Abstract
Background & Aims:
Obesity is established as a major risk factor for the development of nonalcoholic fatty liver disease (NAFLD). However, the influence of dynamic changes in adiposity over the life course on NAFLD risk remains poorly understood.
Methods:
We collected data from 110,054 women enrolled in the Nurses’ Health Study II cohort. Early adulthood weight was ascertained at age 18 years, and weight gain since early adulthood was defined prospectively every 2 years. We used a group-based modeling approach to identify five trajectories of body shape from age 5 years up to age 50 years. NAFLD was defined by physician-confirmed diagnoses of fatty liver, after excluding excess alcohol intake and viral hepatitis, using validated approaches.
Results:
We documented 3,798 NAFLD cases over a total of 20 years of follow-up. Compared to women who maintained stable weight (+/−2 kg), women with ≥20 kg of adulthood weight gain had the multivariable aHR of 6.96 (95% CI, 5.27-9.18), and this remained significant after further adjusting for early adultood BMI and updated BMI (both P trend <0.0001). Compared to women with a medium-stable body shape trajectory, the multivariable aHRs for NAFLD were, 2.84 (95% CI, 2.50-3.22) for lean-marked increase, 2.60 (95% CI, 2.27-2.98) for medium-moderate increase, and 3.39 (95% CI, 2.95-3.89) for medium-marked increase.
Conclusions:
Both early adulthood weight gain and lifetime body shape trajectory were significantly and independently associated with excess risk of developing NAFLD in mid-life. Maintaining both lean and stable weight throughout life may offer the greatest benefit for the prevention of NAFLD.
Keywords: obesity, weight change, trajectory, NAFLD
Introduction
It is estimated that 50-80 million Americans have nonalcoholic fatty liver disease (NAFLD), defined by fatty infiltration of the liver (steatosis) in the absence of excess alcohol consumption.[1] Among them, nearly 25% develop progressive steatohepatitis with fibrosis, which can lead to cirrhosis, hepatocellular carcinoma (HCC) and death.[2–4] Obesity is an established risk factor for NAFLD, and it is estimated that up to 80% of adults with obesity have NAFLD.[5–7] Despite a growing awareness of both diseases, the prevalence of and mortality from obesity and NAFLD continue to increase at alarming rates,[8, 9] underscoring the importance of identifying effective population-level prevention strategies that target weight control.
Increasing data suggest that weight change over the life course plays a vital role in long-term disease outcomes, independently of baseline body weight.[10] Specifically, early adulthood weight gain has been associated with an increased risk of type 2 diabetes,[11] coronary heart disease,[11] hypertension,[11] cholelithiasis,[12] and certain types of cancer.[13] Early adulthood weight gain may be particularly relevant to NAFLD because the incidence of NAFLD typically increases with age, and excess adiposity tends to accrue during early and middle adulthood. However, the precise influence of early adulthood weight gain on the risk of developing NAFLD in mid-life remains unclear. To date, most prior studies of adiposity and NAFLD risk have focused on static measures of attained body mass index (BMI), weight, or waist/hip circumference, primarily in adulthood.[14, 15] In contrast, weight changes during adolescence, early adulthood and adulthood may better capture the dynamic effects of excess body fat and lean body mass during the life course, thereby enabling formulation of public health recommendations for weight control at different ages.
In addition to dynamic assessments of weight change, a group-based trajectory modeling (GBTM) approach has also demonstrated utility for studying the long-term health impacts of adiposity across the life course.[16–19] This GBTM approach is particularly useful because it respects the continuity of body growth and fat accumulation over time. Compared to traditional weight change analyses that partition time intervals, GBTM allows for closer scrutiny of heterogeneity in changes of body fatness over the life course, and permits a more direct comparison of disease risk across different trajectory groups.[17] In doing so, it addresses a different but more tangible question about the relative disease risk of people who experience different patterns of changes in body fatness over the life-course. To our knowledge, no study has reported on how group-based trajectories of body shape from childhood to middle age are related to subsequent risk of NAFLD.
Thus, we conducted a comprehensive evaluation of early adulthood weight change and the trajectory of body shape changes from childhood through mid-life, in relation to the incidence of NAFLD, within a large, prospective cohort of US women from the Nurses’ Health Study II (NHSII) cohort.
Methods
Study population
The Nurses’ Health Study II (NHSII) is an ongoing, prospective US cohort study that began in 1989 with the enrollment of 116,430 US female registered nurses, aged 25 to 42 years. Every 2 years since enrollment, participants have been mailed self-administered questionnaires to provide detailed data regarding demographics, lifestyle factors, medical history, disease outcomes, and other health-related information, with overall follow-up rates of approximately 90% for all questionnaires.[20] For the current study, we excluded women with a diagnosis of NAFLD at baseline, which was set as 1995, the first year that detailed information regarding NAFLD was available. We also excluded anyone at baseline with a diagnosis of hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, or excess alcohol intake (defined as weekly ethanol consumption of more than 140 g[21]). Consistent with prior work[22], we further excluded participants with missing weight at baseline in 1995 or age 18 years, and anyone with a recorded BMI of less than 10 either in 1995 or at age 18 years. After these exclusions, a total of 110,054 participants were eligible for this study. Participants were followed prospectively through June 30, 2015.
The NHSII cohort study was approved by the Institutional Review Board of the Brigham and Women’s Hospital. Informed consent was indicated by questionnaire return.
Ascertainment and Validation of NAFLD
The primary endpoint was a physician-confirmed diagnosis of NAFLD. In 2013 and 2015, participants were asked if they had received a diagnosis from a physician of fatty liver disease. If yes, participants were subsequently asked the year of first diagnosis, dating back to before 1995, as well as the method of diagnosis, as well as details regarding the presence or absence of cirrhosis, and diagnoses of either HBV or HCV infection. In a validation study within our analogous cohort of older female nurses (the NHSI cohort), a blinded study physician reviewed 33 randomly-selected medical records from women who met our criteria for NAFLD, and applied validated radiographic and/or histological criteria to identify hepatic steatosis, and excluded alternative etiologies of liver disease[23]. With that algorithm, 29/33 cases were confirmed to be true NAFLD (positive predictive value, 88%).
Assessment of anthropomorphic measures
Participants were asked to report their height and weight at baseline, and weight was prospectively updated thereafter with biennial questionnaires. The high validity and reliability of self-reported body weight has previously been demonstrated in the NHSI cohort, in which self-reported and technician-measured weights were highly correlated (correlation coefficient, 0.97).[24] Using these data, we calculated prospectively-updated BMI every two years, and this was as, 18.5 to 20.9, 21.0 to 22.9, 23.0 to 24.9, 25.0 to 29.9, or 30.0 or greater.[25, 26] Recalled weight at age 18 years was queried in 1989, and a prior validation study in this cohort demonstrated a correlation coefficient of 0.87 between recalled weight and recorded weight at age 18 years.[27] In 1989, participants were also asked to measure their waist and hip circumferences, and these data were updated again in 1993 and in 1995, permitting calculation of the waist-to-hip ratio (WHR).
Early adulthood weight change was defined as the difference in kg between early adulthood weight (age 18 years) and the weight reported in the current questionnaire cycle, and these data were updated prospectively, every 2 years,[10, 13] and subsequently partitioned into 6 categories: weight loss ≥ 2.0 kg; loss or gain within 2 kg; gain 2.0-5.9 kg; gain 6.0-9.9 kg; gain 10.0-19.9 kg; and gain ≥ 20.0 kg, consistent with prior work.[28]
Body shape and trajectories
In 1989, participants selected their body shape in early and middle life (ages 5, 10, 20, 30, and 40 years) among one of nine pictorial body diagrams (somatotypes) developed by Stunkard et al[29] (Figure 1). The validity of these somatotypes as a marker of adiposity in early life was investigated among 181 participants aged 71-76 years in the Third Harvard Growth Study.[30] In that study, participants’ recalled body shapes were compared with their measured BMI at approximately the same ages. The Pearson correlation coefficients for women aged 5, 10, and 20 years were 0.60, 0.65, and 0.66, respectively.[30]
Figure 1.
Figure drawing used to assess body shape at ages 5, 10, 20, 30, and 40 years among women from the Nurses’ Health Study II. (Reproduced from Stunkard et al. with permission from Lippincott Williams & Wilkins, Philadelphia, Pennsylvania).
To estimate trajectories of body shape over the life-course, we first calculated BMI at ages 40, 45, and 50 years, using the average BMI at age 50 ± 3 years, to minimize random variation.[17] Next, we converted BMI to the same scale as the somatotypes, using an established proportional odds mixed effects model, by linking BMI to the somatotype data at younger ages, and we used this model to predict the somatotype at ages 45 and 50 on the basis of the BMI at that age,[17] consistent with prior studies.[16, 17, 19] Because the trajectory analysis required a participant to survive at least to age 50 without having NAFLD, for this analysis we additionally excluded persons who died or who had a diagnosis of NAFLD before or at the age of 50.
Assessment of covariates
We assessed total caloric and alcohol consumption using semiquantitative food frequency questionnaires every 4 years, since 1995.[31] Diet quality was defined using the Alternative Healthy Eating Index 2010, with a higher score reflecting a healthier overall diet.[32–34] Validated measures of weekly physical activity, in metabolic equivalent of tasks (MET) hours, was ascertained and updated every 2 to 4 years.[35] Smoking status was updated every 2 years.[36] Participants also provided information on race and ethnicity, and detailed, biennially-updated information regarding diabetes, hypertension, hypercholesterolemia, regular use of aspirin, menopausal status, and use of menopausal hormone therapy.[37–40]
Statistical Analysis
Among women from the NHSII who provided somatotype data for at least 4 different ages, we used a GBTM approach implemented by SAS Trajectory Procedure (Proc Traj) to identify subgroups within each cohort who shared a similar body shape trajectory from age 5 to 50 years.[17] This method represents an application of finite mixture modeling and is designed to identify relatively homogeneous clusters of developmental trajectories within the population.[41] Participants were assigned to one of the trajectories to which they had the highest estimated probability of belonging. Details of trajectory analyses have been described in prior literature, including studies conducted within this cohort.[16] Briefly, the optimal number of groups and the shapes of trajectories were determined in a two-stage approach, based on the change in the Bayesian Information Criterion.[42] For the current study, the model with five trajectories and a cubic function of age demonstrated the best fit to our data. The name of each trajectory was defined on the basis of the visual pattern of changes in body shape over age and estimated the mean body shape levels for each trajectory at each age from the final model. Next, the posterior predicted probability for each participant of being a member of each of the five trajectories was calculated and participants were assigned into the trajectory to which their posterior probability of membership was greatest.[17] The average posterior probability for each trajectory was 0.98, 0.90, 0.95, 0.88, and 0.96, indicating excellent discrimination of trajectory assignments. Based on the results from the trajectory analyses and the interpretability of the groups, we included five trajectories in subsequent analyses: lean-moderate increase; lean-marked increase; medium-stable; medium-moderate increase; and medium-marked increase.
Person-time accrued from the return of the baseline questionnaire (1995) until the date of NAFLD diagnosis, death from any cause, or the end of follow-up (2015), whichever came first. We used Cox proportional hazards regression models stratified by age (years) and study period (2-year intervals) to estimate multivariable adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs). Covariates were selected a priori based on established risk factors for NAFLD. We constructed two multivariable models: the first model accounted for age and early adulthood BMI (at age 18 years). The fully-adjusted multivariable model further accounted for race (white/non-white) and time-varying covariates, including diabetes (yes/no), hypertension (yes/no), hypercholesterolemia (yes/no), smoking status (never, past, current), physical activity (quintiles), regular use of aspirin use (yes/no), menopausal status (premenopausal or postmenopausal), menopausal hormone use (never, past, or current), and diet (total calories and Alternate Healthy Eating Index 2010, both continuous). Tests for linear trend were performed using continuous variables.
To provide a comprehensive evaluation of adiposity in relation to incident NAFLD, we conducted secondary analyses focusing on early adulthood BMI, middle adulthood BMI, updated BMI throughout adulthood, and visceral adiposity (using waist circumference [WC] and the WHR). Additionally, we sought to define whether obesity in early adulthood or in middle adulthood might play a more important role in the development of NAFLD in mid-life. Thus, we constructed nested models to directly compare models that included only early adulthood BMI, to models including both early adulthood and mid-life BMI. Additionally, we compared nested models that included early adulthood weight change, to models that included both early adulthood weight change and middle adulthood weight change. Nested model analyses to compare only early adulthood BMI or mid-life BMI, with models that included both early adulthood BMI and body shape trajectories or both mid-life BMI and body shape trajectories were also done. For all nested models, we tested the significance of associations using the log-likelihood ratio test. We conducted several sensitivity analyses to test the robustness of our results. First, we repeated our primary analyses after excluding any participant who reported an incident diagnosis of viral hepatitis B or C infection during follow-up. Second, to address potential confounding related to being underweight, we excluded anyone with BMI <17 kg/m2 at age 18 years or during follow-up. Finally, to assess the robustness of our defined body shape trajectories, we repeated the trajectory analysis after excluding participants whose trajectory assignment probability was less than 75%.
Statistical significance was set as a value of P ≤.05 in two-tailed tests. Statistical analysis was conducted with SAS software, version 9.4 (SAS Institute Inc., NC).
Results
Early adulthood weight change and the risk of NAFLD
Over a total of 1,842,560 person-years, we documented 3,798 incident cases of NAFLD. We present baseline (in 1995) characteristics according to early adulthood weight change categories in Table 1. Women with a greater amount of early-adulthood weight gain were more likely to have a higher BMI in early adulthood and during follow-up, and more likely to have diabetes, hypertension, and hypercholesterolemia.
Table 1.
Age-adjusted baseline characteristics according to early adulthood weight change
| Weight loss, ≥2.0 kg | Weight change, within 2.0 kg | Weight gain, 2.0-5.9 kg | Weight gain, 6.0-9.9 kg | Weight gain, 10.0-19.9 kg | Weight gain, ≥20.0 kg | |
|---|---|---|---|---|---|---|
| Age, years* | 39.3 (4.7) | 39.2 (4.7) | 39.5 (4.6) | 40.0 (4.6) | 40.6 (4.6) | 41.2 (4.5) |
| Body mass index, kg/m2 | 22.0 (3.6) | 21.2 (2.7) | 21.9 (2.5) | 23.4 (2.7) | 26.1 (3.3) | 33.7 (6.0) |
| BMI at age 18 | 24.5 (4.8) | 21.1 (2.7) | 20.4 (2.5) | 20.4 (2.6) | 20.7 (3.0) | 22.3 (3.7) |
| Physical activity, METsa | 26.8 (32.9) | 25.1 (31.3) | 23.2 (28.8) | 21.3 (26.8) | 19.4 (25.5) | 16.4 (22.8) |
| Smoking status | ||||||
| - never, % | 61.7 | 67.8 | 68.9 | 68.0 | 68.1 | 69.4 |
| - past, % | 25.3 | 24.3 | 23.7 | 24.8 | 24.2 | 23.9 |
| - current, % | 13.0 | 7.9 | 7.4 | 7.2 | 7.7 | 6.7 |
| History of diabetes, % | 1.4 | 0.8 | 0.9 | 0.8 | 1.3 | 3.2 |
| History of hypertension, % | 2.4 | 2.1 | 2.2 | 3.1 | 5.3 | 13.4 |
| History of hypercholesterolemia, % | 5.9 | 5.7 | 6.1 | 7.7 | 10.9 | 17.4 |
| Alternate Healthy Eating Index score | 53.1 (11.3) | 52.1 (11.2) | 51.0 (10.8) | 49.9 (10.5) | 48.6 (10.3) | 46.3 (10.2) |
| Regular aspirin use, 1995,† % | 11.5 | 10.8 | 10.8 | 10.3 | 10.8 | 12.0 |
| Premenopausal, % | 93.2 | 94.0 | 94.0 | 93.8 | 92.6 | 91.4 |
| Current menopausal hormone,‡ % therapy,‡ % | 4.8 | 4.5 | 4.5 | 4.7 | 5.7 | 6.5 |
| Race (white), % | 96.9 | 96.7 | 96.3 | 95.6 | 94.7 | 95.1 |
BMI, body mass index; MET, metabolic equivalent of tasks.
All values other than age are standardized to the age distrubition of the study population. Values are means (SD) unless stated otherwise.
Physical activity was defined according to expended MET-hours/week (see Methods).
Regular aspirin use was defined as the regular use of at least 2 aspirin pills per week.
Among postmenopausal participants.
Table 2 outlines the association between early adulthood weight change and risk of incident NAFLD. Compared with women who maintained a stable weight (i.e. weight change within 2 kg since age 18 years), the HRs after adjusting for age and BMI at age 18 years, were 1.30 (95% CI, 0.94-1.80) with gain of 2-5.9 kg, 1.72 (95% CI, 1.26-2.34) with gain of 6-9.9 kg, 3.51 (95% CI, 2.64-4.65) with gain of 10-19.9 kg, and 9.71 (95% CI, 7.38-12.78) with ≥20 kg weight gain since early adulthood (P trend<0.0001) (Table 2). These associations were robust after further accounting for clinical, lifestyle and dietary risk factors for NAFLD, with corresponding multivariable aHRs of 1.26 (95% CI, 0.91-1.74), 1.59 (95% CI, 1.17-2.16), 2.99 (95% CI, 2.26-3.97) and 6.96 (95% CI, 5.27-9.18), respectively (P trend<0.0001) (Table 2). After further adjusting for updated BMI, this positive association remained statistically significant (P trend<0.0001).
Table 2.
Early adulthood weight change and risk of NAFLD
| Weight change |
|||||||
|---|---|---|---|---|---|---|---|
| Weight loss, ≥2.0 kg | Weight change, within 2.0 kg | Weight gain, 2.0-5.9 kg | Weight gain, 6.0-9.9 kg | Weight gain, 10.0-19.9 kg | Weight gain, ≥20.0 kg | P for trend | |
| Cases, No. | 65 | 52 | 121 | 179 | 750 | 2631 | |
| Person-years | 105976 | 131341 | 249129 | 274192 | 514226 | 567697 | |
| Model 1, HR (95% CI) † | 1.19 (0.82-1.71) | 1 (Reference) | 1.30 (0.94-1.80) | 1.72 (1.26-2.34) | 3.51 (2.65-4.65) | 9.71 (7.38-12.78) | <0.0001 |
| Model 2, HR (95% CI) ‡ | 1.26 (0.88-1.82) | 1 (Reference) | 1.26 (0.91-1.74) | 1.59 (1.17-2.16) | 2.99 (2.26-3.97) | 6.96 (5.27-9.18) | <0.0001 |
NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; HR, hazard ratio; CI, confidence interval.
Model 1 was adjusted for age and bmi at age 18 years.
Model 2 was further Adjusted for smoking status (never, past, current), total calories (kcal), menopausal hormone therapy (never, past, current, premenopausal), Alternate Healthy Eating Index score (quintiles, missing), physical activity (quintiles), type 2 diabetes (yes or no), hypertension (yes/no), dyslipidemia (yes or no), physical activity (MET-hours/week, quintiles), regular use of aspirin (yes/no), and race (white/non-white).
Attained adiposity and NAFLD risk
The risk of incident NAFLD increased linearly with increasing updated early adulthood BMI (P trend <0.0001) (Supplementary table 1). Overall, each 5 kg/m2 increase in early adulthood BMI contributed to a 1.2-fold increased risk of incident NAFLD in mid-life (multivariable aHR, 1.18; 95% CI, 1.13-1.24). The strong, positive gradient of increasing risk persisted in analyses focused on updated adulthood BMI and baseline BMI (in 1995) (both P trend < 0.0001) (Supplementary table 1). Compared to women with an early adulthood BMI< 22.5 kg/m2, the multivariable aHRs were 1.30 (95% CI, 1.19-1.41) for BMI of 22.5-24.9 kg/m2, 1.34 (95% CI, 1.19-1.51) for BMI of 25.0-27.4 kg/m2, 1.31 (95% CI, 1.10-1.57) for BMI of 27.5-29.9 kg/m2, 1.42 (95% CI, 1.18-1.70) for BMI of 30.0-34.9 kg/m2, and 1.37 (95% CI, 1.01-1.86) for BMI of ≥35.0 kg/m2.
In nested analyses comparing attained BMI in early adulthood, mid-life and updated throughout adulthood, mid-life and updated BMI significantly modified the association between early adulthood BMI and incident NAFLD risk (both log-likelihood P<0.001). The association between mid-life weight change and incident NAFLD risk was significantly impacted by early adulthood weight change (log-likelihood P<0.001).
Trajectory of body shape and the risk of NAFLD
We identified five distinct body shape trajectories between ages 5 years to 50 years (Figure 2): 28.1% of women had a medium-stable trajectory, in which they maintained a medium body shape throughout life; 34.7% of women had a lean-moderate increase trajectory, in which they were lean in early adulthood and subsequently gained a moderate amount of weight; 16.7% of women had lean-marked increase trajectory, in which they were lean in early adulthood and subsequently gained a substantial amount of weight; and 11.7% and 8.7% of women started at a medium weight and subsequently gained either a moderate or a substantial amount of weight (medium-moderate increase/medium-marked increase). Overall, BMI in each category tracked well with the predicted trajectories from early to late adulthood (Table 3). For example, the mean BMI in the lean-moderate increase group remained below 23 kg/m2 over the life course, whereas the mean BMI in the lean-marked increase group increased from 21.1 at age 18 years to 30.5 kg/m2 at age 50 years. We also noted that women in the five trajectory groups showed distinctive lifestyle patterns: those in the medium-stable and lean-moderate increase groups were more physically active, were less likely to have hypertension or hypercholesterolemia, or to be aspirin users (Table 3).
Figure 2.
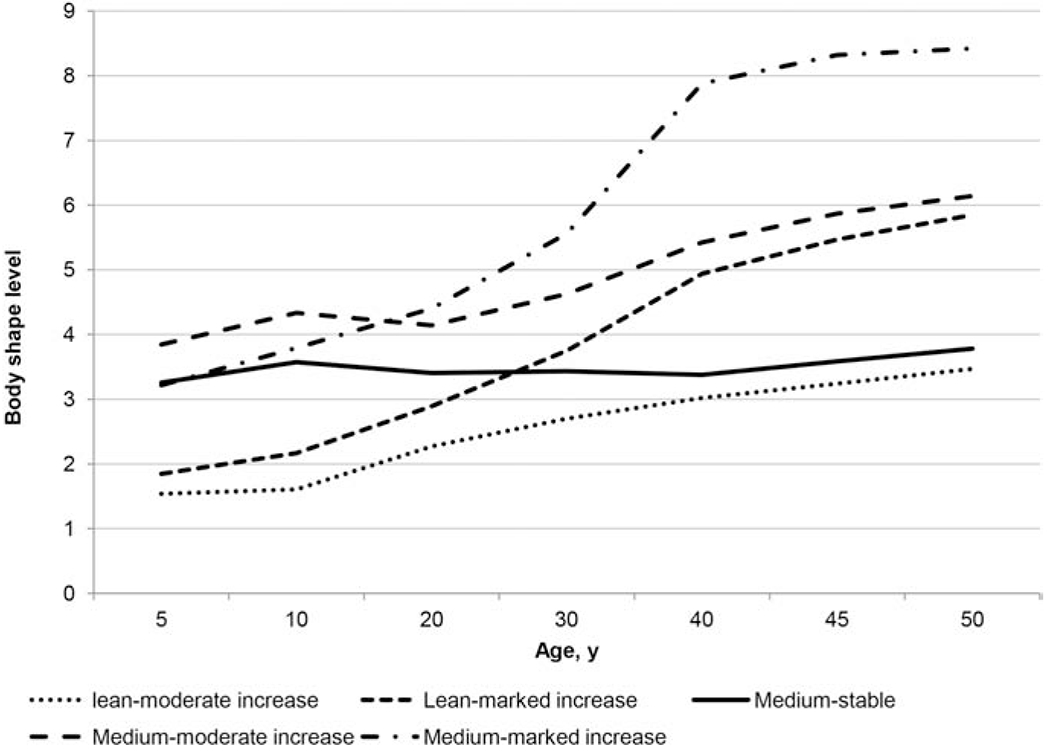
Trajectories of body shape by age among women from the Nurses’ Health Study II. We identified distinct trajectories of body shape across the life span using a group-based modeling approach.
Table 3.
Baseline characteristics of study participants at age 50 according to trajectories of body shape
| Variable | Medium-stable | Lean-moderate increase | Lean-marked increase | Medium-moderate increase | Medium-marked increase |
|---|---|---|---|---|---|
| No. of participants (%) | 17994 (28.1) | 22184 (34.7) | 10705 (16.7) | 7464 (11.7) | 5588 (8.7) |
| BMI at age 18, kg/m2 | 21.1 (2.2) | 19.2 (1.7) | 21.1 (2.2) | 23.7 (3.3) | 25.9 (4.7) |
| BMI at age 50, kg/m2 | 24.0 (2.4) | 23.0 (2.4) | 30.5 (2.9) | 31.4 (3.1) | 40.7 (5.4) |
| Physical activity, METs | 23.4 (23.2) | 23.4 (23.2) | 17.9 (17.7) | 18.0 (18.2) | 13.5 (13.6) |
| Current smoker, % | 7.8 | 6.7 | 6.8 | 8.5 | 6.3 |
| History of diabetes, % | 35.3 | 27.8 | 26.9 | 32.8 | 49.3 |
| History of hypertension, % | 24.7 | 26.3 | 51.2 | 50.7 | 71.1 |
| History of hypercholesterolemia, % | 46.4 | 48.3 | 64.2 | 64.9 | 61.5 |
| Alternate Healthy Eating Index score | 53.9 (10.6) | 51.2 (9.8) | 55.8 (10.6) | 53.0 (9.9) | 49.5 (9.6) |
| Regular aspirin use, 1995,† % | 11.8 | 11.2 | 13.3 | 13.4 | 15.5 |
| Current menopausal hormone therapy,‡ % | 19.5 | 20.4 | 20.8 | 18.7 | 15.8 |
| Race (white),% | 97.3 | 95.8 | 95.3 | 97.4 | 96.4 |
BMI, body mass index; MET, metabolic equivalent of tasks.
All values are standardized to the age distribution of the study population. Values are means (SD) unless stated otherwise.
Regular aspirin use was defined as the regular use of at least 2 aspirin pills per week.
Among postmenopausal participants.
Table 4 presents the HRs for incident NAFLD according to body shape trajectories over the lifespan. Compared to women with a medium-stable trajectory, the age-adjusted HRs were, 1.11 (95% CI, 0.98-1.27) for those with a lean-moderate increase trajectory, 3.61 (95% CI, 3.19-4.08) for lean-marked increase, 3.21 (95% CI, 2.81-3.66) for medium-moderate increase, and 4.68 (95% CI, 4.10-5.34) for medium-marked increase. These associations were consistent after multivariable adjustment (corresponding aHRs of, 1.08 [95% CI, 0.94-1.23] for lean-moderate increase, 2.84 [95% CI, 2.50-3.22] for lean-marked increase, 2.60 [95% CI, 2.27-2.98] for medium-moderate increase, and 3.39 [95% CI, 2.95-3.89] for medium-marked increase) (Table 4). In the nested models, body shape trajectories were found to significantly modify the associations between early adulthood BMI (at age 18 years) or mid-life BMI (at age 50 years) and incident NAFLD risk (both log-likelihood P <0.0001).
Table 4.
Relative risk of NAFLD according to trajectories of body shape
| Medium-stable | Lean-moderate increase | Lean-marked increase | Medium-moderate increase | Medium-marked increase | |
|---|---|---|---|---|---|
| Cases, No. | 378 | 522 | 807 | 511 | 551 |
| Age-adjusted HR (95% CI) | 1 (Reference) | 1.11 (0.98-1.27) | 3.61 (3.19-4.08) | 3.21 (2.81-3.66) | 4.68 (4.10-5.34) |
| Multivariable HR (95% CI)† | 1 (Reference) | 1.08 (0.94-1.23) | 2.84 (2.50-3.22) | 2.60 (2.27-2.98) | 3.39 (2.95-3.89) |
Adjusted for smoking status (never or past/current), total calories (kcal, quintiles), menopausal hormone therapy (never, past, current, premenopausal), Alternate Healthy Eating Index score (quintiles, missing), physical activity (quintiles), type 2 diabetes (yes or no), hypertension (yes/no), dyslipidemia (yes or no), physical activity (MET-hours/week, quintiles), regular use of aspirin (yes/no), and race (white/non-white).
Visceral adiposity
Increasing WC and WHR were both significantly and positively associated with the risk of incident NAFLD (Supplementary table 2). Compared to women in the lowest quintile, the multivariable aHR in the highest WC and WHR quintiles were 5.26 (95% CI, 4.51-6.14; P for trend < 0.0001), and 2.94 (95% CI, 2.59-3.33; P trend < 0.0001), respectively. These significant positive associations were not materially altered after further adjusting for updated BMI (both P for trend <0.0001).
To directly evaluate the relative importance of attained BMI compared to visceral adiposity, we classified participants according to combined categories of updated BMI (< 25 vs ≥ 25 kg/m2) and WC (<35 vs. ≥35 inches) or WHR (<0.8 vs ≥0.8).[28] Compared to women with BMI < 25 kg/m2 and WC < 35 inches or WHR < 0.8, the highest NAFLD risk was observed in those with BMI ≥ 25 kg/m2 and either WC ≥35 inches (multivariable aHR 5.44, 95% CI 4.90-6.05) or and WHR ≥ 0.8 (multivariable aHR 6.00, 95% CI 5.28-6.81) (Supplementary table 3). Significant excess risk was also observed among women who were lean (BMI <25 kg/m2) but who had an elevated WC (multivariable aHR 2.27, 95% CI 1.79-2.89) or WHR (multivariable aHR 1.85, 95% CI 1.56-2.19) (Supplementary table 3).
Sensitivity Analyses
Our findings were consistent across all sensitivity analyses, including: (1) after excluding anyone reporting incident viral hepatitis B or C infections during follow-up (Supplementary table 4); (2) after excluding participants who were underweight (BMI <17 kg/m2) at age 18 years or during follow-up (Supplementary table 5); and (3) after excluding participants with <75% likelihood of being in a given body shape trajectory category (Supplementary table 6).
Discussion
In this large prospective cohort of US women, both excess adiposity and weight gain since early adulthood were associated with a significantly higher risk of developing incident NAFLD in mid-life. The significant, positive association between early adulthood weight gain and NAFLD risk was independent of attained BMI, and it was evident even with as little as 6 kg of weight gain since early adulthood. Furthermore, using a trajectory approach, we identified five distinct subgroups of participants according to their body-shape evolution over the life course. We found that women with a medium body shape in early life and who subsequently gained marked weight between ages 5 and 50 years had the highest NAFLD risk, whereas women who maintained a medium body shape throughout life has the lowest overall risk. Importantly, being lean in early life did not counteract the adverse effects of subsequent weight gain; compared to adults who maintained a medium body shape throughout life, those who were initially lean in early life but subsequently gained substantial weight had a 2.8-fold higher risk of developing NAFLD, later in life. Together, our findings demonstrate that dynamic patterns of weight are significant, modifiable risk factors for NAFLD, which are independent of traditional measures of attained adiposity. These data, in turn, support the development of public health strategies focused on maintaining a stable body weight and shape throughout life, by preventing weight gain, for the prevention of NAFLD.
In the general population, both obesity in early adulthood and weight gain since early adulthood have been related to premature morbidity and mortality.[43, 44] However, much less is known about the impact of dynamic weight changes during on the subsequent risk of NAFLD. In this cohort, both a higher early adulthood BMI and subsequent adulthood weight gain were each independent predictors of NAFLD. The highest NAFLD risk was found in women who had an elevated BMI and who gained substantial weight during adulthood. However, early adulthood weight gain was independently and significantly associated with excess risk of developing NAFLD, across all levels of underlying BMI. Moreover, even women who were lean in early adulthood but who subsequently gained significant weight were found to be at increased risk. Thus, our findings demonstrate that dynamic, long-term patterns of weight change add significant, additional information about NAFLD risk, beyond traditional measures of attained adiposity.
Although BMI is the most commonly studied measurement of attained adiposity, it is an imperfect marker because people with similar BMI values can have substantially different risks of adiposity-related morbidity and mortality.[45] In contrast, visceral adiposity might better capture metabolic and cardiovascular risk[46, 47], particularly among patients who are lean. Visceral fat is closely correlated with hepatic steatosis, as visceral adipose tissue is more lipolytically active than subcutaneous fat[48], and contributing to chronic inflammation[49] and to the pathogenesis of NAFLD.[15] Despite this, to date, prospective, population-based data that comprehensively evaluate the relative impact of visceral adiposity (i.e. WC or WHR) and BMI on the risk of NAFLD accounting for key risk factors including diet and lifestyle factors are limited. In the current study, we found that both WC and WHR were significantly associated with the risk of NAFLD, regardless of underlying BMI. Even among women with a normal BMI, the risk of NAFLD was significantly elevated in those who also had an elevated WC or an elevated WHR. These findings lend further support to the hypothesis that fat distribution and visceral adipose tissue play a key role in the pathogenesis of NAFLD.
Our study is strengthened by the prospective design, large sample size, and high rates of follow-up over 20 years. The prospective design minimized recall bias, and any errors in recall would most likely have attenuated rather than exaggerated a true association. Including repeated anthropometric measurements across adulthood provided a unique opportunity to prospectively examine the long-term influence of adiposity and dynamic weight changes on subsequent NAFLD risk. We have also validated NAFLD cases against established radiographic and/or histologic criteria, with a positive predictive value was 88%. Furthermore, the cohort benefits from detailed, well-validated and updated information on numerous known and putative NAFLD risk factors, including diet, lifestyle and menopausal status, which minimize potential residual confounding.
Certain limitations of this study should be noted. First, participants in this study were all female health professionals, and the majority were white. Thus, the generalizability of our findings to other groups may be limited, and this underscores the need for additional large-scale, prospective studies in racially and ethnically diverse populations of both men and women. Second, despite our validation of NAFLD diagnoses, misclassification is possible because the ascertainment of NAFLD was based on self-reported and recalled information. However, the participants were health care professionals who are more likely to accurately report the information, and the high validity of their self-reported medical information has been confirmed in various studies.[50–52] Furthermore, the relative homogeneity of this cohort could limit confounding by social class, which is an increasingly important factor for both obesity and NAFLD risk. Third, we lacked detailed information regarding NAFLD fibrosis stages, and further research will be needed to assess long-term changes in body weight and BMI in relation to NAFLD fibrosis severity. Fourth, while reverse causation is possible, our results were similar in analyses that included long durations of elapsed time between exposure assessment and the development of NAFLD. Finally, anthropometric measurements including early adulthood weights were also self-reported or recalled; however, the high validity and reproducibility of these measurements in this cohort has previously been established.[24, 27]
In conclusion, within a large, nationwide cohort of women, early adulthood weight gain was significantly and independently associated with an increased risk of developing NAFLD in mid-life. Furthermore, the trajectory of body shape between early- and mid-life was also significantly associated with incident NAFLD, regardless of underlying BMI. Given the increasing prevalence of NAFLD, our findings support the development of public health strategies to maintain a healthy weight and body shape throughout life for the prevention of NAFLD.
Supplementary Material
Highlights.
The influence of dynamic changes in adiposity over the life course on NAFLD risk remains poorly understood.
Early adulthood weight gain was independently associated with an increased risk of developing NAFLD in mid-life.
The trajectory of body shape between early- and mid-life was significantly associated with incident NAFLD, regardless of underlying BMI.
Maintaining both lean and stable weight throughout life may offer the greatest benefit for the prevention of NAFLD.
Acknowledgments
Grant support
UM1 CA186107 (Nurses’ Health Study infrastructure grant)
K24 DK 098311 (ATC)
K23 DK122104 (TGS)
ATC is a Stuart and Suzanne Steele MGH Research Scholar
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Chan has previously served as a consultant for Bayer Pharma AG for work unrelated to this manuscript. The remaining authors have no disclosures and no conflicts of interest to disclose.
References
- [1].Wong RJ, Liu B, Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross-sectional analysis of 2011-2014 National Health and Nutrition Examination Survey. Alimentary pharmacology & therapeutics. 2017;46:974–80. [DOI] [PubMed] [Google Scholar]
- [2].Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–55. [DOI] [PubMed] [Google Scholar]
- [3].Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology (Baltimore, Md). 2015;62:1723–30. [DOI] [PubMed] [Google Scholar]
- [4].Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology (Baltimore, Md). 2013;57:1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. [DOI] [PubMed] [Google Scholar]
- [6].O’Brien J, Powell LW. Non-alcoholic fatty liver disease: is iron relevant? Hepatology international. 2012;6:332–41. [DOI] [PubMed] [Google Scholar]
- [7].Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism: clinical and experimental. 2019;92:82–97. [DOI] [PubMed] [Google Scholar]
- [8].Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism: clinical and experimental. 2020:154170. [DOI] [PubMed] [Google Scholar]
- [9].Yoo JJ, Kim W, Kim MY, Jun DW, Kim SG, Yeon JE, et al. Recent research trends and updates on nonalcoholic fatty liver disease. Clinical and molecular hepatology. 2019;25:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, et al. Associations of Weight Gain From Early to Middle Adulthood With Major Health Outcomes Later in Life. JAMA. 2017;318:255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Obesity Epidemiology. New York. NY: Oxford University Press; 2008. [Google Scholar]
- [12].Maclure KM, Hayes KC, Colditz GA, Stampfer MJ, Speizer FE, Willett WC. Weight, diet, and the risk of symptomatic gallstones in middle-aged women. The New England journal of medicine. 1989;321:563–9. [DOI] [PubMed] [Google Scholar]
- [13].Song M, Hu FB, Spiegelman D, Chan AT, Wu K, Ogino S, et al. Adulthood Weight Change and Risk of Colorectal Cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Cancer Prev Res (Phila). 2015;8:620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md). 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- [15].van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology (Baltimore, Md). 2008;48:449–57. [DOI] [PubMed] [Google Scholar]
- [16].Song M, Willett WC, Hu FB, Spiegelman D, Must A, Wu K, et al. Trajectory of body shape across the lifespan and cancer risk. International journal of cancer. 2016;138:2383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Song M, Hu FB, Wu K, Must A, Chan AT, Willett WC, et al. Trajectory of body shape in early and middle life and all cause and cause specific mortality: results from two prospective US cohort studies. BMJ (Clinical research ed). 2016;353:i2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].His M, Le Guelennec M, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F, Fagherazzi G. Life course evolution of body size and breast cancer survival in the E3N cohort. 2018;142:1542–53. [DOI] [PubMed] [Google Scholar]
- [19].Zheng Y, Song M, Manson JE, Giovannucci EL, Hu FB. Group-Based Trajectory of Body Shape From Ages 5 to 55 Years and Cardiometabolic Disease Risk in 2 US Cohorts. American journal of epidemiology. 2017;186:1246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. Jama. 2011;306:1884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–97.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu PH, Burke KE, Ananthakrishnan AN, Lochhead P, Olen O, Ludvigsson JF, et al. Obesity and Weight Gain Since Early Adulthood Are Associated With a Lower Risk of Microscopic Colitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2019;17:2523–32.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chalasani N, Younossi Z. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- [24].Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology (Cambridge, Mass). 1990;1:466–73. [DOI] [PubMed] [Google Scholar]
- [25].Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell reports. 2016;15:857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chiuve SE, Sun Q, Sandhu RK, Tedrow U, Cook NR, Manson JE, et al. Adiposity throughout adulthood and risk of sudden cardiac death in women. JACC Clinical electrophysiology. 2015;1:520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19:570–2. [PubMed] [Google Scholar]
- [28].Ma W, Jovani M, Liu PH, Nguyen LH, Cao Y, Tam I, et al. Association Between Obesity and Weight Change and Risk of Diverticulitis in Women. Gastroenterology. 2018;155:58–66.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Research publications - Association for Research in Nervous and Mental Disease. 1983;60:115–20. [PubMed] [Google Scholar]
- [30].Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. American journal of epidemiology. 1993;138:56–64. [DOI] [PubMed] [Google Scholar]
- [31].Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. American journal of epidemiology. 2017;185:570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. The American journal of clinical nutrition. 2002;76:1261–71. [DOI] [PubMed] [Google Scholar]
- [33].Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. The Journal of nutrition. 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bullon-Vela V, Abete I, Tur JA, Pinto X, Corbella E, Martinez-Gonzalez MA, et al. Influence of lifestyle factors and staple foods from the Mediterranean diet on non-alcoholic fatty liver disease among older individuals with metabolic syndrome features. Nutrition (Burbank, Los Angeles County, Calif). 2019;71:110620. [DOI] [PubMed] [Google Scholar]
- [35].Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. International journal of epidemiology. 1994;23:991–9. [DOI] [PubMed] [Google Scholar]
- [36].Higuchi LM, Khalili H, Chan AT, Richter JM, Bousvaros A, Fuchs CS. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. The American journal of gastroenterology. 2012;107:1399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, et al. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. American journal of epidemiology. 1987;126:319–25. [DOI] [PubMed] [Google Scholar]
- [38].Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. The New England journal of medicine. 2012;367:1596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, et al. Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Annals of internal medicine. 1998;129:517–24. [DOI] [PubMed] [Google Scholar]
- [40].Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. CA: a cancer journal for clinicians. 2010;60:207–21. [DOI] [PubMed] [Google Scholar]
- [41].Song M Trajectory analysis in obesity epidemiology: a promising life course approach. Current opinion in endocrine and metabolic research. 2019;4:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].DS. N. Group-based modeling of development. Harvard University Press; 2005. [Google Scholar]
- [43].Engeland A, Bjorge T, Tverdal A, Sogaard AJ. Obesity in adolescence and adulthood and the risk of adult mortality. Epidemiology (Cambridge, Mass). 2004;15:79–85. [DOI] [PubMed] [Google Scholar]
- [44].Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. The New England journal of medicine. 1992;327:1350–5. [DOI] [PubMed] [Google Scholar]
- [45].Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, Loos RJF, et al. Obesity. Nature reviews Disease primers. 2017;3:17034. [DOI] [PubMed] [Google Scholar]
- [46].Patel NS, Doycheva I, Peterson MR, Hooker J, Kisselva T, Schnabl B, et al. Effect of weight loss on magnetic resonance imaging estimation of liver fat and volume in patients with nonalcoholic steatohepatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015;13:561–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Alimentary pharmacology & therapeutics. 2012;36:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Einstein FH, Atzmon G, Yang XM, Ma XH, Rincon M, Rudin E, et al. Differential responses of visceral and subcutaneous fat depots to nutrients. Diabetes. 2005;54:672–8. [DOI] [PubMed] [Google Scholar]
- [49].Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003;112:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Strate LL, Liu YL, Aldoori WH, Giovannucci EL. Physical activity decreases diverticular complications. The American journal of gastroenterology. 2009;104:1221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Strate LL, Liu YL, Aldoori WH, Syngal S, Giovannucci EL. Obesity increases the risks of diverticulitis and diverticular bleeding. Gastroenterology. 2009;136:115–22.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Strate LL, Liu YL, Syngal S, Aldoori WH, Giovannucci EL. Nut, corn, and popcorn consumption and the incidence of diverticular disease. Jama. 2008;300:907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


