Abstract
Reversible addition−fragmentation chain-transfer (RAFT) polymerization is a commonly used polymerization methodology to generate synthetic polymers. The product of RAFT polymerization, i.e. RAFT polymers, have been widely employed in several biologically relevant areas, including drug delivery, biomedical imaging, and tissue engineering. In this protocol, we summarize a synthetic methodology to display an azide group at the chain end of a RAFT polymer, presenting a reactive site on the polymer terminus. This platform enables the click reaction between azide-terminated polymers and alkyne-containing molecules, providing a broadly applicable scaffold for chemical and bioconjugation reactions on RAFT polymers. We also highlight the applications of these azide-terminated RAFT polymers in fluorophore labeling and promoting the organelle targeting capability.
Basic Protocol 1: Synthesis of the azide derivatives of chain transfer agent and radical initiator
Basic Protocol 2: Installation of an azide group on the α-end of RAFT polymers
Alternate Protocol: Installation of an azide group on the ω-end of RAFT polymers
Basic Protocol 3: Click reaction between azide-terminated RAFT polymers and alkyne derivatives
Keywords: RAFT polymerization, end-group modification, click reaction, conjugation, labeling
INTRODUCTION
Reversible addition−fragmentation chain-transfer (RAFT) polymerization is a controlled radical polymerization process (Chiefari et al., 1998). As one of the most commonly used techniques for polymer synthesis, RAFT polymerization generates synthetic polymers with technical advances, including controllable molecular weight with low dispersity (Đ) and capability of continued chain growth (i.e. living polymerization) (Moad et al., 2012; Perrier, 2017). In addition, the structure of RAFT polymers can be conveniently tuned with a versatility that matches the broad needs of materials science (Boyer et al., 2009; Perrier et al., 2005; Wei et al., 2017). For example, in the biomaterials field, RAFT polymers have been widely employed as carriers for therapeutic delivery (Jiang, Cui, Mager, et al., 2019; Jiang, Cui, Prasad, et al., 2019; Jiang & Thayumanavan, 2020a, 2020b; Ryu et al., 2010), imaging probes for diagnostics (Y. Li et al., 2012; Munkhbat et al., 2019; Thurecht et al., 2010), and artificial extracellular matrices for tissue engineering (Dong et al., 2015; Duque Sánchez et al., 2016).
From the structural design perspective, decorating RAFT polymers with azide groups extends the structural variations of RAFT polymers. By incorporating click reactions (Jewett et al., 2010; Kolb et al., 2001), azide-reactive species such as alkyne-containing molecules can be readily attached to azide-containing RAFT polymers (Golas et al., 2010; Sumerlin et al., 2010). Azide groups can be installed on either the side chains or the polymeric chain end of RAFT polymers. For the side-chain installation, the azide-containing polymers can be achieved by co-polymerizing with azide-containing monomers (Chen et al., 2010; G. Li et al., 2009), or post-functionalizing reactive side chains on a polymer (Sun et al., 2012). The azide distribution within the resultant polymers is generally stochastic in polymers that contain multiple side chain functionalities. In contrast, the end-group approach installs the azide group on a polymer chain with precise location and number, i.e. one azide on the chain end (Gondi et al., 2007; Jiang, He, et al., 2019; Quémener et al., 2006).
While both the approaches are applicable to obtain azide-containing RAFT polymers, we will herein summarize a methodology to functionalize the RAFT polymer chain end with an azide group. In detail, this protocol will first describe the synthesis of the azide derivatives of two essential reactants for RAFT polymerization: the chain transfer agent and the radical initiator (Basic Protocol 1). Next, the protocol will present the installation of an azide group on the α-end (Basic Protocol 2) or the ω-end (Alternate Protocol) of a RAFT polymer, respectively. Finally, the protocol will highlight the click reaction between azide-terminated RAFT polymers and alkyne derivatives (Basic Protocol 3).
STRATEGIC PLANNING
A general planning on RAFT polymerization includes the choice of radical initiator, chain transfer agent, and monomers. To understand the workflow on selecting these components, the mechanism as well as the product analysis of RAFT polymerization are briefly introduced. The rationale of how azide-terminus is incorporated in RAFT polymers is provided alongside.
Mechanism of RAFT polymerization.
The mechanism of RAFT polymerization guides the design of azide-terminated RAFT polymers (Figure 1) (Barner-Kowollik et al., 2003; Moad, Rizzardo, et al., 2005; Moad et al., 2008). The basic reactants for RAFT polymerization include a radical initiator, a chain transfer agent (CTA, also called RAFT agent), and monomer(s). During the RAFT polymerization, radical initiators provide the free-radical source to start the process. The resultant free-radical reacts with one monomer and forms a propagating radical, followed by repeatedly reacting with another monomer to proceed the chain growth. In the presence of CTA, such a propagating radical adds to the CTA to initiate the reversible RAFT pre-equilibrium, producing a polymeric CTA and a new radical. While the newly generated radical is available to afford another propagating radical, the polymeric CTA participates in the main RAFT equilibrium by reacting with the existing propagating radicals, eventually resulting in the desired RAFT polymerization product. Note that in an on-going RAFT polymerization, undesired bimolecular termination occurs among the radical species, leading to the formation of “dead” polymers as minor products.
Figure 1.
Proposed mechanism of RAFT polymerization. Adapted with permission (York et al., 2008). Copyright 2008, Elsevier.
Living and dead polymers.
In RAFT polymerization, an effective CTA warrants that the rate of addition/fragmentation equilibrium is faster than the chain propagation process, ensuring that less than one monomer is consumed within each activation cycle (Keddie et al., 2012). Therefore, the CTA plays an important role in determining the degree of polymerization (DP), offering a control over the molecular weight of RAFT polymers (Perrier, 2017). Meanwhile, the involvement of CTA maintains the “livingness” of the desired polymerization product, i.e. RAFT polymers are capable of further extension with monomers. For example, dithioesters are a representative class of CTAs, in which the substituent groups on each side of the thioester bond are referred as the R and Z group, respectively (Chiefari et al., 2003). As the RAFT polymerization proceeds with the addition of monomer units, the main RAFT product maintains the R group on the α-end and the thiocarbonylthio group (containing Z group) on the ω-end (Moad, Chong, et al., 2005). The polymers with the thiocarbonylthio group at the ω-end are considered as living polymers, whereas the ones without such an end-group are considered as dead polymers (Figure 2).
Figure 2.
Products of RAFT polymerization. Although dead chains are minorities among the products, their possible structures are not limited to the ones illustrated.
Product analysis of RAFT polymerization.
With the thiocarbonylthio group at the ω-end, the living polymer products from RAFT polymerization can be divided into two species, depending on the difference in the α-end (Keddie, 2014). The R group in these living polymers originates either from the radical initiator or the CTA. For the dead polymers, the number of chains directly correlates to the number of radicals initially introduced in the system by the decomposition of the radical initiator (Pereira et al., 2018; Perrier, 2017). Therefore, to reduce the percentage of dead polymers within the polymerization products, the feed amount of radical initiator is typically maintained lower than the CTA (Moad, Rizzardo, et al., 2005; Moad et al., 2009). Consequently, the chains with CTA-originated R group are the major species in living products. Meanwhile, the dead polymers account for a relatively small portion within the polymerization products, however, cannot be avoided (Keddie, 2014; Perrier et al., 2005). Overall, the ratio between living and dead polymer products can also be predicted, primarily based on the molar ratio between the CTA and the radicals that are generated by the radical initiator (Keddie, 2014; Perrier, 2017). The protocol herein is designed to display an azide group on each of the living polymeric chain.
BASIC PROTOCOL 1
Synthesis of the azide derivatives of chain transfer agent and radical initiator
To obtain an azide moiety on the α-end of RAFT polymers, the azide is incorporated into the chain transfer agent (CTA) and the radical initiator. Because the origin of the α-end in living polymers comes from either the CTA or the initiator, the products will share the same α-end structure if both the CTA and the initiator generate identical radicals during the reaction. Here, we add 3-azido-1-propanol to 4-cyano-4-(thiobenzoylthio)pentanoic acid (CTPA, a CTA) and 4,4’-azobis(4-cyanovaleric acid) (ACVA, a radical-initiator) to obtain Az-CTPA and Az-ACVA (Figure 3; (Jiang, He, et al., 2019).
Figure 3.
Chemical structure of the azide-derivatives of chain transfer agent (Az-CTPA) and radical initiator (Az-ACVA). Adapted with permission (Jiang, He, et al., 2019). Copyright 2019, American Chemical Society.
Materials
3-Azido-1-propanol (e.g., Sigma-Aldrich, cat. no. 776130)
4,4’-Azobis(4-cyanovaleric acid) (ACVA, e.g., Sigma-Aldrich, cat. no. 11590)
4-Cyano-4-(thiobenzoylthio)pentanoic acid (e.g., Sigma-Aldrich, cat. no. 722995)
4-(Dimethylamino)pyridine (DMAP, e.g., Sigma-Aldrich, cat. no. 107700)
Methylene chloride (DCM, e.g., Fisher Scientific, cat. no. D37–20)
Tetrahydrofuran (THF, e.g., Fisher Scientific, cat. no. S271–1)
EDC hydrochloride (EDC·HCl, e.g., Chem-Impex, cat. no. 00050)
Chloroform (CHCl3, e.g., Fisher Scientific, cat. no. C607SK-4)
Ethyl acetate (EtOAc, e.g., Fisher Scientific, cat. no. E145–20)
Potassium permanganate (KMnO4, e.g., Fisher Scientific, cat. no. P279–500)
Sodium hydroxide (NaOH, e.g., Fisher Scientific, cat. no. S318–100)
KMnO4-TLC stain solution [Dissolve 1 g KMnO4 and 2 g NaOH in 100 mL deionized water. Keep in an amber glass bottle (e.g., Fisher Scientific, cat. no. FB02911433A)]
n-Hexanes (e.g., Fisher Scientific, cat. no. H292–20)
Sodium chloride (NaCl, e.g., Fisher Scientific, cat. no. S271–1)
Sodium sulfate, anhydrous (Na2SO4, e.g., Fisher Scientific, cat. no. S421–1)
Cotton (e.g., Fisher Scientific, cat. no. 22–456-880)
Sand (e.g., Fisher Scientific, cat. no. S25–500)
Silica gel (e.g., Sorbent Technologies, cat. no. 30930M)
Deuterated NMR solvents
Round-bottom flasks (e.g., Synthware Glass, cat. no. F304250)
Septum stopper (e.g., Fisher Scientific, cat. no. FB57875)
Magnetic stir bar, egg-shaped (e.g., Fisher Scientific, cat. no. 14–512-120)
Disposable syringes, all-plastic (e.g., Fisher Scientific, cat. no. 03–377-23)
Hypodermic needles (e.g., BD, cat. no. 305196)
Balloons (e.g., Fisher Scientific, cat. no. S04187)
Glass scintillation vials (e.g., Fisher Scientific, cat. no. 03–337-4)
Ultrasonic bath (e.g., Branson, cat. no. CPX-952–219R)
Stirring hotplate (e.g., Fisher Scientific, cat. no. SP88857200)
Foam Dewar flask (e.g., Chemglass, cat. no. CG-1590-F800)
Glass thin-layer chromatography (TLC) plate (e.g., Millipore-Sigma, cat. no. 1057210001)
TLC developing tank (e.g., Synthware Glass, cat. no. T216105; Fisher Scientific, cat. no. FB02911458A)
Microcapillary pipettes (e.g., Sigma-Aldrich, cat. no. Z543241)
Separatory funnel (e.g., Synthware Glass, cat. no. F472250)
Erlenmeyer flask (e.g., Synthware Glass, cat. no. F664125)
Filter funnel (e.g., Synthware Glass, cat. no. F752450M)
Rotary evaporator, joint clips, circulating chiller, and diaphragm pump
Chromatography column (e.g., Chemglass, cat. no. CG-1202–26)
Flow-control adapters (e.g., Chemglass, cat. no. CG-1201–09)
Vacuum pump (e.g., Fisher Scientific, cat. no. 01–184-204)
Nuclear magnetic resonance (NMR) spectrometer
Electrospray ionization mass spectrometer (ESI-MS)
Synthesis of Az-ACVA, an azide-derivative of 4,4’-azobis(4-cyanovaleric acid)
- Dissolve ACVA (1 equiv.), 3-azido-1-propanol (3 equiv.), and DMAP (0.8 equiv.) in a mixture of DCM/THF (v/v = 1:1, 14 mL per mmol of ACVA). Place the mixture in a round-bottom flask pre-loaded with a magnetic stir bar. Seal the flask with a septum stopper. Attach a balloon to the top of the stopper through a hypodermic needle to connect the inner space of the balloon and the flask.
- The overall solvent volume should not exceed the half-maximal capacity of the round-bottom flask. The balloon does not need to be inflated with any extra gas. It serves as a reservoir to accommodate the pressure change within the round-bottom flask during the reaction. (b) A suggested scale for the reaction is to start the synthesis with 2 g (7.1 mmol) ACVA.
Prepare an ice water bath in a Dewar flask and place the Dewar on top of a stirring plate. Dip the round-bottom flask into the ice bath. Start stirring the mixture.
Suspend EDC·HCl (2.4 equiv.) in DCM (one-tenth of the volume of DCM/THF mixture above) with sonication for 2~3 minutes. Add the suspension dropwise into the reaction mixture through a plastic syringe via a hypodermic needle.
-
Keep stirring the mixture in the ice bath for another 3 hours. Remove the Dewar flask and allow the mixture to warm up to room temperature. Keep stirring the mixture for another hour.
More ice may need to be supplied into the ice bath during the initial 3 hours to maintain the temperature.
-
Monitor the reaction progress using thin-layer chromatography (TLC) until the reaction is completed.
The optimal mobile phase for the TLC analysis of this reaction is CHCl3–EtOAc (v/v = 94:6). The spots on the TLC plate require staining with KMnO4-TLC stain solution. ACVA should be completely consumed at the 4-hour time point.
-
6. Transfer the mixture into a separatory funnel and wash the mixture three times with saturated NaCl solution. Collect the organic layer.
Because the density of DCM is higher than the density of H2O, the organic layer will be below the aqueous layer after the phase separation.
-
Mix the organic layer with anhydrous Na2SO4 to remove the water residue within the mixture. Filter the solid with a filter funnel and collect the organic solution in a round-bottom flask.
The Na2SO4 (white solid) should be added with a spatula in small portions. After each addition, swirl the solution to disperse the white solid and allow it to settle for a few minutes. Repeat this process until fine granule-like white solid is visible in the solution.
-
Evaporate the organic solvent in vacuo using a rotary evaporator.
To prevent bumping when using the rotary evaporator, the volume of the mixture should not exceed the half-maximal capacity of the round-bottom flask.
-
Purify the concentrated crude product by column chromatography.
The mobile phase (or eluent) for this reaction has been optimized as CHCl3–EtOAc (v/v = 94:6). When using the wet packing method to prepare the silica gel for the column, use the same eluent to disperse the silica gel. See the “Internet Resources” section on how to pack a silica gel column.
-
After TLC analysis, collect and combine the fractions that contain the desired product. Evaporate the solvent in vacuo with a rotary evaporator.
Az-ACVA is a colorless oil. The solvent residue may still remain if the rotary evaporator lacks efficiency. If so, the organic solvent can be removed by attaching to a vacuum pump.
- Characterize the product with 1H NMR and ESI-MS. Calculate the yield of the reaction.
- An example of the 1H NMR chemical shift assignments for Az-ACVA: 1H NMR (400 MHz, CDCl3) δ 4.21 (tdd, J = 6.1, 3.8, 1.8 Hz, 4H, -OCH2-), 3.40 (td, J = 6.6, 2.9 Hz, 4H, -CH2N3), 2.60 – 2.30 (m, 8H, -CH2CH2COO-), 1.93 (pd, J = 6.4, 2.7 Hz, 4H, -CH2-), 1.73 (s, 3H, -CH3), 1.68 (s, 3H, -CH3). (b) For Az-ACVA, the theoretical ESI-MS result is 469.20, representing the sodium adduct of Az-ACVA ([M+Na]+). (c) If the synthesis is properly conducted, the percent yield of the reaction is ~89%.
Store the compound under inert atmosphere (Ar or N2) at −20 °C.
Synthesis of Az-CTPA, an azide-derivative of 4-cyano-4-(thiobenzoylthio)pentanoic acid
The synthesis of Az-CTPA follows a similar procedure as above, with slight modifications in the reactants and the mobile phase for TLC and column chromatography (Gondi et al., 2007; Jiang, He, et al., 2019).
- Dissolve 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid (1 equiv.), 3-azido-1-propanol (5 equiv.), and DMAP (0.05 equiv.) in DCM [17.5 mL per mmol of 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid]. Place the mixture in a round-bottom flask pre-loaded with a magnetic stir bar. Seal the flask with a septum stopper. Attach a balloon to the top of the stopper through a hypodermic needle to connect the inner space of the balloon and the flask.
- The overall solvent volume should not exceed the half-maximal capacity of the round-bottom flask. The balloon does not need to be inflated with any extra gas. It serves as a reservoir to accommodate the pressure change within the round-bottom flask during the reaction. b) A suggested scale for the reaction is to start the synthesis with 0.96 g (3.42 mmol) 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid.
Prepare an ice water bath in a Dewar flask and place the Dewar on top of a stirring plate. Dip the round-bottom flask into the ice bath. Start stirring the mixture.
Suspend EDC·HCl (2.9 equiv.) in DCM (one-tenth of the volume of DCM above) with sonication for 2~3 minutes. Add the suspension dropwise into the reaction mixture through a plastic syringe via a hypodermic needle.
-
Keep stirring the mixture in the ice bath for another 2 hours. Remove the Dewar flask and allow the mixture to warm up to room temperature. Keep stirring the mixture overnight.
More ice may need to be supplied into the ice bath during the initial 2 hours to maintain the temperature.
-
Monitor the reaction progress using thin-layer chromatography (TLC) until the reaction is completed.
The optimal mobile phase for the TLC analysis of this reaction is n-hexanes–EtOAc (v/v = 5:1). 4-Cyano-4-(phenylcarbonothioylthio)pentanoic acid should be completely consumed overnight.
-
Transfer the mixture into a separatory funnel and wash the mixture three times with saturated NaCl solution. Collect the organic layer.
Because the density of DCM is higher than the density of H2O, the organic layer will be below the aqueous layer after the phase separation.
-
Mix the organic layer with anhydrous Na2SO4 to remove the water residue within the mixture. Filter the solid with a filter funnel and collect the organic solution in a round-bottom flask.
The Na2SO4 (white solid) should be added with a spatula in small portions. After each addition, swirl the solution to disperse the white solid and allow it to settle for a few minutes. Repeat this process until fine granule-like white solid is visible in the solution.
-
Evaporate the organic solvent in vacuo using a rotary evaporator.
To prevent bumping when using the rotary evaporator, the volume of the mixture should not exceed the half-maximal capacity of the round-bottom flask.
-
Purify the concentrated crude product by column chromatography.
The mobile phase (or eluent) for this reaction has been optimized as n-hexanes–EtOAc (v/v = 5:1). When using the wet packing method to prepare the silica gel for the column, use the same eluent to disperse the silica gel. See the “Internet Resources” section on how to pack a silica gel column.
-
After TLC analysis, collect and combine the fractions that contain the desired product. Evaporate the solvent in vacuo with a rotary evaporator.
Az-CTPA is a dark-red oil. The solvent residue may still remain if the rotary evaporator lacks efficiency. If so, the organic solvent can be removed by attaching to a vacuum pump.
- Characterize the product with 1H NMR and ESI-MS. Calculate the yield of the reaction.
- An example of the 1H NMR chemical shift assignments for Az-CTPA: 1H NMR (400 MHz, CDCl3) δ 7.93 – 7.88 (m, 2H, Ar), 7.60 – 7.54 (m, 1H, Ar), 7.43 – 7.37 (m, 2H, Ar), 4.21 (t, J = 6.2 Hz, 2H,-OCH2-), 3.41 (t, J = 6.6 Hz, 2H, -CH2N3), 2.74 – 2.58 (m, 3H, -CH2CH2COO-), 2.49 – 2.38 (m, 1H, -CH2COO-), 1.97 – 1.88 (m, 5H, -CH2- and -CH3). (b) If the synthesis is properly conducted, the percent yield of the reaction is ~70%.
Store the compound under inert atmosphere (Ar or N2) at −20 °C.
BASIC PROTOCOL 2
Installation of an azide group on the α-end of RAFT polymers
This protocol prepares RAFT polymers with an azide group installed on the α-end using the CTA (Az-CTPA) and the radical-initiator (Az-ACVA) available from Basic Protocol 1. Chemical modifications on the R group (Figure 1, Step ii) of CTPA do not affect its fragmentation ability during RAFT polymerization (Keddie et al., 2012); therefore, any monomer that is compatible with CTPA is also compatible with Az-CTPA. Typically used monomers are methacrylates and methacrylamides. The desired product of this step is an azide-terminated RAFT polymer, consisting of a C-C bond-based backbone and repeated side chains (RM) of interest (Figure 4).
Figure 4.
(a) Installation of an azide group on the α-end of polymers through RAFT polymerization. (b) An example of RAFT polymerization to generate azide-terminated homopolymers. (c) A list of compatible methacrylate monomers for Basic Protocol 2 that have been reported.
Materials
Az-ACVA (product of Basic Protocol 1)
Az-CTPA (product of Basic Protocol 1)
Monomer (e.g., methacrylates)
Tetrahydrofuran, anhydrous (THF, e.g., Sigma-Aldrich, cat. no. 900636)
2,2,2-Trifluoroethanol (TFE, e.g., Sigma-Aldrich, cat. no. T63002)
Liquid nitrogen
Methylene chloride (DCM, e.g., Fisher Scientific, cat. no. D37–20)
Acetone (CHCl3, e.g., Fisher Scientific, cat. no. A18–20)
Methanol (MeOH, e.g., Fisher Scientific, cat. no. A412–4)
Deuterated NMR solvents
Schlenk tube (e.g., Synthware Glass, cat. no. F891410)
Glass stopper (e.g., DWK Life Sciences, cat. no. 850500–1420)
High-vacuum silicone grease (e.g., Dow Corning, cat. no. 1597418)
PTFE seal tape (e.g., Fisher Scientific, cat. no. 15–078-260)
Parafilm (e.g., Fisher Scientific, cat. no. 13–374-12)
Magnetic stir bar (e.g., Fisher Scientific, cat. no. 14–513-63)
Silicone oil (e.g., Alfa Aesar, cat. no. A12728–36)
Glass Pasteur pipets (e.g., Fisher Scientific, cat. no. 13–678-20C)
Pipet filling bulbs (e.g., Fisher Scientific, cat. no. 15–000-506)
Stirring hotplate (e.g., Fisher Scientific, cat. no. SP88857200)
Foam Dewar flask (e.g., Chemglass, cat. no. CG-1590-F800)
Double vacuum manifold (i.e. Schlenk line, e.g., Synthware Glass, cat. no. M360004)
Inert gas cylinders (e.g., Ar or N2)
Vacuum pump (e.g., Fisher Scientific, cat. no. 01–184-204)
Plastic beaker (e.g., Fisher Scientific, cat. no. 02–591-10H)
Dialysis membrane tubing (e.g., Spectrum Chemical, cat. no. 132104)
Dialysis clips (e.g., Fisher Scientific, cat. no. PI68011)
Hypodermic needles (e.g., BD, cat. no. 305196)
Nuclear magnetic resonance (NMR) spectrometer
Gel permeation chromatography (GPC) instrument
Infrared spectrometer (IR)
Synthesis of RAFT polymers with an azide group installed on the α-end
-
Calculate the equiv. of monomers and the volume of solvent for the polymerization.
-
The equiv. of monomers depends on the target molecular weight of the polymer (Mn). A simplified method for the calculation follows. For homopolymers, if the molecular weight of the monomer is Mm, the equiv. of the monomer can be determined as Mn/Mm. For random copolymers that contain two or more different repeating units, a similar calculation can be applied after obtaining the average molecular weight of all monomers. Such an average molecular weight is the weighted arithmetic mean of all monomers, based on the target molar ratio of each monomer in the product. For example, when a RAFT polymerization involves two different monomers the equiv. of monomer A and monomer B can be determined as:
Equiv. of monomer A = a · Mn / (a · MmA + b · MmB); Equiv. of monomer B = b · Mn / (a · MmA + b · MmB), where the targeted molar ratio between A and B is a:b in the copolymer; MmA is the molecular weight of monomer A and MmB is the molecular weight of monomer B).
-
The solvent volume is suggested to be:
2 mL·g−1 × the sum of the weight of all reactants.
The rate of polymerization can be adjusted by varying the concentration of the reactants.
-
-
Dissolve Az-ACVA (0.2 equiv.), Az-CTPA (1 equiv.), and methacrylate monomer(s) (pre-calculated equiv.) in a suitable solvent. Place the mixture in a Schlenk tube pre-loaded with a magnetic stir bar.
The solvent should completely dissolve all the reactants. THF dissolves both Az-ACVA and Az-CTPA, and monomers with moderate polarity, e.g., pyridyl disulfide ethyl methacrylate, polyethylene glycol methyl ether methacrylate (Mn ~500). THF should either be freshly distilled or taken from a new ampule. For charged monomers (e.g., 3-sulfopropyl methacrylate potassium salt, 2-methacryloyloxyethyl phosphorylcholine), TFE is suggested as the solvent.
-
Seal the Schlenk tube with a greased glass stopper. Apply vacuum grease to the stopcock of the side arm.
Complete sealing of the tube is critical for properly conducting the polymerization. PTFE seal tape or parafilm can be wrapped on the glassware joint for further protection.
-
Fill a Dewar flask with liquid nitrogen and place the Dewar on top of a stirring plate. Set up the vacuum pump and the inert gas supply to the Schlenk line. Connect the side arm of the Schlenk tube to the Schlenk line.
CAUTION: Running the reaction in regular glass vials instead of a Schlenk tube is strongly discouraged due to safety concerns.
-
Dip the Schlenk tube into the liquid nitrogen-filled Dewar flask. Once the reaction mixture is completely frozen, keep the tube in liquid nitrogen and apply vacuum to the Schlenk tube for 10 minutes. Next, turn off the vacuum connection through the stopcock of the Schlenk tube. Remove the Schlenk tube from liquid nitrogen and let the mixture thaw completely. Repeat such a freeze-pump-thaw cycle for another two to three times.
The freeze-pump-thaw cycle is an efficient method to remove the oxygen within the Schlenk tube. Dipping the Schlenk tube in water can accelerate the thawing process.
-
After the third or fourth freeze-pump-thaw cycle, apply inert gas (Ar or N2) into the Schlenk tube and turn off the stopcock.
To ensure the sealing of the Schlenk tube, both the stopcock and the side arm end can be wrapped with parafilm.
-
Dip the completely sealed Schlenk tube into a pre-heated oil bath (~60 °C) on top of a stirring hotplate. Stir the mixture for 20 hours.
For polymers targeting at the molecular weight of less than 50 kDa, 20-hour is typically an ample amount of time for polymerization in our experience. Different monomers with varied reactivities (Mayo et al., 1950), as well as different target molecular weights of final polymers may require optimization on the reaction time (Konkolewicz et al., 2008).
-
Quench the polymerization by dipping the Schlenk tube into liquid nitrogen. Once the mixture is completely frozen, expose the mixture to air by opening the Schlenk tube.
Terminating all the chain growth at the same time by immersing the mixture in liquid nitrogen is helpful in generating a narrow dispersity value for the polymers.
- Dilute the resultant mixture to reduce its viscosity. Dialyze the mixture against a solvent that dissolves both the reactants and the products.
- For example, both the RAFT polymer shown in Figure 5a (PEG-PDS-N3) and its monomeric reactants are soluble in DCM. Therefore, DCM is an ideal dialysis solvent to purify PEG-PDS-N3. (b) The molecular weight cut-off (MWCO) for the dialysis tubing needs to be properly chosen to remove the unreacted small molecules from the mixture. The dialysis tubing should be carefully sealed with the dialysis clips to prevent leakage. A mixed solvent can be applied for dialysis to efficiently purify the polymers. The dialysis solvent should be changed two or three times within 24 hours.
-
Collect the purified solution from the dialysis tubing and evaporate the solvent.
Two options are recommended for drying: (a) put solution in a large enough flask that is it less than 1/3 full and dry on a rotovap. If the solvent of the polymer solution exceeds one-third of the flask/vial, using a rotary evaporator may cause bumping and lead to substantial loss of the product. (b) add the sample to a flask and dry the sample with air flow until a majority of the solvent is evaporated, followed by evaporation in vacuo overnight. During the evaporation in vacuo, an extra step facilitates the removal of solvent: If the product is in solid form, grind the polymers into powder; If the product is oily and viscous, swirl the product with a hypodermic needle.
-
Characterize the polymers with NMR, gel permeation chromatography (GPC), and infrared spectroscopy (IR). See sample data in Figure 5.
1H NMR demonstrates the structural assignments and the molar ratio of the repeating units. GPC provides the average molecular weight and the molecular weight distribution (dispersity, Đ) of the polymeric products. IR is required to identify the presence of the azide group within the polymer (~2100 cm−1).
Figure 5.
(a) 1H NMR spectrum of an azide-terminated (α-end) random copolymer (PEG-PDS-N3) synthesized via RAFT polymerization. (b) Comparison of FTIR spectra demonstrates the appearance of azide groups (~2100 cm−1) in PEG-PDS-N3, compared to a structural analog without azide group on the α-end (PEG-PDS). Adapted with permission (Jiang, He, et al., 2019). Copyright 2019, American Chemical Society. (c) Gel permeation chromatography (GPC) curve of PEG-PDS-N3 using THF as the eluent. Molecular weight is versus poly(methylmethacrylate) standards.
ALTERNATE PROTOCOL
Installation of an azide group on the ω-end of RAFT polymers
As an alternate protocol for Basic Protocol 2, when it is not feasible to use Az-CTPA as the CTA for polymerization, this step demonstrates the procedures to install an azide group on the ω-end of RAFT polymers. This post-modification approach utilizes an excess amount of Az-ACVA, generating radicals to replace the thiocarbonylthio group on the ω-end of the polymer (Figure 6). One example is the synthesis of block copolymers using a commercially available macro-CTA, and such macro-CTAs generally do not have an azide group on the α-end (Jiang, Liu, He, Ribbe, et al., 2020). Note that for RAFT polymers, the Z group attached to the thiocarbonylthio is designed to activate the C=S moiety upon the addition of propagating radicals, as well as determine the fragmentation rate of the intermediate radical in each RAFT equilibrium (Moad et al., 2006; Perrier, 2017; Willcock et al., 2010). Therefore, the polymer chain will not be able to extend (i.e. dead polymer) through radical polymerization after the replacement on the ω-end.
Figure 6.
(a) Installation of an azide group on the ω-end of RAFT polymers. (b) An example of replacing the thiocarbonylthio group on a block copolymer with radicals generated by Az-ACVA. Adapted with permission (Jiang, Liu, He, Ribbe, et al., 2020). Copyright 2020, American Chemical Society.
Synthesis of RAFT polymers with an azide group installed on the ω-end
- Calculate the amount of Az-ACVA (20 equiv. vs. the number of polymer chains) and the volume of solvent for the polymerization.
- The reaction stoichiometry is calculated based on the number of polymer chains, i.e., the number of ω-end groups. The calculation of polymeric chain number can be simplified as (the weight of the polymer to be modified / the number average molecular weight of the polymer). The number average molecular weight of a polymer can be obtained by GPC, defined as the total weight of polymer being divided by the total number of polymer chains (Izunobi et al., 2011). (b) The overall solvent volume is suggested to be 3.6 mL per mmol of Az-ACVA.
-
Dissolve Az-ACVA and RAFT polymers in a suitable solvent. Place the mixture in a Schlenk tube pre-loaded with a magnetic stir bar.
The solvent should completely dissolve both the Az-ACVA and the polymers (e.g., THF or TFE).
-
Seal the Schlenk tube with a greased glass stopper. Apply vacuum grease to the stopcock of the side arm.
Complete sealing of the tube is critical for properly conducting the polymerization. PTFE seal tape or parafilm can be wrapped on the glassware joint for further protection.
-
Fill a Dewar flask with liquid nitrogen and place the Dewar flask on top of a stirring plate. Set up the vacuum pump and the inert gas supply to the Schlenk line. Connect the side arm of the Schlenk tube to the Schlenk line.
CAUTION: Running the reaction in regular glass vials instead of a Schlenk tube is strongly discouraged due to safety concerns.
-
Dip the Schlenk tube into the liquid nitrogen-filled Dewar flask. Once the reaction mixture is completely frozen, keep the tube in liquid nitrogen and apply vacuum to the Schlenk tube for 10 minutes. Next, turn off the vacuum connection through the stopcock of the Schlenk tube. Remove the Schlenk tube from liquid nitrogen and let the mixture thaw completely. Repeat such a freeze-pump-thaw cycle for another two to three times.
The freeze-pump-thaw cycle is an efficient method to remove the oxygen within the Schlenk tube. Dipping the Schlenk tube in water can accelerate the thawing process.
-
After the third or fourth freeze-pump-thaw cycle, apply inert gas (Ar or N2) into the Schlenk tube and turn off the stopcock.
To ensure the sealing of the Schlenk tube, both the stopcock and the side arm end can be wrapped with parafilm.
-
Dip the completely sealed Schlenk tube into a pre-heated oil bath (~60 °C) on top of a stirring hotplate. Stir the mixture for 4 hours.
For RAFT polymers with benzyl thiocarbonylthio as the ω-end-group, 4-hour is typically an ample amount of time for the end-group modification. Different end-groups with varied reactivities may require optimization on the reaction time.
-
Quench the polymerization by dipping the Schlenk tube into liquid nitrogen. Once the mixture is completely frozen, expose the mixture to air by opening the Schlenk tube.
Terminating all the chain growth at the same time by immersing the mixture in liquid nitrogen is helpful in generating a narrow dispersity value for the polymers.
-
Dilute the resultant mixture to reduce its viscosity. Dialyze the mixture against a solvent that dissolves both the reactants and the products.
The molecular weight cut-off (MWCO) for the dialysis tubing needs to be properly chosen to remove the unreacted small molecules from the mixture. The dialysis tubing should be carefully sealed with the dialysis clips to prevent leakage. A mixed solvent can be applied for dialysis to efficiently purify the polymers. The dialysis solvent should be changed two or three times within 24 hours.
-
Collect the purified solution from the dialysis tubing and evaporate the solvent.
Two options are recommended for drying: a) put solution in a large enough flask that is it less than 1/3 full and dry on a rotovap. If the solvent of the polymer solution exceeds one-third of the flask/vial, using a rotary evaporator may cause bumping and lead to substantial loss of the product. b) add the sample to a flask and dry the sample with air flow until a majority of the solvent is evaporated, followed by evaporation in vacuo overnight. During the evaporation in vacuo, an extra step facilitates the removal of solvent: If the product is in solid form, grind the polymers into powder; If the product is oily and viscous, swirl the product with a hypodermic needle.
- Characterize the polymers with NMR, GPC, and IR (see sample data in Figure 7).
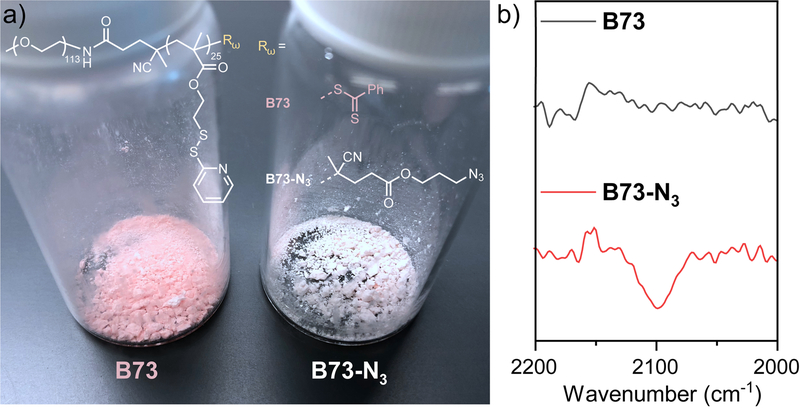
- Note that ineffective radical trapping and undesired termination may happen with the Alternate Protocol, resulting in an unwanted shoulder peak in the GPC result (Chong et al., 2007). IR is required to identify the presence of the azide group within the polymer (~2100 cm−1). (b) For polymers originally with the benzyl thiocarbonylthio end-group, the modification with azide group on the ω-end will lead to the color fading of the polymers, typically from pink to white/colorless. Disappearance of benzyl group from the 1H NMR will provide further structural confirmation. (c) If it is feasible to involve Az-CTPA in a polymerization reaction, Basic Protocol 2 is recommended for the direct synthesis of azide-terminated RAFT polymers.Ineffective radical trapping may occur in the Alternate Protocol, causing the formation of unwanted termination product (dimer) (Chong et al., 2007).
Figure 7.
(a) Replacing the thiocarbonylthio group with an azide group at the ω-end of a RAFT polymer (B73). The color of polymer changes from pink (B73) to white (B73-N3) upon the removal of the thiocarbonylthio group. (b) FTIR spectra comparison between B73 and B73-N3, demonstrating the presence of the azide group (~2100 cm−1) in B73-N3. Adapted with permission (Jiang, Liu, He, Ribbe, et al., 2020). Copyright 2020, American Chemical Society.
BASIC PROTOCOL 3
Click reaction between the azide-terminated RAFT polymers and alkyne derivatives
This step describes the procedures for the click reaction between alkyne derivatives and the azide-terminated RAFT polymers obtained from Basic Protocol 2 (or Alternate Protocol). Click reaction allows a straightforward and efficient functionalization on the azide-end of RAFT polymers (Golas et al., 2010; Gregory et al., 2012). In detail, the protocol demonstrates an example of the copper-free conjugation between an azide-terminated random copolymer and a DBCO derivative of cyanine-3 (DBCO-Cy3). Labeling polymers with fluorescent molecules such as Cy3 is frequently used to visualize polymers in biological applications. This protocol is applicable for the conjugation of various alkynes that are compatible with organic solvents (Figure 8). The desired product of this step is a RAFT polymer with the alkyne of interest functionalized on the azide-containing end of the polymer.
Figure 8.
Click reaction between azide-terminated polymers and DBCO derivatives. DBCO, dibenzocyclooctyne.
Materials
Azide-terminated RAFT polymers (product of Protocol 2 or Alternate Protocol)
Dibenzocyclooctyne (DBCO) derivatives (e.g., Lumiprobe, cat. no. C10F0)
2,2,2-Trifluoroethanol (TFE, e.g., Sigma-Aldrich, cat. no. T63002)
Methylene chloride (DCM, e.g., Fisher Scientific, cat. no. D37–20)
Acetone (CHCl3, e.g., Fisher Scientific, cat. no. A18–20)
Chloroform (CHCl3, e.g., Fisher Scientific, cat. no. C607SK-4)
Methanol (MeOH, e.g., Fisher Scientific, cat. no. A412–4)
Cotton (e.g., Fisher Scientific, cat. no. 22–456-880)
Deuterated NMR solvents
Glass scintillation vials (e.g., Fisher Scientific, cat. no. 03–337-4)
Magnetic stir bar (e.g., Fisher Scientific, cat. no. 14–513-63)
Glass Pasteur pipets (e.g., Fisher Scientific, cat. no. 13–678-20C)
Pipet filling bulbs (e.g., Fisher Scientific, cat. no. 15–000-506)
Stirring hotplate (e.g., Fisher Scientific, cat. no. SP88857200)
Lipophilic hydrophilic gel filtration matrix (e.g., Sorbent Technologies, cat. no. 801009)
Chromatography column (e.g., Chemglass, cat. no. CG-1186–10)
Plastic beaker (e.g., Fisher Scientific, cat. no. 02–591-10H)
Vacuum pump (e.g., Fisher Scientific, cat. no. 01–184-204)
Copper-free click reaction between the azide-terminated RAFT polymers and DBCO-Cy3
- Calculate the amount of DBCO-Cy3 (1.05 equiv. vs. the number of polymer chains) for the click reaction.
- The reaction stoichiometry is calculated based on the number of polymer chains, i.e., the number of azide groups. The calculation of the azide group can be simplified as (the weight of the polymer to be conjugated / the number average molecular weight of the polymer). (b) The overall solvent volume is suggested to be 40 μL per mg of polymers. (c) A suggested scale for the reaction is to start the synthesis with 20 mg azide-terminated polymers, followed by the calculation of the number of polymer chains.
-
Dissolve azide-terminated polymers and DBCO-Cy3 in a suitable solvent such as TFE. Place the mixture in a glass vial pre-loaded with a magnetic stir bar.
The solvent should completely dissolve both the reactants. TFE is commonly used.
Stir the mixture in the capped vial on a stirring plate for 24 hours at room temperature.
Evaporate the solvent.
-
Disperse the gel filtration matrix for size exclusion chromatography (SEC) in the eluent at least one hour before packing the matrix in the column. Transfer the matrix-containing slurry into a glass column and wait until the matrix is completely settled by gravity.
The eluent for size exclusion chromatography is not suggested to contain more than 75 vol% halogenated solvent. For example, after reacting PEG-PDS-N3 (Figure 5a) with DBCO-Cy3, the suggested eluent for the SEC purification is a mixture of CHCl3/MeOH (v:v = 3:1).
-
Purify the product by the freshly packed SEC (see sample data in Figure 9a) and collect the fractions that contain high-molecular-weight products.
Run the eluent through the column only by gravity. For example, when conjugating DBCO-Cy3 (903 Da) to azide-terminated polymers that are ~10 kDa (number average molecular weight), the Cy3-conjugated polymers should run faster as the high-molecular-weight fraction, while the free DBCO-Cy3 runs slower as the low-molecular-weight fraction.
-
Collect the fractions that contain the desired products. Evaporate the solvent.
CAUTION: Purifying the polymers with centrifugal filters may not completely remove the free fluorophores.
-
Characterize the resultant polymers with NMR, GPC, and IR (see sample data in Figure 9b).
Both the 1H NMR and the GPC results of the product should demonstrate negligible changes compared to the azide-terminated polymeric reactant. IR is required to identify the disappearance of the azide group from the product (~2100 cm−1).
Figure 9.
(a) A representative purification process for Cy3-conjugated RAFT polymers by size-exclusion chromatography. Reprinted with permission (Jiang, He, et al., 2019). Copyright 2019, American Chemical Society. (b) Comparison of FTIR spectra for azide-terminated polymers before and after reacting with a DBCO derivative, demonstrating the consumption of azide groups from the polymers after the conjugation with DBCO derivatives. Reprinted with permission (Jiang, Liu, He, Yadava, et al., 2020). Copyright 2020, American Chemical Society.
COMMENTARY
BACKGROUND INFORMATION
RAFT polymerization was first introduced by the Commonwealth Scientific and Industrial Research Organization (CSIRO) in 1998 (Chiefari et al., 1998). The emergence of RAFT polymerization has initiated numerous research efforts in polymer chemistry and materials science. Modifications on the end-group of RAFT polymers result in precisely located reactive sites, readily for the post-functionalization of polymers. The first post-functionalization approaches for displaying an azide-terminal on the α-end of RAFT polymers used azide-modified dithioester-based CTAs for polymerization (Gondi et al., 2007; Quémener et al., 2006). Our later report further optimized the approach by incorporating azide-modified radical-initiators in the RAFT polymerization (Jiang, He, et al., 2019). To display an azide group on the ω-end of RAFT polymers, previous reports on radical-induced removal of the thiocarbonylthio end-group laid the foundation for the end-group modification with azide-based radicals (Chong et al., 2007; Willcock et al., 2010). The azide-derivative of 4,4’-azobis(4-cyanovaleric acid) has been successfully utilized to install an azide terminus on the ω-end of RAFT polymers (Gauthier et al., 2009; Jiang, Liu, He, Ribbe, et al., 2020).
CRITICAL PARAMETERS & TROUBLESHOOTING
The critical parameters within the protocols and the corresponding troubleshooting guide are listed in Table 1.
Table 1.
Troubleshooting guide for common problems encountered from the protocols, with possible causes and their potential solutions
| Problem | Possible cause | Solution |
|---|---|---|
| Polymerization does not occur | Poor airtightness of Schlenk tube | Homogeneously apply vacuum grease on the glassware joint and the stopcock |
| Tightly wrap the potential leaking sites on the Schlenk tube with parafilm | ||
| Impurities in the radical initiator or monomers | Purify the radical initiator using column chromatography | |
| Remove existing stabilizers within the monomers by a neutral alumina column | ||
| Insufficient vacuum level provided during freeze-pump-thaw cycles | Homogenously apply vacuum grease on the stopcocks of the Schlenk line | |
| Replace aged rubber tubing for glassware connections | ||
| Slowly rotate the stopcock when switching vacuum to inert gas after freeze-pump-thaw cycles, preventing air suction through the air bubbler | ||
| Change vacuum pump oil | ||
| Fix or replace vacuum pump if vacuum gauge does not show sufficient vacuum level after changing pump oil | ||
| Polymer products do not reach the target molecular weight | Insufficient reaction time | Run time-dependent GPC measurement during the reaction to evaluate the degree of polymerization and obtain the optimal reaction time length |
| Inefficient monomer conversion | Optimize the concentration of reactants | |
| Targeted degree of polymerization is too high | Generally, target the product with less than 50 kDa average molecular weight | |
| Polymer products exceed the target molecular weight, or gelation occurs after polymerization reaction | Loss of chain transfer agents during mixture transfer | Divide the calculated solvent into three portions, take two portions to rinse the weighing containers (e.g., glass vials) and combine the solutions together |
| Impurities in chain transfer agents or radical initiators | Store the reagents in an inert atmosphere (purging Ar or N2) at −20 °C after purification with column chromatography | |
| Dissolve the reactants via sonication | Use a vortex mixer or shake to dissolve the reactants | |
| Use a glass vial with a rubber septum stopper as the reaction vessel instead of a Schlenk tube | Use a Schlenk tube/flask that is dedicated for polymerization | |
| Insufficient stirring during polymerization due to high viscosity | Optimize (e.g., slightly increase) the solvent volume for polymerization | |
| Inefficient purification of click-reaction product | Low bed volume for size exclusion chromatography | Increase the amount of gel filtration matrix and choose a suitable chromatography column to increase the bed volume |
| Overloaded sample for purification in size exclusion chromatography | Divide the crude samples into smaller portions for purification (the sample volume should be in the range of 1~2% of the total bed volume) | |
| Bubbles in the column | Extend the equilibration time of gel filtration matrix in the eluent before packing the column; Make sure the eluent does not dry out during purification | |
| Gel filtration matrix floating in the column | Make sure the eluent does not contain more than 75 vol% halogenated solvent | |
| Fast flow rate during purification | Switch to an automated size exclusion chromatography with precisely controlled flow rate (e.g., ÄKTA pure chromatography system) |
UNDERSTANDING RESULTS
With the described protocols, one can expect the synthesis of azide-terminated RAFT polymers. The variations on the choice of monomers, the sequence of the polymers, and the conjugated moieties with the azide group ensure a large scope of biological applications for this platform. Herein we will briefly summarize a few recent examples from our group that utilize these azide-terminated RAFT polymers.
Fluorescently labeled RAFT polymers.
Visualization of RAFT polymers during biological applications is regularly assisted by the attachment of fluorescent molecules. The platform of azide-terminated polymers allows a facile variation on the choice of fluorophores for labeling. Because only one fluorophore is covalently attached to each polymer chain, compared to multi-fluorophore labeling (e.g., stochastic labeling on polymer side chains), the conjugation reaction on the end-group leads to minimal changes to the overall polymer structure. We recently demonstrated the labeling of these azide-terminated polymers by different fluorophores (Figure 10a), presenting a wide range of emission signature for the labeled RAFT polymers (Jiang, Liu, He, Yadava, et al., 2020). These azide-terminated polymers are expected to be adaptable to multi-color imaging systems, especially when it is difficult to tune the fluorescence properties of other objects in the same system of interest.
Figure 10.
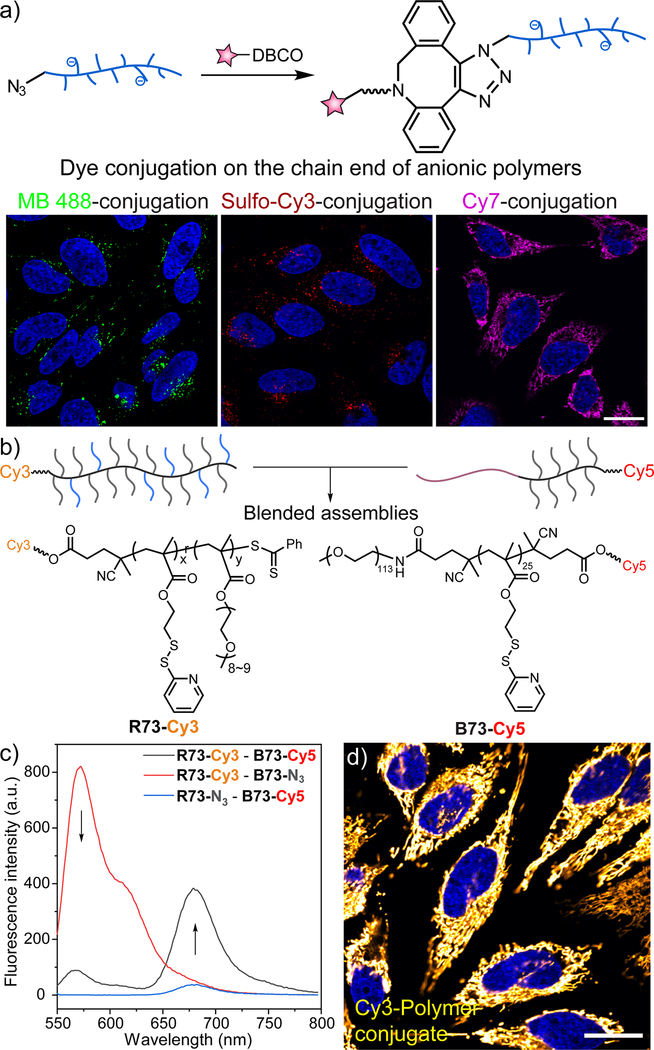
(a) Labeling azide-terminated anionic polymers with different fluorophores, resulting in the intracellular visualization of these fluorescently labeled polymers. Adapted with permission (Jiang, Liu, He, Yadava, et al., 2020). Copyright 2020, American Chemical Society. (b~c) Blending a pair of fluorescently labeled amphiphilic polymers enables the evaluation of their proximity through FRET. Adapted with permission (Jiang, Liu, He, Ribbe, et al., 2020). Copyright 2020, American Chemical Society. (d) Conjugation between azide-terminated anionic polymers and Cy3 leads to an efficient mitochondrial targeting capability of RAFT polymers. In all the confocal microscopy images, scale bars represent 20 μm. Adapted with permission (Jiang, Liu, He, Yadava, et al., 2020). Copyright 2020, American Chemical Society.
Toolkit for evaluating macromolecular interactions.
The end-group labeling on these azide-terminated polymers avoids the potential complications that can be brought up by multi-fluorophore labeling. Such an advantage is especially beneficial for amphiphilic polymers. As a majority of fluorescent molecules are lipophilic, multi-fluorophore labeling could possibly affect the hydrophilic-lipophilic balance (HLB) of an amphiphilic polymer. The HLB of amphiphilic polymers has been shown to be a determining factor for the self-assembly properties of these polymers in aqueous solutions (Hirai et al., 2016). Azide-terminated polymers are therefore suitable for the labeling of amphiphilic polymers. We have utilized such a platform to validate the interaction between amphiphilic random copolymers and block copolymers (Figure 10b,c) (Jiang, Liu, He, Ribbe, et al., 2020). Because Cy3/Cy5 is a pair of fluorophores known for fluorescence resonance energy transfer (FRET) (Rowland et al., 2015), the donor Cy3 was conjugated on the α-end of random copolymers while the acceptor Cy5 was conjugated on the ω-end of block copolymers. The occurrence of FRET between the labeled polymers demonstrated that the pair of polymers are in proximity within the blended polymeric assemblies.
Organelle-targeting of RAFT polymers.
The conjugation on the azide-terminal also provides opportunities to understand the effect of organelle-targeting moieties when conjugated to polymers. Organelle-targeting moieties facilitate the development of imaging and drug delivery (Louzoun-Zada et al., 2019; Xu et al., 2016). For example, different analogs of delocalized lipophilic cations have been utilized to direct therapeutics to the mitochondria, as well as expand the library of imaging probes for the mitochondria (Johnson et al., 1980; Smiley et al., 1991; Zielonka et al., 2017). Meanwhile, a benzyl boronate moiety has also been shown as an targeting signal to transport proteins into the cell nucleus (Tang et al., 2017). The azide-terminated polymer can integrate such organelle-targeting moieties through the end-group conjugation. Recently, we conjugated cyanine derivatives to a series of azide-terminated RAFT polymers, revealing that only one Cy-molecule for each polymer chain is sufficient to target anionic polymers to the mitochondria (Figure 10d) (Jiang, Liu, He, Yadava, et al., 2020). The study also implies that because certain organelle-targeting moieties are fluorophores (e.g., cyanine and rhodamine derivatives), when incorporating such moieties into a delivery system for cellular uptake evaluation, extra validation needs to be conducted before drawing conclusions (Bujold et al., 2018).
TIME CONSIDERATIONS
Depending on the user’s expertise with synthetic chemistry, the total time to synthesize and characterize Az-CTPA and Az-ACVA is approximately one week. RAFT polymerization or end-group modification can be accomplished in one day and purified with another day. Click reactions on the azide-terminal can be completed in one day, with the purification taking another day.
SIGNIFICANCE STATEMENT.
This protocol describes a synthetic methodology to display an azide group at the chain end of a reversible addition−fragmentation chain-transfer (RAFT) polymer, presenting a reactive site on the polymer terminus as a broadly applicable scaffold for bioconjugation reactions on RAFT polymers.
ACKNOWLEDGEMENTS
We thank the NIGMS of the National Institutes of Health (GM-136395) for support.
Footnotes
INTERNET RESOURCES
Detailed instructions on the preparation of a silica gel column: https://www.jove.com/t/10217/column-chromatography.
LITERATURE CITED
- Barner-Kowollik C, Davis TP, Heuts JPA, Stenzel MH, Vana P, & Whittaker M (2003). RAFTing down under: Tales of missing radicals, fancy architectures, and mysterious holes. Journal of Polymer Science Part A: Polymer Chemistry, 41(3), 365–375. doi: 10.1002/pola.10567 [DOI] [Google Scholar]
- Boyer C, Bulmus V, Davis TP, Ladmiral V, Liu J, & Perrier S (2009). Bioapplications of RAFT Polymerization. Chemical Reviews, 109(11), 5402–5436. doi: 10.1021/cr9001403 [DOI] [PubMed] [Google Scholar]
- Bujold KE, Lacroix A, & Sleiman HF (2018). DNA Nanostructures at the Interface with Biology. Chem, 4(3), 495–521. doi: 10.1016/j.chempr.2018.02.005 [DOI] [Google Scholar]
- Chen X, & Ayres N (2010). Synthesis of Novel Polymer/Urea Peptoid Conjugates Using RAFT Polymerization. Macromolecules, 43(3), 1341–1348. doi: 10.1021/ma902427m [DOI] [Google Scholar]
- Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, … Thang SH (1998). Living Free-Radical Polymerization by Reversible Addition−Fragmentation Chain Transfer: The RAFT Process. Macromolecules, 31(16), 5559–5562. doi: 10.1021/ma9804951 [DOI] [Google Scholar]
- Chiefari J, Mayadunne RTA, Moad CL, Moad G, Rizzardo E, Postma A, & Thang SH (2003). Thiocarbonylthio Compounds (SC(Z)S−R) in Free Radical Polymerization with Reversible Addition-Fragmentation Chain Transfer (RAFT Polymerization). Effect of the Activating Group Z. Macromolecules, 36(7), 2273–2283. doi: 10.1021/ma020883+ [DOI] [Google Scholar]
- Chong YK, Moad G, Rizzardo E, & Thang SH (2007). Thiocarbonylthio End Group Removal from RAFT-Synthesized Polymers by Radical-Induced Reduction. Macromolecules, 40(13), 4446–4455. doi: 10.1021/ma062919u [DOI] [Google Scholar]
- Dong Y, Qin Y, Dubaa M, Killion J, Gao Y, Zhao T, … Wang W (2015). A rapid crosslinking injectable hydrogel for stem cell delivery, from multifunctional hyperbranched polymers via RAFT homopolymerization of PEGDA. Polymer Chemistry, 6(34), 6182–6192. doi: 10.1039/C5PY00678C [DOI] [Google Scholar]
- Duque Sánchez L, Brack N, Postma A, Pigram PJ, & Meagher L (2016). Surface modification of electrospun fibres for biomedical applications: A focus on radical polymerization methods. Biomaterials, 106, 24–45. doi: 10.1016/j.biomaterials.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Gauthier MA, Gibson MI, & Klok H-A (2009). Synthesis of Functional Polymers by Post-Polymerization Modification. Angewandte Chemie International Edition, 48(1), 48–58. doi: 10.1002/anie.200801951 [DOI] [PubMed] [Google Scholar]
- Golas PL, & Matyjaszewski K (2010). Marrying click chemistry with polymerization: expanding the scope of polymeric materials. Chemical Society Reviews, 39(4), 1338–1354. doi: 10.1039/b901978m [DOI] [PubMed] [Google Scholar]
- Gondi SR, Vogt AP, & Sumerlin BS (2007). Versatile Pathway to Functional Telechelics via RAFT Polymerization and Click Chemistry. Macromolecules, 40(3), 474–481. doi: 10.1021/ma061959v [DOI] [Google Scholar]
- Gregory A, & Stenzel MH (2012). Complex polymer architectures via RAFT polymerization: From fundamental process to extending the scope using click chemistry and nature’s building blocks. Progress in Polymer Science, 37(1), 38–105. doi: 10.1016/j.progpolymsci.2011.08.004 [DOI] [Google Scholar]
- Hirai Y, Terashima T, Takenaka M, & Sawamoto M (2016). Precision Self-Assembly of Amphiphilic Random Copolymers into Uniform and Self-Sorting Nanocompartments in Water. Macromolecules, 49(14), 5084–5091. doi: 10.1021/acs.macromol.6b01085 [DOI] [Google Scholar]
- Izunobi JU, & Higginbotham CL (2011). Polymer Molecular Weight Analysis by 1H NMR Spectroscopy. Journal of Chemical Education, 88(8), 1098–1104. doi: 10.1021/ed100461v [DOI] [Google Scholar]
- Jewett JC, Sletten EM, & Bertozzi CR (2010). Rapid Cu-Free Click Chemistry with Readily Synthesized Biarylazacyclooctynones. Journal of the American Chemical Society, 132(11), 3688–3690. doi: 10.1021/ja100014q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Cui W, Mager J, & Thayumanavan S (2019). Postfunctionalization of Noncationic RNA–Polymer Complexes for RNA Delivery. Industrial & Engineering Chemistry Research, 58(17), 6982–6991. doi: 10.1021/acs.iecr.9b00666 [DOI] [Google Scholar]
- Jiang Z, Cui W, Prasad P, Touve MA, Gianneschi NC, Mager J, & Thayumanavan S (2019). Bait-and-Switch Supramolecular Strategy To Generate Noncationic RNA–Polymer Complexes for RNA Delivery. Biomacromolecules, 20(1), 435–442. doi: 10.1021/acs.biomac.8b01321 [DOI] [PubMed] [Google Scholar]
- Jiang Z, He H, Liu H, & Thayumanavan S (2019). Cellular Uptake Evaluation of Amphiphilic Polymer Assemblies: Importance of Interplay between Pharmacological and Genetic Approaches. Biomacromolecules, 20(12), 4407–4418. doi: 10.1021/acs.biomac.9b01073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Liu H, He H, Ribbe AE, & Thayumanavan S (2020). Blended Assemblies of Amphiphilic Random and Block Copolymers for Tunable Encapsulation and Release of Hydrophobic Guest Molecules. Macromolecules, 53(7), 2713–2723. doi: 10.1021/acs.macromol.9b02595 [DOI] [Google Scholar]
- Jiang Z, Liu H, He H, Yadava N, Chambers JJ, & Thayumanavan S (2020). Anionic Polymers Promote Mitochondrial Targeting of Delocalized Lipophilic Cations. Bioconjugate Chemistry, 31(5), 1344–1353. doi: 10.1021/acs.bioconjchem.0c00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, & Thayumanavan S (2020a). Disulfide-Containing Macromolecules for Therapeutic Delivery. Israel Journal of Chemistry, 60(1–2), 132–139. doi: 10.1002/ijch.201900160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, & Thayumanavan S (2020b). Noncationic Material Design for Nucleic Acid Delivery. Advanced Therapeutics, 3(3), 1900206. doi: 10.1002/adtp.201900206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Walsh ML, & Chen LB (1980). Localization of mitochondria in living cells with rhodamine 123. Proceedings of the National Academy of Sciences, 77(2), 990–994. doi: 10.1073/pnas.77.2.990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keddie DJ (2014). A guide to the synthesis of block copolymers using reversible-addition fragmentation chain transfer (RAFT) polymerization. Chemical Society Reviews, 43(2), 496–505. doi: 10.1039/C3CS60290G [DOI] [PubMed] [Google Scholar]
- Keddie DJ, Moad G, Rizzardo E, & Thang SH (2012). RAFT Agent Design and Synthesis. Macromolecules, 45(13), 5321–5342. doi: 10.1021/ma300410v [DOI] [Google Scholar]
- Kolb HC, Finn MG, & Sharpless KB (2001). Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angewandte Chemie International Edition, 40(11), 2004–2021. doi: [DOI] [PubMed] [Google Scholar]
- Konkolewicz D, Hawkett BS, Gray-Weale A, & Perrier S (2008). RAFT Polymerization Kinetics: Combination of Apparently Conflicting Models. Macromolecules, 41(17), 6400–6412. doi: 10.1021/ma800388c [DOI] [Google Scholar]
- Li G, Zheng H, & Bai R (2009). A Facile Strategy for the Preparation of Azide Polymers via Room Temperature RAFT Polymerization by Redox Initiation. Macromolecular Rapid Communications, 30(6), 442–447. doi: 10.1002/marc.200800666 [DOI] [PubMed] [Google Scholar]
- Li Y, Beija M, Laurent S, Elst L. v., Muller RN, Duong HTT, … Boyer C (2012). Macromolecular Ligands for Gadolinium MRI Contrast Agents. Macromolecules, 45(10), 4196–4204. doi: 10.1021/ma300521c [DOI] [Google Scholar]
- Louzoun-Zada S, Jaber QZ, & Fridman M (2019). Guiding Drugs to Target-Harboring Organelles: Stretching Drug-Delivery to a Higher Level of Resolution. Angewandte Chemie International Edition, 58(44), 15584–15594. doi: 10.1002/anie.201906284 [DOI] [PubMed] [Google Scholar]
- Mayo FR, & Walling C (1950). Copolymerization. Chemical Reviews, 46(2), 191–287. doi: 10.1021/cr60144a001 [DOI] [PubMed] [Google Scholar]
- Moad G, Chong YK, Postma A, Rizzardo E, & Thang SH (2005). Advances in RAFT polymerization: the synthesis of polymers with defined end-groups. Polymer, 46(19), 8458–8468. doi: 10.1016/j.polymer.2004.12.061 [DOI] [Google Scholar]
- Moad G, Rizzardo E, & Thang SH (2005). Living Radical Polymerization by the RAFT Process. Australian Journal of Chemistry, 58(6), 379–410. [Google Scholar]
- Moad G, Rizzardo E, & Thang SH (2006). Living Radical Polymerization by the RAFT Process A First Update. Australian Journal of Chemistry, 59(10), 669–692. [Google Scholar]
- Moad G, Rizzardo E, & Thang SH (2008). Toward Living Radical Polymerization. Accounts of Chemical Research, 41(9), 1133–1142. doi: 10.1021/ar800075n [DOI] [PubMed] [Google Scholar]
- Moad G, Rizzardo E, & Thang SH (2009). Living Radical Polymerization by the RAFT Process A Second Update. Australian Journal of Chemistry, 62(11), 1402–1472. [Google Scholar]
- Moad G, Rizzardo E, & Thang SH (2012). Living Radical Polymerization by the RAFT Process – A Third Update. Australian Journal of Chemistry, 65(8), 985–1076. [Google Scholar]
- Munkhbat O, Canakci M, Zheng S, Hu W, Osborne B, Bogdanov AA, & Thayumanavan S (2019). 19F MRI of Polymer Nanogels Aided by Improved Segmental Mobility of Embedded Fluorine Moieties. Biomacromolecules, 20(2), 790–800. doi: 10.1021/acs.biomac.8b01383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira OS, Barros-Timmons A, & Trindade T (2018). Polymer@gold Nanoparticles Prepared via RAFT Polymerization for Opto-Biodetection. Polymers, 10(2). doi: 10.3390/polym10020189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier S (2017). 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules, 50(19), 7433–7447. doi: 10.1021/acs.macromol.7b00767 [DOI] [Google Scholar]
- Perrier S, & Takolpuckdee P (2005). Macromolecular design via reversible addition–fragmentation chain transfer (RAFT)/xanthates (MADIX) polymerization. Journal of Polymer Science Part A: Polymer Chemistry, 43(22), 5347–5393. doi: 10.1002/pola.20986 [DOI] [Google Scholar]
- Quémener D, Davis TP, Barner-Kowollik C, & Stenzel MH (2006). RAFT and click chemistry: A versatile approach to well-defined block copolymers. Chemical Communications(48), 5051–5053. doi: 10.1039/B611224B [DOI] [PubMed] [Google Scholar]
- Rowland CE, Brown CW, Medintz IL, & Delehanty JB (2015). Intracellular FRET-based probes: a review. Methods and Applications in Fluorescence, 3(4), 042006. doi: 10.1088/2050-6120/3/4/042006 [DOI] [PubMed] [Google Scholar]
- Ryu J-H, Chacko RT, Jiwpanich S, Bickerton S, Babu RP, & Thayumanavan S (2010). Self-Cross-Linked Polymer Nanogels: A Versatile Nanoscopic Drug Delivery Platform. Journal of the American Chemical Society, 132(48), 17227–17235. doi: 10.1021/ja1069932 [DOI] [PubMed] [Google Scholar]
- Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, … Chen, L. B. (1991). Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proceedings of the National Academy of Sciences, 88(9), 3671–3675. doi: 10.1073/pnas.88.9.3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumerlin BS, & Vogt AP (2010). Macromolecular Engineering through Click Chemistry and Other Efficient Transformations. Macromolecules, 43(1), 1–13. doi: 10.1021/ma901447e [DOI] [Google Scholar]
- Sun Y, Chen Z, Puodziukynaite E, Jenkins DM, Reynolds JR, & Schanze KS (2012). Light Harvesting Arrays of Polypyridine Ruthenium(II) Chromophores Prepared by Reversible Addition–Fragmentation Chain Transfer Polymerization. Macromolecules, 45(6), 2632–2642. doi: 10.1021/ma202804u [DOI] [Google Scholar]
- Tang R, Wang M, Ray M, Jiang Y, Jiang Z, Xu Q, & Rotello VM (2017). Active Targeting of the Nucleus Using Nonpeptidic Boronate Tags. Journal of the American Chemical Society, 139(25), 8547–8551. doi: 10.1021/jacs.7b02801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurecht KJ, Blakey I, Peng H, Squires O, Hsu S, Alexander C, & Whittaker AK (2010). Functional Hyperbranched Polymers: Toward Targeted in Vivo 19F Magnetic Resonance Imaging Using Designed Macromolecules. Journal of the American Chemical Society, 132(15), 5336–5337. doi: 10.1021/ja100252y [DOI] [PubMed] [Google Scholar]
- Wei M, Gao Y, Li X, & Serpe MJ (2017). Stimuli-responsive polymers and their applications. Polymer Chemistry, 8(1), 127–143. doi: 10.1039/C6PY01585A [DOI] [Google Scholar]
- Willcock H, & O’Reilly RK (2010). End group removal and modification of RAFT polymers. Polymer Chemistry, 1(2), 149–157. doi: 10.1039/B9PY00340A [DOI] [Google Scholar]
- Xu W, Zeng Z, Jiang J-H, Chang Y-T, & Yuan L (2016). Discerning the Chemistry in Individual Organelles with Small-Molecule Fluorescent Probes. Angewandte Chemie International Edition, 55(44), 13658–13699. doi: 10.1002/anie.201510721 [DOI] [PubMed] [Google Scholar]
- York AW, Kirkland SE, & McCormick CL (2008). Advances in the synthesis of amphiphilic block copolymers via RAFT polymerization: Stimuli-responsive drug and gene delivery. Advanced Drug Delivery Reviews, 60(9), 1018–1036. doi: 10.1016/j.addr.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O, Vasquez-Vivar J, … Kalyanaraman B (2017). Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chemical Reviews, 117(15), 10043–10120. doi: 10.1021/acs.chemrev.7b00042 [DOI] [PMC free article] [PubMed] [Google Scholar]