Abstract
Clinicians and scientists have begun to recognize that chronic pain and its treatment with opioid analgesics may occasion hedonic dysregulation—a blunting of physiological and positive emotional responses to rewarding objects and events in the social environment. Conversely, pain relief can be rewarding [83,84]. Yet, this relation is not unidirectional; experimental manipulations and clinical observational studies demonstrate the analgesic effects of positive affect and reward [32,86]. Although positive emotions and rewarding experiences can produce analgesia, how might they be intentionally cultivated for their therapeutic value? To answer this research question, this topical review proposes a mechanistic model (Figure 1) for optimizing psychosocial interventions by leveraging positive affective/reward-related mechanisms as means of addressing chronic pain and opioid misuse.
Keywords: CBT, hedonic, Mindfulness-Oriented Recovery Enhancement, opioid, positive affect, reward
1. Conceptual and Empirical Background
In classical Western philosophy, pleasure and pain were considered opposites on a hedonic balance that propelled behavior [9]. Modern neurobiology complements this view by demonstrating that pain and pleasure operate through a common emotional currency in the brain [17] – mediated by mesocorticolimbic dopamine circuity [23,72,92,95,105,116] and the endogenous opioid system [15,19,28,79,96,121] – the neural substrates of reward. This common neurobiological architecture underlying hedonic experience integrates pain and reward to motivate homeostatic goal attainment [7]. When the decision to avoid potential injury is outweighed by the motivation to pursue a conflicting goal (e.g., a significant reward), top-down processes in the brain inhibit nociception via release of opioid peptides; conversely, if the decision is to ignore the conflicting goal and instead to respond to the noxious stimulus, top-down facilitation of nociception occurs [31]. As a result of this motivational-decisional process, pain may subvert enjoyment of reward via multiple mechanisms. The attention-grabbing quality of pain can shift attentional resources away from reward processing [26]. Stress produced by pain can also result in reward devaluation [87]. Pain-induced reward devaluation is coupled with dampened neural responding during reward anticipation in the ventral striatum [100]. Furthermore, chronic pain may blunt the motivation to seek reward via neuroplasticity in dopamine neurons [91] and produce anhedonia by reducing endogenous opioid signaling [60,79] and opioid receptor availability [101] in reward-related brain regions. These neurobiological alterations may underlie impaired reward learning [3] and reduced reward responsiveness [30,66] observed among individuals with chronic pain.
Because exogenous opioids interact with endogenous opioid and dopamine systems [63,95] involved in the regulation of pain and reward, prolonged opioid use is theorized to blunt reward processing by modulating dopaminergic and opioidergic mechanisms integral to hedonic function [110]. Overuse and misuse of opioid analgesics can exacerbate pain-related hedonic dysregulation [46,68,94] by (a) producing neuroadaptations in corticostriatal reward systems and (b) by magnifying antireward processes instantiated by limbic systems (e.g., extended amygdala) that mediate release of signaling molecules including corticotropin-releasing factor (CRF), dynorphin, and substance P [29]—neuromolecular mediators of stress and inflammation implicated in pain chronification [12] and opioid-induced hyperalgesia [2]. As such, opioid misusing chronic pain patients demonstrate increased pain sensitivity [27], increased anhedonia [54], and decreased autonomic and attentional responses to naturally rewarding stimuli [40,43] relative to medication-adherent pain patients and healthy controls.
Despite basic science suggesting that chronic pain and opioid misuse are linked with a dearth of positive affect and undergirded by reward system dysfunction, current psychosocial pain treatments (e.g., cognitive-behavioral therapy – CBT) focus primarily on ameliorating negative affective reactions to pain. Though CBT is efficacious [16] and includes techniques that might affect reward mechanisms, few studies have gathered evidence on how CBT or other psychosocial interventions can modulate reward system function in ways that both reduce pain and opioid misuse. To address this gap we present a model that can guide efforts in this area.
2. Conceptual Model
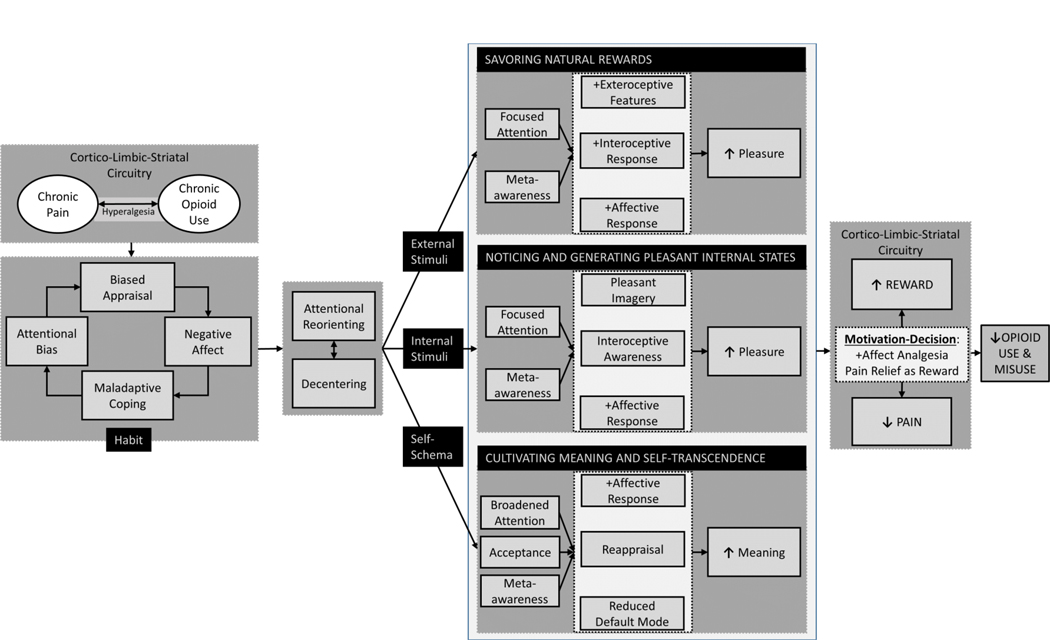
Chronic pain is fueled by a habit loop of attentional bias towards pain, biased pain appraisals (e.g., catastrophizing), negative affect, and maladaptive coping behaviors (e.g., overuse of opioids—which in turn may further exacerbate pain chronicity via opioid-induced neuroadaptations). As shown in Figure 1, psychosocial interventions can be used to disrupt the habit loop by first re-orienting attention from pain sensations and decentering from catastrophic appraisals, and then activating three core positive affective/reward mechanisms: (1) savoring natural rewards by focusing on pleasant features of naturally rewarding stimuli and the resulting positive emotions and bodily responses that occur while doing so; (2) noticing and generating pleasant internal states in spite of painful sensations by cultivating interoceptive awareness to delimit pain, increasing awareness of pleasurable sensations, and using imagination to superimpose pleasure on painful body sites; and (3) cultivating meaning and self-transcendence to disrupt cognitive biases by shifting patients’ thinking from a narrowed focus on pain to a much broader awareness of how pleasant and painful life experiences interact to produce meaning in life. By virtue of their potential effects on cortico-limbic-striatal circuitry function, these three therapeutic mechanisms may alter the motivational-decisional balance via top-down modulatory processes to disengage from pain and engage salient life goals, thereby reducing pain experience and enhancing reward. Ultimately, this process may reduce the drive towards opioid dose escalation and opioid misuse.
Figure 1. Conceptual model depicting positive affective/reward-related processes to be targeted by psychosocial interventions for chronic pain and opioid misuse.
Chronic pain and chronic opioid use/misuse amplify one another through reward/antireward processes kindled by opioid-induced neuroplasticity in cortio-limbic-striatal circuitry. Psychosocial interventions may leverage positive affective/reward-related processes to ameliorate chronic pain and opioid misuse. Standard CBT techniques are well-suited to interrupt the habit loop of attentional bias and hypervigilance towards pain, reducing biased pain appraisals (e.g., pain catastrophizing) and thereby decreasing negative affect and maladaptive coping behaviors (e.g., avoidance). Mindfulness techniques can then be used to decenter from dysfunctional pain-related cognitive processing and re-orient attention from pain sensations and catastrophic appraisals to focus on three therapeutic targets: positive external stimuli, positive internal stimuli, or self-schemas. Focused attention and meta-awareness facilitates the practice of savoring natural rewards, involving attending the pleasant exteroceptive (i.e., perceptual) features of a naturally rewarding stimulus while cultivating meta-awareness of the positive emotions and pleasant interoceptive sensations occasioned by that stimulus. Through focused attention and meta-awareness, one may notice and generate pleasant internal states (e.g., positive affect and pleasurable sensations) in spite of painful sensations. This process may be accomplished by fine-grained interoceptive awareness to delimit pain and increase appreciation of pleasure, as well as the use of imagination to superimpose pleasure on body sites proximal and distal to the painful body part. Finally, meta-awareness and acceptance may disrupt biased appraisals and allow one to integrate a widened array of pleasant, neutral, and painful life experiences within the broadened scope of attention, thereby attenuating default model processing and fueling adaptive reappraisals of pain-laden self-schemas. This process ultimately results in cultivating meaning and self-transcendence in the face of adversity. By virtue of their effects on corticostriatal circuitry function, these three processes in turn shift the motivation-decision balance to disengage from pain and engage salient life goals, resulting in enhanced reward and reduced pain experience. Ultimately, this process may reduce the drive towards opioid dose escalation and opioid misuse.
Mechanism 1: Savoring Natural Rewards
Attention allocation to pain comes at the expense of processing non-painful stimuli [26], and in particular, diverts attentional resources from noticing and appreciating pleasant, naturally rewarding objects and events in the social environment. Similarly, by virtue of sensitization of the mesocorticolimbic dopamine system, repeated opioid use imbues opioid-related cues with incentive salience [11], resulting in an attentional bias towards opioid cues [47] that promotes opioid misuse [51] and diverts attention from natural rewards. Countering this process by re-orienting attention away from pain and opioid-related cues might be therapeutic. Diverting attention from chronic pain may be optimized by attending to motivationally-salient exteroceptive stimuli [107]. In that regard, attending to pleasant visual [115] and auditory stimuli [89] modulates nociception at spinal and supraspinal levels [88]; the analgesic effect of rewarding stimuli appears to be mediated by connectivity between nucleus accumbens and corticostriatal circuitry [93]. Among people with chronic pain, attending to pleasant visual stimuli reduces chronic pain intensity [102].
Decades ago, Fordyce made similar observations and clarified the role of operant conditioning and reward contingencies in the maintenance of chronic pain, and recommended leveraging natural rewards to reinforce behavioral activation as a treatment for chronic pain [34]. This approach is integrated into modern CBT approaches that emphasize behavioral activation and pleasant activity scheduling [102]. Insofar as these techniques increase motivation to engage in, and exposure to, naturally rewarding events, they may boost activation in brain reward circuity [82]. Yet, merely being in the presence of a natural reinforcer may not be sufficient to overcome a chronic pain patient’s negative attentional bias. A recent review found no evidence that behavioral activation (or CBT as a whole) can significantly improve anhedonia [65]. Additional psychosocial approaches may be needed to disrupt maladaptive cognitive-attentional styles and increase reward responsiveness.
One novel approach consists of attentional retraining via savoring – the practice of attending to the pleasant sensory features of a naturally rewarding stimulus while cultivating meta-awareness of the positive emotions and pleasant sensations occasioned by that stimulus [42]. Savoring can be taught through instructions to focus attention on a generally pleasant or personally meaningful object in the present. For instance, in savoring a flower, one might attend to its pleasant colors, textures, and scents, as well as the touch of its petals against the skin, and then appreciate the embodied pleasure and emotions of contentment and joy occasioned by this process. Or, in savoring the warmth and sense of connection from holding hands with a loved one, one might magnify the analgesia produced by affective touch [55]. Also, given that exposure to nature and other enriched environments can reduce pain and post-operative analgesic requirements [77,104], savoring pleasant sensory features of the environmental context may amplify positive affect [90] and thereby improve analgesia—for instance, when in pain, one could shift attention away from the body and towards a beautiful skyline view, focusing on the pleasing hues and contours of the open expanse while deepening awareness of awe and calm.
Mindfulness has been theorized to promote savoring by first stabilizing and reorienting attention from distraction onto the pleasant stimulus, and then by deepening meta-awareness of positive emotional and sensory responses to the stimulus [41]. In support of this contention, mindfulness increases pleasure derived from eating and thereby decreases caloric intake [4,62]. Given hedonic dysregulation of the reward system in opioid misuse, I previously proposed the reward restructuring hypothesis that posits that psychosocial interventions may increase responsiveness to natural rewards by shifting reward processing from valuing drug-related rewards back towards much greater valuing of natural rewards, and thereby reduce addictive behavior [36].
2.1. Mechanism 2: Noticing and Generating Pleasant Internal States
From the perspective of “pain perception as inference” [99,113], pain is shaped by inferences and predictions derived from past pain episodes [71,114]. Over time, pain episodes are associated with deeply held and biased ways of attending and viewing the world (cognitive schema) that may obscure patients’ interoceptive awareness of non-painful sensations [20,25,106,108] – including pleasant sensations [18]. Opioid craving may result from a similar inferential process; misinterpretations of interoceptive signals magnify the discrepancy between predicted versus actual internal states of the body (“Will this opioid make me feel better?”), resulting in reward prediction errors that drive craving and blunted responsivity to natural rewards [97].
In contrast, nuanced and fine-grained use of interoceptive attention to delimit pain (i.e., notice the precise location and boundary of pain sensations) may enable patients to become more aware of pleasant sensations occurring contemporaneously with painful ones. To test this hypothesis, we recently developed a digital sensation manikin capable of quantifying the spatial distribution of pleasant and unpleasant sensations, and found that chronic pain patients who reported a greater distribution of pleasant sensations relative to unpleasant sensations experienced less pain-related functional interference, even after controlling for pain severity [58] – suggesting that awareness of embodied pleasure might be therapeutic. These results are congruent with preclinical data demonstrating that sexual pleasure in the context of painful stimulation reduces pain sensitivity via endogenous opioid release [56,98], and data from humans showing that sexual pleasure [112] and affective touch [69] during painful stimulation modulates pain experience. Similarly, consumptive pleasure from palatable sweet foods during painful stimulation increases pain tolerance [80]. Beyond externally-stimulated pleasant sensations, internally generated pleasant sensations produced by psychosocial pain interventions like hypnosis and mindfulness meditation [38] may also be analgesic and reduce the desire for opioids. In that regard, these mind-body therapies have been shown to reduce pain severity, opioid dose, and craving [39].
Hypothetically, activation in orbitofrontal, limbic, and striatal brain circuits [8,85,93,109,118] observed to mediate analgesia from reward and positive affect might underlie relief of pain and craving stemming from the use of psychosocial interventions to induce pleasant sensations. These circuit functions may be undergirded by increased endogenous dopamine and opioid release [78,83,121], and if so, inducing pleasant sensations might provide a non-drug means of reward and thereby reduce craving among opioid misusing chronic pain patients. That said, naloxone blockade studies indicate that that pain relief via pleasant imagery [10], hypnosis [81], and mindfulness [119] is not mediated by endogenous opioids, suggesting that at least among healthy individuals, non-opioid neurochemical mediators including dopamine, endocannabinoids, GABA, and serotonin may be at play.
2.2. Mechanism 3: Cultivating Meaning and Self-Transcendence
The ability to generate positive meaning might be an important protective factor against the adverse sequelae of pain [61] and opioid misuse [42]. According to the Mindfulness-to-Meaning Theory [41], meaning can arise from mindful acceptance of painful life experiences, which disrupts negative, self-relevant appraisals (e.g., “Pain and opioids ruined my life”) and broadens awareness to encompass an expanded set of contextual information from which adaptive reappraisals can be generated. Integrating a widened array of pleasant, neutral, and painful situational features within a broadened scope of awareness can reconfigure appraisals within working memory, leading to a shift in perspective on difficult life circumstances that promotes positive meaning in the face of adversity (“Pain has taught me to be more compassionate and to appreciate the good things in my life”).
On the psychological level, cultivating meaning may foster resilience to pain and addiction in much the same way that it fosters resilience to other stressors – by reappraising adversity as a source of personal growth [1,31,98]. To that point, meaning in life predicts lower levels of pain intensity, depressive symptoms, and analgesic medication use [21], and less attenuation of positive affect among people with chronic pain [57]. On a neural level, reappraisal recruits corticostriatal and limbic circuitry to produce lasting changes in amygdala reactivity to negative experiences [111] and activates nodes of the corticostriatal reward circuit, including ventral striatum and mPFC [22]. Such neural-functional effects are also evident when attributing positive meaning to pain; in a recent experiment, experiencing increased pain for the benefit of one’s romantic partner increased mPFC activation coupled with decreased neural pain processing and reduced pain unpleasantness [74]. Similarly, reappraising the meaning of drug-related stimuli decreases craving by modulating corticostriatal circuitry function [67].
Ultimately, meaning-making may reach its zenith in the experience of self-transcendence – the profound sense of connection or oneness with something greater than the self. Though self-transcendence can emerge naturally during intense experiences of awe, flow, and other peak experiences [117], it might also be induced by psychosocial interventions, like mindfulness meditation. Indeed, the original purpose of mindfulness and other contemplative practices was to foster self-transcendence through the emergence of a form of non-dual awareness [24]– a temporary experiential unification of the subject-object dichotomy that structures ordinary human consciousness. During meditation practices focused on self-transcendence, neural activity in nodes of the default mode network (DMN) decreases [13] while DMN connectivity increases with neural networks involved in exteroceptive stimulus processing [64], including the dorsal attentional and salience networks [35]. In view of the central role of the DMN in pain chronification [5,6,70] and opioid addiction [73,75,76], modulating default mode processing by evoking self-transcendence might transform the maladaptive cognitive-affective habits underlying chronic pain and opioid misuse.
3. MORE as an Example
How might behavioral treatment development research leverage these therapeutic mechanisms to generate novel psychosocial interventions? As one example, here I briefly describe how Mindfulness-Oriented Recovery Enhancement (MORE), a therapy I developed that has been shown to significantly decrease chronic pain, opioid misuse, and craving in several RCTs [48,49,53], integrates all three therapeutic mechanisms. Research on MORE advances prior work in this area by being theory-based and programmatic, with a focus on examining mechanisms, and findings to date that support proposed conceptual model (Fig. 1).
3.1. Savoring natural rewards.
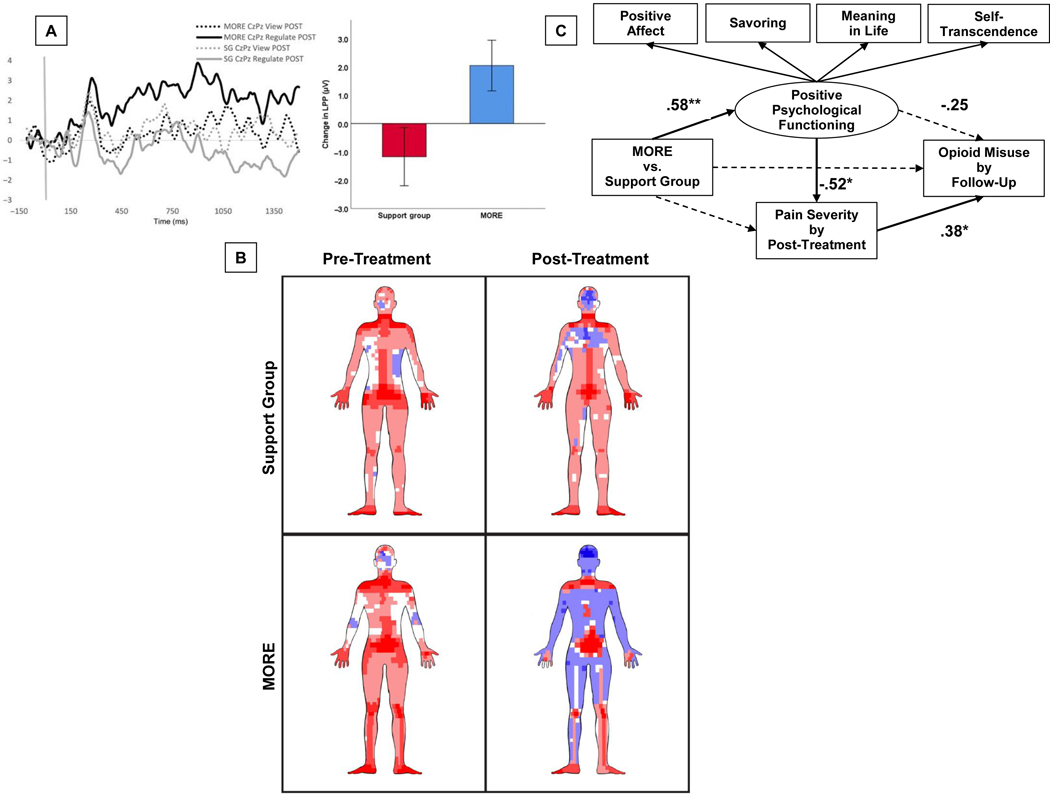
MORE, which integrates training in mindfulness and savoring, has been shown in opioid-treated chronic pain patients to increase autonomic [44] and neurophysiological responses [45] to natural reward stimuli which were associated with improvements in pain severity and pain catastrophizing, and predicted less pain interference at 3-month follow-up [50], suggesting that training attention towards natural rewards may decrease pain experience. MORE’s effects on increasing autonomic and neurophysiological responsiveness to natural reward were also associated with decreased opioid craving [44,45]. Further, minutes of savoring practice predicted decreases in opioid dose following treatment with MORE [52]. More recently, using EEG we found that MORE decreased opioid cue-reactivity while simultaneously increasing the capacity to upregulate neurophysiological reward responses during savoring; and increased responsivity to natural rewards mediated the effect of MORE on decreasing opioid misuse by 3-month follow-up [37] (Figure 2a). Taken together, these data provide robust support for my aforementioned restructuring reward hypothesis.
Figure 2. Effects of MORE on positive affective and reward-related processes.
2A. Evidence in support of mechanism 1: savoring natural rewards. Findings from [21] depicting effects of Mindfulness-Oriented Recovery Enhancement (MORE) vs. a social support group control condition on pre-post treatment change in event-related potentials of the EEG during savoring (i.e., up-regulating) vs. viewing of natural reward cues. 2B. Evidence in support of mechanism 2: noticing and self-generating pleasant internal states. Rendering of sensation reports on a computerized sensation manikin for participants treated with Mindfulness-Oriented Recovery Enhancement (MORE) or a social support group control condition in [35]. Blue represents pleasant sensations and red represents unpleasant sensations. The density of the hue reflects the frequency of pleasant or unpleasant sensation reports at a given sensation pixel. 2C. Evidence in support of mechanism 3: cultivating meaning and self-transcendence. Findings from [31] indicating that the effect of MORE on reducing pain severity and opioid misuse risk was associated with increases in these positive affective/reward-related psychological functions.
3.2. Noticing and generating pleasant internal states.
In MORE, patients are taught unique mindful breathing and body scan meditations designed to decompose pain experience into its constituent sensations (e.g., heat, tightness, tingling), as well as to increase awareness of the center, edges, and permeability (versus solidity) of these sensations, and any adjacent or distal pleasant sensations. In a RCT using the digital sensation manikin described above [58], after intervention, chronic pain patients participating in MORE reported a 7.5 fold increase in the ratio of pleasant to unpleasant sensations over the course of treatment (Figure 2b). No such increase in pleasant sensations was observed in the social support control condition, suggesting that these effects were not due to non-specific therapeutic factors, but rather stemmed from specific cognitive training provided in MORE.
3.3. Cultivating meaning.
In MORE, patients use mindfulness to encourage psychological flexibility and perspective shifting, facilitating reappraisal as a means of generating positive meaning in the face of adversity. Also, mindfulness training in MORE involves instructions to foster self-transcendence by cultivating non-dual awareness of the field of consciousness in which mental contents are experienced [59]. In a RCT of opioid-treated chronic pain patients in primary care (N=95), MORE significantly improved a latent positive psychological functioning variable comprised of positive affect, savoring, meaning in life, and self-transcendence measures. Importantly changes in this measure over the course of treatment predicted decreases in pain severity and opioid misuse risk [49] (Figure 2c). Among the factors included in the positive psychological functioning variable, increases in self-transcendence showed the largest effect size on reductions in pain severity.
4. Conclusion
Studies have taken important first steps to test the proposed model. Though MORE has been shown to modulate the three mechanisms delineated above in RCTs, extant investigations are subject to limitations including the (a) absence of a sham mindfulness condition [120] to provide a formal placebo control; (b) inability to identify the neural mechanisms undergirding the observed effects; (c) uncertainty regarding the duration of these effects, in light of hedonic adaptation/habituation [14]; and (d) use of a multi-modal treatment approach that combines mindfulness, reappraisal, and savoring skills, precluding dismantling of the independent contributions of these techniques on positive affect and reward-related processes. To remediate these limitations and advance the field, specific research recommendations are presented in Table 1.
Table 1.
Recommendations to advance research on the positive affective/reward mechanisms of psychosocial interventions for chronic pain and opioid misuse.
| Recommendation | Detail |
|---|---|
| Dismantle effects of various techniques | Studies should optimize the next generation of psychosocial pain therapies by using micro RCTs and factorial designs (e.g., multiphase optimization strategy) to determine the independent and interactive effects of an array of techniques for stimulating the reward system. |
| Quantify the phenomenological and physiological experience of pleasure as a mediator of treatment outcomes | Studies should quantify and examine interoceptive awareness of pleasure elicited by psychosocial interventions as a mediator of treatment outcomes. |
| Employ biobehavioral measures of reward processing | Studies should employ behavioral (e.g., eye-tracking) and neuroimaging (e.g., fMRI) measures to assess effects of psychosocial intervention on reward processing of exteroceptive stimuli, and whether increased reward responsiveness predicts reduced pain and opioid (mis)use. |
| Decipher intervention-induced dynamics of change | Studies should employ high-density ecological momentary assessments (EMA) of positive emotions, savoring, reappraisal, and other processes to analyze complex chains of causes and effects that synergistically emerge during chronic pain treatment. |
| Use placebo controls | Studies should use sham intervention arms (e.g., sham mindfulness [112]) to parse effects of expectancy and social desirability from mechanisms of action. |
| Determine the duration of effects on reward system function | Studies should collect longitudinal data to ascertain the duration of psychosocial intervention effects on reward-related mechanisms, and to determine whether such interventions can overcome the hedonic treadmill effect [12] to produce trait-like changes in the propensity towards positive affective states. |
| Experimentally stimulate meaning and self-transcendence | With the re-emergence of psychedelic research, pharmacological probes to reliably stimulate meaning and self-transcendent states are becoming available [49]. Studies with psychedelics, alone or in combination with psychosocial interventions like MBIs, may test whether self-transcendent experiences produce relief of pain and opioid craving by modulating reward system function. |
Ultimately, rigorous scientific pursuit of a multifaceted research program into these domains of inquiry may generate a “fourth wave” of CBT and boost the known efficacy of mind-body therapies [39] to help stem the tide of the opioid crisis.
Acknowledgements:
E.L.G. was supported by R01DA042033 (PI: Garland) from the National Institute on Drug Abuse during the preparation of this manuscript. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. I would like to thank Francis J. Keefe for his comments on a previous version of this manuscript. I would also like to thank Mathias Sanyer for his artistic rendering of scientific data in Figure 3.
Footnotes
Conflict of Interest: Eric Garland, PhD, LCSW is the Director of the Center on Mindfulness and Integrative Health Intervention Development. The Center provides Mindfulness-Oriented Recovery Enhancement (MORE), mindfulness-based therapy, and cognitive behavioral therapy in the context of research trials for no cost to research participants; however, Dr. Garland has received honoraria and payment for delivering seminars, lectures, and teaching engagements (related to training clinicians in mindfulness) sponsored by institutions of higher education, government agencies, academic teaching hospitals, and medical centers. Dr. Garland also receives royalties from the sale of books related to MORE.
References
- [1].Affleck G, Tennen H. Construing benefits from adversity: adaptational significance and dispositional underpinnings. Journal of Personality 1996;64:899–922. [DOI] [PubMed] [Google Scholar]
- [2].Angst MS, Clark JD. Opioid-induced HyperalgesiaA Qualitative Systematic Review. Anesthesiology 2006;104:570–587. [DOI] [PubMed] [Google Scholar]
- [3].Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain 2004;108:129–136. [DOI] [PubMed] [Google Scholar]
- [4].Arch JJ, Brown KW, Goodman RJ, Della Porta MD, Kiken LG, Tillman S. Enjoying food without caloric cost: The impact of brief mindfulness on laboratory eating outcomes. Behaviour Research and Therapy 2016;79:23–34. [DOI] [PubMed] [Google Scholar]
- [5].Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. The Journal of Neuroscience 2008;28:1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PloS one 2014;9:e106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ballantyne JC, Sullivan MD. Discovery of endogenous opioid systems: what it has meant for the clinician’s understanding of pain and its treatment. PAIN 2017;158:2290–2300. [DOI] [PubMed] [Google Scholar]
- [8].Becker S, Gandhi W, Pomares F, Wager TD, Schweinhardt P. Orbitofrontal cortex mediates pain inhibition by monetary reward. Social cognitive and affective neuroscience 2017;12:651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bentham J An introduction to the principles of morals and legislation. Library of Economics and Liberty, 1789 p. Available: http://www.econlib.org/library/Betham/bnthPML1.html. Accessed 16 Sep 2005. [Google Scholar]
- [10].Berna C, Leknes S, Ahmad AH, Mhuircheartaigh RN, Goodwin GM, Tracey I. Opioid-Independent and Opioid-Mediated Modes of Pain Modulation. J Neurosci 2018;38:9047–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. American Psychologist 2016;71:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. Reward deficiency and antireward in pain chronification. Neuroscience & Biobehavioral Reviews 2016;68:282–297. [DOI] [PubMed] [Google Scholar]
- [13].Brewer JA, Worhunsky PD, Gray JR, Tang Y-Y, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. PNAS 2011;108:20254–20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brickman P, Campbell DT. Hedonic relativism and planning the good science In. Appley MH (Ed.), Adaptation level theory: A symposium (pp. 287–302). New York: Academic Press, 1971. p. [Google Scholar]
- [15].Buchel C, Miedl S, Sprenger C. Hedonic processing in humans is mediated by an opioidergic mechanism in a mesocorticolimbic system. eLife 2018;7:e39648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].de C Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane database of systematic reviews 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cabanac M The dialectics of pleasure. Pleasures of the brain 2010:113–124. [Google Scholar]
- [18].Case LK, Čeko M, Gracely JL, Richards EA, Olausson H, Bushnell MC. Touch Perception Altered by Chronic Pain and by Opioid Blockade. eNeuro 2016;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chelnokova O, Laeng B, Eikemo M, Riegels J, Løseth G, Maurud H, Willoch F, Leknes S. Rewards of beauty: the opioid system mediates social motivation in humans. Molecular psychiatry 2014;19:746. [DOI] [PubMed] [Google Scholar]
- [20].Clauwaert A, Torta DM, Danneels L, Van Damme S. Attentional modulation of somatosensory processing during the anticipation of movements accompanying pain: an event-related potential study. The Journal of Pain 2018;19:219–227. [DOI] [PubMed] [Google Scholar]
- [21].Dezutter J, Luyckx K, Wachholtz A. Meaning in life in chronic pain patients over time: associations with pain experience and psychological well-being. Journal of Behavioral Medicine 2015;38:384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Doré BP, Boccagno C, Burr D, Hubbard A, Long K, Weber J, Stern Y, Ochsner KN. Finding positive meaning in negative experiences engages ventral striatal and ventromedial prefrontal regions associated with reward valuation. Journal of cognitive neuroscience 2017;29:235–244. [DOI] [PubMed] [Google Scholar]
- [23].Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry 2001;49:81–96. [DOI] [PubMed] [Google Scholar]
- [24].Dunne J Toward an understanding of non-dual mindfulness. Contemporary Buddhism 2011;12:71–88. [Google Scholar]
- [25].Durnez W, Van Damme S. Trying to fix a painful problem: the impact of pain control attempts on the attentional prioritization of a threatened body location. The Journal of Pain 2015;16:135–143. [DOI] [PubMed] [Google Scholar]
- [26].Eccleston C, Crombez G. Pain demands attention: A cognitive–affective model of the interruptive function of pain. Psychological Bulletin 1999;125:356–366. [DOI] [PubMed] [Google Scholar]
- [27].Edwards RR, Wasan AD, Michna E, Greenbaum S, Ross E, Jamison RN. Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. The Journal of Pain 2011;12:953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Eikemo M, Løseth GE, Johnstone T, Gjerstad J, Willoch F, Leknes S. Sweet taste pleasantness is modulated by morphine and naltrexone. Psychopharmacology 2016;233:3711–3723. [DOI] [PubMed] [Google Scholar]
- [29].Elman I, Borsook D. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron 2016;89:11–36. [DOI] [PubMed] [Google Scholar]
- [30].Elvemo NA, Landrø NI, Borchgrevink PC, Håberg AK. Reward responsiveness in patients with chronic pain. European Journal of Pain 2015;19:1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fields H State-dependent opioid control of pain. Nature Reviews Neuroscience 2004;5:565–575. [DOI] [PubMed] [Google Scholar]
- [32].Finan PH, Garland EL. The role of positive affect in pain and its treatment. The Clinical journal of pain 2015;31:177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Folkman S, Moskowitz JT. Positive affect and the other side of coping. American Psychologist 2000;55:647–654. [DOI] [PubMed] [Google Scholar]
- [34].Fordyce WE. Behavioral methods for chronic pain and illness. CV Mosby, 1976. p. [Google Scholar]
- [35].Froeliger B, Garland EL, Kozink RV, Modlin LA, Chen N-K, McClernon FJ, Greeson JM, Sobin P. Meditation-state functional connectivity (msfc): strengthening of the dorsal attention network and beyond. Evidence-Based Complementary and Alternative Medicine 2012;2012. Available: http://www.hindawi.com.proxy.lib.fsu.edu/journals/ecam/aip/680407/. Accessed 20 May 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Garland EL. Restructuring reward processing with Mindfulness-Oriented Recovery Enhancement: novel therapeutic mechanisms to remediate hedonic dysregulation in addiction, stress, and pain. Annals of the New York Academy of Sciences 2016;1373:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Garland EL, Atchley RM, Hanley AW, Zubieta J-K, Froeliger B. Mindfulness-Oriented Recovery Enhancement remediates hedonic dysregulation in opioid users: Neural and affective evidence of target engagement. Science Advances 2019;5:eaax1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Garland EL, Baker AK, Larsen P, Riquino MR, Priddy SE, Thomas E, Hanley AW, Galbraith P, Wanner N, Nakamura Y. Randomized controlled trial of brief mindfulness training and hypnotic suggestion for acute pain relief in the hospital setting. Journal of general internal medicine 2017;32:1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Garland EL, Brintz CE, Hanley AW, Roseen EJ, Atchley RM, Gaylord SA, Faurot KR, Yaffe J, Fiander M, Keefe FJ. Mind-Body Therapies for Opioid-Treated Pain: A Systematic Review and Meta-analysis. JAMA Intern Med 2019. doi: 10.1001/jamainternmed.2019.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Garland EL, Bryan CJ, Nakamura Y, Froeliger B, Howard MO. Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology 2017;234:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Garland EL, Farb NA, Goldin PR, Fredrickson BL. Mindfulness Broadens Awareness and Builds Eudaimonic Meaning: A Process Model of Mindful Positive Emotion Regulation. Psychological Inquiry 2015;26:293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Garland EL, Fredrickson BL. Positive psychological states in the arc from mindfulness to self-transcendence: extensions of the Mindfulness-to-Meaning Theory and applications to addiction and chronic pain treatment. Current Opinion in Psychology 2019;28:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Garland EL, Froeliger B, Howard MO. Allostatic dysregulation of natural reward processing in prescription opioid misuse: Autonomic and attentional evidence. Biological psychology 2015;105:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Garland EL, Froeliger B, Howard MO. Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology 2014;231:3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Garland EL, Froeliger B, Howard MO. Neurophysiological evidence for remediation of reward processing deficits in chronic pain and opioid misuse following treatment with Mindfulness-Oriented Recovery Enhancement: exploratory ERP findings from a pilot RCT. Journal of behavioral medicine 2015;38:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Garland EL, Froeliger B, Zeidan F, Partin K, Howard MO. The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neuroscience & Biobehavioral Reviews 2013;37:2597–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Garland EL, Froeliger BE, Passik SD, Howard MO. Attentional bias for prescription opioid cues among opioid dependent chronic pain patients. Journal of behavioral medicine 2013;36:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Garland EL, Hanley AW, Kline A, Cooperman NA. Mindfulness-Oriented Recovery Enhancement reduces opioid craving among individuals with opioid use disorder and chronic pain in medication assisted treatment: Ecological momentary assessments from a stage 1 randomized controlled trial. Drug and Alcohol Dependence 2019;203:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Garland EL, Hanley AW, Riquino MR, Reese SE, Baker AK, Salas K, Yack BP, Bedford CE, Bryan MA, Atchley R, Nakamura Y, Froeliger B, Howard MO. Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial. Journal of Consulting and Clinical Psychology 2019;87:927–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Garland EL, Howard MO. Enhancing natural reward responsiveness among opioid users predicts chronic pain relief: EEG analyses from a trial of Mindfulness-Oriented Recovery Enhancement. Journal of the Society for Social Work and Research 2018;9:285–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Garland EL, Howard MO. Opioid attentional bias and cue-elicited craving predict future risk of prescription opioid misuse among chronic pain patients. Drug and Alcohol Dependence 2014;144:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Garland EL, Hudak J, Hanley AW, Nakamura Y. Mindfulness-Oriented Recovery Enhancement Reduces Opioid Dose Among Chronic Pain Patients in Primary Care by Strengthening Autonomic Regulation During Meditation. American Psychologist 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. Journal of Consulting and Clinical Psychology 2014;82:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Garland EL, Trøstheim M, Eikemo M, Ernst G, Leknes S. Anhedonia in chronic pain and prescription opioid misuse. Psychol Med 2019:1–12. [DOI] [PubMed] [Google Scholar]
- [55].Goldstein P, Shamay-Tsoory SG, Yellinek S, Weissman-Fogel I. Empathy predicts an experimental pain reduction during touch. The Journal of Pain 2016;17:1049–1057. [DOI] [PubMed] [Google Scholar]
- [56].Gomora P, Beyer C, Gonzalez-Mariscal G, Komisaruk BR. Momentary analgesia produced by copulation in female rats. Brain research 1994;656:52–58. [DOI] [PubMed] [Google Scholar]
- [57].Gruszczyńska E, Knoll N. Meaning-focused coping, pain, and affect: a diary study of hospitalized women with rheumatoid arthritis. Qual Life Res 2015;24:2873–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hanley AL, Garland EL. Mapping the Affective Dimension of Embodiment with the Sensation Manikin: Validation Among Chronic Pain Patients and Modification by Mindfulness-Oriented Recovery Enhancement. Psychosomatic Medicine 2019. [DOI] [PubMed] [Google Scholar]
- [59].Hanley AW, Nakamura Y, Garland EL. The Nondual Awareness Dimensional Assessment (NADA): New tools to assess nondual traits and states of consciousness occurring within and beyond the context of meditation. Psychological assessment 2018;30:1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta J-K. Decreased central μ-opioid receptor availability in fibromyalgia. The Journal of Neuroscience 2007;27:10000–10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hassett AL, Finan PH. The role of resilience in the clinical management of chronic pain. Current pain and headache reports 2016;20:39. [DOI] [PubMed] [Google Scholar]
- [62].Hong PY, Lishner DA, Han KH. Mindfulness and eating: An experiment examining the effect of mindful raisin eating on the enjoyment of sampled food. Mindfulness 2014;5:80–87. [Google Scholar]
- [63].Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 1992;12:483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Josipovic Z Neural correlates of nondual awareness in meditation. Annals of the New York Academy of Sciences 2014;1307:9–18. [DOI] [PubMed] [Google Scholar]
- [65].Kiluk BD, Yip SW, DeVito EE, Carroll KM, Sofuoglu M. Anhedonia as a Key Clinical Feature in the Maintenance and Treatment of Opioid Use Disorder. Clinical Psychological Science 2019:2167702619855659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kim M, Mawla I, Albrecht DS, Admon R, Torrado-Carvajal A, Bergan C, Protsenko E, Kumar P, Edwards RR, Saha A, Napadow V, Pizzagalli DA, Loggia ML. Striatal hypofunction as a neural correlate of mood alterations in chronic pain patients. NeuroImage 2020;211:116656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences 2010;107:14811–14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Koob GF. Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia and Negative Reinforcement. Biological Psychiatry 2019. doi: 10.1016/j.biopsych.2019.05.023. [DOI] [PubMed] [Google Scholar]
- [69].Krahé C, Drabek MM, Paloyelis Y, Fotopoulou A. Affective touch and attachment style modulate pain: a laser-evoked potentials study. Philosophical Transactions of the Royal Society B: Biological Sciences 2016;371:20160009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. Journal of Neuroscience 2014;34:3969–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Labrenz F, Icenhour A, Schlamann M, Forsting M, Bingel U, Elsenbruch S. From Pavlov to pain: How predictability affects the anticipation and processing of visceral pain in a fear conditioning paradigm. NeuroImage 2016;130:104–114. [DOI] [PubMed] [Google Scholar]
- [72].Ledermann K, Jenewein J, Sprott H, Hasler G, Schnyder U, Warnock G, Johayem A, Kollias S, Buck A, Martin-Soelch C. Altered Dopamine Responses to Monetary Rewards in Female Fibromyalgia Patients with and without Depression: A [11C]Raclopride Bolus-plus-Infusion PET Study. PPS 2017;86:181–182. [DOI] [PubMed] [Google Scholar]
- [73].Li W, Li Q, Wang D, Xiao W, Liu K, Shi L, Zhu J, Li Y, Yan X, Chen J, Ye J, Li Z, Wang Y, Wang W. Dysfunctional Default Mode Network in Methadone Treated Patients Who Have a Higher Heroin Relapse Risk. Sci Rep 2015;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].López-Solà M, Koban L, Wager TD. Transforming pain with prosocial meaning: an fMRI study. Psychosom Med 2018;80:814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ma N, Liu Y, Fu X-M, Li N, Wang C- X, Zhang H, Qian R- B, Xu H- S, Hu X, Zhang D- R. Abnormal brain default-mode network functional connectivity in drug addicts. PloS one 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ma X, Qiu Y, Tian J, Wang J, Li S, Zhan W, Wang T, Zeng S, Jiang G, Xu Y. Aberrant default-mode functional and structural connectivity in heroin-dependent individuals. PLoS One 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Malenbaum S, Keefe FJ, Williams A, Ulrich R, Somers TJ. Pain in its Environmental Context: Implications for Designing Environments to Enhance Pain Control. Pain 2008;134:241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Martikainen IK, Hagelberg N, Jääskeläinen SK, Hietala J, Pertovaara A. Dopaminergic and serotonergic mechanisms in the modulation of pain: In vivo studies in human brain. European Journal of Pharmacology 2018;834:337–345. [DOI] [PubMed] [Google Scholar]
- [79].Martikainen IK, Peciña M, Love TM, Nuechterlein EB, Cummiford CM, Green CR, Harris RE, Stohler CS, Zubieta J-K. Alterations in endogenous opioid functional measures in chronic back pain. The Journal of Neuroscience 2013;33:14729–14737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mercer ME, Holder MD. Antinociceptive Effects of Palatable Sweet Ingesta on Human Responsivity to Pressure Pain. Physiology & Behavior 1997;61:311–318. [DOI] [PubMed] [Google Scholar]
- [81].Moret V, Forster A, Laverrière M-C, Lambert H, Gaillard RC, Bourgeois P, Haynal A, Gemperle M, Buchser E. Mechanism of analgesia induced by hypnosis and acupuncture: is there a difference? Pain 1991;45:135–140. [DOI] [PubMed] [Google Scholar]
- [82].Mori A, Okamoto Y, Okada G, Takagaki K, Takamura M, Jinnin R, Ichikawa N, Yamamura T, Yokoyama S, Shiota S, Yoshino A, Miyake Y, Okamoto Y, Matsumoto M, Matsumoto K, Yamawaki S. Effects of behavioural activation on the neural circuit related to intrinsic motivation. BJPsych Open 2018;4:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci 2014;17:1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward–valuation circuitry. Proceedings of the National Academy of Sciences 2012;109:20709–20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Nees F, Usai K, Löffler M, Flor H. The evaluation and brain representation of pleasant touch in chronic and subacute back pain. Neurobiology of Pain 2019;5:100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ong A, Thoemmes F, Ratner K, Ghezzi-Kopel K, Reid MC. Positive affect and chronic pain: A preregistered systematic review and meta-analysis. PsyArXiv 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Porcelli AJ, Delgado MR. Stress and decision making: effects on valuation, learning, and risk-taking. Current opinion in behavioral sciences 2017;14:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rhudy JL, Williams AE, McCabe KM, Nguyêñ MATV, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology 2005;42:579–587. [DOI] [PubMed] [Google Scholar]
- [89].Roy M, Peretz I, Rainville P. Emotional valence contributes to music-induced analgesia. Pain 2008;134:140–147. [DOI] [PubMed] [Google Scholar]
- [90].Sato I, Jose PE, Conner TS. Savoring mediates the effect of nature on positive affect. International Journal of Wellbeing 2018;8. doi: 10.5502/ijw.v8i1.621. [DOI] [Google Scholar]
- [91].Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 2014;345:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta J-K. Individual Differences in Reward Responding Explain Placebo-Induced Expectations and Effects. Neuron 2007;55:325–336. [DOI] [PubMed] [Google Scholar]
- [93].Seminowicz DA, Remeniuk B, Krimmel SR, Smith MT, Barrett FS, Wulff AB, Furman AJ, Geuter S, Lindquist MA, Irwin MR. Pain-related nucleus accumbens function: modulation by reward and sleep disruption. Pain 2019;160:1196–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shurman J, Koob GF, Gutstein HB. Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Med 2010;11:1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Spagnolo PA, Kimes A, Schwandt ML, Shokri-Kojori E, Thada S, Phillips KA, Diazgranados N, Preston KL, Herscovitch P, Tomasi D, Ramchandani VA, Heilig M. Striatal Dopamine Release in Response to Morphine: A [11C]Raclopride Positron Emission Tomography Study in Healthy Men. Biological Psychiatry 2019;86:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sprenger C, Eichler I-C, Eichler L, Zöllner C, Büchel C. Altered Signaling in the Descending Pain-modulatory System after Short-Term Infusion of the μ-Opioid Agonist Remifentanil. J Neurosci 2018;38:2454–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Stewart JL, Khalsa SS, Kuplicki R, Puhl M, Investigators T, Paulus MP. Interoceptive attention in opioid and stimulant use disorder. Addiction Biology n.d.;n/a:e12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Szechtman H, Hershkowitz M, Simantov R. Sexual behavior decreases pain sensitivity and stimulates endogenous opioids in male rats. European Journal of Pharmacology 1981;70:279–285. [DOI] [PubMed] [Google Scholar]
- [99].Tabor A, Keogh E, Eccleston C. Embodied pain—negotiating the boundaries of possible action. Pain 2017;158:1007–1011. [DOI] [PubMed] [Google Scholar]
- [100].Talmi D, Dayan P, Kiebel SJ, Frith CD, Dolan RJ. How humans integrate the prospects of pain and reward during choice. Journal of Neuroscience 2009;29:14617–14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Thompson SJ, Pitcher MH, Stone LS, Tarum F, Niu G, Chen X, Kiesewetter DO, Schweinhardt P, Bushnell MC. Chronic neuropathic pain reduces opioid receptor availability with associated anhedonia in rat. Pain 2018;159:1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Thorn BE, Eyer JC, Van Dyke BP, Torres CA, Burns JW, Kim M, Newman AK, Campbell LC, Anderson B, Block PR. Literacy-adapted cognitive behavioral therapy versus education for chronic pain at low-income clinics: a randomized controlled trial. Annals of internal medicine 2018;168:471–480. [DOI] [PubMed] [Google Scholar]
- [103].Tugade MM, Fredrickson BL. Regulation of positive emotions: Emotion regulation strategies that promote resilience. Journal of happiness studies 2007;8:311–333. [Google Scholar]
- [104].Ulrich R View through a window may influence recovery. Science 1984;224:224–225. [DOI] [PubMed] [Google Scholar]
- [105].Ungless MA, Magill PJ, Bolam JP. Uniform Inhibition of Dopamine Neurons in the Ventral Tegmental Area by Aversive Stimuli. Science 2004;303:2040–2042. [DOI] [PubMed] [Google Scholar]
- [106].Van Damme S, Bulcke CV, Van Den Berghe L, Poppe L, Crombez G. Do patients with chronic unilateral orofacial pain due to a temporomandibular disorder show increased attending to somatosensory input at the painful side of the jaw? PeerJ 2018;6:e4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Van Ryckeghem DM, Van Damme S, Eccleston C, Crombez G. The efficacy of attentional distraction and sensory monitoring in chronic pain patients: A meta-analysis. Clinical Psychology Review 2018;59:16–29. [DOI] [PubMed] [Google Scholar]
- [108].Vanden Bulcke C, Van Damme S, Durnez W, Crombez G. The anticipation of pain at a specific location of the body prioritizes tactile stimuli at that location. PAIN® 2013;154:1464–1468. [DOI] [PubMed] [Google Scholar]
- [109].Villemure C, Laferrière AC, Bushnell MC. The ventral striatum is implicated in the analgesic effect of mood changes. Pain Research and Management 2012;17:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Volkow ND, McLellan AT. Opioid Abuse in Chronic Pain — Misconceptions and Mitigation Strategies. New England Journal of Medicine 2016;374:1253–1263. [DOI] [PubMed] [Google Scholar]
- [111].Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 2008;59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Whipple B, Komisaruk BR. Analgesia produced in women by genital self‐stimulation. The Journal of Sex Research 1988;24:130–140. [DOI] [PubMed] [Google Scholar]
- [113].Wiech K Deconstructing the sensation of pain: The influence of cognitive processes on pain perception. Science 2016;354:584–587. [DOI] [PubMed] [Google Scholar]
- [114].Wiech K, Vandekerckhove J, Zaman J, Tuerlinckx F, Vlaeyen JW, Tracey I. Influence of prior information on pain involves biased perceptual decision-making. Current Biology 2014;24:R679–R681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].de Wied M, Verbaten MN. Affective pictures processing, attention, and pain tolerance. Pain 2001;90:163–172. [DOI] [PubMed] [Google Scholar]
- [116].Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA. Fibromyalgia patients show an abnormal dopamine response to pain. European Journal of Neuroscience 2007;25:3576–3582. [DOI] [PubMed] [Google Scholar]
- [117].Yaden DB, Haidt J, Hood RW Jr., Vago DR, Newberg AB. The varieties of self-transcendent experience. Review of General Psychology 2017;21:143–160. [Google Scholar]
- [118].Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: Involvement of neural reward systems. PloS one 2010;5:e13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Zeidan F, Adler-Neal AL, Wells RE, Stagnaro E, May LM, Eisenach JC, McHaffie JG, Coghill RC. Mindfulness-Meditation-Based Pain Relief Is Not Mediated by Endogenous Opioids. The Journal of Neuroscience 2016;36:3391–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, Coghill RC. Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. Journal of Neuroscience 2015;35:15307–15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zubieta J-K, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 2001;293:311–315. [DOI] [PubMed] [Google Scholar]