Abstract
Background
Electronic cigarettes (e-cigarettes) are widely promoted as harm-reduction products for smokers, and smokers commonly perceive them as less harmful than combustible cigarettes. One of the key questions regarding public health consequences of e-cigarettes is the magnitude of harm reduction achievable by smokers who switch from combustible cigarettes to e-cigarettes. We conducted a systematic literature review of epidemiological studies that estimated odds of respiratory and cardiovascular outcomes among former smokers who use e-cigarettes compared to current smokers.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement. We searched the PubMed and Embase databases in September 2020 to identify epidemiological studies that compared odds of cardiovascular and respiratory outcomes among former smokers who transitioned to e-cigarettes relative to odds among current smokers not using e-cigarettes (current exclusive smokers). We included studies that provided direct estimates of relevant odds ratios (ORs). We also included studies where indirect estimates of relevant ORs could be calculated based on published results. Two reviewers independently extracted data and conducted quality appraisals.
Results
Six population-based studies with sample sizes ranging from 19,475 to 161,529 respondents met review inclusion criteria, five of which were cross-sectional and one longitudinal. Three studies reported respiratory outcomes and three reported cardiovascular outcomes. ORs of respiratory outcomes (including chronic obstructive pulmonary disease, chronic bronchitis, emphysema, asthma, and wheezing) in former smokers who transitioned to e-cigarettes versus current exclusive smokers were below 1.0, ranging from 0.58 (95%CI 0.36–0.94) to 0.66 (95%CI 0.50–0.87; all p < 0.05). All ORs for cardiovascular outcomes (including stroke, myocardial infarction, and coronary heart disease) did not differ significantly from 1.0.
Conclusion
Though our review included a small number of studies, it provided consistent results. Former smokers who transitioned to e-cigarettes showed ~ 40% lower odds of respiratory outcomes compared to current exclusive smokers. Switching from smoking to e-cigarette does not appear to significantly lower odds of cardiovascular outcomes. Since the utility of cross-sectional studies for causal inference remains limited, both randomized controlled trials and prospective cohort studies are needed to better evaluate contributions of e-cigarettes as harm reduction tools for smokers.
Keywords: Electronic cigarettes, e-cigarettes, Vaping, Respiratory diseases, Cardiovascular diseases, Harm reduction, Relative risk, Smoking
Background
A limited number of studies have evaluated associations between e-cigarette use (vaping) and various health outcomes. Most studies thus far have been cross-sectional due to the novelty of e-cigarettes, and many have focused exclusively on e-cigarette users who have never smoked [1–6]. Since some youth have taken up vaping [7–9], it is important to evaluate potential absolute health risk associated with vaping among e-cigarette users who have never smoked tobacco cigarettes. However, since the vast majority of adult e-cigarette users are former smokers [10–13], it is important to consider e-cigarette use in the context of smoking (i.e., relative harm) [14, 15].
Numerous in vitro and in vivo laboratory studies have investigated relative harm of e-cigarettes compared to combustible cigarettes. Overall, laboratory studies have demonstrated that aerosols emitted from e-cigarettes contain fewer amounts and lower concentrations of toxicants than combustible cigarettes [16–19]. In vitro studies and in vivo animal models also suggest lower toxicity of e-cigarette compared to combustible cigarettes [20–26]. While laboratory studies provide important insights into relative toxicity of e-cigarettes compared to combustible cigarettes, human studies provide further evidence of how the reduced toxicity of e-cigarettes correlates with a potential reduction of health risk among smokers who transitioned from smoking to vaping. Although cross-sectional and longitudinal studies have shown that exposure to selected toxicants in exclusive e-cigarette users is substantially lower as compared to exclusive cigarette smokers [27–30], those studies are not suited to directly evaluate potential reduction in health risk among smokers who transitioned to e-cigarettes.
Randomized controlled trials (RCTs) assessing clinically relevant health outcomes among smokers who switched to e-cigarettes compared to smokers who continue to smoke likely offer the most comprehensive evaluation of the harm reduction potential of vaping. Indeed, some of the strongest evidence regarding relative cardiovascular health effects of vaping has come from the VESUVIUS Trial, an RCT conducted between 2016 and 2018 that observed improvements to vascular health over a one-month period among participants who switched from smoking to vaping [31]. However, such RCTs require considerable resources and extensive time, as many relevant clinical outcomes manifest over relatively long periods of time (often several years). In the absence of considerable evidence from RCTs, large population-based observational studies can offer meaningful information about relative health risk of vaping compared to smoking. Epidemiological cross-sectional studies that compare the odds of health outcomes among former smokers who switched to e-cigarettes versus those who continue to smoke may provide a crude estimation of harm reduction potential of vaping. Although several cross-sectional studies have been published, those studies have not been systematically reviewed, critically evaluated, and their outcomes have not been summarized. As such, we aimed to conduct a systematic literature review of epidemiological studies that have estimated the odds of key respiratory and cardiovascular outcomes among e-cigarette users who formerly smoked tobacco cigarettes compared with current cigarette smokers who do not use e-cigarettes.
Methods
Data sources and search strategy
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) protocol was used to guide the design of this systematic review [32]. On September 17, 2020, we completed literature searches of MEDLINE’s PubMed (1946 to present) and EMBASE (1974 to present). The searches included text words to capture concepts associated with e-cigarettes, respiratory outcomes, and cardiovascular outcomes published from database inception to the date of search. We chose not to include any terms limiting participant age, language, study design, or year of publication in the search strategy, to minimize unintentional exclusions. Title/abstract search fields were used for each search. Full details of the search strategy are provided in Additional file 1 (Table S1. Summary of Search Results; Table S2. Details of the PubMed run (conducted September 17th, 2020); Table S3. Details of the Embase run (conducted September 17th, 2020); Figure S1. Screenshot depicting the Embase run (conducted September 17th, 2020).
Study selection criteria
We included studies that modeled smoking and vaping as a composite variable, providing direct estimates of prevalence odds ratios (ORs) for specified health outcomes among former smokers currently using e-cigarettes compared to current smokers not using e-cigarettes (current exclusive smokers). We also included studies that modeled smoking and vaping as independent factors (i.e., current smoker vs. never smoker, current vaper vs. never vaper). For these, we calculated ORs for former smokers who switched to e-cigarettes compared to current smokers, assuming independent associations of smoking and e-cigarette use with each health outcome. Details regarding calculations are provided in Additional file 1 (Appendix 1. Calculation of Odds Ratios (ORs) for Composite Smoking and Vaping Variables; Appendix 2. Calculation of Odds Ratios (ORs) for Separate Smoking and Vaping Variable). Cross-sectional and longitudinal studies were included in the review.
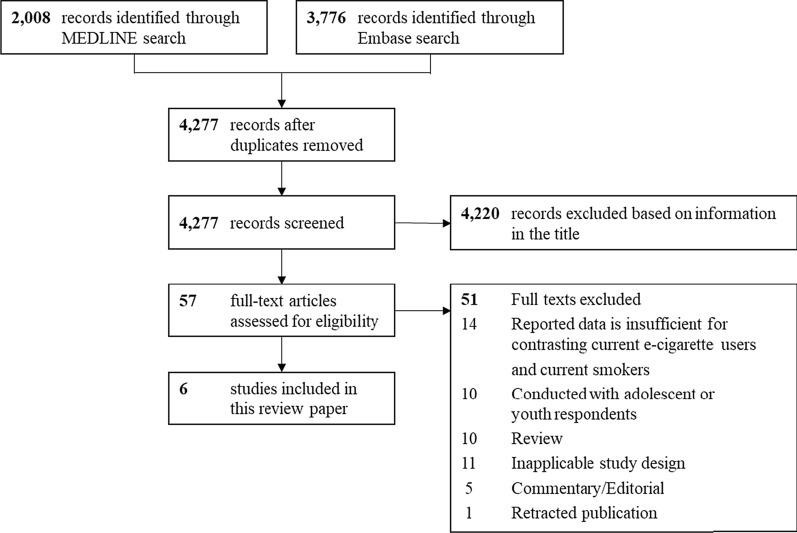
As our primary interest was to examine potential harm reduction among current e-cigarette users who were former smokers, we excluded studies where current e-cigarette users were youth or never smokers. Two investigators (C.R.M. and E.S.) independently reviewed the title, abstract, and full text of 57 publications that met screening criteria (Fig. 1). In case of disagreement between the two investigators, inclusion of studies in a final review was independently decided by a third investigator (M.L.G.). Methodological approach for systematic review and article selection are presented in Fig. 1.
Fig. 1.
Methodological approach for systematic review and article selection (PRISMA diagram)
Data extraction
Data extracted for each study included study design; data source; geographic location of study sample; sample size; age of study participants; e-cigarette use; cigarette use; cardiorespiratory outcomes; covariates accounted for in adjusted models; and adjusted odds ratios (aORs) with 95% CIs. Authors who did not report odds ratios were contacted if the results they did report suggested that a relevant measure of association, although not published, had likely been calculated. Each corresponding author of these papers (n = 5) confirmed they had not calculated the requisite aORs for inclusion in the review. Individual study data were extracted by a designated reviewer (C.R.M. or E.S.) and subsequently verified by a second reviewer.
Methodological quality appraisal
The quality of each study was assessed using the Appraisal Tool for Cross-Sectional Studies (AXIS tool) [33]. The AXIS tool assesses a number of factors related to study quality, including the study design’s suitability for stated research aims, justification for sample size, reliability of survey items, and the appropriateness of authors’ interpretation of results. Studies were independently evaluated by two reviewers (C.R.M. and E.S.), and in case of disagreement between the two investigators, a final grade was independently decided by a third reviewer (M.L.G.).
Results
Summary of study search and selection results
Of 4277 unique publications identified through the database search, 57 were classified as potentially eligible for inclusion in the systematic review based on their titles (Fig. 1). After exclusion of 51 studies through full-text screening, 6 studies were included in the systematic review (see Additional file 1 for the reference list of the 51 studies excluded (Appendix 3. Reference list of 51 studies excluded after full text screening).
Description of included studies
The included studies are described in Table 1. Almost all studies were surveyed on the United States general adult population, while one study surveyed the Swedish general adult population. Five studies were cross-sectional and one was longitudinal in design. The sample size for each study ranged from 19,475 to 161,529. Each of the reviewed studies included self-reported health outcomes. Half of the included studies (n = 3) reported respiratory outcomes (e.g., chronic obstructive pulmonary disease, chronic bronchitis, emphysema, asthma, wheezing) [34–36], while the other half (n = 3) reported cardiovascular outcomes (e.g., stroke, myocardial infarction, coronary heart disease) [37–39].
Table 1.
Contrasting the odds of self-reported health outcomes between current e-cigarette users and current smokers in cross-sectional studies
| Study information | Study results | |||||
|---|---|---|---|---|---|---|
| References | Data source and study design | Analytic sample | Outcome | Covariates | Statistical modeling approach* | aOR (95% CI) |
| Respiratory | ||||||
| Hedman et al. [33] |
OLIN and WSAS (2016) Cross-sectional |
Age range: 20–75 years Total sample: n = 30,272 Exclusive vapers who were former smokers: n = 79 |
Respiratory symptoms |
Sociodemographics OLIN or WSAS survey respondent |
Composite smoking and vaping variable | 0.58 (0.36–0.94)† |
| Li et al. [34] |
PATH W2 (2015) Cross-sectional |
Age range: ≥ 18 years Total sample: n = 28,171 Exclusive vapers who were former smokers: n = 471 |
Wheezing |
Sociodemographics Weight status Secondhand smoke Asthma Perceived health |
Composite smoking and vaping variable |
0.66 (0.50–0.87)‡ |
| Bhatta and Glantz [35] |
PATH W1–W3 (2014–2016) Longitudinal |
Age range: ≥ 18 years Total sample: n = 19,475 All vapers: n = 2059 (1.4%)§ |
COPD, chronic bronchitis, emphysema, or asthma |
Sociodemographics Weight status High blood pressure High cholesterol Diabetes mellitus |
Separate smoking and vaping variables |
0.58 (0.37–0.93) |
| Cardiovascular | ||||||
|
Alzahrani et al (2018) [36] |
NHIS (Pooled 2014 and 2016) Cross-sectional |
Age range: ≥ 18 years Total sample: n = 69,905 All everyday vapers: n = 776 (5.3%)§ All someday vapers: n = 1483 (9.2%)§ |
History of MI |
Sociodemographics Weight status High blood pressure High cholesterol Diabetes mellitus |
Separate smoking and vaping variables |
1.12 (0.72–1.76)|| 0.83 (0.53–1.31)¶ |
| Farsalinos et al. [37] |
NHIS (Pooled 2016 and 2017) Cross-sectional |
Age range: ≥ 18 years Total sample: n = 59,770 All everyday vapers: n = 714 (9.1%)§ All someday vapers: n = 1009 (17.9%)§ |
[A] History of MI [B] History of CHD |
Sociodemographics Weight status High blood pressure High cholesterol Diabetes mellitus |
Separate smoking and vaping variables |
[A] 1.22 (0.70–2.10)|| [A] 1.39 (0.76–2.54)¶ [B] 1.48 (0.83–2.64)|| [B] 1.26(0.70–2.30)¶ |
| Parekh et al. [38] |
BRFSS (Pooled 2016 and 2017) Cross-sectional |
Age range: 18–44 years Total sample: n = 161,529 Exclusive vapers who were former smokers: n = 13,318 |
History of stroke |
Sociodemographics Weight status Physical activity Binge drinking Diabetes mellitus |
Composite smoking and vaping variable |
1.60 (0.69–3.71)‡ |
aOR adjusted odds ratio, CI confidence interval, COPD chronic obstructive pulmonary disease, CHD coronary heart disease, MI myocardial infarction, BRFSS Behavioral Risk Factor Surveillance System, NHIS National Health Interview Survey, OLIN Obstructive Lung Disease in Northern Sweden Study, WSAS West Sweden Asthma Study, PATH Population Assessment of Tobacco and Health Study, W1 Wave 1, W2 Wave 2, W3 Wave 3
*For composite smoking and vaping variable, exclusive vapers only include former smokers
†Exclusive smokers only include never vapers
‡Exclusive smokers may include never or former vapers
§Weighted percent of current vapers who are never smokers
||Everyday vapers vs. everyday smokers
¶Someday vapers vs. someday smokers
Three studies used a composite smoking and vaping variable [34, 35, 39], with the remaining three using separate smoking and vaping variables [36–38] (Table 1). Of the three studies which utilized separate smoking and vaping variables, two reported odds ratios for ‘every day’ and ‘some days’ users [37, 38], while one study pooled both user groups as ‘current users’ [36]. In addition, the studies were generally deemed acceptable quality in accordance with the AXIS tool, as 5 of the 6 reviewed studies met at least 16 of the 20 AXIS tool criteria [33] (Table 2). However, it is important to recognize that ability to evaluate associations for causality is drastically limited for the 5 cross-sectional studies [40].
Table 2.
Appraisal of reviewed studies using the AXIS tool
| Hedman et al. [33] | Li et al. [34] | Bhatta and Glantz [35] | Alzahrani et al. [37] | Farsalinos et al. [37] | Parekh et al. [38] | |
|---|---|---|---|---|---|---|
| Were the aims/objectives of the study clear? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the study design appropriate for the stated aim(s)? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the sample size justified? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the target/reference population clearly defined? (Is it clear who the research was about?) | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the sample frame taken from an appropriate population base so that it closely represented the target/reference population under investigation? | Don't know | Yes | Yes | Yes | Yes | Yes |
| Was the selection process likely to select subjects/participants that were representative of the target/reference population under investigation? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were measures undertaken to address and categorize non-responders? | Yes | Don't know | Don't know | Don't know | Don't know | Don't know |
| Were the risk factor and outcome variables measured appropriate to the aims of the study? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the risk factor and outcome variables measured correctly using instruments/measurements that have been trialed, piloted, or published previously? | Yes | Yes | Yes | Yes | Yes | Yes |
| Is it clear what was used to determine statistical significance and/or precision estimates? (e.g., p values, Cis) | Yes | Yes | Yes | No | Yes | Yes |
| Were the methods (including statistical methods) sufficiently described to enable them to be repeated? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the basic data adequately described? | Yes | Yes | Yes | Yes | Yes | Yes |
| Does the response rate raise concerns about non-response bias?* | Yes | Don't know | Don't know | Don't know | Don't know | Don't know |
| If appropriate, was information about non-responders described? | Yes | Don't know | Don't know | Don't know | Don't know | Don't know |
| Were the results internally consistent? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the results for the analyses described in the methods presented? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the authors discussions and conclusions justified by the results? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the limitations of the study discussed? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were there any funding sources or conflicts of interest that may affect the authors' interpretation of the results?* | Don't know | Don't know | Don't know | Don't know | Don't know | No |
| Was ethnical approval or consent of participants attained? | Yes | Yes | Yes | Yes | Yes | Yes |
| Overall | 17 | 16 | 16 | 15 | 16 | 17 |
Synthesis of findings
Overall, ORs of respiratory outcomes (including chronic obstructive pulmonary disease, chronic bronchitis, emphysema, asthma, and wheezing) in former smokers who transitioned to e-cigarettes versus current exclusive smokers were below 1.0, ranging from 0.58 (95%CI 0.36–0.94) to 0.66 (95%CI 0.50–0.87; all p < 0.05) (Table 1). No ORs for cardiovascular outcomes (including stroke, myocardial infarction, and coronary heart disease) differed significantly from 1.0 (Table 1).
Discussions
In summary, epidemiologic studies have observed ~ 40% lower odds of respiratory outcomes for former smokers’ currently using e-cigarettes compared to current exclusive smokers, yet no difference for cardiovascular outcomes. While the utility of cross-sectional studies for causal inference remains limited at best, especially considering unmeasured confounders, these findings offer some quantitative insight regarding harm reduction applications of e-cigarettes. In particular, consistency between cross-sectional and longitudinal study results increases our confidence in estimates for respiratory outcomes. Whereas respiratory outcomes ranged in severity, only major adverse cardiovascular events were assessed in the reviewed studies. As interest in harm reduction might be greater among smokers who have experienced a major clinical event, concerns of reverse causality and potential selection bias are especially warranted for these cardiovascular publications. Therefore, both randomized controlled trials and prospective cohort studies are needed to better evaluate contributions of e-cigarettes to respiratory or cardiovascular risks in patients who would quit smoking using those devices compared to those who would quit without any intervention or with support of approved smoking-cessation medications. Additionally, future epidemiologic studies evaluating subclinical and preclinical risk factors (i.e., hypertension, hyperlipidemia, etc.) are needed, particularly in light of recent randomized trial results showing smokers who switched completely to e-cigarettes saw clinically significant reductions in flow-mediated dilation [31], an important marker of endothelial dysfunction. Similar markers of potential harm can be measured in the biospecimens collected from vapers and smokers. Those markers are useful for early detection of ongoing pathological processes in the body and, if sensitive enough, could serve as potential indicators of health risk before clinical symptoms are observed.
Only two groups of health outcomes, respiratory and cardiovascular, were assessed in this review as both are primary contributors to overall mortality associated with smoking [41, 42]. During preliminary literature searches, we did not identify any epidemiological studies that evaluated associations between vaping and cancer outcomes. Cancer outcomes would be expected to be seen later than acute respiratory and cardiovascular events, as their induction time is lengthy. One may expect potential harm reduction in cancer to be more substantial due to a stronger correlation between exposure to carcinogenic substances in combustible cigarettes and risk of neoplastic diseases [43–45].
It should be emphasized that the potential for harm reduction may differ according to comorbid status, and no studies conducted stratified analyses separating respondents with other relevant clinical diseases from ‘healthy’ subjects. Thus, the potential beneficial effect of switching to e-cigarettes for smokers with existing respiratory and cardiovascular diseases may be different than our estimates. An important limitation of the studies included in our review is that the time from quitting smoking and switching to vaping among former smokers may have been relatively short. One may expect that potential benefits of switching from smoking to vaping may change over time. Additionally, all studies included in our review were solely based on self-reported symptoms. It is important that future studies also include objective measures of participants’ health status and a comprehensive clinical evaluation of the potential symptoms observed among vapers and smokers. Finally, some studies included in our review were restricted to younger vapers and smokers. As the risk of many cardiovascular and respiratory diseases increases with aging, the relative risk of vaping compared to smoking among older subjects could differ from our estimates. While the reviewed studies all controlled for important sociodemographic factors as potential confounding variables, future studies aiming to examine differences by age and sex through stratification methods would be a strong addition to the literature, particularly as longitudinal studies become more feasible.
Though our estimates are based on a small number of epidemiological studies, they could be used by health care providers in their discussions with smokers about relative harm of e-cigarettes. We also encourage other researchers evaluating potential links between e-cigarettes and health outcomes to include comparisons to long-term smokers in their analyses. Robust evidence is needed by health organizations, public health advocates, and regulators that currently consider endorsing or discouraging e-cigarettes as harm reduction tools for smokers.
Conclusions
Although our systematic review showed ~ 40% lower odds of respiratory outcomes and no difference of cardiovascular outcomes for former smokers who transitioned to e-cigarettes compared to current exclusive smokers, these estimates of relative risk of vaping compared to smoking are primarily based on a limited number of epidemiological studies with several important limitations. Both randomized controlled trials and prospective cohort studies are needed to better evaluate contributions of e-cigarettes as harm reduction tools for smokers.
Supplementary information
Additional file 1: Table S1. Summary of Search Results. Table S2. Details of the PubMed run (conducted September 17th, 2020). Table S3. Details of the Embase run (conducted September 17th, 2020). Figure S1. Screenshot depicting the Embase run (conducted September 17th, 2020). Appendix 1. Calculation of Odds Ratios (ORs) for Composite Smoking and Vaping Variables. Appendix 2. Calculation of Odds Ratios (ORs) for Separate Smoking and Vaping Variable. Appendix 3. Reference list of 51 studies excluded after full text screening.
Acknowledgements
Not applicable.
Abbreviations
- aOR
Adjusted odds ratio
- AXIS
Appraisal tool for cross-sectional studies
- BRFSS
Behavioral risk factor surveillance system
- CHD
Coronary heart disease
- CI
Confidence interval
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- MI
Myocardial infarction
- NHIS
National Health Interview Survey
- OLIN
Obstructive Lung Disease in Northern Sweden Study
- OR
Odds ratio
- PATH
Population assessment of tobacco and health study
- RCTs
Randomized controlled trials
- W1
Wave 1
- W2
Wave 2
- W3
Wave 3
- WSAS
West Sweden Asthma Study
Authors’ contributions
MLG contributed to study concept, data interpretation and writing a manuscript. CRM and ES contributed to literature search, data analysis, and writing a manuscript. DL contributed to data analysis and writing a manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the US Food and Drug Administration and National Cancer Institute under grant award U54CA238110. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Food and Drug Association.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr Goniewicz received research grant from Pfizer and personal fees from Johnson and Johnson, outside of this work. Other authors have nothing to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maciej L. Goniewicz, Email: maciej.goniewicz@roswellpark.org
Connor R. Miller, Email: connor.miller@roswellpark.org
Edward Sutanto, Email: edward.sutanto@roswellpark.org.
Dongmei Li, Email: dongmei_li@urmc.rochester.edu.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12954-020-00440-w.
References
- 1.Wang MP, Ho SY, Leung LT, et al. Electronic cigarette use and respiratory symptoms in Chinese adolescents in Hong Kong. JAMA Pediatr. 2016;170:89–91. doi: 10.1001/jamapediatrics.2015.3024. [DOI] [PubMed] [Google Scholar]
- 2.McConnell R, Barrington-Trimis JL, Wang K, et al. Electronic cigarette use and respiratory symptoms in adolescents. Am J Respir Crit Care Med. 2017;195:1043–1049. doi: 10.1164/rccm.201604-0804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schweitzer RJ, Wills TA, Tam E, et al. E-cigarette use and asthma in a multiethnic sample of adolescents. Prev Med. 2017;105:226–231. doi: 10.1016/j.ypmed.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi K, Bernat D. E-cigarette use among Florida youth with and without asthma. Am J Prev Med. 2016;51:446–453. doi: 10.1016/j.amepre.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho JH, Paik SY. Association between electronic cigarette use and asthma among high school students in South Korea. PLoS ONE. 2016;11:e0151022. doi: 10.1371/journal.pone.0151022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SY, Sim S, Choi HG. Active, passive, and electronic cigarette smoking is associated with asthma in adolescents. Sci Rep. 2017;7:1–8. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentzke AS, Creamer M, Cullen KA, et al. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. Morb Mortal Wkly Rep. 2019;68:157. doi: 10.15585/mmwr.mm6806e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond D, Reid JL, Rynard VL, et al. Prevalence of vaping and smoking among adolescents in Canada, England, and the United States: repeat national cross sectional surveys. BMJ. 2019;365 [DOI] [PMC free article] [PubMed]
- 9.Kasza KA, Edwards KC, Tang Z, et al. Correlates of tobacco product initiation among youth and adults in the USA: findings from the PATH Study Waves 1–3 (2013–2016) Tob Control. 2020;29:s191–s202. doi: 10.1136/tobaccocontrol-2020-055671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obisesan OH, Osei AD, Uddin SI, et al. Trends in e-cigarette use in adults in the United States, 2016–2018. JAMA Int Med. 2020;180:1394–1398. doi: 10.1001/jamainternmed.2020.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei L, Muhammad-Kah RS, Hannel T, et al. The impact of cigarette and e-cigarette use history on transition patterns: a longitudinal analysis of the population assessment of tobacco and health (PATH) study, 2013–2015. Harm Reduct J. 2020;17:1–12. doi: 10.1186/s12954-020-00386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen R, Pierce JP, Leas EC, et al. E-cigarette use to aid long-term smoking cessation in the US: prospective evidence from the PATH Cohort Study. Am J Epidemiol. 2020. [DOI] [PMC free article] [PubMed]
- 13.Kyriakos CN, Filippidis FT, Hitchman S, et al. Characteristics and correlates of electronic cigarette product attributes and undesirable events during e-cigarette use in six countries of the EUREST-PLUS ITC Europe Surveys. Tob Induc Dis. 2018;16:A1. [DOI] [PMC free article] [PubMed]
- 14.Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5:67–86. doi: 10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Academies of Sciences, Engineering, and Medicine . Public health consequences of e-cigarettes. Washington: National Academies Press; 2018. [PubMed] [Google Scholar]
- 16.Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward AM, Yaman R, Ebbert JO. Electronic nicotine delivery system design and aerosol toxicants: a systematic review. PLoS ONE. 2020;15:e0234189. doi: 10.1371/journal.pone.0234189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23:ii11–ii17. doi: 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farsalinos KE, Gillman G. Carbonyl emissions in e-cigarette aerosol: a systematic review and methodological considerations. Front Physiol. 2018;8:1119. doi: 10.3389/fphys.2017.01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G, Liu W, Song W. Toxicity assessment of electronic cigarettes. Inhal Toxicol. 2019;31:259–273. doi: 10.1080/08958378.2019.1671558. [DOI] [PubMed] [Google Scholar]
- 21.Scheffler S, Dieken H, Krischenowski O, et al. Evaluation of e-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int J Environ Res Public Health. 2015;12:3915–3925. doi: 10.3390/ijerph120403915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romagna G, Allifranchini E, Bocchietto E, et al. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol. 2013;25:354–361. doi: 10.3109/08958378.2013.793439. [DOI] [PubMed] [Google Scholar]
- 23.Cervellati F, Muresan XM, Sticozzi C, et al. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicol In Vitro. 2014;28:999–1005. doi: 10.1016/j.tiv.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiemstra PS, Bals R. Basic science of electronic cigarettes: assessment in cell culture and in vivo models. Respir Res. 2016;17:1–5. doi: 10.1186/s12931-016-0447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Husari A, Shihadeh A, Talih S, et al. Acute exposure to electronic and combustible cigarette aerosols: effects in an animal model and in human alveolar cells. Nicotine Tob Res. 2016;18:613–619. doi: 10.1093/ntr/ntv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merecz-Sadowska A, Sitarek P, Zielinska-Blizniewska H, et al. A Summary of In Vitro and In Vivo studies evaluating the impact of e-cigarette exposure on living organisms and the environment. Int J Mol Sci. 2020;21:652. doi: 10.3390/ijms21020652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goniewicz ML, Smith DM, Edwards KC, et al. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw Open. 2018;1:e185937–e185937. doi: 10.1001/jamanetworkopen.2018.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpenter MJ, Heckman BW, Wahlquist AE, et al. A naturalistic, randomized pilot trial of e-cigarettes: uptake, exposure, and behavioral effects. Cancer Epidemiol Prev Biomark. 2017;26:1795–1803. doi: 10.1158/1055-9965.EPI-17-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahab L, Goniewicz ML, Blount BC, et al. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med. 2017;166:390–400. doi: 10.7326/M16-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goniewicz ML, Gawron M, Smith DM, et al. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine Tob Res. 2017;19:160–167. doi: 10.1093/ntr/ntw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George J, Hussain M, Vadiveloo T, et al. Cardiovascular effects of switching from tobacco cigarettes to electronic cigarettes. J Am Coll Cardiol. 2019;74:3112–3120. doi: 10.1016/j.jacc.2019.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Downes MJ, Brennan ML, Williams HC, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) BMJ Open. 2016;6:e011458. doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedman L, Backman H, Stridsman C, et al. Association of electronic cigarette use with smoking habits, demographic factors, and respiratory symptoms. JAMA Netw Open. 2018;1:e180789–e180789. doi: 10.1001/jamanetworkopen.2018.0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D, Sundar IK, McIntosh S, et al. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: cross-sectional results from the Population Assessment of Tobacco and Health (PATH) study, wave 2. Tob Control. 2020;29:140–147. doi: 10.1136/tobaccocontrol-2018-054694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatta DN, Glantz SA. Association of e-cigarette use with respiratory disease among adults: a longitudinal analysis. Am J Prev Med. 2020;58:182–190. doi: 10.1016/j.amepre.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alzahrani T, Pena I, Temesgen N, et al. Association between electronic cigarette use and myocardial infarction. Am J Prev Med. 2018;55:455–461. doi: 10.1016/j.amepre.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farsalinos KE, Polosa R, Cibella F, et al. Is e-cigarette use associated with coronary heart disease and myocardial infarction? Insights from the 2016 and 2017 National Health Interview Surveys. Ther Adv Chronic Dis. 2019;10:1–10. doi: 10.1177/2040622319877741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parekh T, Pemmasani S, Desai R. Risk of stroke with e-cigarette and combustible cigarette use in young adults. Am J Prev Med. 2020;58:446–452. doi: 10.1016/j.amepre.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Reichenheim ME, Coutinho ES. Measures and models for causal inference in cross-sectional studies: arguments for the appropriateness of the prevalence odds ratio and related logistic regression. BMC Med Res Methodol. 2010;10:66. doi: 10.1186/1471-2288-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.US Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, National Center on Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010 [PubMed]
- 42.US Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the surgeon general. Atlanta, GA: Centers for Disease Control and Prevention, National Center on Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014
- 43.Thomas DC. Models for exposure-time-response relationships with applications to cancer epidemiology. Annu Rev Public Health. 1988;9:451–482. doi: 10.1146/annurev.pu.09.050188.002315. [DOI] [PubMed] [Google Scholar]
- 44.Wu S, Zhu W, Thompson P, et al. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat Commun. 2018;9:1–12. doi: 10.1038/s41467-017-02088-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo J, Hendryx M, Ducatman A. Association between six environmental chemicals and lung cancer incidence in the United States. J Environ Public Health;2011;2011:463701. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Summary of Search Results. Table S2. Details of the PubMed run (conducted September 17th, 2020). Table S3. Details of the Embase run (conducted September 17th, 2020). Figure S1. Screenshot depicting the Embase run (conducted September 17th, 2020). Appendix 1. Calculation of Odds Ratios (ORs) for Composite Smoking and Vaping Variables. Appendix 2. Calculation of Odds Ratios (ORs) for Separate Smoking and Vaping Variable. Appendix 3. Reference list of 51 studies excluded after full text screening.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.