Abstract
The single-cell green alga Chlamydomonas reinhardtii possesses two α-tubulin genes (tua1 and tua2) and two β-tubulin genes (tub1 and tub2), with the two genes in each pair encoding identical amino acid sequences. Here, we screened an insertional library to establish eight disruptants with defective tua2, tub1, or tub2 expression. Most of the disruptants did not exhibit major defects in cell growth, flagellar length, or flagellar regeneration after amputation. Because few tubulin mutants of C. reinhardtii have been reported to date, we then used our disruptants, together with a tua1 disruptant obtained from the Chlamydomonas Library Project (CLiP), to isolate tubulin-mutants resistant to the anti-tubulin agents propyzamide (pronamide) or oryzalin. As a result of several trials, we obtained 8 strains bearing 7 different α-tubulin mutations and 12 strains bearing 7 different β-tubulin mutations. One of the mutations is at a residue similar to that of a mutation site known to confer drug resistance in human cancer cells. Some strains had the same amino acid substitutions as those reported previously in C. reinhardtii; however, the mutants with single tubulin genes showed slightly stronger drug-resistance than the previous mutants that express the mutated tubulin in addition to the wild-type tubulin. Such increased drug-resistance may have facilitated sensitive detection of tubulin mutation. Single-tubulin-gene disruptants are thus an efficient background of generating tubulin mutants for the study of the structure–function relationship of tubulin.
Introduction
Microtubules are fundamental cytoskeletal filaments that play pivotal roles in eukaryotic cell functions such as cell division, intra-cellular transport, cell shape development, and cilia and flagella assembly. Microtubules are produced by polymerization of α/β-tubulin heterodimers. Most eukaryotic cells possess multiple genes encoding α- and β-tubulin. For example, humans possess seven genes that encode α-tubulin and eight genes that encode β-tubulin, with each gene encoding a slightly different amino acid sequence. The presence of multiple genes for the two types of tubulin makes it difficult to study the properties of a particular tubulin species by genetic analysis, because the effects arising from mutation of one of the genes can be masked by the expression of the remaining intact genes.
The single-cell green alga Chlamydomonas reinhardtii is a useful experimental organism for studying tubulin function because it possesses a small number of tubulin genes and it produces microtubule-based organelles, flagella. In addition, there is a wide range of genetic tools available and a large amount of biological data has been accumulated for this species. In contrast to the majority of eukaryotes, C. reinhardtii possesses only two genes (tua1 and tua2) encoding α-tubulin and two genes (tub1 and tub2) encoding β-tubulin [1, 2]. The two genes for each type of tubulin encode the same amino acid sequence [2, 3], and the expression of all four genes is up-regulated after flagellar excision [4]. Whether the two genes in each pair are expressed independently of each other has not yet been firmly established, but the genes do appear to be similarly expressed during flagella formation [4].
Although C. reinhardtii possess only two genes for each tubulin, the presence of more than one gene expressing the same protein still makes it difficult to isolate tubulin mutants. To date, only five tubulin mutations have been reported: a tua1 mutation (Y24H) that confers amiprophos-methyl (APM) and oryzalin resistance (upA12) [3]; two kinds of mutations in tua2 (D205N and A208T) that confer colchicine hypersensitivity (tua2-1 etc., suppressors of uni-3-1, a mutant lacking δ-tubulin) [5]; and two mutations in tub2 (K350E and K350M) that confer colchicine resistance (colR4 and colR15) [6]. APM, oryzalin, colchicine, and propyzamide (also known as pronamide) are compounds that inhibit tubulin polymerization. These compounds other than colchicine inhibit plant tubulin polymerization at low concentrations and are used as herbicides.
Here, we isolated eight tua2, tub1, or tub2 disruptants from an insertional library comprising around 8000 clones prepared based on ref [7]. We also obtained a tua1 disruptant from the Chlamydomonas Library Project (CLiP) [8]. We then used one of the tub2 disruptants and two double-disruptants possessing only one α-tubulin gene and one β-tubulin gene as parent strains for the production of 20 mutants showing various degrees of resistance to propyzamide and oryzalin. Thus, the use of single-tubulin-gene C. reinhardtii disruptants enabled efficient isolation of a large number of tubulin mutants resistant to anti-tubulin agents. An early version of this paper has been published as a preprint in bioRxiv (doi: https://doi.org/10.1101/2020.04.07.031005). However, after its publication, 12 of the 32 isolated mutants were accidentally lost. The present paper reports only about the surviving 20 strains.
Materials and methods
Isolation of tubulin-gene disruptants
A library of Chlamydomonas mutants was prepared by inserting the aphVIII gene (paromomycin resistance gene) into the genome [7]. In total, eight tubulin gene disruptants were obtained from this library. Six disruptants, tua2-B, tua2-C, tub1-B, tub2-A, tub2-B, and tub2-C were isolated by performing PCR on the pooled transformants using primers targeting the aphVIII sequence (PSI103-F2 and RB02) and two tubulin consensus sequences (3'-Tus1891g and 3'-Tus1803g). A disruptant, tub1-A, was isolated using two alternative tubulin consensus primers (5'-Tus1082c and 5'-Tus1596g). A disruptant, tua2-A, was isolated using RB02 and an alternative consensus primer (3'-TuA2-3254g). S1 File shows the primers used in the present study. After screening, the disruptants were sequenced in the vicinity of their disrupted tubulin gene (Macrogen Japan Co., Japan).
In addition, a tua1 disruptant (LMJ.RY0402.158052; referred to as tua1-A in the present study) was obtained from the Chlamydomonas Library Project [8]; this disruptant has a long insertion composed of two facing paromomycin-resistant CIB1cassettes immediately before the stop codon in tua1 [8].
The disruptants were backcrossed with wild-type C. reinhardtii (CC-125) and selected for tubulin-gene disruption by PCR before use. Double disruptants were constructed by standard methods [9], and selected from tetrads by PCR analysis.
Semi-quantitative real-time PCR
For disruptants tua1-A, tua2-A, tub1-B, and tub2-A, semi-quantitative real-time PCR was performed by using TB Green Premix Ex Taq GC (Perfect Real Time) and a Thermal Cycler Dice Real-Time System II (Takara, Japan) in accordance with the manufacturer’s instructions.
Assessment of tubulin protein level and flagellar length during reflagellation
Cells were grown in Tris–acetate–phosphate (TAP) medium [10] under a 12-h light/12-h dark cycle. Deflagellation was induced by pH shock [11]. A second deflagellation was carried out 2 h after the first deflagellation. Before the first deflagellation and after each deflagellation, whole-cell lysates were prepared by following the methods of Wakabayashi et al. [12]. Samples were electrophoresed on 5–20% acrylamide gradient gel [13] and stained with silver [14]. To assess reflagellation activity, aliquots of cells were fixed with formaldehyde/glutaraldehyde solution, and video-recorded under a dark-field microscope. Their flagellar lengths were measured using ImageJ software [15]. More than 50 flagella were measured for each sample. The percentage of cells that had two flagella was 85–100% in pre-deflagellation, 81–100% 2 h after the first deflagellation, and 70–97% 2 h after the second deflagellation.
Isolation of anti-tubulin drug resistant mutants
Propyzamide Reference Material, Oryzalin Standard, and colchicine were obtained from Fujifilm Wako Pure Chemical Co., Japan. The strains tub2-A, tua1-A x tub1-B (double disruptant) or tua2-A x tub1-B (double disruptant) were grown to the mid-log phase and then irradiated by ultraviolet light until about 50% of the cells were killed. The culture was spread on TAP/agar plates containing 20 μM propyzamide or 10 μM oryzalin, kept in the dark for 12 h, and then incubated under light for 5–10 days. Colonies that appeared were transferred to liquid medium in 96-well plates containing the same concentration of propyzamide or oryzalin. From each culture that grew, genomic DNA was extracted and subjected to PCR using the following primers: 5'-ChlaTuA1_long969 and 3'-TuA6260 (for tua1), 5'-Tua2-10g and 3'-TuA2-3288g (for tua2), 5'-tub1-33c and 3'-tub1-1667c (for tub1), and 5'-EcoTuB2-upper and 3'-XhoTuB2-lower (for tub2). The PCR products were processed for DNA sequencing (Macrogen Japan Co.).
Drug-resistance test
C. reinhardtii strains were grown in liquid medium until the mid-log phase, and then diluted to 5 × 104 cells/mL. Then, 30 μL of culture was mixed with 270 μL of growth medium containing propyzamide (0–40 μM) or oryzalin (0–20 μM) in each well of 96-well plates, and cultured for 8 days at 26°C under 12-h light/12-h dark conditions. As a measure of cell proliferation, absorbance at 595 nm was measured 3 h after light onset every day. A wild-type strain (CC-124), an oryzalin-resistant mutant (upA12) [3], or colchicine-resistant mutants (colR4 and colR15) [6, 16] were used as references. This assay was not applicable to the evaluation of colchicine resistance, as TAP media containing colchicine (> 1 mM) often became turbid during culture. The optical density of cells in liquid cultures as well as on the agar plates allowed only qualitative or semi-quantitative assessment of cell proliferation. This is because, in the presence of a high concentration of anti-tubulin drugs, some cells became huge in size while a small fraction of cells assumed an almost normal size [16]. Possibly because of such anomalous growth, some mutants showed slightly higher optical density at higher drug concentrations. Even so, assessment by optical density measurements allowed us to reproducibly assess the drug-resistance of each isolate. The criteria for assessment scores are as follows. For propyzamide: ++, a strain that grew at 40 μM; +, a strain that grew at 4 μM but not 40 μM; +/-, a strain that grew at 2 μM but not > 4 μM (the level exhibited by parental strains); -, a strain that did not grow at 2 μM. For oryzalin: ++, a strain that grew at 20 μM; +, a strain that grew at 5 and10 μM but not at 20 μM; +/-, a strain that did not grow at 5 μM (the level exhibited by parental strains).
As an alternative assessment of cell growth, an equal number of cells from each strain was spotted in a dilution series on agar plates containing different concentrations of propyzamide, oryzalin and colchicine and cultured under the same conditions as above. In this case, the effect of each drug was visually assessed from the density of cells on the plate after 1 week of culture. The two methods yielded qualitatively similar results. The colchicine resistance given in Table 1 was based on the following criteria. +: a strain whose density cultured at 4 mM colchicine was similar to that of its parental strain cultured without colchicine, and much higher than that of the parental strain cultured at 4 mM colchicine. +/-: a strain whose density at 2 mM colchicine was similar to that of the parental strains cultured without colchicine, but much lower than the latter when cultured at 4 mM colchicine. -: a strain whose density was similar to that of the parental strain cultured without colchicine but lower when cultured at 1–4 mM colchicine.
Table 1. Tubulin missense mutants isolated in this study.
| Strain | Parent strain | Gene altered | Mutation (DNA) | Mutation (protein) | Growth | Resistance to | ||
|---|---|---|---|---|---|---|---|---|
| propyzamide | oryzalin | colchicined | ||||||
| tub2-A | normalc | +/- | +/- | +/- | ||||
| tua1-A x tub1-B | normalc | +/- | +/- | +/- | ||||
| tua2-A x tub1-B | normalc | +/- | +/- | +/- | ||||
| ory2 | tua2-A x tub1-B | tua1 | TAC->CACa1 | Y24Ha1 | normal | - | ++ | +/- |
| ory3 | TTC->CTC | F52L | normal | + | ++ | +/- | ||
| ory4 | TCC->GCC | S165A | normal | +/- | ++ | +/- | ||
| ory5 | normal | +/- | ++ | +/- | ||||
| pyz9 | tub2-A | TTC->TTA | F351L | normal | + | +/- | - | |
| ory6 | tua1-A x tub1-B (E2) | tua2 | TAC->AAC | Y24N | normal | +/- | + | +/- |
| ory7 | TTC->TGC | F49C | normal | +/- | + | +/- | ||
| ory8 | TTC->GTC | F138V | slow | +/- | ++ | +/- | ||
| pyz10 | tub2-A | tub1 | GAG->AAG | E198K | normal | ++ | +/- | +/- |
| pyz11 | normal | ++ | + | +/- | ||||
| pyz12 | TTC->TGC | F266C | normal | ++ | +/- | + | ||
| pyz13 | normal | ++ | +/- | + | ||||
| pyz14 | normal | ++ | +/- | + | ||||
| pyz2 | tua2-A x tub1-B | tub2 | CAG->CAT | Q134H | normal | + | +/- | +/- |
| pyz3 | slow | + | +/- | - | ||||
| pyz4 | GAG->AAG | E198K | normal | ++ | + | + | ||
| pyz5 | ATC->AAT | I236Nb | normal | ++ | + | + | ||
| pyz6 | AAG->GAGa2 | K350Ea2 | slow | ++ | + | + | ||
| pyz7 | ATC->TTC | I368F | normal | ++ | +/- | - | ||
| pyz8 | normal | ++ | +/- | - | ||||
++, highly resistant; +, resistant; +/-, parent-strain level; -, hypersensitive. For criteria, see text.
a1Mutation previously reported for upA12 [3].
a2Mutation previously reported for colR4 [6].
bMutation found in human cancer cells resistant to 2-methoxyestradiol [36].
cCompared with a wild type (CC-124).
dAssessed by visual inspection of 7-d cultures on agar plates containing 0–4 mM colchicine.
Three-dimensional structure prediction of C. reinhardtii α/β-tubulin heterodimer
The three-dimensional structure of the C. reinhardtii α/β-tubulin heterodimer was predicted by using FAMS software [17] based on a known tubulin tetramer structure obtained from the Protein Data Bank (PDB ID: 1Z2B [18]). To determine the amino acids that most likely interacted with the examined drugs, in silico molecular docking analyses were performed using the ChooseLD program [19].
2D-PAGE of isolated axonemes
Axonemes were isolated from the C. reinhardtii strains by using standard procedures [11]. A small aliquot of axonemal precipitate (~2 or 10 μg) was extracted with a buffer containing 5 M urea and 2 M thiourea and analyzed by 2D-PAGE as described previously [20]. Since α- and β-tubulin are modified post-translationally, the loading amount was adjusted so that their major forms only were detectable by silver staining. The predicted pI values of the wild-type and mutant tubulins were calculated by using the EMBOSS database and the Sequence Manipulation Suite, which is a collection of JavaScript programs for examining short protein sequences (https://www.bioinformatics.org/sms2/protein_iep.html) [21].
Results
Isolation of tubulin-gene disruptants
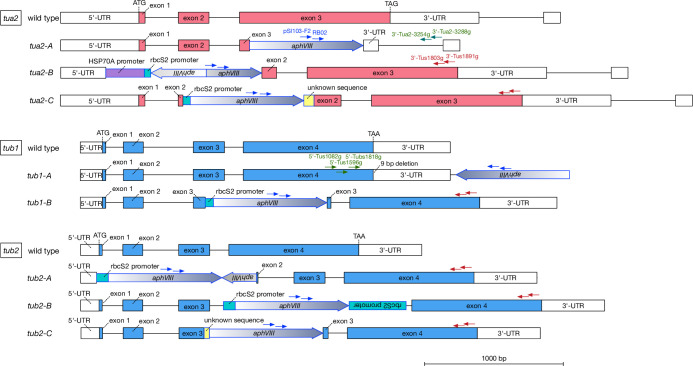
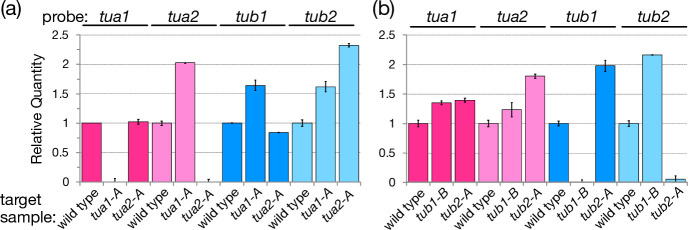
A library of around 8000 clones was prepared by aphVIII gene cassette insertion into the genome according to the methods reported previously [7]. The library was then screened by PCR using primer pairs consisting of one primer targeting a consensus sequence of the four tubulin genes and another primer targeting the aphVIII fragment. As a result, we isolated eight tubulin gene disruptants: three showing tua2 disruption (tua2-A, tua2-B, tua2-C), two showing tub1 disruption (tub1-A, tub1-B), and three showing tub2 disruption (tub2-A, tub2-B, tub2-C). Fig 1 shows the sites of the aphVIII cassette insertion in the eight disruptants, and S1 Fig shows the PCR confirmation of the structure of the disrupted genes. In six of the disruptants (tua2-A, tua2-B, tua2-C, tub1-B, tub2-A, tub2-C), the aphVIII cassette was inserted into the gene. In the remaining two disruptants, the aphVIII cassette was inserted after the open reading frame (tub1-A) or within an intron (tub2-B). In all eight disruptants, AphVIII cassette insertion resulted in complete absence of mRNA expression of the affected tubulin gene, as confirmed by northern blot analysis (S2 Fig). For tua1-A, tua2-A, tub1-B, and tub2-A, semi-quantitative real-time PCR was performed and again no expression of mRNA from the tubulin genes was detected (Fig 2).
Fig 1. Structures of the tua2, tub1, and tub2 genes in wild type and the eight tubulin-gene disruptants.
Structures of the tubulin genes in wild type and disruptants. The aphVIII insertion sites in the disrupted genes are shown as large gray arrows. Exons are shown as boxes and introns as lines between boxes. Start and stop codons are indicated in the diagrams of the wild-type genes. Arrows show the primers used for screening. Blue arrows indicate primers targeting the aphVIII sequence (PSI103-F2 and RB02). Red arrows indicate tubulin consensus sequences (3'-Tus1891g and 3'-Tus1803g). Green arrows with labels indicate other tubulin-specific primers used for detecting gene disruption in some strains. Primers and their sequences are listed in S1 File.
Fig 2. Semi-quantitative real-time polymerase chain reaction in the tubulin-gene disruptants.
The transcription levels of the four tubulin genes were analyzed by using total RNA extracted from (a) wild type (CC-125) and two α-tubulin gene disruptants (tua1-A and tua2-A), and (b) from wild type and two β-tubulin gene disruptants (tub1-B and tub2-A). Data are means ± SD (n = 3).
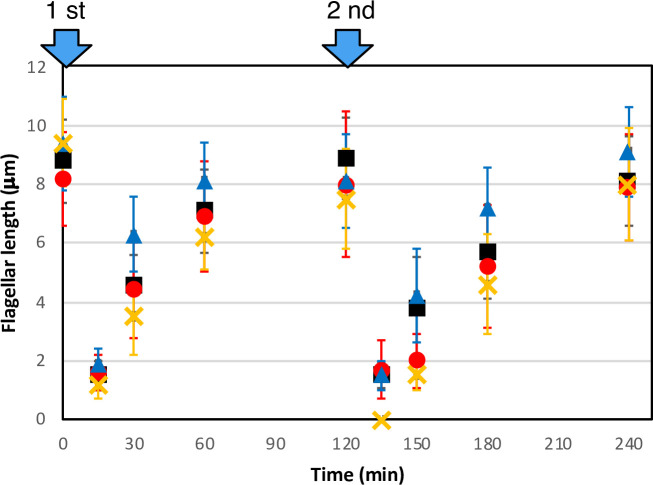
The growth rate of the eight disruptants was reduced to varying degrees; while five disruptants grew at almost the wild-type rate, tua2-B and tub1-A displayed very slow growth rates and tub2-B displayed intermediate rates. These slow-growing strains were not used for subsequent studies. The two double disruptants used as parent strains for isolation of drug-resistant mutants grew at almost the normal rate (S1 Table, S3 Fig). For tua2-A, tub1-B, and the double disruptant tua2-A×tub1-B, tubulin expression (Fig 3), and the time course of flagellar regeneration after amputation (Fig 4) were normal, suggesting that the disruptants still produced sufficient α/β-tubulin heterodimer for their cellular functions via the remaining intact genes. The mean flagellar length was comparable among the disruptants (see Fig 4). The tubulin disruptants showed some difference in their sensitivity to anti-tubulin drugs, although the sensitivity somewhat varied among alleles (S1 Table, S3 Fig). Therefore, the tubulin missense mutants obtained in this study, as shown below, were examined for their drug sensitivity relative to the sensitivity of each parental strain.
Fig 3. Tubulin protein expression in the tubulin-gene disruptants.
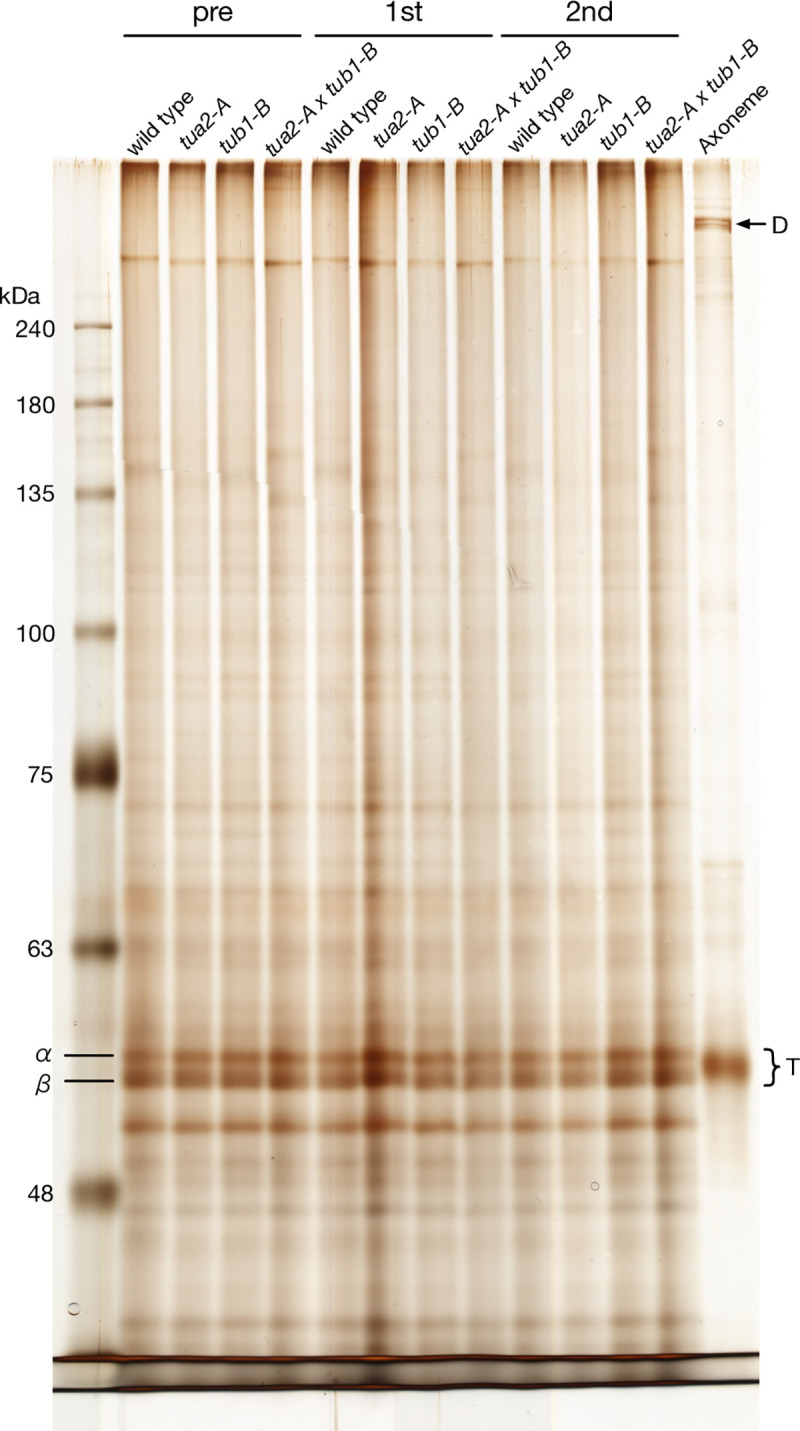
All of the disruptants showed tubulin expression at the normal level. Whole-cell lysates from wild type (CC-125), tua2-A, tub1-B, and a tua2-A x tub1-B double mutant were prepared before deflagellation (pre), after the 1st deflagellation (1st), and after the 2nd deflagellation (2nd) and electrophoresed. Wild-type axoneme (Axoneme) was loaded for comparison. D indicates bands with axonemal dynein heavy chains (~500 kDa). T indicates α and β-tubulin.
Fig 4. Change in flagellar length after repeated deflagellation in the tubulin-gene disruptants.
Horizontal axis indicates time after the 1st deflagellation. Arrows indicate the timing of the 1st and 2nd deflagellation by acid treatment followed by neutralization. Black squares, wild type (CC-125); red circles; tua2-A, blue triangles; tub1-B; yellow crosses; a tua2-A x tub1-B double mutant. Error bars: SDs (n = 50).
Mutant isolation using tubulin-gene disruptants
We used the disruptants to isolate C. reinhardtii strains expressing tubulins with missense mutations that confers resistance to propyzamide or oryzalin. Three parent strains were used: the single disruptant tub2-A, a double disruptant generated by crossing tua1-A with tub1-B, and a double disruptant generated by crossing tua2-A and tub1-B. As detailed in Materials and Methods, these strains were first mutagenized by UV and inoculated on drug-containing agar plates, and colonies that appeared were cultured in drug-containing liquid media. The clones that grew were saved as drug-resistant mutants, and the sequence of their tubulin genes were determined.
As a result of 1–3 trials with each parental strain against oryzalin or propyzamide, 20 strains showing a total of 14 different tubulin missense mutations were isolated. Mutants first selected for oryzalin-resistance were given strain names with ory, while those selected for propyzamide-resistance were given names with pyz. Specifically, PCR and DNA sequencing identified 1 tua and 5 tub mutants in 8 isolates from the tub2-A disruptant, 3 tua and 8 tub mutants in 22 isolates from the tua1-A×tub1-B double disruptant, and 4 tua mutants and 11 tub mutants in 23 isolates from the tua2-A×tub1-B double disruptant. The overall probability of an isolate having a mutation in one of the tubulin genes was ~60% (however, 12 of the 32 isolated were accidentally lost). We assessed the sensitivity of each isolate to propyzamide and oryzalin by measuring the culture's density at 595 nm every 24 h in the presence of these drugs (S2 Table). At the same time, each isolate was cultured on agar plates containing various concentrations of these drugs and colchicine, and the density of cells after 1 week of culture was regarded as a measure of resistance (S4 Fig).
Table 1 shows the obtained mutants classified by the gene affected, as well as the results of a semi-quantitative assessment of each strain’s resistance to oryzalin and propyzamide. Most of the ory strains and a pyz strain (pyz9) had a missense mutation in an α-tubulin gene. In contrast, most of the pyz strains, other than pyz9, had mutations in a β-tubulin gene.
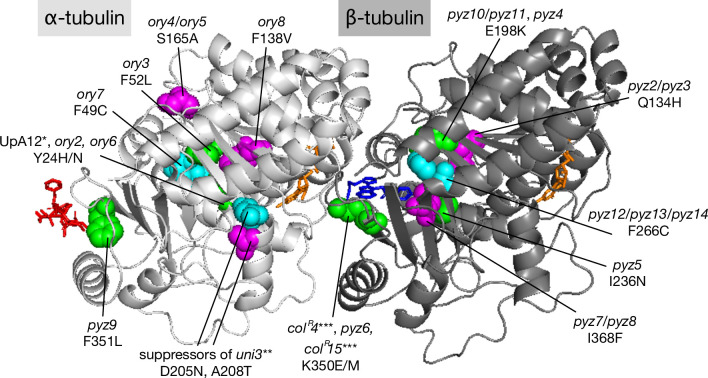
Fig 5 shows a predicted three-dimensional structure of C. reinhardtii α/β-tubulin heterodimer labeled with the site of each missense mutation reported here and in previous studies [3, 5, 6]. Two of the isolates had mutations that have been reported previously: ory2 had a tua1 Y24H mutation as did upA12 [3]; pyz6 had a tub2 K350E mutation as did colR4 [6]. Interestingly these mutants displayed a slightly stronger drug resistance than the previously-reported corresponding mutants; ory2 exhibited a stronger oryzalin-resistance than upA12, and pyz6 exhibited a stronger propyzamide-resistance than colR4 (S4 Fig). Their stronger drug-resistances possibly reflects the fact that the mutants isolated here express only mutated tubulin from a single gene, whereas the previously-reported mutants express a mutated tubulin together with a wild-type counterpart.
Fig 5. Predicted three-dimensional structure of Chlamydomonas reinhardtii α/β-tubulin heterodimer showing the mutations reported in the present and previous studies.
Light gray, α-tubulin; dark gray, β-tubulin. Altered amino acids are shown as sphere representations. The binding sites of tubulysin M (red, an oryzalin-like compound) and 2RR (blue, 3-[(4-{1-[2-(4-aminophenyl)-2-oxoethyl]-1H-benzimidazol-2-yl}-1,2,5-oxadiazol-3-yl)amino]propanenitrile, a propyzamide-like compound) were determined by applying the alignment command in MacPyMol software to the tubulin structures 4ZOL and 4O2A reported in the presence of these compounds [25, 26]. The orange stick representations show GTP (in α-tubulin) and GDP (in β-tubulin). Mutations identical to previously reported mutations are marked with asterisks: *, [3]; **, [5]; and ***, [6].
Some of the identified mutations involved the substitution of amino acids with different charges. For example, pyz2/pyz3 (we use a slash to separate strains that have the same tubulin genotypes) expressed β-tubulin with the mutation Q134H and pyz4 and pyz10/pyz11 expressed β-tubulin with the mutation E198K. The isoelectric point (pI) values of these β-tubulins predicted from their amino acid sequences were 4.59 and 4.63, respectively, which were greater than the pI of wild-type β-tubulin (4.55). We confirmed the expression of β-tubulins with different pIs in those strains by two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) of axonemal proteins from the mutants and wild type (Fig 6). As expected, the spot of β-tubulin appeared at higher pH values in the order pyz10 (E198K) > pyz2 (Q134H) > wild type. The 2D-PAGE analysis also verified that each mutant expressed β-tubulin from only a single gene, since it detected no β-tubulin spots with the wild-type pI in mutant samples.
Fig 6. Two-dimensional polyacrylamide gel electrophoresis analysis of axonemes from strains pyz2 and pyz10.
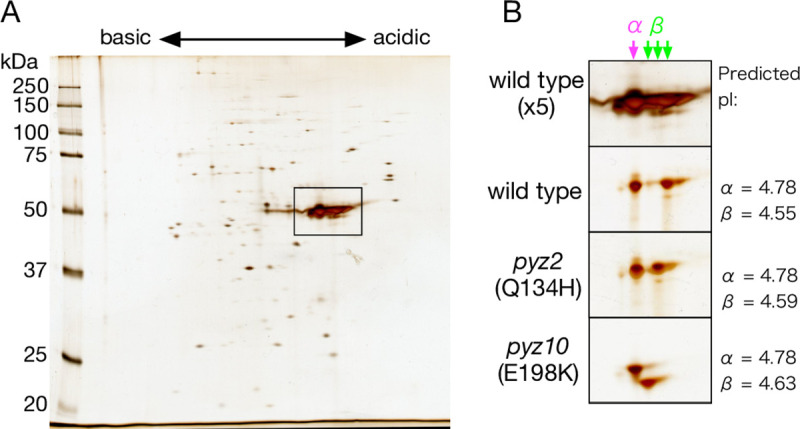
Protein extracts of axonemes of wild type (CC-125), pyz2 and pyz10 were loaded on a two-dimensional polyacrylamide gel and stained with silver. pH range: 4.0–7.0. (A) Electrophoresis pattern of wild-type axoneme (~10 μg loaded). (B) Portions of polyacrylamide gels showing the major spots of α- and β-tubulin. Upper panel shows a close-up of the area indicated by the box in (A). The lower three panels show the polyacrylamide gels after loading approximately 2 μg of axoneme. The predicted pIs of the wild-type and three mutant α- and β-tubulins are indicated to the right of the panels.
Novel tubulin mutant strains exhibited various sensitivities to anti-tubulin agents
The mutant strains obtained, most of which are novel in Chlamydomonas, displayed various patterns of sensitivity to anti-tubulin agents (Table 1 and Fig 7). Most of the mutants selected in oryzalin-containing media, ory2, ory3, ory4, ory5, and ory8, naturally showed high oryzalin resistance and grew well in the presence of 20 μM oryzalin. Of these, ory2 showed hypersensitivity to propyzamide. In contrast, ory3 was slightly resistant to propyzamide. Likewise, ten strains of mutants first selected on propyzamide-containing media, pyz4, pyz5, pyz6, pyz7, pyz8, pyz10, pyz11, pyz12, pyz13, and pyz14, showed particularly strong propyzamide resistance and grew well in growth medium containing 40 μM propyzamide, where parental strains (tub2-A, tua1-A✕tub1-B, tua2-A✕ tub1-B) were barely viable (Fig 7, S2 Table). Many pyz strains (those with the mutation F266C, I236N, or K350E) exhibited strong resistance to colchicine. Such cross-resistance has been observed in K350E [16]. In contrast, strains having Q134H or I368F mutation displayed no resistance despite their strong resistance to propyzamide (S4 Fig). The different sensitivities to colchicine and propyzamide in these mutants are interesting because the two agents bind to almost the same position on the tubulin heterodimer [22].
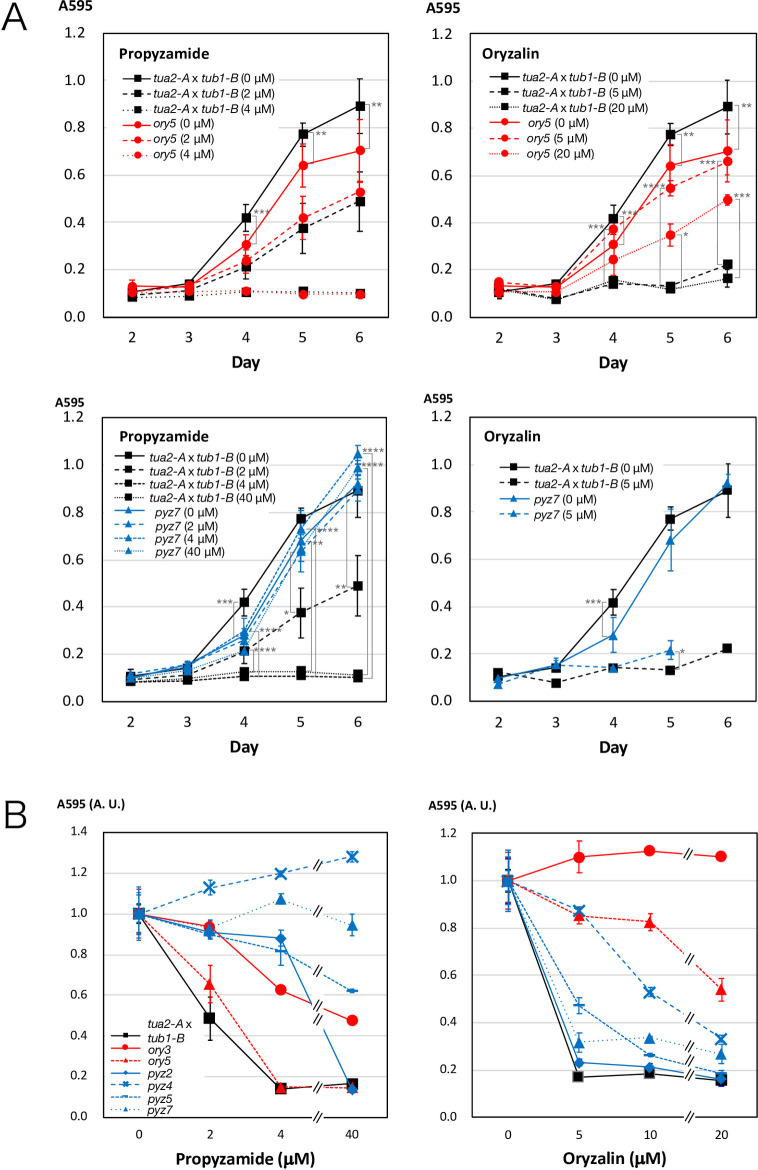
Fig 7. Growth profiles of representative missense mutants in the presence of propyzamide and oryzalin.
Cells were cultured in growth medium containing different concentrations of propyzamide and oryzalin. Absorbance of the culture was measured at 595 nm every day. Error bars represent standard deviations in three independent measurements. (A) Growth curves of two representative strains, ory5 and pyz7, compared with the parent strain tua2-A x tub1-B at two or three concentrations of drugs. ory5 is resistant to oryzalin but not to propyzamide. In contrast, pyz7 is resistant to propyzamide but not to oryzalin. *, P<0.05; **, P<0.01; ***, P<0.0001; ****, P<0.00001 (ANOVA test). (B) Relative optical densities 5 days after the initiation of culture are plotted against the concentration of propyzamide or oryzalin. Seven strains, ory3, ory5, pyz2, pyz4, pyz5, pyz7, and the parent strain tua2-A x tub1-B are compared. Optical densities at 0 concentration are normalized to 1.
Discussion
By screening an AphVIII insertional library, we isolated three disruptants lacking tua2, two lacking tub1, and three lacking tub2. All were most likely null mutants (S2 Fig). Although these disruptants lacked one of their tubulin-encoding genes, their cytoplasmic tubulin levels remained normal (Fig 3), suggesting the presence of an auto-regulatory mechanism that maintains the tubulin mRNA level, as observed in other eukaryotic cells [23]. Indeed, in tua1-A, tub1-B, and tub2-A, the mRNA expression level of the remaining α- or β-tubulin gene was increased approximately 2-fold compared with wild type (Fig 2). Also, flagellar length, ability to produce flagella after amputation (Fig 4), and in most of the disruptants, overall cell growth rate did not greatly differ from the wild-type growth rate (S3 Fig). Thus, although C. reinhardtii possesses two α-tubulin genes and two β-tubulin genes, a single gene for each type is enough to supply the tubulin necessary for its cellular functions. However, the present findings do not mean that the two genes for each tubulin have exactly the same function; rather, the two genes may differ from each other in a subtle manner. For example, the sensitivity to anti-tubulin drugs varied among the disruptants (S3 Fig). Although it could be due to some non-tubulin mutation(s) unintentionally introduced in these disruptants, there may be some difference in the regulation of gene expression that is dependent on the concentration of free tubulin in the cytoplasm [24]. Thus, how the two genes encoding the two tubulins differ in their function and regulation warrants further investigation, and our single-tubulin-gene mutants established here should be useful for such investigations.
Next, we used the disruptants to obtain mutants with resistance to two anti-tubulin agents, propyzamide and oryzalin. Several rounds of trial to isolate mutants resistant to one or both of the agents afforded 8 mutants with 7 different α-tubulin gene missense mutations and 12 mutants with 7 different β-tubulin gene missense mutations (Table 1). The number of mutations obtained is much larger than the total number that has been reported previously (i.e., 3 α-tubulin mutations and 2 β-tubulin mutations) [3, 5, 6]. In addition, we found that about 60% of the clones isolated on the basis of drug resistance harbored a mutation in a tubulin gene. Together, these findings suggest that our approach of using single-tubulin-gene disruptants is a highly efficient means of obtaining tubulin mutant strains. The high efficiency may be partly due to the stronger effect of tubulin mutations in single-tubulin-gene strains than in ordinary strains (S4 Fig, S2 Table), and partly due to the easier detection of tubulin mutations in strains with fewer tubulin genes.
How the sensitivity to anti-tubulin agents varied in the mutants is an important issue that warrants clarification. Some of the tubulin mutants that conferred resistance to the anti-tubulin agents had a mutation near to where the anti-tubulin agents bind to the α/β-tubulin heterodimer (Fig 5). The binding site of oryzalin, inferred from that of an analogous compound, tubulysin M, is at the intra-dimer interface [25] close to the α-tubulin mutation F351L (in strain pyz9). Other ory mutants whose mutation sites occur independently of the tubulysin M-binding site may confer oryzalin resistance by modulating the three-dimensional structure of α-tubulin. Likewise, a propyzamide-like compound, 2RR, is known to bind at the inter-dimer interface [26] close to several of the identified β-tubulin mutation sites: E198K (in pyz4 and pyz10/pyz11), I236N (in pyz5), K350E (in colR4 and pyz6), and I368F (in pyz7/pyz8). Other mutations may confer propyzamide resistance through some structural change in α/β-tubulin.
Several of the mutations detected in the present study are similar to those reported in other organisms (S3 Table). For α-tubulin, mutation F49C in ory7, F52L in ory3, and S165A in ory4/ory5 are analogous to the mutations reported in a Toxoplasma gondii oryzalin-resistant mutants [27, 28]. For β-tubulin, mutation Q134H in pyz2/pyz3 is analogous to the mutation in a Beauveria bassiana benzimidazole-resistant mutant [29]; mutation E198K in pyz4 and pyz10/pyz11 corresponds to the mutation in fungi and nematodes that confers benzimidazole resistance and phenylcarbamate hypersensitivities [30–35]; I236N in pyz5 is analogous to the mutation responsible for resistance to the anti-cancer drug 2-methoxyestradiol in human epithelial cancer cells [36]. Mutations Y24N, F138V, and F351L in α-tubulin (ory6, ory8, and pyz9) and mutations F266C and I368F in β-tubulin (pyz12/pyz13/pyz14 and pyz7/pyz8) are being reported here for the first time; further investigations are needed to examine whether mutations analogous to these mutations cause altered drug sensitivity in other organisms.
Although the present study selected mutants based only on their resistance to two anti-tubulin agents, use of other agents such as the microtubule-stabilizing agent taxol, or screening for other properties such as hypersensitivity to drugs, resistance to low temperature, or deficiency in flagellar formation and motility will lead to the isolation of a greater variety of mutants. Detailed analyses of many such mutants will deepen our understanding of the structure–function relation of tubulins. Since one of the mutations identified in the present study corresponded to mutation found in human tubulins that confer drug resistance in cancer cells, we expect that studies of Chlamydomonas tubulin mutants will also contribute to the development of improved cancer therapies.
Supporting information
(PDF)
In each disruptant, the structure of the affected tubulin gene was confirmed by PCR using primers specific to AphVIII (RB02) and the target tubulin gene. A wild type (CC-125) genomic DNA was used as the template for negative controls. PCR-amplified gene fragments contained the inserted AphVIII cassette from the genomic DNA templates extracted from the disruptants but not the wild type. The sizes of the amplified fragments matched those predicted from the manner of AphVIII gene insertion (Fig 1), which was determined by sequencing in the vicinity of their disrupted tubulin gene.
(TIF)
(a) tua1 transcripts. Total RNA from a wild type (CC-125), tua1-A, tua2-A, tua2-B, and tua2-C were hybridized with tua1 mRNA-specific probes. (b) tua2 transcripts. Total RNA from tua2-A, tua2-B, tua2-C, and wild type were hybridized with tua2 mRNA-specific probes. (c) tub1 transcripts. Total RNA from wild type, tub1-A, tub1-B, tub2-A, tub2-B, and tub2-C were hybridized with tub1 mRNA-specific probes. (d) tub2 transcripts. Total RNA from wild type, tub1-A, tub1-B, tub2-A, tub2-B, and tub2-C were hybridized with tub2 mRNA-specific probes.
(TIF)
Cells were cultured in growth medium containing different concentrations of anti-tubulin drugs. Absorbance of the culture was measured at 595 nm every day. For comparison, data of a wild type (CC-124) was also collected. Error bars represent standard deviations of three different measurements. A: Growth rate of the tubulin disruptants. B: Drug sensitivities. Relative optical densities on day 5 are shown for all strains except for slow-growing strains, tua2-B, tub1-A, and tub2-B. For the slow-growing strains, data on day 8 are shown. In each case, the optical density observed without a drug is normalized to 1.
(TIF)
Serial dilutions of the cells were inoculated onto TAP/agar medium containing various concentrations of propyzamide, oryzalin and colchicine, and cultured for 7 days. The names of strains are given in the table (upper right corner) in different colors reflecting their parent strains. Three parent strains and three previously reported strains, upA12, colR4, and colR15, were also cultured and compared with ory2 and pyz6 having the same mutations (boxed). The latter single-tubulin-gene mutants show slightly stronger drug resistance as seen at high drug concentrations.
(TIF)
Cells were cultured in growth medium containing various concentrations of anti-tubulin drugs. Absorbance of the culture was measured at 595 nm every day. For comparison, data of a wild type (CC-124) were also collected. Standard deviations in three different measurements are also shown. The green background shows a rough measure of cell density for qualitative comparison of growth extent.
(XLSX)
Absorbance at 595 nm was measured as in S1 Table. For comparison, data of parent strains and previously reported mutants, upA12, colR4, and colR15, were also collected.
(XLSX)
(XLSX)
(JPEG)
(PDF)
(JPEG)
(PDF)
Acknowledgments
We thank Ai Tashiro, Haruka Fujisawa, and Tomoko Kurihara (Chuo University, Tokyo, Japan) for conducting the tubulin disruptant screening, and Ryosuke Suzuki, Hinano Sugai, Mami Ishigaki, and Yidong Tang (Chuo University) for performing the gene analysis of the tubulin mutants. We also thank Mitsuo Iwadate (Chuo University) for conducting the three-dimensional structure prediction and in silico molecular docking analyses, and Hiroko Kawai-Toyooka (The Univ. of Tokyo, Tokyo, Japan) for her technical support with the real-time polymerase chain reaction analyses.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by grants from Chuo University (Joint Research Grant and Grant for Special Research) to T K-M. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Silflow CD, Chisholm RL, Conner TW, Ranum LP. The two alpha-tubulin genes of Chlamydomonas reinhardi code for slightly different proteins. Mol Cell Biol. 1985;5(9):2389–98. 10.1128/mcb.5.9.2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youngblom J, Schloss JA, Silflow CD. The two beta-tubulin genes of Chlamydomonas reinhardtii code for identical proteins. Mol Cell Biol. 1984;4(12):2686–96. 10.1128/mcb.4.12.2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James SW, Silflow CD, Stroom P, Lefebvre PA. A mutation in the a1-tubulin gene of Chlamydomonas reinhardtii confers resistance to anti-microtubule herbicides. J Cell Sci. 1993:209–18. [DOI] [PubMed] [Google Scholar]
- 4.Brunke KJ, Young EE, Buchbinder BU, Weeks DP. Coordinate regulation of the four tubulin genes of Chlamydomonas reinhardi. Nucleic Acids Res. 1982;10(4):1295–310. Epub 1982/02/25. 10.1093/nar/10.4.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fromherz S, Giddings TH Jr., Gomez-Ospina N, Dutcher SK. Mutations in alpha-tubulin promote basal body maturation and flagellar assembly in the absence of delta-tubulin. J Cell Sci. 2004;117(Pt 2):303–14. Epub 2003/12/17. 10.1242/jcs.00859 . [DOI] [PubMed] [Google Scholar]
- 6.Lee VD, Huang B. Missense mutations at lysine 350 in beta 2-tubulin confer altered sensitivity to microtubule inhibitors in Chlamydomonas. Plant Cell. 1990;2(11):1051–7. Epub 1990/11/01. 10.1105/tpc.2.11.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamano T, Sato E, Iguchi H, Fukuda Y, Fukuzawa H. Characterization of cooperative bicarbonate uptake into chloroplast stroma in the green alga Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2015;112(23):7315–20. Epub 2015/05/28. 10.1073/pnas.1501659112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Zhang R, Patena W, Gang SS, Blum SR, Ivanova N, et al. An indexed, mapped mutant library enables reverse genetics studies of biological processes in Chlamydomonas reinhardtii. Plant Cell. 2016;28(2):367–87. Epub 2016/01/15. 10.1105/tpc.15.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris EH, Stern DB, Witman GB. The Chlamydomonas sourcebook: Elsevier San Diego, CA; 2009. [Google Scholar]
- 10.Gorman DS, Levine RP. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci USA. 1965;54(6):1665–9. Epub 1965/12/01. 10.1073/pnas.54.6.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witman GB. Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol. 1986;134(280):280–90. 10.1016/0076-6879(86)34096-5 [DOI] [PubMed] [Google Scholar]
- 12.Wakabayashi K, Takada S, Witman GB, Kamiya R. Transport and arrangement of the outer-dynein-arm docking complex in the flagella of Chlamydomonas mutants that lack outer dynein arms. Cell Motil Cytoskel. 2001;48(4):277–86. 10.1002/cm.1015 . [DOI] [PubMed] [Google Scholar]
- 13.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond). 1970;227:680–5. 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 14.Blum H, Beier H, Gross HJ. Improved silver staining of plant-proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–9. [Google Scholar]
- 15.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5. Epub 2012/08/30. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schibler MJ, Huang B. The colR4 and colR15 beta-tubulin mutations in Chlamydomonas reinhardtii confer altered sensitivities to microtubule inhibitors and herbicides by enhancing microtubule stability. J Cell Biol. 1991;113(3):605–14. Epub 1991/05/01. 10.1083/jcb.113.3.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umeyama H, Iwadate M. FAMS and FAMSBASE for protein structure. Curr Protoc Bioinformatics. 2004;Chapter 5:Unit5 2. Epub 2008/04/23. 10.1002/0471250953.bi0502s04 . [DOI] [PubMed] [Google Scholar]
- 18.Gigant B, Wang C, Ravelli RB, Roussi F, Steinmetz MO, Curmi PA, et al. Structural basis for the regulation of tubulin by vinblastine. Nature. 2005;435(7041):519–22. Epub 2005/05/27. 10.1038/nature03566 . [DOI] [PubMed] [Google Scholar]
- 19.Takaya D, Takeda-Shitaka M, Terashi G, Kanou K, Iwadate M, Umeyama H. Bioinformatics based Ligand-Docking and in-silico screening. Chem Pharm Bull (Tokyo). 2008;56(5):742–4. Epub 2008/05/03. 10.1248/cpb.56.742 . [DOI] [PubMed] [Google Scholar]
- 20.Kato-Minoura T, Hirono M, Kamiya R. Chlamydomonas inner-arm dynein mutant, ida5, has a mutation in an actin-encoding gene. J Cell Biol. 1997;137(3):649–56. 10.1083/jcb.137.3.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stothard P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques. 2000;28(6):1102, 4. Epub 2000/06/27. 10.2144/00286ir01 . [DOI] [PubMed] [Google Scholar]
- 22.Silflow CD, Sun X, Haas NA, Foley JW, Lefebvre PA. The Hsp70 and Hsp40 chaperones influence microtubule stability in Chlamydomonas. Genetics. 2011;189(4):1249–60. Epub 2011/09/24. 10.1534/genetics.111.133587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleveland DW, Havercroft JC. Is apparent autoregulatory control of tubulin synthesis nontranscriptionally regulated? J Cell Biol. 1983;97(3):919–24. Epub 1983/09/01. 10.1083/jcb.97.3.919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cleveland DW, Lopata MA, Sherline P, Kirschner MW. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981;25(2):537–46. Epub 1981/08/01. 10.1016/0092-8674(81)90072-6 . [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Benz FW, Wu Y, Wang Q, Chen Y, Chen X, et al. Structural insights into the pharmacophore of vinca domain inhibitors of microtubules. Mol Pharmacol. 2016;89(2):233–42. Epub 2015/12/15. 10.1124/mol.115.100149 . [DOI] [PubMed] [Google Scholar]
- 26.Prota AE, Danel F, Bachmann F, Bargsten K, Buey RM, Pohlmann J, et al. The novel microtubule-destabilizing drug BAL27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization. J Mol Biol. 2014;426(8):1848–60. Epub 2014/02/18. 10.1016/j.jmb.2014.02.005 . [DOI] [PubMed] [Google Scholar]
- 27.Morrissette NS, Mitra A, Sept D, Sibley LD. Dinitroanilines bind alpha-tubulin to disrupt microtubules. Mol Biol Cell. 2004;15(4):1960–8. Epub 2004/01/27. 10.1091/mbc.e03-07-0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma C, Li C, Ganesan L, Oak J, Tsai S, Sept D, et al. Mutations in alpha-tubulin confer dinitroaniline resistance at a cost to microtubule function. Mol Biol Cell. 2007;18(12):4711–20. Epub 2007/09/21. 10.1091/mbc.e07-04-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou G, Ying SH, Shen ZC, Feng MG. Multi-sited mutations of beta-tubulin are involved in benzimidazole resistance and thermotolerance of fungal biocontrol agent Beauveria bassiana. Environ Microbiol. 2006;8(12):2096–105. Epub 2006/11/17. 10.1111/j.1462-2920.2006.01086.x . [DOI] [PubMed] [Google Scholar]
- 30.Jung MK, Wilder IB, Oakley BR. Amino acid alterations in the benA (beta-tubulin) gene of Aspergillus nidulans that confer benomyl resistance. Cell Motil Cytoskel. 1992;22(3):170–4. Epub 1992/01/01. 10.1002/cm.970220304 . [DOI] [PubMed] [Google Scholar]
- 31.Cai M, Lin D, Chen L, Bi Y, Xiao L, Liu XL. M233I mutation in the beta-tubulin of Botrytis cinerea confers resistance to zoxamide. Sci Rep. 2015;5:16881 Epub 2015/11/26. 10.1038/srep16881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright AJ, Hunter CP. Mutations in a beta-tubulin disrupt spindle orientation and microtubule dynamics in the early Caenorhabditis elegans embryo. Mol Biol Cell. 2003;14(11):4512–25. Epub 2003/08/26. 10.1091/mbc.e03-01-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buhr TL, Dickman MB. Isolation, characterization, and expression of a second beta-tubulin-encoding gene from Colletotrichum gloeosporioides f. sp. aeschynomene. Appl Environ Microbiol. 1994;60(11):4155–9. Epub 1994/11/01. 10.1128/AEM.60.11.4155-4159.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujimura M, Kamakura T, Inoue H, Yamaguchi I. Amino-acid alterations in the beta-tubulin gene of Neurospora crassa that confer resistance to carbendazim and diethofencarb. Curr Genet. 1994;25(5):418–22. Epub 1994/05/01. 10.1007/BF00351780 . [DOI] [PubMed] [Google Scholar]
- 35.Chen CJ, Yu JJ, Bi CW, Zhang YN, Xu JQ, Wang JX, et al. Mutations in a beta-tubulin confer resistance of Gibberella zeae to benzimidazole fungicides. Phytopathology. 2009;99(12):1403–11. Epub 2009/11/11. 10.1094/PHYTO-99-12-1403 . [DOI] [PubMed] [Google Scholar]
- 36.Escuin D, Burke PA, McMahon-Tobin G, Hembrough T, Wang Y, Alcaraz AA, et al. The hematopoietic-specific beta1-tubulin is naturally resistant to 2-methoxyestradiol and protects patients from drug-induced myelosuppression. Cell Cycle. 2009;8(23):3914–24. Epub 2009/11/11. 10.4161/cc.8.23.10105 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
In each disruptant, the structure of the affected tubulin gene was confirmed by PCR using primers specific to AphVIII (RB02) and the target tubulin gene. A wild type (CC-125) genomic DNA was used as the template for negative controls. PCR-amplified gene fragments contained the inserted AphVIII cassette from the genomic DNA templates extracted from the disruptants but not the wild type. The sizes of the amplified fragments matched those predicted from the manner of AphVIII gene insertion (Fig 1), which was determined by sequencing in the vicinity of their disrupted tubulin gene.
(TIF)
(a) tua1 transcripts. Total RNA from a wild type (CC-125), tua1-A, tua2-A, tua2-B, and tua2-C were hybridized with tua1 mRNA-specific probes. (b) tua2 transcripts. Total RNA from tua2-A, tua2-B, tua2-C, and wild type were hybridized with tua2 mRNA-specific probes. (c) tub1 transcripts. Total RNA from wild type, tub1-A, tub1-B, tub2-A, tub2-B, and tub2-C were hybridized with tub1 mRNA-specific probes. (d) tub2 transcripts. Total RNA from wild type, tub1-A, tub1-B, tub2-A, tub2-B, and tub2-C were hybridized with tub2 mRNA-specific probes.
(TIF)
Cells were cultured in growth medium containing different concentrations of anti-tubulin drugs. Absorbance of the culture was measured at 595 nm every day. For comparison, data of a wild type (CC-124) was also collected. Error bars represent standard deviations of three different measurements. A: Growth rate of the tubulin disruptants. B: Drug sensitivities. Relative optical densities on day 5 are shown for all strains except for slow-growing strains, tua2-B, tub1-A, and tub2-B. For the slow-growing strains, data on day 8 are shown. In each case, the optical density observed without a drug is normalized to 1.
(TIF)
Serial dilutions of the cells were inoculated onto TAP/agar medium containing various concentrations of propyzamide, oryzalin and colchicine, and cultured for 7 days. The names of strains are given in the table (upper right corner) in different colors reflecting their parent strains. Three parent strains and three previously reported strains, upA12, colR4, and colR15, were also cultured and compared with ory2 and pyz6 having the same mutations (boxed). The latter single-tubulin-gene mutants show slightly stronger drug resistance as seen at high drug concentrations.
(TIF)
Cells were cultured in growth medium containing various concentrations of anti-tubulin drugs. Absorbance of the culture was measured at 595 nm every day. For comparison, data of a wild type (CC-124) were also collected. Standard deviations in three different measurements are also shown. The green background shows a rough measure of cell density for qualitative comparison of growth extent.
(XLSX)
Absorbance at 595 nm was measured as in S1 Table. For comparison, data of parent strains and previously reported mutants, upA12, colR4, and colR15, were also collected.
(XLSX)
(XLSX)
(JPEG)
(PDF)
(JPEG)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.