Abstract
Impatiens capensis is an annual plant native to eastern North America that is currently spreading across Europe. In Poland, due to this plant’s rapid spread in the secondary range and high competitiveness in relation to native species, it is considered a locally invasive species. The microstructure of seeds is an important tool for solving various taxonomic problems and also provides data useful for determining the impact of various environmental factors on the phenotypic variability of species. This issue is particularly important in regard to invasive species which occupy a wide range of habitats in the invaded range. There are few reports on seed size and thus far no descriptions of the seed ultrastructure of I. capensis in the analyzed literature. We present new data on the seed morphology of I. capensis growing in different habitats and conditions in the secondary range of the species. The studied populations differed significantly in each of the investigated traits (seed length, width, circumference, area, roundness, and mass). Our findings showed that anthropogenic disturbances in habitats and some soil parameters (presence of carbonates, potassium, loose sand, and moisture) were statistically significant with various seed sizes and morphology in the studied populations of I. capensis. Moreover, our studies showed maximum seed length (5.74 mm) and width (3.21 mm) exceeding those values given in the available literature. For the first time, we also provide a detailed SEM study of the ultrastructure of the seed coat of I. capensis. There are two types of epidermal cells on the seeds: (a) between the ribs (elongated with straight anticlinal walls, slightly concave outer periclinal walls, and micropapillate secondary sculpture on the edges with anticyclic walls), and (b) on the ribs (isodiametric cells with straight anticlinal walls and concave outer periclinal walls). Unlike the variability of size and weight of seeds, the coat ornamentation has turned out to be a steady feature within the studied secondary range of I. capensis.
Keywords: Balsaminaceae, Environmental factors, Invasive species, Scanning electron microscope (SEM), Seed coat, Variability, 3D ultrastructure
Introduction
The genus Impatiens is the most species-rich within the family Balsaminaceae, with ca. 1,000 species distributed primarily in the Old World tropics and subtropics (Grey-Wilson, 1980; Yu et al., 2015).
Impatiens has been a subject of numerous studies regarding distribution (Zhou et al., 2019), ecology (Abrahamson & Hershey, 1977; Boyer et al., 2016), physiology (Nanda & Kumar, 1983; Tooke et al., 2005), biochemistry (Sreelakshmi et al., 2018), biology (Jacquemart et al., 2015), pollination (Abrahamczyk et al., 2017), morphology (Akiyama & Ohba, 2000; Janssens et al., 2018), systematics (Chen et al., 2007; Chen, Akiyama & Ohba, 2007; Gogoi et al., 2018), phylogeny and evolution (Janssens et al., 2007; Ruchisansakun et al., 2015), and other (see Adamowski, 2016–2020). Despite the plethora of publications on various attributes of Impatiens, this genus requires further attention and research. Impatiens is taxonomically one of the most difficult groups to classify and remains a major challenge due to the enormous species richness and extraordinary morphological variability, with plants ranging from annuals growing only several centimeters high and bearing a single flower to subshrubs four meters high (Hooker, 1904–1906; Grey-Wilson, 1980; Gogoi et al., 2018; Ruchisansakun et al., 2018).
The majority of balsam species grow in hardly accessible mountain ranges and have delicate flowers with complex morphology (Bhaskar, 2012; Yu, 2012; Rahelivololona et al., 2018). Herbarium specimens of balsams are difficult to prepare due to the succulent nature of the stems. Specimens need special preparations such as floral dissections (Shui et al., 2011) and extensive field notes, otherwise they are of limited value. Flower colors fade quickly and the position of the individual flower parts is often impossible to determine from traditionally prepared specimens. One of the taxonomically important features within the genus Impatiens is related to the morphology of seeds. First information on the diversity of the seed coat of Impatiens was reported by Hooker & Thomson (1859) and Warburg & Reiche (1895). Other works were concerned mostly with the shape and size of seeds rather than details of their surface ornamentation (Shimizu, 1977).
The development of new imaging methods enables the observation and study of ultra-small-sized structures. Scanning electron microscopy (SEM) has allowed a detailed analysis of seed coat micromorphology of Impatiens seeds (Song, Yuan & Kupfer, 2005; Chen et al., 2007; Zhang et al., 2016). Earlier works focused on seed dimensions were rarely devoted to the ultrastructure of seeds (Shimizu, 1979; Lu & Chen, 1991). The sculpture on seed coats offers a set of characters which can be used to identify a species, and in combination with other morphological data, can provide crucial evidence towards the taxonomy of a genus (Lu & Chen, 1991; Song, Yuan & Kupfer, 2005; Utami & Shimizu, 2005; Cai et al., 2013; Yu et al., 2015).
Seed morphological features of Impatiens have not only been used for solving various taxonomic problems within the genus but also prove to be useful for determining the impact of various environmental factors on the phenotypic variability of balsam species (Argyres & Schmitt, 1991; Schmitt, 1993; Maciejewska-Rutkowska & Janczak, 2016). The understanding of environmentally induced variation in an individual plant phenotype is crucial for predicting population responses to environmental changes. This issue is particularly important regarding invasive species which occupy a wide range of habitats in the invaded range (Richards et al., 2006).
Despite an increasing number of publications on the surface of Impatiens seeds by SEM (e.g., Shimizu, 1979; Yu, Chen & Qin, 2007; Shui et al., 2011; Xia et al., 2019 a.o.), there is still a lack of information on the seed micromorphology of the majority of species. In fact, a detailed understanding of the seed morphology of the entire genus Impatiens is missing, despite major studies using novel imaging methods (e.g., Yuan et al., 2004; Ruchisansakun et al., 2015; Rahelivololona et al., 2018). As yet, only about 170 species have been investigated, which is about one fifth of all known balsams (Maciejewska-Rutkowska & Janczak, 2016).
One of the species with morphologically undescribed seeds is Impatiens capensis (jewelweed, orange balsam), an annual plant native to eastern North America (Meusel et al., 1978), which is currently spreading across Europe. Today I. capensis is considered as naturalized in several European countries (Matthews et al., 2015), including Poland, where the species is locally established and invasive due to its rapid spread in the secondary range and high competitiveness in relation to native species, even perennials (Tokarska-Guzik et al., 2012). In Poland, it was found for the first time in 1987 (Pawlaczyk & Adamowski, 1991), and it is currently spreading in the Western Pomerania region (Popiela et al., 2015; M Myśliwy, pers. obs., 2017). The species occurs in the area of the Szczecin Lagoon and enters alder forests, willow shrubs, rushes and riparian tall herb fringe communities (Pawlaczyk & Adamowski, 1991; Myśliwy, Ciaciura & Hryniewicz, 2009; M Myśliwy, pers. obs., 2014). It also appears in moist anthropogenic habitats, e.g., along roadside ditches (M Myśliwy, pers. obs., 2017).
Impatiens capensis is an annual plant growing from 0.5–1.5 m or more in height. The flowers are 2.5–3.0 cm long and orange with darker patches in the most common f. capensis. The lower sepal forms a light-orange nectar spur, 5–9 mm long, which is bent at 180° to lie parallel to the sepal-sac (Zika, 2006). Besides color, it differs from the predominantly Eurasiatic I. noli-tangere in that the lower sepal is more rapidly constricted into the spur and the position of the spur (Zika, 2009). The fruit is a five-valved capsule, 2.0–2.5 cm long and 0.3–0.5 cm wide, with explosive dehiscence ejecting the seeds (Moore, 1968; Gleason & Cronquist, 1991; Day, Pellicer & Kynast, 2012). The seeds are laterally compressed, prolate spheroid, with four strong ribs of 5–5.6 × 2.7–3.1 mm (Bojňanský & Fargašová, 2007). The weight ranges from 6.4 to 26.9 mg (Simpson, Leck & Parker, 1985). Schemske (1978) recorded 11.5 mg for cleistogamous seeds and 13.3 mg for chasmogamous ones, and Waller (1982) 10.6 mg. The seed surface is wrinkled or rough, lusterless, dark-brown, with some roundish and paler spots (Bojňanský & Fargašová, 2007).
Numerous studies (several hundred; see Adamowski, 2016–2020 and the literature cited therein) have been devoted to the ecology, biology, and genetics of this species (e.g., Antlfinger, 1989; Schmitt, Ehrhardt & Swartz, 1985; Donohue & Schmitt, 1999; Donohue et al., 2000; Zika, 2009; Tabak & Von Wettberg, 2008; Day, Pellicer & Kynast, 2012). However, a review of the available literature showed a scarcity of data on seed size and a complete lack of information describing the morphological variation of the seed coat of I. capensis (Schemske, 1978; Waller, 1982; Simpson, Leck & Parker, 1985; Bojňanský & Fargašová, 2007).
The aim of our work has been to characterize the micromorphological traits and ultrastructure of I. capensis seeds from various habitats and growing conditions and their morphological variability. Anthropogenic changes in habitats were expected as important factors affecting seed micromorphology and ultrastructure.
Materials and Methods
Study sites
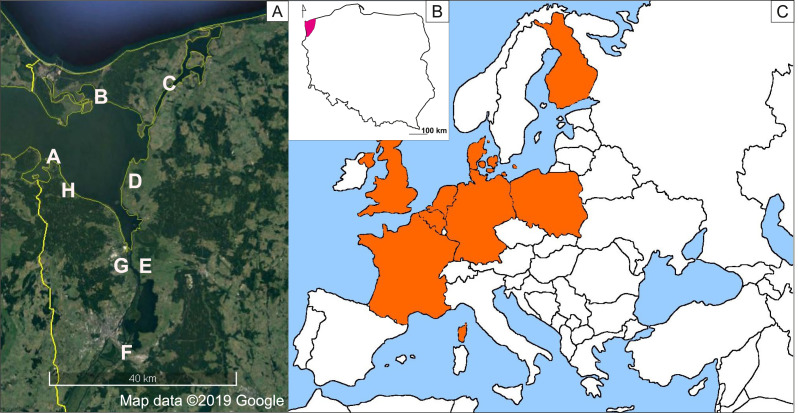
Seeds were collected from August to September 2018 (to avoid seasonal variability) from eight populations of I. capensis in Poland. We sampled the entire Polish range of this species from all types of habitats, from natural (alder carrs, hydrophilous tall herb communities along rivers, near water seepages, and along the banks of the Szczecin Lagoon) to anthropogenic (tall herb communities along roadside ditches, transformed forests along artificial canals) (Table 1, Fig. 1). The studied populations were also subject to different lighting conditions, which were scored using a 3-point scale: plants which grew in willow forests and the understory of alder carrs were strongly shaded (3), while those from tall herb communities were partly shaded by solitary trees (2) or exposed to full sun (1). As the height of I. capensis specimens, the location of capsules within the plant (main stem vs. branches), and their derivation from flowers of various types (cleistogamous vs. chasmogamous) may affect seeds weight (Waller, 1982), the seeds for our study were collected always from the main stems of 8–10 plants of similar (average for the population) height and from capsules derived from chasmogamous flowers, to minimize the bias. Species nomenclature was adopted from Euro+Med PlantBase (Euro+Med PlantBase, 2019).
Table 1. List of the studied populations of Impatiens capensis Meerb. in Poland.
| Code | Locality | Latitude | Longitude | Habitat | Average plant height [cm] | Number of analyzed seeds | Population size (mature individuals) |
|---|---|---|---|---|---|---|---|
| A | Podgrodzie | 53.740222° | 14.306667° | tall herbs on the bank of Szczecin Lagoon | 130 | 29 | 20–30 |
| B | Lubin | 53.865056° | 14.426778° | tall herbs and grasses near water seeps | 50 | 24 | >20 |
| C | Unin | 53.894806° | 14.634444° | tall herbs along the river | 120 | 27 | 20–30 |
| D | Czarnocin | 53.722306° | 14.549167° | tall herbs on the bank of Szczecin Lagoon | 130 | 30 | >50 |
| E | Święta | 53.559861° | 14.659083° | tall herbs along roadside ditch | 165 | 27 | >100 |
| F | Szczecin-Zdroje | 53.382861° | 14.614944° | tall herbs along the river | 120 | 29 | >50 |
| G | Police | 53.573194° | 14.581472° | willow forest along artificial canal | 145 | 28 | >100+ |
| H | Trzebieradz | 53.675417° | 14.441444° | alder carr | 70 | 30 | >100+ |
Figure 1. Distribution map of Impatiens capensis Meerb. in Europe (C), range in Poland (B), sites of the studied populations (A) (prepared by Adamowski & Myśliwy).
Satellite map data ©2019 Google, Modifed using CorelDRAW 18. For explanation of symbols, see Table 1.
Biometric and SEM analysis
From 24 to 30 mature seeds were used from each population for biometric analysis. We measured four quantified seed traits: seed length (SL), seed width (SW), seed circumference (SC), and seed area (SA). The seeds were measured as previously described in Rewicz et al. (2017). In order to describe the seed mass, we used 15 seeds from each population. The seeds were weighed with an Ohaus PA 21. We determined roundness by the formula: R = 4 × area/π [Major axis]2 as defined by Ferreira & Wayne (2010).
We used eight seeds from each population for SEM. The seeds were air-dried and sputter-coated with a 4-nm-thick layer of gold (Leica EM ACE200). The SEM work was performed on a Phenom Pro X Scanning Electron Microscope at the Department of Invertebrate Zoology and Hydrobiology, University of Lodz, Poland. The 3D models of the seed surface were generated using the dedicated software 3D Roughness Reconstruction for Phenom. SEM micrographs were analyzed as previously described in Rewicz et al. (2017). Seed shape terminology and types of seed surfaces were adopted from Barthlott (1981).
Soil properties
In order to characterize habitat conditions at each locality, five soil core samples (0–20 cm depth) were collected and then mixed together into one composite sample. Soil samples were dried at room temperature, and passed through a sieve to remove fractions larger than one mm. The following physicochemical soil parameters were determined (Bednarek et al., 2011), as first described by Myśliwy (2019): organic matter content defined as the loss on ignition (LOI)–soil samples annealed at 550 °C (%); grain composition (the content of sand, silt, clay)–Bouyoucos’s sedimentation method, modified by Casagrande and Prószyński; granulometric categories according to the Polish Society of Soil Science (2009) classification; soil reaction (pH)–the potentiometric method, in 1-M solution of KCl; soil calcium carbonate (CaCO3) content (%)–the Scheibler’s method; organic carbon (Corg) content (%), and total nitrogen (Ntot) content (%) were determined by an elemental analyzer CHNS/O FlashSmart (Thermo Scientific), and the C/N ratio; the content of available forms of soil nutrients (mg/100 g soil): calcium (Ca) and sodium (Na) were determined spectrophotometrically (Ca–AAS and Na–EAS) on ICE3000; potassium (K) and phosphorus (P)–measured according to the Egner-Riehm method; magnesium (Mg)–measured by Schachtschabel’s method; soil moisture content, hand-felt assessed directly in the field using a 4-point scale recommended by the Soil Science Society of Poland (2017): (1) dry (the soil crumbles and turns to dust, it is neither cool nor moist to touch; it darkens visibly after wetting), (2) fresh (the soil feels cool, but no moisture is felt; darkens after wetting), (3) moist (the soil moistens fingers and tissue paper, but water does not leak when squeezed; clayey, loamy, and some dusty soils are plastic; does not darken after wetting), (4) wet (water leaks from the soil when squeezed, aggregates, soil smears).
Data analysis
The five following basic characteristic traits were calculated: arithmetic average (x), minimum and maximum values (min/max), coefficient of variation (CV), and standard deviation (SD). The distribution of the data was not normal; statistical analysis was based on the Kruskal-Wallis test (for p ≤ 0.05), which is a nonparametric alternative to ANOVA (Zar, 1984). Correlation between pairs of morphological characters was evaluated using Spearman’s correlation coefficient and the values were adopted after Meissner (2010), (correlation: less than 0.20–very poor; 0.21–0.39–weak; 0.40–0.69–moderate; 0.70–0.89–strong; and above 0.89–very strong).
The cluster analysis based on the nearest neighbor method was performed using the matrix on the population’s mean values. As the dataset required a linear response model (Jongman et al., 1995), the Redundancy Analysis (RDA) was used to relate the variability of morphological traits of seeds to environmental variables. The variables Corg and Ntot were excluded from the RDA as they were strongly correlated with organic matter content (LOI). The Monte Carlo permutation test with the forward selection of environmental variables was applied to determine the importance and statistical significance of variables in explaining the variability in seeds. The software packages Canoco v.4.5 (Ter Braak & Šmilauer, 2002), MVSP 3.2 (Kovach, 2010), and STATISTICA PL. ver. 13.1 (StatSoft Inc, 2011) were used for all analyses (Van Emden, 2008; Lepš & Šmilauer, 2003).
Results
Biometric analysis
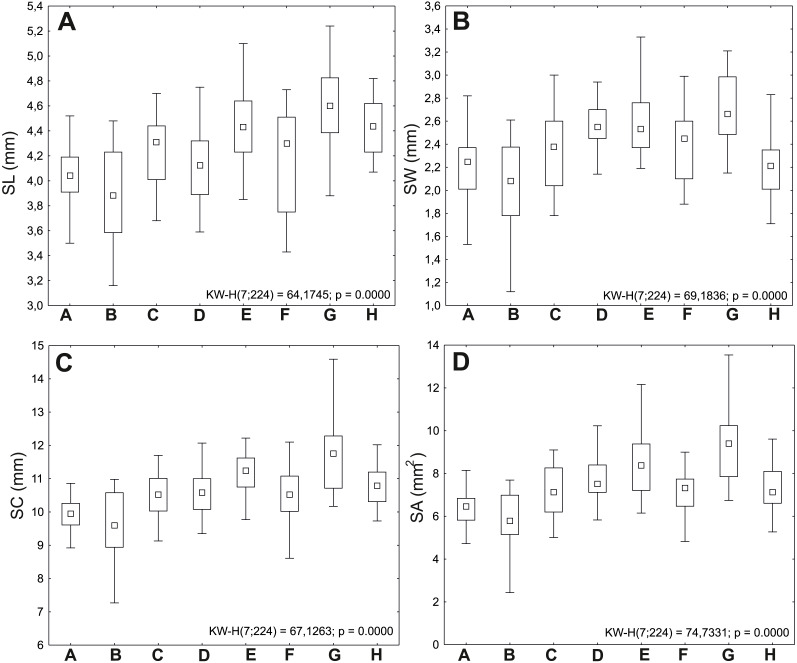
Seeds from the G (Police) population were the largest, with average values of length (SL) 4.60 mm, width (SW) 2.71 mm, circumference (SC) 11.65 mm, and area (SA) 9.26 mm2; comparatively large seeds were also obtained from the E population (Święta); the B (Lubin) population had the shortest (mean SL 3.88 mm) and narrowest seeds (mean SW 2.03 mm) (Table 2).
Table 2. Biometric comparison of seed traits of Impatiens capensis Meerb.
Seed length (SL), seed width (SW), seed circumference (SC), seed area (SA), variation coefficient (CV), standard deviation (SD), minimum/maximum (Min/Max), arithmetic average (X), A–H as in Table 1.
| A | B | C | D | E | F | G | H | x | |
|---|---|---|---|---|---|---|---|---|---|
| Weight (mg) | 7.66 | 6.52 | 8.16 | 7.82 | 9.82 | 8.62 | 11.42 | 6.92 | 8.37 |
| SL (mm) | 4.05 | 3.88 | 4.23 | 4.11 | 4.46 | 4.17 | 4.60 | 4.41 | 4.24 |
| Min-max | 3.50-4.64 | 3.16-4.48 | 3.68-4.70 | 3.59-4.75 | 3.85-5.26 | 3.43-4.73 | 3.88-5.74 | 3.59-4.82 | 3.16-5.74 |
| SD | 0.26 | 0.40 | 0.29 | 0.30 | 0.32 | 0.40 | 0.40 | 0.27 | 0.40 |
| CV | 6.50 | 10.28 | 6.95 | 7.35 | 7.21 | 9.71 | 8.75 | 6.19 | 9.34 |
| SW (mm) | 2.23 | 2.03 | 2.36 | 2.56 | 2.60 | 2.40 | 2.71 | 2.23 | 2.39 |
| Min-max | 1.53-2.82 | 1.12-2.61 | 1.78-3.00 | 2.14-2.94 | 2.19-3.33 | 1.88-2.99 | 2.15-3.21 | 1.71-2.93 | 1.12-3.33 |
| SD | 0.28 | 0.42 | 0.33 | 0.19 | 0.31 | 0.32 | 0.30 | 0.30 | 0.37 |
| CV | 12.50 | 20.57 | 13.98 | 7.30 | 12.02 | 13.19 | 11.04 | 13.51 | 15.35 |
| SC (mm) | 10.00 | 9.55 | 10.51 | 10.61 | 11.21 | 10.49 | 11.65 | 10.75 | 10.61 |
| Min-max | 8.63-11.94 | 7.27-10.98 | 9.13-11.70 | 9.35-12.70 | 9.77-13.27 | 8.61-12.10 | 10.17-14.59 | 8.9-12.20 | 7.27-14.59 |
| SD | 0.77 | 1.09 | 0.67 | 0.69 | 0.89 | 0.89 | 1.04 | 0.67 | 1.02 |
| CV | 7.65 | 11.42 | 6.39 | 6.50 | 7.90 | 8.53 | 8.97 | 6.22 | 9.64 |
| SA (mm2) | 6.52 | 5.79 | 7.15 | 7.71 | 8.42 | 6.74 | 9.26 | 7.25 | 7.44 |
| Min-max | 4.72-9.53 | 2.43-7.69 | 5.10-9.10 | 5.83-1.23 | 6.15-12.16 | 4.82-1.21 | 6.74-13.54 | 5.27-9.61 | 2.43-13.54 |
| SD | 1.14 | 1.51 | 1.21 | 1.07 | 1.48 | 1.27 | 1.63 | 1.08 | 1.62 |
| CV | 17.54 | 26.06 | 16.92 | 13.88 | 17.63 | 17.58 | 17.65 | 14.96 | 21.76 |
The minimum values of analyzed traits were also recorded in the B (Lubin) population (SL 3.16 mm, SW 1.12 mm, SC 7.27 mm, SA 2.43 mm2). The maximum values of length (5.74 mm), circumference (14.59 mm), and area (13.54 mm2) were recorded in the G population (Police).
A very strong Spearman correlation (r = 0.94) was observed between the seed area and circumference (Table 3). The most variable features were the seed area (CV = 21.76%) and width (CV = 15.35%). The variation of seed traits ranged insignificantly from 6.19% (H population) to 10.28% (B) for SL; from 7.30% (D) to 20.57% (B) for SW; from 6.22% (H) to 11.42% (B) for SC; and from 13.88% (D) to 26.06% (B) for SA, respectively.
Table 3. Spearman correlation values for seed traits of Impatiens capensis Meerb.
All values with significance of p < 0.05.
| Length | Width | Circumference | Area | |
|---|---|---|---|---|
| Length | 1.00 | 0.47 | 0.85 | 0.72 |
| Width | 1.00 | 0.73 | 0.84 | |
| Circumference | 1.00 | 0.94 | ||
| Area | 1.00 |
The G (Police: 11.42 mg) and E (Święta: 9.82 mg) populations are characterized by the heaviest seeds. The lightest seeds were observed in the following populations: B (Lubin: 6.52 mg) and H (Trzebieradz: 6.92 mg) (Fig. 2, Table 2).
Figure 2. Ranges of variation of seed traits of Impatiens capensis Meerb.
The boxes represent the 25th–75th percentiles; the upper and lower whiskers extend the minimum and maximum data point; the square inside the box indicates median. (A) Seed length (SL); (B) seed width (SW); (C) seed circumference (SC); (D) seed area (SA).
The Kruskal-Wallis test showed that the I. capensis populations differed significantly in each of the analyzed traits. The conducted post hoc test (DunnTest) showed that the populations from: Police (G), followed by Czarnocin (D), Święta (E), and Trzebieradz (H) showed the greatest variation in terms of studied traits among all the populations (Table 4).
Table 4. Interpopulation variability for length (A), width (B), circumference (C), and area (D) of seeds of Impatiens capensis.
Asterisk next to letter indicates significance at p < 0.05.
| Podgrodzie | Lubin | Unin | Czarnocin | Święta | Szczecin-Zdroje | Police | Trzebieradz | |
|---|---|---|---|---|---|---|---|---|
| Podgrodzie | ABCD | ABCD | AB*CD* | A*B*C*D* | ABCD | A*B*C*D* | A*BC*D | |
| Lubin | ABCD | AB*C*D* | A*B*C*D* | AB*CD | A*B*C*D* | A*BC*D | ||
| Unin | ABCD | ABCD | ABCD | A*B*C*D* | ABCD | |||
| Czarnocin | A*BCD | ABCD | A*BC*D | A*B*CD | ||||
| Święta | ABCD | ABCD | AB*CD | |||||
| Szczecin-Zdroje | A*BC*D* | ABCD | ||||||
| Police | AB*CD* | |||||||
| Trzebieradz |
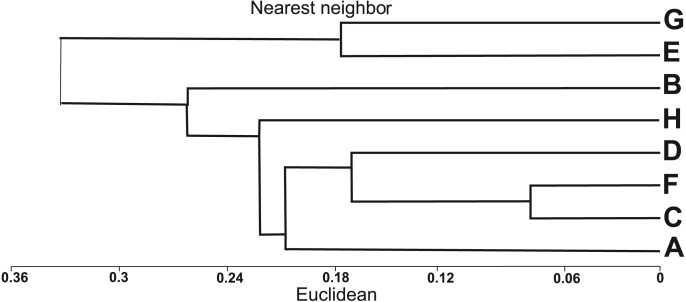
The similarity analysis using Euclidean’s distances showed two main clusters (Fig. 3). The first cluster included six populations of I. capensis (A–D, F, H), all derived from natural habitats, while the other cluster groups two populations (E, G) from anthropogenic habitats, where the examined plants were the highest (Table 1). According to the dendrogram (Fig. 3), populations C and F are the closest to each other; both were associated with river valleys (Dziwna and Oder rivers, respectively) and close to the river bed, hence under the influence of flooding. The D and A populations were growing in tall herb communities on the banks of the Szczecin Lagoon. The most distinct populations in the first cluster (H and B) were also found on the banks of the Szczecin Lagoon, but they had the lowest average height and differed in habitat conditions from the other populations of this cluster (Table 1).
Figure 3. Dendrogram of similarities of populations of Impatiens capensis Meerb. in Poland, obtained by the nearest neighbor method.
The investigated morphological parameter of seed shape, roundness, showed statistically significant differences between the populations (p < 0.05). For roundness, the highest value was recorded at D: Czarnocin (0.58) (tall herbs on the bank of the Szczecin Lagoon) and the lowest at H: Trzebieradz (0.47) (alder carr) (Table 5).
Table 5. Seed roundness (R) comparison between populations of Impatiens capensis Meerb. in Poland.
| R | SD | A | B | C | D | E | F | G | H | |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.50 | 0.06 | a | a | a | p= 0.0023 | a | a | a | a |
| B | 0.48 | 0.08 | a | a | p= 0.0000 | a | p= 0.0087 | a | ||
| C | 0.51 | 0.09 | p= 0.0033 | a | a | a | a | |||
| D | 0.58 | 0.06 | a | a | a | p= 0.0000 | ||||
| E | 0.54 | 0.07 | a | a | p= 0.0197 | |||||
| F | 0.53 | 0.07 | a | a | ||||||
| G | 0.56 | 0.07 | p= 0.0002 | |||||||
| H | 0.47 | 0.05 |
Notes.
SD, standard deviation, A–H, in Table 1; the same capital letters mean the values do not differ significantly.
Biometric variability of seeds and its relationship with environmental conditions
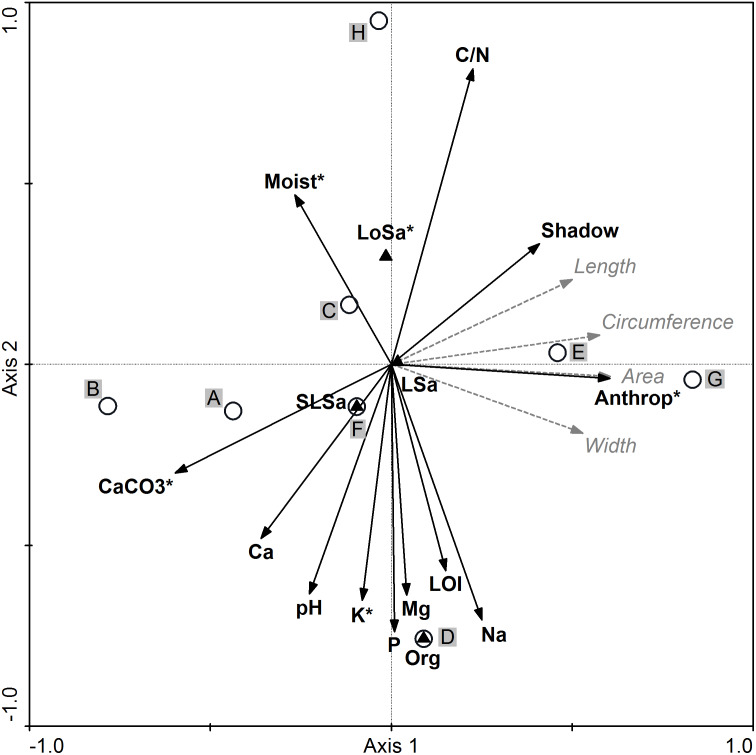
All environmental variables included in the RDA accounted for 35.6% of the total variation. The results of stepwise forward selection of variables indicated that five variables: anthropogenic disturbances (Anthrop), carbonates (CaCO3), loose sand presence (LoSa), potassium (K), and soil moisture content (Moist) were statistically significant and varied between the studied populations of I. capensis (Fig. 4). Along the gradient represented by Axis I, the highest correlation between the sample position and environmental variables (the so-called interset correlation) was typical of anthropogenic disturbances and CaCO3, followed by the degree of shading and soil Ca, while the C/N ratio was most closely correlated with Axis II, followed by soil content of P, Na, K, and organic soil.
Figure 4. Ordination diagram of populations of Impatiens capensis Meerb.
(A–H) Seed biometric traits (dotted gray arrows) and environmental variables (solid black arrows) along the first two RDA axes. * Statistically significant variables; Anthrop, anthropogenic disturbances; shadow, degree of shading. For codes of populations and soil properties see Tables 1 and 6, respectively.
The location of population H (Trzebieradz) in the ordination space (the upper part of the RDA diagram) was associated with the highest C/N ratio, the highest soil moisture and shading, as well as with the lowest soil pH and the lowest soil content of CaCO3, Ca, P, and K (Fig. 4, Table 6). At the same time, the H population was dominated by short specimens (Table 1), with one of the lightest seeds and average values of biometric traits (Table 2). In contrast, populations characterized by the longest, widest, and heaviest seeds, from E (Święta) and G (Police), located in the right-hand side of the RDA diagram, were also related to a relatively high C/N ratio, but unlike the previous population, they were associated with a low level of soil moisture as well as the highest anthropogenic disturbances (Fig. 4), and consisted of the tallest specimens (Table 1). Population D (Czarnocin) occupied the bottom part of the diagram and was distinct in its organic soil, with the highest content of organic matter (LOI), as well as P, K, Mg, and Na content in the soil, while having the lowest C/N ratio (Table 6). The lowest values of the seed biometric traits were found for population B (Lubin), located in the left-hand part of the RDA diagram (Fig. 4, Table 2), and associated with high soil pH and the highest content of soil carbonates and calcium, as well as a low level of soil moisture (Table 6). The other populations (A: Podgrodzie; C: Unin; F: Szczecin-Zdroje) were also on the left side of the diagram, but closer to the center (Fig. 4). Neither their seed biometric traits nor habitat conditions were distinct (Tables 2, 6).
Table 6. Soil conditions at the investigated sites of occurrence of Impatiens capensis Meerb. in Poland.
| Code/ Locality | Soil group | LOI | Corg | Ntot | C/N | pH | CaCO3 | Ca | P | K | Mg | Na | Moist |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | LoSa | 11.6 | 7.11 | 0.52 | 13.61 | 6.6 | 0.00 | 3007 | 60.2 | 68.3 | 378.0 | 405.4 | 3 |
| B | LSa | 15.8 | 9.98 | 0.65 | 15.37 | 7.4 | 3.54 | 30769 | 194.5 | 171.8 | 258.0 | 181.6 | 2 |
| C | LSa | 23.4 | 12.63 | 0.95 | 13.25 | 7.3 | 2.31 | 18476 | 130.4 | 206.4 | 325.0 | 177.9 | 3 |
| D | Org | 65.3 | 31.90 | 2.54 | 12.54 | 6.7 | 1.47 | 21816 | 653.6 | 310.0 | 1796.0 | 1152.8 | 3 |
| E | LoSa | 19.5 | 13.18 | 0.81 | 16.25 | 6.3 | 0.00 | 9142 | 67.6 | 187.9 | 204.0 | 62.2 | 2 |
| F | SLSa | 6.0 | 3.70 | 0.27 | 13.49 | 7.5 | 1.09 | 5792 | 111.6 | 85.9 | 89.0 | 44.0 | 3 |
| G | LSa | 17.3 | 8.77 | 0.55 | 15.84 | 6.8 | 0.00 | 7801 | 117.7 | 47.4 | 264.0 | 638.5 | 2 |
| H | LoSa | 16.5 | 7.95 | 0.43 | 18.69 | 5.1 | 0.00 | 2705 | 31.4 | 33.7 | 262.0 | 57.6 | 4 |
Notes.
- LoSa
- loose sand
- LSa
- loamy sand
- SLSa
- slightly loamy sand
- Org
- organic soil
- LOI
- organic matter content
- Corg
- organic carbon
- Ntot
- total nitrogen
- C/N
- C/N ratio
- pH
- soil pH
- CaCO3
- carbonates
- Ca
- calcium
- P
- phosphorus
- K
- potassium
- Mg
- magnesium
- Na
- sodium
- Moist
- soil moisture content
Seed surface ultrastructure
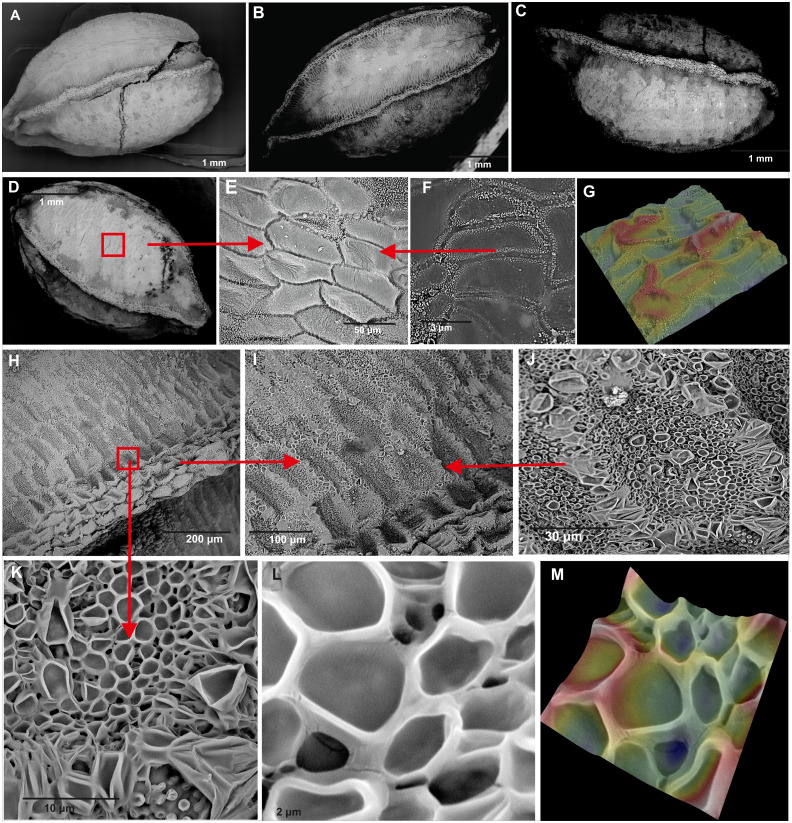
The studied seeds of I. capensis were round in shape, with a lusterless, rough, and dark-brown surface, without roundish and paler spots (Fig. 5). The seeds had four strong, clear ribs, the apex and bottom narrowed. Each rib was built of rows of 4–5 cells and had a darker color than the surface between them (Fig. 5H). The seed coat is composed of two types of epidermal cells (Figs. 5E, 5H) creating a net-like pattern. The cells of the seed surface between the ribs were: elongated with straight anticlinal walls (Fig. 5E), raised cell boundaries between the cells (Fig. 5G), slightly concave outer periclinal walls (Figs. 5F, 5G) and a micropapillate secondary sculpture on the edges of anticyclic walls (Fig. 5F). Near the ribs, there were rows of 4–7 isodiametric cells (Figs. 5I, 5K) with straight anticlinal walls (Figs. 5L), with raised cell boundaries (Fig. 5M) and concave outer periclinal walls. Seeds from all studied populations did not differ in their ultrastructure (Figs. 5A–5D).
Figure 5. Comparison of seeds of Impatiens capensis Meerb.
(A–D) General view of seeds from (A) Czarnocin, (B) Police, (C) Lublin, (D) Podgrodzie; (E–F) seed sculpture; (G) seed surface 3D ultrastructure between the ribs; (H) rib; (I–L) cells near rib; (M) 3D ultrastructure of cells near rib.
Discussion
SEM gives us the means for studying the morphological characters of seeds and their ultrastructural characteristics which helps or identifying and determining the taxonomic delimitation of various angiosperm groups, as demonstrated for Brasssicaceae (Tantaway et al., 2004), Caryophyllaceae (Ullah et al., 2019a; Ullah et al., 2019b), Poaceae (Martín-Gómez et al., 2019), Cyperaceae (Więcław et al., 2017), Ranunculaceae (Constantinidis, Psaras & Kamari, 2001; Rewicz et al., 2017; Martín-Gómez, Rewicz & CerVantes, 2019; Hadidchi, Attar & Ullah, 2020), Rosaceae (Ballian & Mujagić-Pašić, 2013), Cervantes (Akbari & Azizian, 2006), and Orchidaceae (Gamarra et al. 2007; Gamarra et al., 2010; Rewicz, Kołodziejek & Jakubska-Busse, 2016). Although seed morphology alone does not provide universally applicable key characters for species identification, it can be as helpful as many other characters used in taxonomy.
Members of Balsaminaceae have a diverse and elaborately sculptured seed coat. Unfortunately, till now seed morphology has been observed only for a small number of Impatiens species, which has limited the use of the morphological traits of seeds in taxonomy and classification (e.g., Song, Yuan & Kupfer, 2005; Utami & Shimizu, 2005; Chen et al., 2007; Yu, Chen & Qin, 2007; Jin et al., 2008; Shui et al., 2011).
We here provide new data about the seed morphology and seed coat sculpture of I. capensis, as well as new information about the area of its distribution in Poland. Our studies have shown maximum seed length (5.74 mm) and width (3.21 mm) beyond the values reported elsewhere. Bojňanský & Fargašová (2007) found seeds of I. capensis to be 5-5.6 mm long and 2.7–3.1 mm wide. Our ultrastructural studies have shown two types of cells on between the ribs and on the ribs, that have previously not been described (Fig. 5). The occurrence of several types of epidermal cells on the seeds of members of Impatiens was previously noted, for instance, three types of epidermal cells have been reported in Impatiens aconitoides by Shui et al. (2011). We were not able to confirm in any of our studied populations the presence of roundish spots on the surface of seeds (Fig. 6) as reported by Bojňanský & Fargašová (2007), which may be due to the different geographical origin of the examined seeds: our seeds of I. capensis are from wild-growing populations from various habitats, while those studied by Bojňanský & Fargašová (2007) were obtained from cultivation and of unknown origin.
Figure 6. (A) Drawing of seed of Impatiens capensis. (based on Bojňanský & Fargašová, 2007), seed of Impatiens capensis under the light microscope; (B) general view; (C) rib.
The analysis of SEM micrographs of I. noli-tangere seeds closely related to I. capensis (Yu et al., 2015) has shown that seed coats of this species vary significantly depending on the geographical origin of the seeds (Utami & Shimizu, 2005; Chen et al., 2007a; Jin et al., 2008). On the other hand, the comparison of the seed micromorphology of I. capensis has not shown similarity to seed coat ornamentation of the aforementioned I. noli-tangere (with narrow and ellipsoid seeds, fine reticulate subtype, testa cells with reticulate thickened outer walls; Song, Yuan & Kupfer, 2005; Utami & Shimizu, 2005; Chen et al., 2007; Jin et al., 2008). Despite the fact that both species are closely related and may be confused (Zika, 2009; Yu et al., 2015), their seeds clearly differ morphologically. The new data presented here may be useful in the identification of these species. In turn, there is no information about the seed morphology of I. pallida, which is sympatric and synchronic species to I. capensis (Rust, 1977), which makes this subject even more difficult. Elucidating the overall variation in seed coat micromorphology and to implement this feature to taxonomy of I. capensis will require more samplings, also within the native range of orange jewelweed as well as other closely related species and this eventually should become the basis for further comparisons and studies. Seed ultrastructure appears to be a constant feature within a taxonomic unit (Stace, 1992) and, as morphological studies show, seed shape and size are highly diverse at the genus and species levels (Yu, Chen & Qin, 2007; Jin et al., 2008; Shui et al., 2011; Ullah et al., 2019a; Ullah et al., 2019b; Hadidchi, Attar & Ullah, 2020). Both statements have been proven for I. capensis in Poland.
Data concerning the size, shape and structure of seeds not only have been used as an important tool for solving various taxonomic problems within the genus Impatiens but also provide results useful for determining the impact of various environmental factors on the phenotypic variability of these species (Bell, Lechowicz & Schoen, 1991; Argyres & Schmitt, 1991; Schmitt, 1993; Chmura, Csontos & Sendek, 2013; Maciejewska-Rutkowska & Janczak, 2016).
Environmental heterogeneity is indicated as a major factor driving morphological changes (Nakazato, Bogonovich & Moyle, 2008). Seeds are sensitive to changes in biotic and abiotic conditions (Moles et al., 2005). According to Silvertown (1989), the correlation between seed size and the place where plant is growing is an adaptative feature. Bigger seeds occur in habitats with stable environmental conditions, where seedlings may grow slowly. Small seeds are generally produced by plants with a short life cycle, growing mainly in disturbed habitats.
Orange balsam is known for colonizing a wide range of habitats (Schemske, 1978; Waller, 1980). Moreover, Simpson, Leck & Parker (1985) have shown that I. capensis vegetative and reproductive growth parameters reflect habitat differences. Light availability (Simpson, Leck & Parker, 1985) as well as soil moisture and pH (Waller, 1980) have been reported to affect its growth patterns. Our studies indicate that five environmental variables were statistically significant and were able to serve to discern the studied populations in terms of seed size and weight: anthropogenic disturbances (which may serve as a proxy for habitat fertility), carbonates (CaCO3), loose sand presence, potassium (K), and soil moisture (Fig. 4). Populations G (Police) and E (Święta), occurring in the most disturbed anthropogenic habitats (artificial canal and roadside), have the heaviest seeds as a result of growth under favorable environmental conditions (neutral or slightly acidic soil with a relatively high C/N ratio). In turn, population B (Lubin) with the smallest and lightest seeds was associated with high soil pH, and the highest content of soil carbonates and calcium. Interestingly, Waller (1982) reported that the higher nodes of I. capensis individuals tended to produce heavier seeds. In Waller’s (1982) opinion, the position effect probably leads to a greater mean seed size for higher plants. Werner & Platt (1976) stated that populations growing at higher plant densities often produce larger seeds. Our results are consistent with both studies, as the largest and heaviest seeds were obtained from populations G (Police) and E (Święta), formed by the highest plants, growing in large numbers and densities.
Another important factor shaping a diverse array of plant traits, including morphological features, is climate (Nakazato, Bogonovich & Moyle, 2008; Colautti & Barrett, 2013; Van Boheemen, Atwater & Hodgins, 2019). Temperature and precipitation gradients are the main climatic factors driving the adaptive diversification of species (Nakazato, Bogonovich & Moyle, 2008). As it seems, climatic conditions have had a limited effect on the investigated seed parameters till now, due to a small area of secondary distribution of I. capensis in Poland (Adamowski, Myśliwy & Dajdok, 2018; Fig. 1) and short time of residence of little over 30 years. Although this investigated plant has only a few localities inhabiting only a relatively small area in Poland, rapid expansion across environmental gradients has been reported for several plants introduced to a new area and species can evolve quite quickly driven by environmental factors (Dlugosh & Parker, 2008; Colautti & Barrett, 2013; Molina-Montenegro et al., 2018; Van Boheemen, Atwater & Hodgins, 2019).
Phenotypic plasticity has been considered to be the primary feature enabling aliens to colonize new, environmentally diverse areas (Richards et al., 2006; Molina-Montenegro, Atala & Gianoli, 2010). However, recent research has indicated that alien plants can evolve quickly in newly occupied areas, so both phenotypic plasticity and evolution of reproductive features could be relevant factors for successful invasions (Geng et al., 2007; Molina-Montenegro et al., 2018).
An evolutionary explanation for plant invasiveness implies that seed and fruit traits are crucial for invasive plants since they are related to dispersal strategies and mechanisms to cope with environmental stress. Some research reports have indicated that native and invasive populations employ different strategies for growth and reproduction (Chun et al., 2007; Molina-Montenegro, Atala & Gianoli, 2010; Molina-Montenegro et al., 2018). Results by Molina-Montenegro et al. (2018) suggest that some seed traits of invasive plant species with rapid adaptive capacity can evolve leading to maximizing their establishment in new environments and such features can be heritable.
Due to the scarcity of data we could not point out the presence of morphological differentiation between native and invasive populations of I. capensis, and we have not been able to determine whether the seed traits are evolving. However, I. capensis, classified as an invasive species in Poland, can be suspected, while adapting and occupying new territories and competing with native species, to develop specific adaptations, contributing to its success and spread in the new environments.
Conclusions
New data on seed morphology and seed coat sculpture of I. capensis is provided. The presented results are useful for the identification of this species when occurring together with other closely related species. These details on seed coat ornamentation are here described for the first time.
Further studies on the developmental variation of seed coat sculpture, especially of species closely related to I. capensis, may provide a better understanding of the evolutionary relationships of the different types of sculpture.
We provide new information on the plasticity of seeds of I. capensis. There are only few papers on the phenotypic variability of species of Impatiens. Data on the morphology of seeds can prove useful for determining the impact of various environmental factors on morphological traits and show whether a given feature is stable or susceptible to environmental change.
Our results suggest that certain habitat variables, especially anthropogenic disturbances and individual soil properties, contribute in shaping the morphological variation of seeds of I. capensis. In turn, the seed coat sculpture has turned out to be a stable feature within the secondary range of this species in Poland.
Supplemental Information
Acknowledgments
The authors wish to express their gratitude to Theodor C.H. Cole, Dipl. rer. nat. (FU Berlin) for English language editing and valuable comments.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Agnieszka Rewicz and Monika Myśliwy conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Wojciech Adamowski conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Marek Podlasiński analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Anna Bomanowska analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are provided in the Supplementary File.
References
- Abrahamczyk et al. (2017).Abrahamczyk S, Lozada-Gobilard S, Ackermann M, Fischer E, Krieger V, Redling A, Weigend M. A question of data quality—testing pollination syndromes in Balsaminaceae. PLOS ONE. 2017;12(10):e0186125. doi: 10.1371/journal.pone.0186125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson & Hershey (1977).Abrahamson WG, Hershey BJ. Resource allocation and growth of Impatiens capensis (Balsaminaceae) in two habitats. Bulletin of the Torrey Botanical Club. 1977;104:160–164. doi: 10.2307/2484362. [DOI] [Google Scholar]
- Adamowski (2016–2020).Adamowski W. Balsaminaceae information center. 2016–2020. https://www.researchgate.net/project/Balsaminaceae-Information-Center. [12 January 2020]. https://www.researchgate.net/project/Balsaminaceae-Information-Center
- Adamowski, Myśliwy & Dajdok (2018).Adamowski W, Myśliwy M, Dajdok Z. Ankieta oceny stopnia inwazyjności Impatiens capensis Meerb. w Polsce, na podstawie protokołu Harmonia +PL –procedura oceny ryzyka negatywnego oddziaływania inwazyjnych i potencjalnie inwazyjnych gatunków obcych w Polsce. [Questionnaire for assessing the degree of invasiveness of Impatiens capensis Meerb. in Poland, based on the protocol Harmonia +PL –procedure for negative impact risk assessment for invasive alien species and potentially invasive alien species in Poland]. Generalna Dyrekcja Ochrony Środowiska. 2018. http://projekty.gdos.gov.pl/igo. [20 January 2020]. http://projekty.gdos.gov.pl/igo
- Akbari & Azizian (2006).Akbari RS, Azizian D. Seed morphology and seed coat sculpturing of Epilobium L. species (Onagraceae Juss.) from Iran. Turkish Journal of Botany. 2006;30:435–440. [Google Scholar]
- Akiyama & Ohba (2000).Akiyama S, Ohba H. Inflorescences of the Himalayan species of Impatiens (Balsaminaceae) Journal of Japanese Botany. 2000;75(4):226–240. [Google Scholar]
- Antlfinger (1989).Antlfinger AE. Seed bank, survivorship and size distribution of a Nebraska population of Impatiens capensis (Balsaminaceae) American Journal of Botany. 1989;76(2):222–230. doi: 10.1002/j.1537-2197.1989.tb11305.x. [DOI] [Google Scholar]
- Argyres & Schmitt (1991).Argyres A, Schmitt J. Microgeographic genetic structure of morphological and life history traits in a natural population of Impatiens capensis. Evolution. 1991;45:178–189. doi: 10.1111/j.1558-5646.1991.tb05276.x. [DOI] [PubMed] [Google Scholar]
- Ballian & Mujagić-Pašić (2013).Ballian D, Mujagić-Pašić A. Morphological variability of the fruit and seed of wild cherry (Prunus avium L.) in a part of its natural distribution in Bosnia and Herzegovina. Biologica Nyssana. 2013;4(1–2):15–17. [Google Scholar]
- Barthlott (1981).Barthlott W. Epidermal and seed surface characters of plants: systematic applicability and some evolutionary aspects. Nordic Journal of Botany. 1981;1(3):345–355. doi: 10.1111/j.1756-1051.1981.tb00704.x. [DOI] [Google Scholar]
- Bednarek et al. (2011).Bednarek R, Dziadowiec H, Pokojska U, Prusinkiewicz Z. Badania ekologiczno-gleboznawcze. WN PWN; Warszawa: 2011. [Google Scholar]
- Bell, Lechowicz & Schoen (1991).Bell G, Lechowicz MJ, Schoen DJ. The ecology and genetics of fitness in forest plants. III. Environmental variance in natural populations of Impatiens pallida. Journal of Ecology. 1991;79:697–714. doi: 10.2307/2260662. [DOI] [Google Scholar]
- Bhaskar (2012).Bhaskar V. Taxonomic monograph on Impatiens L. (Balsaminaceae) of Western Ghats –the key genus for endemism. Centre for Plant Taxonomic Studies; Bangalore: 2012. [Google Scholar]
- Bojňanský & Fargašová (2007).Bojňanský V, Fargašová A. Atlas of seeds and fruits of Central and East-European flora. The Carpathian Mountains Region. Springer; Dordrecht: 2007. [Google Scholar]
- Boyer et al. (2016).Boyer MDH, Soper Gorden NL, Barber NA, Adler LS. Floral damage induces resistance to florivory in Impatiens capensis. Arthropod-Plant Interactions. 2016;10(2):121–131. doi: 10.1007/s11829-015-9411-y. [DOI] [Google Scholar]
- Cai et al. (2013).Cai X-Z, Yi R-Y, Zhuang Y-H, Cong Y-Y, Kuang R-P, Liu K-M. Seed coat micromorphology characteristics of Impatiens L. and its systematic significance. Acta Horticulturae Sinica. 2013;407:1337–1348. [Google Scholar]
- Chen, Akiyama & Ohba (2007).Chen YL, Akiyama S, Ohba H. Balsaminaceae. In: Wu ZY, Raven PH, editors. Flora of China. vol. 12. Beijing: Science Press; St. Louis: Missouri Botanical Garden Press; 2007. pp. 43–113. [Google Scholar]
- Chen et al. (2007).Chen W, Liu K-M, Cai X-Z, Cong Y-Y. Micromorphological features of seed surface of fourteen species in Impatiens (Balsaminaceae) in relation to their taxonomic significance. Acta Botanica Yunnanica. 2007;29(6):625–631. [Google Scholar]
- Chmura, Csontos & Sendek (2013).Chmura D, Csontos P, Sendek A. Seed mass variation in Central European populations of invasive Impatiens glandulifera Royle. Polish Journal of Ecology. 2013;61(4):805–809. [Google Scholar]
- Chun et al. (2007).Chun JJ, Michael L, Collyer ML, Kirk A, Moloney KA, Nason JD. Phenotypic plasticity of native vs. invasive purple loosestrife: a two-state multivariate approach. Ecology. 2007;88(6):1499–1512. doi: 10.1890/06-0856. [DOI] [PubMed] [Google Scholar]
- Colautti & Barrett (2013).Colautti RI, Barrett SCH. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science. 2013;342:364–366. doi: 10.1126/science.1242121. [DOI] [PubMed] [Google Scholar]
- Constantinidis, Psaras & Kamari (2001).Constantinidis T, Psaras GK, Kamari G. Seed morphology in relation to infrageneric classification of Consolida (DC.) Gray (Ranunculaceae) Flora. 2001;196(2):81–100. doi: 10.1016/S0367-2530(17)30024-5. [DOI] [Google Scholar]
- Day, Pellicer & Kynast (2012).Day PD, Pellicer J, Kynast RG. Orange balsam (Impatiens capensis Meerb. Balsaminaceae): a re-evaluation by chromosome number and genome size. Journal of the Torrey Botanical Society. 2012;139(1):26–33. doi: 10.2307/41475119. [DOI] [Google Scholar]
- Dlugosh & Parker (2008).Dlugosh KM, Parker IM. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Molecular Ecology. 2008;17:431–449. doi: 10.1111/j.1365-294X.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- Donohue et al. (2000).Donohue K, Hammond Pyle E, Messiqua D, Heschel MS, Schmitt J. Density dependence and population differentiation of genetic architecture in Impatiens capensis in natural environments. Evolution. 2000;54(6):1969–1981. doi: 10.1111/j.0014-3820.2000.tb01241.x. [DOI] [PubMed] [Google Scholar]
- Donohue & Schmitt (1999).Donohue K, Schmitt J. The genetic architecture of plasticity to density in Impatiens capensis. Evolution. 1999;53(5):1377–1386. doi: 10.1111/j.1558-5646.1999.tb05402.x. [DOI] [PubMed] [Google Scholar]
- Euro+Med PlantBase (2019).Euro+Med PlantBase The information resource for Euro-Mediterranean plant diversity. 2019. http://www.emplantbase.org/home.html. [10 December 2019]. http://www.emplantbase.org/home.html
- Ferreira & Wayne (2010).Ferreira T, Wayne R. The Image J User Guide. First edition: v 1.43http://imagej.nih.gov/ij/docs/guide/index.html#. [15 December 2019];2010
- Gamarra et al. (2007).Gamarra R, Dorda E, Scrugli A, Galán P, Ortúñez E. Seed micromorphology in the genus Neotinea Rchb. f. (Orchidaceae, Orchidinae). Botanical Journal of the Linnean Society. 2007;153(2):133–140. doi: 10.1111/j.1095-8339.2006.00603.x. [DOI] [Google Scholar]
- Gamarra et al. (2010).Gamarra R, Ortúñez E, Sanz E, Esparza I, Galán P. Seeds in subtribe Orchidinae (Orchidaceae): the best morphological tool to support molecular analyses. In: Nimis PL, Vignes Lebbe R, editors. Tools for identifying biodiversity: progress and problems. 2010. pp. 323–326. [Google Scholar]
- Geng et al. (2007).Geng Y-P, Pan X-Y, Xu CH-Y, Zhang W-J, Li B, Chen J-K, Lu B-R, Son Z-P. Phenotypic plasticity rather than locally adapted ecotypes allows the invasive alligator weed to colonize a wide range of habitats. Biological Invasions. 2007;9:245–256. doi: 10.1007/s10530-006-9029-1. [DOI] [Google Scholar]
- Gleason & Cronquist (1991).Gleason HA, Cronquist A. Manual of vascular plants of Northeastern United States and adjacent Canada. Van Nostrand; Toronto: 1991. [Google Scholar]
- Gogoi et al. (2018).Gogoi R, Borah S, Dash SS, Singh P. Balsams of Eastern Himalaya. Botanical Survey of India; Kolkata: 2018. [Google Scholar]
- Grey-Wilson (1980).Grey-Wilson C. Impatiens of Africa: morphology; pollination and pollinators; ecology; phytogeography; hybridisation; keys and a systematic treatment of all African species; with a note on collecting and cultivation. A. A. Balkema; Rotterdam: 1980. p. 235. [Google Scholar]
- Hadidchi, Attar & Ullah (2020).Hadidchi A, Attar F, Ullah F. Using microscopic techniques for taxonomic implications of seed and fruits of Delphinium L. (sensu lato) (Ranunculaceae) Microscopy Research and Technique. 2020;83(2):99–117. doi: 10.1002/jemt.23393. [DOI] [PubMed] [Google Scholar]
- Hooker (1904–1906).Hooker JD. An epitome of the British Indian Species of Impatiens. Records of the Botanical Survey of India. 1904–1906;4:1–58. [Google Scholar]
- Hooker & Thomson (1859).Hooker JD, Thomson T. Precursores ad floram indicam: Balsamineae. Journal of the Linnean Society. 1859;4:106–157. [Google Scholar]
- Jacquemart et al. (2015).Jacquemart AL, Somme L, Colin C, Quinet M. Floral biology and breeding system of Impatiens balfourii (Balsaminaceae): an exotic species in extension in temperate areas. Flora. 2015;214:70–75. doi: 10.1016/j.flora.2015.06.001. [DOI] [Google Scholar]
- Janssens et al. (2007).Janssens S, Geuten K, Viaene T, Yuan Y-M, Song Y, Smets E. Phylogenetic utility of the AP3/DEF K-domain and its molecular evolution in Impatiens (Balsaminaceae) Molecular Phylogenetics and Evolution. 2007;43(1):225–239. doi: 10.1016/j.ympev.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Janssens et al. (2018).Janssens SB, Vinckier S, Bosselaers K, Smets EF, Huysmans S. Palynology of African Impatiens (Balsaminaceae) Palynology. 2018;43(4):621–630. doi: 10.1080/01916122.2018.1509149. [DOI] [Google Scholar]
- Jin et al. (2008).Jin XF, Yang SZ, Chen ZH, Quian L. Impatiens yilingiana sp. nov. and I. huangyanensis subsp. attenuata subsp. nov. (Balsaminaceae) from Zhejiang, eastern China. Nordic Journal of Botany. 2008;26(3–4):207–213. doi: 10.1111/j.1756-1051.2008.00325.x. [DOI] [Google Scholar]
- Jongman et al. (1995).Jongman RHG, Ter Braak CJF, Van Tongeren OFR, editors. Data analysis in community and landscape ecology. Cambridge University Press; Cambridge: 1995. [Google Scholar]
- Kovach (2010).Kovach WL. Kovach Computing Services; Pentraeth, Wales: 2010. [Google Scholar]
- Lepš & Šmilauer (2003).Lepš J, Šmilauer P. Multivariate analysis of ecological data using CANOCO. Cambridge University Press; Cambridge: 2003. [Google Scholar]
- Lu & Chen (1991).Lu Y-Q, Chen Y-L. Seed morphology of Impatiens L. (Balsaminaceae) and its taxonomic significance. Acta Phytotaxonomica Sinica. 1991;29:252–257. [Google Scholar]
- Maciejewska-Rutkowska & Janczak (2016).Maciejewska-Rutkowska I, Janczak B. Variability of seeds of the invasive species Impatiens glandulifera Royle (Balsaminaceae) and their micromorphology. Steciana. 2016;20(4):183–190. doi: 10.12657/steciana.020.019. [DOI] [Google Scholar]
- Martín-Gómez, Rewicz & CerVantes (2019).Martín-Gómez JJ, Rewicz A, Cervantes E. Seed shape diversity in families of the Order Ranunculales. Phytotaxa. 2019;425(4):193–207. doi: 10.11646/phytotaxa.425.4.1. [DOI] [Google Scholar]
- Martín-Gómez et al. (2019).Martín-Gómez JJ, Rewicz A, Goriewa-Duba K, Wiwart M, Tocino Á, Cervantes E. Morphological description and classification of wheat kernels based on geometric models. Agronomy. 2019;9(7):399. doi: 10.3390/agronomy9070399. [DOI] [Google Scholar]
- Matthews et al. (2015).Matthews J, Beringen R, Boer E, Duistermaat H, Odé B, Van Valkenburg JLCH, Van der Velde G, Leuven RSEW. 2015. [12 December 2019]. Risks and management of non-native Impatiens species in the Netherlands. Radboud University, FLORON, Naturalis Biodiversity Center, The Netherlands. http://repository.ubn.ru.nl/handle/2066/149286
- Meissner (2010).Meissner W. Przewodnik do ćwiczeń z przedmiotu metody statystyczne w biologii. Wydawnictwo Uniwersytetu Gdańskiego; Gdańsk: 2010. [Google Scholar]
- Meusel et al. (1978).Meusel H, Jager E, Rauschert S, Weinert E. Vergleichende Chorologie der Zentral-europäischen Flora 2. Gustav Fischer Verl; Jena: 1978. [Google Scholar]
- Moles et al. (2005).Moles AT, Ackerly DD, Webb CO, Tweddle JC, Dickie JB, Pitman AJ, Westoby M. Factors that shape seed mass evolution. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10540–10544. doi: 10.1073/pnas.0501473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Montenegro, Atala & Gianoli (2010).Molina-Montenegro MA, Atala C, Gianoli E. Phenotypic plasticity and performance of Taraxacum officinale (dandelion) in habitats of contrasting environmental heterogeneity. Biological Invasions. 2010;12:2277–2284. doi: 10.1007/s10530-009-9638-6. [DOI] [Google Scholar]
- Molina-Montenegro et al. (2018).Molina-Montenegro MA, Acuña Rodríguez IS, Flores TSM, Hereme R, Lafon A, Atala C, Torres-Díaz C. Is the success of plant invasions the result of rapid adaptive evolution in seed traits? Evidence from a latitudinal rainfall gradient. Frontiers in Plant Science. 2018;9 doi: 10.3389/fpls.2018.00208. Article 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore (1968).Moore DM. Impatiens L. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA, editors. Flora Europaea. Vol. 2. Cambridge Univ. Press; Cambridge: 1968. pp. 240–242. [Google Scholar]
- Myśliwy (2019).Myśliwy M. vol. 108. Polish Botanical Society; Wrocław: 2019. Diversity and environmental variability of riparian tall herb fringe communities of the order Convolvuletalia sepium in Polish river valleys. (Monographiae Botanicae). [DOI] [Google Scholar]
- Myśliwy, Ciaciura & Hryniewicz (2009).Myśliwy M, Ciaciura M, Hryniewicz M. Charakterystyka populacji Impatiens capensis Meerb. nad Zalewem Szczecińskim. In: Ciaciura M, editor. Flora roślin naczyniowych województwa zachodniopomorskiego. Cz. II: 225-246. Katedra Taksonomii Roślin i Fitogeografii Uniwersytetu Szczecińskiego; Szczecin: 2009. [Google Scholar]
- Nakazato, Bogonovich & Moyle (2008).Nakazato T, Bogonovich M, Moyle LC. Environmental factors predict adaptive phenotypic differentiation within and between two wild Andean tomatoes. Evolution. 2008;62(4):774–792. doi: 10.1111/j.1558-5646.2008.00332.x. [DOI] [PubMed] [Google Scholar]
- Nanda & Kumar (1983).Nanda KK, Kumar S. Some spectacular responses of flowering in Impatiens balsamina L. cv. Rose. Current Science. 1983;52:571–576. [Google Scholar]
- Pawlaczyk & Adamowski (1991).Pawlaczyk P, Adamowski W. Impatiens capensis (Balsaminaceae)—nowy gatunek we florze Polski. Fragmenta Floristica et Geobotanica. 1991;35:225–232. [Google Scholar]
- Popiela et al. (2015).Popiela A, Łysko A, Sotek Z, Ziarnek K. Preliminary results of studies on the distribution of invasive alien vascular plant species occurring in semi-natural and natural habitats in NW Poland. Biodiversity: Research and Conservation. 2015;37:21–35. doi: 10.1515/biorc-2015-0003. [DOI] [Google Scholar]
- Polish Society of Soil Science (2009).Polish Society of Soil Science Particle size distribution and textural classes of soils and mineral materials –classification of Polish Society of Soil Science 2008. Roczniki Gleboznawcze. 2009;60(2):5–16. [Google Scholar]
- Rahelivololona et al. (2018).Rahelivololona EM, Fischer E, Janssens SB, Razafimandimbison SG. Phylogeny, infrageneric classification and species delimitation in the Malagasy Impatiens (Balsaminaceae) PhytoKeys. 2018;110:51–67. doi: 10.3897/phytokeys.110.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewicz et al. (2017).Rewicz A, Bomanowska A, Magda J, Rewicz T. Morphological variability of Consolida regalis seeds of south-eastern and central Europe. Systematics and Biodiversity. 2017;15(1):25–34. doi: 10.1080/14772000.2016.1216017. [DOI] [Google Scholar]
- Rewicz, Kołodziejek & Jakubska-Busse (2016).Rewicz A, Kołodziejek J, Jakubska-Busse A. The role of anthropogenic habitats as substitutes for natural habitats: a case study on Epipactis helleborine (L.) Crantz (Orchidaceae, Neottieae). Variations in size and nutrient composition of seed. Turkish Journal of Botany. 2016;40(3):258–268. doi: 10.3906/bot-1404-69. [DOI] [Google Scholar]
- Richards et al. (2006).Richards CL, Bossdorf O, Muth NZ, Gurevith J, Pigliucci M. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecological Letters. 2006;9:981–993. doi: 10.1111/j.1461-0248.2006.00950.x. [DOI] [PubMed] [Google Scholar]
- Ruchisansakun et al. (2018).Ruchisansakun S, Suksathan P, Van der Niet T, Smets EF, Saw-Lwin, Janssens SB. Balsaminaceae of Myanmar. Blumea. 2018;63:199–267. doi: 10.3767/blumea.2018.63.03.01. [DOI] [Google Scholar]
- Ruchisansakun et al. (2015).Ruchisansakun S, Van der Niet T, Janssens SB, Triboun P, Techaprasan J, Jenjittikul T, Suksathan P. Phylogenetic analyses of molecular data and reconstruction of morphological character evolution in Asian Impatiens Section Semeiocardium (Balsaminaceae) Systematic Botany. 2015;40(4):1063–1074. doi: 10.1600/036364415X690102. [DOI] [Google Scholar]
- Rust (1977).Rust RW. Pollination in Impatiens capensis and Impatiens pallida (Balsaminaceae) Bulletin of Torrey Botanical Club. 1977;104:361–367. doi: 10.2307/2484781. [DOI] [Google Scholar]
- Schemske (1978).Schemske DW. Evolution of reproductive characteristics in Impatiens (Balsaminaceae): the significance of cleistogamy and chasmogamy. Ecology. 1978;59:596–613. doi: 10.2307/1936588. [DOI] [Google Scholar]
- Schmitt (1993).Schmitt J. Reaction norms of morphological and life-history traits to light availability in Impatiens capensis. Evolution. 1993;47(6):1654–1668. doi: 10.1111/j.1558-5646.1993.tb01258.x. [DOI] [PubMed] [Google Scholar]
- Schmitt, Ehrhardt & Swartz (1985).Schmitt J, Ehrhardt DW, Swartz D. Differential dispersal of self-fertilized and outcrossed progeny in jewelweed (Impatiens capensis) American Naturalist. 1985;126:570–575. doi: 10.1086/284439. [DOI] [Google Scholar]
- Shimizu (1977).Shimizu T. Some additional notes on Impatiens (Balsaminaceae) of Thailand. Acta Phytotaxonomica et Geobotanica. 1977;28:31–34. [Google Scholar]
- Shimizu (1979).Shimizu T. A comment on the limestone flora of Thailand, with special reference to Impatiens. Acta Phytotaxonomica et Geobotanica. 1979;30:180–188. [Google Scholar]
- Shui et al. (2011).Shui Y-M, Janssens S, Huang S-H, Chen W-H, Yang Z-G. Three new species of Impatiens L. from China and Vietnam: preparation of flowers and morphology of pollen and seeds. Systematic Botany. 2011;36(2):428–439. doi: 10.1600/036364411X569615. [DOI] [Google Scholar]
- Silvertown (1989).Silvertown J. The paradox of seed size and adaptation. Trends in Ecology and Evolution. 1989;4:24–26. doi: 10.1016/0169-5347(89)90013-X. [DOI] [PubMed] [Google Scholar]
- Simpson, Leck & Parker (1985).Simpson RL, Leck MA, Parker TV. The comparative ecology of Impatiens capensis Meerb. (Balsaminaceae) in central New Jersey. Bulletin of Torrey Botanical Club. 1985;112:295–311. doi: 10.2307/2996545. [DOI] [Google Scholar]
- Soil Science Society of Poland (2017).Soil Science Society of Poland . Fieldguide for soil description. Polskie Towarzystwo Gleboznawcze; Warszawa: 2017. [Google Scholar]
- Song, Yuan & Kupfer (2005).Song Y, Yuan Y-M, Kupfer P. Seed coat micromorphology of Impatiens (Balsaminaceae) from China. Botanical Journal of the Linnean Society. 2005;149:195–208. doi: 10.1111/j.1095-8339.2005.00436.x. [DOI] [Google Scholar]
- Sreelakshmi et al. (2018).Sreelakshmi V, PrabhuRamya R, Arya VK, Athira VM, Ayeasha M, Shaninas S, Subhandra Vishnu C. Phytochemical screening and evaluation of antioxidant potential of Impatiens balsamina L. flowers in vitro. Trends in Biosciences. 2018;11:1412–1416. [Google Scholar]
- Stace (1992).Stace CA. Plant taxonomy and biosystematics. Cambridge University Press; Cambridge: 1992. [Google Scholar]
- StatSoft Inc (2011).StatSoft Inc STATISTICA (data analysis software system) Version 13.1http://www.statsoft.com. [10 December 2019];2011
- Tabak & Von Wettberg (2008).Tabak NM, Von Wettberg E. Native and introduced jewelweeds of the Northeast. Northeastern Naturalist. 2008;15:159–176. doi: 10.1656/1092-6194(2008)15[159:NAIJOT]2.0.CO;2. [DOI] [Google Scholar]
- Tantaway et al. (2004).Tantaway M, Sayed F, Soad A, Ghalia T. Seed exomorphic characters of some Brassicaceae (LM and SEM study) International Journal of Agriculture and Biology. 2004;1560:821–830. [Google Scholar]
- Ter Braak & Šmilauer (2002).Ter Braak CJF, Šmilauer P. CANOCO reference manual and CanoDraw for windows user’s guide: software for canonical community ordination (version 4.5) Microcomputer Power; Ithaca: 2002. [Google Scholar]
- Tokarska-Guzik et al. (2012).Tokarska-Guzik B, Dajdok Z, Zając M, Zając A, Urbisz A, Danielewicz W, Hołdyński C. Rośliny obcego pochodzenia w Polsce ze szczególnym uwzględnieniem gatunków inwazyjnych. Generalna Dyrekcja Ochrony Środowiska; Warszawa: 2012. [Google Scholar]
- Tooke et al. (2005).Tooke F, Ordidge M, Chiurugwi T, Battey N. Mechanisms and function of flower and inflorescence reversion. Journal of Experimental Botany. 2005;56:2587–2599. doi: 10.1093/jxb/eri254. [DOI] [PubMed] [Google Scholar]
- Ullah et al. (2019a).Ullah F, Papini A, Shah SN, Zaman W, Sohail A, Iqbal M. Seed micromorphology and its taxonomic evidence in subfamily Alsinoideae (Caryophyllaceae) Microscopy Research and Technique. 2019a;82:250–259. doi: 10.1002/jemt.23167. [DOI] [PubMed] [Google Scholar]
- Ullah et al. (2019b).Ullah F, Zaman W, Papini A, Zafar M, Shah SN, Ahmad M, Saqib S, Gul S, Sohail A. Using multiple microscopic techniques for the comparative systematic of Spergula fallax and Spergula arvensis (Caryophyllaceae) Microscopy Research and Technique. 2019b;82:352–360. doi: 10.1002/jemt.23176. [DOI] [PubMed] [Google Scholar]
- Utami & Shimizu (2005).Utami N, Shimizu T. Seed morphology and classification of Impatiens (Balsaminaceae) Blumea. 2005;50:447–456. doi: 10.3767/000651905X622699. [DOI] [Google Scholar]
- Van Boheemen, Atwater & Hodgins (2019).Van Boheemen LA, Atwater DZ, Hodgins KA. Rapid and repeated local adaptation to climate in an invasive plant. New Phytologist. 2019;222:614–627. doi: 10.1111/nph.15564. [DOI] [PubMed] [Google Scholar]
- Van Emden (2008).Van Emden H. Statistics for terrified biologists. Blackwell Publishing; Oxford: 2008. p. 360pp. [Google Scholar]
- Waller (1980).Waller D. Environmental determinants of outcrossing in Impatiens capensis (Balsaminaceae) Evolution. 1980;34:747–761. doi: 10.1111/j.1558-5646.1980.tb04014.x. [DOI] [PubMed] [Google Scholar]
- Waller (1982).Waller DM. Factors influencing seed weight in Impatiens capensis (Balsaminaceae) American Journal of Botany. 1982;69(9):1470–1475. doi: 10.1002/j.1537-2197.1982.tb13395.x. [DOI] [Google Scholar]
- Warburg & Reiche (1895).Warburg O, Reiche K. Balsaminaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. Vol. 3. Engelmann; Leipzig: 1895. pp. 390–392. [Google Scholar]
- Werner & Platt (1976).Werner FA, Platt WJ. Ecological relationships of co-occurring goldenrods (Solidago: Compositae) The American Naturalist. 1976;110:959–971. doi: 10.1086/283120. [DOI] [Google Scholar]
- Więcław et al. (2017).Więcław H, Kurnicki B, Bihun M, Białecka B, Koopman J. Carex section Racemosae (Cyperaceae) in Europe: morphological diversity, taxonomy and phylogenetic relationships. Botanical Journal of the Linnean Society. 2017;183:124–145. [Google Scholar]
- Xia et al. (2019).Xia C-Y, Gadagkar SR, Zhao X-L, Van Do Truong, Zhu X-Y, Qin Y, Deng HP, Yu S-X. Impatiens maculifera sp. nov. (Balsaminaceae) Yunnan, China. Nordic Journal of Botany. 2019;2019:e02422. doi: 10.1111/njb.02422. [DOI] [Google Scholar]
- Yu (2012).Yu SX. Balsaminaceae of China. Peking University Press; Beijing: 2012. [Google Scholar]
- Yu, Chen & Qin (2007).Yu S-X, Chen Y-L, Qin H-N. Impatiens macrovexilla var. yaoshanensis S. X. Yu, Y. L. Chen & H. N. Qin, a new variety of Balsaminaceae from Guangxi, China. Acta Phytotaxonomica Sinica. 2007;45(5):708–712. doi: 10.1360/aps06037. [DOI] [Google Scholar]
- Yu et al. (2015).Yu SX, Janssens SB, Zhu XY, Lidén M, Gao TG, Wang W. Phylogeny of Impatiens (Balsaminaceae): integrating molecular and morphological evidence into a new classification. Cladistics. 2015;32:179–197. doi: 10.1111/cla.12119. [DOI] [PubMed] [Google Scholar]
- Yuan et al. (2004).Yuan Y-M, Song Y, Geuten K, Rahelivololona E, Wohlhauser S, Fischer E, Smets E, Küpfer P. Phylogeny and biogeography of Balsaminaceae inferred from ITS sequences. Taxon. 2004;53(2):391–403. doi: 10.2307/4135617. [DOI] [Google Scholar]
- Zar (1984).Zar JH. Biostatistical analysis. 2nd edition Prentice-Hall, Inc; Englewood Cliffs: 1984. p. 718. [Google Scholar]
- Zhang et al. (2016).Zhang LJ, Guo H, Li XH, Liang TJ, Zhang M, Yu SX. Observation research of the seedcoat micromorphology of Impatiens sect. Racemasae. Acta Horticulturae Sinica. 2016;40:1337–1348. doi: 10.16420/j.issn.0513-353x.2016-0097. [DOI] [Google Scholar]
- Zhou et al. (2019).Zhou L, Tian J, Wu Y, Li S, Wu Y, Kuang R, Liu K, Liu K. Newly recorded plants from Hunan Province of China (VIII) Life Science Research. 2019;23:35–38. doi: 10.16605/j.cnki.1007-7847.2019.01.005. [DOI] [Google Scholar]
- Zika (2006).Zika PF. The status of Impatiens capensis (Balsaminaceae) on the Pacific Northwest coast. The Journal of the Torrey Botanical Society. 2006;133(4):593–600. doi: 10.3159/1095-5674(2006)133[593:TSOICB]2.0.CO;2. [DOI] [Google Scholar]
- Zika (2009).Zika PF. Jewelweeds and Touch-Me-Nots (Impatiens, Balsaminaceae) in the Pacific Northwest of North America. 2009. https://www.ou.edu/cas/botany-micro/ben/ben408.html. [10 January 2020]. https://www.ou.edu/cas/botany-micro/ben/ben408.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Matthews J, Beringen R, Boer E, Duistermaat H, Odé B, Van Valkenburg JLCH, Van der Velde G, Leuven RSEW. 2015. [12 December 2019]. Risks and management of non-native Impatiens species in the Netherlands. Radboud University, FLORON, Naturalis Biodiversity Center, The Netherlands. http://repository.ubn.ru.nl/handle/2066/149286
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are provided in the Supplementary File.