Abstract
There is a considerable periprocedural risk of thromboembolic events in atrial fibrillation patients undergoing cardioversion, and treatment with anticoagulants is therefore a hallmark of cardioversion safety. Based on retrospective subgroup analyses and prospective studies, non-vitamin K anticoagulants are at least as efficient as vitamin K-antagonists in preventing thromboembolic complications after cardioversion. The risk of thromboembolic complications after cardioversion very much depends on the comorbidities in a given patient, and especially heart failure, diabetes, and age >75 years carry a markedly increased risk. Cardioversion has been considered safe within a 48-h time window after onset of atrial fibrillation without prior treatment with anticoagulants, but recent studies have set this practice into question based on e.g. erratic debut assessment of atrial fibrillation. Therefore, a simple and more practical approach is here suggested, where early cardioversion is performed only in hemodynamically unstable patients.
Keywords: Cardioversion, Safety, Atrial fibrillation, Anticoagulation, Stroke prevention
Introduction
Cardioversion of patients with atrial fibrillation (AF) is a procedure that is commonly utilized in clinical praxis [1]. The evolving guidelines on AF since 2010 have progressively emphasized a rhythm-control approach as preferred initial treatment strategy in many patients with new-onset AF, a development triggered by the successful implementation of ablation procedures shown to be applicable in a variety of AF patients [2, 3, 4]. Very often, the initial step in rhythm control is the conversion of new-onset or recurrent AF to normal sinus rhythm.
The current appraisal is focusing on the most prevalent complications of direct-current and pharmacological cardioversion: embolic strokes. We here examine the literature on documented periprocedural stroke rates in cardioversion. We then propose increased scrutiny on several cardioversion-related risk factors in order to minimize the occurrence of such iatrogenic strokes, ultimately aiming at increasing the safety of patients.
Current Recommendations
To prevent thromboembolic complications, current European Society of Cardiology (ECS) and American College of Cardiology/American Heart Association (ACC/AHA) guidelines on AF management recommend anticoagulation for at least 3 weeks prior to cardioversion and at least 4 weeks post cardioversion, irrespective whether the procedure is carried out as direct-current (DC-cardioversion, electrical cardioversion) or as pharmacological cardioversion [4, 5]. This recommendation applies for all patients with an unknown duration of AF, or a duration exceeding 48 h. In cases where new-onset AF is clarified to have lasted less than 48 h, cardioversion without prior anticoagulation is acceptable, with anticoagulation being initiated as soon as possible and continued for at least 4 weeks after the procedure. In patients at increased risk of stroke, anticoagulation treatment should be lifelong.
Of note, the recommendation related to the so-called safe time window of 48 h is arbitrary, a fact that is explicitly stated in the latest ESC guidelines on AF under “gaps of evidence.” Indeed, this recommendation is primarily based on consensus derived from older studies, and it has never been validated in contemporary controlled clinical trials.
Incidence of Thromboembolic Complications in Cardioversion Using Vitamin-K Antagonists
Periprocedural risk of thromboembolic events during cardioversion when there is suboptimal or no anticoagulation is staggering, with stroke rates up to 3.4–6.8% [6, 7]. Observational data have shown that vitamin-K antagonist (VKA) therapy reduces the incidence to 0.5–1.6% [8]. One reliable source to determine stroke rates in patients treated with VKA are the head-to-head comparator (i.e., VKA) arms of the pivotal trials investigating non-vitamin-K oral anticoagulants (NOACs) in stroke prevention in AF.
Each of the four pivotal trials of NOACs provided a post-hoc analysis of those patients who underwent cardioversion. In the RE-LY trial investigating Dabigatran versus VKA, the incidence of stroke and systemic embolism in the VKA group was 0.6% [9]. In the ARISTOTLE study, VKA had no recorded strokes after cardioversion [10]. In the ROCKET-AF trial, the incidence of stroke or systemic embolism after cardioversion was 1.9% in the VKA treated group [11]. Finally, in the ENGAGE-AF study, there were no recorded strokes after cardioversion in the VKA study arm [12].
Of course, prospective trials have contributed to our knowledge in the cardioversion context as well. In the X-VeRT trial comparing rivaroxaban to VKA in early and delayed cardioversion, the rate of thromboembolic complications was 1.0% in the VKA study arm [13]. In the ENSURE-AF trial investigating edoxaban versus VKA, the primary endpoint was 1.0% for VKA, which included stroke, systemic embolism, myocardial infarction, and cardiovascular mortality. For stroke and systemic embolism alone, the incidence was 0.3% [14]. In the EMANATE study in early and delayed cardioversion, comparing apixaban to VKA, the overall incidence of thromboembolic complications was 0.8% in the VKA arm [15].
Incidence of Thromboembolic Complications in Cardioversion Using NOACs
Very much as elucidated for the VKAs, data for cardioversion safety can be extracted from the pertinent retrospective subgroup analyses derived from the four pivotal trials, as well as the few prospective head-to-head comparison trials in cardioversion.
In the RE-LY study, cardioversion during NOAC treatment had a 0.3% post-procedural stroke rate in the dabigatran group (150 mg b.i.d.) [9]. In the ARISTOTLE study, apixaban had no recorded strokes after cardioversion [10]. In the ROCKET-AF trial, rivaroxaban had a post-cardioversion stroke rate or systemic embolism rate of 1.9% [11]. Finally, in the ENGAGE-AF study, there were no recorded strokes after cardioversion in the full-dose (60 mg) edoxaban study arm [12].
In addition to these retrospective data, again one can add data from the prospective trials in the cardioversion context. The X-VeRT study showed a 0.5% stroke rate after cardioversion in the rivaroxaban arm [13]. In the ENSURE-AF study, the rate of stroke and systemic embolism in the edoxaban arm was 0.2% [14]. Finally, in the EMANATE trial, cardioversion performed in the group randomized to apixaban triggered no strokes; i.e., displaying a 0.0% incidence [15].
Taken together, it appears safe to state that the combined knowledge base shows at least the same protection provided by NOACs as compared to VKAs against stroke and systemic embolism after cardioversion [16].
Predictors of Thromboembolic Complications in Cardioversion
Thromboembolic complications depend on a number of factors. One is the practice of the 48 h safety time window, another pertains to the duration of pre- and post-cardioversion anticoagulation, and a third has to do with comorbidities. Let us scrutinize why the time window of less than 48 h of AF duration is problematic for decision-making about early or delayed cardioversion:
Firstly, one has to be confident about the exact debut time of an AF episode. In clinical practice, the estimation of debut is largely based on the onset of symptoms, in some cases also on a clinical examination, ECG findings, or telemetric evidence. Since most patients experience a new-onset AF episode outside a medical setting, the simple onset of symptoms is used as a time-hallmark to estimate the start of AF. However, as shown in a variety of AF populations, more than 50% of AF-episodes are asymptomatic.
Important insights into this topic can be gained from studies in patients having rhythm monitoring devices, although the risks linked to such detection are definitely different from spontaneous reporting of AF episodes. In the TRENDS study, a prospective, observational investigation of patients with a rhythm device, an AF-burden of more than 5.5 h doubled the annual thromboembolic risk from 1.1% in the low-risk group to 2.2% [17]. Further, in the ASSERT study, it was demonstrated that subclinical AF is common in pacemaker patients and is associated with an increased risk for ischemic stroke. Patients with AF-episodes lasting more than 24 h approximately had a 3.1% absolute risk increase for stroke per year [18]. Interestingly, stroke risk was not significantly different between patients with AF durations less than 24 h compared with patients without AF episodes [18]. Yet another study, the KP-RHYTHM study, analyzed the AF-burden as defined as the percentage of AF during a continuous 2-weeks-monitoring of patients with paroxysmal AF [19]. Here, an AF burden of more than 11.4% was associated with a three times higher risk for stroke [19].
Secondly, another thought-provoking aspect pertains to transesophageal echocardiography (TEE) studies performed in emergency departments. Two studies revealed the presence of left atrial thrombus in 4.0% of the patients who had presented within 48 h of AF-onset, and in 14.0% of the patients after 72 h of AF-duration [20, 21].
Thirdly, the FinCV (Finnish Cardio Version) cohort study in more than 10,000 cardioverted patients showed an interesting time relationship of estimated AF duration and strokes. Although the overall risk of thromboembolism after acute cardioversion without anticoagulation was very low for all patients with an AF duration of less than 48 h, there was a differential in the timing. Cardioversions performed within less than 12 h had a post-procedural stroke/embolism rate of 0.3%, while cardioversions performed within less than 48 h but more than 24 h had a stroke/embolism rate of 1.1% [22], almost four times higher than the earlier group. Taken together, these data suggest that onset of symptoms is not sufficiently reliable when determining the time of AF onset in the context of cardioversion and the inherent peri-procedural risk of stroke, and that burden of AF is associated with a higher risk of stroke.
On a different pathway of thinking, one also has to keep in mind additional factors that increase the risk of thromboembolism, particularly comorbidities. The mentioned FinCV cardioversion registry stratified thromboembolic events according to classical stroke risk factors in AF: while the overall stroke rate in the entire population undergoing cardioversions was 0.7%, it was dramatically higher in patients with concomitant heart failure and diabetes, namely 9.8%. On the other hand, patients without heart failure and under 60 years of age had a very low stroke risk of 0.2%. This study also confirmed the relationship of the CHA2DS2-VASc score to higher thromboembolic event rates in cardioversion [22]. Similarly, a retrospective study of 16,274 patients in Denmark demonstrated that a first-time DC-cardioversion without prior anticoagulation was associated with a higher risk of thromboembolism compared to cardioversions in anticoagulated patients (HR 2.25). The predictors of thromboembolic events were age, previous thromboembolism, and rehospitalization for AF [23]. Yet another retrospective study from Sweden evaluated the effect of VKAs in acute cardioversion when AF-duration was below 48 hours. Thromboembolic events occurred in 0.9% of patients with a CHA2DS2-VASc score of >1 with no anticoagulation, three times higher than among those pre-treated with a VKA (0.3%). The authors concluded that one should not perform acute cardioversions in patients without prior anticoagulation who have a CHA2DS2-VASc score of >1 [24]. Finally, in a prospective study in 484 cardioversion patients it was shown that cardioversion of acute-onset AF without prior anticoagulation in patients with CHA2DS2-VASc scores of 2–3 increased thromboembolic complications almost 5 times compared with those patients who had therapeutic anticoagulation [25]. Taken together, while the CHA2DS2-VASc score is reliable to define the long-term risk for thromboembolism in the general AF population, the weight of the individual score factors appears different in the setting of cardioversion. Here, particularly the factors heart failure, diabetes, and age over 75 years carry a markedly increased peri-procedural risk not reflected in the even distribution of the eight CHA2DS2-VASc factors.
Recommendations to Increase Safety in Cardioversion
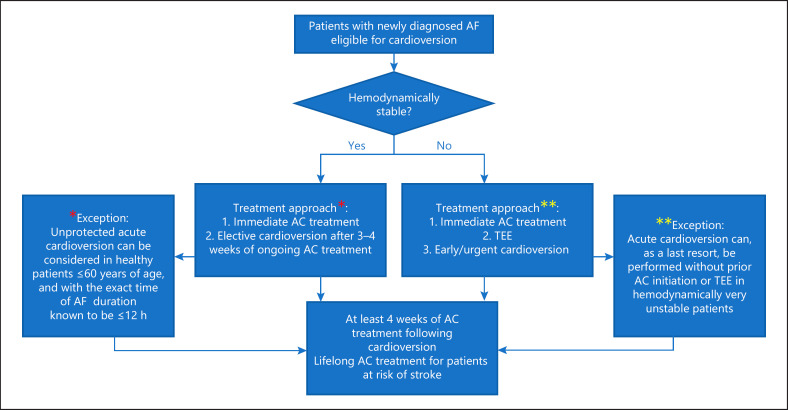
Given the evidence discussed above, we are herewith proposing a number of recommendations, which in our view are reasonable to be incorporated into clinical practice (Fig. 1):
Fig. 1.
Proposed flowchart of cardioversion strategies. AC, anticoagulation; TEE, transesophageal echocardiogram. The * denotes an exception to the proposed treatment strategy in hemodynamically stable patients (left panel). The ** denotes an exception to the proposed treatment strategy in hemodynamically unstable patients (right panel).
We endorse abandoning the 48-h time window for decision-making of unprotected AF cardioversion. Instead, we suggest a simple and more practical approach by dividing recent-onset AF patients into the following groups:
(a) Hemodynamically stable patients should receive immediate anticoagulation, preferably a NOAC, and frequency-control therapy should be commenced if indicated (i.e., if patients present with initial tachycardia). Subsequently an elective cardioversion should be planned after 3–4 weeks of ongoing (and reliable) anticoagulation. This approach has the advantage to allow for a possible spontaneous termination of AF, which indeed often occurs [26]. With the utilization of one of the NOACs, which is more convenient for patients and preferred in every respect, it is of course particularly important that optimal compliance is assessed. On a sidenote, in the previously mentioned XVeRT study, the authors accepted a pill intake based on a pill count of >80% as adequate compliance. This translates into one to four forgotten or omitted pills for the once-daily dosages, or two to eight omitted pills for the twice-daily dosages in any given 3-week treatment period prior to cardioversion. This policy is difficult to defend and in fact blameworthy, and we recommend that even one single forgotten pill should disqualify the patient for a scheduled cardioversion; i.e., it should lead to a postponement of the cardioversion. In the same context, patients should provide a log-book to document that no single pill had been forgotten. They should also be instructed to use the medication in a correct mode; for example, rivaroxaban once daily should be taken with a meal and at the same time of the day.
(b) If patients are hemodynamically unstable; e.g., with therapy-resistant tachycardia and after excluding any correctable conditions, one should start with anticoagulation with a NOAC, perform TEE to exclude left atrium thrombus, and then proceed to early/urgent cardioversion.
(c) In very rare cases and as a last resort, cardioversion can be performed as an acute cardioversion without prior OAC initiation or TEE in hemodynamically very unstable patients.
(d) In patients under the age of 60 years and without serious comorbidities, preferably with a CHA2DS2-VASc score ≤1, and with knowledge about the exact time of AF duration being less than 12 h, it is reasonably safe to perform an unprotected acute cardioversion.
2 All patients should use oral anticoagulation for at least 4 weeks after cardioversion. For patients with an increased risk of stroke, treatment should be lifelong.
The findings in the recently presented RACE 7-ACWAS study support the above-mentioned strategic choices. The study demonstrated impressively that in recent-onset, symptomatic AF, a wait-and-see approach was non-inferior to early cardioversion in achieving a return to sinus rhythm at 4 weeks [26].
With the present article, we hope that our work may become a primer for further scrutiny into the important topic of cardioversion safety. Some of the suggestions put forward are not necessarily based on solid evidence but might in the future turn out to be reasonable strategies, as improved evidence will be emerging in the wake of our increased focus on rhythm control strategies in AF.
Statement of Ethics
This opinion paper has solely been based on literature review. No ethics committee submission was required.
Conflict of Interest Statement
M.K., M.K.P., S.L.P., P.R., V.S., and I.G. have no disclosures. D.A. has received honoraria from Boehringer-Ingelheim, Bayer, BMS/Pfizer, MSD, and AstraZeneca, and research grants to the institution from BMS/Pfizer and Medtronic.
Funding Sources
The authors did not receive any funding.
Author Contributions
M.K: performed the literature search, drafted the manuscript, contributed to data analysis and to intellectual content, and approved the final version. M.K.P: performed the literature search together with the first author, helped drafting the manuscript, contributed to intellectual content, and approved the final version. S.L.P: contributed to data analysis, contributed to intellectual content, and approved the final version. P.R: contributed to data analysis, contributed to intellectual content, and approved the final version. V.S: contributed to data analysis, contributed to intellectual content, and approved the final version. I.G: contributed to data analysis, contributed to intellectual content, and approved the final version. D.A: supervised the group, contributed to data analysis, contributed to intellectual content, and approved the final version.
References
- 1.Hernández-Madrid A, Svendsen JH, Lip GY, Van Gelder IC, Dobreanu D, Blomstrom-Lundqvist C, Scientific Initiatives Committee, European Heart Rhythm Association (EHRA) Cardioversion for atrial fibrillation in current European practice: results of the European Heart Rhythm Association survey. Europace. 2013 Jun;15((6)):915–8. doi: 10.1093/europace/eut143. [DOI] [PubMed] [Google Scholar]
- 2.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. European Heart Rhythm Association. European Association for Cardio-Thoracic Surgery Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010 Oct;31((19)):2369–429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 3.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. ESC Committee for Practice Guidelines (CPG) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012 Nov;33((21)):2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. ESC Scientific Document Group 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016 Oct;37((38)):2893–962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 5.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Jr, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019 Jul;74((1)):104–32. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Arnold AZ, Mick MJ, Mazurek RP, Loop FD, Trohman RG. Role of prophylactic anticoagulation for direct current cardioversion in patients with atrial fibrillation or atrial flutter. J Am Coll Cardiol. 1992 Mar;19((4)):851–5. doi: 10.1016/0735-1097(92)90530-z. [DOI] [PubMed] [Google Scholar]
- 7.Bjerkelund CJ, Orning OM. The efficacy of anticoagulant therapy in preventing embolism related to D.C. electrical conversion of atrial fibrillation. Am J Cardiol. 1969 Feb;23((2)):208–16. doi: 10.1016/0002-9149(69)90068-x. [DOI] [PubMed] [Google Scholar]
- 8.Stellbrink C, Nixdorff U, Hofmann T, Lehmacher W, Daniel WG, Hanrath P, et al. ACE (Anticoagulation in Cardioversion using Enoxaparin) Study Group Safety and efficacy of enoxaparin compared with unfractionated heparin and oral anticoagulants for prevention of thromboembolic complications in cardioversion of nonvalvular atrial fibrillation: the Anticoagulation in Cardioversion using Enoxaparin (ACE) trial. Circulation. 2004 Mar;109((8)):997–1003. doi: 10.1161/01.CIR.0000120509.64740.DC. [DOI] [PubMed] [Google Scholar]
- 9.Nagarakanti R, Ezekowitz MD, Oldgren J, Yang S, Chernick M, Aikens TH, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011 Jan;123((2)):131–6. doi: 10.1161/CIRCULATIONAHA.110.977546. [DOI] [PubMed] [Google Scholar]
- 10.Flaker G, Lopes RD, Al-Khatib SM, Hermosillo AG, Hohnloser SH, Tinga B, et al. ARISTOTLE Committees and Investigators Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) J Am Coll Cardiol. 2014 Mar;63((11)):1082–7. doi: 10.1016/j.jacc.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 11.Piccini JP, Stevens SR, Lokhnygina Y, Patel MR, Halperin JL, Singer DE, et al. ROCKET AF Steering Committee & Investigators Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. J Am Coll Cardiol. 2013 May;61((19)):1998–2006. doi: 10.1016/j.jacc.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Plitt A, Ezekowitz MD, De Caterina R, Nordio F, Peterson N, Giugliano RP, ENGAGE AF-TIMI 48 Investigators Cardioversion of Atrial Fibrillation in ENGAGE AF-TIMI 48. Clin Cardiol. 2016 Jun;39((6)):345–6. doi: 10.1002/clc.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cappato R, Ezekowitz MD, Klein AL, Camm AJ, Ma CS, Le Heuzey JY, et al. X-VeRT Investigators Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J. 2014 Dec;35((47)):3346–55. doi: 10.1093/eurheartj/ehu367. [DOI] [PubMed] [Google Scholar]
- 14.Goette A, Merino JL, Ezekowitz MD, Zamoryakhin D, Melino M, Jin J, et al. ENSURE-AF investigators Edoxaban versus enoxaparin-warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): a randomised, open-label, phase 3b trial. Lancet. 2016 Oct;388((10055)):1995–2003. doi: 10.1016/S0140-6736(16)31474-X. [DOI] [PubMed] [Google Scholar]
- 15.Ezekowitz MD, Pollack CV, Jr, Halperin JL, England RD, VanPelt Nguyen S, Spahr J, et al. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: the EMANATE trial. Eur Heart J. 2018 Aug;39((32)):2959–71. doi: 10.1093/eurheartj/ehy148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Telles-Garcia N, Dahal K, Kocherla C, Lip GY, Reddy P, Dominic P. Non-vitamin K antagonists oral anticoagulants are as safe and effective as warfarin for cardioversion of atrial fibrillation: A systematic review and meta-analysis. Int J Cardiol. 2018 Oct;268:143–8. doi: 10.1016/j.ijcard.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009 Oct;2((5)):474–80. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 18.Van Gelder IC, Healey JS, Crijns HJ, Wang J, Hohnloser SH, Gold MR, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017 May;38((17)):1339–44. doi: 10.1093/eurheartj/ehx042. [DOI] [PubMed] [Google Scholar]
- 19.Go AS, Reynolds K, Yang J, Gupta N, Lenane J, Sung SH, et al. Association of Burden of Atrial Fibrillation With Risk of Ischemic Stroke in Adults With Paroxysmal Atrial Fibrillation: the KP-RHYTHM Study. JAMA Cardiol. 2018 Jul;3((7)):601–8. doi: 10.1001/jamacardio.2018.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleemann T, Becker T, Strauss M, Schneider S, Seidl K. Prevalence of left atrial thrombus and dense spontaneous echo contrast in patients with short-term atrial fibrillation [{LT}] 48 hours undergoing cardioversion: value of transesophageal echocardiography to guide cardioversion. J Am Soc Echocardiogr. 2009 Dec;22((12)):1403–8. doi: 10.1016/j.echo.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995 Feb;25((2)):452–9. doi: 10.1016/0735-1097(94)00396-8. [DOI] [PubMed] [Google Scholar]
- 22.Airaksinen KE, Grönberg T, Nuotio I, Nikkinen M, Ylitalo A, Biancari F, et al. Thromboembolic complications after cardioversion of acute atrial fibrillation: the FinCV (Finnish CardioVersion) study. J Am Coll Cardiol. 2013 Sep;62((13)):1187–92. doi: 10.1016/j.jacc.2013.04.089. [DOI] [PubMed] [Google Scholar]
- 23.Hansen ML, Jepsen RM, Olesen JB, Ruwald MH, Karasoy D, Gislason GH, et al. Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. Europace. 2015 Jan;17((1)):18–23. doi: 10.1093/europace/euu189. [DOI] [PubMed] [Google Scholar]
- 24.Själander S, Svensson PJ, Friberg L. Atrial fibrillation patients with CHA2DS2-VASc [{GT}]1 benefit from oral anticoagulation prior to cardioversion. Int J Cardiol. 2016 Jul;215:360–3. doi: 10.1016/j.ijcard.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Garg A, Khunger M, Seicean S, Chung MK, Tchou PJ. Incidence of Thromboembolic Complications Within 30 Days of Electrical Cardioversion Performed Within 48 Hours of Atrial Fibrillation Onset. JACC Clin Electrophysiol. 2016 Aug;2((4)):487–94. doi: 10.1016/j.jacep.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pluymaekers NA, Dudink EA, Luermans JG, Meeder JG, Lenderink T, Widdershoven J, et al. RACE 7 ACWAS Investigators Early or Delayed Cardioversion in Recent-Onset Atrial Fibrillation. N Engl J Med. 2019 Apr;380((16)):1499–508. doi: 10.1056/NEJMoa1900353. [DOI] [PubMed] [Google Scholar]