owing to the collagen orientation along the longitudinal direction of TSs, horizontal and vertical aligned collagen fibers were present in the corresponding layers in the assembled tissue. Non-linear second harmonic generation could be performed for future work to better characterize the collagen alignment in the 3D construct. Also, it has been observed that TSs fused rapidly and tightly, and the bioprinted tissues did not exhibit structural disintegration during transportation and handling, demonstrating the tight fusion of TSs between layers. Although some gaps between TSs were observed in histological sections (Figure 6e), they can be minimized by the further optimization of the pin array in future work.
In this research, scaffold-free zonally-stratified articular cartilage was engineered using human ADSCs obtained from adipose tissue removal process. The collagen expression in bioprinted tissues was less compared to that of native tissues in histological images as ADSCs were cultured for only five weeks in total in the induction media (three weeks before bioprinting and two weeks after bioprinting). Longer term culture of bioprinted tissues could enhance further collagen synthesis. Bioprinting of scalable TSs enabled the fabrication of cartilage at the clinically-relevant volumes demonstrating its clinical translation potential for treatment of chondral injury and osteoarthritis using cells from the same patient. In future work, cells in different zones could be cultured with different biochemical stimulation to induce the zone-specific expression of different proteins, such as lubricin in the superficial zone and type X collagen in the deep zone.
The bioprinted tissues could be a substitute of conventional chondral plug which is used in the osteochondral mosaicplasty.[47] As compared to mosaicplasty of autograft or allograft, the use of bioprinted tissues can avoid drawbacks include donor-site soreness and limited availability of donor tissues.[47] In addition, the tissues could be customized based on the defect size, eliminating multiple sample extraction due to the fixed size of surgical instrument. Currently, TSs in bioprinted tissues were tightly fused resulting in very stable constructs for transportation, handling and mechanical testing. Indeed, mechanical testing study revealed a compression modulus of ~1.1 MPa, which is very similar to the compression modulus of human articular cartilage. Tissues are expected to be immobilized by press-fit effect.[48] Space between the tissues and the defect, if any, could be filled with a small amount of a surgical glue to facilitate tissue-host adhesion. As their mechanical properties were similar to that of nature human articular cartilage, we expect that they can be sutured.[49] However, such implantation is out of the scope of this study, which could be explored in the future.
4. Conclusion
Herein, we reported for the first time a novel strategy in scaffold-free bioprinting of zonally-stratified articular cartilage with horizontally and vertically aligned TSs. TSs made of pre-differentiated chondrocytes from human ADSCs demonstrated greater chondrogenic functionality, in terms of biological and mechanical properties, as compared to ADSCs differentiated to chondrocytes after being compacted into TSs. Post bioprinting, TSs of pre-differentiated chondrocytes were able to self-assemble into an integrated tissue patch. Cellular and collagenous orientation in individual TSs was maintained in bioprinted tissues with a zone-specific organization, which imitated the matrix arrangement in native articular cartilage. Additionally, this study offers a close-looped practical strategy for regenerative medicine, which uses autologous stem cells from surgical waste (fat tissue in this case) to fabricate tissue substitutes with clinically-amenable scale and biologically-relevant microstructure.
5. Materials and methods
5.1. Fabrication of tubular alginate capsules
Tubular alginate capsules were fabricated using a coaxial nozzle apparatus as described in our previous work.[18] Briefly, sterilized sodium alginate powder (Sigma Aldrich, MO) was dissolved in sterile deionized (DI) water and subjected to magnetic stirring overnight to obtain 4% (w/v) alginate solution. 4% calcium chloride (CaCl2) solution in sterile DI water was used as a crosslinker. The coaxial nozzle was comprised of a 22 G inner nozzle for CaCl2 deposition and a 14 G outer nozzle for alginate extrusion. The dispensing pressure of alginate was 82.7 kPa while the CaCl2 dispensing rate was 16 mL/min.[19] Crosslinked alginate capsules were collected into a CaCl2 pool for further crosslinking.
5.2. Fabrication of TSs
To obtain human ADSCs, surgically discarded adipose tissues were obtained from patients, who underwent an elective adipose tissue removal process (e.g., panniculectomy) at the Pennsylvania State University (Hershey, PA) with patients’ consent and approved by the Institutional Review Board (IRB protocol #4972). Isolation of ADSCs was performed according to our previously published work [22] with verification of flow cytometry against CD73 and CD90. The sorted ADSCs were cultured in DMEM/F12 supplement with 20% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C with 5% CO2. Cell medium was changed every three days.
In order to obtain cartilage TSs, three groups were designed and compared. In Group 1, ADSCs were expanded and differentiated into chondrocytes for 3 weeks using Human Chondrocyte Differentiation Media (Cell Applications, CA). Chondrocytes were then trypsinized and centrifuged to form cellular pellets at the bottom of 1.5 mL tubes, and the cell medium was removed to obtain concentrated cell pellets. Next, cell pellet in each tube was aspirated using a syringe (Hamilton Company, NV) and injected into each alginate capsule, followed by closing both ends of the capsule using vascular clamps (Thomas Scientific, NJ). Samples were further cultured for 3 weeks. In Group 2, ADSCs were injected into capsules in the same manner but differentiated after they were injected into capsules using the same differentiation media for 3 weeks. In Group 3 (control), ADSCs were injected into capsules and cultured with ADSC culture media for 3 weeks. After maturation of strands, alginate capsules were de-crosslinked using sodium citrate solution (4% (w/v) in sterile DI water) leaving TSs behind. TSs were rinsed with phosphate buffer saline (PBS) twice for 10 min after removing sodium citrate solution. Cell density of solid TSs was ~1.6×105 cells/mm. The morphology of TSs, which were cultured in alginate capsules, were observed using an EVOS® microscope (Invitrogen, MA), and the diameter of TSs were measured at different time points using ImageJ software (National Institutes of Health, MD).
5.3. Cell viability before and after microinjection for the TS formation
Differentiated ADSCs in monolayer culture were trypsinized and collected on Day 21. In order to determine cell viability, Trypan blue staining was performed.[50] The collected cells were aspirated using the syringe and injected into alginate capsules as described in Section 5.2. Thereafter, the alginate capsules were placed in 4% sodium citrate solution until alginate was completely dissolved. The cells were then suspended again to determine their viability after the microinjection process using Trypan blue staining. Three samples (n=3) were used and the data was obtained by normalizing cell viability after microinjection process with respect to that before injection.
5.4. Water content measurement
In order to determine their wet weight, TSs cultured for 3 weeks were de-crosslinked and then washed with PBS. Next, PBS was removed and TSs were lightly blotted to remove surface moisture. In order to facilitate sample handling, samples were immediately transferred to a small piece of weighing paper, which was dried at 67 °C in a vacuum oven (Model 282A, Isotemp™, PA). The weight of each sample and dried paper was measured using a scale (ML104T, Mettler Toledo, OH) and wet weight (Wwet) was calculated by subtracting the weight of dried paper from the reading. The sample with weighing paper was dried at 67 °C in a vacuum oven [51] and the combined weight was measured every 30 min until obtaining a constant weight. Dry weight (Wdry) was calculated by subtracting the weight of paper from the reading. Water content (WC) was calculated using Eq. (1):
| (1) |
5.5. Histology analysis
All groups of TSs were cultured for 3 weeks, de-crosslinked, fixed with 4% paraformaldehyde, and embedded in paraffin using an automatic tissue processor (TP 1020, Leica, Germany). Next, TSs were gradually dehydrated in alcohol, cut into 8 μm sections, and placed onto charged slides. Sections were then dewaxed using Leica Autostainer XL (Leica, Germany) and underwent Toluidine Blue O and Picrosirius Red/Fast Green staining according to standard protocols for detection of GAGs and collagen, separately. Briefly, for Toluidine Blue O staining, sections were incubated in a Toluidine Blue solution (0.1% in DI water, Sigma Aldrich, MO) at room temperature for 2 min.[52] The dye was then removed and samples were washed twice with DI water. After dehydration with ascending alcohol and clearing with xylene, coverslips were mounted to the slides using Xylene Substitute Mountant (Thermo Fisher Scientific Inc., PA). Picrosirius Red/Fast Green staining solution was prepared by dissolving 0.1% direct Red 80 and 0.1% Fast Green FCF in saturated aqueous picric acid (1.2% picric acid in water, Sigma Aldrich, MO). Picrosirius Red/Fast Green solution was then applied to the sections and sections were incubated for 1 h.[53] Samples were rinsed with DI water, dehydrated with alcohol, cleaned with xylene, and then mounted. Finally, samples were imaged using a BX51 microscope (Olympus, Japan).
5.6. Immunohistochemistry assay
All primary monoclonal antibodies were purchased from Abcam (MA) and florescence-conjugated secondary antibodies were purchased from Life Technologies (CA). Sections of TSs in all groups were treated with Triton-X 100 (0.1 % in PBS, 10 min) followed by blocking them with normal goat serum (NGS, 10 % in PBS, 1 h). Samples were then incubated with monoclonal rabbit anti-human collagen type II (COL-II, 1:200), mouse anti-human aggrecan (1:50), and NGS (negative control) for 1 h. Samples were then washed twice with PBS and incubated with secondary antibodies (goat anti-rabbit IgG (H+L)-Alexa Fluor 647 for COL-II, and goat anti-mouse IgG (H+L)-Alexa Fluor 488 for Aggrecan, both at 1:200 dilution) for another hour. After rinsing thrice with PBS, samples were finally incubated with Hoechst 33258 (1:200, 5 min) for nucleus visualization and mounted with Fluoromount-G™ (Invitrogen, MA). Images for each marker were taken by a Zeiss Axiozoom microscope (Carl Zeiss Microscope LLC, NY).
5.7. Protein content evaluation
sGAG content was determined by DMMB dye-binding assay. All groups cultured for 3 weeks were washed and digested in 500 μL solution of 0.1 mg/mL papain extraction reagent at 65 °C in water bath for 18 h. 20 μL of the digested samples were mixed with 200 μL DMMB solution and the absorbance was measured at 525 nm using a microplate reader (PowerWaveX, BioTek, Winooski, VT). Serially diluted solution of chondroitin 4 sulfate was prepared as the standard and the sGAG content was calculated according to the standard curve.[19] The DNA content of same samples was also measured by a DNA quantitation assay. A Quant-iT™ PicoGreen dsDNA Assay Kit (Molecular Probes Inc., Eugene, OR) was used according to the manufacturer’s instructions. Fluorescence intensity was determined by a SpectraMax multidetection microplate reader (Molecular Devices, Inc., Sunnyvale, CA) using a wavelength of 480 nm (excitation) and 520 nm (emission). sGAG content from each sample was normalized to dsDNA content.
A PIIANP ELISA Kit (Millipore, Germany) was used to quantitatively determine the level of PIIANP released into the media by cells. To facilitate the release of PIIANP, TSs were segregated by pipette aspiration using 0.5 mL 0.25% Typsin (Corning, Manassas, VA) and incubated for 5 min at 37 °C with 5% CO2. Segregated samples were centrifuged and cultured in 1 mL ADSC culture media for 24 h. 5 μL of each cell culture media was used as samples for the PIIANP ELISA Kit and the assay was conducted according to the manufacturer’s instructions. The absorbance was measured at a primary wavelength of 450 nm with a reference wavelength of 590 nm. The PIIANP content was calculated according to the standard curve and normalized to the DNA content of each sample.
5.8. Gene expression analysis through real time polymerase chain reaction (RT-PCR)
In order to determine the expression of cartilage specific genes, TSs in all groups were homogenized in TRIzol reagent (Life Technologies, CA), followed by adding 0.2 ml chloroform per 1 ml TRIzol reagent and centrifuging the mixture at 12,000 g for 15 min at 4 °C. The upper aqueous phase with RNA was transferred and the RNA was then precipitated by adding 0.5 ml isopropyl alcohol per 1 ml TRIzol reagent, followed by centrifuging at 12,000 g for 10 min at 4 °C. Subsequently, the precipitated RNA was rinsed twice by 75% ethanol, air dried for 5 min, and dissolved in 50 μl diethyl pyrocarbonate (DEPC)-treated water. RNA concentration was measured using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, MA). All RNA samples were reconstituted with the nuclease-free DI water to obtain an identical concentration of 20 ng/μL, followed by reverse transcription using AccuPower® CycleScript RT Premix (BIONEER, Korea) following the manufacturer’s instructions. Gene expression was analyzed quantitatively with SYBR Green using a 7500 RT-PCR system (Applied Biosystems®, Life Technologies, CA) to analyze the transcription levels of cartilage matrix-related genes, including collagen type II (COL2A1), Aggrecan and chondrogenic transcription factor SOX9, and all values were normalized by GAPDH. For gene sequences, the reader is referred to Table 2. For all genes, the fold change of Group 3 (control group) was set as 1-fold and values in Groups 1 and 2 were normalized with respect to those in Group 3.
Table 2:
Primer information for qRT-PCR
| Gene | Primer | |
|---|---|---|
| GAPDH | Forward | 5’-CACATGGCCTCCAAGGAGTA-3’ |
| Reverse | 5’-GTACATGACAAGGTGCGGCT-3’ | |
| Aggrecan | Forward | 5’-TCCCCTGCTATTTCATCGAC-3’ |
| Reverse | 5’-CCAGCAGCACTACCTCCTTC-3’ | |
| SOX9 | Forward | 5’-AGCGAACGCACATCAAGAC-3’ |
| Reverse | 5’-CTGTAGGCGATCTGTTGGGG-3’ | |
| COL2A1 | Forward | 5’-CCAGATGACCTTCCTACGCC-3’ |
| Reverse | 5’-TTCAGGGCAGTGTACGTGAAC-3’ |
5.9. Mechanical testing of TSs
After de-crosslinking of alginate capsules following 3 weeks of culture, TSs were immediately transferred to a BioTense Bioreactor (ADMET, Inc., MA) to evaluate their tensile strength characteristics. Each sample, at a maximum of 10 mm in length, was loaded with a rectangular mini sandpaper at both ends to prevent slippage during testing. Tensile testing was carried out at a strain rate of 3 mm/min. Upon applying the mechanical load, TSs were ruptured in the middle. Displacement and load data were recorded by a data acquisition system (MTestQuattro System, MA). Young’s Modulus, ultimate tensile stress and strain were determined from the stress-strain curves. TSs in Group 1 were further cultured for three more weeks (i.e., six weeks in total) and mechanical properties were determined as explained above.
5.10. Characterization of cell elongation and alignment
Fluorescent images of cell nuclei in TSs were used to analyze cell elongation and alignment using a built-in function of ImageJ software.[38, 54] Two regions were defined within the strand domain based on the distance from edges (i.e., 0-50 μm as “edge” and the rest region as “core”). Cell orientation angle was defined as the orientation of the major elliptic axis of individual nucleus. A preferential orientation was defined as the longitudinal direction of the strand in each image, which was set to be 90°. Cell nuclei falling within 60 to 120° were considered to be aligned longitudinally and those falling within 0 to 30° or 150 to 180° were considered to be aligned transversely. The alignment efficiency was presented as a percentage of nuclei oriented in certain ranges. Nucleus elongation was determined by the circularity of ellipse.
5.11. Bioprinting of zonally-stratified articular cartilage
In this study, the zonally-stratified cartilage, which was comprised of superficial and bottom zones, was fabricated using a hybrid process. For the bottom zone, a home-made pin array was used to guide the vertical placement of TSs. The pin base was created by a 3D printer (Ultimaker, Geldermalsen, Netherlands) with an inter-hole distance of ~700 μm, a hole diameter of ~200 μm, and a thickness of ~1 mm. In order to immobilize the pins on the base, 3 mL of 2% agarose (in DI water) was suspended in a 35 X 10 mm tissue culture dish (Corning, NY) and then solidified in 4 °C for 20 min. The base was then press-fitted into the solidified agarose substrate. Stainless steel pins with a diameter of 150 μm (Entomoravia, Slavkov u Brna, Czech Republic) was cut to ~5 mm in length, and inserted into the needle base to create a 4 X 4 pin array (Figure 1). The pin array was washed twice with 70% ethanol for 10 min followed by being exposed to ultraviolet (UV) light for 30 min for sterilization. TSs in Group 1 after 3 weeks of culture were used for cartilage bioprinting. The bottom zone was bioprinted into the pin array using an AAB technique described in our recent work.[25] TSs were cut using a surgical blade into 2.5 mm in length and transferred with a pipette to a reservoir, which contained cell culture media. In order to fabricate custom-made glass pipettes, borosilicate Pasteur pipettes (VWR, PA) were pulled using a P-2000 Flaming/Brown micropipette puller (Sutter Instrument, Novato, CA). The customized glass nozzle with a diameter of ~80 μm and vacuum pressure of ~300 mmHg was used to aspirate TSs from the cell media. By applying vacuum pressure, each TS was attracted and picked by the pipette, then lifted and transferred, at a speed of 75 mm/s, to the pin array (which was placed next to the reservoir). Each TS was then slowly inserted at a speed of 10 mm/s into the space between pins. With real-time monitoring using CCD cameras, the position of TS could be fine-tuned during the process by adjusting XYZ axes to ensure the smooth insertion of TS without any damage. When TSs reached the designated position, the vacuum pressure was terminated and the pipette was moved up. The process was repeated as needed until the designed tissue was constructed. Droplets of cell medium were added to prevent dehydration after bioprinting of each TS. Since unmodified alginate is inert to cell adhesion due to the lack of cell receptors,[55] it was used as a supporting material to maintain the position of TSs throughout the bioprinting process. A minimum amount of 2% alginate (in DI water) was gently suspended around the bioprinted construct by manual pipetting, and then crosslinked with 4% CaCl2. The agarose substrate was then gently removed, leaving the needle base and alginate-supported construct behind. The pins were then removed followed by detaching the alginate shell from the pin base (Figure 1). The construct was then cultured with chondrogenic media supplemented with 2.5 μg/mL fungizone (Life Technologies, CA) for a week, which was changed every other day.
In order to bioprint the superficial zone, MABP with a customized nozzle system [19] was utilized. Before bioprinting, alginate capsules were de-crosslinked with 4% sodium citrate and TSs were loaded into an interchangeable nozzle under sterile conditions. Droplets of cell medium were added to prevent dehydration. Next, the nozzle was mounted onto the MABP. Filter paper, which was hydrated with cell culture media, was used as a substrate. A robot printing speed of 50 mm/min and extrusion speed of 25 mm/min were used. The minimum amount of 2% alginate was gently suspended around the bioprinted TSs and crosslinked with 4% CaCl2, in order to maintain their position. After crosslinking, alginate-supported constructs were detached from the filter paper and incubated with chondrogenic media (supplemented with 2.5 μg/mL fungizone) for a week, which was changed every other day.
After one week of culture, TSs in both zones fused and sodium alginate around the superficial zone was de-crosslinked. The superficial zone was then transferred and placed on top of the bottom zone (Figure 1). In order to stabilize the assembled tissue, a minimum amount of 2% sodium alginate was suspended to coat the entire construct, which was crosslinked with 4% CaCl2 and cultured under chondrogenic media for another week until superficial and bottom zone were fused completely.
5.12. Cell viability on TSs before and after extrusion
In order to investigate cell viability before and after extrusion, TSs in Group 1 was loaded into the customized print head and extruded through the mechanical-extrusion system of the multi-arm bioprinter (MABP) at a speed of 25 mm/min. Thereafter, TSs, before and after extrusion process, were stained by 2 μM calcein AM (Life Technologies, MA) and 1 μM ethidium homodimer-1 (Invitrogen, MA) solutions according to our previous work.[50] After 30 min of incubation, the stained TSs were washed with PBS thrice for 15 min, followed by mounting with Neo-Mount® anhydrous mounting medium (Millipore, Germany). The stained TSs were imaged using the Zeiss LSM880 confocal microscope. ImageJ software was used for quantitative analysis for red- and green-fluorescent cells. Data were obtained from three measurements per sample, and three independent samples (n=3) were used for both groups.
5.13. Mechanical test of zonally-stratified articular cartilage
Mechanical properties of bioprinted tissues were measured by a Thermomechanical Analyzer (TMA) (TMA 402 F1/F3 Hyperion, Netzsch, Germany). The fused silica sample fixture for expansion measurements was employed to conduct a quasi-static compression testing on the prepared specimens with cross-sectional dimensions of 3.13 × 3.13 mm. Since the TMA 402 F1/F3 Hyperion was a force control instrument, a ramp force signal changing from 1 to 1.98 N over the temperature range of 21-22 °C with heating rate of 0.10 K/min was implemented into the test protocol to simulate the quasi-static compressive loading. Force and strain data collected from the experiment were used to determine the compressive stress-strain curves. The linear elastic region was selected using the first 6-7 data points in stress-strain curves. Elastic modulus of the samples was determined according to the linear elastic region.
5.14. Histological analysis of zonally-stratified articular cartilage
Fabricated constructs were fixed, embedded in paraffin, and sectioned. Sections were then stained with H&E using Leica Autostainer XL and mounted. Nucleus staining was performed as described in Section 5.9 in order to quantify the cell orientation and elongation. On sections of the superficial zone, regions within 50 μm from the edges of TSs were defined as “edge” region, while the rest regions were defined as the “core” region. Samples were imaged using the EVOS® fluorescence microscope. Picrosirius Red staining was performed on the sagittal sections of tissues to observe the orientation of collagen fibers as mentioned in Section 5.4. Samples were imaged using the Olympus BX51 microscope. Orientation angles and the area occupied by collagen fibers at bottom and superficial zones were measured using the built-in function of ImageJ software, respectively. Collagen fibers oriented within 60 to 120° were considered aligned vertically and those oriented within 0 to 30° or 150 to 180° were considered aligned horizontally. The collagen alignment efficiency was presented using Eq. (2):
| (2) |
In order to investigate collagen orientation at the boundaries of fused TSs, zones with the area of 0.5 mm × 0.4 mm near the boundaries of TSs were imaged and analyzed in the same way.
5.15. Procurement and treatment of human cartilage
An Institutional Review Board-approved protocol was executed to collect discarded de-identified cartilage from patients. The normal human cartilage was obtained from amputees’ knees. Cartilage plugs were fixed for 7 days in 10% neutral buffered formalin (NBF), decalcified for 2 weeks in 10% w/v EDTA, embedded in paraffin, and 5-μm sections were cut and mounted for histological staining. Picrosirius Red staining was performed on the sections to observe the collagen as mentioned in Section 5.4.
5.16. Statistical analysis
Data were presented as mean ± standard deviation and analyzed using Student’s t test and one-way analysis of variance (ANOVA) to test for significance when comparing the data. Post-hoc Tukey’s multiple comparison test was used to determine the individual differences among the groups. Differences were considered significant at *p < 0.05, **p < 0.01 and ***p < 0.001. Statistical analysis was performed using Statistical Product and Service Solutions software (SPSS, IBM, USA). In particular, for cell viability analysis before and after TSs extrusion or before and after microinjection, Mann-Whitney non-parametric test, which is an alternative to parametric two-tailed Student t-test, was used assuming not equal variances at 95% of confidence level using Minitab software.
Acknowledgement
This work has been supported by National Science Foundation Award # 1624515 and the Hartz Professorship awarded to I.T.O. The authors also acknowledge the support from Engineering Science and Mechanics Department, the Huck Institutes of Life Sciences and Materials Research Institutes at the Pennsylvania State University.
Contributor Information
Yang Wu, Engineering Science and Mechanics Department, Penn State University, University Park, PA, USA; The Huck Institutes of the Life Sciences, Penn State University, University Park, PA, USA.
Bugra Ayan, Engineering Science and Mechanics Department, Penn State University, University Park, PA, USA; The Huck Institutes of the Life Sciences, Penn State University, University Park, PA, USA.
Kazim K. Moncal, Engineering Science and Mechanics Department, Penn State University, University Park, PA, USA; The Huck Institutes of the Life Sciences, Penn State University, University Park, PA, USA
Youngnam Kang, Engineering Science and Mechanics Department, Penn State University, University Park, PA, USA; The Huck Institutes of the Life Sciences, Penn State University, University Park, PA, USA.
Aman Dhawan, Department of Orthopaedics and Rehabilitation, Penn State College of Medicine, Milton S. Hershey Medical Center, Hershey, PA, USA.
Srinivas Koduru, Department of Surgery, Penn State Health Milton S. Hershey Medical Center, Hershey, PA, USA.
Dino J. Ravnic, Department of Surgery, Penn State Health Milton S. Hershey Medical Center, Hershey, PA, USA
Fadia Kamal, Department of Orthopaedics and Rehabilitation, Penn State College of Medicine, Milton S. Hershey Medical Center, Hershey, PA, USA.
Ibrahim T. Ozbolat, Engineering Science and Mechanics Department, Penn State University, University Park, PA, USA; The Huck Institutes of the Life Sciences, Penn State University, University Park, PA, USA; Biomedical Engineering Department, Penn State University, University Park, PA, USA; Materials Research Institute, Penn State University, University Park, PA, USA; W313 Millennium Science Complex, Penn State University, University Park, PA 16802
References
- [1].Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG, Arthroscopy 1997, 13, 456. [DOI] [PubMed] [Google Scholar]
- [2].Farr J, Cole B, Dhawan A, Kercher J, Sherman S, Clin. Orthop. Relat. Res 2011, 469, 2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang L, Hu J, Athanasiou KA, Crit. Rev. Bioeng 2009, 37, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Laurencin CT, Ambrosio A, Borden M, Cooper J Jr, Annu. Rev. Biomed. Eng 1999, 1, 19. [DOI] [PubMed] [Google Scholar]
- [5].Temenoff JS, Mikos AG, Biomaterials 2000, 21, 431. [DOI] [PubMed] [Google Scholar]
- [6].Harris J, Siston R, Brophy R, Lattermann C, Carey J, Flanigan D, Osteoarthr. Cartil 2011, 19, 779. [DOI] [PubMed] [Google Scholar]
- [7].Rai V, Dilisio MF, Dietz NE, Agrawal DK, J. Biomed. Mater. Res. A 2017, 105, 2343. [DOI] [PubMed] [Google Scholar]
- [8].Caldwell KL, Wang J, Osteoarthr. Cartil 2015, 23, 351. [Google Scholar]
- [9].Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA, Nat. Rev. Rheumatol 2015, 11, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hunziker E, Quinn T, Häuselmann H-J, Osteoarthr. Cartil 2002, 10, 564. [DOI] [PubMed] [Google Scholar]
- [11].Boschetti F, Pennati G, Gervaso F, Peretti GM, Dubini G, Biorheology 2004, 41, 159. [PubMed] [Google Scholar]
- [12].Chen S, Falcovitz Y, Schneiderman R, Maroudas A, Sah R, Osteoarthr. Cartil 2001, 9, 561. [DOI] [PubMed] [Google Scholar]
- [13].Ofek G, Athanasiou K, J. Mech. Mater. Struct 2007, 2, 1059. [Google Scholar]
- [14].MacBarb RF, Chen AL, Hu JC, Athanasiou KA, Biomaterials 2013, 34, 9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Catros S, Fricain J-C, Guillotin B, Pippenger B, Bareille R, Remy M, Lebraud E, Desbat B, Amédée J, Guillemot F, Biofabrication 2011, 3, 025001. [DOI] [PubMed] [Google Scholar]
- [16].Datta P, Dhawan A, Yu Y, Hayes D, Gudapati H, Ozbolat IT, Int. J. Bioprint 2017, 3, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wu Y, Kennedy P, Bonazza N, Yu Y, Dhawan A, Ozbolat I, Cartilage 2018, 1947603518809410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Akkouch A, Yu Y, Ozbolat IT, Biofabrication 2015, 7, 031002. [DOI] [PubMed] [Google Scholar]
- [19].Yu Y, Moncal KK, Li J, Peng W, Rivero I, Martin JA, Ozbolat IT, Scientific Reports 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lin RZ, Chang HY, Biotechnology journal 2008, 3, 1172. [DOI] [PubMed] [Google Scholar]
- [21].Sart S, Tsai AC, Li Y, Ma T, Tissue engineering. Part B, Reviews 2014, 20, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu Y, Hospodiuk M, Peng W, Gudapati H, Neuberger T, Koduru S, Ravnic DJ, Ozbolat IT, Biofabrication 2018, 11, 015009. [DOI] [PubMed] [Google Scholar]
- [23].Mow VC, Ratcliffe A, Poole AR, Biomaterials 1992, 13, 67. [DOI] [PubMed] [Google Scholar]
- [24].Kiani C, Liwen C, Wu YJ, Albert JY, Burton BY, Cell research 2002, 12, 19.11942407 [Google Scholar]
- [25].Ayan B, Nyoung Heo D, Zhang Z, Dey M, Povilianskas A, Drapaca C, Ozbolat IT, Sci. Adv 2019, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Little CJ, Bawolin NK, Chen X, Tissue Engineering Part B: Reviews 2011, 17, 213. [DOI] [PubMed] [Google Scholar]
- [27].Beck EC, Barragan M, Tadros MH, Gehrke SH, Detamore MS, Acta biomaterialia 2016, 38, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Depalle B, Qin Z, Shefelbine SJ, Buehler MJ, Journal of the mechanical behavior of biomedical materials 2015, 52, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu Y, Han Y, Wong YS, Fuh JYH, Journal of tissue engineering and regenerative medicine 2018, 12, 1798. [DOI] [PubMed] [Google Scholar]
- [30].Roberts S, Weightman B, Urban J, Chappell D, The Journal of bone and joint surgery. British volume 1986, 68, 278. [DOI] [PubMed] [Google Scholar]
- [31].Akizuki S, Mow VC, Müller F, Pita JC, Howell DS, Manicourt DH, Journal of Orthopaedic Research 1986, 4, 379. [DOI] [PubMed] [Google Scholar]
- [32].Hwang NS, Kim MS, Sampattavanich S, Baek JH, Zhang Z, Elisseeff J, Stem cells 2006, 24, 284. [DOI] [PubMed] [Google Scholar]
- [33].Xu J, Wang W, Ludeman M, Cheng K, Hayami T, Lotz JC, Kapila S, Tissue Engineering Part A 2008, 14, 667. [DOI] [PubMed] [Google Scholar]
- [34].Yoon HH, Bhang SH, Shin J-Y, Shin J, Kim B-S, Tissue Eng. Part A 2012, 18, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hubka KM, Dahlin RL, Meretoja VV, Kasper FK, Mikos AG, Tissue Engineering Part B: Reviews 2014, 20, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rubbens MP, Driessen-Mol A, Boerboom RA, Koppert MM, Van Assen HC, Romeny BMT, Baaijens FP, Bouten CV, Ann. Biomed. Eng 2009, 37, 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang JH, Jia F, Gilbert TW, Woo SL, Journal of biomechanics 2003, 36, 97. [DOI] [PubMed] [Google Scholar]
- [38].Wu Y, Wang Z, Fuh JYH, Wong YS, Wang W, Thian ES, Journal of Biomedical Materials Research Part B: Applied Biomaterials 2017, 105, 616. [DOI] [PubMed] [Google Scholar]
- [39].Wang JH-C, Grood ES, Florer J, Wenstrup R, Journal of Biomechanics 2000, 33, 729. [DOI] [PubMed] [Google Scholar]
- [40].Wang Z, Lee W, Koh B, Hong M, Wang W, Lim P, Feng J, Park L, Kim M, Thian E, Science advances 2018, 4, eaat4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhu B, Lu Q, Yin J, Hu J, Wang Z, Tissue Eng. 2005, 11, 825. [DOI] [PubMed] [Google Scholar]
- [42].Eastwood M, Mudera V, McGrouther D, Brown R, Cell motility and the cytoskeleton 1998, 40, 13. [DOI] [PubMed] [Google Scholar]
- [43].Pins GD, Christiansen DL, Patel R, Silver FH, Biophysical journal 1997, 73, 2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sophia Fox AJ, Bedi A, Rodeo SA, Sports health 2009, 1, 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ren X, Wang F, Chen C, Gong X, Yin L, Yang L, BMC musculoskeletal disorders 2016, 17, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Daly AC, Kelly DJ, Biomaterials 2019, 197, 194. [DOI] [PubMed] [Google Scholar]
- [47].Medvedeva EV, Grebenik EA, Gornostaeva SN, Telpuhov VI, Lychagin AV, Timashev PS, Chagin AS, International journal of molecular sciences 2018, 19, 2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Robert H, Orthopaedics & Traumatology: Surgery & Research 2011, 97, 418. [DOI] [PubMed] [Google Scholar]
- [49].Hunziker EB, Stähli A, Osteoarthritis and cartilage 2008, 16, 1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Moncal KK, Ozbolat V, Datta P, Heo DN, Ozbolat IT, Journal of Materials Science: Materials in Medicine 2019, 30, 55. [DOI] [PubMed] [Google Scholar]
- [51].Venn M, Maroudas A, Annals of the rheumatic diseases 1977, 36, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bergholt NL, Lysdahl H, Lind M, Foldager CB, Cartilage 2018, 1947603518764262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Segnani C, Ippolito C, Antonioli L, Pellegrini C, Blandizzi C, Dolfi A, Bernardini N, PloS one 2015, 10, e0144630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wu Y, Wang Z, Fuh JYH, San Wong Y, Wang W, San Thian E, J. Mater. Sci. Mater. Med 2016, 27, 115. [DOI] [PubMed] [Google Scholar]
- [55].Lee KY, Mooney DJ, Prog. Polym. Sci 2012, 37, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]


