Abstract
Background
Antimicrobial resistance (AMR) is presently considered an emergent major global public health concern and excessive and/or inappropriate use of broad-spectrum antimicrobials contribute to the development of AMR.
Objective
To evaluate the appropriateness of carbapenems and piperacillin-tazobactam use in a tertiary care hospital.
Methods
A retrospective, observational, cross-sectional, drug-utilization study was conducted. The study included all adult hospitalized patients who had received at least one dose of the antimicrobials during their admission for the period between 1 January 2016 and 31 December 2017. The appropriateness of antimicrobial therapy was evaluated according to the Infectious Diseases Society of America (IDSA) guidelines with the consideration of the institutional antibiogram.
Results
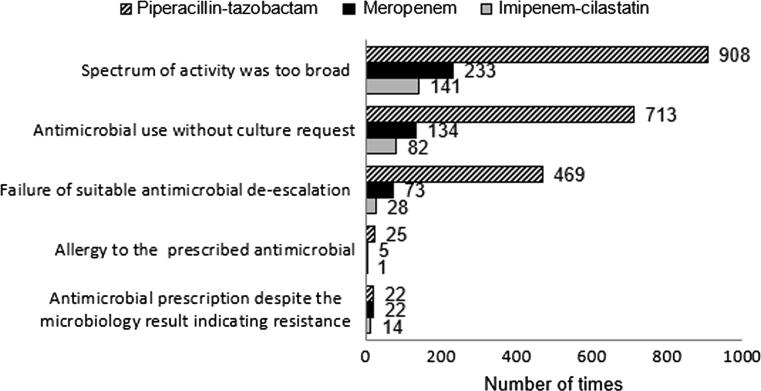
Overall, 2731 patients received 5005 courses with one of the antimicrobials, for a total of 5045.9 defined daily doses (DDD) of imipenem-cilastatin, 6492.3 of meropenem and 15,595 of piperacillin-tazobactam (4.93, 6.34 and 15.24 DDD/100 bed days, respectively). The mean age of the patients who received either antimicrobial was 55.5 ± 20.3 years, with a 14-day average length of hospital stay. About half (52%) of the prescriptions were written for patients treated in the medical ward. Pneumonia (26.6%) and sepsis (24.9%) were the most common indication for the initiation of antimicrobial therapy. Of the assessed prescriptions, only 2787 (56.5%) were prescribed appropriately, with 2142 (43.5%) deemed inappropriate. The three most common reasons for inappropriate prescription were: the spectrum of activity was too broad (44.6%), followed by antimicrobial use without culture request (32.4%), and failure of suitable antimicrobial de-escalation (19.9%).
Conclusions
The study indicates that the overall rate of inappropriateness was high, emphasizing the need to develop initiatives to effectively improve broad-spectrum antimicrobial prescribing.
Keywords: Carbapenem, Imipenem-cilastatin, Meropenem, Piperacillin-tazobactam, Prescribing patterns, Antimicrobial stewardship
1. Introduction
In the last decade, there has been a substantial increase in bacterial organisms resistant to multiple antimicrobial drugs (World Health Organization, 2017). At present, the World Health Organization (WHO) considers AMR as a significant global public health crisis (WHO, 2017). Infections caused by resistant bacteria are not only difficult to treat (Frieri et al., 2017) but also can prolong patient hospitalization, increase mortality, morbidity, and the cost of care (Cosgrove, 2006). Excessive antimicrobial use has been associated with superinfection and also disease associated with antimicrobial use, for example, Clostridium difficile, both of which increase morbidity and mortality in hospitalized patients (Dial et al., 2008, Wenisch et al., 2012). There is no reliable estimation of AMR cases worldwide, primarily due to inadequate surveillance. Limitations of any surveillance system or AMR research are those characteristics associated with the assumptions, design, methodology and data used that influence the findings explanation and interpretation (World Health Organization, 2018). The development and implementation of a standard method for estimating AMR would generate a reliable estimate of AMR globally (Limmathurotsakul et al., 2019). Despite inadequate surveillance, some evidence suggests that the incidence is much higher in developing countries (Ayukekbong et al., 2017). By 2050, it is estimated that 10 million people will die annually from AMR-related infections (O’Neill, 2016). Inappropriate use of broad-spectrum antimicrobials contributes to the emergence of AMR (Ventola, 2015). Studies have shown that up to 50% of antimicrobial use in clinical settings were inappropriate (Dellit et al., 2007, Hecker et al., 2003). Several studies have demonstrated a correlation between antimicrobial use and the emergence of antimicrobial-resistant organisms in hospital settings (Bell et al., 2014, Fishman, 2006). Pseudomonas aeruginosa, and extended-spectrum beta-lactamases (ESBL) producing gram-negative bacteria are often resistant to multiple antimicrobials presenting healthcare professionals with limited therapeutic options (Fatima et al., 2012).
Broad-spectrum antimicrobials such as piperacillin-tazobactam, imipenem-cilastatin, and meropenem have excellent activity against many gram-positive, anaerobic, and gram-negative organisms including many multidrug-resistant strains of Pseudomonas aeruginosa and Enterobacteriaceae species (Perry and Markham, 1999, Wilson, 2017). These antimicrobials are often considered the last resort in treating multi-drug-resistant bacterial infections and have been classified by the WHO as critically essential antimicrobials since 2005 (WHO, 2016). Evidence has shown that disproportionate use of piperacillin-tazobactam has been associated with the isolation of piperacillin-tazobactam-resistant Pseudomonas aeruginosa (Harris et al., 2002, Patel et al., 2008, Sonmezer et al., 2016). Moreover, increased use of carbapenems has been linked to carbapenem-resistant Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter species (Mladenovic-Antic et al., 2016, WHO, 2016). The studies have shown that the rates of carbapenem-resistant Pseudomonas aeruginosa, Acinetobacter baumannii, and ESBL-producing Enterobacteriaceae are generally high in Saudi Arabian hospital settings (Zowawi, 2016, Yezli et al., 2014).
Strategies to reduce inappropriate utilization of broad-spectrum antimicrobials can minimize the emergence of AMR (Drew, 2009). Studying antimicrobial utilization can assist in developing strategies to improve the local prescribing practice for antimicrobials, help tailor evidence-based antimicrobial prescribing, develop antimicrobial prescribing policies and augment antimicrobial stewardship (AMS) program to control AMR (WHO, 2003). Limited information is currently available in the literature about carbapenems and piperacillin-tazobactam prescribing in Saudi Arabia. Previous studies have either included hospitalized patients in specific departments (Balkhy et al., 2018, Huwait et al., 2019, Youssif et al., 2018) or focused in narrow patient population (Balkhy et al., 2019). The aim of this study was to assess the appropriateness of carbapenems (imipenem-cilastatin or meropenem) and piperacillin-tazobactam prescribing in Saudi adult hospitalized patients including all hospital departments and to identify reasons, if any, for their inappropriate use.
2. Methods
2.1. Ethical approval
Ethical approval was obtained from the Institutional Review Board, King Saud University College of Medicine (IRB number: E18-2869).
2.2. Study design and setting
A retrospective, observational, cross-sectional, drug-utilisation study was conducted in an 1100-bed tertiary care teaching hospital. The study included all adult hospitalized patients aged 18 years and older who had received at least one dose of imipenem-cilastatin, meropenem, or piperacillin-tazobactam during their inpatient or emergency room admission for the period between 1 January 2016 and 31 December 2017. All neonates, pediatric or any adult patient who received the studied antimicrobials in home healthcare settings were excluded from the study. During the study period, there was no antimicrobial stewardship program in place.
2.3. Data collection
Data were retrieved from the hospital electronic medical records (EMRs) and exported to an Excel® spreadsheet. The retrieved data included: patient demographics; indication for imipenem-cilastatin, meropenem or piperacillin-tazobactam; the use and duration of antimicrobial therapy; culture and sensitivity results if available; antimicrobial prescriber specialty and all antimicrobials prescribed during the patient’s admission.
2.4. Definitions and classifications of antimicrobial prescribing
Antimicrobial therapy was categorized according to the type of therapy into three main categories: empirical, targeted and prophylactic. Empirical antimicrobial therapy was defined as antimicrobials prescribed for a patient suspected to have an infection of unknown pathogen(s) before the availability of culture and susceptibility results. Targeted antimicrobial therapy was defined as antimicrobial therapy prescribed for a patient with identified infection documented in culture and sensitivity results. Prophylactic antimicrobial therapy was defined as antimicrobial therapy prescribed for a patient to prevent an infection or for a patient undergoing a surgical procedure to avoid surgical site infection (Leekha et al., 2011). In addition to the number of prescriptions, the total defined daily doses (DDD) antimicrobial utilization metric was used to report the antimicrobial use (World Health Organization, 2020).
2.5. Assessment of antimicrobial appropriateness
The appropriateness of antimicrobial therapy was evaluated according to IDSA guidelines (IDSA, 2018) with the consideration of the institutional antibiogram and microbiological findings for each patient. If the indication of antimicrobial use was empiric therapy, all cases were reviewed to identify if a microbiology culture had been requested. If a microbiology culture had been requested, each case was reviewed for the follow-up of antimicrobial therapy (continuation, discontinuation, escalation or de-escalation) based on reported results. If the indication for treatment was targeted therapy and the antimicrobial was not used initially as empiric therapy, culture and susceptibility results were reviewed to check whether the targeted therapy was in accord with the susceptibility data and the organism was not susceptible to a narrow-spectrum antimicrobial. Some patients had more than one admission during the study period. In addition, some patients may have been prescribed more than one antimicrobial or the same antimicrobial but at different times during the same admission period. Each antimicrobial prescription was assessed independently, according to its appropriateness. Some patients died or were discharged before the culture results were obtained. In such cases, the result was categorized under patient died before the culture results and was excluded from the analysis.
2.6. Reasons for inappropriateness
Reasons for inappropriateness were defined after determining the appropriateness criteria. Table S1 illustrates the definition of each reason for inappropriateness of antimicrobial therapy. Some antimicrobial prescriptions had more than one reason for inappropriateness.
2.7. Statistical analysis
The Shapiro-Wilk test of normality was carried out on all data to verify the fit of the data to a normal distribution. Categorical variables were expressed as frequencies and percentages, while continuous variables were expressed as means, standard deviation (SD), median, and range where appropriate. To assess whether there was an association between independent factors and inappropriate antimicrobial prescribing we subtracted the percentage with inappropriate prescribing in the reference category from the percentage in the exposed category to obtain the percentage risk difference. Confidence intervals were calculated using the normal approximation to the binomial distribution. All statistical analyses were performed with IBM SPSS Statistics for Windows, version 24.0 (Armonk, NY: IBM Corp, 2016).
3. Results
3.1. Patient demographics
A total of 2871 patients received 5250 courses of antimicrobial treatment with at least one of the studied broad-spectrum antimicrobials during 3671 patient admissions over a two-year period. Six hundred and sixty-eight patients were prescribed two courses of antimicrobials, and 1219 patients were prescribed more than two courses. One hundred and forty patients who received 245 (4.7%) courses of antimicrobials during 167 visits were excluded from the assessment due to a lack of documentation. The mean age of the patients was 55.5 years (SD = ±20.25, range 18 - 108 years), and 1389 (50.9%) were male. During the study period, 377 (13.8%) patients died, and 885 (32.4%) had surgery. The demographic characteristics of the patients are summarized in Table 1.
Table 1.
Demographic characteristics of all adult patients prescribed imipenem-cilastatin, meropenem, or piperacillin-tazobactam during the study period.
| Characteristics | N (%) |
|---|---|
| Sex: | |
| Male | 1389 (50.9%) |
| Female | 1342 (49.1%) |
| Age (years) Mean ± SD | 55.5 ± 20.3 |
| Length of stay Median (range) | 14 (1–3185) |
| Allergy status: | |
| Documented drug allergy | 91 (3.3%) |
| Documented antimicrobial allergy | 48 (1.8%) |
| Co-morbidities: | |
| Diabetes mellitus | 1079 (39.5%) |
| Cardiovascular disease | 702 (25.7%) |
| Hypertension | 767 (28.1%) |
| Respiratory disease | 355 (13%) |
| Dyslipidaemia | 526 (19.3%) |
| Psychiatric disorder | 226 (8.3%) |
| Musculoskeletal disorder | 220 (8.1%) |
| Haematological disorder | 359 (13.1%) |
| Gastrointestinal disease | 232 (8.5%) |
| Liver disease | 154 (5.6%) |
| Neurological disorders | 303 (11.1%) |
| Thyroid disorder | 178 (6.5%) |
| Kidney disease | 365 (13.4%) |
| Cancer | 510 (18.7%) |
| Vitamin D deficiency | 215 (7.9%) |
3.2. Antimicrobial use
Overall, on 4106 (82%) occasions antimicrobials were prescribed as empiric therapy, 757 (15.2%) were as targeted therapy after a pathogen had been identified in a clinical specimen, and 142 (2.8%) were as prophylactic therapy. Table 2 shows the types of prescriptions and the duration of treatment for imipenem-cilastatin, meropenem and piperacillin-tazobactam.
Table 2.
Type of prescriptions for imipenem-cilastatin, meropenem and piperacillin-tazobactam.
| Variable | Antimicrobial agent |
||
|---|---|---|---|
| Imipenem-cilastatin | Meropenem | Piperacillin-tazobactam | |
| Total antimicrobial use (DDD) | 5045.87 | 6492.25 | 15594.99 |
| DDD per 100 bed days | 4.93 | 6.34 | 15.24 |
| Total courses (n) | 776 | 1021 | 3208 |
| Type of prescriptions | |||
| Empiric | 476 (61.3%) | 708 (69.3%) | 2922 (91.1%) |
| Targeted | 294 (38%) | 292 (28.6%) | 171 (5.3%) |
| Prophylaxis | 6 (0.7%) | 21 (2.1%) | 115 (3.6%) |
| Duration of treatment (days), Median (range) | 7 (1–53) | 7 (1–64) | 7 (1–48) |
The highest proportion of prescriptions were written for the patients treated in a medical ward (52%) followed by surgical wards (23.4%), intensive care unit (ICU, 15.3%), and emergency department (ED, 2.2%). Pneumonia (26.6%) was the most common indication for initiation of antimicrobial therapy, followed by sepsis (24.9%), urinary tract infections (17.3%), skin and soft tissue infections (14.8%), intra-abdominal infections (8.6%) and febrile neutropenia (4%). Table 3 summarizes the main indications for imipenem-cilastatin, meropenem and piperacillin-tazobactam. Medical residents prescribed the majority of antimicrobial therapy (64.7%), followed by internal medicine specialists (17%) and ICU specialist (11%). Culture and sensitivity tests were ordered on 3408 (68%) occasions before prescribing antimicrobials; 2651 (52.9%) were before initiating empiric antimicrobial therapy.
Table 3.
Indications of antimicrobial therapy.
| Indication | Number | Imipenem-cilastatin n = 776 |
Meropenem n = 1021 |
Piperacillin-tazobactam n = 3208 |
|---|---|---|---|---|
| Febrile neutropenia | 199 | 21 (2.7%) | 27 (2.6%) | 151 (4.7%) |
| Gynaecological infection | 5 | 0 (0%) | 0 (0%) | 5 (0.2%) |
| Intra-abdominal infection | 431 | 54 (7%) | 53 (5.2%) | 324 (10.1%) |
| Meningitis | 8 | 0 (0%) | 3 (0.2%) | 5 (0.2%) |
| Osteomyelitis | 25 | 5 (0.6%) | 9 (0.9%) | 11 (0.3%) |
| Pneumonia | 1333 | 122 (15.7%) | 203 (19.9%) | 1008 (31.4%) |
| Sepsis | 1248 | 257 (33.1%) | 359 (35.2%) | 632 (19.7%) |
| Skin and soft tissue infection | 743 | 130 (16.8%) | 130 (12.7%) | 483 (15.1%) |
| Urinary tract infection | 864 | 181 (23.3%) | 213 (20.9%) | 470 (14.7%) |
| aOther | 7 | 0 (0%) | 3 (0.3%) | 4 (0.1%) |
| Prophylaxis | ||||
| Surgical prophylaxis | 102 | 3 (0.4%) | 8 (0.8%) | 91 (2.8%) |
| bOther prophylaxis | 40 | 3 (0.4%) | 13 (1.3%) | 24 (0.7%) |
Other includes endocarditis and acute otitis externa.
Other prophylaxis includes trauma, burn and bite.
3.3. Appropriateness of antimicrobial therapy
Overall, 4929 (98.5%) of the prescribed antimicrobials were assessed for their appropriateness of prescribing. A small number (76, 1.5%) of the prescribed antimicrobials could not be assessed as some patients were either discharged or died before culture results became available. The results showed that only 2787 (56.5%) of antimicrobial orders were prescribed appropriately, with 2142 (43.5%) inappropriate. An empirical initiation of piperacillin-tazobactam was only appropriate in 52.3% of the total piperacillin-tazobactam prescriptions. Almost all carbapenems and piperacillin-tazobactam prescribed as prophylactic therapy were inappropriate. Table 4 illustrates the appropriateness of imipenem-cilastatin, meropenem and piperacillin-tazobactam prescriptions.
Table 4.
Appropriateness of imipenem-cilastatin, meropenem and piperacillin-tazobactam prescriptions.
| Antimicrobial | Type of indication | Appropriate | Inappropriate |
|---|---|---|---|
| Imipenem-cilastatin | Empiric (n = 466) |
63.5% | 36.5% |
| Targeted (n = 294) |
87% | 13% | |
| Prophylaxis (n = 6) |
0% | 100% | |
| Meropenem | Empiric (n = 691) |
57.9% | 42.1% |
| Targeted (n = 291) |
79.4% | 20.6% | |
| Prophylaxis (n = 21) |
0% | 100% | |
| Piperacillin-tazobactam | Empiric (n = 2874) |
52.3% | 47.7% |
| Targeted (n = 171) |
57.9% | 42.1% | |
| Prophylaxis (n = 115) |
0.9% | 99.1% |
The most common reasons for inappropriate use of antimicrobials were: spectrum of activity was too broad (44.6%), antimicrobial use without culture request (32.4%), failure of suitable antimicrobial de-escalation (19.9%), continuation of antimicrobial prescribing despite microbiology results indicating resistance (2%), and known allergy to the prescribed antimicrobial (1.1%). As some antimicrobial prescriptions had more than one reason for inappropriateness, the total number for the identified reasons were 2870. Fig. 1 outlines the reason why antimicrobials were inappropriate for of imipenem-cilastatin, meropenem and piperacillin-tazobactam.
Fig. 1.
Reasons for the inappropriate prescribing for imipenem-cilastatin, meropenem and piperacillin-tazobactam.
There were no significant differences in the incidence of appropriate antimicrobial prescribing based on age, gender and patient location (ICU versus non-ICU). Patients with two or more co-morbidities were more likely to be prescribed inappropriate antimicrobial than patients with no co-morbidities (51.2% versus 42.9%; risk difference 8.3%; 95% CI, 2.8% to 13.8%) and (51.7% versus 42.9%; risk difference 8.8%; 95% CI, 4.5% to 13.1%). Prescriptions that were for targeted therapy were less likely to be inappropriate than prescriptions that were for empiric therapy (22.5% versus 45.4%; risk difference –22.9%; 95% CI, −26.3%, to 19.5%). Prescriptions that were for prophylaxis therapy were more likely to be inappropriate than prescriptions that were for empiric therapy (99.3% versus 45.4%; risk difference 53.9%; 95% CI, 51.8% to 56%). Table 5 shows the characteristics of patients prescribed both appropriate and inappropriate antimicrobials. Table 6 illustrates the appropriateness of antimicrobials according to the indication.
Table 5.
Demographics of patient and prescription characteristics.
| Characteristic | Appropriate n/N (%) N= (2787) |
Inappropriate n/N (%) N= (2142) |
Percentage risk difference (95%CI)* |
|---|---|---|---|
| Age | |||
| 18–44 | 715/1345 (53.2%) | 630/1345 (46.8%) | Reference |
| 45–64 | 905/1553 (58.3%) | 648/1553 (41.7%) | −5.1% (−8.7 to 1.5) |
| 65–84 | 1016/1766 (57.5%) | 750/1766 (42.5%) | −4.5% (−7.9 to 0.8) |
| ≥85 | 151/265 (57.0%) | 114/265 (43.0%) | −3.8% (−10.4 to 2.7) |
| Gender | |||
| Female | 1403/2445 (57.4%) | 1042/2445 (42.6%) | Reference |
| Male | 1384/2484 (55.7%) | 1100/2484 (44.3%) | 1.7% (−1.1 to 4.4) |
| Co-morbidities | |||
| 0 | 394/690 (57.1%) | 296/690 (42.9%) | Reference |
| 1 | 1155/1680 (68.8%) | 525/1680 (31.2%) | −11.7% (−16 to 7.3) |
| 2 | 281/576 (48.8%) | 295/576 (51.2%) | 8.3% (2.8 to 13.8) |
| ≥3 | 957/1983 (48.3%) | 1026/1983 (51.7%) | 8.8% (4.5 to 13.1) |
| Location | |||
| Non-ICU | 2368/4203 (56.3%) | 1835/4203 (43.7%) | Reference |
| ICU | 419/726 (57.7%) | 307/726 (42.3%) | −1.4% (−5.3 to 2.5) |
| Reason for antimicrobial therapy | |||
| Empiric | 2200/4031 (54.6%) | 1831/4031 (45.4%) | Reference |
| Targeted | 586/756 (77.5%) | 170/756 (22.5%) | –22.9% (−26.3 to −19.5) |
| Prophylaxis | 1/142 (0.7%) | 141/142 (99.3%) | 53.9% (51.8 to 56) |
Values in bold represent data where there is a significant difference with p ≤ 0.05.
Table 6.
Appropriateness of antimicrobials according to the indication.
| Indication | Appropriate n (%) |
Inappropriate n (%) |
Not assessed n (%) |
|---|---|---|---|
| Treatment (empiric and targeted) | |||
| Febrile neutropenia | 63/199 (31.7%) | 134/199 (67.3%) | 2/199 (1%) |
| Gynaecological infection | 0/5 (0%) | 5/5 (100%) | 0/5 (0%) |
| Intra-abdominal infection | 183/431 (42.5%) | 243/431 (56.4%) | 5/431 (1.1%) |
| Meningitis | 3/8 (37.5%) | 5/8 (62.5%) | 0/8 (0%) |
| Osteomyelitis | 17/25 (68%) | 8/25 (32%) | 0/25 (0%) |
| Pneumonia | 612/1333 (45.9%) | 703/1333 (52.7%) | 18/1333 (1.4%) |
| Sepsis | 873/1248 (70%) | 340/1248 (27.2%) | 35/1248 (2.8%) |
| Skin and soft tissue infections | 492/743 (66.2%) | 248/743 (33.4%) | 3/743 (0.4%) |
| Urinary tract infection | 539/864 (62.4%) | 312/864 (36.1%) | 13/864 (1.5%) |
| aOther | 4/7 (57.1%) | 3/7 (42.9%) | 0/7 (0%) |
| Prophylaxis | |||
| Surgical prophylaxis | 0/102 (0%) | 102/102 (100%) | 0/102 (0%) |
| bOther prophylaxis | 1/40 (2.5%) | 39/40 (97.5%) | 0/40 (0%) |
Other includes endocarditis and acute otitis externa.
Other prophylaxis includes trauma, burn and bite.
4. Discussion
This study provides important insights into the current prescribing practices of three broad-spectrum antimicrobials at a tertiary hospital within Saudi Arabia. Inappropriate use of antimicrobials is a common practice in hospital settings. Epidemiological studies have shown that there is an association between antimicrobial consumption and the emergence and spreading of bacteria resistance (Bell et al., 2014, Fishman, 2006). Drug utilization studies are useful in identifying current practice in hospital settings (WHO, 2003). The results of this study may be useful in assisting healthcare providers to improve the current practice and, therefore, reduce the emergence of resistant bacteria.
The consumption of piperacillin/tazobactam in this study was 15.24 DDDs per 100 bed days which was similar to previously reported Saudi (13.4 DDDs per 100 bed days; Balkhy et al., 2018), Australian & New Zealand studies (12.4 (13.4 DDDs per 100 bed days; Dulhunty et al., 2011) and was higher than reported in an Indian study (3.04 DDDs per 100 bed days; Ray and Datta, 2018). Unlike piperacillin/tazobactam, the consumption of carbapenem was 11.27 DDDs per 100 bed days which was less than previously reported in local (25.6 DDDs per 100 bed days; Balkhy et al., 2018) and international studies (67.6 DDDs per 100 bed days, Ray and Datta, 2018 and 25.7 DDDs per 100 bed days, Dulhunty et al., 2011).
In this study, broad-spectrum antimicrobials were commonly prescribed in the internal medicine wards. The most common indication for prescribing carbapenems and piperacillin-tazobactam was pneumonia, followed by sepsis. A study conducted by Youssif et al., found that the most common indication for prescribing carbapenems and piperacillin-tazobactam was skin and soft tissue infections, followed by intra-abdominal infections (Youssif et al., 2018). Reported differences may be due to the inclusion of surgical floors in the later study.
In this study, we have reported the results as the proportion of inappropriate prescriptions instead of the proportion of patients for the reason that 65.7% of the patients received more than one antimicrobial prescription during the evaluation period. The study found that the overall appropriate use of the three broad-spectrum antimicrobials was 56.5%. Most antimicrobials were prescribed for empirical indication (82%) and only 15.2% were post-culture results; the rate of appropriateness of empiric therapy was significantly lower than targeted therapy. Empiric indication of piperacillin-tazobactam was appropriate in 52.3% of the empiric courses. The current results are similar to the study by Khan et al. in evaluating piperacillin-tazobactam use in an adult population, which found that piperacillin-tazobactam was appropriately initiated in 57% of courses (Khan et al., 2012). In contrast, the results of the current study differ from other studies, where it was calculated that piperacillin-tazobactam was appropriately initiated on 71.5–93% occasions (Janowski et al., 2016, Raveh et al., 2006, Shah and Ryzner, 2013, Zeenny et al., 2014). The differences may be due to the variation in indication between pediatric versus adult populations, variations in prescriber education or the status of AMS in each hospital. The rate of appropriateness of empiric indication of meropenem and imipenem was 57.9% and 63.5%, respectively. A higher rate of imipenem appropriateness was demonstrated in another study using different methodology (Kabbara et al., 2015). Overall, the low rate of appropriateness observed in this study may have been for several reasons, including a lack of institutional guidelines and prescriber education.
The most common diagnosis in the category of inappropriateness was pneumonia and sepsis. In sepsis, a delay in antimicrobial prescribing is associated with increased mortality (Levy et al., 2018). The initial step in sepsis management is identification of patients with sepsis, which can be clinically challenging. Sepsis diagnosis is extremely subjective and identification can be complex, particularly in the early stages when presenting symptoms are non-specific and test results are pending. This means that a prescriber must make early decisions regarding antimicrobials usage (Vincent, 2016). In this period of uncertainty, prescribers need to act, balancing the risks of failing to treat sepsis versus overprescribing, and the risk of increasing AMR. To avoid potential consequences from inappropriate antimicrobial prescribing, including AMR and infections such as Clostridium difficile infection, AMS strategies, including guidelines and de-escalation protocols, should be implemented (Davey et al., 2017).
The most common reason given for the assessment of inappropriateness was that the spectrum of activity was too broad (44.6%). The high percentage of inappropriate initial selection of these broad-spectrum antimicrobials is concerning. Such overprescribing is associated with an increased risk of AMR, adverse effects, opportunistic infections and increased healthcare costs (Tamma et al., 2017). We, therefore, propose the need for AMS. Possible AMS strategies include guideline implementation and staff education (Davey et al., 2017).
In terms of microbiological tests, culture tests were requested to guide therapy in 52.9% of prescriptions. Of these prescriptions, failure of de-escalation, although warranted, was shown in 19.9% of cases. The failure of de-escalation may be due to the reluctance of prescribers to make modifications to a clinically unwell patient’s therapy or the tendency toward continuation of a therapy that appears to be effective (Paterson, 2006). Nevertheless, this rate and duration of therapy was concerning and is considered a focus area for a future intervention in antimicrobial prescribing practice.
Analysis of antimicrobial appropriateness for pre-defined subgroups of patients and prescriptions showed that patients who had a higher number of co-morbidities were more likely to be prescribed inappropriate antimicrobials than those who had fewer or no co-morbidities. We assume that for these patients, prescribers might prescribe broad-spectrum antimicrobials inappropriately in order to prevent the decompensation of co-morbidities related to sepsis. In addition, we found a significant association between empiric treatment and inappropriate antimicrobial prescribing. This implies that the empiric use of carbapenems and piperacillin-tazobactam was significantly associated with their inappropriate use.
The hospital does not currently have treatment guidelines regarding appropriate indications for the use of carbapenems and piperacillin-tazobactam. Establishing and implementing antimicrobial guidelines and in parallel educating and supporting prescribers in their adoption would be a feasible intervention that has been shown to be a cost-effective moderate impact activity (Robson et al., 2018). Another antimicrobial utilization evaluation could be conducted after these interventions have been implemented to evaluate their impact and to additionally direct AMS activities.
4.1. Strengths and limitations
To the best of our knowledge, this is the first study in the Gulf countries that has evaluated the appropriateness of the three broad-spectrum antimicrobials, including all hospitalized adult patients in tertiary care hospitals. The strength of the study was the large sample size. The study had several limitations. The inherent limitation lies in employing a retrospective study design, even though this is a common and acceptable method for evaluating appropriateness of antimicrobial therapy in hospital settings. In addition, as the assessment did not occur at the time of prescribing, the degree of accuracy of interpretations of information extracted from the hospital electronic database in the assessment of prescriptions relies on how much information had been recorded. However, the electronic chart captured comprehensive information on the course during hospitalization and physician notes and reports on microbiological findings. A further limitation is that the study was conducted in a single academic tertiary care hospital, which may limit the generalizability of the study. It should be noted, though, that all hospital wards were included, which assisted representative results being recorded across a range of disciplines. Finally, a lack of local guidelines prevailed. Hospital antibiograms were considered in the evaluation, involving the use of international guidelines. Therefore, the results can be compared with those in the literature.
5. Conclusion
This study has provided important insights into the use and appropriateness of broad-spectrum antimicrobials. The study shows that there is considerable inappropriate prescribing of broad-spectrum antimicrobials. Our study implies that AMS is needed to optimize their use in hospital settings to reduce the emergence of resistant organisms and to optimize patient safety and outcomes. Future research is needed to further explore factors associated with inappropriate antimicrobial prescribing practices as well as the impact of interventions on improving antimicrobial prescribing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2020.09.015.
Contributor Information
Nada A Alsaleh, Email: Nada.alsaleh@strath.ac.uk.
Hussain A Al-Omar, Email: halomar@ksu.edu.sa.
Ahmed Y Mayet, Email: iymayet@ksu.edu.sa.
Alexander B Mullen, Email: a.mullen@strath.ac.uk.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ayukekbong J.A., Ntemgwa M., Atabe A.N. The threat of AMR in developing countries: causes and control strategies. AMR Infect. Control. 2017;6:47. doi: 10.1186/s13756-017-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkhy H.H., El-Saed A., El-Metwally A., Arabi Y.M., Aljohany S.M., Al Zaibag M., Baharoon S., Alothman A.F. Antimicrobial consumption in five adult intensive care units: a 33-month surveillance study. Antimicrobial Resist. Infect. Control. 2018;7(1):156. doi: 10.1186/s13756-018-0451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkhy H.H., El-Saed A., AlShehri A., Alshaalan M., Hijazi O., El-Metwally A., Aljohany S.M., Al Saif S. Antimicrobial consumption in three pediatric and neonatal intensive care units in Saudi Arabia: 33-month surveillance study. Ann. Clin. Microbiol. Antimicrobials. 2019;18(1):20. doi: 10.1186/s12941-019-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell B.G., Schellevis F., Stobberingh E., Goossens H., Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014;14:13. doi: 10.1186/1471-2334-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove S.E. The relationship between AMR and patient outcomes: mortality, length of hospital stay, and health care costs. Clin. Infect. Dis. 2006;42:S82–S89. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- Davey P., Marwick C.A., Scott C.L., Charani E., McNeil K., Brown E. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017;(2):CD003543. doi: 10.1002/14651858.CD003543.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellit T.H., Owens R.C., McGowan J.E., Gerding D.N., Weinstein R.A., Burke J.P. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- Dial S., Kezouh A., Dascal A., Barkun A., Suissa S. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. CMAJ. 2008;179:767–772. doi: 10.1503/cmaj.071812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew R.H. Antimicrobial stewardship programs: how to start and steer a successful program. J. Manag. Care Pharm. 2009;15:S18–S23. doi: 10.18553/jmcp.2009.15.s2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty J.M., Paterson D., Webb S.A., Lipman J. Antimicrobial utilisation in 37 Australian and New Zealand intensive care units. Anaesth. Intensive Care. 2011;39(2):231–237. doi: 10.1177/0310057X1103900212. [DOI] [PubMed] [Google Scholar]
- Fatima A., Naqvi S.B., Khaliq S.A., Perveen S., Jabeen S. Antimicrobial susceptibility pattern of clinical isolates of Pseudomonas aeruginosa isolated from patients of lower respiratory tract infections. Springerplus. 2012;1:70. doi: 10.1186/2193-1801-1-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman N. Antimicrobial stewardship. Am. J. Infect. Control. 2006;34:S55–S63. doi: 10.1016/j.ajic.2006.05.237. [DOI] [PubMed] [Google Scholar]
- Frieri M., Kumar K., Boutin A. Antibiotic resistance. J. Infect. Publ. Health. 2017;10:369–378. doi: 10.1016/j.jiph.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Harris A.D., Perencevich E., Roghmann M.C., Morris G., Kaye K.S., Johnson J.A. Risk factors for piperacillin-tazobactam-resistant Pseudomonas aeruginosa among hospitalized patients. Antimicrob. Agents Chemother. 2002;46:854–858. doi: 10.1128/aac.46.3.854-858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M.T., Aron D.C., Patel N.P., Lehmann M.K., Doneskey C.J. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch. Intern. Med. 2003;163:972–978. doi: 10.1001/archinte.163.8.972. [DOI] [PubMed] [Google Scholar]
- Huwait B., Rahman B., Ramadan O., Aletreby W.T., Mady A.F., Harthy A.M.A. A pilot study to evaluate appropriateness of empirical antibiotic use in intensive care unit of King Saud Medical City, Riyadh, Saudi Arabia. Gen. Med. (Los Angeles) 2019;7:323. https://doi:10.24105/2327-5146.7.323 [Google Scholar]
- Infectious Diseases Society of America. practice guideline, 2018. https://www.idsociety.org/practice-guideline/alphabetical-guidelines/ (accessed January 2, 2018).
- Janowski A.B., Michaels M.G., Martin J.M., Green M.D. Piperacillin-Tazobactam usage at a tertiary pediatric hospital: an antimicrobial stewardship review. J. Pediatric Infect. Dis. Soc. 2016;5:342–345. doi: 10.1093/jpids/piv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbara W.K., Nawas G.T., Ramadan W.H. Evaluation of the appropriateness of imipenem/cilastatin prescription and dosing in a tertiary care hospital. Infect. Drug Resist. 2015;8:31–38. doi: 10.2147/IDR.S78633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F.Y., Elhiday A., Khudair I.F., Yousef H., Omran A.H., Alsamman S.H. Evaluation of the use of piperacillin/tazobactam (Tazocin) at Hamad General Hospital, Qatar: are there unjustified prescriptions? Infect Drug Resist. 2012;5:17–21. doi: 10.2147/IDR.S27965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekha S., Terrell C.L., Edson R.S. General principles of antimicrobial therapy. Mayo Clin. Proc. 2011;86:156–167. doi: 10.4065/mcp.2010.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M.M., Evans L.E., Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit. Care Med. 2018;46:997–1000. doi: 10.1097/CCM.0000000000003119. [DOI] [PubMed] [Google Scholar]
- Limmathurotsakul D., Dunachie S., Fukuda K., Feasey N.A., Okeke I.N., Holmes A.H., Moore C.E., Dolecek C., van Doorn H.R., Shetty N., Lopez A.D. Improving the estimation of the global burden of antimicrobial resistant infections. Lancet Infect. Dis. 2019;19(11):e392–e398. doi: 10.1016/S1473-3099(19)30276-2. [DOI] [PubMed] [Google Scholar]
- Mladenovic-Antic S., Kocic B., Velickovic-Radovanovic R., Dinic M., Petrovic J., Randjelovic G. Correlation between antimicrobial consumption and AMR of Pseudomonas aeruginosa in a hospital setting: a 10-year study. J. Clin. Pharm. Ther. 2016;41:532–537. doi: 10.1111/jcpt.12432. [DOI] [PubMed] [Google Scholar]
- O’Neill, J., 2016. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations, May 2016. https://amr-review.org/ (accessed November 19, 2019). https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
- Patel N., McNutt L.A., Lodise T.P. Relationship between various definitions of prior antibiotic exposure and piperacillin-tazobactam resistance among patients with respiratory tract infections caused by Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008;52:2933–2936. doi: 10.1128/AAC.00456-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D.L. The role of antimicrobial management programs in optimizing antibiotic prescribing within hospitals. Clin. Infect. Dis. 2006;42:S90–S95. doi: 10.1086/499407. [DOI] [PubMed] [Google Scholar]
- Perry C.M., Markham A. Piperacillin/tazobactam: an updated review of its use in the treatment of bacterial infections. Drugs. 1999;57:805–843. doi: 10.2165/00003495-199957050-00017. [DOI] [PubMed] [Google Scholar]
- Raveh D., Muallem-Zilcha E., Greenberg A., Wiener-Well Y., Schlesinger Y., Yinnon A.M. Prospective drug utilisation evaluation of three broad-spectrum antimicrobials: cefepime, piperacillin-tazobactam and meropenem. QJM. 2006;99:397–406. doi: 10.1093/qjmed/hcl050. [DOI] [PubMed] [Google Scholar]
- Ray D., Datta S. A study on antimicrobial agents utilization pattern using anatomical therapeutic chemical/daily defined dose system and adverse drug reaction pattern in the intensive care unit of a tertiary care teaching hospital in North Eastern state of India. Int. J. Basic Clin. Pharmacol. 2018;7:1612–1619. doi: 10.18203/2319-2003.ijbcp20183032. [DOI] [Google Scholar]
- Robson S.E., Cockburn A., Sneddon J., Mohana A., Bennie M., Mullen A.B. Optimizing carbapenem use through a national quality improvement programme. J. Antimicrob. Chemother. 2018;73:2223–2230. doi: 10.1093/jac/dky171. [DOI] [PubMed] [Google Scholar]
- Shah P.J., Ryzner K.L. Evaluating the appropriate use of piperacillin/tazobactam in a community health system: a retrospective chart review. P T. 2013;38:462–483. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3814439/ [PMC free article] [PubMed] [Google Scholar]
- Sonmezer M.C., Ertem G., Erdinc F.S., Kilic E., Tulek N., Adiloglu A. Evaluation of risk factors for antibiotic resistance in patients with nosocomial infections caused by Pseudomonas aeruginosa. Can. J. Infect. Dis. Med. Microbiol. 2016;2016:ID1321487. doi: 10.1155/2016/1321487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamma P.D., Avdic E., Li D.X., Dzintars K., Cosgrove S.E. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern. Med. 2017;177:1308–1315. doi: 10.1001/jamainternmed.2017.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola C.L. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40:277–283. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4378521/ [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L. The clinical challenge of sepsis identification and monitoring. PLoS Med. 2016;13(e1002022) doi: 10.1371/journal.pmed.1002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenisch J.M., Schmid D., Tucek G., Kuo W.H., Allerberger F., Michl V. A prospective cohort study on hospital mortality due to Clostridium difficile infection. Infection. 2012;40:479–484. doi: 10.1007/s15010-012-0258-1. [DOI] [PubMed] [Google Scholar]
- Wilson A.P.R. Sparing carbapenem usage. J. Antimicrob. Chemother. 2017;72:2410–2417. doi: 10.1093/jac/dkx181. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2003. Introduction to drug utilisation research. WHO International Working Group for Drug Statistics Methodology, WHO Collaborating Centre for Drug Statistics Methodology, WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services. ISBN 92 4 156234 X (NLM classification: WB 330); (cited 2019 Dec10). https://www.who.int/medicines/areas/quality_safety/safety_efficacy/Drug%20utilization%20research.pdf. (accessed December 10, 2019).
- World Health Organization. Antibiotic resistance 2017. http://www.who.int/mediacentre/factsheets/antibiotic-resistance/en/ (accessed December 10, 2019).
- World Health Organization, 2018. Global antimicrobial resistance surveillance system (GLASS) report: early implementation 2017–2018. https://www.who.int/docs/default-source/searo/amr/global-antimicrobial-resistance-surveillance-system---glass-report-early-implementation-2017-2018.pdf?sfvrsn=7e629fec_6 (accessed September 7, 2020).
- World Health Organization, 2020. ATC/DDD Index 2020 (2020) https://www.whocc.no/atc_ddd_index/ (accessed September 5, 2020).
- World Health Organization, 2016. Critically Important Antimicrobials for Human Medicine, 5th Revision 2016: Ranking of Antimicrobial Agents for Risk Management of AMR Due to Non-Human Use. Geneva, Switzerland, 2016. https://apps.who.int/iris/bitstream/handle/10665/255027/9789241512220-eng.pdf;jsessionid=28CA2D411CEACA85B2851142284F7C20?sequence=1 (accessed December 10, 2019).
- Yezli S., Shibl A.M., Livermore D.M., Memish Z.A. Prevalence and AMR among Gram-negative pathogens in Saudi Arabia. J. Chemother. 2014;26:257–272. doi: 10.1179/1973947814Y.0000000185. [DOI] [PubMed] [Google Scholar]
- Youssif E., Aseeri M., Khoshhal S. Retrospective evaluation of piperacillin–tazobactam, imipenem-cilastatin and meropenem used on surgical floors at a tertiary care hospital in Saudi Arabia. J. Infect. Publ. Health. 2018;11:486–490. doi: 10.1016/j.jiph.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Zeenny R., Nasr Z., Adaimy I. Retrospective evaluation of the appropriate use of piperacillin/tazobactam in a tertiary care teaching hospital in Lebanon. Acta Medica Mediterranea. 2014;30:655–663. http://www.actamedicamediterranea.com/archive/2014/medica-3/retrospective-evaluation-of-the-appropriate-use-of-piperacillintazobactam-in-a-tertiary-care-teaching-hospital-in-lebanon [Google Scholar]
- Zowawi H.M. AMR in Saudi Arabia. An urgent call for an immediate action. Saudi Med. J. 2016;37:935–940. doi: 10.15537/smj.2016.9.16139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.