Abstract
Background
Evidence-based international expert consensus regarding anaesthetic practice in hip/knee arthroplasty surgery is needed for improved healthcare outcomes.
Methods
The International Consensus on Anaesthesia-Related Outcomes after Surgery group (ICAROS) systematic review, including randomised controlled and observational studies comparing neuraxial to general anaesthesia regarding major complications, including mortality, cardiac, pulmonary, gastrointestinal, renal, genitourinary, thromboembolic, neurological, infectious, and bleeding complications. Medline, PubMed, Embase, and Cochrane Library including Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, NHS Economic Evaluation Database, from 1946 to May 17, 2018 were queried. Meta-analysis and Grading of Recommendations Assessment, Development and Evaluation approach was utilised to assess evidence quality and to develop recommendations.
Results
The analysis of 94 studies revealed that neuraxial anaesthesia was associated with lower odds or no difference in virtually all reported complications, except for urinary retention. Excerpt of complications for neuraxial vs general anaesthesia in hip/knee arthroplasty, respectively: mortality odds ratio (OR): 0.67, 95% confidence interval (CI): 0.57–0.80/OR: 0.83, 95% CI: 0.60–1.15; pulmonary OR: 0.65, 95% CI: 0.52–0.80/OR: 0.69, 95% CI: 0.58–0.81; acute renal failure OR: 0.69, 95% CI: 0.59–0.81/OR: 0.73, 95% CI: 0.65–0.82; deep venous thrombosis OR: 0.52, 95% CI: 0.42–0.65/OR: 0.77, 95% CI: 0.64–0.93; infections OR: 0.73, 95% CI: 0.67–0.79/OR: 0.80, 95% CI: 0.76–0.85; and blood transfusion OR: 0.85, 95% CI: 0.82–0.89/OR: 0.84, 95% CI: 0.82–0.87.
Conclusions
Recommendation: primary neuraxial anaesthesia is preferred for knee arthroplasty, given several positive postoperative outcome benefits; evidence level: low, weak recommendation. Recommendation: neuraxial anaesthesia is recommended for hip arthroplasty given associated outcome benefits; evidence level: moderate-low, strong recommendation. Based on current evidence, the consensus group recommends neuraxial over general anaesthesia for hip/knee arthroplasty.
Trial registry number
PROSPERO CRD42018099935.
Keywords: anaesthesia, epidural, anaesthesia, general, anaesthesia, spinal, arthroplasty, replacement, hip, arthroplasty, replacement, knee, assessment, outcomes
Editor's key points.
-
•
In this state-of-the-art systematic review and analysis of the literature, a multinational expert group reached a consensus on the optimal anaesthetic approach for patients undergoing lower-limb arthroplasty.
-
•
Considering multiple perioperative outcomes, the consensus was that neuraxial anaesthesia is the preferred anaesthetic technique (when no contraindications exist), and that this reduces the risk of most (but not all) complications.
-
•
Neuraxial anaesthesia, which remains underutilised in many countries, may be used to improve perioperative outcomes, although limitations of the current literature may mandate the revision of these recommendations when new data become available.
Total joint arthroplasty (TJA) is amongst the most commonly performed surgical procedures in the developed world.1 Globally, millions of patients receive total hip and knee arthroplasties every year with large projected increases as the population ages.2 Despite the fact that TJA represents a value-based solution to end-stage arthritis of the hip and knee,3 the procedure is associated with a moderate risk for complications. Complications affecting major organ systems have been reported to occur in approximately 8% of patients undergoing either hip or knee arthroplasty.4 The identification of risk-modifying perioperative interventions represents an attractive target, given the large burden of resources required for the management of complications on a population-health level.
In this context, a number of recently published population-based studies have supported findings of earlier clinical trials, indicating that the type of anaesthetic technique may influence perioperative outcomes.5, 6 Whilst earlier RCTs suggested a potential benefit of neuraxial anaesthesia (NA) for outcomes, such as blood loss and thromboembolic events, these investigations were not sufficiently powered to study low-incidence outcomes, such as mortality, infectious, or cardiovascular complications.7 Furthermore, earlier clinical trials were primarily conducted before the widespread use of chemical thromboembolic prophylaxis and contemporary blood-loss prevention practices.8 The advent of population-based scientific approaches utilising large data sets that encompass healthcare information from hundreds of thousands of patients in actual practice environments has allowed researchers to add to the available knowledge in this field. Guided by a series of publications suggesting better outcomes with NA, a number of healthcare entities have developed policies encouraging the use of this anaesthetic type for TJA.9 Despite this development, definitive evidence in the form of large RCTs or pragmatic, multicentre trials is lacking. Moreover, it is questionable whether such studies will ever exist, given the challenges of feasibility and cost. As population-level data suggesting cost and outcome benefits of neuraxial approaches across a wide range of patient characteristics continue to emerge,10, 11, 12 it is also unclear if the necessary pre-RCT condition of equipoise can exist to support an experimental trial design.
In light of these factors and given that the utilisation of NA remains low in many countries,13 this international group of perioperative clinicians, researchers, quality experts, librarians, educators, and administrators assembled to (i) systematically investigate current published evidence to determine whether the type of anaesthesia technique can influence perioperative outcomes in patients undergoing total hip and knee arthroplasty; (ii) grade the level of evidence quality; and (iii) develop and formulate clinical practice recommendations, each with its own rating of strength.
The aim of the present consensus project was to systematically analyse and interpret current research evidence with regard to the impact of regional, and specifically neuraxial, anaesthesia in comparison to general anaesthesia (GA) on major perioperative outcomes for patients undergoing total hip or knee arthroplasties.
Methods
Consensus group
The International Consensus on Anaesthesia-Related Outcomes after Surgery (ICAROS) consensus group included 50 individuals with extensive expertise in the perioperative care of orthopaedic surgery patients. Included in this multidisciplinary group were anaesthesiologists, orthopaedic surgeons, healthcare outcomes and quality researchers, administrators, librarians, and methodologists from North America, Europe, and Oceania representing 19 nationalities, working in 10 countries. A 10-member steering committee was formed and tasked with overseeing day-to-day aspects of the project.
Study plan and healthcare question
A study plan was specified in advance, defining the healthcare questions and basic parameters, including intervention (NA) and alternative management strategy (GA), population, outcomes of interest, and inclusion and exclusion criteria. The detailed respective protocol, including analyses conducted for this project was registered on the International Prospective Register of Systematic Reviews (protocol number: CRD42018099935).14 An institutional review board approval was not required because of the analysis of previously published data.
The healthcare questions posed to the group were:
-
(i)
Does NA vs GA influence perioperative outcomes in patients undergoing total hip arthroplasty (THA)?
-
(ii)
Does NA vs GA influence perioperative outcomes in patients undergoing total knee arthroplasty (TKA)?
The predefined outcomes of interest included the following major perioperative complications: mortality, cardiac (with and without myocardial infarction), pulmonary (including pneumonia), gastrointestinal, renal (including acute renal failure), genitourinary (including urinary retention and urinary tract infection), thromboembolic (DVT and pulmonary embolism [PE]), neurological (including CNS complications and stroke), infectious, and wound complications, as well as blood loss (in ml), transfusion requirements (both binary and in ml), and inpatient falls. To account for resource utilisation, the study plan also included outcomes, such as cost of care, length of hospitalisation, and admission to critical care settings. However, because of the lack of studies on cost of care, the outcome could de facto not be included in the quantitative meta-analysis.15, 16
As specified in the study protocol, the consensus group will also address the impact of peripheral nerve block utilisation in patients undergoing total hip and knee arthroplasty. This healthcare question is currently being investigated and will be the focus of a subsequent analysis.
Selection criteria
Eligible studies included RCTs and observational prospective or retrospective studies in adult patients primarily undergoing elective total hip or knee arthroplasties. We included only studies directly comparing perioperative outcomes amongst patients who received NA vs those under GA. GA was defined as total intravenous, inhalational, or combination thereof, or when termed specifically as ‘general anaesthesia’ by the study authors. NA was defined as spinal, extradural, combined spinal and extradural, and caudal anaesthesia. Exclusion criteria encompassed patients under 18 yr, studies not reporting on postoperative outcomes of interest, case reports, and case series, and also studies without control groups.
Search strategy
A systematic literature search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
The search strategy, including Medical Subject Headings (MeSH), keywords, and controlled vocabulary terms, was crafted and validated by the expert group in collaboration with two institutional librarians. Medline, PubMed, Embase, and Cochrane Library, including Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Health Technology Assessment Database, and NHS Economic Evaluation Database, were queried from database inception (1946) to May 17, 2018. The search cross-referenced MeSH terms, keywords, and controlled vocabulary terms for the predefined areas of interest according to the healthcare question.
The following is the excerpt of respective search terms: arthroplasty, replacement, hip, total hip arthroplasty, total hip arthroplasties, hip prosthesis, total joint replacement, knee, knee replacement arthroplasty, knee replacement arthroplasties, total knee arthroplasty, knee prosthesis, total knee replacements, lower extremity, lower joints, anaesthesia, neuraxial, spinal, epidural, conduction, regional anaesthesia, intrathecal, peridural, and combined spinal epidural.
The full search strategy is reported in Supplementary materials and can be found in Supplementary Appendix A1. The search yielded 8985 studies. In addition to the electronic search, a manual search of previously published corresponding systematic reviews was performed for the purpose of completeness.
Study identification and data extraction
After deduplication, abstracts of 5553 studies were extracted and imported into the Covidence platform. Covidence is a web tool that provides a comprehensive framework for the complete process of a systematic literature review, including the steps of title and abstract screening, full-text review, data extraction, and quality assessment (risk of bias).17 As required, each step was performed independently by two reviewers. In case of a disagreement, a third reviewer was consulted for resolution.
After the title and abstract screening, full-text articles of 956 studies were imported into Covidence for a detailed review and data extraction. Extracted data were categorised according to the predefined outcomes. Furthermore, within the Covidence platform, the risk of bias for each individual study was assessed and established as high, low, or unknown, according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria for RCTs and observational studies.18
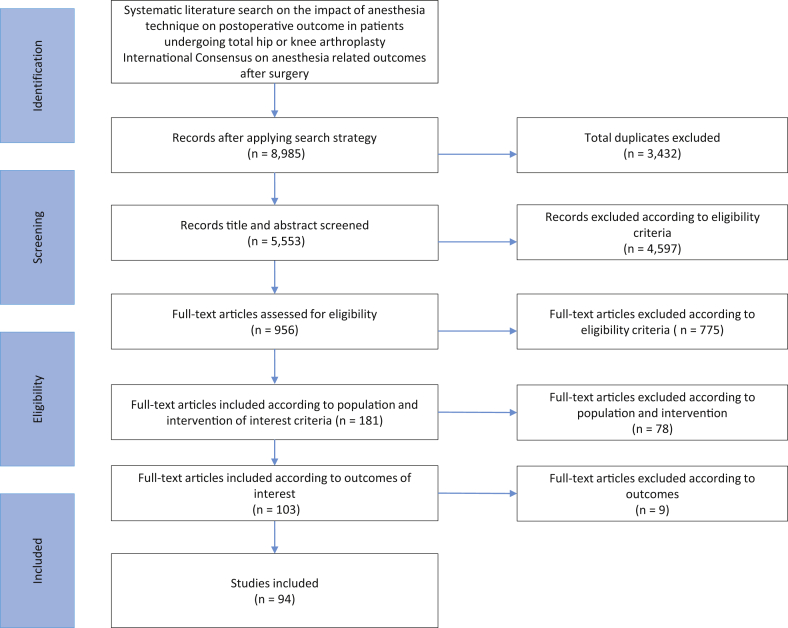
A flow chart describing the complete literature search process is depicted in Figure 1.
Fig 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Quantitative analysis
To provide estimates of intervention effects for each outcome of interest,19 RCT and observational data were pooled by meta-analysis. Review Manager software (Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was utilised to facilitate data analysis and graphic presentation as is commonly used for preparing Cochrane reviews.20 Summary estimates were calculated separately for each outcome (odds ratios [ORs] and 95% confidence intervals [CIs]), whilst heterogeneity utilising (I2 statistic) was also determined in quantitative analysis. For binary outcomes, group-specific risk was presented in events per 1000, whilst the relative effect was presented in ORs. For continuous variables, risk was presented as mean difference.
The primary analysis was performed including all eligible studies for both types of surgery, respectively (n=27 for TKA; n=49 for THA). A separate analysis was performed amongst studies that reported on THA/TKA mixed populations (n=21).
Secondary analyses were performed to test the influence of combined NA+GA compared with GA on perioperative outcomes in THA and TKA separately (n=12 and n=4, respectively), and also in the mixed THA/TKA surgical cohort (n=8).
The following are the additional sensitivity analyses:
-
(i)
Sensitivity analysis to investigate outcomes when only including evidence from RCTs (n=25 for THA; n=12 for TKA; n=2 for THA/TKA)
-
(ii)
Sensitivity analysis to investigate outcomes when removing studies that did not explicitly exclude all revision/trauma-related surgery or bilateral arthroplasties in their cohorts (n=46 for THA; n=25 for TKA; n=17 for THA/TKA)
-
(iii)
Sensitivity analysis to investigate the potential impact of recent changes in utilisation of perioperative thromboembolic prophylaxis protocols on the outcome of thromboembolic complications (DVT+PE).
Qualitative analysis
To provide useful recommendations for the practice of evidence-based treatment at the point of care, we utilised the GRADE system.15, 16 This methodology for rating the quality of evidence and grading the strength of recommendations has been widely adopted for the purpose of providing high-quality summaries of research evidence in systematic reviews and for standardised guideline development. Subsequent to data collection and quantitative analysis, GRADE offers a comprehensive framework for assessing the quality of the body of evidence and for carrying out steps required for developing recommendations.21 The concept of the certainty or quality of evidence represents the confidence in effect estimates and the extent to which they are sufficiently credible to support a particular recommendation. GRADE specifies four levels of certainty: high, moderate, low, and very low. This rating is determined for each relevant outcome by the systematic and transparent assessment of study design, limitations of the body of evidence, and special circumstances that increase the quality of evidence. Explicit criteria according to GRADE that were utilised for downgrading the quality of evidence included risk of bias according to study design and study conduct, inconsistency or heterogeneity (lack of similarity of point estimates and overlap of CIs; determination of I2 statistic), imprecision (optimal information size for adequate power), indirectness (strength of association to the healthcare question), and publication bias (utilising funnel plots).15 These criteria were assessed for each reported outcome of interest across informing studies. However, risk of bias was also assessed previously for each individual study, whilst in qualitative analysis the impact of risk of bias on cumulative evidence for each outcome was determined. The rationale for upgrading the quality of evidence, particularly for methodologically rigorous observational studies, includes large effect size, presence of a dose–response relationship, or when all plausible confounders or biases would decrease an apparent treatment effect.22 Utilising the GRADEpro software (McMaster University and Evidence Prime Inc.),23 final results, including the pooled estimates of effect and the quality of evidence, are presented in summary of findings (Table 1, Table 2 for THA and TKA, respectively).
Table 1.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) summary of findings for total hip arthroplasty (THA). CI, confidence interval; GA, general anaesthesia; MI, myocardial infarction; NA, neuraxial anaesthesia; OR, odds ratio. GRADE Working Group grades of evidence: high certainty (we are very confident that the true effect lies close to that of the estimate of the effect), moderate certainty (we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different), low certainty (our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect), and very low certainty (we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect). ∗The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). †Publication bias: funnel plot not symmetric. ‡Heterogeneity: widely differing estimates of effect.
| Summary of findings | ||||||
|---|---|---|---|---|---|---|
| NA compared with GA for THA | ||||||
| Patient or population: THA Setting: perioperative care Intervention: NA Comparison: GA | ||||||
| Outcomes/complications | Anticipated absolute effects∗ (95% CI) |
Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with GA | Risk with NA | |||||
| Mortality | 2 per 1000 | 1 per 1000 (1–2) | OR: 0.67 (0.57–0.80) | (3 RCTs, 4 observational studies) | ⊕⊕◯◯ Low |
|
| Cardiac including MI | 57 per 1000 | 53 per 1000 (50–58) | OR: 0.94 (0.88–1.02) | (3 RCTs, 3 observational studies) | ⊕⊕◯◯ Low |
|
| Cardiac excluding MI | 48 per 1000 | 47 per 1000 (43–50) | OR: 0.96 (0.88–1.03) | (2 RCTs, 3 observational studies) | ⊕◯◯◯ Very low† |
|
| MI | 3 per 1000 | 3 per 1000 (2–4) | OR: 0.94 (0.71–1.24) | (2 RCTs, 2 observational studies) | ⊕⊕◯◯ Low |
|
| Pulmonary | 7 per 1000 | 4 per 1000 (3–5) | OR: 0.65 (0.52–0.80) | (3 observational studies) | ⊕⊕⊕◯ Moderate |
|
| Pneumonia | 10 per 1000 | 7 per 1000 (5–8) | OR: 0.69 (0.56–0.84) | (2 RCTs, 2 observational studies) | ⊕⊕⊕◯ Moderate |
|
| Gastrointestinal | 10 per 1000 | 8 per 1000 (7–10) | OR: 0.83 (0.67–1.02) | 109 732 (1 observational study) | ⊕⊕◯◯ Low |
|
| Acute renal failure | 15 per 1000 | 10 per 1000 (9–12) | OR: 0.69 (0.59–0.81) | (1 RCT, 5 observational studies) | ⊕⊕◯◯ Low‡ |
|
| Urinary retention | 111 per 1000 | 277 per 1000 (199–370) | OR: 3.05 (1.98–4.69) | (3 RCTs, 3 observational studies) | ⊕⊕⊕◯ Moderate |
|
| Urinary tract infection | 15 per 1000 | 13 per 1000 (10–15) | OR: 0.86 (0.70–1.06) | (2 observational studies) | ⊕⊕◯◯ Low |
|
| DVT | 15 per 1000 | 8 per 1000 (6–10) | OR: 0.52 (0.42–0.65) | (5 RCTs, 8 observational studies) | ⊕⊕⊕◯ Moderate |
|
| Pulmonary embolism (PE) | 3 per 1000 | 2 per 1000 (2–2) | OR: 0.63 (0.50–0.81) | (7 RCTs, 6 observational studies) | ⊕⊕◯◯ Low |
|
| Thromboembolism (DVT+PE) | 5 per 1000 | 3 per 1000 (3–4) | OR: 0.61 (0.53–0.71) | (15 RCTs, 16 observational studies) | ⊕⊕⊕◯ Moderate |
|
| CNS | 2 per 1000 | 1 per 1000 (0–1) | OR: 0.39 (0.23–0.65) | (3 observational studies) | ⊕⊕⊕◯ Moderate |
|
| Stroke | 2 per 1000 | 1 per 1000 (0–1) | OR: 0.37 (0.22–0.64) | (2 observational studies) | ⊕⊕⊕◯ Moderate |
|
| All infections (including pneumonia and sepsis) | 25 per 1000 | 19 per 1000 (17–20) | OR: 0.73 (0.67–0.79) | (2 RCTs, 7 observational studies) | ⊕⊕◯◯ Low |
|
| Wound superficial infection | 8 per 1000 | 9 per 1000 (7–12) | OR: 1.21 (0.93–1.56) | (1 RCT, 2 observational studies) | ⊕⊕◯◯ Low |
|
| Wound deep infection | 7 per 1000 | 6 per 1000 (5–7) | OR: 0.86 (0.70–1.06) | (3 observational studies) | ⊕⊕◯◯ Low |
|
| Blood transfusion | 224 per 1000 | 197 per 1000 (192–205) | OR: 0.85 (0.82–0.89) | (8 RCTs, 9 observational studies) | ⊕◯◯◯ Very low† |
|
| Critical care admission | 2 per 1000 | 1 per 1000 (1–2) | OR: 0.80 (0.49–1.32) | (2 observational studies) | ⊕⊕◯◯ Low |
|
| Readmission | 38 per 1000 | 34 per 1000 (30–39) | OR: 0.91 (0.80–1.04) | 28 857 (1 observational study) | ⊕⊕◯◯ Low |
|
| Nerve injury | 2 per 1000 | 2 per 1000 (1–3) | OR: 0.81 (0.56–1.18) | (1 RCT, 4 observational studies) | ⊕⊕◯◯ Low |
|
| Falls | 16 per 1000 | 13 per 1000 (12–15) | OR: 0.81 (0.72–0.92) | 166 871 (1 observational study) | ⊕⊕◯◯ Low |
|
| Blood loss (ml) | The mean blood loss was 0. | The mean blood loss in the intervention group was 146.12 lower (173.73 lower to 118.51 lower). | — | 1546 (12 RCTs, 4 observational studies) | ⊕⊕⊕◯ Moderate‡ |
|
| Length of stay (days) | The mean length of hospital stay (LOS) was 0. | The mean LOS in the intervention group was 0.16 lower (0.22 lower to 0.1 lower). | — | (1 RCT, 1 observational study) | ⊕⊕◯◯ Low |
|
| Blood transfusion (ml) | The mean blood transfusion was 0. | The mean blood transfusion in the intervention group was 187.83 lower (272.29 lower to 103.38 lower). | — | (2 RCTs, 3 observational studies) | ⊕⊕◯◯ Low |
|
Table 2.
GRADE summary of findings for total knee arthroplasty (TKA). CI, confidence interval; GA, general anaesthesia; GRADE, Grading of Recommendations Assessment, Development and Evaluation; MI, myocardial infarction; NA, neuraxial anaesthesia; OR, odds ratio. GRADE Working Group grades of evidence: high certainty (we are very confident that the true effect lies close to that of the estimate of the effect), moderate certainty (we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different), low certainty (our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect), and very low certainty (we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect). ∗The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). †Imprecision. ‡Risk of bias: random sequence generation.
| Summary of findings: | ||||||
|---|---|---|---|---|---|---|
| NA compared with GA for TKA | ||||||
| Patient or population: TKA Setting: perioperative care Intervention: NA Comparison: GA | ||||||
| Outcomes/complications | Absolute effects∗ (95% CI) |
Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with GA | Risk with NA | |||||
| Mortality | 1 per 1000 | 1 per 1000 (1–1) | OR: 0.83 (0.60–1.15) | 259 847 (2 RCTs, 4 observational studies) | ⊕⊕◯◯ Low |
|
| Cardiac including MI | 59 per 1000 | 60 per 1000 (58–63) | OR: 1.03 (0.98–1.08) | 261 695 (1 RCT, 6 observational studies) | ⊕⊕◯◯ Low |
|
| Cardiac excluding MI | 57 per 1000 | 58 per 1000 (55–61) | OR: 1.02 (0.97–1.08) | 259 332 (4 observational studies) | ⊕⊕◯◯ Low |
|
| MI | 2 per 1000 | 2 per 1000 (2–3) | OR: 0.99 (0.80–1.22) | 261 695 (1 RCT, 6 observational studies) | ⊕⊕◯◯ Low |
|
| Pulmonary | 6 per 1000 | 4 per 1000 (4–5) | OR: 0.69 (0.58–0.81) | 259 392 (1 RCT, 4 observational studies) | ⊕⊕⊕◯ Moderate |
|
| Pneumonia | 8 per 1000 | 6 per 1000 (6–7) | OR: 0.82 (0.72–0.94) | 275 947 (1 RCT, 5 observational studies) | ⊕⊕◯◯ Low |
|
| Gastrointestinal | 7 per 1000 | 7 per 1000 (6–8) | OR: 0.99 (0.85–1.15) | 223 108 (1 observational study) | ⊕⊕◯◯ Low |
|
| Acute renal failure | 14 per 1000 | 10 per 1000 (9–11) | OR: 0.73 (0.65–0.82) | 273 384 (5 observational studies) | ⊕⊕◯◯ Low |
|
| Urinary retention | 235 per 1000 | 203 per 1000 (121–317) | OR: 0.83 (0.45–1.51) | 277 (2 RCTs, 1 observational study) | ⊕⊕⊕◯ Moderate† |
|
| Urinary tract infection | 15 per 1000 | 12 per 1000 (11–14) | OR: 0.82 (0.71–0.96) | 52 779 (4 observational studies) | ⊕⊕◯◯ Low |
|
| DVT | 36 per 1000 | 27 per 1000 (22–32) | OR: 0.77 (0.64–0.93) | 19 756 (6 RCTs, 6 observational studies) | ⊕⊕◯◯ Low |
|
| Pulmonary embolism (PE) | 6 per 1000 | 4 per 1000 (4–5) | OR: 0.79 (0.67–0.94) | 238 066 (3 RCTs, 4 observational studies) | ⊕⊕◯◯ Low |
|
| Thromboembolism (DVT+PE) | 7 per 1000 | 5 per 1000 (5–6) | OR: 0.77 (0.68–0.88) | 257 793 (8 RCTs, 10 observational studies) | ⊕⊕◯◯ Low |
|
| CNS | 1 per 1000 | 1 per 1000 (1–1) | OR: 0.77 (0.55–1.08) | 259 594 (1 RCT, 3 observational studies) | ⊕⊕◯◯ Low |
|
| Stroke | 1 per 1000 | 1 per 1000 (1–1) | OR: 0.70 (0.49–1.01) | 259 585 (1 RCT, 4 observational studies) | ⊕⊕◯◯ Low |
|
| All infections | 22 per 1000 | 17 per 1000 (16–18) | OR: 0.80 (0.76–0.85) | 571 503 (1 RCT, 12 observational studies) | ⊕⊕◯◯ Low |
|
| Wound superficial infection | 6 per 1000 | 4 per 1000 (3–6) | OR: 0.77 (0.60–0.98) | 52 839 (1 RCT, 4 observational studies) | ⊕⊕◯◯ Low |
|
| Wound deep infection | 2 per 1000 | 2 per 1000 (1–3) | OR: 1.01 (0.60–1.69) | 31 843 (3 observational studies) | ⊕⊕◯◯ Low |
|
| Blood transfusion | 165 per 1000 | 142 per 1000 (139–146) | OR: 0.84 (0.82–0.87) | 259 332 (4 observational studies) | ⊕⊕◯◯ Low |
|
| Critical care admission | 1 per 1000 | 0 per 1000 (0–1) | OR: 0.17 (0.04–0.75) | 20 936 (1 observational study) | ⊕⊕⊕◯ Moderate† |
|
| Readmission | 76 per 1000 | 38 per 1000 (23–59) | OR: 0.48 (0.29–0.77) | 1629 (1 observational study) | ⊕⊕⊕◯ Moderate |
|
| Nerve injury | 4 per 1000 | 5 per 1000 (2–10) | OR: 1.16 (0.58–2.32) | 25 243 (4 observational studies) | ⊕⊕◯◯ Low |
|
| Falls | 0 per 1000 | 0 per 1000 (0–0) | OR: 0.00 (–0.03 to 0.03) | 118 (1 observational study) | ⊕⊕◯◯ Low |
Not estimable |
| Blood loss (ml) | The mean blood loss was 0. | The mean blood loss in the intervention group was 13.54 higher (25.75 lower to 52.83 higher). | — | 130 (1 RCT) | ⊕⊕⊕◯ Moderate‡ |
|
| Length of stay (days) | The mean length of hospital stay (LOS) was 0. | The mean LOS in the intervention group was 0.09 lower (0.15 lower to 0.02 lower). | — | 36 956 (3 RCTs, 5 observational studies) | ⊕⊕◯◯ Low |
|
Recommendations
The assessment of the quality of evidence, the formulation of recommendations, and the determination of their strength are separate processes. When moving from evidence to recommendations, the GRADE strategy focuses on integrating factors that are basic for the formulation of guidelines or recommendations.19, 24 Thus, critical factors beyond the quality of evidence include the balance between benefits and harms; patient values and preferences; resource considerations; and issues pertaining to feasibility, equity, and acceptability of recommendations.19 GRADE distinguishes between strong and weak recommendations.
The balance between desirable and undesirable outcomes and the application of patients' values determine the direction of the recommendation. Moreover, these factors, along with the quality of evidence, resource implications, and clinical feasibility considerations, determine the strength or grade of recommendations.
Strong recommendations reflect a clear preference for one alternative and should apply to almost all eligible patients. Weak recommendations are appropriate when there is a close balance between desirable and undesirable consequences or alternative management strategies, uncertainty regarding the effects of the alternatives, uncertainty or variability in patient's values and preferences, or questionable cost-effectiveness. Weak recommendations usually require accessing the underlying evidence and a shared decision-making approach.15, 19, 21 In certain circumstances, a strong recommendation is based on low-quality evidence.16
Modified Delphi process and consensus meeting
Subsequent to analyses completion, two pairs of participants were tasked with summarising the evidence, formulating conclusions, and suggesting recommendations. This work was distributed in the form of white papers for the THA and TKA cohorts separately. The white papers, together with detailed files and summary tables of analysis results, were distributed to the entire group with the request for anonymous edits and comments according to the modified Delphi process,25 and repeated after revisions.26
Finally, the group met in person on December 8, 2018, in New York, NY, USA, to review the process; discuss results; and reach a consensus on conclusions, recommendations, and their strength. Approval was assessed in an anonymous vote after statements were finalised as facilitated by a group discussion.
Results
A summary of findings for patients undergoing THA and TKA, including the estimates of effect and the quality of evidence by outcomes, is found in Table 1, Table 2, respectively.
Additional in-depth quantitative and qualitative analysis data and figures are provided as Supplementary material.
Impact of the type of anaesthesia in total hip arthroplasties
Primary analyses (NA vs GA)
Amongst all hip arthroplasty patients, NA without GA was associated with fewer complications in most categories, except for urinary retention, when compared with patients who received GA (Table 3).
Table 3.
Influence of anaesthesia type on perioperative outcomes in total hip arthroplasty. CI, confidence interval; GA, general anaesthesia; MI, myocardial infarction; NA, neuraxial anaesthesia; OR, odds ratio; PE, pulmonary embolism. All infections, including pneumonia and sepsis; pulmonary complications, excluding pneumonia; n (NA/GA): total number of patients with NA/GA.
| Complication | NA vs GA |
NA+GA vs GA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies | OR (95% CI) | n (NA) | n (GA) | P-value | Studies | OR (95% CI) | n (NA) | n (GA) | P-value | |
| Mortality | Various authors5, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 | 0.67 (0.57–0.80) | 60 499 | 148 583 | <0.0001 | Various authors5, 30 | 0.58 (0.38–0.89) | 15 331 | 98 230 | 0.014 |
| Cardiac including MI | Various authors5, 28, 32, 33, 34, 37 | 0.94 (0.88–1.02) | 28 182 | 121 215 | 0.135 | Various authors5, 38 | 0.76 (0.54–1.07) | 15 281 | 98 139 | 0.113 |
| Cardiac excluding MI | Various authors5, 27, 32, 33, 34 | 0.96 (0.88–1.03) | 32 639 | 133 832 | 0.255 | Memtsoudis and colleagues5 | 1.01 (0.95–1.09) | 15 261 | 98 122 | 0.689 |
| MI | Various authors5, 33, 34, 37 | 0.94 (0.71–1.24) | 23 022 | 115 759 | 0.647 | Various authors5, 38 | 0.76 (0.54–1.07) | 15 281 | 98 139 | 0.113 |
| Pulmonary | Various authors5, 28, 33 | 0.65 (0.52–0.80) | 28 029 | 121 058 | <0.0001 | Memtsoudis and colleagues5 | 0.66 (0.52–0.84) | 15 261 | 98 122 | 0.001 |
| Pneumonia | Various authors5, 33, 34, 37 | 0.69 (0.56–0.84) | 23 022 | 115 759 | <0.0001 | Memtsoudis and colleagues5 | 0.88 (0.74–1.05) | 15 261 | 98 122 | 0.165 |
| Gastrointestinal | Memtsoudis and colleagues5 | 0.83 (0.67–1.02) | 11 610 | 98 122 | 0.078 | Memtsoudis and colleagues5 | 0.79 (0.65–0.95) | 15 261 | 98 122 | 0.013 |
| Acute renal failure | Various authors5, 33, 37, 39, 40 | 0.69 (0.59–0.81) | 34 366 | 133 687 | <0.0001 | Memtsoudis and colleagues5 | 0.75 (0.65–0.86) | 15 261 | 98 122 | <0.0001 |
| Urinary retention | Various authors34, 39, 40, 41, 42, 43 | 3.05 (1.98–4.69) | 252 | 628 | <0.0001 | Various authors44, 45 | 1.91 (1.05–3.48) | 123 | 163 | 0.035 |
| Urinary tract infection | Various authors30, 33 | 0.86 (0.70–1.06) | 11 334 | 17 648 | 0.164 | Brinker and colleagues30 | 1.14 (0.43–2.99) | 70 | 108 | 0.793 |
| DVT | Various authors30, 33, 36, 41, 43, 46, 47, 48, 49, 50, 51, 52, 53 | 0.52 (0.42–0.65) | 15 688 | 20 477 | <0.0001 | Various authors30, 38 | 0.81 (0.17–3.89) | 90 | 125 | 0.795 |
| PE | Various authors5, 33, 34, 35, 36, 37, 41, 48, 49, 50, 51, 52, 53 | 0.63 (0.50–0.81) | 34 875 | 123 934 | <0.0001 | Memtsoudis and colleagues5 | 0.68 (0.46–1.03) | 15 261 | 98 122 | 0.066 |
| DVT+PE | Various authors5, 30, 33, 34, 35, 36, 37, 41, 43, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 | 0.61 (0.53–0.71) | 59 573 | 157 731 | <0.0001 | Various authors5, 30, 38 | 0.69 (0.47–1.02) | 15 351 | 98 247 | 0.065 |
| CNS | Various authors5, 33, 58 | 0.39 (0.23–0.65) | 22 977 | 115 712 | <0.0001 | Various authors5, 34, 59 | 0.68 (0.42–1.09) | 15 306 | 98 162 | 0.112 |
| Stroke | Various authors5, 33 | 0.37 (0.22–0.64) | 22 927 | 115 662 | <0.0001 | Memtsoudis and colleagues5 | 0.71 (0.44–1.16) | 15 261 | 98 122 | 0.176 |
| All infections | Various authors5, 28, 30, 33, 34, 37 | 0.73 (0.67–0.79) | 62 385 | 254 465 | <0.0001 | Various authors5, 30 | 0.86 (0.79–0.92) | 30 592 | 196 352 | <0.0001 |
| Wound (superficial) | Various authors30, 33, 34 | 1.21 (0.93–1.56) | 11 363 | 17 679 | 0.152 | Brinker and colleagues30 | 1.56 (0.21–11.33) | 70 | 108 | 0.661 |
| Wound (deep) | Various authors28, 33, 54 | 0.86 (0.70–1.06) | 24 603 | 35 688 | 0.159 | |||||
| Blood transfusion | Various authors5, 30, 33, 34, 37, 41, 43, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 | 0.85 (0.82–0.89) | 25 033 | 117 443 | <0.0001 | Various authors5, 30, 38, 61 | 0.78 (0.75–0.82) | 15 421 | 98 317 | <0.0001 |
| Critical care | Various authors27, 33 | 0.80 (0.49–1.32) | 20 690 | 33 125 | 0.387 | |||||
| Readmission | Haughom and colleagues33 | 0.91 (0.80–1.04) | 11 317 | 17 540 | 0.161 | |||||
| Nerve injury | Various authors30, 33, 34, 69 | 0.81 (0.56–1.18) | 19 842 | 27 106 | 0.278 | Brinker and colleagues30 | 0.30 (0.01–6.39) | 70 | 108 | 0.442 |
| Falls | Kendrišić and colleagues71 | 0.81 (0.72–0.92) | 20 985 | 145 886 | 0.001 | |||||
| Blood loss (ml) | Various authors36, 43, 50, 51, 52, 60, 61, 62, 66, 68, 72, 73, 74, 75, 76, 77 | –146.12 (–173.73 to –118.51) | 902 | 644 | <0.0001 | Various authors38, 59, 61, 74, 77, 78, 79 | –20.13 (–50.10 to 9.83) | 226 | 216 | 0.188 |
| Length of stay (days) | Various authors28, 80 | –0.16 (–0.22 to –0.10) | 5146 | 5442 | <0.0001 | Benson and colleagues59 | –6.00 (–14.77 to 2.77) | 16 | 9 | 0.18 |
| Blood transfusion (ml) | Various authors43, 50, 51, 60, 66 | –187.83 (–272.29 to –103.38) | 310 | 195 | <0.0001 | |||||
NA was associated with decreased odds for all-cause mortality (OR: 0.67, 95% CI: 0.57, 0.80; absolute effect: 2 per 1000 with GA vs 1 per 1000 with NA, 95% CI: 1, 2), pulmonary complications (OR: 0.65; 95% CI: 0.52, 0.80), pneumonia (OR: 0.69; 95% CI; 0.56, 0.84), and acute renal failure (OR: 0.69; 95% CI: 0.59, 0.81). NA was also associated with fewer thromboembolic events compared with GA, including DVT (OR: 0.52; 95% CI: 0.42, 0.65) and PE (OR: 0.63; 95% CI: 0.50–0.81). Furthermore, CNS complications (OR: 0.39; 95% CI: 0.23, 0.65), stroke (OR: 0.37; 95% CI: 0.22, 0.64), all-cause infections (OR: 0.73; 95% CI: 0.67, 0.79), blood transfusion requirements (OR: 0.85; 95% CI: 0.82, 0.89), and postoperative falls (OR: 0.81; 95% CI: 0.72, 0.92) were reduced with NA vs GA.
We did not identify any differences in cardiac, gastrointestinal, or wound complications; critical care admissions; readmissions; and nerve injuries between NA and GA amongst hip arthroplasty patients.
Impact of the type of anaesthesia in total knee arthroplasties
Primary analyses (NA vs GA)
Amongst patients undergoing total knee arthroplasties, the utilisation of NA in comparison to GA was associated with improved outcomes with regard to multiple complications (Table 4). Amongst patients who received NA, reduced odds were observed for pulmonary complications (OR: 0.69; 95% CI: 0.58–0.81), pneumonia (OR: 0.82; 95% CI: 0.72, 0.94), acute renal failure (OR: 0.73; 95% CI: 0.65, 0.82), urinary tract infection (OR: 0.82; 95% CI: 0.71, 0.96), DVT (OR: 0.77; 95% CI: 0.64, 0.93), PE (OR: 0.79; 95% CI: 0.67, 0.94), all-cause infections (OR: 0.80; 95% CI: 0.76, 0.85), superficial wound infections (OR: 0.77; 95% CI: 0.60, 0.98), blood transfusions (OR: 0.84; 95% CI: 0.82, 0.87), critical care admissions (OR: 0.17; 95% CI: 0.04, 0.75), and readmissions (OR: 0.48; 95% CI: 0.29, 0.77).
Table 4.
Influence of anaesthesia type on perioperative outcomes in total knee arthroplasty. CI, confidence interval; GA, general anaesthesia; MI, myocardial infarction; NA, neuraxial anaesthesia; OR, odds ratio; PE, pulmonary embolism. All infections, including pneumonia and sepsis; pulmonary complications, excluding pneumonia; n (NA/GA): total number of patients with NA/GA.
| Complication | NA vs GA |
NA+GA vs GA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies | OR (95% CI) | n (NA) | n (GA) | P-value | Studies | OR (95% CI) | n (NA) | n (GA) | P-value | |
| Mortality | Various authors5, 6, 54, 57, 81, 82 | 0.83 (0.60–1.15) | 43 653 | 216 194 | 0.261 | Memtsoudis and colleagues5 | 0.73 (0.51–1.05) | 34 135 | 194 682 | 0.094 |
| Cardiac including MI | Various authors5, 6, 54, 57, 81, 83, 84 | 1.03 (0.98–1.08) | 44 831 | 216 864 | 0.324 | Various authors5, 85 | 1.07 (0.85–1.34) | 34 165 | 194 715 | 0.553 |
| Cardiac excluding MI | Various authors5, 6, 54, 57 | 1.02 (0.97–1.08) | 43 386 | 215 946 | 0.349 | Memtsoudis and colleagues5 | 1.07 (1.02–1.12) | 34 135 | 194 682 | 0.007 |
| MI | Various authors5, 6, 54, 57, 81, 83, 84 | 0.99 (0.80–1.22) | 44 831 | 216 864 | 0.896 | Various authors5, 85 | 1.07 (0.85–1.34) | 34 165 | 194 715 | 0.553 |
| Pulmonary | Various authors5, 6, 54, 57, 86 | 0.69 (0.58–0.81) | 43 416 | 215 976 | <0.0001 | Various authors5, 85 | 0.89 (0.77–1.03) | 34 165 | 194 715 | 0.132 |
| Pneumonia | Various authors5, 6, 54, 57, 86, 87 | 0.82 (0.72–0.94) | 50 804 | 225 143 | 0.003 | Memtsoudis and colleagues5 | 1.02 (0.90–1.16) | 34 135 | 194 682 | 0.727 |
| Gastrointestinal | Memtsoudis and colleagues5 | 0.99 (0.85–1.15) | 28 426 | 194 682 | 0.855 | Various authors5, 85 | 1.07 (0.93–1.22) | 34 165 | 194 715 | 0.344 |
| Acute renal failure | Various authors5, 6, 54, 57 | 0.73 (0.65–0.82) | 49 416 | 223 968 | <0.0001 | Memtsoudis and colleagues5 | 0.96 (0.87–1.05) | 34 135 | 194 682 | 0.377 |
| Urinary retention | Various authors32, 86, 88 | 0.83 (0.45–1.51) | 111 | 166 | 0.537 | |||||
| Urinary tract infection | Various authors6, 54, 57, 87 | 0.82 (0.71–0.96) | 22 348 | 30 431 | 0.011 | |||||
| DVT | Various authors6, 47, 56, 82, 83, 89, 90, 91, 92, 93, 94, 95 | 0.77 (0.64–0.93) | 9466 | 10 222 | 0.005 | 85 | 0.53 (0.05–6.21) | 30 | 33 | 0.617 |
| PE | Various authors5, 6, 42, 82, 90, 92, 94 | 0.79 (0.67–0.94) | 34 890 | 203 176 | 0.007 | Memtsoudis and colleagues5 | 0.78 (0.66–0.93) | 34 135 | 194 682 | 0.006 |
| DVT+PE | Various authors5, 6, 42, 47, 56, 82, 83, 89, 90, 92, 93, 94, 95 | 0.77 (0.68–0.88) | 44 373 | 213 420 | <0.0001 | Various authors5, 85 | 0.78 (0.66–0.93) | 34 165 | 194 715 | 0.005 |
| CNS | Various authors5, 6, 54, 57, 81 | 0.77 (0.55–1.08) | 43 520 | 216 074 | 0.133 | Various authors5, 85, 96 | 1.03 (0.75–1.43) | 34 270 | 194 823 | 0.84 |
| Stroke | Various authors5, 6, 54, 57, 82 | 0.70 (0.49–1.01) | 43 519 | 216 066 | 0.059 | Various authors5, 85 | 1.06 (0.76–1.49) | 34 165 | 194 715 | 0.72 |
| All infections | Various authors5, 6, 54, 57, 86, 87 | 0.80 (0.76–0.85) | 109 150 | 462 353 | <0.0001 | Memtsoudis and colleagues5 | 0.98 (0.93–1.03) | 68 270 | 389 364 | 0.464 |
| Wound (superficial) | Various authors6, 54, 57, 86, 87 | 0.77 (0.60–0.98) | 22 378 | 30 461 | 0.034 | |||||
| Wound (deep) | Various authors6, 57, 87 | 1.01 (0.60–1.69) | 14 164 | 17 679 | 0.982 | |||||
| Blood transfusion | Various authors5, 6, 54, 57 | 0.84 (0.82–0.87) | 43 386 | 215 946 | <0.0001 | Memtsoudis and colleagues5 | 1.02 (0.99–1.05) | 34 135 | 194 682 | 0.197 |
| Critical care | Basques and colleagues54 | 0.17 (0.04–0.75) | 8184 | 12 752 | 0.019 | |||||
| Readmission | Belmont and colleagues97 | 0.48 (0.29–0.77) | 586 | 1043 | 0.003 | |||||
| Nerve injury | Various authors6, 57, 70 | 1.16 (0.58–2.32) | 7304 | 17 939 | 0.665 | |||||
| Falls | Harsten and colleagues32 | 0.00 (0.00–0.00) | 58 | 60 | <0.0001 | Kendrišić and colleagues71 | 0.91 (0.81–1.02) | 24 699 | 145 886 | 0.092 |
| Blood loss (ml) | Zhou and colleagues95 | 13.54 (–25.75 to 52.83) | 63 | 67 | 0.499 | Kudoh and colleagues96 | 13.10 (–18.99 to 45.19) | 75 | 75 | 0.424 |
| Length of stay (days) | Various authors6, 54, 57, 81, 82, 98, 99, 100 | –0.09 (–0.15 to –0.02) | 15 326 | 21 630 | 0.009 | |||||
Impact of the type of anaesthesia in studies reporting outcomes in mixed total knee/hip arthroplasties
Primary analyses (NA vs GA)
The results are presented in Supplementary Table A5. Overall, improved outcomes were seen in association with the use of NA vs GA in this cohort of studies.
Secondary analyses (NA+GA vs GA)
In a secondary analysis, we compared the utilisation of combined NA+GA vs GA only to assess the impact on studied outcomes in patients undergoing THA and TKA (Table 3, Table 4, and Supplementary Table A5). The output indicated a similar trend as observed in the NA vs GA analysis. The outcomes with significantly reduced odds for combined NA+GA vs GA included mortality, pulmonary complications, gastrointestinal complications, acute renal failure, all-cause infections, and blood transfusions, whilst the odds for urinary retention were increased as seen in the NA vs GA comparison.
Sensitivity analyses
Randomised clinical trials only
The first sensitivity analysis focused on RCTs only and verified that NA was associated with fewer thromboembolic events than GA (Table 5, Table 6, and Supplementary Table A6). NA patients also had less blood loss and received lower blood transfusion volumes (Table 5). This analysis did not present statistically significant differences in other complications, which may be attributable to the much smaller sample size in RCTs compared with population-based analyses.
Table 5.
Subgroup RCTs: influence of anaesthesia type on perioperative outcomes in total hip arthroplasty. CI, confidence interval; GA, general anaesthesia; MI, myocardial infarction; NA, neuraxial anaesthesia; OR, odds ratio; PE, pulmonary embolism. All infections, including pneumonia and sepsis; pulmonary complications, excluding pneumonia; n (NA/GA): total number of patients with NA/GA.
| Complication | NA vs GA |
NA+GA vs GA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies | OR (95% CI) | n (NA) | n (GA) | P-value | Studies | OR (95% CI) | n (NA) | n (GA) | P-value | |
| Mortality | Various authors32, 34, 36 | 0.34 (0.01–8.80) | 135 | 137 | 0.519 | |||||
| Cardiac including MI | Various authors32, 34, 37 | 0.82 (0.19–3.48) | 153 | 157 | 0.783 | Dauphin and colleagues38 | 1.78 (0.15–21.51) | 20 | 17 | 0.651 |
| Cardiac excluding MI | Various authors32, 34 | 0.65 (0.08–5.38) | 87 | 91 | 0.687 | |||||
| MI | Various authors34, 37 | 0.75 (0.14–4.07) | 95 | 97 | 0.736 | Dauphin and colleagues38 | 1.78 (0.15–21.51) | 20 | 17 | 0.651 |
| Pneumonia | Various authors34, 37 | 1.03 (0.14–7.53) | 95 | 97 | 0.973 | |||||
| Acute renal failure | Liang and colleagues37 | 0.33 (0.01–8.21) | 66 | 66 | 0.498 | |||||
| Urinary retention | Various authors34, 41, 42 | 1.65 (0.89–3.05) | 158 | 162 | 0.113 | |||||
| DVT | Various authors36, 41, 50, 51 | 0.33 (0.20–0.55) | 177 | 174 | <0.0001 | Dauphin and colleagues38 | 0.81 (0.17–3.89) | 20 | 17 | 0.795 |
| PE | Various authors34, 36, 37, 41, 50, 51 | 0.40 (0.20–0.79) | 255 | 257 | 0.008 | |||||
| DVT+PE | Various authors34, 36, 37, 41, 50, 51, 55, 56 | 0.43 (0.30–0.63) | 482 | 479 | <0.0001 | Dauphin and colleagues38 | 0.81 (0.17–3.89) | 20 | 17 | 0.795 |
| CNS | Various authors34, 59 | 0.26 (0.03–2.28) | 45 | 40 | 0.222 | |||||
| All infections | Various authors34, 37 | 1.03 (0.14–7.53) | 95 | 97 | 0.973 | |||||
| Wound (superficial) | 34 | 0.33 (0.03–3.40) | 29 | 31 | 0.354 | |||||
| Blood transfusion | Various authors34, 37, 41, 61, 62, 63, 67, 68 | 0.43 (0.28–0.65) | 357 | 364 | <0.0001 | Various authors38, 61 | 0.50 (0.24–1.05) | 90 | 87 | 0.067 |
| Nerve injury | Hole and colleagues34 | 0.34 (0.01–8.80) | 29 | 31 | 0.519 | |||||
| Blood loss (ml) | Various authors36, 50, 51, 61, 62, 68, 72, 73, 74, 75, 77 | –121.82 (–152.22 to –91.42) | 334 | 335 | <0.0001 | Various authors38, 59, 61, 74, 77, 78, 79 | –20.13 (–50.10 to 9.83) | 226 | 216 | 0.188 |
| Length of stay (days) | Williams-Russo and colleagues80 | –3.00 (–6.25 to 0.25) | 44 | 46 | 0.07 | Benson and colleagues59 | –6.00 (–14.77 to 2.77) | 16 | 9 | 0.18 |
| Blood transfusion (ml) | Various authors50, 51 | –542.64 (–771.95 to –313.32) | 45 | 45 | <0.0001 | |||||
Table 6.
Subgroup RCTs: influence of anaesthesia type on perioperative outcomes in total knee arthroplasty. CI, confidence interval; GA, general anaesthesia; MI, myocardial infarction; NA, neuraxial anaesthesia; OR, odds ratio; PE, pulmonary embolism. All infections, including pneumonia and sepsis; pulmonary complications, excluding pneumonia; n (NA/GA): total number of patients with NA/GA.
| Complication | NA vs GA |
NA+GA vs GA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies | OR (95% CI) | n (NA) | n (GA) | P-value | Studies | OR (95% CI) | n (NA) | n (GA) | P-value | |
| Mortality | Various authors81, 82 | 0.93 (0.13–6.64) | 267 | 248 | 0.941 | |||||
| Cardiac including MI | Williams-Russo and colleagues81 | 1.28 (0.28–5.84) | 134 | 128 | 0.748 | |||||
| MI | Williams-Russo and colleagues81 | 0.95 (0.19–4.82) | 134 | 128 | 0.955 | |||||
| Pulmonary | Chu and colleagues86 | 0.48 (0.04–5.63) | 30 | 30 | 0.561 | |||||
| Pneumonia | Chu and colleagues86 | 0.19 (0.01–4.06) | 30 | 30 | 0.286 | |||||
| Urinary retention | Various authors32, 86 | 0.86 (0.47–1.59) | 88 | 90 | 0.628 | |||||
| DVT | Various authors56, 82, 89, 90, 91, 95 | 0.82 (0.56–1.18) | 256 | 327 | 0.283 | |||||
| PE | Various authors42, 82, 90 | 1.17 (0.45–3.03) | 163 | 149 | 0.748 | |||||
| DVT+PE | Various authors42, 56, 82, 89, 90, 95 | 0.78 (0.56–1.10) | 436 | 498 | 0.157 | |||||
| CNS | Williams-Russo and colleagues81 | 1.31 (0.59–2.89) | 134 | 128 | 0.503 | Kudoh and colleagues96 | 0.74 (0.16–3.42) | 75 | 75 | 0.7 |
| Stroke | Williams-Russo and colleagues82 | 2.73 (0.11–67.61) | 133 | 120 | 0.54 | |||||
| All infections | Chu and colleagues86 | 0.19 (0.01–4.06) | 30 | 30 | 0.286 | |||||
| Wound (superficial) | Chu and colleagues86 | 0.48 (0.04–5.63) | 30 | 30 | 0.561 | |||||
| Falls | Harsten and colleagues32 | 0.00 (0.00–0.00) | 58 | 60 | <0.0001 | |||||
| Blood loss (ml) | Zhou and colleagues95 | 13.54 (–25.75 to 52.83) | 63 | 67 | 0.499 | Kudoh and colleagues96 | 13.10 (–18.99 to 45.19) | 75 | 75 | 0.424 |
| Length of stay (days) | Various authors81, 82, 98 | –0.14 (–0.56 to 0.28) | 308 | 295 | 0.512 | |||||
Exclusion of studies likely containing a minority of revision/trauma surgery or bilateral arthroplasty cases
Our primary analysis included all patients from all candidate studies, which encompassed RCTs and observational studies. In some of these investigations, revision/trauma-related arthroplasty patients could not be excluded with certainty. To test the potential effect that this patient population may have on outcomes, we excluded them in a sensitivity analysis. The relationship between anaesthetic type and outcomes when excluding revision/trauma arthroplasty was nearly identical compared with the primary inclusive analysis (Supplementary Tables A2–A4).
Sensitivity analysis: thromboembolic complications (DVT+PE)
To account for potential prognostic imbalance as a result of recent emerging differences in perioperative care with regard to the implementation of thrombosis prophylaxis in recent years, we performed a further sensitivity analysis. Estimates of intervention effects were established for the outcome of thromboembolic complications (DVT+PE) when including all eligible studies, when excluding studies without thrombosis prophylaxis, and when excluding studies published before 1995.
NA was associated with a 24% reduction in thromboembolic events when including all studies (n=37; OR: 0.76; 95% CI: 0.71, 0.83), a 14% reduction when excluding studies lacking thromboembolic prophylaxis (n=9; OR: 0.86; 95% CI: 0.79, 0.92), and a 16% reduction when excluding studies before 1995 (n=14; OR: 0.84; 95% CI: 0.78, 0.90).
Discussion
Recommendations and comments
Does type of anaesthesia influence perioperative outcomes in THA?
The utilisation of NA over GA for THA was associated with lower complication odds for most studied outcomes. The utilisation of combined NA and GA was also associated with better perioperative outcomes compared with GA alone, although the magnitude and diversity of benefits were decreased compared with using NA alone (Table 1, Table 3).
-
(i)
Level of evidence: low to moderate
-
(ii)
Recommendation: NA is recommended for primary unilateral THA when there is no significant contraindication or special circumstance to preclude its use.
-
(iii)
Strength of recommendation: strong
-
(iv)
Rationale: Based on the findings of our analysis and the grading of evidence, the group reached a unanimous decision on the aforementioned recommendation. The results of all analyses showed improvement in outcomes with NA compared with GA in most cases, or no impact, with the sole exception of urinary retention, albeit the latter is a known, expected side-effect of NA.101
The level of evidence underlying the individual analyses by outcome was low to moderate. When considering the factors integrated by the GRADE approach for the development of recommendations,19 the majority of the group (n=33 out of 43 votes) determined it to be overall strong.
The latter conclusion was based on the observations that: (i) the evidence was largely in favour of the intervention, (ii) the desirable effects of the intervention outweigh the undesirable ones, (iii) the intervention was associated with neutral to beneficial resource utilisation, (iv) the intervention is acceptable to stakeholders, and (v) the intervention is feasible.
Does type of anaesthesia influence perioperative outcomes in TKA?
Compared with GA, NA was associated with fewer complications or no difference in complications in all reported outcomes after TKA (Table 2, Table 4).
NA was associated with lower odds of thromboembolic events and blood transfusion, and also infectious complications, including pneumonia and all-cause infections. Furthermore, lower odds for acute renal failure and respiratory complications were found amongst patients receiving NA for TKA. With regard to outcomes of resource utilisation, NA was associated with fewer admissions to critical care units, lower rates of hospital readmissions, and a shorter length of hospital stay (mean difference: –0.08; 95% CI: –0.15 to 0.01 days).
Our analysis failed to find any significant differences in the odds for mortality, composite CNS complications, or stroke. There was also no effect of anaesthetic type on cardiac or gastrointestinal complications.
-
(i)
Level of evidence: low
-
(ii)
Recommendation: Provided no contraindication, a primary neuraxial anaesthetic technique is preferred for TKA, given several positive benefits of NA on important post-TKA outcomes, together with no evidence of worse outcomes.
-
(iii)
Strength of recommendation: weak
-
(iv)
Rationale: Based on the findings of our analysis and the grading of the level of evidence, the group reached a majority (n=42 out of 43 votes) decision on the aforementioned recommendation. The results of all analyses showed improvement with NA for outcomes compared with GA for some but not all outcomes. The effect was smaller than that seen in the larger THA cohort.
The level of evidence underlying the individual analyses by outcome was low.
When considering the factors integrated by the GRADE approach for the development of recommendations, the majority of the group (n=31 out of 43 votes) determined it to be overall weak.
The latter conclusion was based on the observations that the evidence was in favour of the intervention, but to a lesser extent than that observed in the THA cohort. However, the group believed that the desirable effects of the intervention outweigh the undesirable effects, and that the intervention was associated with beneficial resource utilisation. Further, the intervention is acceptable to stakeholders and is clinically feasible.
Comments
Several limitations to our consensus approach have to be considered. Perioperative care has evolved significantly over years and decades, including surgical techniques. This may be a source of unmeasured or unknown confounding that is not adequately balanced by randomisation.
Further, the group discussed extensively the lack of detailed information regarding the potentially wide variability in the conduct of GA and the potential influence of GA technique on outcomes. Whilst NA as a technique may vary to certain degrees (type of local anaesthetic used, use of spinal vs extradural vs combined spinal/extradural, and level of neuraxial block), the group agreed that the conduct of the technique and its major characteristics are standardised and have been in place for many decades. In contrast, the conduct of GA has evolved significantly over time with changes in pharmacological agents (both intravenous and inhalational), assistive technology (target-controlled infusion), airway devices, monitoring, and ventilation equipment, and also care strategies.
Therefore, it seems appropriate to re-evaluate the differential impact of modern general anaesthetic techniques in this context once such granular information becomes reliably available in the future.
Additional factors that may influence outcomes include the use of procedural sedation and its depth, which may, in practice, approach levels seen with GA.102 However, at this time, such an analysis is not feasible because of the lack of adequate data. In addition, the inherent anaesthetic-related risks of each technique (GA or NA) were not considered in this analysis, but are rare for either approach.
In the last decade, advances in regional anaesthesia, such as the utilisation of ultrasound-guided peripheral nerve block techniques, have gained significant popularity in the clinical setting. Thus, our research group is currently reviewing evidence regarding the perioperative impact of peripheral nerve blocks. However, given the numerous options and combinations of various anaesthesia-related procedures, further studies are needed to address specifically the impact of peripheral nerve blocks as adjuncts to GA when compared with NA.
Further, the group discussed what future research would be needed to derive definitive data on the questions addressed in this consensus article. Whilst large, multicentre RCTs or pragmatic trials may provide definitive evidence, they are not and may never be available. Future studies are indicated to better evaluate the mechanisms by which the observed beneficial effects associated with NA are realised. The group acknowledged that, whilst a plausible mechanism for improved outcomes is likely related to NA-associated reductions in stress response, the body of evidence establishing this link is scarce.103 Further, it was determined that future research is needed to elucidate the relationship between anaesthetic type and outcomes in the ever-increasing commonality of high-risk patient populations presenting for joint arthroplasty. Moreover, comparative literature for some complications, such as postoperative cognitive dysfunction, is rare, and these topics require more scientific investigations to allow robust analysis and conclusions in the context of anaesthesia practice.58, 81 Finally, the group commented that, given the potential benefits and relative under-utilisation of NA, research with focus on identification and amelioration of barriers to the widespread implementation of NA techniques is needed.
Executive summary
Does type of anaesthesia influence perioperative outcomes in THA?
The utilisation of NA over GA for THA was associated with lower complication risk for most studied outcomes. Furthermore, the utilisation of combined NA and GA was also associated with better perioperative outcomes compared with GA alone, although the magnitude and diversity of benefits were decreased compared with using NA alone (Table 1, Table 3).
-
(i)
Level of evidence: low to moderate
-
(ii)
Recommendation: NA is recommended for primary unilateral THA when there is no significant contraindication or special circumstance to preclude its use.
-
(iii)
Strength of recommendation: strong
Does type of anaesthesia influence perioperative outcomes in TKA?
-
(i)
Level of evidence: low
-
(ii)
Recommendation: Provided no contraindication, a primary neuraxial anaesthetic technique is preferred for TKA, given several positive benefits of NA on important post-TKA outcomes, together with no evidence of worse outcomes.
-
(iii)
Strength of recommendation: weak
Authors' contributions
Study conception: SGM
Study design/planning/execution: SGM, NES, CC, JB, JL, EMS, ERM, RLJ, MJH, GG
Reviewing/expanding study plan: EA, MJB, AB, JDA, NE, PEG, PG, AGDV, EG, PK, SLK, PL’H, CHML, CBM, DM, AM, JMN, MP, JPa, LP, JPo, LAP, BDS, OS, ECS, ERV, EGV-V, CW, JTYD
Literature search: CC, RG, BJ, LP, BHL, PW, MB, GG, SJK, LB, DW, GH
Data extraction: JB, DB, CC, BHL, PW, MB, GG, SJK, LB, DW, GH, JL, SGM
Data analysis: CC, SGM, NES, JPo, JB, JL, ES, ERM, RLJ, MJH, GG
Reviewing results of data analysis: EA, MJB, AB, JDA, NE, PEG, PG, AGDV, EG, PK, SLK, PL’H, CHML, CBM, DM, AM, JMN, MP, JPa, LP, JPo, LAP, BDS, OS, ECS, ERV, EGV-V, CLW, JTYD
Interpreting results: CC, SGM, NES, JPo, JB, JL, ES, ERM, RLJ, MJH, GG
Reviewing/editing white papers: EA, MJB, AB, JDA, NE, PEG, PG, AGDV, EG, PK, SLK, PL’H, CHML, CBM, DM, AM, JMN, MP, JPa, LP, JPo, LAP, BDS, OS, ECS, ERV, EGV-V, CLW, JTYD
Writing paper: SGM, CC, NES, JB, JL, EMS, ERM, RLJ, MJH, GG
All authors reviewed, commented on, and approved the study plan; reviewed the data and the analysis results; commented on and gave feedback to the interpretation of results, including quantitative and qualitative analyses; and convened in person or were given the opportunity to join remotely in an all-day consensus conference held on December 8, 2018 at the Hospital for Special Surgery, New York, NY, USA, where the entire analysis steps and results were presented, and the GRADE approach was utilised for the interpretation of the body of evidence and the formation of recommendations.
SGM had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declarations of interest
SGM is a director of the boards of the American Society of Regional Anesthesia and Pain Medicine and the president of the Society of Anesthesia and Sleep Medicine. He is a one-time consultant for Teikoku, Sandoz Inc. and a consultant/investor for HATH. Furthermore, SGM has a US Patent application pending for a Multicatheter Infusion System (US-2017-0361063). He is the owner of SGM Consulting, LLC, and co-owner of FC Monmouth, LLC. None of these relations influenced the conduct of the present project. ERM is a director of the board of the American Society of Regional Anesthesia and Pain Medicine and an officer of the California Society of Anesthesiologists. ERM is also an employee of the United States government, and his contribution to this project is supported with resources based at the Veterans Affairs (VA) Palo Alto Health Care System (Palo Alto, CA, USA). The contents do not represent the views of VA or the United States Government. NE is a board member of the American Society of Regional Anesthesia and Pain Medicine. NE is also a consultant for Foundry Therapeutics, but declared no conflict of interest. ECS reports consulting fees from Egalet, Inc. and the Mission Lisa foundation and acknowledges funding from the National Institute on Drug Abuse (K08DA042314). which are unrelated to this work. The other authors declare that they have no conflicts of interest.
Funding
Department of Anesthesiology, Critical Care, and Pain Management, Hospital for Special Surgery, New York, NY, USA.
Disclaimer
The conclusions and recommendations resulting from this project are not intended to establish practice guidelines or standards, nor can they—if followed—guarantee successful outcomes. Many adequate reasons exist why a clinician or patient may deviate from the recommendations in this article, including, but not limited to, medical circumstances, individual patient and clinician preferences, and the availability of resources. The present conclusions and recommendations are based on the currently available literature, established in a systematic review process; thus, reassessment and revisions are required as new or different evidence emerges.
Handling editor: J.G. Hardman
Editorial decision: 20 May 2019
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2019.05.042.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Pabinger C., Geissler A. Utilization rates of hip arthroplasty in OECD countries. Osteoarthritis Cartilage. 2014;22:734–741. doi: 10.1016/j.joca.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Jt Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 3.Ethgen O., Bruyere O., Richy F., Dardennes C., Reginster J.Y. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Jt Surg Am. 2004;86:963–974. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Memtsoudis S.G., Ma Y., Gonzalez Della Valle A. Demographics, outcomes, and risk factors for adverse events associated with primary and revision total hip arthroplasties in the United States. Am J Orthop. 2010;39:E72–E77. [PubMed] [Google Scholar]
- 5.Memtsoudis S.G., Sun X., Chiu Y.L. Perioperative comparative effectiveness of anesthetic technique in orthopedic patients. Anesthesiology. 2013;118:1046–1058. doi: 10.1097/ALN.0b013e318286061d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pugely A.J., Martin C.T., Gao Y., Mendoza-Lattes S., Callaghan J.J. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Jt Surg Am. 2013;95:193–199. doi: 10.2106/JBJS.K.01682. [DOI] [PubMed] [Google Scholar]
- 7.Mauermann W.J., Shilling A.M., Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;103:1018–1025. doi: 10.1213/01.ane.0000237267.75543.59. [DOI] [PubMed] [Google Scholar]
- 8.Johnson R., Kopp S., Burkle C. Neuraxial vs general anaesthesia for total hip and total knee arthroplasty: a systematic review of comparative-effectiveness research. Br J Anaesth. 2016;116:163–176. doi: 10.1093/bja/aev455. [DOI] [PubMed] [Google Scholar]
- 9.Health Quality Ontario & Ministry of Health and Long-Term Care . 2014. Quality-based procedures: clinical handbook for primary hip and knee replacement.http://www.health.gov.on.ca/en/pro/programs/ecfa/docs/qbp_prihipknee.pdf 1–95. [Google Scholar]
- 10.Memtsoudis S.G., Poeran J., Zubizarreta N. Do hospitals performing frequent neuraxial anesthesia for hip and knee replacements have better outcomes? Anesthesiology. 2018;129:428–439. doi: 10.1097/ALN.0000000000002299. [DOI] [PubMed] [Google Scholar]
- 11.Memtsoudis S.G., Poeran J., Zubizarreta N., Rasul R., Opperer M., Mazumdar M. Anesthetic care for orthopedic patients: is there a potential for differences in care? Anesthesiology. 2016;124:608–623. doi: 10.1097/ALN.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 12.Memtsoudis S.G., Rasul R., Suzuki S. Does the impact of the type of anesthesia on outcomes differ by patient age and comorbidity burden? Reg Anesth Pain Med. 2014;39:112–119. doi: 10.1097/AAP.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 13.Cozowicz C., Poeran J., Memtsoudis S.G. Epidemiology, trends, and disparities in regional anaesthesia for orthopaedic surgery. Br J Anaesth. 2015;115 doi: 10.1093/bja/aev381. ii57–67. [DOI] [PubMed] [Google Scholar]
- 14.International Prospective Register of Systematic Reviews. https://www.crd.york.ac.uk/prospero/
- 15.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balshem H., Helfand M., Schünemann H.J. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org (please see https://support.covidence.org/help/how-can-i-cite-covidence)
- 18.Guyatt G.H., Oxman A.D., Vist G. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Neumann I., Santesso N., Akl E.A. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016;72:45–55. doi: 10.1016/j.jclinepi.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Cochrane Community. RevMan 5. https://community.cochrane.org/help/tools-and-software/revman-5
- 21.Guyatt G., Oxman A.D., Akl E.A. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt G.H., Oxman A.D., Sultan S. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–1316. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 23.McMaster University . 2015. GRADEpro GDT: GRADEpro Guideline Development Tool [software]https://gradepro.org/ [Google Scholar]
- 24.Woolf S.H., Grol R., Hutchinson A., Eccles M., Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318:527–530. doi: 10.1136/bmj.318.7182.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Colla L., Albertin A., La Colla G. No adjustment vs. adjustment formula as input weight for propofol target-controlled infusion in morbidly obese patients. Eur J Anaesthesiol. 2009;26:362–369. doi: 10.1097/EJA.0b013e328326f7d0. [DOI] [PubMed] [Google Scholar]
- 26.Jones J., Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311:376–380. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabriel R.A., Kaye A.D., Jones M.R., Dutton R.P., Urman R.D. Practice variations in anesthetic care and its effect on clinical outcomes for primary total hip arthroplasties. J Arthroplasty. 2016;31:918–922. doi: 10.1016/j.arth.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 28.Helwani M.A., Avidan M.S., Ben Abdallah A. Effects of regional versus general anesthesia on outcomes after total hip arthroplasty: a retrospective propensity-matched cohort study. J Bone Jt Surg Am. 2015;97:186–193. doi: 10.2106/JBJS.N.00612. [DOI] [PubMed] [Google Scholar]
- 29.Aynardi M., Jacovides C.L., Huang R., Mortazavi S.M., Parvizi J. Risk factors for early mortality following modern total hip arthroplasty. J Arthroplasty. 2013;28:517–520. doi: 10.1016/j.arth.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 30.Brinker M.R., Reuben J.D., Mull J.R., Cox D.D., Daum W.J., Parker J.R. Comparison of general and epidural anesthesia in patients undergoing primary unilateral THR. Orthopedics. 1997;20:109–115. doi: 10.3928/0147-7447-19970201-06. [DOI] [PubMed] [Google Scholar]
- 31.Chen W.H., Hung K.C., Tan P.H., Shi H.Y. Neuraxial anesthesia improves long-term survival after total joint replacement: a retrospective nationwide population-based study in Taiwan. Can J Anaesth. 2015;62:369–376. doi: 10.1007/s12630-015-0316-0. [DOI] [PubMed] [Google Scholar]
- 32.Harsten A., Kehlet H., Ljung P., Toksvig-Larsen S. Total intravenous general anaesthesia vs. spinal anaesthesia for total hip arthroplasty: a randomised, controlled trial. Acta Anaesthesiol Scand. 2015;59:298–309. doi: 10.1111/aas.12456. [DOI] [PubMed] [Google Scholar]
- 33.Haughom B.D., Schairer W.W., Nwachukwu B.U., Hellman M.D., Levine B.R. Does neuraxial anesthesia decrease transfusion rates following total hip arthroplasty? J Arthroplasty. 2015;30:116–120. doi: 10.1016/j.arth.2015.01.058. [DOI] [PubMed] [Google Scholar]
- 34.Hole A., Terjesen T., Breivik H. Epidural versus general anaesthesia for total hip arthroplasty in elderly patients. Acta Anaesthesiol Scand. 1980;24:279–287. doi: 10.1111/j.1399-6576.1980.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 35.Khatod M., Inacio M.C., Bini S.A., Paxton E.W. Prophylaxis against pulmonary embolism in patients undergoing total hip arthroplasty. J Bone Jt Surg Am. 2011;93:1767–1772. doi: 10.2106/JBJS.J.01130. [DOI] [PubMed] [Google Scholar]
- 36.Modig J., Maripuu E., Sahlstedt B. Thromboembolism following total hip replacement: a prospective investigation of 94 patients with emphasis on the efficacy of lumbar epidural anesthesia in prophylaxis. Reg Anesth Pain Med. 1986;11:72–79. [Google Scholar]
- 37.Liang C., Wei J., Cai X., Lin W., Fan Y., Yang F. Efficacy and safety of 3 different anesthesia techniques used in total hip arthroplasty. Med Sci Monit. 2017;23:3752–3759. doi: 10.12659/MSM.902768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dauphin A., Raymer K.E., Stanton E.B., Fuller H.D. Comparison of general anesthesia with and without lumbar epidural for total hip arthroplasty: effects of epidural block on hip arthroplasty. J Clin Anesth. 1997;9:200–203. doi: 10.1016/s0952-8180(97)00035-4. [DOI] [PubMed] [Google Scholar]
- 39.Walton J.K., Robinson R.G. Some observations on urinary retention and deep hip sepsis following total hip replacement. Aust N Z J Surg. 1982;52:130–133. doi: 10.1111/j.1445-2197.1982.tb06086.x. [DOI] [PubMed] [Google Scholar]
- 40.Walts L.F., Kaufman R.D., Moreland J.R., Weiskopf M. Total hip arthroplasty. An investigation of factors related to postoperative urinary retention. Clin Orthop Relat Res. 1985;194:280–282. [PubMed] [Google Scholar]
- 41.Davis F.M., Laurenson V.G., Gillespie W.J., Wells J.E., Foate J., Newman E. Deep vein thrombosis after total hip replacement. A comparison between spinal and general anaesthesia. J Bone Jt Surg Br. 1989;71:181–185. doi: 10.1302/0301-620X.71B2.2925731. [DOI] [PubMed] [Google Scholar]
- 42.Harsten A., Kehlet H., Toksvig-Larsen S. Recovery after total intravenous general anaesthesia or spinal anaesthesia for total knee arthroplasty: a randomized trial. Br J Anaesth. 2013;111:391–399. doi: 10.1093/bja/aet104. [DOI] [PubMed] [Google Scholar]
- 43.Thorburn J., Louden J., Vallance R. Spinal and general anaesthesia in total hip replacement: frequency of deep vein thrombosis. Br J Anaesth. 1980;52:1117–1121. doi: 10.1093/bja/52.11.1117. [DOI] [PubMed] [Google Scholar]
- 44.Macdowell A.D., Robinson A.H., Hill D.J., Villar R.N. Is epidural anaesthesia acceptable at total hip arthroplasty? A study of the rates of urinary catheterisation. J Bone Jt Surg Br. 2004;86:1115–1117. doi: 10.1302/0301-620x.86b8.14240. [DOI] [PubMed] [Google Scholar]
- 45.Williams A., Price N., Willett K. Epidural anaesthesia and urinary dysfunction: the risks in total hip replacement. J R Soc Med. 1995;88:699–701. doi: 10.1177/014107689508801218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feller J.A., Parkin J.D., Phillips G.W., Hannon P.J., Hennessy O., Huggins R.M. Prophylaxis against venous thrombosis after total hip arthroplasty. Aust N Z J Surg. 1992;62:606–610. doi: 10.1111/j.1445-2197.1992.tb07530.x. [DOI] [PubMed] [Google Scholar]
- 47.Haas S., Holberg G., Kreutz R. The effects of timing of prophylaxis, type of anesthesia, and use of mechanical methods on outcome in major orthopedic surgery—subgroup analyses from 17,701 patients in the XAMOS study. Vasc Health Risk Manag. 2016;12:209–218. doi: 10.2147/VHRM.S100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarmiento A., Goswami A. Thromboembolic disease prophylaxis in total hip arthroplasty. Clin Orthop Relat Res. 2005;436:138–143. doi: 10.1097/01.blo.0000161824.52515.31. [DOI] [PubMed] [Google Scholar]
- 49.Dalldorf P.G., Perkins F.M., Totterman S., Pellegrini V.D., Jr. Deep venous thrombosis following total hip arthroplasty. Effects of prolonged postoperative epidural anesthesia. J Arthroplasty. 1994;9:611–616. doi: 10.1016/0883-5403(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 50.Fredin H., Rosberg B. Anaesthetic techniques and thromboembolism in total hip arthroplasty. Eur J Anaesthesiol. 1986;3:273–281. [PubMed] [Google Scholar]
- 51.Modig J., Hjelmstedt A., Sahlstedt B., Maripuu E. Comparative influences of epidural and general anaesthesia on deep venous thrombosis and pulmonary embolism after total hip replacement. Acta Chir Scand. 1981;147:125–130. [PubMed] [Google Scholar]
- 52.Modig J., Borg T., Karlstrom G., Maripuu E., Sahlstedt B. Thromboembolism after total hip replacement: role of epidural and general anesthesia. Anesth Analg. 1983;62:174–180. [PubMed] [Google Scholar]
- 53.Wille-Jorgensen P., Christensen S.W., Bjerg-Nielsen A., Stadeager C., Kjaer L. Prevention of thromboembolism following elective hip surgery. The value of regional anesthesia and graded compression stockings. Clin Orthop Relat Res. 1989;247:163–167. [PubMed] [Google Scholar]
- 54.Basques B.A., Toy J.O., Bohl D.D., Golinvaux N.S., Grauer J.N. General compared with spinal anesthesia for total hip arthroplasty. J Bone Jt Surg Am. 2015;97:455–461. doi: 10.2106/JBJS.N.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brueckner S., Reinke U., Roth-Isigkeit A., Eleftheriadis S., Schmucker P., Siemens H.J. Comparison of general and spinal anesthesia and their influence on hemostatic markers in patients undergoing total hip arthroplasty. J Clin Anesth. 2003;15:433–440. doi: 10.1016/s0952-8180(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell D., Friedman R.J., Baker J.D., 3rd, Cooke J.E., Darcy M.D., Miller M.C., 3rd Prevention of thromboembolic disease following total knee arthroplasty. Epidural versus general anesthesia. Clin Orthop Relat Res. 1991;269:109–112. [PubMed] [Google Scholar]
- 57.Park Y.B., Chae W.S., Park S.H., Yu J.S., Lee S.G., Yim S.J. Comparison of short-term complications of general and spinal anesthesia for primary unilateral total knee arthroplasty. Knee Surg Relat Res. 2017;29:96–103. doi: 10.5792/ksrr.16.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi H.J., Xue X.H., Wang Y.L., Zhang W.S., Wang Z.S., Yu A.L. Effects of different anesthesia methods on cognitive dysfunction after hip replacement operation in elder patients. Int J Clin Exp Med. 2015;8:3883–3888. [PMC free article] [PubMed] [Google Scholar]
- 59.Kita T., Maki N., Song Y.S., Arai F., Nakai T. Caudal epidural anesthesia administered intraoperatively provides for effective postoperative analgesia after total hip arthroplasty. J Clin Anesth. 2007;19:204–208. doi: 10.1016/j.jclinane.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Benson M., Hartmann B., Junger A., Dietrich G., Böttger S., Hempelmann G. Causes of higher blood loss during general anesthesia compared to spinal anesthesia in total hip replacement—a retrospective analysis of data collected online. Transfus Med Hemother. 2000;27:311–316. [Google Scholar]
- 61.Borghi B., Casati A., Iuorio S. Frequency of hypotension and bradycardia during general anesthesia, epidural anesthesia, or integrated epidural-general anesthesia for total hip replacement. J Clin Anesth. 2002;14:102–106. doi: 10.1016/s0952-8180(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 62.Davis F.M., McDermott E., Hickton C. Influence of spinal and general anaesthesia on haemostasis during total hip arthroplasty. Br J Anaesth. 1987;59:561–571. doi: 10.1093/bja/59.5.561. [DOI] [PubMed] [Google Scholar]
- 63.Eroglu A., Uzunlar H., Erciyes N. Comparison of hypotensive epidural anesthesia and hypotensive total intravenous anesthesia on intraoperative blood loss during total hip replacement. J Clin Anesth. 2005;17:420–425. doi: 10.1016/j.jclinane.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Flordal P.A., Neander G. Blood loss in total hip replacement. A retrospective study. Arch Orthop Trauma Surg. 1991;111:34–38. doi: 10.1007/BF00390191. [DOI] [PubMed] [Google Scholar]
- 65.Hamilton H., Jamieson J. Deep infection in total hip arthroplasty. Can J Surg. 2008;51:111–117. [PMC free article] [PubMed] [Google Scholar]
- 66.Hartmann B., Junger A., Benson M. Comparison of blood loss using fluorescein flow cytometry during total hip replacement under general or spinal anesthesia. Transfus Med Hemother. 2003;30:20–26. [Google Scholar]
- 67.Jones M.J., Piggott S.E., Vaughan R.S. Cognitive and functional competence after anaesthesia in patients aged over 60: controlled trial of general and regional anaesthesia for elective hip or knee replacement. BMJ. 1990;300:1683–1687. doi: 10.1136/bmj.300.6741.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keith I. Anaesthesia and blood loss in total hip replacement. Anaesthesia. 1977;32:444–450. doi: 10.1111/j.1365-2044.1977.tb09981.x. [DOI] [PubMed] [Google Scholar]
- 69.Wong S., Tang H., de Steiger R. Blood management in total hip replacement: an analysis of factors associated with allogenic blood transfusion. ANZ J Surg. 2015;85:461–465. doi: 10.1111/ans.13048. [DOI] [PubMed] [Google Scholar]
- 70.Jacob A.K., Mantilla C.B., Sviggum H.P., Schroeder D.R., Pagnano M.W., Hebl J.R. Perioperative nerve injury after total knee arthroplasty: regional anesthesia risk during a 20-year cohort study. Anesthesiology. 2011;114:311–317. doi: 10.1097/ALN.0b013e3182039f5d. [DOI] [PubMed] [Google Scholar]
- 71.Memtsoudis S.G., Danninger T., Rasul R. Inpatient falls after total knee arthroplasty: the role of anesthesia type and peripheral nerve blocks. Anesthesiology. 2014;120:551–563. doi: 10.1097/ALN.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 72.Kendrišić M., Šurbatović M., Đorđević D., Trifunović B., Jevđić J. Analgesic efficacy and safety of four different anesthesia/postoperative analgesia protocols in patients following total hip arthroplasty. Vojnosanit Pregl. 2017;74:814–820. [Google Scholar]
- 73.Bonnet F., Harari A., Thibonnier M., Viars P. Suppression of antidiuretic hormone hypersecretion during surgery by extradural anaesthesia. Br J Anaesth. 1982;54:29–36. doi: 10.1093/bja/54.1.29. [DOI] [PubMed] [Google Scholar]
- 74.Borghi B., Casati A., Iuorio S. Effect of different anesthesia techniques on red blood cell endogenous recovery in hip arthroplasty. J Clin Anesth. 2005;17:96–101. doi: 10.1016/j.jclinane.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 75.Modig J., Malmberg P., Karlstrom G. Effect of epidural versus general anaesthesia on calf blood flow. Acta Anaesthesiol Scand. 1980;24:305–309. doi: 10.1111/j.1399-6576.1980.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 76.Modig J., Borg T., Bagge L., Saldeen T. Role of extradural and of general anaesthesia in fibrinolysis and coagulation after total hip replacement. Br J Anaesth. 1983;55:625–629. doi: 10.1093/bja/55.7.625. [DOI] [PubMed] [Google Scholar]
- 77.Modig J., Karlstrom G. Intra- and post-operative blood loss and haemodynamics in total hip replacement when performed under lumbar epidural versus general anaesthesia. Eur J Anaesthesiol. 1987;4:345–355. [PubMed] [Google Scholar]
- 78.D’Ambrosio A., Borghi B., Damato A., D’Amato G., Antonacci D., Valeri F. Reducing perioperative blood loss in patients undergoing total hip arthroplasty. Int J Artif Organs. 1999;22:47–51. [PubMed] [Google Scholar]
- 79.Chin S.P., Abou-Madi M.N., Eurin B., Witvoet J., Montagne J. Blood loss in total hip replacement: extradural v. phenoperidine analgesia. Br J Anaesth. 1982;54:491–495. doi: 10.1093/bja/54.5.491. [DOI] [PubMed] [Google Scholar]
- 80.Wulf H., Biscoping J., Beland B., Bachmann-Mennenga B., Motsch J. Ropivacaine epidural anesthesia and analgesia versus general anesthesia and intravenous patient-controlled analgesia with morphine in the perioperative management of hip replacement. Ropivacaine Hip Replacement Multicenter Study Group. Anesth Analg. 1999;89:111–116. doi: 10.1097/00000539-199907000-00019. [DOI] [PubMed] [Google Scholar]
- 81.Williams-Russo P., Sharrock N.E., Mattis S., Szatrowski T.P., Charlson M.E. Cognitive effects after epidural vs general anesthesia in older adults. A randomized trial. JAMA. 1995;274:44–50. [PubMed] [Google Scholar]
- 82.Williams-Russo P, Sharrock NE, Haas SB Randomized trial of epidural versus general anesthesia: outcomes after primary total knee replacement. Clin Orthop Relat Res. 1996;331:199–208. doi: 10.1097/00003086-199610000-00028. [DOI] [PubMed] [Google Scholar]
- 83.Bugada D., Allegri M., Gemma M. Effects of anaesthesia and analgesia on long-term outcome after total knee replacement: a prospective, observational, multicentre study. Eur J Anaesthesiol. 2017;34:665–672. doi: 10.1097/EJA.0000000000000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hsieh J.Y., Lin H.W. Less incidence of coronary artery disease in general anesthesia compared to spinal-epidural anesthesia after total knee replacement: 90-day follow-up period by a population-based dataset. Eur J Orthop Surg Traumatol. 2015;25:927–932. doi: 10.1007/s00590-015-1616-3. [DOI] [PubMed] [Google Scholar]
- 85.Chelly J.E., Greger J., Gebhard R. Continuous femoral blocks improve recovery and outcome of patients undergoing total knee arthroplasty. J Arthroplasty. 2001;16:436–445. doi: 10.1054/arth.2001.23622. [DOI] [PubMed] [Google Scholar]
- 86.Chu C.P., Yap J.C., Chen P.P., Hung H.H. Postoperative outcome in Chinese patients having primary total knee arthroplasty under general anaesthesia/intravenous patient-controlled analgesia compared to spinal-epidural anaesthesia/analgesia. Hong Kong Med J. 2006;12:442–447. [PubMed] [Google Scholar]
- 87.Liu J., Ma C., Elkassabany N., Fleisher L.A., Neuman M.D. Neuraxial anesthesia decreases postoperative systemic infection risk compared with general anesthesia in knee arthroplasty. Anesth Analg. 2013;117:1010–1016. doi: 10.1213/ANE.0b013e3182a1bf1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lingaraj K., Ruben M., Chan Y., Das S.D. Identification of risk factors for urinary retention following total knee arthroplasty: a Singapore hospital experience. Singapore Med J. 2007;48:213. [PubMed] [Google Scholar]
- 89.Haas S.B. Effects of epidural anesthesia on incidence of venous thromboembolism following joint replacement. Orthopedics. 1994;17:18–20. doi: 10.3928/0147-7447-19940702-08. [DOI] [PubMed] [Google Scholar]
- 90.Jorgensen L.N., Rasmussen L.S., Nielsen P.T., Leffers A., Albrecht-Beste E. Antithrombotic efficacy of continuous extradural analgesia after knee replacement. Br J Anaesth. 1991;66:8–12. doi: 10.1093/bja/66.1.8. [DOI] [PubMed] [Google Scholar]
- 91.Nielsen P.T., Jorgensen L.N., Albrecht-Beste E., Leffers A.M., Rasmussen L.S. Lower thrombosis risk with epidural blockade in knee arthroplasty. Acta Orthop Scand. 1990;61:29–31. doi: 10.3109/17453679008993060. [DOI] [PubMed] [Google Scholar]
- 92.Ishii Y., Noguchi H., Sato J., Takayama S., Okada Y., Toyabe S.I. Impact of anesthesia modality and mechanical venous thromboembolism prophylaxis on the incidence of symptomatic deep venous thrombosis after TKA. J Clin Orthop Trauma. 2018;9:142–145. doi: 10.1016/j.jcot.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nathan S., Aleem M.A., Thiagarajan P., Das De S. The incidence of proximal deep vein thrombosis following total knee arthroplasty in an Asian population: a Doppler ultrasound study. J Orthop Surg (Hong Kong) 2003;11:184–189. doi: 10.1177/230949900301100214. [DOI] [PubMed] [Google Scholar]
- 94.Sharrock N.E., Haas S.B., Hargett M.J., Urquhart B., Insall J.N., Scuderi G. Effects of epidural anesthesia on the incidence of deep-vein thrombosis after total knee arthroplasty. J Bone Jt Surg Am. 1991;73:502–506. [PubMed] [Google Scholar]
- 95.Zhou L.Y., Gu W., Liu Y., Ma Z.L. Effects of inhalation anesthesia vs. total intravenous anesthesia (TIVA) vs. spinal-epidural anesthesia on deep vein thrombosis after total knee arthroplasty. Med Sci Monit. 2018;24:67–75. doi: 10.12659/MSM.904378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kudoh A., Takase H., Takazawa T. A comparison of anesthetic quality in propofol-spinal anesthesia and propofol-fentanyl anesthesia for total knee arthroplasty in elderly patients. J Clin Anesth. 2004;16:405–410. doi: 10.1016/j.jclinane.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 97.Belmont P.J., Jr., Goodman G.P., Rodriguez M., Bader J.O., Waterman B.R., Schoenfeld A.J. Predictors of hospital readmission following revision total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24:3329–3338. doi: 10.1007/s00167-015-3782-6. [DOI] [PubMed] [Google Scholar]
- 98.Kayupov E., Okroj K., Young A.C. Continuous adductor canal blocks provide superior ambulation and pain control compared to epidural analgesia for primary knee arthroplasty: a randomized, controlled trial. J Arthroplasty. 2018;33:1040–1044.e1. doi: 10.1016/j.arth.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 99.Napier D.E., Bass S.S. Postoperative benefits of intrathecal injection for patients undergoing total knee arthroplasty. Orthop Nurs. 2007;26:374–378. doi: 10.1097/01.NOR.0000300950.96117.0f. [DOI] [PubMed] [Google Scholar]
- 100.Pope D., El-Othmani M.M., Manning B.T., Sepula M., Markwell S.J., Saleh K.J. Impact of age, gender and anesthesia modality on post-operative pain in total knee arthroplasty patients. Iowa Orthop J. 2015;35:92–98. [PMC free article] [PubMed] [Google Scholar]
- 101.Lawrie C.M., Ong A.C., Hernandez V.H., Rosas S., Post Z.D., Orozco F.R. Incidence and risk factors for postoperative urinary retention in total hip arthroplasty performed under spinal anesthesia. J Arthroplasty. 2017;32:3748–3751. doi: 10.1016/j.arth.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 102.Williams M.R., McKeown A., Dexter F. Efficacy outcome measures for procedural sedation clinical trials in adults: an ACTTION systematic review. Anesth Analg. 2016;122:152–170. doi: 10.1213/ANE.0000000000000934. [DOI] [PubMed] [Google Scholar]
- 103.Kehlet H. The stress response to surgery: release mechanisms and the modifying effect of pain relief. Acta Chir Scand Suppl. 1989;550:22–28. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.