Abstract
Background
Recent reports of patients with coronavirus disease 2019 (COVID-19) developing pneumothorax correspond mainly to case reports describing mechanically ventilated patients. The real incidence, clinical characteristics, and outcome of spontaneous pneumothorax (SP) as a form of COVID-19 presentation remain to be defined.
Research Question
Do the incidence, risk factors, clinical characteristics, and outcomes of SP in patients with COVID-19 attending EDs differ compared with COVID-19 patients without SP and non-COVID-19 patients with SP?
Study Design and Methods
This case-control study retrospectively reviewed all patients with COVID-19 diagnosed with SP (case group) in 61 Spanish EDs (20% of Spanish EDs) and compared them with two control groups: COVID-19 patients without SP and non-COVID-19 patients with SP. The relative frequencies of SP were estimated in COVID-19 and non-COVID-19 patients in the ED, and annual standardized incidences were estimated for both populations. Comparisons between case subjects and control subjects included 52 clinical, analytical, and radiologic characteristics and four outcomes.
Results
We identified 40 occurrences of SP in 71,904 patients with COVID-19 attending EDs (0.56‰; 95% CI, 0.40‰-0.76‰). This relative frequency was higher than that among non-COVID-19 patients (387 of 1,358,134, 0.28‰; 95% CI, 0.26‰-0.32‰; OR, 1.93; 95% CI, 1.41-2.71). The standardized incidence of SP was also higher in patients with COVID-19 (34.2 vs 8.2/100,000/year; OR, 4.19; 95% CI, 3.64-4.81). Compared with COVID-19 patients without SP, COVID-19 patients developing SP more frequently had dyspnea and chest pain, low pulse oximetry readings, tachypnea, and increased leukocyte count. Compared with non-COVID-19 patients with SP, case subjects differed in 19 clinical variables, the most prominent being a higher frequency of dysgeusia/anosmia, headache, diarrhea, fever, and lymphopenia (all with OR > 10). All the outcomes measured, including in-hospital death, were worse in case subjects than in both control groups.
Interpretation
SP as a form of COVID-19 presentation at the ED is unusual (< 1‰ cases) but is more frequent than in the non-COVID-19 population and could be associated with worse outcomes than SP in non-COVID-19 patients and COVID-19 patients without SP.
Key Words: clinical characteristics, COVID-19, incidence, outcome, risk factors, SARS-CoV-2, spontaneous pneumothorax
Abbreviations: COVID-19, coronavirus disease 2019; RT-PCR, reverse transcriptase-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome-coronavirus 2; SP, spontaneous pneumothorax; UMC-19, Unusual Manifestations of COVID-19 project; UMC-19-S7, UMC-19 Study 7
Infection with severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) is characterized mainly by fever and respiratory symptoms, with dyspnea and lung infiltrates being present in more than 50% of hospitalized cases.1 A significant number of other signs and symptoms can be present, involving the GI tract, hepatic inflammation, myalgia and rhabdomyolysis, neurologic symptoms such as dysgeusia and anosmia, or a procoagulant state, biochemically detected by increased D-dimers and related to complications and worse prognosis.1, 2, 3, 4 In some patients, some of these entities appear after the patient has been admitted and, to some extent, represent the increased number of complications that may be presented by patients who are bedridden, multidrug treated, and/or in very poor condition. In this scenario, it is difficult to quantify the real association of a certain manifestation with the pathogenesis of the disease caused by SARS-CoV-2 infection.
Spontaneous pneumothorax (SP) is a potential complication in some pulmonary infections, and it is especially frequent in Pneumocystis jirovecii pneumonia.5 The real incidence of SP in patients with coronavirus disease 2019 (COVID-19) is currently unknown. Some sporadic cases of patients with COVID-19 developing pneumothorax have been reported.2 , 6, 7, 8, 9, 10, 11, 12, 13, 14 In some of these cases, invasive or noninvasive mechanical ventilation was applied before the development of pneumothorax,6 , 9 , 12, 13, 14 whereas in other cases it appeared after several weeks of pulmonary involvement, with large inflammatory infiltration and cyst formation in the pulmonary parenchyma.6 , 9 , 10 Indeed, only a few case series have described the frequency of pneumothorax in COVID-19, being reported as present in 1% of 99 hospitalized patients,2 in 3% of patients hospitalized with pneumonia,14 in 6% of 202 mechanically ventilated patients,15 and in 1% of 91 deceased patients.16 For most of these patients, noninvasive or invasive mechanical ventilation probably contributed to this relatively high incidence. In the present study, we aimed to investigate the frequency of SP in patients attended in the ED, before hospitalization and treatment with specific drugs for SARS-CoV-2 infection and before the initiation of ventilatory support. The specific objectives were as follows: (1) to determine the relative frequency of SP in patients with COVID-19 coming to the ED as well as estimate the annual standardized incidence; (2) to uncover the risk factors associated with the development of SP in patients with COVID-19; (3) to describe whether these patients have any distinctive clinical characteristic compared with SP observed in non-COVID-19 patients; and (4) to investigate the outcomes of patients with COVID-19 presenting with SP.
Methods
Study Design and Setting
The present study forms part of the Unusual Manifestations of COVID-19 (UMC-19) project, which was designed to investigate the potential relationship between COVID-19 and 10 different entities that could be influenced by SARS-CoV-2 infection itself: SP, acute pancreatitis, meningoencephalitis, Guillain-Barré syndrome, (myo)pericarditis, acute coronary syndrome, DVT, pulmonary embolism, ictus, and GI bleeding. The main objectives of the UMC-19 project were common for all of the entities and consisted in the description of the incidence, risk factors, clinical characteristics, and outcomes for each particular entity, using patients with COVID-19 who did not develop these entities as well as non-COVID-19 patients who presented these entities as comparators. Complete details of the UMC-19 project have been published elsewhere.17
In Spain, the first case of SARS-CoV-2 infection was detected on January 31, 2020 and, accordingly, the COVID-19 period for the inclusion of case subjects in the present UMC-19 study project was set from March 1 to April 30, 2020. For the recruitment of control subjects, the UMC-19 project selected patients from two different periods: one corresponding to the same dates as the case subjects (from March 1 to April 30, 2020) and one corresponding to the same period of the previous year (from March 1 to April 30, 2019).
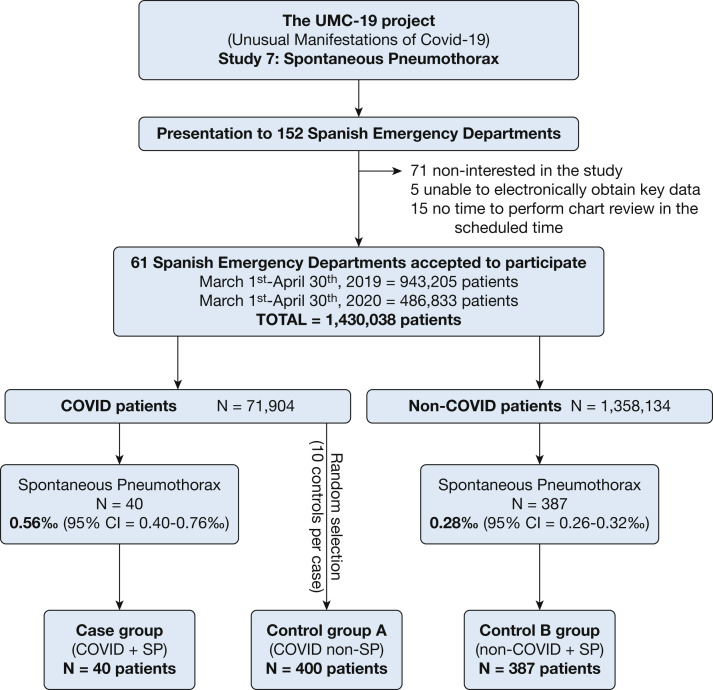
The investigators of the UMC-19 project initially contacted 152 Spanish EDs, which constitute roughly one-half of the 312 hospital EDs of the Spanish public health network. Of these, 81 considered participation and analyzed the protocol, and finally 61 (20% of Spanish EDs) consented to participate and duly sent all the data required (Fig 1 ). Altogether, these 61 hospitals provide health coverage to 14,537,000 citizens (31% of the population of 46.9 million of Spain) and make up a balanced representation of the Spanish territory (representing 12 of the 17 Spanish autonomous communities), type of hospital (community, reference, and high-technology university hospitals were included), and involvement in the pandemic (with EDs attending from 1% to 47% of the ED census during the COVID-19 outbreak period corresponding to patients with COVID-19).17
Figure 1.
Study design and inclusion flow chart. COVID-19 = coronavirus disease 2019; SP = spontaneous pneumothorax; UMC-19 = Unusual Manifestations of COVID-19 project.
The investigation of SP in patients with COVID-19, one of the entities included in the UMC-19 project, was labeled as UMC-19 Study 7 (UMC-19-S7) and consisted of a retrospective, case-control, multicenter study that reviewed the medical reports of patients with COVID-19 attended and diagnosed with SP during ED assessment and treatment in Spanish EDs before hospitalization. As the UMC-19-S7 was conceived as an exploratory study, sample size was not predetermined.
Case Subjects in UMC-19-S7
The case group was formed by patients with COVID-19, with the diagnosis of SP being made on the basis of chest radiography or thoracic CT imaging. All SPs were confirmed by radiologists and/or thoracic surgeons. Patients in whom pneumothorax developed as a consequence of trauma, manipulations, or other secondary causes were excluded. On the other hand, the diagnosis of COVID-19 was accepted on the basis of SARS-CoV-2 antigen detection in a nasopharyngeal swab by reverse transcriptase-polymerase chain reaction (RT-PCR), a clinically compatible clinical picture (including at least malaise, fever, and cough) or the presence of typical lung parenchymal infiltrates on chest radiography (bilateral interstitial lung infiltrates and ground-glass infiltrates) in patients with some clinical symptoms attributable to COVID-19. Diagnostic adjudication was done by the principal investigator of each center without external review.
Control Subjects in UMC-19-S7
We defined two different control groups. One group was formed by patients with COVID-19 (without SP) attending the ED during the same COVID-19 outbreak period used for case inclusion (March 1 to April 30, 2020). As the number of COVID-19 patients with SP included in the UMC-19-S7 was expected to be very low, we planned to select 10 patients with COVID-19 for every case detected by each center, to maximize the statistical power as much as possible. Selection was performed by inclusion of the 10 patients with COVID-19 seen immediately before (five patients) and after (five patients) each case subject included by the center. This group, named control group A, was specifically designed to uncover the risk factors associated with SP development in patients with COVID-19.
The second control group was made up of non-COVID-19 patients with a diagnosis of SP attending the ED during the same period as the case subjects (March 1 to April 30, 2020), which was defined in the same terms as for the case subjects. To avoid the possibility that some of these control case subjects could eventually have been inadvertently infected with SARS-CoV-2, or that some with mild symptoms could have remained at home during the COVID-19 outbreak due to fear of COVID-19 contagion, we also included patients with SP diagnosed in the ED from March 1 to April 30, 2019, just 1 year before the COVID-19 pandemic. On the basis of the same principle of maximizing the statistical power as much as possible, we planned to include and review all non-COVID-19 patients with SP identified in these two periods (the COVID-19 period in 2020 and the pre-COVID-19 period in 2019). This group, named control group B, was specifically designed to uncover the particular distinctive clinical characteristics of SP developed in COVID-19 patients with respect to SP developed in the general population.
Independent Variables
We collected 52 independent variables, which included two demographic data (age, sex), 12 comorbidities (COPD, asthma, active smoker, hypertension, dyslipidemia, diabetes mellitus, coronary artery disease, obesity [clinically estimated], cerebrovascular disease, chronic kidney disease [creatinine > 2 mg/dL], dementia, active cancer), 16 related to symptomatology (time elapsed from symptom onset to ED attendance, fever, rhinorrhea, cough, expectoration, dyspnea, chest pain, syncope, hemoptysis, abdominal pain, vomiting, diarrhea, confusion, headache, anosmia, dysgeusia), five vital signs at ED arrival (temperature, systolic BP, heart rate, respiratory rate, room air pulse oximetry reading), nine laboratory parameters (C-reactive protein, creatinine, aspartate aminotransferase, lactate dehydrogenase, procalcitonin, hemoglobin, leukocytes, lymphocytes, D-dimer), and eight radiologic findings on chest radiography (cardiomegaly, pleural effusion, interstitial lung infiltrates, and ground-glass opacities; and location, extension, and accompanying pneumomediastinum and subcutaneous emphysema in patients with pneumothorax). All these variables were collected by retrospective review of all patient medical reports obtained during ED and hospital stay.
Outcomes
We defined four different outcomes for case subjects and control subjects, which consisted of (1) the need for hospitalization, (2) the need for admission to intensive care, (3) prolonged hospitalization (defined as a length of stay > 7 days, which is the median length of stay of hospitalized patients in Spain), and (4) in-hospital all-cause mortality. Outcomes were determined after retrospective review of all patient medical reports obtained during ED and hospital stay. Outcomes adjudication was done by the principal investigator of each center without external review.
Statistical Analysis
Discrete variables were expressed as absolute values and percentages, and continuous variables as mean and SD or median and interquartile range if not normally distributed. The relative frequency of SP was expressed per thousand (‰) COVID-19 or non-COVID-19 patients coming to the ED, and the annual standardized incidence was expressed per 100,000 COVID-19 or non-COVID-19 individuals. Both estimations were made with 95% CIs that were calculated using the exact method for binomial distributions. To estimate the COVID-19 and non-COVID-19 population in each ED catchment area, we used the seroprevalence of SARS-CoV-2 in the province where the ED was located. These detailed seroprevalences were determined in a wide Spanish study performed between April 27 and May 11, 2020, and have recently been reported.18
Differences between the case and the control groups were assessed by the χ2 test (or Fisher exact test if needed) for qualitative variables, and the Student t test (or the Mann-Whitney nonparametric test if nonnormally distributed) for quantitative variables. Correction for multiple comparisons was performed by the Bonferroni method. The magnitude of associations remaining statistically significant after this correction was expressed as unadjusted ORs with 95% CIs, which were calculated by logistic regression independently comparing all case subjects with all control A patients, and all case subjects with all control B patients. Continuous variables were dichotomized using clinically meaningful cutoffs or around the median of distribution. As the number of patients with SP we expected to identify was not large, we did not plan to go further in the investigation of the significant relationships of risk factors and clinical characteristics identified in the unadjusted analysis using adjusted models. On the other hand, outcomes analysis is presented as unadjusted and adjusted for age and sex and, in addition, we also added center to obtain further adjustment. Sensitivity analysis for the comparison between case subjects and control A patients, using only those with microbiologic confirmation of SARS-CoV-2 infection, was performed for the clinical characteristics found to be statistically significant in the main analysis and for the outcomes.
In all comparisons, statistical significance was accepted if the P value was < .05 or if the 95% CI of the risk estimations excluded the value 1. All the analyses were performed with the SPSS (version 24) statistical software package (IBM).
Ethics
The UMC-19 project was approved by the Ethics Committee of the Hospital Clínic of Barcelona (Spain) (reference number HCB/2020/0534), which acted as the central ethics committee. Under the exceptional circumstances generated by the COVID-19 pandemic, the urgent need to obtain feasible data related to this new disease, and the noninterventional and retrospective nature of the project, the requirement that written patient consent be obtained to be included in the study was waived. All patients were codified by investigators of the participating centers before entering their data into the general database, thereby ensuring patient anonymity to investigators analyzing the database. The UMC-19-S7 was carried out in strict compliance with the principles of the Declaration of Helsinki. The authors designed the study, gathered and analyzed the data, vouched for the data and analysis, wrote the article, and decided to publish.
Results
Relative Frequency and Standardized Incidence
A total of 71,904 patients with COVID-19 were seen in the 61 Spanish EDs participating in the UMC-19-S7 (Fig 1) during the 61-day study period. Forty of these patients presented SP (0.56‰; 95% CI, 0.40‰-0.76‰) and constituted the case group. Control group A was formed by 400 selected patients without SP (COVID-19 non-SP) during the same period. Confirmation of SARS-CoV-2 infection by RT-PCR was performed in 75.0% and 74.8% of patients, respectively. On the other hand, 1,358,134 non-COVID-19 patients were seen during the 122-day period (414,929 during the 61 days in the 2020 COVID-19 period, and 943,205 during the 61 days of the 2019 pre-COVID-19 period). Of these, 387 were diagnosed with SP (134 in the COVID-19 period and 253 in the pre-COVID-19 period). Accordingly, the overall relative frequency for the whole period was 0.28‰ (95% CI, 0.26‰-0.32‰), with relative frequencies during the COVID-19 and pre-COVID-19 periods of 0.32‰ (95% CI, 0.27‰-0.38‰) and 0.27‰ (95% CI, 0.24‰-0.30‰), respectively. These 387 patients constituted control group B. A pleural tube for pneumothorax drainage was placed in 29 of 40 patients of the case group (73%) and in 306 of the 387 patients in control B group (79.1%; P = .32).
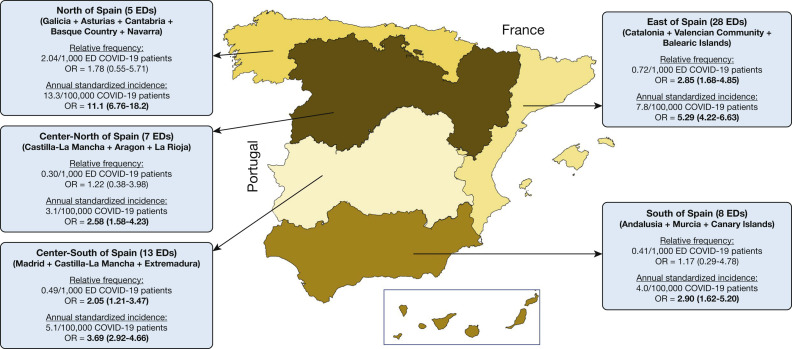
The relative frequency of SP in COVID-19 compared with non-COVID-19 patients coming to the ED resulted in an OR of 1.93 (95% CI, 1.41-2.71), with ORs of 1.72 (95% CI, 1.21-2.45) and 2.07 (95% CI, 1.49-2.90) when comparisons were made with non-COVID-19 patients diagnosed during the COVID-19 and pre-COVID-19 periods separately, respectively. On the other hand, the standardized incidences of SP were 34.2 per 100,000 COVID-19 individuals per year (95% CI, 30.0-38.9), and 8.2 per 100,000 non-COVID-19 individuals per year (95% CI, 7.7-8.7; with partial standard incidences of 5.8 [95% CI, 5.4-6.2] in the COVID-19 period and 10.4 [95% CI, 9.9-11.0] in the pre-COVID-19 period). Accordingly, the OR for the standardized incidence in COVID-19 patients compared with non-COVID-19 patients was 4.19 (95% CI, 3.64-4.81); with an OR with respect to the COVID-19 period of 5.89 (95% CI, 5.10-6.81) and an OR with respect to the pre-COVID-19 period of 3.28 (95% CI, 2.86-3.76).The analysis of relative frequencies and standardized incidences by Spanish geographical regions showed some variability, although most ORs remained statistically significant (Fig 2 ).
Figure 2.
Relative frequencies and annual standardized incidence of spontaneous pneumothorax for patients with COVID-19 in the various Spanish regions. ORs were determined for COVID-19 patients with respect to non-COVID-19 patients; ORs in boldface denote statistical significance (P < .05). COVID-19 = coronavirus disease 2019.
Clinical Characteristics
The mean age of the COVID-19 patients with SP (case subjects) was 66 years, 73% were male, 20% had asthma, 10% had COPD, and 10% were active smokers. The most frequent symptomatology was dyspnea (88%), cough (53%), chest pain (40%), and fever (38%), and the median time from symptom onset to ED consultation was 5 days. The remaining clinical characteristics, as well as the vital signs at ED arrival and laboratory findings, are presented in Table 1 . Cardiomegaly and pleural effusion were rarely seen in these patients (in 11% and 3% of radiographs, respectively), but interstitial lung infiltrates and ground-glass opacities were frequently observed (in about one-half of the patients). The location of SP was in the right lung in 81% of cases, with an extension ranging from minimal to massive, and was accompanied by pneumomediastinum and subcutaneous emphysema in 16% of cases (Table 1). In three case subjects and 15 patients in control group B, chest radiography was not performed in the ED, and SP diagnosis was made by thoracic CT imaging.
Table 1.
Baseline Characteristics of COVID-19 Patients With Spontaneous Pneumothorax and Comparison With COVID-19 Patients Without Spontaneous Pneumothorax (Control Group A) and With Non-COVID-19 Patients With Spontaneous Pneumothorax (Control Group B)
| Characteristic | Case Subjects (COVID-19-SP) (n = 40) |
Comparison With Control Group A |
Comparison With Control Group B |
||||
|---|---|---|---|---|---|---|---|
| Control Group A (COVID-19-Non-SP) (n = 400) |
P Valuea | P Value (Corrected)b | Control Group B (Non-COVID-19-SP) (n = 387) |
P Valuec | P Value (Corrected)d | ||
| Demographics | |||||||
| Age, median (IQR), y | 66 (47-74) | 61 (46-77) | .93 | ... | 36 (22-57) | < .001 | < .001 |
| Sex, male | 29 (72.5) | 205 (51.3) | .012 | > .05 | 303 (78.3) | .43 | ... |
| Pulmonary comorbidities | |||||||
| COPD | 4 (10.0) | 33 (8.3) | .76 | ... | 58 (15.0) | .49 | ... |
| Asthma | 8 (20.0) | 27 (6.8) | .009 | > .05 | 22 (5.7) | .004 | > .05 |
| Active smoker | 4 (10.0) | 26 (6.5) | .34 | ... | 146 (37.7) | < .001 | .02 |
| Other comorbidities | |||||||
| Hypertension | 15 (37.5) | 168 (42.0) | .62 | ... | 48 (12.4) | < .001 | .001 |
| Diabetes mellitus | 7 (17.5) | 74 (18.5) | 1.00 | ... | 23 (5.9) | .02 | > .05 |
| Active cancer | 5 (12.5) | 38 (9.5) | .57 | ... | 41 (10.6) | .79 | ... |
| Coronary artery disease | 4 (10.0) | 30 (7.5) | .54 | ... | 8 (2.1) | .02 | > .05 |
| Obesity (clinically estimated) | 3 (7.5) | 57 (14.3) | .33 | ... | 8 (2.1) | .07 | ... |
| Chronic kidney disease | 1 (2.5) | 34 (8.5) | .35 | ... | 14 (3.6) | 1.00 | ... |
| Cerebrovascular disease | 1 (2.5) | 29 (7.3) | .50 | ... | 13 (3.4) | 1.00 | ... |
| Dementia | 1 (2.5) | 35 (8.8) | .23 | ... | 7 (1.8) | .55 | ... |
| Peripheral arterial disease | 1 (2.5) | 17 (4.3) | 1.00 | ... | 9 (2.3) | 1.00 | ... |
| Symptoms related to COVID-19 | |||||||
| Lasting symptoms, median (IQR), d | 5 (2-11) | 7 (4-10) | .16 | ... | 1 (1-2) | < .001 | < .001 |
| Fever (> 38°C) | 15 (37.5) | 247 (61.8) | .004 | > .05 | 9 (2.3) | < .001 | < .001 |
| Rhinorrhea | 2 (5.0) | 33 (8.3) | .76 | ... | 6 (1.6) | .17 | ... |
| Cough | 21 (52.5) | 242 (60.5) | .40 | ... | 47 (12.1) | < .001 | < .001 |
| Expectoration | 6 (15.0) | 59 (14.8) | 1.00 | ... | 24 (6.2) | .05 | ... |
| Dyspnea | 35 (87.5) | 217 (54.3) | < .001 | .002 | 227 (58.7) | < .001 | .02 |
| Abdominal pain | 0 (0) | 22 (5.5) | .25 | ... | 8 (2.1) | 1.00 | ... |
| Vomiting | 0 (0) | 25 (6.3) | .15 | ... | 3 (0.8) | 1.00 | ... |
| Diarrhea | 9 (22.5) | 74 (18.5) | .53 | ... | 3 (0.8) | < .001 | < .001 |
| Confusion | 2 (5.0) | 27 (6.8) | 1.00 | ... | 4 (1.0) | .10 | ... |
| Headache | 3 (7.5) | 47 (11.8) | .60 | ... | 0 (0) | < .001 | .04 |
| Anosmia | 5 (12.5) | 31 (7.8) | .36 | ... | 2 (0.5) | < .001 | < .001 |
| Dysgeusia | 7 (17.5) | 36 (9.0) | .09 | ... | 0 (0) | < .001 | < .001 |
| Symptoms related to pneumothorax | |||||||
| Lasting symptoms, median (IQR), d | 5 (2-11) | 7 (4-10) | .16 | ... | 1 (1-2) | < .001 | < .001 |
| Dyspnea | 35 (87.5) | 217 (54.3) | < .001 | .002 | 227 (58.7) | < .001 | .02 |
| Chest pain | 16 (40.0) | 57 (14.3) | < .001 | .001 | 329 (85.0) | < .001 | < .001 |
| Syncope | 1 (2.5) | 13 (3.3) | 1.00 | ... | 7 (1.8) | .55 | ... |
| Hemoptysis | 0 (0) | 3 (0.8) | 1.00 | ... | 3 (0.8) | 1.00 | ... |
| Vital signs at ED arrival | |||||||
| Temperature, median (IQR), °C | 36.8 (36.0-37.1) | 36.6 (36.0-37.3) | .67 | ... | 36.0 (36.0-36.5) | < .001 | < .001 |
| SBP, median (IQR), mm Hg | 132 (116-143) | 123 (115-138) | .41 | ... | 125 (115-138) | .48 | ... |
| Heart rate, median (IQR), beats/min | 90 (80-107) | 89 (79-100) | .30 | ... | 86 (73-99) | .04 | > .05 |
| Respiratory rate, median (IQR), breaths/min | 24 (20-30) | 18 (16-22) | < .001 | < .001 | 18 (15-20) | < .001 | < .001 |
| Room air pulse oximetry, median (IQR), % | 92 (87-97) | 96 (93-98) | < .001 | < .001 | 97 (95-99) | < .001 | < .001 |
| Laboratory findings, median (IQR) | |||||||
| Hemoglobin, g/L | 143 (134-152) | 138 (127-148) | .07 | ... | 148 (136-157) | .10 | ... |
| Leukocyte count, cells/μL | 9.81 (7.80-12.2) | 6.65 (5.02-9.00) | < .001 | < .001 | 9.54 (7.24-11.90) | .74 | ... |
| Lymphocyte count, cells/μL | 0.79 (0.54-1.20) | 1.16 (0.80-1.60) | .002 | > .05 | 1.97 (1.30-2.54) | < .001 | < .001 |
| D-dimer, ng/mL | 1,483 (606-4,201) | 620 (354-1,220) | .001 | > .05 | 420 (200-996) | .001 | > .05 |
| Creatinine, mg/dL | 0.85 (0.73-1.05) | 0.86 (0.71-1.12) | .95 | ... | 0.85 (0.71-0.98) | .48 | ... |
| Aspartate aminotransferase, IU/L | 34 (25-67) | 30 (22-44) | .14 | ... | 20 (17-30) | .001 | > .05 |
| Lactate dehydrogenase, IU/L | 384 (252-586) | 269 (207-354) | .002 | > .05 | 215 (166-306) | < .001 | .003 |
| C-reactive protein, mg/dL | 4.68 (1.65-21.3) | 5.40 (1.69-11.3) | .45 | ... | 0.40 (0.19-1.51) | < .001 | < .001 |
| Procalcitonin, ng/mL | 0.15 (0.03-0.54) | 0.09 (0.05-0.21) | .88 | ... | 0.06 (0.04-0.20) | .42 | ... |
| Chest radiograph findingse | |||||||
| Cardiomegaly | 4 (10.8) | 35 (9.0) | .76 | ... | 10 (2.7) | .03 | > .05 |
| Pleura effusion | 1 (2.7) | 10 (2.6) | 1.00 | ... | 28 (7.5) | .50 | ... |
| Lung interstitial infiltrates | 18 (48.6) | 148 (38.2) | .22 | ... | NA | NA | … |
| Lung ground-glass opacities | 22 (59.5) | 225 (58.1) | .88 | ... | NA | NA | … |
| Location of pneumothorax | NA | < .001 | < .001 | ||||
| Right | 30 (81.1) | NA | ... | 196 (52.7) | … | ||
| Left | 7 (18.9) | NA | ... | 176 (47.3) | … | ||
| Extension of pneumothorax | NA | .43f | ... | ||||
| < 10% | 11 (29.7) | NA | ... | 72 (19.4) | … | ||
| 10%-50% | 13 (35.1) | NA | ... | 171 (46.0) | … | ||
| > 50% | 13 (35.1) | NA | ... | 129 (34.7) | … | ||
| Pneumomediastinum | 6 (16.2) | NA | NA | ... | 12 (3.2) | .003 | > .05 |
| Subcutaneous emphysema | 6 (16.2) | NA | NA | ... | 31 (8.3) | .13 | ... |
Boldface P values denote statistical significance. COVID-19 = coronavirus disease 2019; COVID-19-non-SP = COVID-19 patients without spontaneous pneumothorax; COVID-19-SP = COVID-2019 patients with spontaneous pneumothorax; IQR = interquartile range; NA = not applied; non-COVID-19-SP = non-COVID-19 patients with spontaneous pneumothorax; SBP = systolic BP.
P values refer to comparison between case subjects and control group A.
P values refer to comparison of statistically significant variables between case subjects and control group A after Bonferroni correction for multiple comparisons.
P values refer to comparison between case subjects and control group B.
P values refer to comparison of statistically significant variables between case subjects and control group B after Bonferroni correction for multiple comparisons.
There were no chest radiographs for three case subjects and for 13 and 15 patients in control groups A and B, respectively
Calculated by χ2 for trend.
Some statistically significant differences were found when case subjects were compared with control subjects (Table 1), and the magnitudes of these associations are shown in Table 2 . It should be noted that the complaint of dyspnea at ED arrival was the clinical characteristic of patients with COVID-19 associated with the highest risk of presenting concomitant SP (OR, 5.90; 95% CI, 2.27-15.4). In addition, SP was also found to be associated with more chest pain, tachypnea, and hypoxia, and a higher leukocyte count. All these associations remained statistically significant in the sensitivity analysis including only COVID-19 patients with SARS-CoV-2 infection confirmed by RT-PCR (Table 3 ). COVID-19 patients with SP statistically differed from non-COVID-19 patients with SP in 19 variables, with the most distinctive findings being dysgeusia (OR, 174; 95% CI, 9.69-3,106), headache (OR, 72.3; 95% CI, 3.66-1,428), and fever at ED arrival (OR, 42.4; 95% CI, 4.64-392) (Table 2).
Table 2.
Magnitude of Statistically Significant Associations for Variables Found to Be Unequally Distributed in the Univariable Analysis After Correction for Multiple Comparisons (Ordered by Magnitude)
| Variable | OR (95% CI) |
|---|---|
| Distinctive characteristics of patients with COVID-19 developing spontaneous pneumothorax (with respect to patients with COVID-19 not developing spontaneous pneumothorax) | |
| Complaining of dyspnea | 5.90 (2.27-15.4) |
| Respiratory rate > 20 bpm | 5.37 (2.62-11.0) |
| Room air pulse oximetry < 95% | 4.28 (2.14-8.58) |
| Leukocytes > 10 cells/μL | 4.21 (2.02-8.75) |
| Complaining of chest pain | 4.01 (2.00-8.01) |
| Distinctive characteristics of patients with COVID-19 developing spontaneous pneumothorax (with respect to non-COVID-19 patients developing spontaneous pneumothorax) | |
| Dysgeusia | 174 (9.69-3,106)a |
| Headache | 72.3 (3.66-1,428)a |
| Temperature at ED arrival > 38°C | 42.4 (4.64-392) |
| Diarrhea | 37.2 (9.57-144) |
| Anosmia | 27.5 (5.15-147) |
| Complaining of fever | 25.2 (10.0-63.2) |
| Lymphocytes < 1 cell/μL | 12.7 (5.9-27.6) |
| Lactate dehydrogenase > 350 IU/L | 9.92 (3.20-30.7) |
| Not complaining of chest pain | 8.47 (4.26-16.9) |
| Cough | 8.00 (4.00-16.0) |
| Respiratory rate at ED arrival > 20 bpm | 7.25 (3.51-15.0) |
| C-reactive protein > 5 mg/dL | 7.06 (3.02-16.5) |
| Room air pulse oximetry at ED arrival < 95% | 6.45 (3.20-13.0) |
| Not being active smoker | 5.46 (1.90-15.6) |
| Age > 60 y | 5.33 (2.71-10.5) |
| Complaining of dyspnea | 4.93 (1.89-12.9) |
| Symptoms lasting ≥ 7 d | 4.81 (2.31-10.0) |
| Hypertension | 4.24 (2.09-8.60) |
| Pneumothorax located at right lung/pleura | 3.85 (1.65-8.98) |
bpm = breaths per minute; COVID-19 = coronavirus disease 2019.
Calculated by Fisher exact test, using the approximation of Woolf.
Table 3.
Sensitivity Analysis for Comparison Between Case Subjects (COVID-19 Patients With Spontaneous Pneumothorax) and Control A Patients (COVID-19 Patients Without Spontaneous Pneumothorax) With Respect to the Significant Clinical Differences and Outcomes Using Only Case Subjects and Control Subjects With Microbiologic Confirmation of SARS-CoV-2 Infection
| Main Analysisa |
Sensitivity Analysisb |
|
|---|---|---|
| OR (95% CI) for Case Subjects With Respect to Control A Patients | OR (95% CI) for Case Subjects With Respect to Control A Patients | |
| Clinical characteristics | ||
| Complaining of dyspnea | 5.90 (2.27-15.4) | 11.4 (2.66-48.6) |
| Respiratory rate > 20 bpm | 5.37 (2.62-11.0) | 8.52 (3.14-23.1) |
| Room air pulse oximetry < 95% | 4.28 (2.14-8.58) | 5.44 (2.34-12.7) |
| Leukocytes > 10 cells/μL | 4.21 (2.02-8.75) | 3.93 (1.71-9.05) |
| Complaining of chest pain | 4.01 (2.00-8.01) | 5.03 (2.24-11.3) |
| Outcomes | ||
| Hospitalization | ||
| Unadjusted | 30.8 (1.88-506) | 16.1 (0.97-266) |
| Adjusted by age and sex | Not calculable | Not calculable |
| Adjusted by age, sex, and center | Not calculable | Not calculable |
| Admission to ICU | ||
| Unadjusted | 27.0 (9.96-73.3) | 31.9 (11.7-90.3) |
| Adjusted by age and sex | 26.2 (9.40-72.9) | 31.8 (10.9-92.8) |
| Adjusted by age, sex, and center | 25.9 (9.30-72.3) | 32.6 (11.1-95.9) |
| Prolonged hospitalization | ||
| Unadjusted | 8.79 (4.10-18.8) | 11.3 (3.70-34.7) |
| Adjusted by age and sex | 9.21 (4.20-20.2) | 11.2 (3.64-34.6) |
| Adjusted by age, sex, and center | 9.27 (4.22-20.4) | 11.2 (3.64-34.6) |
| In-hospital mortality | ||
| Unadjusted | 3.02 (1.47-6.21) | 3.24 (1.47-7.14) |
| Adjusted by age and sex | 4.14 (1.79-9.74) | 5.49 (2.09-14.4) |
| Adjusted by age, sex, and center | 4.07 (1.73-9.59) | 5.47 (2.08-14.4) |
COVID-19 = coronavirus disease 2019; SARS-CoV-2 = severe acute respiratory syndrome coronavirus type 2.
This analysis included 40 case subjects and 400 control group A patients.
This analysis included 30 case subjects and 299 control group A patients.
Outcomes
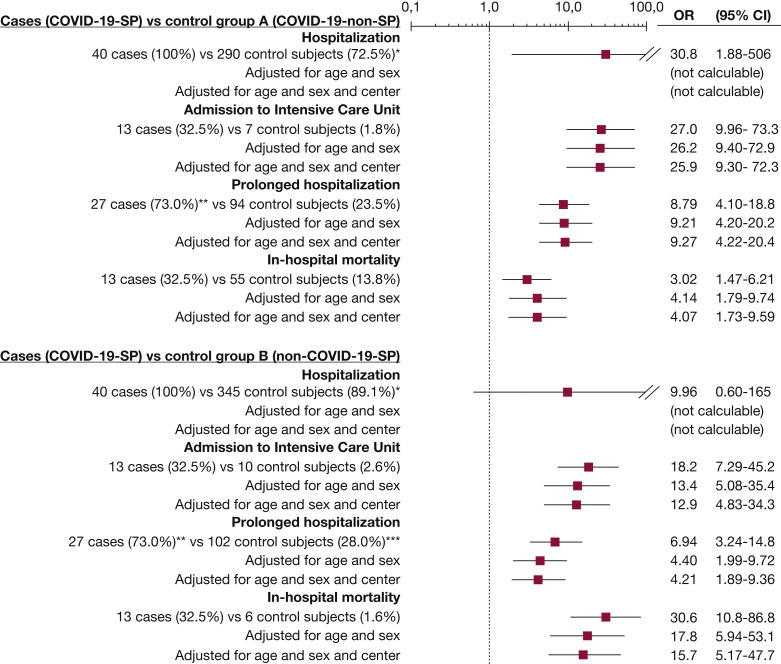
Nearly all the outcomes measured were worse in the case subjects than in the two control groups (Fig 3 ). Specifically, COVID-19 patients with SP had an adjusted OR (for age, sex, and center) for in-hospital mortality of 4.07 (95% CI, 1.73-9.59) compared with COVID-19 patients without SP (control group A), and of 15.7 (95% CI, 5.17-47.7) compared with non-COVID-19 patients with SP (control group B). The estimation of outcomes in COVID-19 patients with SP (case subjects) with respect to COVID-19 patients without SP (control group A) was very similar in the sensitivity analysis using only COVID-19 patients with SARS-CoV-2 infection confirmed by RT-PCR (Table 3).
Figure 3.
Unadjusted and adjusted outcomes of patients with COVID-19 and spontaneous pneumothorax compared with patients with COVID-19 without spontaneous pneumothorax (control group A) and with non-COVID-19 patients with spontaneous pneumothorax (control group B). ∗ Calculated by Fisher exact test, using the approximation of Woolf. ∗∗ The length of hospitalization was not obtained in three of 40 case subjects. ∗∗∗ The length of hospitalization was not obtained for 23 of 387 control group B patients. COVID-19 = coronavirus disease 2019; COVID-19-non-SP = COVID-19 patients without spontaneous pneumothorax; COVID-19-SP = COVID-19 patients with spontaneous pneumothorax; non-COVID-19-SP = non-COVID-19 patients with spontaneous pneumothorax.
Discussion
We found that 0.56‰ of patients with COVID-19 coming to the ED had SP, and we estimated an annual standardized incidence of 34.2 SP per 100,000 patients with COVID-19. Both the relative frequency among patients coming to the ED and the standardized incidence found during the 2-month period of the COVID-19 outbreak in Spain were higher than those observed in the non-COVID-19 population. We consider our results to be quite reliable, as some of our figures found in the general population (not infected with SARS-CoV-2) match previously reported data. On the one hand, the relative frequency of SP among patients coming to the ED reported in a previous Italian study was 0.42‰,19 which is similar to that found in the non-COVID-19 general population in the Spanish EDs participating in the present study (0.28‰). On the other hand, the standardized incidence per 100,000 population each year of primary SP has been reported to be between 7.4 and 18 cases in men, and between 1.2 and 6 cases in women,20 , 21 values that are also close to the standardized incidence of 8.2 found in the present study. It should be noted that our study did not include cases of SP appearing after the initiation of invasive or noninvasive mechanical ventilation, a circumstance that increases the risk of pneumothorax in patients in general and in patients with lung infections in particular.22 , 23 Therefore, the increased ORs of 1.93 for the relative frequency of SP in the ED and 4.19 for the standardized incidence in the general population found for patients with COVID-19 in the UMC-19-S7 represent the first evidence that SP is probably a consequence related to SARS-CoV-2 infection and, in fact, that COVID-19 should be added to the list of etiologies of secondary SP. The main facilitating entity of secondary SP is COPD, and especially lung emphysema, which are present in more than 50% of cases.24, 25, 26 Of note, with respect to the control groups, this entity was not overrepresented in our case subjects, reinforcing the role of SARS-CoV-2 infection itself in SP development. In fact, SP can complicate the course of necrotizing pneumonia due to P. jirovecii, lung TB, and, less often, fungi or other microorganisms.5 , 25 , 27
The presumed common mechanism underlying SP in patients with lung infection is direct invasion and necrosis of lung tissue including the pleura by the microorganism itself. However, other factors could facilitate SP development. In patients with HIV-related P. jirovecii, in whom the frequency of SP is about 10‰ (more than 10-fold higher than what we reported for patients with COVID-19), it has been hypothesized that the administration of aerosolized pentamidine may increase the likelihood of P. jirovecii pneumonia to grow and cause cavitation in the peripheral parts of the upper lobe, thereby increasing the risk of pneumothorax.5 , 27 In addition, an inflammatory reaction could also contribute to SP during lung infections. Some studies have reported that SARS might independently result in cyst formation, even in the absence of mechanical ventilation, and inflammatory exudate could play a relevant role in this.28 , 29 The pathologic features in lungs of patients with COVID-19-related pneumonia greatly resemble those seen in SARS and Middle East respiratory syndrome coronavirus infection.30 , 31 Histologic examinations reported diffuse bilateral alveolar damage with cellular fibromyxoid exudates in COVID-19, and pulmonary cystic lesions. Pulmonary cystic lesions may develop in response to cellular fibromyxoid exudates, which form a valve in the bronchus.32 In fact, the cytokine storm syndrome, a critical clinical condition induced by a cascade of cytokine activation, characterized by overwhelming systemic inflammation, hyperferritinemia, hemodynamic instability, and multiple organ failure, is now recognized as being a main cause of the severity of SARS-CoV-2 infection.33 It could, therefore, be hypothesized that patients with a higher inflammatory response in SARS-CoV-2 infection could be at higher risk of developing lung damage favoring SP. Our study measured a reduced number of inflammatory biomarkers (as this was not its main objective) and, although we found significantly higher leukocyte blood counts in patients with COVID-19 who developed SP, C-reactive protein, procalcitonin, and D-dimers did not significantly differ. Therefore, further studies specifically designed to test this hypothesis are needed.
Patients with COVID-19 developing SP more frequently presented with dyspnea, tachypnea, and hypoxia, but all these features are very common in patients with severe COVID-19. Interestingly, chest pain was present markedly more often in connection with SP development. On the other hand, the distinctive clinical characteristics of SP in patients with COVID-19 with respect to SP in the general non-COVID-19 population are older age, and a higher frequency of diabetes mellitus and hypertension (probably related to the more advanced age of the COVID-19 population). Regarding the clinical picture, symptoms derived from SARS-CoV-2 infection itself were by and large the most distinctive features (including dysgeusia, headache, fever, diarrhea, and anosmia). On the other hand, symptoms lasted longer when patients went to the ED (a median of 5 days for COVID-19 patients vs 1 day for non-COVID-19 patients, again in connection with COVID-19 rather than with SP), and although dyspnea, tachycardia, tachypnea, and hypoxemia were more frequent in patients with COVID-19, chest pain was a less frequent complaint in this population.
All the outcomes assessed in the UMC-19-S7 were worse for COVID-19 patients with SP compared with the two control groups (with the exception of the need for hospitalization with respect to control group B). COVID-19 itself was responsible for the poorer prognosis of SP in patients with COVID-19 compared with the general population, especially in terms of the huge increase observed in in-hospital mortality (OR, 15). In addition, it should also be taken into account that the former group was older than the latter (median age, 66 vs 36 years), and age is always a main driver of mortality. Conversely, it is difficult to attribute SP to the increment of in-hospital mortality observed in COVID-19 patients with SP compared with COVID-19 patients without SP. Patients with COVID-19 developing SP may have been the most severely ill patients, in terms of viral infection and, especially, in terms of inflammatory response. The relatively small sample size of our study precludes adjustment for variables linked to the severity of COVID-19. Therefore, despite finding statistically significant differences, we could not rule out that this apparent increase in risk for patients with COVID-19 developing SP was due to the clinical characteristics of COVID-19 itself rather than to SP, and caution should be taken when interpreting differences in outcomes among COVID-19 patients with and without SP.
Limitations
This study has several limitations. First, SP was detected only if chest radiography or CT imaging was performed in the ED. However, during the COVID-19 pandemic, emergency physicians had a very low threshold for ordering chest imaging, and radiography or CT imaging was performed in the vast majority of patients with COVID-19, especially if respiratory symptoms were manifested. Second, inadvertent SP might have occurred, although in many centers a senior radiologist reviewed the imaging studies. Third, some mildly symptomatic patients with SP, especially among non-COVID-19 patients, could have remained at home during the COVID-19 outbreak because of fear of COVID-19 contagion. The standardized incidence of SP in non-COVID-19 patients during the pre-COVID-19 period was almost double that found during the COVID-19 period, suggesting that this probably occurred. Nonetheless, even when the relative frequency of SP in the ED and the standardized incidence in the general population for SP in patients with COVID-19 were specifically compared with those found in non-COVID-19 patients during the pre-COVID-19 period, the differences still remained statistically significant. Fourth, we did not adjust the incidence of SP in COVID-19 for all relevant patient-related or disease-related factors influencing the relative frequency of SP presentation and outcomes, and this could somewhat alter the estimations presented in the current study. Fifth, in about one-quarter of the cases the COVID-19 diagnosis was based on clinical and/or radiologic findings with no microbiologic confirmation, due to a shortage of tests.34 , 35 Although these figures matched those in most countries during the outbreak, there could be a bias of misclassification in the COVID-19 group for some of these cases. Nonetheless, as the sensitivity analysis that compared only patients with COVID-19 (with and without SP) with microbiologic confirmation of SARS-CoV-2 infection provided very similar results as the main analysis, we believe that this bias was not large in magnitude. In addition, some COVID-19 patients with SP who were asymptomatic with respect to SARS-CoV-2 infection could have been misclassified into the non-COVID-19 group. Sixth, although 61 EDs participated in the present study, the number of cases (COVID-19 patients with SP) was very low. This is a major limiting factor to this study (although not preventable) and means that all the factors associated with SP in patients with COVID-19 were very imprecisely estimated, as the CIs were very wide. Seventh, as a retrospective study, although the case record form was standardized, there was no monitoring of data collection methods, and diagnosis and outcome adjudication were done locally. Finally, although the UMC-19-S7 involved 61 EDs, it was carried out in a single country, and external validation in other countries is needed before generalizing our findings.
Conclusions
Despite these limitations, we conclude that the incidence of SP in patients with COVID-19 attending the ED is low but increased with respect to non-COVID-19 patients. Therefore, SARS-CoV-2 infection may have a direct role in this increased incidence. Patients with COVID-19 complaining of dyspnea and chest pain, and exhibiting tachycardia, tachypnea, and hypoxemia, should be assessed to rule out SP.
Acknowledgments
Author contributions: O. M. is the guarantor of the article, taking responsibility for the integrity of the work as a whole, from inception to publication of the article. All the authors discussed the idea and design of this study and provided patients. Data analysis and first draft writing were done by O. M. All the authors have read this draft and provided insight for the final version.
Financial/nonfinancial disclosures: None declared.
∗Spanish Investigators on Emergency Situations Team (SIESTA) Network Collaborators:Steering Committee: Òscar Miró, Sònia Jiménez (Hospital Clínic, Barcelona), Juan González del Castillo, Francisco Javier Martín-Sánchez (Hospital Clínico San Carlos, Madrid), Pere Llorens (Hospital General de Alicante), Guillermo Burillo-Putze (Hospital Universitario de Canarias, Tenerife), Alfonso Martín (Hospital Universitario Severo Ochoa de Leganés, Madrid), Pascual Piñera Salmerón (Hospital General Universitario Reina Sofía, Murcia), E. Jorge García Lamberechts (Hospital Clínico San Carlos, Madrid), Javier Jacob (Hospital Universitario de Bellvitge, L’Hospitalet de Llobregat, Barcelona), Aitor Alquézar-Arbé (Hospital de la Santa Creu i Sant Pau, Barcelona). Participating centers: (1) Hospital Universitario Doctor Peset Aleixandre de Valencia: María Luisa López Grima, María Ángeles Juan Gómez; (2) Hospital Universitario y Politécnico La Fe de Valencia: Javier Millán, Leticia Serrano Lázaro; (3) Hospital Universitario General de Alicante: Bárbara Peña, Francisco Román; (4) Hospital Clínico Universitario de Valencia: José Noceda; (5) Hospital Arnau de Vilanova de Valencia: María José Cano Cano, Rosa Sorando Serra; (6) Hospital Francesc de Borja de Gandía, Valencia: María José Fortuny Bayarri, Francisco José Salvador Suárez; (7) Hospital General Universitario de Elche, Alicante: Matilde González Tejera; (8) Hospital Marina Baixa de Villajoyosa de Alicante: Liced Aguilar Herrera, Ana María Caballero Mateos; (9) Hospital Virgen de los Lirios, Alcoy Alicante: Napoleón Meléndez, Patricia Borrás Albero; (10) Hospital Universitario Vinalopó de Elche (Alicante): Blas Jiménez; (11) Hospital Universitario de Torrevieja de Alicante: Rigoberto del Río, Blas Jiménez; (12) Hospital Lluís Alcanyís de Xàtiva: Carles Pérez García, Pilar Sánchez Amador; (13) Hospital Universitario de La Ribera de Valencia: José Vicente Brasó Aznar, José Luis Ruiz López; (14) Hospital de la Vega Baja Orihuela de Alicante: María Carmen Ponce, Elena Díaz Fernández; (15) Hospital Universitario Sant Joan Alicante: Elena Díaz Fernández; (16) Hospital General de Requena de Valencia: Laura Ejarque Martínez, Marisa de Reynoso Rodríguez; (17) Hospital de Llíria de Valencia: Ana Peiró Gómez, Elena Gonzalo Bellver; (18) Hospital de la Santa Creu i Sant Pau (Barcelona): Miguel Rizzi, Carla Cabrera Suarez, Laura Balcells Argilag; (19) Hospital Clinic (Barcelona): Carlos Cardozo; (20) Hospital Universitari de Bellvitge de Hospitalet de Llobregat (Barcelona): Alejandro Roset-Rigat, Antonio Haro-Bosch; (21) Hospital Universitari Germans Trias i Pujol de Badalona (Barcelona): Marta Alujas Rovira, Pepe Ferrer Arbaizar; (22) Hospital de Terrassa (Barcelona): Josep Tost; (23) Hospital del Mar (Barcelona): Isabel Cirera Lorenzo, Silvia Mínguez Masó; (24) Hospital Universitari Joan XXIII (Tarragona): Anna Palau, Ruth Gaya Tur; (25) Hospital Universitari de Girona Dr. Josep Trueta (Girona): Maria Adroher Muñoz, Ester Soy Ferrer; (26) Hospital Universitari de Vic (Barcelona): Lluís Llauger García; (27) Hospital de Sant Pau i Santa Tecla (Tarragona): Brigitte Silvana Alarcón Jiménez, Silvia Flores Quesada; (28) Clinica Sagrada Familia (Barcelona): Arturo Huerta; (29) Hospital Clínico San Carlos (Madrid): Marcos Fragiel; (30) Hospital Universitario La Paz (Madrid): Susana Martínez Álvarez, Ana María Martínez Virto; (31) Hospital Universitario de la Princesa (Madrid): Carmen del Arco Galán, Guillermo Fernández Jiménez; (32) Hospital Universitario Severo Ochoa de Leganés (Madrid): Esther Álvarez Rodríguez, Teresa Agudo Villa; (33) Hospital Universitario Rey Juan Carlos (Madrid): María José Venegas de L’Hotellerie, Verónica Prieto Cabezas; (34) Hospital Universitario del Henares (Madrid): Catalina Mocanu, Patricia Gantes Nieto; (35) Hospital Universitario de Fuenlabrada (Madrid): Marta Álvarez Alonso, Cristina Latorre Marco; (36) Hospital Universitario Infanta Cristina de Parla (Madrid): Alicia Fuente Gaforio, Beatriz Honrado Galán; (37) Hospital Comarcal El Escorial (Madrid): Sara Gayoso Martín, Frida Vallejo Somohano; (38) Clínica Universidad Navarra de Madrid: Nieves López Laguna, Raquel Piñero Panadero; (39) Hospital Universitario de Salamanca: Marta Fuentes de Frutos, Cristina Gil Castillo; (40) Complejo Asistencial Universitario de León: Ana Barrientos Castañeda, Susana Garcia Escudero; (41) Hospital Universitario de Burgos: María Pilar López Díez; (42) Hospital Universitario Rio Hortega (Valladolid): Jose Ramón Oliva Ramos, Daniel Serrano Herrero, Rosa Castellanos Flórez; (43) Complejo Asistencial de Soria: Fadh Beddar Chaib, Ikram Samira Mohamedi Abdelkader; (44) Hospital Universitario Regional de Málaga: Ana Pérez Tornero, Alberto Núñez Chia; (45) Hospital Universitario Juan Ramón Jiménez: Esther Maldonado Pérez, Verónica Rodríguez Martín; (46) Hospital Costa del Sol de Marbella: Ana Belen García Soto, Elisa Delgado Padial; (47) Hospital Valle de los Pedroches de Pozoblanco (Córdoba): Jorge Pedraza García; (48) Hospital Virgen del Rocío de Sevilla: Amparo Fernández de Simón Almela; (49) Complejo Hospitalario Universitario de A Coruña: Ricardo Calvo López; (50) Hospital Universitario Lucus Augusti Lugo: Juan José López Díaz; (51) Complejo Hospitalario Universitario de Vigo, Hospital Álvaro Cunqueiro: María Teresa Maza Vera, Raquel Rodríguez Calveiro; (52) Hospital Universitario General de Albacete: Francisco Javier Lucas-Galán, María Ruipérez Moreno; (53) Hospital Virgen de la Luz (Cuenca): Félix González Martínez, Diana Moya Olmeda; (54) Hospital Nuestra Señora del Prado de Talavera de la Reina (Toledo): Ricardo Juárez; (55) Hospital Universitario de Canarias (Tenerife): Marcos Expósito Rodríguez, José Francisco Fernández Rodríguez; (56) Hospital Universitario de Gran Canaria Dr. Negrín: José Pavón Monzo, Nayra Cabrera González; (57) Hospital Universitario Central Asturias: Desiré María Velarde Herrera, Beatriz María Martínez Bautista; (58) Hospital Universitario de Cabueñes (Gijón): Ana Patricia Niembro Valdés, Lorena Arboleya Álvarez; (59) Hospital Clínico Universitario Virgen de la Arrixaca: Eva Quero Motto, Nuria Tomas García; (60) Hospital General Universitario Reina Sofía de Murcia: José Andrés Sánchez Nicolás, Paula Lázaro Aragües; (61) Hospital San Pedro de Logroño: Noemí Ruiz de Lobera; (62) Hospital Clínico Universitario Lozano Blesa: José María Ferreras Amez.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Contributor Information
Spanish Investigators on Emergency Situations Team (SIESTA) Network:
Òscar Miró, Sònia Jiménez, Juan González del Castillo, Francisco Javier Martín-Sánchez, Pere Llorens, Guillermo Burillo-Putze, Alfonso Martín, Pascual Piñera Salmerón, E. Jorge García Lamberechts, Javier Jacob, Aitor Alquézar-Arbé, María Luisa López Grima, María Ángeles Juan Gómez, Javier Millán, Leticia Serrano Lázaro, Bárbara Peña, Francisco Román, José Noceda, María José Cano Cano, Rosa Sorando Serra, María José Fortuny Bayarri, Francisco José Salvador Suárez, Matilde González Tejera, Liced Aguilar Herrera, Ana María Caballero Mateos, Napoleón Meléndez, Patricia Borrás Albero, Blas Jiménez, Rigoberto del Río, Blas Jiménez, Carles Pérez García, Pilar Sánchez Amador, José Vicente Brasó Aznar, José Luis Ruiz López, María Carmen Ponce, Elena Díaz Fernández, Elena Díaz Fernández, Laura Ejarque Martínez, Marisa de Reynoso Rodríguez, Ana Peiró Gómez, Elena Gonzalo Bellver, Miguel Rizzi, Carla Cabrera Suarez, Laura Balcells Argilag, Carlos Cardozo, Alejandro Roset-Rigat, Antonio Haro-Bosch, Marta Alujas Rovira, Pepe Ferrer Arbaizar, Josep Tost, Isabel Cirera Lorenzo, Silvia Mínguez Masó, Anna Palau, Ruth Gaya Tur, Maria Adroher Muñoz, Ester Soy Ferrer, Lluís Llauger García, Brigitte Silvana Alarcón Jiménez, Silvia Flores Quesada, Arturo Huerta, Marcos Fragiel, Susana Martínez Álvarez, Ana María Martínez Virto, Carmen del Arco Galán, Guillermo Fernández Jiménez, Esther Álvarez Rodríguez, Teresa Agudo Villa, María José Venegas de L’Hotellerie, Verónica Prieto Cabezas, Catalina Mocanu, Patricia Gantes Nieto, Marta Álvarez Alonso, Cristina Latorre Marco, Alicia Fuente Gaforio, Beatriz Honrado Galán, Sara Gayoso Martín, Frida Vallejo Somohano, Nieves López Laguna, Raquel Piñero Panadero, Marta Fuentes de Frutos, Cristina Gil Castillo, Ana Barrientos Castañeda, Susana Garcia Escudero, María Pilar López Díez, Jose Ramón Oliva Ramos, Daniel Serrano Herrero, Rosa Castellanos Flórez, Fadh Beddar Chaib, Ikram Samira Mohamedi Abdelkader, Ana Pérez Tornero, Alberto Núñez Chia, Esther Maldonado Pérez, Verónica Rodríguez Martín, Ana Belen García Soto, Elisa Delgado Padial, Jorge Pedraza García, Amparo Fernández de Simón Almela, Ricardo Calvo López, Juan José López Díaz, María Teresa Maza Vera, Raquel Rodríguez Calveiro, Francisco Javier Lucas-Galán, María Ruipérez Moreno, Félix González Martínez, Diana Moya Olmeda, Ricardo Juárez, Marcos Expósito Rodríguez, José Francisco Fernández Rodríguez, José Pavón Monzo, Nayra Cabrera González, Desiré María Velarde Herrera, Beatriz María Martínez Bautista, Ana Patricia Niembro Valdés, Lorena Arboleya Álvarez, Eva Quero Motto, Nuria Tomas García, José Andrés Sánchez Nicolás, Paula Lázaro Aragües, Noemí Ruiz de Lobera, and José María Ferreras Amez
References
- 1.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 323(20):2052-2059. [DOI] [PMC free article] [PubMed]
- 4.Lodigiani C., Iapichino G., Carenzo L., et al. Humanitas COVID-19 Task Force Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivero A., Perez-Camacho I., Lozano F., et al. Etiology of spontaneous pneumothorax in 105 HIV-infected patients without highly active antiretroviral therapy. Eur J Radiol. 2009;71(2):264–268. doi: 10.1016/j.ejrad.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Liu K., Zeng Y., Xie P., et al. COVID-19 with cystic features on computed tomography: a case report. Medicine (Baltimore) 2020;99(18) doi: 10.1097/MD.0000000000020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W., Gao R., Zheng Y., Jiang L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med. 2020;27(5) doi: 10.1093/jtm/taaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J., Su X., Zhang T., Zheng C. Spontaneous pneumomediastinum: a probable unusual complication of coronavirus disease 2019 (COVID-19) pneumonia. Korean J Radiol. 2020;21(5):627–628. doi: 10.3348/kjr.2020.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiolfi A., Biraghi T., Montisci A., et al. Management of persistent pneumothorax with thoracoscopy and bleb resection in COVID-19 patients. Ann Thorac Surg. 2020;110(5):e413–e415. doi: 10.1016/j.athoracsur.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun R., Liu H., Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. 2020;21(5):541–544. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López Zúñiga M.A., López Zúñiga D., Martínez Colmenero J., Rodríguez Sánchez A., Gutiérrez Lara G., López Ruz M.A. Spontaneous mediastinal emphysema in patients with COVID-19. Emergencias. 2020;32(4):298–299. [PubMed] [Google Scholar]
- 12.Janssen M.L., van Manen M.J.G., Cretier S.E., Braunstahl G.J. Pneumothorax in patients with prior or current COVID-19 pneumonia. Respir Med Case Rep. 2020;31:101187. doi: 10.1016/j.rmcr.2020.101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpi S., Ali J.M., Suleman A., Ahmed R.N. Pneumomediastinum in COVID-19 patients: a case series of a rare complication. Eur J Cardiothorac Surg. 2020;58(3):646–647. doi: 10.1093/ejcts/ezaa222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamous F., Meyer N., Buus D., et al. Critical illness due to Covid-19: a description of the surge in a single center in Sioux Falls. S D Med. 2020;73(7):312–317. [PubMed] [Google Scholar]
- 15.Yao W., Wang T., Jiang B., et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020;125(1):e28–e37. doi: 10.1016/j.bja.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Analysis of 92 deceased patients with COVID-19. J Med Virol. 2020;92(11):2511-2515. [DOI] [PMC free article] [PubMed]
- 17.Miró O., González del Castillo J. Collaboration among Spanish emergency departments to promote research: on the creation of the SIESTA (Spanish Investigators in Emergency Situations Team) Network and the coordination of the UMC-19 (Unusual Manifestations of COVID-19) microproject. Emergencias. 2020;32(4):269–277. [PubMed] [Google Scholar]
- 18.Pollán M., Pérez-Gómez B., Pastor-Barriuso R., et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comelli I., Bologna A., Ticinesi A., et al. Incidence of primary spontaneous pneumothorax is not associated with microclimatic variations: results of a seven-year survey in a temperate climate area. Monaldi Arch Chest Dis. 2017;87(1):793. doi: 10.4081/monaldi.2017.793. [DOI] [PubMed] [Google Scholar]
- 20.Baumann M.H., Noppen M. Pneumothorax. Respirology. 2004;9(2):157–164. doi: 10.1111/j.1440-1843.2004.00577.x. [DOI] [PubMed] [Google Scholar]
- 21.Bense L., Eklund G., Wilman L.G. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest. 1987;92(6):1009–1012. doi: 10.1378/chest.92.6.1009. [DOI] [PubMed] [Google Scholar]
- 22.Roberts D.J., Leigh-Smith S., Faris P.D., et al. Clinical presentation of patients with tension pneumothorax: a systematic review. Ann Surg. 2015;261(6):1068–1078. doi: 10.1097/SLA.0000000000001073. [DOI] [PubMed] [Google Scholar]
- 23.Kao H.K., Wang J.H., Sung C.S., Huang Y.C., Lien T.C. Pneumothorax and mortality in the mechanically ventilated SARS patients: a prospective clinical study. Crit Care. 2005;9(4):R440–R445. doi: 10.1186/cc3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C.H., Liao W.C., Liu Y.H., et al. Secondary spontaneous pneumothorax: which associated conditions benefit from pigtail catheter treatment? Am J Emerg Med. 2012;30:45–50. doi: 10.1016/j.ajem.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Onuki T., Ueda S., Yamaoka M., et al. Primary and secondary spontaneous pneumothorax: prevalence, clinical features, and in-hospital mortality. Can Respir J. 2017;2017:6014967. doi: 10.1155/2017/6014967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi F., Takihara T., Nakamura N., et al. Etiology and prognosis of spontaneous pneumothorax in the elderly. Geriatr Gerontol Int. 2020;20(10):878–884. doi: 10.1111/ggi.13996. [DOI] [PubMed] [Google Scholar]
- 27.Afessa B. Pleural effusion and pneumothorax in hospitalized patients with HIV infection: the Pulmonary Complications, ICU support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest. 2000;117(4):1031–1037. doi: 10.1378/chest.117.4.1031. [DOI] [PubMed] [Google Scholar]
- 28.Joynt G.M., Antonio G.E., Lam P., et al. Late-stage adult respiratory distress syndrome caused by severe acute respiratory syndrome: abnormal findings at thin-section CT. Radiology. 2004;230(2):339–346. doi: 10.1148/radiol.2303030894. [DOI] [PubMed] [Google Scholar]
- 29.Desai S.R. Acute respiratory distress syndrome: imaging of the injured lung. Clin Radiol. 2002;57(1):8–17. doi: 10.1053/crad.2001.0889. [DOI] [PubMed] [Google Scholar]
- 30.Ding Y., Wang H., Shen H., et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng D.L., Al Hosani F., Keating M.K., et al. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol. 2016;186(3):652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao YM, Xu G, Wang B, Liu BC. Cytokine storm syndrome in coronavirus disease 2019: a narrative review. [published online ahead of print July 22, 2020]. J Intern Med. 10.1111/joim.13144. [DOI] [PMC free article] [PubMed]
- 34.Gil-Rodrigo A., Miró O., Piñera P., et al. Analysis of clinical characteristics and outcomes in patients with COVID-19 based on a series of 1000 patients treated in Spanish emergency departments. Emergencias. 2020;32(4):233–241. [PubMed] [Google Scholar]
- 35.Martín-Sánchez F.J., González del Castillo J., Valls Cargó A., et al. Diagnostic groups and short-term outcomes in suspected COVID-19 cases treated in an emergency department. Emergencias. 2020;32(4):242–252. [PubMed] [Google Scholar]