Abstract
The human gastrointestinal tract is home to an incredibly dense population of microbes. These microbes employ unique strategies to capture energy in this largely anaerobic environment. In the process of breaking down dietary-and host-derived substrates, the gut microbiota produce a broad range of metabolic products that accumulate to high levels in the gut. Increasingly, studies are revealing that these chemicals impact host biology, either by acting on cells within the gastrointestinal tract or entering circulation and exerting their effects at distal sites within the body. Given the high level of functional diversity in the gut microbiome and the varied diets that we consume, the repertoire of microbiota-derived molecules within our bodies varies dramatically across individuals. Thus, the microbes in our gut and the metabolic end products they produce represent a phenotypic lever that we can potentially control to develop new therapeutics for personalized medicine. Here, we review current understanding of how microbes in the gastrointestinal tract contribute to the molecules within our gut and those that circulate within our bodies. We also highlight examples of how these molecules affect host physiology and discuss potential strategies for controlling their production to promote human health and to treat disease.
Keywords: human microbiome, human metabolome, metabolomics
1. INTRODUCTION
In recent years, our conventionally held view of microbes as pathogens has fundamentally changed. We now appreciate that the enormous population of microbes colonizing our intestine is critical to many aspects of normal human physiology. One of the most important ways bacteria influence our biology is through the production of small molecules that accumulate in our gut and circulate throughout our body. Although it has been known for more than 100 years that microbial metabolites circulate in our body (1), only recently have host-microbe metabolic interactions garnered broad interest from the scientific community. Driven by advances in mass spectrometry, the burgeoning field of metabolomics has begun to uncover the broad impact microbes have on the human metabolome. Numerous studies have shown that microbial metabolites engage specific host receptors and activate downstream signaling cascades that ultimately change cellular physiology. In addition to acting locally on cells in the intestine, where they modulate barrier function and immune activation, they are also absorbed into the circulation and impact cellular processes throughout the body.
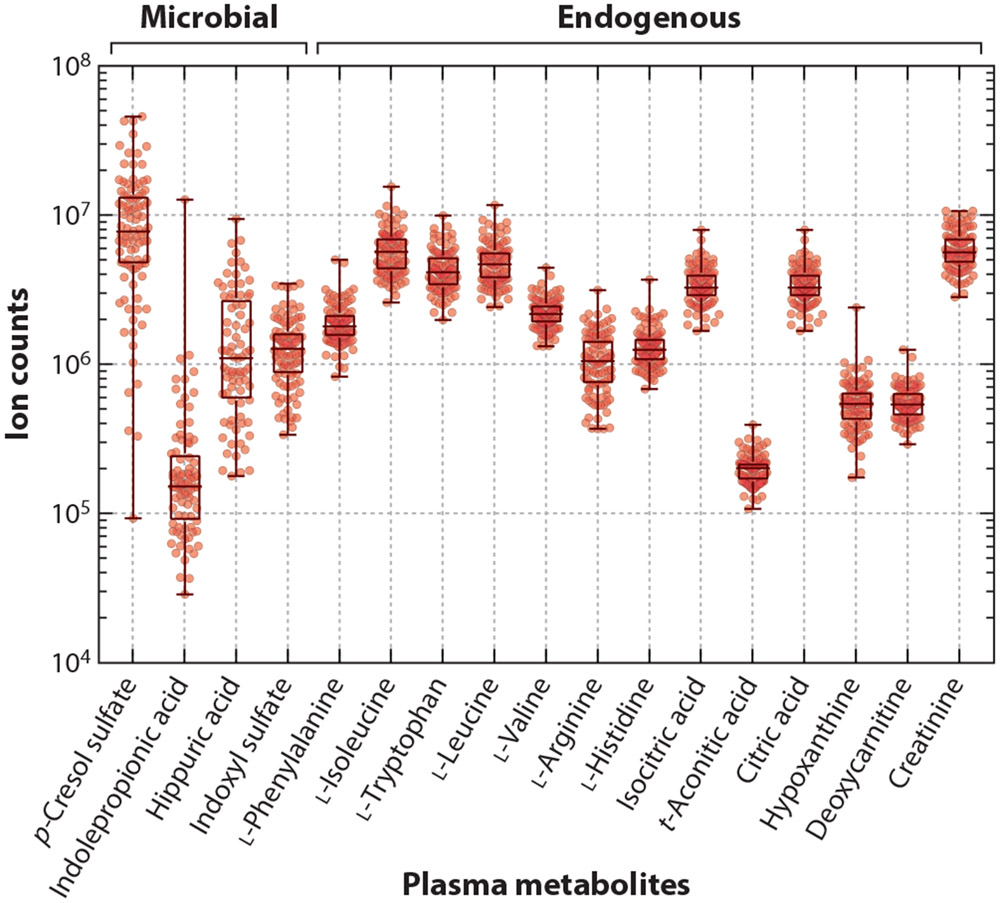
The repertoire of endogenous (host-derived) circulating metabolites is held fairly constant across individuals, reflecting tight homeostatic control of biochemical pathways for production, recycling, and elimination. In contrast, the levels of microbiota-derived molecules vary widely across individuals (Figure 1). For example, indolepropionic acid is undetectable in 25% of healthy individuals, and in the remaining 75%, its plasma levels vary by nearly three orders of magnitude. This variation mirrors the phenotypic variation seen in the microbiome and emphasizes the personalized nature of our gut microbial communities. Due to the relative plasticity of the microbiome, altering the species composition in the gut has the potential to control the production of these drug-like molecules. To achieve this goal, we first need to understand (a) the factors that influence nutrient availability to the gut microbiome, (b) the biochemical and genetic factors that dictate microbial metabolism in the gut, and (c) the mechanisms for host metabolism and elimination of microbiota-derived metabolites.
Figure 1.
Metabolomics analysis of small molecules from the plasma of 100 healthy blood donors. Figure from unpublished data from the Stanford Microbiome-ChEM-H collaborative.
In this review, we first present a brief introduction to the human metabolome, focusing on the chemical signatures of human health and disease. We then summarize our current understanding of the contribution made by gut microbes to the human metabolome, with an emphasis on the functional organization of the human gastrointestinal tract (GIT) and pathways for anaerobic metabolism. Finally, we describe the impact that microbial metabolites have on human physiology and disease and describe microbiome interventions that will likely become an integral part of medicine in the future.
2. THE HUMAN METABOLOME IN HEALTH AND DISEASE
2.1. Molecules in Human Blood
Human blood can be divided into two parts: (a) a cellular component that includes erythrocytes, leukocytes, and platelets; and (b) an aqueous component, known as plasma. Plasma serves as the conduit through which small molecules travel between organs in the body. It provides a sampling of normal human metabolism and can serve as a marker for cellular or organ dysfunction. In addition to small molecules, plasma also contains clotting factors and fibrinogen, which are preserved if blood is collected in the presence of an anticoagulant. In contrast, serum is the liquid component of blood that remains following activation of the clotting cascade. Apart from clear differences in the levels of clotting factors and fibrinogen, the complement of small molecules present in plasma and serum is largely indistinguishable.
As of March 2018, the Human Metabolome Database (HMDB) estimated that ~4,600 named compounds had been detected in human serum (2). These compounds represent more than 50 distinct chemical classes, with phospholipids and glycerolipids comprising roughly 75% of the individual metabolites. Excluding these two compound classes, the HMDB lists just more than 1,000 compounds in human serum. Notable chemical classes include hydroxyl acids, aromatic acids, fatty acids, amino acids, steroids and steroid derivatives, biogenic amines, alcohols, and polyols. Armed with the relatively comprehensive catalog of metabolites identified in serum, we can ask what this catalog reveals about human physiology and disease.
2.2. Chemical Signatures of Human Health and Disease
Metabolomics was first adopted for clinical diagnosis by biochemical geneticists seeking to identify metabolic biomarkers for inborn errors of metabolism (IEM). IEMs arise from mutations in genes that encode enzymes involved in specific biochemical pathways. Metabolic blockade of endogenous pathways often results in aberrant patterns of molecules upstream of the block in the pathway, which can be detected in blood, cerebrospinal fluid, or urine. Since the late 1970s, gas chromatography–mass spectrometry (GC-MS) technologies have been used to identify metabolite patterns that are diagnostic of a particular biochemical genetic disease (3-8). Examples of diseases diagnosed by GC-MS analysis of human urine organic acids include propionic acidemia, methylmalonic acidemia, maple syrup urine disease, and isovaleric acidemia (9).This methodology continues to be the gold standard for the confirmatory diagnosis of many IEMs.
Additional diagnostic modalities employed in the diagnosis of IEMs include the analysis of amino acids and acylcarnitines in plasma. Acylcarnitine profiles in plasma provide a readout of mitochondrial function, with mutations in fatty acid oxidation blocking certain fatty acid metabolic pathways and resulting in characteristic patterns of elevated acylcarnitine species. Examples of these disorders include medium-chain acyl-coenzyme A (CoA) deficiency, carnitine palmitoyltransferase I deficiency, and carnitine–acylcarnitine translocase deficiency (10). Mutations in genes involved in amino acid metabolism lead to aberrancies in plasma amino acid levels. Disorders for which patterns of plasma amino acids are relevant to diagnosis include phenylketonuria, tyrosinemia, alkaptonuria, and maple syrup urine disease (11).
For as long as biochemical geneticists have been using GC-MS to detect abnormal metabolic patterns in urine, they have observed exogenous molecules arising from bacterial metabolism in the gut. In some cases, these compounds are so abundant that they mask diagnostic peaks and complicate the analysis of patients’ samples (12). Owing to a lack of understanding of the potential roles of these high-abundance microbial metabolites in human health and disease, these chemicals have been largely ignored.
3. THE HUMAN MICROBIOME
3.1. Composition and Function of the Human Microbiome
The human GIT is colonized with microbes along its entire length, from the oral cavity to the rectum. The vast majority of these microbes reside in the large intestine, with significant populations in both the distal ileum and the oral cavity (Table 1). They are predominately bacteria, although archaea may represent a sizable (>10%) fraction of the colonic population in some individuals (13). Fungi and other eukaryotes likely represent a much smaller proportion, while viruses and phages are as abundant as bacteria, but their metabolic output is likely much lower (14). Here, we focus on the bacteria of the large intestine (and refer to them as the microbiome) because of their dominant mass and metabolism, although we caution that abundance has not been established as a proxy for importance in host–microbe interactions.
Table 1.
Microbial density across sites in the gastrointestinal tracta
| Structure | Substructure | Volume (L) | Surface area (m2) |
Retention time (h) |
Microbial density (number of bacteria/mL) |
References |
|---|---|---|---|---|---|---|
| Oral cavity | ||||||
| Saliva, buccal mucosa | (0.871 to 1.19) × 10−3 | 1 × 109 | 151,b 152e | |||
| Tongue | 29.5 × 10−4 | 153c | ||||
| Dental plaque | (0 to 1) × 10−3 | 42.94 × 10−4 | 1 × 1011 | 151,b 154,c 152e | ||
| Stomach | 0.25 to 0.9 | 0.053 | 1 to 4 | 103 to 104 | 14,b,e 155,d 156,c,d | |
| Small intestine | 200 | 3 to 4 | Varies geographically | 156c,d | ||
| Duodenum | 103 to 104 | 14e | ||||
| Jejunum | 103 to 104 | 14e | ||||
| Ileum | 0.4 | 108 | 14b,e | |||
| Large intestine | 0.4 to 0.6 | 0.35 | 25 to 40 | 0.9 × 1011 | 14,b,d,e 156,c 159c | |
| Cecum | 0.006 | 157,b 158c | ||||
| Ascending colon | 0.203 | 0.024 | 157,b 158c | |||
| Transverse colon | 0.198 | 0.051 | 157,b 158c | |||
| Descending colon | 0.16 | 0.019 | 157,b 158c | |||
| Sigmoid rectum | 0.02 | 157,b 158c |
Data are approximations based on labeling studies (volume, surface area, retention time) and culture-based enumeration methods (microbial density). Empty cells indicate that no data were found or that it would be inappropriate to extrapolate beyond known data.
Reference refers to volume.
Refers to surface area.
Refers to retention time.
Refers to microbial density.
The healthy adult intestine is one of the most diverse microbial ecosystems known, with studies reporting from hundreds to thousands of species. Estimates of the abundance of different taxonomic groups of microbes (e.g., phyla) vary widely by population, diet, and technical factors (e.g., DNA extraction procedure) (15, 16). Because of this environmental and technical variability, assigning mean proportions to any bacterial group can be misleading. With this in mind, it is generally accepted that Bacteroidetes (abundant genera: Bacteroides, Parabacteroides, Alistipes, and Prevotella) and Firmicutes (abundant genera: Eubacterium, Clostridium, and Ruminococcus) comprise roughly 90% of the microbiome of the large intestine, with the balance composed of members of Actinobacteria (abundant genera: Collinsella and Bifidobacterium) and Proteobacteria (abundant genera: Escherichia) (17-21). With respect to nontaxonomic groupings, it is harder to evaluate what an average GIT microbiome looks like. Using gene content (frequently in metagenomic analyses) to assess microbiome composition is a popular alternative to taxonomic approaches, but comes with its own caveats (18, 19). Most importantly, the definition of sameness (or cluster inclusion) for arbitrary genes is much less well understood than for taxonomic marker genes, making groupings based on gene content frequently either overbroad or too specific (22).
An important reemerging method of classifyingmicrobes (and, consequently, the collective behavior of microbiomes) is functional. The functional approach links microbes to phenotypic traits (e.g., colonymorphology), molecular or chemical output (e.g., butyrate production), or immediate consequences to the host (e.g., immunostimulatory effects). In the clinical setting, the functional classification paradigm has always been important because it helps determine treatment decisions for microbial infections (23). For example, bacteria producing a β-lactamase require different antibiotic treatment than those that are β-lactamase negative. Similarly, the definition of new bacterial species has frequently used a polyphasic approach that delineates species on the basis of genetic and biochemical distinctness (23). Even though there are numerical approaches to creating phenotypic and molecular taxonomies from polyphasic data, they have not been favored in the study of the microbiome. Recent microbiome work has been dominated by 16S ribosomal RNA gene surveys (and, more recently, metagenomic surveys), as discussed above. While these are powerful techniques, they cannot engender a molecular taxonomy because they do not assay phenotypic or biochemical behavior: They must infer the biochemical behavior from host or environmental covariates.
Because of this recent focus, the functional consequences of any nonpathogenic microbe or particular microbiome composition are not well understood. However, some broad patterns have emerged. First, the GIT microbiome plays an important role in host immune homeostasis throughout life (24). In addition to direct immune signaling, data suggest that microbes alter the barrier function of the intestine. Barrier integrity influences the rate at which metabolites and bacteria enter privileged host spaces (e.g., the bloodstream, epithelial tissue) and, thus, strongly influences host response (25-27). Second, most GIT microbiomes appear to share a core set of metabolic activities, most notably the degradation of complex polysaccharides, fermentation of amino acids, and production of short-chain fatty acids (SCFAs) (28, 29). Some of the main sources of food for colonic microbes are resistant starch, nonstarch polysaccharides, host glycans, and dietary protein. The fermentation of starches and nonstarch polysaccharides likely occurs first, predominantly in the cecum and ascending colon. Dietary protein and amino acid fermentation occur after starch exhaustion, mostly in the descending colon (30). Host mucus likely plays a critical role in maintaining a carbohydrate source for microbes during times of starvation or reduced intake of complex polysaccharides (31, 32). SCFAs are exclusively produced by bacteria, and their concentrations can exceed 100 mM in the gut, making them some of the most relevant molecules to host-microbe interactions (30, 33). Two of the best-studied and most important functions of SCFAs are their roles as energy sources for colonocytes and capacity to modulate immune state and barrier function (34).
The time variance of microbiome composition with respect to each method of classification (i.e., taxonomic, metagenomic, or functional) is an important focus of ongoing research. Published data measures temporal variation of the microbiome primarily through taxonomic and metagenomic markers. In these measures, the microbiome varies daily, likely reflecting gut environment differences due to both host and bacterial activity (35, 36). This variance is usually small, with the core set of microbes that comprises most of the mass of the community remaining stable and with low-abundance members appearing or disappearing transiently (37). Substantial changes in the gut environment (e.g., caused by antibiotic usage, inflammatory illness, weight change, or dietary change) can significantly alter the microbiome, producing either a transient perturbed state that relaxes to the previous composition or a new stable composition (35, 38). The factors that produce the bacterial composition of any particular gut are extremely complex and poorly understood. At a high level, diversity is positively correlated with a healthy diet, dietary fiber intake, age, and general good health, and it is negatively correlated with inflammation, dietary intake of simple carbohydrates, and antibiotic use (16, 39-42). Evidence suggests that both dietary protein and microbially accessible carbohydrates strongly influence the composition of the microbiome. Using gnotobiotic mice fed isocaloric diets with different macronutrient contents, Faith et al. (43) determined that the strongest predictor of change from baseline microbial composition was protein content. In humans, these results have been somewhat recapitulated. Omnivores who switched to meat-rich diets experienced substantially larger alterations to their microbiome than those who switched to plant-rich diets (44). In contrast, studies in mice with transplanted human microbial communities suggest that microbially accessible carbohydrates are essential to maintain diversity over generations (39). Controlled feeding experiments in humans have isolated specific high-abundance polysaccharides—notably resistant starch—that are sufficient (necessity is unclear) to alter the abundances of specific high-abundance microbes (45). In total, we do not yet understand the function that maps host and environmental inputs to microbial composition, but we know dietary flux must play a key role.
3.2. Metabolism in an Anaerobic World
The lumen of the distal gastrointestinal tract is deeply anaerobic (46, 47), and metabolic pathways in gut bacteria are different from human metabolism. Indeed, many of these anaerobic processes are ancient, having roots in the origins of life (48, 49). In aerobic environments, molecular oxygen serves as a high-redox-potential electron acceptor, enabling efficient energy capture during substrate oxidation. For example, the complete oxidation of glucose in the presence of oxygen has a Gibbs free energy change of −2,870 kJ/mol, and cells typically capture around 30 ATP molecules/mol of glucose. In the anaerobic environment of the gut, microbes use alternative electron acceptors (i.e., nitrate, sulfate, fumarate, trimethylamine-N-oxide, and other organic molecules) to drive energy-forming reactions (50-54). Anaerobic redox reactions involving these alternative electron acceptors operate much nearer to the thermodynamic limit, and, consequently, anaerobic microbes harvest less energy from growth substrates compared with their aerobic counterparts (typically 2–3 mol ATP molecules/mol of glucose). Gastrointestinal communities are characterized by diverse pathways for energy capture and involve complex syntrophic interactions among phylogenetically diverse microbial members (54, 55). Below, we detail two examples of anaerobic metabolism that are relevant to the production of high-abundance metabolites in the gut.
Butyrate is an SCFA produced by certain genera of human gut bacteria (56-58). An important reason why bacteria make butyrate is to maintain the redox state inside the cell, using butyrate as an electron sink. But these organisms can also generate energy from butyrate-producing pathways. Four unique pathways have been described for butyrate production by gut bacteria, involving the metabolism of pyruvate, glutarate, 4-aminobutyrate, and lysine (59, 60). These pathways converge on a single enzymatic step involving the reduction of crotonyl-CoA to butyryl-CoA by an electron-bifurcating butyryl-CoA dehydrogenase that couples reductive metabolism to the generation of a proton motive force (e.g., anaerobic respiration) (61-63). Additionally, many organisms capture energy during butyrate fermentation from substrate-level phosphorylation using butyrate kinase. Thus, the anaerobic production of butyrate by gut bacteria helps maintain redox balance, and it also contributes to energy stores within the cells. Butyrate is not typically degraded under anaerobic conditions in the gut due to unfavorable thermodynamics that would require slower gut transit times. However, in the oxygenated tissue of the gut, butyrate is rapidly metabolized via β-oxidation and serves as the preferred energy source for the epithelial cells lining the distal GIT (64). This intriguing metabolic hand off between microbe and host illustrates the dichotomy between anaerobic and aerobic metabolic strategies. It also typifies many of the metabolic interactions between gut bacteria and the host, in which a large number of reduced end products of anaerobic metabolism serve as a source of energy for aerobic metabolism in the host.
Syntrophy is a situation in which by combining metabolic functions, two or more organisms carry out a metabolic function that neither could achieve on its own (65, 66). A classic example of this is the situation of the purported organismMethanobacterium omelianskii. Originally isolated by Barker (67) from an enrichment culture of marine mud, the organism catalyzed the oxidation of ethanol to acetate and the conversion of carbon dioxide to methane (67). Following the development of improved anaerobic culture techniques, Bryant and colleagues (68) found that this organism was in fact a coculture of two distinct organisms: a bacterium metabolizing ethanol to acetate and hydrogen and a methanogen that consumed hydrogen and converted carbon dioxide into methane. Hydrogen inhibited the growth of the fermentative bacterium, and by consuming the hydrogen, the methanogen kept the partial pressure of hydrogen low enough to enable the growth of the bacterium. Also known as interspecies hydrogen transfer (69), this mechanism for metabolic cross-feeding across microbes in the human gut is a critical aspect of the normally functioning gut microbial ecosystem (54, 55). In the gut, hydrogenotrophic organisms—such as sulfate-reducing bacteria, acetogens, and methanogens—consume hydrogen, keeping the hydrogen partial pressure low in most individuals, despite an estimated production from carbohydrate metabolism in a typical diet of nearly 14 L (54). Most of the hydrogen is converted to methane, acetate, or hydrogen sulfide by hydrogenotrophic microbes, which are absorbed and either detoxified (e.g., hydrogen sulfide), metabolized peripherally (e.g., acetate), or exhaled in the breath (e.g., methane). Through interspecies hydrogen transfer, hydrogenotrophic microbes in the gut exert considerable influence on the metabolic output of the community. In gnotobiotic mice colonized by Bacteroides thetaiotaomicron, adding the methanogenic archaeon Methanobrevibacter smithii promoted the fermentation of dietary polysaccharides, leading to an increase in serum acetate, liver triglycerides, and fat stores (70).
3.3. Effect of the Microbiome on Circulating Metabolites
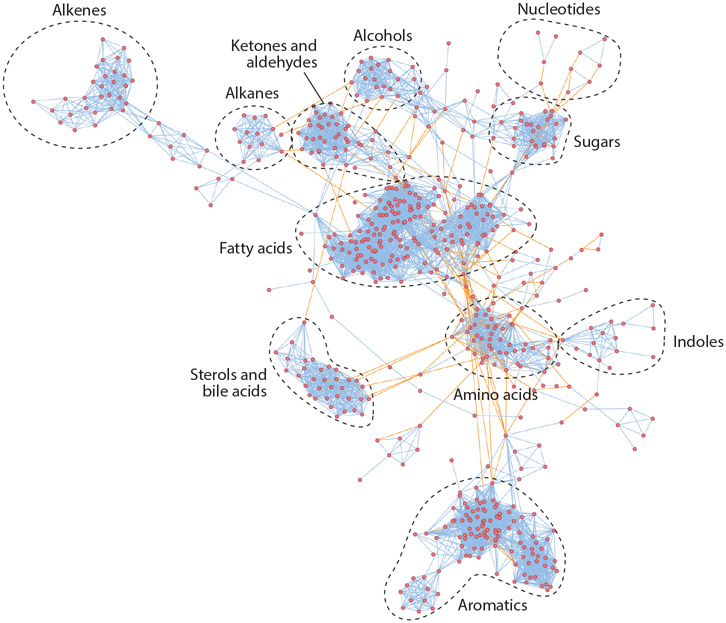
The microbiome impacts numerous aspects of normal human health and disease. One the most concrete ways that the microbiome influences host health is through the production of small molecules. Correspondingly, there has been an explosion of interest in understanding the contribution of gut microbes to the human metabolome. The HMDB lists ~6,000 metabolites that have been identified in human feces (71) (Figure 2). Despite this rich data set, understanding which host metabolites are influenced by the microbiome is complicated by the following key factors: (a) Anaerobic microbial metabolites and pathways are underrepresented in databases; (b) microbial metabolites are absent in some individuals, and they vary by several orders of magnitude in others; (c) microbial metabolites are acted on by host pathways, potentially masking their origin; and (d) diet- and host-derived components that become substrates for microbes are poorly characterized. Despite these limitations, several strategies have been employed by investigators to illuminate the contribution of the gut microbiota to the host metabolome.
Figure 2.
Chemical similarity map of metabolites detected in human feces. Nodes represent metabolites; blue lines represent chemical similarity; orange lines represent KEGG (Kyoto Encyclopedia of Genes and Genomes) reactions. Figure adapted from an image created with MetaMapp (http://metamapp.fiehnlab.ucdavis.edu).
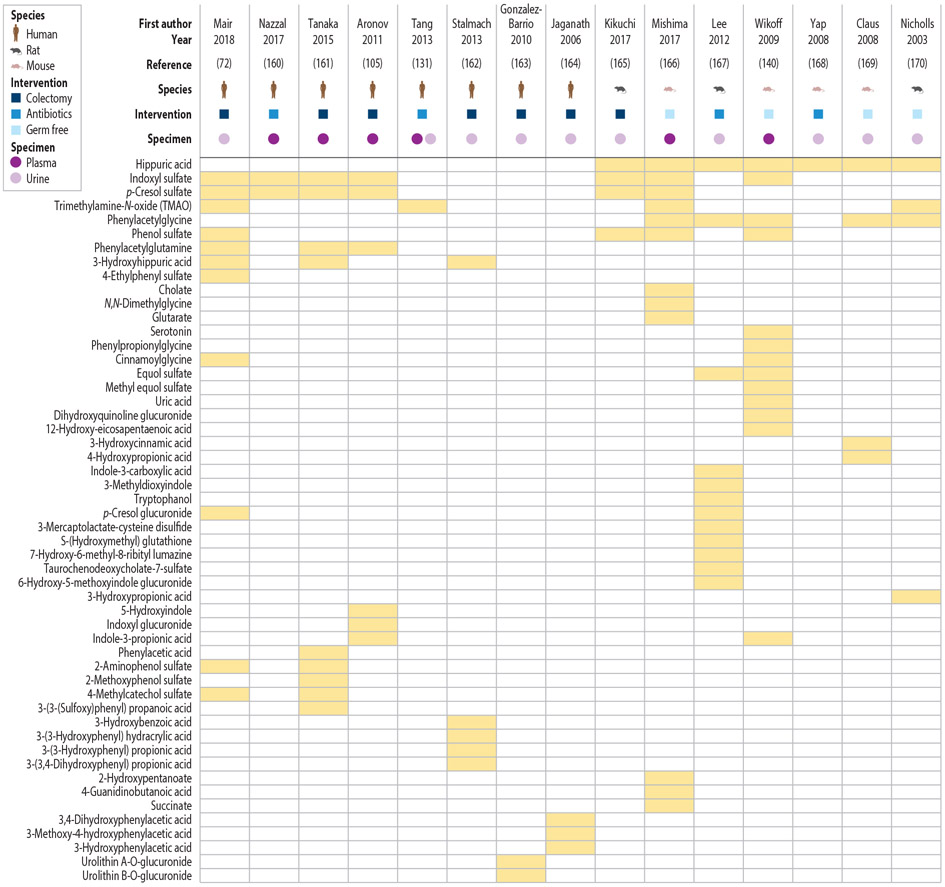
Three strategies are primarily used in studies exploring the role of microbes in the host metabolome: (a) comparison of germ-free rodents with their conventionally housed counterparts, (b) comparison of animal models pre- and post-exposure to antibiotics, and (c) comparison of humans with intact colons with those lacking a colon. Studies that provide high-quality support for the microbial contribution to specific host metabolites are few and far between, making it difficult to comprehensively assess how the microbiome contributes to the host metabolome. A recent study of humans with or without colons identified a large number of new colon-derived solutes and carefully reviewed the literature to summarize what is currently known about microbiota-derived metabolites (72). As of March 2019, the number of named metabolites that circulate in the host and that have been experimentally demonstrated to be influenced by colonic microbes is approximately 52 (data summarized in Figure 3). This is likely a significant underestimation since metabolomics techniques do not comprehensively survey the entire suite of chemical classes. Most notably, microbiota-derived SCFAs and branched-chain fatty acids (BCFAs) are not routinely measured by liquid chromatography–mass spectrometry–based metabolomics. In addition, only a small proportion of the total metabolites in a given metabolomics study can be assigned identities, leading to what some refer to as the “dark matter” of metabolism (73).
Figure 3.
Microbiota-dependent metabolites reported by metabolomics studies. Adapted with permission from Reference 72.
In the following sections, we summarize results that implicate the microbiome in the production of various classes of compounds that contribute to the metabolome.
3.3.1. Short-chain fatty acids.
Although the contribution of SCFAs to the circulating human metabolome is limited, these molecules represent one of the most important host–microbe metabolic interactions. Substrates for the growth of human colonic microbiota include endogenous mucins and glycoproteins, proteins, oligopeptides, and dietary polysaccharides that escape digestion by the host. The major products of microbial fermentation within the human colon are the SCFAs acetate, propionate, and butyrate. Most measurements suggest a ratio of 60:20:20 (acetate:propionate:butyrate) in the gut (74, 75). Collectively, these compounds accumulate to more than 100 mM in the lumen of the distal GIT (74), and nearly 95% of the SCFAs produced are absorbed by the host (76-79). In total, the contribution of SCFAs produced by the microbial metabolism of dietary and endogenous colonic substrates to the energy requirements of humans is estimated to be 6–10% (80, 81).
Acetate and propionate are rapidly absorbed in the colon and transported to the liver via the hepatic portal vein (82). Isotope labeling suggests that acetate is rapidly metabolized and contributes to gluconeogenesis, enters the tricarboxylic acid (or TCA) cycle, and is a substrate for lipogenesis (83, 84). Acetate is metabolized in tissues throughout the body. Conversely, propionate is substantially metabolized in the liver, where it acts as a gluconeogenic substrate. The degree of propionate conversion by the liver is high, but absolute values are unclear; isotope labeling in mice suggests that 62% of gut-derived propionate is converted to glucose (83), while data from human sudden-death victims suggests there is ~30% propionate extraction (69). The distal nature of acetate and propionate metabolism is also supported by data in living surgical patients in whom levels of hepatic SCFA consumption were significantly higher for propionate than acetate or butyrate (85). However, despite the high extraction rate, the absolute contribution of propionate to glucose requirements in humans is likely small, especially in comparison with ruminant animals, in which it may account for more than 50% (86-88).
In contrast, butyrate is the preferred substrate for proximal metabolism. Butyrate is preferentially absorbed and undergoes β-oxidation by colonocytes, a process that consumes oxygen and fuels the colonocyte. Studies in isolated rat intestinal cells show that 70–80% of oxygen utilization by colonocytes is due to β-oxidation of butyrate, and the cells’ uptake rate for butyrate is higher than for any other catabolic substrate tested (89-91). The importance of butyrate metabolism to colonocytes has also been demonstrated: Germ-free mice are chronically deficient in butyrate, and they show impaired metabolism and increased rates of autophagy that can be rescued with the addition of butyrate or butyrate-producing microbes (92). At an organismal level, the consequences of reduced butyrate have been studied for more than 40 years (64), but direct evidence of pathogenesis is limited.
In addition to the stoichiometric contributions of SCFAs to energy capture from the diet, these molecules also modulate aspects of human metabolism through their activity on G protein–coupled receptors, inhibition of histone deacetylase activity, and alterations to fatty acid oxidation and lipolysis (28). Because of the rapid consumption of SCFAs at both proximal and distal sites, the signaling and effector functions of SCFAs may be more important than their contribution to energy stores (84).
3.3.2. Branched-chain fatty acids.
BCFAs are produced by the degradation of the amino acids valine, leucine, and isoleucine. The end products of these pathways include isobutyrate, methylbutyrate, isovalerate, and isocaproate (93, 94). The abundance of these compounds in feces is lower than that of SCFAs, but their concentrations are still physiologically relevant (e.g., in the high micromolar to low millimolar range) (74). Recent studies have revealed that these compounds act locally on cells in the GIT and influence host physiology. For example, isovaleric acid is specifically recognized by chemosensory receptors on enterochromaffin cells in the gut, which, in turn, activate neurons (95). This represents a mechanism by which metabolites produced within the gut can be sensed by the enteric nervous system and alter processes such as gastrointestinal motility. The fate of microbial-derived BCFAs in the host remains largely un-studied. Evidence suggests that BCFAs accumulate in host serum, and in the case of isovaleric acid, its circulating levels are nearly 40 μM (96). It can be assumed that this value reflects a purely microbial contribution because the isovaleric acid intermediate in human leucine metabolism is thought to be sequestered in the mitochondria as isovaleryl-CoA. Human mitochondrial isovaleric acid is not thought to contribute to circulating pools in healthy individuals; however, patients with mutations in isovaleryl-CoA dehydrogenase experience significant isovaleric acidemia (96). Studies have suggested that microbial BCFAs, especially isovaleric acid, are important for cholesterol synthesis (97), modulate mitochondrial β-oxidation of pyruvate (98), and alter lipogenesis in adipocytes (99). Branched-chain amino acid metabolism in the host has been implicated in obesity, metabolic syndrome, and diabetes, and it is intriguing to speculate that BCFAs from the microbiome could influence human metabolism in currently unknown but potentially important ways.
3.3.3. Aromatic acids.
Metabolomics studies aimed at identifying the contribution of the microbiome to the host metabolome have consistently identified numerous aromatic compounds as microbiota dependent (72). Microbiota-dependent aromatic compounds contain indoles, phenols, and phenyl groups (Figure 4). These compounds are subject to extensive host metabolism (e.g., sulfation, glucuronidation, amino acid conjugation), and in many cases, the original bacterial product giving rise to the host metabolite cannot be inferred. Sources for the microbial production of aromatic metabolites include aromatic amino acids present in nondigested protein, mucus, intestinal secretions, and shed epithelial cells (83, 85, 92), as well as dietary polyphenols and plant secondary metabolites derived from fruits, vegetables, coffee, tea, and wine (86-88).
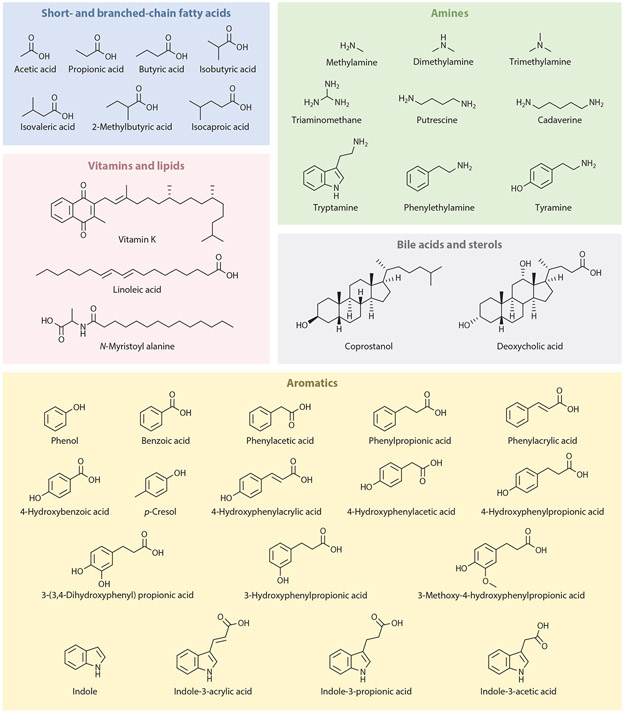
Figure 4.
Classes of metabolites produced by gut bacteria. The gut microbiota produce short-chain fatty acids, branched-chain fatty acids, vitamins, lipids, amines, and aromatics, and modify bile acids and sterols.
The metabolism of aromatic amino acids gives rise to the uremic toxins indoxyl sulfate, p-cresol sulfate (pCS), and phenylacetylglutamine, so called because their levels rise as the kidneys fail (84), and data suggest that they contribute to cardiovascular disease (CVD) in the context of renal insufficiency (100). pCS is a prototype of this class of molecules, discussed in more detail in Section 3.4.1. Indolepropionic acid is a tryptophan metabolite produced by a small subset of gut bacteria, and it has important effects on host gut barrier function. Other tryptophan metabolites, collectively referred to as indoles, appear to have important roles in modulating tissue repair after injury and immune homeostasis in the gut (see Section 3.4.4.).
A number of dietary polyphenolic compounds that are modified by the microbiome have demonstrated antioxidant activity or protection from cancer in cell lines or mouse models (101). While these studies are potentially of interest to human health, the direct connection of these molecules to cancer development in humans has not been established (102). Some of the most abundant urine organic acids found in humans and other mammals (namely, hippuric acid and 3-hydroxyhippuric acid) are thought to be derived from the microbial metabolism of dietary phenolics (103, 104). Recent studies have complicated this picture, however, as colonic microbes appear to be essential for hippuric acid production in mice and rats, but not in humans (72, 105). This suggests that for humans, endogenous sources of hippuric acid may be more important than for rodents. These results also reveal that host-related differences in the metabolism of microbial metabolites are important considerations when generalizing data from animal models to human physiology.
3.3.4. Biogenic amines.
Biogenic amines (BAs) found in humans have endogenous, dietary, and microbial origins, and they mediate a wide variety of biological activities. For example, histamine, an endogenously produced BA, is a potent vasodilator released by degranulating mast cells activated by allergens binding immunoglobulin E receptors. Tyramine is a BA found in cheese that is normally metabolized by monoamine oxidases (MAOs). In patients taking an MAO inhibitor (MAOi) for depression or Parkinson’s disease, the ingestion of large amounts of tyramine can trigger a life-threatening hypertensive crisis (106). Several gut-resident bacteria can produce tyramine as a decarboxylation product of tyrosine, and strains producing and consuming tyramine have been routinely cultured from human feces (107, 108). What effect, if any, the microbial production of tyramine has on the human body is unknown, but the possibility that tyramine might contribute to hypertension (possibly in an MAOi treatment background) is a tantalizing open research issue (109). Spermine, a BA of dietary, endogenous, and bacterial origins, has been implicated in immune homeostasis and the maintenance of a healthy microbiome (110). Fifty percent of the dominant Firmicutes in the gut appear to be auxotrophic for spermine and may rely on cocolonization with spermine-producing bugs (111). Collectively, the microbiota-dependent production of BAs has been recognized for 60 years (112), but the health consequences are still poorly understood.
3.4. Effect of Microbial Metabolites on Health and Disease
The end products of bacterial fermentation in the large intestine are either excreted through feces or absorbed by the host (Figure 5). A critical open question for human microbiology is to understand which molecules are absorbed and how they interact with proximal and distal human tissues. For absorbed compounds there are broadly two fates: energy metabolism or detoxification and excretion. Molecules that are absorbed can be directly metabolized (as discussed for the SCFAs in Section 3.3.1) or they can be detoxified by the host and excreted. Here, we briefly describe chemical modifications catalyzed by the host (detoxification or biotransformation) and then introduce examples of well-studied host–microbe metabolic interactions.
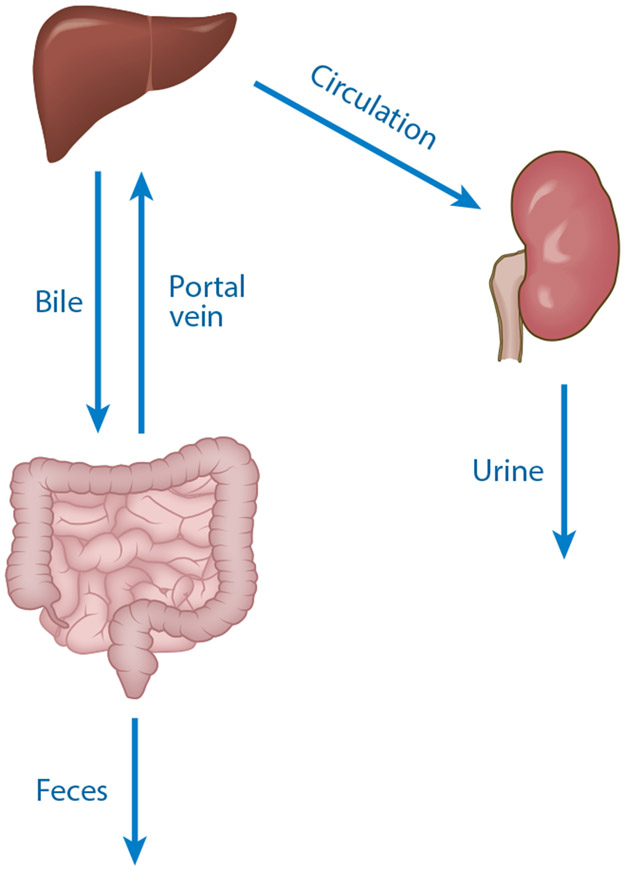
Figure 5.
Routes of absorption and elimination of microbial metabolites. Molecules are produced in the intestines and absorbed into portal circulation. Following metabolism in the liver, compounds are either secreted through the bile back into the intestine (enterohepatic circulation) or they enter the circulation, where they are eliminated in urine by the kidney.
Host modifications to xenobiotics (e.g., pharmaceuticals) that enter circulation have traditionally been called collectively detoxification or biotransformation reactions and divided into phase I or phase II reactions. These reactions operate on diet- and microbe-derived molecules in addition to endogenously produced compounds (e.g., steroids). Both phase I and II enzymes are expressed in tissues across the body, where they fill both biosynthetic and detoxification roles. The expression of these enzymes is highest in the liver (although certain enzymes and isoforms are expressed more highly in the intestinal epithelium, kidney, and other tissues), allowing the body the chance to detoxify ingested metabolites before they reach systemic circulation (so-called first-pass elimination). The primary events in the detoxification of gut microbial metabolites can be described as follows. First, metabolites are absorbed at the apical surface of the intestinal epithelial cells and transported into portal venous circulation. Biotransformation (both phase I and II) occurs in both intestinal and liver cells. The balance of each phase in each location is actively debated (113). At the liver sinusoids, hepatocytes actively and passively transport the metabolites across their basolateral surface. Phase I and II reactions (as well as general metabolism) occur in the cytosol, endoplasmic reticulum, and mitochondria of the hepatocytes. Modified compounds are then returned to either (a) the sinusoidal lumen, where they enter circulation and may be renally cleared, or (b) the canalicular space across the apical membrane, where they drain into the biliary tree and then into the duodenum. Whether a compound is cleared in the kidney or the biliary tree is determined in large part by the substrate specificity of transporters within the polarized hepatocyte (114). Generally, the larger compounds are excreted in bile and smaller compounds enter the circulation, destined to be cleared by the kidneys (115).
The reactions of phase I are predominately intracellular, concentrated in the cytosol and endoplasmic reticulum. These reactions attempt to introduce a reactive polar group to the target molecule (usually a hydroxyl, sulfhydryl, or amine). This can be done through hydrolysis, oxidation, or reduction; all three transformations are common in human phase I metabolism. The most well-known phase I enzymes are the cytochrome P450s, a family of monooxygenases capable of a staggering array of transformations. Additionally, alcohol dehydrogenases, amine oxidases, carboxyesterases, flavin monooxygenases, and others play critical roles for certain classes of compounds.
The phase II enzymes conjugate a hydrophilic group to a target molecule with an exposed functional group (whether preexisting or made available by the activity of a phase I enzyme), increasing water solubility and aiding renal and biliary transport. Similar to phase I enzymes, these enzymes are found throughout the body but concentrated in the liver and kidneys, with the majority of reactions occurring in the cytosol, endoplasmic reticulum, and mitochondria. Two phase II reactions result in an amino acid conjugate of the target molecule. In the first, target carboxylic acids are conjugated to taurine or glycine. In the second, glutathione conjugation results in the production of a thioether linkage between the target and a cysteine. Glutathione conjugates undergo further processing, with cleavage of the glutamic acid and glycine, and the N-acetylation of the remaining cysteine (forming a mercapturic acid) in both the liver and kidneys (116). Three other phase II reactions conjugate non-amino-acid molecules, including sulfates to alcohols and some amines (i.e., sulfation), glucuronic acids to nucleophilic atoms (i.e., oxygen, nitrogen, sulfur; glucuronidation), and acetyl groups to aromatic amines (i.e., acetylation). Sulfation, glucuronidation, acetylation, and methylation require an activated cofactor. In contrast, amino acid conjugations require activation of the target molecule by acetyl-CoA or an aminoacyl-tRNA-synthetase.
Against this background of phase I and II metabolism, we present examples of microbially derived molecules (or chemistries) that interact with host metabolism and physiology.
3.4.1. p-Cresol sulfate.
Chronic kidney disease (CKD) is characterized by a progressive reduction in the glomerular filtration rate of the kidneys, and it is associated with substantial increases in all-cause mortality (117). There are multiple etiologies of CKD, but emerging evidence suggests that the progression of CKD may be potentiated by the microbiome through the production of protein-derived uremic toxins (118-120). One of the best-studied microbe-derived uremic toxins is pCS, a host-microbe cometabolite produced from tyrosine by a limited number of gut bacteria (121, 122). The pathway for p-cresol production begins with the production of p-hydroxyphenylacetic acid, involving first, a transamination, followed by an oxidative decarboxylation. These first two steps are catalyzed by a fairly large number of gut bacteria (123); however, the final step involving p-hydroxyphenylacetate decarboxylase is restricted to a small number of gut bacteria, mainly from two genera, Peptostreptococcus and Clostridium. Once released from microbes, p-cresol is absorbed by the intestine and sulfated (predominantly) or glucuronidated in the liver and intestinalmucosa. Serum pCS circulates bound to albumin, and in dialysis-dependent patients with end-stage renal disease, this protein-bound molecule reaches superphysiological levels (i.e., 10- to 100-fold elevations) (124). Animal models and cell-based assays indicate that at these high concentrations, pCS may contribute to cardiovascular and kidney damage (125).
3.4.2. Irinotecan.
A second example of microbial cometabolism illustrates enterohepatic circulation and its consequences for the host. Irinotecan is an anticancer agent that is taken as an intravenous prodrug. After administration, it is cleaved by a phase I carboxylesterase to produce the active form of the drug (called SN-38), with some portion being inactivated by glucuronidation (SN-38G). The SN-38G is excreted in bile, and following intestinal transit, bacterial β-glucuronidases (BGs) cleave the glucuronide and produce active SN-38. In the intestine, microbially activated SN-38 causes dose-limiting diarrhea in up to 40% of patients. In an attempt to reduce the GIT toxicity of this drug without using broad spectrum antibiotics, Wallace et al. (126) designed a BG inhibitor that is active against gut-resident microbes. In amousemodel, coad-ministration of this BG inhibitor with irinotecan substantially ameliorated treatment-associated diarrhea. Therefore, the specific inhibition of microbial enzymes may be a valuable strategy to limit side effects and improve the therapeutic index of certain drugs (127).
3.4.3. Trimethylamine-N-oxide.
Trimethylamine (TMA) enters the body either through the consumption of fish (high in TMA) or by microbiota-dependent conversion of choline and carnitine from dietary sources. The pathway for choline conversion involves the enzyme choline TMA-lyase, encoded by the cutC and cutD genes of anaerobic bacteria (128). The resulting TMA is rapidly absorbed and converted to trimethylamine-N-oxide (TMAO) by liver flavin monooxygenases (129); then, it enters the circulation destined for renal excretion. Bacteria-dependent TMA production in the gut (and its relationship to kidney disease) has been recognized since the 1970s (130), but there has been renewed focus on TMA recently, with a wealth of studies implicating its role in CVD.
Epidemiological studies have revealed a strong association between serum TMAO levels and CVD (131, 132). A comprehensive meta-analysis of studies of TMAO(19,256 participants over all studies) and major adverse cardiovascular events (MACE) showed a relative risk ratio of MACE of 1.63 for high serum levels of TMAO compared with low serum levels (133). Due to methodological differences in the studies, the classification of elevated TMAO ranged almost 20-fold, but a sensitivity analysis showed that the general conclusion—high TMAO is correlated withMACE—was quite robust across all studies. In addition, the serum levels of TMA precursors, including choline and carnitine, had a relative risk ratio slightly under that of TMA (range, 1.3 to 1.4), even after adjusting for comorbidities, suggesting either unexplored covariates or a toxicant effect of TMAO occurring below detection limits. There is debate about the effects of these precursors, however, as several recent studies that included a large number of participants failed to find risks associated with precursor abundance in serum (134).
It is still unclear whether TMAO is causal or just an excellent marker for CVD (135). At the molecular level, TMAO appears to be an important toxicant, but the cellular receptor or target for TMAO is unknown (134). In mouse models of dyslipidemia, TMAO has demonstrated a proatherogenic effect, increasing foam cell deposition, inflammation in arterial endothelia, leukocyte adhesion, inflammasome activation, and mitogen-activated protein kinase induction (136, 137). Data from mouse and human studies also show that TMAO is associated with thrombosis risk and enhances platelet aggregation (138, 139). However, a few important questions remain unanswered regarding the causality of TMAO in CVD, and these warrant further investigation: (a) Why do populations who consume large amounts of fish (high in TMA) not have increased risk for CVD? (b) Since TMAO is renally cleared and its levels rise as the kidneys fail, could elevated TMAO be a result of decreased kidney function due to renal atherosclerosis rather than a cause of atherosclerosis? Given how the story of TMAO and CVD has captivated physicians, nutritionists, and microbiologists alike, there is no doubt that future studies will seek to address these questions.
3.4.4. Indoles.
Indolepropionic acid (IPA) is a tryptophan derivative that circulates in the blood of mammals; its synthesis depends on gut bacteria (140). IPA has potent radical scavenging activity (141) and has received considerable attention for its neuroprotective properties. Recently, a study demonstrated that IPA specifically engages the pregnane X receptor (PXR), leading to the upregulation of genes that regulate intestinal permeability and to the downregulation of tumor necrosis factor (TNF)-α expression by enterocytes (142). The addition of an IPA-producing commensal bacterium (C. sporogenes) to antibiotic-treated mice replenished plasma IPA levels and reduced indomethacin-mediated colitis in a PXR-dependent manner (142). These results indicate that IPA is a commensal-derived small molecule that modulates the mucosal immune system and represents a potentially important therapeutic target for inflammatory bowel disease. The biosynthetic machinery for IPA synthesis is encoded by a discrete gene cluster that is required for reductive metabolism of all three aromatic amino acids (25). In one study, toggling IPA on and off in gnotobiotic mice enabled modulation of gastrointestinal permeability in the mice and altered systemic immune cell profiles (25).
Several othermicrobially derived indoles are characterized by their ability to engage the arylhydrocarbon receptor (AhR). These molecules include indole, indoxyl sulfate, indoleacetic acid, in-dolecarboxaldehyde, indoleacetaldehyde, 3-methylindole, and tryptamine. The activation of AhR by these molecules promotes tissue repair and homeostasis involving interleukin (IL)-22 (143). Recently, several studies have revealed that in the gut microbiota of patients with inflammatory bowel disease, the levels of several AhR ligands, including indoleacetic acid, are decreased, and this decrease correlates with a decreased activation of AhR in these patients (144). Thus, results indicate that these commensal-derived small molecules modulate the mucosal immune system, and they represent a potentially important therapeutic target for inflammatory bowel disease that functions independently of IPA and PXR.
3.5. Promoting Health Through the Gut Microbiota
Increasingly, functional studies of the microbiome are revealing the specific effects that metabolites have on human health and disease. As knowledge of these molecules expands and biological mechanisms are elucidated, a new set of therapeutic targets will emerge. We envision that precision health strategies will entail measuring a person’s metabolome and coupling these data with species-level analysis of the microbiome. These data will then guide strategies to harness the relative plasticity of the microbiome to modulate microbial metabolites and promote health. Already, investigators are developing strategies to control microbial metabolites including (a) altering substrate availability for microbial metabolism, (b) modulating species composition through the diet, (c) using the so-called drugging-the-bug approaches, (d) developing designer probiotics, (e) assembling microbial consortia, and (f) performing fecal microbiota transplants. These strategies exist on a continuum of molecular specificity, ranging from drugging-the-bug approaches that target perhaps a single enzyme in a single microbe to fecal transplants that replace a large portion of the metabolic activity of a microbiome. Finding the strategies that work will depend on knowledge of the metabolism of both microbe and host. This is particularly relevant for host-modified compounds because those that control metabolite flux in animal models may not do so in humans due to differing phase I and II metabolism (see Section 3.3.3). There are a few strategies for which there are compelling data showing that the modulation of microbial metabolism can be used to treat disease.
In the context of TMAO production (see Section 3.4.3.), two separate groups have reported that inhibitors of choline TMA-lyase (145-147) show in vitro activity on TMA-producing microbes, on fecal suspensions, and in animal models of CVD and thrombosis. By reducing microbial TMAO production, these compounds could potentially reduce adverse cardiovascular events in CVD patients with elevated TMAO levels. An advantage to this drugging-the-bug approach is that the inhibitors function selectively on the pathway of interest, and unlike broad spectrum antibiotics, they would leave other microbes in the community intact.
Indoxyl sulfate is a uremic toxin derived from microbial indole, and it accumulates at high levels in patients with deteriorating renal function. Devlin et al. (148) used the gnotobiotic mouse model as a proof of concept that indole-producing microbes could be modulated through diet or through the administration of probiotics. Administering polysaccharides that improve the fitness of a non-indole-producing bacterial strain brought urinary indoxyl sulfate levels down. Strategies to modulate indoxyl sulfate levels were also developed in the context of a conventional mouse microbiota, indicating that it is possible to reprogram indoxyl sulfate levels in a complex microbiota.
Synthetic biologists are tackling several IEMs by harnessing the gut microbiome. Two recent papers from Synlogic report animal studies and human safety studies for products designed to treat phenylketonuria and hyperammonemia (149, 150). Using Escherichia coli as a scaffold, this group engineered entire pathways (including transporters, regulators, and catalytic enzymes) for the metabolism of phenylalanine to trans-cinnamic acid (for phenylketonuria) or ammonia to arginine (for hyperammonemia). As a biocontainment strategy, these strains were engineered to be auxotrophic for thymidine. Oral administration of synthetic bacteria led to dose-dependent decreases in target molecules (ammonia or phenylalanine) in mouse models of metabolic disease. Furthermore, when administered to healthy humans, these probiotics were generally well tolerated, with side effects at the highest doses including mild-to-moderate nausea and vomiting. These studies demonstrate the power of harnessing microbial metabolism in the gut to treat human metabolic diseases.
ACKNOWLEDGMENTS
We thank our colleagues at Stanford, especially Michael Fischbach, Robert Mair, Tim Meyer, Tammy Sirich, and Justin Sonnenburg for their encouragement and discussions. The writing of this manuscript was made possible in part by a grant from the US National Institutes of Health (DK110335 to D.D.).
Footnotes
DISCLOSURE STATEMENT
D.D. is a cofounder of Federation Bio, a microbiome therapeutics company.
LITERATURE CITED
- 1.Jaffe M 1877. Ueber die Ausscheidung des Indicans unter physiologischen und pathologischen Verhält-nissen. Virchow’s Arch. 70:72–111 [Google Scholar]
- 2.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, et al. 2011. The human serum metabolome. PLOS ONE 6:e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gates SC, Sweeley CC, Krivit W, DeWitt D, Blaisdell BE. 1978. Automated metabolic profiling of organic acids in human urine. II. Analysis of urine samples from “healthy” adults, sick children, and children with neuroblastoma. Clin. Chem 24:1680–89 [PubMed] [Google Scholar]
- 4.Gates SC, Dendramis N, Sweeley CC. 1978. Automated metabolic profiling of organic acids in human urine. I. Description of methods. Clin. Chem 24:1674–79 [PubMed] [Google Scholar]
- 5.Zlatkis A, Liebich HM. 1971. Profile of volatile metabolites in human urine. Clin. Chem 17:592–94 [PubMed] [Google Scholar]
- 6.Horning EC, Horning MG. 1971. Metabolic profiles: gas-phase methods for analysis of metabolites. Clin. Chem 17:802–9 [PubMed] [Google Scholar]
- 7.Mamer OA, Crawhall JC, Tjoa SS. 1971. The identification of urinary acids by coupled gas chromatography–mass spectrometry. Clin. Chim. Acta 32:171–84 [DOI] [PubMed] [Google Scholar]
- 8.Crawhall JC, Mamer O, Tjoa S, Claveau JC. 1971. Urinary phenolic acids in tyrosinemia. Identification and quantitation by gas chromatography-mass spectrometry. Clin. Chim. Acta 34:47–54 [DOI] [PubMed] [Google Scholar]
- 9.Pasquali M, Longo N. 2018. Newborn screening and inborn errors of metabolism In Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, ed. Rifai N, pp. 1697–730. St. Louis, Mo: Elsevier; 6th ed. [Google Scholar]
- 10.Rinaldo P, Cowan TM,Matern D. 2008. Acylcarnitine profile analysis. Genet. Med 10:151–56 [DOI] [PubMed] [Google Scholar]
- 11.Nyhan WL, Barshop BA, Al-Aqeel AI, Hoffmann GF. 2012. Atlas of Inherited Metabolic Diseases. London, UK: Hodder Arnold [Google Scholar]
- 12.Kumps A, Duez P, Mardens Y. 2002Metabolic, nutritional, iatrogenic, and artifactual sources of urinary organic acids: a comprehensive table. Clin. Chem 48:708–17 [PubMed] [Google Scholar]
- 13.Dridi B, Raoult D, Drancourt M. 2011. Archaea as emerging organisms in complex human microbiomes. Anaerobe 17:56–63 [DOI] [PubMed] [Google Scholar]
- 14.Sender R, Fuchs S, Milo R. 2016. Revised estimates for the number of human and bacteria cells in the body. PLOS Biol. 14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinha R, Abu-Ali G, Vogtmann E, Fodor AA, Ren B, et al. 2017. Assessment of variation in microbial community amplicon sequencing by the Microbiome Quality Control (MBQC) project consortium. Nat. Biotechnol 35:1077–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, et al. 2012. Human gut microbiome viewed across age and geography. Nature 486:222–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, et al. 2011. Enterotypes of the human gut microbiome. Nature 473:174–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hum. Microbiome Proj. Consort. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, et al. 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savage DC. 1977. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol 31:107–33 [DOI] [PubMed] [Google Scholar]
- 21.Walter J, Ley R. 2011. The human gut microbiome: ecology and recent evolutionary changes. Annu. Rev. Microbiol 65:411–29 [DOI] [PubMed] [Google Scholar]
- 22.Heintz-Buschart A, Wilmes P.2018. Human gut microbiome: function matters. Trends Microbiol. 26:563–74 [DOI] [PubMed] [Google Scholar]
- 23.Oren A, Garrity GM. 2014. Then and now: a systematic review of the systematics of prokaryotes in the last 80 years. Antonie Van Leeuwenhoek 106:43–56 [DOI] [PubMed] [Google Scholar]
- 24.Belkaid Y, Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell 157:121–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, et al. 2017A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551:648–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwabe RF,Jobin C. 2013. The microbiome and cancer. Nat. Rev. Cancer 13:800–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnabl B, Brenner DA. 2014. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146:1513–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. 2016. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165:1332–45 [DOI] [PubMed] [Google Scholar]
- 29.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. 2012. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3:289–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings JH, Macfarlane GT. 1991. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol 70:443–59 [DOI] [PubMed] [Google Scholar]
- 31.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, et al. 2016A dietary fiber–deprived gutmicrobiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167:1339–53.e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Earle KA, Billings G, Sigal M, Lichtman JS, Hansson GC, et al. 2015. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe 18:478–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macfarlane GT, Gibson GR, Cummings JH. 1992. Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol 72:57–64 [DOI] [PubMed] [Google Scholar]
- 34.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, et al. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504:446–50 [DOI] [PubMed] [Google Scholar]
- 35.David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, et al. 2014. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 15:R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flores GE, Caporaso JG, Henley JB, Rideout JR, Domogala D, et al. 2014. Temporal variability is a personalized feature of the human microbiome. Genome Biol. 15:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, et al. 2013. The long-term stability of the human gut microbiota. Science 341:1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. 2012. The application of ecological theory toward an understanding of the human microbiome. Science 336:1255–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. 2016. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529:212–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dethlefsen L, Relman DA. 2011. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. PNAS 108(Suppl. 1):4554–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, et al. 2014. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15:382–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walters WA, Xu Z, Knight R. 2014. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 588:4223–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faith JJ, McNulty NP, Rey FE, Gordon JI. 2011. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science 333:101–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, et al. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, et al. 2011. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 5:220–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman ES, Bittinger K, Esipova TV, Hou L, Chau L, et al. 2018. Microbes versus chemistry in the origin of the anaerobic gut lumen. PNAS 115:4170–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albenberg L, Esipova TV,Judge CP, Bittinger K, Chen J, et al. 2014. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 147:1055–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Vladar HP. 2012. Amino acid fermentation at the origin of the genetic code. Biol. Direct 7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canfield DE, Rosing MT, Bjerrum C. 2006. Early anaerobic metabolisms. Philos. Trans. R. Soc. Lond. B 361:1819–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischbach MA, Sonnenburg JL. 2011. Eating for two: how metabolism establishes interspecies interactions in the gut. CellHostMicrobe 10:336–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoyles L, Jimenez-Pranteda ML, Chilloux J, Brial F, Myridakis A,et al. 2018Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome 6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiso M, Schechter AN. 2015. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLOS ONE 10:e0119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, et al. 2013. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339:708–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura N, Lin HC, McSweeney CS, Mackie RI, Gaskins HR. 2010. Mechanisms of microbial hydrogen disposal in the human colon and implications for health and disease. Annu. Rev. Food Sci. Technol 1:363–95 [DOI] [PubMed] [Google Scholar]
- 55.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. 2008. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat. Rev. Microbiol 6:121–31 [DOI] [PubMed] [Google Scholar]
- 56.Barcenilla A, Pryde SE,Martin JC, Duncan SH, Stewart CS, et al. 2000. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol 66:1654–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Louis P, Flint HJ. 2009. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett 294:1–8 [DOI] [PubMed] [Google Scholar]
- 58.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. 2002. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett 217:133–39 [DOI] [PubMed] [Google Scholar]
- 59.Vital M, Howe AC, Tiedje JM. 2014. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 5:e00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anand S, Kaur H, Mande SS. 2016. Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Front. Microbiol 7:1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chowdhury NP, Mowafy AM, Demmer JK, Upadhyay V, Koelzer S, et al. 2014. Studies on the mechanism of electron bifurcation catalyzed by electron transferring flavoprotein (Etf) and butyryl-CoA dehydrogenase (Bcd) of Acidaminococcus fermentans. J. Biol. Chem 289:5145–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herrmann G, Jayamani E, Mai G, Buckel W. 2008. Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J. Bacteriol 190:784–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buckel W, Thauer RK. 2013. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim. Biophys. Acta Bioenerg 1827:94–113 [DOI] [PubMed] [Google Scholar]
- 64.Roediger WE. 1980. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 21:793–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sieber JR, McInerney MJ, Gunsalus RP. 2012. Genomic insights into syntrophy: the paradigm for anaerobic metabolic cooperation. Annu. Rev. Microbiol 66:429–52 [DOI] [PubMed] [Google Scholar]
- 66.Morris BE, Henneberger R, Huber H, Moissl-Eichinger C. 2013Microbial syntrophy: interaction for the common good. FEMS Microbiol. Rev 37:384–406 [DOI] [PubMed] [Google Scholar]
- 67.Barker HA. 1939. Studies upon the methane fermentation. IV The isolation and culture ofMethanobacterium Omelianskii. Antonie Van Leeuwenhoek 6:201–20 [Google Scholar]
- 68.Bryant MP, Wolin EA, Wolin MJ, Wolfe RS. 1967. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch. Mikrobiol 59:20–31 [DOI] [PubMed] [Google Scholar]
- 69.Iannotti EL,Kafkewitz D, Wolin MJ, Bryant MP. 1973. Glucose fermentation products in Ruminococcus albus grown in continuous culture with Vibrio succinogenes: changes caused by interspecies transfer of H2. J. Bacteriol 114:1231–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samuel BS, Gordon JI. 2006. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. PNAS 103:10011–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karu N, Deng L, Slae M, Guo AC, Sajed T, et al. 2018A review on human fecal metabolomics: methods, applications and the Human Fecal Metabolome Database. Anal. Chim. Acta 1030:1–24 [DOI] [PubMed] [Google Scholar]
- 72.Mair RD, Sirich TL, Plummer NS, Meyer TW. 2018. Characteristics of colon-derived uremic solutes. Clin. J. Am. Soc. Nephrol 13:1398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.da Silva RR, Dorrestein PC, Quinn RA. 2015. Illuminating the dark matter in metabolomics. PNAS 112:12549–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. 1987. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28:1221–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.den Besten G, Lange K, Havinga R, van Dijk TH, Gerding A,et al. 2013. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol 305:G900–10 [DOI] [PubMed] [Google Scholar]
- 76.Topping DL, Clifton PM. 2001. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev 81:1031–64 [DOI] [PubMed] [Google Scholar]
- 77.Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG Jr. 1980. Absorption of short-chain fatty acids by the colon. Gastroenterology 78:1500–7 [PubMed] [Google Scholar]
- 78.Dawson AM, Holdsworth CD, Webb J. 1964. Absorption of short chain fatty acids in man. Proc. Soc. Exp. Biol. Med 117:97–100 [DOI] [PubMed] [Google Scholar]
- 79.Rechkemmer G, Ronnau K, von Engelhardt W. 1988. Fermentation of polysaccharides and absorption of short chain fatty acids in the mammalian hindgut. Comp. Biochem. Physiol. A 90:563–68 [DOI] [PubMed] [Google Scholar]
- 80.Bergman EN. 1990. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev 70:567–90 [DOI] [PubMed] [Google Scholar]
- 81.McNeil NI. 1984. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr 39:338–42 [DOI] [PubMed] [Google Scholar]
- 82.Skutches CL, Holroyde CP, Myers RN, Paul P, Reichard GA. 1979. Plasma acetate turnover and oxidation. J. Clin. Investig 64:708–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adibi SA, Mercer DW. 1973. Protein digestion in human intestine as reflected in luminal, mucosal, and plasma amino acid concentrations after meals. J. Clin. Investig 52:1586–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyer TW, Hostetter TH. 2012. Uremic solutes from colon microbes. Kidney Int. 81:949–54 [DOI] [PubMed] [Google Scholar]
- 85.Cummings JH, Hill MJ, Bone ES, Branch WJ, Jenkins DJ. 1979. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am. J. Clin. Nutr 32:2094–101 [DOI] [PubMed] [Google Scholar]
- 86.Ross JA, Kasum CM. 2002. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr 22:19–34 [DOI] [PubMed] [Google Scholar]
- 87.Gonthier MP, Cheynier V, Donovan JL, Manach C, Morand C, et al. 2003. Microbial aromatic acid metabolites formed in the gut account for a major fraction of the polyphenols excreted in urine of rats fed red wine polyphenols. J. Nutr 133:461–67 [DOI] [PubMed] [Google Scholar]
- 88.Clifford MN. 1999. Chlorogenic acids and other cinnamates—nature, occurrence and dietary burden. J. Sci. Food Agric 79:362–72 [Google Scholar]
- 89.Scheppach W 1994. Effects of short chain fatty acids on gut morphology and function. Gut 35(Suppl.):S35–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fleming SE, Fitch MD, DeVries S, Liu ML, Kight C. 1991. Nutrient utilization by cells isolated from rat jejunum, cecum and colon. J. Nutr 121:869–78 [DOI] [PubMed] [Google Scholar]
- 91.Roediger WE. 1982. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 83:424–29 [PubMed] [Google Scholar]
- 92.Chacko A, Cummings JH. 1988. Nitrogen losses from the human small bowel: obligatory losses and the effect of physical form of food. Gut 29:809–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith EA, Macfarlane GT. 1997. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe 3:327–37 [DOI] [PubMed] [Google Scholar]
- 94.Elsden SR, Hilton MG. 1978. Volatile acid production from threonine, valine, leucine and isoleucine by Clostridia. Arch. Microbiol 117:165–72 [DOI] [PubMed] [Google Scholar]
- 95.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, et al. 2017. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170:185–98.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jakobsdottir G, Bjerregaard JH, Skovbjerg H, Nyman M. 2013. Fasting serum concentration of short-chain fatty acids in subjects with microscopic colitis and celiac disease: no difference compared with controls, but between genders. Scand. J. Gastroenterol 48:696–701 [DOI] [PubMed] [Google Scholar]
- 97.Zabin I, Bloch K. 1951. Studies on the utilization of isovaleric acid in cholesterol synthesis. J. Biol. Chem 192:267–73 [PubMed] [Google Scholar]
- 98.Gregersen N 1979. Studies on the effects of saturated and unsaturated short-chain monocarboxylic acids on the energy metabolism of rat liver mitochondria. Pediatr. Res 13:1227–30 [DOI] [PubMed] [Google Scholar]
- 99.Heimann E, Nyman M, Palbrink AK, Lindkvist-Petersson K, Degerman E. 2016. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 5:359–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mair RD, Sirich TL, Meyer TW. 2018. Uremic toxin clearance and cardiovascular toxicities. Toxins 10:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bultman SJ. 2016. The microbiome and its potential as a cancer preventive intervention. Semin. Oncol 43:97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cardona F, Andres-Lacueva C,Tulipani S,Tinahones FJ, Queipo-Ortuno MI. 2013. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem 24:1415–22 [DOI] [PubMed] [Google Scholar]
- 103.Gonthier MP, Verny MA, Besson C, Remesy C, Scalbert A. 2003. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr 133:1853–59 [DOI] [PubMed] [Google Scholar]
- 104.Rechner AR, Kuhnle G, Bremner P, Hubbard GP, Moore KP, Rice-Evans CA. 2002. The metabolic fate of dietary polyphenols in humans. Free Radic. Biol. Med 33:220–35 [DOI] [PubMed] [Google Scholar]
- 105.Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S,et al. 2011. Colonic contribution to uremic solutes. J. Am. Soc. Nephrol 22:1769–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown C, Taniguchi G, Yip K. 1989. The monoamine oxidase inhibitor-tyramine interaction. J. Clin. Pharmacol 29:529–32 [DOI] [PubMed] [Google Scholar]
- 107.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, et al. 2015. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161:264–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pugin B, Barcik W, Westermann P, Heider A, Wawrzyniak M, et al. 2017. A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microb. Ecol. Health Dis 28:1353881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aydin S, Ugur K, Aydin S. 2018. Could excessive production of tyramine by the microbiota be a reason for essential hypertension? Biosci. Microbiota Food Health 37:77–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Levy M, Thaiss CA, Zeevi D, Dohnalova L, Zilberman-Schapira G, et al. 2015. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 163:1428–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hanfrey CC, Pearson BM, Hazeldine S, Lee J, Gaskin DJ, et al. 2011. Alternative spermidine biosynthetic route is critical for growth of Campylobacter jejuni and is the dominant polyamine pathway in human gut microbiota. J. Biol. Chem 286:43301–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perry TL, Hestrin M, MacDougall L, Hansen S. 1966. Urinary amines of intestinal bacterial origin. Clin. Chim. Acta 14:116–23 [DOI] [PubMed] [Google Scholar]
- 113.Lin JH, Chiba M, Baillie TA. 1999. Is the role of the small intestine in first-pass metabolism overem-phasized? Pharmacol. Rev 51:135–58 [PubMed] [Google Scholar]
- 114.Treyer A,Musch A. 2013. Hepatocyte polarity. Compr. Physiol 3:243–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Faber KN,Muller M, Jansen PL. 2003. Drug transport proteins in the liver. Adv. Drug Deliv. Rev 55:107–24 [DOI] [PubMed] [Google Scholar]
- 116.Hinchman CA, Ballatori N. 1994. Glutathione conjugation and conversion to mercapturic acids can occur as an intrahepatic process. J. Toxicol. Environ. Health 41:387–409 [DOI] [PubMed] [Google Scholar]
- 117.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. 2004. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med 351:1296–305 [DOI] [PubMed] [Google Scholar]
- 118.Ramezani A, Raj DS. 2014. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol 25:657–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Niwa T 1996. Organic acids and the uremic syndrome: proteinmetabolite hypothesis in the progression of chronic renal failure. Semin. Nephrol 16:167–82 [PubMed] [Google Scholar]
- 120.Evenepoel P, Meijers BK, Bammens BR, Verbeke K. 2009. Uremic toxins originating from colonic microbial metabolism. Kidney Int. 76(Suppl. 114):S12–19 [DOI] [PubMed] [Google Scholar]
- 121.Saito Y, Sato T, Nomoto K, Tsuji H. 2018. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol. Ecol 94:fiy125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Selvaraj B, Buckel W, Golding BT, Ullmann GM, Martins BM. 2016. Structure and function of 4-hydroxyphenylacetate decarboxylase and its cognate activating enzyme. J. Mol. Microbiol. Biotechnol 26:76–91 [DOI] [PubMed] [Google Scholar]
- 123.Russell WR, Duncan SH, Scobbie L, Duncan G, Cantlay L, et al. 2013Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol. Nutr. Food Res 57:523–35 [DOI] [PubMed] [Google Scholar]
- 124.Gryp T, Vanholder R, Vaneechoutte M, Glorieux G. 2017p-Cresyl sulfate. Toxins (Basel) 9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. 2014. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J. Am. Soc. Nephrol 25:1897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wallace BD, Wang H, Lane KT, Scott JE, Orans J, et al. 2010. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330:831–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spanogiannopoulos P, Bess EN, Carmody RN, Turnbaugh PJ. 2016. The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat. Rev. Microbiol 14:273–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Craciun S, Balskus EP 2012. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. PNAS 109:21307–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, et al. 2013. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 17:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Simenhoff ML, Saukkonen JJ, Burke JF, Wesson LG, Schaedler RW. 1976. Amine metabolism and the small bowel in uraemia. Lancet 2:818–21 [DOI] [PubMed] [Google Scholar]
- 131.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, et al. 2013. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med 368:1575–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tang WH, Kitai T, Hazen SL. 2017. Gut microbiota in cardiovascular health and disease. Circ. Res 120:1183–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heianza Y,Ma W, Manson JE, Rexrode KM, Qi L. 2017. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J. Am. Heart Assoc 6:e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brown JM, Hazen SL. 2018. Microbial modulation of cardiovascular disease. Nat. Rev. Microbiol 16:171–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zeisel SH, Warrier M. 2017. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu. Rev. Nutr 37:157–81 [DOI] [PubMed] [Google Scholar]
- 136.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, et al. 2011. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, et al. 2016. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Heart Assoc 5:e002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhu W, Wang Z, Tang WHW, Hazen SL. 2017. Gut microbe-generated trimethylamine N-oxide from dietary choline is prothrombotic in subjects. Circulation 135:1671–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, et al. 2016. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165:111–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, et al. 2009. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. PNAS 106:3698–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, et al. 1999. Potent neuroprotective properties against the Alzheimer β-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J. Biol. Chem 274:21937–42 [DOI] [PubMed] [Google Scholar]
- 142.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, et al. 2014. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41:296–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, et al. 2013. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39:372–85 [DOI] [PubMed] [Google Scholar]
- 144.Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, et al. 2016. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med 22:598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, et al. 2018. Development of a gut microbe–targeted nonlethal therapeutic to inhibit thrombosis potential. Nat. Med 24:1407–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, et al. 2015. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163:1585–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Orman M, Bodea S, Funk MA, Campo AM, Bollenbach M, et al. 2018. Structure-guided identification of a small molecule that inhibits anaerobic choline metabolism by human gut bacteria. J. Am. Chem. Soc 141:33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Devlin AS,Marcobal A, Dodd D,Nayfach S, Plummer N,et al. 2016Modulation of a circulating uremic solute via rational genetic manipulation of the gut microbiota. Cell Host Microbe 20:709–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kurtz CB, Millet YA, Puurunen MK, Perreault M, Charbonneau MR, et al. 2019. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci. Transl. Med 11:eaau7975. [DOI] [PubMed] [Google Scholar]
- 150.Isabella VM, Ha BN, Castillo MJ, Lubkowicz DJ, Rowe SE, et al. 2018. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat. Biotechnol 36:857–64 [DOI] [PubMed] [Google Scholar]
- 151.Lagerlöf F, Dawes C. 1984. The volume of saliva in the mouth before and after swallowing. J. Dent. Res 198463:618–21 [DOI] [PubMed] [Google Scholar]
- 152.Gibbons RJ, Houte JV 1975. Bacterial adherence in oral microbial ecology. Annu. Rev. Microbiol 29:19–42 [DOI] [PubMed] [Google Scholar]
- 153.Liégeois F, Albert A, Limme M. 2010. Comparison between tongue volume from magnetic resonance images and tongue area from profile cephalograms. Eur.J. Orthod 32:381–86 [DOI] [PubMed] [Google Scholar]
- 154.Collins LM, Dawes C. 1987. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J. Dent. Res 66:1300–2 [DOI] [PubMed] [Google Scholar]
- 155.Schiller C, Fröhlich CP, Giessmann T, Siegmund W, Mönnikes H, et al. 2005. Intestinal fluid volumes and transit of dosage forms as assessed bymagnetic resonance imaging. Aliment. Pharmacol. Ther 22:971–79 [DOI] [PubMed] [Google Scholar]
- 156.DeSesso JM, Jacobson CF. 2001. Anatomical and physiological parameters affecting gastrointestinal absorption in humans and rats. Food Chem. Toxicol 39:209–28 [DOI] [PubMed] [Google Scholar]
- 157.Pritchard SE,Marciani L, Garsed KC, Hoad CL, Thongborisute W, et al. 2014. Fasting and postprandial volumes of the undisturbed colon: normal values and changes in diarrhea-predominant irritable bowel syndrome measured using serial MRI. Neurogastroenterol. Motil 26:124–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sadahiro S, Ohmura T, Yamada Y, Saito T, Taki Y. 1992. Analysis of length and surface area of each segment of the large intestine according to age, sex and physique. Surg. Radiol. Anat 14:251–57 [DOI] [PubMed] [Google Scholar]
- 159.Int. Commis. Radiol. Prot. 1975. Report of the Task Group on Reference Man Oxford: Pergamon Press [Google Scholar]
- 160.Nazzal L, Roberts J, Singh P, Jhawar S, Matalon A, et al. 2017. Microbiome perturbation by oral vancomycin reduces plasma concentration of two gut-derived uremic solutes, indoxyl sulfate and p-cresyl sulfate, in end-stage renal disease. Nephrol. Dial. Transplant 32:1809–17 [DOI] [PubMed] [Google Scholar]
- 161.Tanaka H, Sirich TL, Plummer N S, Weaver DS,Meyer TW. 2015. An enlarged profile of uremic solutes. PLOS ONE 10:e0135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stalmach A, Edwards CA, Wightman JD, Crozier A. 2013. Colonic catabolism of dietary phenolic and polyphenolic compounds from Concord grape juice. Food Funct. 4:52–62 [DOI] [PubMed] [Google Scholar]
- 163.Gonzalez-Barrio R, Borges G, Mullen W, Crozier A. 2010. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J. Agric. Food Chem 58:3933–39 [DOI] [PubMed] [Google Scholar]
- 164.Jaganath IB, Mullen W, Edwards CA, Crozier A. 2006. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic. Res 40:1035–46 [DOI] [PubMed] [Google Scholar]
- 165.Kikuchi M, Ueno M, Itoh Y, Suda W, Hattori M. 2017. Uremic toxin-producing gut microbiota in rats with chronic kidney disease. Nephron 135:51–60 [DOI] [PubMed] [Google Scholar]
- 166.Mishima E, Fukuda S, Mukawa C, Yuri A, Kanemitsu Y, et al. 2017. Evaluation of the impact of gut microbiota on uremic solute accumulation by a CE-TOFMS–based metabolomics approach. Kidney Int. 92:634–45 [DOI] [PubMed] [Google Scholar]
- 167.Lee SH, An JH, Park HM,Jung BH. 2012. Investigation of endogenous metabolic changes in the urine of pseudo germ-free rats using a metabolomic approach. J. Chromatogr. B 887–888:8–18 [DOI] [PubMed] [Google Scholar]
- 168.Yap IK, Li JV, Saric J, Martin FP, Davies H, et al. 2008. Metabonomic and microbiological analysis of the dynamic effect of vancomycin-induced gut microbiota modification in the mouse. J. Proteome Res 7:3718–28 [DOI] [PubMed] [Google Scholar]
- 169.Claus SP, Tsang TM, Wang Y, Cloarec O, Skordi E, et al. 2008. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol. Syst. Biol 4:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nicholls AW,Mortishire-Smith RJ, Nicholson JK. 2003NMRspectroscopic–basedmetabonomic studies of urinary metabolite variation in acclimatizing germ-free rats. Chem. Res. Toxicol 16:1395–404 [DOI] [PubMed] [Google Scholar]