Abstract
Bacterial efflux pumps are an important pathogenicity trait because they extrude a variety of xenobiotics. Our laboratory previously identified in silico Burkholderia collagen-like protein 8 (Bucl8) in the hazardous pathogens Burkholderia pseudomallei and Burkholderia mallei. We hypothesize that Bucl8, which contains two predicted tandem outer membrane efflux pump domains, is a component of a putative efflux pump. Unique to Bucl8, as compared to other outer membrane proteins, is the presence of an extended extracellular region containing a collagen-like (CL) domain and a non-collagenous C-terminus (Ct). Molecular modeling and circular dichroism spectroscopy with a recombinant protein, corresponding to this extracellular CL-Ct portion of Bucl8, demonstrated that it adopts a collagen triple helix, whereas functional assays screening for Bucl8 ligands identified binding to fibrinogen. Bioinformatic analysis of the bucl8 gene locus revealed it resembles a classical efflux-pump operon. The bucl8 gene is co-localized with downstream fusCDE genes encoding fusaric acid (FA) resistance, and with an upstream gene, designated as fusR, encoding a LysR-type transcriptional regulator. Using reverse transcriptase (RT)-qPCR, we defined the boundaries and transcriptional organization of the fusR-bucl8-fusCDE operon. We found exogenous FA induced bucl8 transcription over 80-fold in B. pseudomallei, while deletion of the entire bucl8 locus decreased the minimum inhibitory concentration of FA 4-fold in its isogenic mutant. We furthermore showed that the putative Bucl8-associated pump expressed in the heterologous Escherichia coli host confers FA resistance. On the contrary, the Bucl8-associated pump did not confer resistance to a panel of clinically-relevant antimicrobials in Burkholderia and E. coli. We finally demonstrated that deletion of the bucl8-locus drastically affects the growth of the mutant in L-broth. We determined that Bucl8 is a component of a novel tetrapartite efflux pump, which confers FA resistance, fibrinogen binding, and optimal growth.
Introduction
Burkholderia pseudomallei and Burkholderia mallei are Gram-negative bacteria that are the etiological agents of melioidosis and glanders, respectively [1]. Both pathogens are highly virulent and easily aerosolized, therefore they are classified as Tier one select agents by both the U.S. Department of Health and Human Services and the U.S. Department of Agriculture. In addition to being a biodefense concern, the bacteria are highly resistant to antibiotics and currently there is no licensed vaccine for either pathogen. Increasing global investigation into melioidosis has indicated that the disease may be more widespread than originally reported [2], and it has one of the highest disability-adjusted life years (DALYs) of neglected tropical diseases at 4.6 million [3].
B. pseudomallei is a soil saprophyte that can infect humans, resulting in symptoms ranging from localized infections, including swelling or ulcerations, to systemic infections that can lead to septic shock [4]. Treatment includes an extensive two-part chemotherapeutic regimen, most commonly using ceftazidime intravenously and then following it with an oral antibiotic eradication therapy of trimethoprim/sulfamethoxazole [5, 6]. B. mallei is a clonal derivative of B. pseudomallei that has undergone significant genomic reduction and rearrangement. This genomic evolution is attributed to the species transition from being a soil saprophyte to an obligate host pathogen, selecting for genes advantageous for host-survival [7]. Glanders primarily affects equines, but can infect other livestock such as donkeys and goats. Although uncommon in humans, this zoonotic disease is often fatal if left untreated [4]. Symptoms typically affect the pulmonary system, including pneumonia and lung abscess, but may also present as cutaneous ulceration following direct inoculation.
Several classes of efflux pumps are expressed in multidrug resistant Gram-negative bacteria, such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Burkholderia spp., and are at least partly responsible for their intrinsic antimicrobial resistance, including resistance-nodulation division (RND) efflux pumps [8]. Burkholderia are notorious for being resistant to an array of antibiotics, such as β-lactams, aminoglycosides, tetracyclines, fluoroquinolones, macrolides, polymyxins, and trimethoprim [9], resulting in serious infections that are hard to treat [10]. Bioinformatic analyses of the B. pseudomallei genomes have identified at least ten RND efflux pumps [11], although only three systems were characterized in more detail, e.g., AmrAB-OprA, BpeAB-OprB, and BpeEF-OprC [12]; this gap in knowledge underscores a need for more studies of drug efflux pumps in Burkholderia [13]. Importantly, a large body of evidence indicates that efflux pumps also contribute to resistance to a variety of host-defense molecules, biofilm formation, regulation of quorum sensing and balanced metabolism, and overall pathogenesis [14], which further accentuate the importance of the efflux systems in bacteria.
Our previous studies have identified 13 novel Burkholderia collagen-like (CL) proteins (Bucl) containing collagen-like Gly-Xaa-Yaa (GXY) repeats, as well as non-collagen domains, some of which had predicted functions: Talin-1 cytoskeletal integrin-binding domain, Bac_export_1 domain found in inner-membrane protein components of a type III secretion system, or Bac_export_3 domain of solute-binding proteins often associated with ABC-type transporters [15]. Specifically, Bucl8 was predicted to be an outer membrane protein, containing tandem efflux pump OEP1 and OEP2 (outer membrane efflux protein; PF02321) domains. Unique to Bucl8, as compared to typical outer membrane proteins with OEP domains, is the presence of an extended extracellular portion of unknown function that contains a presumed collagen-like (CL) domain, followed by a non-collagen C-terminal (Ct) region. This Bucl8 variant was present only in B. pseudomallei and B. mallei. In addition, the collagen domain, which is broadly characterized as a stretch of repeating GXY motifs [16], in Bucl8 is composed of an uncommon repeating (Gly-Ala-Ser or GAS)n collagen-like sequence.
Here, our objectives are to characterize the structure and function of the Bucl8 extracellular domain, define the bucl8 locus, and identify substrates and potential function(s) of the putative Bucl8-associated efflux pump. We demonstrate that the collagen-like domain indeed adopts the characteristic collagen triple-helical structure. In addition, the recombinant extracellular portion of Bucl8 can bind to fibrinogen. We find that Bucl8 is the outer membrane component of an efflux pump responsible for fusaric acid (FA) resistance, a potent mycotoxin produced by Fusarium species that cohabitate the soil environment with Burkholderia [17, 18]. We further identify bucl8-associated genes, designated fusCDE, encoding the remaining components of the putative Bucl8-efflux pump. Transcripts of the bucl8-operon were upregulated in B. pseudomallei and B. mallei by exogenous FA, as well as by FA-derivative p-hydroxybenzoic acid (pHBA), which is involved in regulation of balanced metabolism in E. coli. FA resistance was diminished in a B. pseudomallei isogenic deletion mutant without the bucl8 locus and could also be transferred to a FA-sensitive E. coli strain. Lastly, we found that the mutant grew at a significantly reduced rate, suggesting that under laboratory conditions the pump is important for the cell’s physiology. Here, we describe a previously unreported putative efflux pump with unique structure and functional implications in the biology of B. pseudomallei and B. mallei species.
Materials and methods
Bacterial strains and growth
Two BSL2 Burkholderia strains exempt from the Select Agents list were used in this study: (i) B. pseudomallei strain Bp82 is an avirulent ΔpurM mutant of strain 1026b [19], which was obtained from Christopher Cote (US AMRIID, Frederick, MD) and (ii) B. mallei CLH001 ΔtonBΔhcp1 mutant originates from the strain Bm ATCC23344 [20], which was obtained from Alfredo Torres (UTMB, Galveston, TX) (Table 1). Strain Bp82 was routinely grown in Luria broth-Miller (LBM) with shaking at 37°C and on Luria agar (LA) solid medium at 37°C. Strain CLH001 was grown under the same conditions, but the broth medium was supplemented with 4% glycerol. E. coli strains JM109 (Promega) and S17-1λpir/pLFX (E. coli Genetic Stock Center, Yale University) were cultured in LBM media and on LA. Antimicrobials were used in selective media and in susceptibility/ resistance assays, as described in the methods below.
Table 1. Bacterial strains and plasmids.
| Strains and Plasmids | Description/ Characteristics | Source | |
|---|---|---|---|
| B. pseudomallei | Bp 1026b (genomic DNA) | Blood culture from 29-year-old female rice farmer with diabetes mellitus, Northeast Thailand, Sappasithiprasong hospital; 1993 | BEI Resources |
| Bp K96243 (genomic DNA) | Female diabetic patient- Khon Kaen hospital, Northeast Thailand; 1996 | BEI Resources | |
| Bp82 | Attenuated 1026b strain with a partial deletion of the purM gene resulting in adenine and thiamine auxotrophy | USAMRIID, Frederick, MD | |
| Bp82Δbucl8-fusE | Bucl8-associated pump deletion mutant | This study | |
| B. mallei | CLH001 | Attenuated Bm ATCC23344 mutant with genes tonB (iron acquisition) and hcp1 (type 6 secretory system structural protein) deleted | UTMB, Galveston, TX |
| E.coli | JM109 | Host strain; ΔendA1, ΔrecA1, ΔlacZ gene | Promega |
| JM109::525 | JM109 with pSL525 plasmid containing the Bucl8-pump locus from Bp 1026b/Bp82 | This study | |
| JM109::529 | JM109 with pSL529 plasmid containing the Bucl8-pump locus from Bp K96243 | This study | |
| S17-1λpir/pLFX | Mobilization host | E. coli Genetic Stock Center, Yale University | |
| Plasmids | pQE-30 | E. coli expression vector for proteins with N-terminal 6xHis-tag; T5 promoter; ampicillin resistance | Qiagen |
| pUC18T-mini-Tn7T-Tp | Mobilizable TpR mini-Tn7 vector; trimethoprim and ampicillin resistance | [21] | |
| pMo130 | Mobilizable E. coli vector that is suicide in Burkholderia | [22] | |
| pSL520 | pQE-30-based plasmid for expression of rBucl8-Ct protein | This study | |
| pSL521 | pQE-30-based plasmid for expression of rBucl8-CL-Ct protein | This study | |
| pSL522 | pMo130-based plasmid with fusR for generating chromosomal deletion of Bucl8-pump. | This study | |
| pSL524 | pMo130-based plasmid with fusR and tar for generating chromosomal deletion of Bucl8-pump | This study | |
| pSL525 | pUC18T-mini-Tn7T-Tp based plasmid with Bucl8-pump locus of Bp 1026b/Bp82 | This study | |
| pSL529 | pUC18T-mini-Tn7T-Tp based plasmid with Bucl8-pump locus of Bp K96243 | This study | |
Bioinformatic analyses of the bucl8 locus
Annotation of transcriptional and translational signals
The promoter regions of fusR and bucl8 were defined by combining public transcriptome data and computational prediction. Briefly, strand-specific RNA-Seq data of B. pseudomallei [23] was downloaded from National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under BioProject accession PRJNA398168. The RNA-Seq read distribution across the genome was visualized by the UCSC genome browser [24], which includes a reference strain for 1106a. The genomic region spanning genes fusR to tar is highly similar between strain Bp 1106a and our target strain Bp 1026b (identity = 99.4%). The RNA-Seq reads were pooled and then mapped to the genome of strain 1106a using Bowtie2, which allows two base-pair mismatch [25]. The RNA-Seq read density at each genomic position was visualized by the UCSC genome browser [24] to determine putative transcription boundaries of fusR and bucl8. Sigma 70 promoters (-10 and -35) were predicted by BPROM [26]. Translation initiation sites (TISs) were predicted by TriTISA with default parameters [27]. The Shine-Dalgarno (SD) translation initiation signals were manually annotated within 20 bps upstream to TISs by considering “GGAG”, a SD consensus sequence annotated for Burkholderia [28]. The gene and protein designation were adopted according to Crutcher et al. 2017.
Prediction of FusR putative binding sites
The positions of the predicted FusR binding sites, a LysR-type transcriptional regulator, were determined using the University of Braunschweig Virtual Footprint Promoter analysis tool v3.0 [29]. Known LysR regulators were used as models to predict binding, including CysB, MetR, and OxyR from E. coli, GltC from Bacillus subtilis, and OxyR from P. aeruginosa. Standard settings were used to run the prediction (sensitivity = 0.8, core sensitivity = 0.9, and size = 5) on the 500-bp region upstream from the translational start site of bucl8.
Genetic and molecular biology methods
Construction of an unmarked isogenic deletion mutant of bucl8 locus in Bp82
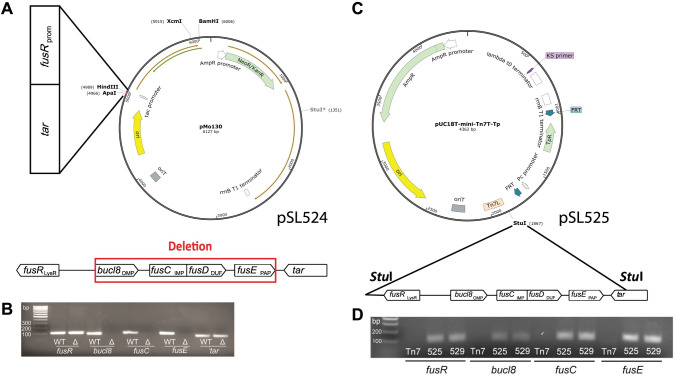
The chromosomal region in Bp82, encompassing genes bucl8-fusCD-fusE, was deleted using suicide plasmid pSL524 constructed in vector pMo130 (Addgene), as described previously [22]. Two Bp82-DNA fragments of about 1 kb each were sequentially cloned within the multiple cloning site of pMo130: (i) pSL522 construct, containing fusR gene located upstream of bucl8 was PCR-amplified with primers pSL522-ApaI-F and pSL522-HindIII-R, was cloned between ApaI-HindIII sites of the vector; and (ii) pSL524, containing tar gene located downstream of fusE was cloned at ApaI site, following amplification with primers pSL523-ApaI-2F and pSL523-ApaI-2R.
Plasmid pSL524 was introduced by conjugation into Bp82 via biparental mating with a donor strain E. coli S17-1λpir/pLFX::pSL524 on LA medium overnight. The mating bacteria were then scraped off and plated onto selective LA medium supplemented with 200 μg/mL kanamycin, to counter-select WT Bp82, and 50 μg/mL zeocin, to counter-select E. coli. Merodiploid colonies resulting from the single cross-over event, were sprayed with 0.45 M pyrocatechol (Sigma-Aldrich) to detect yellow transconjugants [22]. Several yellow colonies were streaked onto YT medium (10 mg/mL yeast extract, 10 mg/mL tryptone) containing 15% sucrose to force the excision of the bucl8-fusE locus and pMo130 sequence from Bp82 merodiploids. Colonies were grown for 48 hours. Successful excision produces deletion mutants as white colonies identified by spraying with pyrocatechol. White colonies were isolated and characterized by PCR and sequencing to confirm the deletion of the bucl8-fusCD-fusE genes.
Cloning of bucl8 locus in E. coli JM109
The cloning strategy was based on the genomic sequence of the Bp82 parent strain B. pseudomallei 1026b, which identified a ~8.2-kb StuI-StuI fragment, encompassing the entire fusR-bucl8-fusCD-fusE locus. Bp82 gDNA was digested with StuI and DNA species of about 8–10 kb were isolated from the gel and ligated to StuI-cleaved vector pUC18T-mini-Tn7T-Tp (pUC18T-mini-Tn7T-Tp was a gift from Heath Damron, Addgene plasmid # 65024) [21].The E. coli JM109 transformants were isolated on a LA medium containing 100 μg/mL FA. Plasmid pSL525 was isolated from several colonies and analyzed by restriction digestion. Junctions between vector and insert sequences were sequenced to establish insert orientation. The presence of fusR-bucl8-fusC-fusE genes was verified by PCR and sequencing.
The plasmid construct pSL529, containing the bucl8 sequence with extended CL region from Bp K96243, was also generated based on pSL525. An internal Bucl8 fragment from Bp K9264 (~1.4-kb) was PCR-amplified (using primers Bucl8-1F and BurkhFusBCD-1R) and cloned between two unique sites in bucl8, XcmI, and FseI, of pSL525. E. coli JM109 transformants were isolated on a LA medium containing 100 μg/mL ampicillin. The plasmid sequence was verified as before.
Cloning, expression and purification of Bucl8-derived recombinant proteins
Two recombinant polypeptides, derived from the presumed extracellular portions of Bucl8 variant in Bp K96243, were generated for this study: (i) pSL521-encoded rBucl8-CL-Ct polypeptide, containing both the collagen-like region and the non-collagen C-terminal region and (ii) pSL520-encoded rBucl8-Ct, which only includes the C-terminal region.
For cloning, gBlocks (Integrated DNA Technologies) were designed, encoding two recombinant constructs (S3 Table), as described [30]. gBlocks were used as templates to produce cloned DNA inserts using primers pSL521-F and pSL521-2R for pSL521 construct, and pSL520-F and pSL520-R for pSL520. gBlock DNA fragments were inserted between HindIII and BamHI sites of the pQE-30 vector, resulting in an N-terminal 6xHis-tag (Qiagen) for each construct and were then cloned into E. coli JM109. Plasmid constructs pSL520 and pSL521 were confirmed by sequencing (Primers pQE30-F, pQE30-2R).
For protein expression, E. coli JM109 with pSL520 or pSL521 constructs were grown in LBM plus 100 μg/mL ampicillin with shaking at 37⁰C overnight, and then 10 mL cultures were used to inoculate 1 L batches of the same media. The protein expression was induced in cultures at OD600 ~0.5 with 1 mM isopropyl β-d-1-thiogalactopyranoside for 3 hours and then bacterial cells were pelleted and frozen at -20°C overnight. Cell pellets were thawed and suspended in 10 mL of lysis buffer (50 mM Tris buffer, 50 mM NaCl, 2 mM MgCl2, 2% Triton X-100, 10 mM β-mercaptoethanol, 0.2 mg/mL lysozyme, 1 mL of Protease inhibitor (Pierce), 1 mM PMSF, 10 μg/mL). The samples were vortexed, placed on ice for 20 minutes, and then centrifuged. The supernatants were applied onto affinity columns with HisPurTM Cobalt Resins (Thermo Fisher Scientific) and purification was carried out according to manufacturer’s protocol. The eluted proteins were analyzed by 4–20% SDS-PAGE to assess the overall integrity and purity. The proteins were dialyzed in 25 mM HEPES and stored at -20°C.
Ligand binding assay to rBucl8-CL-Ct and rBucl8-Ct
In the initial screening assay, binding of the rBucl8-CL-Ct to different extracellular matrix (ECM) ligands was assessed by ELISA [31]. Wells were coated overnight with 1 μg of each ligand dissolved in bicarbonate buffer: collagen type I and IV (Sigma), elastin (Sigma), fibrinogen (Enzyme Research), plasma fibronectin (Sigma), cellular fibronectin (Sigma), laminin (Gibco), and vitronectin (Sigma). Next, 1 μg per well of rBucl8-CL-Ct in TBS, 1% BSA was added and incubated for two hours at 37°C. Wells were washed with TBS and bound rBucl8-CL-Ct was detected with anti-6His-tag mouse mAb (Proteintech) in TBS-1% BSA and a secondary goat anti-mouse HRP-conjugated Ab (Jackson Immuno Research Laboratories Inc.); immunoreactivity was detected with ABTS substrate and measured spectrophotometrically at OD415. Data represent the mean ±SE of three independent experiments (n = 3), each performed in triplicate wells. Concentration-dependent binding was assessed in a similar manner, however with varying concentrations (0–10 μM) of rBucl8-CL-Ct.
Structural characterization of Bucl8
Homology modelling of the periplasmic/outer membrane component of Bucl8 was performed using the software MODELLER [32] and the structure of VceC from V. cholerae as a template (PDB code 1yc9). For the collagen-like (CL) region of Bucl8, homology modelling was performed with MODELLER [32] using the high-resolution structure of a collagen-like peptide (PDB code 1k6f) [33] as a template. The Ct random coil region was generated using the Molefacture plugin of VMD [34]. Electrostatic potential surface was computed using the software Chimera [35].
Circular dichroism spectroscopy (CD) of rBucl8-derived polypeptides was performed as previously described [30]. Briefly, protein samples were dialyzed against 1x Dulbecco’s phosphate buffered saline, pH 7.4. CD spectra were taken with a Jasco 810 spectropolarimeter, in a thermostatically controlled cuvette, with a path length of 0.5 cm. Data were acquired at 10 nm per minute. Wavelength scans were performed from 240 nm to 190 nm at either 25°C or 50°C for unfolded triple helix in rBucl8-CL-Ct construct.
Gene transcription by RT-qPCR
Duplicated bacterial cultures of Bp82 and CLH001 were grown in broth media at 37°C with shaking till early logarithmic phase (OD600 ~0.4), then, FA was added to one of each culture at sub-inhibitory concentrations and incubated for one hour. Cultures were mixed with a 1:2 ratio of RNA Protect reagent, incubated for five minutes, then centrifuged and decanted. Pellets were suspended in lysing buffer (1.3 μg/μL proteinase K, 0.65 mg/mL lysozyme, TE; 10 mM Tris, 1mM EDTA, pH 7) and incubated for ten minutes. Total RNA was isolated using RNeasy Protect Bacteria Mini kit (Qiagen). TurboDNase enzyme [36] was used to remove traces of genomic DNA. RNA was either used immediately for cDNA synthesis or stored at −80°C for no more than one week. cDNA was generated using iScript cDNA synthesis kit (Bio-Rad).
RT-qPCR was performed using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad), with primers listed in S1 Table. Transcript levels were normalized to 16S rRNA [37]. Transcription fold change was calculated as relative to non-FA conditions, using the 2-ΔΔCT method. Technical and experimental replicates were done in triplicate.
Determination of antimicrobial susceptibility/resistance
Antimicrobial susceptibility by broth dilution method
Minimum inhibitory concentration (MIC) testing was performed in liquid and on solid media. Initially, Bp82 and CLH001 were grown overnight at 37°C with shaking to inoculate fresh media with varying concentrations of FA (32 μM to 8000 μM), as described [38]. The optical density was recorded after overnight incubation and colony forming units (CFU) were calculated after plating serially diluted samples on LA media.
Antimicrobial sensitivity on agar
Strains were also tested for growth on LA media supplemented with differing concentrations of antimicrobials. Bacterial cultures were grown to an optical density of ~0.4 and plated on agar, and incubated at 37°C for 48 hours. The following concentrations of antimicrobials were used: fusaric acid (FA), 100–800 μg/mL [39]; para-hydroxybenzoic acid (pHBA), 0.5–2.5 mg/mL [40]; and chloramphenicol (CHL), 2–32 μg/mL serially diluted [41].
Antimicrobial susceptibility in clinical laboratory
Strains were tested with antimicrobials in a clinical laboratory using Thermo Sensititre GNX3F dehydrated 96-well plates (TREK Diagnostic Systems). Bacterial cultures were grown on LA medium and cells were emulsified in sterile water to turbidity of 0.5 McFarland. The suspension was then diluted in cation adjusted Mueller-Hinton broth with TES buffer before inoculation of 100uL (approximately 5x105 CFU) into each antimicrobial test well. Plates were incubated for 24 hours at 34–36°C in a non-CO2 incubator. Results were read and interpreted based on manufacturer’s protocol and CLSI MIC interpretive guidelines. Antimicrobials tested included: amikacin, doxycycline, gentamicin, minocycline, tobramycin, tigecycline, ciprofloxacin, trimethoprim/sulfamethoxazole, levofloxacin, aztreonam, imipenem, cefepime, meropenem, colistin, polymyxin, ceftazidime, cefotaxime, ampicillin/sulbactam, doripenem, piperacillin/tazobactam, ticarcillin/clavulanate.
Antimicrobial susceptibility was also assessed by disk diffusion using the following antimicrobials: ampicillin (Am 10), ciprofloxacin (CIP 5), doxycycline (D 30), gentamycin (GM), trimethoprim/sulfamethoxazole (SXT), tetracycline (TE 30), tobramycin (NN 10), levofloxacin (LVX 5).
Statistical analysis
Statistics were performed using GraphPad Prism software for two‐tailed paired Student's t ‐test, one‐way and two‐way ANOVA, pending the experiment. For gene expression of the bucl8 operon in Bp82, statistical analysis was applied to log-transformed fold changes to account for the phenomena of heteroscedasticity. Significance was denoted at levels of *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Error bars represent standard error measurements (SEM) with analyses based on three independent experimental repeats (n = 3), each performed in triplicate technical replicates, unless otherwise noted.
Results
Structural and functional characterization of the extended extracellular domain of Bucl8
Bucl8 is a homotrimeric molecule, with each mature monomer comprised of two tandem outer membrane efflux protein domains (OEP1 and OEP2), and a rare repetitive region consisting of glycine, alanine, and serine (GAS)n triplet repeats, here denoted as the CL domain, which is followed by a non-collagen carboxyl-terminal (Ct) region (Fig 1A). We homology-modelled the structure of Bucl8’s periplasmic/outer-membrane component, with the program MODELLER, using the crystal structure of the outer membrane channel protein VceC from V. cholerae (35.8% sequence identity; Table 2) as a template. In the homology model, the OEP1 domain is a typical α-barrel, formed by twelve short helices and six long helices, spanning the periplasmic space. The OEP2 domain forms a β-barrel, which crosses the outer membrane outwards (Fig 1B). Bucl8’s homology model can be superimposed over the structure of OprM (RMSD between the two structures of 2.0 Å), which is an outer membrane component of the tripartite efflux pump complex MexAB-OprM.
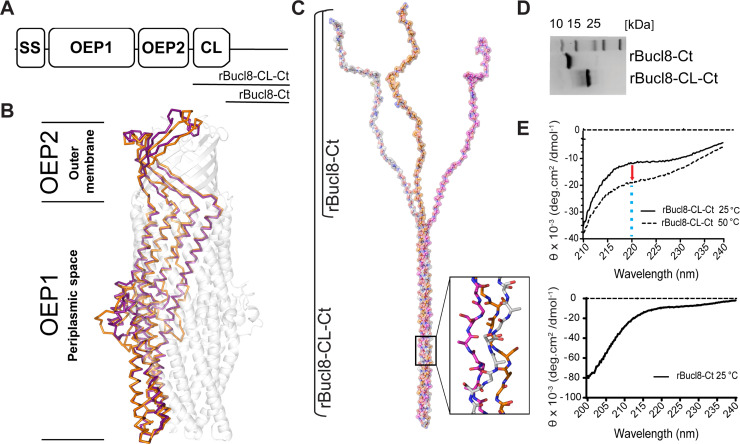
Fig 1. Structure analysis of the Bucl8 outer membrane protein.
(A) Schematic organization of Bucl8 structural organization (not to scale). Bucl8 domains include: signal sequence (SS), outer membrane efflux protein domains 1 and 2 (OEP1, OEP2), collagen-like region (CL) and the C-terminus (Ct). The Bucl8 regions represented in recombinant proteins rBucl8-CL-Ct and rBucl8-Ct are depicted. (B) Homology modeling of Bucl8 intracellular OEP1-OEP2 region. Bucl8, modelled off the VceC structure (pdb code 1YC9), is shown in transparent grey. Alpha chains of Bucl8 periplasmic/outer-membrane domains (purple) superimposed onto OprM (1wp1) α-chains (orange) are shown in solid ribbon representations. (C) Structural modeling of Bucl8 extracellular CL-Ct region. Model depicts a homotrimeric polypeptide consisting of triple-helical CL domain of rBucl8-CL-Ct and unstructured C-terminus (rBucl8-Ct). The stick model in the inset depicts the triple helical fold of repeating (GAS)n collagen sequence of Bucl8-CL. (D) 4–20% SDS-PAGE analysis of recombinant Bucl8-derived constructs. rBucl8-CL-Ct and rBucl8-Ct polypeptides were expressed in E. coli and purified via His-tag affinity chromatography (original, uncropped gel image is shown in S1 Raw image). (E) Circular dichroism (CD) spectroscopy. (Upper plot) Wavelength scans of rBucl8-CL-Ct were performed at 25°C (solid line) and 50°C (dashed line). A drop in molar ellipticity maximum at 220 nm (Θ220) is observed in the CD spectra, indicating the transition from triple-helical (25°C) to unfolded form (50°C). (Bottom plot) CD spectrum of rBucl8-Ct at 25°C indicates an unstructured form.
Table 2. Sequence identities between Bucl8-associated efflux pump components and known corresponding proteins.
| Protein | Species | PDB code | Sequence identity (%) | Query cover (%) | |
|---|---|---|---|---|---|
| Bucl8* | VceC | Vibrio cholerae | 1YC9 | 35.8 | 63 |
| NodT | Burkholderia mallei | 6U94 | 31.6 | 61 | |
| CusC | Escherichia coli | 3PIK | 28.7 | 61 | |
| OprJ | Pseudomonas aeruginosa PAO1 | 5AZS | 29.9 | 61 | |
| OprM | Pseudomonas aeruginosa | 1WP1 | 29.0 | 61 | |
| MtrE | Neisseria gonorrhoeae | 4MT0 | 26.9 | 61 | |
| TolC | Escherichia coli | 1EK9 | 24.7 | 38 | |
| FusC | FuaA | Stenotrophomonas maltophilia | 41.9 | 44 | |
| AaeB | Klebsiella pneumoniae | 24.62 | 60 | ||
| MexB | Pseudomonas aeruginosa | 3W9I | NS | ||
| AcrB | Escherichia coli | 4ZLJ | NS | ||
| CusA | Escherichia coli | 3K07 | NS | ||
| FusD | AcrZ | Escherichia coli | 4C48 | NS | |
| CusF | Escherichia coli | 3E6Z | NS | ||
| YajC | Escherichia coli | 2RDD (in complex) | NS | ||
| FusE | MexA | Pseudomonas aeruginosa | 30.99 | 48 | |
| AcrA | Escherichia coli | 2F1M | 27.27 | 48 | |
| BesA | Borreliella afzelii | 4KKS | 26.56 | 48 | |
| EmrA | Klebsiella pneumoniae | 4TKO | 26.73 | 86 | |
| CusB | Escherichia coli | 3H94 | NS |
NS = No significant similarity
*Comparison to Bucl8 protein sequence without CL-Ct domains.
Following OEP2, towards the extracellular space, the trimeric structure of the molecule supports a triple-helical structure of the extracellular CL-(GAS)n domain (Fig 1C), although, the specific (GAS)n sequence has not been studied previously for triple helix formation. The number of consecutive (GAS)n repeats present fluctuates between Bucl8 variants from different B. pseudomallei isolates. Analysis of ~100 bucl8 alleles showed (GAS)n numbers ranging from 6 to 38 repeats (mode: 20). Notably, 21 consecutive GAS repeats characterize the Bucl8 of B. pseudomallei model strain K96243, while the Bucl8 variants of the strains utilized in this study have fewer (GAS)n numbers, e.g., Bp 1026b/Bp82 has six and B. mallei strain Bm ATCC 23344/CLH001 has eight. Following the CL-(GAS)n domain is a Ct region of 72 amino acids that are conserved among B. pseudomallei and B. mallei strains (S1 File).
Here, we homology-modelled a representative (GAS)19 sequence using the structure of the collagen peptide (PPG10) 3 as a template (PDB code 1k6f, seqid 36%) [33] and the software MODELLER (Fig 1C). This structure formed a triple helix of about 163 Å in length. On its C-terminal end, the Ct domain of each chain is predicted by JPRED to be unfolded and was modeled in a random coil conformation (Fig 1C). Consistent with the sequence composition of the (GAS)n repetitive domain, its electrostatic potential surface appears neutral, with only a few positive charges due to the presence of arginine residues in the unstructured Ct regions of the molecule.
To experimentally validate this homology-modelled structure of the extracellular part of Bucl8, two recombinant proteins, derived from the extracellular portion of Bucl8 variant in strain Bp K96243, were designed and expressed in E. coli. The construct rBucl8-CL-Ct includes the CL-(GAS)19 domain and adjacent unstructured C-terminus (Ct), while construct rBucl8-Ct encompasses the Ct region only. Both Bucl8-derived polypeptides migrate aberrantly in SDS-PAGE in relation to molecular weight standards, e.g., rBucl8-CL-Ct of expected 11.7 kDa and rBucl8-Ct of 7.8 kDa (Fig 1D). Structural analysis of rBucl8-CL-Ct rendered at 25°C, using circular dichroism spectroscopy, confirmed a triple helical structure, demonstrated by a shallow peak at 220 nm (Fig 1E). As a control, denatured rBucl8-CL-Ct (50°C line) displayed a further-depressed peak at 220 nm that no longer held a triple-helical collagen structure. The 220 nm peak in rBucl8-CL-Ct is less pronounced when compared to typical triple helices formed by perfect GPP collagen repeats. This feature suggests the coexistence of both triple helix and random coil structures and/or the contribution of the non-collagen Ct region to the spectrum; such effects on CD spectra were previously reported for streptococcal collagen-like rScl constructs [42]. Additionally, the rBucl8-Ct structure was also analyzed by circular dichroism spectroscopy. The absence of ellipticity maxima and/or minima of known structures, e.g., α-helices or β-strands [43], indicates an unstructured protein (Fig 1E). Altogether, using in silico modeling and experimental CD spectroscopic analyses of the representative recombinant protein, we demonstrated that repeating (GAS)n of the predicted Bucl8-CL region from B. pseudomallei and B. mallei can form a stable collagen triple helix; to our knowledge, this is the first such demonstration obtained for the unusual repeating (GAS)n collagen-like sequence.
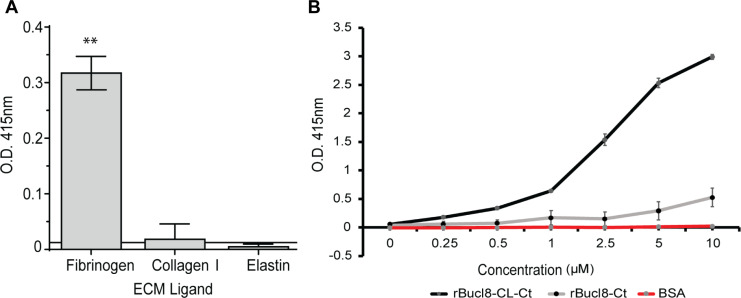
Bacterial proteins harboring CL domains from diverse genera have been demonstrated to bind ligands, including extracellular matrix proteins (ECM), and have been shown to participate in pathogenesis [44–46]. Here, we screened several human compounds by ELISA to ascertain a potential ligand binding function of Bucl8’s extracellular region, rBucl8-CL-Ct; ligands included fibrinogen, collagen-I and IV, elastin, plasma and cellular fibronectin, and vitronectin. Of the ligands tested, rBucl8-CL-Ct construct showed significant binding to fibrinogen, but not to collagen I and elastin (Fig 2A), while binding to other ligands tested was also not significant (not shown). rBucl8-CL-Ct binding to fibrinogen-coated wells was concentration-dependent in contrast to control BSA-coated wells. In addition, rBucl8-Ct construct showed limited level of binding to fibrinogen in this assay (Fig 2B).
Fig 2. Binding of rBucl8-derived constructs to extracellular matrix proteins.
(A) Screening assay for rBucl8-CL-Ct binding to extracellular matrix proteins. Ligand binding was tested by ELISA; representative examples of rBucl8-CL-Ct-binding-positive and binding-negative ligands are shown. rBucl8-CL-Ct binding was compared statistically with binding to BSA-coated wells plus two standard deviations; Student’s t-test, **p ≤ 0.01. (B) Concentration-dependent binding of rBucl8-CL-Ct and rBucl8-Ct to fibrinogen. Wells were coated with fibrinogen and either recombinant Bucl8-derived protein was added at increasing concentrations. Data represents the mean ±SEM of three independent experiments (n = 3), each performed in triplicate wells. Binding was detected with an anti-His-tag mAb.
Identification of bucl8 operon in Burkholderia pseudomallei and Burkholderia mallei
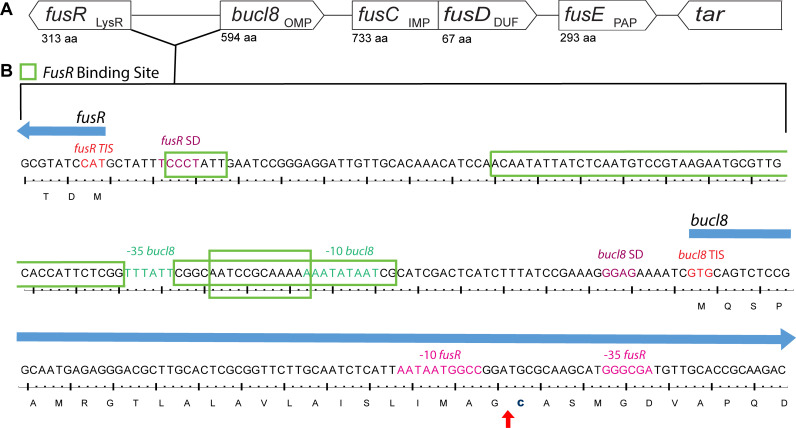
Previously, we identified two tandem outer-membrane-efflux-protein domains in Bucl8 [15], leading to the current hypothesis that Bucl8 is the outer membrane component of an efflux pump. Genes encoding efflux pumps are often clustered in operons that are controlled in cis by transcriptional regulators, such as MexR of P. aeruginosa and AmrR of B. pseudomallei [47–49]. For this reason, we examined the genes surrounding bucl8, which are described in Table 3 and depicted in Fig 3A. The locus contains additional efflux-pump associated genes, annotated in the NCBI database to be involved in fusaric acid (FA) resistance, which we designated here as ‘fus’, as previously proposed [39]. In agreement with genomic annotations, we recognize that Bucl8 is an outer membrane lipoprotein with a lipid moiety attached via the N-terminal Cys residue of the mature protein (Fig 3B; residue No. 24). In the genome of B. pseudomallei 1026b, downstream of bucl8 (OMP; 594 aa) are: fusC, presumably encoding the inner membrane protein of the pump (IMP; 733 aa), fusD, encoding a small protein with domain of unknown function (DUF; 67 aa), and fusE encoding the periplasmic adaptor protein (PAP; 293 aa). The ATG start codon of fusD overlaps with a stop TGA codon of fusC. The direction of the next downstream gene, tar, is opposite to bucl8-fusCDE and was presumed by definition to be outside of this operon. Flanking the locus at the 5’ end of bucl8 is a divergently-oriented gene, encoding a LysR-type transcriptional regulator (LysR; 313 aa) [50], designated here as fusR. The proximity and opposite orientation of fusR gene in relation to the bucl8-fusE genes resembled the typical gene organization described in tripartite efflux pumps with LysR-type regulators; therefore, we hypothesized bucl8 transcription to be regulated by the fusR product. Using predictive software and analysis of transcriptome data, the promoters, transcription initiation sites (TIS), and FusR binding sites were identified in the intergenic region between fusR and bucl8 (Fig 3B). FusR was predicted to have four binding sites, depicted in the green boxes that overlap with the bucl8–10 and -35 sites. The consensus sequence for B. pseudomallei is “GGAG”, according to the ProTISA database [27], which matches bucl8’s predicted Shine-Dalgarno sequence. Thus, fusR-bucl8-fusCD-fusE constitute a regulon, likely involved in FA resistance. The bucl8 locus was also conserved in Bp strain K96243 and Bm ATTC 23344; however, transcriptional units of bucl8-fusE were on the positive strand in the genome of K96243 strain, and on the negative strand in Bp 1026b and Bm ATTC 23344 (Table 3).
Table 3. Genes and associated identification numbers of bucl8 locus.
| Bp 1026b | Bp K96243 | Bm ATTC 23344 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Product Annotation | Locus tag | Protein ID | Genomic position | Locus tag | Protein ID | Genomic position | Locus tag | Protein ID | Genomic position |
| fusR | Transcriptional regulator | BP1026B_I1940 | AFI66557.1 | 2150545–2151486 | BPS_RS10485 | WP_004534689.1 | 2345922–2346863 | BMA_RS04430 | WP_004191155.1 | 987878–988819 |
| bucl8 | RND efflux system, outer membrane lipoprotein, NodT family protein | BP1026B_I1941 | AFI66559.1 | 2151644–2153428 | BPS_RS10490 | WP_162486666.1 | 2347036–2348973 | BMA_RS04425 | WP_024900385.1 | 985939–987705 |
| Fusc | Fusaric acid resistance protein | BP1026B_I1942 | AFI66560.1 | 2153445–2155646 | BPS_RS10495 | WP_009937757.1 | 2348990–2351191 | BMA_RS04420 | WP_004192976.1 | 983721–985922 |
| fusD | Hypothetical protein | BP1026B_I1943 | AFI66561.1 | 2155643–2155846 | BPS_RS10500 | WP_004191885.1 | 2351188–2351391 | BMA_RS04415 | WP_004191885.1 | 983521–983724 |
| fusE | Fusaric acid resistance protein fusE | BP1026B_I1944 | AFI66562.1 | 2155860–2156741 | BPS_RS10505 | WP_004534908.1 | 2351405–2352286 | BMA_RS04410 | WP_004191342.1 | 982626–983507 |
| Tar | Methyl-accepting chemotaxis protein | BP1026B_I1945 | AFI66563.1 | 2157092–2158768 | BPS_RS10510 | WP_004196082.1 | 2352657–2354333 | BMA_RS04405 | WP_004196082.1 | 980610–982286 |
Data were retrieved from NCBI for B. pseudomallei 1026b, B. pseudomallei K96243, and B. mallei ATTC 23344 reference genomes.
Fig 3. Chromosomal locus surrounding bucl8 gene in B. pseudomallei and B. mallei.
(A) Schematic of bucl8-associated locus with presumed protein function (subscript) and amino acid length (aa). Upstream of bucl8 is gene fusR, while downstream are genes fusCD and fusE. Flanking the bucl8 operon is unrelated downstream gene tar. LysR, LysR-type transcriptional regulator; OMP, Outer membrane protein; IMP, Inner membrane protein; DUF, Domain of unknown function; and PAP, periplasmic adaptor protein. (B) Regulatory intergenic region between fusR and bucl8. Both nucleotide and translated sequence are shown. Red arrow indicates cleavage site between the signal peptide and N-terminal cysteine linker (bolded).
Fusaric acid increases relative expression of bucl8-operon transcripts
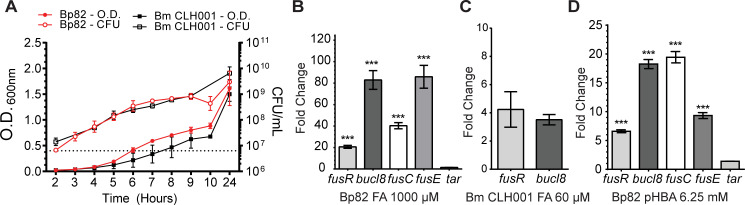
We identified a conserved operon associated with the bucl8 gene that was present in all B. pseudomallei and B. mallei genomes analyzed, including the mutant strains Bp82 and CLH001 used in this study, and had similarity to genes encoding FA resistance found in other Gram-negative bacteria [17, 18, 39]. We consequently tested the predicted FA substrate as a transcriptional inducer for genes associated with the Bucl8-efflux pump. We first examined the FA minimum inhibitory concentration (MIC) in B. pseudomallei (Bp82) and B. mallei (CLH001) strains using a broth dilution method in the range of 32 μM FA to 8000 μM, which was based on an earlier induction data employing GFP reporter construct in P. putida [38]. Here, we established the FA-MIC for Bp82 as 4000 μM (716 μg/mL) and 250 μM (44 μg/mL) for CLH001.
Sub-inhibitory concentrations of FA, e.g., 1000 μM for Bp82 and 60 μM for CLH001 that did not inhibit the growth rates were used in subsequent induction experiments (Fig 4A). Total RNA was isolated from the cultures of Bp82 and CLH001 that were either non-treated or treated with FA (1000 μM or 60 μM, accordingly) at OD600 ~0.4 for one hour. Both fusR and bucl8 genes were expressed in non-treated cultures at basal levels, but transcription of bucl8 in Bp82 was significantly induced with FA by an average 82-fold change in relative expression and a 20-fold change of fusR, using 2ΔΔCt calculations (Fig 4B). CLH001 also demonstrated about a four-fold increase for fusR and bucl8 when induced with 60 μM FA (Fig 4C), although this change is comparatively lower than that recorded in FA-induced Bp82.
Fig 4. Effect of FA and pHBA on gene transcription within fusR-bucl8-fusCD-fusE operon.
(A) Growth curves of B. pseudomallei strain Bp82 and B. mallei strain CLH001. Cultures were grown in strain-specific broth and optical density (O.D.) and colony forming units (CFU) were recorded. Dotted line represents OD of 0.4. Error bars represent ±SEM. (B-D) RT-qPCR was performed on RNA samples isolated from cultures of the indicated strain, untreated and treated with substrate, at an OD600 of ~0.4 for 1 hour. Graph shows fold change of relative gene expression compared to untreated cultures and normalized to transcription of 16S rRNA gene. Technical and experimental replicates were done in triplicate. One-way ANOVA with Tukey’s multiple comparison test of the log10 –transformed fold change. Significance shown is in comparison to tar; ***p < 0.001. Error bars represent ±SEM. (B) Transcription activation of fusR-bucl8-fusCD-fusE genes in Bp82 with 1000 μM FA. The downstream tar gene is assumed outside of the fusR-bucl8 operon. (C) Transcription activation of fusR and bucl8 in CLH001 with 60 μM FA. (D) Transcription activation of Bucl8 regulon in Bp82 with 6.25 mM pHBA.
In a following experiment we confirmed the boundaries of the fusR-bucl8 operon by RT-qPCR. Results show that transcription levels of fusR-bucl8-fusC-fusE were all significantly upregulated in samples treated with FA, compared to non-treated controls (fusR = 20-fold ± 1.37; bucl8 = 82-fold ± 8.73; fusC = 40-fold ± 2.84; fusE = 86-fold ± 10.65; Fig 4B). In contrast, the expression change of tar was significantly lower than genes from the fusR-bucl8-fusC-fusE operon and the associated regulatory gene fusR (1.5-fold ± 0.03. One-way ANOVA with Tukey’s multiple comparison test of the log10-transformed fold change; ***p < 0.001 for all genes compared to tar). This is the first demonstration of FA-inducible efflux pump in B. pseudomallei and B. mallei.
A structural analog of fusaric acid pHBA induces pump expression
Previous work reported that FusC-containing FA-exuding pumps were phylogenetically related to the aromatic carboxylic acid (AaeB) pumps, although it was unknown whether AaeB systems extrude FA [39]. Notably, studies in E. coli show that regulated concentrations of an FA-derivative, para-hydroxybenzoic acid (pHBA), inside bacterial cells is important for balanced metabolism of the aromatic carboxylic acids [40]. Thus, we hypothesized pHBA would also increase the relative expression of the bucl8 operon as FA did. Broth cultures of Bp82Δbucl8-fusE were induced with the sub-inhibitory concentration of 6.25 mM (863 μg/mL) pHBA and compared to non-treated cultures. RT-qPCR data showed in Bp82 pHBA induced a 7-fold ± 0.26 change in fusR, an 18-fold ± 0.78 change in bucl8, a 19-fold ± 0.98 change in fusC, and a 9-fold ± 0.52 change in fusE. Transcription of tar was not significantly affected (1.4 fold ± 0.006 change; One-way ANOVA with Tukey’s multiple comparison test of the log10-transformed fold change; ***p < 0.001 for all genes compared to tar) (Fig 4D). Evidence that aromatic carboxylic acids can induce transcription of this pump may help elucidate the broader function of Bucl8-associated pump in B. pseudomallei and B. mallei.
Deletion of of and complementation with the Bucl8-associated pump affect sensitivity and resistance to FA and pHBA
In order to demonstrate the function of the Bucl8-pump in various physiological roles, we used a genetic approach by generating two strains for assessing (i) loss-of-function and (ii) gain-of function. For loss-of-function, we made an isogenic Bp82 mutant harboring chromosomal deletion of bucl8-fusCD-fusE segment, as described [22]. Plasmid pSL524 (Table 1) was constructed in the E. coli vector pMo130, which is suicidal in Burkholderia, to generate an unmarked deletion mutant (Fig 5A). Construct pSL524, carrying upstream and downstream sequences flanking bucl8 locus was transferred to B. pseudomallei Bp82 via biparental mating. Deletion was achieved in a two-step insertion/excision process, as detailed in Materials and Methods section. Successful deletion of the bucl8-fusCD-fusE segment from the chromosome was confirmed by PCR (Fig 5B) and sequencing. We did not delete the fusR gene on purpose to avoid a possible global regulatory effect associated with unknown FusR function.
Fig 5. Construction of an unmarked Bucl8-associated pump deletion mutant and complementation in a heterologous host.
(A) Strategy for generating an unmarked Bucl8-pump deletion mutant. Construction of the suicide plasmid construct pSL524. Vector pMo130, which is suicide in Burkholderia, was used to generate pSL524 plasmid construct for mutagenesis. HindIII and ApaI sites were utilized to clone flanking regions containing fusR and tar sequences to delete the bucl8-fusE coding region, depicted below. (B) Analysis of the bucl8-fusE deletion mutant of Bp82 by PCR. The presence of bucl8-fusE genes was tested in the genomic DNA isolated from wild type Bp82 (WT) and Bp82 bucl8-fusE mutant (Δ). (C) Cloning of the Bucl8-pump locus for in-trans complementation in E. coli. Vector pUCT18T-mini-Tn7T-Tp was used for cloning of an 8.2-kb genomic Bp82 fragment, flanked by StuI sites, encompassing bucl8 locus. (D) Characterization of the pSL525 and pSL529 constructs. The presence of fusR-fusE genes on pSL525 and pSL529 plasmids was tested by PCR. PCR products shown in B and D were analyzed on 1.3% agarose gel.
To exhibit gain-of function, we complemented a heterologous E. coli host in-trans with a plasmid construct pSL525 (Table 1) harboring the whole bucl8 locus, generated in a mini-transposon vector pUC18T-mini-Tn7T-Tp, as depicted in Fig 5C. JM109::525 transformants were selected on agar containing 100 μg/mL FA and cloning was verified by PCR (Fig 5D) and sequencing. Since Bp82 represents the 1026b strain harboring Bucl8 variant with (GAS)6 repeats in the CL region, we made an additional construct, pSL529, that contains (GAS)21 repeats, to represent the majority of B. pseudomallei strains, by extending the number of GAS triplets in pSL525.
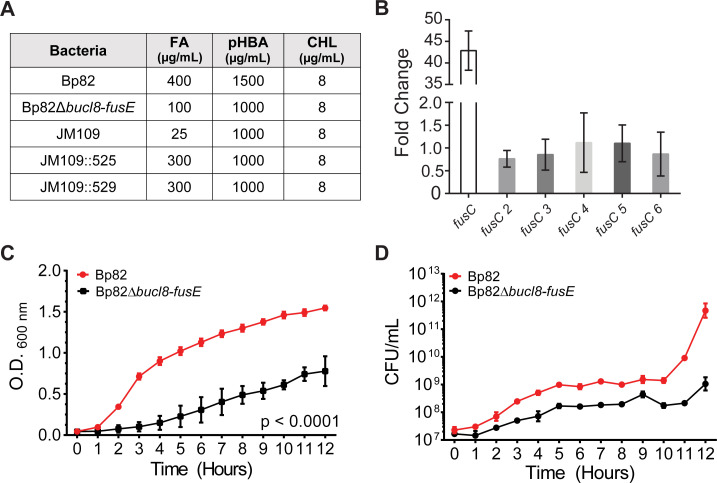
MICs were determined for bacterial growth on LA plates containing FA or pHBA chemicals, ranging from 0 to 800 μg/mL FA and 500–2,500 μg/mL pHBA (Fig 6A). There was a 4-fold decrease in MIC to FA from 400 μg/mL to 100 μg/mL recorded for Bp82Δbucl8-fusE mutant compared to the parental Bp82 strain. A similar effect was observed for pHBA; the MIC for Bp82 was 1500 μg/mL which decreased to 1000 μg/mL in the mutant. A 12-fold increased MIC on the LA medium with FA was recorded in E. coli JM109::525 and JM109::529 (MIC = 300 μg/mL) compared with the JM109 (MIC = 25 μg/mL) recipient. Interestingly, complementation with whole Bucl8-associated pump, however, did not increase the MIC for pHBA above 1000 μg/mL for JM109::525 or JM109:529 strains.
Fig 6. Analysis of loss-of-function and gain-of-function associated with chromosomal deletion and in-trans complementation of Bucl8-pump components.
(A) Changes in sensitivity/resistance patterns in bacterial strains. MIC was determined by plating bacteria on LA containing each substrate. FA, fusaric acid; pHBA, para-hydroxybenzoic acid; CHL, chloramphenicol. (B) Relative expression of fusC genes. RT-qPCR was performed on total mRNA isolated from non-treated and FA-treated (1000 μM, 1 hour) Bp82 cultures (OD600 ~0.4). Graph shows fold change of relative gene expression compared to untreated cultures and normalized to 16S rRNA. Technical and experimental replicates were done in triplicate. (C-D) Effect of chromosomal deletion on growth. Parental strain Bp82 and its bucl8-fusE deletion mutant (Bp82Dbucl8-fusE) were grown in LBM broth at 37°C with shaking. Changes in OD600 (C) were recorded and CFU numbers (D) by plating on LA medium every hour. Data represents the average of three biological replicates. 2-way AVOVA with Tukey multiple comparison test, ***p < 0.001. Error bars represent ±SEM.
Although deletion of the Bucl8-associated pump resulted in a drastically decreased MIC, Bp82Δbucl8-fusE mutant still maintained residual level of FA resistance (100 μg/mL). Therefore, we hypothesized that additional proteins annotated as FusC are contributing to the remaining FA resistance recorded in the Bp82Δbucl8-fusE mutant. Within Bp 1026b and K96243 genomes, there are six genes present that are annotated as FusC-type proteins (Pfam #PF04632), including the protein arbitrarily designated as FusC, which is associated with Bucl8, whereas remaining five were designated FusC 2 thru FusC 6 (S2 Table). These protein sequences ranged roughly in three different lengths: ~200 amino acids for FusC 3, ~350 for FusC 4 and 6, and ~750 amino acids for FusC, FusC 2 and FusC 5. Upon examination, the loci around FusC genes 2 thru 6 were not arranged in as discernable tripartite-pump operons, like FusC, although some were adjacent to either a MFS transporter protein or genes encoding amino acid permeases. To test whether these genes are regulated by FA addition, we performed RT-qPCR on RNA isolated from Bp82 cultures induced with 1000 μM FA and without treatment. The transcription of fusC 2–6 genes showed little to no fold-change (0–2-fold; Fig 6B) when compared to non-treated samples, which contrasts with ~40-fold difference in fusC transcription (Fig 4B). Thus, we conclude that these fusC genes are not inducible by FA.
Bucl8-associated pump does not contribute to the Multidrug Resistance (MDR) phenotype
Efflux pumps contribute to MDR in Gram-negative bacteria [13], including Burkholderia species [9], and are often polyspecific [51]. A study in S. maltophilia concluded that an FA efflux pump did not extrude the antimicrobials tested [52]. Here, we assessed changes in resistance/susceptibility levels between Bp82 and Bp82Δbucl8-fusE, and JM109 and JM109::525 or JM109:529 against variety of antimicrobials.
In the clinical laboratory setting, the Burkholderia failed to grow in commercial medium, and therefore only the E. coli data were generated. Overall, there was not a significant increase in resistance to any of the antibiotics tested; JM109::525/529 showed only increased resistance to the β-lactam antibiotics, which was associated with the resistance gene present on the inserted plasmid. A disc diffusion test, including ampicillin, ciprofloxacin, levofloxacin, tobramycin, gentamicin, tetracycline, doxycycline, and trimethoprim-sulfamethoxazole, resulted in similar zones of inhibition for both Bp82 and Bp82Δbucl8-fusE cultures, as well as E. coli JM109 and JM109::525/529, again with the exception of the plasmid-derived β-lactam resistance determinant.
Microarray data comparing the effect of 84 growth conditions on B. pseudomallei transcriptome showed that chloramphenicol (CHL), which contains an aromatic ring in its structure, induced bucl8 expression, thus, implying CHL might be a substrate for Bucl8-associated pump [53]. Here, we determined the CHL-MICs of our B. pseudomallei and E. coli strains using a growth assay on the LA medium; however, the MIC for all the strains was the same (8 μg/mL; Fig 6A). In addition, the exogenous CHL at 8 μg/mL or 4 μg/mL concentrations did not significantly induced the transcription of bucl8-associated genes (not shown). Thus, our results indicate the Bucl8-associated pump is not needed for CHL resistance in B. pseudomallei [52].
Deletion of Bucl8-associated pump components affects cell growth
Efflux pumps extrude a variety of compounds that are toxic to the cells and play physiological functions linked to pathogenesis [14]. We observed the growth of the BpΔbucl8-fusE mutant was considerably reduced than that of the parent Bp82 and did not reach the same OD600 in the stationary phase (Fig 6C). CFU for Bp82 increased by approximately four logs, while the mutant increased by two logs from hour 0 to 12. (Fig 6D). These results suggest that the pump components are needed for normal growth physiology under laboratory conditions in rich medium.
Discussion
The protein Bucl8 was previously predicted to be the outer membrane protein in B. pseudomallei and B. mallei. Comparative genomics studies between B. mallei and B. pseudomallei have suggested that conserved genes between the species are likely critical for host-survival, while genes useful for saprophytic life-style and adaptability were selected against [7]. The presence of the bucl8 genes, in particular the acquisition and conservation of the extracellular Bucl8-CL-Ct domain, in B. pseudomallei and B. mallei suggests that these genes are selected for because they are useful for bacterial survival in both the environment and in the host. Here, we carried out structure-function studies of the Bucl8 protein and the role of the putative Bucl8-associated efflux pump in antimicrobial resistance, ligand binding, and cell physiology of B. pseudomallei and B. mallei (Fig 7).
Fig 7. Model of assembled Bucl8-associated efflux pump components and substrates.
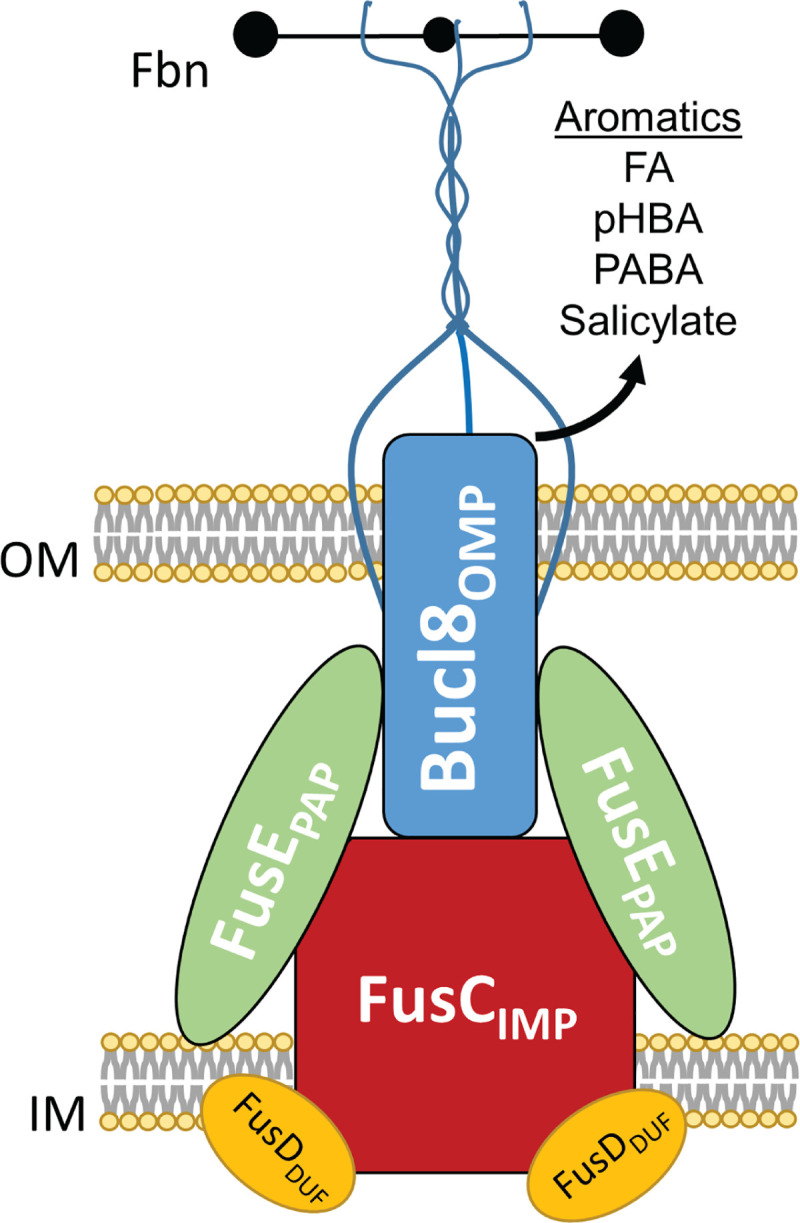
Cartoon representation of assembled the Bucl8-associated efflux pump components: Bucl8OMP, FusCIMP, FusDDUF, and FusEPAP. The extended CL-Ct structure is predicted to transverse the outer membrane into the extracellular space and to bind fibrinogen (Fbn). Aromatic substrates, such as experimentally-confirmed fusaric acid (FA) and p-hydroxybenzoic acid (pHBA), as well as putative p-aminobenzoic acid (PABA) and salicylate, are shown. OM; outer membrane. IM; inner membrane.
Bucl8 is a homotrimeric protein that spans the periplasmic and outer membrane to reach the extracellular region of the cell. Crystal structures of the membrane-spanning region have been reported for a number of homologous proteins, including VceC and OprM (Table 2). Superposition of the homology model of Bucl8, with the crystal structure of OprM from P. aeruginosa shows a strong structural conservation of both OEP1 and OEP2 domains, with root mean square deviation (RMSD) between the two structures of 2.0 Å (Fig 1B). Full structural conservation of all helices of OEP1 provides similar dimensions of the middle bulge of the OEP1 domain that accommodates substrates and suggest a similar mechanism of funnel-channel-structure mediated transport across the outer membrane.
The most puzzling feature of Bucl8 is the extracellular portion, which embeds a collagen like triple helix. In the absence of hydroxyprolines that stabilize the triple helical structure of mammalian collagen, bacterial collagens adopt alternative stabilization mechanisms to form stable triple helices [54]. While some prokaryotic collagens utilize a variety of GXY-repeat types, such as streptococcal collagen-like proteins Scl1 and Scl2 [55], others possess a limited number of triplets, including Bacillus Bcl proteins [56, 57]. The CL regions of various Bucl proteins utilize relatively few distinct triplet types [15]. An extreme case is the Bucl8-CL region, which is exclusively made of a rare repeating (GAS)n sequence. Our results are consistent with studies of triple helix propensity based on host-guest peptide studies, showing reasonable propensities of (GAS)n triplets to form triple helical structures. The Tm value of (GAS)n tripeptide unit in a triple helix is 33.0°C, compared to 47.3°C of (POG)n tripeptide (O is hydroxyproline), although, the physical anchoring of a CL domain increases Tm by additional 2°C [58]. This relatively low Tm may suggest structural flexibility of the Bucl8 extracellular domain under physiological conditions, thus, allowing efflux pump for dual function.
Our laboratory and others have shown that bacterial collagen-like proteins participate in pathogenesis via a variety of functions, including immune evasion, cell adhesion, biofilm formation, and autoaggregation [44–46, 59]. Here, we report that the recombinant rBucl8-CL-Ct polypeptide binds to fibrinogen significantly better than rBucl8-Ct polypeptide. A similar phenomenon was recently reported for Scl1, where the effective binding to fibronectin, directly mediated by the globular V domain, required the presence of adjacent Scl1-CL domain [30]. Fibrinogen is a circulating glycoprotein that is involved in blood clotting and promoting wound healing [60]; we do not know the location of Bucl8 binding site on this multidomain protein. In the scope of pathogenesis, some Gram-negative and Gram-positive bacteria use fibrinogen for biofilm formation and bacterial adhesion. For example, fibrinogen-binding factors and clumping-factors of Staphylococcus aureus have been shown to increase adherence and virulence [61–63]. B. pseudomallei and B. mallei both cause cutaneous infections that lead to lesions and nodules, thus binding to wound factors could increase colonization. In addition, it is likely that unidentified ligand(s), other than fibrinogen, may exist in the environment to support a saprophytic lifestyle of B. pseudomallei.
bucl8-operon expression is regulated by a LysR-type transcriptional regulator, designated here as FusRLysR. LysR-type family regulators are the most abundant class of the prokaryotic transcriptional regulators that monitor the expression of genes involved in pathogenesis, metabolism, quorum sensing and motility, toxin production, and more physiological and virulence traits [50]. LysR proteins are tetrameric and consist of two dimers that bind and bend the DNA within promoter regions, thus, affecting the gene transcription. After the co-inducer binds to the LysR dimers, the DNA is relaxed, allowing one dimer to come into contact with the RNA polymerase to form an active transcription complex. In this study, the FusR binding sites were identified within the intergenic promoter region between bucl8 and fusR in B. pseudomallei and B. mallei. Thus, we hypothesized that FA can act as a co-inducer for the bucl8-operon.
We show that exogenous fusaric acid (FA) induces the transcription of the fusR-bucl8-fusCD-fusE operon, therefore, confirming Bucl8 is a component of a previously unreported FA-inducible efflux pump in B. pseudomallei and B. mallei. Similarly, an inducible FA tripartite efflux pump, encoded by fuaABC operon, was identified in another soil saprophyte S. maltophilia [52]. However, the gene/protein arrangement, e.g. sequence orientation and length, places the bucl8 operon within clade III of a phylogenetic tree of predicted FusC-associated operons, while fuaABC operon is in clade IV [39]. In addition to FA, the FA-derivative pHBA also induced the expression of the bucl8 operon. Interestingly, although the genes and intergenic regions are highly similar, transcription of fusR and bucl8 in FA-induced B. mallei culture is considerably reduced compared to B. pseudomallei. Likewise, the MIC levels for FA and pHBA were lower in B. mallei, although the bucl8 loci are conserved between B. pseudomallei 1026b and B. mallei ATCC23344. There may be other factors affecting transcription, such as additional regulatory circuits for processing FA and similar compounds in both organisms. For similarity, another efflux pump in B. pseudomallei, BpeEF-OprC, is regulated by two highly similar LysR-type transcriptional regulators, BpeT and BpeS [64]. Further studies are needed to identify if there are other regulators or environmental stress/factors that could be affecting upstream/downstream targets.
Efflux systems are categorized into families by sequence similarity, structure–including protein fold, conserved domains, and number of transmembrane spanning regions–as well as by their energy source and substrates [65]. It is not known whether the Bucl8-associated pump relies on ATP hydrolysis to transport FA, but the associated FusCIMP transporter does not contain an ATP-binding domain, therefore, it is an unlikely an ABC-type transporter. In Fusarium, the synthesized intracellular FA is extruded by a predicted MFS-type transporter FUBT [66]. MFS transporters are typically single-component transporters, such as LacY, QacA or NorA [67, 68]; however, some MFS proteins partner with outer membrane and periplasmic adaptor components to form tripartite complexes, such as that of EmrA-EmrB-TolC in E. coli [69]. FusC (733 aa) is likely not an MFS transporter because it is larger than the typical length of MFS proteins (400–600 aa), does not contain well-conserved MFS motifs [68], and as we show is a component of a tetrapartite operon. Furthermore, phylogenetic analysis of bacterial efflux systems implied that FuaABC tripartite FA efflux pump in S. maltophilia forms a separate branch from other bacterial efflux pump families, branching off between the ABC and RND families [52]; of note, the Bucl8-associated FusC in B. pseudomallei and B. mallei has a 41.9% sequence identity to FuaA (Table 2). For these reasons, we think that the Bucl8-associated efflux pump is similar to an RND-type complex.
Here, we adopted the gene designation proposed by Crutcher et al., which also includes a fourth pump component, a small polypeptide DUF, for the Bucl8-associated tetrapartite efflux system. This situation might be more common among known tripartite efflux pumps than currently acknowledged; for example, small polypeptides YajC and AcrZ were reported as accessory components of the “tripartite” RND system AcrAB-TolC [70–72]. Another known tetrapartite RND efflux system is the CusCFBA complex, which transports heavy metals copper and silver [73]. In this system, the small CusF component serves as a periplasmic metal-binding chaperone, which hands over the metal-ion substrate to the IMP transporter [74, 75]. The precise cellular location and function of FusDDUF protein is not known at present.
Early studies reported FA-detoxification genes found in Burkholderia (formerly Pseudomonas) cepacia and Klebsiella oxytoca [17, 18], which were attributed to FA resistance. More recent work identified a tripartite FA efflux pump, FuaABC, in Stenotrophomonas maltophilia [52], while other work distinguished a large number of the phylogenetically related FusC-type proteins, conferring FA resistance, in numerous Gram-negative bacterial species [39]. Not all FusC proteins were predicted components of FA efflux pumps; however they were assumed to be contributing to high levels of FA resistance in some species, including Burkholderia. Crutcher et al. reported positive correlation between the number of putative FusC proteins in bacterial genomes and the level of resistance to FA; for example, Burkholderia cepacia, harboring six predicted FusC proteins, had a FA-MIC of ≥500 μg/mL, whereas Burkholderia glumae had two FusC proteins and a FA-MIC of 200 μg/mL [39]. Strains with 0–1 fusC genes were sensitive to FA with MIC <50 μg/mL. We also observed that our Bp82Δbucl8-fusE mutant retained 100 μg/mL residual resistance to FA. Through transcriptional analysis, we found that the five fusC/FusC genes/proteins outside of the Bucl8-operon showed little to no induction, indicating that the Bucl8-associated efflux pump is the main contributor to FA resistance in B. pseudomallei.
The multidrug resistance in B. pseudomallei is substantially attributed to previously studied RND efflux pumps BpeAB-OprB, AmrAB-OprA, and BpeEF-OprC. At the same time, little is known about the role of FA pumps in resistance against clinically used drugs. In our studies, we assessed the role of Bucl8-associated pump in multidrug resistance in two ways: (i) we compared the spectrum of resistance between parental strain Bp82 and Bucl8-pump deletion mutant, and (ii) we expressed the bucl8-operon in a sensitive E. coli strain. Although MICs for FA changed as predicted, deletion of the Bucl8-pump did not affect MIC values for the clinically-used drugs. This result is comparative to an FA pump in S. maltophilia, which did not determine the resistance to a large panel of therapeutics tested [52]. At the same time, a different study in the same organism showed that deletion of the pcm-tolCsm operon, encoding a different efflux pump, resulted in decreased MICs for several antimicrobials of diverse classes (β-lactams, chloramphenicol, quinolone, tetracycline, aminoglycosides, macrolides), and also decreased FA resistance [76]. Microarray data suggested bucl8 expression was upregulated in the presence of chloramphenicol [53] and deletion of the tolCsm in S. maltophilia resulted in decreased resistance to both CHL and FA [76]. Both CHL and FA harbor aromatic rings in their structures, however, our investigations did not detect bucl8-operon induction by CHL nor changes in CHL resistance levels in Bp82Δbucl8-fusE mutant or complemented E. coli. Altogether, we cannot exclude a possibility that redundant efflux systems are responsible for a lack of change in the drug resistance we recorded in both the Burkholderia isogenic mutant and complemented E. coli. Additional experiments will be needed, employing defined efflux-pump-deletion mutants in both hosts, to fully verify our conclusions presented here.
Efflux pumps support physiological functions [65]. The decrease in bacterial growth of the Bp82Δbucl8-fusE mutant suggests that the Bucl8-associated pump may be involved in modulating essential cellular stresses, both in the environment and in infected human host [14]. Limited studies show that FA repressed quorum sensing genes, expression of stress factors, secretion of siderophores, production of anti-fungal metabolites, and iron uptake [77–80]. Additionally, Bucl8-associated pump may be involved in transport of aromatic carboxylic acid compounds and act as a pHBA-metabolic efflux valve [40]. Other possible substrates include p-aminobenzoic acid (PABA), which is a key component of folate synthesis or salicylate, used in foods and pain-relieving drugs. (Fig 7). Ongoing investigations are aimed to define spectrum specificity of the Bucl8-associated efflux pumps in the context of the human host.
In summary, we conclude that Bucl8 is a component of a previously unreported tetrapartite efflux system that is involved in FA resistance and cell physiology. We have demonstrated that the extracellular Bucl8-CL domain forms the prototypic collagen triple-helix, while the extracellular Bucl8-CL-Ct portion is capable of binding to fibrinogen. Further studies will investigate what role fibrinogen binding plays in pathogenesis. While the Bucl8-pump is likely not be involved in the MDR phenotype of Burkholderia, we have identified FA and pHBA as inducible substrates of the pump and will continue to investigate metabolite analogs that may affect cell function. Importantly, the growth of the Bucl8-pump deletion mutant was significantly affected even in the absence of FA and pHBA. By characterizing the Bucl8-associated efflux system, we can advance therapies and strategies for combating these pathogens, including developing pump inhibitors, targeting transport mechanisms, or identifying potential surface-exposed vaccine targets derived from Bucl8.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(PDF)
Acknowledgments
We would like to thank Heath Damron for providing us pUC18T-mini-Tn7T-Tp vector, Alfredo Torres for providing B. mallei CLH001, Shelby Bradford for her assistance with initial experiments, and Paul Feustel for advice with statistical analyses. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the US Army.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
S.L. was supported by the Vaccine Development Center at WVU-HSC, Research Challenge Grant no.HEPC.dsr.18.6 from the Division of Science and Research, WV Higher Education Policy Commission. G.H. was supported with Bioinformatics Core grants NIH-NIGMS U54 GM- 104942 and P20 GM103434 R.B. acknowledges funding by the project 2017SFBFER provided by the Italian MIUR.
References
- 1.Currie BJ. Burkholderia pseudomallei and Burkholderia mallei: melioidosis and glanders. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases 7th edn Philadelphia: Churchill Livingstone Elsevier; 2010:2869–85. [Google Scholar]
- 2.Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1:15008 10.1038/nmicrobiol.2015.8 . [DOI] [PubMed] [Google Scholar]
- 3.Birnie E, Virk HS, Savelkoel J, Spijker R, Bertherat E, Dance DAB, et al. Global burden of melioidosis in 2015: a systematic review and data synthesis. Lancet Infect Dis. 2019;19(8):892–902. Epub 2019/07/10. 10.1016/S1473-3099(19)30157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan S, Chieng S, Kingsley PV, Mohan A, Podin Y, Ooi MH, et al. Melioidosis in Malaysia: Incidence, clinical challenges, and advances in understanding pathogenesis. Trop Med Infect Dis. 2018;3(1). 10.3390/tropicalmed3010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inglis TJ. The treatment of melioidosis. Pharmaceuticals (Basel). 2010;3(5):1296–303. 10.3390/ph3051296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dance D. Treatment and prophylaxis of melioidosis. Int J Antimicrob Agents. 2014;43(4):310–8. Epub 2014/03/13. 10.1016/j.ijantimicag.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Losada L, Ronning CM, DeShazer D, Woods D, Fedorova N, Kim HS, et al. Continuing evolution of Burkholderia mallei through genome reduction and large-scale rearrangements. Genome Biol Evol. 2010;2:102–16. Epub 2010/03/25. 10.1093/gbe/evq003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X-Z, Plésiat P, Nikaido H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev. 2015;28(2):337–418. 10.1128/CMR.00117-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodes KA, Schweizer HP. Antibiotic resistance in Burkholderia species. Drug Resist Updat. 2016;28:82–90. Epub 2016/09/14. 10.1016/j.drup.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarovich DS, Webb JR, Pitman MC, Viberg LT, Mayo M, Baird RW, et al. Raising the Stakes: Loss of efflux pump regulation decreases meropenem susceptibility in Burkholderia pseudomallei. Clin Infect Dis. 2018;67(2):243–50. Epub 2018/02/03. 10.1093/cid/ciy069 . [DOI] [PubMed] [Google Scholar]
- 11.Guglierame P, Pasca MR, De Rossi E, Buroni S, Arrigo P, Manina G, et al. Efflux pump genes of the resistance-nodulation-division family in Burkholderia cenocepacia genome. BMC Microbiology. 2006;6:66–. 10.1186/1471-2180-6-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podnecky NL, Rhodes KA, Schweizer HP. Efflux pump-mediated drug resistance in Burkholderia. Front Microbiol. 2015;6:305 Epub 2015/04/14. 10.3389/fmicb.2015.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vargiu AV, Pos KM, Poole K, Nikaido H. Editorial: Bad bugs in the XXIst century: Resistance mediated by multi-drug efflux pumps in Gram-negative bacteria. Front Microbiol. 2016;7:833 Epub 2016/06/16. 10.3389/fmicb.2016.00833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piddock LJ. Multidrug-resistance efflux pumps—not just for resistance. Nat Rev Microbiol. 2006;4(8):629–36. Epub 2006/07/18. 10.1038/nrmicro1464 . [DOI] [PubMed] [Google Scholar]
- 15.Bachert BA, Choi SJ, Snyder AK, Rio RVM, Durney BC, Holland LA, et al. A unique set of the Burkholderia collagen-like proteins provides insight into pathogenesis, genome evolution and niche adaptation, and infection detection. PLoS ONE. 2015;10(9):e0137578 Epub 2015/09/15. 10.1371/journal.pone.0137578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramachandran GN. Stereochemistry of collagen. Int J Pept Protein Res. 1988;31(1):1–16. Epub 1988/01/01. . [PubMed] [Google Scholar]
- 17.Utsumi R, Yagi T, Katayama S, Katsuragi K, Tachibana K, Toyoda H, et al. Molecular cloning and characterization of the fusaric acid-resistance gene from Pseudomonas cepacia. Agr Biol Chem. 1991;55(7):1913–8. Epub 2014/09/08. 10.1271/bbb1961.55.1913 . [DOI] [PubMed] [Google Scholar]
- 18.Toyoda H, Katsuragi K, Tamai T, Ouchi S. DNA sequence of genes for detoxification of fusaric acid, a wilt-inducing agent produced by Fusarium species. J Phytopathology. 1991;133(4):265–77. 10.1111/j.1439-0434.1991.tb00162.x .2064443 [DOI] [Google Scholar]
- 19.Propst KL, Mima T, Choi K-H, Dow SW, Schweizer HP. A Burkholderia pseudomallei ΔpurM mutant is avirulent in immunocompetent and immunodeficient animals: candidate strain for exclusion from select-agent lists. Infect Immun. 2010;78(7):3136–43. 10.1128/IAI.01313-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatcher CL, Mott TM, Muruato LA, Sbrana E, Torres AG. Burkholderia mallei CLH001 attenuated vaccine strain is immunogenic and protects against acute respiratory glanders. Infection and Immunity. 2016;84(8):2345–54. 10.1128/IAI.00328-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi KH, Schweizer HP. mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc. 2006;1(1):153–61. Epub 2007/04/05. 10.1038/nprot.2006.24 . [DOI] [PubMed] [Google Scholar]
- 22.Hamad MA, Zajdowicz SL, Holmes RK, Voskuil MI. An allelic exchange system for compliant genetic manipulation of the select agents Burkholderia pseudomallei and Burkholderia mallei. Gene. 2009;430(1):123–31. 10.1016/j.gene.2008.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price EP, Viberg LT, Kidd TJ, Bell SC, Currie BJ, Sarovich DS. Transcriptomic analysis of longitudinal Burkholderia pseudomallei infecting the cystic fibrosis lung. Microb Genom. 2018;4(8):e000194 Epub 2018/07/10. 10.1099/mgen.0.000194 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan PP, Holmes AD, Smith AM, Tran D, Lowe TM. The UCSC Archaeal Genome Browser: 2012 update. Nucleic Acids Res. 2012;40(Database issue):D646–52. Epub 2011/11/15. 10.1093/nar/gkr990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langdon WB. Performance of genetic programming optimised Bowtie2 on genome comparison and analytic testing (GCAT) benchmarks. BioData Min. 2015;8(1):1 Epub 2015/01/27. 10.1186/s13040-014-0034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solovyev V SA. Automatic annotation of microbial genomes and metagenomic sequences. Metagenomics and its applications in agriculture, biomedicine and environmental studies. 2011:61–78. [Google Scholar]
- 27.Hu GQ, Zheng X, Zhu HQ, She ZS. Prediction of translation initiation site for microbial genomes with TriTISA. Bioinformatics. 2009;25(1):123–5. Epub 2008/11/19. 10.1093/bioinformatics/btn576 . [DOI] [PubMed] [Google Scholar]
- 28.Hu GQ, Zheng X, Yang YF, Ortet P, She ZS, Zhu H. ProTISA: a comprehensive resource for translation initiation site annotation in prokaryotic genomes. Nucleic Acids Res. 2008;36(Database issue):D114–9. Epub 2007/10/19. 10.1093/nar/gkm799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Münch R, Hiller K, Grote A, Scheer M, Klein J, Schobert M, et al. Virtual Footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics. 2005;21(22):4187–9. Epub 2005/08/20. 10.1093/bioinformatics/bti635 . [DOI] [PubMed] [Google Scholar]
- 30.McNitt DH, Choi SJ, Keene DR, Van De Water L, Squeglia F, Berisio R, et al. Surface-exposed loops and an acidic patch in the Scl1 protein of group A Streptococcus enable Scl1 binding to wound-associated fibronectin. J Biol Chem. 2018;293(20):7796–810. 10.1074/jbc.RA118.002250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caswell CC, Oliver-Kozup H, Han R, Lukomska E, Lukomski S. Scl1, the multifunctional adhesin of group A Streptococcus, selectively binds cellular fibronectin and laminin, and mediates pathogen internalization by human cells. FEMS Microbiol Lett. 2010;303(1):61–8. Epub 2009/12/17. 10.1111/j.1574-6968.2009.01864.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamin W, Andrej S. Comparative Protein Structure Modeling Using MODELLER. Current Protocols in Bioinformatics. 2014;47(1):5.6.1–5.6.32. 10.1002/0471250953.bi0506s47 . [DOI] [PubMed] [Google Scholar]
- 33.Berisio R, Vitagliano L, Mazzarella L, Zagari A. Crystal structure of the collagen triple helix model [(Pro-Pro-Gly)10]3. Protein Sci. 2002;11(2):262–70. Epub 2002/01/16. 10.1110/ps.32602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yahyavi M, Falsafi-Zadeh S, Karimi Z, Kalatarian G, Galehdari H. VMD-SS: A graphical user interface plug-in to calculate the protein secondary structure in VMD program. Bioinformation. 2014;10(8):548–50. Epub 2014/09/27. 10.6026/97320630010548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–12. Epub 2004/07/21. 10.1002/jcc.20084 . [DOI] [PubMed] [Google Scholar]
- 36.Quoilin S, Lambion N, Mak R, Denis O, Lammens C, Struelens M, et al. Soft tissue infections in Belgian rugby players due to Streptococcus pyogenes emm type 81. Euro Surveill. 2006;11(12):E0612212. 10.2807/esw.11.51.03099-en [DOI] [PubMed] [Google Scholar]
- 37.Sirijant N, Sermswan RW, Wongratanacheewin S. Burkholderia pseudomallei resistance to antibiotics in biofilm-induced conditions is related to efflux pumps. J Med Microbiol. 2016;65(11):1296–306. 10.1099/jmm.0.000358 . [DOI] [PubMed] [Google Scholar]
- 38.Rv R. Fungal Sensing: iGEM Wageningen UR 2014; 2014. Available from: http://2014.igem.org/Team:Wageningen_UR/project/fungal_sensing.
- 39.Crutcher FK, Puckhaber LS, Stipanovic RD, Bell AA, Nichols RL, Lawrence KS, et al. Microbial resistance mechanisms to the antibiotic and phytotoxin fusaric acid. J Chem Ecol. 2017;43(10):996–1006. 10.1007/s10886-017-0889-x . [DOI] [PubMed] [Google Scholar]
- 40.Van Dyk TK, Templeton LJ, Cantera KA, Sharpe PL, Sariaslani FS. Characterization of the Escherichia coli AaeAB efflux pump: a metabolic relief valve? J Bacteriol. 2004;186(21):7196–204. Epub 2004/10/19. 10.1128/JB.186.21.7196-7204.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thibault FM, Hernandez E, Vidal DR, Girardet M, Cavallo J-D. Antibiotic susceptibility of 65 isolates of Burkholderia pseudomallei and Burkholderia mallei to 35 antimicrobial agents. J Antimicrob Chemother. 2004;54(6):1134–8. 10.1093/jac/dkh471 . [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Keene DR, Bujnicki JM, Höök M, Lukomski S. Streptococcal Scl1 and Scl2 proteins form collagen-like triple helices. J Biol Chem. 2002;277(30):27312–8. Epub 2002/04/27. 10.1074/jbc.M201163200 . [DOI] [PubMed] [Google Scholar]
- 43.Brodsky-Doyle B, Leonard KR, Reid KB. Circular-dichroism and electron-microscopy studies of human subcomponent C1q before and after limited proteolysis by pepsin. Biochem J. 1976;159(2):279–86. 10.1042/bj1590279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duncan C, Prashar A, So J, Tang P, Low DE, Terebiznik M, et al. Lcl of Legionella pneumophila is an immunogenic GAG binding adhesin that promotes interactions with lung epithelial cells and plays a crucial role in biofilm formation. Infect Immun. 2011;79(6):2168–81. Epub 2011/03/23. 10.1128/IAI.01304-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paterson GK, Nieminen L, Jefferies JMC, Mitchell TJ. PclA, a pneumococcal collagen-like protein with selected strain distribution, contributes to adherence and invasion of host cells. FEMS Microbiol Lett. 2008;285(2):170–6. 10.1111/j.1574-6968.2008.01217.x [DOI] [PubMed] [Google Scholar]
- 46.Pizarro-Guajardo M, Olguin-Araneda V, Barra-Carrasco J, Brito-Silva C, Sarker MR, Paredes-Sabja D. Characterization of the collagen-like exosporium protein, BclA1, of Clostridium difficile spores. Anaerobe. 2014;25:18–30. Epub 2013/11/26. 10.1016/j.anaerobe.2013.11.003 . [DOI] [PubMed] [Google Scholar]
- 47.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, et al. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21(4):713–24. Epub 1996/08/01. 10.1046/j.1365-2958.1996.281397.x . [DOI] [PubMed] [Google Scholar]
- 48.Moore RA, DeShazer D, Reckseidler S, Weissman A, Woods DE. Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob Agents Chemother. 1999;43(3):465–70. Epub 1999/02/27. 10.1128/AAC.43.3.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poole K, Tetro K, Zhao Q, Neshat S, Heinrichs DE, Bianco N. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob Agents Chemother. 1996;40(9):2021–8. Epub 1996/09/01. 10.1128/AAC.40.9.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maddocks SE, Oyston PC. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology. 2008;154(Pt 12):3609–23. Epub 2008/12/03. 10.1099/mic.0.2008/022772-0 . [DOI] [PubMed] [Google Scholar]
- 51.Nikaido H, Pagès J-M. Broad specificity efflux pumps and their role in multidrug esistance of Gram negative bacteria. FEMS Microbiology Reviews. 2012;36(2):340–63. 10.1111/j.1574-6976.2011.00290.x PMC3546547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu R-M, Liao S-T, Huang C-C, Huang Y-W, Yang T-C. An inducible fusaric acid tripartite efflux pump contributes to the fusaric acid resistance in Stenotrophomonas maltophilia. PLoS ONE. 2012;7(12):e51053 10.1371/journal.pone.0051053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ooi WF, Ong C, Nandi T, Kreisberg JF, Chua HH, Sun G, et al. The condition-dependent transcriptional landscape of Burkholderia pseudomallei. PLoS Genet. 2013;9(9):e1003795 Epub 2013/09/27. 10.1371/journal.pgen.1003795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohs A, Silva T, Yoshida T, Amin R, Lukomski S, Inouye M, et al. Mechanism of stabilization of a bacterial collagen triple helix in the absence of hydroxyproline. J Biol Chem. 2007;282(41):29757–65. 10.1074/jbc.M703991200 [DOI] [PubMed] [Google Scholar]
- 55.Han R, Zwiefka A, Caswell CC, Xu Y, Keene DR, Lukomska E, et al. Assessment of prokaryotic collagen-like sequences derived from streptococcal Scl1 and Scl2 proteins as a source of recombinant GXY polymers. Appl Microbiol Biotechnol. 2006;72(1):109–15. Epub 2006/03/23. 10.1007/s00253-006-0387-5 . [DOI] [PubMed] [Google Scholar]
- 56.Leski TA, Caswell CC, Pawlowski M, Klinke DJ, Bujnicki JM, Hart SJ, et al. Identification and classification of bcl genes and proteins of Bacillus cereus group organisms and their application in Bacillus anthracis detection and fingerprinting. Appl Environ Microbiol. 2009;75(22):7163–72. Epub 2009/09/22. 10.1128/AEM.01069-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sylvestre P, Couture-Tosi E, Mock M. Polymorphism in the collagen-like region of the Bacillus anthracis BclA protein leads to variation in exosporium filament length. J Bacteriol. 2003;185(5):1555–63. Epub 2003/02/20. 10.1128/jb.185.5.1555-1563.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Persikov AV, Ramshaw JAM, Brodsky B. Prediction of collagen stability from amino acid sequence. J Biol Chem. 2005;280(19):19343–9. 10.1074/jbc.M501657200 [DOI] [PubMed] [Google Scholar]
- 59.Gu C, Jenkins SA, Xue Q, Xu Y. Activation of the classical complement pathway by Bacillus anthracis is the primary mechanism for spore phagocytosis and involves the spore surface protein BclA. J Immunol. 2012;188(9):4421–31. Epub 2012/03/24. 10.4049/jimmunol.1102092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herrick S, Blanc-Brude O, Gray A, Laurent G. Fibrinogen. Int J Biochem Cell Biol. 1999;31(7):741–6. Epub 1999/09/01. 10.1016/s1357-2725(99)00032-1 . [DOI] [PubMed] [Google Scholar]
- 61.Vaudaux P, Pittet D, Haeberli A, Huggler E, Nydegger UE, Lew DP, et al. Host factors selectively increase staphylococcal adherence on inserted catheters: a role for fibronectin and fibrinogen or fibrin. J Infect Dis. 1989;160(5):865–75. Epub 1989/11/01. 10.1093/infdis/160.5.865 . [DOI] [PubMed] [Google Scholar]
- 62.Ko YP, Flick MJ. Fibrinogen is at the interface of host defense and pathogen virulence in Staphylococcus aureus infection. Semin Thromb Hemost. 2016;42(4):408–21. Epub 2016/04/09. 10.1055/s-0036-1579635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fitzgerald JR, Loughman A, Keane F, Brennan M, Knobel M, Higgins J, et al. Fibronectin-binding proteins of Staphylococcus aureus mediate activation of human platelets via fibrinogen and fibronectin bridges to integrin GPIIb/IIIa and IgG binding to the FcgammaRIIa receptor. Mol Microbiol. 2006;59(1):212–30. Epub 2005/12/20. 10.1111/j.1365-2958.2005.04922.x . [DOI] [PubMed] [Google Scholar]
- 64.Rhodes KA, Somprasong N, Podnecky NL, Mima T, Chirakul S, Schweizer HP. Molecular determinants of Burkholderia pseudomallei BpeEF-OprC efflux pump expression. Microbiology. 2018;164(9):1156–67. Epub 2018/07/20. 10.1099/mic.0.000691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teelucksingh T, Thompson LK, Cox G. The evolutionary conservation of Escherichia coli drug efflux pumps supports physiological functions. J Bacteriol. 2020;202(22). Epub 2020/08/26. 10.1128/JB.00367-20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crutcher FK, Liu J, Puckhaber LS, Stipanovic RD, Bell AA, Nichols RL. FUBT, a putative MFS transporter, promotes secretion of fusaric acid in the cotton pathogen Fusarium oxysporum f. sp. vasinfectum. Microbiology. 2015;161(Pt 4):875–83. Epub 2015/01/30. 10.1099/mic.0.000043 . [DOI] [PubMed] [Google Scholar]
- 67.Madej MG. Function, structure, and evolution of the major facilitator superfamily: The LacY manifesto. Advances in Biology. 2014;2014(Article ID 523591):20 10.1155/2014/523591. [DOI] [Google Scholar]
- 68.Pasqua M, Grossi M, Zennaro A, Fanelli G, Micheli G, Barras F, et al. The varied role of efflux pumps of the MFS family in the interplay of bacteria with animal and plant cells. Microorganisms. 2019;7(9). Epub 2019/08/25. 10.3390/microorganisms7090285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hinchliffe P, Greene NP, Paterson NG, Crow A, Hughes C, Koronakis V. Structure of the periplasmic adaptor protein from a major facilitator superfamily (MFS) multidrug efflux pump. FEBS Lett. 2014;588(17):3147–53. Epub 2014/07/06. 10.1016/j.febslet.2014.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tornroth-Horsefield S, Gourdon P, Horsefield R, Brive L, Yamamoto N, Mori H, et al. Crystal structure of AcrB in complex with a single transmembrane subunit reveals another twist. Structure. 2007;15(12):1663–73. Epub 2007/12/13. 10.1016/j.str.2007.09.023 . [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Fan G, Hryc CF, Blaza JN, Serysheva II, Schmid MF, et al. An allosteric transport mechanism for the AcrAB-TolC multidrug efflux pump. Elife. 2017;6 Epub 2017/03/30. 10.7554/eLife.24905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hobbs EC, Yin X, Paul BJ, Astarita JL, Storz G. Conserved small protein associates with the multidrug efflux pump AcrB and differentially affects antibiotic resistance. Proc Natl Acad Sci U S A. 2012;109(41):16696–701. Epub 2012/09/27. 10.1073/pnas.1210093109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delmar JA, Su CC, Yu EW. Bacterial multidrug efflux transporters. Annu Rev Biophys. 2014;43:93–117. Epub 2014/04/08. 10.1146/annurev-biophys-051013-022855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loftin IR, Franke S, Roberts SA, Weichsel A, Heroux A, Montfort WR, et al. A novel copper-binding fold for the periplasmic copper resistance protein CusF. Biochemistry. 2005;44(31):10533–40. Epub 2005/08/03. 10.1021/bi050827b . [DOI] [PubMed] [Google Scholar]
- 75.Loftin IR, Franke S, Blackburn NJ, McEvoy MM. Unusual Cu(I)/Ag(I) coordination of Escherichia coli CusF as revealed by atomic resolution crystallography and X-ray absorption spectroscopy. Protein Sci. 2007;16(10):2287–93. Epub 2007/09/26. 10.1110/ps.073021307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang YW, Hu RM, Yang TC. Role of the pcm-tolCsm operon in the multidrug resistance of Stenotrophomonas maltophilia. J Antimicrob Chemother. 2013;68(9):1987–93. Epub 2013/05/01. 10.1093/jac/dkt148 . [DOI] [PubMed] [Google Scholar]
- 77.Quecine MC, Kidarsa TA, Goebel NC, Shaffer BT, Henkels MD, Zabriskie TM, et al. An interspecies signaling system mediated by fusaric acid has parallel effects on antifungal metabolite production by Pseudomonas protegens strain Pf-5 and antibiosis of Fusarium spp. Appl Environ Microbiol. 2015;82(5):1372–82. Epub 2015/12/15. 10.1128/AEM.02574-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tung TT, Jakobsen TH, Dao TT, Fuglsang AT, Givskov M, Christensen SB, et al. Fusaric acid and analogues as Gram-negative bacterial quorum sensing inhibitors. Eur J Med Chem. 2017;126:1011–20. 10.1016/j.ejmech.2016.11.044 [DOI] [PubMed] [Google Scholar]
- 79.Ruiz J, M Bernar E, Jung K. Production of siderophores increases resistance to fusaric acid in Pseudomonas protegens Pf-52015. e0117040 p. [DOI] [PMC free article] [PubMed]
- 80.van Rij ET, Girard G, Lugtenberg BJJ, Bloemberg GV. Influence of fusaric acid on phenazine-1-carboxamide synthesis and gene expression of Pseudomonas chlororaphis strain PCL1391. Microbiology. 2005;151(Pt 8):2805–14. Epub 2005/08/05. 10.1099/mic.0.28063-0 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.