Abstract
Identification of bacterial virulence factors is critical for understanding disease pathogenesis, drug discovery and vaccine development. In this study we used two approaches to predict virulence factors of Burkholderia pseudomallei, the Gram-negative bacterium that causes melioidosis. B. pseudomallei is naturally antibiotic resistant and there are no clinically available melioidosis vaccines. To identify B. pseudomallei protein targets for drug discovery and vaccine development, we chose to search for substrates of the B. pseudomallei periplasmic disulfide bond forming protein A (DsbA). DsbA introduces disulfide bonds into extra-cytoplasmic proteins and is essential for virulence in many Gram-negative organism, including B. pseudomallei. The first approach to identify B. pseudomallei DsbA virulence factor substrates was a large-scale genomic analysis of 511 unique B. pseudomallei disease-associated strains. This yielded 4,496 core gene products, of which we hypothesise 263 are DsbA substrates. Manual curation and database screening of the 263 mature proteins yielded 81 associated with disease pathogenesis or virulence. These were screened for structural homologues to predict potential B-cell epitopes. In the second approach, we searched the B. pseudomallei genome for homologues of the more than 90 known DsbA substrates in other bacteria. Using this approach, we identified 15 putative B. pseudomallei DsbA virulence factor substrates, with two of these previously identified in the genomic approach, bringing the total number of putative DsbA virulence factor substrates to 94. The two putative B. pseudomallei virulence factors identified by both methods are homologues of PenI family β-lactamase and a molecular chaperone. These two proteins could serve as high priority targets for future B. pseudomallei virulence factor characterization.
Introduction
Burkholderia pseudomallei is a Gram-negative soil dwelling saprophyte, and an opportunistic pathogen responsible for the severe tropical disease melioidosis [1]. B. pseudomallei infections are difficult to treat [2–4] and are intrinsically resistant to almost all available antibiotics [5–8]. Predominant resistance factors utilised by B. pseudomallei include a thick, impermeable cell wall combined with efficient efflux pumps that interfere with drug activity [9]. Furthermore, B. pseudomallei infections are difficult to diagnose as melioidosis symptoms vary significantly, ranging from fever, pneumonia, urinary tract infections, and on rare occasions encephalomyelitis [3]. Standard treatment consists of a combination of intravenous antibiotic for two weeks to stop septicaemia, followed by a second eradication phase that can last for up to six months, with no guarantee of success [10].
More generally, antibiotic resistance is increasing at an accelerating rate among pathogenic bacteria [11]. New approaches and treatment strategies are needed including vaccination [12] novel antimicrobial compounds [13], and anti-virulence strategies [14]. There is currently no successful, persistent vaccine against B. pseudomallei [15]. However several vaccine candidates have shown promising results in mice, for example the BatA autotransporter protein expressed in virus was efficient at preventing infection by inhaled B. pseudomallei [16]. Moreover vaccines which use a combination of different antigens have also yielded promising candidates (thoroughly reviewed in [17]). For example Burtnick et. al. [18] combined capsular polysaccharides, diphtheria toxin mutant and Type VI secretion system component Hcp1 from B. pseudomallei to protect mice from inhaled bacteria. Other combinations of capsular polysaccharides and/or B. pseudomallei antigenic proteins (such as flagellar proteins, Type 3 secretion system (T3SS) and outer membrane proteins) have also shown encouraging results [19–22].
Using attenuated or inactivated B. pseudomallei strains lacking essential virulence genes to vaccinate mice has also produced excellent results. B. pseudomallei strains with deletion of a gene such as purM [20, 23], hcp1, tonB [20, 24], aroC [20, 25] and others (see [17, 20] for a recent review) have successfully protected mice against melioidosis.
For both these vaccination strategies (combined antigenic components, or attenuated live strain), identification of new B. pseudomallei virulence factors would increase options for vaccination. Identification of virulence factors would also contribute to a better understanding of B. pseudomallei pathogenesis [26].
Targeting virulence rather than viability is an approach that is hypothesized to have a number of benefits including an increased range of possible anti-virulence mechanisms compared to antimicrobial compounds, as well as the possibility of reducing selection pressure [27, 28]. Both vaccine development and novel anti-virulence approaches could reduce selection pressure and potentially reduce resistance development [14, 27, 28].
The formation of correct disulfide bonds is critical for the proper folding and function of proteins [29]. In bacteria, the introduction of disulfide bonds is mediated by the DiSufide Bond-forming proteins (DSB). The DSB proteins are of particular interest as an anti-virulence strategy, because many virulence factors contain disulfide bonds [28, 30–32]. The Disulfide bond forming protein A (DsbA) is a periplasmic protein found in most Gram-negative bacteria and incorporates a thioredoxin fold with two cysteines which introduce disulfide bonds into substrate proteins via a redox transfer reaction [33].
Mice infected with B. pseudomallei DsbA knockouts (or of its redox partner DsbB) have an increased rate of survival compared with mice infected with wild type B. pseudomallei [34, 35]. These findings suggest that many B. pseudomallei virulence factors are substrates of DsbA, as is also observed in Escherichia coli [36, 37], Klebsiella pneumoniae [38], Salmonella enterica [39], Francisella tularensis [40] and many more [30, 31, 41]. However, the full extent of B. pseudomallei DsbA substrates has not been investigated. Identification of B. pseudomallei DsbA substrates would help identification of infection mechanisms, and could lead to the discovery of key virulence factors and potential drug and vaccine targets. Finding potential DsbA substrates is assisted by the observation that: (i) DsbA is located in the periplasm, and thus its substrates are likely to have a secretion signal sequence; and (ii) proteins containing disulfide bonds may have an even rather than an odd number of cysteines in their sequence. This last point is thought to have evolved to limit formation of mis-matched disulfide bonds and therefore misfolded proteins [42, 43].
In the present study, we used two approaches to identify potential B. pseudomallei DsbA substrates for further study as virulence factors. In one approach, we used computational methods to generate a curated list of 263 putatively extra-cytoplasmic proteins from the core genome of 511 disease-associated isolates of B. pseudomallei, 81 of which were predicted to be virulence-associated. In the second approach, 15 candidate DsbA virulence factor substrates were identified by sequence homology to known DsbA virulence factor substrates in other bacteria.
Results
Genomic analysis to predict B. pseudomallei DsbA virulence factor substrates
In this approach, our strategy was to cast a wide net initially, by determining the pangenome of disease-associated isolates of B. pseudomallei, and then filtering from that the core genome (i.e. the highly conserved genes). The disease-associated B. pseudomallei core genome should then be enriched in conserved virulence factors. At the time of this analysis the NCBI database [44] contained 1577 B. pseudomallei isolates. Metadata notation allowed selection of 512 isolates associated with disease (i.e. isolates from swabs/clinical isolates: accession numbers of these are given in S1 Data); other genomes were discarded. We note that only 355 of the 512 isolates were tagged ‘pathogen’ in the NCBI database indicating a discrepancy between NCBI assignment and user-uploaded metadata. Analysis of the pangenome, that is the core, accessory and unique genes of these 512 B. pseudomallei isolates (see Table 1), revealed two identical strains. Therefore for the remainder of this analysis, only the 511 unique strains were used.
Table 1. Pangenome results of 511 disease-associated B. pseudomallei strains.
| Pangenome breakdown | Classification | Number of genes | Percent of pangenome (%) |
|---|---|---|---|
| Core genes | (99% < = strains < = 100%) | 4,496 | 22.49 |
| Soft core genes | (95% < = strains < 99%) | 517 | 2.59 |
| Shell genes | (15% < = strains < 95%) | 965 | 4.83 |
| Cloud genes | (0% < = strains < 15%) | 14,013 | 70.10 |
| Total pangenome | (0% < = strains < = 100%) | 19,991 | 100 |
The pangenome is subdivided into the core (found in every strain), soft shell core (found in 95–99% of strains), shell (found in 15–95% of strains), and cloud (found in 0–15% of strains) genes. The total number of genes is shown, along with the percentage of total pangenome.
We found that the core genome consisted of 4,496 genes (see S2 Data) or 22.49% of the total 19,991 pangenome. This analysis largely agrees with a previous pangenomic analysis which extrapolated a modelled core genome of 4,568±16 genes from a much smaller set of 37 isolate genomes [45]. In that approach, modelling was used to predict the core genome if the number of isolates was expanded. Our approach gives an exact number because all 4,496 genes were found in all 511 genomes. Notably, the dithiol oxidase redox enzyme pair DsbA and DsbB and the disulfide isomerase redox relay enzymes DsbC and DsbD were all identified as core genes.
We then used the B. pseudomallei core genome for further analysis, because it encodes highly conserved proteins—a key criteria for selecting vaccine or anti-virulence targets.
From these 4,496 core genes, 726 were predicted to encode proteins with a signal sequence and which are therefore likely to be exported out of the cytoplasm and into the periplasm where DsbA is localised. Of these 726 proteins, 263 have an even number of cysteines, indicating the likelihood that the proteins form intramolecular disulfide bonds (see S3 Data). We predict that these 263 proteins are substrates of B. pseudomallei DsbA. The workflow for this analysis is shown in Fig 1.
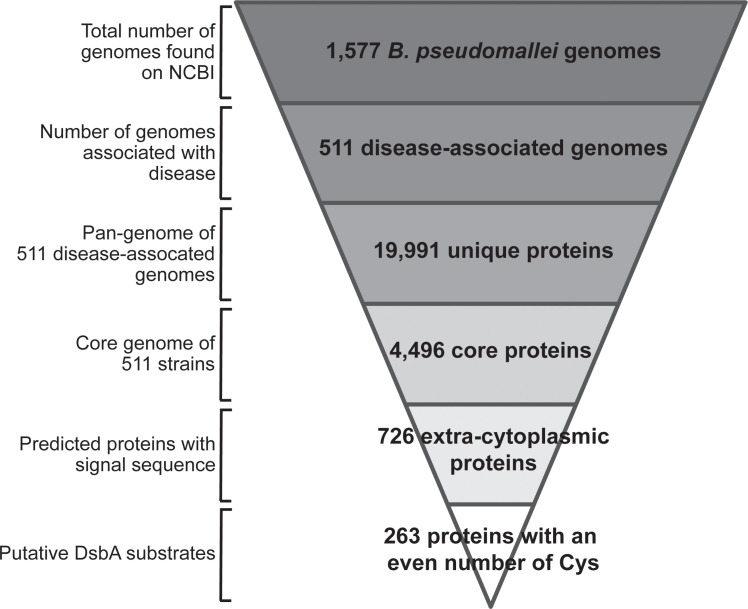
Fig 1. Bioinformatic workflow.
From the 1,577 B. pseudomallei genomes found on NCBI, 511 were unique and associated with disease and these were used for further analysis. The pangenome of these 511 genomes comprised 19,991 unique genes. 4,496 of these were classified as core genes. Predicted translation of these genes gave 726 predicted extra-cytoplasmic proteins. Of these extra-cytoplasmic proteins, 263 were predicted to contain an even number of cysteines. We predict that these 263 proteins are substrates of B. pseudomallei DsbA.
Distribution of cysteines in the core genome of disease-related B. pseudomallei
Many bacterial extra-cytoplasmic (periplasmic and extracellular) proteins have a strong preference for an even number of cysteines, which is thought to minimise non-native disulfide bond formation [42]. This point could be of interest as a means to reduce false positive DsbA substrates by filtering out proteins with an odd number of cysteines. We examined the cysteine distribution of encoded proteins in the B. pseudomallei pangenome to investigate whether the previously demonstrated enrichment of an even number of cysteines in extra-cytoplasmic proteins in other Gram-negative bacteria [42] was also true for B. pseudomallei.
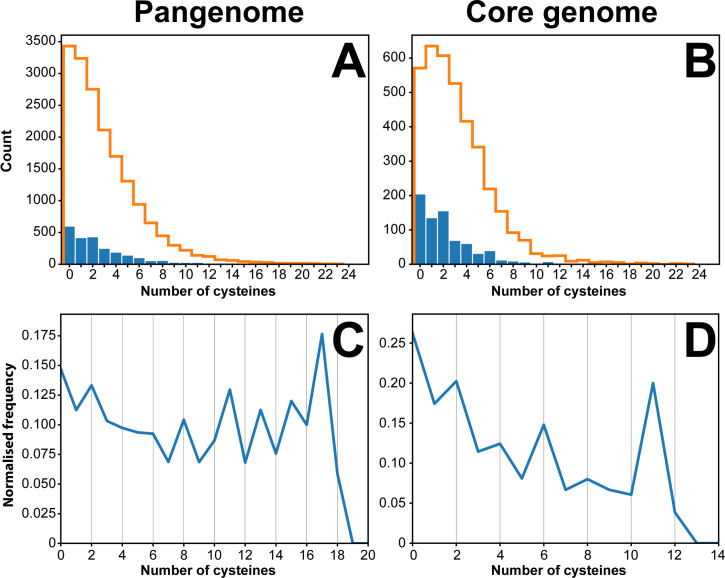
The distribution of cysteines in B. pseudomallei cytoplasmic and extra-cytoplasmic proteins was calculated for the pangenome (total of 19,991 genes) and the core genome (4,496 genes) (refer to Table 1). In cytoplasmic B. pseudomallei proteins, cysteine distribution followed a Poisson law peaking at zero for the pangenome and at one for the core genome (denoted by the orange lines in the histograms on Fig 2A and 2B). This distribution changed for extra-cytoplasmic B. pseudomallei proteins. For the core genome (blue bars Fig 2B), B. pseudomallei proteins with an even number of cysteines were over-represented compared to a typical Poisson distribution. As extra-cytoplasmic proteins represent a small fraction of the total number of the translated core genome and pangenome (16% and 11.5% of all proteins, respectively), we also analysed the normalised frequency (Fig 2C and 2D). The core genome normalised cysteine distribution reveals a sawtooth pattern with a preference for even number of cysteines with peaks for two, four, six and eight cysteines (Fig 2D). In contrast, the pangenomic normalised cysteine distribution for extra-cytoplasmic B. pseudomallei proteins does not indicate a strong preference for even number of cysteines (Fig 2C). Overall, the saw-tooth pattern observed in Fig 2B and 2D is similar to that described for E. coli exported proteins [42] although not as pronounced.
Fig 2. Cysteine distribution in the translated genome of B. pseudomallei.
Panel A shows the distribution of cysteines in the pangenome (19,991 proteins). Panel B represents the same analysis for the core genome, comprising 4,496 translated genes. Predicted number of extra-cytoplasmic proteins for each number of cysteines are represented as blue bars. Similarly, predicted cytoplasmic proteins are represented as orange lines. Panels C and D represent the normalised frequency of cysteine-containing extra-cytoplasmic proteins. The blue line in panel D peaks for proteins with 2, 4, 6 and 8 cysteines suggesting a preference for an even number of cysteines. This trend is not observed as strongly in panel C, where a clear peak can only be seen for two and eight cysteines. The normalised frequency was calculated by dividing the number of extra-cytoplasmic proteins (having N number of cysteines) by the total number of proteins with N cysteines (N being a number between 0–20 as per the data points in C and D above).
Functional assignment of core, extra-cytoplasmic, putative DsbA substrates
The next step in the genomic analysis was to predict which of the 263 putative DsbA substrates are associated with virulence. Of the 263 selected proteins, 44 were annotated as hypothetical/uncharacterised. The remaining 219 proteins include ABC transporter-related proteins, housekeeping proteins like cytochrome C, proteins required for motility such as flagellar and fimbrial proteins, enzymes such as collagenase, peptidases and proteases, as well as antibiotic resistance enzymes, β-lactamases. Many oxidoreductases were also present including DsbA, DsbD and others such as Gfo/Idh/MocA family, glycerol-3-phosphate dehydrogenase GpsA and thioredoxin-like TlpA oxidoreductases. Redox enzymes such as DsbB and DsbC are core genes with signal sequences, and they have catalytic rather than structural disulfides. These two enzymes are not identified as DsbA substrates in our filter as they have an odd number of cysteines.
The list of 263 proteins with an even number of cysteines was initially screened against the Virulence Factor DataBase (VFDB) [46], the Burkholderia Genome Database (BGD) [47] and against a list of B. pseudomallei virulence genes identified by previous studies [48, 49]. Of the 263 putative DsbA substrates two are closely related to virulence factors from the VFDB (flagellar proteins FlgA and FlhG), six are close homologues to proteins identified previously by Moule et al. [48] five reported by Holden et. al. [49] and one identified from the BGD, giving a total of 14 virulence factors identified through cross-analysis (see S1 File for a full list). It was also noted that two of the 14 identified putative virulence factors, were homologous to the same collagenase (BPSS0666).
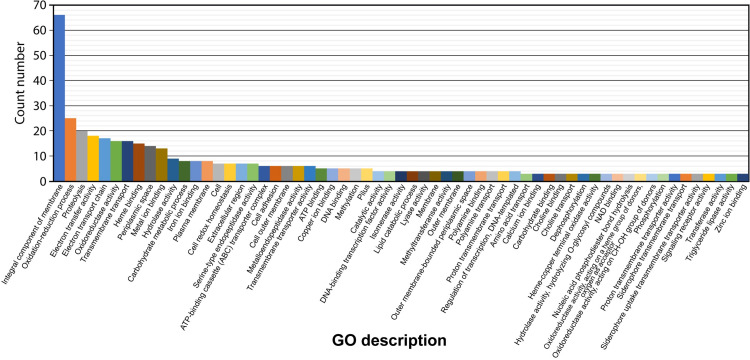
Gene Ontology (GO) classification of the gene and gene-product function of the 263 proteins revealed a variety of functions, totalling 223 GO descriptions (Fig 3) (see S1 File). The highest frequency are integral components of the membrane (66 proteins), followed by proteins involved in redox processes (25 proteins). Of particular interest due to their putative involvement in virulence, are proteins associated with: proteolysis (20), heme binding (15), hydrolase activity (9), carbohydrate metabolism (8), serine-type endopeptidase activity (7), cell adhesion (6), metallo-endopeptidase activity (6), pilus formation and organisation (6), copper binding (5), lipid catabolism (4), choline binding (3), triglyceride lipase activity (3), aminopeptidase activity (2), porin activity (OmpA family proteins) (2), chitin catabolism (1), N-carbamoylputrescine amidase activity (1) and toxin activity (Tat pathway signal protein) (1).
Fig 3. Gene Ontology (GO) descriptions of predicted extra-cytoplasmic proteins with an even number of cysteines.
The highest frequency of proteins with an even number of cysteines are integral components of membranes (66 proteins), followed by proteins involved in redox (oxidation-reduction) processes (25 proteins) and proteolysis (20 proteins). For ease of representation and clarity, GO descriptors with less than three counts were excluded from this graph. A complete graph, along with raw values can be found in S1 File.
By further inspection of the 263 core, putatively extra-cytoplasmic DsbA substrates, and by using the GO descriptions to aid in predicting protein functions, 73 sequences were identified which were virulence-associated (Table 2). These include serine-type endopeptidases [50] associated with adherence, choline binding proteins [51], N-carbamoylputrescine amidase, essential for production of putrescine, a component of Gram-negative cell walls of pathogens and key virulence [52–55], and many proteases and peptidases.
Table 2. Predicted virulence-associated core, extra-cytoplasmic proteins.
| Virulence-associated GO description | Accession numbers |
|---|---|
| Aminopeptidase activity | ABA50277.1; WP_053292838.1 |
| Bacterial-type flagellum assembly | WP_004525898.1 |
| β-lactamase activity | KGV04506.1 |
| Carbohydrate metabolic processes | ABA52198.1; EDO83218.1; EEH25224.1; WP_004526045.1; WP_004526830.1; WP_004553625.1; WP_053293009.1 |
| Cell adhesion/lipid metabolic/catabolic process/chitinase | WP_004193933.1 |
| Cell adhesion/pillus | EDU07436.1; WP_004193385.1; WP_038760383.1; WP_038765499.1; WP_063597677.1 |
| Chitin catabolic process | WP_076802983.1 |
| Choline binding and transport | ABA51731.1; ABN86005.1; ABN92885.1 |
| Copper ion binding | WP_004529973.1; WP_004546221.1 |
| Heme binding | WP_004194773.1; WP_004535805.1; WP_004536717.1; WP_004538457.1; WP_004538458.1; WP_038730764.1; WP_041189005.1; WP_043304483.1; WP_076903047.1; WP_139900217.1; WP_151277731.1 |
| Heme binding/copper ion binding | WP_029671417.1; WP_122827599.1 |
| Heme binding/proteolysis | WP_009981622.1 |
| Heme bindingcopper ion binding | WP_080248664.1 |
| Hydrolase activity | CFL10512.1; EEC34719.1; WP_004525656.1; WP_024428578.1; WP_024429096.1; WP_080300428.1 |
| Lipid metabolic/catabolic process | WP_009956690.1; WP_080248725.1 |
| Metallopeptidase/metalloendopeptidase activity | AFR18870.1; WP_004548157.1; WP_011204325.1; WP_038708181.1; WP_038730428.1; WP_076887541.1 |
| N-carbamoylputrescine amidase activity | WP_045597613.1 |
| Penicillin binding/β-lactamase activity | EDO89205.1 |
| Pillus and pillus organisation | WP_151269450.1 |
| Porin activity | WP_004189892.1; WP_011205039.1 |
| Proteolysis/hydrolase activity | WP_011204795.1; WP_076852667.1 |
| Serine-type endopeptidase/carboxypeptidase activity | ABA50268.1; ACQ98979.1; AFR20596.1; WP_004528537.1; WP_004529035.1; WP_004553586.1; WP_011852052.1; WP_024428782.1; WP_038778478.1 |
| Toxin activity | WP_038707916.1 |
| Triglyceride lipase activity | EEH28759.1; WP_038741497.1; WP_038775093.1 |
| Xenobiotic transmembrane transporter activity | WP_004534049.1 |
| Putative virulence factors identified from literature and VFDB | Accession numbers |
| Acid phosphatase activity | WP_122651768.1 |
| Endoribonuclease activity | WP_004194152.1 |
| Catalytic activity | WP_065793661.1 |
| DNA-binding transcription factor activity | WP_004524330.1 |
| Methylation | AHE31311.1 |
| NAD Binding | WP_004527508.1 |
| N/A | OMW33686.1 |
| Bacterial-type flagellum assembly | WP_004198637.1 |
Analysis of the 263 putative DsbA substrates revealed 73 proteins associated with virulence, based on GO descriptions. In addition, 8 proteins were identified as potential virulence factors from literature or from database screening. Accession numbers from B. pseudomallei are shown, separated by a semicolon.
Our GO description analysis identified many more potential virulence associated genes (73 in total) as compared to the 14 found on the VFDB, BGD and through literature [46–49]. Six of the putative virulence factors were common to both our GO analysis and to previous analyses (See S1 and S2 Files for full lists), to give a total of 81 identified putative virulence factors.
The 73 putative virulence factors sequences identified by our GO analysis, along with the 8 additional sequences found in the literature and databases, are grouped in Table 2 (the six common sequences found on both lists are displayed with the GO analysis results and underlined). Interestingly, one protein is annotated as a DNA transcriptional regulator from the AraC family (WP_004524330.1) a suspected cytoplasmic protein, although no experimental subcellular localisation information can be found [47]. As a cytoplasmic protein cannot be a substrate of the periplasmic DsbA protein, further experimental studies are needed to confirm the localisation.
Sequence homology prediction of B. pseudomallei DsbA virulence factor substrates
To complement the genomic analysis described above we used a second approach to identify DsbA substrates, by screening all B. pseudomallei genomes uploaded on NCBI [56] (taxid 28450) for homologues of known DsbA substrates. We implemented this approach because some DsbA substrates might be filtered out using the genomic approach described above if the substrates are not encoded by core genes, or if the gene product has an odd number of cysteines.
Over 90 DsbA substrates have been reported in the literature. We searched for B. pseudomallei homologues of these DsbA substrates using the following criteria: (i) presence of secretion signal, (ii) at least two cysteines in the mature sequence, (iii) at least 20% identity and (iv) 50% coverage to a known DsbA substrate sequence. After removing duplicates, our analysis found that B. pseudomallei encodes homologues of 15 DsbA substrates (Table 3). Two of these 15 are DsbA substrates in other Burkholderia species B. cepacia and B. cenocepacia [57–60]: a metalloproteases, ZmpA and a sulfatase-like hydrolase transferase. In B. cenocepacia, ZmpA is a wide spectrum metalloprotease, thought to cause tissue damage during infection [61].
Table 3. List of B. pseudomallei proteins homologous to previously reported DsbA substrates.
| Accession Number (DsbA substrate) | Organism | Reference | B. pseudomallei homologue | Identity / coverage (%) | Protein function | Cys # |
|---|---|---|---|---|---|---|
| WP_059237834 | B. cepacia | [57] | WP_076835606.1 | 89 /100 | Sulfatase like hydrolase /transferase | 3 |
| WP_006481898 | B. cenocepacia | [58, 59] | WP_139900467 | 87/100 | M4 family metallopeptidase | 4 |
| gi|89255876 | F. tularensis | [43] | WP_050859308 | 24/92 | lytic transglycosylase | 3 |
| gi|89255615 | F. tularensis | [43] | WP_080367462 | 40/51 | Pilin | 2 |
| gi|89255615 | F. tularensis | [43] | WP_076953316 | 27/92 | Pilin | 2 |
| gi|89256194 | F. tularensis | [43] | WP_041862011 | 30/83 | Molybdopterin synthase adenyl transferase (MoeB) | 13 |
| gi|89256236 | F. tularensis | [43] | WP_064459078 | 34/53 | DNA/RNA endonuclease | 2 |
| gi|89256237 | F. tularensis | [43] | WP_050772403 | 31/90 | PenI family β-lactamase | 4 |
| gi|89256856 | F. tularensis | [43] | WP_044360358 | 21/80 | hypothetical protein | 4 |
| gi|89256859 | F. tularensis | [43] | WP_058035453 | 39/80 | Polyamine ABC transporter substrate binding protein | 3 |
| gi|89257049 | F. tularensis | [43] | WP_009915682 | 54/99 | Succinate dehydrogenase | 6 |
| WP_001363619 | E. coli | [31] | WP_102811167 | 38/88 | Molecular chaperone | 3 |
| AAC38377 | E. coli | [31] | WP_082252625 | 44/93 | T3SS outer membrane ring protein | 4 |
| AAA24962 | Heamophilus Influenza | [31] | WP_053293022 | 47/92 | ABC transporter substrate binding protein | 4 |
| CAA43967 | Yersinia pestis | [31] | WP_085538626 | 32/83 | Pilus assembly protein PapD | 2 |
The accession number of the known DsbA substrate (in an organism other than B. pseudomallei), the organism and the publication reference are given in the first three columns. The corresponding B. pseudomallei homologue is given in the fourth column. The identity and coverage (number of residues in the result sequence that overlap with the search sequence) is given in percent in the column “identity/coverage”. The final two columns provide the protein function and the number of cysteines in the predicted mature sequence. All proteins in this table are known or predicted to be secreted or periplasmic.
Over 50 DsbA substrates in Francisella tularensis were identified by trapping and co-purifying substrates bound to a DsbA variant [43]. Of these 50, we found nine homologues encoded in B. pseudomallei (see Table 3). These include homologues of the lytic transglycosylase domain containing protein (implicated in peptidoglycan rearrangement) and homologues of two pilin proteins involved in the formation of pilus and flagella. Also present is an MoeB homologue; MoeB is a molybdopterin synthase adenyl transferase (cytoplasmic in E. coli but likely periplasmic in B. pseudomallei due to the twin-arginine translocation (TAT) signal sequence). A PenI family β-lactamase homologue is also found in B. pseudomallei; this is a class A β-lactamase that confers resistance to β-lactams including, in rare cases, ceftazidime (commonly used to treat melioidosis) [62]. A succinate dehydrogenase flavoprotein subunit homologue, found in the bacterial inner membrane and part of the electron transport chain, is also encoded in B. pseudomallei. This protein is cytoplasmically oriented in E. coli, though again the B. pseudomallei version has a TAT signal sequence suggesting a possible periplasmic localisation.
A number of DsbA substrates identified in E. coli (reviewed in [31]) have B. pseudomallei homologues including a molecular chaperone homologous to PapD and EscC, involved in the formation of the Type III secretion system (T3SS). The T3SS assembly requires DsbA activity in many Gram-negative bacteria, including E. coli and S. typhimurium [63, 64]. Finally, a B. pseudomallei protein homologous to the Y. pestis pilus assembly protein Caf1M (a molecular chaperone involved with assembly of the surface capsule of the bacterium) was also identified.
Of the 15 putative B. pseudomallei DsbA substrates identified using this substrate homology method, two were also identified in the genomic pipeline method. These are the PenI (WP_050772403) and a molecular chaperon (WP_102811167).
We then aligned the sequences of the Table 3 B. pseudomallei proteins to identify any possible sequence conservation around the cysteine residues, but no pattern was identified. This lack of peptide sequence motif in DsbA substrates has also been observed in E.coli, demonstrating the difficulty of DsbA substrate prediction [65].
Epitope prediction of virulence-associated proteins
To determine whether the DsbA substrates identified in the two methods above could contribute to vaccination efforts against B. pseudomallei, we also predicted B-cell epitopes, using a structure-informed approach. The sequences of the 81 putative, extra-cytoplasmic DsbA substrates (predicted virulence factors, Table 2) along with the 13 unique, homologous DsbA substrates (Table 3) were screened against the Protein Data Bank (PDB) [66], to identify structurally characterised homologues. Seven of the 94 proteins were found to have at least 80% similarity to a structurally characterised protein. Three of these seven protein structures were from Pseudomonas species, while the other four were from Burkholderia species. Similarity was used rather than identity to account for mutations of functionally similar residues. The seven protein structures were then used as models to predict structurally-informed B-cell epitopes of length 10–32 residues (Table 4 and Fig 4) using the SEPPA 3.0 server. While SEPPA 3.0 is considered the foremost B-cell epitope predictor, the software also accounts for potential glycosylation of the peptide [67], a feature that is mostly absent from bacterial proteins. To ensure that the epitopes identified by SEPPA 3.0 were not the result of erroneous glycosylation interpretation, the epitopes were cross-validated using ElliPro software that does not rely on glycosylation patterns [68]. All hits obtained with SEPPA 3.0 were also identified with ElliPro, with 1–3 residue differences in the starting and ending residues, suggesting that they were not based on wrongly attributed glycosylation patterns. However, we recommend using the more stringent list of epitopes identified with SEPPA 3.0 over the much longer list of potential epitopes and antigenic determinants identified with ElliPro.
Table 4. B-cell epitope prediction.
| Gene name | Predicted epitopes | Homologue PDB code | Accession number |
|---|---|---|---|
| β-lactamase Toho-1 | RREPELNTALPGDER; TTMRNPNAQARDDVIA | 3W4O | KGV04506.1 |
| type 1 fimbrial protein | SSKAYTIAEGDNTF | 5N2B | WP_063597677.1 |
| triacylglycerol lipase | SSTNNTNQDALA; AYVQQVLAATGASK | 1HQD | WP_038741497.1 |
| class D β-lactamase | VSGDPGQNNGLDR | 6NI0 | EDO89205.1 |
| triacylglycerol lipase | QQVLAVTGAQK; SHTHNTNQDAIA | 1HQD | WP_038775093.1 |
| S8 family serine peptidase | SGDEGVYECNNRGYPDGSNYTV; SNETVWNEGLDGNGKLW; YECNNRGYPDGSNYTV; MADLDASGNTGLTQ; QTNGSGGNYSDDQEG; GYSGYGYKASTGWDY | 1GA1/1NLU | WP_004553586.1 |
| UPD-glucose dehydrogenase | DVDQAKIDIlNNGGVPIHEPGlKEVIARNRSA | 2Y0E | WP_004527508.1 |
The virulence-associated putative DsbA substrates (Table 2) were screened for ≥80% similarity to proteins within the PDB to account for substitution of functionally similar residues. The structures were then screened for epitopes using SEPPA 3.0. Fourteen B-cell epitopes of 10 to 32 residues were predicted.
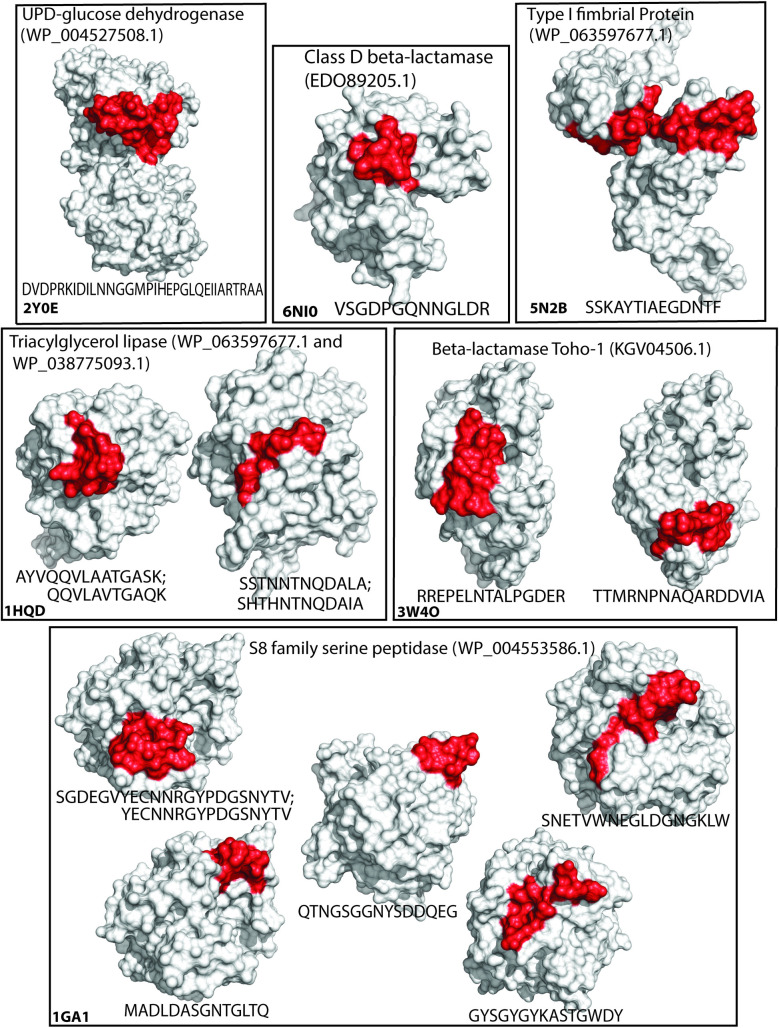
Fig 4. Predicted B-cell epitopes.
Graphical representation of B-cell epitopes found in Table 4. Proteins are shown as white surfaces and their respective PDB ID is given in the bottom left corner of each box. The epitope region is highlighted in red and the corresponding homologous sequences found in B. pseudomallei are given in one letter code under each respective structure and separated by semicolon when more than one sequence pointed to the same epitope.
These epitopes provide an interesting list for further evaluation. For example, epitopes from β-lactamase Toho-1 and class D β-lactamase could provide a useful vaccination approach for B. pseudomallei because these directly target antibiotic resistance proteins. Similar approaches have conferred protection against other bacteria in animal models [69–72].
Vaccination targeting adhesion proteins and essential virulence factors such as FimA [73] and type 1 fimbrial protein [74] is a commonly used approach due to the external localisation of these proteins and their exposure to host immune systems. Anti-fimbrial antibodies have been shown to interfere with function and reduce disease [75, 76] and a FimA vaccine provided protection against Streptococcus parasanguis, Streptococcus mitis, Streptococcus mutans and Streptococcus salivarius in rats [77–79].
Vaccination against conserved, secreted enzymes such as the triacylglycerol lipase (EstA) and S8 family serine peptidase enzymes may also be a useful strategy. Secreted peptidases are known virulence factors in many pathogenic bacteria [50, 80] and vaccines targeting them have attenuated disease in animal models [81, 82]. Two triacylglycerol lipases (WP_038741497.1 and WP_038775093.1) were identified as having a structural homologue in the PDB. These two lipases are both core genes and share 78% similarity (72% identity, 87% query cover) and their sequences were both aligned to the same PDB code, resulting in epitope variants of similar sequences.
Finally the UDP-glucose dehydrogenase appears to be a key player in the synthesis of exopolysaccharide in the B. cepacia complex [83], and is suspected to contribute to virulence and cystic fibrosis.
Discussion
In the present study, we analysed genomes from 512 B. pseudomallei isolates specifically associated with disease to identify core putative DsbA substrates and virulence factors. Pangenomic analysis of B. pseudomallei has previously been performed utilising 37 isolates from a variety of isolation sources [45] and concluded the pangenome to be ‘open’, indicating that new isolates will continually increase the number of total genes, which we found to be the case, based on a pangenome of 19,991 genes from 512 isolates. Previous studies comparing the B. pseudomallei genome with the obligate pathogen Burkholderia mallei (responsible for glanders) and the generally non-pathogenic Burkholderia thailandensis [84–87], identified several loci likely to be involved in B. pseudomallei virulence. These include the capsular polysaccharide gene cluster and Type III secretion needle complex [87], which were not considered core genes, demonstrating the importance of large-scale analysis.
In the present study, we used two orthogonal approaches to identify a total of 278 putative DsbA substrates, with 94 predicted to be virulence factors (S2 File). Of these, 73 were identified by the genome analysis approach, 8 more via comparison to previous studies and 15 were identified by the DsbA substrate homology approach, with two of the putative 94 DsbA virulence factor substrates identified in both genomic and homology analysis. These two are the experimentally validated bacterial virulence factors and DsbA substrates—a molecular chaperon (reported to be an E. coli DsbA substrate [31]), and a PenI family β-lactamase (reported to be a F. tularensis DsbA substrates) [43].
Delving deeper into the results presents some curious outcomes. For example, the well-characterised E.coli DsbA substrate and virulence factor FlgI [36, 88] was not picked up as a potential B. pseudomallei DsbA substrate by either method, though B. pseudomallei encodes FlgI. The B. pseudomallei FlgI sequence has 4 cysteines in the translated gene product but the predicted mature sequence after cleavage of the signal sequence has just one cysteine. Generally, DsbA does not interact with proteins having just one cysteine. If B. pseudomallei FlgI is a DsbA substrate (that is yet to be tested), then the most likely reasons that it was not identified as a substrate by either of the two methods we used are that (i) the predicted signal peptide is incorrect and/or (ii) the single cysteine of B. pseudomallei FlgI forms an inter-molecular disulfide bond.
The finding that the two orthogonal approaches identified the same two target proteins suggests that there is merit in using different theoretical approaches to select high priority targets for further evaluation (in this case, the PenI family β-lactamase and the molecular chaperon). On the other hand, the fact that there were so few overlaps in the predicted substrates from the two methods raises questions about the filters we applied. Specifically, we found that of the 15 potential substrates identified by the substrate homology method, 5 had an odd numbers of cysteines, whereas the genomic analysis filtered these proteins out of consideration to reduce the number of false negatives. We applied the even cysteine filter because previous reports showed that E. coli exported proteins have a strong preference for an even number of cysteines [42]. This even number of cysteine preference is present in B. pseudomallei exported proteins (Fig 2) though is not as pronounced as in E. coli. By restricting our genomic analysis to core, extra-cytoplasmic B. pseudomallei proteins with an even number of cysteines, some DsbA substrates may therefore have been missed. There is considerable evidence that many virulence factors such as adhesion and motility proteins, toxins and enzymes are extra-cytoplasmic proteins in both Gram-positive and Gram-negative bacteria [31, 32, 89]. Given that extra-cytoplasmic proteins in the translated core genome of B. pseudomallei have a slight preference for even number of cysteines (Fig 2) and the identification of many virulence-associated proteins within the 263 proteins in the list, the approach taken in this analysis (Fig 1) to identify DsbA substrates was justified. Further, the genomic analysis focused on highly conserved proteins from the core genome; accessory proteins associated with virulence would not be identified using this approach. Nevertheless, the genomic analysis identified homologues of known DsbA substrates in other bacteria, such as the OmpA porin, supporting the use of this approach. However, attempting to identify epitopes from proteins which are not found in every disease-causing isolate may present challenges for anti-virulence and vaccination attempts.
In addition, the genomic analysis identified several proteins of unknown function which could represent novel virulence factors for future studies. Importantly, our theoretical approach was extended to predict structurally-informed surface epitopes for several core gene DsbA substrates for potential vaccine or antibody development (Table 4).
In summary, our in silico analysis combined a substrate homology approach and a genomic analysis approach to identify more than 90 potential B. pseudomallei DsbA virulence factor substrates, two of which we mark as high priority for experimental validation. Future characterization of these proteins will aid our understanding of B. pseudomallei virulence and could provide new targets for anti-virulence drug discovery and vaccine development. The approaches we report here could also be applied to identify potential DsbA virulence factor substrates in other pathogenic bacteria.
Methods
Data acquisition and filtering of core, extra-cytoplasmic, putative DsbA substrates
1577 B. pseudomallei genomes were obtained from the genome information table from NCBI (https://www.ncbi.nlm.nih.gov/genome/genomes/476) (date accessed: 1/2/20). The biosample accession numbers were batch downloaded using Entrez. A list of assembly accession numbers can be found in S1 Data. Metadata was then scraped for disease association using grep with the following command:
grep -A 1 "disease"
The assemblies were then downloaded using Entrez and annotated using a prokka (version 1.14.5) [90] for loop with the following command:
for file in *.fna; do tag = ${file%.fna}; prokka—prefix "$tag"—locustag "$tag"—genus Burkholderia—species pseudomallei—strain "$tag"—outdir "$tag"_prokka—force—addgenes "$file"; done
The.gff files were used as input for roary (version 3.11.2) [91] without splitting paralogues via the following command:
roary -e—mafft -i 90 -v -p 72 -z -s -o output -f *.gff
The roary output file was altered from interleaved fasta to one line per sequence
awk '{if(NR = = 1) {print $0} else {if($0 ~ /^>/) {print "\n"$0} else {printf $0}}}' input.fa > output.fa
The core genome was then used in the remaining analysis and core DNA sequences were translated into protein sequences using transeq [92] with the following command:
transeq -sequence input.fasta -outseq output.fasta -table 11 -frame 1
The core genome was then filtered based on signal sequence and then the sequence of the mature exported protein, as predicted utilising SignalP 5.0 [93, 94].
signalp -fasta prot_core_genome_complete.fasta -format short -mature -org gram- -verbose
These sequences were then filtered for genes containing even numbers of cysteines
awk -F \C 'NF % 2' < input.fasta | awk "/C.*C/" | sed '/>/{$!N;/\n.*>/!P;D}' > output.fasta
This list was then annotated via screening sequences against NCBI and Gene Ontology [95] using the PANNZER2 server [96].
Identification of DsbA substrate homologues in B. pseudomallei
DsbA substrates were also predicted using a substrate homology search. This approach may identify proteins not encoded in the core genome. The B. pseudomallei genome was screened for homologues of known DsbA substrates using BLASTP. A starting list of confirmed DsbA substrates was extracted from the literature [31, 43, 57–61], and their amino acid sequences used in BLAST searches [97] against the NCBI protein database [56] for homologues in B. pseudomallei using default search parameters. In some cases two search proteins identified the same homologue in B. pseudomallei. In these cases only the search protein most similar to the B. pseudomallei homologue is given in Table 3. The results were filtered to select proteins with at least 20% sequence identity and a sequence coverage of at least 50%. Protein sequences with fewer than two cysteines were removed. Exported proteins were selected on the basis of predicted signal sequence (SignalP 5.0 [93]) or experimental evidence of extra-cytoplasmic localisation for the reported DsbA substrate in another Burkholderia species.
Identification of putative virulence factors
ABRicate version 1.0.1 (https://github.com/tseemann/abricate) [98] was used, along with the virulence factor database (VFDB) [46] to identify the presence of putative virulence factors of the putative paired cysteine gene list. 244 genes identified as virulence-related, on the basis of mutagenesis studies [49, 99, 100] were also screened against the paired cysteine gene list using blastp version 2.9.0+ [97, 101] and results were filtered for ≥90% coverage and ≥80% similarity/positives to be considered a putative virulence factor. Additionally, the burkholderia.com virulence database [47] was downloaded and screened against gene lists using blastp version 2.9.0+ with the same filtering conditions.
Cysteine distribution analysis
Fasta files containing either the 19,991 pan genes or the 4,496 core gene of B. pseudomallei with their corresponding amino acid sequences and descriptors were utilised to calculate the distribution of cysteines with a custom Python 3.0 script (available on Github: (https://github.com/gpetit99/cysteineCount_bPseudomallei/blob/master/CysCountFrequency.py”). Briefly, lists of the extra-cytoplasmic protein sequences with signal peptides removed were compared to lists of the protein sequences from the whole genome to create dataframes with either cytoplasmic or extra-cytoplasmic proteins. Proteins were grouped based on the presence or absence of SP, and based on the number of cysteines in the mature protein. To calculate the normalised frequency of cysteines for extra-cytoplasmic proteins, we divided the number of extra-cytoplasmic proteins having N cysteines by the total number of proteins having N cysteines (N being an integer from 0 to 73 –No protein has more than 73 cysteines in the B. pseudomallei translated genome). This analysis was run for the core genome and pangenome independently. Other statistics (e.g. number of proteins in each group) were extracted from the dataframes.
Epitope prediction
The metadata for each of the 263 proteins in the annotated list was manually inspected to select for further analysis a total of 81 proteins likely related to virulence. The sequences of these 81 selected proteins were combined with the 13 unique proteins from the homology analysis (to give 94 unique protein sequences). These were screened against the protein data bank using BLAST (criteria: ≥80% positive substitutions/similarity used as a threshold) to find structurally characterised homologues. These structural homologues were then used to predict B-cell epitopes using SEPPA 3.0 (http://www.badd-cao.net/seppa3/index.html) with a threshold of 0.1 [67]. Similarity was used rather than identity to account for mutations of functionally similar residues. Predicted B-cell epitopes were accepted if they were 10–32 residues in length, as described in [102]. The same structural homologues were also tested with the ElliPro server [68] and the resulting epitope sequences compared with the results from SEPPA 3.0 to ensure that the results were redundant and method independent.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Acknowledgments
We gratefully acknowledge the support of the eResearch Services Team at Griffith University and the use of the High Performance Computing Cluster "Gowonda" to complete this research.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by an Australian National Health and Medical Research Council (www.nhmrc.gov.au) Project Grant (GRT1144046) to JLM; GAP and MAH were also supported by this award. BV was in receipt of a Research Fellowship from Griffith University. GAP is supported by a Griffith University Post-Graduate Research Scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.White NJ. Melioidosis. The Lancet 2003;361(9370):1715–22. 10.1016/s0140-6736(03)13374-0 [DOI] [PubMed] [Google Scholar]
- 2.Chakravorty A, Heath CH. Melioidosis: An updated review. Aust J Gen Pract 2019;48(5):327–32. 10.31128/AJGP-04-18-4558 [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga WJ, Virk HS, Torres AG, Currie BJ, Peacock SJ, Dance DAB, et al. Melioidosis. Nat Rev Dis Primers 2018;4:17107 10.1038/nrdp.2017.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willcocks SJ, Denman CC, Atkins HS, Wren BW. Intracellular replication of the well-armed pathogen Burkholderia pseudomallei. Curr Opin Microbiol 2016;29:94–103. 10.1016/j.mib.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 5.Held K, Gasper J, Morgan S, Siehnel R, Singh P, Manoil C. Determinants of extreme β-Lactam tolerance in the Burkholderia pseudomallei complex. Antimicrob Agents Chemother 2018;62(4). 10.1128/AAC.00068-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Podnecky NL, Rhodes KA, Mima T, Drew HR, Chirakul S, Wuthiekanun V, et al. Mechanisms of resistance to folate pathway inhibitors in Burkholderia pseudomallei: Deviation from the norm. mBio 2017;8(5). 10.1128/mBio.01357-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podnecky NL, Rhodes KA, Schweizer HP. Efflux pump-mediated drug resistance in Burkholderia. Front Microbiol 2015;6:305 10.3389/fmicb.2015.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes KA, Schweizer HP. Antibiotic resistance in Burkholderia species. Drug Resist Updat 2016;28:82–90. 10.1016/j.drup.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweizer HP. Mechanisms of antibiotic resistance in Burkholderia pseudomallei: implications for treatment of melioidosis. Future Microbiol 2012;7(12):1389–99. 10.2217/fmb.12.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treatment Dance D. and prophylaxis of melioidosis. Int J Antimicrob Agents 2014;43(4):310–8. 10.1016/j.ijantimicag.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Antimicrobial resistance: Global report on surveillance. France; 2014 [cited 22 April, 2020]. Available from www.who.int/antimicrobial-resistance/publications/surveillancereport/en
- 12.Kennedy DA, Read AF. Why the evolution of vaccine resistance is less of a concern than the evolution of drug resistance. Proc Natl Acad Sci U S A 2018;115(51):12878–86. 10.1073/pnas.1717159115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thabit AK, Crandon JL, Nicolau DP. Antimicrobial resistance: impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opin Pharmacother 2015;16(2):159–77. 10.1517/14656566.2015.993381 [DOI] [PubMed] [Google Scholar]
- 14.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov 2010;9(2):117–28. 10.1038/nrd3013 [DOI] [PubMed] [Google Scholar]
- 15.Johnson MM, Ainslie KM. Vaccines for the prevention of melioidosis and glanders. Curr Trop Med Rep 2017;4(3):136–45. 10.1007/s40475-017-0121-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lafontaine ER, Chen Z, Huertas-Diaz MC, Dyke JS, Jelesijevic TP, Michel F, et al. The autotransponrter protein BatA is a protective antigen against lethal aerosol infection with Burkholderia mallei and Burkholderia pseudomallei. Vaccine: X 2019;1 10.1016/j.jvacx.2018.100002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morici L, Torres AG, Titball RW. Novel multi-component vaccine approaches for Burkholderia pseudomallei. Clin Exp Immunol 2019;196(2):178–88. 10.1111/cei.13286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burtnick MN, Shaffer TL, Ross BN, Muruato LA, Sbrana E, DeShazer D, et al. Development of subunit vaccines that provide high-level protection and sterilizing immunity against acute inhalational melioidosis. Infect Immun 2018;86(1). 10.1128/IAI.00724-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casey WT, Spink N, Cia F, Collins C, Romano M, Berisio R, et al. Identification of an OmpW homologue in Burkholderia pseudomallei, a protective vaccine antigen against melioidosis. Vaccine 2016;34(23):2616–21. 10.1016/j.vaccine.2016.03.088 [DOI] [PubMed] [Google Scholar]
- 20.Muruato LA, Tapia D, Hatcher CL, Kalita M, Brett PJ, Gregory AE, et al. Use of reverse vaccinology in the design and construction of nanoglycoconjugate vaccines against Burkholderia pseudomallei. Clin Vaccine Immunol 2017;24(11). 10.1128/CVI.00206-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Champion OL, Gourlay LJ, Scott AE, Lassaux P, Conejero L, Perletti L, et al. Immunisation with proteins expressed during chronic murine melioidosis provides enhanced protection against disease. Vaccine 2016;34(14):1665–71. 10.1016/j.vaccine.2016.02.038 [DOI] [PubMed] [Google Scholar]
- 22.Hara Y, Mohamed R, Nathan S. Immunogenic Burkholderia pseudomallei outer membrane proteins as potential candidate vaccine targets. PLoS One 2009;4(8):e6496 10.1371/journal.pone.0006496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva EB, Goodyear A, Sutherland MD, Podnecky NL, Gonzalez-Juarrero M, Schweizer HP, et al. Correlates of immune protection following cutaneous immunization with an attenuated Burkholderia pseudomallei vaccine. Infect Immun 2013;81(12):4626–34. 10.1128/IAI.00915-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khakhum N, Bharaj P, Myers JN, Tapia D, Kilgore PB, Ross BN, et al. Burkholderia pseudomallei ΔtonB Δhcp1 live attenuated vaccine strain elicits full protective immunity against aerosolized melioidosis infection. mSphere 2019;4(1). 10.1128/mSphere.00570-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srilunchang T, Proungvitaya T, Wongratanacheewin S, Strugnell R, Homchampa P. Construction and characterization of an unmarked aroC deletion mutant of Burkholderia pseudomallei strain A2. Southeast Asian J Trop Med Public Health 2009;40(1):123–30. [PubMed] [Google Scholar]
- 26.Nagpal G, Usmani SS, Raghava GPS. A web resource for designing subunit vaccine against major pathogenic species of bacteria. Front Immunol 2018;9:2280 10.3389/fimmu.2018.02280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muhlen S, Dersch P. Anti-virulence strategies to target bacterial infections. Curr Top Microbiol Immunol 2016;398:147–83. 10.1007/82_2015_490 [DOI] [PubMed] [Google Scholar]
- 28.Heras B, Scanlon MJ, Martin JL. Targeting virulence not viability in the search for future antibacterials. Br J Clin Pharmacol 2015;79(2):208–15. 10.1111/bcp.12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anfinsen CB. Principles that govern the folding of protein chains. Science (New York, NY) 1973;181(4096):223–30. 10.1126/science.181.4096.223 [DOI] [PubMed] [Google Scholar]
- 30.Bocian-Ostrzycka KM, Grzeszczuk MJ, Banas AM, Jagusztyn-Krynicka EK. Bacterial thiol oxidoreductases—from basic research to new antibacterial strategies. Appl Microbiol Biotechnol 2017;101(10):3977–89. 10.1007/s00253-017-8291-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heras B, Shouldice SR, Totsika M, Scanlon MJ, Schembri MA, Martin JL. DSB proteins and bacterial pathogenicity. Nat Rev Microbiol 2009;7(3):215–25. 10.1038/nrmicro2087 [DOI] [PubMed] [Google Scholar]
- 32.Smith RP, Paxman JJ, Scanlon MJ, Heras B. Targeting bacterial Dsb proteins for the development of anti-virulence agents. Molecules 2016;21(7). 10.3390/molecules21070811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shouldice SR, Heras B, Walden PM, Totsika M, Schembri MA, Martin JL. Structure and function of DsbA, a key bacterial oxidative folding catalyst. Antioxid Redox Signal 2011;14(9):1729–60. 10.1089/ars.2010.3344 [DOI] [PubMed] [Google Scholar]
- 34.Ireland PM, McMahon RM, Marshall LE, Halili M, Furlong E, Tay S, et al. Disarming Burkholderia pseudomallei: structural and functional characterization of a disulfide oxidoreductase (DsbA) required for virulence in vivo. Antioxid Redox Signal 2014;20(4):606–17. 10.1089/ars.2013.5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahon RM, Ireland PM, Sarovich DS, Petit G, Jenkins CH, Sarkar-Tyson M, et al. Virulence of the melioidosis pathogen Burkholderia pseudomallei requires the oxidoreductase membrane protein DsbB. Infect Immun 2018;86(5). 10.1128/IAI.00938-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dailey FE, Berg HC. Mutants in disulfide bond formation that disrupt flagellar assembly in Escherichia coli. Proc Natl Acad Sci U S A 1993;90(3):1043–7. 10.1073/pnas.90.3.1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Totsika M, Heras B, Wurpel DJ, Schembri MA. Characterization of two homologous disulfide bond systems involved in virulence factor biogenesis in uropathogenic Escherichia coli CFT073. J Bacteriol 2009;191(12):3901–8. 10.1128/JB.00143-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurth F, Rimmer K, Premkumar L, Mohanty B, Duprez W, Halili MA, et al. Comparative sequence, structure and redox analyses of Klebsiella pneumoniae DsbA show that anti-virulence target DsbA enzymes fall into distinct classes. PLoS One 2013;8(11):e80210 10.1371/journal.pone.0080210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heras B, Totsika M, Jarrott R, Shouldice SR, Guncar G, Achard ME, et al. Structural and functional characterization of three DsbA paralogues from Salmonella enterica serovar typhimurium. J Biol Chem 2010;285(24):18423–32. 10.1074/jbc.M110.101360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straskova A, Pavkova I, Link M, Forslund A-L, Kuoppa K, Noppa L, et al. Proteome analysis of an attenuated Francisella tularensis dsbA mutant: Identification of potential DsbA substrate proteins. J Proteome Res 2009;8(11):5336–46. 10.1021/pr900570b [DOI] [PubMed] [Google Scholar]
- 41.Hatahet F, Boyd D, Beckwith J. Disulfide bond formation in prokaryotes: history, diversity and design. Biochim Biophys Acta 2014;1844(8):1402–14. 10.1016/j.bbapap.2014.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dutton RJ, Boyd D, Berkmen M, Beckwith J. Bacterial species exhibit diversity in their mechanisms and capacity for protein disulfide bond formation. Proc Natl Acad Sci U S A 2008;105(33):11933–8. 10.1073/pnas.0804621105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren G, Champion MM, Huntley JF. Identification of disulfide bond isomerase substrates reveals bacterial virulence factors. Mol Microbiol 2014;94(4):926–44. 10.1111/mmi.12808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benson DA, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res 2015;43(Database issue):D30–5. 10.1093/nar/gku1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spring-Pearson SM, Stone JK, Doyle A, Allender CJ, Okinaka RT, Mayo M, et al. Pangenome analysis of Burkholderia pseudomallei: Genome evolution preserves gene order despite high recombination Rates. PLoS One 2015;10(10):e0140274 10.1371/journal.pone.0140274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen L, Zheng D, Liu B, Yang J, Jin Q. VFDB 2016: hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res 2016;44(D1):D694–7. 10.1093/nar/gkv1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winsor GL, Khaira B, Van Rossum T, Lo R, Whiteside MD, Brinkman FS. The Burkholderia Genome Database: facilitating flexible queries and comparative analyses. Bioinformatics 2008;24(23):2803–4. 10.1093/bioinformatics/btn524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moule MG, Spink N, Willcocks S, Lim J, Guerra-Assuncao JA, Cia F, et al. Characterization of new virulence factors involved in the intracellular growth and survival of Burkholderia pseudomallei. Infect Immun 2015;84(3):701–10. 10.1128/IAI.01102-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holden MT, Titball RW, Peacock SJ, Cerdeno-Tarraga AM, Atkins T, Crossman LC, et al. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc Natl Acad Sci U S A 2004;101(39):14240–5. 10.1073/pnas.0403302101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Backert S, Bernegger S, Skorko-Glonek J, Wessler S. Extracellular HtrA serine proteases: An emerging new strategy in bacterial pathogenesis. Cell Microbiol 2018;20(6):e12845 10.1111/cmi.12845 [DOI] [PubMed] [Google Scholar]
- 51.Gosink KK, Mann ER, Guglielmo C, Tuomanen EI, Masure HR. Role of novel choline binding proteins in virulence of Streptococcus pneumoniae. Infect Immun 2000;68(10):5690–5. 10.1128/iai.68.10.5690-5695.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamya MF, Ayoola MB, Park S, Shack LA, Swiatlo E, Nanduri B. The Role of Cadaverine Synthesis on Pneumococcal Capsule and Protein Expression. Med Sci (Basel) 2018;6(1). 10.3390/medsci6010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koski P, Vaara M. Polyamines as constituents of the outer membranes of Escherichia coli and Salmonella typhimurium. J Bacteriol 1991;173(12):3695–9. 10.1128/jb.173.12.3695-3699.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yethon JA, Vinogradov E, Perry MB, Whitfield C. Mutation of the lipopolysaccharide core glycosyltransferase encoded by waaG destabilizes the outer membrane of Escherichia coli by interfering with core phosphorylation. J Bacteriol 2000;182(19):5620–3. 10.1128/jb.182.19.5620-5623.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wortham BW, Oliveira MA, Fetherston JD, Perry RD. Polyamines are required for the expression of key Hms proteins important for Yersinia pestis biofilm formation. Environ Microbiol 2010;12(7):2034–47. 10.1111/j.1462-2920.2010.02219.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coordinators NR. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 2016;44(D1):D7–19. 10.1093/nar/gkv1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi S, Abe M, Kimoto M, Furukawa S, Nakazawa T. The DsbA‐DsbB disulfide bond formation system of Burkholderia cepacia is involved in the production of protease and alkaline phosphatase, motility, metal resistance, and multi‐drug resistance. Microbiol Immunol 2000;44(1):41–50. 10.1111/j.1348-0421.2000.tb01244.x [DOI] [PubMed] [Google Scholar]
- 58.Corbett CR, Burtnick MN, Kooi C, Woods DE, Sokol PA. An extracellular zinc metalloprotease gene of Burkholderia cepacia. Microbiology (Reading) 2003;149(Pt 8):2263–71. 10.1099/mic.0.26243-0 [DOI] [PubMed] [Google Scholar]
- 59.Abe M, Nakazawa T. The dsbB gene product is required for protease production by Burkholderia cepacia. Infect Immun 1996;64(10):4378–80. 10.1128/IAI.64.10.4378-4380.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kooi C, Subsin B, Chen R, Pohorelic B, Sokol PA. Burkholderia cenocepacia ZmpB is a broad-specificity zinc metalloprotease involved in virulence. Infect Immun 2006;74(7):4083–93. 10.1128/IAI.00297-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kooi C, Corbett CR, Sokol PA. Functional analysis of the Burkholderia cenocepacia ZmpA metalloprotease. J Bacteriol 2005;187(13):4421–9. 10.1128/JB.187.13.4421-4429.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papp-Wallace KM, Becka SA, Taracila MA, Winkler ML, Gatta JA, Rholl DA, et al. Exposing a β-lactamase "Twist": the mechanistic basis for the high level of ceftazidime resistance in the C69F variant of the Burkholderia pseudomallei PenI β-lactamase. Antimicrob Agents Chemother 2016;60(2):777–88. 10.1128/AAC.02073-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miki T, Okada N, Danbara H. Two periplasmic disulfide oxidoreductases, DsbA and SrgA, target outer membrane protein SpiA, a component of the Salmonella pathogenicity island 2 type III secretion system. J Biol Chem 2004;279(33):34631–42. 10.1074/jbc.M402760200 [DOI] [PubMed] [Google Scholar]
- 64.Miki T, Okada N, Kim Y, Abe A, Danbara H. DsbA directs efficient expression of outer membrane secretin EscC of the enteropathogenic Escherichia coli type III secretion apparatus. Microb Pathog 2008;44(2):151–8. 10.1016/j.micpath.2007.09.001 [DOI] [PubMed] [Google Scholar]
- 65.Paxman JJ, Borg NA, Horne J, Thompson PE, Chin Y, Sharma P, et al. The structure of the bacterial oxidoreductase enzyme DsbA in complex with a peptide reveals a basis for substrate specificity in the catalytic cycle of DsbA enzymes. J Biol Chem 2009;284(26):17835–45. 10.1074/jbc.M109.011502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The Protein Data Bank. Nucleic Acids Res 2000;28(1):235–42. 10.1093/nar/28.1.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou C, Chen Z, Zhang L, Yan D, Mao T, Tang K, et al. SEPPA 3.0-enhanced spatial epitope prediction enabling glycoprotein antigens. Nucleic Acids Res 2019;47(W1):W388–W94. 10.1093/nar/gkz413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ponomarenko J, Bui HH, Li W, Fusseder N, Bourne PE, Sette A, et al. ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC Bioinformatics 2008;9:514 10.1186/1471-2105-9-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lipsitch M, Siber GR. How can vaccines contribute to solving the antimicrobial resistance problem? mBio 2016;7(3). 10.1128/mBio.00428-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Senna JP, Roth DM, Oliveira JS, Machado DC, Santos DS. Protective immune response against methicillin resistant Staphylococcus aureus in a murine model using a DNA vaccine approach. Vaccine 2003;21(19–20):2661–6. 10.1016/s0264-410x(02)00738-7 [DOI] [PubMed] [Google Scholar]
- 71.Zarantonelli ML, Antignac A, Lancellotti M, Guiyoule A, Alonso JM, Taha MK. Immunogenicity of meningococcal PBP2 during natural infection and protective activity of anti-PBP2 antibodies against meningococcal bacteraemia in mice. J Antimicrob Chemother 2006;57(5):924–30. 10.1093/jac/dkl066 [DOI] [PubMed] [Google Scholar]
- 72.Ciofu O, Bagge N, Hoiby N. Antibodies against β-lactamase can improve ceftazidime treatment of lung infection with β-lactam-resistant Pseudomonas aeruginosa in a rat model of chronic lung infection. APMIS 2002;110(12):881–91. 10.1034/j.1600-0463.2002.1101207.x [DOI] [PubMed] [Google Scholar]
- 73.Fenno JC, Shaikh A, Spatafora G, Fives-Taylor P. The fimA locus of Streptococcus parasanguis encodes an ATP-binding membrane transport system. Mol Microbiol 1995;15(5):849–63. 10.1111/j.1365-2958.1995.tb02355.x [DOI] [PubMed] [Google Scholar]
- 74.Liu CC, Ou SC, Tan DH, Hsieh MK, Shien JH, Chang PC. The fimbrial protein is a virulence factor and potential vaccine antigen of Avibacterium paragallinarum. Avian Dis 2016;60(3):649–55. 10.1637/11410-031316-Reg.1 [DOI] [PubMed] [Google Scholar]
- 75.Singh B, Mortezaei N, Savarino SJ, Uhlin BE, Bullitt E, Andersson M. Antibodies damage the resilience of fimbriae, causing them to be stiff and tangled. J Bacteriol 2017;199(1). 10.1128/JB.00665-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmgren J, Svennerholm AM. Vaccines against mucosal infections. Curr Opin Immunol 2012;24(3):343–53. 10.1016/j.coi.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 77.Kitten T, Munro CL, Wang A, Macrina FL. Vaccination with FimA from Streptococcus parasanguis protects rats from endocarditis caused by other viridans streptococci. Infect Immun 2002;70(1):422–5. 10.1128/iai.70.1.422-425.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vandemaele F, Ververken C, Bleyen N, Geys J, D'Hulst C, Addwebi T, et al. Immunization with the binding domain of FimH, the adhesin of type 1 fimbriae, does not protect chickens against avian pathogenic Escherichia coli. Avian Pathol 2005;34(3):264–72. 10.1080/03079450500112682 [DOI] [PubMed] [Google Scholar]
- 79.Viscount HB, Munro CL, Burnette-Curley D, Peterson DL, Macrina FL. Immunization with FimA protects against Streptococcus parasanguis endocarditis in rats. Infect Immun 1997;65(3):994–1002. 10.1128/IAI.65.3.994-1002.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hritonenko V, Stathopoulos C. Omptin proteins: an expanding family of outer membrane proteases in Gram-negative Enterobacteriaceae. Mol Membr Biol 2007;24(5–6):395–406. 10.1080/09687680701443822 [DOI] [PubMed] [Google Scholar]
- 81.Marana MH, Jorgensen LV, Skov J, Chettri JK, Holm Mattsson A, Dalsgaard I, et al. Subunit vaccine candidates against Aeromonas salmonicida in rainbow trout Oncorhynchus mykiss. PLoS One 2017;12(2):e0171944 10.1371/journal.pone.0171944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santillan DA, Andracki ME, Hunter SK. Protective immunization in mice against group B streptococci using encapsulated C5a peptidase. Am J Obstet Gynecol 2008;198(1):114 e1–6. 10.1016/j.ajog.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 83.Rocha J, Popescu AO, Borges P, Mil-Homens D, Moreira LM, Sa-Correia I, et al. Structure of Burkholderia cepacia UDP-glucose dehydrogenase (UGD) BceC and role of Tyr10 in final hydrolysis of UGD thioester intermediate. J Bacteriol 2011;193(15):3978–87. 10.1128/JB.01076-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ong C, Ooi CH, Wang D, Chong H, Ng KC, Rodrigues F, et al. Patterns of large-scale genomic variation in virulent and avirulent Burkholderia species. Genome Res 2004;14(11):2295–307. 10.1101/gr.1608904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim HS, Schell MA, Yu Y, Ulrich RL, Sarria SH, Nierman WC, et al. Bacterial genome adaptation to niches: divergence of the potential virulence genes in three Burkholderia species of different survival strategies. BMC Genomics 2005;6:174 10.1186/1471-2164-6-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Majerczyk CD, Brittnacher MJ, Jacobs MA, Armour CD, Radey MC, Bunt R, et al. Cross-species comparison of the Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia mallei quorum-sensing regulons. J Bacteriol 2014;196(22):3862–71. 10.1128/JB.01974-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu Y, Kim HS, Chua HH, Lin CH, Sim SH, Lin D, et al. Genomic patterns of pathogen evolution revealed by comparison of Burkholderia pseudomallei, the causative agent of melioidosis, to avirulent Burkholderia thailandensis. BMC Microbiol 2006;6:46 10.1186/1471-2180-6-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hizukuri Y, Yakushi T, Kawagishi I, Homma M. Role of the intramolecular disulfide bond in FlgI, the flagellar P-ring component of Escherichia coli. J Bacteriol 2006;188(12):4190–7. 10.1128/JB.01896-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allen RC, Popat R, Diggle SP, Brown SP. Targeting virulence: can we make evolution-proof drugs? Nat Rev Microbiol 2014;12(4):300–8. 10.1038/nrmicro3232 [DOI] [PubMed] [Google Scholar]
- 90.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 2014;30(14):2068–9. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 91.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015;31(22):3691–3. 10.1093/bioinformatics/btv421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 2019;47(W1):W636–W41. 10.1093/nar/gkz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Almagro Armenteros JJ, Tsirigos KD, Sonderby CK, Petersen TN, Winther O, Brunak S, et al. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol 2019;37(4):420–3. 10.1038/s41587-019-0036-z [DOI] [PubMed] [Google Scholar]
- 94.Kall L, Krogh A, Sonnhammer EL. Advantages of combined transmembrane topology and signal peptide prediction—the Phobius web server. Nucleic Acids Res 2007;35(Web Server issue):W429–32. 10.1093/nar/gkm256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harris MA, Clark J, Ireland A, Lomax J, Ashburner M, Foulger R, et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res 2004;32(Database issue):D258–61. 10.1093/nar/gkh036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Toronen P, Medlar A, Holm L. PANNZER2: a rapid functional annotation web server. Nucleic Acids Res 2018;46(W1):W84–W8. 10.1093/nar/gky350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. NCBI BLAST: a better web interface. Nucleic Acids Res 2008;36(Web Server issue):W5–9. 10.1093/nar/gkn201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seemann T, Gruning B. [Available from: https://github.com/tseemann/abricate].
- 99.Moule MG, Hemsley CM, Seet Q, Guerra-Assuncao JA, Lim J, Sarkar-Tyson M, et al. Genome-wide saturation mutagenesis of Burkholderia pseudomallei K96243 predicts essential genes and novel targets for antimicrobial development. mBio 2014;5(1):e00926–13. 10.1128/mBio.00926-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cuccui J, Easton A, Chu KK, Bancroft GJ, Oyston PC, Titball RW, et al. Development of signature-tagged mutagenesis in Burkholderia pseudomallei to identify genes important in survival and pathogenesis. Infect Immun 2007;75(3):1186–95. 10.1128/IAI.01240-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinformatics 2009;10:421 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shey RA, Ghogomu SM, Esoh KK, Nebangwa ND, Shintouo CM, Nongley NF, et al. In-silico design of a multi-epitope vaccine candidate against onchocerciasis and related filarial diseases. Sci Rep 2019;9(1):4409 10.1038/s41598-019-40833-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.