Abstract
Background:
Alterations in major stress response systems are present during the immediate aftermath of trauma and may play a role in determining risk for developing posttraumatic stress disorder (PTSD). However, the dynamics and determinants of stress responses during this acute recovery phase, and their relevance for longitudinal clinical course and prognosis, have yet to be fully examined. The objectives of the present study were to characterize stress response and habituation patterns to repeated social stressors in women who recently experienced interpersonal trauma and to determine the extent to which these stress responses were associated with PTSD during prospective follow-up.
Method:
This longitudinal study examined salivary cortisol and alpha-amylase and heart rate (HR) responses to repeated stressors in 98 young women (ages 18 to 30). Participants included women who had experienced an incident of interpersonal trauma (i.e., physical and/or sexual assault) in the three months prior to their baseline assessment (n=58) and a comparison group of healthy, non-traumatized women (n=40). Women completed the Trier Social Stress Test (TSST), clinical interviews to evaluate posttraumatic stress symptom severity at the baseline assessment and again at 1-, 3-, and 6-month follow-ups.
Results:
Multilevel models revealed a pattern of robust initial cortisol TSST responses and habituation across successive TSSTs; alpha-amylase and HR responses showed no evidence of habituation across TSSTs. Among interpersonal trauma survivors, current PTSD status was associated with more pronounced cortisol responses to the first TSST. Survivors exhibited similarly blunted cortisol responses across follow-up TSSTs regardless of PTSD status, suggesting habituation of cortisol responses among survivors who developed PTSD. PTSD re-experiencing symptoms were uniquely associated with blunting of cortisol TSST responses.
Conclusion:
Findings suggest that PTSD as a diagnostic entity is meaningfully associated with cortisol responses to repeated social stressors. Social-evaluative threat is a salient form of danger for interpersonal trauma survivors. Identifying the determinants of cortisol (non)habituation to repeated social-evaluative threat among interpersonal trauma survivors could inform the development of early interventions for PTSD.
Keywords: PTSD, stress response, interpersonal trauma, cortisol, alpha-amylase, habituation
1. Introduction
Young women who experience interpersonal traumatic events, including physical or sexual assault, are at increased risk for developing posttraumatic stress disorder (PTSD) compared to those exposed to non-interpersonal traumatic events and to their male counterparts with interpersonal trauma (Shalev et al., 2019). Alterations in major stress response systems, including the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS), are common among individuals with chronic PTSD (Morris et al., 2012; Pole, 2007) and may contribute to higher risk among women (Olff et al., 2007). Theoretical models posit that HPA and SNS activity influence the development and maintenance of PTSD in recent trauma survivors (Liberzon and Abelson, 2016). However, the dynamics and determinants of stress responses in the immediate aftermath of trauma have yet to be fully examined.
Individuals with PTSD often exhibit dysregulated HPA function in the form of lower circulating cortisol levels and enhanced glucocorticoid negative feedback compared to non-traumatized controls (Yehuda, 2002). Lower circulating cortisol appears to distinguish these individuals from trauma-exposed individuals without PTSD, whereas enhanced HPA negative feedback does not (Morris et al., 2012). This work suggests diminished cortisol is the aspect of HPA function most closely linked to development of PTSD after trauma exposure and prospective studies further suggest that diminished cortisol secretion in the early aftermath of trauma exposure increases risk for PTSD (Morris et al., 2016). Social-evaluative threat is a salient form of danger for interpersonal trauma survivors. Psychosocial stress paradigms, such as the Trier Social Stress Test (TSST), complement pharmacologic/neuroendocrine challenges by probing the supra-hypothalamic circuits involved in HPA reactivity to social-evaluative threat (Kirschbaum et al., 1993). Studies of social-evaluative threat generally report lower HPA reactivity in individuals with PTSD compared to healthy controls (Metz et al., 2020; Pierce and Pritchard, 2016; Wichmann et al., 2017; Zaba et al., 2015; see also Simeon et al., 2007). TSST studies of trauma-exposed individuals without PTSD found blunted HPA reactivity compared to healthy controls (Elzinga et al., 2008; MacMillan et al., 2009).
Individuals with PTSD frequently exhibit altered SNS function in the form of elevated resting heart rate and heart rate reactivity to startling sounds and trauma cues (Pole, 2007). Salivary alpha-amylase secretion is considered to be a non-invasive indicator of SNS activity (Nater and Rohleder, 2009). Whereas healthy individuals typically show increases in salivary alpha-amylase levels immediately post-TSST (Gordis et al., 2006), these responses are blunted in women with a history of childhood trauma (Mielock et al., 2017).
One corollary of context-processing PTSD models (Liberzon and Abelson, 2016) is that individuals who have difficulty processing safety cues may fail to show normative habituation to repeated social stressors. Difficulty adjusting to repeated stressors can lead to allostatic load (McEwen, 2004) and contribute to sustained arousal and reactivity symptoms characteristic of PTSD. A pattern of reduced responses across repeated exposures to a particular stressor is generally considered adaptive (McEwen, 2004). However, other response patterns are possible, including increased responses (i.e., sensitization) and lack of habituation. Whereas healthy individuals typically exhibit HPA habituation across repeated psychosocial stressors, 30–40% show a lack of habituation (Gerra et al., 2001; Kirschbaum et al., 1995; Schommer et al., 2003) and 16% show sensitization effects (Wust et al., 2005). Notably, norepinephrine and epinephrine stress responses do not appear to habituate across repeated TSSTs (Schommer et al., 2003), which suggests that the HPA axis and SNS could serve different functions in adaptation to recurring stressors. Whether alterations in HPA, alpha-amylase, and/or HR reactivity emerge shortly after interpersonal trauma exposure and are associated with PTSD symptoms remains uncertain. Understanding the factors influencing non-habituation to social stressors could provide directions for secondary prevention research in recent interpersonal trauma survivors.
This study sought to evaluate HPA and SNS response patterns by measuring changes in cortisol, alpha-amylase, and heart rate stress reactivity and recovery patterns across repeated TSSTs in recent interpersonal trauma survivors. Additionally, we examined changes in cortisol and alpha-amylase levels for 90 minutes preceding each TSST, which may be considered an indicator of habituation to the laboratory environment and referred to as the ‘arrival index’ (Balodis et al., 2010). The goal of this study was to compare and contrast stress response patterns in trauma survivors with and without PTSD and healthy women. We anticipated that baseline cortisol reactivity would be stronger for trauma survivors with current PTSD compared to those without PTSD and healthy controls. We based this hypothesis on evidence for heightened cortisol reactivity to personalized trauma scripts in women with PTSD (Elzinga et al., 2003), which we expect would correspond more closely to the assessment of stress reactivity during the acute aftermath of trauma than TSST studies conducted long after trauma exposure. We further anticipated that cortisol reactivity would exhibit reduced/delayed habituation among trauma survivors with PTSD over follow-up compared to those without PTSD and healthy controls. This hypothesis was based on evidence that individuals with PTSD have difficulty processing contextual safety cues (Liberzon and Abelson, 2016), which should interfere with normative habituation to repeated social stressors. We anticipated dynamic changes in cortisol responses to repeated TSSTs but a relatively stable pattern of alpha-amylase and HR reactivity in both trauma survivors and healthy controls. This hypothesis was based on prior work showing habituation of salivary and plasma cortisol responses but a maintenance of noradrenergic reactivity to repeated TSSTs (Schommer et al., 2003). We also explored whether PTSD symptom clusters (i.e., re-experiencing, avoidance, hyperarousal) were differentially associated with stress response habituation in trauma survivors.
2. Method
2.1. Participants
Women (n=58) between ages 18 and 30 (inclusive) completed their baseline assessment within 90 days of experiencing an incident of interpersonal trauma (e.g., physical and/or sexual assault). Participants in the IPV group were excluded if they reported any of the following psychiatric conditions: current major depressive disorder (MDD) that preceded their index trauma; PTSD resulting from an index trauma that occurred prior to the 90-day window; current substance use disorder; or any history of bipolar or psychotic disorder. Diagnoses for mood, PTSD, substance use, and psychotic disorders were based on the Structured Clinical Interview (SCID) for DSM-IV (First et al., 2005); PTSD diagnoses were based on the Clinician Administered PTSD Scale (CAPS) for DSM-IV (Blake et al., 1995). In addition, participants were excluded if they reported serious health conditions known to influence HPA or SNS activity (e.g., Cushing’s or Addison’s Disease, hyperthyroidism), pregnancy, or current use of prescription (e.g., corticosteroids, antidepressant medication) or non-prescription drugs known to affect HPA or SNS activity. IPV survivors with past diagnoses of MDD or PTSD, exposure to traumatic events prior to their recent index event, or those using oral contraceptives, were not excluded due to concerns regarding recruitment feasibility and external validity. A non-traumatized comparison (NTC) group of women (n=40), between ages 18 and 30, was recruited with no lifetime history of interpersonal trauma, no potentially traumatic events in the past year, and no history of mood or anxiety disorders or PTSD. Men were excluded from this study due to evidence for sex differences in rates of PTSD and levels of neuroendocrine risk markers for PTSD (Olff et al., 2007; Shalev et al., 2019).
2.2. Protocol
Recruitment for IPV and NTC groups occurred through online advertisements and research participant registries. The IPV group also was recruited from a local hospital through a team of nurse practitioners providing medical legal exams to rape survivors as well as from local agencies coordinating services for survivors of domestic violence and sexual assault. Interpersonal violence/trauma (IPV) survivors were first screened over the phone for a criterion A event according to the Diagnostic and Statistical Manual for Mental Disorders, 4th Edition Text Revision (DSM-IV-TR) (American Psychiatric Association, 2000), which was later confirmed at baseline using the Life Events Checklist (LEC) (Gray et al., 2004). Screening and assessment measures were based on DSM-IV because data collection was initiated prior to the introduction of DSM-5 criteria and creation of validated DSM-5 assessment tools (recruitment was conducted from 2013 to 2019).
All participants completed assessments at baseline, and at 1-, 3-, and 6-month follow-up intervals. For the IPV group, the baseline assessment occurred within 90 days of index traumatic event. Each assessment included a first visit during which the CAPS interview (Blake et al., 1995) was administered along with mood and anxiety modules from the SCID (First et al., 2005), questionnaires administered via a secure web-based Research Electronic Data Capture (REDCap) platform (Harris et al., 2009), and a second visit during which participants completed a modified version of the TSST (Kirschbaum et al., 1993). The present study reports data on cortisol, alpha-amylase, and heart rate responses to the four TSSTs, along with a subset of demographic, life stress, cognitive, and clinical factors assessed via clinical interview and self-report measures (see below). All participants provided written informed consent following approval by the institutional review board.
2.3. Measures
Prior trauma exposure.
Exposure to childhood abuse and neglect was determined for both IPV and NTC groups using the 28-item, self-report Childhood Trauma Questionnaire (CTQ); individual items were rated according to frequency of exposure on a 5-point scale and a total score was computed (Bernstein et al., 1994). Coefficient alpha for the CTQ in this study was .87.
Depressive symptoms.
The 21-item, self-report Beck Depression Inventory-second edition (BDI-II) (Beck et al., 1996) was used to assess depression symptom severity. Coefficient alphas in the full sample ranged from .92 to .94.
Posttraumatic Stress Symptoms and Disorder.
Posttraumatic stress symptoms were determined at each assessment using the CAPS interview for DSM-IV (Blake et al., 1995). The CAPS-IV has excellent psychometric properties (Weathers et al., 2001) and was either administered by a licensed clinical psychologist (MCM) or by a research assistant under his supervision. The CAPS-IV interview was used to determine (1) overall posttraumatic stress symptom severity, (2) symptom cluster severity (i.e., re-experiencing, avoidance, hyperarousal), and (3) PTSD diagnostic status (using the ‘F1/I2’ rule) at each assessment (Weathers et al., 1999).
Trier Social Stress Test.
A modified TSST (Kirschbaum et al., 1993) was used to assess cortisol, alpha-amylase, and heart rate reactivity to a social stressor at the baseline assessment and again at 1-, 3-, and 6-month follow-ups. Participants were scheduled for each TSST between 3:00 and 7:00 pm to control for circadian variation in stress hormone levels. For each TSST, participants were instructed to sit quietly in a room for 90 minutes while they provided four saliva samples at 30-minute intervals. Participants were then provided instructions for the 5-minute speech task (first TSST topic = “applying for your ideal job”) and given 5 minutes to prepare. After the preparation period, participants were led into another room with an evaluator and video camera, given 5 minutes to complete the speech task, and were subsequently instructed to perform a 5-minute serial subtraction task. The topic of the speech task and starting number for the serial subtraction task differed for the TSST administered at each assessment to avoid the possibility that participants would give a rehearsed speech or memorize the correct sequence of numbers. Following each TSST, participants were asked to provide seven saliva samples at 10-minute intervals. Participants were debriefed regarding the procedure after their final TSST.
Cortisol and Alpha-Amylase Levels.
Salivary cortisol and alpha-amylase levels throughout the four TSSTs were determined by saliva samples collected using cotton swabs (Sarstedt Inc., Netwon, NC). Participants were instructed to refrain from brushing teeth, eating, drinking caffeine, or engaging in rigorous exercise within 30 minutes of arrival for their TSST visit. Participants were also instructed not to eat or drink anything other than water until the TSST visit was complete. Free cortisol levels were determined by commercial chemiluminescence immunoassay (Kirschbaum and Hellhammer, 1989) and alpha-amylase levels were determined by a quantitative enzyme kinetic method (Nater et al., 2007). Intra- and inter-assay coefficients of variation for these assays were below 6%.
Heart rate reactivity and recovery.
Heart rate (HR) was recorded every second using a Polar H7 Bluetooth chest-strap (Polar, Finland) paired with an Actigraph wrist watch. Recording started 30 minutes prior to the TSST and ended 60 minutes after the TSST. Mean HR was computed for a 20-minute pre-TSST resting period, the 10-minute TSST period, and a 20-minute post-TSST recovery period. A stress reactivity index was computed as the difference in mean HR from resting to TSST; stress recovery was computed as the difference in mean HR from TSST to recovery.
2.4. Data Analysis
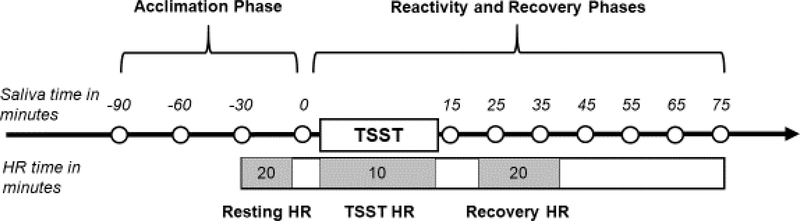
All variables were examined for distributional properties and cases were screened for univariate outliers. Missing cortisol and alpha-amylase data were handled using maximum-likelihood estimation. Three-level piecewise multilevel models (MLM) were specified using hierarchical linear models (HLM v. 8) to examine within-individual (level 1), between-TSST session (level 2), and between-individual (level 3) changes in cortisol and alpha-amylase responses (Raudenbush et al., 2019). The level 1 model examined linear change across four cortisol/alpha-amylase samples during the 90-minute acclimation to the laboratory environment, instantaneous rate of change in cortisol/alpha-amylase levels at the final pre-TSST sample, and quadratic change in cortisol/alpha-amylase levels from the final pre-TSST sample across the 7 post-TSST samples (see Figure 1 for saliva sampling timeline). A piecewise MLM approach was used to simultaneously model these three response indices (MacMillan et al., 2009; Morris et al., 2017). A typical response pattern would be reflected in declining cortisol levels from arrival until the start of the TSST (or acclimation), linear increases in cortisol levels reaching a peak between 20 and 40 minutes after TSST onset (or reactivity), followed by a deceleration in cortisol change resulting in a return to pre-TSST levels within 60 minutes (or recovery). Habituation would be reflected in a pattern of (1) flattening arrival index (due to lower initial levels presenting to the laboratory), (2) decreasing anticipatory reactivity, and/or (3) less quadratic change across repeated TSSTs. For HR analyses, MLMs were specified to examine between-TSST (level 1) and between-individual (level 2) changes in HR reactivity and recovery.
Figure 1.
TSST saliva and heart rate (HR) sampling timeline.
Preliminary analyses examined whether stress responses (cortisol, alpha-amylase, HR) changed across repeated TSSTs in the full sample. Preliminary analyses further examined whether age (at enrollment), childhood trauma exposure (assessed at baseline), and depressive symptoms (assessed across all time points) were associated with stress responses across TSSTs (i.e., main effects) and/or predicted changes in stress responses across TSSTs (i.e., interactive effects). To account for multiple testing of moderators in preliminary analyses (3 interactions for each stress response index), we used the Benjamini-Hochberg false discovery rate correction to control for the rate of Type I errors by adjusting the p-value based on the number of significant results in a family of tests (Benjamini & Hochberg, 1995). Primary analyses examined whether current diagnostic status at each TSST session (i.e., PTSD, IPV without PTSD, or NTC status) was associated with – and predicted changes in – stress responses across TSSTs. Within the IPV group, we further examined whether changes in overall posttraumatic symptom severity and/or symptom cluster severity were associated with – and predicted changes in – stress responses across TSSTs. All MLMs included TSST session as a moderator.
3. Results
3.1. Participant characteristics
Demographic and clinical characteristics for IPV and NTC groups are presented separately in Table 1. The groups did not differ with regard to age or ethnicity, but the IPV group reported fewer years of education and more racial diversity. MLMs revealed that more years of education was associated with more rapid cortisol acclimation (b=−.004, SE=.002, p=.032) and more blunted cortisol responses, as evident in lower anticipatory reactivity levels (b=−.017, SE=.007, p=.011) and quadratic change (b=.0002, SE=.0001, p=.042). Years of education were not, however, associated with any of the alpha-amylase or HR response indices. Additionally, the IPV group reported higher levels of depressive symptoms and greater exposure to childhood trauma at the baseline assessment. Among the 58 interpersonal trauma survivors, 15 (26%) met PTSD diagnostic criteria at baseline and 7 (12%) developed new onset PTSD over follow-up. The mean duration from index trauma to baseline assessment was 45.1 days (SD = 24.6 days). MLMs revealed that time elapsed since interpersonal trauma was not significantly associated with any of the cortisol, alpha-amylase, or HR response indices.
Table 1.
Descriptive and clinical characteristics at baseline assessment of women with recent interpersonal trauma (IPV) and a non-traumatized comparison (NTC) group.
| Mean (SD) or n (%) | IPV vs. NTC | ||
|---|---|---|---|
| IPV (n = 58) |
NTC (n = 40) |
t or χ2 |
|
| Sociodemographic | |||
| Age (years) | 23.8 (3.3) | 24.1 (3.3) | 0.41 |
| Race | 6.75* | ||
| White/Caucasian | 33 (57%) | 32 (80%) | |
| Black/African American | 19 (33%) | 5 (13%) | |
| Asian | 6 (10%) | 3 (7%) | |
| Hispanic | 4 (7%) | 0 (0%) | 1.32 |
| Education (years) | 14.7 (1.9) | 16.7 (2.3) | 4.54*** |
| Marital Status | 9.69* | ||
| Single | 51 (88%) | 32 (80%) | |
| Married | 1 (2%) | 6 (10%) | |
| Engaged | 2 (3%) | 0 (0%) | |
| Living with partner | 4 (7%) | 1 (3%) | |
| Divorced | 0 (0%) | 1 (3%) | |
| Depressive symptoms (BDI-II) | 15.6 (11.1) | 5.0 (5.8) | 5.95*** |
| Childhood trauma (CTQ) | 48.5 (18.5) | 30.9 (6.4) | 6.39*** |
| Prior traumatic events (LEC) | 7.3 (3.3) | - | - |
| CAPS-IV total severity | 49.3 (24.6) | - | - |
| CAPS-IV re-experiencing severity | 16.4 (7.1) | - | - |
| CAPS-IV avoidance severity | 17.5 (10.2) | - | - |
| CAPS-IV hyperarousal severity | 15.7 (10.4) | - | - |
p < .001
p < .01
p <.05.
Note: IPV = interpersonal violence; BDI-II = Beck Depression Inventory second edition; CTQ = Childhood Trauma Questionnaire; LEC = Life Events Checklist; CAPS-IV = Clinician Administered PTSD Scale for DSM-IV.
3.2. Cortisol, alpha-amylase, and HR responses to repeated psychosocial stressors
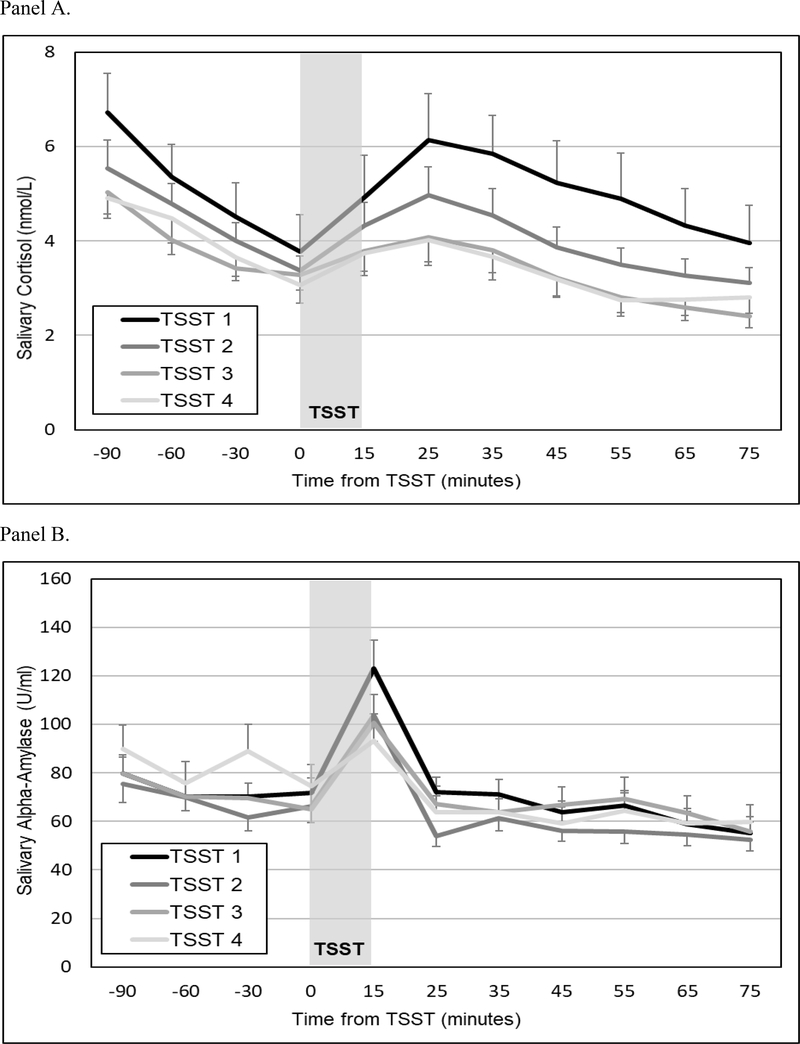
Mean salivary cortisol levels for all participants (± SEM) throughout the four TSSTs are presented in Figure 2 (Panel A). MLMs indicated that across all participants and TSSTs, cortisol levels declined significantly during the acclimation phase (b=−.023, SE=.002, p<.001). The TSSTs triggered robust cortisol responses, as evident in increasing cortisol levels at the start of the tasks (b=.058, SE=.006, p<.001) and significant decelerations during the recovery period (b=−.001, SE=.0001, p<.001). Moreover, cortisol responses exhibited habituation across successive TSSTs, as evident in flattening arrival indices (b=.004, SE=.002, p=.023) and diminishing anticipatory reactivity (b=−.026, SE=.006, p<.001) and quadratic change (b=.0003, SE=.0001, p<.001).
Figure 2.
Mean (± SEM) salivary cortisol (Panel A) and alpha-amylase (Panel B) concentrations for all participants across four TSSTs.
Mean salivary alpha-amylase levels for all participants (± SEM) throughout the four TSSTs are presented in Figure 2 (Panel B). MLMs indicated that across all participants and TSSTs, alpha-amylase levels exhibited quadratic change (b=−.004, SE=.001, p=.002) but no evidence of acclimation (b=.016, SE=.031, p=.613) or anticipatory reactivity (b=−.017, SE=.107, p=.870). Moreover, alpha-amylase responses showed no evidence of habituation across TSSTs, as evident in non-significant changes in acclimation (b=−.047, SE=.028, p=.094), anticipatory reactivity (b=−.131, SE=.096, p=.173), or quadratic change (b=.002, SE=.001, p=.057).
MLMs revealed that across all participants, neither HR reactivity (b=−1.455, SE=.928, p=.119) nor HR recovery (b=−.569, SE=.668, p=.395) changed across successive TSSTs.
3.3. Diagnostic group differences in responses and habituation to repeated psychosocial stressors
Diagnostic groups differed significantly in their acclimation of pre-TSST cortisol levels (b=.008, SE=.003, p=.009), anticipatory cortisol reactivity (b=−.040, SE=.010, p<.001), and quadratic change in cortisol levels (b=.0006, SE=.0001, p<.001).1 IPV participants with current PTSD exhibited slower acclimation of pre-TSST cortisol levels and more blunted cortisol TSST responses compared to their counterparts without PTSD. IPV participants without PTSD, in turn, exhibited slower acclimation of pre-TSST cortisol levels and more blunted cortisol TSST responses compared to the NTC group. Diagnostic group status was not significantly associated with any of the alpha-amylase or HR response indices.
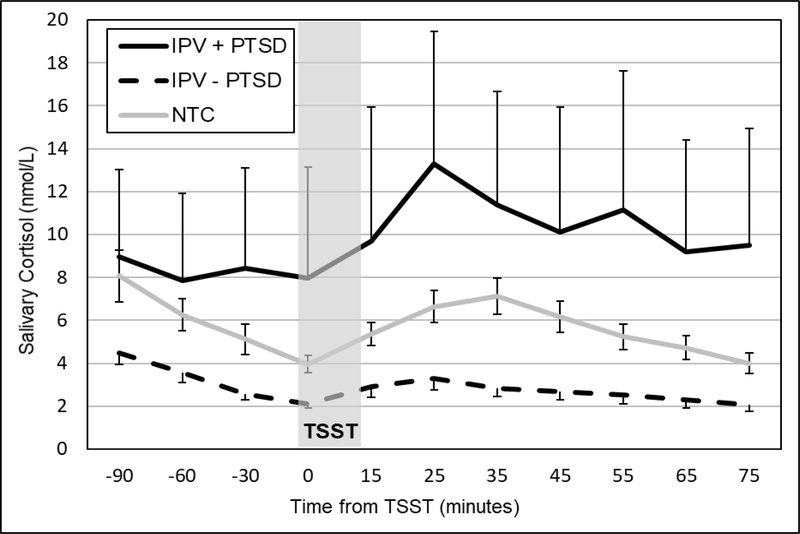
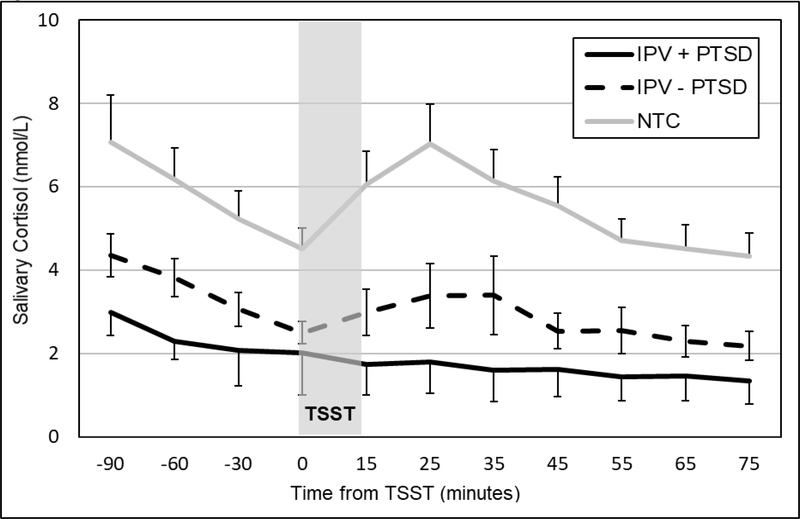
Cortisol – but not alpha-amylase or HR – responses exhibited significant changes across TSSTs. Therefore, diagnostic group influences on cortisol response habituation were examined through interactions with TSST session. Diagnostic groups differed significantly in their patterns of changes in anticipatory reactivity (b=−.073, SE=.026, p=.006) and quadratic change (b=.0009, SE=.0003, p=.011) across TSSTs.2 Whereas IPV survivors with current PTSD exhibited heightened cortisol responses at baseline (Figure 3) and a progressive blunting of cortisol responses at 1-month (Figure 4) and across subsequent TSSTs, IPV survivors without current PTSD exhibited blunted cortisol responses to all TSSTs. The NTC group exhibited less robust cortisol responses to the first TSST and slower habituation across TSSTs compared to those with current PTSD group. Supplemental Figures 1–2 show diagnostic group differences in cortisol responses for TSSTs 3 and 4.
Figure 3.
Mean (± SEM) salivary cortisol concentrations for women with (IPV+PTSD) and without (IPV – PTSD) and non-traumatic control (NTC) groups at the baseline TSST.
Figure 4.
Mean (± SEM) salivary cortisol concentrations for women with (IPV+PTSD) and without (IPV – PTSD) and non-traumatic control (NTC) groups at the second TSST (1-month follow-up).
3.4. Moderators of physiological responses and habituation to repeated psychosocial stressors across all diagnostic groups
MLMs examined moderators of cortisol, alpha-amylase, and HR TSST responses in the full sample, controlling for TSST session. These analyses assessed concurrent associations between moderators and TSST responses. Age was significantly associated with cortisol responses: older participants exhibited lower pre-TSST levels (b=−.505, SE= .181, p=.006) and more blunted cortisol responses compared to younger participants, as evident in lower anticipatory reactivity levels (b=−.008, SE=.002, p<.001) and quadratic change (b=.0001, SE=.00003, p<.001). Higher levels of depressive symptoms were associated with slower acclimation of pre-TSST cortisol levels (b=.0005, SE=.0002, p=.008) and more blunted cortisol responses, as evident in lower anticipatory reactivity (b=−.001, SE=.001, p=.041) and quadratic change (b=.00002, SE= .00001, p=.018). Childhood trauma exposure was not significantly associated with any of the cortisol response indices.
Greater childhood trauma exposure was associated with slower alpha-amylase acclimation (b=.006, SE=.002, p=.007); none of the remaining moderators (i.e., age, depressive symptoms) were significantly associated with any of the alpha-amylase response indices. Higher depressive symptom levels were associated with diminished HR reactivity (b=−.233, SE=.116, p=.047) and slower HR recovery (b=−.211, SE=.087, p=.016) across TSSTs. Neither age nor childhood trauma exposure were associated with HR response indices.
Analyses of habituation in the full sample assessed the extent to which moderators were associated with different patterns of change in TSST cortisol responses over time. None of the moderators (i.e., age, depressive symptoms, childhood trauma exposure) were significantly associated with changes in cortisol response indices across TSSTs.
3.5. Moderators of physiological responses and habituation to repeated psychosocial stressors within the IPV group
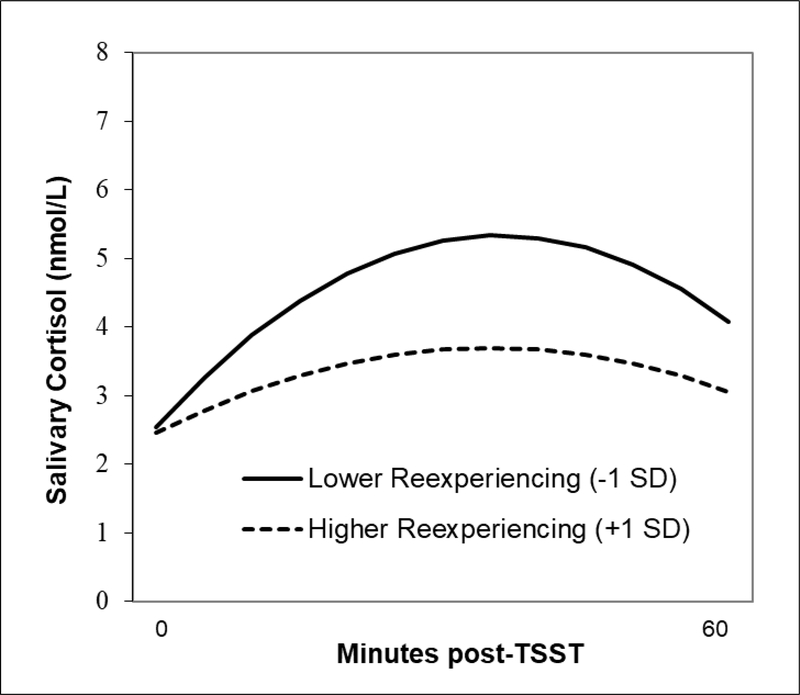
Within the IPV group, greater posttraumatic stress symptom severity (CAPS-IV total severity scores) was associated with slower cortisol acclimation (b=.0002, SE=.0001, p=.038), but was not associated with anticipatory reactivity (b=−.0003, SE=.0004, p=.423) or quadratic change (b=.00001, SE=.00001, p=.345). Exploratory analyses examined posttraumatic stress symptom clusters separately as moderators of cortisol response indices. The association between re-experiencing symptom severity and cortisol responses to the baseline TSST is presented in Figure 5. MLMs revealed that higher re-experiencing symptom severity was associated with more blunted cortisol TSST responses among IPV survivors, as evident in lower anticipatory reactivity (b=−.003, SE=.001, p=.007) and quadratic change (b=.00004, SE=.00002, p=.006). In contrast, neither avoidance nor hyperarousal symptom severity was significantly associated with any of the cortisol response indices. Greater posttraumatic stress symptom severity was associated with slower HR recovery across TSSTs (b=−.096, SE=.047, p=.044), but was not associated with HR reactivity (b=−.113, SE=.070, p=.110). Similarly, greater hyperarousal symptom severity was associated with slower HR recovery across TSSTs (b=−.245, SE=.118, p=.041), but was not associated with HR reactivity (b=−.132, SE=.176, p=.453). Neither re-experiencing nor avoidance symptom severity was significantly associated with any of the HR response indices. None of the moderators were significantly associated with any of the alpha-amylase response indices.
Figure 5.
Multilevel model of the association between PTSD re-experiencing sympom severity and quadratic change in salivary cortisol levels at the baseline TSST.
Analyses of habituation in the IPV group assessed the extent to which moderators were associated with different patterns of change in TSST cortisol responses over time. Neither overall posttraumatic stress symptom severity (CAPS-IV total severity scores) nor symptom cluster severity scores were significantly associated with changes in cortisol response indices across TSSTs.
4. Discussion
Alterations in major stress response systems are present during the acute aftermath of trauma and may play a role in determining risk for developing PTSD (Morris et al., 2016; Morris and Rao, 2013). The present study sought to characterize stress response and habituation patterns to repeated social stressors (TSST) over 6 months in young women who had experienced potentially traumatic interpersonal violence in the 90 days prior to baseline, as compared to a group of non-traumatized women. In the full sample, salivary cortisol levels declined between arrival for a study visit and TSST task instructions (acclimation), increased at the start of the TSST (anticipation), and decelerated after the TSST (recovery). Changes in cortisol responses to the TSST across study visits (baseline, 1-, 3-, and 6-months) were observed, with evidence of decreasing cortisol reactivity over time. In contrast, alpha-amylase levels showed deceleration during the recovery period but no evidence of habituation across study visits. Similarly, neither HR reactivity nor recovery changed across TSSTs. These findings are broadly consistent with prior research showing a dissociation between HPA and SNS responses to repeated psychosocial stressors in healthy individuals (Gerra et al., 2001; Kirschbaum et al., 1995; Schommer et al., 2003). Cortisol responses to the first TSST were more pronounced among trauma survivors with current PTSD compared to those without PTSD and non-traumatized controls. Survivors exhibited similarly blunted cortisol responses across follow-up TSSTs regardless of PTSD status, suggesting habituation of cortisol responses among survivors who developed PTSD. Identifying the factors that contribute to these disparate stress response patterns could shed light on mechanisms of risk and resilience to PTSD during the acute posttraumatic recovery phase.
Theoretical models propose that a progressive divergence in the activity of the HPA and SNS systems following trauma exposure may contribute to the onset and maintenance of PTSD (Pervanidou, 2008). Noradrenergic hyperactivity, amplified by reduced cortisol-mediated restraint, could trigger PTSD re-experiencing and hyperarousal symptoms via interactions with memory, attentional, and arousal processes (Pitman et al., 2012). In the present study, trauma survivors with current PTSD were characterized by more pronounced cortisol responses to the first TSST compared to IPV survivors without PTSD and healthy controls. This result is consistent with our hypothesis and prior work on heightened responses to personalized trauma scripts among women with PTSD compared to those without PTSD (Elzinga et al., 2003). Contrary to expectation, survivors with current PTSD showed blunted cortisol responses to follow-up TSSTs, which suggests more rapid – instead of slower - cortisol habituation compared to the other groups. Notably, IPV survivors without PTSD exhibited blunted cortisol responses across all four TSSTs; blunted cortisol responses to the first TSST precluded habituation (i.e., floor effect). These findings extend prior cross-sectional work showing associations between blunted cortisol TSST responses and childhood maltreatment (MacMillan et al., 2009) as well as lifetime trauma exposure (Elzinga et al., 2008). The present findings further suggest a pattern of transition from acute cortisol hyper-reactivity to prolonged hypo-reactivity among female interpersonal trauma survivors with PTSD - similar to that observed for diurnal cortisol secretion in chronic stress (Miller et al., 2007). This could explain why studies examining cortisol response differences long after the index trauma find similarly blunted cortisol responses among individuals with and without PTSD (Pierce and Pritchard, 2016).
Blunted cortisol responses were observed among interpersonal trauma survivors but were not significantly associated with childhood trauma exposure. Enhanced cortisol responses to a novel social-evaluative threat among women with PTSD could reflect a ‘stress sensitization’ process (Stam, 2007). Whether or not elevated cortisol responses to acute social stressors are adaptive in recent interpersonal violence survivors likely depends on their context and chronicity (Dickerson et al., 2009). Greater pre-deployment cortisol TSST reactivity predicted higher risk for developing PTSD among soldiers exposed to traumatic events during deployment (Steudte-Schmiedgen et al., 2015). Heightened cortisol reactivity to a novel social stressor could also reflect an adaptive mechanism to recruit glucocorticoid receptors in neural circuits critical for contextual processing (Kaouane et al., 2012).
Exploratory analyses revealed that greater severity of re-experiencing symptoms appeared to be driving the blunting of cortisol TSST responses. Changes in cortisol responses across TSSTs (i.e., anticipatory reactivity, quadratic change) were not associated with changes in avoidance, hyperarousal, or total PTSD symptom severity. Consistent with these findings, prior work has shown that women with PTSD who were classified as TSST “non-responders” (due to blunted cortisol reactivity) did not differ from TSST “responders” based on overall PTSD symptom severity (Zaba et al., 2015). The latter study identified elevated dissociative symptoms and attenuated peripheral FKBP5 (a co-chaperone of the glucocorticoid receptor) expression in the non-responder group and speculated that these characteristics may “constitute a clinically and biologically distinct subtype of PTSD” (Zaba et al., 2015). The present findings add to this growing literature by demonstrating an inverse relation between re-experiencing symptom severity and cortisol reactivity. It is important to note that IPV survivors without PTSD were non-responders to all four TSSTs. While it is tempting to speculate that blunted cortisol responses to a novel social-evaluative threat is a marker of resilience against the development of PTSD, we cannot rule out the possibility that blunted stress responses could render survivors more vulnerable to developing PTSD in the face of future traumatic events. Recent work in healthy individuals who were administered the TSST and then shown a film depicting interpersonal violence revealed that cortisol responders reported more intrusive memories over a week-long follow-up than non-responders (Schultebraucks et al., 2019). In summary, the clinical relevance of initially elevated cortisol responses among the PTSD group relative to the IPV (without manifest PTSD) and NTC groups remains unclear.
Current PTSD was associated with more rapid cortisol habituation to repeated TSSTs compared to the non-traumatized control group. Hence, we did not find support for our hypothesis that individuals with current PTSD would exhibit reduced or slower cortisol habituation when confronted with repeated social-evaluative stressors. Instead, our findings are in line with neuroimaging studies showing stronger amygdala habituation to repeated fearful faces among women with higher PTSD symptom severity (Kim et al., 2019). HPA feedback function (the process through which circulating cortisol inhibits the HPA axis) is one neuroendocrine mechanism that could account, in part, for habituation to repeated social stressors. Enhanced HPA negative feedback has been found in trauma-exposed individuals with and without PTSD compared to non-traumatized controls (Morris et al., 2012). One possibility is that heightened sensitivity to social-evaluative threat during the acute aftermath of IPV overwhelmed HPA negative feedback at baseline, resulting in a pattern of initially elevated cortisol responses among women with PTSD that rapidly diminished across successive TSSTs. A second possibility is that decreasing cortisol responses across repeated social stressors are linked to symptomatic recovery. Habituation is proposed as a key mechanism of change in evidence-based therapies for PTSD: larger between-session decreases in self-reported distress during prolonged exposure therapy are associated with lower post-treatment PTSD symptoms (Sripada and Rauch, 2015). However, within-person changes in total PTSD symptom severity were not significantly associated with changes in cortisol reactivity or recovery over time. A third possibility is that more rapid declines in cortisol TSST responses among survivors with PTSD may be driven by higher cortisol responses to the first TSST rather than a learned adaption or habituation to repeated TSSTs. An argument for habituation could be made if changes in putative psychological mechanisms of PTSD symptom change, including posttraumatic cognitions, were associated with cortisol response indices.
Limitations of the present study provide directions for future research. Only a minority of recent trauma survivors develop PTSD (McNally, 2003). Larger samples will be needed to examine neuroendocrine correlates and predictors of chronic PTSD and other trajectories of symptom change. Women with IPV varied in the number of days elapsed from their traumatic event at the baseline assessment; however, this variable was not significantly associated with any of the cortisol, alpha-amylase, or HR response indices. Subjective measures of perceived stress and emotional responses to the TSSTs were not included. Although these self-report measures do not typically covary with physiological stress responses (Campbell & Ehlert, 2012), their omission prevents examination of diagnostic group differences in perceived stress responses to the TSST over time. Notably, heightened perceived stress responses to the TSST have recently been reported for women with PTSD compared to healthy women (Metz et al., 2020). Finally, results of moderator analyses within the IPV group should be interpreted with caution due to power considerations for cross-level interactions (Mathieu et al., 2012).
The factors that shape response patterns to repeated stressors in healthy individuals and recent interpersonal trauma survivors are not well understood. Findings of the current study suggest that PTSD as a diagnostic entity is meaningfully associated with cortisol responses to repeated social stressors. Blunted cortisol responses were specifically associated with elevated PTSD re-experiencing symptoms over time, which complements evidence that cortisol impairs memory retrieval (de Quervain et al., 2017). Prior work suggests that pre-traumatic alterations in cortisol reactivity are associated with risk for developing PTSD (Steudte-Schmiedgen et al., 2015) and could be used to identify high-risk individuals who may benefit from preventive interventions. Evaluating the determinants of cortisol (non)habituation to repeated social-evaluative threat among recent interpersonal trauma survivors represents an important avenue for future research that could inform the development of early interventions.
Supplementary Material
Acknowledgements
This work was supported, in part, by grants from the National Institutes of Health (K01 MH101403, U54 MD007593, U54MD007586, R01MH108155, R01MD010757 and R01DA040966). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors would like to thank all the women who participated in this study.
Footnotes
Competing Interests
The authors report no conflicts of interest.
These results were unchanged when including years of education – which differed between diagnostic groups – as a covariate.
These results were unchanged when including years of education – which differed between diagnostic groups – as a covariate.
References
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders, 4th, Text Revision ed. Author, Washington, DC,. [Google Scholar]
- Balodis IM, Wynne-Edwards KE, Olmstead MC, 2010. The other side of the curve: examining the relationship between pre-stressor physiological responses and stress reactivity. Psychoneuroendocrinology 35, 1363–1373. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G, 1996. Manual for Beck Depression Inventory II (BDI-II). San Antonio, TX, Psychology Corporation. [Google Scholar]
- Benjamini Y, & Hochberg Y, 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society 57, 289–300. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J, 1994. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry 151, 1132–1136. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM, 1995. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress 8, 75–90. [DOI] [PubMed] [Google Scholar]
- Campbell J, & Ehlert U, 2012. Acute psychosocial stress: Does the emotional stress response correspond with physiological responses. Psychoneuroendocrinology 37, 1111–1134. [DOI] [PubMed] [Google Scholar]
- de Quervain D, Schwabe L, Roozendaal B, 2017. Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nature Reviews Neuroscience 18, 7–19. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME, 2009. Psychobiological Responses to Social Self Threat: Functional or Detrimental? Self and Identity 8, 270–285. [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P, 2008. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology 33, 227–237. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, Van Dyck R, Bremner JD, 2003. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology 28, 1656–1665. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 2005. Structured clinical interview for DSM-IV-TR Axis I disorders: patient edition Biometrics Research Department, Columbia University; New York, NY. [Google Scholar]
- Gerra G, Zaimovic A, Mascetti G, Gardini S, Zambelli U, Timpano M, Raggi M, Brambilla F, 2001. Neuroendocrine responses to experimentally-induced psychological stress in healthy humans. Psychoneuroendocrinology 26, 91–107. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK, 2006. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology 31, 976–987. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Litz BT, Hsu JL, Lombardo TW, 2004. Psychometric properties of the life events checklist. Assessment 11, 330–341. [DOI] [PubMed] [Google Scholar]
- Kaouane N, Porte Y, Vallée M, Brayda-Bruno L, Mons N, Calandreau L, Marighetto A, Piazza PV, Desmedt A, 2012. Glucocorticoids can induce PTSD-like memory impairments in mice. Science 335, 1510–1513. [DOI] [PubMed] [Google Scholar]
- Kim YJ, van Rooij SJ, Ely TD, Fani N, Ressler KJ, Jovanovic T, Stevens JS, 2019. Association between posttraumatic stress disorder severity and amygdala habituation to fearful stimuli. Depression and anxiety 36, 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH, 1989. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology 22, 150–169. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH, 1993. The Trier Social Stress Test: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28, 76–81. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH, 1995. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic medicine 57, 468–474. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Abelson JL, 2016. Context processing and the neurobiology of post-traumatic stress disorder. Neuron 92, 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, 2009. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biological psychiatry 66, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu JE, Aguinis H, Culpepper SA, & Chen G, 2012. Understanding and estimating the power to detect cross-level interaction effects in multilevel modeling. Journal of Applied Psychology 97, 951–966. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2004. Protection and damage from acute and chronic stress - Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders, in: Yehuda R, McEwen B (Eds.), Biobehavioral Stress Response: Protective and Damaging Effects, pp. 1–7. [DOI] [PubMed] [Google Scholar]
- McNally RJ, 2003. Psychological mechanisms in acute response to trauma. Biological Psychiatry 53, 779–788. [DOI] [PubMed] [Google Scholar]
- Metz S, Duesenberg M, Hellman-Regen J, Wolf OT, Roepke S, Otte C, Wingenfeld K, 2020. Blunted salivary cortisol response to psychosocial stress in women with posttraumatic stress disorder. Journal of Psychiatric Research 10.1016/j.jpsychires.2020.07.014 [DOI] [PubMed] [Google Scholar]
- Mielock AS, Morris MC, Rao U, 2017. Patterns of cortisol and alpha-amylase reactivity to psychosocial stress in maltreated women. Journal of Affective Disorders 209, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES, 2007. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin 133, 25–45. [DOI] [PubMed] [Google Scholar]
- Morris MC, Compas BE, Garber J, 2012. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clinical Psychology Review 32, 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Hellman N, Abelson JL, Rao U, 2016. Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clinical Psychology Review 49, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Kouros CD, Mielock AS, Rao U, 2017. Depressive symptom composites associated with cortisol stress reactivity in adolescents. Journal of Affective Disorders 210, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Rao U, 2013. Psychobiology of PTSD in the acute aftermath of trauma: Integrating research on coping, HPA function and sympathetic nervous system activity. Asian J Psychiatr 6, 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Rao U, 2014. Cortisol response to psychosocial stress during a depressive episode and remission. Stress-the International Journal on the Biology of Stress 17, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, 2009. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology 34, 486–496. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C, 2007. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology 32, 392–401. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BP, 2007. Gender differences in posttraumatic stress disorder. Psychol Bull 133, 183–204. [DOI] [PubMed] [Google Scholar]
- Pervanidou P, 2008. Biology of post‐traumatic stress disorder in childhood and adolescence. Journal of neuroendocrinology 20, 632–638. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Pritchard LM, 2016. Lower stress-reactive cortisol in female veterans associated with military status but not PTSD. Stress 19, 486–491 [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I, 2012. Biological studies of post-traumatic stress disorder. nature Reviews neuroscience 13, 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pole N, 2007. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull 133, 725–746. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Cheong Y, Congdon R, du Toit M, 2019. HLM 8: Hierarchical linear and nonlinear modeling. Skokie, IL: Scientific Software International. [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C, 2003. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosomatic medicine 65, 450–460. [DOI] [PubMed] [Google Scholar]
- Schultebraucks K, Rombold-Bruehl F, Wingenfeld K, Hellman-Regen J, Otte C, Roepke S, 2019. Heightened biological stress response during exposure to a trauma film predicts an increase in intrusive memories. Journal of Abnormal Psychology 128, 645–657. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Gevonden M, Ratanatharathorn A, Laska E, Van Der Mei WF, Qi W, Lowe S, Lai BS, Bryant RA, Delahanty D, 2019. Estimating the risk of PTSD in recent trauma survivors: results of the International Consortium to Predict PTSD (ICPP). World Psychiatry 18, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J, Smith LM, 2007. Hypothalamic-pituitary-adrenal axis function in dissociative disorders, post-traumatic stress disorder, and healthy volunteers. Biol Psychiatry 61, 966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Rauch SA, 2015. Between-session and within-session habituation in Prolonged Exposure Therapy for posttraumatic stress disorder: A hierarchical linear modeling approach. Journal of Anxiety Disorders 30, 81–87. [DOI] [PubMed] [Google Scholar]
- Stam R, 2007. PTSD and stress sensitisation: A tale of brain and body: Part 1: Human studies. Neuroscience & Biobehavioral Reviews 31, 530–557. [DOI] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S, Stalder T, Schonfeld S, Wittchen HU, Trautmann S, Alexander N, Miller R, Kirschbaum C, 2015. Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology 59, 123–133. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR, 2001. Clinician‐Administered PTSD Scale: A review of the first ten years of research. Depression and anxiety 13, 132–156. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Ruscio AM, Keane TM, 1999. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychological assessment 11, 124. [Google Scholar]
- Wichmann S, Kirschbaum C, Böhme C, Petrowski K, 2017. Cortisol stress response in post-traumatic stress disorder, panic disorder, and major depressive disorder patients. Psychoneuroendocrinology 83, 135–141. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko IS, van Rossum EF, Koper JW, Hellhammer DH, 2005. Habituation of cortisol responses to repeated psychosocial stress-further characterization and impact of genetic factors. Psychoneuroendocrinology 30, 199–211. [DOI] [PubMed] [Google Scholar]
- Yehuda R, 2002. Clinical relevance of biologic findings in PTSD. Psychiatric Quarterly 73, 123–133. [DOI] [PubMed] [Google Scholar]
- Zaba M, Kirmeier T, Ionescu IA, Wollweber B, Buell DR, Gall-Kleebach DJ, Schubert CF, Novak B, Huber C, Köhler K, 2015. Identification and characterization of HPA-axis reactivity endophenotypes in a cohort of female PTSD patients. Psychoneuroendocrinology 55, 102–115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.