Abstract
When oral bacteria accidentally enter the bloodstream due to transient tissue damage during dental procedures, they have the potential to attach to the endocardium or an equivalent surface of an indwelling prosthesis and cause infection. Many bacterial species produce extracellular vesicles (EVs) as part of normal physiology, but also use it as a virulence strategy. In this study, it was hypothesized that Granulicatella adiacens produce EVs that possibly help it in virulence. Therefore, the objectives were to isolate and characterize EVs produced by G. adiacens and to investigate its immune-stimulatory effects. The reference strain G. adiacens CCUG 27809 was cultured on chocolate blood agar for 2 days. From subsequent broth culture, the EVs were isolated using differential centrifugation and filtration protocol and then observed using scanning electron microscopy. Proteins in the vesicle preparation were identified by nano LC-ESI-MS/MS. The EVs proteome was analyzed and characterized using different bioinformatics tools. The immune-stimulatory effect of the EVs was studied via ELISA quantification of IL-8, IL-1β and CCL5, major proinflammatory cytokines, produced from stimulated human PBMCs. It was revealed that G. adiacens produced EVs, ranging in diameter from 30 to 250 nm. Overall, G. adiacens EVs contained 112 proteins. The proteome consists of several ribosomal proteins, DNA associated proteins, binding proteins, and metabolic enzymes. It was also shown that these EVs carry putative virulence factors including moonlighting proteins. These EVs were able to induce the production of IL-8, IL-1β and CCL5 from human PBMCs. Further functional characterization of the G. adiacens EVs may provide new insights into virulence mechanisms of this important but less studied oral bacterial species.
Introduction
Granulicatella species, formerly known as nutritionally variant streptococci based on their characteristic dependence on pyridoxal or cysteine supplementation for their growth in standard media [1], are catalase and oxidase negative, non-motile, non-spore-forming, facultatively anaerobic Gram-positive cocci [2, 3]. They are part of the normal oral flora [4], but cause serious infections such as infective endocarditis. The genus Granulicatella consists of 3 species: Granulicatella adiacens, Granulicatella elegans and Granulicatella balaenopterae [3]. The species G. balaenopterae has not been isolated from human samples, whereas both G. adiacens and G. elegans have been reported from IE cases [5, 6]. In addition, these oral commensal cocci have been associated with endodontic infections [7, 8], dental caries [9], and periodontitis [8, 10] via DNA-based studies. Although this association does not substantiate the role of Granulicatella species in dental diseases, the fact that these species are causative agents in infective endocarditis implies that they might exert similar pathogenic potential also in the oral cavity.
Many bacterial species routinely produce extracellular vesicles (EVs) during normal growth [11]. Gram-negative bacteria are commonly found to produce such vesicles, which are derived from blebbing of the outer membrane and thus are called outer membrane vesicles (OMVs) [11]. Generally, these OMVs contain outer membrane proteins, lipopolysaccharides, glycerophospholipids in addition to enclosed periplasmic components and bacterial nucleic acids [11–13]. The study of the EVs was initially limited to Gram-negative bacteria, as it was thought that the rigidity of the Gram-positive cell wall, which is rich in peptidoglycans, would not allow vesicle blebbing [11]. However, the production of EVs was also observed in some Gram-positive bacteria [14, 15]. Current studies [16, 17] showed that the activity of cell wall-degrading enzymes, which weaken the peptidoglycan layer and thus facilitate the release of Gram-positive EVs, could probably explain such phenomena in Gram-positive bacteria. Similar to Gram-negative OMVs, these EVs contain proteins, lipids, enzymes, toxins and bacterial nucleic acids [18]. However, Gram-positive EVs can still be distinguished from OMVs as the former lack lipopolysaccharide and enclosed periplasmic components [18].
Several studies [13, 14, 16] showed that bacteria exploit vesicle production as a virulence strategy. Bacterial components, including virulence factors, are packed in the vesicles and delivered to the host cells and tissues. The vesicle-derived virulence factors play an important role in bacterial pathogenicity, e.g., by eliciting an inflammatory response, manipulating the host's immunity, eliminating the competing commensal microorganisms, relieving internal stress, mediating biofilm formation, and acting as decoys absorbing and blocking cell wall-lytic compounds and membrane-disrupting antimicrobial peptides produced by other commensals and host innate immune cells [13, 14, 16]. Protein secretion in Granulicatella species has been studied [19], but vesicle production in these species has not been investigated yet. In this preliminary exploratory study, we isolated EVs from G. adiacens and acquired information on the EV proteome by proteomics approach. Initial functional analyses of the EVs showed the immunostimulatory potential against human PBMCs.
Materials and methods
Bacterial strains and culture conditions
The reference strain G. adiacens CCUG 27809 was cultured on chocolate blood agar (CBA) with 0.001% pyridoxal hydrochloride at 37°C and in 5% CO2 in air for 2 days. A loop-full of colonies from the CBA plates was inoculated into brucella broth supplemented with 0.001% pyridoxal hydrochloride and incubated as above for 2 days.
Isolation of EVs
The EVs were isolated using a previously described centrifugation and filtration protocol [20], with slight modifications. Briefly, for pelleting the bacteria, the broth culture was centrifuged at 5000 × g at room temperature for 10 minutes (Centrifuge 5430 R, Eppendorf AG, Germany). For removing any remnants of intact bacterial cells, the supernatant was filtered through a 0.22 μm sterile syringe filter (Millipore, Germany). The filtrate was then re-centrifuged at 125000 × g at 4° C for 3 hours (Optima™ L-XP ultracentrifuge, Beckman, USA). The obtained pellet was suspended in 300 μl sterile phosphate-buffered saline (PBS). The EVs samples were stored at -20° C until used.
Preparation of whole cell protein (WCP)
A loop full of colonies from the CBA plates was suspended in 2 ml sterile PBS. The bacterial suspension was centrifuged at 5000 ×g at room temperature for 5 minutes (Centrifuge 5430 R, Eppendorf AG, Germany). Then, after discarding the supernatant, the pellet was washed with 2 ml sterile PBS. The bacterial whole cell protein (WCP) was obtained by ultra-sonicating bacterial cells at 40 pulse rate on ice for 8 cycles (1 minute sonication followed by 1 minute rest per cycle) (Omni Sonic Ruptor 4000, Omni International, USA) followed by centrifugation at 7000 ×g at 4° C for 10 minutes (Centrifuge 5430 R, Eppendorf AG, Germany). The resulting supernatant was used as the WCP sample and stored at -20° C until used.
Characterization of EVs
Scanning electron microscopy (SEM)
The obtained vesicle preparations were suspended in sterile PBS containing 3% glutaraldehyde for 2 hours on a rotator and then kept in a refrigerator overnight. For staining, the vesicle samples were incubated in 1% osmium tetroxide for 2 hours. For dehydration, the samples were kept in increasing concentrations of acetone from 30 to 100%, 10 minutes in each, on a rotator. The samples were then placed in a critical point dryer for complete drying, mounted on stubs with carbon double adhesive tape and finally coated with gold and stored in a desiccator until observation. The samples were observed on Zeiss Leo Supra 50VP field emission scanning electron microscope (Carl Zeiss, Germany). For comparison, SEM analysis of bacterial whole cells was also performed using the same previous biological sample preparation protocol.
Determination of protein concentration and SDS-PAGE
Protein concentrations in the EVs and WCP samples were determined by Quick StartTM Bradford protein microplate standard assay (Bio-Rad, USA). For protein separation, the samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using the mini-PROTEAN II cell electrophoresis system (Bio-Rad, USA). The proteins were denatured in 2× loading buffer at 100°C for 5 minutes, followed by centrifugation at 5000 ×g for 5 minutes. 20 μl of proteins loaded in each well of the gel were separated on 12% SDS-PAGE at a constant 120 V. After the run was completed, protein bands were detected using silver stain. Gel images were visualized in G: Box Imaging System (Syngene, India). Protein banding patterns and molecular weights of the bands were determined using GeneSys tools software.
Identification of EVs proteins by nano-LC-ESI-MS/MS
For the identification of EVs proteins, mass spectrometry was performed by Proteome Factory (Proteome Factory AG, Berlin, Germany) using nano-liquid chromatography-electrospray ionization-tandem mass spectrometry (nano-LC-ESI-MS/MS). With an Agilent 1100 nanoHPLC system (Agilent, Waldbronn, Germany) interfaced to an Orbitrap Velos (Thermo Scientific, Bremen, Germany) via a nanoelectrospray ion source. After pooling replicate samples from EVs preparations, proteins were reduced, alkylated and digested by trypsin (Promega, Mannheim, Germany). Then, 400 ng of the resulting peptides were subjected to the nanoLC-ESI-MS/MS. 1% acetonitrile/0.5% formic acid was used as eluent for 5 minutes to trap and desalt the peptides on the enrichment column (Zorbax 300SB-C18, 0.3 × 5 mm, Agilent). A water/acetonitrile (both supplemented with 0.1% formic acid) gradient from 5% to 40% acetonitrile was then used within 120 minutes to separate the peptides on a Zorbax 300SB-C18, 75 μm x 150 mm column (Agilent). The mass spectrometer automatically recorded mass spectra, and tandem mass spectra were data-dependently acquired for multiply charged ions. Protein identification was made using the Mascot search engine (Matrix Science, London, England) against the bacterial subset of the RefSeq protein database (National Center for Biotechnology Information), (downloaded on 1st July 2016, 49867978 entries, NCBI, Bethesda, USA) and a database with common protein contaminants. For MS/MS spectra where assignment of the precursor ion’s charge state was missing, search parameters for ions from ESI-MS/MS data acquisition was set to "2+, 3+ or 4+" according to the instrument's and method's standard charge state distribution. The search parameters were: Fixed modifications: Carbamidomethyl (C); variable modifications: Deamidated (NQ), Oxidation (M); Peptide Mass Tolerance: ± 3 ppm; Fragment Mass Tolerance: ± 0.6 Da; Missed Cleavages: 2. The inclusion criterion was: peptides that match with a score of 20 or above. Mass spectrometry data, with the project acession number PXD021414, has been deposited at PRIDE archive (https://www.ebi.ac.uk/pride/archive/) repository.
Bioinformatic analysis
Protein sequences from the liquid chromatography-mass spectrometer (LC-MS) analysis of the EVs proteome was analyzed by an in silico 2-dimensional electrophoresis (2-DE) tool. For this, the software JVirGel, version 2.0 (http://www.jvirgel.de/index.html), was used to obtain a theoretical (2-DE) image of the EVs proteins by uploading protein sequences to the software [18]. The subcellular localization of the EVs proteins detected with LC-MS/MS was predicted using the PSORTb tool, version 3.0.2 (https://www.psort.org/psortb/) [21]. To determine if any of the secreted proteins are packed into the vesicles, the prediction tool SignalP, version 5.0 (http://www.cbs.dtu.dk/services/SignalP/abstract.php), was utilized to predict proteins secreted via the general Secretion route (Sec-pathway) [22]. In addition to that, the prediction tool TatP (http://www.cbs.dtu.dk/services/TatP/), was used to predict proteins secreted via the Twin-arginine translocation pathway (Tat-pathway) [23]. To identify lipoproteins, lipoboxes were searched using the prediction tools LipoP (http://www.cbs.dtu.dk/services/LipoP/) and PRED-LIPO (http://bioinformatics.biol.uoa.gr/PRED-LIPO/input.jsp) [24].
Function prediction analysis
Proteins with multiple functions, known as “moonlighting proteins”, were identified using the prediction tool moonprot, version 2.0 (http://www.moonlightingproteins.org/) [25], and searching the database Multitask ProtDB (http://wallace.uab.es/multitaskII/) [26]. Gene Ontology (GO) analysis of the EVs proteome was performed using the amino acid FASTA sequences of G. adiacens. For this, GO annotations were analyzed and plotted using the tools OmicsBox version 1.3.11 (https://www.biobam.com/download-omicsbox/) [27], and WEGO, version 2.0 (http://wego.genomics.org.cn/) [28]. The EVs proteins were grouped based on functional association networks using the tool STRING (https://string-db.org/) [29]. Minimum interaction scores were set at a strong confidence level of 0.7. The EVs proteins were also grouped based on different biological pathways. For this, all protein sequences from G. adiacens EVs proteome were analyzed by the Kyoto Encyclopedia of Genes and Genome (KEGG) (https://www.genome.jp/kegg/pathway.html) pathway analysis tool using the genus “Streptococcus” as reference [30].
Prediction of virulence factors in the EVs proteomes
To predict virulence proteins in the EVs proteome, the tool VirulentPred (http://203.92.44.117/virulent/) [31], along with the Virulence Factor Data Base (VFDB; http://www.mgc.ac.cn/VFs/) were used. Proteins predicted to be virulent by the previous tools were manually searched in the literature for experimental evidence on their virulence properties.
Cytokine induction of human PBMCs by EVs
Isolation of human PBMCs
PBMCs from the blood of a healthy human volunteer were isolated using Ficoll-Paque density gradient centrifugation method [32]. After obtaining written informed consent from the donor, blood was collected by venipuncture into vacutainer heparin tubes (3 ml per tube). The blood was then carefully layered onto 3.5 ml Ficoll-Paque media solution (GE Healthcare, USA) in a sterile centrifugation tube. For separating mononuclear cells, the tubes were centrifuged at 3400 ×g at room temperature with the brakes off for 10 minutes. The layer of PBMCs, the buffy coat layer, was then transferred to another sterile centrifugation tube. The cell isolate was washed twice by resuspending it in 5 ml RPMI medium followed by centrifugation at 2000 rpm at room temperature with the brakes on for 5 minutes. The supernatant was discarded, and the cell pellet was finally resuspended in 1 ml RPMI medium supplemented with 10% heat-inactivated fetal bovine serum and 2% GibcoTM 100× antibiotic-antimycotic solution. Cell concentration in the PBMCs sample was estimated by loading 10 μl aliquot on a hemocytometer under 400× magnification.
Stimulation of human PBMCs with EVs and WCP
Isolated human PBMCs were stimulated with different concentrations (10, 25, 50, and 100 μg/ml) of G. adiacens EVs and WCP for 24 hours. For this, in a 24-well plate, 480 μl supplemented RPMI medium containing PBMCs (106 cells per ml) was added to each well and stimulated with 20 μl of bacterial EVs or WCP. The plate was incubated at 37°C and in 5% CO2 in air for 24 hours. Well with 20 μl sterile PBS and 480 μl RPMI medium containing PBMCs was used as negative control.
Quantitative determination of selected cytokines
The quantitative sandwich enzyme-linked immunosorbent assay (ELISA) technique was used to quantify the production of the human cytokines IL-8, IL-1β, and CCL5 (RANTES) from the stimulated PBMCs. For this, ELISA immunoassay kits (Quantikine® ELISA R&D systems, Bio-Techne, USA) were used according to the manufacturer's instructions. Briefly, standards, samples, and controls were added to the wells of a 96-well microplate pre-coated with a monoclonal antibody specific for the cytokine of interest. To allow the specific cytokine in the sample to be bound by the specific immobilized antibody, the plate was incubated at room temperature for 2 hours. To remove any unbound substances, the wells were washed with wash buffer using ImmunoWashTM 1575 microplate washer (Bio-Rad, USA). Then, an enzyme-linked polyclonal antibody for the specific cytokine was added to each well. After an incubation period of one hour at room temperature, the wells were washed again with wash buffer to remove any unbound antibody-enzyme reagent. A substrate solution was then added to each well, and the microplate was incubated at room temperature for 20–30 minutes while being protected from light. To terminate the colorful enzyme-substrate reaction, a stop solution was added to each well. Finally, iMarkTM microplate reader was used to measure the intensity of the color developed.
Statistical analysis
All experiments were repeated twice. Statistical Package for Social Sciences Software (SPSS), version 25, was used for data analysis. Descriptive statistics were presented using mean ± standard deviation (SD). Independent-samples T test and Mann Whitney U test were used to analyze differences between groups. A critical probability value (P value) of < 0.05 was used as the cut-off level for statistical significance.
Ethical considerations
This study was approved by the ethical committee of the Health Sciences Center, Kuwait University (DR/EC/3413), and has been carried out in full accordance with the World Medical Association Declaration of Helsinki. The blood donor received written information about the nature and purposes of the study and a written informed consent was obtained upon his/her approval to participate.
Results and discussion
Isolation of EVs
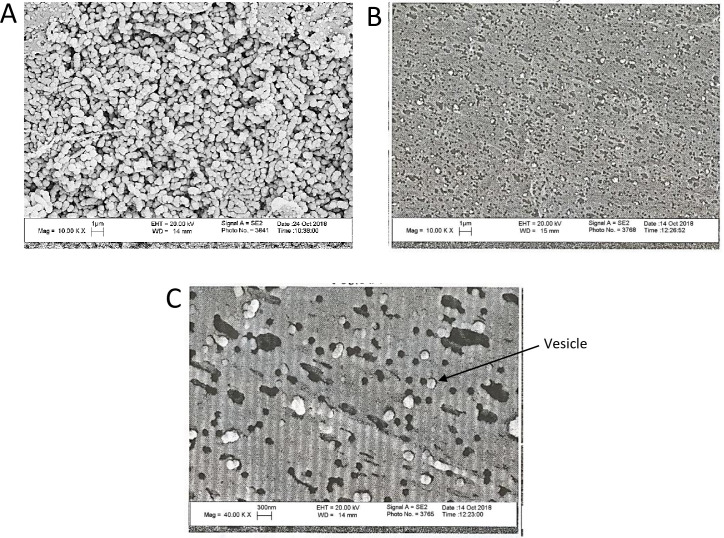
It was revealed by the current study that G. adiacens produce EVs. Vesicles of varying sizes, ranging from 30 to 250 nm in diameter, were seen in the electron micrographs. This nano-scale range size was consistent with other bacterial EVs [14, 15]. For comparison, images of bacterial whole cells (Fig 1A) and the vesicle preparations (Fig 1B) were captured at the same magnification of ×10000. Vesicle shape and size could be visualized better at a higher magnification of ×40000 (Fig 1C).
Fig 1. SEM images of G. adiacens whole cells and the EVs preparation.
SEM images of bacterial whole cells (A) and the EVs preparation (B) captured at the magnification ×10000. (C) SEM images of the EVs acquired at ×40000.
Characterization of EVs
Determination of protein concentration and SDS-PAGE
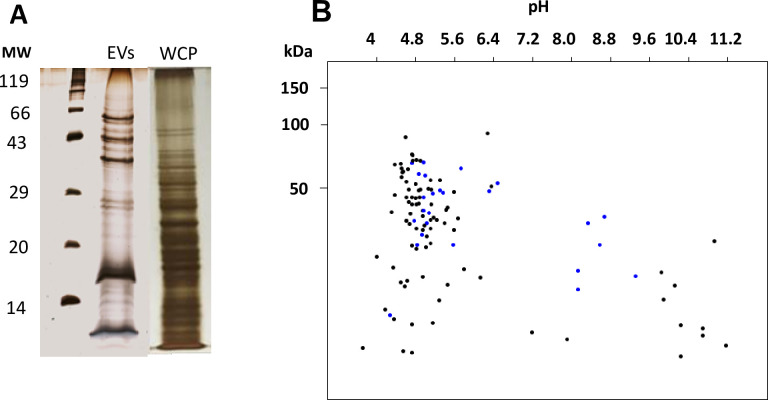
Protein concentration in the EVs sample from G. adiacens, 1337 μg/ml, was much lower compared to its respective WCP sample, 3102 μg/ml. Consistently, SDS-PAGE analysis revealed that the EVs preparation from G. adiacens showed much fewer bands on gel than its respective WCP preparation (Fig 2A).
Fig 2. Analysis of the proteome of G. adiacens EVs.
(A) SDS-PAGE gel showing protein bands from EVs and WCP preparations. (B) Protein sequences from LC-MS analysis of the vesicle proteome analyzed by an in silico 2-DE tool. Blue spots indicate proteins with transmembrane domains.
Identification of EVs proteins by NanoLC-ESI-MS/MS
In total, 112 proteins detected by NanoLC-ESI-MS/MS in EVs preparations of G. adiacens, were analyzed and defined as the EVs proteome in the present study (S1 File). These numbers were within the range of proteins identified in previous analyses of other bacterial vesicle proteomes [14, 15].
Bioinformatic analysis
In silico 2-DE analysis of the EVs proteome showed that the molecular mass of the proteins ranged between 20.16 kDa and 91.73 kDa (Fig 2B). Most proteins from G. adiacens EVs were found to be in the predicted isoelectric point (pI) range of 3.99 and 5.6 (Fig 2B). In silico 2D gel analysis is a helpful tool when proteins with transmembrane helices on their membrane spanning region possess hydrophobic characters and thefore can not be separated by electrophoresis and therefore are not visible on 2D gels ☯23].
According to the PSORTb subcellular localization prediction tool analysis, G. adiacens EVs proteome was predicted to contain 74 cytoplasmic proteins, 8 cytoplasmic membrane proteins, and 2 cell-wall anchored proteins; whereas the localization of 25 proteins could not be predicted. As predicted in this study, the majority of EVs proteins were cytoplasmic in G. adiacens (66%). Cytoplasmic proteins located in other bacterial vesicles have been reported in several earlier studies [33, 34]. Existing evidence suggests that the enormous location of cytoplasmic proteins into vesicles is due to specific sorting mechanisms, and not due to lysis of dead cells [35]. Importantly, cytoplasmic proteins released as part of vesicles are known to function as adhesins, contribute to biofilm matrix formation, and help bacteria in evading the immune system [36].
As predicted in our study by the SignalP and TatP tools, secretory proteins were packed into the EVs of G. adiacens. According to the SignalP prediction tool, 24 proteins of G. adiacens EVs proteome were found to contain a signal sequence where 3 proteins were Signal peptide (Sec/SPI) type and 21 were Lipoprotein signal peptide (Sec/SPII) type (S2 File). The list of proteins predicted by SignalP and LipoP included several ABC transporter proteins, extracellular solute-binding proteins, and CHAP-domain containing proteins. The TatP prediction tool showed that none of the proteins of the G. adiacens EVs proteome contained TatP signal sequence. Both the Sec and Tat pathways are major pathways that exist in bacteria for proteins secretion across the cytoplasmic membrane [37, 38]. The former pathway is well known to translocate proteins in their unfolded conformation, while the latter catalyzes the secretion of proteins that fold before their translocation [38]. It is well-established that protein secretion is an essential strategy in the pathogenesis of bacterial infections [37]. Lipoprotein prediction tools (Pred-Lipo, LipoP) revealed that there were 21 lipoproteins in the G. adiacens EVs proteome.
Function prediction analysis
The present study showed that EVs from G. adiacens carry proteins predicted to exhibit multitasking capabilities. Table 1 lists the 15 proteins from the G. adiacens EVs proteome that were identified as “moonlighting proteins”. Major proteins predicted as multifunctional proteins were ribosomal proteins and molecular chaperones. Additionally, a glycolytic enzyme, glyceraldehyde-3-phosphate-dehydrogenase, and a few putative virulent proteins such as transketolase and thioredoxin were also identified. Such multifunctional bacterial proteins were found to play a role in the virulence of several other human pathogenic bacteria; e.g., Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae, Helicobacter pylori, and Mycobacterium tuberculosis [39–41].
Table 1. Predicted moonlighting proteins from G. adiacens EVs proteome.
| GI Number | Protein |
|---|---|
| gi|491800925 | Chaperonin GroEL |
| gi|491800793 | Triose-phosphate isomerase |
| gi|491800797 | Glyceraldehyde-3-phosphate dehydrogenase |
| gi|491797953 | Molecular chaperone DnaK |
| gi|491800498 | Glucose-6-phosphate isomerase |
| gi|491801148 | Elongation factor Tu |
| gi|491798679 | 6-phosphofructokinase |
| gi|491797130 | Transketolase |
| gi|1489647615 | Pyruvate kinase |
| gi|259035990 | Phosphoglycerate kinase |
| gi|491801605 | 50S ribosomal protein L10 |
| gi|1489648176 | NADP-dependent phosphogluconate dehydrogenase |
| gi|259036192 | Thioredoxin |
| gi|1686099964 | Translation superoxide dismutase |
| gi|259035743 | Ribosomal protein L2 |
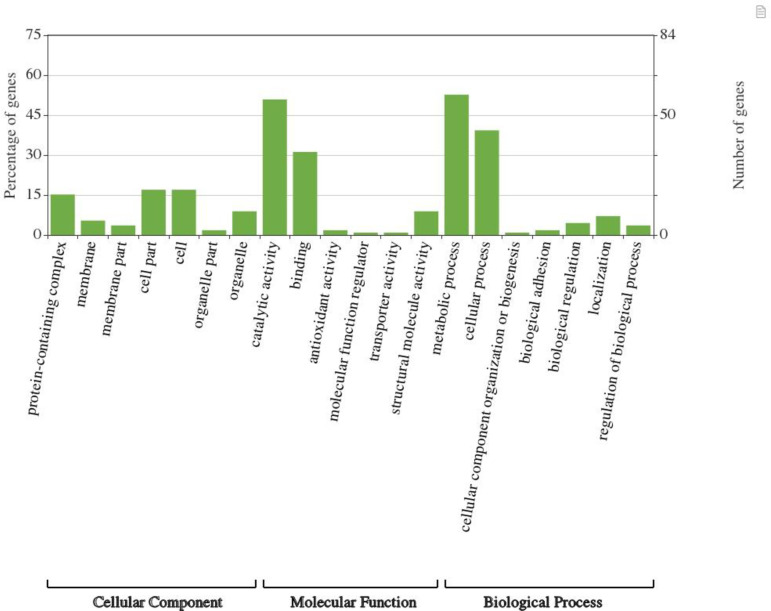
Fig 3 summarizes the Gene Ontology analysis of the EVs proteome. Overall, 112 of the G. adiacens sequences were assigned with GO annotation. For G. adiacens, the proteins were divided into 3 groups based on GO terms: 69 proteins in “biological process” group, 21 proteins in the “cellular component” group, and 77 proteins in the “molecular function” group. According to the Gene Ontology analysis conducted in the present study, most proteins in G. adiacens EVs proteome were predicted to be involved in molecular functions, particularly catalytic and binding functions, followed by biological processes, mainly metabolic and cellular processes. It is possible that these species might utilize nutrients in the environment by using the metabolism-mediator proteins in the EVs [42]. Only 21 proteins in the proteome were annotated for cellular components. Similar to other bacterial EVs, G. adiacens EVs contained several ribosomal proteins, DNA associated proteins, binding proteins, and metabolic enzymes, indicating that bacterial EVs might facilitate the transfer of functional proteins [14, 16].
Fig 3. Gene Ontology analysis of the proteome of G. adiacens EVs preparations.
Gene ontology annotation was achieved using OmicsBox and an online software “WEGO”. Protein sequences were grouped into 3 categories based on their properties and functions.
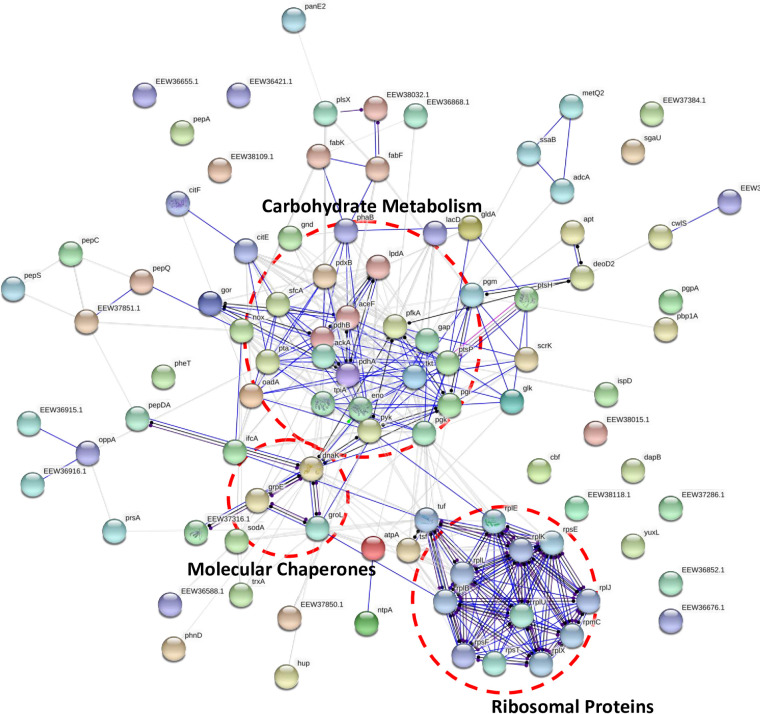
Fig 4 demonstrate a STRING functional protein association network analysis of G. adiacens EVs proteome. As demonstrated in our study, G. adiacens EVs proteome formed three distinct protein groups based on their functional associations. These groups were carbohydrate metabolism, ribosomal proteins, and heat shock proteins/chaperones. Components of the carbohydrate metabolism network were: glyceraldehyde-3-phosphate dehydrogenase, phosphoenolpyruvate-protein phosphotransferase, glucose-6-phosphate isomerase, phosphoglycerate kinase, Pyruvate kinase, ATP-dependent 6-phosphofructokinase, transketolase, pyruvate dehydrogenase E1 component, and dihydrolipoamide acetyltransferase component of pyruvate dehydrogenase complex. The ribosomal protein group consisted mainly of the secreted ribosomal proteins: 30S ribosomal protein S20, 50S ribosomal protein L10, 30S ribosomal protein S5, 50S ribosomal protein L5, 50S ribosomal protein L7/L12, 30S ribosomal protein S6; Binds together with S18 to 16S ribosomal RNA, 50S ribosomal protein L11, 30S ribosomal protein S7, 50S ribosomal protein L2, Ribosome-recycling factor, and 50S ribosomal protein L1. The molecular chaperones (DnaK, GroL, and GrpE) and superoxide dismutase formed another cluster. A growing body of literature [40, 41] has shown that a number of enzymes involved in the glycolytic pathway as well as molecular chaperones are recognized as moonlighting proteins and thus could play a role in the pathogenesis of bacterial infection. Of the glycolytic enzymes detected in EVs proteomes in this study, glyceraldehyde-3-phosphate dehydrogenase, glucose-6-phosphate isomerase, phosphoglycerate kinase, pyruvate kinase, and ATP-dependent 6-phosphofructokinase were found to possess moonlighting properties. These enzymes could function as transferrin receptor, cell signaling kinase, neutrophil evasion protein, immunomodulator, plasminogen binding protein, fibrinogen binding protein, actin binding protein, and has a role in NAD-ribosylation activity and extracellular polysaccharide synthesis [40]. Moreover, the molecular chaperone DnaK was found to act as a multifunctional protein, which could stimulate CD8 lymphocyte and monocyte chemokines production, compete with HIV for binding to CCR5 receptors, and bind plasminogen [40]. In addition, it was concluded by a previous study [43] that many bacterial ribosomal proteins could function beyond their primary role as ribosomes, integral components of protein synthesis machinery. These proteins could also modulate different cell processes, such as transcription, regulation of the mRNA stability, DNA repair and replication, and phage RNA replication [43]. Furthermore, the L7/L12 ribosomal protein was experimentally proven to elicit a cell-mediated immune response in mice [44].
Fig 4. Functional protein association networks of G. adiacens EVs proteome.
The online tool STRING was used for grouping the EVs proteins based on functional networks. Minimum interaction scores were set at a strong confidence level of 0.7. The three major network groups formed are shown in dotted circles. Seven different colors link a number of nodes and represent seven types of evidence used in predicting associations. A red line indicates the presence of fusion evidence; a green line represents neighborhood evidence; a blue line represents co-occurrence evidence; a purple line represents experimental evidence; a yellow line represents text mining evidence; a light blue line represents database evidence and a black line represents co-expression evidence.
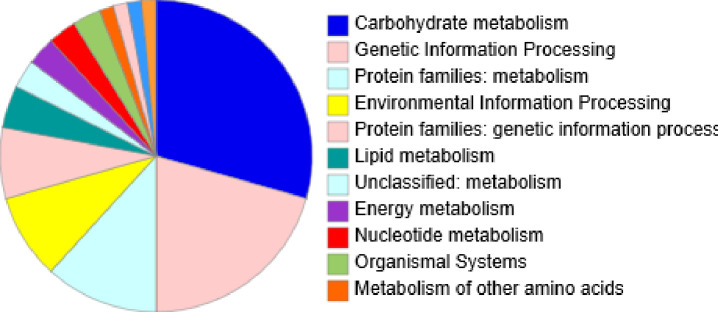
KEGG pathway analysis of the EVs proteome is depicted in Fig 5. Proteins belonging to carbohydrate metabolism and genetic information processing were found to be the most predominant in G. adiacens EVs. About 29.4% of the proteins in G. adiacens EVs proteome was predicted to be involved in the carbohydrate metabolism and 21% in genetic information processing. Other 12%, 9% and 7.4% of proteins were involved in protein families: metabolism, environmental information processing and protein families: genetic information processing respectively. As predicted by the pathway tool, a few proteins were also implicated in amino acid metabolism, lipid metabolism, glycan metabolism, and energy metabolism. Vesicles equipped with metabolic machineries can help bacterial colonization and host cell invasion. For example, ATP generated in vesicles might regulate the activity of virulence factors and facilitate cell-cell communication of bacteria [45]. Overall, metabolism related proteins in the EVs might facilitate long-term contact between the bacterium and the epithelial cells, causing increased epithelial cell/tissue damage.
Fig 5. KEGG pathway analysis of G. adiacens EVs proteome.
All protein sequences from G. adiacens vesicle proteome were subject to KEGG pathway analysis using the genus “streptococcus” as reference.
Prediction of virulence proteins in G. adiacens EVs proteome
Our study revealed that EVs produced by G. adiacens contained proteins that were predicted to carry virulent properties. This finding overemphasizes the role of EVs in the pathogenesis of Granulicatella infections. Table 2 show the list of 26 proteins that were predicted to be virulent from EVs proteome of G. adiacens. The major proteins with demonstrated evidence on their virulence properties in other bacterial species were: thioredoxin [46], aminopeptidase [47], molecular chaperones DnaK and GroES [48, 49], Superoxide dismutase [50], Glyceraldehyde-3-phosphate dehydrogenase [51], phosphoglycerate kinase [52], and acyl carrier protein [53]. A vast literature on membrane vesicles has demonstrated that a number of well-known and extensively studied toxins and non-toxin virulence factors are secreted via vesicles [54]. Unlike virulence factors secreted in soluble form, vesicle-associated virulence factors are provided with a unique benefit of being protected from host proteases [13]. Moreover, vesicle-virulence factors are delivered to host cells/tissues as concentrated packages, increasing the damage level at specific target sites. Vesicle-mediated delivery of virulence factors is a widespread mechanism across bacterial species and genera. Similar to other oral bacteria such as Aggregatibacter actinomycetemcomitans [55], Kingella kingae [56] and others that are also implicated in infective endocarditis, G. adiacens possibly use its EVs filled with numerous putative virulent proteins in the pathogenesis of this infection.
Table 2. Putative virulence factors predicted in G. adiacens EVs proteome.
| GI Number | Protein | Literature evidence |
|---|---|---|
| gi|491800219 | CHAP domain-containing protein | [57] |
| gi|491799853 | DNA starvation/stationary phase protection protein | [58] |
| gi|491800704 | Aminopeptidase | [47] |
| gi|491797310 | Acyl carrier protein | [53] |
| gi|259035608| | peptidase, C69 family | [59] |
| gi|1489650858| | oligoendopeptidase F | [60] |
| gi|1489651594| | 30S ribosomal protein S20 | [61] |
| gi|491798621| | hypothetical protein | [62] |
| gi|1489651148| | 2-C-methyl-D-erythritol 4-phosphate | [63] |
| gi|1489650155| | phosphonate ABC transporter | [64] |
| gi|491798879| | hypothetical protein | [65] |
| gi|1489650855| | toxic anion resistance protein | [66] |
| gi|763046713 | Copper resistance protein CopC | [67] |
| gi|1489647414| | DUF1307 domain-containing protein | [68, 69] |
| gi|1489650843| | hypothetical protein D8B48_01700 | [70] |
| gi|259035675| | 3D domain protein | [71] |
| gi|491798643| | LysM peptidoglycan-binding | [72] |
| gi|1489647413| | DUF1307 domain-containing protein | [68, 69] |
| gi|1489650906| | thiol reductase thioredoxin, partial | [60] |
| gi|491797269| | extracellular solute-binding protein | [73] |
| gi|491796985| | YlbF family regulator | [74] |
| gi|1489650842| | hypothetical protein D8B48_01695 | [70] |
| gi|1489649584| | DUF1002 domain-containing protein | [68, 69] |
| gi|491801129| | 50S ribosomal protein | [75] |
| gi|259035743| | ribosomal protein L2 | [76] |
| gi|491801123| | 50S ribosomal protein L24 | [77] |
ELISA quantification of selected cytokines produced from stimulated human PBMCs with EVs and WCP
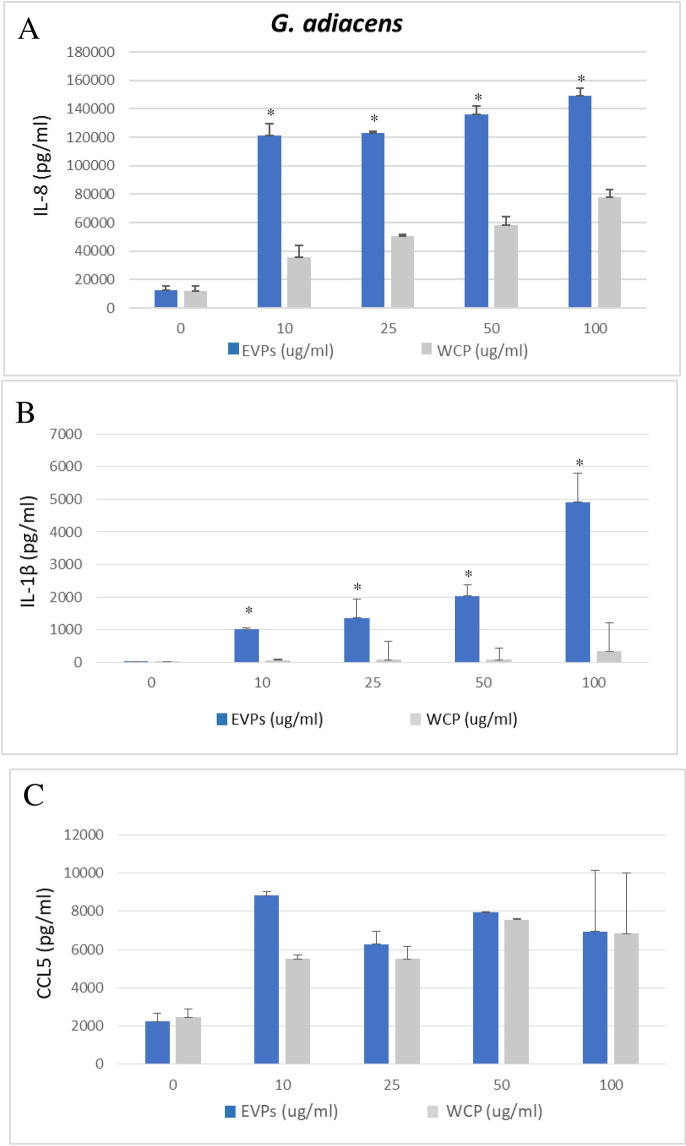
As shown in Fig 6, all concentrations (10, 25, 50, and 100 μg/ml) of G. adiacens EVs triggered the production of the selected potent proinflammatory cytokines from human PBMCs as compared to the controls (0 μg/ml). Our study demonstrated that G. adiacens EVs were able to stimulate cytokine release from human PBMCs and thus could play a role in the induction of an inflammatory response. This finding is in accordance with previous studies [11, 14, 16] that revealed the immuno-modulatory effects of EVs in other bacteria. In the current study, EVs from G. adiacens induced IL-8 and IL-1β, but not CCL5, in a dose-dependent manner. G. adiacens EVs induced the release of IL-8 and IL-1β to significantly (P < 0.05) higher levels compared to WCP. These observations overemphasize the importance of bacterial vesicle production in the activation of inflammation and thus pathogenesis of bacterial infections. The ability of bacterial vesicles to trigger host inflammatory response is a well-established phenomenon. When host epithelial cells encounter or take up the vesicles, an immediate innate immune response begins. IL-8 and IL-1β are prominent cytokines in infective endocarditis [78], but also in oral infections [79, 80]. IL-1β has a wide range of actions mediating inflammatory host response. At low concentrations, it mediates local inflammation while at high concentrations it possesses endocrine effects. Due to its neutrophil recruiting property, IL-8 is a major inflammatory cytokine induced by a variety of microbial components [81, 82].
Fig 6.
ELISA quantification of IL-8 (A), IL-1β (B), and CCL5 (C) production by human PBMCs stimulated with G. adiacens EVs and WCP (10, 25, 50, and 100 μg/ml). Cytokine induction from the EVs was considered significantly different from WCP at *p < 0.05.
Conclusion
To the best of our knowledge, this is the first research that presented evidence for the hypothesis that G. adiacens release EVs. In this preliminary exploratory study, we found that the EVs proteome of G. adiacens was enriched with a large number of predicted putative virulence factors. The diversity of proteins in EVs suggests possible roles of these vesicles in bacterial survival, invasion, host immune modulation as well as infection, as is the case for a number of other bacterial species. Moreover, EVs of G. adiacens were demonstrated to be potent inducers of proinflammatory cytokines, and importantly, the EVs were significantly more potent than the whole cell proteins in eliciting inflammatory response. These EVs may play an important role in the activation of inflammation and thus pathogenesis of Granulicatella infections. Further functional characterization of the G. adiacens EVs may throw more light on how this species may utilize vesicles to orchestrate events that may lead it from being silent normal flora species towards infection-causing ones.
Supporting information
(XLSX)
(XLSX)
(PDF)
(PDF)
Acknowledgments
We acknowledge the Research Sector, Kuwait University (Project SRUL 01/14) and the College of Graduate Studies, Kuwait University. Special gratitude is given to the Research Core Facility (SRUL 02/13), The Nanoscopy Science Center (GE01/07) and National Unit for Environmental Research and Services, (SRUL 01/13), Kuwait University. We are very grateful to Prof. Raj Raghupathy, at the Department of Microbiology, Faculty of Medicine, Kuwait University for kind help with the laboratory work concerning fractionation of human peripheral blood mononuclear cells.
Data Availability
Mass spectrometry data is deposited in the PRIDE archive under accession number PXD021414 and publicly accessible via the following URL: https://www.ebi.ac.uk/pride/archive/projects/PXD021414.
Funding Statement
MK received funding from Kuwait University Grant Number SRUL 01/14. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ruoff KL. Nutritionally variant streptococci. Clin Microbiol Rev. 1991;4(2):184–90. 10.1128/cmr.4.2.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cargill JS, Scott KS, Gascoyne-Binzi D, Sandoe JA. Granulicatella infection: diagnosis and management. J Med Microbiol. 2012;61(Pt 6):755–61. 10.1099/jmm.0.039693-0 [DOI] [PubMed] [Google Scholar]
- 3.Collins MD, Lawson PA. The genus Abiotrophia (Kawamura et al.) is not monophyletic: proposal of Granulicatella gen. nov., Granulicatella adiacens comb. nov., Granulicatella elegans comb. nov. and Granulicatella balaenopterae comb. nov. Int J Syst Evol Microbiol. 2000;50 Pt 1:365–9. [DOI] [PubMed] [Google Scholar]
- 4.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721–32. 10.1128/JCM.43.11.5721-5732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adam EL, Siciliano RF, Gualandro DM, Calderaro D, Issa VS, Rossi F, et al. Case series of infective endocarditis caused by Granulicatella species. Int J Infect Dis. 2015;31:56–8. 10.1016/j.ijid.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 6.Giuliano S, Caccese R, Carfagna P, Vena A, Falcone M, Venditti M. Endocarditis caused by nutritionally variant streptococci: a case report and literature review. Infez Med. 2012;20(2):67–74. [PubMed] [Google Scholar]
- 7.Hsiao WW, Li KL, Liu Z, Jones C, Fraser-Liggett CM, Fouad AF. Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics. 2012;13:345 10.1186/1471-2164-13-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siqueira JF Jr., Rocas IN. Catonella morbi and Granulicatella adiacens: new species in endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(2):259–64. 10.1016/j.tripleo.2005.09.021 [DOI] [PubMed] [Google Scholar]
- 9.Kanasi E, Dewhirst FE, Chalmers NI, Kent R Jr., Moore A, Hughes CV, et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010;44(5):485–97. 10.1159/000320158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asikainen S, Dogan B, Turgut Z, Paster BJ, Bodur A, Oscarsson J. Specified species in gingival crevicular fluid predict bacterial diversity. PLoS One. 2010;5(10):e13589 10.1371/journal.pone.0013589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwab A, Meyering SS, Lepene B, Iordanskiy S, van Hoek ML, Hakami RM, et al. Extracellular vesicles from infected cells: potential for direct pathogenesis. Front Microbiol. 2015;6:1132 10.3389/fmicb.2015.01132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74(1):81–94. 10.1128/MMBR.00031-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19(22):2645–55. 10.1101/gad.1299905 [DOI] [PubMed] [Google Scholar]
- 14.Avila-Calderon ED, Araiza-Villanueva MG, Cancino-Diaz JC, Lopez-Villegas EO, Sriranganathan N, Boyle SM, et al. Roles of bacterial membrane vesicles. Arch Microbiol. 2015;197(1):1–10. 10.1007/s00203-014-1042-7 [DOI] [PubMed] [Google Scholar]
- 15.Kim GH, Choi CW, Park EC, Lee SY, Kim SI. Isolation and proteomic characterization of bacterial extracellular membrane vesicles. Curr Protein Pept Sci. 2014;15(7):719–31 10.2174/1573403x10666140505163121 [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Defourny KAY, Smid EJ, Abee T. Gram-Positive Bacterial Extracellular Vesicles and Their Impact on Health and Disease. Front Microbiol. 2018;9:1502 10.3389/fmicb.2018.01502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyofuku M, Carcamo-Oyarce G, Yamamoto T, Eisenstein F, Hsiao CC, Kurosawa M, et al. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat Commun. 2017;8(1):481 10.1038/s41467-017-00492-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiller K, Schobert M, Hundertmark C, Jahn D, Munch R. JVirGel: Calculation of virtual two-dimensional protein gels. Nucleic Acids Res. 2003;31(13):3862–5. 10.1093/nar/gkg536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karched M, Bhardwaj RG, Tiss A, Asikainen S. Proteomic Analysis and Virulence Assessment of Granulicatella adiacens Secretome. 2019;9(104). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karched M, Ihalin R, Eneslatt K, Zhong D, Oscarsson J, Wai SN, et al. Vesicle-independent extracellular release of a proinflammatory outer membrane lipoprotein in free-soluble form. BMC Microbiol. 2008;8:18 10.1186/1471-2180-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, et al. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26(13):1608–15. 10.1093/bioinformatics/btq249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almagro Armenteros JJ, Tsirigos KD, Sonderby CK, Petersen TN, Winther O, Brunak S, et al. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol. 2019;37(4):420–3. 10.1038/s41587-019-0036-z [DOI] [PubMed] [Google Scholar]
- 23.Bendtsen JD, Nielsen H, Widdick D, Palmer T, Brunak S. Prediction of twin-arginine signal peptides. BMC Bioinformatics. 2005;6:167 10.1186/1471-2105-6-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagos PG, Tsirigos KD, Liakopoulos TD, Hamodrakas SJ. Prediction of lipoprotein signal peptides in Gram-positive bacteria with a Hidden Markov Model. J Proteome Res. 2008;7(12):5082–93. 10.1021/pr800162c [DOI] [PubMed] [Google Scholar]
- 25.Chen C, Zabad S, Liu H, Wang W, Jeffery C. MoonProt 2.0: an expansion and update of the moonlighting proteins database. Nucleic Acids Res. 2018;46(D1):D640–d4. 10.1093/nar/gkx1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franco-Serrano L, Hernandez S, Calvo A, Severi MA, Ferragut G, Perez-Pons J, et al. MultitaskProtDB-II: an update of a database of multitasking/moonlighting proteins. Nucleic Acids Res. 2018;46(D1):D645–d8. 10.1093/nar/gkx1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6. 10.1093/bioinformatics/bti610 [DOI] [PubMed] [Google Scholar]
- 28.Ye J, Zhang Y, Cui H, Liu J, Wu Y, Cheng Y, et al. WEGO 2.0: a web tool for analyzing and plotting GO annotations, 2018 update. Nucleic Acids Res. 2018;46(W1):W71–w5. 10.1093/nar/gky400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Mering C, Jensen LJ, Snel B, Hooper SD, Krupp M, Foglierini M, et al. STRING: known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2005;33(Database issue):D433–7. 10.1093/nar/gki005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du J, Yuan Z, Ma Z, Song J, Xie X, Chen Y. KEGG-PATH: Kyoto encyclopedia of genes and genomes-based pathway analysis using a path analysis model. Mol Biosyst. 2014;10(9):2441–7. 10.1039/c4mb00287c [DOI] [PubMed] [Google Scholar]
- 31.Garg A, Gupta D. VirulentPred: a SVM based prediction method for virulent proteins in bacterial pathogens. BMC Bioinformatics. 2008;9:62 10.1186/1471-2105-9-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhardwaj RG, Al-Khabbaz A, Karched M. Cytokine induction of peripheral blood mononuclear cells by biofilms and biofilm supernatants of Granulicatella and Abiotrophia spp. Microb Pathog. 2018;114:90–4. 10.1016/j.micpath.2017.11.037 [DOI] [PubMed] [Google Scholar]
- 33.Haas B, Grenier D. Isolation, Characterization and Biological Properties of Membrane Vesicles Produced by the Swine Pathogen Streptococcus suis. PLoS One. 2015;10(6):e0130528 10.1371/journal.pone.0130528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Resch U, Tsatsaronis JA, Le Rhun A, Stübiger G, Rohde M, Kasvandik S, et al. A Two-Component Regulatory System Impacts Extracellular Membrane-Derived Vesicle Production in Group A Streptococcus. MBio. 2016;7(6). 10.1128/mBio.00207-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bai J, Kim SI, Ryu S, Yoon H. Identification and characterization of outer membrane vesicle-associated proteins in Salmonella enterica serovar Typhimurium. Infect Immun. 2014;82(10):4001–10. 10.1128/IAI.01416-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebner P, Gotz F. Bacterial Excretion of Cytoplasmic Proteins (ECP): Occurrence, Mechanism, and Function. Trends Microbiol. 2019;27(2):176–87. 10.1016/j.tim.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 37.Green ER, Mecsas J. Bacterial Secretion Systems: An Overview. Microbiol Spectr. 2016;4(1). 10.1128/microbiolspec.VMBF-0012-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natale P, Bruser T, Driessen AJ. Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane—distinct translocases and mechanisms. Biochim Biophys Acta. 2008;1778(9):1735–56. 10.1016/j.bbamem.2007.07.015 [DOI] [PubMed] [Google Scholar]
- 39.Henderson B. An overview of protein moonlighting in bacterial infection. Biochem Soc Trans. 2014;42(6):1720–7. 10.1042/BST20140236 [DOI] [PubMed] [Google Scholar]
- 40.Henderson B, Martin A. Bacterial virulence in the moonlight: multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect Immun. 2011;79(9):3476–91. 10.1128/IAI.00179-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson B, Martin A. Bacterial moonlighting proteins and bacterial virulence. Curr Top Microbiol Immunol. 2013;358:155–213. 10.1007/82_2011_188 [DOI] [PubMed] [Google Scholar]
- 42.Cezairliyan B, Ausubel FM. Investment in secreted enzymes during nutrient-limited growth is utility dependent. Proc Natl Acad Sci U S A. 2017;114(37):E7796–e802. 10.1073/pnas.1708580114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aseev LV, Boni IVJMB. Extraribosomal functions of bacterial ribosomal proteins. 2011;45(5):739. [PubMed] [Google Scholar]
- 44.Oliveira SC, Harms JS, Banai M, Splitter GA. Recombinant Brucella abortus proteins that induce proliferation and gamma-interferon secretion by CD4+ T cells from Brucella-vaccinated mice and delayed-type hypersensitivity in sensitized guinea pigs. Cell Immunol. 1996;172(2):262–8. 10.1006/cimm.1996.0241 [DOI] [PubMed] [Google Scholar]
- 45.Zakharzhevskaya NB, Vanyushkina AA, Altukhov IA, Shavarda AL, Butenko IO, Rakitina DV, et al. Outer membrane vesicles secreted by pathogenic and nonpathogenic Bacteroides fragilis represent different metabolic activities. Sci Rep. 2017;7(1):5008 10.1038/s41598-017-05264-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bjur E, Eriksson-Ygberg S, Aslund F, Rhen M. Thioredoxin 1 promotes intracellular replication and virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2006;74(9):5140–51. 10.1128/IAI.00449-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carroll RK, Robison TM, Rivera FE, Davenport JE, Jonsson IM, Florczyk D, et al. Identification of an intracellular M17 family leucine aminopeptidase that is required for virulence in Staphylococcus aureus. Microbes Infect. 2012;14(11):989–99. 10.1016/j.micinf.2012.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goulhen F, Hafezi A, Uitto VJ, Hinode D, Nakamura R, Grenier D, et al. Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect Immun. 1998;66(11):5307–13. 10.1128/IAI.66.11.5307-5313.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinode D, Grenier D, Mayrand D. Purification and characterization of a DnaK-like and a GroEL-like protein from Porphyromonas gingivalis. Anaerobe. 1995;1(5):283–90. 10.1006/anae.1995.1028 [DOI] [PubMed] [Google Scholar]
- 50.Gerlach D, Reichardt W, Vettermann S. Extracellular superoxide dismutase from Streptococcus pyogenes type 12 strain is manganese-dependent. FEMS Microbiol Lett. 1998;160(2):217–24. 10.1111/j.1574-6968.1998.tb12914.x [DOI] [PubMed] [Google Scholar]
- 51.Lu GT, Xie JR, Chen L, Hu JR, An SQ, Su HZ, et al. Glyceraldehyde-3-phosphate dehydrogenase of Xanthomonas campestris pv. campestris is required for extracellular polysaccharide production and full virulence. Microbiology. 2009;155(Pt 5):1602–12. 10.1099/mic.0.023762-0 [DOI] [PubMed] [Google Scholar]
- 52.Baska P, Norbury LJ, Wisniewski M, Januszkiewicz K, Wedrychowicz H. Excretory/secretory products of Fasciola hepatica but not recombinant phosphoglycerate kinase induce death of human hepatocyte cells. Acta Parasitol. 2013;58(2):215–7. 10.2478/s11686-013-0126-x [DOI] [PubMed] [Google Scholar]
- 53.Taguchi F, Ogawa Y, Takeuchi K, Suzuki T, Toyoda K, Shiraishi T, et al. A homologue of the 3-oxoacyl-(acyl carrier protein) synthase III gene located in the glycosylation island of Pseudomonas syringae pv. tabaci regulates virulence factors via N-acyl homoserine lactone and fatty acid synthesis. J Bacteriol. 2006;188(24):8376–84. 10.1128/JB.00763-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13(10):605–19. 10.1038/nrmicro3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thay B, Damm A, Kufer TA, Wai SN, Oscarsson J. Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger NOD1- and NOD2-dependent NF-kappaB activation. Infect Immun. 2014;82(10):4034–46. 10.1128/IAI.01980-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maldonado R, Wei R, Kachlany SC, Kazi M, Balashova NV. Cytotoxic effects of Kingella kingae outer membrane vesicles on human cells. Microb Pathog. 2011;51(1–2):22–30. 10.1016/j.micpath.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong Q, Zhao Y, Chen T, Yin S, Yao X, Wang J, et al. A functional peptidoglycan hydrolase characterized from T4SS in 89K pathogenicity island of epidemic Streptococcus suis serotype 2. BMC Microbiol. 2014;14:73 10.1186/1471-2180-14-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loprasert S, Whangsuk W, Sallabhan R, Mongkolsuk S. DpsA protects the human pathogen Burkholderia pseudomallei against organic hydroperoxide. Arch Microbiol. 2004;182(1):96–101. 10.1007/s00203-004-0694-0 [DOI] [PubMed] [Google Scholar]
- 59.Li J, Guo M, Cao Y, Xia Y. Disruption of a C69-Family Cysteine Dipeptidase Gene Enhances Heat Shock and UV-B Tolerances in Metarhizium acridum. Front Microbiol. 2020;11:849 10.3389/fmicb.2020.00849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reardon-Robinson ME, Osipiuk J, Jooya N, Chang C, Joachimiak A, Das A, et al. A thiol-disulfide oxidoreductase of the Gram-positive pathogen Corynebacterium diphtheriae is essential for viability, pilus assembly, toxin production and virulence. Mol Microbiol. 2015;98(6):1037–50. 10.1111/mmi.13172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nasher F, Heller M, Hathaway LJ. Streptococcus pneumoniae Proteins AmiA, AliA, and AliB Bind Peptides Found in Ribosomal Proteins of Other Bacterial Species. Front Microbiol. 2017;8:2688 10.3389/fmicb.2017.02688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jose L, Ramachandran R, Bhagavat R, Gomez RL, Chandran A, Raghunandanan S, et al. Hypothetical protein Rv3423.1 of Mycobacterium tuberculosis is a histone acetyltransferase. FEBS J. 2016;283(2):265–81. 10.1111/febs.13566 [DOI] [PubMed] [Google Scholar]
- 63.Shi W, Feng J, Zhang M, Lai X, Xu S, Zhang X, et al. Biosynthesis of isoprenoids: characterization of a functionally active recombinant 2-C-methyl-D-erythritol 4-phosphate cytidyltransferase (IspD) from Mycobacterium tuberculosis H37Rv. J Biochem Mol Biol. 2007;40(6):911–20. 10.5483/bmbrep.2007.40.6.911 [DOI] [PubMed] [Google Scholar]
- 64.Gebhard S, Tran SL, Cook GM. The Phn system of Mycobacterium smegmatis: a second high-affinity ABC-transporter for phosphate. Microbiology. 2006;152(Pt 11):3453–65. 10.1099/mic.0.29201-0 [DOI] [PubMed] [Google Scholar]
- 65.Shankar M, Hossain MS, Biswas I. Pleiotropic Regulation of Virulence Genes in Streptococcus mutans by the Conserved Small Protein SprV. J Bacteriol. 2017;199(8). 10.1128/JB.00847-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suprenant KA, Bloom N, Fang J, Lushington G. The major vault protein is related to the toxic anion resistance protein (TelA) family. J Exp Biol. 2007;210(Pt 6):946–55. 10.1242/jeb.001800 [DOI] [PubMed] [Google Scholar]
- 67.Ladomersky E, Petris MJ. Copper tolerance and virulence in bacteria. Metallomics. 2015;7(6):957–64. 10.1039/c4mt00327f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noar RD, Daub ME. Transcriptome sequencing of Mycosphaerella fijiensis during association with Musa acuminata reveals candidate pathogenicity genes. BMC Genomics. 2016;17:690 10.1186/s12864-016-3031-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dang G, Chen L, Li Z, Deng X, Cui Y, Cao J, et al. Expression, Purification and Characterisation of Secreted Esterase Rv2525c from Mycobacterium tuberculosis. Appl Biochem Biotechnol. 2015;176(1):1–12. 10.1007/s12010-015-1555-9 [DOI] [PubMed] [Google Scholar]
- 70.Garg R, Tripathi D, Kant S, Chandra H, Bhatnagar R, Banerjee N. The conserved hypothetical protein Rv0574c is required for cell wall integrity, stress tolerance, and virulence of Mycobacterium tuberculosis. Infect Immun. 2015;83(1):120–9. 10.1128/IAI.02274-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herrera AI, Ploscariu NT, Geisbrecht BV, Prakash O. (1)H, (15)N, and (13)C resonance assignments of the third domain from the S. aureus innate immune evasion protein Eap. Biomol NMR Assign. 2018;12(1):175–8. 10.1007/s12104-018-9804-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mesnage S, Dellarole M, Baxter NJ, Rouget JB, Dimitrov JD, Wang N, et al. Molecular basis for bacterial peptidoglycan recognition by LysM domains. Nat Commun. 2014;5:4269 10.1038/ncomms5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Troxell B, Hassan HM. Transcriptional regulation by Ferric Uptake Regulator (Fur) in pathogenic bacteria. Front Cell Infect Microbiol. 2013;3:59 10.3389/fcimb.2013.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tortosa P, Albano M, Dubnau D. Characterization of ylbF, a new gene involved in competence development and sporulation in Bacillus subtilis. Mol Microbiol. 2000;35(5):1110–9. 10.1046/j.1365-2958.2000.01779.x [DOI] [PubMed] [Google Scholar]
- 75.Lanotte P, Perivier M, Haguenoer E, Mereghetti L, Burucoa C, Claverol S, et al. Proteomic biomarkers associated with Streptococcus agalactiae invasive genogroups. PLoS One. 2013;8(1):e54393 10.1371/journal.pone.0054393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh I, Yadav AR, Mohanty KK, Katoch K, Sharma P, Mishra B, et al. Molecular mimicry between Mycobacterium leprae proteins (50S ribosomal protein L2 and Lysyl-tRNA synthetase) and myelin basic protein: a possible mechanism of nerve damage in leprosy. Microbes Infect. 2015;17(4):247–57. 10.1016/j.micinf.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 77.Durighello E, Bellanger L, Ezan E, Armengaud J. Proteogenomic biomarkers for identification of Francisella species and subspecies by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. Anal Chem. 2014;86(19):9394–8. 10.1021/ac501840g [DOI] [PubMed] [Google Scholar]
- 78.Araujo IR, Ferrari TC, Teixeira-Carvalho A, Campi-Azevedo AC, Rodrigues LV, Guimaraes Junior MH, et al. Cytokine Signature in Infective Endocarditis. PLoS One. 2015;10(7):e0133631 10.1371/journal.pone.0133631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamaji Y, Kubota T, Sasaguri K, Sato S, Suzuki Y, Kumada H, et al. Inflammatory cytokine gene expression in human periodontal ligament fibroblasts stimulated with bacterial lipopolysaccharides. Infect Immun. 1995;63(9):3576–81. 10.1128/IAI.63.9.3576-3581.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamamoto T, Kita M, Oseko F, Nakamura T, Imanishi J, Kanamura N. Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J Periodontal Res. 2006;41(6):554–9. 10.1111/j.1600-0765.2006.00905.x [DOI] [PubMed] [Google Scholar]
- 81.Jiang Y, Russell TR, Schilder H, Graves DT. Endodontic pathogens stimulate monocyte chemoattractant protein-1 and interleukin-8 in mononuclear cells. J Endod. 1998;24(2):86–90. 10.1016/S0099-2399(98)80083-6 [DOI] [PubMed] [Google Scholar]
- 82.Nagaoka S, Tokuda M, Sakuta T, Taketoshi Y, Tamura M, Takada H, et al. Interleukin-8 gene expression by human dental pulp fibroblast in cultures stimulated with Prevotella intermedia lipopolysaccharide. J Endod. 1996;22(1):9–12. 10.1016/S0099-2399(96)80228-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(PDF)
(PDF)
Data Availability Statement
Mass spectrometry data is deposited in the PRIDE archive under accession number PXD021414 and publicly accessible via the following URL: https://www.ebi.ac.uk/pride/archive/projects/PXD021414.