Abstract
Intestinal fibrosis is a common complication of inflammatory bowel disease (IBD) that is usually the consequence of chronic inflammation. Although the currently available anti-inflammatory therapies have had little impact on intestinal fibrosis in Crohn’s disease (CD), increased understanding of the pathophysiology and the development of therapies targeting fibrogenic pathways hold promise for the future. One of the critical challenges is how reduction or reversal of intestinal fibrosis should be defined and measured in the setting of clinical trials and drug approval.
The International Organization for Inflammatory Bowel Disease (IOIBD) organized a workshop in Amsterdam, The Netherlands, on December 19th and 20th, 2018 in an attempt to review the current knowledge of the biological background, diagnosis, treatment of intestinal fibrosis and clinical trial endpoints. Basic and clinical scientists discussed the pathophysiology of intestinal fibrosis, the current status of biomarkers and imaging modalities in stenosing CD, and recent clinical studies in this area. Researchers from outside of the IBD field presented advances in the understanding of fibrotic processes in other organs, such as the skin, liver and lungs. Lastly, the design of clinical trials with antifibrotic therapy for IBD was discussed, with priority on patient populations, patient reported outcomes (PROs) and imaging.
This report summarizes the key findings, discussions and conclusions of the workshop.
Pathophysiology of intestinal fibrosis
Fibrosis represents excessive production of extracellular matrix (ECM) by activated mesenchymal cells. ECM-producing cells are predominantly myofibroblasts, that differentiate from epithelial, endothelial and stellate cells, as well as from fibroblasts and bone marrow-derived stem cells (Figure 1).1 Luminal microorganisms and bacterial products, in addition to growth factors and cytokines released from immune and non-immune cells, are the main drivers of mesenchymal cell activation and differentiation that ultimately result in fibrosis.
Figure 1.
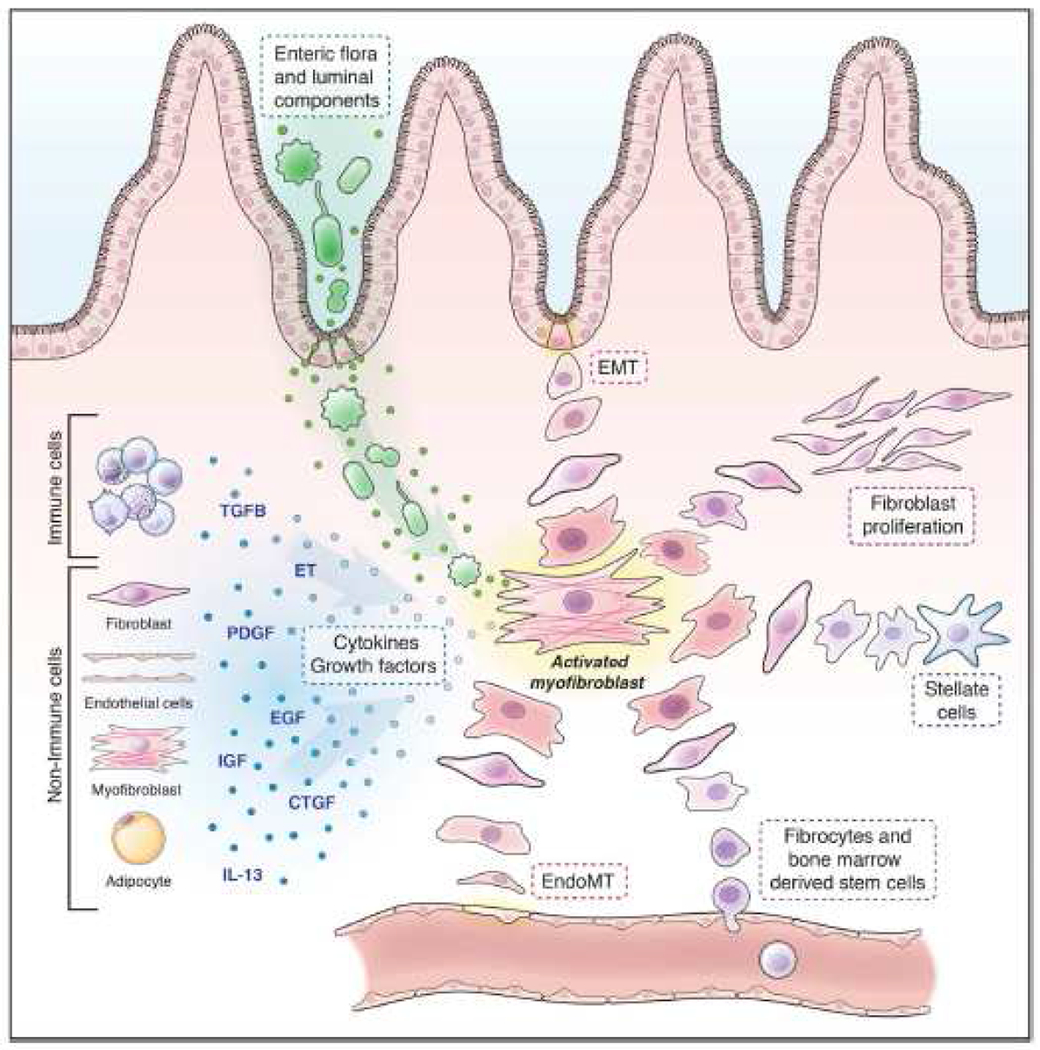
Pathophysiology of intestinal fibrosis: Soluble factors (red) and different origins of mesenchymal cells (blue).1
CTGF: connective tissue growth factor; EGF, epidermal growth factor; EndoMT: endothelial-to-mesenchymal transition; ET: endothelins; PDGF: platelet-derived growth factor
Preclinical models of intestinal fibrosis have recently been developed to better understand the pathophysiology, including a heterologous transplant model in rats and mice. For example, it was demonstrated that the bacteria-responsive adaptor protein myeloid differentiation primary response 88 (MYD88) and the cytokine interleukin 10 (IL-10) do not play a critical role in intestinal fibrosis, despite the theoretical plausibility of this interaction. Pirfenidone and antibodies against matrix metalloproteinase 9 (MMP-9), agents currently approved for the treatment of idiopathic pulmonary fibrosis (IPF), prevented the development of experimental intestinal fibrosis. Other translational studies indicated that inhibition of the pH-sensing ovarian cancer G-protein-coupled receptor 1 (OGR1) and the apoptosis regulator B-cell lymphoma 2 (BCL2) are potential approaches to prevent fibrosis in IBD.
Biomarkers for intestinal fibrosis
Prediction of the development and progression of intestinal fibrosis and stricture formation is of great importance in the management of IBD. Multiple studies have tried to identify markers that can stratify patients in fibrotic risk groups, detect early stages of fibrosis before the onset of symptoms, and/or predict the outcomes of therapy. This search resulted in the identification of several phenotypic characteristics and serologic and genetic markers associated with stenotic complications.
Of the >200 genes connected to IBD, several have been associated with fibrostenotic CD, such as variants of nucleotide-binding oligomerization domain-containing protein 2 (NOD2) gene and MMP-3. Epigenetic regulation of the genes encoding wingless-type mouse mammary tumor virus integration site family 2B (WNT2B), and two eicosanoid synthesis pathway enzymes was also associated with CD fibrosis. In addition, several serologic parameters, including ECM molecules, growth factors and antibodies against microbial products, are associated with the development of IBD, and in some cases with fibrosis.
In the Risk Stratification and Identification of Immunogenetic and Microbial Markers of Rapid Disease Progression in Children with Crohn’s Disease (RISK) study that included more than 900 children/adolescents with newly diagnosed IBD, a competing-risk model based upon demographics, clinical, serologic and genetic markers could predict a complicated disease course and response to anti-tumor necrosis factor (TNF) therapy.2 Other risk models, such as the Bacardi model, have been developed to facilitate therapeutic decisions.
Histopathological analysis of intestinal fibrosis may provide critical information beyond that available from these clinical phenotype-based instruments. Smooth muscle hyperplasia of the submucosa, hypertrophy of the muscularis propria and chronic inflammation are the most prominent changes in CD strictures.3 Since the overall muscular hyperplasia/hypertrophy correlated positively with chronic inflammation and negatively with fibrosis, the ‘inflammation-smooth muscle hyperplasia axis’ appears to play a dominant role in the pathogenesis of CD-associated strictures.
Evaluation of stenosis in CD
Sensitive and specific diagnostic methods, including imaging modalities and biomarkers, are required to quantify different components of stenosis. An ideal instrument must be valid, reliable and responsive. Outcome measures in clinical trials should also be non-invasive, widely available, inexpensive and, if possible, radiation-free. An instrument with all these desirable properties could become validated as a surrogate endpoint in clinical trials in stenosing CD. A number of tools are currently available as potential outcome measures in stenosing CD, each of which has specific advantages and disadvantages. Critical components are the assessment of bowel wall thickness, mechanical and vascular characteristics, infiltration of immune cells, and the expression of genes and proteins. However, it should be recognized that none of these candidate modalities has undergone the comprehensive validation process required for a robust outcome measure.
Cross-sectional imaging techniques, such as magnetic resonance imaging (MRI) and computer tomography scanning, are the most widely explored methods to differentiate fibrosis and inflammation. For instance, whereas inflammation in CD is associated with increased T2 hyperintensity, mucosal enhancement, ulcerations, and blurred margins, fibrosis is rather characterized by abnormalities in contrast uptake (‘enhancement’). Diffusion-weighted MRI (DWI) and magnetization transfer MRI are potentially useful for quantification of fibrosis. Other promising modalities include T2* mapping, intravoxel incoherent motion DWI, MRI–defined motility, and magnetic resonance elastography. Intestinal ultrasound (IUS) is increasingly being used for the evaluation of CD, the assessment of complications and treatment response. IUS is non-invasive, convenient for the patient and physician, inexpensive and associated with an overall sensitivity and specificity for strictures of 80% and 95%, respectively.4 The use of contrast media can further increase the sensitivity and specificity. Limitations of IUS include the lack of a ‘panoramic view’ of the whole bowel, the currently missing standard for image documentation, and the difficulty of assessing the proximal ileum and jejunum. Innovative imaging modalities are contrast-enhanced ultrasonography (CEUS) and elastography. CEUS is time consuming, relatively expensive and not yet validated.
Elastography, including quantitative shear wave elastography and qualitative strain elastography, might be especially useful to distinguish fibrotic from inflammatory stenosis, and to discriminate low-grade from high-grade fibrosis. Limitations include variability in manual compression and lack of standardization.
Photoacoustic imaging, which uses specific laser wavelengths to produce detectable vibrations in specific molecules such as hemoglobin and collagen, is currently also being developed as a tool to quantify intestinal inflammation and fibrosis.
Lessons from fibrosis in other organs
Fibrosis is a complication of many chronic diseases and aging, and occurs in virtually all organs. Accumulating evidence indicates that the pathogenic pathways involved in systemic sclerosis (SSc) and hepatic fibrosis display similarities with intestinal fibrosis.
Systemic sclerosis
SSc is an autoimmune disorder characterized by extensive fibrosis, vasculopathy, and immune dysfunction. Although the pathogenesis of SSc is largely unknown, translational studies have revealed altered fibroblast biology in addition to activation and accumulation of immune cells. CXC-chemokine ligand 4 (CXCL4), a chemokine secreted by plasmacytoid dendritic cells, is highly overexpressed in SSc, and correlates with the severity of pulmonary fibrosis and pulmonary arterial hypertension, the two most serious complications of the disease.
Transforming growth factor β (TGFβ)-dependent signaling through ‘signal transducer and activator of transcription 3’ (STAT3) is also up-regulated in SSc. STAT3 inhibitors which are currently being tested for other indications offer an attractive approach in SSc and other fibrotic conditions. The inhibition of serotonin signaling is another potential approach given that inhibition of serotonin receptors prevents the onset of experimental fibrosis and reduces established fibrosis in animal models.
Clinical trials in SSc assess disease modification in skin or lungs, using both composite clinical endpoints and/or other organ-specific read-outs. For instance, the modified Rodnan skin score is used for the clinical assessment of skin sclerosis, the forced vital capacity measures lung function in pulmonary fibrosis, and the Composite Response Index in SSc (CRISS) is commonly used to evaluate disease activity in early diffuse cutaneous SSc.
At present, tocilizumab, nintedanib, riociguat and abatacept have been approved in various jurisdictions for the treatment of SSc. In addition, many other agents, including inhibitors of adhesion molecules, growth factors, and cytokines and their receptors, are being evaluated as mono- or combination therapy. Myeloablative autologous hematopoietic stem cell transplantation has also been of benefit to patients with severe SSc.
Hepatic fibrosis
Hepatic fibrosis is characterized by excessive accumulation of ECM components, resulting from chronic liver injury associated with non-alcoholic steatohepatitis (NASH), viral hepatitis or alcoholic liver disease. Several methods are available to quantify the degree of hepatic fibrosis in non-alcoholic fatty liver disease, (NAFLD) of which NASH is the advanced stage. These include the fibrosis 4 score, the enhanced liver fibrosis test, fibroscan, (serum) biomarkers and magnetic resonance elastography.
A central factor in the pathobiology of hepatic fibrosis is the activation of hepatic stellate cells with subsequent transformation into myofibroblasts, cells that have both fibrogenic and immunomodulatory capacity. Antifibrotic therapies that deactivate, silence or eliminate hepatic stellate cells that have been explored include the peroxisome proliferator-activated receptor (PPAR) gamma agonist pioglitazone and the farnesoid X receptor (FXR) agonist obeticholic acid. Alternative strategies have interfered with biogenesis, remodeling of connective tissue or specific inflammatory processes. Although no anti-fibrotic agents have been approved for NASH, a number of agents have shown encouraging results in phase 2 and 3 trials. Examples include the FXR agonist cilofexor, the antioxidant vitamin E, the chemokine receptor inhibitor cenicriviroc, and the apoptosis signal–regulating kinase 1 (ASK-1) inhibitor selonsertib. Innovative 3D-models composed of human liver tissue or cells are anticipated to boost the development of effective and clinically relevant therapies for liver fibrosis.
Idiopathic pulmonary fibrosis
In IPF, agents that inhibit activation and/or differentiation of fibroblasts and/or immune cells or decrease the production of ECM molecules have shown promising results. Of these, the immunosuppressive agent pirfenidone and the tyrosine kinase inhibitor nintedanib have been approved in selected jurisdictions. Although treatment with these agents resulted in short-term improvement in lung function, existing data do not support benefit on overall prognosis. Other effective agents in development include the connective tissue growth factor (CTGF) inhibitor FG3019, recombinant pentraxin 2 (PTX-2), the lysophosphatidic acid receptor (LPAR) antagonist BMS-986020, and the αvβ6 integrin inhibitor BG00011.
Treatment of intestinal fibrosis
No antifibrotic therapies have been approved for IBD, but several agents showed promising results in preclinical studies. A recent study demonstrated that an antibody to the IL-36 receptor reduced fibrosis in a murine model of chronic intestinal inflammation (Table 1).5 Other potentially interesting agents include inhibitors of/antibodies against TNF-like cytokine 1A (TL1A) and agents that are used for the treatment of fibrosis in other organs (Table 1). Blockade of the fibrosis-inducing cytokine Oncostatin M with the monoclonal GSK2330811, offers an attractive strategy as well. Topical Rho kinase inhibition may also be effective by reducing myocardin-related transcription factor (MRTF) and p38 mitogen-activated protein kinase (MAPK) activation, and activation of fibroblast autophagy.
Table 1.
Potential agents for the treatment of intestinal fibrosis.
| Agent | Class | Original Indication | Status |
|---|---|---|---|
|
| |||
| spesolimab | IL-36Ri | UC | phase 2 |
| PF-06480605 | TL1Ai | UC | phase 2 |
| GSK2330811 | oncostatin Mi | cut SSc | phase 2 |
| pirfenidone | unknown | IPF | registered* |
| nintedanib | TKI | IPF | registered* |
| FG-3019 | CTGFi | IPF | phase 2b |
| PRM-151 | rhPTX-2 | IPF, HF | phase 2 |
| lebrikizumab | IL-13i | IPF | phase 2 |
| SAR-156597 | IL-4/13i | IPF | phase 2 |
| BG-00011 | αvβ6i | IPF | phase 2 |
| BMS-986020 | LPARi | IPF | phase 2 |
| pioglitazone | PPARγa | NASH | phase 3 |
| elafibranor | PPARγa | NASH | phase 3 |
| GS-9674 | FXRa | NASH | phase 2 |
| vitamin E | anti-oxidant | NASH | phase 3 |
| emricasan | caspase i | NASH | phase 2b |
| cenicriviroc | CCR2/5i | NASH | phase 3 |
| selonsertib | ASK-1i | NASH | phase 3 |
| GR-MD-02 | galectin 3i | NASH | phase 2b |
registered by FDA and EMA
a, agonist; ASK-1, apoptotic signal regulating kinase 1; CCR, CC chemokine receptor; CTGF, connective tissue growth factor; cut SSc, cutaneous systemic sclerosis; FXR, farnesoid X receptor; HF, hepatic fibrosis; i, inhibitor; IL, interleukin; IPF, idiopathic pulmonary fibrosis; LPAR, lysophosphatidic acid receptor; NASH, non-alcoholic steatohepatitis; PTX-2, pentraxin 2; R, receptor; rh, recombinant human; TKI, tyrosine kinase inhibitor; TL1A, tumor necrosis factor-like cytokine 1A; UC, ulcerative colitis.
Clinical trial design
No anti-fibrotic agents have been approved for CD, which is partially due to a lack of standardized definitions, diagnostic modalities, and validated treatment endpoints. The interdisciplinary Crohn’s Disease Anti-fibrotic Stricture Therapies (CONSTRICT) Group used a modified RAND Corporation/University of California Los Angeles (RAND/UCLA) appropriateness methodology trying to reach consensus definitions for small bowel strictures, outcome measures and treatment endpoints in stricturing CD.6 Consensus was reached on MRI being the preferred imaging technique to define strictures and assess response to therapy, and 24-48 weeks of therapy was considered appropriate for pharmacologic trials.
The only large prospective trial that evaluated the efficacy of treatment for stenosing CD was CREOLE. This cohort study assessed the efficacy of adalimumab in symptomatic CD small bowel strictures using a composite endpoint of treatment success at week 24, defined as continuation of adalimumab without prohibited treatment, endoscopic dilation or bowel resection.7 However, the ‘Crohn’s disease obstructive score’ used was designed by physicians and hence not a valid PRO. Sixty-four per cent of the patients met the endpoint. A prognostic score was proposed to define patients with a good, intermediate and poor prognosis.
For trials in symptomatic CD patients with fibrosis, the identification of the most optimal study population with radiological confirmation will be essential.
Measurement of stenotic fibrosis by validated magnetic resonance/computed tomography criteria is likely to be preferred for assessment of treatment efficacy. Nevertheless, evaluation of symptoms by rigorously developed PRO is also necessary to show the value of new treatments to patients’ well-being and for regulatory approval. Expert members of the IOIBD recently proposed 13 endpoints for use in clinical trials that assess the efficacy and safety of antifibrotic agents in CD.8
Currently, the international Stenosis Therapy and Anti-fibrotic Research (STAR) consortium is developing a PRO instrument for stricturing CD according to Food and Drug Administration-recommended methodology and internationally-developed best practices.
Discussion and disagreement
Most likely, antifibrotic treatment in isolation has relatively little chance of success if not combined with anti-inflammatory treatment. It remains unclear and a matter of discussion what this component of a ‘combined intervention’ should look like. Moreover, it remains unclear if smooth muscle hyperplasia observed in the submucosa, and hypertrophy of the muscularis propria would need different targeted treatment.
Also, the ideal study population remains uncertain: should it be patients who have had a complete bowel obstruction? Can they have a balloon dilatation or stricturoplasty prior to entering an antifibrotic trial? How can dietary changes be controlled? Patients tend to alter their intake based on symptoms caused by specific food products, which may interfere with subjective well being and even with prestenotic dilatation as documented on imaging. Although MR enterography was proposed as the most attractive imaging modality, validated and reliable scores for fibrostenotic IBD are lacking. Moreover, waiting times for MR can be challenging and therefore less invasive ultrasound-based technologies warrant further investigation.
Conclusions
During this IOIBD workshop the pathophysiology, diagnosis and potential therapies for intestinal fibrosis and other chronic fibrotic diseases were reviewed. The pathways of fibrosis analyzed and compared, and potential therapeutic targets were identified. Currently approved and investigational therapies for systemic sclerosis and pulmonary and hepatic fibrosis were discussed. The workshop fueled continuing efforts to formulate definitions, endpoints and trial designs that are highly needed for the optimal evaluation of antifibrotic agents in IBD. Progress in the development of a PRO for stricturing CD is anticipated to further facilitate clinical studies in this field. Although intestinal fibrosis is a complex and challenging disorder, numerous preclinical and clinical efforts have resulted in promising advances.
Acknowledgments
Conflicts of interest
Geert D’Haens has served as advisor for Abbvie, Ablynx, Allergan, Alphabiomics, Amakem, Amgen, AM Pharma, Arena Pharmaceuticals, AstraZeneca, Avaxia, Biogen, Bristol Meiers Squibb, Boehringer Ingelheim, Celgene/Receptos, Celltrion, Cosmo, Echo Pharmaceuticals, Eli Lilly, Engene, Ferring, DrFALK Pharma, Galapagos, Genentech/Roche, Gilead, Glaxo Smith Kline, Gossamerbio, Hospira/Pfizer, Immunic, Johnson and Johnson, Kintai Therapeutics, Lycera, Medimetrics, Millenium/Takeda, Medtronics, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Nextbiotics, Novonordisk, Otsuka, Pfizer/Hospira, Photopill, Prodigest, Prometheus laboratories/Nestle, Progenity, Protagonist, RedHill; Robarts Clinical Trials, Salix, Samsung Bioepis, Sandoz, Seres/Nestle, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant and Vifor; and received speaker fees from Abbvie, Biogen, Ferring, Johnson and Johnson, Merck Sharp Dome, Mundipharma, Norgine, Pfizer, Samsung Bioepis, Shire, Millenium/Takeda, Tillotts and Vifor. Florian Rieder received consulting fees from Allergan, AbbVie, Boehringer Ingelheim, Celgene, Cowen, Gilead, Gossamer, Helmsley, Janssen, Koutif, Metacrine, Pliant, Pfizer, Receptos, RedX, Roche, Samsung, Takeda, Thetis, and UCB; and research funding from Boehringer Ingelheim, UCB, Celgene, and Pliant. Brian Feagan received grants and/or research support from AbbVie Inc., Amgen Inc., AstraZeneca/MedImmune Ltd., Atlantic Pharmaceuticals Ltd., Boehringer-Ingelheim, Celgene Corporation, Celltech, Genentech Inc/Hoffmann-La Roche Ltd., Gilead Sciences Inc., GlaxoSmithKline, Janssen Research & Development LLC., Pfizer Inc., Receptos Inc./Celgene International, Sanofi, Santarus Inc., Takeda Development Center Americas Inc., Tillotts Pharma AG, and UCB; is a consultant for Abbott/AbbVie, Akebia Therapeutics, Allergan, Amgen, Applied Molecular Transport Inc., Aptevo Therapeutics, Astra Zeneca, Atlantic Pharma, Avir Pharma, Biogen Idec, BioMx Israel, Boehringer-Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, Galapagos, Galen/Atlantica, GiCare Pharma, Gilead, Gossamer Pharma, GSK, Inception IBD Inc, Intact Therapeutics, JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nestles, Nextbiotix, Novonordisk, ParImmune, Parvus Therapeutics Inc., Pfizer, Prometheus Therapeutics and Diagnostics, Progenity, Protagonist, Qu Biologics, Receptos, Salix Pharma, Shire, Sienna Biologics, Sigmoid Pharma, Sterna Biologicals, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, Vivelix Pharma, VHsquared Ltd., and Zyngenia; affiliated with the speakers bureau of Abbott/AbbVie, JnJ/Janssen, Lilly, Takeda, Tillotts, UCB Pharma; a member of the scientific advisory board of Abbott/AbbVie, Allergan, Amgen, Astra Zeneca, Atlantic Pharma, Avaxia Biologics Inc., Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Galapagos, Genentech/Roche, JnJ/Janssen, Merck, Nestles, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Sterna Biologicals, Takeda, Teva, TiGenix, Tillotts Pharma AG, UCB Pharma; is a Senior Scientific Officer of Robarts Clinical Trials Inc. (London). Peter Higgins has served as a consultant for Abbvie, Genentech, UCB, Takeda, and has received educational grants and research grants from Abbvie, Janssen, Pfizer, and Takeda. Julian Panés received consulting fees from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Ferring, Genentech, GlaxoSmithKline, Janssen, MSD, Nestle, Oppilan, Pfizer, Progenity, Robarts, Roche, Takeda, Theravance, and TiGenix; speaker fees from AbbVie, Janssen, MSD, and Takeda; and research funding from AbbVie and MSD. Christian Maaser has served as a speaker, consultant and/or advisory board member for Abbvie, Celgene, Falk Foundation, Ferring, Janssen, MSD, Pfizer, Takeda and Vifor. Gerhard Rogler has consulted to Abbvie, Augurix, BMS, Boehringer, Calypso, Celgene, FALK, Ferring, Fisher, Genentech, Gilead, Janssen, MSD, Novartis, Pfizer, Phadia, Roche, UCB, Takeda, Tillots, Vifor, Vital Solutions and Zeller; received speaker’s honoraria from Astra Zeneca, Abbvie, FALK, Janssen, MSD, Pfizer, Phadia, Takeda, Tillots, UCB, Vifor and Zeller; and educational grants and research grants from Abbvie, Ardeypharm, Augurix, Calypso, FALK, Flamentera, MSD, Novartis, Pfizer, Roche, Takeda, Tillots, UCB and Zeller. Mark Löwenberg has served as speaker, consultant or principal investigator for Abbvie, Celgene, Covidien, Dr. Falk, Ferring Pharmaceuticals, Gilead, GlaxoSmithKline, Janssen-Cilag, Merck Sharp & Dohme, Pfizer, Protagonist therapeutics, Receptos, Takeda, Tillotts, and Tramedico; and received research grants from AbbVie, Merck Sharp & Dohme, Achmea healthcare, Dr Falk and ZonMW. Robbert van der Voort has no conflicts of interest to disclose. Massimo Pinzani is an inventor and patent holder (ELF test) for Siemens; and served as a speaker or consultant for Echosens, Promethera, Neurovive, Chemomab, Median Technology, UCB Cell Tech, Boehringer Ingelheim, Engitix Ltd, 3P-Sense Ltd and Hepatotargets. Laurent Peyrin-Biroulet has served as a speaker for Merck, Abbvie, Janssen, Genentech, Ferring, Tillots, Vifor, Pharmacosmos, Celltrion, Takeda, Boerhinger-Ingelheim, Pfizer, Amgen, Biogen, Samsung Bioepis; and as a consultant for Merck, Abbvie, Janssen, Genentech, Ferring, Tillots, Vifor, Pharmacosmos, Celltrion, Takeda, Biogaran, Boerhinger-Ingelheim, Lilly, Pfizer, Index Pharmaceuticals, Amgen, Sandoz, Celgene, Biogen, Samsung Bioepis, Alma, Sterna, Nestlé, Enterome, Mylan, HAC-Pharma. Silvio Danese has served as a speaker, a consultant and an advisory board member for Abbvie, Allergan, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Hospira, Johnson & Johnson, Merck, MSD, Takeda, Mundipharma, Pfizer, Sandoz, Tigenix, UCB Pharma and Vifor.
Funding
This meeting was supported through unrestricted grants from Celgene, Gilead, GlaxoSmithKline, Pfizer, Roche, and Union Chimique Belge (UCB) Pharma.
Abbreviations used in this paper
- CD
Crohn’s disease
- CEUS
contrast-enhanced ultrasonography
- DWI
diffusion-weighted MRI
- ECM
extracellular matrix
- IBD
inflammatory bowel disease
- IOIBD
International Organization for Inflammatory Bowel Disease
- IPF
idiopathic pulmonary fibrosis
- IUS
intestinal ultrasound
- MMP
matrix metalloproteinase
- MRI
magnetic resonance imaging
- NASH
non-alcoholic steatohepatitis
- PRO
patient reported outcome
- SSc
systemic sclerosis
- STAT
signal transducer and activator of transcription
- TNF
tumor necrosis factor
APPENDIX
Acknowledgements
*IOIBD Fibrosis Working Group:
Mariangela Allocca, IBD Center, Humanitas Clinical and Research Hospital, Humanitas University, Milan, Italy
Gert De Hertogh, University Hospitals Leuven, Department of Pathology, Leuven, Belgium
Chris Denton, Royal Free Hospital, Centre for Rheumatology and Connective Tissue Diseases, UCL, London, UK
Jörg Distler, Friedrich-Alexander-University (FAU) Erlangen-Nürnberg and Universitätsklinikum Erlangen, Department of Internal Medicine 3 - Rheumatology and Immunology, Erlangen, Germany
Kelly McCarrier, Pharmerit, Bethesda, USA
Dermot McGovern, Cedars-Sinai Medical Center, Medical Genetics Research Institute, Department of Medicine, Los Angeles, California, USA
Tim Radstake, University Medical Center Utrecht, Utrecht University, Laboratory of Translational Immunology, and Department of Rheumatology and Clinical Immunology, Utrecht, The Netherlands
Daniel Serrano, Pharmerit, Bethesda, USA
Jaap Stoker, Cancer Center Amsterdam, Amsterdam University Medical Center, Academic Medical Center, Departments of Radiology and Nuclear Medicine, Amsterdam, The Netherlands
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Author names in bold designate shared co-first authorship.
- 1.Rieder F, Fiocchi C, and Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology 2017; 152: 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 2017; 389: 1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Lu C, Hirota C, et al. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohns Colitis 2017; 11: 92–104. [DOI] [PubMed] [Google Scholar]
- 4.Kucharzik T, and Maaser C. Intestinal ultrasound and management of small bowel Crohn’s disease. Therap Adv Gastroenterol. 2018. May 1; 11: 1756284818771367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheibe K, Kersten C, Schmied A, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology 2019; 156: 1082–1097. [DOI] [PubMed] [Google Scholar]
- 6.Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment Pharmacol Ther 2018; 48: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouhnik Y, Carbonnel F, Laharie D, et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut 2018; 67: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danese S, Bonovas S, Lopez A, et al. Identification of endpoints for development of antifibrosis drugs for treatment of Crohn’s disease. Gastroenterology 2018; 155: 76–87. [DOI] [PubMed] [Google Scholar]


