Abstract
Today, persistent and uncontrolled inflammation is appreciated to play a pivotal role in many diseases, such as cardiovascular disease, neurodegenerative diseases, metabolic syndrome, and many other diseases of public health concern (e.g. COVID-19 and periodontal disease). The ideal response to initial challenge in humans is a self-limited inflammatory response leading to complete resolution. The resolution phase is now widely recognized as a biosynthetically active process, governed by a superfamily of endogenous chemical mediators that stimulate resolution of inflammatory responses, namely specialized proresolving mediators (SPMs). Because resolution is the natural ideal response, the SPMs have gained attention. SPMs are mediators that include omege-6 arachidonic acid-derived lipoxins, omega-3 EPA and DHA derived resolvins, protectins and maresins, cysteinyl-SPMs, as well as n-3 DPA derived SPMs. These novel immunoresolvents, their biosynthetic pathways and receptors have proven to promote resolution of inflammation, clearance of microbes, reduce pain and promote tissue regeneration via specific cellular and molecular mechanisms. As of July 20, 2020, PubMed.gov reports >1,150 publications for resolvins, confirming their potent protective actions from many laboratories worldwide. Since this field is rapidly expanding, we provide a short update of advances within 2–3 years from human and preclinical animal studies, together with the structural-functional elucidation of SPMs and identification of novel SPM receptors. These new discoveries indicate that SPMs, their pathways and receptors could provide a basis for new approaches to treating inflammation-associated diseases and to stimulating tissue regeneration via resolution pharmacology.
Introduction
Uncontrolled inflammation is now widely recognized as a critical component of many pathologies including cancer, arthritis, metabolic syndromes, chronic pain, periodontal, cardiovascular and neurological diseases, as well as bacterial and viral infections, such as COVID-19 (1–3). These are significant public health concerns, thus emphasizing the urgent need to understand the mechanisms controlling inflammation and its timely resolution, in order to develop new treatments (4–6). The acute inflammatory response is normally protective and self-limited, permitting repair of injured tissues and eliminating invading organisms, thus leading to complete resolution of leukocyte infiltrates and clearance of cellular debris and microbes enabling homeostasis (7). Although resolution of disease is appreciated by clinicians, it was considered a passive process (7), until we (2) and now many others (8, 9) obtained new evidence that resolution of self-limited inflammation is an active process. An early consensus report provided the definition and underscored the potential of this new field of resolution with impact in modern medicine and surgery (10).
Focusing on fundamental mechanisms in the resolution responses, the authors’ laboratory uncovered several novel families of pro-resolving lipid mediators (LM) of inflammation that are biosynthesized from essential polyunsaturated fatty acid precursors, e.g. eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and n-3 docosapentaenoic (DPA). These potent bioactive molecules were named resolvins (Rv), protectins (PD), their aspirin-triggered (AT) isomers, and more recently maresins (MaR) as well as cysteinyl-conjugated SPMs (cys-SPMs), a superfamily collectively termed specialized pro-resolving mediators (SPMs). SPMs function as potent local resolution agonists, providing the first evidence that resolution is actively “turned on” and not simply a passive process. The complete structural elucidations of most SPMs are established (See Table 1 for the complete stereochemical names) and total organic synthesis achieved, which have enabled the confirmation of their potent bioactions and pro-resolving mechanisms at picomolar to low nanomolar concentrations in many cell types, and picogram to nanogram range in pre-clinical in vivo disease systems by researchers worldwide (2). Interested readers are directed to earlier reviews covering the original research (see 1, 2, 3, 10–12).
Table 1.
SPMs and cys-SPMs: Stereochemical names and assignments
| Substrate | SPM | Chemical name | PubChem**** CID: | Lipid Maps***** LM_ID |
|---|---|---|---|---|
| EPA | *E-series Resolvins | |||
| RvE1 | 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid | 10473088 | LMFA03140003 | |
| RvE2 | 5S,18R-dihydroxy-6E,8Z,11Z,14Z,16E-eicosapentaenoic acid | 16061125 | LMFA03140011 | |
| RvE3 | 17R,18R-dihydroxy-5Z,8Z,11Z,13E,15E-eicosapentaenoic acid | 60150429 | LMFA03140006 | |
| RvE4 | 5S,15S-dihydroxy-6E,8Z,11Z,13E,17Z-eicosapentaenoic acid | |||
| DHA | **D-series Resolvins | |||
| RvD1 | 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid | 44251266 | LMFA04030011 | |
| RvD2 | 7S,16R,17S-trihydroxy-4Z,8E,10Z,12E,14E,19Z-docosahexaenoic acid | 11383310 | LMFA04030001 | |
| RvD3 | 4S,11R,17S-trihydroxy-5Z,7E,9E,13Z,15E,19Z-docosahexaenoic acid | 71665428 | LMFA04030012 | |
| RvD4 | 4S,5R,17S-trihydroxydocosa-6E,8E,10Z,13Z,15E,19Z hexaenoic acid | 16061138 | LMFA04030002 | |
| RvD5 | 7S,17S-dihydroxy-4Z,8E,10Z,13Z,15Z,19E-docosahexaenoic acid | 16061139 | LMFA04030003 | |
| RvD6 | 4S,17S-dihydroxy-5E,7E,10Z,13Z,15E,19Z-docosahexaenoic acid | 25073193 | LMFA04030004 | |
| Protectins | ||||
| NPD1/PD1 | 10R,17S-dihydroxy-4Z,7Z,11E,13E,15Z,19Z-docosahexaenoic acid | 16042541 | LMFA04040001 | |
| Maresins | ||||
| MaR1 | 7R,14S-dihydroxy-4Z,8E,10E,12Z,16Z,19Z-docosahexaenoic acid | 60201795 | LMFA04050001 | |
| MaR2 | 13R,14S-dihydroxy-4Z,7Z,9E,11E,16Z,19Z-docosahexaenoic acid | 101894912 | LMFA04050004 | |
| eMaR | 13S,14S-epoxy-docosa-4Z,7Z,9E,11E,16Z,19Z-hexaenoic acid | 72204813 | LMFA04050002 | |
| Cysteinyl SPMs | ||||
| MCTR1 | 13R-glutathionyl-14S-hydroxy-4Z,7Z,9E,11E,16Z,19Z-docosahexaenoic acid | 122368871 | ||
| MCTR2 | 13R-cysteinylglycinyl-14S-hydroxy-4Z,7Z,9E,11E,16Z,19Z-docosahexaenoic acid | 122368872 | ||
| MCTR3 | 13R-cysteinyl-14S-hydroxy-4Z,7Z,9E,11E,16Z,19Z-docosahexaenoic acid | 122368873 | ||
| PCTR1 | 16R-glutathionyl-17S-hydroxy-4Z,7Z,10Z,12E,14E,19Z-docosahexaenoic acid | 132472316 | ||
| PCTR2 | 16R-cysteinylglycinyl-17S-hydroxy-4Z,7Z,10Z,12E,14E,19Z-docosahexaenoic acid | 132472317 | ||
| PCTR3 | 16R-cysteinyl-17S-hydroxy-4Z,7Z,10Z,12E,14E,19Z-docosahexaenoic acid | 132472318 | ||
| RCTR1 | 8R-glutathionyl-7S,17S-dihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid | 132472320 | ||
| RCTR2 | 8R-cysteinylglycinyl-7S,17S-dihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid | 132472321 | ||
| RCTR3 | 8R-cysteinyl-7S,17S-dihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid | 132472322 | ||
| n-3 DPA | n-3 DPA-derived SPMs | |||
| RvD1 n-3 DPA | 7S,8R,17S-trihydroxy-9E,11E,13Z,15E,19Z-docosapentaenoic acid | |||
| RvD2 n-3 DPA | 7,16,17-trihydroxy-8,10,12,14,19-docosapentaenoic acid | |||
| RvD5 n-3 DPA | 7S,17S-dihydroxy-8E,10Z,13Z,15Z,19E-docosapentaenoic acid | |||
| PD1 n-3 DPA | 10R,17S-dihydroxy-7Z,11E,13E,15Z,19Z-docosapentaenoic acid | |||
| PD2 n-3 DPA | 16,17-dihydroxy-7Z,10,13,14,19-docosapentaenoic acid | |||
| MaR1 n-3 DPA | 7R,14S-dihydroxy-8E,10E,12Z,16Z,19Z-docosapentaenoic acid | |||
| MaR2 n-3 DPA | 13,14-dihydroxy-7,9,11,16,19-docosapentaenoic acid | |||
| MaR3 n-3 DPA | 14, 21-dihydroxy-7,10,12,16,19-docosapentaenoic acid | |||
| RvT1 *** | 7S,13R,20S-trihydroxy-8E,10Z,14E,16Z,18E-docosapentaenoic acid | 124202379 | LMFA04000091 | |
| RvT2 | 7,12,13-trihydroxy-8,10,14,16,19-docosapentaenoic acid | 124202381 | LMFA04000092 | |
| RvT3 | 7,8,13-trihydroxy-9,11,14,16,19-docosapentaenoic acid | 124202383 | LMFA04000093 | |
| RvT4 *** | 7S,13R-dihydroxy-8E,10Z,14E,16Z,19Z-docosapentaenoic acid | 124202385 | LMFA04000094 | |
The complete stereochemical assignments listed here were established in earlier publications from the authors’ lab.
18S-RvEs contain 18S-hydroxyl group; see (142);
17R-RvDs contain 17R-hydroxyl group.
See (143) for total synthesis of RvT1 and RvT4
For 2D and 3D structure models of SPMs; see https://pubchem.ncbi.nlm.nih.gov/
Current dogma and innovation:
The currently available pharmacopeia for treating inflammation consists mainly of inhibitors and receptor antagonists to manage hyper-inflammation and/or infectious inflammation, e.g. steroids, non-steroidal anti-inflammatory drugs (cyclooxygenase inhibitors) and the biologics that act by blocking cytokines such as anti-TNF therapies (13). New approaches are still needed to minimize or eliminate unwanted side effects and immune suppression that can accompany prolonged use of these traditional anti-inflammatory therapies. This is most evident in the need to control the cytokine storms and unexpected coagulopathies in COVID-19 patients (3, 14, 15). The emergence of new concepts and novel mediators within the resolution terrain that activate resolution and promote tissue regeneration have given rise to the new field of resolution pharmacology. These are subjects of several in-depth reviews that interested readers are directed to (2, 11, 16–19).
SPM production in humans
(I). SPM production in diseases and on challenge
While the structural elucidation of SPMs used mass spectrometry and other physical and bioactive properties, their production in humans is documented using targeted mass spectrometry-based LM metabololipidomics in many tissues and organs under physiologic and pathologic conditions, and now by many other investigators (Table 2; reviewed earlier in (2, 12)). In several recent human trials, supplementation with omega-3 or marine oil increases select SPMs that correlate with enhanced phagocyte functions (Table 3; ref. (20); reviewed in (2)). SPM biosynthesis is impaired in several diseases, including tuberculous meningitis (21), multiple sclerosis (22), and osteoarthritis (23), suggesting that impaired endogenous resolution pathways might be a pathological etiology in these diseases. The biosynthesis of cys-SPMs and their complete stereochemistry are documented in human tissues, including brain, spleen, lymph nodes and bone marrow (24, 25), as well as in human platelets and macrophages exposed to pathogens (26). For n-3 DPA-derived SPMs in human, 13-series resolvins (RvTs) are increased following strenuous exercise, and RvDn-3 DPA are regulated in a diurnal manner (27, 28).
Table 2.
SPM production in humans: Identification and quantification by mass spectrometry*
| Tissue/organ | SPMs | Quantities | Reference |
|---|---|---|---|
| Serum (Tuberculosis and Type 2 diabetes) | RvE2,3, RvD1,2,4,5,6, PD1, 17R-PD1, MaR1,2, MCTR3, RvTs, RvDn-3 DPA, PD1n-3 DPA, MaR1n-3 DPA | range 0.2–28 pg/ml | (144) |
| Lymph nodes | MCTR1-3, PCTR1-3 | 1–5 pg/500 mg protein | (24) |
| Spleen | MCTR1, MCTR2, PCTR1, RCTR1-3 | 1–5 pg/500 mg protein 60–400 pg/500 mg protein |
(24) |
| Brain | MCTRs, PCTRs, RCTRs | 1–5 pg/500 mg protein | (24) |
| Plasma | RvDn-3 DPA | 10–30 pg/ml | (27) |
| Cerebrospinal fluid | RvT2, RvT4 (Tuberculous meningitis) | 1–2 pg/ml | (21) |
| Synovial fluid | PD1, RvD1, RvD2, RvD5, MaR1 (RA and OA) | ~5 pmol/ml | (23) |
| Bone marrow | RvD4 | (80) | |
| Blister | RvEs, RvDs, PD1, MaR1 AT-RvD1 | 0.1–10 pg/blister | (29) |
| Vagus nerve (electric stimulation) | RvE1-3, RvD3-6, NPD1/PD1, MaR1 | 1–40 pg/3.5 cm of tissue | (33) |
| Metabolic syndrome (weight loss program) | RvE1, RvE3, RvD2, MaR1 (neutrophils+A23187) | 26–1340 pg/4.5 × 106 PMN | (32) |
| Obesity | RvD1-6, MaR1, MaR2, PD1, RvEs (Plasma and leukocytes) | 0.2–200 pg/ml plasma, 0.1–2 pg/3×106 cells | (145) |
| Stenotic aortic valves | RvE1, RvD3 | ~500 – 3500 pg/g tissue | (44) |
| Sputum (cystic fibrosis) | RvD1 | ~200 pg/ml | (120) |
| SARS-CoV-2 infection | RvE3, RvD1-4, PD1 | (139) |
Table 3.
Supplementation: Production and increase of SPMs
| Diseases/conditions | Doses and regimen | SPMs present | SPMs that are increased by supplementation | Reference |
|---|---|---|---|---|
| Chronic kidney disease | n-3 PUFA; 4 g/day; 8 wks | RvE1, RvE2, RvE3, RvD5 (plasma) | RvE1, RvE2, RvE3, RvD5 | (146) |
| Effect of n-3 in pregnancy on offspring | n-3 PUFA ethyl esters with DHA (56.0%) and EPA (27.7 %); 3.7 g/day; from 20 wks gestation until delivery | 18-HEPE, 17-HDHA RvE1, RvE2, RvE3, RvD1, 17R-RvD1, RvD2 (cord blood) | 18-HEPE, 17-HDHA | (147) |
| Healthy individuals (Serum & plasma) | Study A - ω-3 triglyceride form; 900 mg/d (550 mg EPA and 350 mg dHa) or 1800 mg/d (1100 mg EPA and 700 mg DHA); 5 months Study B - 3.4 grams/d EPA and DHA (in the form of four capsules each containing 460 mg of EPA-ethyl ester and 380 mg of DHA-ethyl ester); 8–12 wks | RvE1, RvD1, 17=HDHA, 18-HEPE (plasma and serum) | RvE1, RvD1, 17=HDHA, 18-HEPE | (31) |
| Peripheral artery disease (OMEGA-PAD II trial) | n-3 PUFA; 325 mg EPA and 225 mg DHA per capsule; 4.4 g (4 capsules)/day; 3 months | RvE1, RvE2, RvE3 (plasma) | RvE3 | (148) |
| Peripheral artery disease with marine oil supplementation | PUFA with EPA (≈46%), n-3 DPA (≈18%), and DHA (≈33%); 1.5, 3, and 4.5 g/day; 5 days | RvEs, RvDs, PD, MaR, MCTRs, PCTRs, RvTs, RvDn-3 DPA, PDn-3 DPA, MaRn-3 DPA (plasma) | MaR | (149) |
| Healthy individuals (marine oil supplementation) | PUFA with EPA (≈46%), n-3 DPA (≈18%), and DHA (≈33%); 1.5, 3, and 4.5 g/day; 2 wks | RvEs, RvDs, PD1, MaR1, MCTRs, PCTRs, RvTs, RvDn-3 DPA, PDn-3 DPA, MaRn-3 DPA (plasma) | RvEs, RvDs, PD1, MaR1, MCTRs, PCTRs, RvTs, RvDn-3 DPA, PDn-3 DPA, MaRn-3 DPA | (20) |
| Arthritis | Microalgae oil (Schizochytrium sp); 2.1 g DHA/day; 10 wks | 14-HDHA, 17-HDHA (plasma) | 14-HDHA, 17-HDHA | (150) |
| Diseases/conditions | Doses and regimen | SPMs present | SPMs that are increased by supplementation | Reference |
| Peritonitis and Sepsis (mouse) | omega-3 lipid emulsions; infusion ~2 mg/g/day; 24 hours | 18-HEPE, MaR1, PDx | 18-HEPE, MaR1, PDX | (151) |
| Liver (rat) | DHA; 300 mg/kg; 3 days; | RvE1, RvE2, RvD1, RvD2 | RvD1, RvD2 | (152) |
| Asthma (mouse) | Long-chain PUFA; 1g/kg EPA, 229.6 mg/kg DHA; 24 days | RvEs, RvDs, PD1, MaR2, MCTRs, PCTRs, RCTRs, RvTs, RvDn-3 DPA, PDn-3 DPA, MaRn-3 DPA (lungs) | RvD1, RvD4 | (154) |
| Brain (neonatal piglet) | Herring oil; 40 mg DHA/kg; 10 days | RvD1 | (155) |
In human challenge using a model of UV-killed E. coli-triggered skin inflammation, SPMs are biosynthesized locally at the start of resolution, including LXB4, RvE1, RvD2 and AT-RvD1 (~10 pg/blister). This SPM panel, when given back to skin blisters at the concentrations present within blisters, reduces PMN numbers (29). Alsoin this human model, a synthetic cannabinoid anabasum, given orally as a CB2 agonist, increases RvD1 and RvD3, while reduces proinflammatory leukotriene (LT) B4 and PMN infiltration, demonstrating pro-resolving actions of anabasum (30). With young adults taking ω-3 supplements, LC-MS-MS-based metabololipidomic profiling was verified in two separate laboratories with coded samples for inter-laboratory validation using human plasma and serum and low-dose intravenous lipopolysaccharide (LPS) challenge (31). Results from these laboratories with the same samples gave evidence in humans for temporal production of SPMs, supporting the role of SPMs in inflammation-resolution. Human neutrophils from individuals with metabolic syndrome following weight loss gave increased RvE1 production upon stimulation (32). Also, the human vagus nerve produces SPMs, e.g., RvE1, NPD1/PD1, MaR1, upon electrical stimulation (33) suggesting that this vagus-SPM circuit contributes to a new proresolving vagal reflex and reduction of leukotrienes (LT) and prostaglandins (PG) produced by the vagus nerve.
(II). Lipid mediator class switching and cell type-dependent SPM production
Temporal analyses of LM in experimental exudates indicate early appearance of LT and PG, followed by lipoxin (LX) biosynthesis, which was concurrent with resolution (reviewed in 4). Following these original findings in vivo and with human PMN (4), it was recently reported that E. coli and S. aureus each stimulate predominantly proinflammatory LTB4 and PGE2 in M1-macrophages, while stimulating M2-macrophages (34, 35). Also in human macrophages, high-mobility group box 1 protein (HMGB1) stimulates LT, while HMGB1 plus C1q switch macrophages to produce SPMs, including RvDs (36). Along these lines, a dual inhibitor of 5-LOX and microsomal prostaglandin E2 synthase-1 selectively reduces LT and PG in M1, while enhancing SPMs (i.e. resolvins and maresins) in M2-macrophages (37). These results indicate that M1- and M2-like macrophages respond to pathogenic bacteria and pharmacologic inhibitors differently, biosynthesizing distinct LM profiles that further distinguish inflammatory versus pro-resolving phenotypes of these cells.
(III). Impact of age, sex and race on SPM production
Aging is associated with an overt inflammatory phenotype and physiological decline. In aged mice, resolution of inflammation is delayed and SPM levels reduced (38). Of interest, nano-proresolving medicines carrying SPMs reduce inflammation by promoting efferocytosis, providing evidence for age-dependent resolution pathways and novel means to activate resolution (38). In acute myocardial infarction, RvE1 is significantly lower in Black compared to White patients. In this study, PD1 is lower in White males compared to White female and Black patients (39). The prevalence of coronary heart disease is similar between Black and White patients, but cardiovascular events (including MI), rehospitalization, and mortality are disproportionately higher in Black patients. Whether lower RvE1 is associated with higher cardiovascular events and mortality in Black patients remains of interest. These findings emphasize the importance of assessing the age-, sex- and race-specific LM-SPM profiles in health and disease, in order to develop personalized and precision medicine. Moreover, these are areas of current ongoing research that shall yield important insights.
SPM functions in vitro and in vivo
(I). E-series Resolvins
Resolvin E1 was the first identified SPM derived from EPA (40), isolated from resolving exudates and disease models (Table 4) that reduced PMN infiltration (reviewed in 2), e.g. in carotid artery thrombosis upon fish oil and aspirin supplementation (41). In animal models of inflammatory diseases, RvE1 displays potent actions protecting against leukocyte-mediated tissue injury and excessive pro-inflammatory responses (reviewed earlier in (4)). RvE1 via its receptor ERV1/ChemR23 (Figures 1 & 2; reviewed in (42)) controls vascular inflammation, protecting against atherosclerosis by modifying oxidized LDL uptake and enhancing macrophage phagocytosis (43). In aortic valve stenosis, targeted deletion of ChemR23 in mice heightens disease progression (44). RvE1 also reduces neuroinflammation in a murine model of Alzheimer’s disease (45), attenuates murine psoriatic dermatitis (46) and promotes intestinal epithelial wound healing in colitis (47). RvE1 also reduces tumor burden and enhances clearance of tumor cell debris (48). Since certain chemotherapeutic agents, e.g. cisplatin, trigger formation of tumor cell debris that stimulates growth of living tumor cells (48), SPMs provide a new therapeutic target that may be used in combination with chemotherapy to reduce cytotoxicity.
Table 4.
Updates on SPM actions in animal models of diseases from recent 3 years
| Diseases/conditions | SPMs that resolve inflammation and reduce disease severity | Reference |
|---|---|---|
| Infection | Bacterial: MCTRs, PCTR1, RCTRs | (24, 25) |
| Acute lung injury | MCTRs | (100, 102) |
| ARDS | PCTR1 | (103) |
| Skin and burn wound | RvD2 | (70, 72) |
| Colitis/IBD | RvE1 | (47) |
| Arthritis | RvD1 | (59) |
| Periodontitis | RvD2 | (156) |
| Regeneration | Planaria head regeneration: MaR1, RvE1, MACTRs, PCTRs, RCTRs | (24, 25) |
| Alzheimer’s disease | RvE1 | (45) |
| Neuroinflammation | Neurocognitive disorders: MaR1 | (91) |
| Dermatitis | RvE1 | (46) |
| Multiple sclerosis | RvD1, PD1 | (22) |
| Cancer | RvE1, RvD1, RvD2 | (48) |
| Atherosclerosis | RvE1 | (43) |
| Aortic aneurysm | RvD1 | (136) |
| Depression | RvE3 | (52) |
| Aging | RvD1 | (58) |
| Parkinson’s disease | RvD1 | (60) |
| Sickle cell disease | AT-RvD1 | (61) |
| Ischemia-reperfusion kidney injury | RvD1 | (67) |
| Deep vein thrombosis | RvD4 | (81) |
| Neuropathic and inflammatory pain | RvD5 | (83) |
| Traumatic brain injury | NPD1 | (85) |
| Stroke | NPD1, 17R-NPD1 | (86, 87) |
| Skin inflammation | MaR1 | (94, 95) |
| Cystic fibrosis | RvD1 | (120) |
Figure 1. Illustration of resolution metabolome: SPM biosynthesis, receptors and inactivation.
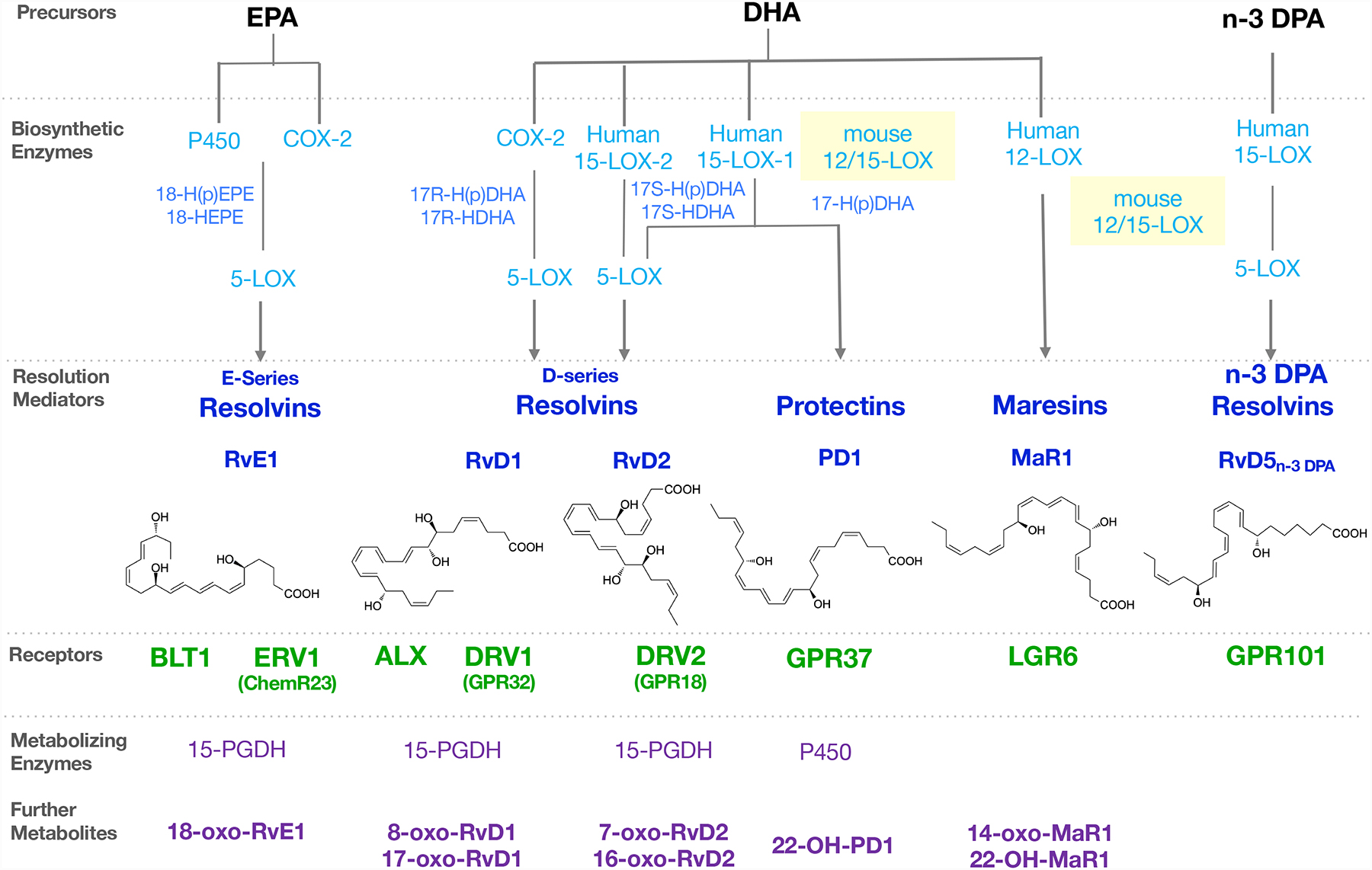
Precursors EPA, DHA and n-3 DPA are converted via biosynthetic enzymes to SPMs, which activate their specific receptors to stimulate pro-resolving immune functions. The SPMs are rapidly enzymatically converted to metabolites with diminished or reduced activity.
Figure 2. SPM receptor networks.
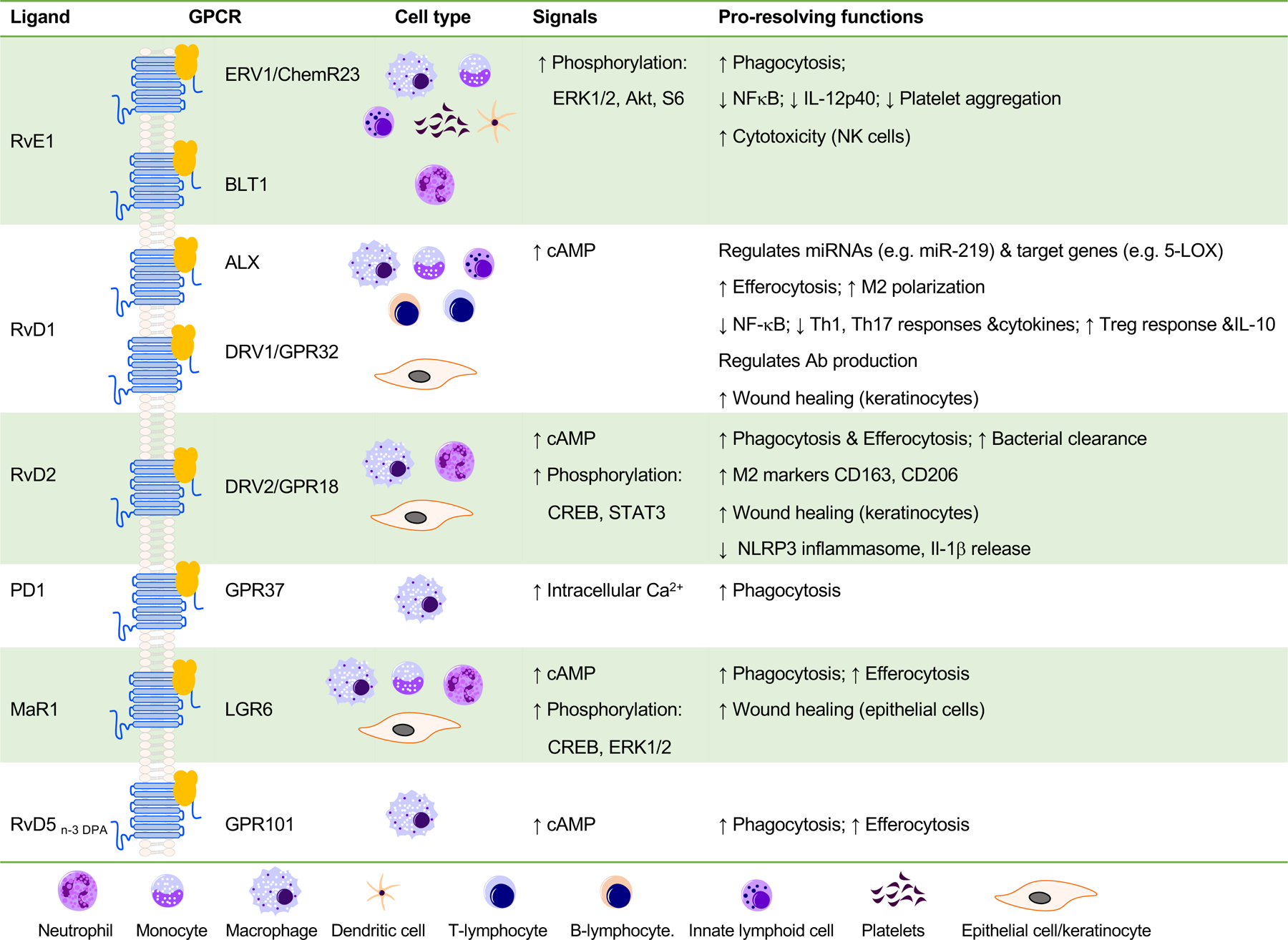
SPMs stimulate resolution via activating cell surface GPCRs. RvE1 activates ERV1/ChemR23, leading to phosphorylation of Akt/S6 protein pathways that stimulates macrophage phagocytosis. RvD1 activates both DRV1/GPR32 and ALX, leading to regulation of microRNAs and their target genes, increases of efferocytosis (macrophage phagocytosis of apoptotic PMN) and M2 macrophage polarization. RvD2 activates GPR18, leading to cAMP release and phosphorylation of select kinases and transcription factors, essential for macrophage phagocytosis. Besides phagocytes, SPMs also display cell type-specific actions. For example, RvE1 activates both ERV1/ChemR23 and BLT1 on human conjunctival goblet cells to stimulate mucin secretion (158). 17R-RvD1 attenuates vascular smooth muscle cell migration via ALX/FPR2 and cAMP/PKA activity (159). RvD2-GPR18 axis enhances wound repair with human on keratinocytes (70). See text and earlier reviews (42, 112) for further details.
Current members of the E-series resolvins include RvE1, RvE2 and RvE3, with the recent addition and elucidation of RvE4 (49). RvE2 stops zymosan-induced PMN infiltration in murine peritonitis (50). RvE3 attenuates depression-like behavior in mice and airway allergic inflammation via IL23-IL17A pathway (51–53). RvE4, identified in physiologic hypoxia condition with M2-macrophages, stimulates efferocytosis of senescent erythrocytes in vitro and reduces mouse hemorrhagic exudates in vivo (49). Collectively, these results demonstrate tissue/organ-and cell type-specific actions of E-series resolvins in controlling acute and chronic inflammation, neurological disorders, cancer as well as stimulating tissue repair (Figure 3).
Figure 3. Temporal phases in inflammation-resolution and SPM actions in preclinical animal disease models.
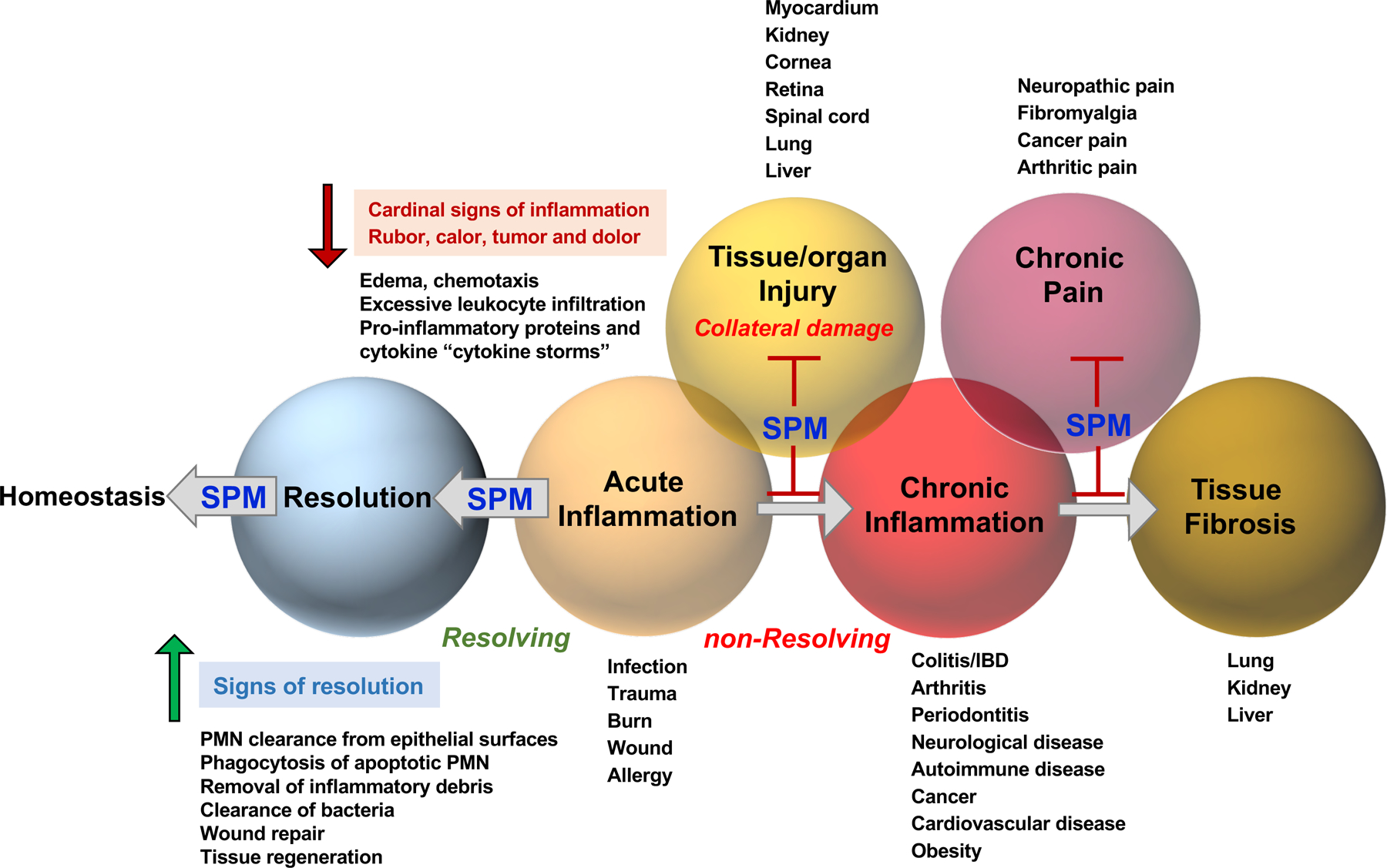
In in vivo animal diseases, select SPMs not only control classic non-resolving inflammation conditions, but also reduce collateral damages such as tissue and organ injuries, chronic pain and tissue fibrosis. They are normally active in picogram to low nanogram quantities, that is 100–1000x more potent than widely used classic NSAID. In the case of controlling pain, RvE1 is 1000X more potent than morphine (160). As potent agonists i.e. immunoresolvents, SPMs promote resolution and homeostasis via limiting excessive inflammatory responses (e.g. limiting PMN infiltration and cytokine storm), and enhancing efferocytosis, bacterial clearance, wound repair as well as tissue regeneration, defining the signs of resolution.
(II). D-series Resolvins
Using the mediator lipidomics-informatics approach, several bioactive compounds derived from DHA were isolated and identified, including both 17R alcohol-containing resolvins (17R-RvDs or AT-RvDs; via aspirin and COX-2 initiated mechanisms (54)) and 17S alcohol-containing resolvins (RvDs; via LOX-initiated mechanisms (55); see Figure 1). Aspirin acetylates COX-2 to produce17R-alcohol-containing intermediate 17R-H(p)DHA that is converted by 5-LOX to produce 17R-isomers of RvDs (ref. (54); Figure 1). In addition to aspirin, N-acetyl sphingosine (N-AS), generated from acetyl-CoA and sphingosine via sphingosine kinase1 (SphK1), also acetylates COX2 and increases RvE1 and 17R-RvD1 (56).
(a). Resolvin D1 and 17R-resolvin D1
Both RvD1 and 17R-RvD1 are potent regulators of human and murine phagocytes at picomolar to low nanomolar concentrations (54, 55). They stimulate macrophage phagocytosis of microbes, efferocytosis of apoptotic PMN and tumor cell debris (57) as well as enhance efferocytosis in aging (58). In obesity-associated osteoarthritis, intra-articular treatment with RvD1 diminishes the progression of osteoarthritis in the knee joint (59). In a rat model of Parkinson’s disease, RvD1 prevents central and peripheral inflammation (Table 4), as well as neuronal dysfunction and motor deficits (60). In a sickle cell disease mouse model, 17R-RvD1 promotes efferocytosis of PMN and erythrocytes (61). Also, 17R-RvD1 restores TLR9-impaired PMN phagocytosis and accelerates resolution of bacterial DNA-induced lung inflammation (62). Further, RvD1 promotes clearance of necroptotic cells by activating CDC42-dependent “eat-me signals” (63). RvD1 controls macrophage polarization; it increases pro-resolving markers such as arginase 1 and mannose receptor C-type 1, inhibiting tumor-associated macrophage (TAM), and regulates cardiac fibroblast plasticity (64, 65). RvD1 also induces a pro-revascularization phenotype in macrophages during tissue ischemia via its receptor, ALX/FPR2, that enhances tissue perfusion (66). In ischemia-reperfusion kidney injury, RvD1 increases Treg population via ALX/FPR2 pathways (67). Thus, RvD1 and 17R-RvD1 regulate leukocyte functions and plasticity, via activating their receptors ALX/FPR2 and DRV1/GPR32; see Figure 2 and for review (42).
(b). Resolvin D2
RvD2 is a potent regulator of bacterial sepsis (nanogram quantities) via its receptor DRV2/GPR18 in mice (Figure 2). RvD2 suppresses tumor growth and enhances clearance of tumor cell debris, while DRV2/GPR18-deficient mice display defective tumor clearance (48). Along these lines, the higher percentages of GPR18-positive PMN are associated with lower mortality in human sepsis (68). In several types of human cancers, GPR18 expression levels on tumor-infiltrating B lymphocytes are prognostic in that higher GPR18 levels are associated with higher percentages of overall survival (69). Both RvD1 and RvD2 are tissue/organ protective; RvD2 promotes keratinocyte repair in DRV2-dependent manner (70) and stimulates muscle regeneration (71), as well as limits tissue necrosis in burn wound (72). In periapical lesion of periodontitis, RvD2 increases DRV2 expression, and enhances pulp-like tissue regeneration and healing of periapical lesion (73). With conjunctival goblet cells, RvD2 stimulates mucin secretion via elevated cAMP (74). Thus, in addition to its potent actions on phagocytes, RvD2 displays cell type-and organ-specific actions promoting tissue protection, repair and regeneration.
(c). Resolvin D3 and 17R-resolvin D3
Both RvD3 and 17R-RvD3 potently regulate leukocyte-directed actions in vitro and in vivo ((75); reviewed in (25)). In lung epithelial cells, RvD3 increases NF-κB counter-regulators (76). RvD3 and 17-RvD3 both display anti-cancer activity in murine systems (57, 77), raising the possibility of targeting and stimulating resolution pathways for treatment of cancer.
(d). Resolvin D4
Several different total organic syntheses of RvD4 were achieved. The first total synthesis of RvD4 established its stereochemical configuration, which permits uncovering RvD4’s ability to clear S. aureus skin infections and stimulate efferocytosis by dermal fibroblasts (78). A second stereoselective synthesis of RvD4 was reported (79). And recently, RvD4 was scaled-up in a new commercial synthesis, and this synthetic RvD4 displays organ protection in two mouse ischemic injury models (80). In a murine model of deep vein thrombosis, RvD4 reduces thrombus burden and decreases the release of neutrophil extracellular traps (NETs), i.e. NETosis, a critical component for thrombosis (Figure 4) development (81). These findings and new roles of RvD4 suggest that SPMs could provide an effective strategy in controlling thrombo-inflammatory disease.
Figure 4. Microvascular thrombosis and the roles of SPMs.
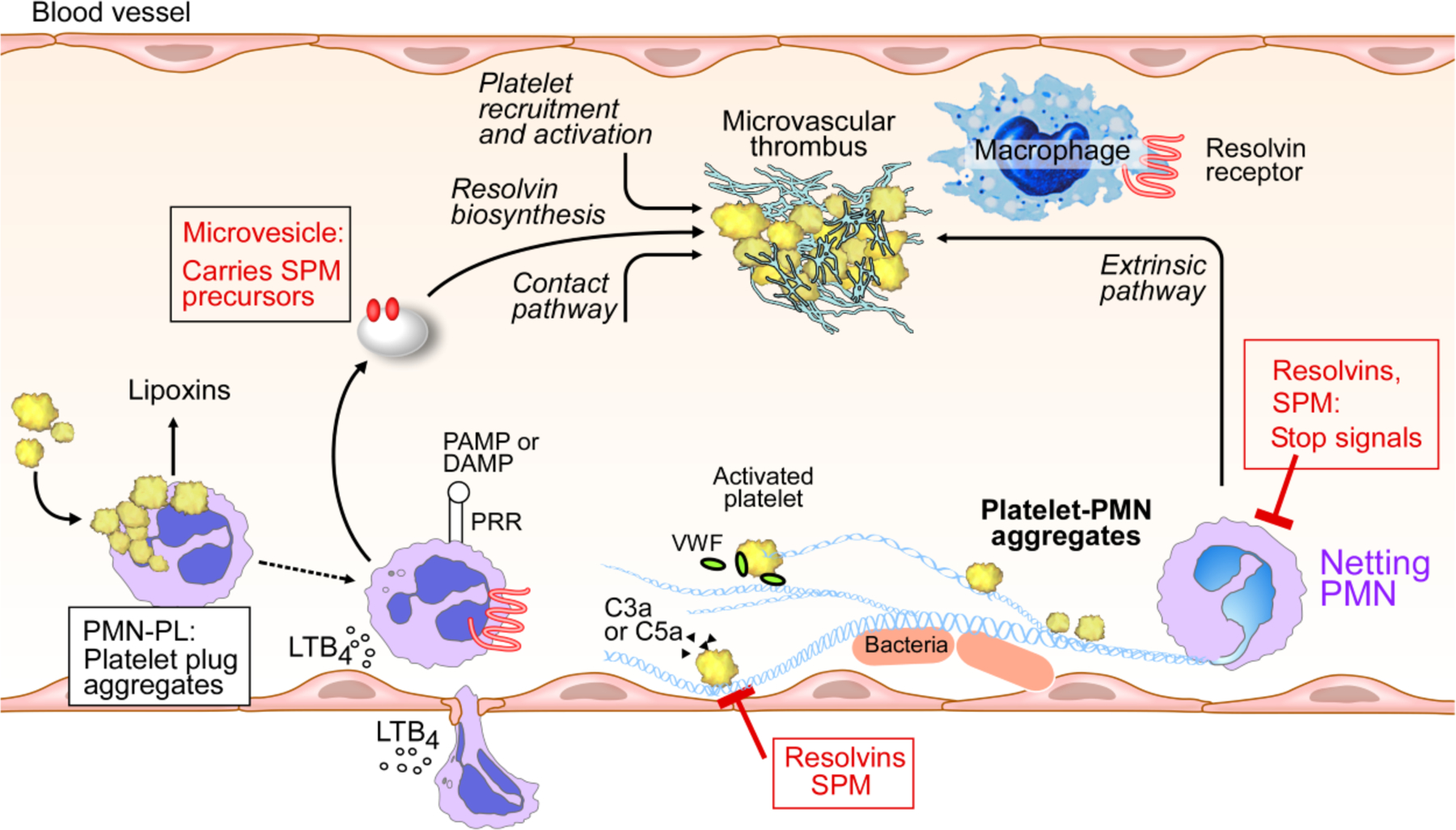
Illustration of the intraluminal production and action of SPMs; PMN-platelet interactions initiate production of lipoxins and other SPMs, e.g. MaR1 (reviewed in 4). Microvesicles released from PMN also contribute to SPM formation (reviewed in 25). Select SPMs including LXA4, MaR1, RvD1 and RvD4 enhance thrombus resolution, and RvD4 reduces NETosis (81).
(e). Resolvin D5
RvD5 controls E. coli and S aureus infection, lowering antibiotic requirements for bacterial clearance (82). With human cells, RvD5 stimulates phagocytosis of E. coli by both M1- and M2-like human macrophages (35). Of interest, RvD5 is the first SPM that shows sex dimorphism in pain regulation, inhibiting both neuropathic and inflammatory pain in male but not with female mice (83).
(III). Protectins
Protectin D1/Neuroprotectin D1 is biosynthesized from DHA via 15-LOX-initiated mechanism in several human cell types, murine exudates, and brain tissues (55). Establishment of its complete stereochemical assignments ((84) and reviewed in (12)) enabled the demonstration of its potent actions in many disease systems. When of neural origin, NPD1 is used, and PD1 is used to denote its peripheral actions. PD1/NPD1 displays potent neuroprotective actions in brain, retina and central nervous system, e.g. protecting from ischemic stroke, retina degenerative disease (reviewed in 12) and traumatic brain injury (85). The aspirin-triggered epimer 17R-NPD1 shares the action of NPD1 in controlling PMN, enhancing macrophage functions and attenuating experimental stroke (86, 87).
(IV). Maresins
The Macrophage mediator in resolving inflammation, denoted maresins (MaR), were first identified in human macrophages (MΦ) via 12-LOX-initiated mechanisms (88). Next, the complete stereochemistry of maresin 1 (MaR1) was determined, its total organic synthesis was achieved and confirmed by several independent teams (reviewed in 12). We investigated MaR1’s ability in stimulating tissue regeneration using a freshwater flatworm, namely planaria, as a primordial model organism, since it possesses robust regenerative capability. The stereochemically defined synthetic MaR1 accelerates planaria regeneration following head resection, reduces pain and is neuroprotective (12). MaR1 is also biosynthesized during platelet-PMN interactions (25), and MaR1, RvD2 and RvD1 each reduce cold-stored platelet activation (89), which may be important for long-term cold platelet storage needed for transfusion. Recently,MaR1 was found to improve functional neurological recovery after spinal cord injury (90), attenuate neuroinflammation in perioperative neurocognitive disorders in mice (91), as well as reduce mechanical and thermal hyperalgesia (i.e. enhanced pain sensitivity) (92). Following tooth extractions, MaR1 reduces post-operative pain, accelerates wound healing and promotes socket bone regeneration (93). In skin, MaR1 reduces psoriasis-like inflammation and UVB irradiation damage (94, 95). Thus, MaR1 is pro-regenerative, pro-repair and neuroprotective in a wide range of tissues and organs across phyla. MaR1 activates two different classes of receptors, namely leucine-rich repeat-containing G protein–coupled receptor 6 (LGR6), a cell surface G protein-coupled receptor on phagocytes (96), and retinoic acid-related orphan receptor α (ROR-α), a nuclear receptor on liver macrophages that might be relevant in liver pathology (97). These findings highlight the cell-type specific and receptor-dependent actions of MaR1.
(V). Cysteinyl SPMs (cys-SPMs)
Three new series of peptide-lipid conjugated SPMs were recently identified, including maresin conjugates in tissue regeneration (MCTR), protectin conjugates in tissue regeneration (PCTR) and resolvin conjugates in tissue regeneration (RCTR), collectively coined cysteinyl-SPMs (cys-SPMs) (98) (reviewed in 25). Each series contains 3 bioactive members that display potent (picogram to nanogram range) pro-regenerative and pro-repair actions, including stimulating regeneration of freshwater planaria and promoting tissue repair in acute lung injury (24, 98). In addition, MCTRs exhibit cardiovascular protection in mice and in primordial sea squirt, as well as counter LTD4-initiated signals and vascular response (99). In murine allergic airway inflammation, MCTRs block LTD4-induced airway contraction (100, 101). MCTR1 and PCTR1 also prevent LPS-induced acute respiratory distress syndrome and multiple organ damage (102, 103). Thus, the organ-protective actions of cys-SPMs are evolutionarily conserved across phyla, from primordial lower-phylum species such as planaria and sea squirt to mice and humans.
(VI). n-3 DPA-derived SPMs
In addition to EPA and DHA, n-3 DPA is also converted to novel SPMs, including RvDn-3 DPA, MaRn-3 DPA and PDn-3 DPA, as well as 13-series resolvins (RvTs) (28, 104). Biosynthesis of RvTs is increased by atorvastatin via S-nitrosylation of COX-2 during neutrophil-endothelial cell interactions. RvTs and atorvastatin demonstrate additive protection, increasing survival during E. coli infections (28). Stereochemistries of some of the n-3 DPA-derived SPMs are established using total organic synthesis (105, 106). Using the synthetic authentic compounds, these n-3 DPA-derived SPMs share the potent protective actions of the EPA-and DHA-derived SPMs in regulating key innate protective responses, dampening GI, joint, cardiovascular and neuroinflammation as well as promoting timely resolution (27, 107–110).
SPM Receptors and mimetics
SPMs stimulate resolution via activating cell surface GPCRs. Each SPM demonstrates stereoselective activation of its own specific GPCR, leading to some overlapping downstream signals, pathways and pro-resolving functions. The affinities of SPMs for their respective GPCRs (i.e. Kd values) are in the low nanomolar range, consistent with their bioactions in vitro and in vivo. Deficit of ALX/FPR2, a RvD1 receptor, in mice amplifies leukocyte-directed endothelial dysfunction, cardiomyopathy and age-related obesity (111). See Figures 2 and 3, and recent reviews (42, 112) for details on mechanisms and roles in cardiovascular pathologies.
(I). Newly discovered SPM receptors
Several new SPM receptors were recently uncovered. NPD1/PD1 activates GPR37, increasing intracellular Ca2+ and phagocytosis with macrophages. Mice lacking Gpr37 display defects in macrophage phagocytic activity and delayed resolution of inflammatory pain (113). Using an unbiased screening of > 200 GPCRs, MaR1 was identified as a stereoselective activator for human leucine-rich repeat containing G protein-coupled receptor 6 (LGR6), expressed in phagocytes. MaR1-specific binding to LGR6 was confirmed using 3H-labeled MaR1. With human and mouse phagocytes, MaR1 stimulates phagocytosis, efferocytosis, and phosphorylation of select proteins in a LGR6-dependent manner. MaR1 is therefore an endogenous activator of human LGR6, stimulating MaR1’s key proresolving functions of phagocytes (96). The first GPCR for n-3 DPA-derived resolvins was recently identified. Using a similar GPCR screening approach and radioactive ligand, RvD5n-3 DPA was shown to bind to an orphan receptor GPR101 with high selectivity and stereospecificity, as well as RvD5. Knockdown of GPR101 in vivo reverses the protective actions of RvD5n-3 DPA in limiting joint and gut inflammation during inflammatory arthritis (114). Taken together, these newly identified SPM receptors together with ALX/FPR2, ERV1/ChemR23, DRV1/GPR32 and DRV2/GPR18 display potent pro-resolving properties with ligand-receptor specificity, as evidenced by structure-function activity (SAR) established for each receptor. In addition, receptor activation by each SPM initiates overlapping (e.g. cAMP signal for ALX with calcium mobilization, GPR18, LGR6 and GPR101) and distinct signals (e.g. intracellular [Ca2+] for PD1) on phagocytes, likely acting in tandem to govern host defense against injury, inflammation and infection to ensure timely resolution.
(II). SPM Mimetics
SPMs are subject to rapid further metabolism via enzymatic inactivation (Figure 1). Metabolically stable analogs that resist enzymatic inactivation were designed and synthesized (115), and new benzo-diacetylenic 17R-RvD1 methyl ester (BDA-RvD1) mimetic with reduced chiral centers and steps in organic synthesis shares defining pro-resolving actions with RvD1 (116). Using a high-throughput screening of small-molecule libraries (>48,000 compounds), several chemotypes were identified to activate recombinant DRV1 and stimulate macrophage phagocytosis of live E. coli in DRV1-dependent manner (117). These molecules act as RvD1 mimetics, offering templates for potentially more cost-effective synthesis to facilitate clinical development of therapeutics that stimulate resolution pathways. Also, a benzo-RvE2 analog was prepared, showing amazing femtomolar potency in reducing inflammation in vivo (118).
SPMs control infections
Select SPMs exhibit potent host-protective actions in bacterial, parasite and viral infections (2, 5). The anti-inflammatory and pro-resolving actions of SPMs were proven. Their ability to clear live microbes was unexpected. We first uncovered resolvins’ ability to enhance bacterial killing and clearance while maintaining limited collateral tissue damage (82); these unanticipated actions of resolvins opened the study of SPMs in microbial control.
(I). Bacterial infection
In a microbial sepsis, SPMs display multi-level mechanisms, controlling excessive inflammatory responses, reducing bacterial loads and increasing survival (25). In E. coli and S. aureus murine infections, RvDs and PD1 reduce pro-inflammatory cytokines IL-1beta and IL-6, while increasing anti-inflammatory IL-10 and interferon (IFN)-gamma (82). Select cys-SPMs also accelerate resolution of E. coli infection, shortening resolution intervals (2, 24). Also, RvD1 and MaR1 control M. tuberculosis infection, increasing expression of antibacterial peptides (119). In P. aeruginosa lung infection, RvD1 reduces lung bacterial load, inflammation, and tissue damage relevant in cystic fibrosis patients (120).
(II). Parasitic infection
Chagas disease, transmitted by a protozoan named Trypanosoma cruzi, is a major public health issue worldwide. From patients with Chagas disease, 17R-RvD1 significantly reduces T. cruzi antigen-stimulated PBMC proliferation (121), and RvD1 in a murine model of T. cruzi infection increases survival, controls parasite replication and prevents cardiac fibrosis (122).
(III). Viral infection
In a murine model of stromal keratitis (SK), a common cause of blindness caused by herpes simplex virus (HSV)-1 infection, RvE1 and AT-RvD1 reduce HSV-1 ocular infection (42). In lethal influenza H5N1 virus infection, a PD1 isomer protects mice via inhibiting viral replication (123). Also, 17-HDHA, a pathway marker and precursor to D-series Rvs, enhances the antibody-mediated immune response against influenza virus H1N1, exhibiting an adjuvant-like property (124). In viral and bacterial coinfection (pneumonia with Streptococcus pneumoniae and influenza A virus), 17R-RvD1 significantly reduces neutrophil elastase (NE) activity, while accelerating clearance of pneumococci in the lungs (125). Thus, 17R-RvD1 could be harnessed for the treatment of chronic obstructive pulmonary disease and other diseases that impact lungs such as COVID-19 and cystic fibrosis that are typically complicated by both viral and bacterial respiratory pathogens.
(IV). SPMs and SARS-CoV-2 infection
Severe acute respiratory syndrome (SARS) is a viral respiratory illness caused by a single-stranded RNA virus, namely SARS-associated coronavirus (SARS-CoV), reported in 2003. The current Coronavirus Disease 2019 (COVID-19) pandemic is caused by a new strain SARS-CoV-2 (https://www.cdc.gov/sars/index.html). In light of COVID-19 pathologies with inflammation of the respiratory and cardiovascular systems (126–131), anti-inflammatory, pro-resolving, microbial clearance and organ-protective actions of SPMs may be useful in controlling disease severity. These include:
Hyper-inflammation; SARS-CoV-2 infection is associated with hyper-inflammatory status, including excessive inflammatory cell infiltration, inflammasome activation and the “cytokine storms” (131). Intravenous administration of fish oil emulsions has been proposed as a parenteral supplementation for critically ill COVID-19 patients, because the high content of EPA and DHA (precursors of SPMs) could help to reduce cytokine storms (132, 133). In this context, SPMs limit neutrophil infiltration and reduce pro-inflammatory cytokines in lungs and other organs during bacterial and viral infections (38; see “bacterial infection” above). In addition, select SPM including RvTs, RvD2 and MaR1 block inflammasome components leading to reduced IL-1b release (28, 134, 135). Thus, SPM may be useful in controlling cytokine storms in COVID-19 patients as proposed in (3, 14).
NETs, venous thrombosis and hypercoagulation; aberrant NETosis, i.e. the process of neutrophil extracellular trap (NET) formation (Figure 4), is observed in many patients with COVID-19, which may lead to acute respiratory distress syndrome (ARDS) (126, 130). As demonstrated in deep vein thrombosis model, RvD1, RvD4 and MaR1 reduce venous thrombus burden and RvD4 decreases the release of NETs, i.e. NETosis (81). Also, RvD1 inhibits markers of NETosis in a murine model of aortic aneurysm (136). RvE4 stimulates efferocytosis of senescent erythrocytes in hemorrhagic exudates in hypoxic conditions (49), such as those in COVID-19 patients (137).
ARDS; the pathology of ARDS has been associated with severe COVID-19 patients, possibly triggered by NETosis and cytokine storm (126–128). In this regard, SPMs are potent regulators of acute lung injury including ARDS, reducing leukocyte accumulation in lungs and protecting from lung damage (138).
Neurological disorder; COVID-19 can cause neurological disorders, including stroke, blood-brain barrier disruption, and endothelial cell damage in the brain (129). In this regard, NPD1 and 17R-NPD1 protect from ischemic stroke (12, 86, 87); RvD1 and PD1 attenuate inflammation-induced blood-brain barrier dysfunction (22).
LM including SPMs were identified in serums from COVID-19 patients (139). There is distinct separation between SPMs and prostanoids (prostaglandins and thromboxane), and SPMs change during disease courses. For example, RvE3 is reduced in severe compared to moderate COVID-19 patients (139). Treatment of COVID-19 patients with dexamethasone lowered 28-day mortality among those who received respiratory support (140). Along these lines, in murine allergic airway inflammation, dexamethasone increases SPMs, particularly protectins (141). Therefore, it is possible that targeting SPM pathways and metabolomes (Figure 1) could provide a novel therapeutic approach for treating SARS-CoV-2 infections (Table 4).
Summary Points.
Resolution of inflammation is an active biosynthetic process that connects the first response of the innate immune system to biosynthesis of the specialized proresolving mediators (SPMs) including resolvins, protectins and maresins, as well as novel cys-SPMs.
Mass spectrometry-based profiling approaches for the resolution metabolome have documented the temporal production of SPMs in humans and preclinical animal systems. Today, hundreds of independent investigators confirm and extend the potent actions of each of the SPM pathway bioactive metabolomes (e.g. resolvins, maresins, lipoxins, (neuro)protectins and cys-SPMs).
SPMs evoke proresolving responses via activating their G protein-coupled receptors and downstream intracellular mechanisms in the concentrations at which they are produced locally.
The structural elucidation and complete stereochemical assignments of each SPM enable confirmation of their potent actions in controlling inflammatory response, promoting resolution and tissue repair, thus opening the opportunity for studying resolution physiology and pharmacology.
The goal of resolution pharmacology is to stimulate the host innate response to accelerate microbial clearance, limit collateral tissue damage and stimulate tissue regeneration. This might be achieved in part via personalized profiling of resolution metabolomes and following specific SPM treatment to provide precision medicine and nutrition support.
Acknowledgements
We thank Mary H. Small for expert assistance in manuscript preparation. We also thank our coauthors from the original studies reviewed here, as well as our colleagues for their original contributions. CNS’s research is supported by National Institutes of Health USA (grant numbers R01GM38765 and P01GM095467).
Footnotes
Delivered in a recent lecture in honor of the 75th Anniversary of Instituto de Biofísica Carlos Chagas Filho da Universidade Federal do Rio de Janeiro, Brazil June 10th, 2020 https://www.youtube.com/watch?v=nNjwnWYUEO4
Competing interests:
The authors declare no competing financial interest.
References
- 1.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serhan CN. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol Aspects Med. 2017;58:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panigrahy D, Gilligan MM, Huang S, Gartung A, Cortés-Puch I, Sime PJ, et al. Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128:2657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CR, Zeldin DC. Resolvin Infectious Inflammation by Targeting the Host Response. N Engl J Med. 2015;373(22):2183–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotran RS, Kumar V, Collins T, editors. Robbins Pathologic Basis of Disease. 6th ed. Philadelphia: W.B. Saunders Co.; 1999. [Google Scholar]
- 8.Dakin SG, Coles M, Sherlock JP, Powrie F, Carr AJ, Buckley CD. Pathogenic stromal cells as therapeutic targets in joint inflammation. Nat Rev Rheumatol. 2018;14(12):714–26. [DOI] [PubMed] [Google Scholar]
- 9.Doran AC, Yurdagul A Jr., Tabas I. Efferocytosis in health and disease. Nat Rev Immunol. 2020;20:254–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LAJ, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551–67. [DOI] [PubMed] [Google Scholar]
- 12.Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta. 2015;1851:397–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Dyke TE. Shifting the paradigm from inhibitors of inflammation to resolvers of inflammation in periodontitis. J Periodontol. 2020:doi: 10.1002/jper.20-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorokin AV, Karathanasis SK, Yang ZH, Freeman L, Kotani K, Remaley AT. COVID-19 associated dyslipidemia: implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 2020:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei J, Gronert K. The role of pro-resolving lipid mediators in ocular diseases. Mol Aspects Med. 2017;58:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredman G, Spite M. Specialized pro-resolving mediators in cardiovascular diseases. Mol Aspects Med. 2017;58:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perretti M, Cooper D, Dalli J, Norling LV. Immune resolution mechanisms in inflammatory arthritis. Nat Rev Rheumatol. 2017;13(2):87–99. [DOI] [PubMed] [Google Scholar]
- 19.Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin Immunol. 2016;28(2):137–45. [DOI] [PubMed] [Google Scholar]
- 20.Souza PR, Marques RM, Gomez EA, Colas RA, De Matteis R, Zak A, et al. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study. Circ Res. 2020;126:75–90. [DOI] [PubMed] [Google Scholar]
- 21.Colas RA, Nhat LTH, Thuong NTT, Gomez EA, Ly L, Thanh HH, et al. Proresolving mediator profiles in cerebrospinal fluid are linked with disease severity and outcome in adults with tuberculous meningitis. FASEB J. 2019;33:13028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kooij G, Derada Troletti C, Leuti A, Norris PC, Riley I, Albanese M, et al. Specialized pro-resolving lipid mediators are differentially altered in peripheral blood of patients with multiple sclerosis and attenuate monocyte and blood-brain barrier dysfunction. Haematologica. 2019:doi: 10.3324/haematol.2019.219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sano Y, Toyoshima S, Miki Y, Taketomi Y, Ito M, Lee H, et al. Activation of inflammation and resolution pathways of lipid mediators in synovial fluid from patients with severe rheumatoid arthritis compared with severe osteoarthritis. Asia Pac Allergy. 2020;10(2):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de la Rosa X, Norris PC, Chiang N, Rodriguez AR, Spur BW, Serhan CN. Identification and complete stereochemical assignments of the new Resolvin Conjugates in Tissue Regeneration (RCTR) in human tissues that stimulate proresolving phagocyte functions and tissue regeneration. Am J Pathol. 2018;188:950–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serhan CN, Chiang N, Dalli J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol Aspects Med. 2018;64:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liening S, Romp E, Werz O, Scriba GKE, Garscha U. Liquid chromatography-coupled mass spectrometry analysis of glutathione conjugates of oxygenated polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2019;144:106350. [DOI] [PubMed] [Google Scholar]
- 27.Colas RA, Souza PR, Walker ME, Burton M, Zaslona Z, Curtis AM, et al. Impaired Production and Diurnal Regulation of Vascular RvDn-3 DPA Increase Systemic Inflammation and Cardiovascular Disease. Circ Res. 2018;122(6):855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalli J, Chiang N, Serhan CN. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat Med. 2015;21:1071–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motwani MP, Colas RA, George MJ, Flint JD, Dalli J, Richard-Loendt A, et al. Pro-resolving mediators promote resolution in a human skin model of UV-killed Escherichia coli-driven acute inflammation. JCI Insight. 2018;3:e94463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motwani MP, Bennett F, Norris PC, Maini AA, George MJ, Newson J, et al. Potent anti-inflammatory and pro-resolving effects of anabasum in a human model of self-resolving acute inflammation. Clin Pharmacol Ther. 2018;104:675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norris PC, Skulas-Ray AC, Riley I, Richter CK, Kris-Etherton PM, Jensen GL, et al. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: a methodological validation. Sci Rep. 2018;8(1):18050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barden A, Shinde S, Tsai IJ, Croft KD, Beilin LJ, Puddey IB, et al. Effect of weight loss on neutrophil resolvins in the metabolic syndrome. Prostaglandins Leukot Essent Fatty Acids. 2019;148:25–9. [DOI] [PubMed] [Google Scholar]
- 33.Serhan CN, De la Rosa X, Jouvene CC. Cutting Edge: Human vagus produces specialized pro-resolving mediators of inflammation with electrical stimulation reducing pro-inflammatory eicosanoids. J Immunol. 2018;201:3161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner M, Jordan PM, Romp E, Czapka A, Rao Z, Kretzer C, et al. Targeting biosynthetic networks of the proinflammatory and proresolving lipid metabolome. FASEB J. 2019;33(5):6140–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werz O, Gerstmeier J, Libreros S, De la Rosa X, Werner M, Norris PC, et al. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat Commun. 2018;9(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T, Xiang A, Peng T, Doran AC, Tracey KJ, Barnes BJ, et al. HMGB1-C1q complexes regulate macrophage function by switching between leukotriene and specialized proresolving mediator biosynthesis. Proc Natl Acad Sci U S A. 2019;116(46):23254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung SY, Werner M, Esposito L, Troisi F, Cantone V, Liening S, et al. Discovery of a benzenesulfonamide-based dual inhibitor of microsomal prostaglandin E(2) synthase-1 and 5-lipoxygenase that favorably modulates lipid mediator biosynthesis in inflammation. Eur J Med Chem. 2018;156:815–30. [DOI] [PubMed] [Google Scholar]
- 38.Arnardottir HH, Dalli J, Colas RA, Shinohara M, Serhan CN. Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines. J Immunol. 2014;193(8):4235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halade GV, Kain V, Dillion C, Beasley M, Dudenbostel T, Oparil S, et al. Race-based and sex-based differences in bioactive lipid mediators after myocardial infarction. ESC Heart Fail. 2020:doi: 10.1002/ehf2.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong Y, Lin M, Piao L, Li X, Yang F, Zhang J, et al. Aspirin enhances protective effect of fish oil against thrombosis and injury-induced vascular remodelling. Br J Pharmacol. 2015;172(23):5647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med. 2017;58:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laguna-Fernandez A, Checa A, Carracedo M, Artiach G, Petri MH, Baumgartner R, et al. ERV1/ChemR23 Signaling Protects Against Atherosclerosis by Modifying Oxidized Low-Density Lipoprotein Uptake and Phagocytosis in Macrophages. Circulation. 2018;138(16):1693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Artiach G, Carracedo M, Plunde O, Wheelock CE, Thul S, Sjövall P, et al. Omega-3 Polyunsaturated Fatty Acids Decrease Aortic Valve Disease through the Resolvin E1 and ChemR23 Axis. Circulation. 2020:doi: 10.1161/circulationaha.119.041868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kantarci A, Aytan N, Palaska I, Stephens D, Crabtree L, Benincasa C, et al. Combined administration of resolvin E1 and lipoxin A4 resolves inflammation in a murine model of Alzheimer’s disease. Exp Neurol. 2018;300:111–20. [DOI] [PubMed] [Google Scholar]
- 46.Sawada Y, Honda T, Nakamizo S, Otsuka A, Ogawa N, Kobayashi Y, et al. Resolvin E1 attenuates murine psoriatic dermatitis. Sci Rep. 2018;8(1):11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quiros M, Feier D, Birkl D, Agarwal R, Zhou DW, Garcia AJ, et al. Resolvin E1 is a pro-repair molecule that promotes intestinal epithelial wound healing. Proc Natl Acad Sci U S A. 2020:doi: 10.1073/pnas.1921335117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sulciner ML, Serhan CN, Gilligan MM, Mudge DK, Chang J, Gartung A, et al. Resolvins suppress tumor growth and enhance cancer therapy. J Exp Med. 2018;215(1):115–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norris PC, Libreros S, Serhan CN. Resolution metabolomes activated by hypoxic environment. Sci Adv. 2019;5:eaax4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, et al. Resolvin E2: Identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–202. [DOI] [PubMed] [Google Scholar]
- 51.Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J Biol Chem. 2012;287(13):10525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deyama S, Shimoda K, Ikeda H, Fukuda H, Shuto S, Minami M. Resolvin E3 attenuates lipopolysaccharide-induced depression-like behavior in mice. J Pharmacol Sci. 2018;138(1):86–8. [DOI] [PubMed] [Google Scholar]
- 53.Sato M, Aoki-Saito H, Fukuda H, Ikeda H, Koga Y, Yatomi M, et al. Resolvin E3 attenuates allergic airway inflammation via the interleukin-23-interleukin-17A pathway. FASEB J. 2019;33(11):12750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong S, Gronert K, Devchand P, Moussignac R-L, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–87. [DOI] [PubMed] [Google Scholar]
- 56.Lee JY, Han SH, Park MH, Song IS, Choi MK, Yu E, et al. N-AS-triggered SPMs are direct regulators of microglia in a model of Alzheimer’s disease. Nat Commun. 2020;11(1):2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilligan MM, Gartung A, Sulciner ML, Norris PC, Sukhatme VP, Bielenberg DR, et al. Aspirin-triggered proresolving mediators stimulate resolution in cancer. Proc Natl Acad Sci U S A. 2019;116(13):6292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rymut N, Heinz J, Sadhu S, Hosseini Z, Riley CO, Marinello M, et al. Resolvin D1 promotes efferocytosis in aging by limiting senescent cell-induced MerTK cleavage. FASEB J. 2020;34(1):597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun AR, Wu X, Liu B, Chen Y, Armitage CW, Kollipara A, et al. Pro-resolving lipid mediator ameliorates obesity induced osteoarthritis by regulating synovial macrophage polarisation. Sci Rep. 2019;9(1):426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krashia P, Cordella A, Nobili A, La Barbera L, Federici M, Leuti A, et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat Commun. 2019;10(1):3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matte A, Recchiuti A, Federti E, Koehl B, Mintz T, El Nemer W, et al. Resolution of sickle cell disease associated inflammation and tissue damage with 17R-Resolvin D1. Blood. 2019;133:252–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sekheri M, El Kebir D, Edner N, Filep JG. 15-Epi-LXA(4) and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation. Proc Natl Acad Sci U S A. 2020;117(14):7971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerlach BD, Marinello M, Heinz J, Rymut N, Sansbury BE, Riley CO, et al. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ. 2020;27:525–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kain V, Halade GV. Immune responsive resolvin D1 programs peritoneal macrophages and cardiac fibroblast phenotypes in diversified metabolic microenvironment. J Cell Physiol. 2019;234(4):3910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shan K, Feng N, Cui J, Wang S, Qu H, Fu G, et al. Resolvin D1 and D2 inhibit tumour growth and inflammation via modulating macrophage polarization. J Cell Mol Med. 2020:doi: 10.1111/jcmm.15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sansbury BE, Li X, Wong B, Patsalos A, Giannakis N, Zhang MJ, et al. Myeloid ALX/FPR2 regulates vascularization following tissue injury. Proc Natl Acad Sci U S A. 2020:doi: 10.1073/pnas.1918163117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luan H, Wang C, Sun J, Zhao L, Li L, Zhou B, et al. Resolvin D1 Protects Against Ischemia/Reperfusion-Induced Acute Kidney Injury by Increasing Treg Percentages via the ALX/FPR2 Pathway. Front Physiol. 2020;11:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang L, Qiu C, Yang L, Zhang Z, Zhang Q, Wang B, et al. GPR18 expression on PMNs as biomarker for outcome in patient with sepsis. Life Sci. 2019;217:49–56. [DOI] [PubMed] [Google Scholar]
- 69.Liu Y, Wang L, Lo KW, Lui VWY. Omics-wide quantitative B-cell infiltration analyses identify GPR18 for human cancer prognosis with superiority over CD20. Commun Biol. 2020;3(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hellmann J, Sansbury BE, Wong B, Li X, Singh M, Nuutila K, et al. Biosynthesis of D-Series Resolvins in Skin Provides Insights into their Role in Tissue Repair. J Invest Dermatol. 2018;138:2051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giannakis N, Sansbury BE, Patsalos A, Hays TT, Riley CO, Han X, et al. Dynamic changes to lipid mediators support transitions among macrophage subtypes during muscle regeneration. Nat Immunol. 2019;20(5):626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inoue Y, Liu YM, Otawara M, Chico Calero I, Stephanie Nam A, Yu YM, et al. Resolvin D2 Limits Secondary Tissue Necrosis After Burn Wounds in Rats. J Burn Care Res. 2018;39(3):423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siddiqui YD, Omori K, Ito T, Yamashiro K, Nakamura S, Okamoto K, et al. Resolvin D2 Induces Resolution of Periapical Inflammation and Promotes Healing of Periapical Lesions in Rat Periapical Periodontitis. Front Immunol. 2019;10:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Botten N, Hodges RR, Li D, Bair JA, Shatos MA, Utheim TP, et al. Resolvin D2 elevates cAMP to increase intracellular [Ca(2+)] and stimulate secretion from conjunctival goblet cells. FASEB J. 2019;33(7):8468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CYC, Chiang N, et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013;20:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sham HP, Walker KH, Abdulnour RE, Krishnamoorthy N, Douda DN, Norris PC, et al. 15-epi-Lipoxin A4, Resolvin D2, and Resolvin D3 Induce NF-kappaB Regulators in Bacterial Pneumonia. J Immunol. 2018;200(8):2757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panigrahy D, Gartung A, Yang J, Yang H, Gilligan MM, Sulciner ML, et al. Preoperative stimulation of resolution and inflammation blockade eradicates micrometastases. J Clin Invest. 2019;129(7):2964–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winkler JW, Orr SK, Dalli J, Cheng CY, Sanger JM, Chiang N, et al. Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci Rep. 2016;6:18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morita M, Kobayashi Y. Stereocontrolled Synthesis of Resolvin D4. J Org Chem. 2018;83(7):3906–14. [DOI] [PubMed] [Google Scholar]
- 80.Winkler JW, Libreros S, De La Rosa X, Sansbury BE, Norris PC, Chiang N, et al. Structural insights into Resolvin D4 actions and further metabolites via a new total organic synthesis and validation. J Leukoc Biol. 2018;103:995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cherpokova D, Jouvene CC, Libreros S, DeRoo E, Chu L, de la Rosa X, et al. Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood. 2019;134:1458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery TW, Schmidt BA, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo X, Gu Y, Tao X, Serhan CN, Ji RR. Resolvin D5 Inhibits Neuropathic and Inflammatory Pain in Male But Not Female Mice: Distinct Actions of D-Series Resolvins in Chemotherapy-Induced Peripheral Neuropathy. Front Pharmacol. 2019;10:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–59. [DOI] [PubMed] [Google Scholar]
- 85.Berg RWV, Davidsson J, Lidin E, Angéria M, Risling M, Günther M. Brain tissue saving effects by single-dose intralesional administration of Neuroprotectin D1 on experimental focal penetrating brain injury in rats. J Clin Neurosci. 2019;64:227–33. [DOI] [PubMed] [Google Scholar]
- 86.Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, et al. Novel proresolving aspirin-triggered DHA pathway. Chem Biol. 2011;18:976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bazan NG, Eady TN, Khoutorova L, Atkins KD, Hong S, Lu Y, et al. Novel aspirin-triggered neuroprotectin D1 attenuates cerebral ischemic injury after experimental stroke. Exp Neurol. 2012;236(1):122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, et al. Maresins: novel macrophage mediators with potent anti-inflammatory and pro-resolving actions. J Exp Med. 2009;206:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reddoch-Cardenas KM, Sharma U, Salgado CL, Cantu C, Darlington DN, Pidcoke HF, et al. Use of Specialized Pro-Resolving Mediators to Alleviate Cold Platelet Storage Lesion. Transfusion (Paris). 2020;60 Suppl 3:S112–s8. [DOI] [PubMed] [Google Scholar]
- 90.Francos-Quijorna I, Santos-Nogueira E, Gronert K, Sullivan AB, Kopp MA, Brommer B, et al. Maresin 1 Promotes Inflammatory Resolution, Neuroprotection, and Functional Neurological Recovery After Spinal Cord Injury. J Neurosci. 2017;37(48):11731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang T, Xu G, Newton PT, Chagin AS, Mkrtchian S, Carlstrom M, et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br J Anaesth. 2019;122(3):350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fattori V, Pinho-Ribeiro FA, Staurengo-Ferrari L, Borghi SM, Rossaneis AC, Casagrande R, et al. The specialised pro-resolving lipid mediator maresin 1 reduces inflammatory pain with a long-lasting analgesic effect. Br J Pharmacol. 2019;176(11):1728–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang CW, Yu SH, Fretwurst T, Larsson L, Sugai JV, Oh J, et al. Maresin 1 Promotes Wound Healing and Socket Bone Regeneration for Alveolar Ridge Preservation. J Dent Res. 2020;99(8):930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saito-Sasaki N, Sawada Y, Mashima E, Yamaguchi T, Ohmori S, Yoshioka H, et al. Maresin-1 suppresses imiquimod-induced skin inflammation by regulating IL-23 receptor expression. Sci Rep. 2018;8(1):5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cezar TLC, Martinez RM, Rocha C, Melo CPB, Vale DL, Borghi SM, et al. Treatment with maresin 1, a docosahexaenoic acid-derived pro-resolution lipid, protects skin from inflammation and oxidative stress caused by UVB irradiation. Sci Rep. 2019;9:3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chiang N, Libreros S, Norris PC, de la Rosa X, Serhan CN. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J Clin Invest. 2019: doi: 10.1172/jci129448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han YH, Shin KO, Kim JY, Khadka DB, Kim HJ, Lee YM, et al. A maresin 1/RORalpha/12-lipoxygenase autoregulatory circuit prevents inflammation and progression of nonalcoholic steatohepatitis. J Clin Invest. 2019;130:1684–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dalli J, Chiang N, Serhan CN. Identification of sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc Natl Acad Sci USA. 2014;111:E4753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chiang N, Riley IR, Dalli J, Rodriguez AR, Spur BW, Serhan CN. New maresin conjugates in tissue regeneration pathway counters leukotriene D4-stimulated vascular responses. FASEB J. 2018;32(7):4043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Levy BD, Abdulnour RE, Tavares A, Brüggemann TR, Norris PC, Bai Y, et al. Cysteinyl maresins regulate the prophlogistic lung actions of cysteinyl leukotrienes. J Allergy Clin Immunol. 2020;145(1):335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Godson C. Balancing the Effect of Leukotrienes in Asthma. N Engl J Med. 2020;382(15):1472–5. [DOI] [PubMed] [Google Scholar]
- 102.Li H, Hao Y, Yang LL, Wang XY, Li XY, Bhandari S, et al. MCTR1 alleviates lipopolysaccharide-induced acute lung injury by protecting lung endothelial glycocalyx. J Cell Physiol. 2020:doi: 10.1002/jcp.29628. [DOI] [PubMed] [Google Scholar]
- 103.Zhang PH, Han J, Cao F, Liu YJ, Tian C, Wu CH, et al. PCTR1 improves pulmonary edema fluid clearance through activating the sodium channel and lymphatic drainage in lipopolysaccharide-induced ARDS. J Cell Physiol. 2020:doi: 10.1002/jcp.29758. [DOI] [PubMed] [Google Scholar]
- 104.Dalli J, Colas RA, Serhan CN. Novel n-3 immunoresolvents: structures and actions. Sci Rep. 2013;3:1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Primdahl KG, Aursnes M, Walker ME, Colas RA, Serhan CN, Dalli J, et al. Synthesis of 13(R)-Hydroxy-7Z,10Z,13R,14E,16Z,19Z Docosapentaenoic Acid (13R-HDPA) and Its Biosynthetic Conversion to the 13-Series Resolvins. J Nat Prod. 2016:doi: 10.1021/acs.jnatprod.6b00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vik A, Dalli J, Hansen TV. Recent advances in the chemistry and biology of anti-inflammatory and specialized pro-resolving mediators biosynthesized from n-3 docosapentaenoic acid. Bioorg Med Chem Lett. 2017;27(11):2259–66. [DOI] [PubMed] [Google Scholar]
- 107.Walker ME, Souza PR, Colas RA, Dalli J. 13-Series resolvins mediate the leukocyte-platelet actions of atorvastatin and pravastatin in inflammatory arthritis. FASEB J. 2017;31(8):3636–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gobbetti T, Dalli J, Colas RA, Federici Canova D, Aursnes M, Bonnet D, et al. Protectin D1n-3 DPA and resolvin D5n-3 DPA are effectors of intestinal protection. Proc Natl Acad Sci U S A. 2017;114(15):3963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frigerio F, Pasqualini G, Craparotta I, Marchini S, van Vliet EA, Foerch P, et al. n-3 Docosapentaenoic acid-derived protectin D1 promotes resolution of neuroinflammation and arrests epileptogenesis. Brain. 2018;141(11):3130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Flak MB, Colas RA, Munoz-Atienza E, Curtis MA, Dalli J, Pitzalis C. Inflammatory arthritis disrupts gut resolution mechanisms, promoting barrier breakdown by Porphyromonas gingivalis. JCI Insight. 2019;4(13):e125191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tourki B, Kain V, Shaikh SR, Leroy X, Serhan CN, Halade GV. Deficit of resolution receptor magnifies inflammatory leukocyte directed cardiorenal and endothelial dysfunction with signs of cardiomyopathy of obesity. FASEB J. 2020:doi: 10.1096/fj.202000495RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pirault J, Back M. Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front Pharmacol. 2018;9:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji RR. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest. 2018;128(8):3568–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Flak MB, Koenis DS, Sobrino A, Smith J, Pistorius K, Palmas F, et al. GPR101 mediates the pro-resolving actions of RvD5n-3 DPA in arthritis and infections. J Clin Invest. 2020;130:359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arita M, Oh S, Chonan T, Hong S, Elangovan S, Sun Y-P, et al. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J Biol Chem. 2006;281:22847–54. [DOI] [PubMed] [Google Scholar]
- 116.Orr SK, Colas RA, Dalli J, Chiang N, Serhan CN. Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent. Am J Physiol Lung Cell Mol Physiol. 2015;308(9):L904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chiang N, Barnaeva E, Hu X, Marugan J, Southall N, Ferrer M, et al. Identification of Chemotype Agonists for Human Resolvin D1 Receptor DRV1 with Pro-Resolving Functions. Cell Chem Biol. 2019;26(2):244–54.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Murakami Y, Fukuda H, Muromoto R, Hirashima K, Ishimura K, Fujiwara K, et al. Design and Synthesis of Benzene Congeners of Resolvin E2, a Proresolving Lipid Mediator, as Its Stable Equivalents. ACS Med Chem Lett. 2020;11(4):479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ruiz A, Sarabia C, Torres M, Juarez E. Resolvin D1 (RvD1) and maresin 1 (Mar1) contribute to human macrophage control of M. tuberculosis infection while resolving inflammation. Int Immunopharmacol. 2019;74:105694. [DOI] [PubMed] [Google Scholar]
- 120.Isopi E, Mattoscio D, Codagnone M, Mari VC, Lamolinara A, Patruno S, et al. Resolvin D1 Reduces Lung Infection and Inflammation Activating Resolution in Cystic Fibrosis. Front Immunol. 2020;11:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ogata H, Teixeira MM, Sousa RC, Silva MV, Correia D, Rodrigues Junior V, et al. Effects of aspirin-triggered resolvin D1 on peripheral blood mononuclear cells from patients with Chagas’ heart disease. Eur J Pharmacol. 2016;777:26–32. [DOI] [PubMed] [Google Scholar]
- 122.Horta AL, Williams T, Han B, Ma Y, Menezes APJ, Tu V, et al. Resolvin D1 Administration Is Beneficial in Trypanosoma cruzi Infection. Infect Immun. 2020;88(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–25. [DOI] [PubMed] [Google Scholar]
- 124.Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, Serhan CN, et al. The Specialized Proresolving Mediator 17-HDHA Enhances the Antibody-Mediated Immune Response against Influenza Virus: A New Class of Adjuvant? J Immunol. 2014;193:6031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang H, Anthony D, Yatmaz S, Wijburg O, Satzke C, Levy B, et al. Aspirin-triggered resolvin D1 reduces pneumococcal lung infection and inflammation in a viral and bacterial coinfection pneumonia model. Clin Sci (Lond). 2017;131(18):2347–62. [DOI] [PubMed] [Google Scholar]
- 126.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hariri L, Hardin CC. Covid-19, Angiogenesis, and ARDS Endotypes. N Engl J Med. 2020;383(2):182–3. [DOI] [PubMed] [Google Scholar]
- 129.Kempuraj D, Selvakumar GP, Ahmed ME, Raikwar SP, Thangavel R, Khan A, et al. COVID-19, Mast Cells, Cytokine Storm, Psychological Stress, and Neuroinflammation. Neuroscientist. 2020:1073858420941476. [DOI] [PubMed] [Google Scholar]
- 130.Nathan C. Neutrophils and COVID-19: Nots, NETs, and knots. J Exp Med. 2020;217(9):doi: 10.1084/jem.20201439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bistrian BR. Parenteral Fish-Oil Emulsions in Critically Ill COVID-19 Emulsions. JPEN J Parenter Enteral Nutr. 2020:doi: 10.1002/jpen.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Torrinhas RS, Calder PC, Waitzberg DL. Letter to the Editor in relation to Bistrian BR. Parenteral fish oil emulsions in critically ill COVID-19 emulsions [published online ahead of print, 2020 May 8]. JPEN J Parenter Enteral Nutr. 2020; 10.1002/jpen.1871. JPEN J Parenter Enteral Nutr. 2020:doi: 10.1002/jpen.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang YH, Li Y, Wang JN, Zhao QX, Wen S, Wang SC, et al. A Novel Mechanism of Specialized Proresolving Lipid Mediators Mitigating Radicular Pain: The Negative Interaction with NLRP3 Inflammasome. Neurochem Res. 2020;45(8):1860–9. [DOI] [PubMed] [Google Scholar]
- 135.Lopategi A, Flores-Costa R, Rius B, Lopez-Vicario C, Alcaraz-Quiles J, Titos E, et al. Frontline Science: Specialized proresolving lipid mediators inhibit the priming and activation of the macrophage NLRP3 inflammasome. J Leukoc Biol. 2019;105:25–36. [DOI] [PubMed] [Google Scholar]
- 136.Spinosa M, Su G, Salmon MD, Lu G, Cullen JM, Fashandi AZ, et al. Resolvin D1 decreases abdominal aortic aneurysm formation by inhibiting NETosis in a mouse model. J Vasc Surg. 2018;68(6s):93s–103s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leyfman Y, Erick TK, Reddy SS, Galwankar S, Nanayakkara PWB, Di Somma S, et al. Potential Immunotherapeutic Targets For Hypoxia Due to COVI-FLU. Shock. 2020. [DOI] [PubMed] [Google Scholar]
- 138.Duvall MG, Bruggemann TR, Levy BD. Bronchoprotective mechanisms for specialized pro-resolving mediators in the resolution of lung inflammation. Mol Aspects Med. 2017;58:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schwarz B, Sharma L, Roberts L, Peng X, Bermejo S, Leighton I, et al. Severe SARS-CoV-2 infection in humans is defined by a shift in the serum lipidome resulting in dysregulation of eicosanoid immune mediators. Preprint. medRxiv. 2020. July 13:doi: 10.1101/2020.07.09.20149849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020:doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pyrillou K, Chairakaki AD, Tamvakopoulos C, Andreakos E. Dexamethasone induces ω3-derived immunoresolvents driving resolution of allergic airway inflammation. J Allergy Clin Immunol. 2018;142(2):691–5.e4. [DOI] [PubMed] [Google Scholar]
- 142.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rodriguez AR, Spur BW. First total syntheses of the pro-resolving lipid mediators 7(S),13(R),20(S)-Resolvin T1 and 7(S),13(R)-Resolvin T4. Tetrahedron Lett. 2020;61:151473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shivakoti R, Dalli J, Kadam D, Gaikwad S, Barthwal M, Colas RA, et al. Lipid mediators of inflammation and Resolution in individuals with tuberculosis and tuberculosis-Diabetes. Prostaglandins Other Lipid Mediat. 2020;147:106398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.López-Vicario C, Titos E, Walker ME, Alcaraz-Quiles J, Casulleras M, Durán-Güell M, et al. Leukocytes from obese individuals exhibit an impaired SPM signature. FASEB J. 2019;33(6):7072–83. [DOI] [PubMed] [Google Scholar]
- 146.Barden AE, Shinde S, Burke V, Puddey IB, Beilin LJ, Irish AB, et al. The effect of n-3 fatty acids and coenzyme Q10 supplementation on neutrophil leukotrienes, mediators of inflammation resolution and myeloperoxidase in chronic kidney disease. Prostaglandins Other Lipid Mediat. 2018;136:1–8. [DOI] [PubMed] [Google Scholar]
- 147.See VHL, Mas E, Prescott SL, Beilin LJ, Burrows S, Barden AE, et al. Effects of prenatal n-3 fatty acid supplementation on offspring resolvins at birth and 12 years of age: a double-blind, randomised controlled clinical trial. Br J Nutr. 2017;118:971–80. [DOI] [PubMed] [Google Scholar]
- 148.Ramirez JL, Gasper WJ, Khetani SA, Zahner GJ, Hills NK, Mitchell PT, et al. Fish Oil Increases Specialized Pro-resolving Lipid Mediators in PAD (The OMEGA-PAD II Trial). J Surg Res. 2019;238:164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schaller MS, Chen M, Colas RA, Sorrentino TA, Lazar AA, Grenon SM, et al. Treatment with a marine oil supplement alters lipid mediators and leukocyte phenotype in healthy subjects and those with peripheral arterial disease. J Am Heart Assoc. 2020:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dawczynski C, Dittrich M, Neumann T, Goetze K, Welzel A, Oelzner P, et al. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin Nutr. 2018;37:494–504. [DOI] [PubMed] [Google Scholar]
- 151.Korner A, Schlegel M, Theurer J, Frohnmeyer H, Adolph M, Heijink M, et al. Resolution of inflammation and sepsis survival are improved by dietary Omega-3 fatty acids. Cell Death Differ. 2018;25(2):421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Videla LA, Vargas R, Valenzuela R, Muñoz P, Corbari A, Hernandez-Rodas MC. Combined administration of docosahexaenoic acid and thyroid hormone synergistically enhances rat liver levels of resolvins RvD1 and RvD2. Prostaglandins Leukot Essent Fatty Acids. 2019;140:42–6. [DOI] [PubMed] [Google Scholar]
- 153.Echeverría F, Valenzuela R, Espinosa A, Bustamante A, Álvarez D, Gonzalez-Mañan D, et al. Reduction of high-fat diet-induced liver proinflammatory state by eicosapentaenoic acid plus hydroxytyrosol supplementation: involvement of resolvins RvE1/2 and RvD1/2. J Nutr Biochem. 2019;63:35–43. [DOI] [PubMed] [Google Scholar]
- 154.Fussbroich D, Colas RA, Eickmeier O, Trischler J, Jerkic SP, Zimmermann K, et al. A combination of LCPUFA ameliorates airway inflammation in asthmatic mice by promoting pro-resolving effects and reducing adverse effects of EPA. Mucosal Immunol. 2020;13(3):481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Caputo MP, Radlowski EC, Lawson MA, Antonson AM, Watson JE, Matt SM, et al. Herring roe oil supplementation alters microglial cell gene expression and reduces peripheral inflammation after immune activation in a neonatal piglet model. Brain Behav Immun. 2019;81:455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mizraji G, Heyman O, Van Dyke TE, Wilensky A. Resolvin D2 Restrains Th1 Immunity and Prevents Alveolar Bone Loss in Murine Periodontitis. Front Immunol. 2018;9:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nguyen-Chi M, Luz-Crawford P, Balas L, Sipka T, Contreras-López R, Barthelaix A, et al. Pro-resolving mediator protectin D1 promotes epimorphic regeneration by controlling immune cell function in vertebrates. Br J Pharmacol. 2020:doi: 10.1111/bph.15156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang M, Lippestad M, Hodges RR, Fjærvoll HK, Fjærvoll KA, Bair JA, et al. RvE1 uses the LTB(4) receptor BLT1 to increase [Ca(2+)](i) and stimulate mucin secretion in cultured rat and human conjunctival goblet cells. Ocul Surf. 2020;18(3):470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mottola G, Chatterjee A, Wu B, Chen M, Conte MS. Aspirin-triggered resolvin D1 attenuates PDGF-induced vascular smooth muscle cell migration via the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) pathway. PLoS One. 2017;12(3):e0174936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron. 2018;100(6):1292–311. [DOI] [PMC free article] [PubMed] [Google Scholar]


