Abstract
Background
A large proportion of people with COPD are not referred to pulmonary rehabilitation (PR) despite its proven benefits. No previous studies have examined predictors of referral to PR.
Objective
To determine the characteristics of people with COPD associated with referral to PR.
Methods
Cross-sectional analysis of a primary care cohort of 82,696 Welsh people with COPD generated as part of a UK national audit of COPD care. Data represent care received by patients as of 31/03/2017. Referral to PR was defined as any code in the patient record indicating referral to PR in the last 3 years. Potential predictors of referral to PR were chosen based on clinical judgement and data availability. Independent predictors of PR referral were determined using backward stepwise mixed-effects logistic regression with a random effect for practice. Variables assessed were: age, gender, deprivation, MRC recorded in past year, MRC grade, smoking status recorded in past year, smoking status, number of exacerbations in past year, inhaled therapy prescription, influenza vaccination, and comorbidities of diabetes, hypertension, coronary heart disease, stroke, heart failure, lung cancer, asthma, bronchiectasis, depression, anxiety, severe mental illness, osteoporosis, and painful condition.
Results
A total of 13,297 people (16%) with COPD were referred from primary care for PR. Patients with a comorbidity of bronchiectasis or depression, MRC recorded in the last year, higher MRC grade, more exacerbations in the last year, a greater level of inhaled therapy, an influenza vaccination, or were an ex-smoker had significantly higher odds of referral to PR. Patients that were older, female, more deprived, or had a comorbidity of diabetes, asthma, or painful condition had significantly lower odds of referral to PR.
Conclusion
Generally appropriate patients are being prioritised for PR referral; however, it is concerning that women, current smokers, and more deprived patients appear to have lower odds of referral.
Keywords: COPD, chronic obstructive pulmonary disease, GP, general practitioner, observational study
Introduction
Pulmonary rehabilitation (PR) has been shown to improve dyspnoea, fatigue, quality of life, and exercise capacity in individuals with COPD.1,2 The quality of evidence for these benefits has been declared such that no further randomised controlled trials (RCTs) comparing PR and usual care are required to demonstrate its benefits.1,3
While the strength of evidence for the benefits of PR is high and the referral criteria are well defined,4,5 the proportion of patients being referred to PR remains low. A systematic review6 of rates of referral to PR in 10 different countries found referral rates of less than 35% in 93% of the included studies. In the UK, roughly half of PR referrals are from primary care,7,8 however, between 2004 and 2014 only 9% of eligible COPD patients in England were referred to PR from primary care.9 In Wales, the picture is better with 35% of eligible patients referred in 201510 and 50% in 2017.11 However, this still means that half of all eligible COPD patients are missing out on this important intervention.
No large studies of predictors of referral to PR have previously been completed. We therefore aimed to use a national primary care cohort to determine the patient characteristics associated with referral to PR. This will allow us to discover the individuals that require better targeting in primary care.
Methods
Database/Population
To investigate COPD patient factors associated with referral to PR from primary care, we used data from the NCAP 2017 primary care audit. This is a cross-sectional study of all 82,696 currently registered people with COPD in 407 general practices in Wales, UK (94% of all Welsh practices) that examines care received in the 2 years prior to the audit date of 31/03/2017. The dataset was generated following a direct extraction from general practice patient record systems in June 2017 by NHS Wales Informatics Service (NWIS). No identifying information was collected from practices, with identifiable information being pseudonymised at source. Data were only extracted for patients with a diagnosis of COPD, defined using a validated definition (Read diagnosis codes) in UK primary care data.12 Read codes13 are a clinical terminology used to record clinical events in UK primary care, similar to International Classification of Primary Care (ICPC)14 or SNOMED CT15 codes. Data were not extracted for patients that had opted-out of usage of their pseudonymised data for audit or other analysis. Practice participation in the audit was on an opt-in basis and all practices in Wales were eligible. Full details of the primary care audit and the methodology used to create the dataset can be found in the audit data report.11
Variables
The outcome of the analysis – referral to PR – was defined as any person with COPD with a Read code in their patient record indicating referral to PR in the 3 years prior to the audit date (01/04/2014 to 31/03/2017). In the UK referral is recommended for people with COPD who have frequent exacerbations or an MRC grade of 3 or more,4 however ultimately the referral decision is that of the patient’s GP. Read codes used to define pulmonary rehabilitation referral and all other events in the patient record can be found on the audit resources webpage.16 Twenty-three exposures (age, gender, socioeconomic status (SES), diabetes, hypertension, coronary heart disease, stroke, heart failure, lung cancer, asthma, bronchiectasis, depression, anxiety, severe mental illness, osteoporosis, painful condition, MRC grade recorded, MRC grade, smoking status recorded, smoking status, exacerbations, inhaled therapy, and influenza vaccination) were used as potential predictors of referral to PR. Exposures were chosen based on clinical judgement and data availability. Patients aged under 35 years, and without any events recorded in their patient file in the past 4 years were excluded.
SES was defined using the 2014 Welsh Index of Multiple Deprivation (WIMD). WIMD is a measure of deprivation that ranks the relative deprivation between small areas (or neighbourhoods) of Wales. Values for WIMD are derived by assessing the income, employment, health, education, access to services, community safety, physical environment, and housing in a particular small area.17 WIMD data were provided to us by NWIS split in to 5 categories: 10% most deprived, 10–20% most deprived, 20–30% most deprived, 30–50% most deprived, 50% least deprived. Category of WIMD was derived using the patient’s home postcode. Further variable definitions can be found in Supplementary Methods.
Statistical Analysis
All data management and statistical analyses were performed using Stata 15 (StataCorp, College Station, TX, USA). Data were first summarised using means and proportions, where appropriate. Age was discretised to produce a categorical variable as its relationship with PR referral was non-linear. Where variables had no more than 5% missing data, complete-case analysis was used; otherwise additional missing data categories were added to preserve sample size. To account for clustering of patients at practice level, mixed-effects logistic regression (xtlogit command, re option) was used to investigate association between each of the twenty-three exposures and referral to PR with a random effect for each practice. Odds ratios with 95% confidence intervals were generated for each exposure.
After univariate analyses predictors of referral to PR were determined using mixed-effects logistic regression. The model was built using a backward stepwise regression, adding significant and then removing non-significant variables until there were no further changes. A p-value of <0.05 was regarded as statistically significant. Significance of categorical variables was tested using the likelihood ratio test.
Multicollinearity of predictors included in the final model was assessed using the Stata “collin” command. A variance inflation factor (VIF) of 10 was defined as indicating problematic multicollinearity. All variables had VIFs well below 10 indicating multicollinearity was not an issue in the final model. Odds ratio graphs were generated using coefplot.18
We wondered if certain groups of patients may be more likely to refuse PR than others, so we performed a sensitivity analysis where we repeated the analysis including exceptions for PR as well as referrals for PR in the outcome. This would demonstrate that a GP has considered the patient’s suitability for PR and then either deemed them unsuitable, offered them PR and they declined, not had an available PR programme to refer them to, or referred them; thus changing the outcome to “considered for PR” rather than “referred for PR”.
Ethical Approval
The Healthcare Quality Improvement Partnership (HQIP) is data controller for the National Clinical Audit and Patient Outcomes Programme (NCAPOP) projects. An HQIP extended output scope form was completed for the audit data set used in this analysis. Formal approval from the HQIP Data Access Request Group (DARG)19 was not required as the dataset uses de-identified pseudonymised data for a purpose deemed to be in line with primary audit data collection.
Results
A total of 13,297 people (16%) with COPD were referred from primary care for PR (Table 1). Patients with an MRC grade of 3 or higher, patients with 2 or more exacerbations, and patients on triple therapy had the highest proportion of PR referrals. Patients with an MRC grade of 1 had the lowest proportion of PR referrals.
Table 1.
Characteristics of Patients Referred and Not Referred for Pulmonary Rehabilitation. Proportions Shown are Row Percentages
| Not referred for PR (%) N = 69,399 | Referred for PR (%) N = 13,297 | |
|---|---|---|
| Age (years) | ||
| 35–59 | 11,598 (85.8%) | 1,922 (14.2%) |
| 60–64 | 7,615 (81.5%) | 1,729 (18.5%) |
| 65–69 | 10,842 (81.3%) | 2,492 (18.7%) |
| 70–74 | 12,406 (81.8%) | 2,754 (18.2%) |
| 75–80 | 10,579 (82.7%) | 2,221 (17.4%) |
| ≥80 | 16,359 (88.3%) | 2,179 (11.8%) |
| Gender | N = 69,396 | N = 13,297 |
| Male | 34,877 (83.6%) | 6,857 (16.4%) |
| Female | 34,519 (84.3%) | 6,440 (15.7%) |
| Welsh Index of Multiple Deprivation (WIMD) | N = 68,736 | N = 13,207 |
| 10% most deprived | 18,156 (83.3%) | 3,650 (16.7%) |
| 10–20% most deprived | 16,459 (83.8%) | 3,179 (16.2%) |
| 20–30% most deprived | 13,851 (83.9%) | 2,666 (16.1%) |
| 30–50% most deprived | 12,000 (84.7%) | 2,172 (15.3%) |
| 50% least deprived | 8,270 (84.3%) | 1,540 (15.7%) |
| Comorbidities | ||
| Diabetes | 15,732 (84.2%) | 2,953 (15.8%) |
| Hypertension | 36,610 (84.0%) | 6,978 (16.0%) |
| Coronary heart disease | 27,381 (82.8%) | 5,673 (17.2%) |
| Stroke | 7,320 (84.9%) | 1,303 (15.1%) |
| Heart failure | 6,180 (83.0%) | 1,263 (17.0%) |
| Lung cancer | 1,626 (84.6%) | 295 (15.4%) |
| Asthma | 29,203 (84.4%) | 5,419 (15.7%) |
| Bronchiectasis | 3,062 (77.6%) | 884 (22.4%) |
| Depression | 20,451 (82.3%) | 4,410 (17.7%) |
| Anxiety | 20,829 (82.7%) | 4,351 (17.3%) |
| Severe mental illnessA | 5,403 (83.8%) | 1,045 (16.2%) |
| Osteoporosis | 8,751 (82.1%) | 1,906 (17.9%) |
| Painful conditionB | 8,521 (81.5%) | 1,929 (18.5%) |
| MRC grade recorded in the past year | 39,290 (78.0%) | 11,111 (22.1%) |
| MRC grade (latest recorded) | ||
| 1 | 9,741 (96.2%) | 388 (3.8%) |
| 2 | 28,829 (91.4%) | 2,724 (8.6%) |
| 3 | 14,221 (70.7%) | 5,881 (29.3%) |
| 4 | 7,665 (67.6%) | 3,679 (32.4%) |
| 5 | 1,674 (74.4%) | 575 (25.6%) |
| Not recorded | 7,269 (99.3%) | 50 (0.7%) |
| Smoking status recorded in the past year | 52,474 (81.9%) | 11,575 (18.1%) |
| Smoking status (latest recorded) | N = 66,422 | N = 13,182 |
| Current smoker | 22,219 (85.1%) | 3,879 (14.9%) |
| Ex-smoker | 34,770 (81.0%) | 8,164 (19.0%) |
| Never smoker | 9,433 (89.2%) | 1,139 (10.8%) |
| Exacerbations in the past year | N = 68,969 | N = 13,164 |
| 0 | 42,540 (89.1%) | 5,184 (10.9%) |
| 1 | 12,456 (83.0%) | 2,561 (17.1%) |
| 2 | 5,725 (77.2%) | 1,687 (22.8%) |
| >2 | 8,248 (68.9%) | 3,732 (31.2%) |
| Inhaled therapy treatment (last 6 months) | ||
| Not on inhaled therapy | 24,852 (91.2%) | 2,410 (8.8%) |
| ICS | 4,105 (91.4%) | 388 (8.6%) |
| LABA | 1,843 (88.8%) | 232 (11.2%) |
| LABA & ICS | 13,843 (84.7%) | 2,508 (15.3%) |
| LAMA | 9,060 (83.1%) | 1,839 (16.9%) |
| LABA & LAMA | 1,300 (76.5%) | 399 (23.5%) |
| Triple therapy | 14,396 (72.3%) | 5,521 (27.7%) |
| Influenza vaccination | 44,345 (81.2%) | 10,257 (18.8%) |
Notes: ASevere mental illness: schizophrenia, bipolar, and other psychotic illness. BPainful condition: 4 or more prescriptions of analgesics or antiepileptics (in the absence of an epilepsy diagnosis) in the past year.
Abbreviations: PR, pulmonary rehabilitation; MRC, Medical Research Council; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist.
In univariate analysis, coronary heart disease (p<0.001), heart failure (p=0.023), bronchiectasis (p<0.001), depression (p<0.001), anxiety (p<0.001), osteoporosis (p<0.001), painful condition (p<0.001), MRC recorded in the last year (p<0.001), and influenza immunisation (p<0.001) were all significantly associated with greater odds of referral to PR. Being female (p=0.011), having had a stroke (p=0.015), and having asthma (p=0.001) were significantly associated with lower odds of referral to PR. Older, more deprived, patients with a higher MRC grade, ex-smokers relative to current smokers, patients with more exacerbations in the last year, and patients on greater levels of inhaled therapy had greater odds of being referred to PR (Table 2).
Table 2.
Odds Ratios for Referral to Pulmonary Rehabilitation (PR) from Primary Care by Patient Characteristics
| Odds ratio (95% CI) | ||
|---|---|---|
| Crude | Adjusted | |
| Age (years) | ||
| 35–59 | 1 | 1 |
| 60–64 | 1.37 (1.27 – 1.48) | 1.02 (0.93 – 1.10) |
| 65–69 | 1.41 (1.32 – 1.51) | 0.96 (0.89 – 1.04) |
| 70–74 | 1.35 (1.27 – 1.45) | 0.87 (0.80 – 0.94) |
| 75–79 | 1.26 (1.18 – 1.35) | 0.76 (0.70 – 0.82) |
| ≥80 | 0.79 (0.73 – 0.84) | 0.51 (0.47 – 0.55) |
| Gender | ||
| Male | 1 | 1 |
| Female | 0.95 (0.92 – 0.99) | 0.93 (0.89 – 0.98) |
| Welsh Index of Multiple Deprivation (WIMD) | ||
| 10% most deprived | 1.14 (1.05 – 1.23) | 0.85 (0.78 – 0.93) |
| 10–20% most deprived | 1.11 (1.03 – 1.20) | 0.89 (0.82 – 0.97) |
| 20–30% most deprived | 1.09 (1.01 – 1.18) | 0.94 (0.86 – 1.03) |
| 30–50% most deprived | 1.01 (0.93 – 1.09) | 0.94 (0.86 – 1.03) |
| 50% least deprived | 1 | 1 |
| Comorbidities | ||
| Diabetes | 1.01 (0.96 – 1.05) | 0.90 (0.85 – 0.95) |
| Hypertension | 0.99 (0.95 – 1.03) | |
| Coronary heart disease | 1.16 (1.11 – 1.20) | |
| Stroke | 0.92 (0.87 – 0.98) | |
| Heart failure | 1.08 (1.01 – 1.15) | |
| Lung cancer | 0.91 (0.80 – 1.03) | |
| Asthma | 0.93 (0.89 – 0.97) | 0.91 (0.87 – 0.95) |
| Bronchiectasis | 1.61 (1.49 – 1.75) | 1.34 (1.22 – 1.48) |
| Depression | 1.20 (1.15 – 1.25) | 1.08 (1.03 – 1.14) |
| Anxiety | 1.14 (1.09 – 1.18) | |
| Severe mental illnessA | 0.97 (0.90 – 1.04) | |
| Osteoporosis | 1.20 (1.13 – 1.27) | |
| Painful conditionB | 1.21 (1.15 – 1.28) | 0.89 (0.84 – 0.95) |
| MRC grade recorded in the past year | 4.22 (4.01 – 4.44) | 2.68 (2.52 – 2.85) |
| MRC grade (latest recorded) | ||
| 1 | 1 | 1 |
| 2 | 2.54 (2.27 – 2.84) | 2.26 (2.01 – 2.54) |
| 3 | 12.26 (10.98 – 13.68) | 11.45 (10.20 – 12.85) |
| 4 | 14.32 (12.79 – 16.03) | 14.11 (12.50 – 15.92) |
| 5 | 9.81 (8.49 – 11.34) | 10.71 (9.14 – 12.55) |
| Not recorded | 0.18 (0.13 – 0.24) | 0.43 (0.31 – 0.59) |
| Smoking status recorded in the past year | 2.27 (2.15 – 2.41) | |
| Smoking status (latest recorded) | ||
| Current smoker | 1 | 1 |
| Ex-smoker | 1.39 (1.33 – 1.45) | 1.41 (1.34 – 1.49) |
| Never smoker | 0.70 (0.66 – 0.76) | 1.06 (0.98 – 1.16) |
| Exacerbations in the past year | ||
| 0 | 1 | 1 |
| 1 | 1.76 (1.66 – 1.85) | 1.22 (1.15 – 1.30) |
| 2 | 2.61 (2.45 – 2.78) | 1.52 (1.42 – 1.64) |
| >2 | 4.13 (3.93 – 4.35) | 1.85 (1.74 – 1.96) |
| Inhaled therapy treatment (last 6 months) | ||
| Not on inhaled therapy | 0.44 (0.41 – 0.47) | 0.81 (0.75 – 0.88) |
| ICS | 0.45 (0.40 – 0.50) | 0.70 (0.61 – 0.80) |
| LABA | 0.59 (0.50 – 0.68) | 0.72 (0.61 – 0.85) |
| LABA & ICS | 0.88 (0.82 – 0.94) | 0.97 (0.89 – 1.05) |
| LAMA | 1 | 1 |
| LABA & LAMA | 1.54 (1.36 – 1.76) | 1.22 (1.05 – 1.40) |
| Triple therapy | 2.06 (1.93 – 2.19) | 1.39 (1.29 – 1.49) |
| Influenza vaccination | 1.94 (1.86 – 2.03) | 1.25 (1.18 – 1.32) |
Notes: Adjusted results represent odds ratios of independent predictors of PR referral included in the final model. ASevere mental illness: schizophrenia, bipolar, and other psychotic illness. BPainful condition: 4 or more prescriptions of analgesics or antiepileptics (in the absence of an epilepsy diagnosis) in the past year.
Abbreviations: CI, confidence interval; MRC, Medical Research Council; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist.
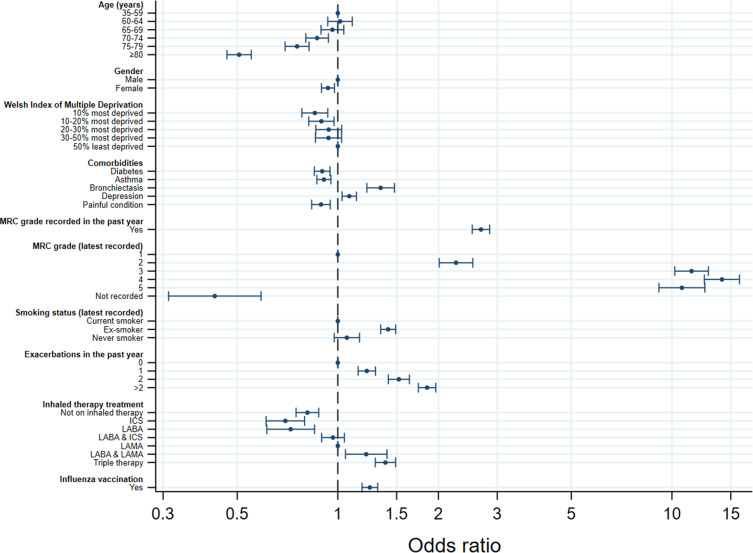
In multivariate analysis, the variables included in the final model and independently associated with referral to PR were age (p<0.0001), gender (p=0.0031), deprivation (p=0.0061), diabetes (p=0.0001), asthma (p=0.0001), bronchiectasis (p<0.0001), depression (p=0.0019), painful condition (p=0.0003), MRC recorded in the last year (p<0.0001), MRC grade (p<0.0001), smoking status (p<0.0001), number of exacerbations in the last year (p<0.0001), inhaled therapy prescription (p<0.0001), and influenza vaccination (p<0.0001). Relative to patients under 60 years old, patients 70 years or older had lower odds of referral to PR. Women had 7% lower odds of referral than men (OR: 0.93 [95% CI: 0.89–0.98]). Relative to the 50% least deprived patients, the 20% most deprived patients had lower odds of referral to PR. Patients with diabetes, asthma, or a painful condition had 10% (OR: 0.90 [95% CI: 0.85–0.95]), 9% (OR: 0.91 [95% CI: 0.87–0.95]), and 11% (OR: 0.89 [95% CI: 0.84–0.95]), respectively, lower odds of referral to PR, and patients with bronchiectasis or depression had 34% (OR: 1.34 [95% CI: 1.22–1.48]) and 8% (OR: 1.08 [95% CI: 1.03–1.14]), respectively, higher odds of referral. Patients with an MRC grade recorded in the last year had more than twice (OR: 2.68 [95% CI: 2.52–2.85]) the odds of referral. Ex-smokers had 41% higher odds (OR: 1.41 [95% CI: 1.34–1.49]) of referral than current smokers. Patients with a higher MRC grade, more exacerbations in the last year, or on higher levels of inhaled therapy had higher odds of referral to PR than those with a lower MRC, fewer exacerbations, or on lower levels of inhaled therapy, respectively (Table 2/Figure 1).
Figure 1.
Plot showing odds ratios with 95% confidence intervals of the independent predictors of referral to pulmonary rehabilitation (PR) from primary care.
Abbreviations: MRC, Medical Research Council; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist.
Sensitivity Analysis
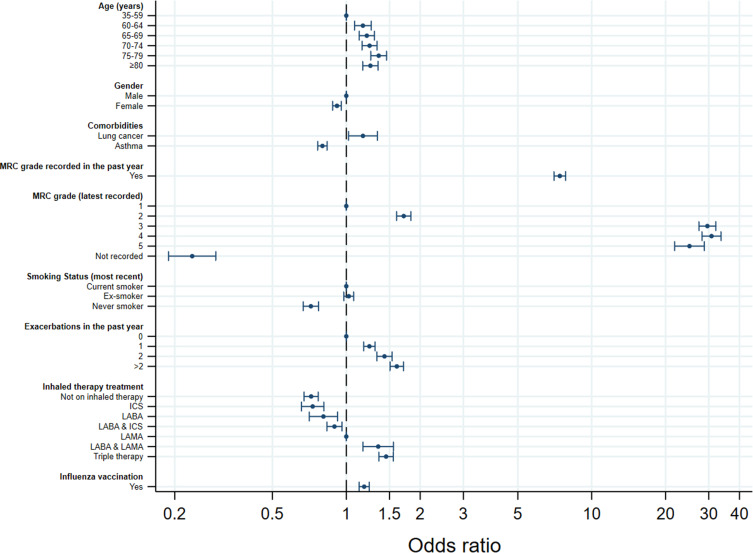
The variables independently associated with consideration for PR were age (p<0.0001), gender (p<0.0001), lung cancer (p=0.0235), asthma (p<0.0001), MRC grade recorded in the last year (p<0.0001), MRC grade (p<0.0001), smoking status (p<0.0001), number of exacerbations in the last year (p<0.0001), inhaled therapy prescription (p<0.0001), and influenza vaccination (p<0.0001). Odds of consideration for PR were similar to the odds of referral to PR for comorbid asthma, having an MRC grade recorded in the last year, the specific MRC grade, number of exacerbations in the last year, prescribed inhaled therapy, influenza vaccination, and women still had 8% lower odds (OR: 0.92 [95% CI: 0.88–0.95]) of consideration for PR than men (Table 3/Figure 2). Unlike in the referral analysis, patients over 60 years old had higher odds of consideration for PR than those under 60. People with comorbid lung cancer had 17% higher odds (OR: 1.17 [95% CI: 1.02–1.34]) of consideration for PR than those without lung cancer. Unlike in the referral analysis, people with comorbidities of diabetes, bronchiectasis, depression, or painful condition did not have significantly different odds of consideration for PR. Deprivation was not a significant factor for consideration for PR. Ex-smokers were not significantly more likely (OR: 1.02 [95% CI: 0.98–1.07]) to be considered for PR than current smokers, however never smokers did have 28% lower odds (OR: 0.72 [95% CI: 0.67–0.77]) of consideration for PR than current smokers.
Table 3.
Odds Ratios for Consideration for Pulmonary Rehabilitation (PR) in Primary Care by Patient Characteristics
| Odds ratio (95% CI) | ||
|---|---|---|
| Crude | Adjusted | |
| Age (years) | ||
| 35–59 | 1 | 1 |
| 60–64 | 1.55 (1.46 – 1.64) | 1.17 (1.08 – 1.26) |
| 65–69 | 1.66 (1.58 – 1.75) | 1.21 (1.13 – 1.30) |
| 70–74 | 1.72 (1.64 – 1.81) | 1.24 (1.16 – 1.33) |
| 75–79 | 1.90 (1.80 – 2.00) | 1.36 (1.26 – 1.46) |
| ≥80 | 1.48 (1.41 – 1.55) | 1.25 (1.17 – 1.35) |
| Gender | ||
| Male | 1 | 1 |
| Female | 0.92 (0.89 – 0.94) | 0.92 (0.88 – 0.95) |
| Welsh Index of Multiple Deprivation (WIMD) | ||
| 10% most deprived | 1.36 (1.28 – 1.44) | |
| 10–20% most deprived | 1.28 (1.21 – 1.36) | |
| 20–30% most deprived | 1.16 (1.10 – 1.24) | |
| 30–50% most deprived | 1.09 (1.03 – 1.16) | |
| 50% least deprived | 1 | |
| Comorbidities | ||
| Diabetes | 1.16 (1.12 – 1.20) | |
| Hypertension | 1.15 (1.12 – 1.18) | |
| Coronary heart disease | 1.22 (1.18 – 1.25) | |
| Stroke | 1.08 (1.03 – 1.13) | |
| Heart failure | 1.32 (1.25 – 1.38) | |
| Lung cancer | 1.05 (0.96 – 1.16) | 1.17 (1.02 – 1.34) |
| Asthma | 0.79 (0.76 – 0.81) | 0.80 (0.76 – 0.84) |
| Bronchiectasis | 1.27 (1.19 – 1.36) | |
| Depression | 1.07 (1.04 – 1.11) | |
| Anxiety | 1.01 (0.98 – 1.04) | |
| Severe mental illnessA | 0.96 (0.91 – 1.01) | |
| Osteoporosis | 1.25 (1.19 – 1.30) | |
| Painful conditionB | 1.29 (1.23 – 1.35) | |
| MRC grade recorded in the past year | 6.40 (6.18 – 6.63) | 7.42 (7.02 – 7.83) |
| MRC grade (latest recorded) | ||
| 1 | 1 | 1 |
| 2 | 2.08 (1.96 – 2.21) | 1.71 (1.60 – 1.83) |
| 3 | 20.13 (18.83 – 21.53) | 29.62 (27.36 – 32.05) |
| 4 | 17.87 (16.62 – 19.23) | 30.79 (28.16 – 33.66) |
| 5 | 11.29 (10.12 – 12.60) | 25.03 (21.78 – 28.77) |
| Not recorded | 0.05 (0.04 – 0.06) | 0.24 (0.19 – 0.29) |
| Smoking status recorded in the past year | 2.70 (2.60 – 2.81) | |
| Smoking status (latest recorded) | ||
| Current smoker | 1 | 1 |
| Ex-smoker | 1.21 (1.17 – 1.25) | 1.02 (0.98 – 1.07) |
| Never smoker | 0.58 (0.55 – 0.61) | 0.72 (0.67 – 0.77) |
| Exacerbations in the past year | ||
| 0 | 1 | 1 |
| 1 | 1.80 (1.73 – 1.87) | 1.24 (1.18 – 1.31) |
| 2 | 2.52 (2.39 – 2.66) | 1.43 (1.33 – 1.54) |
| >2 | 3.96 (3.78 – 4.15) | 1.61 (1.51 – 1.71) |
| Inhaled therapy treatment (last 6 months) | ||
| Not on inhaled therapy | 0.39 (0.37 – 0.40) | 0.72 (0.67 – 0.77) |
| ICS | 0.40 (0.37 – 0.44) | 0.73 (0.66 – 0.81) |
| LABA | 0.65 (0.58 – 0.71) | 0.81 (0.71 – 0.92) |
| LABA & ICS | 0.74 (0.70 – 0.78) | 0.90 (0.83 – 0.96) |
| LAMA | 1 | 1 |
| LABA & LAMA | 1.64 (1.47 – 1.83) | 1.35 (1.17 – 1.56) |
| Triple therapy | 2.01 (1.91 – 2.12) | 1.45 (1.36 – 1.55) |
| Influenza vaccination | 1.95 (1.89 – 2.01) | 1.18 (1.13 – 1.24) |
Notes: Adjusted results represent odds ratios of independent predictors of PR referral included in the final model. ASevere mental illness: schizophrenia, bipolar, and other psychotic illness. BPainful condition: 4 or more prescriptions of analgesics or antiepileptics (in the absence of an epilepsy diagnosis) in the past year.
Abbreviations: CI, confidence interval; MRC, Medical Research Council; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist.
Figure 2.
Plot showing odds ratios with 95% confidence intervals of the independent predictors of consideration for pulmonary rehabilitation (PR) from primary care.
Abbreviations: MRC, Medical Research Council; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist.
Discussion
Eligibility criteria for PR are well defined with both national4 and international guidance.20 With the majority of PR referrals originating from primary care8,21 in the UK, the National COPD Audit has presented a unique opportunity to describe factors that influence referral so that strategies can be developed to ensure equality of access for potential participants.
In this study, we have found that people with COPD with a comorbidity of depression or bronchiectasis, an MRC grade recorded in the last year, a higher MRC grade, more exacerbations in the past year, on higher levels of inhaled therapy, vaccinated for influenza, or who were an ex-smoker had greater odds of referral to PR. These results are encouraging as they seem to indicate that people with more severe symptoms are being appropriately prioritised. From a service delivery perspective, it was interesting to observe that where markers of high-quality care for COPD patients were recorded a greater proportion of patients were referred. For example, where MRC grade had been recorded, patients had an influenza vaccine, or were prescribed either LABA & LAMA or triple therapy compared to LAMA monotherapy they were significantly more likely to be referred. These results suggest that patients are more likely to be referred from general practices that offer higher quality healthcare. However, as we adjusted for clustering of patients within practices, we suspect that this may simply be a sign of greater engagement with primary care, thus providing more opportunities to discuss PR. The finding that people with an MRC grade ≥3 were more than 10 times more likely to be referred to PR than MRC grade 1 patients was unsurprising given that current guidelines recommend PR referral for any COPD patient with an MRC grade ≥3.4 The finding that ex-smokers were more likely to be referred than current or never smokers is potentially concerning as current smokers can benefit from pulmonary rehabilitation in parallel with smoking cessation treatment.
This study also found that people with COPD that were older (≥70 years), female, more deprived, or had a comorbidity of diabetes, asthma or painful condition were less likely to be referred to pulmonary rehabilitation. It was concerning to find that these groups appear to be missing out on best-practice care. It is possible older people are less likely to be referred due to their increased comorbidity and frailty. The most deprived people with COPD may be less likely to be referred than the least deprived due to them being more likely to refuse PR or poorer engagement with primary care. It is disappointing to find that people with comorbid asthma were less likely to be referred as emerging evidence for rehabilitation in participants with asthma22–26 should encourage referral. There is some logic to the finding that people with a comorbidity of diabetes or painful condition were less likely to be referred as NICE guidance4 proposes that significant orthopaedic limitations may well limit participation in PR, although this has not been established in the literature.
It was surprising to find that women were less likely to be referred than men. Reduced access for women has previously been reported in the UK audit for cardiac rehabilitation,27 but there is no obvious reason for it. We wondered if women are perhaps more likely to refuse PR, but our “consideration for PR” sensitivity analysis similarly found that women are less likely to be considered for PR. It is possible an unconscious bias against women exists in treatment of COPD as well as diagnosis.28,29
t was also interesting to note in the “considered for PR” analysis that people ≥70 years old were more likely to be considered for PR despite being less likely to receive a referral. This suggests older people are more likely to decline, be unsuitable for, or not live near an available PR programme. Current smokers being no less likely to be considered for PR than ex-smokers, but being less likely to receive a referral suggests that current smokers are more likely to refuse PR. And the finding that deprivation is not a significant factor in consideration for PR suggests that more deprived patients may be more likely to refuse or not have access to an available PR programme. People with a comorbidity of lung cancer being more likely to be considered for PR but no more likely to be referred is likely a consequence of the Quality and Outcomes Framework (QOF) pay-for-performance scheme. QOF financially incentivises GPs to refer suitable people with COPD to PR. However, as the bonus payment is proportional to the proportion of suitable people referred to PR, GPs are keen to record unsuitable people as such so that the denominator used in the bonus payment calculation is as small as possible. This means that a person with lung cancer that is unsuitable for PR will likely be swiftly recorded as such by their GP, and this record would include them in our “considered for PR” group.
Although several previous studies have investigated uptake of PR (of those referred), only one study has previously quantitatively examined patient factors associated with referral. Li et al30 studied 88 patients hospitalised with COPD and found that patients with hypertension and more respiratory-related hospital bed days in the last 3 years were more likely to be referred. They also claim anxiety and possibly depression were associated with greater likelihood of referral but only present unadjusted results. If we consider both hospital bed days in the last 3 years and number of exacerbations in the last year to be proxies for disease severity, this current study and the Li et al30 study both highlight disease severity and depression as important factors in referral. The different results found by Li et al30 could simply reflect the low power of their study or differing priorities between primary and secondary care.
Strengths and Limitations
The primary strength of this study is its size. It examines referral for 82,696 people with COPD from 94% of Welsh practices, making it the largest study to examine factors associated with PR referral to date. This large sample has allowed us to adjust for multiple variables in our analyses, something that would not been possible in the only previous study30 of predictors of PR referral. Using nearly all people with COPD in Wales also ensures that our results are generalisable.
The analysis is not without limitations though. Our definition of referral to PR was from primary care so we cannot say this represents the characteristics of all COPD patients being referred to PR, as patients referred from secondary care may have substantially different traits. Another weakness of the referral analysis is that it is limited to Wales, which is likely a more homogenous population than the rest of the UK. WIMD is not a perfect definition of SES, as it only signifies the deprivation of an area in which a person lives, not how deprived a person is. This will likely bias results towards the null hypothesis for SES as the deprivation of a local population will appear more homogenous. The National COPD Audit primary care data that we used is also quite limited in the data that it contains in comparison to a patient’s full primary care record. Further details on the severity of a patient’s condition, such as spirometry results, and details on the availability of PR programmes would have been useful additional potential predictors to examine.
In our analysis, we used practice as a random effect in the mixed-effects logistic regression models to account for clustering of patients within practices, but there is a possibility of clustering at the GP level too. We think that clustering at the GP level is likely to be a smaller issue than clustering at the practice level though as patients within a practice are likely to be more similar than the patients assigned to a specific GP within a practice, and care is likely to differ more between practices than between GPs within a specific practice. The use of a cross-sectional study design also adds limitations. In the referral analysis, PR referral is defined as within the last 3 years, but patient characteristics such as smoking status and MRC grade are most recent ever, so it is possible that patient characteristics could reflect those found after, rather than before, completion of PR. This will also likely bias the predictors towards the null hypothesis. This lack of clear temporal pattern in the data also prevents any conclusions or assessment of causal associations in the current analysis. Finally, several significance tests were performed in this study, increasing the probability of some of our significant results being chance findings.
Conclusion
Whilst generally appropriate patients are being prioritised for referral, it is concerning that women, smokers, and more deprived patients are less likely to receive a referral to PR. Ensuring there is easy access to PR programmes in more deprived regions may help to increase referrals among more deprived patients. Useful further work would be to establish if differences in referral between the genders exist in secondary care referrals and other countries.
Acknowledgments
We would like to thank the Healthcare Quality Improvement Partnership for commissioning the National COPD Audit Programme, the National COPD Audit Programme team, and NHS Wales Informatics Service.
Abbreviations
95% CI, 95% confidence interval; AECOPD, acute exacerbation of COPD; COPD, chronic obstructive pulmonary disease; GP, general practitioner; HQIP, Healthcare Quality Improvement Partnership; ICS, inhaled corticosteroid; LABA, long-acting β adrenoceptor agonist; LAMA, long-acting muscarinic antagonist; LRTI, lower respiratory tract infection; MRC, Medical Research Council; NCAP, National COPD Audit Programme; NWIS, NHS Wales Informatics Service; OR, odds ratio; PR, pulmonary rehabilitation; RCT, randomised controlled trial; SES, socioeconomic status; UK, United Kingdom of Great Britain and Northern Ireland; WIMD, Welsh Index of Multiple Deprivation.
Notation of Prior Abstract Publication/Presentation
Unadjusted analyses from this work have previously been presented at the 2019 American Thoracic Society Conference in Dallas, TX, USA.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Guarantor: JKQ.
Disclosure
The authors worked on the National COPD Audit Programme which was funded by the Healthcare Quality Improvement Partnership. Philip Stone reports grants from the Royal College of Physicians, during the conduct of the study. C Michael Roberts reports personal fees from educational lectures on role of audit in COPD care, outside the submitted work. Jennifer Quint reports grants from the Royal College of Physicians, during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
- 1.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2. doi: 10.1002/14651858.CD003793.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steiner M, McMillan V, Lowe D, et al. Pulmonary rehabilitation: beyond breathing better. National chronic obstructive pulmonary disease (COPD) audit programme: outcomes from the clinical audit of pulmonary rehabilitation services in England 2015. Results and Data Analysis. RCP; 2017. Available from: https://www.rcplondon.ac.uk/projects/outputs/pulmonary-rehabilitation-beyond-breathing-better. Accessed October7, 2020.
- 3.Lacasse Y, Cates CJ, McCarthy B, Welsh EJ. This cochrane review is closed: deciding what constitutes enough research and where next for pulmonary rehabilitation in COPD. Cochrane Database Syst Rev. 2015;11. doi: 10.1002/14651858.ED000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Recommendations. NICE guideline (NG115); December 2018. Available from: https://www.nice.org.uk/guidance/ng115/chapter/Recommendations. Accessed February28, 2019. [PubMed]
- 5.Bolton CE, Bevan-Smith EF, Blakey JD, et al. British thoracic society guideline on pulmonary rehabilitation in adults: accredited by NICE. Thorax. 2013;68(Suppl2):ii1–ii30. doi: 10.1136/thoraxjnl-2013-203808 [DOI] [PubMed] [Google Scholar]
- 6.Milner SC, Boruff JT, Beaurepaire C, Ahmed S, Janaudis-Ferreira T. Rate of, and barriers and enablers to, pulmonary rehabilitation referral in COPD: A systematic scoping review. Respir Med. 2018;137:103–114. doi: 10.1016/j.rmed.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 7.Steiner M, Holzhauer-Barrie J, Lowe D, et al. Pulmonary rehabilitation: steps to breathe better. National chronic obstructive pulmonary disease (COPD) audit programme: clinical audit of pulmonary rehabilitation services in England and Wales 2015. National Clinical Audit Report. RCP; 2016. Available from: https://www.rcplondon.ac.uk/projects/outputs/pulmonary-rehabilitation-steps-breathe-better. Accessed October7, 2020.
- 8.Steiner MC, McMillan V, Lowe D, et al. Pulmonary rehabilitation: an exercise in improvement. National chronic obstructive pulmonary disease (COPD) audit programme: clinical and organisational audit of pulmonary rehabilitation services in England and Wales 2017. Clinical audit data analysis and results. RCP; 2018. Available from: https://www.rcplondon.ac.uk/projects/outputs/pulmonary-rehabilitation-exercise-improvement-combined-clinical-and-organisational.
- 9.Moore E, Newson R, Joshi M, et al. Effects of pulmonary rehabilitation on exacerbation number and severity in people with COPD: an historical cohort study using electronic health records. Chest. 2017;152(6):1188–1202. doi: 10.1016/j.chest.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 10.Baxter N, Holzhauer-Barrie J, McMillan V, Saleem Khan M, Skipper E, Roberts CM Time to take a breath. National chronic obstructive pulmonary disease (COPD) audit programme: clinical audit of COPD in primary care in wales 2014–15. National Clinical Audit Report. RCP; 2016. Available from: https://www.rcplondon.ac.uk/projects/outputs/primary-care-time-take-breath. Accessed October7, 2020.
- 11.Baxter N, McMillan V, Holzhauer-Barrie J, et al. Planning for every breath. National chronic obstructive pulmonary disease (COPD) audit programme: primary care audit (Wales) 2015–17. Data analysis and methodology. RCP; 2017. Available from: https://www.rcplondon.ac.uk/projects/outputs/primary-care-audit-wales-2015-17-planning-every-breath. Accessed October7, 2020.
- 12.Quint JK, Müllerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the clinical practice research datalink (CPRD-GOLD). BMJ Open. 2014;4(7):e005540. doi: 10.1136/bmjopen-2014-005540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NHS Digital. Read codes. NHS digital. Available from: https://digital.nhs.uk/services/terminology-and-classifications/read-codes. Accessed May29, 2018.
- 14.World Health Organisation. International classification of primary care, Second edition (ICPC-2). WHO. Available from: http://www.who.int/classifications/icd/adaptations/icpc2/en/. Accessed March25, 2020.
- 15.SNOMED International. SNOMED - 5-step briefing. SNOMED. Available from: https://www.snomed.org/snomed-ct/five-step-briefing. Accessed March25, 2020.
- 16.Royal College of Physicians. National COPD primary care audit (Wales) 2015–17: resources. RCP London; March 29, 2017. Available from: https://www.rcplondon.ac.uk/projects/outputs/national-copd-primary-care-audit-wales-2015-17-resources. Accessed June5, 2018.
- 17.Welsh Government. Welsh index of multiple deprivation (full Index update with ranks): 2014. GOV.WALES; August 12, 2015. Available from: https://gov.wales/welsh-index-multiple-deprivation-full-index-update-ranks-2014. Accessed August14, 2019.
- 18.Jann B COEFPLOT: stata module to plot regression coefficients and other results. Boston College Department of Economics; 2013. Available from: https://ideas.repec.org/c/boc/bocode/s457686.html. Accessed August28, 2019.
- 19.Healthcare Quality Improvement Partnership. Accessing NCAPOP data. HQIP. Available from: https://www.hqip.org.uk/national-programmes/accessing-ncapop-data/. Accessed August15, 2019.
- 20.Rochester CL, Vogiatzis I, Holland AE, et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: enhancing Implementation, Use, and Delivery of Pulmonary Rehabilitation. Am J Respir Crit Care Med. 2015;192(11):1373–1386. doi: 10.1164/rccm.201510-1966ST [DOI] [PubMed] [Google Scholar]
- 21.Hogg L, Garrod R, Thornton H, McDonnell L, Bellas H, White P. Effectiveness, attendance, and completion of an integrated, system-wide pulmonary rehabilitation service for COPD: prospective observational study. COPD J Chronic Obstr Pulm Dis. 2012;9(5):546–554. doi: 10.3109/15412555.2012.707258 [DOI] [PubMed] [Google Scholar]
- 22.Turner S, Eastwood P, Cook A, Jenkins S. Improvements in symptoms and quality of life following exercise training in older adults with moderate/severe persistent asthma. Respiration. 2011;81(4):302–310. doi: 10.1159/000315142 [DOI] [PubMed] [Google Scholar]
- 23.Carson KV, Chandratilleke MG, Picot J, Brinn MP, Esterman AJ, Smith BJ. Physical training for asthma. Cochrane Database Syst Rev. 2013;9. doi: 10.1002/14651858.CD001116.pub4. [DOI] [PubMed] [Google Scholar]
- 24.Lingner H, Ernst S, Groβhennig A, et al. Asthma control and health-related quality of life one year after inpatient pulmonary rehabilitation: the ProKAR study. J Asthma. 2015;52(6):614–621. doi: 10.3109/02770903.2014.996650 [DOI] [PubMed] [Google Scholar]
- 25.Sahin H, Naz I. Comparing the effect of pulmonary rehabilitation in patients with uncontrolled and partially controlled asthma. J Asthma. 2019;56(1):87–94. doi: 10.1080/02770903.2018.1443468 [DOI] [PubMed] [Google Scholar]
- 26.Zampogna E, Centis R, Negri S, et al. Effectiveness of pulmonary rehabilitation in severe asthma: a retrospective data analysis. J Asthma. 2019:1–7. doi: 10.1080/02770903.2019.1646271. [DOI] [PubMed] [Google Scholar]
- 27.National Audit of Cardiac Rehabilitation (NACR). Quality and outcomes report; 2019. Available from: https://www.bhf.org.uk/informationsupport/publications/statistics/national-audit-of-cardiac-rehabilitation-quality-and-outcomes-report-2019. Accessed January20, 2020.
- 28.Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD. CHEST. 2001;119(6):1691–1695. doi: 10.1378/chest.119.6.1691 [DOI] [PubMed] [Google Scholar]
- 29.Delgado A, Saletti-Cuesta L, López-Fernández LA, Gil-Garrido N, Luna Del Castillo J. Gender inequalities in COPD decision-making in primary care. Respir Med. 2016;114:91–96. doi: 10.1016/j.rmed.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 30.Li LSK, Caughey G, Johnston K. Comorbidity associated with referral to pulmonary rehabilitation in people hospitalized with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2014;34(6):430. doi: 10.1097/HCR.0000000000000080 [DOI] [PubMed] [Google Scholar]