Significance
Fibroblast growth factor 21 (FGF21) controls metabolic organ homeostasis and eating behavior via FGF receptor 1/Klothoβ (FGFR1/KLB) complexes. Here we show that a bispecific anti-FGFR1/KLB agonist antibody, BFKB8488A, mimics the actions of FGF21 in monkeys and humans. BFKB8488A induced marked weight loss in obese monkeys while elevating expression of FGFR1 target genes in adipose tissue. A clinical study in overweight human participants demonstrated that a single dose of BFKB8488A caused transient body weight reduction, sustained improvement in cardiometabolic parameters, and a trend toward reduction in preference for sweet taste and carbohydrate intake. These data suggest that antibody-mediated activation of the FGFR1/KLB complex in humans recapitulates the effects of FGF21 and can be used as therapy for obesity-related metabolic defects.
Keywords: obesity, metabolism, weight loss, food preference, FGF21 receptor activation
Abstract
Fibroblast growth factor 21 (FGF21) controls metabolic organ homeostasis and eating/drinking behavior via FGF receptor 1/Klothoβ (FGFR1/KLB) complexes expressed in adipocytes, pancreatic acinar cells, and the nervous system in mice. Chronic administration of recombinant FGF21 or engineered variants improves metabolic health in rodents, nonhuman primates, and humans; however, the rapid turnover of these molecules limits therapeutic utility. Here we show that the bispecific anti-FGFR1/KLB agonist antibody BFKB8488A induced marked weight loss in obese cynomolgus monkeys while elevating serum adiponectin and the adipose expression of FGFR1 target genes, demonstrating its action as an FGF21 mimetic. In a randomized, placebo-controlled, single ascending-dose study in overweight/obese human participants, subcutaneous BFKB8488A injection caused transient body weight reduction, sustained improvement in cardiometabolic parameters, and a trend toward reduction in preference for sweet taste and carbohydrate intake. These data suggest that specific activation of the FGFR1/KLB complex in humans can be used as therapy for obesity-related metabolic defects.
Fibroblast growth factor 21 (FGF21), an endocrine member of the FGF superfamily, controls energy expenditure and nutrient metabolism by stimulating FGF receptor (FGFR) isoforms (1c, 2c, and 3c) bound by the obligatory coreceptor Klothoβ (KLB) (1). While various cell types express FGFR isoforms, KLB is primarily expressed in adipocytes, hepatocytes, pancreatic acinar cells, and within the central nervous system, thus likely limiting the site of FGF21 action (1). Repeated injection or continuous infusion of recombinant FGF21 or modified FGF21 variants into diet-induced obese mice induces weight loss without appetite loss, improves insulin sensitivity, and ameliorates hepatic steatosis, hyperglycemia, and hyperlipidemia (2–4) via stimulation of sympathetic nerves and resulting brown fat thermogenesis, and production of the adipokine, adiponectin (5–7). Many of these FGF21 effects, including weight loss and lipid decreases, appear to be conserved in nonhuman primates (8–11) and humans (11–14). Together, these studies suggest the utility of FGF21 analogs as effective protein drugs for obesity-related disorders, such as nonalcoholic steatohepatitis.
In humans, serum levels of FGF21 were shown to be elevated in obesity, diabetes, and nonalcoholic fatty liver disease, implicating its role in regulating metabolic stress (15, 16). Diurnal variations in serum FGF21 are congruent with levels of circulating free fatty acids, suggesting that free fatty acids may regulate its expression through PPARα (17). In addition, ingestion of high-dose fructose or glucose in humans results in a transient increase in serum FGF21, potentially through the carbohydrate-sensing element within the promoter region (18). Genome-wide association studies have identified FGF21 gene variants associated with higher carbohydrate and lower fat and protein consumption (19–21). Furthermore, several KLB gene variants (rs11940694, rs13130794, rs9991733) were associated with higher alcohol consumption (22–24). In mice, recombinant FGF21 analogs reduced sweetened food/water and alcohol consumption (22, 25, 26), and in monkeys, FGF21 altered sweet preference (25). Collectively, these reports suggest a role for FGF21 in sensing and regulating intake of nutrients.
Therapeutic use of native or engineered FGF21 is limited because of rapid plasma clearance and proteolytic inactivation by the endopeptidase fibroblast activation protein (27–29), which would necessitate frequent dosing (13, 14, 30). As an alternative approach, we generated a humanized, effector-less, bispecific anti-FGFR1/KLB antibody (BFKB8488A, also bFKB1) that selectively activates FGFR1 in a KLB-dependent manner and mimics FGF21 action in mice (4). Anti-FGFR1/KLB antibody enhances dimerization of the c-isoform of FGFR1 (FGFR1c) only when KLB is present on the cell surface and stabilizes the interaction between the extracellular domains of FGFR1c and KLB proteins, as previously observed for FGF21. Despite the functional similarity to FGF21, the BFKB8488A epitope appears to differ from the binding site for FGF21, hence BFKB8488A is not expected to alter endogenous FGF21-mediated signaling (4). Importantly, in contrast to FGF21, BFKB8488A does not signal through FGFR2 and FGFR3, and as such, does not effectively signal through an FGFR/KLB complex in the liver. Whether activation of the FGFR1/KLB complex by a synthetic antibody recapitulates the activity of FGF21 analogs in primate species, especially humans, is unknown.
Here we describe the effect of a single dose of BFKB8488A in obese, nondiabetic cynomolgus monkeys and humans. In monkeys, we examined BFKB8488A effects on FGFR1/KLB receptor activation in adipose tissue and on serum adiponectin, body weight, and food intake. In a first-in-human phase 1 trial, we investigated the safety and pharmacokinetics (PK) of BFKB8448A administered subcutaneously in overweight/obese, but otherwise healthy, participants. We also examined the pharmacodynamic (PD) effects of BFKB8488A on various cardiometabolic parameters, body weight, appetite, and food preference.
Results
Administration of a Humanized Anti-FGFR1/KLB Agonist Antibody Activates the Receptor Complex, Increases Serum Adiponectin, and Decreases Body Weight and Food Intake in Obese Nonhuman Primates.
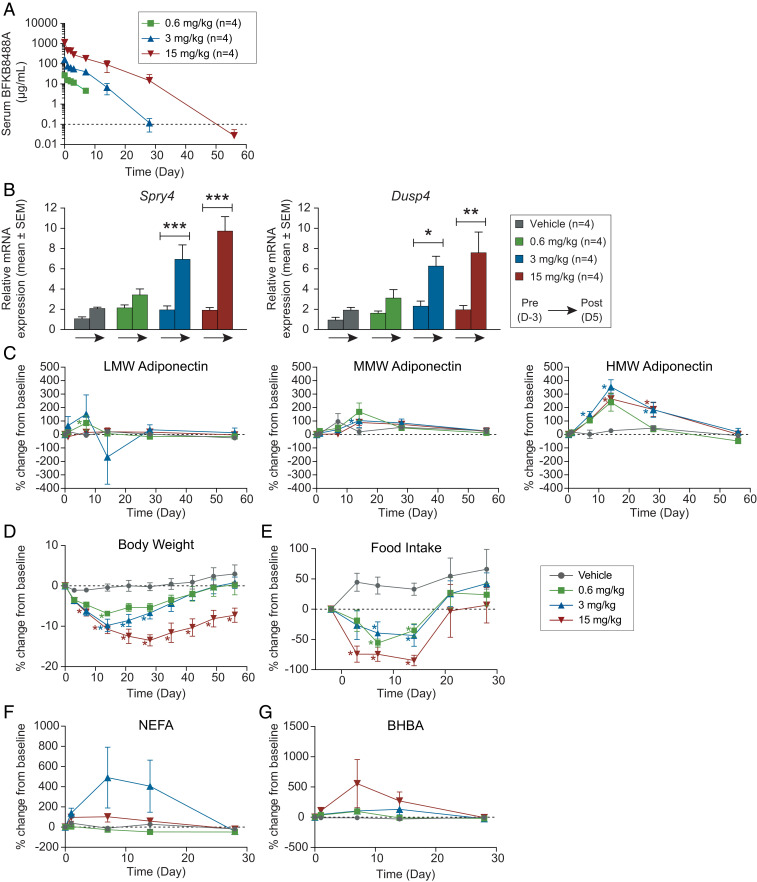
We first assessed the effect of FGFR1/KLB pathway activation by BFKB8488A in obese monkeys. After a single intravenous injection, circulating levels of BFKB8488A increased linearly with dose (Fig. 1A) and remained above the 50% effective concentration (EC50; 0.7 µg/mL, determined by the increase of phosphorylated ERK in primary adipocytes) (SI Appendix, Fig. S1) for at least 7, 14, and 28 d in the 0.6-, 3-, and 15-mg/kg groups, respectively. BFKB8488A target engagement in subcutaneous adipose tissue was demonstrated by elevations in Sprouty 4 (Spry4) and dual-specificity phosphatase 4 (Dusp4) mRNA, genes encoding negative regulators of the FGFR1/KLB pathway that are induced by FGFR activation (4, 7, 31, 32) (Fig. 1B). Consistent with FGF21 pathway activation, a marked dose-related increase in serum high-molecular weight (HMW) adiponectin was observed in all BFKB8488A dose groups with no appreciable changes in medium- (MMW) or low-molecular weight (LMW) adiponectin levels (Fig. 1C). HMW adiponectin is the oligomeric complex predominantly responsible for action in metabolic tissues and has been reported for its insulin-sensitizing and antiinflammatory effects (33).
Fig. 1.
Administration of a humanized anti-FGFR1/KLB agonist antibody activates the receptor complex, increases serum adiponectin, and decreases body weight and food intake in obese nonhuman primates. (A) PKs of BFKB8488A following intravenous administration. Values are mean ± SD. Dotted line indicates minimum quantifiable concentration. (B) Relative Dusp4 and Spry4 mRNA expression (mean ± SEM) in subcutaneous biopsies from day −3 (predose) and day 5 (postdose). *P < 0.05; **P < 0.01; ***P < 0.001. (C) Percent change from predose baseline (mean ± SEM) in LMW, MMW, and HMW adiponectin, (D) body weight, (E) food consumption, (F) NEFA, and (G) BHBA. For C–G, dotted lines indicate baseline; *P ≤ 0.05.
BFKB8488A also caused a significant and profound dose-related body weight loss in all dose groups that began as early as day 3 and reached a nadir between day 15 and day 30 (Fig. 1D). We observed marked weight loss in the highest-dose cohorts, with a mean weight loss at nadir of greater than 15% and sustained for 85 d after drug administration. In the highest-dose cohort, body composition measurements by dual X-ray absorptiometry (DEXA) scans on day 23 revealed that most weight loss derived from a fat mass reduction of ∼30% with minimal effect on lean mass (reduction of ∼7%), similar to other dose cohorts (SI Appendix, Table S1). We also observed a marked reduction in food intake in all animals that received BFKB8488A (Fig. 1E). In particular, food intake was almost completely suppressed for 2 wk in half the animals in the 15-mg/kg group. On day 21, following a substantial decrease in drug exposure, food intake rebounded, and most animals resumed normal feeding by day 30. Despite the marked reduction in food intake, no evidence of emesis was observed. Reduced food consumption was accompanied by increases in serum fasting markers, such as nonesterified free fatty acids (NEFAs) and D-3 (β) hydroxybutyrate (BHBA) (Fig. 1 F and G). However, because of the variable nature of these markers, we could not detect a clear dose–response. Taken together, the observed hypophagia and weight loss in obese primates demonstrate that a single administration of BFKB8488A recapitulates the effects observed following chronic administration of recombinant FGF21 analogs through the specific activation of the FGFR1/KLB receptor complex (11).
First-In-Human Clinical Trial: Participant Disposition and Participant Demographics.
In the first-in-human trial aiming to determine the effects of subcutaneous BFKB8488A administration, 71 eligible overweight or obese participants were randomized into the study from October 2015 to February 2017. All enrolled participants received a single subcutaneous injection of BFKB8488A (3 to 681 mg) or placebo into the thigh or abdomen on day 1 and were included in the safety-evaluable population. Forty-five of 53 BFKB8488A-treated participants (84.9%) and 15 of 18 placebo-treated participants (83.3%) completed the study. Discontinuations were due to subject withdrawal, including noncompliance.
Participant ages ranged from 18 to 64 y, with a median age of 45 y. Despite sample sizes too small to detect any meaningful differences in demographic characteristics, the BFKB8488A 681-mg subcutaneous cohort was notably younger (median age, 28.0 y) than other cohorts. Overall median body mass index (BMI) was 33.4 kg/m2 (Table 1). Median BMI for placebo participants (32.6 kg/m2) and BFKB8488A-treated participants (34.1 kg/m2) fell within the World Health Organization (WHO) obese class I (34). However, median BMI in the 39-mg and 171-mg cohorts fell within WHO obese class II. Additionally, 100% (six of six) of the BFKB8488A 250-mg subcutaneous cohort was female. All other cohorts included at least one participant of each sex.
Table 1.
Participant demographics and baseline characteristics
| BFKB8488A | ||||||||||||
| Abdomen | Thigh | |||||||||||
| Characteristic | Placebo | All BFKB8488A | 3 mg | 10.5 mg | 39 mg | 111 mg | 171 mg | 342 mg | 681 mg | 171 mg | 250 mg | Allparticipants |
| (n = 18) | (n = 53) | (n = 7) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 4) | (n = 6) | (n = 71) | |
| Age (y),mean (SD) | 44.6(10.8) | 44.6(12.3) | 39.1(15.4) | 54.5(6.5) | 46.0(8.4) | 45.7(12.0) | 45.7(10.8) | 44.5(10.0) | 33.3(13.0) | 48.5(13.2) | 46.2(14.1) | 44.6(11.8) |
| Sex, female,n (%) | 1(5.6%) | 29(54.7%) | 1(14.3%) | 3(50.0%) | 4(66.7%) | 2(33.3%) | 3(50.0%) | 5(83.3%) | 2(33.3%) | 3(75.0%) | 6(100.0%) | 30(42.3%) |
| Ethnicity,Hispanic or Latino, n (%) | 8(44.4%) | 24(45.3%) | 3(42.9%) | 3(50.0%) | 3(50.0%) | 2(33.3%) | 4(66.7%) | 3(50.0%) | 3(50.0%) | 0 | 3(50.0%) | 32(45.1%) |
| Race,White | 16(88.9%) | 36(67.9%) | 4(57.1%) | 4(66.7%) | 4(66.7%) | 3(50.0%) | 5(83.3%) | 5(83.3%) | 4(66.7%) | 1(25.0%) | 6(100.0%) | 52(73.2%) |
| BMI (kg/m2),mean (SD) | 33.14(2.43) | 34.11(3.10) | 34.06(3.68) | 35.07(3.11) | 36.17(3.08) | 32.27(1.25) | 34.98(3.38) | 32.30(1.55) | 34.78(2.87) | 35.65(3.03) | 32.27(3.74) | 33.87(2.96) |
| Serumadiponectin(µg/mL), mean (SD) | 5.08(3.52) | 6.26(4.52) | 6.01(5.91) | 7.06(6.70) | 7.03(4.06) | 5.99(5.46) | 5.23(2.64) | 4.54(2.66) | 5.53(2.22) | 10.24(5.62) | 4.20(1.29) | 5.96(4.30) |
| Triglycerides (mg/dL),mean (SD) | 175.79(85.94) | 128.72(74.49) | 138.24(98.56) | 117.88(29.12) | 105.03(38.48) | 135.15(57.35) | 155.96(40.31) | 124.23(58.56) | 186.30(154.71) | 76.84(14.28) | 100.01(47.96) | 140.66(79.63) |
| HDL cholesterol (mg/dL),mean (SD) | 44.67(12.85) | 52.86(14.05) | 50.71(10.43) | 47.11(7.73) | 54.04(8.44) | 46.34(6.70) | 51.98(6.14) | 61.23(24.70) | 43.95(8.65) | 67.82(25.54) | 57.91(12.02) | 50.79(14.13) |
| LDL cholesterol (mg/dL),mean (SD) | 113.07(18.24) | 115.63(24.31) | 120.90(27.80) | 124.55(22.18) | 114.58(18.47) | 112.10(22.44) | 129.38(18.54) | 107.37(35.67) | 109.77(24.28) | 126.40(21.42) | 98.36(21.22) | 114.98(22.83) |
| Blood glucose(mg/dL), mean (SD) | 96.00(10.54) | 94.91(8.25) | 89.14(7.13) | 97.50(12.80) | 97.67(8.87) | 98.00(5.76) | 94.67(6.22) | 94.67(7.39) | 94.00(7.46) | 93.50(13.18) | 95.50(6.25) | 95.18(8.82) |
| Plasma insulin (µU/mL),mean (SD) | 9.78(4.64) | 11.01(7.49) | 7.67(3.60) | 18.30(13.77) | 10.73(9.42) | 13.14(7.23) | 8.08(2.12) | 8.00(4.11) | 13.19(5.70) | 11.90(9.02) | 8.97(4.17) | 10.70(6.87) |
| HOMA-IR, mean (SD) | 2.37(1.54) | 2.50(2.22) | 1.81(1.17) | 5.07(4.43) | 2.87(3.10) | 2.68(1.49) | 1.57(0.65) | 1.40(0.84) | 2.53(1.08) | 2.83(2.26) | 1.96(1.01) | 2.47(2.06) |
HOMA-IR, homeostatic model assessment-insulin resistance.
Safety.
Overall, BFKB8488A was adequately tolerated and manageable; there were no dose-limiting adverse events (DLAEs), deaths, or withdrawals of study treatment due to adverse events (AEs). AEs were mainly mild, and most moderate AEs were gastrointestinal toxicities. No serious AEs occurred. Of those participants receiving BFKB8488A or placebo, 62 of 71 (87%) reported at least one AE (Table 2). A detailed description of events is included in SI Appendix, SI Text.
Table 2.
Total number of adverse events and adverse events reported in more than five participants
| BFKB8488A | ||||||||||||
| Abdomen | Thigh | |||||||||||
| MedDRA SOC | Placebo | All BFKB8488A | 3 mg | 10.5 mg | 39 mg | 111 mg | 171 mg | 342 mg | 681 mg | 171 mg | 250 mg | All participants |
| Preferred term | (n = 18) | (n = 53) | (n = 7) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 6) | (n = 4) | (n = 6) | (n = 71) |
| Total number of subjects with at least one adverse event* | 16 (88.9%) | 46 (86.8%) | 4 (57.1%) | 4 (66.7%) | 6 (100.0%) | 6 (100.0%) | 4 (66.7%) | 6 (100.0%) | 6 (100.0%) | 4 (100.0%) | 6 (100.0%) | 62 (87.3%) |
| Total number of events* | 44 | 260 | 14 | 17 | 36 | 14 | 23 | 42 | 58 | 24 | 32 | 304 |
| Gastrointestinal disorders | ||||||||||||
| Nausea | 3 (16.7%) | 22 (41.5%) | 0 | 1 (16.7%) | 4 (66.7%) | 1 (16.7%) | 0 | 5 (83.3%) | 5 (83.3%) | 2 (50.0%) | 4 (66.7%) | 25 (35.2%) |
| Diarrhea | 2 (11.1%) | 12 (22.6%) | 1 (14.3%) | 1 (16.7%) | 0 | 0 | 1 (16.7%) | 2 (33.3%) | 5 (83.3%) | 1 (25.0%) | 1 (16.7%) | 14 (19.7%) |
| Vomiting | 0 | 12 (22.6%) | 0 | 0 | 0 | 0 | 1 (16.7%) | 3 (50.0%) | 5 (83.3%) | 1 (25.0%) | 2 (33.3%) | 12 (16.9%) |
| Abdominal distension | 2 (11.1%) | 9 (17.0%) | 0 | 0 | 2 (33.3%) | 0 | 1 (16.7%) | 1 (16.7%) | 3 (50.0%) | 1 (25.0%) | 1 (16.7%) | 11 (15.5%) |
| Constipation | 0 | 6 (11.3%) | 0 | 0 | 2 (33.3%) | 0 | 1 (16.7%) | 1 (16.7%) | 1 (16.7%) | 1 (25.0%) | 0 | 6 (8.5%) |
| Dyspepsia | 1 (5.6%) | 5 (9.4%) | 0 | 0 | 1 (16.7%) | 0 | 1 (16.7%) | 2 (33.3%) | 1 (16.7%) | 0 | 0 | 6 (8.5%) |
| Frequent bowel movements | 1 (5.6%) | 5 (9.4%) | 0 | 0 | 0 | 1 (16.7%) | 1 (16.7%) | 1 (16.7%) | 1 (16.7%) | 1 (25.0%) | 0 | 6 (8.5%) |
| General disorders and site administration conditions | ||||||||||||
| Injection site erythema | 2 (11.1%) | 10 (18.9%) | 0 | 0 | 0 | 0 | 0 | 2 (33.3%) | 2 (33.3%) | 1 (25.0%) | 5 (83.3%) | 12 (16.9%) |
| Hunger | 2 (11.1%) | 7 (13.2%) | 0 | 0 | 1 (16.7%) | 2 (33.3%) | 3 (50.0%) | 1 (16.7%) | 0 | 0 | 0 | 9 (12.7%) |
| Fatigue | 1 (5.6%) | 5 (9.4%) | 0 | 0 | 1 (16.7%) | 1 (16.7%) | 0 | 1 (16.7%) | 0 | 2 (50.0%) | 0 | 6 (8.5%) |
| Thirst | 1 (5.6%) | 5 (9.4%) | 1 (14.3%) | 1 (16.7%) | 2 (33.3%) | 0 | 1 (16.7%) | 0 | 0 | 0 | 0 | 6 (8.5%) |
| Infections and infestations | ||||||||||||
| Upper respiratory tract infection | 4 (22.2%) | 8 (15.1%) | 2 (28.6%) | 1 (16.7%) | 1 (16.7%) | 0 | 1 (16.7%) | 1 (16.7%) | 2 (33.3%) | 0 | 0 | 12 (16.9%) |
| Metabolism and nutrition disorders | ||||||||||||
| Decreased appetite | 2 (11.1%) | 9 (17.0%) | 1 (14.3%) | 0 | 0 | 0 | 2 (33.3%) | 2 (33.3%) | 3 (50.0%) | 0 | 1 (16.7%) | 11 (15.5%) |
| Increased appetite | 0 | 7 (13.2%) | 0 | 0 | 0 | 0 | 0 | 1 (16.7%) | 0 | 3 (75.0%) | 3 (50.0%) | 7 (9.9%) |
| Nervous system disorders | ||||||||||||
| Headache | 2 (11.1%) | 6 (11.3%) | 0 | 1 (16.7%) | 2 (33.3%) | 0 | 0 | 0 | 1 (16.7%) | 0 | 2 (33.3%) | 8 (11.3%) |
| Renal and urinary disorders | ||||||||||||
| Pollakiuria | 2 (11.1%) | 4 (7.5%) | 1 (14.3%) | 1 (16.7%) | 1 (16.7%) | 0 | 0 | 0 | 0 | 1 (25.0%) | 0 | 6 (8.5%) |
Data are number of participants (%). MedDRA, Medical Dictionary for Regulatory Activities; SOC, system organ class.
Total number of participants and total number of events are reported for all safety-evaluable participants.
Pharmacokinetics.
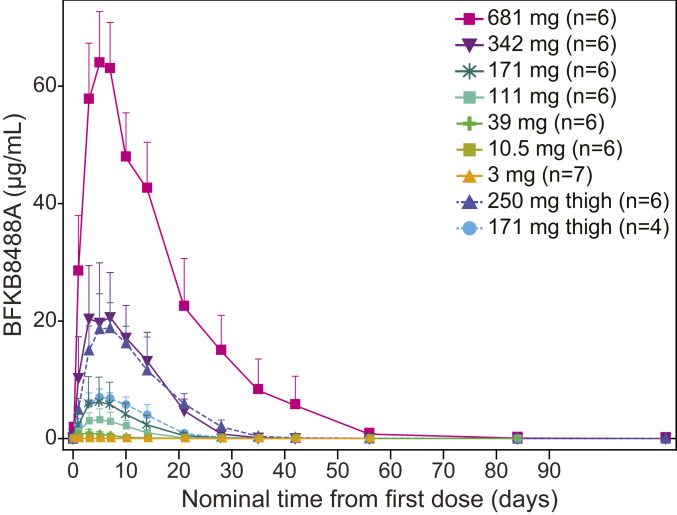
In total, 53 participants received a single subcutaneous dose of BFKB8488A; all were included in the PK analysis. Following subcutaneous administration in the abdomen, serum BFKB8488A concentrations peaked 3.0 to 5.6 d postdose and decreased gradually thereafter (Fig. 2 and SI Appendix, Table S2). Serum BFKB8488A exposures increased greater than dose-proportionally over the dose range evaluated. To explore the impact of injection site on systemic absorption, study drug was injected subcutaneously into the thigh in two cohorts (171 mg and 250 mg). In these cohorts, serum BFKB8488A concentrations peaked 5.4 to 5.9 d postdose and decreased gradually. Overall, elimination was multiphasic, potentially due to a prolonged distribution phase, with an apparent terminal half-life (t1/2) of ∼6 to 7 d, although t1/2 values should be interpreted with caution as concentrations in some participants dropped below the lower limit of quantification before complete characterization of the terminal elimination phase. Data were insufficient to determine whether the site of subcutaneous administration (thigh vs. abdomen) affected BFKB8488A bioavailability.
Fig. 2.
BFKB8488A serum concentration-time profile in obese healthy participants. Values are mean + SD.
Mean total apparent clearance (CL/F) values ranged from 10.1 L/d in the 10.5-mg cohort to 0.56 L/d in the 681-mg cohort (SI Appendix, Table S2), faster than the estimated clearance of 0.21 L/d for endogenous IgG (35). No antidrug antibodies (ADAs) were detected at baseline in either BFKB8488A- or placebo-treated participants (SI Appendix, Table S3). Postbaseline ADAs were detected in 15.0% (8 of 53) of participants in BFKB8488A-treated groups, but none were detected in the placebo group. The limited number of ADA-positive participants prevented forming definitive conclusions about effects on PK or clearance.
BFKB8488A Improved Cardiometabolic Parameters and Reduced Body Weight in Obese Humans.
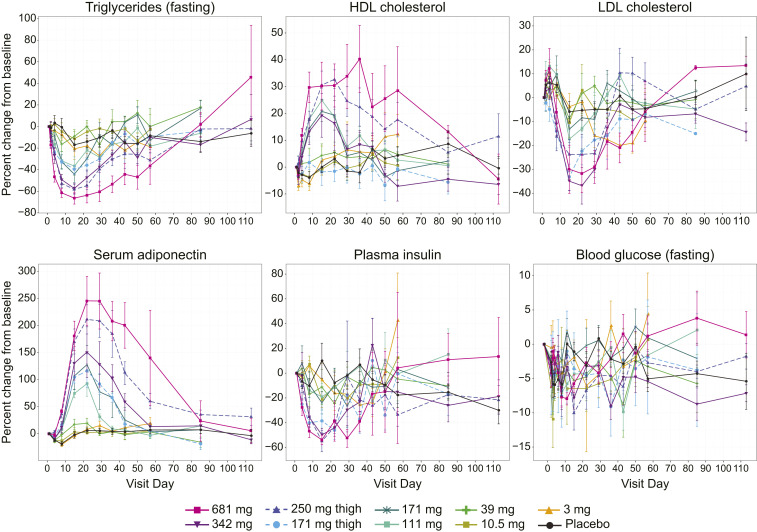
Single administration of BFKB8488A resulted in dose-dependent and metabolically beneficial effects as shown by increases in total serum adiponectin and high-density lipoprotein (HDL) cholesterol and decreases in serum triglycerides, low-density lipoprotein (LDL) cholesterol, and fasting insulin (Fig. 3). Notably, these effects were prolonged, extending up to 60 d for several of the high-dose cohorts.
Fig. 3.
BFKB8488A improved cardiometabolic parameters. Percent change from baseline (mean ± SEM) in triglycerides, HDL cholesterol, LDL cholesterol, serum adiponectin, plasma insulin, and blood glucose from day 0 to day 110. Baseline was defined as the average of results from day −2 and day 1.
BFKB8488A-treated participants had lower mean fasting triglycerides at baseline (Table 1) and decreases of up to ∼66% through day 29 compared with placebo participants (Fig. 3). Although baseline levels were comparable, HDL cholesterol increased up to ∼34%, and LDL cholesterol decreased up to ∼37% through day 29 in BFKB8488A-treated participants. In participants treated with placebo, HDL cholesterol remained constant and LDL cholesterol decreased less than 10% (Fig. 3).
Baseline serum adiponectin and fasting blood glucose levels were comparable between placebo- and BFKB8488A-treated cohorts (Table 1). Increases in mean serum adiponectin in the active cohorts peaked at day 22 and remained at ∼250% relative to baseline in the highest dose cohort at 4 wk after dosing (Fig. 3). Adiponectin levels in the placebo group did not change throughout the study. Participants in both BFKB8488A and placebo cohorts generally experienced mean decreases in fasting blood glucose through day 29, but there were no dose-dependent trends (Fig. 3). For mean plasma insulin, active cohorts showed larger decreases from baseline than the placebo cohort through day 29, with apparent dose-dependent decreases in higher dose cohorts (≥171-mg subcutaneous thigh cohort).
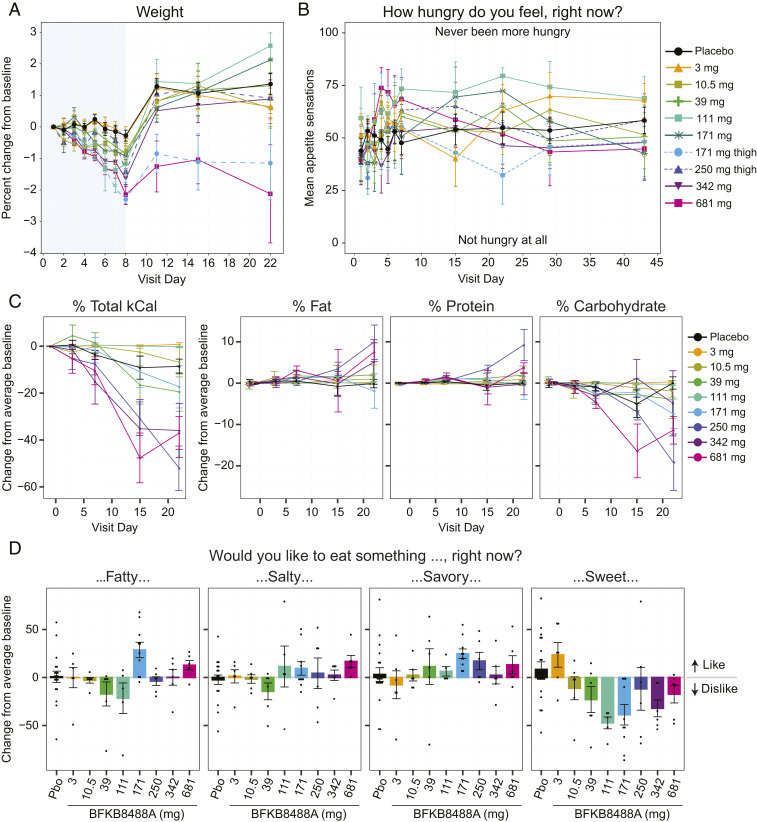
During confinement, when participants only received calorically balanced standardized meals and water, we observed a significant and dose-dependent decrease in body weight after BFKB8488A administration at doses above 3 mg. Mean baseline (day 1) weight was comparable between placebo- (101 kg) and BFKB8488A-treated (98 kg) participants (Fig. 4A; see also SI Appendix, Table S4), but by day 8, body weight had decreased by a mean of 1.20 kg in BFKB8488A-treated participants compared with a mean decrease of 0.28 kg in the placebo group (SI Appendix, Table S4). After confinement, body weights rebounded within 3 d.
Fig. 4.
BFKB8488A treatment led to weight loss, reduced caloric intake, and altered food type preference in obese humans. (A) Percent change from predose baseline (mean ± SEM) in body weight. Blue shaded area indicates the confinement period (day −2 through day 8). (B) Appetite sensations (mean ± SEM) as measured by VAS from predose baseline day 1 through day 43. (C) Change from predose baseline (mean ± SEM) in total food consumption (measured in kCal), and fat, protein, and carbohydrate consumption through day 22. (D) Change from predose baseline (mean ± SEM) in cravings for fatty, salty, savory, and sweet food, measured by VAS on day 22. Positive scores indicate liking a food; negative scores indicate disliking a food. Baseline was defined for each participant as the average of day −1 and day 1 predose. For C and D, the 171-mg thigh and abdomen cohorts were combined. Pbo, placebo.
Throughout the study, participants evaluated their hunger level through a questionnaire, the Appetite Sensations Visual Analog Scales (VAS). In general, the VAS showed little change in participant appetites during or after the confinement period (Fig. 4B).
BFKB8488A Reduced Caloric Intake and Altered Food Type Preference in Obese Humans.
We closely monitored the effect of BFKB8488A on eating behavior, recording the amount and types of food consumed by participants, and calculating consumption by total calories and fat, protein, and carbohydrate calories. Starting on day 7, a dose-related decrease in caloric intake was observed (Fig. 4C). At site visits on days 15 and 22, caloric consumption decreased up to 50% in the higher dose cohorts (≥250 mg) (Fig. 4C). Analysis of the different nutritional elements consumed after each standardized breakfast suggested that carbohydrate intake decreased in the higher-dose cohorts while fat and protein consumption remained approximately constant or increased slightly (Fig. 4C). Consistent with the food consumption data, the VAS data, which evaluated the desire to eat something fatty, salty, savory, or sweet, showed aversion to sweet food in participants receiving 39-mg BFKB8488A or higher doses (Fig. 4D; see also SI Appendix, Fig. S2). Together, these data suggest that BFKB8488A treatment results in weight loss without markedly affecting appetite and may induce an aversion to carbohydrate consumption.
Discussion
While prior clinical trials using repeated administration of recombinant FGF21 analogs have demonstrated a range of beneficial changes in metabolic markers (11–14), the effect of FGF21-class molecules on eating behavior has not yet been reported. Here, we first demonstrated that BFKB8488A, a selective, antibody-based FGFR1/KLB receptor agonist, recapitulates many reported metabolic effects of recombinant FGF21 analogs in nonhuman primates and humans, suggesting that FGFR1, rather than other FGFRs, is the primary effector of recombinant FGF21. We then demonstrated that this long-acting antibody may alter eating behavior and sweet food preference, providing pharmacological evidence for the role of the FGFR1/KLB complex in regulating eating behavior in humans.
In this study, we validated BFKB8488A’s ability to mimic recombinant FGF21 in nonhuman primates. Importantly, we observed elevated Spry4 and Dusp6 gene expression, indicators of in vivo FGFR pathway engagement, in adipose tissue. We also demonstrated robust induction of HMW, but not LMW or MMW, adiponectin by BFKB8488A, potentially suggesting posttranslational control of adiponectin production. Mirroring the anorexic effect of FGF21 analogs in monkeys (9–11), BFKB8488A robustly suppressed food intake in obese monkeys. A previous pair-feeding study suggested that FGF21-induced weight loss in monkeys could be attributed solely to the reduction in food intake (36). Although we did not conduct a similar pair-feeding study, given the degree of the observed anorexic effect, it is highly likely that BFKB8488A reduced body weight in monkeys primarily through the suppression of calorie intake. The apparent delayed recovery in weight gain after food consumption returned to its predose baseline, which suggests that additional factors other than caloric intake may play a role in FGFR1/KLB-mediated weight loss. Drug-related increase in energy expenditure is an appealing possibility, but while FGF21-mediated increase in energy expenditure and the browning of white adipose tissue was extensively reported in rodents, no consistent changes in the expression of thermogenic genes following FGF21 administration were observed in nonhuman primates (37). Another possibility is that drug-related changes in food preference required a longer duration for readaptation relative to food consumption, which returned to baseline more rapidly. Finally, FGF21 has been shown to affect secretion of digestive enzymes in mice (38), thus BFKB8488A may affect calorie absorption in primates as well. Further studies are needed to determine the exact mechanisms governing the marked suppression of food intake following activation of FGFR1/KLB receptor complex in monkeys.
Single administration of BFKB8488A in otherwise healthy obese or overweight humans resulted in profound and sustained dose-related reductions in triglycerides and LDL cholesterol, as well as marked increases in HDL cholesterol. The most sensitive biomarker to BFKB8488A treatment was serum adiponectin, which showed dose-related elevations starting from the 39-mg dose and increases in both the magnitude and duration of response through the highest dose tested. Notably, FGF21 increases adiponectin secretion in human and mouse cultured primary adipocytes as well (6), and adiponectin has been consistently observed as a PD biomarker associated with various FGF21 analogs in mice, monkeys, and humans, further supporting its utilization as a direct readout of pathway engagement in adipose tissue. However, adiponectin has been shown to be dispensable for the metabolic effects of recombinant FGF21 and anti-FGFR1/KLB antibody in mice (4, 39), and the relative contribution of the observed adiponectin induction to the overall metabolic effects in humans has yet to be addressed.
Several recombinant FGF21 analogs reduce food intake in nonhuman primates but not in rodents, and the effect of related molecules on human food intake has not been clear (11, 12). Our careful examination of participant appetite and food intake in a confined clinical study with standardized meals suggests that the dose-dependent weight loss induced by BFKB8488A during the 7 d after dosing cannot be attributed primarily to a reduction in appetite, unlike its action in nonhuman primates. While indications of a weight loss effect were observed as early as day 4 after BFKB8488A administration, the first report of nausea in any dose cohort was on day 7. Therefore, weight loss appeared to precede reports of nausea and emesis. We cannot, however, rule out the possibility that nausea may have affected weight loss and food-consumption parameters. Other factors that may have contributed to BFKB8488A-driven weight loss are increased satiety, reduced caloric intake due to a carbohydrate-to-fat/protein shift in food preference, and altered caloric absorption due to dysregulation of digestive enzyme secretion, as previously suggested in mice (38). Further clinical investigation will be required to address which of these proposed mechanisms actually occur.
Intriguingly, we observed a significant dose-related decrease in standardized meal caloric consumption, particularly of carbohydrates, suggesting an alteration in meal preference. VAS analysis confirmed the effect on food intake, showing that most participants who received doses of 39-mg BFKB8488A and higher also exhibited reduced cravings for sweet food. This effect is consistent with previous rodent studies (25, 26, 40) and human genetic studies that identified variants in the FGF21 gene locus as regulators of carbohydrate consumption (19–21). Altered food preference is likely mediated through a direct effect on the central nervous system, suggesting that despite its HMW, BFKB8488A exposure levels in this compartment were sufficient for FGFR1/KLB activation, as previously shown in mice (41). Further insights into this mechanism were demonstrated in mice, suggesting that FGFR1/KLB activation in hypothalamic glutaminergic neurons may drive suppression of carbohydrate intake (42).
In conclusion, our nonclinical and clinical results suggest that activation of the FGFR1/KLB complex with a bispecific antibody yields similar metabolic benefits to FGF21 treatment in both nonhuman primates and humans. The PK, PD, and safety profiles of BFKB8488A indicate that the molecule is active and well-tolerated in humans, with nausea as the most significant adverse effect. Together, our results support further clinical investigation of this antibody-based therapy as a disease-modifying treatment for obesity-related metabolic defects. Clinical studies with repeated dosing of BFKB8488A (ClinicalTrials.gov: NCT03060538 and NCT04171765) aim to further test this possibility.
Materials and Methods
Nonclinical Study of BFKB8488A.
Study design and animals.
Sixteen male, insulin-independent, drug-naive, obese cynomolgus monkeys (aged 12 to 16 y; body weight, 8.5 to 14.6 kg; Crown Bioscience) were randomized into four treatment groups of four each based on body weight, fasting serum insulin, and fasting triglycerides. Animals received a single intravenous injection of vehicle or BFKB8488A (0.6, 3, or 15 mg/kg; Genentech) and were monitored for 3 mo. Serum samples were collected after ≥12-h fast at baseline and at different time points after dosing.
Cynomolgus monkeys were maintained in accordance with guidelines approved by the Association for Assessment and Accreditation of Laboratory Animal Care (SI Appendix, SI Text). Animal studies followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals Publication 8523, revised 1985 (43). The Genentech Institutional Animal Care and Use Committee reviewed and approved all animal protocols.
Serum BFKB8488A PK and PD biomarker analyses in monkey.
Blood samples were collected from animals after a 12-h fast. Serum was analyzed for NEFAs, BHBA, and adiponectin. Serum BFKB8488A was quantified by a validated generic ELISA sandwich immunoassay (SI Appendix, SI Text). The BFKB8488A EC50 was determined by isolating protein from BFKB8488A-stimulated human adipocytes and performing Western blots for phosphorylated ERK, indicating FGF21 pathway activation (SI Appendix, SI Text).
Subcutaneous tissue biopsies, RNA isolation, and Spry4 and Dusp4 mRNA assays in monkey.
Subcutaneous tissue biopsies were collected from overnight-fasted animals (SI Appendix, SI Text). After tissue homogenization, RNA was isolated, and cDNA was synthesized (SI Appendix, SI Text). Transcripts were quantified by real-time quantitative polymerase reaction (PCR) using predesigned probes for Spry4 and Dusp4 (ViiA 7 Real-Time PCR; DUSP4 [Mf04365382_m1]; SPRY4 [Hs01935412_s1]; Applied Biosystems), with normalization to TATA-box binding protein (TBP; Mf02797119_m1) transcripts. Means of triplicate PCRs performed on each cDNA sample were reported.
Food consumption and body composition assessments in monkey.
Monkeys received body weight-based (20 g/kg per day) portions (20 g/kg per day) of monkey chow, equally divided for twice-daily feeding. Meal start and stop times, and amounts given, consumed, and uneaten were measured. Body composition measurements were assessed by DEXA scans (SI Appendix, SI Text).
Nonclinical statistical analysis.
Statistical analysis for nonclinical studies is provided in SI Appendix, SI Text.
Phase 1 Study Design and Participants.
Study design.
The human trial (ClinicalTrials.gov: NCT02593331) was a phase 1a, randomized, blinded, placebo-controlled, single ascending-dose study conducted at a single clinical trial site in the United States. We enrolled 71 participants (treatment allocation, ∼6 active: 2 placebo) into 7 fixed-dose cohorts (3 mg, 10.5 mg, 39 mg, 111 mg, 171 mg, 342 mg, and 681 mg) who received single subcutaneous BFKB8488A injections into the abdomen and two cohorts (171 mg and 250 mg) who received single subcutaneous BFKB8488A injections into the thigh.
Eligible participants were randomized within 28 d after screening and were followed for 8 to 16 wk after dosing. The study included a run-in period of approximately 1 wk, confinement to the study facility from the morning of day −2 until the morning of day 8, during which participants received a standardized diet, and follow-up visits (with breakfasts provided) on days 15 and 22. Standardized meals (based on daily energy expenditure) were designed to provide sufficient calories to at least maintain body weight during the confinement period. On specific study visits (day −2, day −1, day 3, day 7, day 15, and day 22), the same meals or “identical” meals were provided to further facilitate comparison. Additional details on the study period, randomization and blinding, and study drug are provided in SI Appendix, SI Text.
Participants.
Participants were otherwise healthy adults, 18 to 65 y, either overweight or obese BMI ≥ 30 kg/m2 and ≤40 kg/m2, or with likely insulin resistance defined as one or more of the following: BMI ≥ 30 kg/m2, homeostatic model assessment-insulin resistance > 3.60, waist circumference >100 cm (males) or 88 cm (females), fasting plasma insulin ≥15 mIU/L, fasting plasma glucose ≥100 mg/dL and <126 mg/dL, or hemoglobin A1c (HbA1c) >5.6% (38 mmol/mol) and <6.5% (48 mmol/mol) (44). This study enrolled participants with a BMI of >27.0 kg/m2 but ≤40.00 kg/m2, so permitted participants ranged from preobese (25.00 to 29.99 kg/m2) to obese class II (35.00 to 39.99 kg/m2) per the WHO classification (34).
Exclusion criteria were: Diabetes mellitus; systolic blood pressure ≥ 140 mmHg; diastolic blood pressure ≥ 90 mmHg; triglycerides > 500 mg/dL; or LDL cholesterol > 160 mg/dL at screening; receipt of any investigational treatment 30 d prior to screening evaluation or within five half-lives of the investigational product, whichever was greater; exposure to any biological therapy or investigational biological agent within 90 d before the screening evaluation; active involvement in a weight loss or dietary program, history of surgical procedures for weight loss, or history of an eating disorder.
The phase 1 trial was conducted in full conformance with International Council for Harmonization (ICH) E6 guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki and complied with the requirements of the ICH E2A guideline (Clinical Safety Data Management: Definitions and Standards for Expedited Reporting), US Food and Drug Administration regulations, and applicable local, state, and federal laws. The Schulman Associates Institutional Review Board (Cincinnati, OH) approved this study. All participants provided written, informed consent.
Safety, PK, and immunogenicity outcomes and assessments.
Safety outcomes were the nature, frequency, severity, and timing of all AEs, serious AEs, and DLAEs. Changes in vital signs, physical findings, and clinical laboratory results were monitored. AE classification was according to the Medical Dictionary for Regulatory Activities, v19.0. AE severity was assessed with the WHO Toxicity Grading Scale. Escalation to higher dose cohorts occurred after Safety Review Team review of at least 14 (cohorts A–D) or 21 (cohorts E and F) days of safety data from at least six participants in the preceding cohort.
Serum BKFB8488A concentrations were determined using a validated standard sandwich ELISA using an anti-KLB complementarity determining region monoclonal antibody coated onto microtiter plates as a capture reagent, followed by incubation with appropriately diluted samples. A biotinylated anti-FGFR1 complementarity determining region monoclonal antibody and streptavidin-HRP were used for detection. Assay limit of quantification was determined to be 20 ng/mL in neat serum.
Sampling for ADA assessment was scheduled at baseline and on days 29, 57, 85, and 113. ADA screening in serum used validated bridging screen and confirmatory immunoassays (SI Appendix, SI Text).
PD outcomes and assessments.
All PD marker measurements were performed after an overnight fast. Evaluation of BFKB8488A activity was based on changes in markers of cardiometabolic health: Body weight, serum adiponectin, fasting insulin, fasting glucose, triglycerides, LDL cholesterol, and HDL cholesterol. See SI Appendix, SI Text for assay details.
To assess BFKB8488A activity on aspects of appetite, hunger, and cravings, participants completed questionnaires before clinical measures or drug administration at specified timepoints. The Appetite Sensations VAS comprised items with words anchored at each end that expressed the most positive and negative ratings of hunger, satisfaction, fullness, consumption quantity, and desire to eat something fatty, salty, savory, or sweet (45). Effects on appetite were quantified based on provision of standardized meals to maintain body weight during confinement. At baseline and on days 3, 7, 15, and 22 after BFKB8488A administration, food intake was assessed at breakfast after overnight fasting by measuring pre- and postmeal weights and macronutrient consumption (SI Appendix, SI Text).
Phase 1 clinical trial statistical analysis.
Practical considerations rather than statistical power considerations determined sample size. A sufficient number of participants was screened to ensure at least eight participants in each cohort. It was deemed that six participants dosed with active drug in each cohort would be sufficient to characterize the single-dose safety, tolerability, and PK of BFKB8488A.
Safety analyses included all randomized participants who received a dose of study drug, with participants grouped according to the treatment actually received. Immunogenicity analyses included participants with at least one postdose ADA assessment, with participants grouped according to treatment received.
PK analyses included participants with sufficient data to enable estimation of key PK parameters using Phoenix WinNonlin PK software (Certara USA). Individual and mean serum BFKB8488A concentration versus time data were tabulated and plotted by dose level. BKFB8488A serum PK was characterized by estimating total exposure (area under the concentration-time curve), maximum observed serum concentration (Cmax), total apparent clearance (CL/F), and terminal half-life (t1/2), as appropriate for data collected. Estimates for these parameters were tabulated and summarized (mean, SD, coefficient of variation, median, minimum, and maximum).
Numbers and proportions of ADA-positive and ADA-negative participants were summarized by treatment group. Participants were considered ADA-positive if ADA-negative at baseline but developed an ADA response following study drug administration (treatment-induced ADAs) or if they were ADA-positive at baseline and the titer of ≥one postbaseline samples was ≥fourfold greater (i.e., ≥0.60 titer units) than the baseline titer (treatment-enhanced ADA response).
BFKB8488A PD activity analyses included participants with at least one predose and one postdose activity assessment, with participants grouped by the treatment actually received. Changes from baseline to week 4 (and additional timepoints for the self-rating scales) in activity data were summarized using descriptive statistics. Calculations of changes in taste preferences were performed for each individual participant as the change from their own predose baseline. Mean changes were then calculated as the average of the individual changes from baseline (predose). For the VAS appetite analysis, baseline was measured predose on day 1 unless missing, in which case the patient’s day −1 predose measurement was used. For the VAS taste preference, baseline was defined as the predose average of day −1 and day 1. Drug was administered on day 1 following predose measurements.
Supplementary Material
Acknowledgments
We thank all the participants for their involvement in this study. Editing and writing assistance was provided by Deborah Solymar (Genentech, Inc.). Genentech, Inc. funded this study. P.S.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Portions of this work were presented at the American Diabetes Association 77th Scientific Sessions, San Diego, CA, 9–13 June 2017 and at the 53rd Annual Meeting of the European Association for the Study of Diabetes, Lisbon, Portugal, 11–15 September 2017.
Footnotes
Competing interest statement: All authors, except for L.M., were employees of Genentech, Inc., a member of the Roche group, at the time this work was performed and own/owned Roche stock and/or options at the time of their employment. L.M. is an employee and shareholder of ProSciento, Inc.; ProSciento, Inc. received research funding from Genentech, Inc. A.B. is currently an employee of Calico Life Sciences. J.S. is currently an employee of Regeneron Pharmaceuticals. R.B. is currently an employee of IgGenix. P.S.A. and L.W.C. are currently employees of Principia Biopharma.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2012073117/-/DCSupplemental.
Data Availability.
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
References
- 1.Kliewer S. A., Mangelsdorf D. J., A dozen years of discovery: Insights into the physiology and pharmacology of FGF21. Cell Metab. 29, 246–253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coskun T., et al. , Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Xu J., et al. , Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–259 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolumam G., et al. , Sustained brown fat stimulation and insulin sensitization by a humanized bispecific antibody agonist for fibroblast growth factor receptor 1/βKlotho complex. EBioMedicine 2, 730–743 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Z., et al. , Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 17, 779–789 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Holland W. L., et al. , An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 17, 790–797 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen B. M., et al. , FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 20, 670–677 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kharitonenkov A., et al. , The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148, 774–781 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Véniant M. M., et al. , Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology 153, 4192–4203 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Adams A. C., et al. , LY2405319, an engineered FGF21 variant, improves the metabolic status of diabetic monkeys. PLoS One 8, e65763 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talukdar S., et al. , A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 23, 427–440 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Gaich G., et al. , The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Sanyal A., et al. , Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 392, 2705–2717 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Charles E. D., et al. , Pegbelfermin (BMS-986036), PEGylated FGF21, in patients with obesity and type 2 diabetes: Results from a randomized phase 2 study. Obesity 27, 41–49 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X., et al. , Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57, 1246–1253 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Kralisch S., et al. , Fibroblast growth factor-21 serum concentrations are associated with metabolic and hepatic markers in humans. J. Endocrinol. 216, 135–143 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Yu H., et al. , Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin. Chem. 57, 691–700 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Dushay J. R., et al. , Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol. Metab. 4, 51–57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu A. Y. et al.; CHARGE Nutrition Working Group; DietGen Consortium , Novel locus including FGF21 is associated with dietary macronutrient intake. Hum. Mol. Genet. 22, 1895–1902 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka T., et al. , Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am. J. Clin. Nutr. 97, 1395–1402 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Søberg S., et al. , FGF21 is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab. 25, 1045–1053.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Schumann G., et al. , KLB is associated with alcohol drinking, and its gene product β-Klotho is necessary for FGF21 regulation of alcohol preference. Proc. Natl. Acad. Sci. U.S.A. 113, 14372–14377 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson A., et al. , Functional validity, role, and implications of heavy alcohol consumption genetic loci. Sci. Adv. 6, eaay5034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke T. K., et al. , Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N =112 117). Mol. Psychiatry 22, 1376–1384 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talukdar S., et al. , FGF21 regulates sweet and alcohol preference. Cell Metab. 23, 344–349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Holstein-Rathlou S., et al. , FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 23, 335–343 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunshee D. R., et al. , Fibroblast activation protein cleaves and inactivates fibroblast growth factor 21. J. Biol. Chem. 291, 5986–5996 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhen E. Y., Jin Z., Ackermann B. L., Thomas M. K., Gutierrez J. A., Circulating FGF21 proteolytic processing mediated by fibroblast activation protein. Biochem. J. 473, 605–614 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coppage A. L., et al. , Human FGF-21 is a substrate of fibroblast activation protein. PLoS One 11, e0151269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonoda J, Chen MZ, and Baruch A. FGF21-receptor agonists: An emerging therapeutic class for obesity-related diseases. Horm. Mol. Biol. Clin. Investig. 30, 20170002 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Muise E. S., et al. , Downstream signaling pathways in mouse adipose tissues following acute in vivo administration of fibroblast growth factor 21. PLoS One 8, e73011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ornitz D. M., Itoh N., The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 4, 215–266 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achari A. E., Jain S. K., Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 18, E1321 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization , Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. (World Health Organization, Technical Report Series 894, 2000). [PubMed] [Google Scholar]
- 35.Dirks N. L., Meibohm B., Population pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 49, 633–659 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Thompson W. C., Zhou Y., Talukdar S., Musante C. J., PF-05231023, a long-acting FGF21 analogue, decreases body weight by reduction of food intake in non-human primates. J. Pharmacokinet. Pharmacodyn. 43, 411–425 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray S. A., et al. , Whole transcriptome analysis and validation of metabolic pathways in subcutaneous adipose tissues during FGF21-induced weight loss in non-human primates. Sci. Rep. 10, 7287 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coate K. C., et al. , FGF21 is an exocrine pancreas secretagogue. Cell Metab. 25, 472–480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.BonDurant L. D., et al. , FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 25, 935–944.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen M. Z., et al. , FGF21 mimetic antibody stimulates UCP1-independent brown fat thermogenesis via FGFR1/βKlotho complex in non-adipocytes. Mol. Metab. 6, 1454–1467 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lan T., et al. , FGF19, FGF21, and an FGFR1/β-Klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab. 26, 709–718.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen-Cody S. O., et al. , FGF21 Signals to glutamatergic neurons in the ventromedial hypothalamus to suppress carbohydrate intake. Cell Metab. 32, 273–286.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, 1985). [Google Scholar]
- 44.Stern S. E., et al. , Identification of individuals with insulin resistance using routine clinical measurements. Diabetes 54, 333–339 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Flint A., Raben A., Blundell J. E., Astrup A., Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 24, 38–48 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.