Abstract
Introduction
Bisphosphonates (BPs) are first-line therapy for osteoporosis. Adherence is usually low in chronic, asymptomatic diseases, but gastrointestinal (GI) side-effects can also contribute to low adherence in BP therapy and may necessitate a review by a gastroenterologist with or without gastroscopy.
Aims
Our meta-analysis aims to determine the risk of severe GI adverse events due to oral BP therapy in osteoporotic patients.
Methods
A systematic search was conducted in three databases up to September 2020 for randomized controlled trials (RCTs) detailing GI adverse events in adults with osteoporosis on BP compared to placebo. Risk ratios (RRs) with 95% confidence intervals (CI) were calculated for non-severe and severe adverse events indicating endoscopic procedure with the random-effects model. Statistical heterogeneity was assessed using chi2 and I2 statistics.
Results
Forty-two RCTs with 39,047 patients with 9,999 non-severe and 1,503 severe GI adverse events were included. The incidence of non-severe and severe adverse events ranged between 0.3–54.9 and 0–10.3%, respectively. There was no difference between BP and control groups in terms of the risk of non-severe or severe side effects: RR=1.05 (CI: 0.98–1.12), I2 = 48.1%, and RR=1.01 (CI: 0.92–1.12), I2 = 0.0%, respectively. Subgroup analysis of the most commonly used BP, once-weekly alendronate 70 mg, revealed an association between bisphosphonates and the risk of non-severe GI adverse events, RR=1.16 (CI: 1.00–1.36), I2 = 40.7%, while the risk of severe GI side effects was not increased in this subgroup, RR=1.20 (CI: 0.83–1.74), I2 = 0.0%.
Conclusion
Our results show that bisphosphonates do not increase the risk of severe GI adverse events. However, the marked variability of the screening for side effects in the included studies, and the fact that in most of the studies GI diseases were exclusion criteria limits the strenght of evidence of our results. The conclusions drawn from the meta-analysis are therefore restricted to selected populations, and the results must be interpreted with caution.
Keywords: bisphosphonate, drug safety, gastrointestinal adverse event, gastrointestinal side effect, meta-analysis
Introduction
Osteoporosis is a systemic bone disease with low bone mineral density and poor bone microarchitecture which leads to an increased risk of fracture (1). According to the most recent Osteoporosis Guideline, oral bisphosphonates (BPs) are one of the most commonly used therapeutic agents in patients with osteoporosis (2). Adherence is usually low in chronic, asymptomatic diseases, but gastrointestinal (GI) side-effects can also contribute to low adherence in BP therapy and may necessitate a review by a gastroenterologist with or without gastroscopy (3, 4). A cross-sectional patient survey showed that these GI side effects account for 40% of all discontinuation (5). Most commonly these are reported in the foregut, including heartburn, nausea, vomiting, epigastric pain, esophagitis, gastric ulcer, dyspepsia, and GI bleeding (6).
While the efficacy of BPs is out of debate, previous systematic reviews and meta-analysis investigating the tolerability of bisphosphonates did not determine the risk of severe and non-severe GI side effects of oral bisphosphonates.
There have been studies investigating the bisphosphonates-caused mucosal damage of the upper GI tract since it became an established drug in the treatment of osteoporosis (7–9).
None of the previous meta-analyses in this topic focused on the risks of severe GI side effects. We aimed to differentiate between the mild and severe side effects and determine the risks of these side effects in case of all commonly used oral bisphosphonates for osteoporosis.
Methods
Protocol
Our meta-analysis and systematic review is reported using the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) (10). The project was registered in October 2019 on PROSPERO the registration number is CRD42020147522.
Eligibility Criteria
Our scientific question, using the population-intervention-control-outcomes (PICO) framework was: (P) adult patients with primary osteoporosis, (I) oral bisphosphonates, (C) placebo or vitamin D or calcium, but no other medication for osteoporosis, and (O) severe and non-severe GI adverse events. Articles were included if they provided relevant information about any drug-induced GI adverse event. Only full-text articles and randomized controlled trials (RCTs) were included.
Search Strategy
A systematic search was conducted in 3 databases, MEDLINE (via PubMed), Embase, and Cochrane Central Register of Controlled Trials from inception to 6th September 2020. Keywords for the computer-aided search were ((diphosphonate OR bisphosphonate OR etidron* OR clodron* OR tiludron* OR pamidron* OR neridron* OR olpadron* OR alendron* OR ibandron* OR risedron* OR zoledron*) AND (gastrointestinal OR digestive OR “alimentary tract” OR esophageal OR esophagus OR oesophageal OR oesophagus OR gastric OR stomach OR antrum OR antral OR pylorus OR pyloric OR gastroduodenal OR duodenal OR duodenum OR bowel OR intestine OR intestinal OR colon OR colonic OR viscus OR visceral OR abdomen OR abdominal)), with the “Human” filter applied, but without other restrictions to language or other features.
Study Selection
Records were managed by EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA). After the exclusion of duplicates, the remaining records were screened by title, abstract, and full-text independently by two review authors (ZRD, NV). Additional articles were manually searched and identified from the reference lists of eligible primary studies. Disagreements were resolved by consensus or by the involvement of the senior review author (BE).
Data Extraction
Numeric data were extracted by two review authors (ZRD, NV) and manually populated onto a purpose-designed Excel 2019 sheet (Office 365, Microsoft, Redmond, WA, USA). Data were collected from each paper on the year of publication, study design, country, the number of randomized patients, and baseline patient characteristics (age, sex, race, history of GI, body mass index, tobacco, alcohol, and caffeine usage in both groups). Most importantly, data were collected on the non-severe and severe GI adverse events. To ensure that results of the included studies were uniformly assessed as intention-to-treat protocol, in cases of per-protocol analyses the missing data were imputed, missing subjects were regarded as not having adverse events. Adverse events reported in the original studies were categorized by the review authors following the U.S. Food and Drug Administration criteria (11), detailed in Supplementary Table 1 . Data on type of the bisphosphonate used as treatment and the control treatment, dosage, duration, route, and schedule of administration, follow-up period were also extracted. Disagreements were resolved by consensus or by the involvement of the senior reviewer (BE).
Statistical Analysis
Risk ratios (RRs) with 95% confidence intervals (CI) were calculated for non-severe and severe adverse events with the random-effect model by DerSimonian-Laird (12). Subgroup analyses were performed for the different bisphosphonates (alendronate, risedronate, etidronate, pamidronate, ibandronate), the different dosage of the bisphosphonates and the duration of administration. Statistical heterogeneity was assessed using chi2 and I2 statistics. Statistical heterogeneity was assessed using Cochrane’s Q and the I2 statistics. According to the Cochrane Handbook for Systematic Reviews of Interventions (13), heterogeneity could be interpreted as moderate between 30 and 60%, as substantial between 50 and 90% and as considerable above 75%. The presence of publication bias was assessed by visual inspection of the funnel plots and Egger’s test (14), and the effect of publication bias was evaluated by the trim-and-fill method (15). A significant test result from Egger’s test (p<0.1) indicates the presence of bias. We also performed Trial Sequential Analysis (TSA) for the primary outcomes to evaluate whether further randomized trials are futile to show or discard the anticipated intervention effect. Statistical analyses were performed with Stata 16 (Stata Corp, College Station, TX, USA) and trial sequential analysis program version 0.9 beta (available from www.ctu.dk/tsa).
Risk of Bias Assessment
The quality assessment was done at the study level and then summarized. We used the revised Cochrane Collaboration’s risk-of-bias tool for randomized trials (16) for methodological quality assessment of the individual studies included in our meta-analysis. The risk of bias was assessed independently by three investigators (ZRD, NV, EC). Disagreements were resolved by consensus and the involvement of the corresponding author.
Assessment of the Grade of Evidence
The GRADE approach was used to assess the certainty of evidence regarding the outcomes. GRADE stands for Grades of Recommendation Assessment, Development, and Evaluation (17).
GRADE was assessed independently by two investigators (ZRD, EC). Disagreements were resolved by consensus and with the involvement of the corresponding author.
Results
Results of the Selection Process
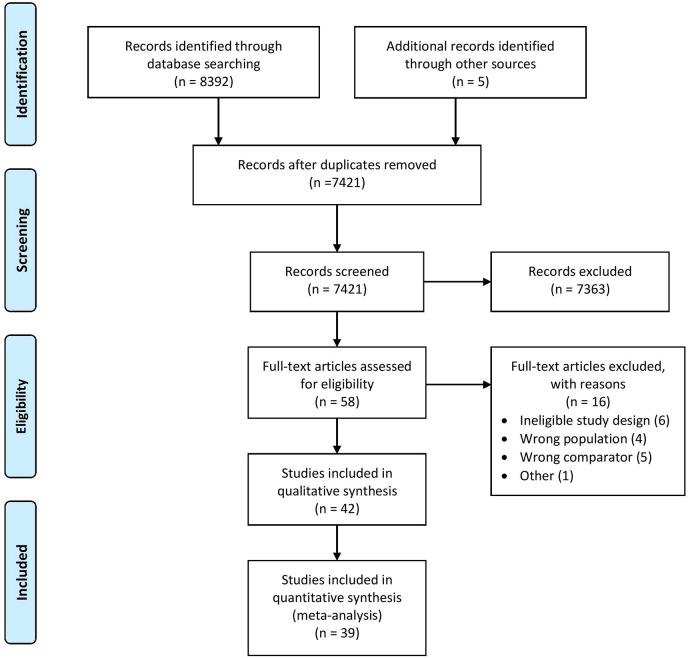
Our search strategy initially identified 8,392 studies, out of those 42 relevant articles were included in the qualitative and 39 in the quantitative synthesis of this meta-analysis. The study selection process is shown in Figure 1 . The summary of the characteristics of the studies included in our analysis is shown in Table 1 . In case of six studies missing data for intention-to-treat analysis were imputed (27, 40, 43, 44, 55, 56).
Figure 1.
PRISMA flowchart.
Table 1.
Baseline characteristics of trials included.
| Author, year/reference no. | Region/country | N° of centers | N° of patients in BP/control group | Age BP/control group (mean years) | Female ratio | Active substance | Dosage (mg) | Control group | Follow-up (months) | Patients with preexisting and/or previous GI diseases excluded | Incidence of nonsevere AE | Incidence of severe AE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adachi et al., 2009 (18) | Canada and Colombia | 34 | 291/147 | 65.4/65.7 | 100% | Alendronate | 10 | Placebo | 3 | No | 5.5% | 0.5% |
| Ascott-Evans 2003 (19) | Argentina, Australia, Brazil, New Zealand, Africa, Europe | 18 | 95/49 | 57.3/57.3 | 100% | Alendronate | 10 | Placebo | 12 | No | 14.6% | 0.0% |
| Bauer et al., 2000 (20) | United States of America | 11 | 3,236/3,223 | 68.6/68.7 | 100% | Alendronate | 5 | Placebo | 45 | Yes | 54.7% | 7.5% |
| Bell et al., 2002 (21) | United States of America | 8 | 33/32 | 66.4/65.9 | 100% | Alendronate | 10 | Placebo | 24 | Yes | 33.8% | 3.1% |
| Black et al., 1996 (22) | United States of America | 11 | 1,022/1,005 | 70.7/71 | 100% | Alendronate | 5, after 2 years 10 | Placebo | 36 | Yes | 42.8% | 5.0% |
| Bone et al., 2000 (23) | United States of America | 18 | 92/50 | 61/62 | 100% | Alendronate | 10 | Placebo | 24 | Yes | 18.3% | 0.0% |
| Boonen et al., 2009 (24) | Eastern and Western Europe, Lebanon, Australia, USA | 24 | 191/93 | 60/62 | 0% | Risedronate | 35 | Placebo | 24 | No | 10.9% | 3.5% |
| Chesnut et al., 2004 (25) | Canada, United States of America, Europe | 73 | 975/977 | 69/69 | 100% | Ibandronate | 2.5 | Placebo | 36 | No | 20.8% | 10.3% |
| Ibandronate | 20 * | 20.9% | 9.9% | |||||||||
| Clemmesen et al., 1997 (26) | Denmark, Belgium | 2 | 44/44 | 67/70 | 100% | Risedronate | 2.5 | Placebo | 36 | NI | 1.1% | 6.8% |
| 68/70 | Risedronate | 2.5⁑ | 3.4% | 6.8% | ||||||||
| Cryer et al., 2005/1 (27) | United States of America | 51 | 224/230 | 64.6/65.8 | 100% | Alendronate | 70 | Placebo | 6 | Yes | 19.8% | 1.3% |
| Cryer et al., 2005/2 (28) | United States of America | 48 | 224/226 | 66.6/66.8 | 92.5% | Alendronate | 70 | Placebo | 3 | Yes | 12.4% | 0.0% |
| Cummings et al., 1998 (29) | United States of America | 11 | 2,214/2,218 | 67.6/67.7 | 100% | Alendronate | 5, after 2 years 10 | Placebo | 24 | Yes | 23.6% | 0.8% |
| Downs et al., 2000 (30) | United States of America | 24 | 118/58 | 64.6/64.6 | 100% | Alendronate | 10 | Placebo | 12 | Only esophageal motility disorders | 18.8% | 0.0% |
| Eisman et al., 2004 (31) | Europe, Australia, USA. Africa, Asia-Pacific | 44 | 225/224 | 63.6/63.6 | 94.2% | Alendronate | 70 | Placebo | 3 | No | 10.0% | 1.1% |
| Felsenberg et al., 1998 (32) | Argentina, Australia, Canada, Colombia, Europe | 62 | 219/223 | 64.1/63.3 | 100% | Alendronate | 10 | Placebo | 12 | Yes | 29.2% | 2.5% |
| Fogelman et al., 2000 (33) | UK, France, Netherlands, Belgium, Germany | 13 | 184/180 | 65/64 | 100% | Risedronate | 2.5 | Placebo | 24 | No | 23.4% | 6.0% |
| 177/180 | Risedronate | 5 | 21.8% | 5.6% | ||||||||
| Greenspan et al., 2002 (34) | United States of America | 48 | 224/226 | 66.6/66.8 | 92.4% | Alendronate | 70 | Placebo | 3 | Only esophageal motility disorders | 14.0% | 1.3% |
| Harris et al., 1999 (35) | North America | 110 | 813/815 | 69/68 | 100% | Risedronate | 5 | Placebo | 36 | No | 24.4% | 5.7% |
| Hosking et al., 2003 (36) | Europe and Brazil | 38 | 222/108 | 68.9/69.6 | 100% | Risedronate | 5 | Placebo | 12 | Only esophageal motility disorders | 0.3% | 1.2% |
| 219/108 | 69,2/69,6 | Alendronate | 70 | 0.6% | 0.9% | |||||||
| Ilter et al., 2006 (37) | Turkey | 1 | 41/41 | 56.4/56.2 | 100% | Risedronate | 5 | Calcium+ Vitamin D | 3 | No | 17.1% | 1.2% |
| 41/41 | 55,9/56,2 | Risedronate | 35 | 15.9% | 0.0% | |||||||
| Iwamoto et al., 2001 (38) | Japan | 1 | 25/24 | 64.3/66 | 100% | Etidronate | 200§ | Calcium lactate | 24 | No | 0.0% | 0.0% |
| Johnell et al., 2002 (39) | Australia, Belgium, Canada, Italy, Mexico, South Africa, Spain, Sweden |
30 | 83/82 | 63.7/63.8 | 100% | Alendronate | 10 | Placebo | 10 | Yes | 8.5% | 0.0% |
| Kung et al., 2000 (40) | China | 1 | 35/35 | 64/65 | 100% | Alendronate | 10 | Placebo | 12 | Yes | 17.1% | 2.9% |
| Kushida et al., 2004 (41) | Japan | 55 | 90/80 | 71.2/72.6 | 100% | Alendronate | 5 | Alfacalcidol | 36 | Yes | 8.2% | 4.7% |
| Lanza et al., 2002 (42) | United States of America | 5 | 126/126 | 54.7/54.7 | ND | Alendronate | 70 | Placebo | 2.5 | Yes | 22.2% | 0.4% |
| Lanza et al., 2000 (43) | United States of America | 4 | 90/36 | 54.3/53.5 | 63.5% | Alendronate | 40 | Placebo | 1 | Yes | ND | 7.1% |
| 89/36 | 63.2% | Risedronate | 30 | ND | 8.8% | |||||||
| Lau et al., 2000 (44) | China | 1 | 53/47 | 74/74 | 100% | Alendronate | 10 | Placebo | 12 | Yes | 12.0% | 0.0% |
| Leung et al., 2005 (45) | China | 4 | 31/34 | 67/67 | 100% | Risedronate | 5 | Placebo | 12 | Yes | 3.1% | 0.0% |
| Liberman et al., 1995 (46) | USA, Canada, Australia, Europe, Israel, New Zealand, Mexico, South America | 28 | 175/355 | 64/64 | 100% | Alendronate | 5 | Placebo | 36 | Yes | 0.0% | ND |
| 175/355 | Alendronate | 10 | 17.2% | ND | ||||||||
| 175/355 | Alendronate | 20 | 0.0% | ND | ||||||||
| McClung et al., 2001 (47) | North America, Europe, New Zealand, Australia | 183 | 3,093/3,134 | ND | 100% | Risedronate | 2.5 | Placebo | 36 | No | 17.0% | 2.1% |
| 3,104/3,134 | Risedronate | 5 | 16.8% | 2.2% | ||||||||
| Miller et al., 2000 (48) | United States of America | 38 | 88/84 | 67/67.1 | 100% | Alendronate | 10 | Placebo | 2 | No | 14.0% | 2.3% |
| Murphy et al., 2001 (49) | United States of America | 10 | 109/36 | 72.9/70.9 | 100% | Alendronate | 10 | Placebo | 18 | Yes | 2.8% | 1.4% |
| Orwoll et al., 2000 (50) | United States of America | 20 | 146/95 | 63/63 | 0% | Alendronate | 10 | Placebo | 24 | Yes | 15.8% | 0.8% |
| Pols et al., 1999 (51) | Europe, Canada, Latin America, Australia, South Africa, China | 153 | 950/958 | 62.8/62.8 | 100% | Alendronate | 10 | Placebo | 12 | Yes | 20.8% | 3.7% |
| Reginster et al., 2000 (52) | Europe, Australia | 80 | 408/407 | 71/71 | 100% | Risedronate | 2.5 | Placebo | 24 | No | 18.4% | 8.0% |
| 407/407 | Risedronate | 5 | 36 | 19.9% | 7.6% | |||||||
| Ryan et al., 2000 (53) | United Kingdom | 2 | 41/41 | 65.6/66.1 | 90.1% | Pamidronate | 150† | Placebo | 24 | Yes | 54.3% | 0.0% |
| 40/41 | 63.8/61.1 | Pamidronate | 300‡ | 36.6% | 0.0% | |||||||
| Seeman et al., 2010 (54) | Argentina, Australia, Canada, France, USA | 9 | 81/83 | 60.7/60.8 | 100% | Alendronate | 70 | Placebo | 12 | No | 54.9% | 0.0% |
| Shiraki et al., 1999 (55) | Japan | 63 | 102/100 | 63.53/63.14 | 100% | Alendronate | 5 | Alfacalcidol | 12 | No | 19.3% | 0.5% |
| Shiraki et al., 2003 (56) | Japan | 70 | 52/54 | 60,7/60,5 | 99% | Risedronate | 1 | Placebo | 3 | 6.1% | 0.0% | |
| 49/54 | 60,6/60,5 | Risedronate | 2.5 | No | 14.6% | 0.0% | ||||||
| 56/54 | 60,2/60,5 | Risedronate | 5 | 14.4% | 0.0% | |||||||
| Tucci et al., 1996 (57) | United States of America | 18 | 98/192 | 66.5/64.2 | 100% | Alendronate | 5 | Placebo | 18 | 12.4% | 1.4% | |
| 94/192 | 63,9/64,2 | Alendronate | 10 | Yes | 14.0% | 0.7% | ||||||
| 94/192 | 63,8/64,2 | Alendronate | 20 | 13.3% | 1.4% | |||||||
| Yan et al., 2009 (58) | China | 7 | 280/280 | 65.19/64.66 | 100% | Alendronate | 70 | Placebo | 12 | No | 16.1% | 0.0% |
| You et al., 2011 (59) | China | 1 | 90/90 | 62.71/61.93 | 100% | Alendronate | 70⁑ | Colecalciferol | 12 | No | ND | ND |
All trials were randomized, controlled multicenter studies, except Ilter, Iwamoto, Lau, You, which are single-center studies. Ten milligrams of Alendronate was taken in a daily regime, and 70 mg was taken once per week. * cyclic intermittent therapy: 20 mg ibadronate every other day for 2 weeks, every 3 months. cyclic intermittent therapy: 2.5 mg risedronate daily for 2 weeks, every 3 months. §Cyclic intermittent therapy: 200 mg etidronate daily for 2 weeks, every 3 months. †Cyclic intermittent therapy: 150 mg pamidronate for 4 weeks, followed by 4 weeks of placebo. ‡Cyclic intermittent therapy: 300 mg pamidronate for 4 weeks, followed by 12 weeks of placebo. ⁑ 70 mg of alendronate every 2 weeks. BP, bisphosphonate; ND, no data; NI, no information.
Adverse Events
The forty-two RCTs included 39,047 patients with 9,999 non-severe and 1,503 severe GI adverse events. The incidence of non-severe and severe adverse events ranged between 0.3–54.9, and 0–10.3%, respectively. The most common non-severe adverse events were nausea, vomiting, dyspepsia, and abdominal pain, while the vast majority of the severe side effects occurred in the esophagus ( Supplementary Table 2 ).
Intervention: Bisphosphonates
Our meta-analysis included data from studies with four bisphosphonates: alendronate, risedronate, etidronate, and ibandronate. One study with pamidronate was included in the qualitative synthesis. All studies used orally administered bisphosphonates. Dosages and other details are shown in Table 1 .
Results of Statistical Analysis
Bisphosphonate Use Is Not Associated With the Risk of Non-Severe Adverse Events
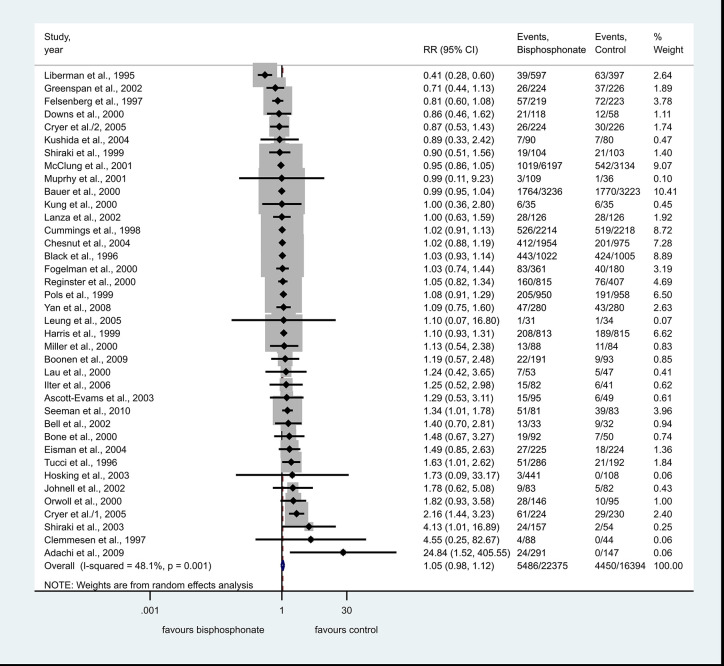
The analysis for non-severe GI adverse events included 39 studies in the quantitative analysis. The number of overall non-severe GI adverse events were 5,486 in the bisphosphonate group and 4,450 in the control group. Compared against controls, the bisphosphonate use was not associated with the risk of non-severe side effects, RR=1.05, CI: 0.98–1.12, p=0.207 the heterogeneity was moderate: I2 = 48.1%, p=0.001 ( Figure 2 ).
Figure 2.
Forest plot of non-severe adverse events.
Among non-serious GI adverse events alendronate, risedronate and ibandronate had 27, ten, and one studies included, respectively. Subgroup analysis for the three different bisphosphonates did not show an association with the risk of non-severe side effects ( Supplementary Figure 1 ).
Bisphosphonate Use Is Not Associated With Increased Risk of Severe Adverse Events
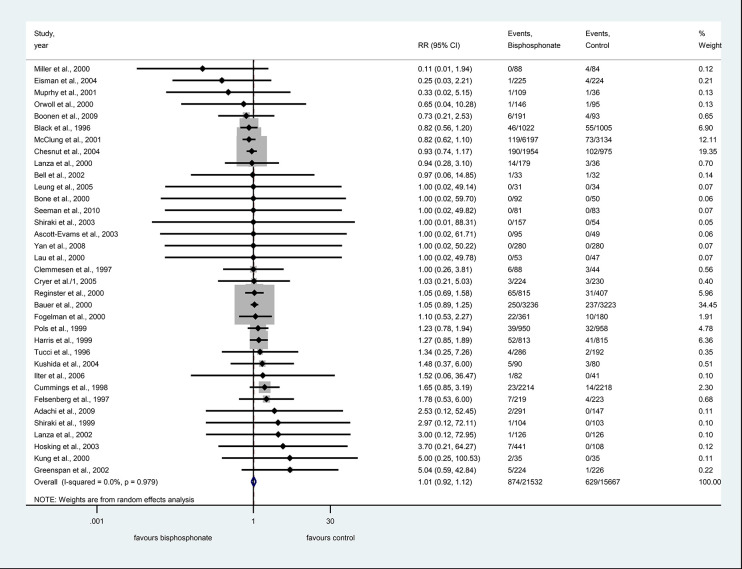
The number of overall severe GI adverse events were 874 in the bisphosphonate group and 629 in the control group.
The bisphosphonate use was not associated with the risk of severe side effects, compared against controls, RR=1.01, CI: 0.92–1.12, p=0.776; there was no significant heterogeneity: I2 = 0.0%, p=0.979 ( Figure 3 ).
Figure 3.
Forest plot of severe adverse events.
Among serious upper GI events alendronate, risedronate, ibandronate, and etidronate had 24, ten, one, and one studies included, respectively. Subgroup analysis for the three different bisphosphonates did not show an association with the risk of non-severe side effects ( Supplementary Figure 2 ).
Subgroup Analysis of Trials With the Primary Outcome of GI Tolerability of BP Therapy Showed No Increased Risk of GI Adverse Events
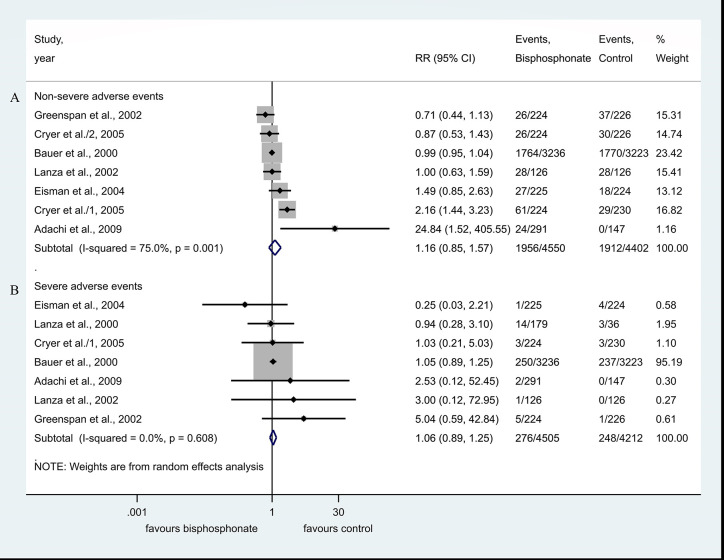
The number of overall non-severe GI adverse events was 1,956 in the bisphosphonate group and 1,912 in the control group. Compared to controls, the BP use was not associated with the risk of non-severe side effects, RR=1.16, CI: 0.85–1.57, p=0.356, with considerable heterogeneity: I2 = 75.0%, p=0.001 ( Figure 4A ).
Figure 4.
(A, B) Subgroup analysis of trials with the primary outcome of GI tolerability of BP therapy.
The number of overall severe GI adverse events was 276 in the bisphosphonate group and 248 in the control group. The BP use was not associated with the risk of severe side effects, compared against controls, RR=1.06, CI: 0.89–1.25, p=0.529; there was no significant heterogeneity: I2 = 0.0%, p=0.608 ( Figure 4B ).
Long-Term Administration of Bisphosphonate Is Not Associated With Increased Risk of Side Effects
In 15 eligible articles, there was no association between BP use and the risk of non-severe side effects in the subgroup of studies, where the treatment was at least 24 months, RR=1.00, CI: 0.93–1.08, p=0.983, heterogeneity was moderate: I2 = 53.9%, p=0.007 ( Supplementary Figure 3 ).
In 14 eligible articles, there was no association with the risk of severe side effects in the subgroup of studies where the treatment was at least 24 months, RR=1.00, CI: 0.90–1.11, p=0.944 there was no significant heterogeneity: I2 = 0.0%, p=0.858 ( Supplementary Figure 4 ).
Non-Severe and Severe Adverse Events in the Context of the Most Commonly Used BP Therapies
Subgroup analysis of the most commonly used BP, alendronate 10 mg/day or once-weekly alendronate 70 mg, revealed an increased risk of non-severe GI adverse events compared against controls, RR=1.16, CI: 1.00–1.36, p=0.056, with moderate heterogeneity: I2 = 40.7%, p=0.031, while the risk of severe adverse events was not increased in this subgroup RR=1.20, CI: 0.83–1.74, p=0.328, without significant heterogeneity: I2 = 0.0%, p=0.897 ( Supplementary Figures 5 , 6 ).
Trial Sequential Analysis
In case of non-severe adverse events, the cumulative z-curve crosses the futility boundary, which provided evidence indicating that no significant difference exists between the groups, and thus, further trials are not required ( Supplementary Figure 7 ). In case of severe adverse events, the same conclusion could not be drawn as the acquired information size was substantially below the required information size (0.76%) by performing the TSA.
Risk of Bias Assessment
According to the Revised Cochrane Risk of Bias Assessment tool for RCTs the risk of bias was low in 25 studies, there were some concerns in 11, and high in six studies. Nearly all studies carried an unknown risk of reporting bias due to the lack of pre-study protocols. The detailed results of the assessment are shown in Supplementary Table 2 .
Publication Bias
In case of non-severe side effects, both the visual assessment of the funnel plot and the Egger’s test, p=0.046, revealed small study effect, so the presence of publication bias was strongly suspected ( Supplementary Figure 8 ). Therefore, the metanalytical pooled estimation was repeated by the use of trim and fill method, which did not change the overall risk association (RR= 0.99, CI: 0.91 – 1.07).
In case of severe side effects, publication bias was undetected by visual inspection of the funnel plot and Egger’s test p = 0.307 ( Supplementary Figure 9 ).
Grade of Evidence
For non-severe GI side effects, the evidence was graded as very low due to inconsistency, indirectness, and publication bias and for severe GI side effects, the evidence was graded moderate due to indirectness ( Table 2 ).
Table 2.
Grade of evidence.
| Summary of findings: | ||||||
|---|---|---|---|---|---|---|
| Gastrointestinal adverse events of bisphosphonates compared to Control | ||||||
| Patient or population: Adult patients with osteoporosisIntervention: BisphosphonateComparison: Control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect
(95% CI) |
№ of participants
(studies) |
Certainty of the evidence
(GRADE) |
Importance | |
| Risk with Control | Risk with Bisphosphonate | |||||
| Non-severe GI adverse events | 271 per 1,000 | 285 per 1,000 (266 to 304) |
RR 1.05 (0.98 to 1.12) |
38,769 (38 RCTs) |
⨁◯◯◯ VERY LOW a,b,c |
IMPORTANT |
| Severe GI adverse events | 40 per 1,000 | 41 per 1,000 (37 to 45) |
RR 1.01 (0.92 to 1.12) |
37199 (35 RCTs) |
⨁⨁⨁◯ MODERATE b |
CRITICAL |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI, confidence interval; RR, risk ratio | ||||||
|
GRADE Working Group grades of evidence
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aHeterogeneity was moderate among the studies included in the analysis of the risk of non-severe GI adverse events (I2 = 48.1%, p = 0.001)
bThere were major differences in the intervention groups of the studies included regarding the used drug (alendronate/ibandronate/risedronate/pamidronate), dosage, and administration intervals.
cThe funnel plot of this outcome revealed asymmetry and Eger’s test suggested small study effect (p = 0.046).
Discussion
The pathophysiology of the bisphosphonate induced esophageal and gastric erosions has not been elucidated. In vitro studies suggest that the mucosal damage is produced through topical irritant effects on the gastric epithelium (60, 61). It is also described that BPs are competitively displacing the phospholipids from the mucus gel layer, therefore the mucosal hydrophobic barrier is attenuated and mucosal healing is hindered (62–64).
Our results from 42 RCTs with nearly 40,000 participants showed that the incidence of non-severe and severe GI side effects ranged between 0–54.9 and 0–10.3%, respectively. Neither the risk of non-severe nor the risk of severe adverse GI events was associated with the oral bisphosphonate use in osteoporotic patients.
Our meta-analysis is the first that objectified the risk of non-severe and severe GI side effects separately. When the use of bisphosphonates became widespread, it was predicted that gastroenterologists would see more patients with consequent GI problems (65).
Two previous reviews assessed the risk of GI side effects of risedronate and ibandronate separately (66, 67). A meta-analysis of nine RCTs focused on the GI tolerability of alendronate (68). A comprehensive network meta-analysis compared the GI safety of BPs, but they did not calculate the risk of side effects of bisphosphonates against placebo (69). The assessment of the risk of severe GI side effects was not based on the true severity of the GI side effects but how they were classified in the original studies.
Studies With a Primary Outcome of Gastrointestinal Adverse Events
Eight out of the included RCTs had GI adverse events as the primary outcome (18, 27, 43–42). Only two of these studies proved that the risk of non-severe GI side effects increased in patients taking BPs (18, 27). None of them showed an association between BPs and severe GI side effects.
While the first trial of Cryer et al. managed to detect an increased risk of non-severe GI side effects, their second trial, in which approximately half of participants took non-steroidal anti-inflammatory drugs on both arms, could not demonstrate this association (27, 28).
A study in 2,000 assessed each participants’ GI side effects through endoscopic inspection of the mucosa at baseline and completion of the study. They concluded that mucosal damage did not translate into clinically significant symptoms or side effects (43).
Miller et al. investigated whether previous GI side effects of BP therapy predisposed to recurrent side effects after rechallenge with alendronate. They found no significant risk of severe or non-severe GI side effects associated with the alendronate use. The incidence of non-severe and severe GI side effects were 14 and 2.3%, respectively (48).
Since the introduction of the BPs in the treatment of osteoporosis multiple studies confirmed their GI tolerability. Even if they have non-severe GI side effects, their use rarely results in severe complications needing the attention of the gastroenterologist.
Oral BPs are nowadays recommended to take with water and to avoid lying down after intake to avoid esophageal irritation. These precautions might reduce the incidence of upper GI side-effects in more recent RCTs and in current clinical practice.
Strength of Our Study
Our work, which includes a large number of RCT-s and participants was conducted following a rigorous methodology. Furthermore, most of the included RCT-s are multinational and multicentric. To date, this is the first meta-analysis which quantified the risk of non-severe and severe GI side effects of oral BP therapy.
Limitations of Our Study
In most of the studies, GI side effects were a secondary outcome and were not powered statistically to reveal a significant difference in that respect. The heterogeneity of the strategy of vigilance for side effects probably explains the wide range of incidence of side effects; however, it did not translate to statistical heterogeneity among the severe side effects. The differences between sexes, ages, length of the studies and various definitions in addition to different approaches of the screening of non-severe side effects resulted in moderate and significant heterogeneity among the studies. Also, the included studies likely used different sets of predetermined GI side effects during the screening for side effects. Another significant limitation of the study is that in 24 of 42 RCTs included in the analysis, pre-existing and/or previous GI diseases were exclusion criteria. The conclusions drawn from the meta-analysis are therefore restricted to selected populations, and the results must be interpreted with caution. These considerations are reflected in Table 2 , in which the grades of evidence were rated very low for non-severe GI side-effects and only moderate for severe GI side-effects.
Risk assessment revealed unclear bias in most of the studies concerning the reporting of the results.
Conclusion
Implications for Research
Although the results suggest that bisphosphonates do not increase the risk of GI side effects in the general osteoporotic population, we cannot conclude whether they are safe to use in a high-risk population with preexisting GI pathologies (e.g. gastroesophageal reflux, peptic ulcer disease, etc.). Therefore future phase III. trials should focus on these high-risk populations.
Implications for Practice
Bisphosphonates seem to be safe in the osteoporotic population concerning the GI side effects, however other factors need to be considered when decisions on treatment are made.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author Contributions
ZD, BE, and PH conceived the study. ZD, NV, and SK wrote the protocol. ZD, EC, and LS did the literature search. ZD and NV screened the records and extracted data. LS and AP validated the extracted data. ZD, NV, and EC assessed the quality of the included studies. LH did the statistical analysis. ZD and NV prepared the tables. BE, ZD, and NV wrote the first draft of this manuscript. PH, SK, and AP supervised the manuscript and approved the submitted draft. BE is the guarantor of this paper. All authors contributed to the article and approved the submitted version.
Funding
Sponsors were not involved in the design, data collection, analysis, interpretation, or preparation of the manuscript. Financial support: Supported by the Economic Development and Innovation Operative Programme Grant (GINOP-2.3.2-15-2016-00048) and the Human Resources Development Operational Programme Grants (EFOP-3.6.2-16-2017-00006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.573976/full#supplementary-material
Non-severe adverse events subgroup by active substance.
Severe adverse events subgroup by active substance.
Non-severe GI side effects subgroup by more than 24 months of treatment.
Severe GI side effects subgroup by more than 24 months of treatment.
Non-severe adverse events in the context of the most commonly used BP therapies: 70 mg/week and 10 mg/day alendronate per os.
Severe adverse events in the context of the most commonly used BP therapies: 70 mg/week and 10 mg/day alendronate per os.
Trial Sequential Analysis of non-severe GI. adverse events
Funnel plot of non-severe GI adverse events.
Funnel plot of severe GI adverse events.
FDA criteria of non-severe and severe GI adverse events.
Risk of Bias Assessment.
References
- 1. Nuti R, Brandi ML, Checchia G, Di Munno O, Dominguez L, Falaschi P, et al. Guidelines for the management of osteoporosis and fragility fractures. Intern Emerg Med (2019) 14(1):85–102. 10.1007/s11739-018-1874-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kanis JA Cooper C Rizzoli R Reginster JY. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int (2019) 30(1):3–44. 10.1007/s00198-018-4704-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kennel KA, Drake MT. Adverse Effects of Bisphosphonates: Implications for Osteoporosis Management. Mayo Cli Proc (2009) 84(7):632–8. 10.4065/84.7.632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Payer J, Killinger Z, Ivana S, Celec P. Therapeutic adherence to bisphosphonates. Biomed Pharmacother = Biomed Pharmacother (2007) 61(4):191–3. 10.1016/j.biopha.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 5. Goldshtein I, Rouach V, Shamir-Stein N, Yu J, Chodick G. Role of Side Effects, Physician Involvement, and Patient Perception in Non-Adherence with Oral Bisphosphonates. Adv Ther (2016) 33(8):1374–84. 10.1007/s12325-016-0360-3 [DOI] [PubMed] [Google Scholar]
- 6. Rossini M, Adami G, Adami S, Viapiana O, Gatti D. Safety issues and adverse reactions with osteoporosis management. Expert Opin Drug safety (2016) 15(3):321–32. 10.1517/14740338.2016.1136287 [DOI] [PubMed] [Google Scholar]
- 7. de Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, et al. Esophagitis Associated with the Use of Alendronate. N Engl J Med (1996) 335(14):1016–21. 10.1056/NEJM199610033351403 [DOI] [PubMed] [Google Scholar]
- 8. Ribeiro A, DeVault KR, Wolfe J, Stark ME. Alendronate-associated esophagitis: endoscopic and pathologic features. Gastrointestinal Endoscopy (1998) 47(6):525–8. 10.1016/S0016-5107(98)70256-1 [DOI] [PubMed] [Google Scholar]
- 9. Nagano Y, Matsui H, Shimokawa O, Hirayama A, Nakamura Y, Tamura M, et al. Bisphosphonate-induced gastrointestinal mucosal injury is mediated by mitochondrial superoxide production and lipid peroxidation. J Clin Biochem Nutr (2012) 51(3):196–203. 10.3164/jcbn.12-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev (2015) 4(1):1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event?fbclid=IwAR2tfSlOW5y4ZsbUjT4D_ky7MV_C8aAamb4oPLQcdAKwS930X2EaWqg73uE.
- 12. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin trials (1986) 7(3):177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 13. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. John Wiley & Sons, Ltd: The Cochrane Collaboration; (2011). Available at: www.handbook.cochrane.org. [Google Scholar]
- 14. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol (2001) 54(10):1046–55. 10.1016/S0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- 15. Shi L, Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Med (Baltimore) (2019) 98(23):e15987–e. 10.1097/MD.0000000000015987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj (2011) 343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (2008) 336(7650):924. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adachi JD, Faraawi RY, O’Mahony MF, Nayar A, Massaad R, Evans JK, et al. Upper gastrointestinal tolerability of alendronate sodium monohydrate 10 mg once daily in postmenopausal women: a 12-week, randomized, double-blind, placebo-controlled, exploratory study. Clin Ther (2009) 31(8):1747–53. 10.1016/j.clinthera.2009.08.016 [DOI] [PubMed] [Google Scholar]
- 19. Ascott-Evans BH, Guañabens N, Kivinen S, Stuckey BGA, Magaril CH, Vandormael K, et al. Alendronate prevents loss of bone density associated with discontinuation of hormone replacement therapy: A randomized controlled trial. Arch Internal Med (2003) 163(7):789–94. 10.1001/archinte.163.7.789 [DOI] [PubMed] [Google Scholar]
- 20. Bauer DC, Black D, Ensrud K, Thompson D, Hochberg M, Nevitt M, et al. Upper gastrointestinal tract safety profile of alendronate: The fracture intervention trial. Arch Internal Med (2000) 160(4):517–25. 10.1001/archinte.160.4.517 [DOI] [PubMed] [Google Scholar]
- 21. Bell NH, Bilezikian JP, Bone HG, Kaur A, Maragoto A, Santora AC. Alendronate increases bone mass and reduces bone markers in postmenopausal African-American women. J Clin Endocrinol Metab (2002) 87(6):2792–7. 10.1210/jcem.87.6.8575 [DOI] [PubMed] [Google Scholar]
- 22. Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet (London England) (1996) 348(9041):1535–41. 10.1016/s0140-6736(96)07088-2 [DOI] [PubMed] [Google Scholar]
- 23. Bone HG, Greenspan SL, McKeever C, Bell N, Davidson M, Downs RW, et al. Alendronate and estrogen effects in postmenopausal women with low bone mineral density. J Clin Endocrinol Metab (2000) 85(2):720–6. 10.1210/jc.85.2.720 [DOI] [PubMed] [Google Scholar]
- 24. Boonen S, Orwoll ES, Wenderoth D, Stoner KJ, Eusebio R, Delmas PD. Once-weekly risedronate in men with osteoporosis: Results of a 2-Year, placebo-controlled, double-blind, multicenter study. J Bone Mineral Res (2009) 24(4):719–25. 10.1359/jbmr.081214 [DOI] [PubMed] [Google Scholar]
- 25. Chesnut CH, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone mineral Res Off J Am Soc Bone Mineral Res (2004) 19(8):1241–9. 10.1359/JBMR.040325 [DOI] [PubMed] [Google Scholar]
- 26. Clemmesen B, Ravn P, Zegels B, Taquet AN, Christiansen C, Reginster JY. A 2-year phase II study with 1-year of follow-up of risedronate (NE-58095) in postmenopausal osteoporosis. Osteoporos Int (1997) 7(5):488–95. 10.1007/PL00004152 [DOI] [PubMed] [Google Scholar]
- 27. Cryer B, Binkley N, Simonelli C, Lewiecki EM, Lanza F, Chen E, et al. A randomized, placebo-controlled, 6-month study of once-weekly alendronate oral solution for postmenopausal osteoporosis. Am J Geriatric Pharmacother (2005) 3(3):127–36. 10.1016/S1543-5946(05)80019-4 [DOI] [PubMed] [Google Scholar]
- 28. Cryer B, Miller P, Petruschke RA, Chen E, Geba GP, De Papp AE. Upper gastrointestinal tolerability of once weekly alendronate 70 mg with concomitant non-steroidal anti-inflammatory drug use. Aliment Pharmacol Ther (2005) 21(5):599–607. 10.1111/j.1365-2036.2005.02378.x [DOI] [PubMed] [Google Scholar]
- 29. Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. Jama (1998) 280(24):2077–82. 10.1001/jama.280.24.2077 [DOI] [PubMed] [Google Scholar]
- 30. Downs RW, Jr., Bell NH, Ettinger MP, Walsh BW, Favus MJ, Mako B, et al. Comparison of alendronate and intranasal calcitonin for treatment of osteoporosis in postmenopausal women. Journal of Clinical. Endocrinol Metab (2000) 85(5):1783–8. 10.1210/jcem.85.5.6606 [DOI] [PubMed] [Google Scholar]
- 31. Eisman JA, Rizzoli R, Roman-Ivorra J, Lipschitz S, Verbruggen N, Gaines KA, et al. Upper gastrointestinal and overall tolerability of alendronate once weekly in patients with osteoporosis: Results of a randomized, double-blind, placebo-controlled study. Curr Med Res Opin (2004) 20(5):699–705. 10.1185/030079904125003548 [DOI] [PubMed] [Google Scholar]
- 32. Felsenberg D, Alenfeld F, Bock O, Hammermeister C, Gowan W, the Fosit-Study-Group Placebo-controlled multicenter study of oral alendronate in postmenopausal osteoporotic women. Maturitas (1998) 31(1):35–44. 10.1016/S0378-5122(98)00050-4 [DOI] [PubMed] [Google Scholar]
- 33. Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY. Risedronate reverses bone loss in postmenopausal women with low bone mass: Results from a multinational, double-blind, placebo-controlled trial. J Clin Endocrinol Metab (2000) 85(5):1895–900. 10.1210/jc.85.5.1895 [DOI] [PubMed] [Google Scholar]
- 34. Greenspan S, Field-Munves E, Tonino R, Smith M, Petruschke R, Wang L, et al. Tolerability of once-weekly alendronate in patients with osteoporosis: A randomized, double-blind, placebo-controlled study. Mayo Cli Proc (2002) 77(10):1044–52. 10.4065/77.10.1044 [DOI] [PubMed] [Google Scholar]
- 35. Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. J Am Med Assoc (1999) 282(14):1344–52. 10.1001/jama.282.14.1344 [DOI] [PubMed] [Google Scholar]
- 36. Hosking D, Adami S, Felsenberg D, Andia JC, Välimäki M, Benhamou L, et al. Comparison of change in bone resorption and bone mineral density with once-weekly alendronate and daily risedronate: A randomised, placebo-controlled study. Curr Med Res Opin (2003) 19(5):383–94. 10.1185/030079903125002009 [DOI] [PubMed] [Google Scholar]
- 37. Ilter E, Karalok H, Tufekci EC, Batur O. Efficacy and acceptability of risedronate 5 mg daily compared with 35 mg once weekly for the treatment of postmenopausal osteoporosis. Climacteric (2006) 9(2):129–34. 10.1080/13697130600652180 [DOI] [PubMed] [Google Scholar]
- 38. Iwamoto J, Takeda T, Ichimura S. Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: A comparison with the effect of etidronate. J Orthopaedic Sci (2001) 6(6):487–92. 10.1007/s007760100002 [DOI] [PubMed] [Google Scholar]
- 39. Johnell O, Scheele WH, Lu Y, Reginster J-Y, Need AG, Seeman E. Additive Effects of Raloxifene and Alendronate on Bone Density and Biochemical Markers of Bone Remodeling in Postmenopausal Women with Osteoporosis. J Clin Endocrinol Metab (2002) 87(3):985–92. 10.1210/jcem.87.3.8325 [DOI] [PubMed] [Google Scholar]
- 40. Kung AWC, Yeung SSC, Chu LW. The efficacy and tolerability of alendronate in postmenopausal osteoporotic Chinese women: A randomized placebo-controlled study. Calcified Tissue Int (2000) 67(4):286–90. 10.1007/s0022330001142 [DOI] [PubMed] [Google Scholar]
- 41. Kushida K, Shiraki M, Nakamura T, Kishimoto H, Morii H, Yamamoto K, et al. Alendronate reduced vertebral fracture risk in postmenopausal Japanese women with osteoporosis: A 3-year follow-up study. J Bone Mineral Metab (2004) 22(5):462–8. 10.1007/s00774-004-0508-0 [DOI] [PubMed] [Google Scholar]
- 42. Lanza F, Sahba B, Schwartz H, Winograd S, Torosis J, Quan H, et al. The upper GI safety and tolerability of oral alendronate at a dose of 70 milligrams once weekly: A placebo-controlled endoscopy study. Am J Gastroenterol (2002) 97(1):58–64. 10.1111/j.1572-0241.2002.05446.x [DOI] [PubMed] [Google Scholar]
- 43. Lanza F, Schwartz H, Sahba B, Malaty HM, Musliner T, Reyes R, et al. An endoscopic comparison of the effects of alendronate and risedronate on upper gastrointestinal mucosae. Am J Gastroenterol (2000) 95(11):3112–7. 10.1016/S0002-9270(00)02051-7 [DOI] [PubMed] [Google Scholar]
- 44. Lau EM, Woo J, Chan YH, Griffith J. Alendronate prevents bone loss in Chinese women with osteoporosis. Bone (2000) 27(5):677–80. 10.1016/s8756-3282(00)00378-1 [DOI] [PubMed] [Google Scholar]
- 45. Leung JYY, Ho AYY, Ip TP, Lee G, Kung AWC. The efficacy and tolerability of risedronate on bone mineral density and bone turnover markers in osteoporotic Chinese women: A randomized placebo-controlled study. Bone (2005) 36(2):358–64. 10.1016/j.bone.2004.10.014 [DOI] [PubMed] [Google Scholar]
- 46. Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med (1995) 333(22):1437–43. 10.1056/NEJM199511303332201 [DOI] [PubMed] [Google Scholar]
- 47. McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med (2001) 344(5):333–40. 10.1056/nejm200102013440503 [DOI] [PubMed] [Google Scholar]
- 48. Miller PD, Woodson G, Licata AA, Ettinger MP, Mako B, Smith ME, et al. Rechallenge of patients who had discontinued alendronate therapy because of upper gastrointestinal symptoms. Clin Ther (2000) 22(12):1433–42. 10.1016/S0149-2918(00)83042-8 [DOI] [PubMed] [Google Scholar]
- 49. Murphy MG, Weiss S, McClung M, Schnitzer T, Cerchio K, Connor J, et al. Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab (2001) 86(3):1116–25. 10.1210/jcem.86.3.7294 [DOI] [PubMed] [Google Scholar]
- 50. Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med (2000) 343(9):604–10. 10.1056/NEJM200008313430902 [DOI] [PubMed] [Google Scholar]
- 51. Pols HAP, Felsenberg D, Hanley DA, Štepán J, Muñoz-Torres M, Wilkin TJ, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: Results of the FOSIT study. Osteoporos Int (1999) 9(5):461–8. 10.1007/PL00004171 [DOI] [PubMed] [Google Scholar]
- 52. Reginster JY, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int (2000) 11(1):83–91. 10.1007/s001980050010 [DOI] [PubMed] [Google Scholar]
- 53. Ryan PJ, Blake GM, Davie M, Haddaway M, Gibson T, Fogelman I. Intermittent oral disodium pamidronate in established osteoporosis: A 2 year double-masked placebo-controlled study of efficacy and safety. Osteoporos Int (2000) 11(2):171–6. 10.1007/PL00004179 [DOI] [PubMed] [Google Scholar]
- 54. Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, et al. Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Mineral Res (2010) 25(8):1886–94. 10.1002/jbmr.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Taga M, Nakamura T, et al. A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. Osteoporos Int (1999) 10(3):183–92. 10.1007/s001980050214 [DOI] [PubMed] [Google Scholar]
- 56. Shiraki M, Fukunaga M, Kushida K, Kishimoto H, Taketani Y, Minaguchi H, et al. A double-blind dose-ranging study of risedronate in Japanese patients with osteoporosis (a study by the Risedronate Late Phase II Research Group). Osteoporos Int (2003) 14(3):225–34. 10.1007/s00198-002-1369-9 [DOI] [PubMed] [Google Scholar]
- 57. Tucci JR, Tonino RP, Emkey RD, Peverly CA, Kher U. Santora 2nd AC. Effect of three years of oral alendronate treatment in postmenopausal women with osteoporosis. Am J Med (1996) 101(5):488–501. 10.1016/S0002-9343(96)00282-3 [DOI] [PubMed] [Google Scholar]
- 58. Yan Y, Wang W, Zhu H, Li M, Liu J, Luo B, et al. The efficacy and tolerability of once-weekly alendronate 70 mg on bone mineral density and bone turnover markers in postmenopausal Chinese women with osteoporosis. J Bone Mineral Metab (2009) 27(4):471–8. 10.1007/s00774-009-0057-7 [DOI] [PubMed] [Google Scholar]
- 59. You L, Sheng ZY, Chen JY, Pan L, Chen L. The safety and efficacy of early-stage bi-weekly alendronate to improve bone mineral density and bone turnover in Chinese post-menopausal women at risk of osteoporosis. J Int Med Res (2011) 39(1):302–10. 10.1177/147323001103900133 [DOI] [PubMed] [Google Scholar]
- 60. Wallace JL, Dicay M, McKnight W, Bastaki S, Blank MA. N-bisphosphonates cause gastric epithelial injury independent of effects on the microcirculation. Aliment Pharmacol Ther (1999) 13(12):1675–82. 10.1046/j.1365-2036.1999.00658.x [DOI] [PubMed] [Google Scholar]
- 61. Elliott SN, McKnight W, Davies NM, MacNaughton WK, Wallace JL. Alendronate induces gastric injury and delays ulcer healing in rodents. Life Sci (1997) 62(1):77–91. 10.1016/S0024-3205(97)01040-0 [DOI] [PubMed] [Google Scholar]
- 62. Thomson ABR, Appleman S, Keelan M, Wallace JL. Role of Gastric Mucosal and Gastric Juice Cytokine Concentrations in Development of Bisphosphonate Damage to Gastric Mucosa. Digestive Dis Sci (2003) 48(2):308–14. 10.1023/A:1021979510860 [DOI] [PubMed] [Google Scholar]
- 63. Lichtenberger LM, Romero JJ, Gibson GW, Blank MA. Effect of bisphosphonates on surface hydrophobicity and phosphatidylcholine concentration of rodent gastric mucosa. Digestive Dis Sci (2000) 45(9):1792–801. 10.1023/A:1005574009856 [DOI] [PubMed] [Google Scholar]
- 64. Pazianas M, Abrahamsen B. Safety of bisphosphonates. Bone (2011) 49(1):103–10. 10.1016/j.bone.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 65. Graham DY. What the gastroenterologist should know about the gastrointestinal safety profiles of bisphosphonates. Digestive Dis Sci (2002) 47(8):1665–78. 10.1023/a:1016495221567 [DOI] [PubMed] [Google Scholar]
- 66. Taggart H, Bolognese MA, Lindsay R, Ettinger MP, Mulder H, Josse RG, et al. Upper gastrointestinal tract safety of risedronate: a pooled analysis of 9 clinical trials. Mayo Clin Proc (2002) 77(3):262–70. 10.4065/77.3.262 [DOI] [PubMed] [Google Scholar]
- 67. Epstein S, Delmas PD, Emkey R, Wilson KM, Hiltbrunner V, Schimmer RC. Oral ibandronate in the management of postmenopausal osteoporosis: Review of upper gastrointestinal safety. Maturitas (2006) 54(1):1–10. 10.1016/j.maturitas.2006.01.011 [DOI] [PubMed] [Google Scholar]
- 68. Zhou M, Zheng Y, Li J, Wu J, Xu W, Cui L, et al. Upper gastrointestinal safety and tolerability of oral alendronate: A meta-analysis. Exp Ther Med (2016) 11(1):289–96. 10.3892/etm.2015.2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tadrous M, Wong L, Mamdani MM, Juurlink DN, Krahn MD, Lévesque LE, et al. Comparative gastrointestinal safety of bisphosphonates in primary osteoporosis: a network meta-analysis. Osteoporos Int (2014) 25(4):1225–35. 10.1007/s00198-013-2576-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Non-severe adverse events subgroup by active substance.
Severe adverse events subgroup by active substance.
Non-severe GI side effects subgroup by more than 24 months of treatment.
Severe GI side effects subgroup by more than 24 months of treatment.
Non-severe adverse events in the context of the most commonly used BP therapies: 70 mg/week and 10 mg/day alendronate per os.
Severe adverse events in the context of the most commonly used BP therapies: 70 mg/week and 10 mg/day alendronate per os.
Trial Sequential Analysis of non-severe GI. adverse events
Funnel plot of non-severe GI adverse events.
Funnel plot of severe GI adverse events.
FDA criteria of non-severe and severe GI adverse events.
Risk of Bias Assessment.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.