INTRODUCTION
Bile acid diarrhea (BAD) can affect up to 33% of patients with diarrhea-predominant irritable bowel syndrome (IBS-D).1 In the United States, the 48-hour fecal BA test is the gold standard diagnostic test for BAD. Fasting serum 7a-hydroxy-4-cholesten-3-one (C4) measures the product of the rate limiting step of hepatic BA synthesis and increases in response to BA loss in the stool. Primary fecal BA [cholic acid (CA) and chenodeoxycholic acid (CDCA)] >10% is equivalent to increased total fecal BA (>2337μmol/48h) in predicting stool weight >400g/48h.2
The aim of this study was to evaluate fasting serum C4 combined with fecal BA from a single, random stool sample compared to 48-hour fecal BA in the diagnosis of BAD.
METHODS
Study Design
In a single-center study, we prospectively collected fasting serum C4, a single, random stool sample, and a 48-hour stool collection in healthy volunteers, patients with IBS-D or with >10cm terminal ileum resection. All participants resided within the catchment area and received their regular healthcare at Mayo Clinic, Rochester, Minnesota.
Diarrhea was defined3 as >3 bowel movements per day and stool consistency >5.5 on the Bristol Stool Form Scale (BSFS). This study was approved by the Mayo Clinic Institutional Review Board (IRB #18–000342).
Diagnostic Tests of Bile Acid Diarrhea
Fasting serum C4 was measured by high performance liquid chromatography (HPLC)/tandem mass spectrometry.4 C4 >52.5ng/mL is suggestive of BAD.4,
Fecal bile acid: Each participant provided a single, random stool sample (with no specification of time or diet) and separately completed a 4-day ingestion of 100 grams fat per day, with a 48-hour stool collection during the last 2 days of the diet which was completed either after or at least 7 days before the 7-day bowel diary (number bowel movements and stool consistency based on BSFS) and random stool sample. Based on our previous study,2 we used 10% cut-off to ascertain the utility of the primary BA % in a single stool sample.
Fecal BAs were measured with HPLC/tandem mass spectrometry.5 Percentages of individual fecal BAs were determined based on concentrations relative to total fecal BAs within the stool sample.
BAD was defined by the 48-hour stool sample results: total fecal BA >2,337μmol/48h, or total fecal BA >1,000μmol/48h with primary BA >4% or primary BA >10%.2
Statistical Analysis
Data are represented as mean ± standard deviation (SD). We utilized the Kruskal Wallis test to compare parameters among healthy volunteers, patients with ileal resection, patients with IBS-D without BAD, and patients with IBS-D with BAD. We utilized logistic regression and receiver operating characteristic (ROC) curves to determine if fasting serum C4 and individual fecal BAs from the single, random stool sample could predict the presence of BAD.
RESULTS
Participants and Demographics
Bowel functions, serum and fecal markers of BAD are reported in Supplemental Table 1; 25 healthy volunteers, 59 patients with IBS-D (34 without BAD and 25 with BAD), and 4 patients with terminal ileum resection completed the study. Only 20% (12/59 IBS-D) had elevated serum C4 >52.5ng/mL, and 12/25 (48%) with BAD (based on 48h fecal BA) had primary BA >10% from a single stool sample.
Fecal Bile Acids Expressed as Percentage or Absolute Concentration from the Random Single and 48-Hour Stool Samples
Bland-Altman plots (data not shown) found no significant differences in the concentrations of individual fecal BAs measured from the random, single stool sample compared to 48-hour stool sample, except for percentage (but not for concentration) of LCA. In single stool sample results, patients with ileum resection had higher primary BA % and BA concentration (μmol/L), and lower deoxycholic acid (DCA) % and lithocholic acid (LCA) % compared to all other groups. IBS-D with BAD had higher primary BA % with lower LCA % compared to patients with IBS-D without BAD and healthy volunteers. There was no significant difference between IBS-D without BAD and healthy volunteers.
Parameters in the Random Single Stool Sample Predicting Clinical Markers of Diarrhea
Combined fasting serum C4 and fecal primary BA was a significant predictor of increased stool frequency: AUC 0.85, sensitivity 79%, specificity of 94%. A 10% increase in fecal primary BA resulted in a 2-fold increase in stool frequency (Figure 1A). In addition, combined serum C4 and % of fecal secretory (CDCA + DCA) BA predicted looser stool consistency: AUC 0.85, sensitivity 78%, specificity 81% (Figure 1B).
Figure 1. ROC curve and odds ratio (95% CI) of significant predictors of clinical markers of diarrhea based on stool frequency (A), stool consistency (B), and diagnosing bile acid diarrhea (C).
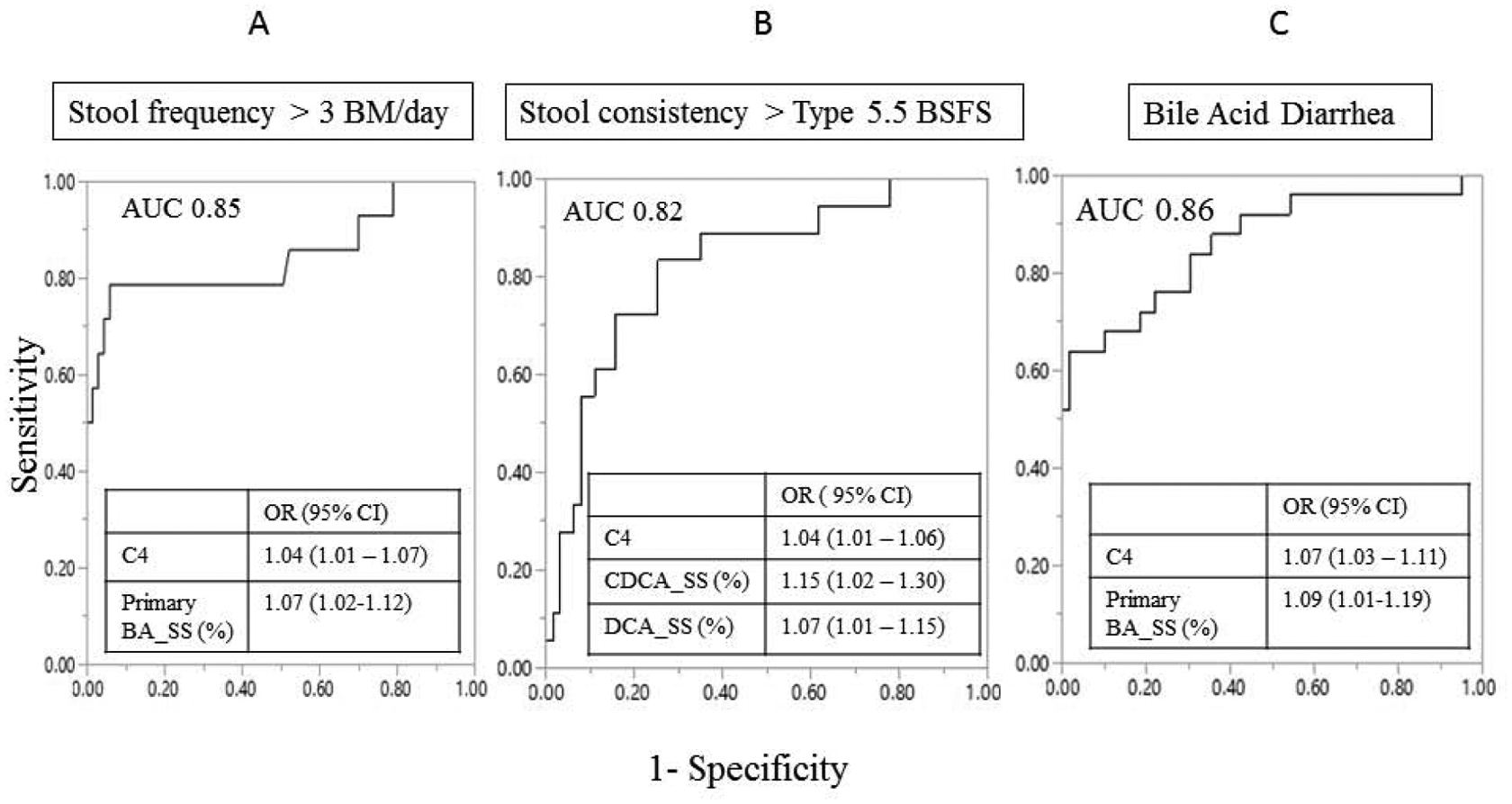
Primary BA-SS=primary bile acid measured from the single stool sample; CDCASS=chenodeoxycholic acid measured from single stool sample; DCA-SS=deoxycholic acid measured from single stool sample; C4=7a-hydroxy-4-cholesten-3-one; BSFS=Bristol Stool Form Scale; ROC=receiver operating characteristic; AUC=area under the curve; OR=odds ratio; CI=confidence interval
Combined serum C4 and % fecal primary BA was a significant predictor of BAD (AUC: 0.86) (Figure 1C). For every increase in 10ng/mL of C4, there was a 2-fold higher odds of diagnosing BAD. For every increase in 10% primary BA in single stool sample, there was a 2.5-fold higher odds of diagnosing BAD.
Using cut-offs for primary BA % of 10%, 15%, and 20% in the healthy subjects and patients with IBS-D did not change the test performance of the combined serum C4 and primary BA in the single stool sample (Supplemental Table 2). For IBS-D patients alone, these combined results revealed sensitivity 60%, specificity 94% with PPV 88% and NPV 76%, consistent with results in the combined healthy and IBS-D patients.
DISCUSSION
The current study shows that combining the results of fasting serum C4 >52.5ng/mL and individual fecal BAs (specifically, primary BA >10%) measured from the single stool sample was a significant predictor of elevated stool frequency, looser stool consistency, and diagnosis of BAD relative to the current gold standard in the U.S. Performance of the combination was greater than each parameter alone. The importance of primary BA in a single stool sample has also been preliminarily reported in a comparison with retention test.6 Further studies are needed to define optimal timing of the single stool collection during the day and the potential impact of dietary fat to optimize test sensitivity (currently 60%) and replicate the results in a larger sample size. Ultimately, the availability of a convenient diagnostic test for diagnosis of BAD based on a fasting serum sample and a single stool sample would enhance the diagnostic algorithm for chronic functional diarrhea and reduce healthcare utilization in patients with chronic, non-bloody diarrhea, as demonstrated for the 75SeHCAT retention test7 and the 48-hour fecal BA excretion test.8
Supplementary Material
Support:
This study was supported by grant R01-DK115950 from NIH to Michael Camilleri; by Department of Laboratory Medicine and Pathology (DLMP) Mayo Clinic intramural grant, 2018 DLMP Small Grant Program; and by 2018 American College of Gastroenterology Clinical Research Pilot Award to Priya Vijayvargiya
Disclosures:
PV, AT, IB, LD – no relevant financial disclosures
MC – research grant from Allergan to study effects of eluxadoline in bile acid diarrhea
EVL – Consulting for AbbVie, Allergan, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion Healthcare, Eli Lilly, Genentech, Gilead, Janssen, Pfizer, Takeda, and UCB;
research support from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Gilead, Janssen, Pfizer, Takeda, and UCB.
Abbreviations:
- BA
bile acid
- BAD
bile acid diarrhea
- BSFS
Bristol Stool Form Scale
- C4
7a-hydroxy-4-cholesten-3-one
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- IBS-D
diarrhea-predominant irritable bowel syndrome
- LCA
lithocholic acid
- 75SeHCAT
75selenium homocholic acid taurine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2009;30:707–717. [DOI] [PubMed] [Google Scholar]
- 2.Vijayvargiya P, Camilleri M, Chedid V, Carlson P, Busciglio I, Burton D, Donato LJ. Analysis of fecal primary bile acids detects increased stool weight and colonic transit in patients with chronic functional diarrhea. Clin Gastroenterol Hepatol 2019;17:922–929, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitsuhashi S, Ballou S, Jiang ZG, Hirsch W, Nee J, Iturrino J, Cheng V, Lembo A. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES). Am J Gastroenterol 2018;113:115–123. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M, Nadeau A, Tremaine WJ, Lamsam J, Burton D, Odunsi S, Sweetser S, Singh R. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil 2009;21:734–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tagliacozzi D, Mozzi AF, Casetta B, Bertucci P, Bernardini S, Di Ilio C, Urbani A, Federici G. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med 2003;41:1633–1641. [DOI] [PubMed] [Google Scholar]
- 6.Walters JR, Sagar N, Duboc H, Arasaradnam R. Primary bile acids in a single fecal sample for the diagnosis of bile acid diarrhea: relationship to SeHCAT testing. Gastroenterology 2019;156:S-774. [Google Scholar]
- 7.Turner JM, Pattni SS, Appleby RN, Walters JR. A positive SeHCAT test results in fewer subsequent investigations in patients with chronic diarrhoea. Frontline Gastroenterol. 2017. October;8(4):279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijayvargiya P, Gonzalez Izundegui D, Calderon G, Tawfic S, Batbold S, Camilleri M. Fecal bile acid testing in assessing patients with chronic unexplained diarrhea: implications for healthcare utilization. Am J Gastroenterol 2020. May 22. doi: 10.14309/ajg.0000000000000637. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


