Supplemental Digital Content is available in the text.
Keywords: aspirin, atherosclerosis, death, population, stroke, ticagrelor, transient ischemic attack
Background and Purpose:
Among patients with a transient ischemic attack or minor ischemic strokes, those with ipsilateral atherosclerotic stenosis of cervicocranial vasculature have the highest risk of recurrent vascular events.
Methods:
In the double-blind THALES (The Acute Stroke or Transient Ischemic Attack Treated With Ticagrelor and ASA for Prevention of Stroke and Death) trial, we randomized patients with a noncardioembolic, nonsevere ischemic stroke, or high-risk transient ischemic attack to ticagrelor (180 mg loading dose on day 1 followed by 90 mg twice daily for days 2–30) or placebo added to aspirin (300–325 mg on day 1 followed by 75–100 mg daily for days 2–30) within 24 hours of symptom onset. The present paper reports a prespecified analysis in patients with and without ipsilateral, potentially causal atherosclerotic stenosis ≥30% of cervicocranial vasculature. The primary end point was time to the occurrence of stroke or death within 30 days.
Results:
Of 11 016 randomized patients, 2351 (21.3%) patients had an ipsilateral atherosclerotic stenosis. After 30 days, a primary end point occurred in 92/1136 (8.1%) patients with ipsilateral stenosis randomized to ticagrelor and in 132/1215 (10.9%) randomized to placebo (hazard ratio 0.73 [95% CI, 0.56–0.96], P=0.023) resulting in a number needed to treat of 34 (95% CI, 19–171). In patients without ipsilateral stenosis, the corresponding event rate was 211/4387 (4.8%) and 230/4278 (5.4%), respectively (hazard ratio, 0.89 [95% CI, 0.74–1.08]; P=0.23, Pinteraction=0.245). Severe bleeding occurred in 4 (0.4%) and 3 (0.2%) patients with ipsilateral atherosclerotic stenosis on ticagrelor and on placebo, respectively (P=NS), and in 24 (0.5%) and 4 (0.1%), respectively, in 8665 patients without ipsilateral stenosis (hazard ratio=5.87 [95% CI, 2.04–16.9], P=0.001).
Conclusions:
In this exploratory analysis comparing ticagrelor added to aspirin to aspirin alone, we found no treatment by ipsilateral atherosclerosis stenosis subgroup interaction but did identify a higher absolute risk and a greater absolute risk reduction of stroke or death at 30 days in patients with ipsilateral atherosclerosis stenosis than in those without. In this easily identified population, ticagrelor added to aspirin provided a clinically meaningful benefit with a number needed to treat of 34 (95% CI, 19–171).
Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT03354429.
Among patients with ischemic stroke, 40% present with ipsilateral stenosis of the cervicocranial vasculature and have the highest risk of recurrence among ischemic stroke etiologic subtypes.1,2 In the SOCRATES trial (Acute Stroke or Transient Ischemic Attack Treated With Aspirin or Ticagrelor and Patient Outcomes), patients with transient ischemic attack (TIA) or minor ischemic strokes, ticagrelor resulted in a 32% relative risk reduction in recurrent stroke and cardiovascular events compared with aspirin among the subgroup of patients with ipsilateral atherosclerotic stenosis of cervicocranial vasculature.3 Because the main trial did not meet its primary hypothesis of a superiority of ticagrelor over aspirin,3 the result in the atherosclerotic subgroup was considered hypothesis generating.
In the THALES trial (Acute Stroke or Transient Ischemic Attack Treated With Ticagrelor and ASA for Prevention of Stroke and Death), we randomized patients with a noncardioembolic, nonsevere ischemic stroke, or high-risk TIA to ticagrelor (180 mg loading dose on day 1 followed by 90 mg twice daily for days 2–30) or placebo within 24 hours of symptom onset. All patients received aspirin (300–325 mg on day 1 followed by 100 mg daily for days 2–30). In THALES, ticagrelor added to aspirin reduced the 30-day risk of stroke or death by 17% relative to placebo and aspirin.4 In a prespecified analysis of the THALES trial, we aimed to evaluate the efficacy and safety of ticagrelor added to aspirin in the first 30 days following a TIA or minor ischemic stroke in patients with or without ipsilateral, potentially causal, ≥30% atherosclerotic stenosis of cervicocranial vasculature.
Methods
Trial Design and Oversight
THALES was a randomized, double-blind, placebo-controlled, multicenter, international, parallel-group trial conducted at 414 sites in 28 countries.4 The Executive Committee designed and oversaw the conduct and analysis of the trial in collaboration with the sponsor, AstraZeneca. Details of the study rationale, design, and methods have been described previously.5 After the trial start, given the results of the POINT trial (Platelet Oriented Inhibition in New TIA and Minor Ischemic Stroke) and CHANCE trial (Clopidogrel in High-Risk Patients With Acute Nondisabling Cerebrovascular Events),6,7 the study assumptions were adjusted to a lower hazard ratio requiring less primary end points and smaller sample size.4
The trial was approved by the relevant ethics committee for each participating site. Descriptions of the trial leadership, committees, and investigators are provided in the Data Supplement, available online with the full text of this article.
An independent Data Monitoring Committee regularly oversaw the safety of the patients and the integrity and conduct of the study based on patient accrual throughout the trial.
The trial analyses were done by the sponsor under the direction of the Executive Committee. The first author, who had full access to the data, wrote the first draft of the article. The article was reviewed, edited, and approved by all authors, who decided to publish the data. The authors vouch for the accuracy and completeness of the data and the adherence to the study protocol and statistical analysis plan, both of which are available online with the full text of this article. Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Patients
Eligible patients enrolled in THALES were ≥40 years of age had a noncardioembolic acute ischemic stroke with a National Institutes of Health Stroke Scale score (range 0–42, higher scores indicate more severe stroke) of ≤5 or high-risk TIA (age, blood pressure, clinical symptoms, diabetes, duration stroke risk score [scores assessing the risk of stroke on the basis of age, blood pressure, clinical features, duration of TIA, and presence or absence of diabetes; range 0 (lowest risk)–7 (highest risk)]) of ≥6 or symptomatic intracranial or extracranial stenosis (≥50% narrowing in the diameter of the lumen of an artery that could account for the TIA). Randomization was required to occur within 24 hours after onset of symptoms. Before randomization patients had undergone a computer tomography or magnetic resonance imaging scan of the brain. In the present subgroup analysis, we included patients with symptomatic intracranial or extracranial arterial stenosis, that is, ≥30% narrowing in the diameter of the lumen of an artery that could account for the clinical presentation (irrespective of >4 mm thick aortic arch plaque). Thirty percent narrowing was chosen as cutoff based on criteria of the atherosclerosis, small vessel disease, cardiac pathology, other disease, dissection (ASCOD) grading system.3,8
Patients were not eligible if there was history of atrial fibrillation, ventricular aneurysm, or suspicion of cardioembolic cause for TIA or stroke; planned carotid endarterectomy that required halting study medication within 3 days of randomization; known bleeding diathesis or coagulation disorder; history of previous symptomatic nontraumatic intracerebral hemorrhage, gastrointestinal bleed within the past 6 months, or major surgery within 30 days. Additional information on inclusion and exclusion criteria is found in the Data Supplement, available with the full text of this article.
Trial Procedures
Written informed consent was provided prior to any study-specific procedures. Following enrollment/randomization, visits were scheduled at 7 (±2) days, 30 (+4) days, and 60 (+4) days. The visits at 7 and 60 days could be telephone visits.
Enrolled, eligible patients were randomly assigned to receive either ticagrelor or matching placebo, in accordance with the sequestered, fixed-randomization schedule, with the use of balanced blocks to ensure an approximate 1:1 ratio of the 2 regimens.
A loading dose of ticagrelor 180 mg (two 90 mg tablets) or matching placebo was to be given as soon as possible after randomization. Subsequent maintenance doses of ticagrelor 90 mg or matching placebo were taken in the morning and evening, at ≈12-hour intervals, for the remainder of the 30-day treatment period.
In addition, and as part of clinical practice, patients received a loading dose with aspirin (recommended 300–325 mg aspirin, taking any dose of aspirin given after symptom onset but before randomization in account) and, thereafter, were treated with a recommended aspirin dose of 75 to 100 mg once daily.
After the 30 days of study treatment, patients were treated according to standard of care at the discretion of the investigator and followed for an additional 30 days with continued collection of end points and safety events.
Atherosclerotic Subgroup
The case report form contained a questionnaire about severity and location of atherosclerosis of the cervicocranial vasculature derived from the ASCOD atherosclerosis phenotype.8 Vascular imaging data were systematically collected in the case report form from computed tomography angiography, magnetic resonance angiography, or ultrasound of both extracranial and intracranial arteries conducted as part of clinical practice for detection of atherosclerotic stenosis and investigated the presence of aortic arch atheroma ≥4 mm in thickness. The arteries supplying and those not supplying the ischemic field were categorized as occlusion with evidence of atherosclerosis, a stenosis with narrowing of the lumen of 70% to 99%, 50% to 69%, 30% to 49%, <30% or plaque, no atherosclerosis and occlusion with no evidence of atherosclerosis, with or without aortic arch atheroma ≥4 mm in thickness. The presence of medical history of peripheral artery disease, coronary artery disease, coronary artery bypass grafting, myocardial infarction, and percutaneous coronary intervention was also recorded.
Outcomes
Outcome events were not adjudicated centrally given a lack of evidence that this improves data quality.9 All efficacy and safety analyses were based on investigator-assessed events. Stroke events, which included both progression of index stroke or new stroke events, were recorded as adverse events and classified by investigators as ischemic, hemorrhagic, or of undetermined cause. Bleeding events were classified by the investigator according to the GUSTO trial (Global Utilization of Streptokinase and Tissue-Type Plasminogen Activator for Occluded Coronary Arteries) bleeding definition as severe, moderate, or mild.10 The definitions of the prespecified end points and GUSTO bleeding classification for this study have been previously described5,11 and are also included in the study protocol available in the Data Supplement. The primary efficacy end point was the time from randomization to the first subsequent event of stroke or death. Secondary end point was time from randomization to first subsequent ischemic stroke. For this analysis, we also evaluated disabling stroke as an exploratory end point, defined as an incident stroke with a modified Rankin Scale score >1 at end of treatment visit 30 to 34 days after randomization. The modified Rankin Scale measures disability as a score of 0 to 6: 0 to 1 no disability, 2 to 5 increasing disability, and 6 death.
Statistical Analyses
Trial assumptions have been reported.4,5 All efficacy and safety analyses were based on the intention-to-treat principle using the full analysis set (including all randomized patients). The time from randomization to the first occurrence of any event for a given end point was compared using the Cox proportional hazards model with a factor for treatment group, using the Efron method for ties. P values and 95% CI for the hazard ratio (HR) were based on the Wald statistic. Since all analyses presented were exploratory, no adjustment for multiple comparisons was made, and P values were nominal. If the total number of events is <15, only the number and percentage of patients with events were presented, but no Kaplan-Meier estimates, HRs, CI, or P values. Interactions between treatment assignment and prespecified subgroups were evaluated by including terms for treatment, subgroup, and treatment-by-subgroup interaction in the Cox model. Interaction terms with a P value of <0.05 were considered statistically significant. With 224 primary events in the ipsilateral stenosis group, the power was 82% assuming a hazard ratio of 0.68 (as found in the SOCRATES trial3).
Results
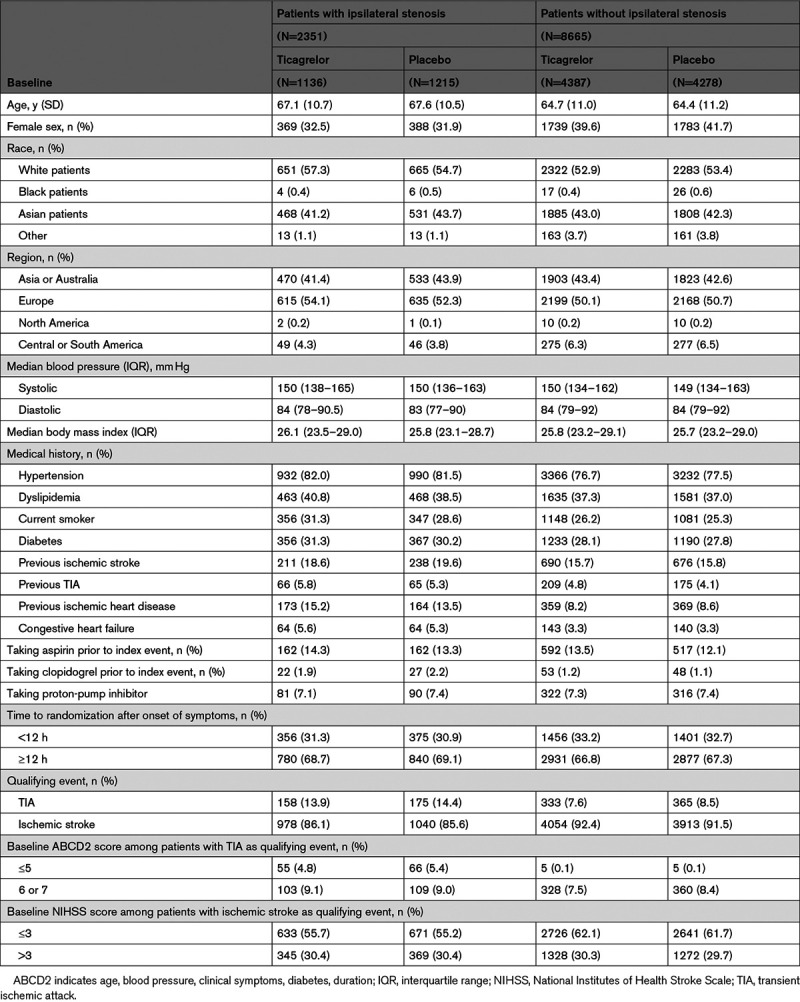
Between January 22, 2018 and October 7, 2019, 2351 patients (21.3% of the overall 11 016 patients in the THALES trial) with ipsilateral stenosis of the cerebral vasculature were randomized and included in the present THALES subgroup analysis (Figure I in the Data Supplement, CONSORT). Among 11 016 patients, 8802 (79.9%) had an imaging of extracranial and intracranial arteries. Four patients with ipsilateral stenosis withdrew their consent during the study; vital status at the end of the study was ascertained for all these patients. Event status for the primary end point was ascertained for 99.7% of the potential patient follow-up time. Baseline characteristics are presented in Table 1; there were no major imbalances between the groups.
Table 1.
Baseline Characteristics of Patients With and Without Ipsilateral Stenosis
In the THALES ipsilateral stenosis population, ticagrelor resulted in fewer primary efficacy outcome events (92/1136, 8.1%) than placebo (132/1215, 10.9%), HR 0.73 (95% CI, 0.56–0.96), P=0.023; Figure 1, Table 2) with a number needed to treat (NNT) of 34 (95% CI, 19–171), whereas in the subgroup with no ipsilateral stenosis 211/4387 patients on ticagrelor and 230/4278 on placebo had a primary outcome event (4.8% versus 5.4%, HR=0.89 [95% CI, 0.74–1.08]; P=0.230; P for interaction=0.245; Figure 1, Table 2). A sensitivity analysis excluding patients with no vascular imaging found the same results.
Figure 1.
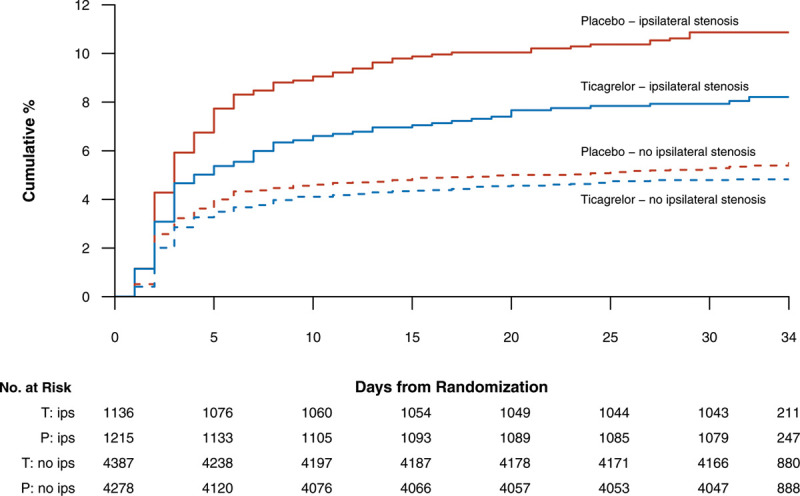
Kaplan-Meier event curves for the primary efficacy end point of stroke or death in patients with ipsilateral atherosclerotic stenosis of cervicocranial vasculature (solid lines, ticagrelor: blue line, placebo: red line) and without (dashed lines).
Table 2.
Outcomes in Patients With or Without Ipsilateral Extracranial or Intracranial Stenosis on Ticagrelor or Placebo
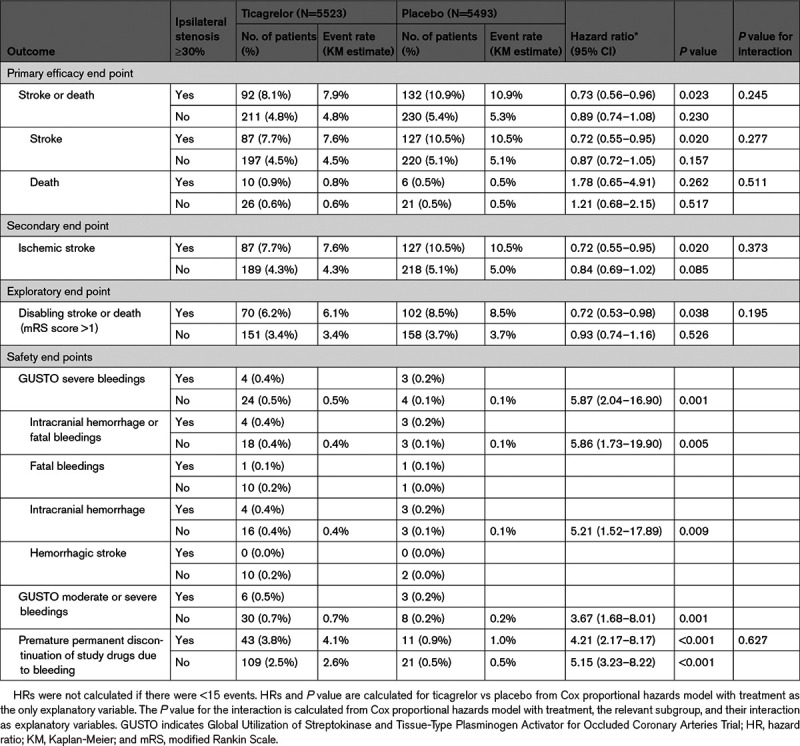
The first secondary end point, ischemic stroke, occurred in 87 (7.7%) patients in the ticagrelor group and 127 (10.5%) in the placebo group, HR 0.72 (0.55–0.95), P=0.020 (Table 2).
Analysis of the primary efficacy outcome including only disabling stroke (modified Rankin Scale score>1 at 30 days) or death showed 2.3% absolute difference between groups (NNT 43; Table 2).
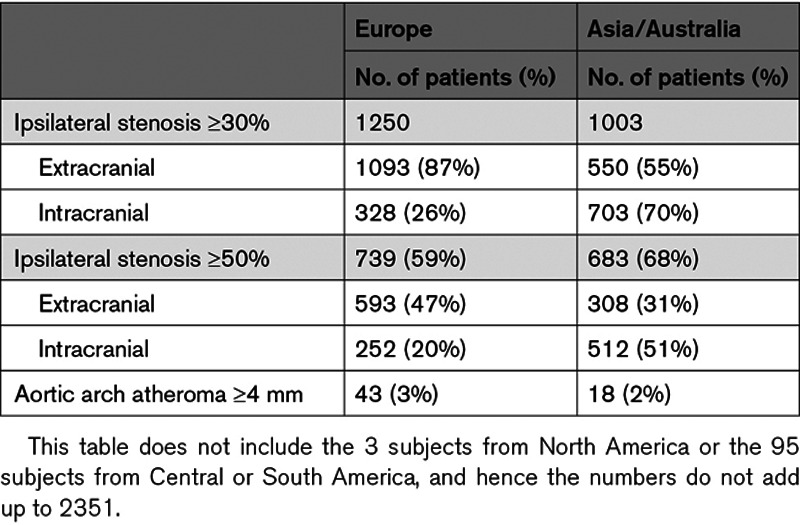
There were no treatment-by-subgroup interactions for the primary end point in the prespecified subgroups at a threshold of P<0.05, except for weight <70 kg (Figure 2). Of note the absolute benefit in Asian patients was more pronounced (10.3 versus 16.2%, HR, 0.61 [95% CI, 0.43–0.87], Pinteraction=0.09, NNT=17) as well as in patients weighting <70 kg (Figure 2). Table 3 shows the distribution of atherosclerotic stenosis in European and Asian patients (showing more intracranial stenosis in Asia and more extracranial stenosis in Europe). However, including geographical region as a factor in the analysis of the primary end point yielded an HR=0.74 (0.57–0.97) for the group with ipsilateral stenosis, and an HR=0.89 (0.74–1.07) for the group without, that is, almost identical results as presented above. Table I in the Data Supplement shows the effect of ticagrelor versus placebo in patients with ipsilateral stenosis according to the extracranial or intracranial site of the stenosis. The effect was significant in patients with intracranial stenosis (9.9% versus 15.2%, HR=0.66 [95% CI, 0.47–0.93], P=0.016; Table I in the Data Supplement).
Figure 2.
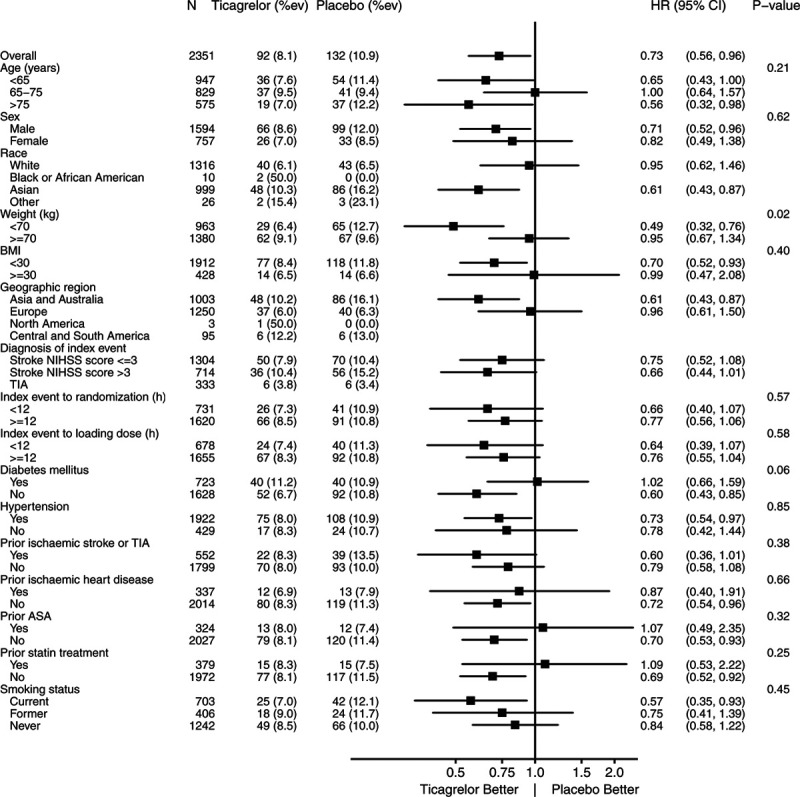
Subgroup analysis in patients with ipsilateral stenosis. Primary end point: ticagrelor added to aspirin versus placebo added to aspirin. BMI indicates body mass index; HR, hazard ratio; NIHSS, National Institutes of Health Stroke Scale; and TIA, transient ischemic attack.
Table 3.
Distribution of Ipsilateral Atherosclerotic Stenosis According to Geographical Regions
The primary safety end point (GUSTO severe bleeding) occurred in 4 patients (0.352%) of the ticagrelor group and 3 (0.247%) of the placebo group with ipsilateral atherosclerotic stenosis (P=NS; a number needed to harm [NNH] of 951 [95% CI, 182 to −296]), while it occurred in 24 (0.5%) and 4 (0.1%) patients respectively in the group with no atherosclerosis (HR 5.87 [95% CI, 2.04–16.9], P=0.001; Table 2). Intracranial hemorrhage occurred in 4 patients (0.4%) in the ticagrelor group versus 3 (0.2%) in the placebo group with ipsilateral atherosclerosis (P=NS), and 16 (0.4%) and 3 (0.1%) patients respectively in the group with no atherosclerosis (HR 5.21 [95% CI, 1.52–17.89], P=0.009). Fatal bleeding occurred in one patient in the ticagrelor group and one in the placebo group among those with atherosclerosis, as compared to 10 and one patients in the ticagrelor and placebo group, respectively, in patients with no atherosclerosis. Permanent discontinuation of study medication due to bleeding in patients with ipsilateral atherosclerotic stenosis occurred in 43 (3.8%) of the ticagrelor group versus 11 (0.9%) of the placebo group (HR=4.21 [95% CI, 2.17–8.17], P<0.001; Table 2). Proton-pump inhibitor was used during the treatment period in 44.9% and 44.7% of patients with ipsilateral stenosis on ticagrelor added to aspirin and aspirin alone, respectively, and in 43.8% and 43.5% in patients without ipsilateral stenosis on ticagrelor added to aspirin, and aspirin alone, respectively.
Patients with postrandomization carotid endarterectomy or stenting had a trend toward fewer primary efficacy outcome events in the ticagrelor group (4/46, 8.7%) than in the placebo group (9/38, 23.7%; P=0.0692), with one GUSTO severe bleedings each.
Discussion
The THALES trial enrolled 2351 patients with ipsilateral atherosclerotic stenosis ≥30% in extracranial or intracranial artery with or without aortic arch plaques ≥4 mm in thickness. Ticagrelor added to aspirin resulted in a significant 27% relative risk reduction of stroke or death as compared to placebo added to aspirin, with an NNT of only 34 (95% CI, 19–171) as compared to a NNT of 92 (95% CI, 51–509) in the overall THALES population.4 The results of the THALES study,4 as well as the subgroup of patients with documented ipsilateral atherosclerosis in THALES and SOCRATES3 may guide treating physicians to a patient population with potentially larger treatment effect. However, contrary to what we observed in the SOCRATES trial atherosclerotic stenosis subanalysis,3 in the THALES trial, the treatment-by-ipsilateral-stenosis ≥30% subgroup interaction was not significant. Indeed, the THALES trial enrolled fewer patients with atherosclerotic stenosis than the SOCRATES trial,3 for 4 reasons. First, THALES made little attempt to enrich this subgroup of patients with the premise that the overall SOCRATES results12 just missed statistical significance, and that the addition of ticagrelor to aspirin would yield a greater relative risk reduction. Second, based on the CHANCE and POINT trials results,6,7 some investigators may have treated their high-risk patients with more severe atherosclerotic stenosis outside the trial with a combination of clopidogrel and aspirin, rather than randomizing them. Third, there were numerically fewer patients enrolled in the THALES trial (11 016)4 than in the SOCRATES trial (13 199).12 Finally, when designing the trial, we did not calculate a specified sample size for this subanalysis and thus did not set targets for enrollment in these subgroups. However, the lack of interaction may be due to the fact that ticagrelor added to aspirin has some beneficial effect also in the subgroup of patients with no ipsilateral stenosis.
Regarding safety, the results in the THALES-atherosclerosis subgroup are similar to the result in the overall population, but the NNH was 951 (95% CI, 182 to −296) as compared to 263 (95% CI, 169–588) in the overall population. However, the number of safety end points is small, and we should be cautious in interpreting them. Three long-term antiplatelet trials with dual therapy have shown an unacceptable increase risk in major bleeding as compared to monotherapy, with a 53%, 52%, and 100% proportion of patients with small vessel disease in MATCH trial (Management of Atherothrombosis With Clopidogrel in High-Risk Patients),13 PRoFESS trial (Prevention Regimen for Effectively Avoiding Secondary Strokes),14 and SPS-3 trial (Secondary Prevention of Small Subcortical Strokes),15 respectively. In THALES, major bleeding was found in 4 patients on ticagrelor and 3 patients on placebo in 2351 patients with ipsilateral stenosis, and in 24 patients on ticagrelor and 4 patients on placebo in 8665 patients without ipsilateral stenosis. In the latter subgroup, small vessel disease was likely highly represented and may account for a large part of excess of bleedings, explaining the difference in bleeding risk between groups with and without ipsilateral stenosis.
In our study, the absolute risk in patients with ipsilateral atherosclerotic stenosis was twice the risk of patients without. A recent registry and several trials found that the ipsilateral atherosclerotic disease subgroup had a much higher absolute risk than other ischemic stroke subtypes in noncardioembolic stroke populations.2–4,6,7,12,16–22 Indeed, the large artery atherosclerosis subgroup of ischemic stroke patients is a logical target for stroke prevention with antiplatelet agents as ruptured atherosclerotic plaques promote thrombosis. In this respect, ticagrelor has shown a high potential beneficial effect in this trial as well as in SOCRATES,3 PRINCE (Platelet Reactivity in Acute Nondisabling Cerebrovascular Events) and trials performed in patients with coronary artery disease.3,23–25 This population is nowadays easily identifiable in clinical practice since imaging of extracranial and intracranial arteries is recommended upon arrival in stroke unit using computed tomography angiography, magnetic resonance angiography, or ultrasonography.26 Given the results of the present analysis with an NNT of 34 (95% CI, 19–171) and an NNH due to bleeding of 951 (95% CI, 182 to −296), patients with ipsilateral stenosis ≥30% of an extracranial or intracranial artery with or without aortic arch plaques ≥4 mm in thickness, this subgroup may be the appropriate target for ticagrelor plus aspirin therapy over a 30-day period after the index stroke. As an indirect comparison, the NNT in the POINT trial was 67, and the NNH was 200 over a 90-day period of treatment.7 In the present trial again, the Kaplan-Meier curves suggest that most of the benefit was front-loaded during the first 10 days.
The limitation of this analysis is that this is a subgroup analysis from the larger trial. While prespecified, it was not selected as a secondary analysis in the hierarchical testing, and thus it should be seen exploratory and hypothesis generating. This analysis was also limited by the low proportion of patients (21.3%) with ipsilateral atherosclerotic stenosis ≥30% with or without aortic arch plaque of ≥4 mm, although in practice it is 40%,1 because some investigators may have treated their patients outside the trial with clopidogrel plus aspirin. It was also limited by the low proportion of patients who underwent a carotid artery revascularization, although our results in these patients suggest a large relative risk reduction in the primary end point and a 15% absolute risk difference without increase GUSTO severe bleedings. Also, in 20% of patients the information on the presence of ipsilateral stenosis was not obtained as data was based on imaging performed as part of clinical practice. Finally, permanent discontinuation of study drug was more common on ticagrelor than on placebo.
In conclusion, in this exploratory analysis comparing ticagrelor added to aspirin to aspirin alone, we found no interaction between treatment group and ipsilateral atherosclerosis stenosis subgroup but did identify a higher absolute risk and a greater absolute risk reduction of stroke or death at 30 days in the ipsilateral atherosclerosis stenosis group than in those without. Taken together with similar subgroup analysis of the SOCRATES trial showing significant interaction, ticagrelor added to aspirin yielded a clinically meaningful relative and absolute risk reduction of stroke and death as compared to aspirin alone with an NNT of 34 (95% CI, 19–171) and an NNH of 951 (95% CI, 182 to −296). These patients form indisputably a group to target with this therapy after a TIA or a minor ischemic stroke.
Sources of Funding
The study was funded by AstraZeneca.
Disclosures
Dr Johnston has received institutional research support from AstraZeneca and drug/placebo from Sanofi for an National Institutes of Health (NIH)-sponsored trial. Dr Amarenco reports receipt of research grant support from Pfizer, Sanofi, Bristol-Myers-Squibb, Merck, AstraZeneca, Boston Scientific, and from the French government, and consulting fees from Pfizer, BMS, Merck, Boehringer Ingelheim, AstraZeneca, Bayer, Daiichi Sankyo, Edwards, Boston Scientific, Kowa, GSK, Fibrogen, Amgen, Shing Poon, Gilead, and lecture fees from Bayer, St-Jude Medical, Amgen, Pfizer, Sanofi. Dr Evans is a statistical consultant to AstraZeneca. Drs Denison, Himmelmann, Knutsson, and Ladenvall are employees of AstraZeneca. Dr James has received institutional research grants from Astra Zeneca, The Medicines Company, Bayer, and Jansen. Dr Molina has received honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Bristol-Myers-, Covidien, Cerevast, and Brainsgate. Dr Wang has received research grants from Sanofi, AstraZeneca and Amgen, and honoraria for participation to advisory board from Sanofi.
Supplemental Materials
Figure I
Table I
Supplementary Material
Appendix
THALES Steering Committee and Investigators: S. Claiborne Johnston, Pierre Amarenco, Scott R. Evans, Anders Himmelmann, Hans Denison, Per Ladenvall, Mikael Knutsson, Yongjun Wang, Stefan K. James, Carlos A. Molina, Kenneth Butcher, Shuya Li, Huaguang Zheng, David Skoloudik, Lawrence Wong, Ketevana Meshkova, Nijasri C. Suwanwela, Sebastian Ameriso, Leonardo Gonzalez, Pablo Ioli, Lorena Jure, Guillermo Povedano, Guadalupe Bruera, Gustavo Herrera, Juan Jose Martin Artesi, Virginia A. Pujol Lereis, Conrado J. Estol, Maia Gomez Schneider, Santiago Pigretti, Fernando Lipovestky, María Cristina Zurru, Stephen Davis, Andrew Wong, Tissa Wijeratne, Arman Sabet, Andrew Lee, Vincent Thijs, Robin Lemmens, Laetitia Yperzeele, Geert Vanhooren, Peter Vanacker, Iris Vansteenkiste, Vicky Maqueda, André Peeters, Marie-Christine Hasenbroekx, Van Daele, Wendy Hasenbroekx, Adinda De Pauw, Regilio Oedit, Wouter De Vooght, Philippe Desfontaines, Yves Vandermeeren, Adinda De Pauw, Marianna D.A. Dracoulakis, Rodrigo Bazan, Octavio M. Pontes Neto, Daniel D.C. Bezerra, Luiz C. Marrone, Pedro A. Kowacs, Carla H.C. Moro, Paulo C.O. Macelino, Marco Tulio A. Pedatella, Ekaterina Titianova, Ivan Staikov, Dimitar Maslarov, Plamen Petkov, Tanya Beleva, Borislav Kralev, Nikolay Sotirov, Dimcho Hristov, Rumeliya M. Ivanova, Margarita V. Mihailova, Ashfaq M. Shuaib, Andrew Demchuk, Michel Beaudry, Anthony R. Winder, Sumiti Nayar, Xingquan Zhao, Guoqiang Wen, Xueshuang Dong, Guozhong Li, Zhaohui Zhang, Huisheng Chen, Dong Wang, Xiaohong Li, Yuncheng Wu, Xu Zhang, Baorong Zhang, Wenke Hong, Xiaogang Li, Lijuan Wang, Li Liu, Xiaolin Xu, Peifu Wang, Weihong Zheng, Jinsheng Zeng, Yukai Wang, Yan Jia, Yongqiu Li, Bo Hu, Wei Shen, Zhi Song, Zhiping Hu, Yunhai Liu, Kaifu Ke, Deqin Geng, Shigang Zhao, Runxiu Zhu, Qiumin Qu, Xiuli Zhao, Qi Wan, Yunhua Yue, Huishan Du, Meiyun Zhang, Yan Wang, Dongfang Li, Dongyu Wang, Yongqiang Li, Xufang Xie, Tingmin Yu, Qi Liu, Mingxiu Yang, Xiaoping Pan, Lijun Xu, Deen Xu, Gang Li, Anding Xu, Martin Roubec, Petr Geier, Daniel Vaclavik, Jiri Neumann, Jana Bednarova, Robert Mikulik, David Hlinovsky, Charlotte Cordonnier, Igor Sibon, Caroline Arquizan, Sonia Alamowitch, Bertrand Lapergue, Jean-Marc Olivot, Nicolas Raposo, Marie-Hélène Mahagne, Emmanuel Touze, Gilles Rodier, Stéphane Vannier, Yves Samson, Michael Obadia, Emmanuel Ellie, Benoît Guillon, Serge Timsit, Yannick Bejot, Valérie Wolff, Didier Smadja, Aude Bagan-Triquenot, Pierre Garnier, Xavier Ducrocq, Peggy Reiner, Thierry Moulin, Frédéric Philippeau, Fernando Pico, Sébastien Richard, Joachim Röther, Jörg Berrouschot, Hassan Soda, Carsten Pohlmann, Christoph Terborg, Darius G. Nabavi, Rainer Dziewas, Martin Köhrmann, Jörg Glahn, Lars Marquardt, Bernd Kallmünzer, Karin Weißenborn, Yannie Oi-Yan Soo, Richard Li, Wing Chi Fong, Siu Hung Li, Raymond Cheung, Kin Keung Yip, Joshua Wai Ming Fok, Michael Y.P. Fu, Norbert Szegedi, Krisztián Pozsegovits, Attila Valikovics, Gyula Pánczél, Csilla Rózsa, Péter Diószeghy, Attila Csányi, Levente Kerényi, Valéria Nagy, László Szapáry, Dániel Bereczki, Sándor Molnár, Gyula Timár, András G. Folyovich, Mária Sátori, Ildikó Vastagh, Praveen S. Kumar, Rajnish Kumar, Atul Prasad, Vikram Sharma, Alok Verma, Indraneel Basu, Abu Z. Ansari, Vijaya Pamidimukkala, Raghavendra B. S., Vivek D. Junewar, Sumit Singh, Advait Prakash Kulkarni, Padma M.V. Srivastava, K. Pramod, Sanjay G. Ramteke, Jaideep Bansal, Kewal Krishan, Hrishikesh Kumar, Priyanka V. Kashyap, T.C.R. Ramakrishnan, Gopal R. Adrasetty, Amit Yeole, Rahul B. Baviskar, Giancarlo Agnelli, Danilo Toni, Stefano Ricci, Rossana Tassi, Giuseppe Micieli, Michelangelo Mancuso, Giovanni Orlandi, Alberto Chiti, Marialuisa Delodovici, Federico Carimati, Alessandro De Vito, Francesco Perini, Cinzia Finocchi, Tiziana Tassinari, Massimo Del Sette, Luisa Roveri, Andrea Zini, Guido Bigliardi, Francesca R. Pezzella, Letizia Cupini, Alessandro Adami, Giampaolo Tomelleri, Carla Zanferrari, Angel A. Arauz Góngora, Minerva López Ruíz, Angelica Ruíz Franco, José O.J. Chacon Romero, Fernando Cruz Castillo, Jose L. Ruiz-Sandoval, Jesús D. López Tapia, Edgar A. Castillo Vargas, Juan F. Gongora Rivera, Guillermo Rivera Martinez, Jorge Villarreal Careaga, Nilton Custodio, Oscar G. Pamo Reyna, Cesar A. Castañeda, Edwin J. Pretell, Nestor Najar, Julio C. Perez, Luisa Cardoza, Carlos Chavez, Maria Reyes, Anna Członkowska, Waldemar Fryze, Piotr Sobolewski, Ryszard Nowak, Dorota Szkopek, Zbigniew Bąk, Sławomir Brzozowski, Waldemar Brola, Marek Zalisz, Konrad Rejdak, Marta Bilik, Małgorzata Fudala, Andrzej Tutaj, Anetta Lasek-Bal, Jan Kochanowicz, Bartosz Karaszewski, Tomasz Berkowicz, Beata Zwiernik, Dorota Różański, Jan P. Mejnartowicz, Szymon Jurga, Jacek Rożniecki, Maciej Świat, Ovidiu A. Bajenaru, Cristina A. Panea, Mihaela A. Simu, Rodica Balasa, Dan I. Cuciureanu, Bogdan O. Popescu, Monica Sabau, Corina Roman-Filip, Liudmila Odnopozova, Oleg Artyukov, Anna Milto, Liudmila V. Stakhovskaya, Sergei Aksentiev, Svetlana E. Chuprina, Elena B. Kuznetsova, Ilya I. Sholomov, Alexander Malygin, Elena Mordvintseva, Rostislav Y. Nilk, Inna Ershova, Dina Khasanova, Leila Akhmadeeva, Aida Iakupova, Ekaterina A. Drozdova, Marine Tanashyan, Evgenij Pudov, Lybov A. Shpagina, Svetlana Berns, Liudmila G. Lenskaya, Konstantin Golikov, Andrey V. Kovalenko, Elena Vasilieva, Elena Reznik, Mikhail Zykov, Evgeniy Kovalchuk, Dmitry Popov, Andrey Belkin, Olga Androfagina, Tatyana Lokshtanova, Elena V. Melnikova, Fahmi Al-Senani, Nouf Almansour, Fawaz Alhussein, Ali Alkhathaami, Saeed Alghamdi, Miroslav Brozman, Marta Mikloskova, Georgi Krastev, Vlastimil Serdahely, Michal Kovacik, Ladislav Gurcik, Miloslav Dvorak, Egon Kurca, Andrea Cimprichova, Marian Kycina, Erika Zacharova, Richard Risnovsky, Hee-Joon Bae, Kyung Bok Lee, Yong-Jin Cho, Jong-Moo Park, Joon-Tae Kim, Jun Lee, Jae-Kwan Cha, Sung-Il Sohn, Dong-Ick Shin, Soo Joo Lee, Byung-Chul Lee, Jay Chol Choi, Moo Seok Park, Dae-Il Chang, Joung-Ho Rha, Sang Min Sung, Yangha Hwang, Jaume Roquer González, Jaime Masjuan Vallejo, Meritxell Gomis Cortina, Francisco Moniche Álvarez, Miguel Ángel Gamero García, Soledad Pérez Sánchez, Francisco Purroy García, Santiago Trillo Senín, Tomás Segura Martín, Joaquín Serena Leal, Juan Arenillas Lara, Joan Martí-Fàbregas, Aida Lago Martín, Carlos Tejero Juste, Javier Marta Moreno, Nicolás López Hernández, Lars Sjöblom, Ann Charlotte Laska, Margarita Callander, Thomas Mooe, Jan-Erik Karlsson, Mihaela Oana Romanitan, Arne Lindgren, Niaz Ahmed, Björn Cederin, Christine Kremer, Tsong-Hai Lee, Jiann-Shing Jeng, Chih-Hung Chen, Helen L. Po, Chia-Wei Liou, Huey-Juan Lin, Ruey-Tay Lin, Hsiu-Fen Lin, Li-Ming Lien, Lung Chan, Wei-Shih Huang, Wen-Yi Huang, Ta-Cheng Chen, Chin-I Chen, Po-Lin Chen, Chun-Pai Yang, Yu Sun, Aurauma Chutinet, Tasanee Tantirittisak, Sombat Muengtaweepongsa, Yongchai Nilanont, Somsak Tiamkao, Chesda Udommongkol, Witoon Jantararotai, Tabtim Chongsuvivatwong, Suwat Srisuwananukorn, Wasutha Khaykhaew, Supachai Paiboonpol, Makorn Limudomporn, Saengduan Mayotarn, Kanoksri Samintharapanya, Arkhom Arayawichanon, Thanoot Thamangraksat, Duangpol Srimanee, Galyna Chmyr, Nataliya Tomakh, Alla Cherkez, Sergii Moskovko, Vadym Nikonov, Svitlana Shkrobot, Lyudmyla Shulga, Hanna Hrebeniuk, Valentyna Yavorska, Nataliia Lytvynenko, Marta Khavunka, Iryna Kobets, Nataliia Chemer, Ivanna Tashchuk, Olha Myronova, Thang H. Nguyen, Tan V. Vo, Thanh T. Tran, Nga T.P. Nguyen, Anh D. Nguyen, Binh T. Nguyen, Thang B. Nguyen, Ngoc H. Nguyen, Quang D. Nguyen, Nhan D. Le, Dai D. Pham.
Nonstandard Abbreviations and Acronyms
- ASCOD
- atherosclerosis, small vessel disease, cardiac pathology, other disease, dissection
- CHANCE
- Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events
- GUSTO
- Global Utilization of Streptokinase and Tissue-Type Plasminogen Activator for Occluded Coronary Arteries Trial
- HR
- hazard ratio
- MATCH
- Management of ATherothrombosis with Clopidogrel in High-risk patients
- NNH
- number needed to harm
- NNT
- number needed to treat
- POINT
- Platelet Oriented Inhibition in New TIA and Minor Ischemic Stroke
- PRINCE
- Platelet Reactivity in Acute Nondisabling Cerebrovascular Events
- PRoFESS
- Prevention Regimen for Effectively Avoiding Secondary Strokes
- SOCRATES
- The Acute Stroke or Transient Ischemic Attack Treated With Aspirin or Ticagrelor and Patient Outcomes
- SPS-3
- Secondary Prevention of Small Subcortical Strokes
- THALES
- The Acute Stroke or Transient Ischemic Attack Treated With Ticagrelor and ASA for Prevention of Stroke and Death
- TIA
- transient ischemic attack
A list of all THALES Steering Committee and Investigators is given in the Appendix.
Presented in part at the American Heart Association's Scientific Sessions, November 16, 2020.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.032239.
For Sources of Funding and Disclosures, see page 3512.
Presented in part at the American Heart Association's Scientific Sessions, November 16, 2020.
Contributor Information
Hans Denison, Email: hans.denison@astrazeneca.com.
Scott R. Evans, Email: sevans@bsc.gwu.edu.
Anders Himmelmann, Email: Anders.himmelmann@astrazeneca.com.
Stefan James, Email: stefan.james@ucr.uu.se.
Mikael Knutsson, Email: Mikael.Knutsson@astrazeneca.com.
Per Ladenvall, Email: per.ladenvall@astrazeneca.com.
Carlos A. Molina, Email: cmolina@vhebron.net.
Yongjun Wang, Email: yongjunwang@ncrcnd.org.cn.
S. Claiborne Johnston, Email: clay.johnston@austin.utexas.edu.
References
- 1.Sirimarco G, Lavallée PC, Labreuche J, Meseguer E, Cabrejo L, Guidoux C, Klein IF, Olivot JM, Abboud H, Adraï V, et al. Overlap of diseases underlying ischemic stroke: the ASCOD phenotyping. Stroke. 2013;44:2427–2433. doi: 10.1161/STROKEAHA.113.001363 [DOI] [PubMed] [Google Scholar]
- 2.Amarenco P, Lavallee PC, Labreuche J, Albers GW, Bornstein NM, Canhao P, Caplan LR, Donnan GA, Ferro JM, Hennerici MG, et al. One-year risk of stroke after transient ischemic attack or minor ischemic stroke. N Engl J Med. 2016;374:1533–1542. doi: 10.1056/NEJMoa1412981 [DOI] [PubMed] [Google Scholar]
- 3.Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Hill MD, Jonasson J, Kasner SE, Ladenvall P, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack of atherosclerotic origin. Lancet Neurol. 2017;16:301–310. [DOI] [PubMed] [Google Scholar]
- 4.Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, Knutsson M, Ladenvall P, Molina CA, Wang Y. Ticagrelor and aspirin versus aspirin in patients with acute ischemic stroke. N Engl J Med. 2020;383:207–217.32668111 [Google Scholar]
- 5.Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, Knutsson M, Ladenvall P, Molina CA, Wang Y; THALES Investigators. The acute stroke or transient ischemic attack treated with ticagrelor and aspirin for prevention of stroke and death (THALES) trial: rationale and design. Int J Stroke. 2019;14:745–751. doi: 10.1177/1747493019830307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, et al. ; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11–19. doi: 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 7.Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, Kim AS, Lindblad AS, Palesch YY; Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215–225. doi: 10.1056/NEJMoa1800410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Wolf ME, Hennerici MG. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36:1–5. doi: 10.1159/000352050 [DOI] [PubMed] [Google Scholar]
- 9.Godolphin PJ, Bath PM, Algra A, Berge E, Brown MM, Chalmers J, Duley L, Eliasziw M, Gregson J, Greving JP, et al. Outcome assessment by central adjudicators versus site investigators in stroke trials: a systematic review and meta-analysis. Stroke. 2019;50:2187–2196. [DOI] [PubMed] [Google Scholar]
- 10.Gusto Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001 [DOI] [PubMed] [Google Scholar]
- 11.Easton JD, Aunes M, Albers GW, Amarenco P, Bokelund-Singh S, Denison H, Evans SR, Held P, Jahreskog M, Jonasson J, et al. ; SOCRATES Steering Committee and Investigators. Risk for major bleeding in patients receiving ticagrelor compared with aspirin after transient ischemic attack or acute ischemic stroke in the SOCRATES study (acute stroke or transient ischemic attack treated with aspirin or ticagrelor and patient outcomes). Circulation. 2017;136:907–916. doi: 10.1161/CIRCULATIONAHA.117.028566 [DOI] [PubMed] [Google Scholar]
- 12.Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Jonasson J, Minematsu K, Molina CA, et al. ; SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35–43. doi: 10.1056/NEJMoa1603060 [DOI] [PubMed] [Google Scholar]
- 13.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias Guiu J, Rupprecht HJ; on behalf of the MATCH Investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomized, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4 [DOI] [PubMed] [Google Scholar]
- 14.Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, et al. Aspirin and extended release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:123812–51. doi: 10.1056/NEJMoa0805002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Perce LA; SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. doi: 10.1056/NEJMoa1204133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarenco P, Lavallée PC, Monteiro Tavares L, Labreuche J, Albers GW, Abboud H, Anticoli S, Audebert H, Bornstein NM, Caplan LR, et al. ; TIAregistry.org Investigators. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. 2018;378:2182–2190. doi: 10.1056/NEJMoa1802712 [DOI] [PubMed] [Google Scholar]
- 17.Amarenco P, Duyckaerts C, Tzourio C, Hénin D, Bousser MG, Hauw JJ. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221–225. doi: 10.1056/NEJM199201233260402 [DOI] [PubMed] [Google Scholar]
- 18.Amarenco P, Cohen A, Tzourio C, Bertrand B, Hommel M, Besson G, Chauvel C, Touboul PJ, Bousser MG. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474–1479. doi: 10.1056/NEJM199412013312202 [DOI] [PubMed] [Google Scholar]
- 19.Amarenco P, Cohen A, Hommel M, Moulin T, Leys D, Bousser MG. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;334:1216–1221. doi: 10.1056/NEJM199605093341902 [DOI] [PubMed] [Google Scholar]
- 20.Amarenco P, Bogousslavsky J, Callahan A, III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, et al. ; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894 [DOI] [PubMed] [Google Scholar]
- 21.Sillesen H, Amarenco P, Hennerici MG, Callahan A, Goldstein LB, Zivin J, Messig M, Welch KM; Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial. Stroke. 2008;39:3297–3302. doi: 10.1161/STROKEAHA.108.516450 [DOI] [PubMed] [Google Scholar]
- 22.Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Béjot Y, Cabrejo L, Cha JK, Ducrocq G, Giroud M, et al. ; Treat Stroke to Target Investigators. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. 2020;382:9 doi: 10.1056/NEJMoa1910355 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Chen W, Lin Y, Meng X, Chen G, Wang Z, Wu J, Wang D, Li J, Cao Y, et al. ; PRINCE Protocol Steering Group. Ticagrelor plus aspirin versus clopidogrel plus aspirin for platelet reactivity in patients with minor stroke or transient ischaemic attack: open label, blinded endpoint, randomised controlled phase II trial. BMJ. 2019;365:l2211 doi: 10.1136/bmj.l2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al. ; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 25.Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA, Held C, Andersson M, Himmelmann A, Ridderstråle W, et al. ; THEMIS Steering Committee and Investigators. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019;381:1309–1320. doi: 10.1056/NEJMoa1908077 [DOI] [PubMed] [Google Scholar]
- 26.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, et al. ; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


