Summary
The parasite Trypanosoma brucei is the causative agent of sleeping sickness and cycles between insect and mammalian hosts. The parasite appears to lack conventional transcriptional regulation of protein coding genes, and mRNAs are processed from polycistronic transcripts by the concerted action of trans-splicing and polyadenylation. Regulation of mRNA function is mediated mainly by RNA binding proteins affecting mRNA stability and translation. In this study, we describe the identification of 62 non-coding (nc) RNAs that are developmentally regulated and/or respond to stress. We characterized two novel anti-sense RNA regulators (TBsRNA-33 and 37) that originate from the rRNA loci, associate with ribosomes and polyribosomes, and interact in vivo with distinct mRNA species to regulate translation. Thus, this study suggests for the first-time anti-sense RNA regulators as an additional layer for controlling gene expression in these parasites.
Subject Areas: Molecular Biology, Omics
Graphical Abstract
Highlights
-
•
Trypanosome non-coding RNAs (ncRNAs) are developmentally regulated during cycling between two hosts
-
•
ncRNAs originate from rRNA locus and associate with the ribosome en route to cytoplasm
-
•
In vivo cross-linking enable identification of target RNA species regulated by ncRNAs
-
•
Trypanosomes possess anti-sense ncRNAs that regulate translation
Molecular Biology; Omics
Introduction
In eukaryotes, non-coding RNAs (ncRNAs) are classified as small (below 200 nt) or long ncRNAs, where the latter harbor a 5′-cap and are polyadenylated (Hombach and Kretz, 2016). The small ncRNAs include RNAs involved in splicing, such as U snRNAs, small nucleolar RNAs (snoRNAs) involved in rRNA processing and modification, 7SL RNA (the signal recognition particle RNA), telomerase RNA, and others (Morris and Mattick, 2014). The best studied small ncRNAs are microRNAs and siRNAs, which are processed by DICER and bind ARGONAUTE (Wilson and Doudna, 2013). MicroRNAs bind to the 3′ UTR by base-pairing, and regulate both mRNA stability and translation (Mohr and Mott, 2015). In prokaryotes, small RNAs utilize anti-sense mechanisms to regulate mRNA function under stress, following metabolic changes, and to control virulence, and they affect translation and/or stability, together with the binding protein Hfq (Wagner and Romby, 2015).
Trypanosoma brucei is the causative agent of sleeping sickness and cycles between two hosts. In the tsetse fly, the parasite propagates in the midgut in the procyclic stage (PCF), and in the mammalian host it transforms to the bloodstream form (BSF) (Rodrigues et al., 2014). These organisms lack conventional promoters for genes encoding mRNA (Clayton, 2016), and mRNAs are transcribed as long polycistronic transcripts that are processed by concerted action of trans-splicing and polyadenylation (Michaeli, 2011). Post-transcriptional regulation is mainly achieved by RNA binding proteins (RBPs), which regulate mRNA stability and translation and control gene expression under stress and during the developmental cycle (Clayton, 2013). For instance, overexpression of a single RBP in PCF, such as RBP6 or RBP10, induces transformation to a form that can initiate infection in the mammalian host (Kolev et al., 2012; Mugo and Clayton, 2017). The reprogramming of gene expression during cycling between the two hosts involves changes in mRNA abundance and in the proteome (Butter et al., 2013; Naguleswaran et al., 2018; Queiroz et al., 2009). Ribosome profiling of the two life stages revealed that thousands of genes show changes in protein synthesis mediated by both mRNA abundance and translational efficiency (Jensen et al., 2014).
Trypanosomes possess all the conventional eukaryotic small RNA types such as U snRNAs (Liang et al., 2003; Rajan et al., 2019a, 2019b), snoRNAs (Chikne et al, 2016, 2019; Michaeli et al., 2012; Rajan et al., 2020), telomerase RNA (Gupta et al., 2013a; Sandhu et al., 2013), 7SL RNA (Michaeli, 2014), and Vault RNA (Kolev et al., 2019). Surprisingly, trypanosomes possess almost double the number of snoRNAs compared to yeast, which have a similar genome-size (Rajan et al., 2019a, 2019b). Pseudouridines guided by H/ACA snoRNAs, are developmentally regulated, with higher levels in the BSF, enabling the parasite to cope with the temperature shift while cycling between the hosts (Chikne et al., 2016). The number of 2′-O-methylations guided by C/D snoRNAs is exceptionally large (∼100) and these modifications are also developmentally regulated but to a lesser extent (Rajan et al., 2020). At least 20 snoRNAs are involved in the complex rRNA processing necessary for forming the fragmented large subunit rRNA (LSU) that is composed of two large molecules and four small ones, termed small rRNA (srRNA or sr) (Chikne et al., 2019).
We previously described the identification of small RNAs enriched by fractionation of RNA protein complexes, which led to the identification of small RNA molecules (TBsRNAs) (Michaeli et al., 2012). The most abundant small RNAs in trypanosomes are siRNAs derived from the retroposon families and from centromeric repeats, but no evidence exists for the presence of canonical microRNAs (miRNAs) bound to the ARGONAUTE protein (Tschudi et al., 2012).
Here, we report the identification of novel ncRNAs that were enriched and sequenced upon removal of ribosomes. Among the 62 ncRNA molecules described, we identified 22 ncRNAs that are developmentally regulated in the two life stages, as well as ncRNAs that change in abundance following stress. We utilized an in vivo UV cross-linking-ligation approach to identify the targets of these ncRNAs, and focused our functional studies on TBsRNA-33 and 37. These RNAs are processed from the internal transcribed spacer (ITS) of pre-rRNA, and appear to move with the ribosomes to the cytoplasm where these interact with distinct subset of mRNAs. We show that the ncRNA can repress/enhance the synthesis of the protein encoded by target mRNAs. This is the first report of a stable anti-sense regulator in trypanosomes that participate in regulating stage-specific gene expression.
Results
Identification of Developmentally Regulated ncRNAs
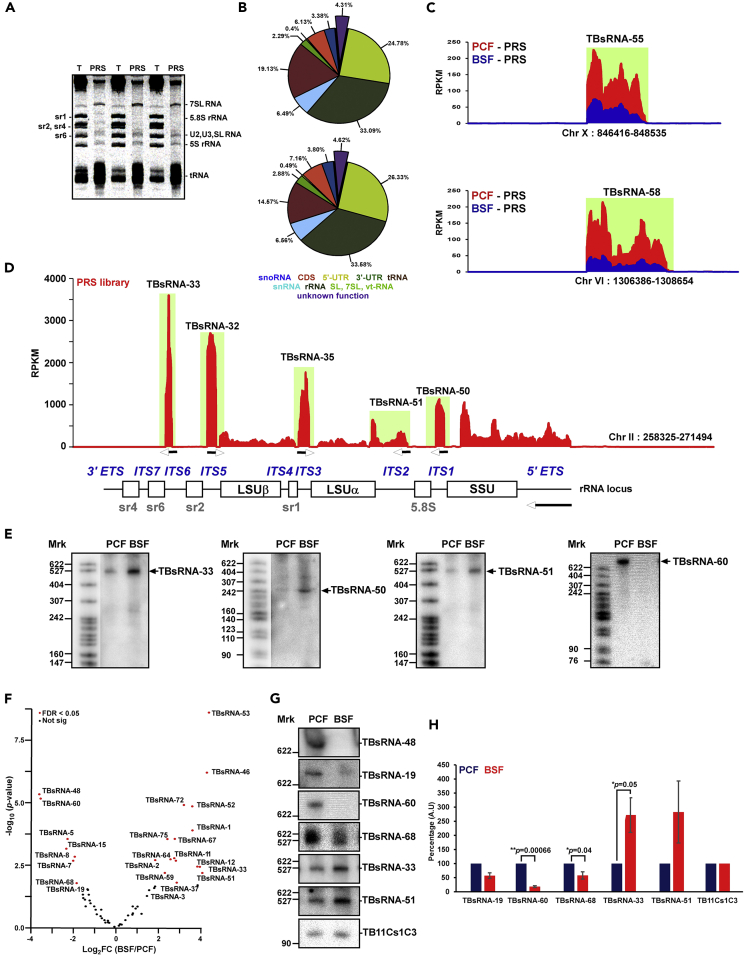
To explore the constellation of ncRNAs in trypanosomes and search for anti-sense regulators, we analyzed by RNA-seq the repertoire of ncRNAs enriched in post-ribosomal supernatant (PRS) (Figure 1A). Total cell lysate was prepared, RNPs were extracted with 300mM KCl, and the ribosomes were removed by ultracentrifugation. RNA from the PRS was used to prepare RNA-seq libraries from both PCF and BSF, as previously described (Chikne et al., 2016; Rajan et al., 2019a, 2019b, 2020). The libraries contained the entire population of ncRNAs (U snRNA, snoRNAs, 7SL RNA, Vault RNA, and tRNAs, Figure 1B) but also ∼62 relatively abundant RNAs with unknown function (Data S1, S2, and S3). The list of these RNAs (Data S2) and their chromosomal location (Data S3) are presented. By inspecting the chromosomal location of these ncRNAs by IGV viewer (Robinson et al., 2011), we found that most of these ncRNAs were located in intergenic regions (Figure 1C) and 3′ UTRs of mRNAs; to our surprise, we also found that five ncRNAs were localized in the ITS, and one in the external transcribed spacer of pre-rRNA (Figure 1D). Four ncRNAs, including TBsRNA-33, are derived from the pre-RNA precursor, but two ncRNAs, TBsRNA-32 and 35, are transcribed from the opposite strand (Figures 1D and 1E), likely by polymerase III, as these are enriched in the ChIP-seq with polymerase III tagged protein (Kolev et al., 2019).
Figure 1.
TBsRNAs Are Developmentally Regulated
(A) Enrichment of ncRNAs from T. brucei cell extracts. Whole cell extracts from 2 × 109 cells were prepared and depleted of ribosomes, as described in (Chikne et al., 2016; Rajan et al., 2019a, 2019b). RNA was extracted from PRS and total cell lysate (T), separated on a 10% denaturing gel, and stained with ethidium bromide.
(B) Example of the RNA composition of the PRS library. Pie diagram depicts the relative abundance of various ncRNA types in two libraries. Approximately, 13 different PRS libraries were used to validate the existence of the 62 ncRNA described in this study.
(C) Representative snapshot coverage of two novel TBsRNAs on their corresponding chromosome locus, given in RPKM.
(D) Distribution of the ncRNA reads across the pre-rRNA given in Reads per kilobase per million (RPKM). The arrows indicate the direction of transcription. The domains of the pre-rRNA are indicated.
(E) Northern analysis of ncRNAs. Total T. brucei RNA (20μg) was separated on denaturing gels and probed with 32P-labeled anti-sense RNA probe. 32P-labeled pBR322 DNA MspI digest was used as a size marker.
(F) TBsRNAs are developmentally regulated. The volcano plot was generated by DESeq2 (Anders and Huber, 2010) using three independent replicates of PRS RNA libraries of both PCF and BSF for the TBsRNA population. TBsRNAs that are developmentally regulated (FDR<0.05, and FC > 2) are indicated in red.
(G) TBsRNA expression analysis. Total RNA (20 μg) from PCF or BSF was separated on a 6% or 10% denaturing polyacrylamide gel and subjected to Northern analysis using anti-sense RNA probes to TBsRNA.
(H) Quantification of TBsRNA expression level based on Northern analysis. Expression data are presented as mean ± S.E.M. Experiments were done in triplicate (n = 3). TB11Cs1C3 was used as loading control. All densitometry data used in this figure were calculated by ImageJ (https://imagej.nih.gov/ij/) software.
Next, we used RNA-seq to examine the expression of a subset of ncRNAs in the two life stages and found 22 developmentally regulated ncRNAs that are preferentially expressed in either the PCF or BSF (Table S1, Figures 1F–1H). DESeq2 (Anders and Huber, 2010) analysis of the TBsRNA population suggested that 15 ncRNAs are upregulated in the BSF, and 7 ncRNAs are downregulated in this form (Tables S1 and S2). Note that among the ncRNAs identified are RNAs ranging from ∼90 to 500 nt in length (Tables S1 and S2) and vary in abundance. The most abundant ncRNAs are TBsRNA-32 and TBsRNA-49 (Tables S1 and S2). The relative expression was confirmed for a subset of ncRNAs by Northern analysis (three replicates) supporting the PRS RNA-seq data (Figures 1F–1H). The quantification of the expression data based on Northern analyses verified distinct ncRNAs that are differentially expressed (p < 0.05) between the two stages (Figures 1G and 1H).
Nuclear Localization and Association of the Novel ncRNAs with RNPs
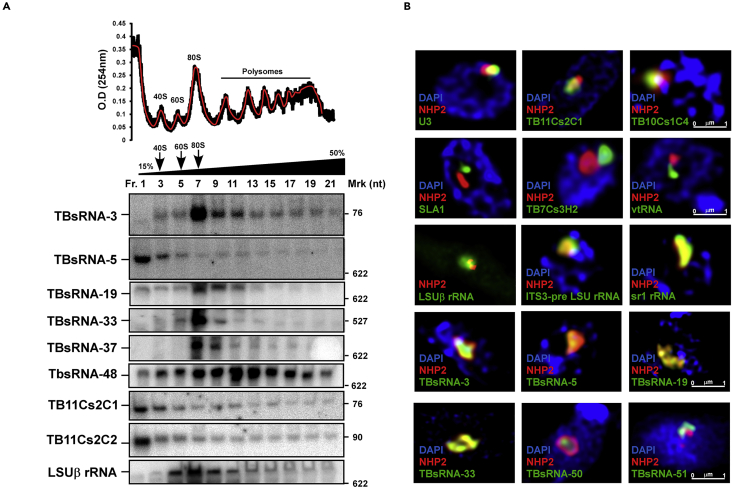
As a first step toward exploring the function of the novel ncRNAs, their association with other RNPs was examined. Two major types of RNPs greater than 80S were described in trypanosomes: polyribosomes and the processome involved in rRNA processing (Rajan et al., 2019a, 2019b). To this end, whole cell extracts were fractionated on 15–45% sucrose gradients, and the RNA was subjected to Northern analysis with gene-specific probes. The results (Figure 2A) indicate that the ncRNAs can be categorized to two groups. One group of ncRNAs are found in small RNPs (peak found in fractions 1–3) that also associate with larger RNP complexes such as the C/D snoRNAs TB11Cs2C2 and C1, which were shown to participate in rRNA processing (Gupta et al., 2010). Indeed, the ncRNA TBsRNA-5 fractionated similarly to C/D snoRNAs involved in rRNA processing (TB11Cs2C2 and C1). However, this RNA lacks the boxes specifying C/D or H/ACA families and therefore is not a member of these families. Interestingly, this RNA is localized in the center of the nucleolus and is not found in all domains containing the NHP2 protein, which is the core protein of the H/ACA RNA (Barth et al., 2005) (Figures 2B and S1). This localization differs from that of U3 involved in SSU processing (Hartshorne and Agabian, 1993), since U3 is not found in the center of the nucleolus. Its possible role in rRNA processing will require further experimentation. Members of the second group (TBsRNA-3, TBsRNA-19, TBsRNA-33, TBsRNA-37) are not present in small distinct RNPs (like TB11Cs2C1, and C2) and their major peak is associated with the 80S ribosomes. The rest of the hybridization signal is distributed on polyribosomes. In the nucleolus, these RNAs are co-localized with NHP2 (Figures 2B and S1). This distribution is distinct from those RNAs involved in rRNA processing and those that guide the modification on snRNAs, such as the spliced leader associated RNA (SLA1), which guides Ψ on SL RNA (Liang et al., 2002), and TB7Cs3H2, which guides Ψ on rRNA and U2 snRNA (Rajan et al., 2019a, 2019b), as well as vault RNA (vtRNA) which we showed recently to affect the trans-splicing process (Kolev et al., 2019) (Figures 2B and S1). Despite the fact that TBsRNA-33 and 37 clearly fractionate with the 80S ribosomes and polysomes, these ncRNAs could not be detected by in situ hybridization in the cytoplasm due to their low abundance compared to rRNA (Figures 2B and S1), which has a dispersed pattern in the cytoplasm and gives a weaker signal compared to the foci in the nucleolus.
Figure 2.
Localization of TBsRNAs
(A) Fractionation of TBsRNA. Whole cell extract from procyclic cells (2 × 109) was fractionated on (15–50%) sucrose gradient at 70,000 rpm for 1.5 hr using a Beckman SW41 rotor. 400 μL fractions were collected using the ISCO gradient fractionation system. The fractions were deproteinized and the RNA was separated on a 6% or 10% polyacrylamide-denaturing gel and subjected to Northern analysis using anti-sense RNA probes hybridizing to TBsRNAs, as indicated. The optical density of the fractions is presented.
(B) High-resolution fluorescence in situ hybridization coupled with immunofluorescence was performed for the indicated RNAs (green) and NHP2 (red). DNA was stained with DAPI (blue). The scale bar is indicated.
“Tell Me Who You Interact with and I Will Tell You Who You Are” mRNAs Associated with Distinct ncRNAs
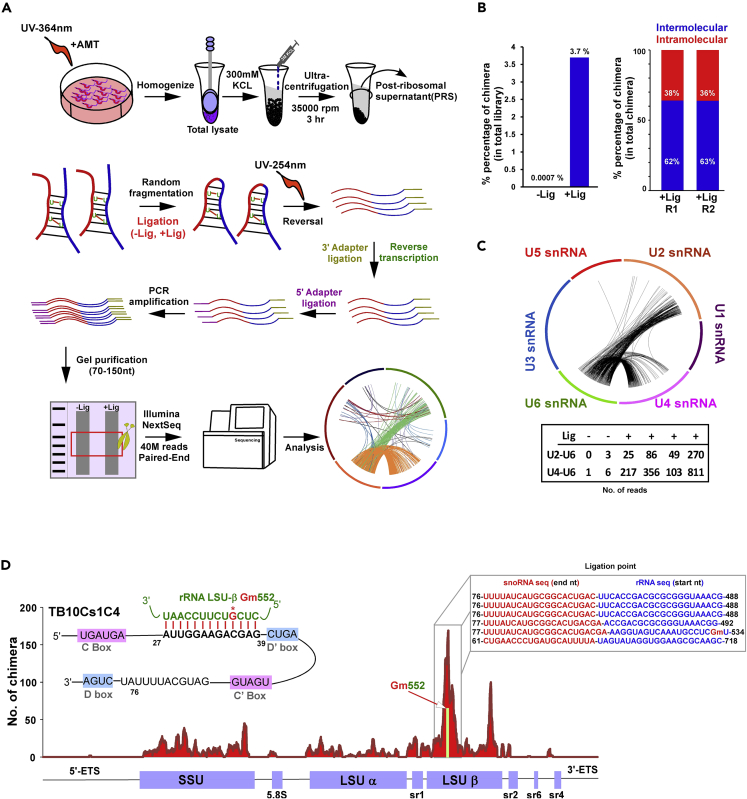
The presence of ncRNAs on 80S ribosomes and polysomes led us to investigate whether these ncRNAs interact with distinct mRNAs to regulate their fate. We employed the methodology recently implemented by us to investigate the small RNA interactome of T. brucei using in vivo cross-linking (Rajan et al., 2019a, 2019b), as depicted in (Figure 3A). The method involves in vivo cross-linking in the presence of psoralen, which increases the efficacy of cross-linking by ten-fold (Garrett-Wheeler et al., 1984), preparation of PRS extracts, extracting the RNA, ligating the interacting RNA, and preparing RNA-seq libraries to detect the chimeric RNA products including the ncRNA and its interacting mRNA (Figure 3A). UV cross-linking enriched for such chimeric RNAs and chimera production was dependent on ligation and increased the detection of intermolecular interactions (Figure 3B) (Table S3). For instance, both U4/U6 and U6/U2 chimeras were highly enriched, as presented by the number of chimeric RNAs detected following ligation. The interactions are also depicted in a Circos plot (Figure 3C). As an example, for the specificity of the method, the chimera generated between the C/D snoRNA TB10Cs1C4 and its rRNA target is shown in Figure 3D. The distribution of the chimeric RNA reads on pre-rRNA indicated that the peak of ligated RNA was found around the interaction domain between the C/D snoRNA and rRNA, guiding 2′-O methylation at Gm552 (Figure 3D). Additional examples are presented in Figure S2, also demonstrating that the peak for the chimeric snoRNA/rRNA is always in the vicinity of the 10–21 bp duplex that is formed between the C/D snoRNA and its rRNA target. The chimeric RNA sequence generated between TB10Cs1C4 and rRNA is presented, demonstrating that the chimeric molecules were generated by ligation within the interaction domain or in its vicinity (Figure 3D). Note that although most of the ligated molecules generated by the cross-linking of the snoRNAs with its target were within the interaction domain, ligations also took place with domains that are as far as ∼400 nt away from the interaction region. Thus, the putative interaction domain can be mapped to the region where most of the chimeric reads were derived, as is generally the case with abundant classes of ncRNA, such as snoRNAs. However, this method cannot unequivocally provide base pair resolution of the interaction domain, as many of the chimeras generated contain regions that are distant from the interaction site.
Figure 3.
T.brucei ncRNA Interactome
(A) Schematic presentation of the ncRNA interactome identification protocol. The scheme illustrates the in vivo AMT-psoralen UV cross-linking treatment, extract preparation, fractionation, RNA extraction, mild fragmentation, ligation, and the RNA library preparation methodology.
(B) Enrichment of intermolecular RNA-RNA hybrids in ncRNA interactome upon ligation. The results show that the percentage of chimeric RNA increase from negligible to nearly 4% following ligation (left) and indicate the percentage of intermolecular cross-links in two replicates (right).
(C) Ciros plot representing the known interactions of U snRNA in T. brucei following ligation of cross-linked RNAs.
(D) Chimeric snoRNA-pre rRNA. The potential for base-pairing of TB10Cs1C4 with the pre-rRNA, and the coverage of ligation products between these RNAs in RPKM across the pre-rRNA are presented. An example of a chimeric RNA sequence and its target snoRNA is shown, demonstrating that ligation between the RNAs takes place close to the interaction domain. The different domains of the pre-rRNA are indicated.
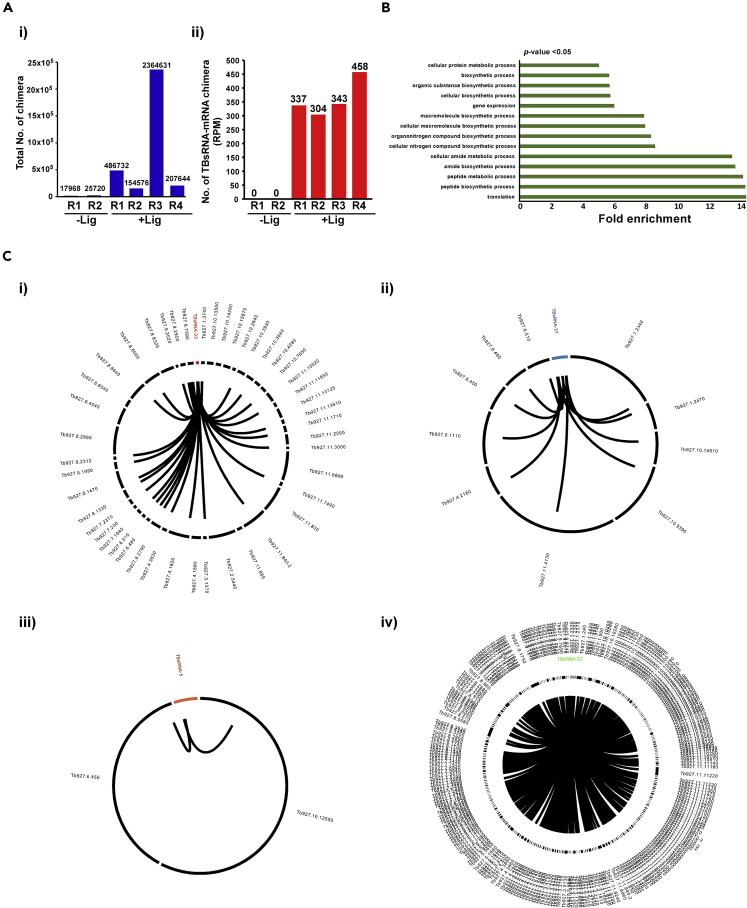
Based on the evidence that this method can identify ncRNA-target interactions, the interaction between the ncRNA and mRNAs was explored in the libraries (Figure 4A). We inspected six libraries specified in Figure 4A, showing that ∼267 mRNAs were cross-linked to 62 ncRNAs (Figure 4A). Note that variation exists in the abundance of chimeric molecules detected in the PRS due to differences in the efficiency of extraction of the RNAs from the different RNPs. The PRS extraction enriches for the ncRNAs and enables the detection of their corresponding targets. To obtain independent evidence for the interactions leading to the formation of the chimeric molecules in vivo, we attempted a reverse experiment, in which we enriched these chimeric molecules by selecting the target mRNA. To this end, cells were treated with AMT psoralen, cross-linked, RNA was extracted with Trizol, and mRNAs were enriched with oligo (dT). After mild alkaline hydrolysis to randomly cleave the mRNA and ncRNA, the cross-linked molecules were ligated, and the cross-linked adducts were released by reversal of cross-linking prior to library preparation. Indeed, 200 potential TBsRNA-mRNA interactions were identified in both these libraries (Table S4, Data S4), suggesting that these reflect genuine in vivo interactions. To control for the specificity of the protocol, we performed a parallel experiment including all steps except the ligation between the RNA molecules, and prepared libraries for RNA-seq. Species that appeared in both PRS and poly(A) selected interactome libraries were considered potential chimeric molecules. In addition, valid chimera was required to be at least 3-fold enriched in the +ligation library compared to the –ligation library (Table S4, Data S4). The –ligation library can produce adducts resulting from ligation events that took place during library preparation, especially from strong base-pair interactions that were not dissociated during RNA extraction.
Figure 4.
T.brucei TBsRNA Interactome
(A) (i) Enrichment of RNA-RNA hybrids in the ncRNA interactome upon ligation in various replicates. (ii) The total number of TBsRNA-mRNA chimeric reads obtained in each library.
(B) Gene ontology of mRNAs cross-linked with TBsRNA.
(C) Circos plots representing the interactions of TBsRNA with mRNAs in T. brucei upon ligation of cross-linked RNAs.
Analyzing the identity of the chimeric mRNAs that passed the above-mentioned criteria indicated that they belong mostly to mRNAs involved in metabolism and protein synthesis (Figure 4B). The Circos plot showed that ncRNAs interact with different mRNAs upon ligation (Figure 4C). The number of chimeric RNAs generated between the ncRNA and mRNA depends on the abundance of the ncRNA. For example, TBsRNA-32 engages in numerous interactions, most likely because it is an abundant RNA in both life stages (Figure 4C). It is important to point out that we cannot rule out the possibility that our analysis missed additional interactions, especially with non-abundant mRNAs.
TBsRNA-33 Is an Anti-sense Repressor of Translation
To further study how these ncRNAs regulate their mRNA interaction partners, we chose to focus on TBsRNA-33. This ncRNA engages in cross-linking with ∼14 mRNAs. Among these are six hypothetical proteins, two ribosomal proteins, and three proteins involved in protein modification (Table S5). Of special interest is the mRNA encoding for the DNA repair and recombination helicase protein, PIF1 encoded by Tb927.11.6890, which is essential because of its role in mitochondrial genome maintenance (Liu et al., 2010).
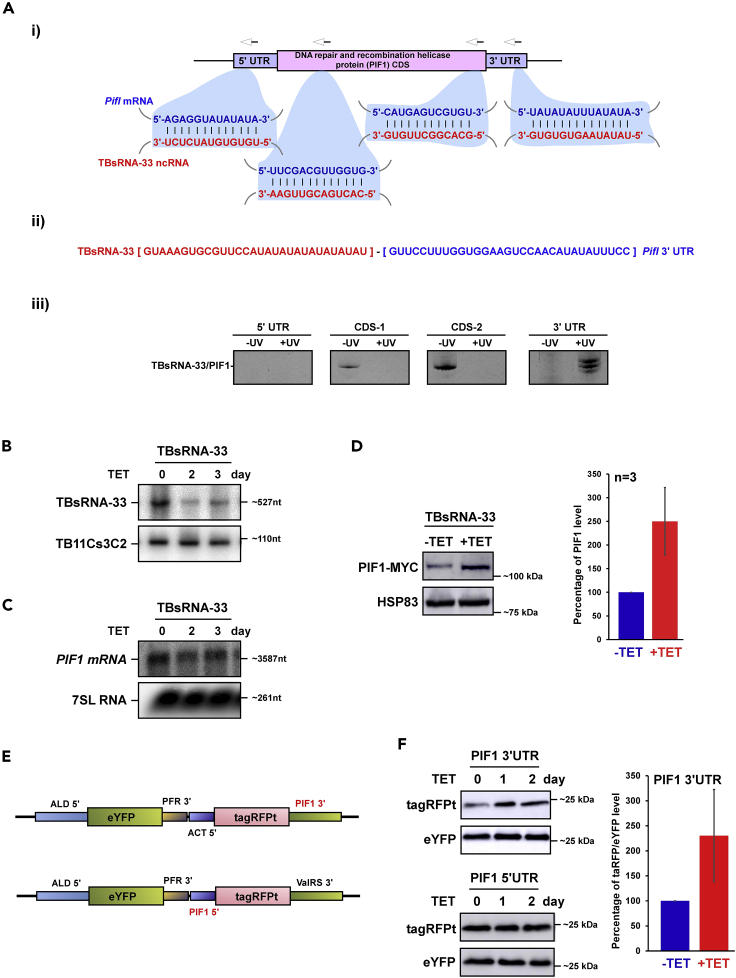
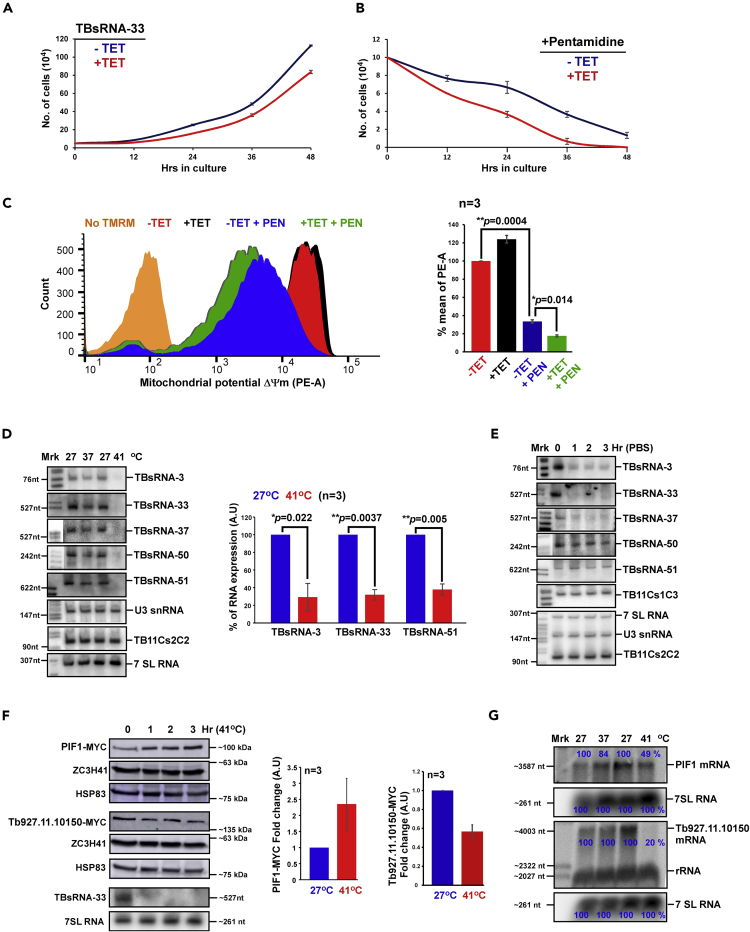
The potential of the ncRNA to interact with Pif1 mRNA is presented in Figure 5Ai, indicating domains of possible interaction within the 5′ and-3′ UTR, and even within the open reading frame. To assess the validity of these interactions, the RNA from the cross-linking and ligation experiment, similar to the one presented in Figure 3A, was used to prepare cDNA, which was amplified with anti-sense primers to the Pif1 mRNA depicted in Figure 5Ai, and sense primer from TBsRNA-33. The results provided evidence that TBsRNA-33 interacts by base-pairing via the 3′ UTR, as can also be seen by inspecting the sequencing of the chimeric molecule (Figures 5Aii and 5Aiii). Note that we also detected the chimeric molecule within the coding sequence (CDS) without UV cross-linking, probably because of strong interaction between TBsRNA-33 and Pif1mRNA CDS. The TBsRNA-33-PifI chimera could not be detected in the poly (A) selected libraries, most likely because these mRNAs are associated with the mitochondrial ribosomes and could not be released without salt extraction. To determine how ncRNAs regulate mRNAs, the TBsRNA-33 was silenced by RNAi using a stem-loop construct (Kalidas et al., 2011). Silencing of the TBsRNA-33 ncRNA was confirmed by Northern analysis (Figure 5B). Next, we examined if silencing of TBsRNA-33 affects the expression of PIF1 protein. First, the level of Pif1 mRNA was examined by Northern analysis over the course of the silencing, and no increase in the level of its mRNA was observed (Figure 5C). Next, the Pif1 gene was tagged with MYC tag and the expression of the tagged protein was examined after silencing. The results indicate ∼2.5-fold increase in the level of the tagged PIF1 protein (n = 3), suggesting that TBsRNA-33 acts as an anti-sense repressor of PIF1 (Figure 5D). To explore the site of regulation by the ncRNA, the 5′ and 3′ UTRs of Pif1 were cloned to flank the tagRFPt gene that is part of dicistronic cassette (Figure 5E). In this dicistronic cassette, the expression of tagRFPt changes as a result of 5′ or 3′ UTR composition, whereas eYFP serves as an internal control for expression. The results (Figure 5F) indicate that the regulation lies in the 3′ UTR of Pif1, supporting the potential interaction domain presented in (Figure 5A). The effect of silencing on cell viability (Figure 6A) showed a mild growth defect but increased sensitivity to pentamidine (Figure 6B) that is known to affect mitochondrial function (Gould and Schnaufer, 2014). The effect on mitochondria was further examined using tetramethyl rhodamine methylester (TMRM) which is a cationic lipophilic dye that enters cells and reversibly accumulates in the negatively charged mitochondrial matrix, depending on mitochondrial membrane potential (Goldshmidt et al., 2010). The silencing of TBsRNA-33 reduced the mitochondrial membrane potential (ΔΨm) upon pentamidine treatment more than in control cells (p < 0.014) (Figure 6C), suggesting that this ncRNA modulates mitochondrial function (see below).
Figure 5.
TBsRNA-33 Is an Anti-Sense Regulator of T. bruceiPif1 mRNA
(A) TBsRNA-33 interacts with Pif1 mRNA. (i) Schematic representation of the potential base-pairing between TBsRNA-33 and Pif1 mRNA. The arrows indicated the positions of primers used in (iii) to amplify the chimeric RNA. (ii) The sequence of chimeric RNA formed by the interaction between TBsRNA-33 and Pif1 mRNA 3′ UTR region is indicated from the PRS interactome library. (iii) Identification of TBsRNA-33 ligated to Pif1 mRNA 3′ UTR by RT-PCR. Total RNA was prepared using the protocol described in (Figure 3A), and the cDNA was subjected to gene specific PCR using a forward primer from TBsRNA-33, and reverse primers corresponding to different regions of Pif1 mRNA as indicated by the arrows in (i).
(B) TBsRNA-33 silencing. Cells carrying the silencing construct for TBsRNA-33 were silenced for the indicated days, and the RNA was subjected to Northern analysis with the indicated anti-sense probes.
(C) Pif1 mRNA is not increased following TBsRNA-33 silencing. Total RNA (20 μg) was prepared from cells before and after TBsRNA-33 silencing, separated on a 1.2% agarose/formaldehyde gel and subjected to Northern analysis using RNA probes, as indicated. 7SL RNA served as a loading control.
(D) PIF1 translation is affected upon TBsRNA-33 silencing. PIF1 was tagged N-terminus with MYC tag in cells carrying the silencing construct for TBsRNA-33 and were silenced for the indicated days. Whole cell lysate from induced (+TET) and uninduced (-TET) was subjected to Western analysis with the indicated antibodies (left). The expression level for PIF1-MYC is presented as mean ± S.E.M (right). Experiments were done in triplicate (n = 3). The expression level of HSP83 was used as a loading control. All densitometry data used in this figure were calculated by ImageJ (https://imagej.nih.gov/ij/) software.
(E) Dicistronic reporter cassette containing PIF1 3′ or 5′ UTR. tagRFPt gene and PIF1 UTR was cloned in PPOTv4 vector carrying eYFP gene (Dean et al., 2015).
(F) TBsRNA-33 regulate PIF1 expression via its 3′ UTR. Dicistronic reporter cassette was introduced in cells carrying the silencing construct for TBsRNA-33 and were silenced for the indicated days. Whole cell lysate from induced (+TET) and uninduced (-TET) cells were subjected to Western analysis with the indicated antibodies (left). The expression level for tagRFPt is presented as mean ± S.E.M (right). Experiments were done in duplicate (n = 2). The expression level of eYFP was used as a loading control. All densitometry data used in this figure was calculated by ImageJ (https://imagej.nih.gov/ij/) software.
Figure 6.
TBsRNA-33 Is an Anti-Sense Regulator of T. brucei Pif1 mRNA
(A) Growth of cells upon TBsRNA-33 silencing. Uninduced cells carrying the silencing construct (-TET) were compared with cells induced for silencing (+TET) at 27°C. Data are presented as mean ± S.E.M. Experiments were done in triplicate (n = 3).
(B) Growth of cells upon TBsRNA-33 silencing and pentamidine treatment. Uninduced cells carrying the silencing construct (-TET) were compared with cells induced for silencing (+TET) upon treatment with 20μM pentamidine. Data are presented as mean ± S.E.M. Experiments were done in triplicate (n = 3).
(C). Silencing of TBsRNA-33 induces loss of mitochondrial membrane potential upon pentamidine treatment. Uninduced cells (-TET) and cells induced for silencing (+TET) upon treatment with 20μM pentamidine were harvested and treated with 150 nM TMRM in PBS with 0.2% glucose. The samples were incubated in the dark for 15 min at 27°C and then analyzed by FACS in PE channel. The cell count of cells with reduced ΔΨm is plotted comparing the cells before and after two days of silencing. Data are presented as mean ± S.E.M (left). Experiments were done in triplicate (n = 3). Student's t-test was performed, and the p value is indicated.
(D) TBsRNAs are reduced upon heat-shock at 41°C. Total RNA (20 μg) from PCF cells subjected to heat-shock for 2 hr at the temperature indicated was separated on a 6% or 10% denaturing polyacrylamide gel and detected by Northern analysis with 32P-labeled anti-sense RNA probes to TBsRNA (left). The quantitative data derived from Northern analysis is presented as mean ± S.E.M (right). Experiments were done in triplicate (n = 3). Student's t-test was performed, and the p value is indicated. 7SL RNA was used as a loading control. 32P-labeled pBR322 DNA MspI digest was used as a size marker.
(E) TBsRNAs are regulated upon starvation. Total RNA (20 μg) from PCF cells upon starvation in 1X PBS for the indicated time points was separated on 6% or 10% denaturing polyacrylamide gels and detected by Northern analysis.
(F) Western analysis of PIF1 protein following heat-shock. Total cell lysates were prepared from an equal number of cells before and after heat-shock and was subjected to western analysis using the indicated antibodies (left). The dilutions used for the antibodies are: (A) MYC (1:10,000), HSP83 (1:10,000), MTAP (1:10,000), and ZCH341 (1:10,000). The RNA derived from the same cells used for Western blot was subjected to Northern analysis. The data for PIF1-MYC is presented as mean ± S.E.M (right). Experiments were done in triplicate (n = 3). The expression level of HSP83 was used as a loading control.
(G) Northern analysis of Pif1 mRNA following heat-shock. Total RNA (20 μg) was prepared under the conditions indicated, separated on a 1.2% agarose/formaldehyde gel and subjected to Northern analysis using RNA probes, as indicated. All densitometry data used in this figure was calculated by ImageJ (https://imagej.nih.gov/ij/) software
To examine if ncRNA level is changed under physiological conditions the level of eight ncRNAs were examined under heat-shock and starvation (Figures 6D, 6E, S3, and S4). The results showed that the level of several ncRNAs changed significantly (p < 0.05), under heat-shock of PCF at 41°C (Figures 6D and S3) or starvation for 1–3 hr (Figures 6E and S4). In particular, the level of TBsRNA-33 is reduced under both heat-shock and starvation. To examine if like silencing of TBsRNA-33 the level of PIF1 protein is increased, the level of the tagged PIF1 protein was examined following incubation at 41°C. Indeed, the level of MYC-tagged PIF1 protein was increased by ∼1.5 fold (n = 3) (Figure 6F), although the level of Pif1 mRNA was reduced by ∼50% as the level of many mRNA under these conditions (Kramer et al., 2008) (Figure 6G).
This is a specific effect, since the level of MYC-tagged protein Enoyl-CoA hydratase/isomerase putative protein encoded by Tb927.11.10150, was reduced by 40% (Figure 6F) as was its mRNA (∼80% reduction) (Figure 6G). Based on these results, TBsRNA-33 is likely to function in repressing the translation of the Pif1 mRNA, since when TBsRNA-33 levels were reduced, the level of MYC-tagged PIF1 protein increased. Interestingly, in BSF the level of TBsRNA-33 is increased by ∼2.7 fold (Figures 1F–1H) and Pif1 mRNA is poorly translated (log2 (−1.78)) (Figure S5) (Jensen et al., 2014), suggesting that this ncRNA contribute to the regulation of mitochondrial mRNA translation in the two life stages of the parasite.
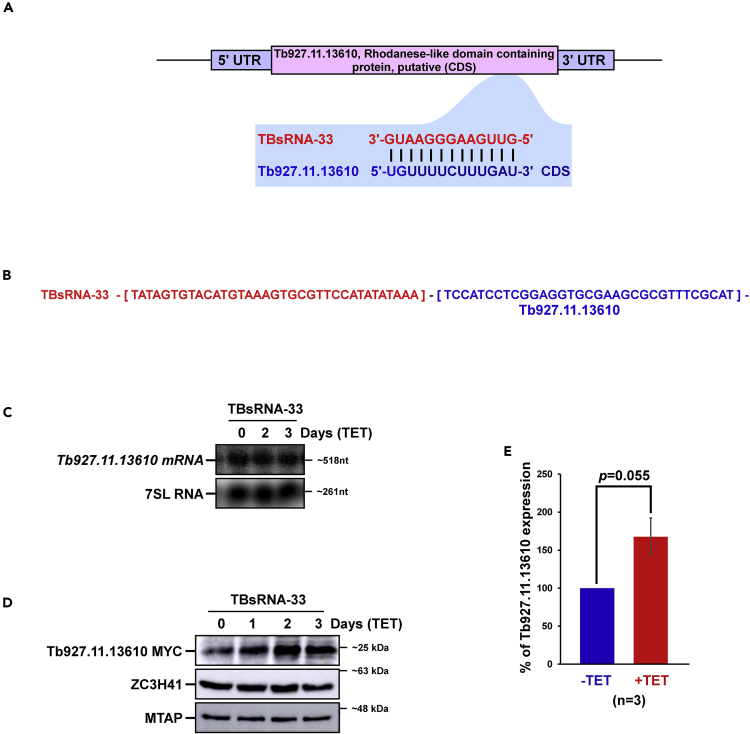
Next, the validity of interaction of TBsRNA-33 with rhodanese-like domain containing protein encoded by Tb927.11.13610 mRNA was examined (Table S5). The potential interaction domain of TBsRNA-33 with the Tb927.11.13610 mRNA target is presented (Figure 7A), and the chimera formed with the target mRNA is indicated (Figure 7B). The level of the Tb927.11.13610 mRNA was examined upon silencing of TBsRNA-33, and no change was observed (Figure 7C). To examine the possible effect on protein expression, the Tb927.11.13610 gene was tagged with the MYC tag, and its expression was examined. The results (Figures 7D and 7E) suggest that the level of rhodanese-like domain-containing protein was increased by ∼1.7 fold upon silencing of TBsRNA-33 (n = 3), suggesting that similar to Pif1 mRNA, the TBsRNA-33 serves as a translational repressor for Tb927.11.13610 mRNA.
Figure 7.
TBsRNA-33 Is an Anti-Sense Regulator of Tb927.11.13610, Rhodanese-like Domain-Containing Protein mRNA
(A) TBsRNA-33 interacts with Tb927.11.13610 mRNA. Schematic representation of the potential base-pairing between TBsRNA-33 and Tb927.11.13610 mRNA.
(B) The sequence of the chimeric RNA formed by the interaction between TBsRNA-33 and the Tb927.11.13610 CDS region is indicated based on the PRS interactome library.
(C) Tb927.11.13610 mRNA is not increased upon TBsRNA-33 silencing. Total RNA (20 μg) was prepared from cells before and after TBsRNA-33 silencing, separated on a 1.2% agarose/formaldehyde gel and subjected to Northern analysis using RNA probes, as indicated. 7SL RNA served as a loading control.
(D) Tb927.11.13610 translation is increased upon TBsRNA-33 silencing. Tb927.11.13610 was tagged with N-terminal MYC tag in cells carrying the silencing construct for TBsRNA-33 and were silenced for the indicated number of days. Whole cell lysates from induced (+TET) and uninduced (-TET) cultures were subjected to western analysis with the indicated antibodies (left). (E) The expression level for Tb927.11.13610-MYC is presented as the mean ± S.E.M. Experiments were performed in triplicate (n = 3). Student's t-test was calculated, and the p value is indicated. The expression level of HSP83 was used as a loading control. All densitometry data were calculated by ImageJ (https://imagej.nih.gov/ij/) software.
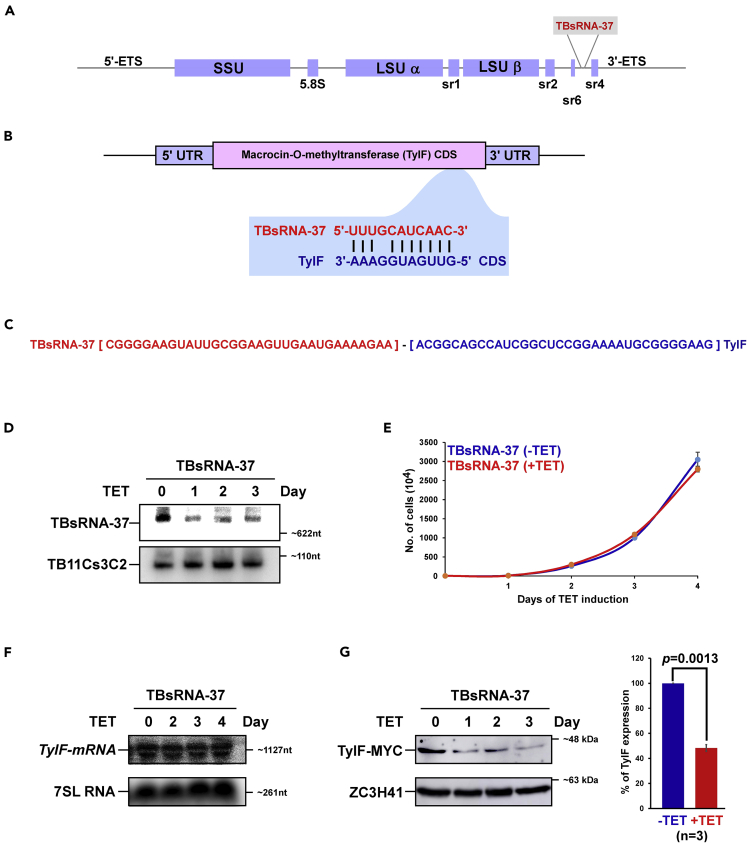
TBsRNA-37 Is an Anti-sense Enhancer of Translation
TBsRNA-37 is encoded in the ITS7 of pre-rRNA (Figure 8A). Inspecting the RNA library of the chimeric RNA described above for interacting RNAs with TBsRNA-37 we identified four mRNAs, including the Macrocin-O-methyltransferase (TyIF) encoded by Tb927.10.9390, which produces tylosin antibiotics active against gram positive bacteria (Seno and Baltz, 1981). The function of this protein is unknown in T. brucei. This chimera was detected in both PRS as well as poly(A) chimeric libraries. Moreover, this chimera was 3-fold enriched in the +ligation library. The potential for base-pairing between the ncRNA and its target mRNA is presented (Figure 8B). This potential interaction can also be seen by inspecting the sequencing of the chimeric molecule (Figure 8C). The ncRNA was silenced using a stem-loop RNAi construct and the RNA was subjected to Northern analysis showing efficient depletion (Figure 8D). Silencing was not associated with a growth defect under normal conditions (Figure 8E). No change in the level of TylF mRNA was observed upon silencing (Figure 8F). Next the TylF gene was tagged with MYC tag at the N-terminus and the expression was examined in the silenced cells, showing 50% reduction in the level of the protein (p < 0.0013) (Figure 8G), suggesting that this ncRNA enhances translation.
Figure 8.
TBsRNA-37 Is an Anti-Sense Regulator of TyIF mRNA
(A) TBsRNA-37 is encoded in the ITS7 of pre-rRNA. Schematic representation of the pre-rRNA locus from which TBsRNA-37 is derived is presented.
(B) TBsRNA-37 interacts with TyIF mRNA. Schematic representation of the potential base-pairing between TBsRNA-37 and TyIF mRNA.
(C) The sequence of chimeric RNA formed by the interaction between TBsRNA-37 and TyIF mRNA CDS region is indicated based on the PRS interactome library.
(D) TBsRNA-37 silencing. Cells carrying the silencing construct for TBsRNA-37 were silenced for the indicated days, and the RNA was subjected to Northern analysis with the indicated anti-sense probes.
(E) Growth of cells upon TBsRNA-37 silencing. Uninduced cells carrying the silencing construct (-TET) were compared with cells induced for silencing (+TET) at 27°C. Data are presented as mean ± S.E.M. Experiments were done in triplicate (n = 3).
(F) TyIF mRNA is not decreased upon TBsRNA-37 silencing. Total RNA (20 μg) was prepared from cells before and after TBsRNA-37 silencing, separated on a 1.2% agarose/formaldehyde gel, and subjected to Northern analysis using RNA probes, as indicated. 7SL RNA served as loading control. (G) TyIF translation is reduced upon TBsRNA-37 silencing. TyIF gene was tagged N-terminus with MYC tag in cells carrying the silencing construct for TBsRNA-37 and were silenced for the indicated days. Whole cell lysate from induced (+TET) and uninduced (-TET) was subjected to Western analysis with the indicated antibodies (left). The expression level for TyIF-MYC is presented as mean ± S.E.M (right). Experiments were done in triplicate (n = 3). Student's t-test was performed, and the p value is indicated. The expression level of ZC3H41 was used as a loading control. All densitometry data used in this figure were calculated by ImageJ (https://imagej.nih.gov/ij/) software.
TBsRNA- 37 Interacts with the Ribosomes: A Model of Its Processing and Function
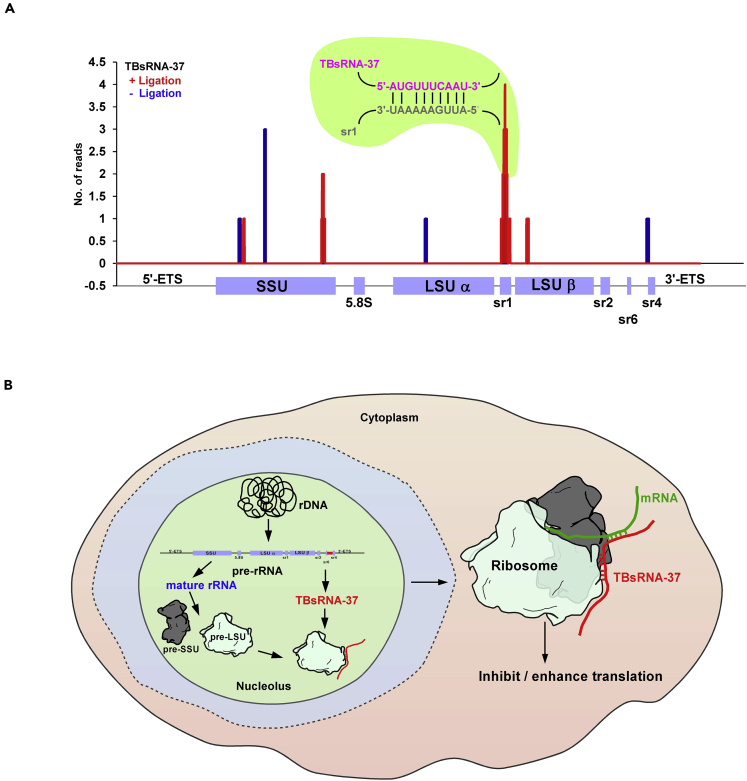
TBsRNA-37 is processed from ITS7 of its pre-rRNA (Figure 8A), but then moves to the cytoplasm, as it co-fractionates with the 80S ribosome. We therefore searched for its cross-linking with a mature rRNA sequence in the chimeric RNA library. TBsRNA-37 has the potential to base-pair with sr1 via 9 nts (Figure 9A), and indeed the majority of the chimeric reads are within the srRNA (sr1) (Figure 9B). Although we have no direct genetic evidence for the validity of such a base-pair interaction, there are no other possible interactions within sr1 apart from this proposed base-pairing. Whether the interaction with sr1 relies solely on base-pairing or also involves adapter protein(s) is currently unknown.
Figure 9.
Proposed Model for the Mechanism of TBsRNA-37 as an Anti-Sense Regulator in T. Brucei
(A) TBsRNA-37 can potentially base-pair with mature rRNA. The coverage of ligation products of TBsRNA-37 across the pre-rRNA is shown from the PRS interactome library. The potential base-pairing of TBsRNA-37 with the srRNA-1 (sr1) is shown. The different domains of the pre-rRNA are indicated.
(B) The proposed mechanism for TBsRNA-37 interaction with its target mRNA. The ncRNA TBsRNA-37 is processed from the pre-rRNA in the nucleolus, potentially base-pairs with sr1 during the biogenesis of pre-LSU and moves together with the LSU to be assembled into a mature ribosome in the cytoplasm. In the cytoplasm, TBsRNA-37 remains base-paired with sr1 and interacts by base pairing to the target mRNA to inhibit or enhance translation.
Discussion
Anti-sense ncRNAs have been demonstrated to regulate gene expression in species ranging from bacteria to humans. These RNAs comprise small RNAs or long ncRNAs, as described above. Long ncRNAs were shown in trypanosomes to undergo trans-splicing and polyadenylation (Kolev et al., 2010). A few of these were shown to associate with ribosomes (Kolev et al., 2010). It was puzzling that neither long nor short anti-sense regulatory RNAs were described to date in trypanosomes, which appear to lack conventional microRNAs (Kolev et al., 2011). Here we focus on such ncRNA molecules that we show to regulate mRNA translation. Strikingly, RNAs TBsRNA-33 and 37 associates with the ribosome, have the potential to base-pair with rRNA, and do not exist as independent small RNA protein complexes like most known ncRNAs such as snoRNPs. We cannot exclude the possibility that these ncRNAs interact with ribosomal or other ribosome-associated protein(s).
Our study using deep sequencing of RNAs enriched in ribosome-free extracts revealed 62 novel ncRNAs and represents first steps in exploring this unique repertoire of novel ncRNAs. Of special interest are those ncRNAs that seem to interact mainly with ribosomes and potentially function as anti-sense regulators, as we show here for TBsRNA-33 and 37.
Among these new ncRNAs are stable RNAs that are processed from the spacers of pre-rRNA such as TBsRNA- 33, 50, 51 that are developmentally regulated and are highly expressed in BSF and their level is differentially regulated under starvation. Many of these ncRNAs listed above are likely to regulate the mRNAs they interact with. These ncRNA seem not to have a global effect on translation, but rather interact with only a small subset of mRNAs. Of special interest is TBsRNA-32 that becomes cross-linked to a variety of mRNAs. Although it is currently unknown how this RNA regulates their target mRNA. The ncRNAs can potentially either interact with 3′ UTR or 5′ UTR and affect stability and/or translation. We can also envision that like microRNAs these ncRNAs can affect the binding of an RBP (Kedde et al., 2007). Differential binding of RBPs was shown to regulate both stability and translation of mRNA in a stage-dependent manner (Clayton, 2019). For instance, T. brucei hnRNPF/H stabilize or de-stabilize the same transcript in the two life stages of the parasite (Gupta et al., 2013b). This is the first study to highlight the presence of ncRNAs that are not derived from other stable RNAs but are generated to regulate the fate of specific mRNAs possibly by either repressing or enhancing stability and/or translation.
The study highlights the function of TBsRNA-33 and -37, which associate with the ribosome in the nucleolus, and likely move to the cytoplasm with the ribosome, where they affect the translation of a distinct set of mRNAs (Figure 9B). TBsRNA-33 is developmentally regulated, and its level is reduced under heat-shock and starvation. The genomic location of TBsRNA-33 and 37 is of special interest. It was previously reported that microRNAs can be processed from the rRNA ITS in Drosophila (Chak et al., 2015). However, here we report that the rDNA locus not only hosts ncRNAs that are processed from pre-rRNA spacers, but also ncRNAs that are transcribed from the anti-sense strand. In higher eukaryotes, microRNAs and snoRNAs are found in introns of pre-mRNA (Hesselberth, 2013). However, trypanosomes only possess two genes containing cis-spliced introns (Mair et al., 2000), which may explain why in trypanosomes, rDNA evolved to become the host of ncRNAs. Note that despite the length of all the TBsRNAs derived from pre-rRNA, which is similar to that of lncRNAs, we have no evidence for the presence of poly(A) at their 3′ end (Chikne et al., 2017) (Data S2). In addition, even the longer ncRNA among the 62 molecules described in this study, do not have significant potential for coding proteins or even small peptides (Table S5) (Kang et al., 2017).
Many of the ncRNAs listed in (Tables S1 and S2) are also highly expressed in the BSF. Since reduction in the level of TBsRNA-33 under heat-shock most likely relieves the inhibition on the translation of Pif1 mRNA (Figure 4), we assume that the ncRNA acts as a repressor, and when its level is reduced during heat-shock, it can no longer repress the translation of its target mRNAs. Although the mechanism of action of TBsRNA-33 is currently unknown, RNA binding to a distinct site on the 3′ UTR can affect the binding of protein(s) that interact with the 43S initiation complex to enhance translation; alternatively the ncRNA may directly or via its binding protein inhibit translation, similar to the mechanism by which microRNAs arrest translation (Rissland, 2017). The mechanism of how TBsRNA-37 enhances translation of TyIF mRNA is currently unknown. The ncRNA can potentially bind a repressor that is bound to the target mRNA and by that relieve repression.
Based on the results obtained, we propose a model for the processing and function of TBsRNA-37 (Figure 9B). We suggest that the ncRNA is processed from pre-rRNA, potentially interacts with sr1, migrates with the ribosome to the cytoplasm, and during translation interacts with specific mRNAs. We currently do not know which protein(s) stabilize TBsRNA-33 and 37, but based on our RNA fractionation experiments, we favor the possibility that the RNA interacts directly with the ribosome and its association with the ribosome protects it from degradation. However, we cannot exclude the possibility that these ncRNA are bound during their biogenesis by protein(s) and that these are dislodged during fractionation. Half tRNA molecules were shown recently to associate with ribosomes in T. brucei. These are produced during nutrient deprivation and stimulate translation by facilitating mRNA loading during stress recovery, once starvation conditions are reversed (Fricker et al., 2019). However, the ncRNAs described here are not derived from stable RNAs.
Very little is known how the translation of proteins translocated to the mitochondria is regulated. Here we show that TBsRNA-33 regulates the translation of the Pif1 mRNA that its protein is translocated to the mitochondria. We suggest a novel mechanism to regulate the mitochondria function in the two life stages of the parasite involving that action of anti-sense regulator. Since in BSF, the mitochondria is smaller and less active, therefore less of mitochondrial proteins should be translated. This is achieved by regulating the ncRNA that controls the translation of mitochondrial proteins in a stage-specific manner. TBsRNA-33 is highly expressed in BSF repressing the synthesis of the mitochondrial PIF1. Indeed, PIF1 is indeed essential for the mitochondrial function in PCF (Liu et al., 2010).
This study describes a novel mechanism to reduce or enhance translation via ncRNAs in T. brucei. This study presents the first report of a previously unknown level of regulation by ncRNAs and is likely only the tip of the iceberg in understanding the role of ncRNA is controlling gene expression during the cycling of the parasite between its hosts, or during its adaption to physiological cues such as heat-shock, starvation, and oxidative stress during infection. Regulation by ncRNAs is quick and economical since it does not require additional synthesis of proteins to control gene expression under ambient conditions. Better understanding the mode of action of these novel ncRNAs can open the way to novel therapeutic approaches against these clinically significant parasites.
Limitations of the Study
The methodology used in this study to reveal ncRNA-target interaction using in vivo cross-linking and ligation of the interacting RNA molecules has limitations. The method requires a fragmentation step to enable ligation of the long target with the ncRNA. Mild fragmentation preserves the ncRNA but may not efficiently cleave longer RNA targets, thereby reducing the number of chimeric RNA molecules formed between the ncRNA and its target. Although most of the detected chimera were found in the interaction domain, other chimeric molecules were formed outside this domain, precluding the ability to unequivocally determine the exact base-pair interaction. The option of validating the interaction domain by mutating the interaction sequence or by introducing a compensatory mutation is not trivial for the ncRNA studied here because these originate from the rRNA locus.
The ultimate proof of our model for the function of TBsRNA-37 would be to find a tri-partite chimeric molecule that includes the ncRNA, the rRNA, and its mRNA target. However, for this RNA, we could not find such molecules, but were able to find examples of other mRNAs that are more abundant. Hence, the ability to find chimera from non-abundant RNAs (ncRNA or mRNA) is limited. Detection of the chimeric molecules varies between experiments and depends on efficiency of extraction of the RNPs (PRS extracts) and their associated RNAs.
Chimeric molecules were also detected in the non-ligated fraction because these could be formed between RNA molecules that are held together strongly by non-covalent interactions, or are very abundant in the pool and become ligated during the ligation step used for library preparation. Taking these limitations into account we focused on non-abundant mRNA targets that were at least 3-fold enriched in the ligation step performed before library preparation. In addition, we mainly considered the chimera that appeared in both types of RNA preparation (PRS and poly(A) selected RNA).
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Prof. Shulamit Michaeli (Shulamit.Michaeli@biu.ac.il).
Materials Availability
All plasmids, cell-lines, and in-house bioinformatics scripts used in this study are available on reasonable requests from the lead contact.
Data and Code Availability
The RNA sequencing data related to this study have been deposited in the NCBI Bioproject database under the accession number PRJNA630014. The data can be accessed using the following link: https://dataview.ncbi.nlm.nih.gov/object/PRJNA630014?reviewer=r8o4gaigdoao6t6nhitl1cku8k. Further information and requests for the bioinformatics codes used in this study should be directed to and will be fulfilled by the Lead Contact, Prof. Shulamit Michaeli (Shulamit.Michaeli@biu.ac.il).
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by a grant from the Israel-US Binational Science Foundation (BSF), and NIH grant R01 AI 056333 to C.T. S.M. holds the David and Inez Myers Chair in RNA silencing of diseases.
Author Contributions
K.S.R: Methodology, visualization, formal analysis, and validation. K.S.R, B.G, S.A: Investigation, validation. S.C.C: Library preparation. K.S.R and T.G: Bioinformatics analysis. R.P: assistance in bioinformatics analysis. KSR, T.G, R.U, C.T, and S.M: Review and editing. R.U, C.T, and S.M: Funding acquisition and writing manuscript.
Declaration of Interests
The authors declare no competing financial interests.
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101780.
Supplemental Information
Listed are those interactions enriched by more than 3 folds (Log2 (1.58)) upon ligation. The Log2-Fold change of chimera sequence in plus ligation (LIGP) vs minus ligation (LIGM) and the normalized read counts in both PRS and poly (A) interactome libraries is indicated. Highlighted in green is the interaction of TBsRNA-37 and Tb927.10.9390 (TyIF), verified in this study
The data were generated using Coding Potential Calculator 2 online program (http://cpc2.cbi.pku.edu.cn/) (Kang et al., 2017)
References
- Anders S., Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth S., Hury A., Liang X., Michaeli S. Elucidating the role of H/ACA-like RNAs in trans -splicing and rRNA processing via RNA interference silencing of the trypanosoma brucei CBF5 pseudouridine synthase. J. Biol. Chem. 2005;280:34558–34568. doi: 10.1074/jbc.M503465200. [DOI] [PubMed] [Google Scholar]
- Butter F., Bucerius F., Michel M., Cicova Z., Mann M., Janzen C.J. Comparative proteomics of two life cycle stages of stable isotope-labeled trypanosoma brucei reveals novel components of the parasite’s host adaptation machinery. Mol. Cell. Proteomics. 2013;12:172–179. doi: 10.1074/mcp.M112.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chak L.-L., Mohammed J., Lai E.C., Tucker-Kellogg G., Okamura K. A deeply conserved, noncanonical miRNA hosted by ribosomal DNA. RNA. 2015;21:375–384. doi: 10.1261/rna.049098.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikne V., Doniger T., Rajan K.S., Bartok O., Eliaz D., Cohen-Chalamish S., Tschudi C., Unger R., Hashem Y., Kadener S., Michaeli S. A pseudouridylation switch in rRNA is implicated in ribosome function during the life cycle of Trypanosoma brucei. Sci. Rep. 2016;6:25296. doi: 10.1038/srep25296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikne V., Gupta S.K., Doniger T., K. S.R., Cohen-Chalamish S., Waldman Ben-Asher H., Kolet L., Yahia N.H., Unger R., Ullu E. The canonical poly (A) polymerase PAP1 polyadenylates non-coding RNAs and is essential for snoRNA biogenesis in trypanosoma brucei. J. Mol. Biol. 2017;429:3301–3318. doi: 10.1016/j.jmb.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Chikne V., Shanmugha Rajan K., Shalev-Benami M., Decker K., Cohen-Chalamish S., Madmoni H., Biswas V.K., Kumar Gupta S., Doniger T., Unger R. Small nucleolar RNAs controlling rRNA processing in Trypanosoma brucei. Nucleic Acids Res. 2019;47:2609–2629. doi: 10.1093/nar/gky1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. Regulation of gene expression in trypanosomatids: living with polycistronic transcription. Open Biol. 2019;9:190072. doi: 10.1098/rsob.190072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. The regulation of trypanosome gene expression by RNA-binding proteins. PLoS Pathog. 2013;9:e1003680. doi: 10.1371/journal.ppat.1003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C.E. Gene expression in kinetoplastids. Curr. Opin. Microbiol. 2016;32:46–51. doi: 10.1016/j.mib.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Dean S., Sunter J., Wheeler R.J., Hodkinson I., Gluenz E., Gull K. A toolkit enabling efficient, scalable and reproducible gene tagging in trypanosomatids. Open Biol. 2015;5:140197. doi: 10.1098/rsob.140197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker R., Brogli R., Luidalepp H., Wyss L., Fasnacht M., Joss O., Zywicki M., Helm M., Schneider A., Cristodero M., Polacek N. A tRNA half modulates translation as stress response in Trypanosoma brucei. Nat. Commun. 2019;10:118. doi: 10.1038/s41467-018-07949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Wheeler E., Lockard R.E., Kumar A. Mapping of psoralen cross-linked nucleotides in RNA. Nucleic Acids Res. 1984;12:3405–3424. doi: 10.1093/nar/12.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmidt H., Matas D., Kabi A., Carmi S., Hope R., Michaeli S. Persistent ER stress induces the spliced leader RNA silencing pathway (SLS), leading to programmed cell death in trypanosoma brucei. PLoS Pathog. 2010;6:e1000731. doi: 10.1371/journal.ppat.1000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould M.K., Schnaufer A. Independence from kinetoplast DNA maintenance and expression is associated with multidrug resistance in trypanosoma brucei in vitro. Antimicrob. Agents Chemother. 2014;58:2925–2928. doi: 10.1128/AAC.00122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.K., Hury A., Ziporen Y., Shi H., Ullu E., Michaeli S. Small nucleolar RNA interference in Trypanosoma brucei: mechanism and utilization for elucidating the function of snoRNAs. Nucleic Acids Res. 2010;38:7236–7247. doi: 10.1093/nar/gkq599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.K., Kolet L., Doniger T., Biswas V.K., Unger R., Tzfati Y., Michaeli S. The Trypanosoma brucei telomerase RNA (TER) homologue binds core proteins of the C/D snoRNA family. FEBS Lett. 2013;587:1399–1404. doi: 10.1016/j.febslet.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Gupta S.K., Kosti I., Plaut G., Pivko A., Tkacz I.D., Cohen-Chalamish S., Biswas D.K., Wachtel C., Waldman Ben-Asher H., Carmi S. The hnRNP F/H homologue of Trypanosoma brucei is differentially expressed in the two life cycle stages of the parasite and regulates splicing and mRNA stability. Nucleic Acids Res. 2013;41:6577–6594. doi: 10.1093/nar/gkt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne T., Agabian N. RNA B is the major nucleolar trimethylguanosine-capped small nuclear RNA associated with fibrillarin and pre-rRNAs in Trypanosoma brucei. Mol. Cell Biol. 1993;13:144–154. doi: 10.1128/mcb.13.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselberth J.R. Lives that introns lead after splicing. Wiley Interdiscip. Rev. RNA. 2013;4:677–691. doi: 10.1002/wrna.1187. [DOI] [PubMed] [Google Scholar]
- Hombach S., Kretz M. Non-coding RNAs: classification, biology and functioning. Adv. Exp. Med. Biol. 2016;937:3–17. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- Jensen B.C., Ramasamy G., Vasconcelos E.J.R., Ingolia N.T., Myler P.J., Parsons M. Extensive stage-regulation of translation revealed by ribosome profiling of Trypanosoma brucei. BMC Genomics. 2014;15:911. doi: 10.1186/1471-2164-15-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalidas S., Li Q., Phillips M.A. A Gateway® compatible vector for gene silencing in bloodstream form Trypanosoma brucei. Mol. Biochem. Parasitol. 2011;178:51–55. doi: 10.1016/j.molbiopara.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.-J., Yang D.-C., Kong L., Hou M., Meng Y.-Q., Wei L., Gao G. CPC2: a fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017;45:W12–W16. doi: 10.1093/nar/gkx428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedde M., Strasser M.J., Boldajipour B., Oude Vrielink J.A.F., Slanchev K., le Sage C., Nagel R., Voorhoeve P.M., van Duijse J., Ørom U.A. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell. 2007;131:1273–1286. doi: 10.1016/j.cell.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Kolev N.G., Franklin J.B., Carmi S., Shi H., Michaeli S., Tschudi C. The transcriptome of the human pathogen trypanosoma brucei at single-nucleotide resolution. PLoS Pathog. 2010;6:e1001090. doi: 10.1371/journal.ppat.1001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev N.G., Rajan K.S., Tycowski K.T., Toh J.Y., Shi H., Lei Y., Michaeli S., Tschudi C. The vault RNA of Trypanosoma brucei plays a role in the production of trans -spliced mRNA. J. Biol. Chem. 2019;294:15559–15574. doi: 10.1074/jbc.RA119.008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev N.G., Ramey-Butler K., Cross G.A.M., Ullu E., Tschudi C. Developmental progression to infectivity in Trypanosoma brucei triggered by an RNA-binding protein. Science. 2012;338:1352–1353. doi: 10.1126/science.1229641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev N.G., Tschudi C., Ullu E. RNA interference in Protozoan parasites: achievements and challenges. Eukaryot. Cell. 2011;10:1156–1163. doi: 10.1128/EC.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S., Queiroz R., Ellis L., Webb H., Hoheisel J.D., Clayton C., Carrington M. Heat shock causes a decrease in polysomes and the appearance of stress granules in trypanosomes independently of eIF2 phosphorylation at Thr169. J. Cell Sci. 2008;121:3002–3014. doi: 10.1242/jcs.031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.-H., Xu Y.-X., Michaeli S. The spliced leader-associated RNA is a trypanosome-specific sn(o) RNA that has the potential to guide pseudouridine formation on the SL RNA. RNA. 2002;8 doi: 10.1017/s1355838202018290. S1355838202018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Haritan A., Uliel S., Michaeli S. Trans and cis splicing in trypanosomatids: mechanism, factors, and regulation. Eukaryot. Cell. 2003;2:830–840. doi: 10.1128/EC.2.5.830-840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Yildirir G., Wang J., Tolun G., Griffith J.D., Englund P.T. TbPIF1, a trypanosoma brucei mitochondrial DNA helicase, is essential for kinetoplast minicircle replication. J. Biol. Chem. 2010;285:7056–7066. doi: 10.1074/jbc.M109.084038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair G., Shi H., Li H., Djikeng A., Aviles H.O., Bishop J.R., Falcone F.H., Gavrilescu C., Montgomery J.L., Santori M.I. A new twist in trypanosome RNA metabolism: cis-splicing of pre-mRNA. RNA. 2000;6:163–169. doi: 10.1017/s135583820099229x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli S. Non-coding RNA and the complex regulation of the trypanosome life cycle. Curr. Opin. Microbiol. 2014;20:146–152. doi: 10.1016/j.mib.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Michaeli S. Trans-splicing in trypanosomes: machinery and its impact on the parasite transcriptome. Future Microbiol. 2011;6:459–474. doi: 10.2217/fmb.11.20. [DOI] [PubMed] [Google Scholar]
- Michaeli S., Doniger T., Gupta S.K., Wurtzel O., Romano M., Visnovezky D., Sorek R., Unger R., Ullu E. RNA-seq analysis of small RNPs in Trypanosoma brucei reveals a rich repertoire of non-coding RNAs. Nucleic Acids Res. 2012;40:1282–1298. doi: 10.1093/nar/gkr786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr A.M., Mott J.L. Overview of microRNA biology. Semin. Liver Dis. 2015;35:3–11. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K.V., Mattick J.S. The rise of regulatory RNA. Nat. Rev. Genet. 2014;15:423–437. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugo E., Clayton C. Expression of the RNA-binding protein RBP10 promotes the bloodstream-form differentiation state in Trypanosoma brucei. PLoS Pathog. 2017;13:e1006560. doi: 10.1371/journal.ppat.1006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguleswaran A., Doiron N., Roditi I. RNA-Seq analysis validates the use of culture-derived Trypanosoma brucei and provides new markers for mammalian and insect life-cycle stages. BMC Genomics. 2018;19:227. doi: 10.1186/s12864-018-4600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz R., Benz C., Fellenberg K., Hoheisel J.D., Clayton C. Transcriptome analysis of differentiating trypanosomes reveals the existence of multiple post-transcriptional regulons. BMC Genomics. 2009;10:495. doi: 10.1186/1471-2164-10-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan K.S., Chikne V., Decker K., Waldman Ben-Asher H., Michaeli S. Unique aspects of rRNA biogenesis in trypanosomatids. Trends Parasitol. 2019;35:778–794. doi: 10.1016/j.pt.2019.07.012. [DOI] [PubMed] [Google Scholar]
- Rajan K.S., Doniger T., Cohen-Chalamish S., Chen D., Semo O., Aryal S., Glick Saar E., Chikne V., Gerber D., Unger R. Pseudouridines on Trypanosoma brucei spliceosomal small nuclear RNAs and their implication for RNA and protein interactions. Nucleic Acids Res. 2019;47:7633–7647. doi: 10.1093/nar/gkz477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan K.S., Zhu Y., Adler K., Doniger T., Cohen-Chalamish S., Srivastava A., Shalev-Benami M., Matzov D., Unger R., Tschudi C. The large repertoire of 2’-O-methylation guided by C/D snoRNAs on Trypanosoma brucei rRNA. RNA Biol. 2020;17:1018–1039. doi: 10.1080/15476286.2020.1750842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissland O.S. The organization and regulation of mRNA-protein complexes. Wiley Interdiscip. Rev. RNA. 2017;8:e1369. doi: 10.1002/wrna.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues J.C.F., Godinho J.L.P., de Souza W. Biology of human pathogenic trypanosomatids: epidemiology, lifecycle and ultrastructure. Subcell. Biochem. 2014;74:1–42. doi: 10.1007/978-94-007-7305-9_1. [DOI] [PubMed] [Google Scholar]
- Sandhu R., Sanford S., Basu S., Park M., Pandya U.M., Li B., Chakrabarti K. A trans-spliced telomerase RNA dictates telomere synthesis in Trypanosoma brucei. Cell Res. 2013;23:537–551. doi: 10.1038/cr.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno E.T., Baltz R.H. Properties of S-adenosyl-L-methionine:macrocin O-methyltransferase in extracts of Streptomyces fradiae strains which produce normal or elevated levels of tylosin and in mutants blocked in specific O-methylations. Antimicrob. Agents Chemother. 1981;20:370–377. doi: 10.1128/aac.20.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Shi H., Franklin J.B., Ullu E. Small interfering RNA-producing loci in the ancient parasitic eukaryote Trypanosoma brucei. BMC Genomics. 2012;13:427. doi: 10.1186/1471-2164-13-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E.G.H., Romby P. Small RNAs in bacteria and archaea: who they are, what they do, and how they do it. Adv. Genet. 2015;90:133–208. doi: 10.1016/bs.adgen.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Wilson R.C., Doudna J.A. Molecular mechanisms of RNA interference. Annu. Rev. Biophys. 2013;42:217–239. doi: 10.1146/annurev-biophys-083012-130404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Listed are those interactions enriched by more than 3 folds (Log2 (1.58)) upon ligation. The Log2-Fold change of chimera sequence in plus ligation (LIGP) vs minus ligation (LIGM) and the normalized read counts in both PRS and poly (A) interactome libraries is indicated. Highlighted in green is the interaction of TBsRNA-37 and Tb927.10.9390 (TyIF), verified in this study
The data were generated using Coding Potential Calculator 2 online program (http://cpc2.cbi.pku.edu.cn/) (Kang et al., 2017)
Data Availability Statement
The RNA sequencing data related to this study have been deposited in the NCBI Bioproject database under the accession number PRJNA630014. The data can be accessed using the following link: https://dataview.ncbi.nlm.nih.gov/object/PRJNA630014?reviewer=r8o4gaigdoao6t6nhitl1cku8k. Further information and requests for the bioinformatics codes used in this study should be directed to and will be fulfilled by the Lead Contact, Prof. Shulamit Michaeli (Shulamit.Michaeli@biu.ac.il).