Abstract
Alkaline phosphatase (AP) is a ubiquitous membrane–bound glycoprotein that catalyzes phosphate monoesters’ hydrolysis from organic compounds, an essential process in cell signaling. Four AP isozymes have been described in humans, placental AP, germ cell AP, tissue nonspecific AP, and intestinal AP (IAP). IAP plays a crucial role in gut microbial homeostasis, nutrient uptake, and local and systemic inflammation, and its dysfunction is associated with persistent inflammatory disorders. AP is a strong predictor of mortality in the general population and patients with cardiovascular and chronic kidney disease (CKD). However, little is known about IAP modulation and its possible consequences in CKD, a disease characterized by gut microbiota imbalance and persistent low-grade inflammation. Mitigating inflammation and dysbiosis can prevent cardiovascular complications in patients with CKD, and monitoring factors such as IAP can be useful for predicting those complications. Here, we review IAP’s role and the results of nutritional interventions targeting IAP in experimental models to prevent alterations in the gut microbiota, which could be a possible target of predictive, preventive, personalized medicine (PPPM) to avoid CKD complications. Microbiota and some nutrients may activate IAP, which seems to have a beneficial impact on health; however, data on CKD remains scarce.
Keywords: Intestinal alkaline phosphatase, Chronic kidney disease, Inflammation, Gut microbiota, Predictive diagnosis, Predictive preventive personalized medicine (PPPM/3 PM)
Introduction
Alkaline phosphatases (APs) are ubiquitous membrane-bound glycoproteins that catalyze phosphate monoesters’ hydrolysis at an alkaline pH optimum, with the highest activity at pH 9.7. In humans, four different alkaline phosphatase isozymes are known, originating from distinct gene loci. Tissue nonspecific AP (tnAP), also known as TNSAP, TNAP, AP-TNAP, expressed mainly in the liver, bone, and kidney, comprising > 90% of circulating AP. Also, there are the placental-type AP (pAP); germ cell AP, known as placental-like AP; GCAP, plAP-like, or AP-1; and the intestinal AP (IAP) [1].
IAP plays a vital role in the intestinal barrier function, affect bicarbonate secretion, duodenal surface pH, nutrient resorption, local intestinal inflammation, and gut microbiota [2, 3]. Disturbances of IAP functions are associated with persistent inflammatory diseases associated with aging (i.e., inflammageing), inflammatory bowel diseases, type 2 diabetes mellitus, obesity, metabolic syndrome, and chronic kidney disease (CKD) [4, 5].
Chronic kidney disease is a growing public health problem affecting over 850 million people worldwide. Many complications of CKD are associated with high cardiovascular morbidity and mortality risk, such as chronic kidney disease–mineral and bone disorders (CKD-MBD) that involve complex, interrelated changes with alterations in calcium and phosphorus balance and, mineral regulating hormones that affect bone turnover and mineralization and, promote the development of vascular calcification [6–8]. Among several markers of MBD, the serum concentration of total AP (tAP) (in the absence of liver disease) is a useful marker of bone turnover, which is widely used together with intact parathyroid hormone (iPTH) to monitor CKD-MBD [8–10]. An elevated AP serum activity is a significant risk factor for all-cause mortality in both the general population and patients with cardiovascular disease (CVD) and CKD [10–12].
Although IAP is not involved directly with MBD, changes on this isoenzyme, such as loss of expression and function, appears to be associated with increased dysbiosis and intestinal and systemic inflammation [13]. Persistent inflammation in the body can be transformed into chronic inflammation. Chronic inflammation is one of the causes of chronic diseases such as diabetes, autoimmune diseases, neurodegenerative disease, CKD, and cancer. Chronic inflammation leads to tissue structure loss, excessive tissue remodeling, and damages of DNA caused by oxidative stress. In this context, many inflammatory factors such as interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α have been used as biomarkers for the prediction of several diseases, including cancer [14].
The gut microbiota is a complex group of microbes located in the distal part of the large intestine that lives in synergy with all body functions. It is known that gut microbiota is relevant in non-communicable diseases’ etiology when there is a disbalance of the microbe’s colonization, called dysbiosis [15]. Indeed, there is a range of physiological and metabolic changes in CKD, including inflammation, oxidative stress, and accumulation of uremic toxins, which may destabilize these patients’ intestinal homeostasis, leading to the development of uremic dysbiosis [16, 17].
Suboptimal health conditions reduce the quality of life, can cause and contribute to several diseases, and inflammatory processes [18–20]. iALP can be extremely useful as an indicator for an in-depth diagnosis of coexisting and health-threatening conditions. Thus, iALP could be used as a predictive diagnosis, targeted prevention, and personalized treatment for CKD individuals. These novel insights have propelled an interest in IAP as a potential target for predicting gut imbalance and preventive, therapeutic interventions. Here, we briefly review aspects of IAP biology and activity and its functions for regulating the intestinal barrier, gut microbiota, transport of nutrients and minerals, and the impact of food components (macronutrients, vitamins, and bioactive compounds) on IAP. With this background, we discuss implications of targeting IAP in CKD, including dietary modulators of IAP gene expression and enzymatic activity that could be of value in CKD as preventive to the development of gut microbiota disorders and inflammation. In this context, predictive, preventive, and personalized medicine (PPPM) can discuss and predict the risk of complications caused by chronic diseases and optimize possible treatments [21]. PPPM is a concept for the implementation of predictive, preventive, and personalized medical interventions. This novel medical approach is more useful to prevent disease development, and it has a more humanized approach to disease control. PPPM prioritizes the attention to all aspects of the tricky parts that define health to change the reactive medical paradigm by all means that can enhance and improve preventive medicine [22].
AP biology and activity
APs belong to a superfamily of metalloenzymes that catalyze the hydrolytic removal of phosphate from various molecules and participate in a wide range of physiological processes [21]. APs are bound to the outer layer of the cell membrane by a glycosylphosphatidylinositol (GPI) anchor. A multigene family and function encode mammalian APs as dimeric molecules. Two Zn2+ ions, one Mg2+, and one Ca2+ ion in the active site’s vicinity are fundamental for the enzymatic activity. Moreover, these metal ions contribute substantially to the AP monomer’s conformation and indirectly control subunit–subunit interactions [23, 24]. tnAP is almost ubiquitously expressed in all tissues, whereas IAP, pAP, and germ cell AP are tissue-specific isozymes [21] (Table 1). For several of these isozymes, different isoforms have been identified, which share the same protein structure, but differ in glycosylation. Although all APs hydrolyze phosphate esters with a catalytic optimum at an alkaline pH, substrate specificity differs between the different isozymes and isoforms. Whereas not all physiological functions of the APs are known, AP’s most well-described functions refer to tnAP and IAP [25]. tnAP plays an important role in bone mineralization, vitamin B6 metabolism, neurogenesis, and influences inflammation through regulation of purinergic signaling [25]. The biological function of pAP, located at the syncytiotrophoblast’s microvillus membrane, is still unclear. However, it is known that during pregnancy, circulating pAP activity increases several-fold and has recently been suggested to play a role in maternal to fetal nutrient transport and may be protective against offspring obesity [26]. While germ cell AP’s functional role is still unclear, circulating germ cell AP is thought to be an early tumor marker for seminomas, but its use in clinical practice has been flawed by methodological difficulties [13].
Table 1.
Characteristic features of human alkaline phosphatases
| Gene | Protein | Tissue distribution | Function |
|---|---|---|---|
| ALPL | Tissue nonspecific (tnAP) | Liver, kidney, bone, nervous system |
- Bone mineralization - Vitamin B6 metabolism |
| ALPI | Intestinal (IAP) | Intestinal epithelial cells |
- Detoxification of LPS - Possible modulation of gut microbiota - Regulation of intestinal lipid absorption - Regulation of bicarbonate secretion and duodenal surface pH |
| ALPP | Placental (pAP) | Syncytiotrophoblast, various tumors | Unknown |
| ALPPL2 | Germ cell (gcAP) | Testis, reproductive tumors | Unknown |
The transition of membrane-bound APs to circulating forms involves phospholipase C (enzymes from the membrane that cleave phospholipids), which cleaves the GPI anchor from the AP molecules. Cell apoptosis and cell destruction have been proposed as additional sources for circulating AP activity. Phospholipase D is present in the circulation and is believed to cleave APs bound to remnant membrane fragments. AP levels in the plasma, more specific the tnAP, have been identified as an independent marker for CVD and mortality in healthy populations and CKD, reflecting its role in a wide range of pathophysiological processes involving bone disease, CKD progression, and cognitive dysfunction, an increase of vascular calcification, chronic inflammation, oxidative stress, and fibrosis [27].
IAP is expressed throughout the gastrointestinal tract, specifically on the microvillus brush-border membrane of enterocytes, and its secretion into the gastrointestinal tract, with the most remarkable expression in the duodenum [28, 29]. The serum half-life of IAP is short, and its secretion into serum is preceded by internalization of apical membrane-bound IAP and by increased transcription of IAP and occurs mainly in the form of membrane-containing surfactant-like particles [28, 30].
As demonstrated in mice, there are three genes expressed in the intestine: Akp3, in the duodenum, Akp5 throughout the intestine, and Akp6 with greater expression in the distal intestine [31]. IAP has been considered a main intestinal mucosal defense factor by maintaining the intestinal homeostasis [32, 33], including regulation of bicarbonate secretion and duodenal surface pH, and detoxification by dephosphorylation of lipopolysaccharides (LPS) and, ultimately, control of intestinal and systemic inflammation as described below [33–35].
The monitoring of IAP concentrations by specific kits for determination and quantification in plasma and urine can be an essential biomarker in predicting intestinal disorders. The used method is by separating total AP through electrophoresis, which electrophoretically is isolated and assayed to determine their biochemical properties, reactivity to anti-intestinal or anti-tissue nonspecific AP antibodies, molecular sizes, and sugar chain heterogeneities [36, 37].
The role of IAP as a predictor of altered barrier intestinal and gut microbiota function
IAP is a part of the first line of defense of the intestine and acts in various ways. IAP stimulates gene expression of critical tight junction molecules and their correct cellular localization [38], regulates the concentration of endotoxins [39, 40], inhibits bacterial DNA and flagellin [30], and inhibits luminal ATP through ATP dephosphorylation [41, 42]. IAP exerts an inhibitory effect on the growth and survival of bacteria present in the gut microbiota, with consequent prevention of bacteria translocation from the intestinal lumen to the bloodstream [43]. Specific bacteria and cells treated with calf IAP (cIAP) presented a reduction of IL-8 response, mainly the gram-negative bacteria that may be related to the dephosphorylation of bacterial components such as LPS, CpG DNA, and flagellin.
LPS is a significant component of the gram-negative bacteria cell wall and is composed of polysaccharide chains covalently attached to a single structure, lipid A, a diglucosamine-based acylated phospholipid. The primary toxic moiety of LPS is located within the lipid A, which has two phosphate groups, essential for many biological activities. Contrary to lipid A, monophosphoryl lipid A (MPLA) is non-toxic and can induce tolerance to subsequent LPS exposure [13, 44]. IAP can remove one of the two phosphate groups from the toxic lipid A fraction of LPS, producing monophosphoryl lipid A (MPLA), which promotes the inactivation of LPS [45].
In sepsis, high circulating levels of LPS (endotoxemia) induce a robust inflammatory response. Also, at lower levels, LPS may stimulate low-grade chronic inflammatory responses, such as those observed in obesity, diabetes, CVD, and CKD. Also, the H. pylori infection can stimulate the secretion of several inflammatory factors since that the cell wall of H. pylori is an LPS by the bond to the transmembrane recognition receptor toll-like receptor 4 (TLR4), which lead to activation of the toll-like receptor signaling pathway [14]. In some intestinal diseases such as colitis and inflammatory bowel disease, IAP mRNA expression is reduced and related to gut inflammation, with a consequent increase of LPS and TLR-4 expression [46, 47]. IAP deficiency also has been associated with ischemic heart disease and type 2 diabetes mellitus [48, 49]. Upon activation by LPS, TLR-4, or MyD88, a standard adaptor downstream of TLRs, mediates the release of proinflammatory cytokines, including IL-6, IL-1, and TNF through the nuclear-kappa B factor (NF-kB) pathway [50–52]. Counteracting these responses, exogenous IAP can inhibit IκBα phosphorylation and DNA-binding activity of NF-κB in peritoneal macrophages, consequently decreasing the inflammatory cytokines in a TLR4-dependent manner (via the TLR4/NF-κB pathway) [53].
Furthermore, about the establishment of the intestinal flora in early childhood, there is an increase in the IAP activity during this time [54]. It is essential to know that IAP also makes part of the gut microbiota modulation since the born [54]. A study using an IAP knockout model in Sprague-Dawley pups increased dysbiosis with bacterial translocation, and inflammation was reported [55]. Malo et al. [56] demonstrated a profound reduction of aerobic and, to a lesser extent, anaerobic bacteria in mice lacking the duodenal IAP gene Akp3.
Transport of IAP-rich membrane vesicles secreted from the microvillus surfaces of duodenal enterocytes might explain the resistance of duodenal IAP to digestion during the passage to the large intestine. Possible mechanisms of microbiota modulation by IAP include changes in inflammatory regulators, promoting mucosal tolerance to commensal bacteria, and regulating pH in the mucosal microenvironment hydrolyzation of nucleotides. In addition to shaping the intestinal microbiota, IAP also detoxifies microbial components and prevents enteric microbial translocation in the body [57–59].
Supplementation of exogenous IAP by gastrointestinal administration including oral supplementation, can reduce intestinal inflammation (decrease of TNF, IL-6, and IL-1β) [46], increase serum AP activity, promote intestinal tissue regeneration, and decrease the abnormally high permeability of the intestinal tract, thereby preventing LPS-mediated inflammatory response, and (in mice) IAP was found to protect against antibiotic-associated bacterial infections [41, 60, 61]. Additionally, IAP supplementation seems to increase the intestinal epithelial barrier in animals with advanced age by increasing rigid junction proteins’ expression. Moreover, IAP supplementation may alleviate the diminished gut integrity associated with aging and extend life-span [62]. Kaliannan et al. [63] reported that both endogenous IAP and oral IAP supplementation could prevent the LPS absorption in rats fed with a high-fat diet and also was able to prevent metabolic syndrome in animals receiving IAP. Economopoulos et al. [64] showed that oral IAP (100 units/ml drinking water) prevented the susceptibility to antibiotic-associated metabolic syndrome in mice treated early in life with azithromycin; IAP decreased total body weight, serum lipids, glucose levels, and liver lipids, and changed the composition of gut microbiota. Oral supplementation of IAP in aging mice regulates the intestinal barrier function, microbial homeostasis, decrease age-related intestinal permeability and systemic inflammation, potentially leading to less fragility and prevention of chronic age-related diseases [65]. Moreover, Singh et al. [57] reported that intestinal IAP treatment (100 U/mL for 14 days) in mice inhibits the LPS-induced IL-1β mRNA expression and activation of NF-κB. They also observed an increase of IAP by inducing autophagy-related genes (Atg5, Atg16, Irgm1, Tlr4, and Lyz) and Atg16 protein expression in the small intestine, and may control intestinal bacterial overgrowth and inflammation.
Regarding sepsis-induced acute kidney injury (AKI), it was observed in the piglet model of AKI that 25 U/kg/h of bovine IAP increased serum and renal tissue AP activity, decreased proximal tubular injury, and prevented AKI [66]. Another study showed preventive and curative effects of treatments with 1 IU/g of calf IAP, which in septic mice decreased the permeability of the small intestine by 50%, and increased mRNA expression levels of claudin-1 and claudin-14, which are main structural tight junction proteins in the intestine [67]. These studies confirmed that IAP influences both inflammatory and intestinal barriers, contributing to intestinal health [66, 67].
Some studies have also used a recombinant human form of AP (IAP and pAP) due to the longest half-life isoenzymes [68], which creates a highly stable, biologically active enzyme compared to isoenzymes separately. Recombinant AP could decrease the inflammatory response by dephosphorylating endotoxin and adenosine triphosphate in human proximal tubular epithelial cells in AKI patients [69]. In another renal ischemia-induced inflammation model in rats, recombinant AP seems to attenuate the renal inflammation and tubular injury provoked by AKI through attenuation of inflammatory and pro-apoptosis markers [70]. In contrast, a randomized, double-blind, placebo-controlled trial using recombinant AP (1.6 mg/kg) for 7 days in AKI patients did not show significant improvement in kidney function [68].
Transport of nutrients and minerals function
IAP has been suggested to be a minute-to-minute regulator of intestinal calcium resorption, as duodenal IAP activity in Sprague-Dawley rats is stimulated by an increased calcium concentration in the duodenal lumen [71]. Increased IAP activity inhibits Ca resorption, hypothetically decreasing luminal pH in the gut, which causes downregulation of TRPV6, a member of the transient receptor potential family of membrane proteins that mediates the uptake of Ca2+ ions [72]. IAP provides a protective mechanism by inhibiting the entry of calcium into enterocytes, thereby preventing calcium overload. A study conducted in mice completely lacking duodenal IAP (Akp3−/−mice) showed mild atrophy of the villi with a lower absorption surface, resulting in higher calcium uptake than in the control mice. During long-term follow-up, mice lacking IAP had better bone properties than controls, providing evidence that IAP regulates calcium absorption [73].
Kuehn et al. [74] investigated the gut-bone axis in mice and observed that IAP-knockout mice presented bone formation changes that were probably associated with dysbiosis and inflammation linked to IAP deficiency. Another study with Akp3−/− mice have shown that the IAP activity in the proximal intestine and 25-hydroxyvitamin D3-24-hydroxylase mRNA levels were decreased, while 1,25 dihydroxy vitamin D3 (1,25(OH)2D3) levels were increased compared with Akp3+/+ mice [75]. Moreover, intestinal inorganic phosphate transport activity was decreased in Akp3−/− mice compared with Akp3+/+ mice. Additionally, Akp3−/−mice with renal failure reduced intestinal inorganic phosphate absorption, leading to an increase in ATP in intestinal epithelial cells. Consequently, there is an increase in entrance calcium into epithelial cells via the P2X7 receptor [75]. Taken together, IAP and Akp3 appear to be essential regulators of intestinal inorganic phosphate [75].
Nutritional strategy as a preventive intervention for IAP modulation
The expression and activity of IAP are directly affected by food intake, i.e., quantity and type of macro- and micronutrients including vitamins and other bioactive nutrients, or by the absence of food, as well as indirectly by the composition of the gut microbiota that in turn is highly dependent on food intake (Table 2). In mice, IAP expression and function are lost with starvation and maintained by enteral feeding [51]. Studies of IAP expression in IAP-knockout vs. wild-type mice and 20 patients post ileostomy in fasted and fed states showed that IAP expression was decreased in patients and mice during fasting with impairment in the barrier function and that IAP supplementation reverted the gut barrier dysfunction [76]. Dietary fat, protein, carbohydrates, vitamins, and minerals are essential dietary modulators that regulate host IAP gene expression and enzymatic activity. This modulation seems to depend on the quantity and type of ingredients in the diet [32, 41].
Table 2.
Effects on IAP of interventions using food compounds in animal experimental studies
| Reference | Study design/sample | Intervention/follow-up | Results |
|---|---|---|---|
| Johnson et al. (1984) [134] | Experimental/20 male Wistar rats |
Group 1: starch or sucrose Group 2: sucrose Group 3: starch, sucrose or guar gum Group 4: starch, sucrose or cellulose for 30 days |
Group 3: ↓ IAP activity Group 4: ↑ IAP activity |
| Montagne et al. (1999) [84] | Experimental/20 Holstein calves | Liquid diet based on skimmed milk powder and soybean protein concentrate for 60 days | ↓ IAP and AP total activities |
| Boudry et al. (2002) [85] | Experimental/45 piglets |
Group 1: Diet based on wheat Group 2: Diet based on barley Group 3: Diet based on milk for 35 days |
↑ activities of IAP and sucrase in both groups Group 1: ↑ dipeptidyl-peptidase IV activity Group 1 and 2: ↑ sodium-dependent glucose absorption, ↓ diarrhea |
| Sogabe et al. (2004) [88] | Experimental/16 Sprague-Dawley strain female rats | Glucose diet and lactose diet for 21 days |
↑ level of IAP activity in the jejunum from the lactose group rats ↑ IAP mRNA expression |
| Montoya et al. (2006) [87] | Experimental/10 female Wistar rats | Protein-free diet, as compared to casein, in slightly energy-restricted rats fed for 10 days |
Protein-free diet—↓villus width, height to crypt depth ratio in the duodenum and ileum ↓ IAP |
| Sogabe et al. (2007) [94] | Experimental/26 Sprague-Dawley rats |
Group 1: vitamin K1 Group 2: vitamin K2 Both orally administered for 90 days |
↑ IAP in both group |
| Kaur et al. (2007) [135] | Experimental/16 Wistar strain rats |
Group 1: coconut oil Group 2: corn oil Group 3: cod liver oil Group 4: Saline Analyses 5 h after oral administration |
Groups 1, 2, and 3: ↑ serum AP activity, ↑ Luminal IAP activity |
| Lynes et al. (2011) [28] | Experimental/adult male C57BL/6 mice | Diet rich in long-chain fatty acids |
↑ IAP in the microvillus brush-border membrane of enterocytes ↑ secretion into the gastrointestinal tract |
| Haraikawa et al. (2011) [95] | Experimental/21 male ICR strain mice |
Group 1: vitamin K1 Group 2: vitamin K2 Analysis 4 h after oral administration |
Group 1: ↑ IAP activity in the jejunum and IAP mRNA expression Group 2: ↑ IAP mRNA expression |
| Brun et al. (2012) [71] | Experimental/18 male Sprague-Dawley inbred rats |
Group 1: 0.2 g % Ca diet Group 2: 1 g % Ca diet Group 3: 2 g % Ca diet Orally supplemented for 3 days |
All groups: ↑ IAP activity in the brush border |
| Riggle et al. (2013) [60] | Experimental/30 newborn Sprague-Dawley rats—necrotizing enterocolitis | 4 or 40 glycine units of bovine IAP intraperitoneally for 3 days | ↓TNF-α; IL-6; IL-1β |
| Ghosh et al. (2014b) [98] | Experimental/ LDLR−/−mice | Western diet for 16 weeks plus 100 mg/kg of curcumin by oral gavage |
Western diet: ↑ LPS levels, glucose intolerance and/or heart disease; ↓ intestinal barrier, ↓ IAP activity, expression of tight junction proteins, ZO-1 and Claudin-1 Curcumin: ↓ LPS levels, ↓ lesions in the aortic arch and entire aorta; ↑ intestinal barrier, IAP activity, glucose tolerance, expression of tight junction proteins, ZO-1 and Claudin-1 |
| Okazaki et al. (2017) [89] | Experimental/male Sprague-Dawley rats fed with diet containing 30% lard | 4% high or low viscous glucomannan for 2 weeks |
↑ colonic AP activity, colonic and gene expression of IAP, fecal levels of IgA, mucins and cecal organic acids ↔ colonic expression of Akp3 |
| Okazaki et al. (2019) [90] | Experimental/42 male Sprague-Dawley rats |
Group 1: Control Group 2: Fructooligosaccharides Group 3: Galactooligosaccharides Group 4: Isomaltooligosaccharides Group 5: Raffinose Group 6: Lactulose |
Groups 2, 3, 5, and 6: ↑ IAP activity, fecal AP activity, colonic IAP gene expression, fecal mucins, fecal IgA, Bifidobacterium spp. ↓ pH of caecal digesta, C. coccoides and C. leptum Groups 2, 3, and 4: ↑ ileum AP activity Groups 2 and 5: ↑ colonic expression on Akp3 |
| Rentea et al. (2019) [77] | Experimental/ newborn Sprague-Dawley rat pups |
Group 1: Vaginal birth breast fed Group 2: Vaginal birth formula fed Group 3: Preterm cesarean breast fed Group 4: Preterm cesarean formula fed Group 5: Term cesarean breast fed Group 6: Term cesarean formula fed |
Group 1: ↑ IAP mRNA expression Formula fed: ↓ IAP mRNA expression Breastfed: ↑ IAP mRNA expression |
IAP, intestinal alcaline phosphatase; TNF-α, tumoral necrosis fator alpha; IL, interleukin; LPS, lipopolysaccharide; ZO-1, tight junction protein 1; Akp3, alkaline phosphatase 3, intestine, not Mn requiring
Rentea et al. [77] have reported that IAP levels of newborn Sprague-Dawley rat pups are influenced by the types of birth (cesarean or vaginal delivery) and feeding (formula or breast). Inhibition of IAP expression and activity was observed in rat pups fed with formula and had a cesarean delivery. Yang et al. [125] have investigated the IAP activity in 122 meconium samples from infants (gestational ages from 24 to 40 weeks). They also analyzed 289 breast milk samples from 78 individual mothers between days 2–49 post-birth to investigate if breast milk serves as a source of exogenous AP to the neonatal intestine. Breast milk of the first-week post-birth had the highest AP activity. These results encourage breast milk feeding and show that preterm birth associates with intestinal disorders, low IAP activity, and decreased detoxification capacity of proinflammatory bacterial products such as LPS [77]. Also, the type of milk formula used for preterm and near-term seems to change the IAP activity. A study showed a beneficial effect of mildly pasteurized whey protein on intestinal integrity and innate defense [78]. The mildly pasteurized whey protein increased IAP activity in the colonic epithelium, improved intestinal maturation, and reduced gut inflammation in preterm and near-term piglets [78]. These studies indicate that the effects on IAP expression and activity differ between food components.
Some studies have currently approached the modulation of gut microbiota and intestinal health as PPPM [79, 80]. Therefore, a personalized and balanced diet contenting macro, micronutrients, and bioactive compounds found in foods can prevent IAP imbalance, improving its activity and expression, including aspects of PPPM, which could, through personalized prevention, avoid complications related to CKD and gut microbiota imbalance.
Fat
The fatty acid composition of oil regulates the synthesis and secretion, and the increased appearance of IAP in serum under physiological conditions seems to be mainly associated with lipid resorption [28, 32, 81]. Using a transgenic mouse model, Kaliannan et al. [82] observed that intake of omega-3 fatty acids increases the expression and activity of IAP, reduces the LPS levels, and improves intestinal permeability. Another study investigating the impact of a high-fat diet during pre- and post-weaning periods on the gut microbiota and IAP activity in male rats showed that pups fed with a high-fat diet had enhanced adiposity and increased IAP activity, as well as higher lactobacillus/enterococcus and lower numbers of bacteroides/prevotella in the jejunum and colon than controls [83].
Protein
IAP metabolism is affected by protein intake. In pre-ruminant calfs treated with soybean diet, most small intestine enzyme activities, including IAP, were reduced [84]. Replacement of a milk-based diet with wheat or barley diets increased IAP in pigs after only 4 days by increasing mucosal enzyme activity, sodium-dependent intestinal absorption, and response to secretagogues [85]. Fermented milk (yogurt) showed to stimulate IAP in the small intestine of mice. Indeed, milk fermentation leads to protein denaturation, which improves free amino acids bioavailability and can induce brush-border enzyme adaptation.
Moreover, the milk fermentation facilitates access to phosphorylated sites, explaining the increase in IAP activity after consumption of yogurt in the mice [32, 86]. Since studies found that a protein-free diet can lead to low IAP activity [32, 87], the effects of a low-protein diet on IAP in patients with CKD need to be studied, mainly because a low-protein diet is recommended to non-dialysis CKD patients. It is believed that the privation or deficiency in amino acids from protein can decrease IAP concentration, possibly, via reduced intestinal epithelial growth [32].
Carbohydrates
Rats fed with a diet containing 10% lactose showed an increase in IAP mRNA expression, suggesting that lactose affects intestinal phosphate metabolism indirectly by regulation of IAP expression [88]. Another study in rats fed a high-fat diet showed that supplementation of glucomannan (a dietary fiber found in the root of the konjac plant) increased the colonic and gene expression of IAP, as well as modulated gut microbiota and increased gut immunoglobulin A, mucins, colonic, and cecal organic acids (n-butyrate, propionate) [89]. A diet with fermentable oligosaccharides increased IAP, IAP gene expression, mucin secretion, and microbial fermentation. There was a significant positive correlation of IAP activity with fecal mucins, caecal lactate, and n-butyrate, as well as Bifidobacterium spp. in rats, fed a high-fat diet [90]. Another study showed that Caco-2 cells treated with sialyl lactose and galactooligosaccharides (human milk oligosaccharides) increased IAP expression [91].
Vitamins
IAP expression is stimulated by 1,25(OH)2 vitamin D, whereas vitamin D deprivation decreased duodenal IAP activity in rats [92]. Nakaoka et al. [93] have shown that vitamin D restriction and high-fat diet together decreased AP activity in the duodenum in an animal model of menopause. These results indicate that vitamin D restriction in a high-fat diet can suppress IAP mRNA expression in the duodenum. Thus, adequate vitamin D levels could be an essential strategy for regulating IAP expression. Besides, it seems that vitamin K1 and K2 may stimulate the IAP gene expression in mice supplemented with both vitamins; however, the mechanism has not yet been defined [94, 95].
Spices
Curcumin increases the catalytic activity and expression of IAP and attenuates the circulating levels of LPS [96, 97]. Indeed, supplementation of 100 mg/kg of curcumin attenuated Western diet damage such as increased LPS production, inflammation, decreased IAP activity and expression, and intestinal barrier alterations. Curcumin increased IAP activity and the expression of tight junction proteins, ZO-1, and Claudin-1 [98]. Ghosh et al. [99] reinforced this idea by showing that intestine-specific expression of human chimeric IAP attenuated Western diet-induced barrier dysfunction and glucose intolerance in transgenic mice.
Dietary intake of other spices such as black pepper, red pepper, ginger, and bioactive compounds (piperine and capsaicin) also increases the IAP levels in rats. Additionally, these spices also induced an increase in the microvilli’s length of the rats’ jejunum [100].
Additives
Dietary additives such as sucrose, titanium dioxide, emulsifier TWEEN 20, and sodium chloride may increase intestinal permeability. A recent study found increased intestinal permeability after treatment with additives, mainly with high sugar, in both Drosophila and a human cell co-culture dramatically reduced the IAP activity in both models [101].
Iron
Iron supplementation can decrease the IAP expression, and other brush-border enzymes such as Lys-Ala-dipeptidyl aminopeptidase, mainly because of the possible deposition of iron duodenum [81], also excess of iron can contribute to oxidative stress and intestinal damage [81, 102].
Probiotics
The consumption of probiotics has been liked with the development of healthy diets, treatment, and prevention of dysbiosis [103]. Also, probiotics and prebiotics can target tissue such as the kidney, brain, conferring health benefits to extraintestinal sites, and gut microbiota by immune-modulating the metabolic activity in the intestine affecting the intestinal barrier [104]. Moreover, evaluating bacteria properties and the host’s phenotype by a panel of biomarkers is essential for the individualized selection of probiotics used [105, 106]. In this context, probiotics therapy can use as a personalized medicine according to the patients’ metabolic patterns in each patient [107]. For example, Borges et al. [108] have observed in a randomized, double-blind, placebo-controlled study with hemodialysis patients that probiotic (Streptococcus thermophilus, Lactobacillus acidophilus, and Bifidobacterialongum, 90 billion colony-forming units per day) increased the serum urea, potassium, and indoxyl sulfate. This demonstrates that the probiotic therapy should be chosen with caution in this kind of patients, and an individual and personalized probiotic therapy is appropriate [106, 107]. Probiotics seem to increase IAP activity [109, 110]. Rats with antibiotic-induced dysbiosis fed with probiotic Lactobacillus fermentum Z showed an increase in IAP activity in the jejunum epithelium [109]. Pre-treatment with Bifidobacterium infantis in mice with intestinal damage induced by Salmonella prevented the reduction of brush-border enzyme activity, including IAP, also presented a reduction in IL-10 and IL-8 expression [110].
Intestinal AP in CKD
CKD is characterized by the irreversible and progressive loss of renal function and the presence of kidney damage that can be measured by the decline of glomerular filtration rate (GFR) and albuminuria [111]. The kidney loss function brings some complications involved with disease progressions such as CVD, metabolic acidosis, hyperkalemia, hyperphosphatemia, sarcopenia, anemia, dyslipidemia, hyperparathyroidism, and bone diseases [112]. CVD is the leading cause of death in patients with CKD and, in addition to traditional risk factors (obesity, hypertension, diabetes, and dyslipidemia) [113], studies have identified intestinal imbalance of microbiota as a novel factor that may contribute to inflammation and CVD in CKD patients [114, 115]. Kidney injury can also be initially caused by changes in the population of intestinal microorganisms that promote increased levels of inflammation, uremic toxins, and blood pressure [115]. Uremia can modify the biochemical milieu of the intestine due to a high amount of urea in the gastrointestinal tract and secretion of uric acid and oxalate by the colonic epithelium [116–118].
An intact intestinal barrier is essential to prevent the influx of microbes, microbial toxins, bacterial products, antigens, digestive enzymes, degraded food products, and other harmful substances from the gastrointestinal lumen internal [119]. Increased permeability leads to the translocation of products generated by intestinal bacteria, mostly LPS, to the bloodstream [120, 121]. Dysbiosis in patients with CKD is associated with various complications, such as the production of uremic toxins (indoxyl sulfate, p-cresyl sulfate, and indole-3-acetic acid), inflammation, oxidative stress, and insulin resistance, which can promote the progression of kidney failure and the modification of the tight epithelial junctions [119, 122, 123].
In acute cases of inflammation, elevated IAP levels may be beneficial to the patient since it is associated with endotoxin detoxification, inactivation of cytokines, and reduction of oxidative stress that may exert nephroprotective and anti-inflammatory effects [1]. As previously mentioned, the most studied pathway concerning the control of inflammation by IAP is through LPS control. It is believed that IAP prevents the activation of TLR4 via LPS and consequent activation of NF-kB and its subsequent translocation to the nucleus [13, 44]. There is a scarcity of studies on the effects of IAP in CKD. However, it is worth remembering that reduced luminal IAP can lead to an imbalance in intestinal functions and gut dysbiosis, with a consequent increase in the risk of developing intestinal inflammation and gut permeability in CKD [124]. Thus, more studies are warranted on IAP’s role in defense of intestinal mucosa and gut microbiota and against local and systemic inflammation in CKD.
In the 1970–1980s, researchers were more interested in IAP; however, recent nephrology studies focus on total AP (Table 3). Some studies showed increased IAP levels in CKD could be due to the liver’s inability to remove this circulating enzyme, especially in liver disease [126]. However, the kidneys can also be a potential source of the IAP to the plasma and be responsible for total serum AP elevations in some patients [127]. Zetterberg et al. [37] observed that peritoneal dialysis patients presented higher IAP levels; however, the mechanism by which this increase of IAP occurs is unknown.
Table 3.
Observational studies on intestinal alkaline phosphatase (IAP) and chronic kidney disease
| References | Patients | Results |
|---|---|---|
| Walker (1974) [136] |
29 HS 44 HD patients 556 other patients (OP) |
IAP bands on electrophoresis (%) HS: 48%, HD patients: 50%, OP: 9% Total AP bands on electrophoresis (%) HS: 5 to 16 IU/L, HD patients: 9 to 57 IU/L, OP: 16 to 542 IU/L |
| De Broe et al. (1974) [137] | 111 HD patients | ↑ IAP in 29.7% of the patients |
| Skillen (1977) [138] |
100 non-dialyzed patients 114 HD patients |
↑ IAP in 15% in HD patients and 10% of non-dialyzed patients. IAP was detected in 36% of those patients with normal serum AP activity |
| Stĕpán et al. (1984) [139] |
21 non-dialyzed patients 52 HD patients |
Patients without hepatopathy: IAP activity was inversely correlated with Ca serum levels and positively correlated with iPTH levels Patients with hepatopathy: No correlation between iPTH and IAP, IAP in serum was influenced by blood group, liver ALP activity in serum and serum GMT activity. |
| Alpers et al. (1988) [127] | 42 HD patients with ↑ total ALP serum levels |
Before HD session: 62% had detectable IAP in plasma (222 IU/L (125–625) IAP measuring by rocket was 17.9 ± 2.3%, and by electrophoresis was 10.2 ± 1.9%. After HD session: IAP of 12.8 ± 3.1% (by rocket), and 9.5 ± 2.1% (by electrophoresis |
| Tibi et al. (1991) [140] | 75 HD patients | ↑ total ALP activity in 28 patients; IAP > 23 UI/L in 8 patients (responsible for increase in total AP in only one patient) |
| Tsumura et al. (2001) [36] |
108 CKD non-dialyzed patients 106 healthy subjects |
With polyacrylamide gel electrophoresis analysis, authors found atypical AP in serum and urine in patients, which could be an intestinal-type AP |
| Zetterberg et al. (2005) [37] | 2 PD patients | ↑ serum total AP and IAP activity |
HS, healthy subjects; HD, hemodialysis; CrCl, creatinine clearance; PD, peritoneal dialysis; iPTH, intact parathyroid hormone; AP, alkaline phosphatase
Relatively little is known regarding the levels and the role of IAP in CKD. A recent review highlights AP’s potential, including IAP, as a novel marker of predictive and treatment targets for CVD in CKD [1]. These observations may open the way for therapeutic strategies involving IAP modulation, aiming to attenuate dysbiosis, improve the gut microbiota, and prevent translocation of LPS and uremic toxins mitigating inflammation [127–131].
It is worth remembering that inflammation in patients with CKD is also linked to increased gut permeability and dysbiosis, which produce high uremic toxin and LPS plasma levels leading to the inflammatory process, making new research on this field essential [16, 17, 132, 133].
Perspectives and preliminary conclusions
Predictive medicine
The measure of IAP concentration in plasma and its gene expression and enzymatic activity can be an essential parameter to predict an imbalance in gut microbiota and intestinal function. As well as, IAP could be used as an inflammatory marker together with other markers, such as interleukins to predict inflammation and diseases that are based on the chronic inflammatory process such as intestinal dysbiosis, CVD, obesity, diabetes mellitus in patients with CKD. Currently, it is not common to measure all AP isozymes in patients with CKD. The isoform usually measured is the tAP. However, this review addresses IAP as a potential marker of gut disorders and inflammation in CKD patients and nutritional strategies to IAP modulation, given predictive diagnostics, preventive and personalized medicine by dietary approach. IAP can be determined and quantified in plasma and urine by separating total AP through electrophoresis, as described in the AP biology and activity topic.
Preventive medicine
Through interactions with diet, gut microbiota, and the intestinal mucosal surface from which it is secreted, IAP plays a pivotal role in preserving the intestine and the gut’s healthy homeostasis microbiota. This is achieved via regulating the microbial ecosystem, maintaining the integrity of the gut wall, and safeguarding the enterocytes’ significant functions, particularly their transport systems for nutrient absorption. At the same time, IAP restricts bacteria’s translocation and disarming inflammatory bacterial components by dephosphorylating toxic microbial ligands, such as LPS.
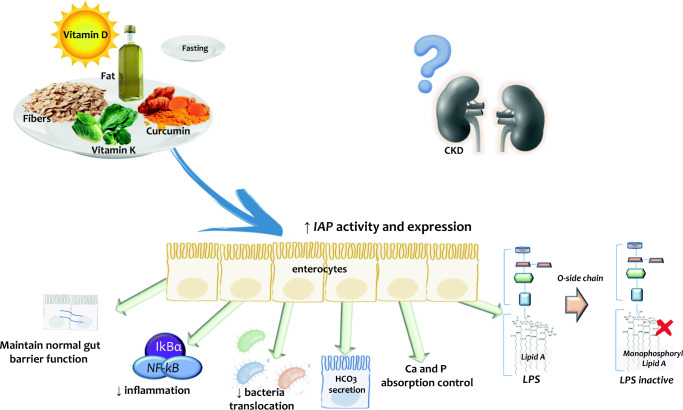
Little is known about the potential consequences of IAP modulation in CKD, but the concept of “food as medicine for CKD” [133] suggests dietary interventions as potentials promoters to target IAP in CKD. The diet’s components promoting detoxification of LPS, leading to prevention or amelioration of intestinal and systemic inflammation processes, and normalization of the gut barrier function are described in Fig. 1.
Fig. 1.
IAP modulation by food components. Macro- as well as micronutrients and bioactive compounds or fasting influence a range of IAP functions that collectively serve to maintain the homeostasis and structural and functional integrity of the intestine and its transport systems for nutrients, while maintaining the gut barrier function that protects against bacteria translocation. Increased IAP gene expression and activity promoting detoxification of LPS may lead to improvement of both intestinal and systemic inflammation, increase the secretion of bicarbonate, reduce the bacteria translocation and maintain the normal gut barrier function. In patients with CKD, studies are needed to evaluate the possible effects of dietary compounds on IAP activity and expression
Future of personalized medicine
Personalized nutrition can be used to prevent diseases or complications related to the disease. Concerning CKD, possibly the dietary treatment, according to the characteristics of the disease and the patients, may benefit the patient about quality of life, prognosis, and mortality decrease. The assessment of nutritional status, biochemical tests, including inflammatory markers, oxidative stress, and gut microbiota assessment can lead to secondary benefits to patients since monitoring these conditions even before the installed disease can prevent the onset of previous intervention. In the future, it is believed that the evaluation of the specific genotype of patients will be of a significant increase in personalized nutrition.
Therefore, further experimental studies are needed using models mimicking CKD to evaluate possible effects of dietary compounds on IAP activity and expression and to study whether and how such interventions may promote intestinal health and reduce local and systemic inflammation in CKD. In this context, PPPM can help identify the problem and find a solution to mitigate CKD complications.
Funding
Conselho Nacional de Pesquisa (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) support Denise Mafra research. The Heart and Lung Foundation, CIMED and “Njurfonden” support Peter Stenvinkel’s research. Baxter Novum is the result of a grant from Baxter Healthcare to Karolinska Institutet. Bengt Lindholm is affiliated with Baxter Healthcare.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haarhaus M, Brandenburg V, Kalantar-Zadeh K, Stenvinkel P, Magnusson P. Alkaline phosphatase: a novel treatment target for cardiovascular disease in CKD. Nat Rev Nephrol. 2017;13(7):429–442. doi: 10.1038/nrneph.2017.60. [DOI] [PubMed] [Google Scholar]
- 2.Buchet R, Millán JL, Magne D. Multisystemic functions of alkaline phosphatases. Methods Mol Biol. 2013;1053:27–51. doi: 10.1007/978-1-62703-562-0_3. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh SS, Wang J, Yannie PJ, Ghosh S. Intestinal barrier dysfunction, LPS translocation, and disease development. J Endocr Soc. 2020;4(2):bvz039. doi: 10.1210/jendso/bvz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parlato M, Charbit-Henrion F, Pan J, Romano C, Duclaux-Loras R, Le Du MH, Warner N, Francalanci P, Bruneau J, Bras M, Zarhrate M, Bègue B, Guegan N, Rakotobe S, Kapel N, De Angelis P, Griffiths AM, Fiedler K, Crowley E, Ruemmele F, Muise AM, Cerf-Bensussan N. Human ALPI deficiency causes inflammatory bowel disease and highlights a key mechanism of gut homeostasis. EMBO Mol Med. 2018;10(4):e8483. doi: 10.15252/emmm.201708483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilski J, Mazur-Bialy A, Wojcik D, Surmiak M, Magierowski M, Sliwowski Z, Pajdo R, Kwiecien S, Danielak A, Ptak-Belowska A, Brzozowski T. Role of obesity, mesenteric adipose tissue, and adipokines in inflammatory bowel diseases. Biomolecules. 2019;9(12):780. doi: 10.3390/biom9120780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai L, Golembiewska E, Lindholm B, Stenvinkel P. End-stage renal disease, inflammation and cardiovascular outcomes. Contrib Nephrol. 2017;191:32–43. doi: 10.1159/000479254. [DOI] [PubMed] [Google Scholar]
- 7.Lundquist AL, Nigwekar SU. Optimal management of bone mineral disorders in chronic kidney disease and end stage renal disease. Curr Opin Nephrol Hypertens. 2016;25(2):120–126. doi: 10.1097/MNH.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nizet A, Cavalier E, Stenvinkel P, Haarhaus M, Magnusson P. Bone alkaline phosphatase: an important biomarker in chronic kidney disease - mineral and bone disorder. Clin Chim Acta. 2020;501:198–206. doi: 10.1016/j.cca.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Vervloet MG, Brandenburg VM, CKD-MBD working group of ERA-EDTA et al. J Nephrol. 2017;30(5):663–670. doi: 10.1007/s40620-017-0408-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Zhang X, Han F, Xie X, Hua Z, Huang X, Lindholm B, Haarhaus M, Stenvinkel P, Qureshi AR, Chen J. High alkaline phosphatase and low intact parathyroid hormone associate with worse clinical outcome in peritoneal dialysis patients. Perit Dial Int. 2020;4:896860820918131. doi: 10.1177/0896860820918131. [DOI] [PubMed] [Google Scholar]
- 11.Fan Y, Jin X, Jiang M, Fang N. Elevated serum alkaline phosphatase and cardiovascular or all-cause mortality risk in dialysis patients: a meta-analysis. Sci Rep. 2017;7(1):13224. doi: 10.1038/s41598-017-13387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumida K, Molnar MZ, Potukuchi PK, Thomas F, Lu JL, Obi Y, Rhee CM, Streja E, Yamagata K, Kalantar-Zadeh K, Kovesdy CP. Prognostic significance of pre-end-stage renal disease serum alkaline phosphatase for post-end-stage renal disease mortality in late-stage chronic kidney disease patients transitioning to dialysis. Nephrol Dial Transplant. 2018;33(2):264–273. doi: 10.1093/ndt/gfw412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fawley J, Gourlay DM. Intestinal alkaline phosphatase: a summary of its role in clinical disease. J Surg Res. 2016;202(1):225–234. doi: 10.1016/j.jss.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian S, Golubnitschaja O, Zhan X. Chronic inflammation: key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019;10(4):365–381. doi: 10.1007/s13167-019-00194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maturo MG, Soligo M, Gibson G, Manni L, Nardini C. The greater inflammatory pathway-high clinical potential by innovative predictive, preventive, and personalized medical approach. EPMA J. 2019;11(1):1–16. doi: 10.1007/s13167-019-00195-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stockler-Pinto MB, Soulage CO, Borges NA, Cardozo LFMF, Dolenga CJ, Nakao LS, Pecoits-Filho R, Fouque D, Mafra D. From bench to the hemodialysis clinic: protein-bound uremic toxins modulate NF-κB/Nrf2 expression. Int Urol Nephrol. 2018;50(2):347–354. doi: 10.1007/s11255-017-1748-y. [DOI] [PubMed] [Google Scholar]
- 17.Borges NA, Barros AF, Nakao LS, Dolenga CJ, Fouque D, Mafra D. Protein-bound uremic toxins from gut microbiota and inflammatory markers in chronic kidney disease. J Ren Nutr. 2016;26(6):396–400. doi: 10.1053/j.jrn.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Kunin A, Polivka J, Jr, Moiseeva N, Golubnitschaja O. “Dry mouth” and “Flammer” syndromes-neglected risks in adolescents and new concepts by predictive, preventive and personalised approach. EPMA J. 2018;9(3):307–317. doi: 10.1007/s13167-018-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thoppay J, Desai B. Oral burning: local and systemic connection for a patient-centric approach. EPMA J. 2019;10(1):1–11. doi: 10.1007/s13167-018-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goncharenko V, Bubnov R, Polivka J, Jr, Zubor P, Biringer K, Bielik T, Kuhn W, Golubnitschaja O. Vaginal dryness: individualised patient profiles, risks and mitigating measures. EPMA J. 2019;10(1):73–79. doi: 10.1007/s13167-019-00164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golubnitschaja O, Kinkorova J, Costigliola V. Predictive, preventive and personalised medicine as the hardcore of ‘Horizon 2020’: EPMA position paper. EPMA J. 2014;5(1):6. doi: 10.1186/1878-5085-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, Krapfenbauer K, Mozaffari MS, Costigliola V. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7(1):23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millán JL. Alkaline phosphatases: structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2006;2(2):335–341. doi: 10.1007/s11302-005-5435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoylaerts MF, Manes T, Millán JL. Mammalian alkaline phosphatases are allosteric enzymes. J Biol Chem. 1997;272(36):22781–22787. doi: 10.1074/jbc.272.36.22781. [DOI] [PubMed] [Google Scholar]
- 25.Rader BA. Alkaline phosphatase, an unconventional immune protein. Front Immunol. 2017;8:897. doi: 10.3389/fimmu.2017.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirschmugl B, Crozier S, Matthews N, Kitzinger E, Klymiuk I, Inskip HM, Harvey NC, Cooper C, Sibley CP, Glazier J, Wadsack C, Godfrey KM, Desoye G, Lewis RM. Relation of placental alkaline phosphatase expression in human term placenta with maternal and offspring fat mass. Int J Obes. 2018;42(6):1202–1210. doi: 10.1038/s41366-018-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haarhaus M, Gilham D, Kulikowski E, Magnusson P, Kalantar-Zadeh K. Pharmacologic epigenetic modulators of alkaline phosphatase in chronic kidney disease. Curr Opin Nephrol Hypertens. 2020;29(1):4–15. doi: 10.1097/MNH.0000000000000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynes M, Narisawa S, Millán JL, Widmaier EP. Interactions between CD36 and global intestinal alkaline phosphatase in mouse small intestine and effects of high-fat diet. Am J Phys Regul Integr Comp Phys. 2011;301(6):R1738–R1747. doi: 10.1152/ajpregu.00235.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young GP, Friedman S, Yedlin ST, Allers DH. Effect of fat feeding on intestinal alkaline phosphatase activity in tissue and serum. Am J Phys. 1981;241(6):G461–G468. doi: 10.1152/ajpgi.1981.241.6.G461. [DOI] [PubMed] [Google Scholar]
- 30.Chen KT, Malo MS, Moss AK, Zeller S, Johnson P, Ebrahimi F, Mostafa G, Alam SN, Ramasamy S, Warren HS, Hohmann EL, Hodin RA. Identification of specific targets for the gut mucosal defense factor intestinal alkaline phosphatase. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G467–G475. doi: 10.1152/ajpgi.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narisawa S, Hoylaerts MF, Doctor KS, Fukuda MN, Alpers DH, Millán JL. A novel phosphatase upregulated in Akp3 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2007;293(5):G1068–G1077. doi: 10.1152/ajpgi.00073.2007. [DOI] [PubMed] [Google Scholar]
- 32.Lallès JP. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev. 2010;68(6):323–332. doi: 10.1111/j.1753-4887.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 33.Lallès JP. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr Rev. 2019;77(10):710–724. doi: 10.1093/nutrit/nuz015. [DOI] [PubMed] [Google Scholar]
- 34.Lallès JP. Luminal ATP: the missing link between intestinal alkaline phosphatase, the gut microbiota, and inflammation? Am J Physiol Gastrointest Liver Physiol. 2014;306(10):G824–G825. doi: 10.1152/ajpgi.00435.2013. [DOI] [PubMed] [Google Scholar]
- 35.Lassenius MI, Fogarty CL, Blaut M, Haimila K, Riittinen L, Paju A, Kirveskari J, Järvelä J, Ahola AJ, Gordin D, Härma MA, Kumar A, Hamarneh SR, Hodin RA, Sorsa T, Tervahartiala T, Hörkkö S, Pussinen PJ, Forsblom C, Jauhiainen M, Taskinen MR, Groop PH, Lehto M, FinnDiane Study Group Intestinal alkaline phosphatase at the crossroad of intestinal health and disease - a putative role in type 1 diabetes. J Intern Med. 2017;281(6):586–600. doi: 10.1111/joim.12607. [DOI] [PubMed] [Google Scholar]
- 36.Tsumura M, Ueno Y, Kinouchi T, Koyama I, Komoda T. Atypical alkaline phosphatase isozymes in serum and urine of patients with renal failure. Clin Chim Acta. 2001;312(1–2):169–178. doi: 10.1016/s0009-8981(01)00606-4. [DOI] [PubMed] [Google Scholar]
- 37.Zetterberg H. Increased serum concentrations of intestinal alkaline phosphatase in peritoneal dialysis. Clin Chem. 2005;51(3):675–676. doi: 10.1373/clinchem.2004.045831. [DOI] [PubMed] [Google Scholar]
- 38.Liu W, Hu D, Huo H, Zhang W, Adiliaghdam F, Morrison S, Ramirez JM, Gul SS, Hamarneh SR, Hodin RA. Intestinal alkaline phosphatase regulates tight junction protein levels. J Am Coll Surg. 2016;222(6):1009–1017. doi: 10.1016/j.jamcollsurg.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brzozowski B, Mazur-Bialy A, Pajdo R, Kwiecien S, Bilski J, Zwolinska-Wcislo M, Mach T, Brzozowski T. Mechanisms by which stress affects the experimental and clinical inflammatory bowel disease (IBD): role of brain-gut axis. Curr Neuropharmacol. 2016;14(8):892–900. doi: 10.2174/1570159x14666160404124127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komazin G, Maybin M, Woodard RW, Scior T, Schwudke D, Schombel U, Gisch N, Mamat U, Meredith TC. Substrate structure-activity relationship reveals a limited lipopolysaccharide chemotype range for intestinal alkaline phosphatase. J Biol Chem. 2019;294(50):19405–19423. doi: 10.1074/jbc.RA119.010836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lallès JP. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr Rev. 2014;72(2):82–94. doi: 10.1111/nure.12082. [DOI] [PubMed] [Google Scholar]
- 42.Malo MS, Moaven O, Muhammad N, Biswas B, Alam SN, Economopoulos KP, Gul SS, Hamarneh SR, Malo NS, Teshager A, Mohamed MM, Tao Q, Narisawa S, Millán JL, Hohmann EL, Warren HS, Robson SC, Hodin RA. Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates. Am J Physiol Gastrointest Liver Physiol. 2014;306(10):G826–G838. doi: 10.1152/ajpgi.00357.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bilski J, Mazur-Bialy A, Wojcik D, Zahradnik-Bilska J, Brzozowski B, Magierowski M, Mach T, Magierowska K, Brzozowski T. The role of intestinal alkaline phosphatase in inflammatory disorders of gastrointestinal tract. Mediat Inflamm. 2017;2017:9074601. doi: 10.1155/2017/9074601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bentala H, Verweij WR, Huizinga-Van der Vlag A, van Loenen-Weemaes AM, Meijer DK, Poelstra K. Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock. 2002;18(6):561–566. doi: 10.1097/00024382-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Peters E, Geraci S, Heemskerk S, Wilmer MJ, Bilos A, Kraenzlin B, Gretz N, Pickkers P, Masereeuw R. Alkaline phosphatase protects against renal inflammation through dephosphorylation of lipopolysaccharide and adenosine triphosphate. Br J Pharmacol. 2015;172(20):4932–4945. doi: 10.1111/bph.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuin A, Poelstra K, de Jager-Krikken A, Bok L, Raaben W, Velders MP, Dijkstra G. Role of alkaline phosphatase in colitis in man and rats. Gut. 2009;58(3):379–387. doi: 10.1136/gut.2007.128868. [DOI] [PubMed] [Google Scholar]
- 47.Molnár K, Vannay A, Szebeni B, Bánki NF, Sziksz E, Cseh A, Győrffy H, Lakatos PL, Papp M, Arató A, Veres G. Intestinal alkaline phosphatase in the colonic mucosa of children with inflammatory bowel disease. World J Gastroenterol. 2012;18(25):3254–3259. doi: 10.3748/wjg.v18.i25.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malo J, Alam MJ, Shahnaz M, Kaliannan K, Chandra G, Aziz T, Sarker T, Bala M, Paul R, Saha CK, Karmakar PK, Malo MS. Intestinal alkaline phosphatase deficiency is associated with ischemic heart disease. Dis Markers. 2019;2019:8473565–8473511. doi: 10.1155/2019/8473565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malo MS. A high level of intestinal alkaline phosphatase is protective against type 2 diabetes mellitus irrespective of obesity. EBioMedicine. 2015;2(12):2016–2023. doi: 10.1016/j.ebiom.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janssens S, Beyaert R. Role of toll-like receptors in pathogen recognition. Clin Microbiol Rev. 2003;16(4):637–646. doi: 10.1128/cmr.16.4.637-646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldberg RF, Austen WG, Jr, Zhang X, Munene G, Mostafa G, Biswas S, McCormack M, Eberlin KR, Nguyen JT, Tatlidede HS, Warren HS, Narisawa S, Millán JL, Hodin RA. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci U S A. 2008;105(9):3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lallès JP. Microbiota-host interplay at the gut epithelial level, health and nutrition. J Anim Sci Biotechnol. 2016;7:66. doi: 10.1186/s40104-016-0123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang SW, Kim JH, Lee C, Im JP, Kim JS. Intestinal alkaline phosphatase ameliorates experimental colitis via toll-like receptor 4-dependent pathway. Eur J Pharmacol. 2018;820:156–166. doi: 10.1016/j.ejphar.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 54.Bates JM, Mittge E, Kuhlman J, Baden KN, Cheesman SE, Guillemin K. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev Biol. 2006;297(2):374–386. doi: 10.1016/j.ydbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Fawley J, Koehler S, Cabrera S, Lam V, Fredrich K, Hessner M, Salzman N, Gourlay D. Intestinal alkaline phosphatase deficiency leads to dysbiosis and bacterial translocation in the newborn intestine. J Surg Res. 2017;218:35–42. doi: 10.1016/j.jss.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 56.Malo MS, Alam SN, Mostafa G, Zeller SJ, Johnson PV, Mohammad N, Chen KT, Moss AK, Ramasamy S, Faruqui A, Hodin S, Malo PS, Ebrahimi F, Biswas B, Narisawa S, Millán JL, Warren HS, Kaplan JB, Kitts CL, Hohmann EL, Hodin RA. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut. 2010;59(11):1476–1484. doi: 10.1136/gut.2010.211706. [DOI] [PubMed] [Google Scholar]
- 57.Singh SB, Carroll-Portillo A, Coffman C, Ritz NL, Lin HC. Intestinal alkaline phosphatase exerts anti-inflammatory effects against lipopolysaccharide by inducing autophagy. Sci Rep. 2020;10(1):3107. doi: 10.1038/s41598-020-59474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lallès JP. Long term effects of pre- and early postnatal nutrition and environment on the gut. J Anim Sci. 2012;90(Suppl 4):421–429. doi: 10.2527/jas.53904. [DOI] [PubMed] [Google Scholar]
- 59.Martínez-Moya P, Ortega-González M, González R, Anzola A, Ocón B, Hernández-Chirlaque C, López-Posadas R, Suárez MD, Zarzuelo A, Martínez-Augustin O, Sánchez de Medina F. Exogenous alkaline phosphatase treatment complements endogenous enzyme protection in colonic inflammation and reduces bacterial translocation in rats. Pharmacol Res. 2012;66(2):144–153. doi: 10.1016/j.phrs.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Riggle KM, Rentea RM, Welak SR, Pritchard KA, Jr, Oldham KT, Gourlay DM. Intestinal alkaline phosphatase prevents the systemic inflammatory response associated with necrotizing enterocolitis. J Surg Res. 2013;180(1):21–26. doi: 10.1016/j.jss.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alam SN, Yammine H, Moaven O, Ahmed R, Moss AK, Biswas B, Muhammad N, Biswas R, Raychowdhury A, Kaliannan K, Ghosh S, Ray M, Hamarneh SR, Barua S, Malo NS, Bhan AK, Malo MS, Hodin RA. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann Surg. 2014;259(4):715–722. doi: 10.1097/SLA.0b013e31828fae14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larrick JW, Mendelsohn AR. Supplementation with brush border enzyme alkaline phosphatase slows aging. Rejuvenation Res. 2020;23(2):171–175. doi: 10.1089/rej.2020.2335. [DOI] [PubMed] [Google Scholar]
- 63.Kaliannan K, Hamarneh SR, Economopoulos KP, Nasrin Alam S, Moaven O, Patel P, Malo NS, Ray M, Abtahi SM, Muhammad N, Raychowdhury A, Teshager A, Mohamed MM, Moss AK, Ahmed R, Hakimian S, Narisawa S, Millán JL, Hohmann E, Warren HS, Bhan AK, Malo MS, Hodin RA. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2013;110(17):7003–7008. doi: 10.1073/pnas.1220180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Economopoulos KP, Ward NL, Phillips CD, Teshager A, Patel P, Mohamed MM, Hakimian S, Cox SB, Ahmed R, Moaven O, Kaliannan K, Alam SN, Haller JF, Goldstein AM, Bhan AK, Malo MS, Hodin RA. Prevention of antibiotic-associated metabolic syndrome in mice by intestinal alkaline phosphatase. Diabetes Obes Metab. 2016;18(5):519–527. doi: 10.1111/dom.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kühn F, Adiliaghdam F, Cavallaro PM, Hamarneh SR, Tsurumi A, Hoda RS, Munoz AR, Dhole Y, Ramirez JM, Liu E, Vasan R, Liu Y, Samarbafzadeh E, Nunez RA, Farber MZ, Chopra V, Malo MS, Rahme LG, Hodin RA. Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight. 2020;5(6):e134049. doi: 10.1172/jci.insight.134049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davidson JA, Khailova L, Treece A, Robison J, Soranno DE, Jaggers J, Ing RJ, Lawson S, Lujan SO. Alkaline phosphatase treatment of acute kidney injury in an infant piglet model of cardiopulmonary bypass with deep hypothermic circulatory arrest. Sci Rep. 2019;9(1):14175. doi: 10.1038/s41598-019-50481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Plaeke P, De Man JG, Smet A, Malhotra-Kumar S, Pintelon I, Timmermans JP, Nullens S, Jorens PG, Hubens G, De Winter BY. Effects of intestinal alkaline phosphatase on intestinal barrier function in a cecal ligation and puncture (CLP)-induced mouse model for sepsis. Neurogastroenterol Motil. 2020;32(3):e13754. doi: 10.1111/nmo.13754. [DOI] [PubMed] [Google Scholar]
- 68.Pickkers P, Mehta RL, Murray PT, Joannidis M, Molitoris BA, Kellum JA, Bachler M, Hoste EAJ, Hoiting O, Krell K, Ostermann M, Rozendaal W, Valkonen M, Brealey D, Beishuizen A, Meziani F, Murugan R, de Geus H, Payen D, van den Berg E, Arend J, STOP-AKI Investigators Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA. 2018;320(19):1998–2009. doi: 10.1001/jama.2018.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peters E, Stevens J, Arend J, Guan Z, Raaben W, Laverman P, Elsas AV, Masereeuw R, Pickkers P. Biodistribution and translational pharmacokinetic modeling of a human recombinant alkaline phosphatase. Int J Pharm. 2015;495(1):122–131. doi: 10.1016/j.ijpharm.2015.08.090. [DOI] [PubMed] [Google Scholar]
- 70.Peters E, Ergin B, Kandil A, Gurel-Gurevin E, van Elsas A, Masereeuw R, Pickkers P, Ince C. Effects of a human recombinant alkaline phosphatase on renal hemodynamics, oxygenation and inflammation in two models of acute kidney injury. Toxicol Appl Pharmacol. 2016;313:88–96. doi: 10.1016/j.taap.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 71.Brun LR, Brance ML, Rigalli A. Luminal calcium concentration controls intestinal calcium absorption by modification of intestinal alkaline phosphatase activity. Br J Nutr. 2012;108(2):229–233. doi: 10.1017/S0007114511005617. [DOI] [PubMed] [Google Scholar]
- 72.Brun LR, Brance ML, Lombarte M, Lupo M, Di Loreto VE, Rigalli A. Regulation of intestinal calcium absorption by luminal calcium content: role of intestinal alkaline phosphatase. Mol Nutr Food Res. 2014;58(7):1546–1551. doi: 10.1002/mnfr.201300686. [DOI] [PubMed] [Google Scholar]
- 73.Brun LR, Lombarte M, Roma S, Perez F, Millán JL, Rigalli A. Increased calcium uptake and improved trabecular bone properties in intestinal alkaline phosphatase knockout mice. J Bone Miner Metab. 2018;36(6):661–667. doi: 10.1007/s00774-017-0887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuehn F, Adiliaghdam F, Hamarneh SR, Vasan R, Liu E, Liu Y, Ramirez JM, Hoda RS, Munoz AR, Ko FC, Armanini M, Brooks DJ, Bouxsein ML, Demay MB, Hodin RA. Loss of intestinal alkaline phosphatase leads to distinct chronic changes in bone phenotype. J Surg Res. 2018;232:325–331. doi: 10.1016/j.jss.2018.06.061. [DOI] [PubMed] [Google Scholar]
- 75.Sasaki S, Segawa H, Hanazaki A, Kirino R, Fujii T, Ikuta K, Noguchi M, Sasaki S, Koike M, Tanifuji K, Shiozaki Y, Kaneko I, Tatsumi S, Shimohata T, Kawai Y, Narisawa S, Millán JL, Miyamoto KI. A role of intestinal alkaline phosphatase 3 (Akp3) in inorganic phosphate homeostasis. Kidney Blood Press Res. 2018;43(5):1409–1424. doi: 10.1159/000493379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamarneh SR, Mohamed MM, Economopoulos KP, Morrison SA, Phupitakphol T, Tantillo TJ, Gul SS, Gharedaghi MH, Tao Q, Kaliannan K, Narisawa S, Millán JL, van der Wilden GM, Fagenholz PJ, Malo MS, Hodin RA. A novel approach to maintain gut mucosal integrity using an oral enzyme supplement. Ann Surg. 2014;260(4):706–714. doi: 10.1097/SLA.0000000000000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rentea RM, Rentea MJ, Biesterveld B, Liedel JL, Gourlay DM. Factors known to influence the development of necrotizing enterocolitis to modify expression and activity of intestinal alkaline phosphatase in a newborn neonatal rat model. Eur J Pediatr Surg. 2019;29(3):290–297. doi: 10.1055/s-0038-1646959. [DOI] [PubMed] [Google Scholar]
- 78.Navis M, Muncan V, Sangild PT, Møller Willumsen L, Koelink PJ, Wildenberg ME, Abrahamse E, Thymann T, van Elburg RM, Renes IB. Beneficial effect of mildly pasteurized whey protein on intestinal integrity and innate defense in preterm and near-term piglets. Nutrients. 2020;12(4):1125. doi: 10.3390/nu12041125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu JC, Khodadadi H, Baban B. Innate immunity and oral microbiome: a personalized, predictive, and preventive approach to the management of oral diseases. EPMA J. 2019;10(1):43–50. doi: 10.1007/s13167-019-00163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Мokrozub VV, Lazarenko LM, Sichel LM, Babenko LP, Lytvyn PM, Demchenko OM, Melnichenko YO, Boyko NV, Biavati B, DiGioia D, Bubnov RV, Spivak MY. The role of beneficial bacteria wall elasticity in regulating innate immune response. EPMA J. 2015;6(1):13. doi: 10.1186/s13167-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vir P, Kaur J, Mahmood A. Effect of chronic iron ingestion on the development of brush border enzymes in rat intestine. Toxicol Mech Methods. 2007;17(7):393–399. doi: 10.1080/15376510601102793. [DOI] [PubMed] [Google Scholar]
- 82.Kaliannan K, Wang B, Li XY, Kim KJ, Kang JX. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci Rep. 2015;5:11276. doi: 10.1038/srep11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Šefčíková Z, Bujňáková D. Effect of pre- and post-weaning high-fat dietary manipulation on intestinal microflora and alkaline phosphatase activity in male rats. Physiol Res. 2017;66(4):677–685. doi: 10.33549/physiolres.933500. [DOI] [PubMed] [Google Scholar]
- 84.Montagne L, Toullec R, Savidge T, Lallès JP. Morphology and enzyme activities of the small intestine are modulated by dietary protein source in the preruminant calf. Reprod Nutr Dev. 1999;39(4):455–466. doi: 10.1051/rnd:19990405. [DOI] [PubMed] [Google Scholar]
- 85.Boudry G, Lallès JP, Malbert CH, Bobillier E, Sève B. Diet-related adaptation of the small intestine at weaning in pigs is functional rather than structural. J Pediatr Gastroenterol Nutr. 2002;34(2):180–187. doi: 10.1097/00005176-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 86.Thoreux K, Balas D, Bouley C, Senegas-Balas F. Diet supplemented with yoghurt or milk fermented by Lactobacillus casei DN-114 001 stimulates growth and brush-border enzyme activities in mouse small intestine. Digestion. 1998;59(4):349–359. doi: 10.1159/000007514. [DOI] [PubMed] [Google Scholar]
- 87.Montoya CA, Leterme P, Lalles JP. A protein-free diet alters small intestinal architecture and digestive enzyme activities in rats. Reprod Nutr Dev. 2006;46(1):49–56. doi: 10.1051/rnd:2005063. [DOI] [PubMed] [Google Scholar]
- 88.Sogabe N, Mizoi L, Asahi K, Ezawa I, Goseki-Sone M. Enhancement by lactose of intestinal alkaline phosphatase expression in rats. Bone. 2004;35(1):249–255. doi: 10.1016/j.bone.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 89.Okazaki Y, Katayama T. Glucomannan consumption elevates colonic alkaline phosphatase activity by up-regulating the expression of IAP-I, which is associated with increased production of protective factors for gut epithelial homeostasis in high-fat diet-fed rats. Nutr Res. 2017;43:43–50. doi: 10.1016/j.nutres.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 90.Okazaki Y, Katayama T. Consumption of non-digestible oligosaccharides elevates colonic alkaline phosphatase activity by up-regulating the expression of IAP-I, with increased mucins and microbial fermentation in rats fed a high-fat diet. Br J Nutr. 2019;121(2):146–154. doi: 10.1017/S0007114518003082. [DOI] [PubMed] [Google Scholar]
- 91.Erdijk O, van Baarlen P, Fernandez-Gutierrez MM, van den Brink E, Schuren FHJ, Brugman S, Savelkoul HFJ, Kleerebezem M, van Neerven RJJ. Sialyllactose and galactooligosaccharides promote epithelial barrier functioning and distinctly modulate microbiota composition and short chain fatty acid production in vitro. Front Immunol. 2019;10:94. doi: 10.3389/fimmu.2019.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakaoka K, Tanabe R, Oku Y. Influences of vitamin D restriction on alkaline phosphatase activity in rats fed a high-fat diet. J Jpn Soc Nutr Food Sci. 2016;69:57–63. doi: 10.4327/jsnfs.69.57. [DOI] [Google Scholar]
- 93.Nakaoka K, Yamada A, Noda S, Goseki-Sone M. Vitamin D-restricted high-fat diet down-regulates expression of intestinal alkaline phosphatase isozymes in ovariectomized rats. Nutr Res. 2018;53:23–31. doi: 10.1016/j.nutres.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 94.Sogabe N, Maruyama R, Hosori T, Goseki-Sone M. Enhancement effects of vitamin K1 (phylloquinone) or vitamin K2 (menaquinone-4) on intestinal alkaline phosphatase activity in rats. J Nutr Sci Vitaminol (Tokyo) 2007;53(3):219–224. doi: 10.3177/jnsv.53.219. [DOI] [PubMed] [Google Scholar]
- 95.Haraikawa M, Sogabe N, Tanabe R, Hosoi T, Goseki-Sone M. Vitamin K1 (phylloquinone) or vitamin K2 (menaquinone-4) induces intestinal alkaline phosphatase gene expression. J Nutr Sci Vitaminol (Tokyo) 2011;57(4):274–279. doi: 10.3177/jnsv.57.274. [DOI] [PubMed] [Google Scholar]
- 96.Ghosh SS, Gehr TW, Ghosh S. Curcumin and chronic kidney disease (CKD): major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules. 2014;19(12):20139–20156. doi: 10.3390/molecules191220139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghosh SS, He H, Wang J, Gehr TW, Ghosh S. Curcumin-mediated regulation of intestinal barrier function: the mechanism underlying its beneficial effects. Tissue Barriers. 2018;6(1):e1425085. doi: 10.1080/21688370.2018.1425085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghosh SS, Bie J, Wang J, Ghosh S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice--role of intestinal permeability and macrophage activation. PLoS One. 2014;9(9):e108577. doi: 10.1371/journal.pone.0108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghosh SS, He H, Wang J, Korzun W, Yannie PJ, Ghosh S. Intestine-specific expression of human chimeric intestinal alkaline phosphatase attenuates Western diet-induced barrier dysfunction and glucose intolerance. Phys Rep. 2018;6(14):e13790. doi: 10.14814/phy2.13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prakash UN, Srinivasan K. Beneficial influence of dietary spices on the ultrastructure and fluidity of the intestinal brush border in rats. Br J Nutr. 2010;104(1):31–39. doi: 10.1017/S0007114510000334. [DOI] [PubMed] [Google Scholar]
- 101.Pereira MT, Malik M, Nostro JA, Mahler GJ, Musselman LP. Effect of dietary additives on intestinal permeability in both Drosophila and a human cell co-culture. Dis Model Mech. 2018;11(12):dmm034520. doi: 10.1242/dmm.034520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Srigiridhar K, Nair KM. Iron-deficient intestine is more susceptible to peroxidative damage during iron supplementation in rats. Free Radic Biol Med. 1998;25(6):660–665. doi: 10.1016/s0891-5849(98)00086-0. [DOI] [PubMed] [Google Scholar]
- 103.Bubnov RV, Spivak MY, Lazarenko LM, Bomba A, Boyko NV. Probiotics and immunity: provisional role for personalized diets and disease prevention. EPMA J. 2015;6(1):14. doi: 10.1186/s13167-015-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reid G, Abrahamsson T, Bailey M, Bindels LB, Bubnov R, Ganguli K, Martoni C, O'Neill C, Savignac HM, Stanton C, Ship N, Surette M, Tuohy K, van Hemert S. How do probiotics and prebiotics function at distant sites? Benefic Microbes. 2017;8(4):521–533. doi: 10.3920/BM2016.0222. [DOI] [PubMed] [Google Scholar]
- 105.Bubnov RV, Babenko LP, Lazarenko LM, Mokrozub VV, Spivak MY. Specific properties of probiotic strains: relevance and benefits for the host. EPMA J. 2018;9(2):205–223. doi: 10.1007/s13167-018-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bubnov R, Babenko L, Lazarenko L, Kryvtsova M, Shcherbakov O, Zholobak N, Golubnitschaja O, Spivak M. Can tailored nanoceria act as a prebiotic? Report on improved lipid profile and gut microbiota in obese mice. EPMA J. 2019;10(4):317–335. doi: 10.1007/s13167-019-00190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abdelhamid AG, El-Masry SS, El-Dougdoug NK. Probiotic Lactobacillus and Bifidobacterium strains possess safety characteristics, antiviral activities and host adherence factors revealed by genome mining. EPMA J. 2019;10(4):337–350. doi: 10.1007/s13167-019-00184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Borges NA, Carmo FL, Stockler-Pinto MB, de Brito JS, Dolenga CJ, Ferreira DC, Nakao LS, Rosado A, Fouque D, Mafra D. Probiotic supplementation in chronic kidney disease: a double-blind, randomized, Placebo-Controlled Trial. J Ren Nutr. 2018;28(1):28–36. doi: 10.1053/j.jrn.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 109.Ermolenko E, Gromova L, Borschev Y, Voeikova A, Karaseva A, Ermolenko K, Gruzdkov A, Suvorov A. Influence of different probiotic lactic acid bacteria on microbiota and metabolism of rats with dysbiosis. Biosci Microbiota Food Health. 2013;32(2):41–49. doi: 10.12938/bmfh.32.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Symonds EL, O'Mahony C, Lapthorne S, O'Mahony D, Sharry JM, O'Mahony L, Shanahan F. Bifidobacterium infantis 35624 protects against salmonella-induced reductions in digestive enzyme activity in mice by attenuation of the host inflammatory response. Clin Transl Gastroenterol. 2012;3(5):e15. doi: 10.1038/ctg.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Said A, Desai C, Lerma EV. Chronic kidney disease. Dis Mon. 2015;61(9):374–377. doi: 10.1016/j.disamonth.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 112.Baumgarten M, Gehr T. Chronic kidney disease: detection and evaluation. Am Fam Physician. 2011;84(10):1138–1148. [PubMed] [Google Scholar]
- 113.Konopelniuk VV, Goloborodko II, Ishchuk TV, Synelnyk TB, Ostapchenko LI, Spivak MY, Bubnov RV. Efficacy of Fenugreek-based bionanocomposite on renal dysfunction and endogenous intoxication in high-calorie diet-induced obesity rat model-comparative study. EPMA J. 2017;8(4):377–390. doi: 10.1007/s13167-017-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mafra D, Lobo JC, Barros AF, Koppe L, Vaziri ND, Fouque D. Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease. Future Microbiol. 2014;9(3):399–410. doi: 10.2217/fmb.13.165. [DOI] [PubMed] [Google Scholar]
- 115.Vaziri ND. CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Curr Opin Nephrol Hypertens. 2012;21(6):587–592. doi: 10.1097/MNH.0b013e328358c8d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vaziri ND, Freel RW, Hatch M. Effect of chronic experimental renal insufficiency on urate metabolism. J Am Soc Nephrol. 1995;6(4):1313–1317. doi: 10.1681/ASN.V641313. [DOI] [PubMed] [Google Scholar]
- 117.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 118.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vaziri ND, Yuan J, Rahimi A, Ni Z, Said H, Subramanian VS. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol Dial Transplant. 2012;27(7):2686–2693. doi: 10.1093/ndt/gfr624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83(2):308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 121.Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31(6):817–844. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 122.Mafra D, Barros AF, Fouque D. Dietary protein metabolism by gut microbiota and its consequences for chronic kidney disease patients. Future Microbiol. 2013;8(10):1317–1323. doi: 10.2217/fmb.13.103. [DOI] [PubMed] [Google Scholar]
- 123.Lau WL, Kalantar-Zadeh K, Vaziri ND. The gut as a source of inflammation in chronic kidney disease. Nephron. 2015;130(2):92–98. doi: 10.1159/000381990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lehto M, Groop PH. The gut-kidney axis: putative interconnections between gastrointestinal and renal disorders. Front Endocrinol (Lausanne) 2018;9:553. doi: 10.3389/fendo.2018.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang Y, Rader E, Peters-Carr M, Bent RC, Smilowitz JT, Guillemin K, Rader B. Ontogeny of alkaline phosphatase activity in infant intestines and breast milk. BMC Pediatr. 2019;19(1):2. doi: 10.1186/s12887-018-1379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van Hoof VO, De Broe ME. Interpretation and clinical significance of alkaline phosphatase isoenzyme patterns. Crit Rev Clin Lab Sci. 1994;31(3):197–293. doi: 10.3109/10408369409084677. [DOI] [PubMed] [Google Scholar]
- 127.Alpers DH, DeSchryver-Kecskemeti K, Goodwin CL, Tindira CA, Harter H, Slatopolsky E. Intestinal alkaline phosphatase in patients with chronic renal failure. Gastroenterology. 1988;94(1):62–67. doi: 10.1016/0016-5085(88)90610-5. [DOI] [PubMed] [Google Scholar]
- 128.Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25(4):657–670. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vaziri ND, Zhao YY, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant. 2016;31(5):737–746. doi: 10.1093/ndt/gfv095. [DOI] [PubMed] [Google Scholar]
- 130.Esgalhado M, Kemp JA, Damasceno NR, Fouque D, Mafra D. Short-chain fatty acids: a link between prebiotics and microbiota in chronic kidney disease. Future Microbiol. 2017;12:1413–1425. doi: 10.2217/fmb-2017-0059. [DOI] [PubMed] [Google Scholar]
- 131.Koppe L, Fouque D. Microbiota and prebiotics modulation of uremic toxin generation. Panminerva Med. 2017;59(2):173–187. doi: 10.23736/S0031-0808.16.03282-1. [DOI] [PubMed] [Google Scholar]
- 132.Mafra D, Borges N, Alvarenga L, Esgalhado M, Cardozo L, Lindholm B, Stenvinkel P. Dietary components that may influence the disturbed gut microbiota in chronic kidney disease. Nutrients. 2019;11(3):496. doi: 10.3390/nu11030496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mafra D, Borges NA, Lindholm B, Shiels PG, Evenepoel P, Stenvinkel P. Food as medicine: targeting the uraemic phenotype in chronic kidney disease. Nat Rev Nephrol. 2020. 10.1038/s41581-020-00345-8. [DOI] [PubMed]
- 134.Johnson IT, Gee JM, Mahoney RR. Effect of dietary supplements of guar gum and cellulose on intestinal cell proliferation, enzyme levels and sugar transport in the rat. Br J Nutr. 1984;52(3):477–487. doi: 10.1079/bjn19840115. [DOI] [PubMed] [Google Scholar]
- 135.Kaur J, Madan S, Hamid A, Singla A, Mahmood A. Intestinal alkaline phosphatase secretion in oil-fed rats. Dig Dis Sci. 2007;52(3):665–670. doi: 10.1007/s10620-006-9384-x. [DOI] [PubMed] [Google Scholar]
- 136.Walker AW. Intestinal alkaline phosphatase in serum of patients on maintenance haemodialysis. Clin Chim Acta. 1974;55(3):399–405. doi: 10.1016/0009-8981(74)90015-1. [DOI] [PubMed] [Google Scholar]
- 137.De Broe ME, Bosteels V, Wieme RJ. Letter: increased intestinal alkaline phosphatase in serum of patients on maintenance haemodialysis. Lancet. 1974;1(7860):753–754. doi: 10.1016/s0140-6736(74)92980-8. [DOI] [PubMed] [Google Scholar]
- 138.Skillen AW, Pierides AM. Serum alkaline phosphatase isoenzyme patterns in patients with chronic renal failure. Clin Chim Acta. 1977;80(2):339–346. doi: 10.1016/0009-8981(77)90042-0. [DOI] [PubMed] [Google Scholar]
- 139.Stĕpán J, Havránek T, Jelínková E, Straková M, Skrha J, Pacovský V. Metabolic implications in the elevation of serum activity of intestinal alkaline phosphatase in chronic renal failure. Experientia. 1984;40(8):896–898. doi: 10.1007/BF01952015. [DOI] [PubMed] [Google Scholar]
- 140.Tibi L, Chhabra SC, Sweeting VM, Winney RJ, Smith AF. Multiple forms of alkaline phosphatase in plasma of hemodialysis patients. Clin Chem. 1991;37(6):815–820. doi: 10.1093/clinchem/37.6.815. [DOI] [PubMed] [Google Scholar]