Abstract
RNAi-based technologies have shown biomedical potential; however, safe and efficient delivery of RNA remains a barrier for their broader clinical applications. Nucleic acid nanoparticles (NANPs) programmed to self-assemble and organize multiple therapeutic nucleic acids (TNAs) also became attractive candidates for diverse therapeutic options. Various synthetic nanocarriers are used to deliver TNAs and NANPs, but their clinical translation is limited due to immunotoxicity. Exosomes are cell-derived nanovesicles involved in cellular communication. Due to their ability to deliver biomolecules, exosomes are a novel delivery choice. In this study, we explored the exosomemediated delivery of NANPs designed to target GFP. We assessed the intracellular uptake, gene silencing efficiency, and immunostimulation of exosomes loaded with NANPs. We also confirmed that interdependent RNA/DNA fibers upon recognition of each other after delivery, can conditionally activate NF-kB decoys and prevent pro-inflammatory cytokines. Our study overcomes challenges in TNA delivery and demonstrates future studies in drug delivery systems.
Keywords: Extracellular vesicles, exosomes, RNA nanotechnology, NANPs, immunostimulation
Graphical abstract
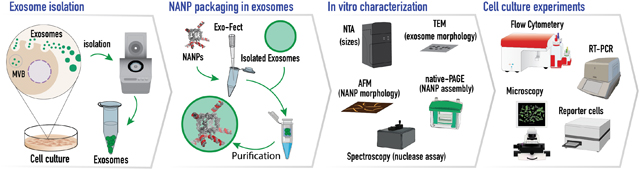
Graphical abstract illustrates the experimental design of the current work. Exosomes were isolated, packaged with functionalized NANPs and characterized in in vitro and cell culture experiments, demonstrating a novel TNA delivery system.
INTRODUCTION
Nucleic acid nanoparticles (NANPs) are modular nanoscaffolds composed of multiple oligonucleotides programmed to self-assemble into precise 3D structures with well-defined properties (1–4). Rationally designed NANPs can be further decorated with cocktails of therapeutic nucleic acids (TNAs), which may differ in composition, secondary structure, and mechanism of action, allowing for synchronized targeting of multiple cellular pathways. This structural versatility, and the ability to finely control the NANPs’ sizes, shapes, composition, multivalences, and therapeutic payloads, makes this technology an attractive option for biomedical applications (5–7). For example, a previously tested combinatorial RNAi strategy of NANPs functionalized with six different Dicer Substrate (DS) RNAs (8) were effective in simultaneous targeting six distinct parts of the HIV-1 genome (1, 9). These NANPs functionalized with DS RNAs relied on the assistance from the intracellular enzyme Dicer that initiated the nuclease-assisted release of siRNAs from the NANPs. Moreover, by simply extending either the 5’- or/and 3′- ends of each strand of the NANP’s composition with its unique functionality, NANPs can be formulated to precisely package not only different TNAs but also fluorophores, targeting agents, small-molecule drugs, proteins, or other therapeutic cargoes (10–12).
A sophisticated approach to the conditional intracellular activation of RNAi was introduced through interdependent RNA/DNA NANPs in which the RNA (and/or DNA) functionalities were split into two inactive NANPs (13–15). Driven by sequence complementarity, the inactive NANPs could recognize each other inside the cells and release the activated functionalities upon isothermal reassociation (2, 9, 16). Using the same approach, RNA/DNA fibers were recently designed for enhanced control of deliverable functionalities (e.g., DS RNAs targeting mutated BRAFV600E in melanoma cells) along with higher stability against enzymatic degradation in human blood and tunable rates of intracellular reassociation (17). Another advantage of this user-friendly approach: the DNA part of the RNA/DNA fibers contained split NF-kB (nuclear factor kappa-light-chain enhancer of activated B cells) decoy sequences, which activate upon intracellular fiber reassociation and restrain the immunostimulatory response. NF-kB is expressed in most mammalian cells and remains sequestered in an inactive state in the cytoplasm with the inhibitory protein IκB. Activation of NF-kB is initiated by a variety of stimuli, resulting in signal-induced degradation of IκB proteins by proteasomes. Subsequently, the NF-κB complex can enter the nucleus and initiate gene expression of pro-inflammatory cytokines. NF-κB decoys (18–20) mimic the κB consensus sequence and upon the decoys binding to NF-κB translocate into the nucleus is prevented.
Since all NANPs are composed of negatively charged, hydrophilic biopolymers, they cannot easily penetrate through biological membranes and mainly rely on the use of different synthetic carriers for efficient cell internalization (17, 21–25). Over the last few decades, extensive research efforts have been undertaken to improve the stability, efficacy, and specificity of TNA delivery systems, such as liposomes (26), micelles (27), nanoparticles (28), hydrogels (29), and viruses (30). In spite of these attempts, most of these systems suffer from immunogenicity, cytotoxicity, rapid blood clearance, and poor biodistribution (31), hindering further clinical translation of therapeutic NANP platforms. Therefore, an urgent need to identify new endogenous sources for potential delivery systems that avoids the complications associated with synthetic materials is vital.
Extracellular vehicles (EVs) are membrane enclosed vesicles ranging from 30 nm to 1,000 nm (or even larger) in size and secreted by cells into the extracellular space (32, 33). EVs comprise of exosomes and microvesicles, which are differentiated by their biogenesis, content, size, release pathways, and function (34). Exosomes have been widely investigated as emerging novel delivery vehicles for biological cargos. These structures range from 30 nm to 150 nm in size (35) and are released into most biological fluids. Biological molecules, such as proteins, lipids, and nucleic acids, undergo transport via exosomes from the cell of origin to recipient cells (36). Exosomes form by inward budding of the cell membrane, a process which generates early endosomes. After the endosomes mature into multivesicular bodies (MVB), they fuse with the cell membrane and exit from the cell; alternatively, they can fuse with a lysosome for degradation. (Fig. 1A) (37). Reflective of the mechanism of biogenesis, exosomes are considered to be the “mini versions” of their parental cells in terms of the specially sorted “cargo” they carry (38). The putative function of exosomes is to perform intercellular communication and trigger physiological responses. Interaction between exosomes and recipient cells is promoted via receptor-mediated endocytosis (39), micropinocytosis (40), or membrane fusion (41). Once internalized, the exosomal contents are released into the cytosol directly or through back-fusion with the endosomal membrane, eliciting the effect on the recipient cell (42). Such features of the exosomes highlight their potential as drug delivery systems to alter gene expression by delivering therapeutic genetic materials. Several studies have investigated their performance as therapeutic vehicles for exogenous genetic material delivery as well as their post-delivery functionality. The first published study showed that exosomes could efficiently deliver exogenous siRNAs to the brains of mice with successful knockdown of mRNA and BACE1 protein with negligible immune response (43). Later, a myriad of studies reported using exosomes to deliver siRNAs and knockdown targeted genes in different diseases. For example, exosome-mediated delivery has shown promise in silencing specific genes, including VEGF, EGFR, AKT, MAPK, and KRAS (44); inhibiting luciferase expression (45); treating hepatitis C infection; causing massive cell death of recipient cells by RAD51 gene suppression (46); and survivin siRNA suppression for the treatment of bladder cancer (47). Recently, exosomes derived from normal fibroblast-like mesenchymal cells were engineered to carry shRNAs or siRNAs against the KrasG12D mutation known for triggering pancreatic cancer growth in multiple mouse models (48). The use of exosomes as delivery vehicles demonstrates a plethora of advantages, including biocompatibility, efficacy, stability, and membrane permeation capability; as well as diminished toxicity; low immunogenicity; low off-target effect; and the ability to cross the blood-brain-barrier (31, 49–53). As a result, exosomes can significantly outperform other delivery methods.
Figure 1:
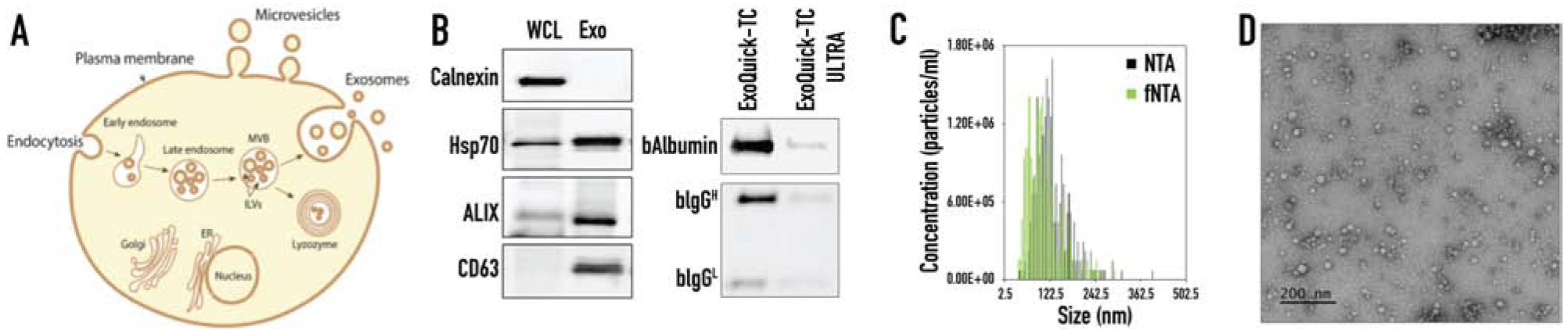
Characterization of exosomes isolated from MDA-MB-231 cells. (A) Schematic representation of exosome formation and release out to the extracellular space. (B) Western blot analysis of exosomal markers for CD63, ALIX and Hsp70, and cellular ER marker Calnexin in the whole cell lysate (WCL) and ExoQuick-TC ULTRA isolated exosomes (Exo). Western Blot analysis of the co-isolating proteins, bovine albumin (bAlbumin) and bovine immunoglobulin (bIgG) after one isolation step of precipitation step with ExoQuick-TC and after two steps of precipitation with ExoQuick-TC ULTRA. (C) The size distribution of isolated exosomes showing concentration in particles/mL. (D) TEM analysis of isolated exosomes.
In this current work, we investigated the use of exosomes as carriers for delivering functional NANPs of different shapes, sizes, and compositions, among which were globular RNA cubes, planar RNA rings, and linear RNA/DNA fibers. We assessed, in vitro, the stability of exosome-loaded NANPs, in addition to their intracellular uptake, silencing efficiency, and immunostimulatory activity.
METHODS
All experimental details and sequences used in this work are listed in the Supplementary Material.
Cell culture
MDA-MB-231-GFP, MDA-MB-231 and HUVEC cells were used in this study. All oligonucleotides were purchased from Integrated DNA Technologies (IDTDNA.com, Coralville, Iowa, USA). All NANP compositions used in this project are listed in the Supplementary Material Table 1.
Assemblies of RNA/DNA fibers, RNA cubes and RNA rings targeting GFP and analysis using native-PAGE
All individual strands were purified by an 8 M urea polyacrylamide gel (8% acrylamide, 19:1), gel extracted, eluted and dissolved in endotoxin-free water. All individual RNA strands for cube and rings were synthesized via in vitro run-off transcription (IVT) assay, purified by an 8 M urea polyacrylamide gel (8% acrylamide, 19:1), gel extracted, eluted and dissolved in endotoxin-free water. RNA/DNA fibers, RNA cubes, and RNA rings were assembled in one-pot by combining individual monomers at equimolar concentrations in an assembly buffer. All NANPs were analyzed on 8% non-denaturing native polyacrylamide gel (19:1 for fibers, 37.5:1 for rings and cubes).
Atomic force microscopy (AFM) imaging of NANP
A freshly cleaved mica surface modified with APS (1-(3-aminopropyl) silatrane) was used for AFM imaging, which was performed on the MultiMode AFM NanoScope IV system (Bruker Instruments, Santa Barbara, CA, USA) in tapping mode.
Nuclease protection assay of DNA duplex
125 nM dsDNA labeled with Alexa Fluor 488 at the 5′ sense strand and an Iowa Black Quencher at the 3′ antisense strand was loaded into exosomes, then treated with RQ1 DNase I. The fluorescence resulting from the digestion of the DNA duplex by RQ1 DNase was monitored at 37°C for a total of 60 minutes with measurements at every 30 seconds. Free fluorescently quenched DNA duplex was used as the control.
Isolation of exosomes
Exosomes were isolated from MDA-MB-231 or HUVEC cells using ExoQuick-TC ULTRA (System Biosciences, Palo Alto, CA, USA) according to the manufacturer’s manual. Protein concentration was measured by Qubit protein assay and all exosomes were stored at −20°C.
Immunoblotting
5 μg of isolated exosomes were run on 4–20% Mini-PROTEAN TGX gel, transferred to PVDF membrane and probed with primary antibodies over-night. Next day, the membranes were washed three times, probed with appropriate secondary antibody and imaged using Molecular Imager ChemiDOC XRS+ Imaging System (Bio-Rad, Hercules, CA, USA).
Nanoparticle tracking analysis and exosomes labeling for f-NTA
Isolated exosomes were labeled with the ExoGlow-NTA fluorescent kit (System Biosciences, Palo Alto, CA, USA). Labeled exosomes were stored at −20°C until the analysis. Unlabeled and labeled samples were analyzed by NTA and f-NTA using ZetaView.
TEM analysis
Isolated exosomes were fixed by addition of 4% paraformaldehyde, 5 μl of the sample was dropped on a carbon coated 400 mesh Cu/Rh grid (Ted Pella, Redding, CA, USA) and stained with 5 μl of 1% uranyl acetate (Polysciences, Warrington, PA, USA) prepared in filtered distilled water. The grids were imaged with a FEI Talos L120C TEM with Gatan 4k × 4 k OneView camera.
Loading of exosomes with NANPs
100 μg of isolated exosomes were loaded with 25 pmoles NANPs using the Exo-Fect siRNA/miRNA transfection kit (System Biosciences, Palo Alto, CA, USA) according to the manufacturer’s manual. The loaded exosomes were cleaned to remove any excess of free NANPs/negative control siRNA, transfection reagent, or complexes. The cleaned NANP loaded exosomes were immediately added onto MDA-MB-231-GFP cells.
RNA purification and quantitative real time PCR (RT-qPCR) analysis
Total RNA was purified using RNeasy Plus Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s manual. Purified total RNA was reverse transcribed using anchor Oligo (dT)20 primer (Thermo Fisher Scientific, Wilmington, DE, USA) and Superscript III reverse transcriptase (Thermo Fisher Scientific, Wilmington, DE, USA). Real time qPCR was performed using TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific, Wilmington, DE, USA) with TaqMan gene expression assay eGFP (Thermo Fisher Scientific, Wilmington, DE, Mr04097229_mr) and with TaqMan gene expression assay Beta-2-microglobulin as a reference gene (Thermo Fisher Scientific, Hs00984230_m1, Wilmington, DE, USA) and the QuantStudio 6 FLEX (Applied Biosystems, Thermo Fisher Scientific, Wilmington, DE, USA).
Flow cytometry
MDA-MB-231-GFP treated cells were trypsinized with 0.25% trypsin-EDTA (Thermo Fisher Scientific, Waltham, MA, USA) for 2 minutes at 37 °C, quenched with DMEM supplemented with 10% FBS, and pelleted by centrifugation at 800xg for 2 minutes. The cell pellet was resuspended with 1xPBS supplemented with 1 % FBS into a 12×75mm test tube with a cell strainer cap (Falcon, Durham, NC, USA) and analyzed with the DxP FACScan (Cytek Biosciences, Fremont, CA, USA). The data was analyzed using FCS Express 5 (De Novo Software, Glendale, CA, USA).
Cell uptake imaging
Cells transfected with NANP loaded exosomes or negative control loaded exosomes were imaged 72 hours post-transfection. For the cancer and primary cells transfected with exosomes loaded with DNA cube-Alexa 488, cells were imaged 24 hours post-transfection. The images were captured using a Leica DMI3000 inverted microscope with a DFC360 FX digital camera (Leica Microsystems, Wetzlar, Germany).
Immunostimulation in vitro
HEK-Blue™ hTLR3 and 7 cells, and THP1-Dual™ cells (Invivogen, San Diego, CA, USA) were used for quantifying the activation pf specific toll-like receptor (TLR) and intracellular signaling pathways, respectively. HEK-Blue™ hTLR 3 and 7 cells are HEK cells engineered to stably co-express human TLR and NF-κB-inducible SEAP (secreted embryonic alkaline phosphatase) reporter genes. The TLR activation results in downstream production of SEAP that can be detected and quantified using QUANTI-Blue™. THP1-Dual™ cells are engineered to express SEAP upon NF-kB stimulation, which can also be assessed by QUANTI-Blue™. In order to investigate the conditional activation of NF-kB decoys, Poly (I:C) (polyinosinic-polycytidylic acid, a synthetic analog of dsRNA) and Pam3CSK4 (a synthetic diacylated lipopeptide) were used to stimulate HEK-Blue™ hTLR3 and THP1-Dual™ cells, respectively. For the experiments with the HEK-Blue™ hTLR7 cells, R848 (resiquimod) was used as a positive control.
RESULTS
Characterization of isolated exosomes.
Extracellular vesicles are cell-derived membrane particles subdivided into: microvesicles, ranging in size from 100–1,000 nm, that are shed from the membrane and exosomes released by fusion of late endosomes with the cell membrane (30–150 nm size range) (Figure 1A) (33–35). Although microvesicles and exosomes are structurally similar and overlap in size, their content and cell origins are different (32). Exosomes secreted by MBA-MD-231(231) were isolated using ExoQuick-TC ULTRA. Prior to the addition of ExoQuick-TC reagent, centrifugation was used to remove cell debris and large particles, such as apoptotic bodies. The isolation method included two steps: (i) precipitation of exosomes with ExoQuick-TC and (ii) their purification with an affinity chromatography column, ULTRA. ExoQuick-TC ULTRA efficiently removes the major non-EV co-isolates, such as bovine albumin and bovine IgG, which usually are overrepresented in the tissue culture media (54). Figure 1B shows high amounts of albumin and IgG after precipitation step and demonstrates significant reduction of the co-isolated after the second step of ULTRA purification. Thus, the use of ExoQuick-TC ULTRA was essential to achieve the higher purity of isolated exosomes.
The ExoQuick-TC ULTRA isolation method yielded a particle concentration of 4.50E+11 ± 6.80E+10 particles/mL as measured by fluorescent NTA (fNTA) and 4.70E+11 ± 5.10E+10 particles/mL as measured by NTA (Figure 1C). Since NTA measures any particle in solution, including protein aggregates and buffer precipitates, we specifically labeled exosomes with a membrane sensor dye and measured the concentration of intact vesicles using fNTA (55, 56). The mean diameter of the isolated exosomes was 103.5 ± 26.4 nm as fNTA and 123.1 ± 8.1 nm as measured by conventional NTA (Figure 1C). A size distribution curve showed a classical distribution of exosomes ranging in size from 30–150 nm. Common exosomal markers Hsp70, ALIX, and tetraspanin, CD63, were identified (Figure 1B). ALIX is an exosomal protein known for its involvement in the MVB biogenesis (32). CD63 was enriched in exosomal membrane from different origins (57, 58). Hsp70 was released into the extracellular space via exosomes as a membrane- bound protein (59). Calnexin is an integral protein of the endoplasmic reticulum (ER) and was used in as a cellular marker. Absence of calnexin marker in the exosome preparation indicated no cellular contamination was present. Transmission electron microscopy (TEM) images of isolated exosomes showed normal morphology without any distortion (Fig. 1D) (60). Altogether, we conclude that the isolated sample was enriched in exosomes based on our results.
Characterization of NANP loaded exosomes and nuclease protection assay.
The NANPs used in this study have distinct, strategic designs that confer different compositions, connectivities, shapes, and sizes (61). Both cube and ring RNA scaffolds are assembled from six individual RNAs; cubes are globular (3D), whereas rings are planar (2D). The hydrodynamic radii (based on DLS results) of cubes and rings functionalized with six anti-GFP DS RNAs were estimated to be ~12 and ~15 nm, respectively (16, 62). DNA/RNA fibers are linear (1D) and composed of both DNA and RNA strands. While RNA cubes and RNA/DNA fibers are assembled via intermolecular Watson-Crick base pairing, assembly of RNA rings requires magnesium-dependent, intramolecular Watson-Crick base pairing to facilitate intermolecular kissing loop interactions (63). Although each of these structures has distinct properties, all of them can be functionalized with DS RNAs to silence any gene through sequence specific mRNA targeting. In addition, DNA/RNA fibers are functionalized with NF-kB decoys, inhibiting immune responses through the NF-kB pathway. All NANPs were analyzed by native-PAGE and AFM, as shown in Figure 2A, to confirm the correct assembly.
Figure 2:
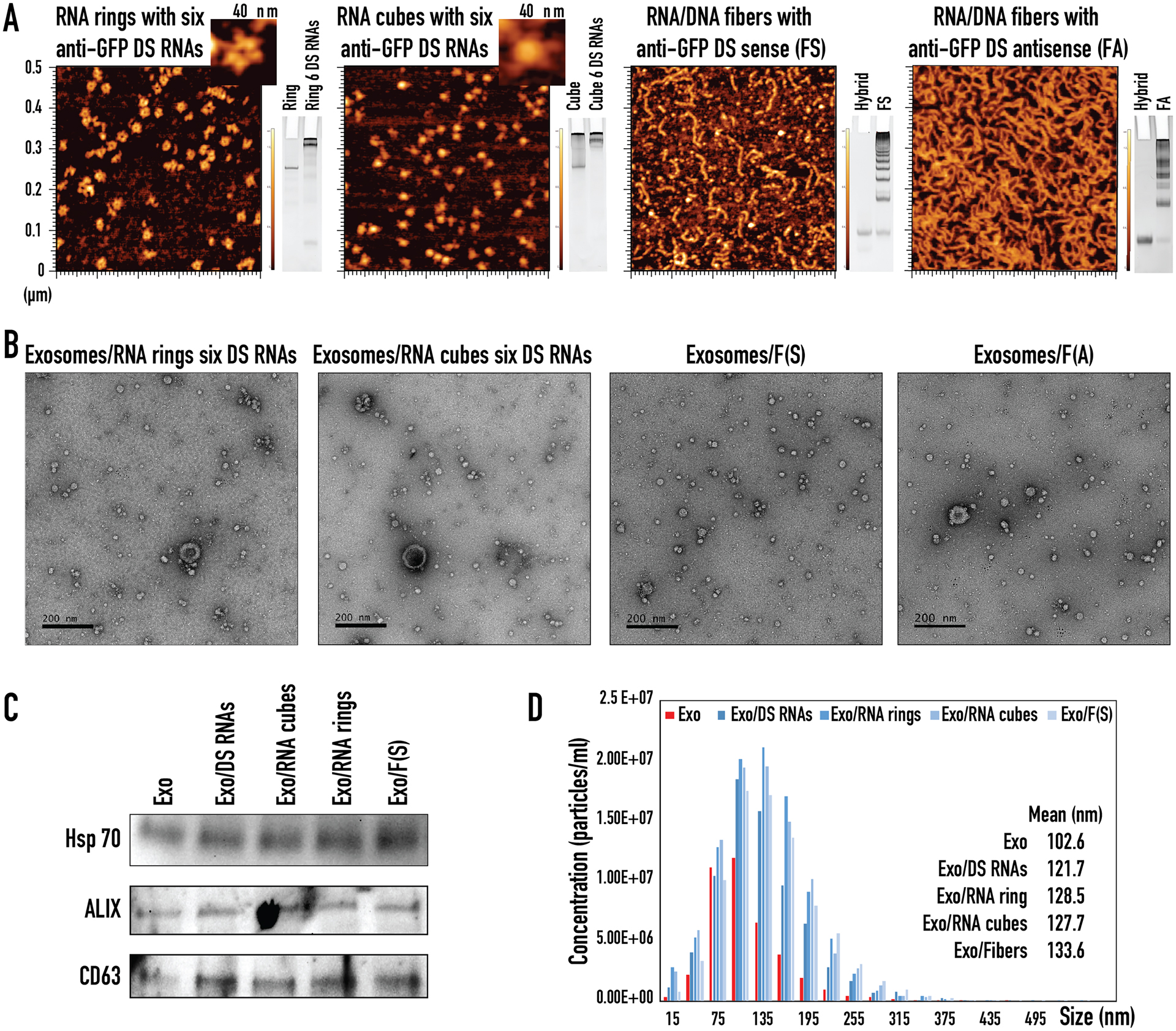
Characterization of various functionalized NANPs and NANP loaded exosomes. (A) AFM images and native-PAGE results of RNA rings with six anti-GFP Dicer Substrate (DS) RNAs, RNA cubes with six anti-GFP DS RNAs, RNA/DNA fibers with anti-GFP DS sense, RNA/DNA fibers with anti-GFP DS antisense. (B) TEM images of exosomes loaded with RNA rings with six anti-GFP DS RNAs, RNA cubes with six anti-GFP DS RNAs, RNA/DNA fibers with anti-GFP DS sense, and RNA/DNA fibers with anti-GFP DS antisense. (C) Western blot analysis of exosomal markers for CD63, ALIX, and Hsp70 in ExoQuick-TC ULTRA isolated exosomes (Exo) taken through the loading steps as negative control and exosomes loaded with anti-GFP DS RNAs (Exo/DS RNAs), RNA cubes with six anti-GFP DS RNAs (Exo/RNA cubes), RNA rings with six anti-GFP DS RNAs (Exo/RNA rings), and RNA/DNA fibers with anti-GFP DS sense (Exo/Fibers). (D) NTA analysis of ExoQuick-TC ULTRA isolated exosomes (Exo) taken through the loading steps as negative control and exosomes loaded with anti-GFP DS RNAs (Exo/DS RNAs), RNA cubes with six anti-GFP DS RNAs (Exo/RNA cubes), RNA rings with six anti-GFP DS RNAs (Exo/RNA rings), and RNA/DNA fibers with anti-GFP DS sense (Exo/Fibers).
To load NANPs into isolated exosomes, Exo-Fect siRNA/miRNA Transfection Kit was used. The Exo-Fect transfection reagent formed a complex with the NANPs and assisted with exosome insertion. Following the transfection, all samples were run through a clean-up column to remove excess Exo-Fect reagent, as well as free NANPs and their complexes. TEM images showed exosomes loaded with functionalized RNA rings, RNA cubes, and RNA/DNA fibers possessed morphology similar to free exosomes (Figure 1D and Figure 2B) with no associated structural changes. To verify the integrity of loaded exosomes, the presence of common exosomal markers (Hsp70, ALIX, and tetraspanin, CD63) was confirmed (Figure 2C). Interestingly, NTA analysis indicated that exosomes originally with mean diameter of ~102–103 nm became larger by ~20–30 nm after loading with NANPs (Figure 2D).
Next, the exosomes’ ability to protect a loaded nucleic acid cargo from nuclease degradation were analyzed using a nuclease protection assay (Figure 3A) developed from previous works (19, 22, 64). As a model system, exosomes loaded with DNA duplexes labeled with Alexa 488 at the 5’-end and Iowa Black quencher at the complementary 3’-end were used. Due to the quencher’s proximity to the fluorophore, the fluorescent signal from the DNA duplex was quenched. However, after RQ1 DNase treatment and subsequent DNA degradation, the fluorophore escaped from the quencher, leading to a progressively increasing fluorescent signal. At the same time, exosome-encapsulated DNA duplexes were protected from DNase digestion and yielded no changes in the fluorescent signal. Indeed, the miniscule increase in fluorescence proves that exosomes can effectively protect nucleic acid cargo from nuclease digestion. Free exosomes were used as controls to show that little to no signal appears in these samples. Results of this experiment suggest that exosomes were able to completely shield their nucleic acid cargos from degradation by enzymatic activity for at least 60 minutes.
Figure 3:
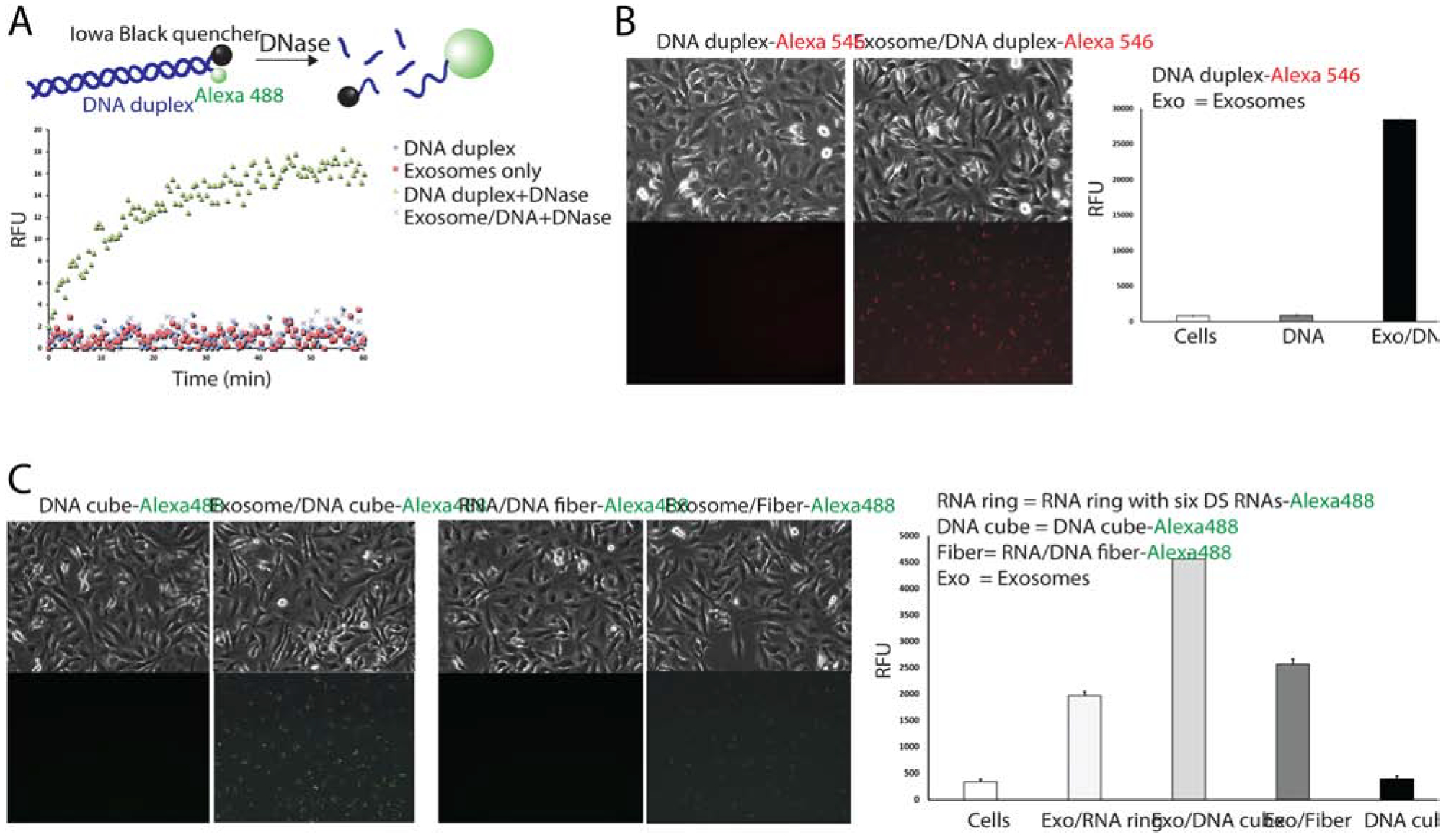
Exosomes protect nucleic acids from enzymatic degradation and promote their cellular uptake. (A) Schematic diagram of nuclease digestion assay and assay results for DNA duplexes loaded exosomes after incubation with RQ1 DNAse. (B–C) Fluorescent microscope images of human breast cancer MDA-MB-231 show cell uptake of fluorescently labeled DNA duplexes (B) and NANPs (C) and the corresponding flow cytometry analysis.
Cellular uptake of exosomes loaded with NANP polyplexes.
To confirm effective delivery of loaded NANPs, exosomes isolated from human breast cancer MDA-MB-231 (231) cells were loaded with either Alexa 488- or Alexa 546-labeled NANPs and added to 231 cells. Fluorescence microscopy images captured 24 hours post-transfection validated the internalization of loaded exosomes into cells (Figure 3B–C). Flow cytometry and microscopy results demonstrated that exosomes loaded with Alexa 546-labeled DNA duplex internalized into the cells and, with no exosomes present, no significant cellular uptake occurred (Figure 3B). Interestingly, flow cytometry results show different transfection efficiencies of exosome-encapsulated Alexa 488-labeled NANPs, indicating that NANP polyplex shapes may potentially affect cellular uptake efficiency (Figure 3C). Overall, data confirmed that NANP-loaded exosomes can be internalized by human cells with visible effects on transfection efficiency. Interestingly, we noticed that the uptake efficiency for the same number of NANP loaded exosomes is significantly lower for primary cell lines when compared to cancer cells (supporting Figure S1). This may offer additional advantages for exosome mediated delivery of NANP, due to the potential reduction in undesired off-target effects in healthy tissues.
GFP gene silencing with functionalized NANP loaded exosomes.
We achieved successful transfection of labeled NANPs into cells using exosomes as delivery vesicles. To confirm retention of the NANPs’ intended function upon delivery, NANPs decorated with DS RNA against GFP (1, 6, 16, 65) were used. Human breast cancer cells MDA-MB-231-GFP expressing GFP (231-GFP) were treated with a panel of exosomes loaded with anti-GFP-functionalized NANP polyplexes (Figure 4A–B). Based on microscopy, flow cytometry, and RT-qPCR results, we found that 231-GFP cells treated with RNA ring- and RNA cube-loaded exosomes demonstrated a marked decrease in GFP expression. Exosomes loaded with sense (FS) or antisense (FA) RNA/DNA fibers did not change GFP expression individually; however, when both exosomes were added onto the same cell, there was a significant decrease in GFP expression. Therefore, fibers can re-associate and release DS RNAs only upon internalization with exosomes. Despite differences in shape, functional RNA cubes, RNA rings, and RNA/DNA fibers performed similarly in GFP silencing experiments (Figure 4A–B), while no cytotoxicity was observed (supporting Figure S2).
Figure 4:
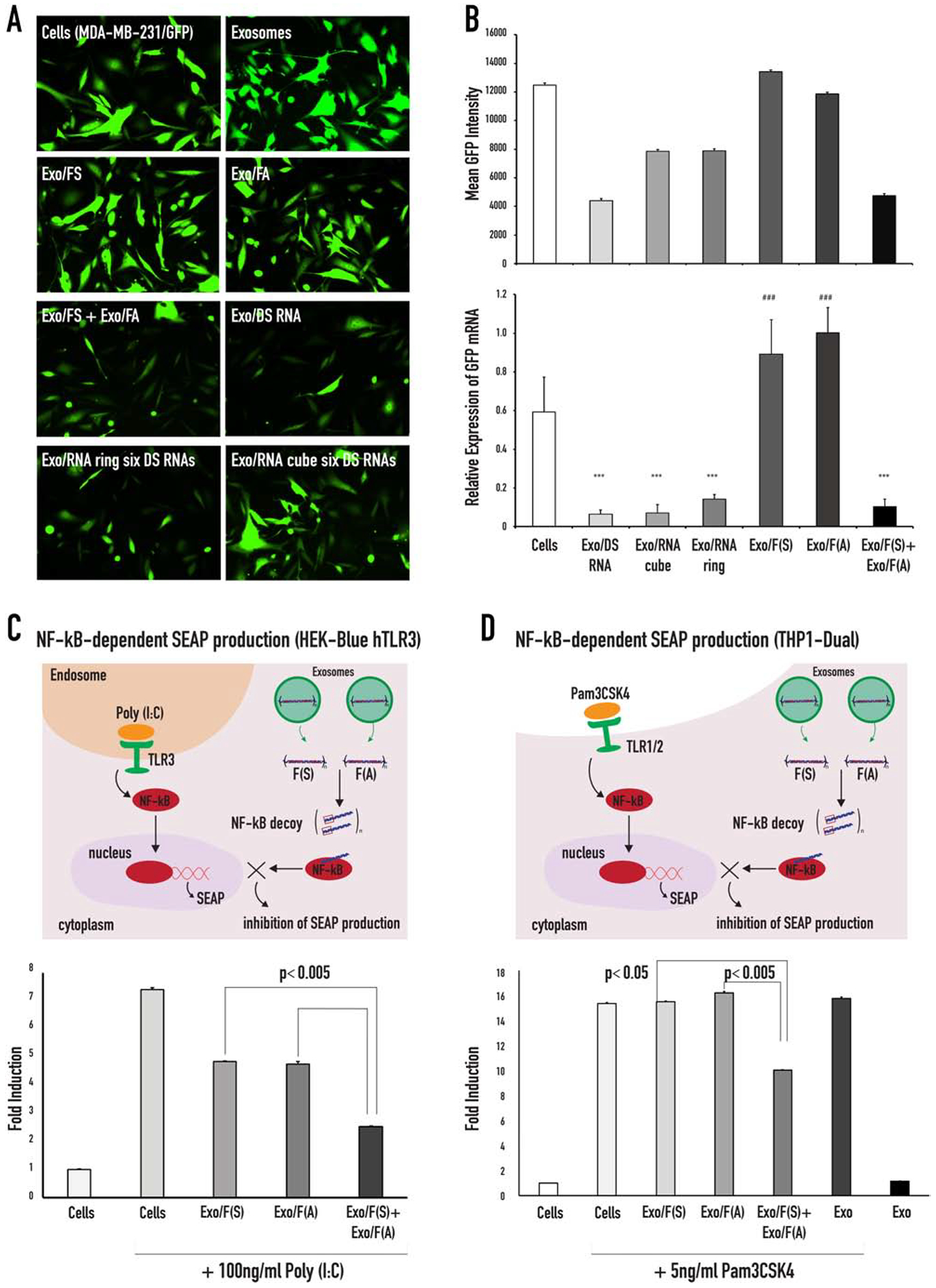
GFP silencing in MDA-MB-231/GFP cells treated with functionalized anti-GFP NANPs loaded into exosomes and inhibition of NF-kB function in HEK-Blue™ hTLR3 and THP1-Dual™ cells. (A) Fluorescent microscope images of MDA-MB-231-GFPs taken 72 hours post treatment with exosomes and exosomes loaded with either NANPs. (B) Flow cytometry data of the mean GFP fluorescence and RT-qPCR analysis of the relative GFP expression of MDA-MB-231-GFP cells after 72 hours of incubation with NANPs loaded exosomes. Statistical significance for the samples compared to the negative control is denoted by * (*** p<0.001). Statistical significance of Exo/FS, Exo/FA compared to Exo/FS+Exo/FA is denoted by # (### p<0.001). (C) Schematic demonstration of the TLR3 signaling that activates the NF-kB pathway and the assembly of the NF-kB decoys (upon re-association of the RNA/DNA fibers) that inhibits the nuclear translocation of the activated NF-kB. Poly (I:C) is used to activate the TLR3 ligand, the TLR3 signal resulted in the activation of NF-kB and subsequently NF-kB entered the nucleus, where it will bind to specific sequences of DNA to promote the downstream transcription and translation of secreted embryonic alkaline phosphatase (SEAP). The reporter cell line HEK-Blue™ hTLR3 was transfected with fibers and stimulated with Poly (I:C). The cells were incubated for 24 hours, and the levels of NF-kB-dependent SEAP were measured in the supernatants. (D) Schematic demonstration of the TLR1/2 signaling that activates the NF-kB pathway that can be inhibited with the NF-kB decoys assembled upon re-association of RNA/DNA fibers (F(S) and F(A)).
Inhibition of NF-kB pathway.
To further verify the intact dual functionality of DNA/RNA fibers that carry NF-kB response specificity and GFP silencing, we conducted a follow-up study using the reporter cell line HEK-Blue™ hTLR3. These cells are designed for the study of human TLR3 stimulation through assessment of NF-kB activation. The latter process induces production of secreted embryonic alkaline phosphatase (SEAP). Poly (I:C), a synthetic mimetic of viral dsRNA, can be used to trigger NF-kB activation and, ultimately, SEAP production (Figure 4C and supporting Figure S3). In this experiment, cognate RNA/DNA fibers (FS and FA) were separately pre-loaded into exosomes which were then mixed together for co-delivery (see FS/Exo+FA/Exo). Individual FS- and FA-loaded exosomes were used as controls (see FS/Exo and FA/Exo). On the same day that the complexes were added to the HEK-Blue™ hTLR3 cells, the cells were challenged with Poly (I:C). Supernatants were then collected and analyzed for presence of secreted alkaline phosphatase (SEAP), which can be easily detected and quantified using QUANTI-Blue™ reagent. Cells treated with poly (I:C) produced significant SEAP signal, and when FS and FA were delivered separately, no inhibition in SEAP production was observed. However, co-delivered of FS and FA significantly reduced SEAP synthesis. To show the generality, another reporter cell line, THP1-Dual™, that also expressed SEAP under the NF-kB promoter was tested (Figure 4D). To induce the activation of the NF-kB pathway, THP1-Dual™ cells were challenged with a TLR agonist (PAM3CSK4). On the same day, exosomes loaded with fibers were added to PAM3CSK4 treated cells and the supernatants were collected and analyzed for the presence of SEAP. The cells treated with Pam3CSK4 induced significant SEAP production, and when FS and FA were delivered separately, no inhibition of SEAP was observed. However, co-delivered FS and FA reduced SEAP production. These results were consistent with Lipofectamine 2000’s actions as a transfection reagent, as described previously (65). When the cells were treated with Poly (I:C) for 24 hours prior to addition of RNA/DNA fibers and then incubated for an additional 24 hours, the extent of SEAP inhibition was decreased (supporting Figure S1). All of these results support intracellular release of NANPs without any loss of their intended function.
Immunostimulation by exosome loaded NANPs.
Another nucleic acid-specific TLR was investigated using HEK-Blue™ hTLR7 cells. Both HEK-Blue™ hTLR 3 and 7 cells were obtained by co-transfection of the hTLR gene into HEK293 cells and engineered to express a single Toll-like receptor. Stimulation of TLR7 with R848, an imidazoquinoline and agonist of TLR7, led to production of SEAP whose levels can be determined using HEK-Blue™ Detection reagent in real time. Here, we used these cells to evaluate the potential immunostimulation by the NANP/exosome complexes (Figure 5). Both TLR3 and TLR7 are responsible for RNA detection, while TLR3 activated by dsRNA and TLR7 detects ssRNA (66). Our data confirmed stimulation from both TLR3 and TLR7 cells to be negligible.
Figure 5:
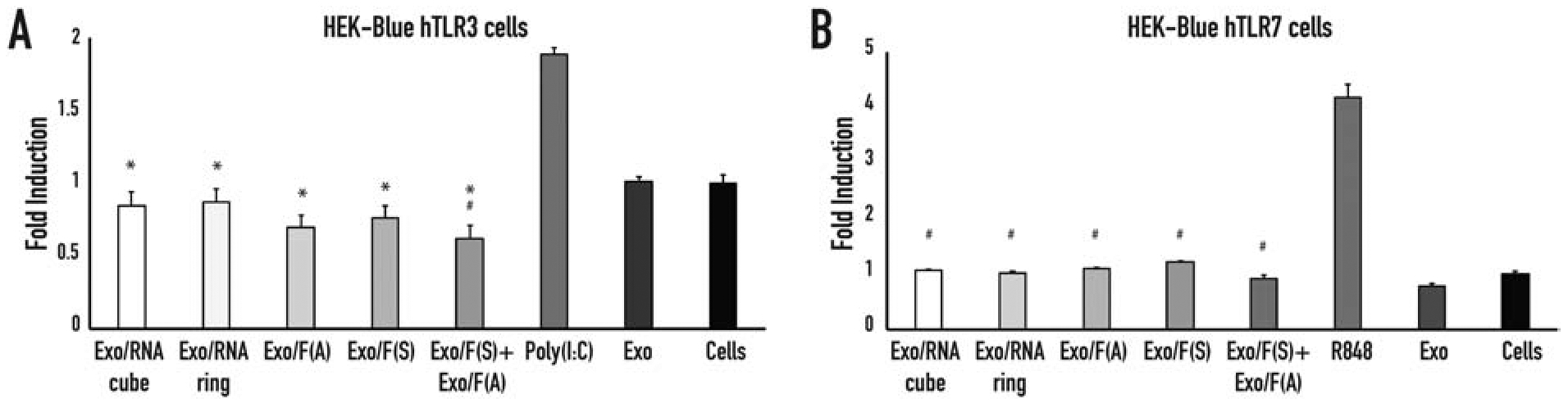
Relative levels of immunostimulation of exosomes loaded with NANPs. TLR stimulation is measured via SEAP secretion from (A) HEK-Blue™ hTLR3 and (B) HEK-Blue™ hTLR7 cells. In (A), statistical significance relevant to the cells is denoted by #, statistical significance relevant to poly I:C is denoted by * (# p<0.05, * p<0.001). In (B), statistical significance relevant to R848 is denoted by # ((# p<0.05).
DISCUSSION
Here, we demonstrated exosome-mediated delivery of different NANPs exhibiting various shapes and structures and investigated their intracellular uptake, post-delivery gene silencing efficiency, and immunostimulation potential. Exosome loading with NANPs of various sizes can be challenging and numerous methods have been developed to efficiently facilitate the transfection. One technique is chemical transfection, shows promising results for direct cargo loading. Exo-Fect™ siRNA/miRNA transfection kit (System Biosciences, Palo Alto, CA, USA) has shown positive results for nucleic acid transfection into exosomes (unpublished results). In order to load the cube, ring, and fiber NANPs into exosomes, Exo-Fect reagent forms a complex with the nucleic acids. We showed that exosomes remain intact post packaging and retain the common exosomal marker such as CD63. Interestingly, we noticed that the exosomes become slightly bigger upon NANPs loading. We demonstrated that NANPs loaded into exosomes with intact shape reassumed their original functionalities upon delivery (Figure 6) and effectively silenced GFP in the cells that constitutively express GFP. In our earlier studies using HEK 293 cells that overexpress human TLR7 and the SEAP genes, we showed that the RNA cubes delivered by L2K and PgP induced SEAP production when placed under the control of an NF-kB and AP-1-inducible promoter (61, 67). In contrast, even for the RNA cubes (known for displaying the greatest immunostimulatory properties) we observed negligible immune response after using exosomes to deliver these structures. Our data suggest that exosome-mediated NANP delivery can potentially “stealth-coat” exosome contents from certain pattern recognition receptors, but further studies are required. In summary, this work may pave the way towards the efficient and safe application of NANPs in personalized medicine by implementing patient-derived exosomes as a novel and promising therapeutic platform.
Figure 6:
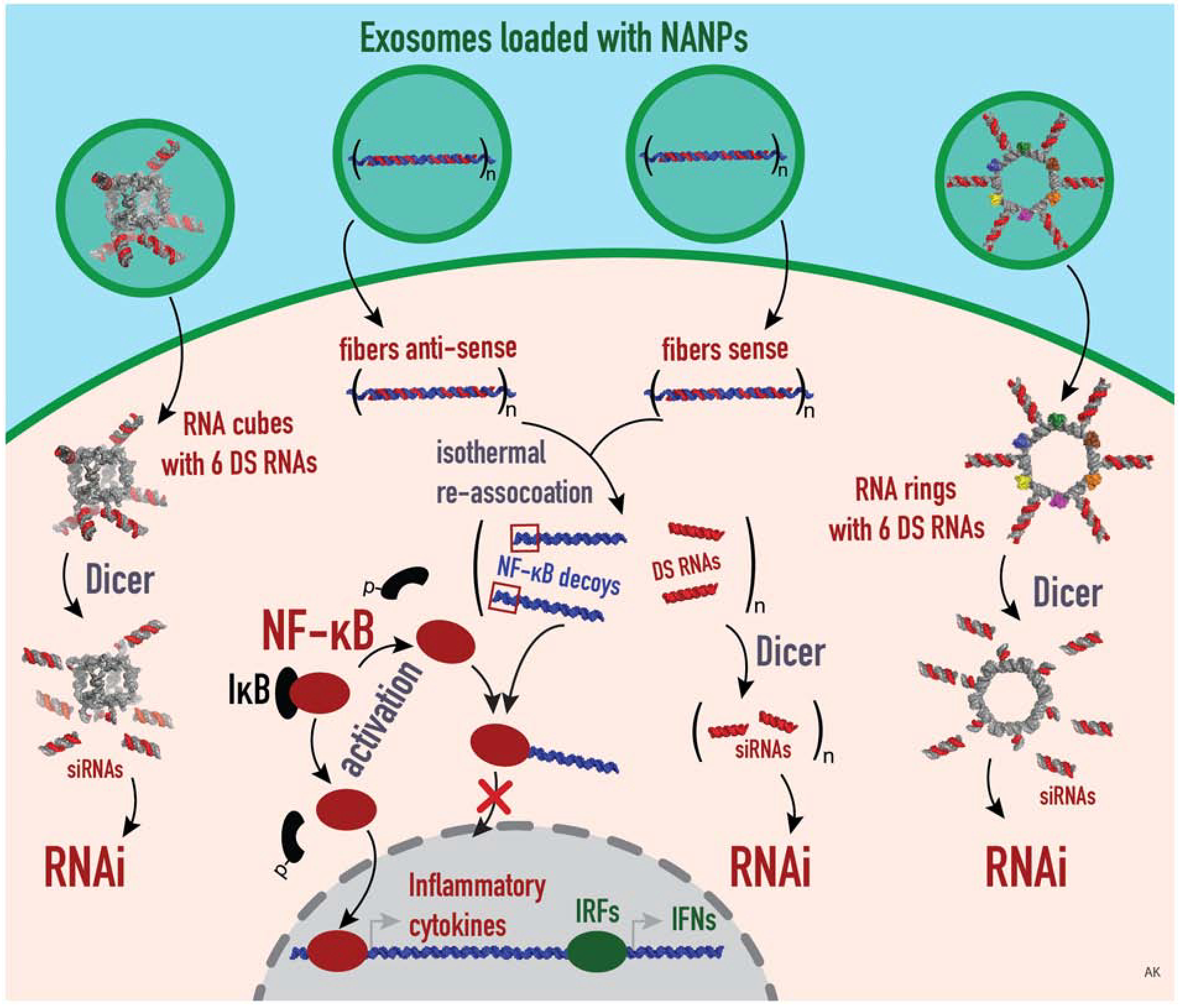
Schematic summary of the proposed mechanism of exosome-mediated delivery of different functionalized NANPs. Exosomes are loaded with NANPs, such as RNA cubes and RNA rings, that are functionalized with 6 DSRNAs, and fiber antisense and fiber sense that carry n numbers of DS RNAs and NF-kB decoys. Those NANPs are delivered by exosomes and released inside the target cells. In the cytosol, the DS RNAs will be further diced by Dicer to result in the generation of siRNAs, by which the RNAi pathway is activated. Meanwhile, the NF-kB decoys are released from the fibers, which in turn bind to NF-kB in order to prevent its nuclear translocation, thus inhibit the downstream production of IRF and IFNs.
Supplementary Material
HIGHLIGHTS.
Exosomes derived from MDA-MB-231 cells delivered NANPs
Exosomes protect NANPs from nuclease degradation
NANPs delivered by exosomes activate RNA interference (RNAi) and NF-kB decoy
Exosome-mediated delivery of NANPs showed high efficiency and low immunostimulation
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM120487 (to K.A.A). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS
The authors report no conflicts of interest in this work.
REFERENCES:
- 1.Afonin KA, Viard M, Koyfman AY, Martins AN, Kasprzak WK, Panigaj M, et al. Multifunctional RNA Nanoparticles. Nano Lett. 2014;14(10):5662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonin Kirill A, Lindsay B, Shapiro Bruce A. Engineered RNA Nanodesigns for Applications in RNA Nanotechnology. DNA and RNA Nanotechnology 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabow WW, Jaeger L. RNA Self-Assembly and RNA Nanotechnology. Accounts of Chemical Research. 2014;47(6):1871–80. [DOI] [PubMed] [Google Scholar]
- 4.Lee H, Lytton-Jean AKR, Chen Y, Love KT, Park AI, Karagiannis ED, et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nature Nanotechnology. 2012;7(6):389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afonin KA, Bindewald E, Yaghoubian AJ, Voss N, Jacovetty E, Shapiro BA, et al. In vitro assembly of cubic RNA-based scaffolds designed in silico. Nature Nanotechnology. 2010;5(9):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rackley L, Stewart JM, Salotti J, Krokhotin A, Shah A, Halman JR, et al. RNA fibers as optimized nanoscaffolds for siRNA coordination and reduced immunological recognition. 2018;28(48):1805959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bui MN, Johnson MB, Viard M, Satterwhite E, Martins AN, Li Z, et al. Versatile RNA tetra-U helix linking motif as a toolkit for nucleic acid nanotechnology. 2017;13(3):1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose SD, Kim DH, Amarzguioui M, Heidel JD, Collingwood MA, Davis ME, et al. Functional polarity is introduced by Dicer processing of short substrate RNAs. Nucleic Acids Res. 2005;33(13):4140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afonin KA, Viard M, Kagiampakis I, Case CL, Dobrovolskaia MA, Hofmann J, et al. Triggering of RNA Interference with RNA-RNA, RNA-DNA, and DNA-RNA Nanoparticles. Acs Nano. 2015;9(1):251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandler M, Panigaj M, Rolband LA, Afonin KA. Challenges to optimizing RNA nanostructures for large scale production and controlled therapeutic properties. Nanomedicine (Lond). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panigaj M, Johnson MB, Ke W, McMillan J, Goncharova EA, Chandler M, et al. Aptamers as Modular Components of Therapeutic Nucleic Acid Nanotechnology. ACS Nano. 2019;13(11):12301–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirill A Afonin MAD, George Church, and Mark Bathe. Opportunities, Barriers, and a Strategy for Overcoming Translational Challenges to Therapeutic Nucleic Acid Nanotechnology. ACS Nano Article ASAP. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afonin KA, Viard M, Martins AN, Lockett SJ, Maciag AE, Freed EO, et al. Activation of different split functionalities on re-association of RNA-DNA hybrids. Nat Nanotechnol. 2013;8(4):296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halman JR, Satterwhite E, Roark B, Chandler M, Viard M, Ivanina A, et al. Functionally-interdependent shape-switching nanoparticles with controllable properties. Nucleic Acids Res. 2017;45(4):2210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandler M, Afonin KA. Smart-Responsive Nucleic Acid Nanoparticles (NANPs) with the Potential to Modulate Immune Behavior. Nanomaterials (Basel). 2019;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afonin KA, Desai R, Viard M, Kireeva ML, Bindewald E, Case CL, et al. Co-transcriptional production of RNA-DNA hybrids for simultaneous release of multiple split functionalities. Nucleic acids research. 2014;42(3):2085–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke W, Hong E, Saito RF, Rangel MC, Wang J, Viard M, et al. RNA-DNA fibers and polygons with controlled immunorecognition activate RNAi, FRET and transcriptional regulation of NF-kappaB in human cells. Nucleic Acids Res. 2019;47(3):1350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porciani D, Tedeschi L, Marchetti L, Citti L, Piazza V, Beltram F, et al. Aptamer-Mediated Codelivery of Doxorubicin and NF-kappaB Decoy Enhances Chemosensitivity of Pancreatic Tumor Cells. Mol Ther Nucleic Acids. 2015;4:e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim KH, Lee ES, Cha SH, Park JH, Park JS, Chang YC, et al. Transcriptional regulation of NF-kappaB by ring type decoy oligodeoxynucleotide in an animal model of nephropathy. Exp Mol Pathol. 2009;86(2):114–20. [DOI] [PubMed] [Google Scholar]
- 20.Griesenbach U, Scheid P, Hillery E, de Martin R, Huang L, Geddes DM, et al. Anti-inflammatory gene therapy directed at the airway epithelium. Gene Ther. 2000;7(4):306–13. [DOI] [PubMed] [Google Scholar]
- 21.Parlea L, Puri A, Kasprzak W, Bindewald E, Zakrevsky P, Satterwhite E, et al. Cellular Delivery of RNA Nanoparticles. ACS Comb Sci. 2016;18(9):527–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halman JR, Kim KT, Gwak SJ, Pace R, Johnson MB, Chandler MR, et al. A cationic amphiphilic co-polymer as a carrier of nucleic acid nanoparticles (Nanps) for controlled gene silencing, immunostimulation, and biodistribution. Nanomedicine. 2020;23:102094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta K, Mattingly SJ, Knipp RJ, Afonin KA, Viard M, Bergman JT, et al. Oxime ether lipids containing hydroxylated head groups are more superior siRNA delivery agents than their nonhydroxylated counterparts. Nanomedicine (Lond). 2015;10(18):2805–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta K, Afonin KA, Viard M, Herrero V, Kasprzak W, Kagiampakis I, et al. Bolaamphiphiles as carriers for siRNA delivery: From chemical syntheses to practical applications. J Control Release. 2015;213:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz-Acuna M, Halman JR, Afonin KA, Dobson J, Rinaldi C. Magnetic nanoparticles loaded with functional RNA nanoparticles. Nanoscale. 2018;10(37):17761–70. [DOI] [PubMed] [Google Scholar]
- 26.Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, et al. Drug delivery systems: An updated review. Int J Pharm Investig. 2012;2(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makhmalzade BS, Chavoshy F. Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J Adv Pharm Technol Res. 2018;9(1):2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhaskar S, Tian F, Stoeger T, Kreyling W, de la Fuente JM, Grazú V, et al. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part Fibre Toxicol. 2010;7:3–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan JF, Zhu JH, Cui MF, Zhang J, Ma F, Su YP, et al. Multifunctional Mineral Hydrogels: Potential in Artificially Intelligent Skins and Drug Delivery. Acs Omega. 2019;4(21):19145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czapar AE, Steinmetz NF. Plant viruses and bacteriophages for drug delivery in medicine and biotechnology. Current Opinion in Chemical Biology. 2017;38:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das CK, Jena BC, Banerjee I, Das S, Parekh A, Bhutia SK, et al. Exosome as a Novel Shuttle for Delivery of Therapeutics across Biological Barriers. Molecular Pharmaceutics. 2019;16(1):24–40. [DOI] [PubMed] [Google Scholar]
- 32.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015;35:69–77. [DOI] [PubMed] [Google Scholar]
- 34.Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol. 2016;36(3):301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575–81. [DOI] [PubMed] [Google Scholar]
- 36.Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharmaceutica Sinica B. 2016;6(4):287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140(1):13–21. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell & Bioscience. 2019;9(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111(2):488–96. [DOI] [PubMed] [Google Scholar]
- 40.Saeedi S, Israel S, Nagy C, Turecki G. The emerging role of exosomes in mental disorders. Translational psychiatry. 2019;9(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. Journal of Biological Chemistry. 2009;284(49):34211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcus ME, Leonard JN. FedExosomes: Engineering Therapeutic Biological Nanoparticles that Truly Deliver. Pharmaceuticals (Basel). 2013;6(5):659–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9(6):654–9. [DOI] [PubMed] [Google Scholar]
- 44.Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Kyakulaga AH, Wilcher SA, et al. Milk exosomes - Natural nanoparticles for siRNA delivery. Cancer Letters. 2019;449:186–95. [DOI] [PubMed] [Google Scholar]
- 45.Banizs AB, Huang T, Dryden K, Berr SS, Stone JR, Nakamoto RK, et al. In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. International Journal of Nanomedicine. 2014;9:4223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang R, Yan X, Zhang S, Guo HJTJoU. MP88–16 targeted exosome-mediated delivery of survivin sirna for the treatment of bladder cancer. 2017.
- 48.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacology & therapeutics. 2017;174:63–78. [DOI] [PubMed] [Google Scholar]
- 50.Ha D, Yang N, Nadithe VJAPSB. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. 2016;6(4):287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kibria G, Ramos EK, Wan Y, Gius DR, Liu H. Exosomes as a Drug Delivery System in Cancer Therapy: Potential and Challenges. Molecular Pharmaceutics. 2018;15(9):3625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Experimental & molecular medicine. 2019;51(3):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Liu Y, Liu H, Tang WHJC, Exosomes:bioscience. Exosomes: biogenesis, biologic function and clinical potential. 2019;9(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrew Malloy DG, Patrick Hole, Bob Carr,. Counting and Sizing of Viral Vector Particles and Aggregates by Nanoparticle Tracking Analysis (NTA). MUSCULO-SKELETAL GENE & CELL THERAPY IIIIIIII. 2010;18(S223). [Google Scholar]
- 56.McNicholas K, Li JY, Michael MZ, Gleadle JM. Albuminuria is not associated with elevated urinary vesicle concentration but can confound nanoparticle tracking analysis. Nephrology (Carlton). 2017;22(11):854–63. [DOI] [PubMed] [Google Scholar]
- 57.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273(32):20121–7. [DOI] [PubMed] [Google Scholar]
- 58.Zoller M Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9(1):40–55. [DOI] [PubMed] [Google Scholar]
- 59.Chanteloup G, Cordonnier M, Isambert N, Bertaut A, Marcion G, Garrido C, et al. Membrane-bound exosomal HSP70 as a biomarker for detection and monitoring of malignant solid tumours: a pilot study. Pilot Feasibility Stud. 2020;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Y, Deng W, Klinke DJ 2nd. Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst. 2015;140(19):6631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong EP, Halman JR, Shah AB, Khisamutdinov EF, Dobrovolskaia MA, Afonin KA. Structure and Composition Define Immunorecognition of Nucleic Acid Nanoparticles. Nano Letters. 2018;18(7):4309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Afonin KA, Grabow WW, Walker FM, Bindewald E, Dobrovolskaia MA, Shapiro BA, et al. Design and self-assembly of siRNA-functionalized RNA nanoparticles for use in automated nanomedicine. Nature Protocols. 2011;6(12):2022–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grabow WW, Zakrevsky P, Afonin KA, Chworos A, Shapiro BA, Jaeger L. Self-assembling RNA nanorings based on RNAI/II inverse kissing complexes. Nano Lett. 2011;11(2):878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim T, Viard M, Afonin KA, Gupta K, Popov M, Salotti J, et al. Characterization of Cationic Bolaamphiphile Vesicles for siRNA Delivery into Tumors and Brain. Mol Ther Nucleic Acids. 2020;20:359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ke W, Hong E, Saito RF, Rangel MC, Wang J, Viard M, et al. RNA-DNA fibers and polygons with controlled immunorecognition activate RNAi, FRET and transcriptional regulation of NF-κB in human cells. Nucleic Acids Research. 2018;47(3):1350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohto U, Shimizu T. Structural aspects of nucleic acid-sensing Toll-like receptors. Biophys Rev. 2016;8(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halman JR, Kim K-T, Gwak S-J, Pace R, Johnson MB, Chandler MR, et al. A cationic amphiphilic co-polymer as a carrier of nucleic acid nanoparticles (Nanps) for controlled gene silencing, immunostimulation, and biodistribution. Nanomedicine: Nanotechnology, Biology and Medicine. 2020;23:102094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


