Abstract
OBJECTIVE:
We evaluate the association between BMI, all-cause and cause-specific mortality in South Africa.
METHODS:
We analysed prospective, population-based observational cohort data from rural South Africa. BMI was measured in 2010. Demographic characteristics were recorded and deaths were verified with verbal autopsy interview. The InterVA-5 tool was used to assign causes of death. HIV testing was conducted annually. Cox proportional hazards models were fit to estimate the effect of BMI on all-cause and cause-specific mortality, accounting for the competing risk of death from other causes. Models were adjusted for sociodemographic characteristics and HIV status and we used inverse probability weighting for survey non-participation.
RESULTS:
Our cohort consisted of 9,728 individuals. In adjusted models, those with a BMI of 25.0 – 29.9 kg/m2 or 30.0 – 34.9 kg/m2 had a lower hazard of death (aHR: 0.80; 95% CI: 0.69 – 0.92 and aHR: 0.75; 95% CI: 0.60 – 0.93, respectively), compared to those with a BMI of 18.5 – 24.9 kg/m2.
CONCLUSIONS:
Individuals in South Africa who meet clinically-defined criteria for overweight or obesity had a lower risk of all-cause mortality than those of normal BMI. These findings were stronger for women and communicable conditions.
Keywords: body mass index, mortality, public health
INTRODUCTION
Overweight and obesity are rapidly increasing in low- and middle-income countries (LMICs).[1, 2] Though the prevalence of clinically-defined obesity has reached epidemic levels in some LMICs, the mortality risk associated with increased body weight remains unknown in these settings. Evidence from high-income countries (HICs) has suggested that mortality risk is increased among those with a BMI 30.0 – 34.9 kg/m2 and a BMI ≥35 kg/m2, respectively.[3–6]
However, the relationship between BMI and mortality for people living in sub-Saharan Africa remains unclear, due to scarce data on body anthropometry measurements and long-term survival. There is good reason to suspect that relationships between body habitus, including both BMI and body composition, and mortality might differ in this setting. For example, relationships between BMI and mortality appear to be different among people living with HIV or cancer, and the prevalence of HIV exceeds 20% in some Southern African countries.[3, 7] Nonetheless, in cross-sectional studies, higher BMI is associated with increased cardiovascular risk, as well as higher rates of hypertension and diabetes.[8, 9] While several studies from sub-Saharan Africa suggest an extremely high prevalence (>67%) of clinically-defined overweight and obesity, [10, 11] particularly in women, there has not yet been a study from this region quantifying the risk posed by overweight or obesity for all-cause or cardiovascular disease-related mortality.
Given the dramatic increases in the prevalence of clinically-defined overweight and obesity in sub-Saharan Africa in recent decades [1, 12, 13], and the lack of evidence about its relationship with mortality in the region, we sought to measure associations between BMI and both all-cause and cause-specific mortality in a population-based cohort in South Africa. To do so, we examined data from a well-characterized, population-based cohort from a demographic health and surveillance site that included BMI measurement in 2010, followed by routine prospective data collection on mortality through verbal autopsy procedures.
METHODS
Study Population and Socio-demographic Data
The African Health Research Institution (AHRI) (formerly the Africa Centre for Health and Population Studies) is a Wellcome Trust-funded research institute in South Africa. Since 2000, AHRI has maintained one of the largest population-based cohorts in the region via periodic household-based surveys. These surveys have been used to collect demographic data from a population of approximately 100,000 individuals living in a rural area of 438 km2 in rural uMkhanyakude District, northern KwaZulu-Natal.[14] Households are surveyed 2–3 times per year, to collect information on birth, deaths and migration patterns for all household members, including non-residents. The participation rate for household surveillance is >99%.[14] In addition, resident household members who are aged ≥15 years are invited to participate in an annual home-based individual survey, which includes an interview on general health and collection of a dried blood spot (DBS) for anonymised HIV testing. Approximately 70% of eligible residents participate in the survey at least once after five rounds, and as of 2017, 80% of individuals had participated in HIV testing at least once.[15, 16]
Body Anthropomorphic Data
In one round of the 2010 survey, all individuals who participated in the home-based individual survey were offered a physical examination in order to determine weight, height and blood pressure, using the World Health Organization STEP-wise approach to surveillance (STEPS) protocol.[17, 18] In brief, body weight was measured on a calibrated scale. Each person was weighed twice with outer clothing removed, and the second measurement was recorded if it fell within 200g of the first. If there was a difference of more than 200g between the first and second measurement, a third measurement was taken and the measurement that was obtained twice within a 200g range was recorded. To measure height, the participant was asked to stand with both feet stepping on flat foot metal and straight knees and a measuring tape was used to assess height in centimetres. BMI was then calculated as weight in kilograms/(height in meters)2.
HIV Infection and Clinical Care Data
In addition to offering annual HIV testing, AHRI has a memorandum of agreement with the South Africa Department of Health to access clinical care data from the local area primary health clinics (PHCs). We link surveillance site data with clinical data from the primary care and HIV care health systems using their surveillance identification numbers which are recorded by data capturers at each clinic and hospital in the DHS catchment area.
Mortality and Cause of Death Data
During each demographic visit, all deaths since the prior survey are recorded, including those of non-resident household members. All deaths are verified by a home-based follow-up verbal autopsy (VA) interview. This interview is conducted by a trained nurse with the closest available relative or caretaker of the deceased. The VA interview includes a qualitative narrative of the circumstances leading up to the death, a checklist of signs and symptoms, and a structured questionnaire, adapted from the World Health Organization (WHO) Verbal Autopsy Questionnaire.[19] The cause of death is then assigned using the InterVA-5 tool, which has been validated in this population previously.[20] Previous research has described the sensitivity and specificity of this tool for cause of death assignment.[20] These causes of death were then categorized into infectious and non-communicable causes using the WHO classification system.
Smoking Status
Smoking data were not collected as part of the 2010 survey. However, there was a recently completed community-based assessment of smoking behaviour in 14,509 individuals in the same population, among which 3,030 had participated in the 2010 survey.[21] In this more recent study, 91% of individuals reported never smoking (98% of women and 76% of men). Moreover, 22% of men and only 1% of women reported that they were current smokers in this same cohort.
Statistical analysis
Individual participants were eligible for inclusion in this analysis if they had their BMI measured in the home-based 2010 survey. We assessed mortality rates over the period from 2010 to 2017. Person-time was defined from the date of the 2010 survey, until the earliest date of death or the date that the individual was last recorded as a member of a household in the surveillance area. Periods of non-residence were included in the analysis if the individual remained a household member because date and cause of death data were available for these individuals.
We first compared sociodemographic characteristics by BMI group and separately, for those who did and did not complete the BMI survey using standard statistical methods. Next, we estimated crude all-cause mortality by sex and BMI category. We then used Kaplan-Meier methods to depict all-cause mortality stratified by BMI group, and Cox proportional hazards regression models to estimate adjusted hazard ratios (aHR) and 95% confidence intervals (CIs) for the effect of BMI on mortality, in the total population and stratified by sex. BMI was modelled as a continuous covariate by restricted cubic splines with 4 knots and aHRs were presented at selected values of BMI, comparing the median value in each BMI group to a BMI of 22.0 as the reference value. BMI groups were defined using standard cut-offs as follows: underweight (<18.5 kg/m2), normal weight (18.5 – 24.9 kg/m2), overweight (25.0 – 29.9 kg/m2), class I obesity (30.0 – 34.9 kg/m2) and class II obesity or great (≥35.0 kg/m2). Models were adjusted for age (as the time scale of the analysis), sex, socioeconomic status (SES) and a composite variable for HIV serostatus, categorized as HIV negative, HIV positive or HIV unknown status. Socioeconomic status and HIV status were treated as time-updated exposures. SES was measured via an asset index, which was constructed using principal component analysis of ownership of common household items, based on information gathered in the household survey.[22] HIV status was assessed using data from the HIV serosurvey and TIER.net. Seroconversion dates were imputed using a random time point along the interval between the last negative test date and the first positive test date (or date of first record in TIER.net).[23, 24] An additional category of HIV unknown was used for individuals for the period before their first HIV test date, and 2 years after their last negative test if they had no record of a positive test, given the high incidence of HIV in this region.[23]
We also estimated the cumulative incidence of cause-specific mortality (infectious disease causes and non-communicable disease causes) stratified by BMI, while accounting for the competing risk of deaths from other causes. We used competing risks proportional hazards regression to estimate the sub-distribution hazard ratio (SHR) for the effect of BMI on cause-specific mortality, adjusted for age, sex, SES and HIV status as described above. These SHRs can be interpreted as an approximation of HRs estimated in standard Cox models, but accounting for the hazard of competing events.[25]
All regression models were weighted to account for non-response in the 2010 survey (when BMI was measured), to augment population representativeness. Response weights were calculated as the inverse probability of participation in the 2010 survey, in strata defined by age group, sex, and place of residence (urban/peri-urban/rural).[26] We assessed assumptions about proportional hazards using scaled Schoenfeld residuals.[27] We conducted the following additional sensitivity analyses: 1) we began observation time two years after the BMI measurement, effectively excluding deaths in the first two years of follow-up; 2) we examined the relationship between BMI and mortality by HIV status (HIV-negative, HIV-positive; 3) we examined the effect of BMI on mortality, unweighted for non-response; and 3) we further stratified the aHR for the normal BMI group into three subgroups (BMI 18.5 – <20.0, 20.0 – <22.5, 22.5 – <25.0), as has been done previously in this literature.
Ethics
Ethical approval for the demographic surveillance surveys, linkage to the government ART records (TIER.net), and analyses of these data were granted by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal, South Africa (reference BE290/16). Separate written informed consent was obtained for the main household survey, the individual general health questionnaires and the HIV serosurvey.
RESULTS
Our cohort consisted of 9,728 individuals who had a BMI measured in the 2010 individual survey. This represents 37.1% of the 26,194 individuals who were eligible for the survey in that round. The median age of participants was 31 years (IQR 20–51); most (64%) were female, married (57%), lived in a rural area (63%) and had less than a secondary school education (54%). These sociodemographic characteristics are provided in Table 1 overall and by BMI group. Additionally, 16,431 (62.7%) of individuals were not available for the survey or declined participation, and another 35 (0.1%) individuals consented but their BMI measurements were not available. The differences in demographic characteristics among those who had their BMI measured and those who did not are provided in the Supplementary Appendix Table 1. In brief, the group that did not participate in BMI measurement included more men, more peri-urban dwellers and more people who were employed than those who did participate.
Table 1.
Baseline characteristics of those with a BMI measurement in the 2010 survey, overall and by BMI group
| All survey participants (N=9728) | BMI <18.5 kg/m (N=861) | BMI 18.5– 24.9 kg/m2 (N=4412) | BMI 25.0–29.9 kg/m2 (N=2167) | BMI 30.0–34.9 kg/m2 (N=1284) | BMI ≥35.0 kg/m2 (N=1004) | |
|---|---|---|---|---|---|---|
| Unweighted N (weighted population proportion)1 | ||||||
| Age group | ||||||
| <30 | 4656 (47.3%) | 609 (69.3%) | 2844 (62.9%) | 813 (35.9%) | 271 (20.5%) | 119 (11.9%) |
| 35–44 | 1817 (20.0%) | 97 (12.5%) | 652 (16.3%) | 478 (23.7%) | 318 (26.3%) | 272 (28.6%) |
| 45–59 | 1771 (17.7%) | 80 (9.5 %) | 474 (10.8%) | 454 (20.8%) | 377 (28.7%) | 386 (37.3%) |
| 60+ | 1484 (15.0%) | 75 (8.8 %) | 442 (10.0%) | 422 (19.5%) | 318 (24.5%) | 227 (22.1%) |
| Sex | ||||||
| Male | 2969 (35.9%) | 528 (67.1%) | 1964 (50.9%) | 325 (18.7%) | 106 (10.5%) | 46 (5.7 %) |
| Female | 6759 (64.1%) | 333 (32.9%) | 2448 (49.1%) | 1842 (81.3%) | 1178 (89.5%) | 958 (94.3%) |
| Marital status | ||||||
| Single (never married) | 3044 (31.7%) | 452 (53.5%) | 1808 (41.2%) | 462 (20.7%) | 200 (15.2%) | 122 (12.1%) |
| Married/informal union | 5436 (56.7%) | 357 (42.3%) | 2284 (52.8%) | 1349 (63.5%) | 813 (64.4%) | 633 (63.8%) |
| Widow/sep/divorced | 1185 (11.5%) | 38 (4.2 %) | 279 (6.0 %) | 351 (15.7%) | 269 (20.4%) | 248 (24.1%) |
| Missing | 63 | 14 | 41 | 5 | 2 | 1 |
| Education | ||||||
| None | 1824 (18.6%) | 154 (18.2%) | 663 (15.0%) | 450 (20.8%) | 304 (23.4%) | 253 (24.7%) |
| Less than complete | 5287 (54.3%) | 531 (62.3%) | 2572 (58.1%) | 1060 (48.3%) | 617 (47.6%) | 507 (50.3%) |
| secondary | ||||||
| Complete secondary/above | 2589 (27.2%) | 167 (19.5%) | 1164 (26.9%) | 653 (30.9%) | 362 (28.9%) | 243 (25.0%) |
| Missing | 28 | 9 | 13 | 4 | 1 | 1 |
| Employed | ||||||
| No | 8164 (83.9%) | 737 (87.2%) | 3718 (84.3%) | 1803 (82.6%) | 1077 (83.3%) | 829 (82.2%) |
| Yes | 1476 (16.1%) | 103 (12.8%) | 642 (15.7%) | 353 (17.4%) | 204 (16.7%) | 174 (17.8%) |
| Missing | 88 | 21 | 52 | 11 | 3 | 1 |
| HIV status2 | ||||||
| Negative | 6843 (70.0%) | 644 (74.9%) | 3053 (68.9%) | 1437 (65.8%) | 933 (72.2%) | 776 (76.9%) |
| Positive not on ART | 1561 (16.0%) | 104 (11.7%) | 713 (16.1%) | 397 (18.4%) | 208 (16.4%) | 139 (14.0%) |
| Positive on ART | 614 (6.5 %) | 60 (7.2 %) | 288 (6.7 %) | 174 (8.1 %) | 62 (5.0 %) | 30 (3.1 %) |
| Unknown3 | 710 (7.5 %) | 53 (6.2 %) | 358 (8.4 %) | 159 (7.6 %) | 81 (6.4 %) | 59 (6.1 %) |
| Residence | ||||||
| Urban | 537 (5.6 %) | 40 (4.7 %) | 249 (5.7 %) | 118 (5.6 %) | 75 (6.0 %) | 55 (5.6 %) |
| Peri-urban | 2980 (31.0%) | 301 (35.4%) | 1376 (31.6%) | 622 (29.0%) | 374 (29.6%) | 307 (30.5%) |
| Rural | 6195 (63.4%) | 515 (59.9%) | 2779 (62.7%) | 1425 (65.4%) | 835 (64.4%) | 641 (63.9%) |
| Missing | 16 | 5 | 8 | 2 | 0 | 1 |
| SES tertile4 | ||||||
| Low | 3451 (36.0%) | 325 (38.3%) | 1664 (38.3%) | 779 (36.1%) | 393 (30.8%) | 290 (29.3%) |
| Middle | 3199 (33.4%) | 278 (33.2%) | 1441 (33.1%) | 700 (32.9%) | 448 (35.3%) | 332 (33.6%) |
| High | 2934 (30.6%) | 244 (28.5%) | 1231 (28.6%) | 662 (31.0%) | 430 (34.0%) | 367 (37.1%) |
| Missing | 144 | 14 | 76 | 26 | 13 | 15 |
N is the actual number of participants, without sampling weights applied. Proportions are weighted to adjust for non-response in the 2010 survey; weights calculated as the inverse probability of participation in the 2010 survey, in strata defined by age group, sex, and residence.
Imputed HIV status in 2010, based on complete history of testing in the DSS, including subsequent years.
Includes 402 individuals who never tested, 138 whose last test was negative but >2 years before the survey, and individuals whose first test was after 2010, and was positive.
Calculated from an asset index derived using principal component analysis, based on ownership of household items as measured in the annual household survey.
In adjusted and weighted Cox proportional hazards models, those with a BMI of 25.0 – 29.9 kg/m2 and those with a BMI of 30.0 – 34.9 kg/m2 had a lower hazard of death (aHR 0.80; 95% CI: 0.69 – 0.92 and aHR 0.75; 95% CI: 0.60 – 0.93, respectively), compared to those with a BMI of 18.5 – 24.9 kg/m2 (Figure 1 and Table 2). Individuals with a BMI ≥35.0 kg/m2 also had a lower hazard ratio of death than those who had a BMI of 18.5 – 24.9 kg/m2 in this context (0.80, 95% CI: 0.64 −1.02). The full unadjusted and adjusted model results including AHRs for all covariates are provided in Supplementary Appendix Table 2. In sex-stratified models, these findings were consistent in women, with an aHR of 0.79 (95% CI: 0.66 – 0.94) for those with a BMI of 25.0 – 29.9 kg/m2, 0.76 (95% CI: 0.58 – 0.94) for those with a BMI of 30.0 – 34.9 kg/m2 and 0.84 (95% CI: 0.64 – 1.10) for those with a BMI ≥35.0 kg/m2, as compared to those with a BMI of 18.5 – 24.9 kg/m2. We found similar effect sizes in men.
Figure 1.
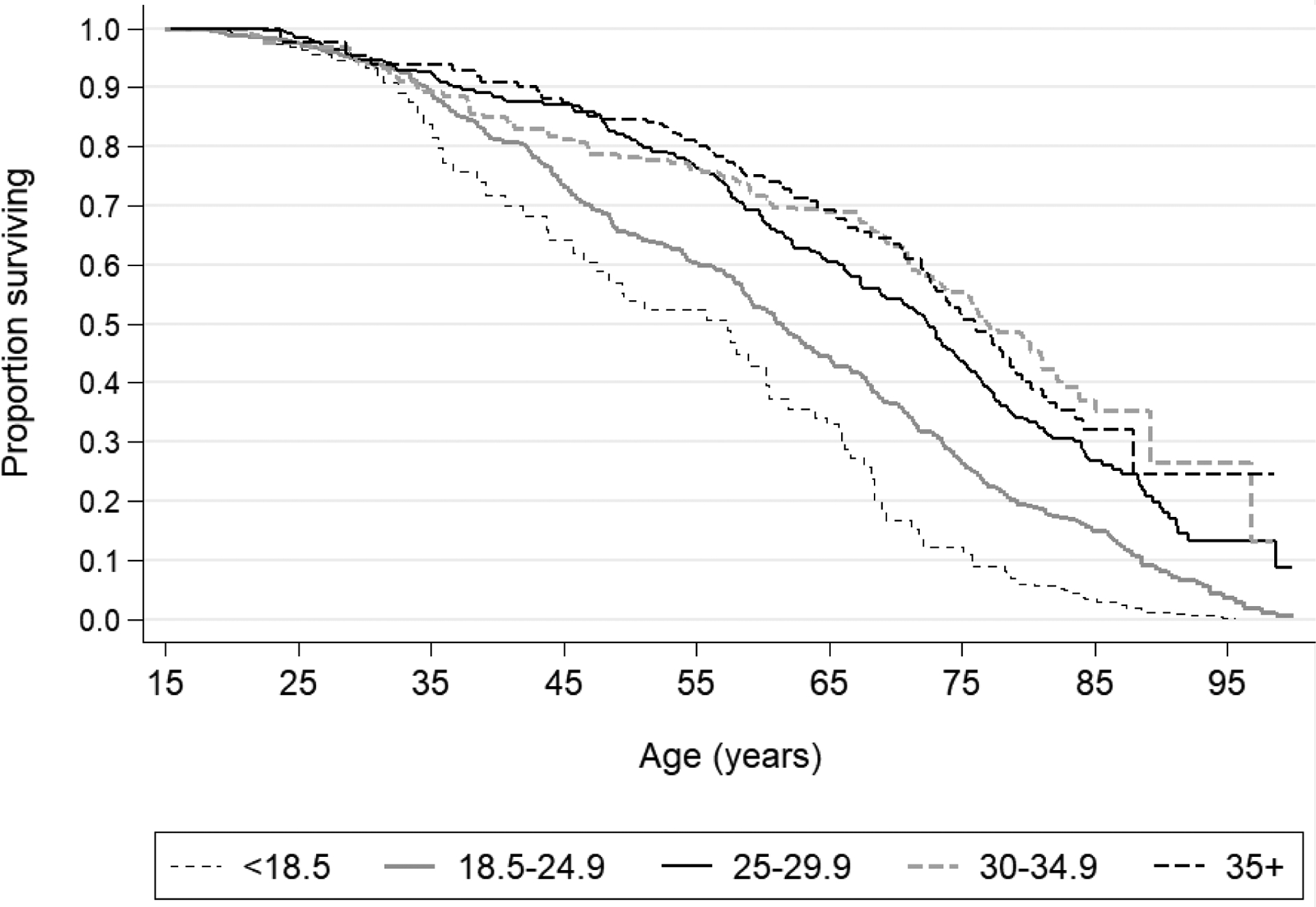
Kaplan-Meier estimates of survival, by BMI group
Table 2.
Association of BMI with all-cause mortality
| BMI (kg/m2)1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25.0–29.9 | 30.0–34.9 | ≥35.0 | ||||||
| All individuals | ||||||||||
| Deaths | 84 | 335 | 184 | 93 | 83 | |||||
| Person-years | 4931 | 25,796 | 12,831 | 7688 | 6056 | |||||
| Individuals | 861 | 4412 | 2167 | 1284 | 1004 | |||||
| HR (95% CI)2 | 1.37 (1.12 –1.69 ) | 1 (reference) | 0.80 (0.69 –0.92 ) | 0.75 (0.60 –0.93 ) | 0.80 (0.64 –1.02 ) | |||||
| Males | ||||||||||
| Deaths | 52 | 179 | 56 | 18 | 5 | |||||
| Person-years | 3073 | 11,423 | 1815 | 608 | 271 | |||||
| Individuals | 528 | 1963 | 325 | 106 | 46 | |||||
| HR (95% CI)2 | 1.27 (0.95 –1.69 ) | 1 (reference) | 0.85 (0.66 –1.11 ) | 0.82 (0.57 –1.19 ) | 0.88 (0.46 –1.69 ) | |||||
| Females | ||||||||||
| Deaths | 32 | 156 | 128 | 75 | 78 | |||||
| Person-years | 1857 | 14,373 | 11,015 | 7080 | 5785 | |||||
| Individuals | 333 | 2449 | 1842 | 1178 | 958 | |||||
| HR (95% CI)2 | 1.64 (1.25 –2.13 ) | 1 (reference) | 0.79 (0.66 –0.94 ) | 0.76 (0.58 –0.99 ) | 0.84 (0.64 –1.10 ) | |||||
| Excluding first 2 years of follow-up | ||||||||||
| Deaths | 84 | 335 | 184 | 93 | 83 | |||||
| Person-years | 4931 | 25,796 | 12,831 | 7688 | 6056 | |||||
| Individuals | 861 | 4412 | 2167 | 1284 | 1004 | |||||
| HR (95% CI)2 | 1.34 (1.04 –1.71 ) | 1 (reference) | 0.85 (0.72 –1.01 ) | 0.84 (0.65 –1.09 ) | 0.93 (0.70 –1.22 ) | |||||
| HIV negative | ||||||||||
| Deaths | 33 | 140 | 82 | 42 | 38 | |||||
| Person-years | 2671 | 12761 | 6996 | 4956 | 4248 | |||||
| Individuals | 644 | 3053 | 1437 | 933 | 776 | |||||
| HR (95% CI)2 | 1.21 (0.89 –1.65 ) | 1 (ref) | 0.76 (0.62 –0.94 ) | 0.67 (0.48 –0.91 ) | 0.65 (0.46 –0.92 ) | |||||
| HIV positive | ||||||||||
| Deaths | 26 | 131 | 52 | 25 | 20 | |||||
| Person-years | 1055 | 6994 | 3850 | 1800 | 1095 | |||||
| Individuals | 211 | 1394 | 720 | 341 | 213 | |||||
| HR (95% CI)2 | 1.33 (0.98 –1.81 ) | 1 (ref) | 0.73 (0.57 –0.94 ) | 0.71 (0.49 –1.03 ) | 0.96 (0.65 –1.41 ) | |||||
| HIV unknown | ||||||||||
| Deaths | 25 | 64 | 50 | 26 | 25 | |||||
| Person-years | 1205 | 6042 | 1985 | 932 | 712 | |||||
| Individuals | 351 | 1676 | 547 | 283 | 206 | |||||
| HR (95% CI)2 | 1.74 (1.21 –2.48 ) | 1 (ref) | 0.94 (0.70 –1.26 ) | 0.99 (0.63–1.55 ) | 1.03 (0.64 –1.64 ) | |||||
| Unweighted for non-response | ||||||||||
| HR (95% CI)3 | 1.39 (1.15 –1.67 ) | 1 (reference) | 0.80 (0.69 –0.91 ) | 0.75 (0.61 –0.92 ) | 0.81 (0.64 –1.01 ) | |||||
| All individuals | <18.5 | 18.5–<20.0 | 20.0– <22.5 | 22.5– <25.0 | 25.0–29.9 | 30.0–34.9 | ≥35 |
|---|---|---|---|---|---|---|---|
| Deaths | 84 | 62 | 145 | 128 | 184 | 93 | 83 |
| Person-years | 4931 | 5005 | 11398 | 9393 | 12831 | 7688 | 6056 |
| Individuals | 861 | 861 | 1938 | 1613 | 2167 | 1284 | 1004 |
| HR (95% CI)2 | 1.37 (1.12 –1.69 ) | 1.20 (1.08 –1.33 ) | 1 (reference) | 0.91 (0.88 –0.95 ) | 0.80 (0.69 –0.92 ) | 0.80 (0.60 –0.93 ) | 0.80 (0.64 –1.02 ) |
BMI modelled as a continuous covariate by restricted cubic splines with 4 knots; deaths / person-years in each group shown for information only. HRs are presented at selected values of BMI, comparing the median value in each BMI group to BMI 22 as the reference.
HRs estimated from Cox regression; adjusted for current age (as timescale), sex, HIV status and socioeconomic status. Models were weighted to account for non-response in the 2010 survey.
HRs estimated from Cox regression as described in footnote 2, but unweighted for non-response.
Those with a BMI ≤18.5 kg/m2 had the highest mortality rate in the total population as compared to those with a BMI of 18.5 – 24.9 kg/m2 overall and when stratified by sex (aHR Overall: 1.37, 95% CI: 1.12 – 1.69; aHR Women: 1.64, 95% CI: 1.25 – 2.13; aHR Men: 1.27, 95% CI: 0.95 – 1.69). The relationship between continuous BMI and the aHRs and 95% confidence intervals for mortality in this cohort are depicted in Figure 2.
Figure 2.
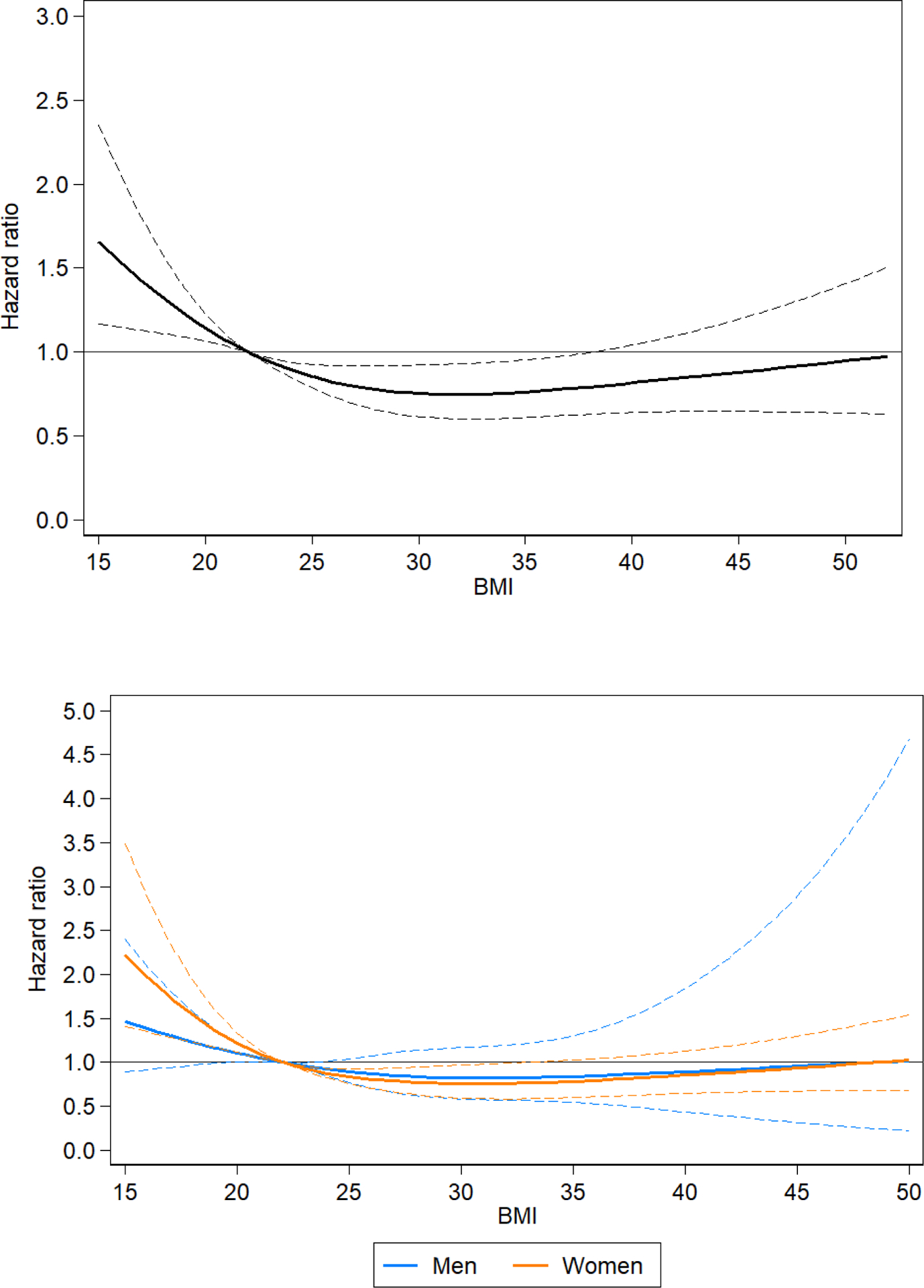
Association of BMI with all-cause mortality (hazard ratios and 95 % confidence intervals), modelled using restricted cubic splines with 4 knots in a Cox regression model, adjusted for age, sex, HIV status and socioeconomic status, in all individuals (2A) and stratified by sex (2B). A BMI of 22 was used as the reference to display the hazard ratios.
In sensitivity analyses, we found that excluding deaths within the first two years of follow-up resulted in similar effect sizes for the mortality risk associated with each BMI category (aHR: 1.34; 95% CI: 1.04 – 1.71, Table 2). Second, when examining these relationships by HIV status, we found that those with a BMI 25.0 – 29.9 kg/m2 had a lower risk of death than those with a BMI of 18.5 – 24.9 kg/m2 in both the HIV-positive (aHR 0.73, 95% CI: 0.57 – 0.94) and HIV-negative groups (aHR 0.76, 95% CI: 0.52 – 0.94) but this relationship was not the case for the HIV unknown group. Those with a BMI of 30.0 – 34.9 kg/m2 (aHR 0.67, 95% CI: 0.48 – 0.91) and ≥35.0 kg/m2 (aHR 0.65, 95% CI: 0.46 – 0.92) also had a lower mortality risk in the HIV-negative group only. The confidence intervals of the HR for these relationships overlapped with 1.0 in the HIV-positive and HIV unknown groups. In a sensitivity analysis that was performed without weights for non-response, we found no differences in the mortality risk by BMI group. Finally, when we stratified the mortality risk for the normal BMI group into three subgroups, and used 20.0 – <22.5 kg/m2 as a referent group, we found that individuals with a BMI of 18.5 – <20.0 kg/m2 had increased mortality (aHR 1.20; 95% CI: 1.08 – 1.33), and that those with a BMI of 22.5 – <25.0 kg/m2 had decreased mortality (aHR 0.91; 95% CI: 0.88 – 0.95). The point estimate and confidence intervals of the lower mortality risk associated with a BMI of 25.0 – 29.9 kg/m2 compared to 20.0 – <22.5 kg/m2 as a referent group were similar to our primary model in this sensitivity analysis. (Table 2)
The sub-distributional hazard ratios describing the relationship between BMI and death from both infectious diseases and non-communicable causes are shown in the Supplementary Appendix Figure 1. In brief, individuals who had a BMI >25 kg/m2 had a lower hazard of infectious causes of mortality across all higher BMI strata. In contrast, relationships between BMI and non-communicable diseases were muted, such that there was no difference in the hazard of mortality between those with BMI 30.0 – 34.9 kg/m2, or ≥35.0 kg/m2 and those with a BMI 18.0 – 24.9 kg/m2. Supplementary Appendix Table 3 provides a detailed list of causes of death by BMI category.
DISCUSSION
In one of the largest population-based cohorts in sub-Saharan Africa, with near complete mortality estimation, we found that all-cause mortality over seven years of observation was lower in those who had a BMI of 25.0 – 29.9 kg/m2 or 30.0 – 34.9 kg/m2, compared to those who had a BMI 18.0 – 24.9 kg/m2, according to standard, clinically-defined BMI definitions. This is consistent with the known J-shaped curve that links BMI and mortality. This pattern was preserved in a sub-analysis of women, though our ability to describe these relationships in men was limited by smaller sample size. The protective effect of overweight and mild obesity was also best demonstrated for infectious causes of death; whereas we found neither a strong protective or harmful effect of higher BMI when restricted to non-communicable causes of death.
The current understanding of relationships between BMI and mortality is primarily based on evidence from HICs. [3, 6] However, this literature has had important clinical implications in terms of recommendations about ideal weight and healthy lifestyle, which have largely been incorporated into global primary and clinical care guidelines around weight loss and obesity prevention. Our study demonstrates that in this South African setting, the relationship between BMI and mortality also conforms to a J-shaped curve in which overweight is not associated with an increased risk of mortality. This was particularly the case for women, such that the lowest risk of short-term mortality might be afforded by a higher BMI, which is clinically defined as overweight or obesity in current guidelines. This was also true in those who were confirmed to be HIV-uninfected in this analysis. One potential hypothesis to explain these findings is that the determinants of higher BMI might be associated with improved access to healthcare, which in turn may be protective against many causes of premature mortality. Alternatively, this finding could be driven by differences in diet quality, or differences in the risk of cardiovascular disease associated with different BMI thresholds, among other factors.
Finally, we observed a lower risk of mortality due to infectious disease causes for those who were overweight versus those of normal weight. This finding was expected given that these deaths are likely driven in part by HIV and tuberculosis, both of which are highly prevalent and associated with wasting in their more advanced stages. In contrast, we were unable to draw definitive conclusions about the relationship between BMI and mortality due to non-communicable diseases, but our preliminary data do not show a strong protective or harmful effect of relatively higher weight in this population.
While data from LMICs are scarce, our findings are consistent with another recent population-based study of the relationship between BMI and cardiovascular outcomes from Chennai, India.[28] In that study, investigators enrolled over 400,000 participants between the ages of 35 and 69 years between 2002 and 2005 and then visited them biennially through 2015. While they uncovered a strong relationship between BMI and systolic blood pressure, they also found a weak relationship between BMI and cardiovascular mortality.[28] After adjusting for systolic blood pressure, BMI was inversely related to cardiac and stroke mortality, with underweight participants having a greater relative risk of cardiac and stroke, when compared with overweight participants.[28] Furthermore, among all participants in that study, as well as in a subset of lifelong non-smoking individuals, those who were overweight had a similarly low risk of mortality to those who were normal weight. This risk of mortality did not increase substantially until a threshold of BMI ≥30.0 kg/m2 was reached.
Efforts to confidently identify the causal framework and quantify the direction of the association between overweight or obesity and mortality have been subject to several theoretical methodological concerns, all of which were carefully considered in the design and execution of this study. We attempted to assess each of these challenges through close attention to methodological details and various sensitivity analyses. First, in a sensitivity analysis, we excluded deaths within the first two years of observation to reduce reverse causation bias. We found the magnitude and direction of relationships were stable, but the confidence intervals around our HR estimates for those with a BMI 18.0 – 24.9 kg/m2, 25.0 – 29.9 kg/m2 or 30.0 – 34.9 kg/m2 all increased, due to reduced power with a smaller number of outcomes in this sub-analysis. Further, we performed a sensitivity analysis in which we stratified the normal BMI group into a low-normal and high-normal BMI and found that those with a low-normal BMI (18.5 – <20.0 kg/m2) had a greater aHR of death, while those who had a high-normal BMI (22.5 – <25.0 kg/m2) had a lower aHR of death, both as compared to those with a BMI of 20 – <22.5 kg/m2. This reinforced our findings from the primary analysis. Finally, while there were no data collected on the smoking status of the participants in this survey, we believe that its influence on these results is likely modest at best, particularly among women, because recent studies have shown extremely low smoking rates. In a survey of smoking that was conducted in this population in 2012, only 4.1% (95% CI: 2.3 – 7.4%) of women in KwaZulu-Natal reported that they smoked and in a recent population-based survey in this area, only 1% of women self-reported that they were current smokers.[29]
There were several additional limitations to this study. First, while the sample size is large at over 9,000 individuals and the data comes from one of the largest population-based cohorts in southern Africa, the rate of participation in the one-time collection of BMI in 2010 was only 37%. Furthermore, participation was greater in women, who represented 62.0% of individuals who were eligible to participate in the study but 69.5% of those who consented to have their BMI measured. The cohort that took part in the data collection was also slightly more likely to be rural (61.6% overall v. 63.8% in participants) and somewhat less likely to be employed (22.7% overall v. 15.3% in participants), than the total eligible sample population. Otherwise, we did not observe major differences between those who did and did not participate in the survey, which somewhat mitigates the risk of selection bias. Moreover, we attempted to address any such differences by weighting all regression models for non-response in the 2010 survey, with weights calculated as the inverse probability of participation in the 2010 survey, in strata defined by age group, sex, and place of residence. We had a single BMI measurement followed by a relatively short follow-up time in this study of 7 years. While the measurement of both height and weight in the survey was a particular strength of the study, it is possible that non-communicable causes of death associated with higher BMI would require a longer period of follow-up to observe, or that these relationships may differ when considering the maximum lifetime or change in BMI.[30] Third, verbal autopsy is imperfect as an assessment of cause of death and thus our death assignment may be subject to misclassification, but this would not affect estimates of all-cause mortality in our primary analyses. Finally, we did not have data on specific chronic diseases co-morbidities. We also did not have data on waist-to-hip ratio and thus were unable to explore this alternative measure of body composition in these analyses; however, this is a potentially important consideration for future investigation.
In summary, we found that those with a BMI 25 – 29.9 kg/m2 or a BMI 30.0 – 34.9 kg/m2 had a lower overall risk of all-cause 7-year mortality in this large prospective cohort in rural South Africa. These findings were strongest for women, and for infectious causes of death, but were consistent in the overall cohort and robust to key sensitivity analyses.[5] In light of the widespread increases in the prevalence of higher BMI in these settings [2], future research should seek to corroborate our findings, while extrapolating the mechanisms by which body weight impacts morbidity and mortality.
Supplementary Material
STUDY IMPORTANCE.
What is already known about this subject?
Previous research regarding the relationship between body mass index (BMI) and mortality has been conducted in high-income settings but there are few studies of this relationship in sub-Saharan Africa.
The implications of clinically-defined BMI thresholds for overweight and obesity are poorly characterized in low-income settings such as South Africa.
What are the new findings in your manuscript?
This study describes the relationship between empirically measured BMI and all-cause mortality over 7 years of follow-up in a large cohort of adults in rural South Africa.
In this setting, the lowest risk of short-term mortality might be afforded by a BMI that has been clinically defined as overweight or Class I obesity in higher income settings. These findings were strongest in women and individuals dying from communicable conditions.
How might your results change the direction of research or the focus of clinical practice?
In light of the widespread increases in the prevalence of higher BMI in sub-Saharan Africa, these findings provide important insight about risk associated with high BMI in this region and specifically suggest that clinically-defined overweight may not confer an increased risk of mortality in this context.
Future research should focus on corroborating these findings, while extrapolating the mechanisms by which body weight impacts morbidity and mortality in sub-Saharan Africa.
ACKNOWLEDGEMENTS
We thank the outstanding field staff at the Africa Health Research Institute for their work in conducting the demographic health survey field work, as well as the community and the study participants for their time and efforts. The population HIV surveillance is a node of the South African Population Research Infrastructure Network (SAPRIN) and funded by the South African Department of Science and Innovation and hosted by the South African Medical Research Council.
FUNDING STATEMENT:
The Africa Health Research Institute receives funding from the UK Wellcome Trust grant 082384/Z/07/Z. MJS receives funding from the National Institutes of Health (R01 AG059504, R01 HL141053 and P30 AI060354). JMG is supported by grant number T32 AI007433 from the National Institute of Allergy and Infectious Diseases. The contents of this research are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.N. C. D. Risk Factor Collaboration, Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet, 2017. 390(10113): p. 2627-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.N. C. D. Risk Factor Collaboration - Africa Working Group, Trends in obesity and diabetes across Africa from 1980 to 2014: an analysis of pooled population-based studies. Int J Epidemiol, 2017. 46(5): p. 1421-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, et al. , Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA, 2013. 309(1): p. 71-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berrington de Gonzalez A, et al. , Body-mass index and mortality among 1.46 million white adults. N Engl J Med, 2010. 363(23): p. 2211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrison RJ and Castelli WP, Weight and thirty-year mortality of men in the Framingham Study. Ann Intern Med, 1985. 103(6 ( Pt 2)): p. 1006-9. [DOI] [PubMed] [Google Scholar]
- 6.Global BMIMC, et al. , Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet, 2016. 388(10046): p. 776-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenlee H, et al. , Association between Body Mass Index and Cancer Survival in a Pooled Analysis of 22 Clinical Trials. Cancer Epidemiol Biomarkers Prev, 2017. 26(1): p. 21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Risk Factor Collaboration NCD, Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet, 2016. 387(10027): p. 1513-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risk Factor Collaboration NCD, Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet, 2017. 389(10064): p. 37-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manne-Goehler J, et al. , Diabetes diagnosis and care in sub-Saharan Africa: pooled analysis of individual data from 12 countries. Lancet Diabetes Endocrinol, 2016. 4(11): p. 903-912. [DOI] [PubMed] [Google Scholar]
- 11.National Department of Health (NDoH), S.S.A., South African Medical Research Council (SAMRC), and ICF, South Africa Demographic and Health Survey 2016. 2019: Pretoria, South Africa [Google Scholar]
- 12.G. B. D. Obesity Collaborators, et al. , Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med, 2017. 377(1): p. 13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The World Health Organization. Obesity and Overweight. 2020. [cited 2020 May 13]; Available from: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight.
- 14.Tanser F, et al. , Cohort Profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol, 2008. 37(5): p. 956-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larmarange J, et al. , Participation dynamics in population-based longitudinal HIV surveillance in rural South Africa. PLoS One, 2015. 10(4): p. e0123345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandormael A, C. D, Kim HK, Bärnighausen T, Tanser F, The state of the HIV epidemic in rural KwaZulu-Natal, South Africa: a novel application of disease metrics to assess trajectories and highlight areas for intervention. International Journal of Epidemiology, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malaza A, et al. , Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PLoS One, 2012. 7(10): p. e47761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siedner MJ, et al. , Linkage to primary care after home-based blood pressure screening in rural KwaZulu-Natal, South Africa: a population-based cohort study. BMJ Open, 2018. 8(12): p. e023369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst AJ, Mafojane T, and Newell ML, Verbal autopsy-based cause-specific mortality trends in rural KwaZulu-Natal, South Africa, 2000–2009. Popul Health Metr, 2011. 9: p. 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byass P, et al. , Comparing verbal autopsy cause of death findings as determined by physician coding and probabilistic modelling: a public health analysis of 54 000 deaths in Africa and Asia. J Glob Health, 2015. 5(1): p. 010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magodoro I, O. S, Gareta D, Modise T, Koole O, Herbst K, Pillay D, Wong E, Siedner M. Engagement in HIV Care as an Avenue to Primary Care in Rural South Africa: Results from the Vukuzazi Population Health Platform in IDWeek 2019. 2019. Washington D.C.: Open Forum Infectious Diseases. [Google Scholar]
- 22.Filmer D and Pritchett LH, Estimating wealth effects without expenditure data--or tears: an application to educational enrollments in states of India. Demography, 2001. 38(1): p. 115-32. [DOI] [PubMed] [Google Scholar]
- 23.Vandormael A, et al. , Declines in HIV incidence among men and women in a South African population-based cohort. Nat Commun, 2019. 10(1): p. 5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandormael A, et al. , Incidence rate estimation, periodic testing and the limitations of the mid-point imputation approach. Int J Epidemiol, 2018. 47(1): p. 236-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC and Fine JP, Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med, 2017. 36(27): p. 4391-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seaman SR and White IR, Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res, 2013. 22(3): p. 278-95. [DOI] [PubMed] [Google Scholar]
- 27.Machin D, C. Y, Parmar MKB, Survival Analysis: a practical approach. 2006, West Sussex, England: John Wiley & Sons, Ltd. [Google Scholar]
- 28.Gajalakshmi V, et al. , Body-mass index, blood pressure, and cause-specific mortality in India: a prospective cohort study of 500 810 adults. Lancet Glob Health, 2018. 6(7): p. e787-e794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy P, et al. , Prevalence of tobacco use among adults in South Africa: Results from the first South African National Health and Nutrition Examination Survey. S Afr Med J, 2015. 105(8): p. 648-55. [DOI] [PubMed] [Google Scholar]
- 30.Yu E, et al. , Weight History and All-Cause and Cause-Specific Mortality in Three Prospective Cohort Studies. Ann Intern Med, 2017. 166(9): p. 613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


