Abstract
Historically and traditionally, it is known that sleep helps in maintaining healthy living. Its duration varies not only among individuals but also in the same individual depending on circumstances, suggesting it is a dynamic and personalized physiological process. It has been divided into rapid eye movement sleep (REMS) and non-REMS (NREMS). The former is unique that adult humans spend the least time in this stage, when although one is physically asleep, the brain behaves as if awake, the dream state. As NREMS is a pre-requisite for appearance of REMS, the latter can be considered a predictive readout of sleep quality and health. It plays a protective role against oxidative, stressful, and psychopathological insults. Several modern lifestyle activities compromise quality and quantity of sleep (including REMS) affecting fundamental physiological and psychopathosomatic processes in a personalized manner. REMS loss–induced elevated brain noradrenaline (NA) causes many associated symptoms, which are ameliorated by preventing NA action. Therefore, we propose that awareness about personalized sleep hygiene (including REMS) and maintaining optimum brain NA level should be of paramount significance for leading physical and mental well-being as well as healthy living. As sleep is a dynamic, multifactorial, homeostatically regulated process, for healthy living, we recommend addressing and treating sleep dysfunctions in a personalized manner by the health professionals, caregivers, family, and other supporting members in the society. We also recommend that maintaining sleep profile, optimum level of NA, and/or prevention of elevation of NA or its action in the brain must be seriously considered for ameliorating lifestyle and REMS disturbance–associated dysfunctions.
Keywords: Biomarkers, Healthy sleep, Neuropathology, Noradrenaline, Predictive preventive personalized medicine, REMS, Sleep loss, Sleep quality
Introduction
Traditional wisdom has suggested that “sound sleep reflects a healthy body, while a disturbed sleep reflects a troubled mind”. It appears to be appropriate and applicable even today on our daily routine to lead healthy life [1]. Sleep is a reversible physiological state, where the consciousness remains in a subdued state. It is a constitutive part of life where the body is relaxed; although the brain is responsive to autonomic stimuli, the threshold of responsiveness to voluntary stimuli is higher. As a matter of fact, optimum sleep can be regarded as an indispensable physiological process necessary for preserving the good health and well-being of an individual; however, its detailed mechanism of action is unknown. Insufficient sleep activates sleep-inducing mechanism(s) as part of homeostatic response that stimulates a compensatory increase in its intensity and duration [2].
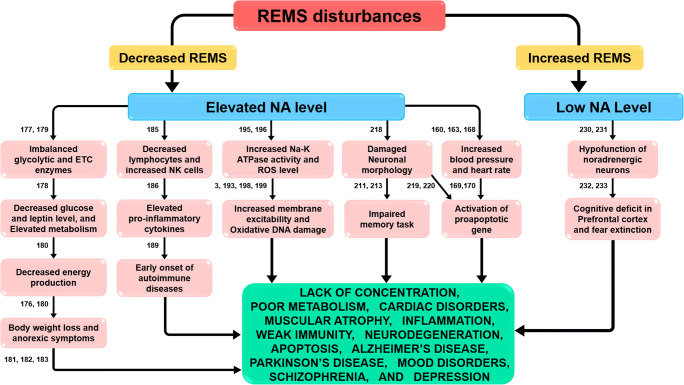
Sleep and wakefulness are subjective phenomena, which have been objectively classified based on the electrophysiological signals recorded from the scalp, eye, and neck muscles. The use of such objective criteria not only rejected the earlier passive theory of appearance of sleep but also established that sleep is a non-homogenous, active state. Based on such criteria, sleep has been objectively classified into rapid eye movement sleep (REMS) and non-REMS (NREMS). REMS is a unique physiological process expressed in all vertebrates studied so far, including humans, who spend the least amount of time in REMS. It plays an important role in maintaining the most physiological processes; it has been suggested to maintain the house-keeping function of the brain [3]. Disturbed REMS is among the common symptoms reported in almost all psychosomatic, neurological, cardiovascular and respiratory, metabolic dysfunctions and acute as well as chronic pathological conditions including Alzheimer’s (AD) and Parkinson’s (PD) diseases, mood disorders, depression, narcolepsy, epilepsy, cognitive impairment, trauma, infections, immune dysfunction, fever, trauma, hypertension, diabetes, endocrine, and other metabolic disorders [4–14] (Fig. 1).
Fig. 1.
REMS loss increases the level of noradrenaline (NA), which then affects several systems and induces several symptoms. The numbers correspond to the citation in the reference list. Abbreviations: ETC electron transport chain, NK cells natural killer cells, ROS reactive oxygen species, DNA deoxyribonucleic acid
Interactions among the cholinergic REM-ON neurons, the noradrenaline (NA)-ergic REM-OFF neurons, and the GABA-ergic neurons in the brainstem form the basic scaffold for the regulation of REMS [15]. The NA-ergic neurons are continuously active and cease activity during REMS, while they continue firing during REMS loss [3]. Upon REMS deprivation (REMSD), the level of NA increases in the brain [16] and that has been shown to induce many REMS loss–associated acute and chronic effects, e.g., hypertension, lack of concentration, confusion, irritability, hyperglycemia, memory loss, psychosomatic disorders, etc. [17–24]. Sleep (including REMS) plays an important role in regulating appetite [25], oxidative stress [26], energy conservation, lethargy [27], behavioral and emotional stability [28], reproduction [29], metabolism [30, 31], cognition [32], endocrine [33], immune function [34], thermoregulation [35], etc. (Table 1). Many of these deficiencies could be due to lack of quality sleep, which should be suspected in case of not maintaining the general health, daytime sleepiness, lack of concentration, psychological dysfunctions, reduced performance, etc. [47].
Table 1.
Recovery from diseases upon improvement in sleep quality. The numbers in parentheses show the cited article in the reference list
| Disorders | Effect upon sleep improvement | References |
|---|---|---|
| Cardiovascular dysfunctions | Lowers the increased heart rate observed upon REMSD and lowers the chances of cardiovascular risks | [36] |
| Metabolic disorders | Glucose level recovered back to normal level, recovery in fatigue | [37] |
| Impaired immunity | Increase in counts of lymphocytes, leukocytes, and natural killer cells after sleep recovery | [38, 39] |
| Oxidative stress | Normalized level of superoxide and stress hormones | [40] |
| Defective blood-brain barrier | Progressive recovery of damaged blood-brain barrier | [41, 42] |
| Neurodegeneration | Improvement of neuronal morphology in brain; sufficient duration of recovery normalizes visual motor task, memory task, and cognitive performances; however, short duration sleep recovery is not effective | [43, 44] |
| Schizophrenia | Improvement in sleep in individuals lessen the psychotic experiences, delusions, and anxiety | [45, 46] |
Sleep quality and sleep monitoring
Sleep quality has been referred to as a “complex phenomenon that is difficult to define and measure objectively” [48]. The sleep quality index is often evaluated by interviewing or asking subjects (or patients) to answer questionnaire about their sleep quality and daily life experiences, for example, the level of tiredness upon waking and through the day, motivation to get up from sleep in the morning, alertness through the day, clear headedness, concentration while working (including driving) throughout the day, sleep onset latency, and frequency of awakening at night [49, 50]. Subsequently, for confirmation and quantification of sleep loss, one’s sleep is monitored by polysomnography. Sleep monitoring by polysomnography is usually done through the night that analyzes snoring, breathing, heart rate, sleep interruptions, sleep phases including REMS, blood oxygenation, leg movements, etc. Of late, instead of polysomnography, several wearable devices have been designed to record several parameters including physical activity, rest, sleep, breathing, heart rate, and blood pressure among others throughout the day and night when people can perform one’s daily routine except while taking bath [51]. The sleep and activity profile are then correlated with other clinical symptoms and behavior of the subject/patient for deciding on the treatment [52, 53].
Role of psychosocial environment
As sleep loss affects many physiological processes, no single process is the primary beneficiary of sleep. In keeping pace with the modern lifestyle, we are constantly exposed to various psychosocial-environmental challenges, which are potentially capable to adversely affect our quality and quantity of sleep. Various psychosocial-environmental factors constantly interact with and often challenge our physiological processes affecting our health. If those factors, which are in dynamic equilibrium, either singly or synergistically overcome the dynamic threshold level to become stressful, various systems in the body react in a non-physiological manner to express acute symptoms. The silver lining is as the intensity of the input factors and the reactions of the body systems are dynamically active, changes in one or more of them may be suitably compensated by that of the others, resulting in reasonable maintenance of one’s health. However, if the stressful input(s) becomes more intense and/or lasts longer so that the cumulative inputs are stronger than the threshold, there may be sustained and/or organic changes in the body’s reacting systems resulting in chronic disorders. In this schema, sleep (which affects almost all the systems in the body) plays a significant role possibly acting as a sink by redistributing and keeping the output of the systems under control.
In addition to natural environmental factors, our interactions with family, friends, coworkers, and other acquaintances are also an important part of the psychosocial environment. A study demonstrated that emotional support from family, friends, and neighbors and quality sleep were negatively associated with insomnia and related symptoms like anxiety, depression, and suicidality [54], suggesting enjoying quality sleep should be a priority to lead a healthy life. Alcohol abuse and suicidality are among the possible obstructions to healthy sleep. Chronic exposure to alcohol increases latency of sleep onset and fragmentation of sleep [55], and chronic sleep disruption has been significantly correlated with an increased risk of suicidal thoughts, its attempt, and committing suicide [56, 57]. Therefore, providing personalized attention towards maintenance of overall healthy sleep habit is likely to offer protection for leading healthy living, and consequently, a record of personal sleep profile is likely to predict disorder-in-creation even before other signs and symptoms get expressed.
Unless consciously aware and careful, one may unknowingly become victim of sleep disturbances, which may tend to knock the body out of its homeostatic balance, resulting in disturbed physiology and disease condition(s). Although sleep loss/disturbance may affect most of the systems in the body, all the systems do not get affected equally and at the same time. Often the early acute signs are impairment of attention [58], concentration [59], and cognition [60]. Several factors like genetic make-up, age, gender, lifestyle, and psychosocial and environmental conditions (which include socio-economic status, eating habits, work environment, exercise, habitat, etc.), which affect an individual’s sleep profile, affect the susceptibility and vulnerability of an individual to several disease(s) [61–65]. The penetrance and expression of symptoms often vary depending on the loss of quality and quantity of total sleep and/or REMS. Normally, as NREMS precedes and is a pre-requisite for appearance of REMS, by-and-large estimation of REMS practically could be of prognostic, preventive, and personalized-predictive importance of multifactorial pathological conditions. Many of the associated symptoms have been proposed as part of predictive, preventive, and personalized medicine (PPPM) [66, 67], and recording and analysis of sleep profile might precede (may be non-specifically) appearance of other classically known symptoms, which may be added.
Personalized susceptibility
Disturbed sleep–related health problems are common; circadian misalignment is often seen in shift workers, nursing personnel, truck and train drivers, frequent travelers particularly due to ignoring circadian shifts and time-zones, etc. Gross estimation is that about one-third of the adult population in developed countries experience insufficient sleep, which can be due to work pressure at schools or offices, leading to increased weekend catching-up with the lost sleep [68]. The weekend sleep recovery process has become a common practice which may tend to overcome sleepiness and other sleep loss–related symptoms. Notwithstanding, the sleep recovery would not only depend on the quantity of lost sleep; more importantly, it would depend on the person’s sensitivity as well as predisposition.
At individual level, the quality and quantity of sleep (including REMS) have been reported to vary with gender and age of the subjects (among other factors). For example, women are more vulnerable to sleep disorders associated with daytime sleepiness and fatigue than are men. Also, sleep latency is longer in women than in men, and older women report about 20 min of less sleep per day than men [69]. Women are at about 40% increased risk of developing insomnia compared with men [70], and depression is more associated with sleep apnea in women than in men [71]. The ability of the brain to initiate and maintain sleep reduces with aging. Newborn human babies spend about 80% of the time in REMS, which gradually reduces with advancing age, and in adults, it occupies about 20% of the total sleep time [72]. Many older people complain of deteriorated sleep quality to various degrees as expressed by increased sleep latency, reduced sleep duration, increased sleep fragmentation, excessive daytime sleepiness, poor performance efficiency, and other disturbed sleep–related symptoms.
The discussions above offer sufficient ground to infer that it is not prudent to trade off sleep for apparent longer working hours keeping in mind the acute and chronic pathophysiological effects associated with direct and indirect costs involved in terms of loss of man-hours, efficiency, material, and finances at personal and at national levels. Despite significant advancement in medical and allied sciences, many questions related to healthy living, particularly related to sleep, REMS loss, and associated disorders, remain unanswered. Experimentally, this issue has been addressed by inducing sleep loss and studying its effects on various behavioral, systemic, cellular, and molecular parameters. However, one of the most important experimental limitations is that as REMS appears only after a period of NREMS, it is almost impossible to induce exclusive loss of NREMS beyond a few hours (depending on the experimental species, circumstances, predisposition, etc.) and to have appropriate control for a meaningful study to arrive at a conclusion. Thus, attempting to induce NREMS loss or total sleep loss effectively induces comparable results, depending on the duration of deprivation. On the other hand, selective REMS loss can be achieved to a reasonable extent. Although there are a few experimental limitations, the advantages are that adequate control experiments may be conducted. The experimental animals can also be deprived of REMS by about 85% [73]; thus, the advantages outweigh the limitations. Earlier, we have discussed in detail the advantages, disadvantages, and limitations of various methods of total sleep deprivation and REMSD. It has been concluded that as of now, experimental REMSD with its various controls is the most effective method to understand the function of REMS, which has advanced our knowledge significantly [74]; however, its detailed mechanism of action is unknown.
Based on current knowledge and our expertise, we expect that REMSD would affect its generating mechanism in the brain and vice versa, which would induce a change in the level of neurotransmitter, and the latter would play a major role in inducing REMSD-associated effects. It is also possible that any factor or input that would affect REMS mechanism or the neurotransmitter(s) might affect the REMS. Accordingly, in this review, we address the following: (a) how REMS maintains optimum level of NA in the brain, which is necessary for the well-being of an individual; (b) REMS loss–associated elevated NA affects physiological processes and induces associated pathophysiological changes; (c) based on these discussions, finally, we would conclude that healthy sleep habit (which includes REMS), by maintaining the level of NA in the brain, prevents imbalances and instabilities among physiological processes and retains physiological equilibrium resulting in healthy and quality life; (d) based on these facts, we recommend that for quality and healthy living, maintenance of record of personalized sleep hygiene and habit should be made a part of national education policy (particularly for training of the health workers and clinicians), and it should be added to the list of clinical history recording of patients [66].
Mechanisms of REMS regulation
Brain areas regulating REMS
To understand better the disturbance in the regulation of REMS, it is important that we describe briefly the basic scaffold of its neural regulation. REMS is characterized by simultaneous presence of desynchronized EEG, rapid eye movements, and muscle atonia. Transection, stimulation, and lesion studies showed that the pontine area is most crucial for the regulation of REMS [75, 76]. Transection made rostral or caudal to the pons showed that characteristic REMS signature signals were expressed only in the portion of the brain which remained connected with the pons [77, 78]. It was therefore concluded that “the pons is both necessary and sufficient to generate the basic phenomenon of REMS” [79]. Interactions of the neurons in the locus coeruleus (LC) and the laterodorsal and pedunculopontine-tegmentum (LDT/PPT) form the basic scaffold that plays a pivotal role in REMS regulation. The neurons from other brain regions like the perifornical area, preoptic area in the hypothalamus [80, 81], basal ganglia [82], nucleus accumbens, ventral tegmental area, amygdala [83], basal forebrain, and the prefrontal cortex [84, 85] modulate REMS possibly through these scaffold nuclei in the brain stem [86].
Based on the firing rate during REMS, the neurons have been classified as REM-ON (active during REMS) or REM-OFF (silent during REMS) [87–89]. The acetylcholine (ACh)-ergic neurons in LDT/PPT become active or increase firing (REM-ON), while the NA-ergic neurons in the LC cease firing (REM-OFF) during REMS. Among the aminergic neurons, isolated reports have shown that the serotonergic neurons in the dorsal raphe also often behaves like REM-OFF type [90–92], while dopaminergic neurons in the substantia nigra do not show a definite trend [93]. Isolated reports have indicated that some of the GABA-ergic neurons in the brainstem also behave like that of REM-ON type [94]. The REM-ON neurons are further classified as phasic and tonic types depending on whether they are active in bursts in association with the phasic appearance of eye movements and/or ponto-geniculo-occipital waves or tonically active throughout the muscle atonia. Although most REM-OFF neurons are usually monoaminergic, isolated reports of possibility of non-monoaminergic REM-OFF neurons have also been proposed [95]. The NA-ergic neurons in the LC cease firing during REMS, and they continue firing during experimental REMSD, while the REM-ON neurons show the opposite behavior [96]. This suggested that possibly the cessation of the LC REM-OFF neurons is a pre-requisite for the withdrawal of inhibition from the REM-ON neurons in the LDT/PPT, resulting in REMS generation [15]. Consistent research over two decades using electrical and chemical stimulation, inhibition of neurons, and behavioral, cellular, neurochemical, and molecular studies [3, 97–107] have confirmed this contention, which has led to construct a comprehensive model of neuronal connections for REMS regulation [15]. These findings from in vivo studies have been used to reconstruct a mathematical model, results of which are complementary [108]. The latter holds prognostic potential as well as it allows predicting neuroanatomical connections associated with normal and abnormal REMS.
LC neurons, their neurotransmitters, and REMS regulation
The LC neurons provide efferent projections to and receive afferent inputs from neurons all over the brain [109]; therefore, the LC neurons may be influenced directly or indirectly by most parts of the brain. These neuro-chemo-anatomical connections support as well as explain why sleep and REMS are affected (increased or decreased) in altered physiological states even before a full-blown disease sets in; REMS disturbance affects most physiological processes [109], while all psychosomatic dysfunctions and disorders are associated with disturbed sleep/REMS [110–113]. The neuronal function(s) depends on the release of neurotransmitters. As we have seen above that LC NA-ergic REM-OFF neurons occupy the pivotal position for REMS regulation and dysregulation, it is expected that disturbed REMS is likely to modulate the level of NA in the brain and vice versa. Further, for confirmation at least as a proof of principle, it needs to be shown if the REMSD-associated changes are mediated by elevated level of NA in the brain.
It has been reported that the level of NA indeed decreased in LC [16] and plasma [114] during REMS as compared with wakefulness, while it increased during REMSD. Upon REMSD, NA and GABA levels increased and decreased in all brain regions except hippocampus, and there was reciprocal relation between them [16]. It was proposed that one of the functions of REMS is to maintain low level of NA in the brain, which then maintains brain excitability and serves housekeeping functions of the brain [3]. Agonist and antagonist studies investigated the effects of various neurotransmitters on the firing of LC neurons. Infusion of GABA-antagonist, picrotoxin [115], baclofen [116], and bicuculine [117] into the LC decreased, while GABA and its agonist, muscimol, into the LC increased [98, 116, 118] the expression of REMS. ACh-ergic perfusion of the LC decreased [119], Orexin (Orx)-ergic agonist into the LC reduced [120], while knockdown of Orx-ergic receptors in the LC increased REMS [121].
Activation of LC neurons in rats by continuous low-frequency, mild electrical stimulation [100] reduced REMS, while upon recovery, there was a rebound increase in REMS. Recently, it has been shown that optogenetic stimulation of LC neurons reduced REMS and enhanced wakefulness [122]. These findings confirm that cessation of LC neurons is a pre-requisite for the appearance of REMS, and their activation results in REMS loss [107]. Stimulation of histaminergic [123], serotonergic [124], and Orx-ergic [125, 126] neurons increased wakefulness along with reduced REMS. The effects were expected to be due to activation of LC-neuronal projections [127–130]. Dopamine may modulate REMS; however, its detailed mechanism, particularly in relation to LC neurons, is not known [131]. The GABA-ergic neurons from the substantia nigra act presynaptically onto the LC NA-ergic terminals to regulate NA release over PPT ACh-ergic neurons and promote REMS initiation [108, 132]. The LC neurons also project onto the ACh-ergic and Orx-ergic neurons and, in turn, modulate physiological processes and REMS [83]. Infusion of serotonin into the LC inhibited the basal neuronal discharge, and the effect appears to be mediated by glutamatergic innervations [103, 130]. Thus, modulation of REMS by several neurotransmitters appears to be mediated at least by acting on the LC neurons and factor(s) which would affect LC neurons and would affect REMS. Additionally, as the LC neurons receive inputs from across the body and the brain, which are person specific, we propose that maintenance of REMS needs to be made part of preventive as well as personalized treatment protocols for healthy living. Further, subject to confirmation, higher level of NA might be suggestive of disturbed REMS.
Risk factors and causes of sleep disorders
Sleep (including REMS) is vital for maintaining normal physiological processes; its deficit and/or disturbances cause short- and long-term health consequences. Several factors, including genetic and environmental, contribute to influencing an individual’s susceptibility to sleep disorders [133, 134]. Sleep loss has been positively associated with cardiovascular and respiratory disorders, diabetes, obesity and metabolic disorders, depression, and Alzheimer’s, Parkinson’s, and neurodegenerative diseases [113]. Several components, like lifestyle changes (food habit, exercise, shift work etc.), environmental factors (light, noise, temperature, family/partner support etc.), drugs/chemicals of abuse (nicotine, caffeine, alcohol etc.), acute and chronic psychosomatic disturbances, and various pathophysiological and diseased conditions, contribute to sleep disruption. The altered level of NA has been observed in most of the cases [23, 135–161] (Table 2). Greater reliance on longer working hours, shift work, increased time spent on electronic accessories (television, internet, games, chatting, etc.) have encroached into our sleep time affecting quality and quantity of sleep, misalignment of circadian rhythm, and other comorbidities [167]. Advancing age, gender, and obesity are some of the risk factors of obstructive sleep apnea [162, 168]. It has been reported that adults with insomnia have higher levels of cortisol and adrenocorticotropic hormone [169] apparently suggesting a person’s sleep and insomnia record could be considered a non-invasive readout of a person’s pathophysiological state and stress level. Since quality and quantity of sleep (inverse of insomnia) are highly dynamic and individualistic expressions, they can serve as reliable personalized parameter for not only prevention and prediction but also for treatment and prognosis of recovery of an individual from acute as well as chronic diseases.
Table 2.
Several factors including social, environmental, lifestyle, habits, and some disorders affecting sleep-including REMS and NA levels. The numbers in parentheses show the cited article in the reference list
| Factors | Symptoms | Changes in NA levels | References | |
|---|---|---|---|---|
| Environmental and Lifestyle changes | Light: Usage of light and electronic appliances/gadgets prior to and during sleep | Affects sleep onset, sleep quality | Increased, in LC and paraventricular hypothalamus | [138, 139, 149] |
| Noise: Fragmented sleep because of noisy appliances or noisy environment | Daytime sleepiness, insomnia | Increased, in Serum and Urine | [146, 150, 151] | |
| Temperature: Extreme temperature disfavors sleep depth | Sleep disruption, especially REM sleep loss | Increased NA level in blood | [20, 147] | |
| Family/social support: Lack of family emotional support, childhood trauma | Psychological symptoms like anxiety, depression, suicidality, further effects sleep quality | Deficiency in NA and 5-hydroxytryptamine (5-HT) | [159] | |
| Food habit: Eating heavy food in night may lead to indigestion and affects sleep | Indigestion, frequent awakening, and insomnia | Increased level in plasma | [143–145, 147, 148] | |
| Shift work: Variable shifts timing in work | Circadian misalignment, insomnia or excessive sleepiness | Increased blood level of NA and cortisol | [149, 160] | |
| Disorders | Cardio-respiratory: Asthma, cough, sputum production | Sleep disturbance, increased insomnia | Increased level | [71, 162–164] |
| Stress, anxiety, and depression: Daily work stress, conditional anxiety | Light and brief episodes of sleep, difficult to achieve restful sleep, loss of interest and appetite, apathy, and suicidal thoughts | Extreme level of NA | [23, 71, 161, 165] | |
| Alzheimer’s: Aging, lifestyle, family history, genetics, and head injuries | loss of interest and appetite, dementia, and sleep/REMS disturbances | Decreased level in many brain areas (difference in depressed and non-depressed AD patients) but Increased level in CSF | [4, 19, 21, 158, 166] | |
| Parkinson’s: Exposure toxins, environmental factors, and genetic mutation | Impairs motor skills, sleep difficulties, cognitive problems | Loss of LC neurons and decreased level of NA in brain | [156, 157] | |
| Drugs/Chemicals abuse | Caffeine: Consumption during bedtime induces alertness, temporarily blocks adenosine receptor | Maintain alertness, decreases sleep quality | Level of noradrenaline increases in brain | [141, 142, 155] |
| Alcohol: Excessive drinking leads to increased awakening, sleep fragmentation | Increase in sleep-onset latency | Decrease in level of NA (however the release of NA is dependent on alcohol dose) | [142, 154] | |
| Nicotine: Intake (smoking) during sleep time lead to alertness | Maintain alertness, decreases sleep quality | Increases level of noradrenaline, inhibits monoamine oxidase in brain | [142, 152, 153] |
LC locus coeruleus, AD Alzheimer’s disease, NA noradrenaline, CSF cerebrospinal fluid
Preventive role of REMS in maintaining normal physiology and associated biomarkers
Circulatory system
Cardiovascular system (CVS) is responsible for transport (import as well as export) of vital molecules including oxygen, nutrients, lymphocytes, etc. throughout the body. It also mediates the absorption and excretion of toxic molecules to and out of the body. As insufficient sleep disturbs cardiovascular functioning, it may lead to several pathological symptoms and conditions [163], severity of which would depend on several factors including susceptibility and chronicity of exposure. In humans, sleep deprivation (SD) has been reported to be an important risk and predisposing factor for CVS disorders compromising longevity [170–172]. For example, patients who suffer from insomnia showed significantly dampened parasympathetic activation and increased sympathovagal imbalance [173] (Table 2). Kakadzic and Dement showed that REMSD induces a significant increase in the heart rate [174] and increased apoptosis of the heart muscles [175]. Additionally, there was lower basal cardiac function and less tolerance to myocardial ischemic-reperfusion injury induced by increased level of nitric oxide and apoptosis [175, 176] (Table 3). Upon REMSD, pain-associated exaggeration of blood pressure and increased heart rate responsiveness has been reported [196]. REMSD also has been shown to cause endothelial dysfunction and increased risk for hypertension [197, 198]; post-ischemic recovery of both systolic and diastolic functions is also affected [199]. In addition to the elevation of heart rate, REMSD also leads to increased respiratory rate [164]. Although sustained and chronic REMS loss affect CVS in otherwise healthy individual, acute REMS loss predisposes susceptible and vulnerable subjects, who may be affected by factors which may not affect otherwise normal subject (may be synergistic effect). As it is not easy to predict the predisposition (a dynamic factor) of an individual, advising maintenance of personal sleep profile (routine/diary) should be practiced by all, more stringently by the otherwise normal elderly as preventive, while by the patients as prognostic value.
Table 3.
Changes observed in biomolecules in association with altered sleep including REMS. The numbers in parentheses show the cited article in the reference list
| Biomolecules studied | Effect on the biomolecules | Tissue sample | Associated disorders | References |
|---|---|---|---|---|
| Caspase-3 | Activation of caspase-3 and downregulation of antiapoptotic gene | Brain | Oxidative DNA damage, ROS-induced carcinogenesis, neurodegeneration | [177, 178] |
| Reactive oxygen species (ROS) | ROS level increases, increased apoptosis | [179–181] | ||
| Na-K ATPase | Increased activity of Na-K ATPase and increased membrane excitability | [182, 183] | ||
| Corticosterone | Increased level | Plasma | [184] | |
| Leucocyte | Increased leucocyte count | Plasma | Higher titer of antinuclear antibodies, worsen immune functions, Early onset of Autoimmune and inflammatory diseases, modulate blood-brain barrier | [184] |
| Lymphocytes | Decreased number of lymphocytes | [184] | ||
| Natural killer (NK) cells | Increased number of NK cells | [185] | ||
| Cytokines | Elevation of pro-inflammatory cytokines (IL-6) | Plasma | [186] | |
| Complement C3 | Increased level | Serum | [184] | |
| Immunoglobulin-M (IgM) | Increased level | [184] | ||
| Immunoglobulin-A (IgA) | Decreased level | [187] | ||
| Nitric oxide | Higher expression of nitric oxide synthase and increased apoptosis in heart muscles | Heart ventricle | Myocardial ischemic-reperfusion injury, cardiac disorders | [175, 176] |
| Hexokinase | Increased activity | Brain | Decreased energy production, body weight loss, lethargy, skeletal muscular atrophy, anorexia | [188] |
| Glucose-6-phosphatase | Decreased activity | [188] | ||
| Glucose, glucose-6-phosphate, and pyruvate | Significant fall in their levels | Brain (cerebral frontal lobe) | [189] | |
| Succinate-CoQ reductase | Decreased in the activity | Brain (mitochondria of hippocampus and striatum) | [190] | |
| CoQH2-cytochrome c reductase | [190] | |||
| Cytochrome c oxidase | Brain (mitochondria of the prefrontal cortex, cerebral cortex, cerebellum, hippocampus, and striatum) | [190] | ||
| Citrate synthase | Increased activity | Brain (prefrontal cortex) | [190] | |
| Leptin | Decreased level | Plasma | Anorexia, depression, and Alzheimer’s disease | [189, 191–193] |
| Gut microbiota | Found in blood | Plasma | Inflammation and damage in the blood-brain barrier | [194, 195] |
| Noradrenaline (NA) | Increased release of NA in different brain areas | Brain, plasma, urine, and cerebrospinal fluid (CSF) | Associated with most of the disease mentioned above | [4, 16, 19, 21, 96, 158, 166] |
| Gamma amino butyric acid (GABA) | Increased level | Brain | [16] | |
| Acetylcholine | Decreased level | Brain | [96] |
A fundamental, dynamic property of the neurons is its excitability that affects the functions and responsiveness of the brain. LC NA-ergic neurons project all over the brain modulating the latter’s excitability and influencing voluntary as well as involuntary physiological processes, including the CVS. Also, NA is an important factor for the regulation of autonomic processes (Table 2). The (over) activation of NA-ergic neurons projecting rostrally from the brainstem is responsible for NA spill-over and that might mediate REMS disturbance-associated sympathetic nervous system activation–induced heart failure [200]. In vivo studies have shown that high dose of NA has a cytotoxic and apoptotic effect on cardiac fibroblast by the activation of caspase-3 and downregulation of the antiapoptotic gene [177, 178] (Table 3). The NA-induced effects were confirmed by the fact that NA-ergic blockers reduced the effects [201]. The dysregulation of CVS-functioning upon REMSD (or vice versa) can subsequently affect other physiological systems and processes. Additionally, recovery from sleep loss has been shown to normalize the increased heart rate and reduces the chances of cardiovascular risk [36] (Table 1). Therefore, we propose that at personal level, REMS disturbance must be consciously and carefully prevented to avoid as well as recover from CVS dysfunction where sympathetic tone is already disturbed. Also, for prevention, one must enjoy adequate sleep (NREMS as well as REMS) to lead healthy living.
Bioenergetics
Sleep, being an essential and natural process, has a fundamental role in the regulation of energy balance of the body. Chronic and acute sleep loss leads to energy balance disturbances [202–204]. Animals upon REMSD have been shown to have a progressive loss of body weight and show hypoglycemic symptoms [205]. REMSD has a major impact on glucose metabolism and mitochondrial electron transport chain and subsequently on the generation of energy in the form of adenosine tri-phosphates (ATPs). The hexokinase (a glycolytic enzyme) activity has been found to be increased upon REMSD; however, glucose-6-phosphatase (a gluconeogenic enzyme) activity was decreased in the brain [188], and these effects may be mediated through NA. These changes in enzyme activities may help in explaining a significant fall in glucose level, glucose 6-phosphate, and pyruvate as observed in cerebral frontal lobe upon REMSD [189]. A study on rats has shown that REMSD caused a significant decrease in the activity of complex II (i.e., succinate-CoQ reductase) and III (i.e., CoQH2-cytochrome c reductase) in the mitochondria of hippocampus and striatum [190]. The activity of complex IV (i.e., cytochrome c oxidase) was also decreased in the mitochondria of the prefrontal cortex, cerebral cortex, cerebellum, hippocampus, and striatum; however, citrate synthase (Kreb’s cycle enzyme) activity was increased in the prefrontal cortex [190]. On the other hand, the oxygen consumption increased upon REMSD, which represent elevated metabolic rate, while serum leptin level decreased and remained suppressed upon REMSD [189]. Studies have also shown that low leptin level in plasma is associated with anorexia, depression, and Alzheimer’s disease [191–193]. Yujra et al. have shown that REMSD causes skeletal muscular atrophy and defects in masticatory muscles [206] (Table 3). Thus, REMS loss may reduce energy production and increase energy consumption, which might explain at least some of the REMSD-associated muscular weakness, lethargy, and tiredness. REMS loss has a global impact on energy metabolic pathways, from digestion to electron transport chain (ETC). Since metabolism is significantly affected by REMS loss, maintaining sleep routine and compensation of lost sleep would bring the metabolism to optimum level and prevent dysregulation. Thus, along with other measures, optimizing sleep (including REMS) of individuals is likely to maintain physiological homeostasis leading to better health and help recovery from sickness. Improved sleep has been shown to help glucose metabolism and recovery from fatigue [37] (Table 1). Importantly, as these effects would be significantly influenced by the person’s predisposition and acclimatization, maintaining proper sleep profiling is strongly recommended to lead healthy living. In other words, it can be suggested that altered sleep and REMS might prove to be of predictive and prognostic value for disturbances in the metabolic machinery (enzymes and biomolecules), while as a corollary, improved sleep/REMS quality should serve as a prognostic value [189, 205].
Immunity
Sleep disturbance disbalances conjoint physiological processes and that may compromise homeostatic processes, including immunity in the body. REMSD has been reported to decrease the percentage of T-lymphocytes and induced a significant increase in natural killer (NK) cells in plasma [185]. This may provoke major changes in the immune system by inducing inflammation. The plasma levels of pro-inflammatory cytokines and markers get significantly elevated in REMS-deprived rats [186]. REMSD leads to increased complement C3 (in serum), corticosterone concentration, spleen weight, total leukocyte counts, and decreased lymphocyte count in plasma. However, the production of a certain class of immunoglobulin, the IgM, increased in the serum [184]. In another study, serum IgA levels decreased during the entire period of REMSD in human volunteers [187]. REMSD exhibits an early onset of an autoimmune disease in mice which resembles systemic lupus erythematosus in humans. The disease onset was reflected by exhibiting high titers of antinuclear antibodies [207] (Table 3). Taken together, the immune responses are improved by REMS, while REMSD-associated worsening of immune function could underlie or aggravate inflammatory and autoimmune diseases, which may be improved by maintaining healthy sleep profile. As many of the immune effects could be modulated and/or induced by NA [18, 208], subject to confirmation, at least some of the REMSD-associated altered immune responses could be due to elevated level of NA.
Thus, sleep loss, including REMS loss, compromises immune responses, which further aggravates the existing disease and/or onset of person-specific autoimmune diseases. The sleep loss–related aggravation may be supported by the fact that recovery of lost sleep has been shown to improve or reset the impaired immune functions as evidenced by regaining towards normal counts of leucocyte, lymphocyte, and NK cells, while reducing the levels of pro-inflammatory cytokines [38, 39] (Table 1). As recent studies have correlated compromised immunity with disturbed sleep, we propose that conscious efforts are needed to spread awareness among both the patients as well as healthy population to maintain healthy sleep hygiene. Although details need to be worked out, personalized sleep monitoring could be used as a predictive and prognostic measure to maintain good health and to recover from diseases associated with compromised immunity; additionally, optimum personal sleep habit would prevent disbalance or maintain homeostasis of personal immunity and immune responses.
Oxidative stress, membrane potential, and brain excitability
REMSD altered neuronal excitability; some reported increase, while other decreased excitability. These may be explained as REMSD could be either a cause or effect of increased neuronal excitability. REMSD indeed increased neuronal depolarization [209], which is likely to cause increased brain excitability. Upon REMSD, neuronal excitability was severely reduced in CA1 neurons, and the production of long-term potentiation of synaptic strength was inhibited [210]. In pyramidal cells of layer V/VI of the medial prefrontal cortex, the miniature excitatory postsynaptic current amplitude was slightly reduced, and intrinsic membrane excitability was increased [182]. REMSD increased the Na-K ATPase enzyme activity in the rat brain; the pons and the medulla (where the REMS regulatory REM-ON and REM-OFF neurons are located) were the first sites to be affected [183]. REMSD induced ROS generation and caused oxidative DNA damage, apoptosis, and ROS-induced carcinogenesis [179–181]. The REMSD-associated increased membrane potential, ROS generation, and apoptosis have been shown to be mediated by the elevated level of NA in the brain [3, 209, 211] (Table 3). Normalized levels of superoxides and stress hormones have been reported upon sleep recovery, which signals the necessity of quality sleep [40] (Table 1). Collectively, loss of NREMS and REMS would affect the brain excitability, which in turn would affect physiological processes and homeostasis. Therefore, the time spent in quality and quantity of sleep is likely to act as a buffer to mitigate the adverse effects including apoptosis. It would also re-align excitability of the neurons in the brain and maintain homeostasis. Thus, the quantity of disturbed REMS (and total sleep for that matter) should be of prognostic value. As a corollary, we expect that enjoying optimum REMS should offer protection and prevent an individual from future disorders. Further, as mechanism of action, we know that many of these changes would be modulated by level of NA in the brain [16, 74, 109].
Blood-brain barrier
Endothelial cells, pericytes, astroglia, and microglia are the major constituents of the blood-brain barrier (BBB). It selectively transports molecules and protects the brain from potentially toxic blood-borne molecules, which may modulate reactivity and induce apoptosis of the neuronal and glial cells. REMS restriction has been shown to increase the caveolae and permeability of the BBB, which has been shown to be restored rapidly and effectively even upon a brief period of sleep recovery [212]. In another study, it was observed that in the hippocampus, the number of pinocytic vesicles had been increased by threefold when animals were subjected to REMSD, which suggests the possibility of compromised integrity of the BBB [41]. Additionally, sleep loss leads to an increase in pro-inflammatory mediators (IL-6) in plasma, which affect the tight junction proteins and promote changes in cellular components of the BBB, particularly in brain endothelial cells [213] (Table 3). Gut microbiota has been detected in blood after sleep loss, which causes induction of inflammation, and the BBB becomes sensitive to these microbiotas [194, 195]. The effect of NA (acting through α-adrenoceptor) on BBB permeability has been studied by stereotactic injection of NA in the rat’s cerebral ventricle. It increased the BBB permeability, which eventually increased the pinocytic activity of endothelial cells in cerebrum [214]. Therefore, REMSD-associated BBB permeability may also be mediated by the elevated level of NA, which needs to be studied. These isolated findings may be supported by the fact that improved sleep has been reported to induce progressive recovery in permeability of BBB [41, 42] (Table 1). In conclusion, a compromised BBB is commonly reported in sleep-deprived subjects. As infiltration of pathogens and toxins into the brain is related to BBB permeability, sleep loss might facilitate the process. Thus, on one hand, for prevention and protection from diseases particularly where the brain may be affected due to compromised BBB, the public at large must be educated about ensuring optimum quality and quantity of sleep. On the other hand, while treating, patients must be advised maintaining proper sleep habit and hygiene for effective treatment of all disorders and comorbidities. Disturbed sleep should ring the initial warning bell of anticipating possible future disorder(s), and thus, it should serve as an important predictor. Therefore, we strongly recommend that in addition to other steps [67], taking care of personal sleep habit and hygiene should be considered as a priority by all, the healthy persons, the patients, and the caregivers for maintaining healthy living and for effective recovery.
Neurodegeneration and cognition
The percentage of REMS remains stable in normal aging; however, it is reduced in Alzheimer’s patients [166, 215]. Sleep plays a crucial role in brain development, memory consolidation after the acquisition of information, and cognition [216, 217]. Total sleep loss and/or REMSD leads to impaired concentration, decision-making, mood disturbances, psychomotor vigilance performance, anxiety-like behavior, and working memory dysfunction [79, 218–221] (Fig. 2). At least some of these effects could be due to changes in neuroinflammation, microglial activation, neuronal apoptosis, and a significant reduction in glucocorticoid-induced neurogenesis [43, 222–224]. Several studies have demonstrated that REMSD drastically impaired object-recognition test [225, 226], decreased motivation for food reward [227], and decreased break-point for sucrose pellet reinforcement [228]. REMSD affects CA1 cholinergic muscarinic receptors, which may be responsible for REMSD-associated amnesia [229] and emotional responsiveness. In a human study, REMSD-induced enhanced emotional reactivity has been found in the visual emotional reactivity task [230], which could be due to the enhanced activity of the brain regions involved in emotional processing, such as occipital and temporal areas and the ventrolateral prefrontal cortex [230] (Table 3). Thus, accumulation of the factors due to aging-associated gradual REMS loss could predispose aging-associated memory as well as other deficits and neurodegenerative disorders. Several studies have shown that sufficient recovery of sleep is positively associated with improvement in damaged neuronal morphology, memory, and cognitive performances [43, 44] (Table 1). Analysis of sleep profile could prove to be of predictive and prognostic value for taking appropriate steps at personal level at all ages, more so for the aged population.
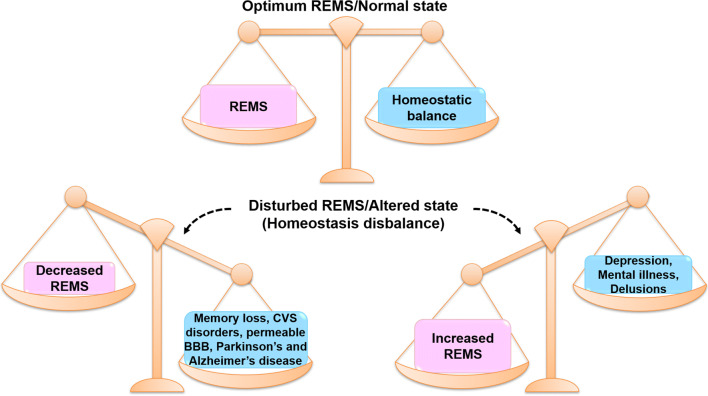
Fig. 2.
This figure shows that the optimum level of REMS maintains physiological homeostasis. Both increased and decreased REMS disbalance the said homeostasis, which results in dysregulation and disorder (pathological condition). Decreased or increased REMS induces different sets of disorders depending on various factors including susceptibility and surrounding conditions. Abbreviations: CVS cardiovascular system, BBB blood-brain barrier
Psychiatric ailments and REMS
Depression
Depression is a common mental health disorder that co-exists with other physical and psychiatric comorbidities. REMS may influence emotions, and disturbed REMS causes emotional distress [28]. There is a strong correlation between altered REMS density, REMS latency, and the onset of depression [165]. Reduced REMS latency, increased duration of REMS, and increased frequency of eye movements are some of the characteristic clinical symptoms of patients with major depressive disorder. Most of the antidepressants target REMS, and thus, REMS suppression might be a key mechanism underlying antidepressant treatment [231]. Depression-associated changes in REMS has been proposed to be mediated by complex interplay of NA-ergic, serotonergic, and cholinergic systems. Reduced activity of the monoaminergic neurons has been traditionally accepted as the cause of depression [232–235]. Antidepressants inhibit re-uptake of the monoamine neurotransmitters, inhibit monoamine oxidase (which degrades NA), and/or antagonize the inhibitory presynaptic NA-ergic auto-receptors [23]. These factors enhance the availability of NA and NA-mediated neurotransmission resulting in amelioration of depressive symptoms. Thus, we propose conducting suitable study to confirm that keeping a record of experiencing quantum of REMS is likely to help in assessing the depth of depression and vice versa, or that the quantity of REMS may have a prognostic value associated with improvement from depression, or that record of optimum REMS might help to protect an individual from depression if the REMS or associated problems are identified and addressed early enough.
Schizophrenia
Schizophrenia is characterized by hallucinations, delusions, and cognitive impairments [236–238]. Deficiency of GABA-ergic neuronal functioning with or without associated dysregulation of other neurotransmitters is thought to be one of the important correlates of this disorder [239–242]. Decreased expression of the glutamic acid decarboxylase-1 gene, which encodes for the 67-kDa glutamate decarboxylase enzyme and is involved in the synthesis of GABA, is commonly seen in schizophrenic postmortem brains [243, 244]. A few studies have reported alterations in slow-wave sleep, along with reduced REMS latency and REMS density [245, 246]. Most of the patients with schizophrenia report abnormal sleep pattern, which includes insomnia, obstructive sleep apnea, restless leg syndrome, or periodic limb movement disorder [247]. Treatment with antipsychotic drugs is associated with an increase in total sleep time and sleep efficiency and is often associated with increased REMS latency [247]. It has been proposed that cholinergic hyperactivity (muscarinic super-sensitivity) could be responsible for the shortened REMS latency in schizophrenic patients [248], while some evidence suggests that schizophrenic patients are at high risk of sleep-related breathing disorder [245]. Mannaa and Walker [249] have proposed that schizophrenia is like a dream-attack of REMS while awake; however, the detailed mechanism needs further study. These contentions may be supported by the report that improvement in sleep in individuals lessens the symptoms associated with psychotic experiences, delusions, and anxiety [45, 46] (Table 1). Thus, due to the clinical association of disturbed sleep in schizophrenia, personalized intervention to improve quality and quantity of sleep and REMS should be seriously considered during assessment of patients [67].
REMS mechanisms for dream episodes
Appearance of vivid dreams is among the most intriguing and fascinating phenomenon yet unexplained aspect of sleep. Dreaming is governed by elements of apparently sleeping brain, which are less responsive to external stimuli. In other words, dreaming may be termed as a cognitive phenomenon of the subconscious mind (!), and it is the activity of the brain, by the brain and for the brain. The field is still open for neurobiological explanation of why and how we dream and what purpose it serves. It has been proposed that the brain interprets the activity of the neuronal network that results into thought during waking, while possibly a similar phenomenon during sleep results into dreaming, which helps memory consolidation. Although both states, REMS or NREMS, can provide the framework for the expression of dreams, usually, subjects report most dreams (at least those we remember upon waking) during REMS.
Diverse emotional content and vivid description associated with REMS are suggestive of the involvement of multiple brain regions and neurotransmitters. It has been reported that subjects awakened during REMS recalled an emotional and vivid description of dreams as compared with those awakened during NREMS [250]. Several studies have reported a positive role of REMS-associated dreams in memory consolidation, mood regulation, and assimilation of new experiences. A relationship among REMS, dreams, and facial expressions during sleep in newborns, normal adults, and patients (adults) with REMS behavior disorder (RBD) was assessed. It was observed that almost half of the facial expressions were temporally associated with rapid eye movements associated with REMS, and the happy facial expression was associated with a happy dreaming scenario [250]. Half of the RBD patients smiled while one-third laughed mostly during REMS. This high frequency of happy emotional expression during RBD suggests that the RBD model can provide insights into the emotional component of REMS. This can also facilitate the understanding of mood regulation and emotional desensitization during sleep.
Almost all psychological and psychiatric disorders are associated with disturbed REMS, and improvement in sleep quality has been reported to improve depression and hallucination. Some of the psychological symptoms, e.g., hallucinations, RLS, etc., are apparently comparable to REMS-associated behavioral expressions, e.g., dreams. Recent studies also show that the brain areas known to modulate hallucinations also modulate REMS, and experimental REMSD may induce hallucination-like symptoms [251, 252]. Therefore, we propose that a record of sleep profile is likely to have predictive and prognostic value of one’s suffering from psychological disorders. Also, appropriate personalized advice to the patients to maintain quality and quantity of sleep should play a significant role in preventing and ameliorating pathophysiological conditions and diseases.
Elevated NA might be the cause of REMS loss–associated effects
We have seen above that acute as well as chronic REMS loss affects most (if not all) physiological processes in the body, and conversely, REMS is affected in most disorders. As a mechanism of action, it has been shown that upon REMSD, at least the level of NA is elevated in the brain, which otherwise is reduced during REMS. Many isolated as well as systematic control and clinical studies have shown that elevated NA is a cause for inducing many of the REMSD-associated symptoms. In support, as a proof of principle, it has been shown that if the NA was not allowed to be synthesized in the brain where REM-OFF neurons are located and then the rats were REMS deprived, the REMSD-associated symptoms were prevented [253]. Notwithstanding, although experimentally we have shown in animal models, it needs to be confirmed in normal humans as well as patients with various comorbidities before applying the knowledge for treatment on humans. Based on these and other related studies in normally behaving animal models, we reiterate our proposition that through evolution, REMS has evolved to maintain the brain level of NA, which protects as well as maintains the housekeeping function(s) of the brain, while REMSD-associated elevated NA is at least one of the primary factors for inducing REMSD-associated disorders and associated symptoms [3, 211, 224].
Conclusion and recommendations
The importance of sleep in maintaining good health has been emphasized in many ways in ancient literatures of all cultures. REMS is an essential component of sleep, at least in higher animals including humans; the appearance of NREMS precedes and is a pre-requisite for appearance of REMS. An adult spends the least amount of time in REMS, and it may be considered an effective readout of sleep status of an individual. Disturbed REMS is a common symptom in most disorders, and many of the symptoms of those disorders can be seen upon experimental REMSD.
We propose that maintenance of optimum quantity of sleep and REMS should be a priority for leading quality life. Furthermore, subject to confirmation in humans, we recommend that evaluation of daily REMS pattern is likely to prove to be of prognostic and predictive significance for many disorders and overall health of an individual. Appropriate personalized advice on maintaining adequate (optimum) sleep paradigm is likely to have synergistic effect along with or without medication for amelioration of comorbidities with or without sleep disturbance.
Based on our review of the literature and expertise, we expect that conscious attention towards experiencing optimum sleep–including REMS might prevent either or several of the following: for example, falling sick, predicting veracity of many diseases, reduce the dose and length of treatment, facilitate one’s recovery from comorbidities and diseases, etc. In fact, even in modern treatment and management of diseases, spending quality time in sleep is being advised in several diseases including viral infections and many psychosomatic disorders, etc. It has been consistently shown in animal studies that the level of NA plays a significant role in regulating REMS, and adequate REMS maintains optimum NA level in the brain. It has been shown that many of the symptoms either associated with diseases where REMS is also affected, or associated with REMSD, are indeed induced by NA. Therefore, subject to confirmation in humans, we propose that REMS loss–associated effects could be ameliorated by reducing the synthesis or elevation of the level of NA and/or by preventing the action of the elevated NA; the former is more desirable though.
As expression of quantity of sleep is a dynamic personalized phenomenon and its disturbance may result in psychosomatic as well as metabolic disturbances (and vice versa) in a person-specific manner, we recommend that personalized sleep issues be essentially considered as a predictive indicator, as well as preventive and protective shield as the case may be. Further, as the effects due to sleep disturbance would affect interpersonal relationships, sleep loss may become a social nuisance, which is not being considered seriously as yet. Hence, awareness about sleep health and sleep hygiene, preferably at personalized level, should be made a part of formal and informal academic courses, curricula, and training at all levels. Such awareness programs among individuals of every section of the society including the primary, middle, and high school students; parents; students for higher studies/trainees; drivers; pilots; nurses; night-shift workers; management bosses; expectant mothers; medical professionals; policymakers; and so on [66], and it should form a part of the national policy. As members of the global society, we appear to be already late in taking up the issue; we must initiate action on a priority basis, comparable to the global concerns raised by other issues, such as environmental pollution, global warming, terrorism, smoking, alcoholism, drug abuse, climatic changes, and so on. Finally, here, we summarize a few recommendations as suggested by many authors in the reviewed literature, for enjoying good sleep, which would encourage healthy living:
Healthy sleep routine must be practiced by all normal individuals of all ages as well as patients.
Routine time schedule for going to bed and waking must be maintained. Routine relaxing activity before bedtime (e.g. reading non-serious books and enjoying TV programs) is advisable.
A quiet, dark, non-cluttered (with unnecessary gadgets e.g. TV, computer, phone, etc.) room with a comfortable bed is needed [136–139].
Strenuous physical exercise and psychological and emotional stress, which may release excess NA and other stress hormone(s), should be avoided until about at least 4 h prior to going to bed [140, 254].
Consumption of caffeine, alcohol, nicotine, and other chemicals that interfere with sleep must be avoided within several hours (depending on one’s sensitivity) before bedtime [141, 142].
Stimulatory and high caloric intake should be avoided close to bedtime [143–145].
We strongly recommend keeping record of sleep profile by every individual.
Also, maintaining sleep profile should be in-built in the course curriculum of every level of health professionals, administrators, managers, etc.
Finally, if the sleep disturbances and difficulties do not improve through good sleep practices, consulting a sleep specialist is highly recommended.
Acknowledgments
Research support to BNM was received from Indian funding agencies viz. J. C. Bose fellowship, Department of Science and Technology (DST), Department of Biotechnology (DBT), and University Grants Commission (UGC) and Institutional Umbrella support over the years under the schemes DBT-BUILDER, DST-Improvement of Science and Technology Infrastructure (FIST) and Promotion of University Research and Scientific Excellence (PURSE), UGC-University with Potential for Excellence in Focus Area (UPEII), Departmental Research Support (DRS) and Networking.
Abbreviations
- ACh
acetylcholine
- AD
Alzheimer’s disease
- GABA
gamma-aminobutyric acid
- IgA
immunoglobulin-A
- IgM
immunoglobulin-M
- LC
locus coeruleus
- NA
noradrenaline
- NK cells
natural killer cells
- NREMS
non-rapid eye movement sleep
- Orx
orexin
- PD
Parkinson’s disease
- PPPM
predictive, preventive, and personalized medicine
- REMS
rapid eye movement sleep
- RBD
REMS behavior disorders
- REMSD
rapid eye movement sleep deprivation
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reynolds CF. 3rd. Troubled sleep, troubled minds, and DSM-5. Arch Gen Psychiatry. 2011;68(10):990–991. doi: 10.1001/archgenpsychiatry.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benington JH. Sleep homeostasis and the function of sleep. Sleep. 2000;23(7):959–966. doi: 10.1093/sleep/23.7.1j. [DOI] [PubMed] [Google Scholar]
- 3.Mallick BN, Singh A. REM sleep loss increases brain excitability: role of noradrenaline and its mechanism of action. Sleep Med Rev. 2011;15(3):165–178. doi: 10.1016/j.smrv.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Bliwise DL. Sleep disorders in Alzheimer’s disease and other dementias. Clin Cornerstone. 2004;6(Suppl 1A):S16–S28. doi: 10.1016/s1098-3597(04)90014-2. [DOI] [PubMed] [Google Scholar]
- 5.Pawlyk AC, Jha SK, Brennan FX, Morrison AR, Ross RJ. A rodent model of sleep disturbances in posttraumatic stress disorder: the role of context after fear conditioning. Biol Psychiatry. 2005;57(3):268–277. doi: 10.1016/j.biopsych.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Breslau N. Neurobiological research on sleep and stress hormones in epidemiological samples. Ann N Y Acad Sci. 2006;1071:221–230. doi: 10.1196/annals.1364.017. [DOI] [PubMed] [Google Scholar]
- 7.Breslau N, Roth T, Burduvali E, Kapke A, Schultz L, Roehrs T. Sleep in lifetime posttraumatic stress disorder: a community-based polysomnographic study. Arch Gen Psychiatry. 2004;61(5):508–516. doi: 10.1001/archpsyc.61.5.508. [DOI] [PubMed] [Google Scholar]
- 8.Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16(4):622–630. doi: 10.1002/mds.1120. [DOI] [PubMed] [Google Scholar]
- 9.Malik S, Boeve BF, Krahn LE, Silber MH. Narcolepsy associated with other central nervous system disorders. Neurology. 2001;57(3):539–541. doi: 10.1212/wnl.57.3.539. [DOI] [PubMed] [Google Scholar]
- 10.Boeve BF, Dickson DW, Olson EJ, Shepard JW, Silber MH, Ferman TJ, et al. Insights into REM sleep behavior disorder pathophysiology in brainstem-predominant Lewy body disease. Sleep Med. 2007;8(1):60–64. doi: 10.1016/j.sleep.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boeve BF, Saper CB. REM sleep behavior disorder: a possible early marker for synucleinopathies. Neurology. 2006;66(6):796–797. doi: 10.1212/01.wnl.0000209264.61035.bb. [DOI] [PubMed] [Google Scholar]
- 12.Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130(Pt 11):2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 13.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson's disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008;79(10):1117–1121. doi: 10.1136/jnnp.2008.149195. [DOI] [PubMed] [Google Scholar]
- 14.Poryazova RG, Zachariev ZI. REM sleep behavior disorder in patients with Parkinson’s disease. Folia Med. 2005;47(1):5–10. [PubMed] [Google Scholar]
- 15.Mallick BN, Singh A, Khanday MA. Activation of inactivation process initiates rapid eye movement sleep. Prog Neurobiol. 2012;97(3):259–276. doi: 10.1016/j.pneurobio.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Mehta R, Singh S, Khanday MA, Mallick BN. Reciprocal changes in noradrenaline and GABA levels in discrete brain regions upon rapid eye movement sleep deprivation in rats. Neurochem Int. 2017;108:190–198. doi: 10.1016/j.neuint.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Asikainen M, Deboer T, Porkka-Heiskanen T, Stenberg D, Tobler I. Sleep deprivation increases brain serotonin turnover in the Djungarian hamster. Neurosci Lett. 1995;198(1):21–24. doi: 10.1016/0304-3940(95)11953-t. [DOI] [PubMed] [Google Scholar]
- 18.Cosentino M, Marino F. Nerve driven immunity: noradrenaline and adrenaline. In: Levite M, editor. Nerve driven immunity: neurotransmitters and neuropeptides in the immune system. Vienna: Springer; 2012. pp. 47–96. [Google Scholar]
- 19.Francis BM, Yang J, Hajderi E, Brown ME, Michalski B, McLaurin J, et al. Reduced tissue levels of noradrenaline are associated with behavioral phenotypes of the TgCRND8 mouse model of Alzheimer's disease. Neuropsychopharmacology. 2012;37(8):1934–1944. doi: 10.1038/npp.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez MM, Debilly G, Valatx JL. Noradrenaline neurotoxin DSP-4 effects on sleep and brain temperature in the rat. Neurosci Lett. 1998;248(2):93–96. doi: 10.1016/s0304-3940(98)00333-4. [DOI] [PubMed] [Google Scholar]
- 21.Adolfsson R, Gottfries CG, Roos BE, Winblad B. Changes in the brain catecholamines in patients with dementia of Alzheimer type. Br J Psychiatry. 1979;135:216–223. doi: 10.1192/bjp.135.3.216. [DOI] [PubMed] [Google Scholar]
- 22.Berridge CW, Arnsten AF, Foote SL. Noradrenergic modulation of cognitive function: clinical implications of anatomical, electrophysiological and behavioural studies in animal models. Psychol Med. 1993;23(3):557–564. doi: 10.1017/s0033291700025332. [DOI] [PubMed] [Google Scholar]
- 23.Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatr Dis Treat. 2011;7(Suppl 1):9–13. doi: 10.2147/NDT.S19619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rommelfanger KS, Weinshenker D. Norepinephrine: the redheaded stepchild of Parkinson's disease. Biochem Pharmacol. 2007;74(2):177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 25.Castro MA, Garcez MR, Pereira JL, Fisberg RM. Eating behaviours and dietary intake associations with self-reported sleep duration of free-living Brazilian adults. Appetite. 2019;137:207–217. doi: 10.1016/j.appet.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Noguti J, Andersen ML, Cirelli C, Ribeiro DA. Oxidative stress, cancer, and sleep deprivation: is there a logical link in this association? Sleep Breath. 2013;17(3):905–910. doi: 10.1007/s11325-012-0797-9. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt MH, Swang TW, Hamilton IM, Best JA. State-dependent metabolic partitioning and energy conservation: a theoretical framework for understanding the function of sleep. PLoS One. 2017;12(10):e0185746. doi: 10.1371/journal.pone.0185746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saghir Z, Syeda JN, Muhammad AS, Balla Abdalla TH. The amygdala, sleep debt, sleep deprivation, and the emotion of anger: a possible connection? Cureus. 2018;10(7):e2912. doi: 10.7759/cureus.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi Y, Sawa R, Ueda M, Shimai S. The relationship between sleep and subjective mental health at one month postpartum in Japanese women. Nihon Koshu Eisei Zasshi. 2018;65(11):646–654. doi: 10.11236/jph.65.11_646. [DOI] [PubMed] [Google Scholar]
- 30.Zhu B, Shi C, Park CG, Zhao X, Reutrakul S. Effects of sleep restriction on metabolism-related parameters in healthy adults: a comprehensive review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2019;45:18–30. doi: 10.1016/j.smrv.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian A, Adderley NJ, Tracy A, Taverner T, Hanif W, Toulis KA, et al. Risk of incident obstructive sleep apnea among patients with type 2 diabetes. Diabetes Care. 2019;42(5):954–963. doi: 10.2337/dc18-2004. [DOI] [PubMed] [Google Scholar]
- 32.Navarrete M, Lewis PA. Cognition: learning while asleep. Curr Biol. 2019;29(5):R164–R1R6. doi: 10.1016/j.cub.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 33.Quera-Salva MA, Claustrat B. Melatonin: physiological and pharmacological aspects related to sleep: the interest of a prolonged-release formulation (Circadin®) in insomnia. L'Encephale. 2018;44(6):548–557. doi: 10.1016/j.encep.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10(3):199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto-Mizuno K, Mizuno K. Effects of thermal environment on sleep and circadian rhythm. J Physiol Anthropol. 2012;31:14. doi: 10.1186/1880-6805-31-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wehrens SM, Hampton SM, Skene DJ. Heart rate variability and endothelial function after sleep deprivation and recovery sleep among male shift and non-shift workers. Scand J Work Environ Health. 2012;38(2):171–181. doi: 10.5271/sjweh.3197. [DOI] [PubMed] [Google Scholar]
- 37.Nedeltcheva AV, Scheer FA. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2014;21(4):293–298. doi: 10.1097/MED.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lasselin J, Rehman JU, Akerstedt T, Lekander M, Axelsson J. Effect of long-term sleep restriction and subsequent recovery sleep on the diurnal rhythms of white blood cell subpopulations. Brain Behav Immun. 2015;47:93–99. doi: 10.1016/j.bbi.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 39.van Leeuwen WM, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4(2):e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jowko E, Rozanski P, Tomczak A. Effects of a 36-h survival training with sleep deprivation on oxidative stress and muscle damage biomarkers in young healthy men. Int J Environ Res Public Health. 2018;15(10). 10.3390/ijerph15102066. [DOI] [PMC free article] [PubMed]
- 41.He J, Hsuchou H, He Y, Kastin AJ, Wang Y, Pan W. Sleep restriction impairs blood-brain barrier function. J Neurosci. 2014;34(44):14697–14706. doi: 10.1523/JNEUROSCI.2111-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hurtado-Alvarado G, Dominguez-Salazar E, Pavon L, Velazquez-Moctezuma J, Gomez-Gonzalez B. Blood-brain barrier disruption induced by chronic sleep loss: low-grade inflammation may be the link. J Immunol Res. 2016;2016:4576012. doi: 10.1155/2016/4576012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majumdar S, Mallick BN. Cytomorphometric changes in rat brain neurons after rapid eye movement sleep deprivation. Neuroscience. 2005;135(3):679–690. doi: 10.1016/j.neuroscience.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 44.Rabat A, Gomez-Merino D, Roca-Paixao L, Bougard C, Van Beers P, Dispersyn G, et al. Differential kinetics in alteration and recovery of cognitive processes from a chronic sleep restriction in young healthy men. Front Behav Neurosci. 2016;10:95. doi: 10.3389/fnbeh.2016.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang ZJ, Chen YC, Wang HN, Wang HH, Xue YY, Feng SF, et al. Electroconvulsive therapy improves antipsychotic and somnographic responses in adolescents with first-episode psychosis--a case-control study. Schizophr Res. 2012;137(1–3):97–103. doi: 10.1016/j.schres.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 46.Yamashita H, Mori K, Nagao M, Okamoto Y, Morinobu S, Yamawaki S. Effects of changing from typical to atypical antipsychotic drugs on subjective sleep quality in patients with schizophrenia in a Japanese population. J Clin Psychiatry. 2004;65(11):1525–1530. doi: 10.4088/jcp.v65n1114. [DOI] [PubMed] [Google Scholar]
- 47.Hyyppa MT, Kronholm E. Quality of sleep and chronic illnesses. J Clin Epidemiol. 1989;42(7):633–638. doi: 10.1016/0895-4356(89)90006-1. [DOI] [PubMed] [Google Scholar]
- 48.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 49.Harvey AG, Stinson K, Whitaker KL, Moskovitz D, Virk H. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31(3):383–393. doi: 10.1093/sleep/31.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akerstedt T, Hume K, Minors D, Waterhouse J. The subjective meaning of good sleep, an intraindividual approach using the Karolinska sleep diary. Percept Mot Skills. 1994;79(1 Pt 1):287–296. doi: 10.2466/pms.1994.79.1.287. [DOI] [PubMed] [Google Scholar]
- 51.Kalkbrenner C, Stark P, Kouemou G, Algorri ME, Brucher R. Sleep monitoring using body sounds and motion tracking. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:6941–6944. doi: 10.1109/EMBC.2014.6945224. [DOI] [PubMed] [Google Scholar]
- 52.Girschik J, Heyworth J, Fritschi L. Reliability of a sleep quality questionnaire for use in epidemiologic studies. J Epidemiol. 2012;22(3):244–250. doi: 10.2188/jea.je20110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valipour A, Lothaller H, Rauscher H, Zwick H, Burghuber OC, Lavie P. Gender-related differences in symptoms of patients with suspected breathing disorders in sleep: a clinical population study using the sleep disorders questionnaire. Sleep. 2007;30(3):312–319. doi: 10.1093/sleep/30.3.312. [DOI] [PubMed] [Google Scholar]
- 54.Hsieh YP, Lu WH, Yen CF. Psychosocial determinants of insomnia in adolescents: roles of mental health, behavioral health, and social environment. Front Neurosci. 2019;13:848. doi: 10.3389/fnins.2019.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thakkar MM, Sharma R, Sahota P. Alcohol disrupts sleep homeostasis. Alcohol. 2015;49(4):299–310. doi: 10.1016/j.alcohol.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(9):e1160–e1167. doi: 10.4088/JCP.11r07586. [DOI] [PubMed] [Google Scholar]
- 57.Bernert RA, Hom MA, Iwata NG, Joiner TE. Objectively assessed sleep variability as an acute warning sign of suicidal ideation in a longitudinal evaluation of young adults at high suicide risk. J Clin Psychiatry. 2017;78(6):e678–ee87. doi: 10.4088/JCP.16m11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3(5):553–567. [PMC free article] [PubMed] [Google Scholar]
- 59.Dinges DF, Rogers NL, Baynard MD. Chronic sleep deprivation. In: Kryger MHRT, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier/ Saunders; 2005. pp. 67–76. [Google Scholar]
- 60.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6(8):e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci U S A. 1996;93(4):1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 63.Meaney MJ, Aitken DH, Bhatnagar S, Sapolsky RM. Postnatal handling attenuates certain neuroendocrine, anatomical, and cognitive dysfunctions associated with aging in female rats. Neurobiol Aging. 1991;12(1):31–38. doi: 10.1016/0197-4580(91)90036-j. [DOI] [PubMed] [Google Scholar]
- 64.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18(3):195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 65.Seckl JR, Meaney MJ. Early life events and later development of ischaemic heart disease. Lancet. 1993;342(8881):1236. doi: 10.1016/0140-6736(93)92215-f. [DOI] [PubMed] [Google Scholar]
- 66.Golubnitschaja O, Costigliola V, EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3(1):14. 10.1186/1878-5085-3-14. [DOI] [PMC free article] [PubMed]
- 67.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Z, Zhao X, Veasey SC. Neural consequences of chronic short sleep: reversible or lasting? Front Neurol. 2017;8:235. doi: 10.3389/fneur.2017.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ohayon MM, Reynolds CF, 3rd, Dauvilliers Y. Excessive sleep duration and quality of life. Ann Neurol. 2013;73(6):785–794. doi: 10.1002/ana.23818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 71.Wheaton AG, Perry GS, Chapman DP, Croft JB. Sleep disordered breathing and depression among U.S. adults: National Health and Nutrition Examination Survey, 2005–2008. Sleep. 2012;35:461–7. [DOI] [PMC free article] [PubMed]
- 72.Mirmiran M, Yolanda MGH. The function of fetal/ neonatal REM sleep. In: Mallick BN, Inoue S, editors. Rapid eye movement sleep. NewYork: Marcel Dekker; 1999. pp. 326–336. [Google Scholar]
- 73.Guzman-Marin R, Suntsova N, Bashir T, Nienhuis R, Szymusiak R, McGinty D. Rapid eye movement sleep deprivation contributes to reduction of neurogenesis in the hippocampal dentate gyrus of the adult rat. Sleep. 2008;31(2):167–175. doi: 10.1093/sleep/31.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehta R, Khan S, Mallick BN. Relevance of deprivation studies in understanding rapid eye movement sleep. Nat Sci Sleep. 2018;10:143–158. doi: 10.2147/NSS.S140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moruzzi G. The sleep-waking cycle. Ergeb Physiol Biol Chem Exp Pharmakol. 1972;64:1–165. doi: 10.1007/3-540-05462-6_1. [DOI] [PubMed] [Google Scholar]
- 76.Siegel J. Brainstem mechanisms generating REM sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 1. Philadelphia: Saunders; 1989. pp. 104–120. [Google Scholar]
- 77.Siegel JM, Tomaszewski KS, Nienhuis R. Behavioral states in the chronic medullary and midpontine cat. Electroencephalogr Clin Neurophysiol. 1986;63(3):274–288. doi: 10.1016/0013-4694(86)90095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siegel JM, Nienhuis R, Tomaszewski KS. Rostral brainstem contributes to medullary inhibition of muscle tone. Brain Res. 1983;268(2):344–348. doi: 10.1016/0006-8993(83)90501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mallick BN, Siegel JM, Fahringer H. Changes in pontine unit activity with REM sleep deprivation. Brain Res. 1990;515(1–2):94–98. doi: 10.1016/0006-8993(90)90581-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538(Pt 2):619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin JS, Sakai K, Jouvet M. Role of hypothalamic histaminergic systems in the regulation of vigilance states in cats. C R Acad Sci III. 1986;303(11):469–474. [PubMed] [Google Scholar]
- 82.Takakusaki K, Saitoh K, Harada H, Okumura T, Sakamoto T. Evidence for a role of basal ganglia in the regulation of rapid eye movement sleep by electrical and chemical stimulation for the pedunculopontine tegmental nucleus and the substantia nigra pars reticulata in decerebrate cats. Neuroscience. 2004;124(1):207–220. doi: 10.1016/j.neuroscience.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 83.Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol. 2008;6(3):254–285. doi: 10.2174/157015908785777193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andrews GD, Lavin A. Methylphenidate increases cortical excitability via activation of alpha-2 noradrenergic receptors. Neuropsychopharmacology. 2006;31(3):594–601. doi: 10.1038/sj.npp.1300818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83(1):63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- 86.Jha SK, Mallick BN. Modulation of REM sleep by non-REM sleep and waking areas in the brain. In: Mallick BN, Pandi-Permual SR, McCarley RW, Morrison AR, editors. Rapid eye movement sleep – regulation and function. New York: Cambridge University Press; 2011. pp. 173–182. [Google Scholar]
- 87.McCarley RW, Hobson JA. Discharge patterns of cat pontine brain stem neurons during desynchronized sleep. J Neurophysiol. 1975;38(4):751–766. doi: 10.1152/jn.1975.38.4.751. [DOI] [PubMed] [Google Scholar]
- 88.Rasmussen K, Morilak DA, Jacobs BL. Single unit activity of locus coeruleus neurons in the freely moving cat. I. during naturalistic behaviors and in response to simple and complex stimuli. Brain Res. 1986;371(2):324–334. doi: 10.1016/0006-8993(86)90370-7. [DOI] [PubMed] [Google Scholar]
- 89.Chu NS, Bloom FE. Activity patterns of catecholamine-containing pontine neurons in the dorso-lateral tegmentum of unrestrained cats. J Neurobiol. 1974;5(6):527–544. doi: 10.1002/neu.480050605. [DOI] [PubMed] [Google Scholar]
- 90.Jacobs BL, Fornal CA. Activity of brain serotonergic neurons in the behaving animal. Pharmacol Rev. 1991;43(4):563–578. [PubMed] [Google Scholar]
- 91.McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101(3):569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 92.Monti JM, Jantos H. Activation of the serotonin 5-HT3 receptor in the dorsal raphe nucleus suppresses REM sleep in the rat. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32(4):940–947. doi: 10.1016/j.pnpbp.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 93.Miller JD, Farber J, Gatz P, Roffwarg H, German DC. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res. 1983;273(1):133–141. doi: 10.1016/0006-8993(83)91101-0. [DOI] [PubMed] [Google Scholar]
- 94.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441(7093):589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 95.Sakai K, Kanamori N. Are there non-monoaminergic paradoxical sleep-off neurons in the brainstem? Sleep Res Online. 1999;2:57–63. [PubMed] [Google Scholar]
- 96.Mallick BN, Fahringer HM, Wu MF, Siegel JM. REM sleep deprivation reduces auditory evoked inhibition of dorsolateral pontine neurons. Brain Res. 1991;552(2):333–337. doi: 10.1016/0006-8993(91)90100-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thakkar M, Portas C, McCarley RW. Chronic low-amplitude electrical stimulation of the laterodorsal tegmental nucleus of freely moving cats increases REM sleep. Brain Res. 1996;723(1–2):223–227. doi: 10.1016/0006-8993(96)00256-9. [DOI] [PubMed] [Google Scholar]
- 98.Kaur S, Saxena RN, Mallick BN. GABAergic neurons in prepositus hypoglossi regulate REM sleep by its action on locus coeruleus in freely moving rats. Synapse. 2001;42(3):141–150. doi: 10.1002/syn.1109. [DOI] [PubMed] [Google Scholar]
- 99.Mallick BN, Kaur S, Saxena RN. Interactions between cholinergic and GABAergic neurotransmitters in and around the locus coeruleus for the induction and maintenance of rapid eye movement sleep in rats. Neuroscience. 2001;104(2):467–485. doi: 10.1016/s0306-4522(01)00062-8. [DOI] [PubMed] [Google Scholar]
- 100.Singh S, Mallick BN. Mild electrical stimulation of pontine tegmentum around locus coeruleus reduces rapid eye movement sleep in rats. Neurosci Res. 1996;24(3):227–235. doi: 10.1016/0168-0102(95)00998-1. [DOI] [PubMed] [Google Scholar]
- 101.Nitz D, Siegel J. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Phys. 1997;273(1 Pt 2):R451–R455. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hobson JA, Goldberg M, Vivaldi E, Riew D. Enhancement of desynchronized sleep signs after pontine microinjection of the muscarinic agonist bethanechol. Brain Res. 1983;275(1):127–136. doi: 10.1016/0006-8993(83)90424-9. [DOI] [PubMed] [Google Scholar]
- 103.Aston-Jones G, Akaoka H, Charlety P, Chouvet G. Serotonin selectively attenuates glutamate-evoked activation of noradrenergic locus coeruleus neurons. J Neurosci. 1991;11(3):760–769. doi: 10.1523/JNEUROSCI.11-03-00760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ennis M, Aston-Jones G. Evidence for self- and neighbor-mediated postactivation inhibition of locus coeruleus neurons. Brain Res. 1986;374(2):299–305. doi: 10.1016/0006-8993(86)90424-5. [DOI] [PubMed] [Google Scholar]
- 105.Gottesmann C. Noradrenaline involvement in basic and higher integrated REM sleep processes. Prog Neurobiol. 2008;85(3):237–272. doi: 10.1016/j.pneurobio.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 106.Jones BE. Arousal and sleep circuits. Neuropsychopharmacology. 2020;45(1):6–20. doi: 10.1038/s41386-019-0444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pal D, Madan V, Mallick BN. Neural mechanism of rapid eye movement sleep generation: cessation of locus coeruleus neurons is a necessity. Sheng Li Xue Bao. 2005;57(4):401–413. [PubMed] [Google Scholar]
- 108.Kumar R, Bose A, Mallick BN. A mathematical model towards understanding the mechanism of neuronal regulation of wake-NREMS-REMS states. PLoS One. 2012;7(8):e42059. doi: 10.1371/journal.pone.0042059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mehta R, Singh A, Mallick BN. Disciplined sleep for healthy living: role of noradrenaline. World J Neurol. 2017;7(1):6–23. doi: 10.5316/wjn.v7.i1.6. [DOI] [Google Scholar]
- 110.Matos G, Tufik S, Scorza FA, Cavalheiro EA, Andersen ML. Sleep and epilepsy: exploring an intriguing relationship with a translational approach. Epilepsy Behav. 2013;26(3):405–409. doi: 10.1016/j.yebeh.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 111.Garcia-Alberca JM, Lara JP, Cruz B, Garrido V, Gris E, Barbancho MA. Sleep disturbances in Alzheimer’s disease are associated with neuropsychiatric symptoms and antidementia treatment. J Nerv Ment Dis. 2013;201(3):251–257. doi: 10.1097/NMD.0b013e3182848d04. [DOI] [PubMed] [Google Scholar]
- 112.Lima MM. Sleep disturbances in Parkinson’s disease: the contribution of dopamine in REM sleep regulation. Sleep Med Rev. 2013;17(5):367–375. doi: 10.1016/j.smrv.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 113.Gagnon JF, Petit D, Latreille V, Montplaisir J. Neurobiology of sleep disturbances in neurodegenerative disorders. Curr Pharm Des. 2008;14(32):3430–3445. doi: 10.2174/138161208786549353. [DOI] [PubMed] [Google Scholar]
- 114.Dodt C, Breckling U, Derad I, Fehm HL, Born J. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension. 1997;30(1 Pt 1):71–76. doi: 10.1161/01.hyp.30.1.71. [DOI] [PubMed] [Google Scholar]
- 115.Kaur S, Saxena RN, Mallick BN. GABA in locus coeruleus regulates spontaneous rapid eye movement sleep by acting on GABAA receptors in freely moving rats. Neurosci Lett. 1997;223(2):105–108. doi: 10.1016/s0304-3940(97)13410-3. [DOI] [PubMed] [Google Scholar]
- 116.Kawahara Y, Kawahara H, Westerink BH. Tonic regulation of the activity of noradrenergic neurons in the locus coeruleus of the conscious rat studied by dual-probe microdialysis. Brain Res. 1999;823(1–2):42–48. doi: 10.1016/s0006-8993(99)01062-8. [DOI] [PubMed] [Google Scholar]
- 117.Pollock MS, Mistlberger RE. Rapid eye movement sleep induction by microinjection of the GABA-A antagonist bicuculline into the dorsal subcoeruleus area of the rat. Brain Res. 2003;962(1–2):68–77. doi: 10.1016/s0006-8993(02)03956-2. [DOI] [PubMed] [Google Scholar]
- 118.Sakai K, Crochet S. A neural mechanism of sleep and wakefulness. Sleep Biol Rhythms. 2003;1:29–42. doi: 10.1046/j.1446-9235.2003.00004.x. [DOI] [Google Scholar]
- 119.Masserano JM, King C. Effects on sleep of acetylcholine perfusion of the locus coeruleus of cats. Neuropharmacology. 1982;21(11):1163–1167. doi: 10.1016/0028-3908(82)90174-5. [DOI] [PubMed] [Google Scholar]
- 120.Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen L, McKenna JT, Bolortuya Y, Winston S, Thakkar MM, Basheer R, et al. Knockdown of orexin type 1 receptor in rat locus coeruleus increases REM sleep during the dark period. Eur J Neurosci. 2010;32(9):1528–1536. doi: 10.1111/j.1460-9568.2010.07401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13(12):1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin JS, Sakai K, Jouvet M. Hypothalamo-preoptic histaminergic projections in sleep-wake control in the cat. Eur J Neurosci. 1994;6(4):618–625. doi: 10.1111/j.1460-9568.1994.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 124.Bjorvatn B, Fagerland S, Eid T, Ursin R. Sleep/waking effects of a selective 5-HT1A receptor agonist given systemically as well as perfused in the dorsal raphe nucleus in rats. Brain Res. 1997;770(1–2):81–88. doi: 10.1016/s0006-8993(97)00758-0. [DOI] [PubMed] [Google Scholar]
- 125.Alam MA, Mallick BN. Glutamic acid stimulation of the perifornical-lateral hypothalamic area promotes arousal and inhibits non-REM/REM sleep. Neurosci Lett. 2008;439(3):281–286. doi: 10.1016/j.neulet.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 126.Nunez A, Moreno-Balandran ME, Rodrigo-Angulo ML, Garzon M, De Andres I. Relationship between the perifornical hypothalamic area and oral pontine reticular nucleus in the rat. Possible implication of the hypocretinergic projection in the control of rapid eye movement sleep. Eur J Neurosci. 2006;24(10):2834–2842. doi: 10.1111/j.1460-9568.2006.05159.x. [DOI] [PubMed] [Google Scholar]
- 127.Choudhary RC, Khanday MA, Mitra A, Mallick BN. Perifornical orexinergic neurons modulate REM sleep by influencing locus coeruleus neurons in rats. Neuroscience. 2014;279:33–43. doi: 10.1016/j.neuroscience.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 128.Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415(2):145–159. doi: 10.1002/(SICI)1096-9861(19991213)415:2<145::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 129.Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28(3):585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- 130.Segal M. Serotonergic innervation of the locus coeruleus from the dorsal raphe and its action on responses to noxious stimuli. J Physiol. 1979;286:401–415. doi: 10.1113/jphysiol.1979.sp012628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev. 2007;11(2):113–133. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 132.Pal D, Mallick BN. GABA in pedunculopontine tegmentum increases rapid eye movement sleep in freely moving rats: possible role of GABA-ergic inputs from substantia nigra pars reticulata. Neuroscience. 2009;164(2):404–414. doi: 10.1016/j.neuroscience.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 133.Mignot E. Genetic and familial aspects of narcolepsy. Neurology. 1998;50(2 Suppl 1):S16–S22. doi: 10.1212/wnl.50.2_suppl_1.s16. [DOI] [PubMed] [Google Scholar]
- 134.Mignot E, Lin L, Rogers W, Honda Y, Qiu X, Lin X, et al. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet. 2001;68(3):686–699. doi: 10.1086/318799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. doi: 10.2147/NSS.S134864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kitsaras G, Goodwin M, Allan J, Kelly MP, Pretty IA. Bedtime routines child wellbeing & development. BMC Public Health. 2018;18(1):386. doi: 10.1186/s12889-018-5290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mindell JA, Telofski LS, Wiegand B, Kurtz ES. A nightly bedtime routine: impact on sleep in young children and maternal mood. Sleep. 2009;32(5):599–606. doi: 10.1093/sleep/32.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burgess HJ, Molina TA. Home lighting before usual bedtime impacts circadian timing: a field study. Photochem Photobiol. 2014;90(3):723–726. doi: 10.1111/php.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96(3):E463–E472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davis SN, Galassetti P, Wasserman DH, Tate D. Effects of gender on neuroendocrine and metabolic counterregulatory responses to exercise in normal man. J Clin Endocrinol Metab. 2000;85(1):224–230. doi: 10.1210/jcem.85.1.6328. [DOI] [PubMed] [Google Scholar]
- 141.Drake C, Roehrs T, Shambroom J, Roth T. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9(11):1195–1200. doi: 10.5664/jcsm.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Spadola CE, Guo N, Johnson DA, Sofer T, Bertisch SM, Jackson CL, et al. Evening intake of alcohol, caffeine, and nicotine: night-to-night associations with sleep duration and continuity among African Americans in the Jackson Heart Sleep Study. Sleep. 2019;42(11). 10.1093/sleep/zsz136. [DOI] [PMC free article] [PubMed]
- 143.Nisar M, Mohammad RM, Arshad A, Hashmi I, Yousuf SM, Baig S. Influence of dietary intake on sleeping patterns of medical students. Cureus. 2019;11(2):e4106. doi: 10.7759/cureus.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zeng Y, Yang J, Du J, Pu X, Yang X, Yang S, et al. Strategies of functional foods promote sleep in human being. Curr Signal Transduct Ther. 2014;9(3):148–155. doi: 10.2174/1574362410666150205165504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.St-Onge MP, Mikic A, Pietrolungo CE. Effects of diet on sleep quality. Adv Nutr. 2016;7(5):938–949. doi: 10.3945/an.116.012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Herzog J, Schmidt FP, Hahad O, Mahmoudpour SH, Mangold AK, Garcia Andreo P, et al. Acute exposure to nocturnal train noise induces endothelial dysfunction and pro-thromboinflammatory changes of the plasma proteome in healthy subjects. Basic Res Cardiol. 2019;114(6):46. doi: 10.1007/s00395-019-0753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bo S, Broglio F, Settanni F, Parasiliti Caprino M, Ianniello A, Mengozzi G, et al. Effects of meal timing on changes in circulating epinephrine, norepinephrine, and acylated ghrelin concentrations: a pilot study. Nutr Diabetes. 2017;7(12):303. doi: 10.1038/s41387-017-0010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wellman PJ. Norepinephrine and the control of food intake. Nutrition. 2000;16(10):837–842. doi: 10.1016/s0899-9007(00)00415-9. [DOI] [PubMed] [Google Scholar]
- 149.Matsumura T, Nakagawa H, Suzuki K, Ninomiya C, Ishiwata T. Influence of circadian disruption on neurotransmitter levels, physiological indexes, and behaviour in rats. Chronobiol Int. 2015;32(10):1449–1457. doi: 10.3109/07420528.2015.1105250. [DOI] [PubMed] [Google Scholar]
- 150.Evans GW, Bullinger M, Hygge S. Chronic noise exposure and physiological response: a prospective study of children living under environmental stress. Psychol Sci. 1998;9(1):75–77. doi: 10.1111/1467-9280.00014. [DOI] [Google Scholar]
- 151.Minami M, Aimoto A, Kabuto M. Individual differences in sympathetic nervous system responses to white noise stimuli: an investigation by finger plethysmogram. Nihon Eiseigaku Zasshi. 1993;48(2):646–654. doi: 10.1265/jjh.48.646. [DOI] [PubMed] [Google Scholar]
- 152.Fu Y, Matta SG, Valentine JD, Sharp BM. Adrenocorticotropin response and nicotine-induced norepinephrine secretion in the rat paraventricular nucleus are mediated through brainstem receptors. Endocrinology. 1997;138(5):1935–1943. doi: 10.1210/endo.138.5.5122. [DOI] [PubMed] [Google Scholar]
- 153.Herraiz T, Chaparro C. Human monoamine oxidase is inhibited by tobacco smoke: beta-carboline alkaloids act as potent and reversible inhibitors. Biochem Biophys Res Commun. 2005;326(2):378–386. doi: 10.1016/j.bbrc.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 154.Brower KJ. Alcohol's effects on sleep in alcoholics. Alcohol Res Health. 2001;25(2):110–125. [PMC free article] [PubMed] [Google Scholar]
- 155.Smith A, Brice C, Nash J, Rich N, Nutt DJ. Caffeine and central noradrenaline: effects on mood, cognitive performance, eye movements and cardiovascular function. J Psychopharmacol. 2003;17(3):283–292. doi: 10.1177/02698811030173010. [DOI] [PubMed] [Google Scholar]
- 156.Selikhova MV, Kogan BM, Serkin GV, Gusev EI. Catecholamine metabolism in different forms of Parkinson's disease. Zh Nevrol Psikhiatr Im S S Korsakova. 2002;102(9):37–40. [PubMed] [Google Scholar]
- 157.Paredes-Rodriguez E, Vegas-Suarez S, Morera-Herreras T, De Deurwaerdere P, Miguelez C. The noradrenergic system in Parkinson’s disease. Front Pharmacol. 2020;11:435. doi: 10.3389/fphar.2020.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chalermpalanupap T, Kinkead B, Hu WT, Kummer MP, Hammerschmidt T, Heneka MT, et al. Targeting norepinephrine in mild cognitive impairment and Alzheimer’s disease. Alzheimers Res Ther. 2013;5(2):21. doi: 10.1186/alzrt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.De Bellis MD, Zisk A. The biological effects of childhood trauma. Child Adolesc Psychiatr Clin N Am. 2014;23(2):185–222. doi: 10.1016/j.chc.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27(8):1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 161.Goekoop JG, de Winter RF, Wolterbeek R, Van Kempen GM, Wiegant VM. Increased plasma norepinephrine concentration in psychotic depression. Ther Adv Psychopharmacol. 2012;2(2):51–63. doi: 10.1177/2045125312436574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 163.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290(14):1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 164.DeMesquita S, Hale GA. Cardiopulmonary regulation after rapid-eye-movement sleep deprivation. J Appl Physiol. 1992;72(3):970–976. doi: 10.1152/jappl.1992.72.3.970. [DOI] [PubMed] [Google Scholar]
- 165.Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: state of the art. Sleep Med Rev. 2013;17(5):377–390. doi: 10.1016/j.smrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 166.Bliwise DL, Tinklenberg J, Yesavage JA, Davies H, Pursley AM, Petta DE, et al. REM latency in Alzheimer’s disease. Biol Psychiatry. 1989;25(3):320–328. doi: 10.1016/0006-3223(89)90179-0. [DOI] [PubMed] [Google Scholar]
- 167.Colten HRAB. Extent and health consequences of chronic sleep loss and sleep disorders. In: Colten HR, Altevogt BM, editors. Sleep disorders and sleep deprivation: an unmet public health problem. Washington (DC): National Academies Press (US); 2006. pp. 55–135. [PubMed] [Google Scholar]
- 168.Edinger JD, Means MK. Cognitive-behavioral therapy for primary insomnia. Clin Psychol Rev. 2005;25(5):539–558. doi: 10.1016/j.cpr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 169.Vgontzas AN, Bixler EO, Wittman AM, Zachman K, Lin HM, Vela-Bueno A, et al. Middle-aged men show higher sensitivity of sleep to the arousing effects of corticotropin-releasing hormone than young men: clinical implications. J Clin Endocrinol Metab. 2001;86(4):1489–1495. doi: 10.1210/jcem.86.4.7370. [DOI] [PubMed] [Google Scholar]
- 170.Schwartz SW, Cornoni-Huntley J, Cole SR, Hays JC, Blazer DG, Schocken DD. Are sleep complaints an independent risk factor for myocardial infarction? Ann Epidemiol. 1998;8(6):384–392. doi: 10.1016/s1047-2797(97)00238-x. [DOI] [PubMed] [Google Scholar]
- 171.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163(2):205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 172.de Souza L, Smaili SS, Ureshino RP, Sinigaglia-Coimbra R, Andersen ML, Lopes GS, et al. Effect of chronic sleep restriction and aging on calcium signaling and apoptosis in the hippocampus of young and aged animals. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(1):23–30. 10.1016/j.pnpbp.2012.01.018. [DOI] [PubMed]
- 173.Jarrin DC, Ivers H, Lamy M, Chen IY, Harvey AG, Morin CM. Cardiovascular autonomic dysfunction in insomnia patients with objective short sleep duration. J Sleep Res. 2018;27(3):e12663. doi: 10.1111/jsr.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Karadzic V, Dement WC. Heart rate changes following selective deprivation of rapid eye movement (REM) sleep. Brain Res. 1967;6(4):786–788. doi: 10.1016/0006-8993(67)90138-2. [DOI] [PubMed] [Google Scholar]
- 175.Jeddi S, Asl AN, Asgari A, Ghasemi A. The effect of sleep deprivation on cardiac function and tolerance to ischemia-reperfusion injury in male rats. Arq Bras Cardiol. 2016;106(1):41–48. doi: 10.5935/abc.20150137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jeddi S, Ghasemi A, Asgari A, Nezami-Asl A. Role of inducible nitric oxide synthase in myocardial ischemia-reperfusion injury in sleep-deprived rats. Sleep Breath. 2018;22(2):353–359. doi: 10.1007/s11325-017-1573-7. [DOI] [PubMed] [Google Scholar]
- 177.Lai KB, Sanderson JE, Yu CM. High dose norepinephrine-induced apoptosis in cultured rat cardiac fibroblast. Int J Cardiol. 2009;136(1):33–39. doi: 10.1016/j.ijcard.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 178.Ma M, Wang L, Ma Y, Yang Y, Chen B, Zhu X. Effects of norepinephrine on proliferation and apoptosis of neonatal cardiac fibroblasts in rats. Zhonghua Xin Xue Guan Bing Za Zhi. 2015;43(6):542–547. [PubMed] [Google Scholar]
- 179.Hipolide DC, D'Almeida V, Raymond R, Tufik S, Nobrega JN. Sleep deprivation does not affect indices of necrosis or apoptosis in rat brain. Int J Neurosci. 2002;112(2):155–166. doi: 10.1080/00207450212022. [DOI] [PubMed] [Google Scholar]
- 180.Breimer LH. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog. 1990;3(4):188–197. doi: 10.1002/mc.2940030405. [DOI] [PubMed] [Google Scholar]
- 181.D'Almeida V, Lobo LL, Hipolide DC, de Oliveira AC, Nobrega JN, Tufik S. Sleep deprivation induces brain region-specific decreases in glutathione levels. Neuroreport. 1998;9(12):2853–2856. doi: 10.1097/00001756-199808240-00031. [DOI] [PubMed] [Google Scholar]
- 182.Winters BD, Huang YH, Dong Y, Krueger JM. Sleep loss alters synaptic and intrinsic neuronal properties in mouse prefrontal cortex. Brain Res. 2011;1420:1–7. doi: 10.1016/j.brainres.2011.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gulyani S, Mallick BN. Effect of rapid eye movement sleep deprivation on rat brain Na-K ATPase activity. J Sleep Res. 1993;2(1):45–50. doi: 10.1111/j.1365-2869.1993.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 184.Zager A, Andersen ML, Ruiz FS, Antunes IB, Tufik S. Effects of acute and chronic sleep loss on immune modulation of rats. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R504–R509. doi: 10.1152/ajpregu.00105.2007. [DOI] [PubMed] [Google Scholar]
- 185.Velazquez-Moctezuma J, Dominguez-Salazar E, Cortes-Barberena E, Najera-Medina O, Retana-Marquez S, Rodriguez-Aguilera E, et al. Differential effects of rapid eye movement sleep deprivation and immobilization stress on blood lymphocyte subsets in rats. Neuroimmunomodulation. 2004;11(4):261–267. doi: 10.1159/000078445. [DOI] [PubMed] [Google Scholar]
- 186.Yehuda S, Sredni B, Carasso RL, Kenigsbuch-Sredni D. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. J Interf Cytokine Res. 2009;29(7):393–398. doi: 10.1089/jir.2008.0080. [DOI] [PubMed] [Google Scholar]
- 187.Ruiz FS, Andersen ML, Martins RC, Zager A, Lopes JD, Tufik S. Immune alterations after selective rapid eye movement or total sleep deprivation in healthy male volunteers. Innate Immunol. 2012;18(1):44–54. doi: 10.1177/1753425910385962. [DOI] [PubMed] [Google Scholar]
- 188.Thakkar M, Mallick BN. Rapid eye movement sleep-deprivation-induced changes in glucose metabolic enzymes in rat brain. Sleep. 1993;16(8):691–694. [PubMed] [Google Scholar]
- 189.Koban M, Swinson KL. Chronic REM-sleep deprivation of rats elevates metabolic rate and increases UCP1 gene expression in brown adipose tissue. Am J Physiol Endocrinol Metab. 2005;289(1):E68–E74. doi: 10.1152/ajpendo.00543.2004. [DOI] [PubMed] [Google Scholar]
- 190.Streck EL, Scaini G, Jeremias GC, Rezin GT, Goncalves CL, Ferreira GK, et al. Effects of mood stabilizers on brain energy metabolism in mice submitted to an animal model of mania induced by paradoxical sleep deprivation. Neurochem Res. 2015;40(6):1144–1152. doi: 10.1007/s11064-015-1575-4. [DOI] [PubMed] [Google Scholar]
- 191.Casanueva FF, Dieguez C, Popovic V, Peino R, Considine RV, Caro JF. Serum immunoreactive leptin concentrations in patients with anorexia nervosa before and after partial weight recovery. Biochem Mol Med. 1997;60(2):116–120. doi: 10.1006/bmme.1996.2564. [DOI] [PubMed] [Google Scholar]
- 192.Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302(23):2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;19(4):1155–1167. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Everson CA, Toth LA. Systemic bacterial invasion induced by sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2000;278(4):R905–R916. doi: 10.1152/ajpregu.2000.278.4.R905. [DOI] [PubMed] [Google Scholar]
- 195.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kassab S, Sachdeva U, Das N, Al-Shaibani T, Nayar U. Cardiovascular responses to tonic pain in REM sleep-deprived rats: role of melatonin and beta endorphin. Sultan Qaboos Univ Med J. 2006;6(1):51–56. [PMC free article] [PubMed] [Google Scholar]
- 197.Jiang J, Gan Z, Li Y, Zhao W, Li H, Zheng JP, et al. REM sleep deprivation induces endothelial dysfunction and hypertension in middle-aged rats: roles of the eNOS/NO/cGMP pathway and supplementation with L-arginine. PLoS One. 2017;12(8):e0182746. doi: 10.1371/journal.pone.0182746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Joukar S, Ghorbani-Shahrbabaki S, Hajali V, Sheibani V, Naghsh N. Susceptibility to life-threatening ventricular arrhythmias in an animal model of paradoxical sleep deprivation. Sleep Med. 2013;14(12):1277–1282. doi: 10.1016/j.sleep.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 199.Zoladz PR, Krivenko A, Eisenmann ED, Bui AD, Seeley SL, Fry ME, et al. Sex-dependent effects of sleep deprivation on myocardial sensitivity to ischemic injury. Stress. 2016;19(2):264–268. doi: 10.3109/10253890.2016.1152469. [DOI] [PubMed] [Google Scholar]
- 200.Aggarwal A, Esler MD, Lambert GW, Hastings J, Johnston L, Kaye DM. Norepinephrine turnover is increased in suprabulbar subcortical brain regions and is related to whole-body sympathetic activity in human heart failure. Circulation. 2002;105(9):1031–1033. doi: 10.1161/hc0902.105724. [DOI] [PubMed] [Google Scholar]
- 201.Bangalore S, Messerli FH, Kostis JB, Pepine CJ. Cardiovascular protection using beta-blockers: a critical review of the evidence. J Am Coll Cardiol. 2007;50(7):563–572. doi: 10.1016/j.jacc.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 202.Vetrivelan R, Fuller PM, Yokota S, Lu J, Saper CB. Metabolic effects of chronic sleep restriction in rats. Sleep. 2012;35(11):1511–1520. doi: 10.5665/sleep.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589(Pt 1):235–244. doi: 10.1113/jphysiol.2010.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, RoyChoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94(2):410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Brianza-Padilla M, Bonilla-Jaime H, Almanza-Perez JC, Lopez-Lopez AL, Sanchez-Munoz F, Vazquez-Palacios G. Effects of different periods of paradoxical sleep deprivation and sleep recovery on lipid and glucose metabolism and appetite hormones in rats. Appl Physiol Nutr Metab. 2016;41(3):235–243. doi: 10.1139/apnm-2015-0337. [DOI] [PubMed] [Google Scholar]
- 206.Yujra VQ, Antunes HKM, Monico-Neto M, Pisani LP, Santamarina AB, Quintana HT, et al. Sleep deprivation induces pathological changes in rat masticatory muscles: role of toll like signaling pathway and atrophy. J Cell Biochem. 2018;119(2):2269–2277. doi: 10.1002/jcb.26389. [DOI] [PubMed] [Google Scholar]
- 207.Palma BD, Gabriel A, Jr, Colugnati FA, Tufik S. Effects of sleep deprivation on the development of autoimmune disease in an experimental model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1527–R1532. doi: 10.1152/ajpregu.00186.2006. [DOI] [PubMed] [Google Scholar]
- 208.Kohm AP, Sanders VM. Norepinephrine: a messenger from the brain to the immune system. Immunol Today. 2000;21(11):539–542. doi: 10.1016/s0167-5699(00)01747-3. [DOI] [PubMed] [Google Scholar]
- 209.Das G, Mallick BN. Noradrenaline acting on alpha1-adrenoceptor mediates REM sleep deprivation-induced increased membrane potential in rat brain synaptosomes. Neurochem Int. 2008;52(4–5):734–740. doi: 10.1016/j.neuint.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 210.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23(29):9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Singh A, Das G, Kaur M, Mallick BN. Noradrenaline acting on Alpha1 adrenoceptor as well as by chelating Iron reduces oxidative burden on the brain: implications with rapid eye movement sleep. Front Mol Neurosci. 2019;12:7. doi: 10.3389/fnmol.2019.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gomez-Gonzalez B, Hurtado-Alvarado G, Esqueda-Leon E, Santana-Miranda R, Rojas-Zamorano JA, Velazquez-Moctezuma J. REM sleep loss and recovery regulates blood-brain barrier function. Curr Neurovasc Res. 2013;10(3):197–207. doi: 10.2174/15672026113109990002. [DOI] [PubMed] [Google Scholar]
- 213.Carreras A, Zhang SX, Peris E, Qiao Z, Gileles-Hillel A, Li RC, et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep. 2014;37(11):1817–1824. doi: 10.5665/sleep.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sarmento A, Borges N, Azevedo I. Adrenergic influences on the control of blood-brain barrier permeability. Naunyn Schmiedeberg's Arch Pharmacol. 1991;343(6):633–637. doi: 10.1007/BF00184295. [DOI] [PubMed] [Google Scholar]
- 215.Petit D, Montplaisir J, Louis EKS, Boeve BF. Alzheimer disease and other dementias. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 6. Philadelphia: Elsevier; 2017. pp. 935–943. [Google Scholar]
- 216.Boyce R, Glasgow SD, Williams S, Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science. 2016;352(6287):812–816. doi: 10.1126/science.aad5252. [DOI] [PubMed] [Google Scholar]
- 217.Dumoulin Bridi MC, Aton SJ, Seibt J, Renouard L, Coleman T, Frank MG. Rapid eye movement sleep promotes cortical plasticity in the developing brain. Sci Adv. 2015;1(6):e1500105. doi: 10.1126/sciadv.1500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Smith CT, Conway JM, Rose GM. Brief paradoxical sleep deprivation impairs reference, but not working, memory in the radial arm maze task. Neurobiol Learn Mem. 1998;69(2):211–217. doi: 10.1006/nlme.1997.3809. [DOI] [PubMed] [Google Scholar]
- 219.Uhde TW, Roy-Byrne P, Gillin JC, Mendelson WB, Boulenger JP, Vittone BJ, et al. The sleep of patients with panic disorder: a preliminary report. Psychiatry Res. 1984;12(3):251–259. doi: 10.1016/0165-1781(84)90030-1. [DOI] [PubMed] [Google Scholar]
- 220.Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, et al. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res. 2011;224(2):233–240. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 221.Silva RH, Kameda SR, Carvalho RC, Takatsu-Coleman AL, Niigaki ST, Abilio VC, et al. Anxiogenic effect of sleep deprivation in the elevated plus-maze test in mice. Psychopharmacology. 2004;176(2):115–122. doi: 10.1007/s00213-004-1873-z. [DOI] [PubMed] [Google Scholar]
- 222.Yin M, Chen Y, Zheng H, Pu T, Marshall C, Wu T, et al. Assessment of mouse cognitive and anxiety-like behaviors and hippocampal inflammation following a repeated and intermittent paradoxical sleep deprivation procedure. Behav Brain Res. 2017;321:69–78. doi: 10.1016/j.bbr.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 223.Biswas S, Mishra P, Mallick BN. Increased apoptosis in rat brain after rapid eye movement sleep loss. Neuroscience. 2006;142(2):315–331. doi: 10.1016/j.neuroscience.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 224.Somarajan BI, Khanday MA, Mallick BN. Rapid eye movement sleep deprivation induces neuronal apoptosis by noradrenaline acting on Alpha1 adrenoceptor and by triggering mitochondrial intrinsic pathway. Front Neurol. 2016;7:25. doi: 10.3389/fneur.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dos Santos AC, Castro MA, Jose EA, Delattre AM, Dombrowski PA, Da Cunha C, et al. REM sleep deprivation generates cognitive and neurochemical disruptions in the intranigral rotenone model of Parkinson's disease. J Neurosci Res. 2013;91(11):1508–1516. doi: 10.1002/jnr.23258. [DOI] [PubMed] [Google Scholar]
- 226.Santos PD, Targa ADS, Noseda ACD, Rodrigues LS, Fagotti J, Lima MMS. Cholinergic oculomotor nucleus activity is induced by REM sleep deprivation negatively impacting on cognition. Mol Neurobiol. 2017;54(7):5721–5729. doi: 10.1007/s12035-016-0112-z. [DOI] [PubMed] [Google Scholar]
- 227.Hanlon EC, Andrzejewski ME, Harder BK, Kelley AE, Benca RM. The effect of REM sleep deprivation on motivation for food reward. Behav Brain Res. 2005;163(1):58–69. doi: 10.1016/j.bbr.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 228.Hanlon EC, Benca RM, Baldo BA, Kelley AE. REM sleep deprivation produces a motivational deficit for food reward that is reversed by intra-accumbens amphetamine in rats. Brain Res Bull. 2010;83(5):245–254. doi: 10.1016/j.brainresbull.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Javad-Moosavi BZ, Vaezi G, Nasehi M, Haeri-Rouhani SA, Zarrindast MR. Critical role of CA1 muscarinic receptors on memory acquisition deficit induced by total (TSD) and REM sleep deprivation (RSD) Prog Neuropsychopharmacol Biol Psychiatry. 2017;79(Pt B):128–135. doi: 10.1016/j.pnpbp.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 230.Rosales-Lagarde A, Armony JL, Del Rio-Portilla Y, Trejo-Martinez D, Conde R, Corsi-Cabrera M. Enhanced emotional reactivity after selective REM sleep deprivation in humans: an fMRI study. Front Behav Neurosci. 2012;6:25. doi: 10.3389/fnbeh.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Vogel GW, Thurmond A, Gibbons P, Sloan K, Walker M. REM sleep reduction effects on depression syndromes. Arch Gen Psychiatry. 1975;32(6):765–777. doi: 10.1001/archpsyc.1975.01760240093007. [DOI] [PubMed] [Google Scholar]
- 232.Hensler JG, Artigas F, Bortolozzi A, Daws LC, De Deurwaerdere P, Milan L, et al. Catecholamine/serotonin interactions: systems thinking for brain function and disease. Adv Pharmacol. 2013;68:167–197. doi: 10.1016/B978-0-12-411512-5.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13(10):1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bhardwaj SK, Tse YC, Ryan R, Wong TP, Srivastava LK. Impaired adrenergic-mediated plasticity of prefrontal cortical glutamate synapses in rats with developmental disruption of the ventral hippocampus. Neuropsychopharmacology. 2014;39(13):2963–2973. doi: 10.1038/npp.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Acheson DT, Geyer MA, Baker DG, Nievergelt CM, Yurgil K, Risbrough VB, et al. Conditioned fear and extinction learning performance and its association with psychiatric symptoms in active duty marines. Psychoneuroendocrinology. 2015;51:495–505. doi: 10.1016/j.psyneuen.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Andreasen NC, Grove WM. Evaluation of positive and negative symptoms in schizophrenia. Psychiatr Psychobiol. 1986;1(2):108–121. doi: 10.1017/S0767399X00003199. [DOI] [Google Scholar]
- 237.Liddle PF. The symptoms of chronic schizophrenia. A re-examination of the positive-negative dichotomy. Br J Psychiatry. 1987;151:145–151. doi: 10.1192/bjp.151.2.145. [DOI] [PubMed] [Google Scholar]
- 238.Strauss ME. Relations of symptoms to cognitive deficits in schizophrenia. Schizophr Bull. 1993;19(2):215–231. doi: 10.1093/schbul/19.2.215. [DOI] [PubMed] [Google Scholar]
- 239.Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62(3):1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.McNally JM, McCarley RW, Brown RE. Impaired GABAergic neurotransmission in schizophrenia underlies impairments in cortical gamma band oscillations. Curr Psychiatry Rep. 2013;15(3):346. doi: 10.1007/s11920-012-0346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Blum BP, Mann JJ. The GABAergic system in schizophrenia. Int J Neuropsychopharmacol. 2002;5(2):159–179. doi: 10.1017/S1461145702002894. [DOI] [PubMed] [Google Scholar]
- 242.Scarr E, Gibbons AS, Neo J, Udawela M, Dean B. Cholinergic connectivity: it’s implications for psychiatric disorders. Front Cell Neurosci. 2013;7:55. doi: 10.3389/fncel.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57(11):1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 244.Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52(2):293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 245.Cohrs S. Sleep disturbances in patients with schizophrenia : impact and effect of antipsychotics. CNS Drugs. 2008;22(11):939–962. doi: 10.2165/00023210-200822110-00004. [DOI] [PubMed] [Google Scholar]
- 246.Monti JM, Monti D. Sleep in schizophrenia patients and the effects of antipsychotic drugs. Sleep Med Rev. 2004;8(2):133–148. doi: 10.1016/S1087-0792(02)00158-2. [DOI] [PubMed] [Google Scholar]
- 247.Kaskie RE, Graziano B, Ferrarelli F. Schizophrenia and sleep disorders: links, risks, and management challenges. Nat Sci Sleep. 2017;9:227–239. doi: 10.2147/NSS.S121076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Das M, Das R, Khastgir U, Goswami U. REM sleep latency and neurocognitive dysfunction in schizophrenia. Indian J Psychiatry. 2005;47(3):133–138. doi: 10.4103/0019-5545.55934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mannaa M, Walker L. Is schizophrenia a REM disorder? Acad J Ped Neonatol. 2017;4(2):1–2. [Google Scholar]
- 250.Cle M, Maranci JB, Weyn Banningh S, Lanfranchi J, Vidailhet M, Arnulf I. Smiling asleep: a study of happy emotional expressions during adult sleep. J Sleep Res. 2019;28(4):e12814. doi: 10.1111/jsr.12814. [DOI] [PubMed] [Google Scholar]
- 251.Reeve S, Sheaves B, Freeman D. The role of sleep dysfunction in the occurrence of delusions and hallucinations: a systematic review. Clin Psychol Rev. 2015;42:96–115. doi: 10.1016/j.cpr.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Waters F, Blom JD, Dang-Vu TT, Cheyne AJ, Alderson-Day B, Woodruff P, et al. What is the link between hallucinations, dreams, and hypnagogic-hypnopompic experiences? Schizophr Bull. 2016;42(5):1098–1109. doi: 10.1093/schbul/sbw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Khanday MA, Somarajan BI, Mehta R, Mallick BN. Noradrenaline from locus coeruleus neurons acts on pedunculo-pontine neurons to prevent REM sleep and induces its loss-associated effects in rats. eNeuro. 2016;3(6). 10.1523/ENEURO.0108-16.2016. [DOI] [PMC free article] [PubMed]
- 254.Myllymaki T, Kyrolainen H, Savolainen K, Hokka L, Jakonen R, Juuti T, et al. Effects of vigorous late-night exercise on sleep quality and cardiac autonomic activity. J Sleep Res. 2011;20(1 Pt 2):146–153. doi: 10.1111/j.1365-2869.2010.00874.x. [DOI] [PubMed] [Google Scholar]