Abstract
Introduction
An increased risk of sarcoidosis and sarcoid-like reactions in subjects with a history of malignancy has been suggested. We assessed the incidence and clinical characteristics of cancer patients with biopsies containing sarcoid-like granulomas on cancer metastasis and patient survival.
Methods
This is a retrospective, multicentre, observational study involving endobronchial ultrasound transbronchial needle aspiration and a melanoma patient dataset at the University of Miami, USA, and a sarcoidosis patient database at Chiba University, Japan. Subjects with a confirmed diagnosis of cancer and who subsequently developed granulomas in different organs were enrolled. The study was registered at Clinicaltrials.gov (NCT03844698).
Results
133 patients met the study's criteria. The most common primary cancer sites were the skin (22.5%), breast (20.3%) and lymph node (12.8%). 24 (18%) patients developed sarcoid-like granulomas within 1 year of cancer diagnosis, 54 (40.6%) between 1 and 5 years and 49 (36.8%) after 5 years. Imaging showed possible sarcoid-like granulomas in lymph nodes in 51 cases (38.3%) and lung tissue and mediastinal lymph nodes in 73 cases (54.9%); some parenchymal reticular opacity and fibrosis was found in 5 (3.7%) and significant parenchymal fibrosis in 2 (1.5%) subjects. According to logistic regression analysis, the frequency of metastatic cancer was significantly lower in patients with sarcoid-like granulomas than in controls. Moreover, multivariate Cox proportional hazard analysis showed a significant survival advantage in those with sarcoid-like granuloma.
Conclusion
Sarcoid-like granulomas are uncommon pathology findings in cancer patients. There is a significant association between the presence of granulomas and reduced metastasis and increased survival. Further study is warranted to understand the protective mechanism involved.
Short abstract
These findings suggest that patients with underlying malignancy who develop sarcoidosis and sarcoid-like reactions have a lower risk of stage 4 metastatic disease and better survival compared to patients who do not develop such granulomatous reactions https://bit.ly/2CNhc9e
Introduction
Sarcoidosis is a multisystem granulomatous disease that predominantly affects the lung but can be seen in any organ system [1]. The fundamental abnormality in sarcoidosis is the development of pathological structures known as noncaseating granulomas [2] consisting of collections of macrophages and epithelioid cells surrounded by lymphocytes and fibroblasts. Although the aetiology of such granuloma formation in sarcoidosis remains unclear, it is believed to be due to an abnormal host immune response to an unknown antigen in genetically susceptible individuals.
Multiple studies have demonstrated an increased risk of cancer following sarcoidosis. In a retrospective study of nearly 9000 sarcoidosis patients, Askling et al. [3] reported an elevated risk of cancer with a standardised incidence of 1.3 (95% CI 1.2–1.4). In a recent meta-analysis of over 25 000 patients, there was a relative risk of 1.19 (95% CI 1.07–1.32) for the development of all types of invasive cancers in patients with sarcoidosis [4].
Less is known about the development of sarcoidosis after the onset of malignancy. Patients with an underlying malignancy develop noncaseating epithelioid cell granulomas in regional and distant lymph nodes and/or parenchyma of various organs [5]. Given the characteristic enlargement of lymph nodes associated with sarcoidosis, the clinical and radiological features of these conditions mimic metastatic cancer and require biopsy. To date, only a few studies have analysed the occurrence of sarcoidosis after cancer diagnosis and most are case reports. We previously described the increased incidence of sarcoidosis among subjects with breast cancer in our registry [6]. Whether the presence of sarcoid-like granulomas affects survival if developed after cancer onset is difficult to assess as different studies have reported conflicting results [7–11].
This study aimed to compare the clinicopathological characteristics and outcomes in cancer patients with and without sarcoid-like granulomas in biopsy specimens. Sarcoid-like granulomas are thought to develop from a persistent immune reaction, and we postulated that cancer patients’ granulomatous reactions indicate a robust immune response and may provide a survival advantage.
Methods
Study design
We conducted a multicentre, retrospective observational study of 1809 subjects at the University of Miami Hospital, Miami Veterans Affairs Medical Center (VAMC) in Florida, USA, and Chiba University in Chiba, Japan. The study was approved by the local Institutional Review Boards (IRBs) with a waiver of informed consent. For better transference, we herein refer to both sarcoidosis and sarcoid reactions occurring in cancer patients as “sarcoid-like granuloma”. We identified each case of sarcoid-like granuloma by manually sorting through prospectively collected datasets of patients who underwent endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) for mediastinal and hilar lymphadenopathy from January 2011 to December 2018 at the University of Miami and Miami VAMC, prospectively collected melanoma dataset of a single provider from January 2014 to December 2018 at the University of Miami and sarcoidosis patient dataset maintained from January 2009 to July 2019 at Chiba University.
Inclusion and exclusion criteria
Any patient with a pathology finding of sarcoid-like granuloma during or after cancer diagnosis was included in the study. Subjects found to have sarcoid-like granuloma before the diagnosis of cancer were excluded. Other causes of granulomatous disease were excluded by mycobacterial and fungal staining of pathology samples and culture, review of the subject history and follow-up. For each patient, demographic data, including age, sex and ethnicity, were collected. Details on cancer as well as sarcoid-like granulomas were also obtained. All cancers were staged according to the American Joint Committee on Cancer (AJCC) guidelines.
Survival analysis
Subject group survival was compared to a control group to determine the effect of sarcoid-like granulomas on cancer survival. For selection of controls, we used the same dataset of prospectively collected EBUS-TBNA patients along with melanoma patients at University of Miami Hospital. No controls were selected from Chiba University, Japan. For the nine most commonly occurring cancers among our subjects (breast, bladder, prostate and pancreatic cancers, lymphoma, renal cell carcinoma, melanoma, colon carcinoma, sarcoma), controls with the same cancer diagnosis were identified and chosen randomly based on the absence of sarcoid-like granuloma. Follow-up was based on information available from the electronic medical record of each subject. Only subjects who developed sarcoid-like granuloma within 3 years of cancer diagnosis were included in survival analysis. The most recent hospital and/or clinic visits were reviewed to analyse the current clinical status of the subject. For both groups, only patients with up-to-date follow-up and for whom their death was documented in the medical records were included for survival analysis. For subjects with more than one cancer diagnosis, cancer that was diagnosed closest to the time of granuloma diagnosis was used for case and control matching.
Statistical analysis
Categorical variables are described as counts and percentages; their distribution was compared using odds ratios and tested using the Chi-squared test and Cochran–Mantel–Haenszel test or exact tests, as indicated. Continuous variables were analysed with independent sample t-tests (two-tailed). Cox proportional hazard analysis was performed for all cancers to identify whether potentially confounding variables affect survival independently. The selection of variables was based on a review of the literature with regards to factors affecting survival among cancer patients. For the four common cancers among our subjects, i.e. lung and breast cancers, melanoma and lymphoma, we performed the log-rank test to predict statistically significant differences in survival. Kaplan–Meier curves were generated to illustrate the ordinality of any differences found. We also applied logistic regression analysis to examine the relationship between various clinical factors and the likelihood of stage 4 metastatic disease. All analyses were carried out using SPSS software for Windows, version 26. For all results, significance was considered at p<0.05.
Results
Patient characteristics
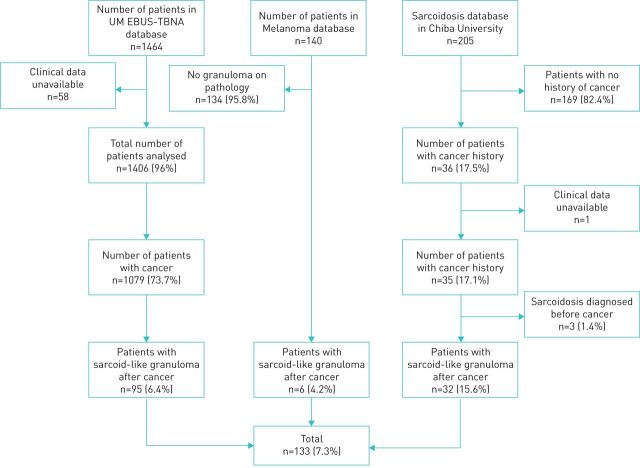
After reviewing 1464 electronic medical records of EBUS-TBNA patients at the University of Miami, 1079 were found to have a history of cancer and among them 95 (8.8%) had sarcoid-like granuloma after cancer diagnosis. From the 140 patients in the melanoma dataset, 6 (4.2%) had sarcoid-like granuloma after cancer diagnosis. Chiba University contributed 32 (15.6%) subjects after screening 205 sarcoidosis patients (figure 1). Among the 133 subjects, 61 (45.9%) were male and 72 (54.1%) female. The ethnic distribution included 35 (26.3%) Asians, 48 (36.1%) European-Americans, 14 (10.5%) African Americans, 31 (23.3%) Latinos and 5 (3.7%) of mixed ethnicity. The mean age of the subjects was 64.5 years (range 22–86 years). Table 1 summarises the subjects’ cancer and sarcoidosis characteristics.
FIGURE 1.
Flowchart showing the selection of cases of cancer with sarcoid-like granuloma. UM: University of Miami; EBUS-TBNA: endobronchial ultrasound transbronchial needle aspiration.
TABLE 1.
Characteristics of patients with cancer and sarcoidosis
| Female n (%) | 72 (54.2) |
| Age at time of sarcoid-like granuloma diagnosis years mean/median | 64.5/65 |
| Underlying cancer n (%) | |
| Skin cancer | 30 (22.5) |
| Breast cancer | 27 (20.3) |
| Lymph cancer | 17 (12.7) |
| Prostate cancer | 14 (10.5) |
| Lung cancer | 12 (9) |
| Colon cancer | 9 (6.7) |
| Urinary bladder cancer | 8(6.0) |
| Cervical cancer | 7 (5.3) |
| Head and neck cancer | 6 (4.5) |
| Sarcoma | 5 (3.8) |
| Kidney cancer | 5 (3.8) |
| Pancreatic cancer | 4 (3.0) |
| Conjunctival cancer | 1 (0.7) |
| Cancer treatment before diagnosis of sarcoidosis n (%) | |
| Surgery | 91 (68.4) |
| Chemotherapy | 54 (40.6) |
| Radiation | 38 (28.6) |
| Chemotherapy agents before diagnosis of sarcoidosis n (%) | |
| Doxorubicin | 15 (11.3) |
| Cisplatin | 11 (8.2) |
| Cyclophosphamide | 11 (8.2) |
| Gemcitabine | 9 (6.7) |
| 5-Fluorouracil | 9 (6.7) |
| Carboplatin | 9 (6.7) |
| Rituximab | 9 (6.7) |
| MAB treatment before cancer diagnosis n (%) | |
| Anti-CD20 | 9 (6.7) |
| PD-1 immune checkpoint | 4 (3) |
| Anti-Her2neu | 2 (1.5) |
| Anti-CTLA4 | 2 (1.5) |
| Anti-VEGF | 1 (0.75) |
| Cancer stage at time of sarcoid-like granuloma diagnosis | |
| Stage 1 | 24 (15.8) |
| Stage 2 | 17 (12) |
| Stage 3 | 18 (12.7) |
| Stage 4 | 12 (7.5) |
| Interval between cancer and sarcoid-like granulomas n(%) | |
| <1 year | 24 (18.9) |
| 1–5 years | 54 (42.5) |
| >5 years | 49 (38.5) |
| Most common symptom at time of sarcoidosis diagnosis n (%) | |
| Dyspnoea | 20 (15) |
| Cough | 18 (13.5) |
| Fatigue | 16 (12) |
| Smoking n (%) | |
| Current | 7 (5.2) |
| Past | 48 (36.1) |
| Never smoker | 76 (57.1) |
| Mean number of years of smoking | 28.5 |
| Sarcoidosis stage at the time of diagnosis n (%) | |
| Stage 1 | 51 (38.3) |
| Stage 2 | 73 (54.9) |
| Stage 3 | 5 (3.7) |
| Stage 4 | 2 (1.5) |
Thirty-nine (29.3%) subjects had a history of more than one cancer before the sarcoid-like granuloma diagnosis. The most common primary cancer sites were the skin (30; 22.5%), breast (27; 20.3%) and lymph node (17; 12.8%). Regarding cancer stages at the time of sarcoid-like granuloma diagnosis, 24 cases (15.8%) were stage 1, 17 cases (12%) were stage 2, 18 cases (12.7%) were stage 3 and 12 cases (7.5%) were stage 4.
Fifty-four patients (40.6%) were treated with chemotherapy, 38 patients (28.6%) were treated with radiation and 91 patients (68.4%) underwent surgery prior to sarcoid-like granuloma diagnosis. The commonly used chemotherapy agents were doxorubicin (15; 11.3%), cisplatin (11; 8.2%) and cyclophosphamide (11; 8.2%). The most commonly used monoclonal antibody therapy classes were anti-CD20 (6.7%), PD-1 immune checkpoint inhibitors (3%), anti-HER2 (1.5%) and anti-CTLA4 (1.5%). Other commonly used agents are listed in table 1. More than half of the subjects (61.4%) developed granulomas within 5 years of their cancer diagnosis. The median interval between cancer and sarcoidosis diagnoses was 3 years (mean 6 years, range 1–37 years).
Radiological, pathological and laboratory characteristics of subjects with confirmed granulomas
The presence of sarcoid-like granulomas in cancer patients led us to define possible sarcoidosis staging by assessing chest imaging via computed tomography (CT) or positron emission tomography/CT. A total of 79% of patients had mediastinal and/or hilar lymph nodes >1 cm at the time of sarcoid-like granuloma diagnosis. The commonly enlarged lymph nodes on imaging were station 7 (85; 64%), 4R (73; 54.9%) and 2R (50; 37.6%). The most common parenchymal lesion was the presence of nodules (in 73 subjects; 54.9%), followed by ground glass opacity (in 13; 9.7%), reticular opacities (in 9; 6.7%), atelectasis (in 8; 6%) and bronchiectasis (in 3; 2.3%). The mean size of pulmonary nodules was 9.7 mm (range 2–30 mm). Thirty-one (23.3%) patients had extrathoracic lymph nodes >10 mm or with standardised uptake value >2.5. Based on Scadding criteria at the time of diagnosis, if defined as sarcoidosis, sarcoidosis staging was as follows: stage 1, 38.3%; stage 2, 54.9%; stage 3, 3.7%; and stage 4, 1.5%.
EBUS-TBNA along with transbronchial lung biopsy/endobronchial lung biopsy was the most common modality of granuloma diagnosis, which was used in 120 (90.2%) subjects. Other modalities include skin biopsy (10; 7.5%), biopsy of extrathoracic nodes (5; 3.7%), liver biopsy (5; 3.7%), muscle biopsy (1; 0.75%) and colonoscopy with biopsy (1; 0.75%). A total of 120 (90.2%) subjects had intrathoracic granuloma among which 89 (66.9%) had granuloma in the mediastinal and hilar lymph nodes only. The most common intrathoracic lymph nodes diagnosed with granuloma were station 7 (51.9%), 4R (23.3%), 11L (15.8%) and 11R (15%). Thirty-one (23.3%) subjects had granulomas in the parenchyma. Twenty-six (19.5%) had extrathoracic granulomas, with skin 10 (7.5%) and liver 5 (3.7%) being the most common sites. Fourteen (10.5%) subjects had necrotising granulomas on pathology. Thirty-four (25.5%) subjects (including lymphoma) had locoregional granuloma. The remaining 99 (74.5%) had granulomas distant from cancer site.
Pulmonary function test results were available for 61 subjects, 35 (26.3%) of which were normal. In those with impaired function, 12 (9%) had restrictive disease and 14 (10.5%) had an obstructive pattern. Angiotensin-converting enzyme levels were available for 54 patients, and the median value was 25.35 U·L−1 (mean=31.92). C-reactive protein was available for 52 patients, and it was elevated in 21 (15.7%). More than half of our subjects (68; 51.2%) were asymptomatic at the time of sarcoid-like granuloma diagnosis. In symptomatic patients, the most common symptoms were dyspnoea (in 20; 15%), cough (in 18; 13.5%) and fatigue (in 16; 12%).
Japanese versus American patients
We also compared the subjects’ nationality to determine whether there is any clinical difference between Japanese or American patients. Japanese subjects were less likely than Americans to have stage 2, 3 and 4 sarcoidosis (OR 0.31, 95% CI 0.13–0.72, p-value=0.007) and were less likely to develop sarcoid-like granulomas within 5 years of cancer diagnosis (OR 0.31, 95% CI 0.13–0.72, p-value=0.006).
Association between sarcoidosis granulomas and cancer stage 4 (metastasis)
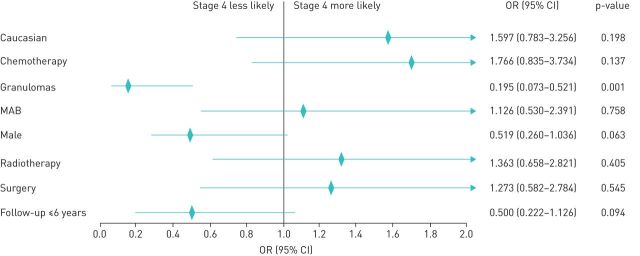
We compared the characteristics of cancer between the case and control groups. Six (13%) of the subjects in the sarcoid-like granuloma group had stage 4 disease compared to 72 (57.6%) subjects in the control group. Figure 2 depicts the forest plot of the estimates and 95% confidence intervals obtained by logistic regression analysis representing independent factors associated with stage 4 disease in patients with cancer. The presence of granuloma (OR=0.195, 95% CI 0.073–0.521, p=0.001) was associated with a lower incidence of stage 4 disease. Of note, the modality of treatment (surgery, chemotherapy, radiotherapy, monoclonal antibody (mAb) therapy) was not independently associated with higher or lower risk of stage 4 disease. Moreover, we did not find any impact on the cancer stage due to the sex and race of the subjects.
FIGURE 2.
Logistic regression with stepwise elimination for factors affecting stage 4 metastasis; p-value from Hosmer and Lemeshow goodness-of-fit test=0.107. The rhombus shape indicates the odds ratio (OR), and the horizontal line indicates the confidence interval (CI). The arrow indicates that the 95% CI is outside the range shown. MAB: monoclonal antibody therapy
Survival analysis
The mean±sd age of cancer diagnosis was 57.95±14.04 years and 58.37±12.9 years in the non-granuloma and granuloma group, respectively (p=0.860). The average number of years of follow-up for the cancer patients with and without sarcoid-like granulomas was 5.54±5.13 years and 6.07±6.49 years, respectively (p=0.615). Patients with both sarcoid-like granulomas and cancer showed greater 2-year (OR 4.6, 95% CI 1.13–18.65, p=0.033), 4-year (OR 3.9, 95% CI 1.33–11.42, p=0.013), 6-year (OR 5.75, 95% CI 2.05–16.1, p=0.001) and 10-year (OR 5.53, 95% CI 2.15–14.18, p≤0.0001) survival times compared to those with cancer without the presence of sarcoid-like granuloma (table 2).
TABLE 2.
Univariate analysis of demographic characteristics and clinical pathological characteristics of cancer subjects with and without sarcoid-like granuloma
| Variable | Cancer only | Cancer and granuloma | p-value | OR (95 CI) |
| Subjects n | 134 | 46 | – | – |
| Age at cancer diagnosis | 57.9±14.3 | 58.3±12.96 | 0.860 | – |
| Average years of follow-up | 6.0±6.4 | 5.5±5.1 | 0.615 | |
| Male | 53 (39.5) | 24 (52.1) | 0.137 | 1.67 (0.85–3.27) |
| European-American | 56 (41.8) | 16 (34.8) | 0.403 | 0.74 (0.37–1.49) |
| Hispanic | 57 (42.5) | 12 (26.1) | 0.050 | 0.48 (0.23–1) |
| African American | 17 (12.7) | 8 (17.4) | 0.428 | 1.45 (0.58–3.62) |
| Asian | 2 (1.4) | 8 (17.4) | 0.001 | 13.90 (2.83–68.2) |
| Smokers | 70 (52.2) | 17 (36.9) | 0.087 | 0.55 (0.27–1.09) |
| Average years of smoking | 30.92 | 36.4 | 0.243 | – |
| Alive <2 years | 20 (46.5) | 12 (80) | 0.033 | 4.6 (1.13–18.65) |
| Alive <4 years | 40 (50.6) | 20 (80) | 0.013 | 3.9 (1.33–11.42) |
| Alive <6 years | 48 (51.1) | 30 (85.7) | 0.001 | 5.75 (2.05–16.1) |
| Alive <10 years | 57 (51.3) | 35 (85.4) | <0.0001 | 5.53 (2.15–14.18) |
| Alive >10 years | 16 (69.6) | 5 (100) | – | – |
| Bladder cancer | 7 (5.2) | 3 (6.5) | 0.741 | 1.27 (0.31–5.11) |
| Breast cancer | 37 (27.7) | 8 (17.4) | 0.171 | 0.55 (0.24–1.3) |
| Colon cancer | 7 (5.2) | 5 (10.9) | 0.195 | 2.21 (0.67–7.35) |
| Lung cancer | 30 (22.4) | 6 (13) | 0.177 | 0.52 (0.2–1.34) |
| Lymphoma | 15 (11.2) | 6 (13) | 0.736 | 1.19 (0.43–3.28) |
| Melanoma | 18 (13.4) | 5 (10.9) | 0.654 | 0.79 (0.27–2.25) |
| Pancreatic cancer | 4 (3) | 3 (6.5) | 0.296 | 2.27 (0.49–10.54) |
| RCC | 8 (6) | 1 (2.2) | 0.329 | 0.35 (0.04–2.88) |
| Sarcoma | 2 (1.5) | 3 (6.5) | 0.100 | 4.61 (0.75–28.48) |
| Prostate cancer | 6 (4.4) | 6 (13) | 0.055 | 3.2 (0.97–10.47) |
| Cancer stage 4 | 72 (57.6) | 6 (13) | <0.0001 | 0.17 (0.07–0.44) |
| Surgery | 86 (67.2) | 26 (56.5) | 0.798 | 0.91 (0.43–1.92) |
| Radiotherapy | 69 (53.9) | 13 (28.3) | 0.020 | 0.41 (0.2–0.87) |
| Chemotherapy | 90 (70.3) | 20 (43.5) | 0.020 | 0.42 (0.20–0.87) |
| mAb therapy | 55 (43.0) | 8 (17.4) | 0.011 | 0.33 (0.14–0.78) |
Data are presented as mean±sd or n (%), unless otherwise stated. RCC: renal cell carcinoma; mAb: monoclonal antibody therapy.
Likely due to the decrease in stage IV cancer diagnoses, we observed a statistically significant difference in the treatment administered, with fewer subjects having sarcoid-like granuloma being treated with chemotherapy (p=0.020), mAb therapy (p=0.011) and radiotherapy (p=0.020) compared to controls. There was a significant difference in the number of Asian and Latino subjects between the two groups, as the control group was obtained from the University of Miami database, which serves a predominantly Latino population with a relatively small Asian community.
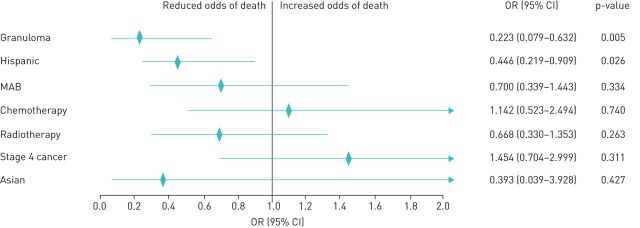
Similarly, a multivariate Cox proportional hazards regression model adjusted for potentially confounding factors of Hispanic and Asian race, treatment including chemotherapy, mAb therapy, radiotherapy and stage 4 cancer showed increased survival among subjects with sarcoid-like granuloma and cancer compared to those with cancer alone (figure 3). Logistic regression analysis showed patients with granuloma (p=0.005) and Hispanic (p=0.026) ethnicity were associated with lower risk of death (figure 4).
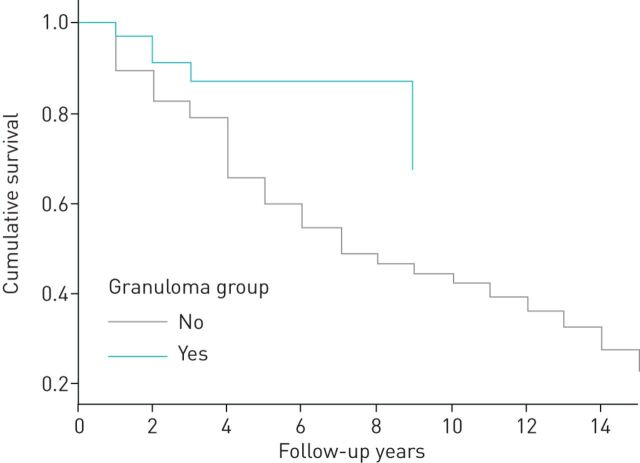
FIGURE 3.
Multivariate Cox proportional hazard analysis of survival of cancer patients with and without sarcoid-like granulomas.
FIGURE 4.
Logistic regression with stepwise elimination for factors affecting survival; p-value from Hosmer and Lemeshow goodness-of-fit test=1.000. The rhombus shape indicates the odds ratio (OR), and the horizontal line indicates the confidence interval (CI). The arrow indicates that the 95% CI is outside the range shown. MAB: monoclonal antibody therapy.
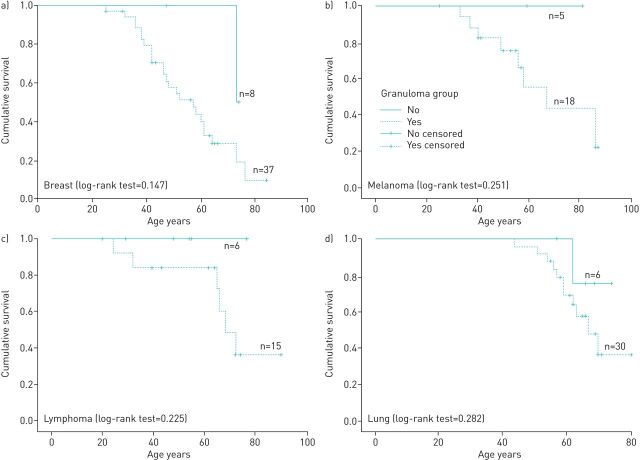
Kaplan–Meier survival curves along with the log-rank test comparing cancer patients with and without sarcoid-like granulomas for the most commonly occurring cancers (breast, lung, lymphoma and melanoma) in our dataset revealed there was no significant survival advantage for those with lung cancer (p=0.282), melanoma (p=0.251), lymphoma (p=0.225) or breast cancer (p=0.147) (figure 5).
FIGURE 5.
Kaplan–Meier survival analysis showing the survival curve for the most common cancers among controls and cases. a) Breast cancer (granuloma=8, no granuloma=37, OR 0.55, 0.24–1.3); b) Melanoma (granuloma=5, no granuloma=18, OR 0.79, 0.27–2.25); c) Lymphoma (granuloma=6, no granuloma=15, OR 1.19, 0.43–3.28); d) Lung cancer (granuloma=6, no granuloma=30, OR 0.52, 0.2–1.34). The curves illustrate the proportion of subjects at each time point who were alive. The p-value was calculated by the log-rank test.
Discussion
Patients with skin, breast and prostate cancers and lymphoma were more likely to develop sarcoid-like granuloma. Of most importance, this study found a decreased incidence of stage 4 cancer in patients with sarcoid-like granulomas compared to controls and led to a significant survival advantage at 2, 4, 6 and 10 years.
Despite the wide range of cancers reported for individuals with sarcoid-like granulomas, breast malignancy is the most commonly occurring cancer in most studies. A study by Keiss et al. [12] that investigated 64 patients with sarcoid-like granuloma after cancer found 17% of the subjects had breast cancer. Furthermore, Butt et al. [13] reviewed 30 subjects with sarcoid-like granuloma following cancer, 33% of whom had breast cancer. A similar predominance of breast cancer has been recorded by several other authors [14–16], and this observation is consistent with our data.
The mean age of onset of sarcoid-like granuloma among our subjects was 64.5 years. This is in agreement with multiple studies describing the occurrence of sarcoid-like granuloma in cancer patients is >50 years instead of the typical ages of sarcoidosis patients who are between 20 and 39 years [7, 12, 15–17]. As suggested by Grados et al. [18] and Hunt et al. [14], the reason may be that cancer occurs more commonly in those of an older age. This discrepancy in age of diagnosis suggests that the malignancy may be providing the antigen that fuels the sarcoid-like granulomatous reaction.
Our findings also suggest a decreased incidence of stage 4 metastatic disease in patients with sarcoid-like granulomas. Grados et al. [18] in their analysis also showed that none of 12 subjects with sarcoid-like granuloma presented with metastatic disease. These findings may suggest a protective role for sarcoid-like granulomas with regard to metastasis in cancer patients. This effect may be due to cancer cells being prevented from evading activated immune cells when there is a high tendency to develop granulomas. Further investigation of this speculation may lead to new therapeutic agents for cancer immunotherapy.
Overall, the prognostic value of sarcoid-like granuloma in cancer requires further investigation. A recent study by Steinfort et al. [7] among subjects with nonsmall cell lung cancer indicated a significant cancer-free survival in those with sarcoid-like granuloma (n=8) who had no recurrence of the disease compared to 44% of controls who had recurrence at a median interval of 11 months after surgery. Similarly, in their study of survival among 19 patients with lung cancer and coexisting granulomatous inflammation, Dagaonkar et al. [19] observed a 3-year survival rate of 21% compared to 6% in those without granulomas. Regardless, several other studies failed to reveal any significant difference in prognosis in patients with sarcoid-like granulomas [8, 20]. One major drawback of these studies is the relatively small sample sizes and univariate analysis of survival, which makes it challenging to predict prognosis accurately.
In our study, the overall survival for all patients with cancer and sarcoid-like granuloma was significantly higher than that of the controls when analysed using the Cox proportional hazards regression model. Significance was not reached in Kaplan–Meier analysis for survival for every individual cancer, which was probably due to the limited sample size; however, the trend for improved survival was apparent. The current data indicated a lower percentage of stage 4 disease in subjects with sarcoid-like granuloma compared to controls, and this, in turn, might be the reason for the observed increase in survival.
Earlier studies have suggested chemotherapeutic agents as potentially inducing sarcoid-like granulomas [21, 22]. We find this suggestion unlikely, as less than half of our subjects were treated with chemotherapy and not one chemotherapeutic agent was used in >12%. Immunotherapy, notably immune checkpoint inhibitors, have recently been reported to be associated with sarcoid-like granulomas [23]. The possibility of such cancer-independent granuloma development including sarcoidosis cannot be ruled out, especially in subjects with longer interval between cancer and sarcoid-like granuloma development.
One question that arises is the mechanism of granuloma formation. The immunology of sarcoid-like reactions occurring in cancer patients remains undiscovered. Nonetheless, it has been suggested that these sarcoid-like granulomas could be an immune reaction to cancer cells [13]. For this to be true, the antigen sustaining the granulomas must be derived from cancer cells. We suggest that vimentin may be a possible candidate antigen. Vimentin, a member of the intermediate filament family of proteins, has been identified in bronchoalveolar lavage (BAL) samples from sarcoidosis subjects [24, 25], and the T-cells derived from these BAL samples have revealed identical T-cell receptor sequences. Interestingly, modelling of these sequences with HLA-DR3 protein sequences showed an ideal fit for a vimentin peptide [26]. In addition, macrophages, an essential part of sarcoid granulomas, have also been shown to secrete vimentin when activated [27]. The Kveim–Siltzbach agent, which was historically used in the diagnosis of sarcoidosis, contained vimentin, further implicating a role in sarcoid-like granuloma formation [28].
Although vimentin is a major component of the cytoskeleton of mesenchymal cells, its expression is upregulated in various cancers of epithelial origin. Vimentin expression has also been reported in malignant lymphomas [29]. Its role in the metastatic cascade has also been well documented [30]. It is possible that vimentin from the primary tumour or metastatic cell triggers an immune response similar to sarcoidosis in genetically susceptible individuals, resulting in the formation of noncaseating granulomas. Currently, no studies assessing the role of vimentin in cancer-related sarcoid reactions have been conducted to corroborate this hypothesis.
Ideally, larger international prospective studies to analyse the incidence and determine the prognostic value of sarcoid-like granulomas more accurately should be conducted. It is also essential to obtain a deeper understanding of the pathophysiology of cancer-related granulomas. Immune profiling of granulomas to assess the presence or absence of various receptors and markers, such as vimentin, may be the first step. If these granulomas are indeed antitumour responses to metastasising cells, studies to molecularly detect neoplastic cells within granulomas using techniques such as PCR can be performed [31].
Limitations
Our study has all the limitations pertinent to retrospective studies. Despite being a relatively large study on this subject, we were unable to perform multivariate Cox survival analysis for every individual cancer due to the small subgroup sample size. One of our datasets consisted of melanoma patients only (140 subjects), which might be the reason for the higher number of subjects with a history of melanoma. Another major limitation of our study is the inability to match the subjects based on the stage of cancer at inclusion and the date of cancer diagnosis due to their limited number.
Conclusion
The findings of our study suggest that cancer patients who develop sarcoid-like granulomas have a survival advantage with lower rates of stage 4 disease. Thus, finding sarcoid-like granulomas could serve as a prognostic biomarker. Although the exact mechanism of granuloma formation is unknown, they are likely to be an immune response to neoplastic components and if proven, could also serve as a therapeutic biomarker for immunotherapy. Larger case–control studies are required to confirm and further assess the increased survival reported herein and evaluate the presence of possible cancer-related biomarkers in these granulomas via molecular-based studies.
Footnotes
Author contributions: M. Murthi and K. Yoshioka conducted chart review and collected data, analysed data, reviewed literature and helped in manuscript preparation. J.H. Cho provided the melanoma dataset and helped to develop first draft of manuscript. S. Arias, E. Danna and G. Holt reviewed the endobronchial ultrasound procedures, and helped in analysing and discussing results, and manuscript preparation. K. Tatsumi and T. Kawasaki assisted in reviewing the subjects’ data accuracy, data analysis and manuscript preparation. M. Mirsaeidi conducted literature review, designed the study, conducted exploratory analysis, performed data analysis and prepared the manuscript.
Conflict of interest: M. Murthi has nothing to disclose.
Conflict of interest: K. Yoshioka has nothing to disclose.
Conflict of interest: J.H. Cho has nothing to disclose.
Conflict of interest: S. Arias has nothing to disclose.
Conflict of interest: E. Donna has nothing to disclose.
Conflict of interest: M. Zaw has nothing to disclose.
Conflict of interest: G. Holt has nothing to disclose.
Conflict of interest: K. Tatsumi has nothing to disclose.
Conflict of interest: T. Kawasaki has nothing to disclose.
Conflict of interest: M. Mirsaeidi has nothing to disclose.
References
- 1.Ma Y, Gal A, Koss MN. The pathology of pulmonary sarcoidosis: update. Semin Diagn Pathol 2007; 24: 150–161. doi: 10.1053/j.semdp.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 2.Soler P, Basset F, Bernaudin JF, et al. . Morphology and distribution of the cells of a sarcoid granuloma: ultrastructural study of serial sections. Ann N Y Acad Sci 1976; 278: 147–160. doi: 10.1111/j.1749-6632.1976.tb47026.x [DOI] [PubMed] [Google Scholar]
- 3.Askling J, Grunewald J, Eklund A, et al. . Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med 1999; 160: 1668–1672. doi: 10.1164/ajrccm.160.5.9904045 [DOI] [PubMed] [Google Scholar]
- 4.Bonifazi M, Bravi F, Gasparini S, et al. . Sarcoidosis and cancer risk: systematic review and meta-analysis of observational studies. Chest 2015; 147: 778–791. doi: 10.1378/chest.14-1475 [DOI] [PubMed] [Google Scholar]
- 5.Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol 2007; 25: 326–333. doi: 10.1016/j.clindermatol.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 6.Schweitzer M, Salamo O, Mirsaeidi M, et al. . Increased risk of sarcoidosis among breast cancer patients; breast cancer – sarcoidosis syndrome. Eur Respir J 2017; 50: Suppl. 61, PA361. [Google Scholar]
- 7.Steinfort DP, Tsui A, Grieve J, et al. . Sarcoidal reactions in regional lymph nodes of patients with early stage non–small cell lung cancer predict improved disease-free survival: a pilot case-control study. Hum Pathol 2012; 43: 333–338. doi: 10.1016/j.humpath.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 8.Tomimaru Y, Higashiyama M, Okami J, et al. . Surgical results of lung cancer with sarcoid reaction in regional lymph nodes. Jpn J Clin Oncol 2007; 37: 90–95. doi: 10.1093/jjco/hyl141 [DOI] [PubMed] [Google Scholar]
- 9.O'Connell MJ, Schimpff SC, Kirschner RH, et al. . Epithelioid granulomas in Hodgkin disease: a favorable prognostic sign? JAMA 1975; 233: 886–889. doi: 10.1001/jama.1975.03260080048020 [DOI] [PubMed] [Google Scholar]
- 10.Sacks EL, Donaldson SS, Gordon J, et al. . Epithelioid granulomas associated with Hodgkin's disease: clinical correlations in 55 previously untreated patients. Cancer 1978; 41: 562–567. doi: [DOI] [PubMed] [Google Scholar]
- 11.Brincker H. Sarcoid reactions and sarcoidosis in Hodgkin's disease and other malignant lymphomata. Br J Cancer 1972; 26: 120–123. doi: 10.1038/bjc.1972.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiess AP, Wang H, Travis WD, et al. . Sarcoid in cancer patients: clinical characteristics and associated disease status. Sarcoidosis Vasc Diffuse Lung Dis 2015; 32: 200–207. [PubMed] [Google Scholar]
- 13.Butt S, Alzebdeh R, Kable T, et al. . Non-caseating granulomas in patients after the diagnosis of cancer: clinical characteristics and outcome. Sarcoidosis Vasc Diffuse Lung Dis 2011; 28: 44–49. [PubMed] [Google Scholar]
- 14.Hunt BM, Vallières E, Buduhan G, et al. . Sarcoidosis as a benign cause of lymphadenopathy in cancer patients. Am J Surg 2009; 197: 629–632. doi: 10.1016/j.amjsurg.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 15.Arish N, Kuint R, Sapir E, et al. . Characteristics of sarcoidosis in patients with previous malignancy: causality or coincidence? Respiration 2017; 93: 247–252. doi: 10.1159/000455877 [DOI] [PubMed] [Google Scholar]
- 16.Spiekermann C, Kuhlencord M, Huss S, et al. . Coexistence of sarcoidosis and metastatic lesions: a diagnostic and therapeutic dilemma. Oncol Lett 2017; 14: 7643–7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357: 2153–2165. doi: 10.1056/NEJMra071714 [DOI] [PubMed] [Google Scholar]
- 18.Grados A, Ebbo M, Bernit E, et al. . Sarcoidosis occurring after solid cancer: a nonfortuitous association: report of 12 cases and review of the literature. Medicine (Baltimore) 2015; 94: e928. doi: 10.1097/MD.0000000000000928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagaonkar RS, Choong CV, Asmat AB, et al. . Significance of coexistent granulomatous inflammation and lung cancer. J Clin Pathol 2017; 70: 337–341. doi: 10.1136/jclinpath-2016-203868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamiyoshihara M, Hirai T, Kawashima O, et al. . Sarcoid reactions in primary pulmonary carcinoma: report of seven cases. Oncol Rep 1998; 5: 177–180. [PubMed] [Google Scholar]
- 21.Merchant TE, Filippa DA, Yahalom J. Sarcoidosis following chemotherapy for Hodgkin's disease. Leuk Lymphoma 1994; 13: 339–347. doi: 10.3109/10428199409056299 [DOI] [PubMed] [Google Scholar]
- 22.Sanan P, Lu Y. Multiorgan involvement of chemotherapy-induced sarcoidosis mimicking progression of lymphoma on FDG PET/CT. Clin Nucl Med 2017; 42: 702–703. doi: 10.1097/RLU.0000000000001735 [DOI] [PubMed] [Google Scholar]
- 23.Gkiozos I, Kopitopoulou A, Kalkanis A, et al. . Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol 2018; 13: 1076–1082. doi: 10.1016/j.jtho.2018.04.031 [DOI] [PubMed] [Google Scholar]
- 24.Heyder T, Kohler M, Tarasova NK, et al. . Approach for identifying human leukocyte antigen (HLA)-DR bound peptides from scarce clinical samples. Mol Cell Proteomics 2016; 15: 3017–3029. doi: 10.1074/mcp.M116.060764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahlström J, Dengjel J, Persson B, et al. . Identification of HLA-DR–bound peptides presented by human bronchoalveolar lavage cells in sarcoidosis. J Clin Invest 2007; 117: 3576–3582. doi: 10.1172/JCI32401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahlström J, Dengjel J, Winqvist O, et al. . Autoimmune T cell responses to antigenic peptides presented by bronchoalveolar lavage cell HLA-DR molecules in sarcoidosis. Clin Immunol 2009; 133: 353–363. doi: 10.1016/j.clim.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 27.Mor-Vaknin N, Punturieri A, Sitwala K, et al. . Vimentin is secreted by activated macrophages. Nat Cell Biol 2003; 5: 59–63. doi: 10.1038/ncb898 [DOI] [PubMed] [Google Scholar]
- 28.Eberhardt C, Thillai M, Parker R, et al. . Proteomic analysis of Kveim reagent identifies targets of cellular immunity in sarcoidosis. PLoS One 2017; 12: e0170285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarker AB, Akagi T, Yoshino T, et al. . Expression of vimentin and epithelial membrane antigen in human malignant lymphomas. Acta Pathol Jpn 1990; 40: 581–587. [DOI] [PubMed] [Google Scholar]
- 30.Kidd ME, Shumaker DK, Ridge KM. The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol 2014; 50: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Cespedes M, Esteller M, Hibi K, et al. . Molecular detection of neoplastic cells in lymph nodes of metastatic colorectal cancer patients predicts recurrence. Clin Cancer Res 1999; 5: 2450–2454. [PubMed] [Google Scholar]