Abstract
Objective
To perform a simultaneous evaluation of potential risk/protective factors of Parkinson disease (PD) to identify independent risk/protective factors, to assess interaction among factors, and to determine whether identified risk factors predict etiologic subtypes of PD.
Methods
We designed a large case-control study assessing 31 protective/risk factors of PD, including environmental and lifestyle factors, comorbid conditions, and drugs. The study enrolled 694 patients with PD and 640 healthy controls from 6 neurologic centers. Data were analyzed by logistic regression models, additive interaction models, and cluster analysis.
Results
The simultaneous assessment of 31 putative risk/protective factors of PD showed that only coffee consumption (odds ratio [OR] 0.6; 95% confidence interval [CI] 0.4–0.9), smoking (OR 0.7, 95% CI 0.6–0.9), physical activity (OR 0.8, 95% CI 0.7–0.9), family history of PD (OR 3.2, 95% CI 2.2–4.8), dyspepsia (OR 1.8, 95% CI 1.3–2.4), and exposure to pesticides (OR 2.3, 95% CI1.3–4.2), oils (OR 5.6, 95% CI 2.3–13.7), metals (OR 2.8, 95% CI 1.5–5.4), and general anesthesia (OR 6.1, 95% CI 2.9–12.7) were independently associated with PD. There was no evidence of interaction among risk/protective factors, but cluster analysis identified 4 subtypes with different risk factor profiles. In group 1, all patients had a family history of PD, while dyspepsia or exposure to toxic agents was present in 30% of patients. In groups 2 and 3, a family history of PD was lacking, while exposure to toxic agents (group 2) and dyspepsia (group 3) played major roles. Group 4 consisted of patients with no risk factors.
Conclusions
This study demonstrated that 9 factors independently modify PD risk by coexisting in the same patient rather than interacting with others. Our study suggests the need for future preventive strategies aimed at reducing the coexistence of different risk factors within the same participant.
A number of previous studies have investigated potential risk and protective factors of Parkinson disease (PD). Although a few factors have been consistently found to reduce or increase PD risk, there is no agreement on most factors that have been investigated.1,2 This inconsistency may depend on several methodologic limitations affecting the epidemiologic plausibility of reported results. These limitations include a variable sample source (there may be a differential association of risk factors in different geographic regions), variable sample size affecting study power (a small sample size may not confidently exclude the contribution of factors unassociated with the outcome or significance bias due to small-study effects), and heterogeneity of the effect size (large differences may be due to differences in exposure assessment or the frequency of exposed control groups). Finally, the lack of simultaneous assessment of a wide range of factors in the same population precludes the assessment and definitive exclusion of potential confounding effects exerted by untested variables.
Although case-control studies may be limited by recall and cause-and-effect biases, the case-control design represents a powerful methodology to challenge most of the aforementioned methodologic issues in a timely manner. We therefore designed a large case-control study aimed at simultaneously evaluating several environmental and lifestyle factors, as well as comorbid conditions and drugs, that may be modifiable protective or risk factors of PD. The recruited population was large enough to provide satisfactory study power for the investigated factors and allow the simultaneous assessment of confounding factors among all study variables. With this approach, we aimed to enhance current knowledge of PD risk factors by identifying some factors as independent modifiable predictive factors of PD, measuring and comparing their relative effect size, and investigating whether risk/protective factors interact to modify PD risk. In addition, we aimed to evaluate the distribution of risk factors in patients with PD, thus identifying etiologic subtypes of PD as determined with a cluster analysis approach.
Methods
Cases were enrolled from among consecutive outpatients with PD who attended follow-up visits at the movement disorders clinic of 6 neurology departments from September 2018 to September 2019.
PD was diagnosed by senior neurologists who were experts in movement disorders according to published standard criteria.3,4 Patients with a diagnosis of monogenic PD were excluded. Healthy controls were recruited among from the relatives of neurologic outpatients without PD who visited participating outpatient neurology departments during the study period. Healthy controls were frequency-matched to cases by 5-year age stratum, sex, and referral center. Potential controls were excluded if they had received treatment with drugs known to induce parkinsonism or were related to a case involved in the study. Cases and controls were not informed of the study hypothesis.
Standard protocol approval, registration, and patient consent
The study received approval from the local ethics committee on human experimentation (Sapienza University of Rome Ethics Committee, No. 4734). Written informed consent was obtained from all patients participating in the study (consent for research).
Definition of variables
Potential risk factors investigated in the present study were chosen through a systematic database search on PubMed to identify the totality of controlled studies examining factors investigated as potential risk factors of PD. Articles were excluded from analysis for any of the following reasons: (1) they were in a language other than English; (2) the disease studied was not specifically designated as PD; or (3) the mentioned studies were uncontrolled. The search strategy used the key words “Parkinson's disease” and “risk factor” or “protective factor” or “environmental” or “education” or “occupation” or “smoking” or “coffee” or “physical activity” or “comorbidities” or “surgery” or “head trauma” or “infections” or “diabetes mellitus” or “hypertension” or “cancer” or “cataract” or “gout” or “nonsteroidal anti-inflammatory agents” (NSAIDs) or “solvents” or “pesticides” or “oils” or “metals” or “paints” or “toxic agents” or “rural living” or “agricultural activity” or “oral contraceptives” or “drugs”. We systematically evaluated each single controlled study examining associations of endogenous and exogenous factors with PD. The full text of potentially eligible articles was closely examined independently by 2 investigators (G.D., R.P). Factors potentially associated with PD according to current literature were included in a semistructured questionnaire (table 1).
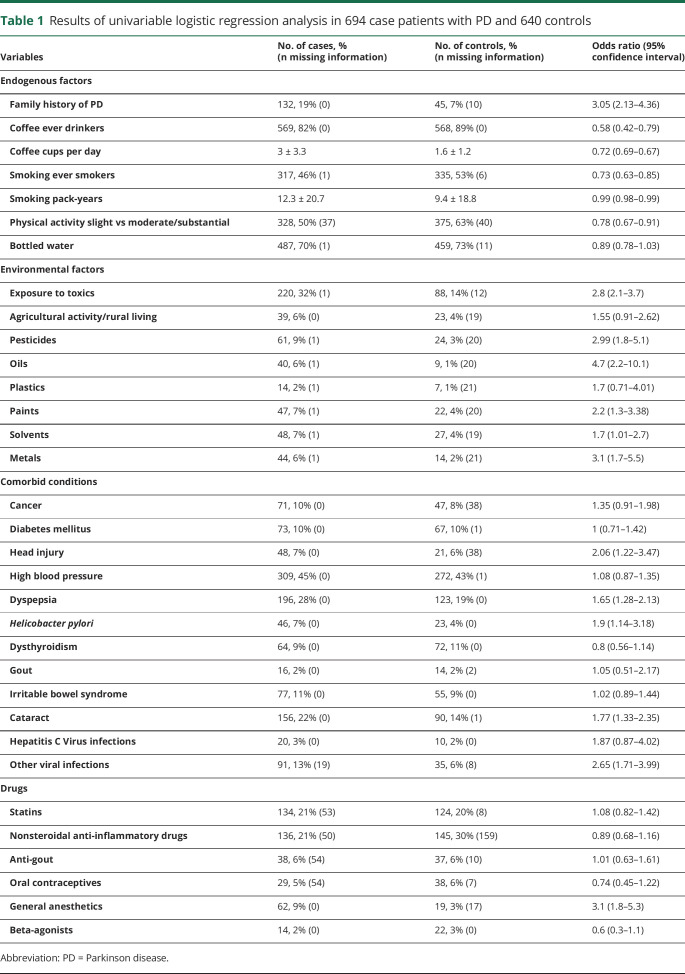
Table 1.
Results of univariable logistic regression analysis in 694 case patients with PD and 640 controls
Data collection
A semistructured questionnaire was administered to all cases and controls enrolled in the study. The questionnaire included information on demographic features, lifestyle factors, physical activity, comorbid conditions, and exposure to toxic substances and drugs previously suggested to be involved in PD development. The questionnaire was administered in person by a medical interviewer in each center. To maximize interpersonal and intrapersonal reliability, interviewers received training before the study. The questionnaire assessed the presence of possible risk/protective factors by interviewing patients about the entire life period preceding PD onset. Controls were interviewed about the same pre-exposure period as their matched cases.
The questionnaire first collected data on demographic features (age, sex, age at motor symptom onset, years of schooling) and family history of parkinsonism (first-degree relatives only). In history taking, we collected demographic (including age) and clinical information on all first-degree relatives. In most cases, participants were able to provide reliable information on first-degree relatives. First-degree relatives were considered to be affected by parkinsonism only if at least 1 other family member provided adequate information (medical report) or one of the investigators personally saw the secondary case. Moreover, participants were asked about their job according to the questions already used in a previous population case-control study.5 Lifestyle factors investigated included cigarette smoking, coffee consumption, bottled water drinking, and physical activity. Smoking and coffee habits were investigated according to a semistructured questionnaire already used in previous studies on dystonia.6 Questions on coffee consumption and cigarette smoking referred to the period preceding PD onset. Participants were classified as never having smoked or drunk (nonsmokers/nondrinkers). Participants were asked about their physical activity before developing PD. Seventeen physical activities were selected and converted into metabolic equivalents of task according to the Pate model.7,8
Previous head or facial trauma (with or without loss of consciousness) that was severe enough to require medical attention but no major surgery was assessed. Other comorbid conditions (including cancer, arterial hypertension, anemia, diabetes mellitus, gout, viral infections, dyspepsia, Helicobacter pylori infection, and dysthyroidism) were included in the questionnaire. To investigate the presence of dyspepsia, we asked participants whether they had postprandial fullness, early satiety, epigastric pain, or epigastric burning.9 Similarly, patients were asked about surgical procedures they underwent before PD diagnosis. Self-reported information on medical history was supported by medical records or detailed reports of specific treatments. Exposure to drugs. including NSAIDs, estrogen, oral contraceptives, statins, β2-adrenoreceptor agonists/antagonists. and general anesthesia, was assessed. Information on the type and timing of medication exposure was obtained through patient interviews and by examining medical reports. For each drug, we obtained information on the year of the first administration and the duration of use.
We also assessed occupational exposure to pesticides or processes involving oils, metals, solvents, and paints. According to a previous study,5 participants were asked about possible workplace exposure to any chemicals, including solvents, oils, plastics, paints, metals, or pesticides.
Factors that did not play a role as protective or risk factors according to previous studies were not assessed in the questionnaire. Moreover, factors that were considered premotor symptoms according to the Braak model10 such as depression, constipation, and REM sleep behavior disorder were not included. Premorbid personality traits, stressful events, magnetic fields, genetic factors, and biomarkers were also excluded because they are not clearly detectable with a semistructured questionnaire. Conversely, dietary factors were included in the questionnaire but were analyzed separately. Ethnicity was included in the questionnaire, although we did not analyze this factor because all participants were White and from Italy.
Data analysis
Data cleaning was performed before the data analysis considering both range and consistence checks. A standard statistical package (STATA 11; StataCorp, College Station, TX) was used for analyses. Data were expressed as a percentage or mean ± SD, and groups were compared by means of the χ2 and t tests and 1-way analysis of variance with the Newman-Keuls post hoc test as appropriate. The association of study variables with outcome was first evaluated by univariate regression analysis, and odds ratios (ORs), 2-sided 95% confidence intervals (95% CIs), and p values (likelihood ratio statistic) were calculated. Most exposure variables were represented in the model by a single indicator variable (1 if the participant was exposed; 0 if not); otherwise, variables were included in the models as continuous variables (age, years of schooling). Case and control records containing missing information were excluded from analysis. To check for type II error, the statistical power relative to each variable was assessed by the equation for a case-control study with an unequal case/control ratio.11
Multivariate analysis was performed after including all study variables in the initial model. As a rule of thumb, the recommended participant-to-variable ratio usually considers the assessment of 10 to 15 patient/control pairs for each assessed variable. Therefore, the recruitment target of >600 cases and controls allowed us to theoretically test >30 variables. After fitting the model containing all selected variables, we deleted the unimportant variables and fitted a new model. The importance of variables was assessed by examining the significance of each p value and comparing the estimated ORs from the new and old models.12 This process of deleting, refitting, and verifying continued until it yielded a model containing only essential variables (main-effects model). Estimates were adjusted for age, sex, years of education, and referral center. To exclude collinearity problems, we examined the correlation coefficient for each pair of selected independent variables. No correlation coefficient near +1 was found.
Thereafter, we tested interactions on the additive scale by estimating the relative excess risk due to interaction (RERI). We focused on additive interaction because this parameter is usually considered more suitable for case-control studies. To obtain the RERI and its 95% CI, we followed the STATA code to estimate additive interaction. Because the interpretation of additive interaction indices is appropriate only for risk factors, we reversed the coding associated with coffee, smoking, and physical activity before calculating the RERI.
Nonhierarchical (k-means) cluster analysis using the Jaccard method for categorical data was performed on patients with PD for 2-, 3-, and 4-cluster solutions.13,14 The variables considered for cluster analysis were selected from among those included in the main-effects model resulting from multivariate analysis and included family history of PD, dyspepsia, and exposure to toxic substances and general anesthesia. The Calinski/Harabasz pseudo-F index (stopping rule)14 was estimated to determine the optimal number of clusters: the higher the Calinski/Harabasz pseudo-F index value, the more distinct the clustering. To test the usefulness of the subgroup classification, we investigated any association between clusters with variables not included within the cluster analysis such as age, sex, age at disease onset, disease duration, and protective lifestyle factors such as coffee consumption, cigarette smoking, and physical activity. Post hoc comparisons of the subgroups generated by cluster analysis were then performed with either a between-group analysis of variance or unpaired t tests for continuous variables.
All statistical tests were 2 tailed, and a value of p < 0.05 was considered statistically significant.
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
A total of 694 patients with PD and 640 healthy controls met the eligibility criteria during the study period and agreed to take part in the study. Cases and controls were similar in terms of sex (407 men and 287 women vs 351 men and 289 women, p = 0.2) but differed significantly in terms of age (67.9 ± 9 years vs 64.7 ± 10.3 years, p < 0.01) and years of schooling (11.2 ± 4.9 vs 12.8 ± 4.7, p < 0.01).
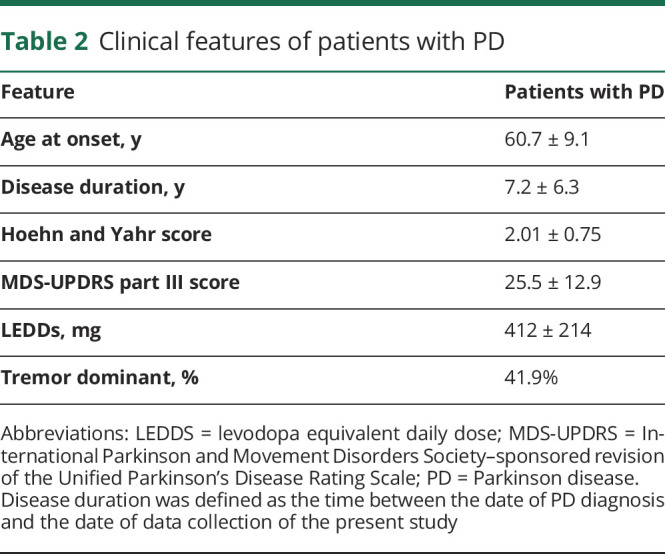
Clinical features of patients with PD are reported in table 2.
Table 2.
Clinical features of patients with PD
Univariate analysis
On logistic regression univariate analysis (table 1), family history of PD, head trauma, dyspepsia, and exposure to pesticides, oils, solvents, paints, metals, chemotherapy, and anesthesia were significantly associated with the outcome as risk factors. Lifestyle factors, including coffee consumption, cigarette smoking, and physical activity, yielded significant inverse associations with PD. Finally, no statistically significant association was found between PD and several comorbid conditions (table 1) or drugs such as NSAIDs, estrogen, oral contraceptives, statins, and calcium channel blockers (table 1). The present study had an estimated >95% chance of detecting a 2-fold modification of the risk of developing PD, with α = 0.05 (2 sided) for all variables that were not associated with PD on univariate analysis except exposure to plastic. However, exposure to plastic yielded satisfactory study power (80%) in detecting a 3-fold modification of the risk of developing PD, with α = 0.05 (2 sided).
Multivariate analysis
The initial model for multivariate analysis included all study variables. The main-effects model (table 3) included family history of PD, dyspepsia, and exposure to pesticides, oils, and metals as independent risk factors and coffee consumption, cigarette smoking, and physical activity as independent protective factors of PD.
Table 3.
Results of multivariable logistic regression analysis in 694 cases with PD and 640 controls (main-effects model)
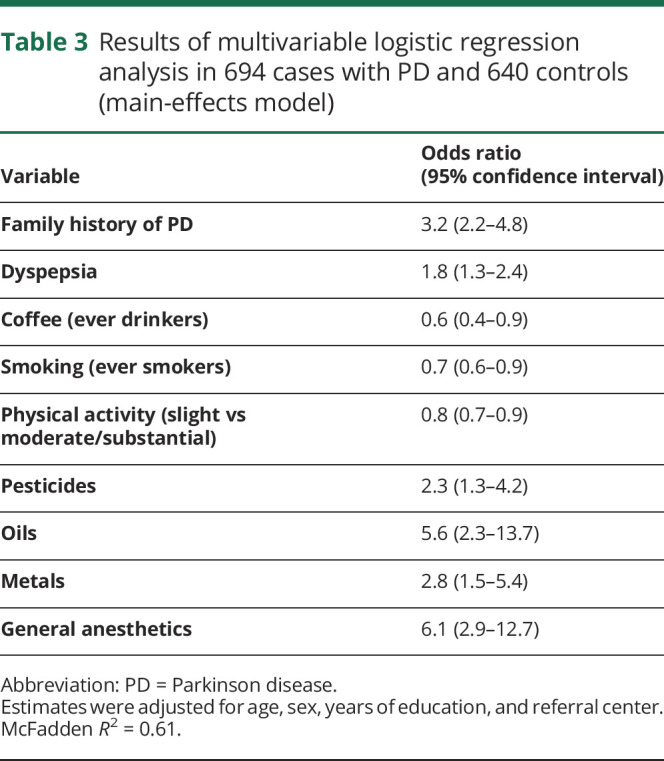
We also fitted another model that initially contained the overall variable exposure to toxins rather than separate variables on exposure to pesticides, oils, solvents, plastics, paints, and metals. In the resulting final model, the variable exposure to toxins yielded an OR of 2.4 (95% CI 1.8–3.2; p = 0.003), while the other estimates remained substantially unchanged (data not shown).
No relationship was found between the variables selected by multivariate analysis and the duration of PD as categorized into 2 levels: >2 and <2 years.
The estimates from the main effects model indicated that general anesthesia and exposure to oils contributed more to the risk of developing PD than other investigated factors, while family history had an intermediate strength.
Additive interaction
No statistically significant additive interaction emerged between family history of PD and any of the risk and protective factors selected by multivariate analysis. Likewise, interaction terms among the identified environmental risk and protective factors never attained significant RERI values (data not shown).
Cluster analysis in patients with PD
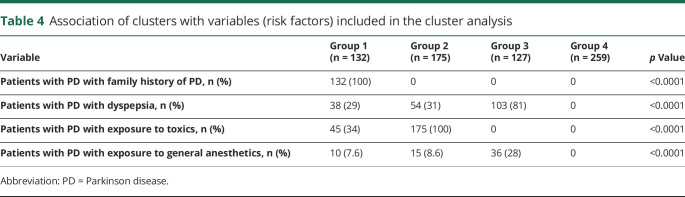
The variables included in generating the cluster solution were the identified risk factors of PD (family history of PD, dyspepsia, exposure to toxins, and general anesthesia). We considered models with 2 to 4 clusters. The Calinski/Harabasz pseudo-F index favored a 4-cluster solution (Calinski/Harabasz pseudo-F index: 2-cluster solution 177.33, 3-cluster solution 330.46, 4-cluster solution 411.15). When we performed k-means cluster analysis using the optimum number of clusters previously determined, the resulting 4 groups contained 132 patients (group 1), 175 patients (group 2), 127 patients (group 3), and 259 patients (group 4).
Regarding variables included in generating the cluster solution (table 4), all 132 patients in group 1 reported a family history of PD. These patients also reported dyspepsia (29%), toxin exposure (34%), and general anesthesia exposure (7.6%). In the remaining 3 groups, no patients reported a family history of PD. In group 2, toxin exposure was reported by all 175 patients, dyspepsia by 54 patients (31%), and general anesthesia exposure by 15 patients (8.6%). In group 3, dyspepsia was reported by 103 of 127 patients (81%) and general anesthesia exposure by 36 of 127 patients (28%), while no participant in this group reported toxin exposure. Finally, group 4 contained only patients who did not report any of the investigated factors.
Table 4.
Association of clusters with variables (risk factors) included in the cluster analysis
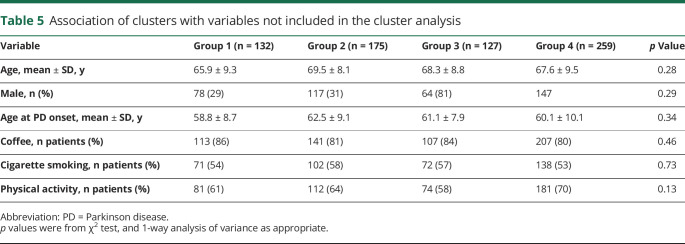
Regarding variables not included in the cluster analysis (table 5), no differences were observed between the 4 groups in terms of age, sex, age at PD onset, and frequency of lifestyle protective factors such as coffee consumption, cigarette smoking, and physical activity.
Table 5.
Association of clusters with variables not included in the cluster analysis
Discussion
The first result of our study was that the simultaneous assessment of 31 putative risk/protective factors of PD showed that family history of PD, dyspepsia, and exposure to pesticides, oils, metals, and general anesthesia were independent risk factors of PD, whereas coffee consumption, smoking, and physical activity were independent protective factors of PD. Conversely, our analysis did not assign any risk-modifying role to several factors, including bottled water drinking, agricultural activity/rural living, exposure to solvents and paints, cancer, diabetes mellitus, head injury, high blood pressure, dyspepsia, Helicobacter pylori, dysthyroidism, gout, irritable bowel syndrome, cataracts, hepatitis C virus infections, other viral infections, statins, NSAIDs, antigout drugs, oral contraceptives, β-agonists, and chemotherapy. Despite the large sample size, interaction analysis did not disclose significant interactions between any of the factors significantly associated with PD. Nevertheless, cluster analysis showed that multiple risk factors could be present in the same group of patients, even though a consistent proportion of patients lacked any risk factor.
This study has several strengths. Even if a possible selection bias due to the hospital-based design cannot be entirely ruled out, it is important to underline that the data obtained in our sample are consistent with data reported in the literature from population-based case-control studies and cohort studies. The sample size was large enough to provide satisfactory study power for all investigated variables, thus confidently supporting the lack of a risk-modifying role for factors excluded by the main-effects model. Recruited patients were relatively homogeneous in terms of geographic origin and ethnicity, which excluded the differential association of risk factors due to different geographic regions. In this regard, it is worth noting that estimates were also adjusted for recruiting center. The simultaneous assessment of 31 risk factors in the same sample by multivariate analysis allowed us to limit possible confounding due to the putative association of several factors with PD and to provide a relative estimation of the effect size. In addition, the results of multivariate analysis disclosed that the 9 factors significantly associated with PD had a level of significance higher than the standard 0.05, allowing us to exclude possible significance bias. Overall, these methodologic strengths support the validity of our findings.
The 9 factors found to be associated with PD have the biological plausibility to potentially prevent or induce PD development. The neuroprotective role of cigarette smoking and coffee consumption may be related to the marked effects exerted by nicotine and caffeine on the CNS, as suggested by animal models.1,2,15–17 Physical exercise has been associated with neuroprotective and neurorestorative effects in the nigrostriatal dopaminergic system in animals.18–20 The association between PD and toxic agents is consistent with animal models showing that the active metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, the structure of which is similar to that of the herbicide paraquat, is involved in the pathogenesis of a subacute form of parkinsonism.1,2 A growing body of evidence suggests that the prodromal phases of PD begin in the gut and are related to peripheral inflammatory/immune responses.20–22 Dyspepsia may reflect gut inflammation that triggers peripheral pathologic processes that lead to PD development. Alternatively, it is possible that dyspepsia represents a prodromal nonmotor symptom of the PD clinical spectrum and is part of the disease rather than an actual risk factor for PD. The relationship between family history of PD and PD development may depend on either a common genetic substrate that predisposes patients to PD development or a family aggregation that implies that members of the same family are exposed to the same environmental conditions. The association between PD and general anesthesia may reflect either a direct effect of these drugs on the CNS or a consequence of the stress of major surgery.
While 9 factors were found to be significantly associated with PD, we did not find any association between PD and the other factors investigated. Previous studies have reported conflicting results on most of the factors we examined; therefore, it is not surprising that we failed to find an association between these factors and PD. Conversely, a few factors that have been consistently reported to be risk factors of PD1,2 had no effect on PD development in our population. Because we obtained a sufficient power and sample size for each factor examined, we can exclude that the lack of association we found was a result of methodologic issues. It is more likely that this finding was dependent on our simultaneous assessment that allowed us to perform multivariate analysis to determine only the protective and risk factors that act independently on PD. For instance, previous case-control studies assessing only 1 or a few factors together have suggested that head injury is a risk factor for PD,22–24 but studies that performed multivariate analysis have excluded this hypothesis.25 Similarly, in our population, univariate analysis showed a significant association between PD and head injury, which was not confirmed with our large multivariate analysis.
A further result of the study was that the 9 risk/protective factors identified did not interact with each other. Although several authors have previously investigated the possible profile of risk/protective factors of PD, only a few previous studies have investigated the possible interactions between PD risk/protective factors. Recently, Kim et al.26 investigated possible interactions between protective factors of PD, including physical activity, coffee consumption, cigarette smoking, and family history of PD, and observed only 2 significant interactions (between family history of PD and caffeine consumption in men and between caffeine consumption and physical activity in women). We believe that the lack of significant interactions we found does not necessarily exclude the coexistence of different risk or protective factors, as supported by the results of cluster analysis.
A further result of our study was that cluster analysis showed 4 different subtypes of patients in our population. In group 1, all patients had a family history of PD, while both dyspepsia and exposure to toxic agents were present in 30% of participants. In groups 2 and 3, a family history of PD was not present as a risk factor, while exposure to toxic agents (group 2) and dyspepsia (group 3) played a major role. Finally, group 4 consisted of patients with no risk/protective factors. The 4 groups did not differ in terms of age, sex, and age at onset. In our patients, OR values demonstrate that the genetic substrate provided by a family history of PD had a lower risk power than environmental factors. Accordingly, group 1 demonstrates that to develop the disease, a family history of PD needs to be combined with other risk factors. This is in line with a recent study performed by Kim et al.,26 who suggested that the presence of multiple protective factors reduces the risk of PD independently from the interactions between factors. Similarly, we observed that the risk score in group 1 was due to the coexistence of different risk factors rather than to the interaction between them. In group 2 some environmental risk factors (toxic substances) and in group 3 some endogenous risk factors (dyspepsia) with a high-risk power may induce the disease without a relevant contribution of other factors. Group 4, which was the largest group identified by cluster analysis, included patients with no PD risk factors. For these patients, unknown genetic and environmental risk factors or random molecular events that have a certain probability of initiating a process of α-synuclein misfolding and prion-like propagation may intervene in determining PD without a genetic predisposition or toxic stimulus. The presence of 4 different etiologic PD subtypes suggests that what is lumped together as PD may result from different combinations of risk factors. In addition, the upstream pathogenesis of the disease process may differ among patients, with implications for presymptomatic testing, neuroprotective treatment, and behavior-based prevention.
A further interesting result of our study was that the distribution of protective factors (cigarette smoking, coffee consumption, and physical activity) was similar in the 4 groups of patients with PD identified by cluster analysis. This would suggest that protective factors exert their effects independently of PD etiology and perhaps through contrasting neurodegenerative processes. In line with this hypothesis, it has been observed that coffee consumption and physical activity also exert a protective role against Alzheimer disease development.27
The present study has some methodologic limitations. To enroll a large number of participants, we used consecutive nonrandom sampling, a cost- and time-effective selection method that may nevertheless introduce selection bias. However, the alternative random-selection method also has several limitations. Because this approach is expensive and time-consuming, it can also be affected by sample selection bias, especially in studies with a multicenter design. It must be noted, however, that the demographic and clinical features of our study population were similar to those of the general PD population, as shown in table 2. The case-control design may result in recall and cause-and-effect bias. In particular, because PD is a chronic long-lasting disease with a prodromal phase of prolonged duration, recall bias may be present. A further possible limitation of our study was that cases and controls differed in terms of age. This difference was <5 years and was therefore consistent with our objective to include cases and controls with a 5-year age stratum. To avoid a possible confounding effect of age on our results, multivariate analysis was adjusted for age. Finally, regarding the association between PD and comorbid conditions, it is important to point out that some of the clinical conditions examined in the study such as Helicobacter pylori may often be underdiagnosed, thus potentially influencing our results.
The present case-control study based on the simultaneous assessment of a large number of risk and protective factors of PD demonstrated that coffee consumption, smoking, physical activity, family history of PD, dyspepsia, and exposure to pesticides, oils, metals, and general anesthesia are associated with disease onset. Risk factors may increase PD risk by coexisting in the same participant rather than interacting with others, while protective factors act independently of the etiologic subtype. Our study suggests the need for future preventive strategies aimed at reducing the coexistence of different risk factors within the same participant.
Acknowledgment
The authors thank Melissa Kerr for English language editing.
Glossary
- CI
confidence interval
- NSAID
nonsteroidal anti-inflammatory drug
- OR
odds ratio
- PD
Parkinson disease
- RERI
relative excess risk due to interaction
Appendix. Authors
Footnotes
Editorial, page 805
CME Course: NPub.org/cmelist
Study funding
The study was supported by the “Accademia Italiana per lo Studio della Malattia di Parkinson e dei Disturbi del Movimento” (accademialimpedismov.it).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol 2016;15:1257–1272. [DOI] [PubMed] [Google Scholar]
- 2.Belvisi D, Pellicciari R, Fabbrini G, Tinazzi M, Berardelli A, Defazio G. Modifiable risk and protective factors in disease development, progression and clinical subtypes of Parkinson's disease: what do prospective studies suggest? Neurobiol Dis 2020;134:104671. [DOI] [PubMed] [Google Scholar]
- 3.Berardelli A, Wenning GK, Antonini A, et al. EFNS/MDS-ES/ENS recommendations for the diagnosis of Parkinson's disease. Eur J Neurol 2013;20:16–34. [DOI] [PubMed] [Google Scholar]
- 4.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591–1601. [DOI] [PubMed] [Google Scholar]
- 5.Rugbjerg K, Harris MA, Shen H, Marion SA, Tsui JKC, Teschke K. Pesticide exposure and risk of Parkinson's disease: a population-based case-control study evaluating the potential for recall bias. Scand J Work Environ Health 2011;37:427–436. [DOI] [PubMed] [Google Scholar]
- 6.Defazio G, Martino D, Abbruzzese G, et al. Influence of coffee drinking and cigarette smoking on the risk of primary late onset blepharospasm: evidence from a multicentre case control study. J Neurol Neurosurg Psychiatry 2007;78:877–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273:402–407. [DOI] [PubMed] [Google Scholar]
- 8.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of Physical Activities: an update of activity codes and MET intensities. Med Sci Sport Exerc 2000;32(suppl):S498–S504. [DOI] [PubMed] [Google Scholar]
- 9.Stanghellini V, Chan FKL, Hasler WL, et al. Gastroduodenal Disord 2016;150:1380–1392. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 11.Schlesselman JJ. Case Control Studies: Design, Conduct, Analysis. Oxford, UK:Oxford University Press Inc; 1982. [Google Scholar]
- 12.Hosmer DW, Lemeshaw S. Applied Logistic Regression. NewYork: John Wiley; 1989. [Google Scholar]
- 13.Everitt BS. Cluster Analysis. New York: Edward Arnold; 1993. [Google Scholar]
- 14.Everitt BS. Commentary: classification and cluster analysis. BMJ 1995;311:535–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagga P, Chugani AN, Patel AB. Neuroprotective effects of caffeine in MPTP model of Parkinson's disease: a (13)C NMR study. Neurochem Int 2016;92:25–34. [DOI] [PubMed] [Google Scholar]
- 16.Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson's disease. Mov Disord 2012;27:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quik M, Bordia T, Zhang D, Perez XA. Nicotine and nicotinic receptor drugs: potential for Parkinson's disease and drug-induced movement disorders. Int Rev Neurobiol 2015;124:247–271. [DOI] [PubMed] [Google Scholar]
- 18.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 2011;77:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petzinger G, Fisher B, McEwen S, Beeler S, Walsh J, Jakowec M. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol 2013;12:716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers 2017;3:17013. [DOI] [PubMed] [Google Scholar]
- 21.Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson's disease pathogenesis? NPJ Parkinsons Dis 2017;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocha NP, de Miranda AS, Teixeira AL. Insights into neuroinflammation in Parkinson's disease: from biomarkers to anti-inflammatory based therapies. Biomed Res Int 2015;2015:628192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Factor SA, Weiner WJ. Prior history of head trauma in Parkinson's disease. Mov Disord 1991;6:225–229. [DOI] [PubMed] [Google Scholar]
- 24.Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson's disease after hospital contact for head injury: population based case-control study. BMJ 2008;337:a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai SW, Liao KF, Lin CL, Lin CC, Sung FC. Hearing loss may be a non-motor feature of Parkinson's disease in older people in Taiwan. Eur J Neurol 2014;21:752–757. [DOI] [PubMed] [Google Scholar]
- 26.Kim IY, O'Reilly ÉJ, Hughes KC, et al. Integration of risk factors for Parkinson disease in 2 large longitudinal cohorts. Neurology 2018;90:e1646–e1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva MVF, Loures CMG, Alves LCV, de Souza LC, Borges KBG, Carvalho MDG. Alzheimer's disease: risk factors and potentially protective measures. J Biomed Sci 2019;26:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.