Abstract
Background:
Previous studies found enlarged striatum and white matter in those with stimulants use disorders. Whether primarily ketamine users (Primarily-K) and ketamine users who co-used stimulants and other substances (K+PolyS) have abnormal brain volumes is unknown. This study aims to evaluate possible brain structural abnormalities, cognitive function and depressive symptoms, between Primarily-K and K+PolyS users.
Methods:
Striatal and white matter volumes were automatically segmented in 39 Primarily-K users, 41 K+PolyS users and 46 non-drug users (ND). Cognitive performance in 7 neurocognitive domains and depressive symptoms were also evaluated.
Results:
Ketamine users had larger caudates than ND-controls (Right: 1-way-ANCOVA-p=0.035; K+PolyS vs. ND, p =0.030; Linear trend for K+PolyS>Primarily-K>ND, p=0.011; Left: 1-way-ANCOVA-p=0.047, Primarily-K vs. ND p=0.051) and larger total white matter (1-way ANCOVA-p=0.009, Poly+K vs. Primarily-K, p=0.05; Poly+K vs. ND p=0.011; Linear trend for K+PolyS>Primarily-K >ND, p=0.004). Across all ketamine users, they performed poorer on Arithmetic, learning and memory tasks, and were more depressed than Non-users (p<0.001 to p=0.001). Greater lifetime ketamine usage correlated with more depressive symptoms (r=0.27, p=0.008). Larger white matter correlated with better learning across all participants (r=0.21, p=0.019), while larger right caudate correlated with lower depression scores in ketamine users (r=−0.28, p=0.013).
Conclusion:
Ketamine users had larger caudates and total white matter than ND-controls. The even larger white matter in K+PolyS users suggests additive effects from co-use of ketamine and stimulants. However, across the ketamine users, since greater volumes were associated with better learning and less depressive symptom, the enlarged caudates and white matter might represent a compensatory response.
Keywords: Ketamine, Stimulants, Caudate, White matter, Cognition, Depression
1. Introduction
Ketamine was synthesized in 1962 as an anesthetic agent, predominantly acting as a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist (Wolff and Winstock, 2006), with weak binding affinity to opioid, muscarinic, and monoamine receptors (Sinner and Graf, 2008; Zanos et al., 2018). Ketamine misuse started soon after its development and is still common among youth (Narcotics Division, 2017; Thames et al., 2016), gay men who frequent dance clubs (McCambridge et al., 2007; Schmidt et al., 2016) and poly-substance users (Morley et al., 2015). Ketamine dependence is especially prevalent in East Asia (Hser et al., 2016; Narcotics Division, 2017), and is currently one of the most commonly abused drugs, 5th in Hong Kong and 3rd in mainland China and Taiwan (Hser et al., 2016). Heavy ketamine users experience strong cravings (Chen et al., 2014; Morgan et al., 2012), and continue to use despite severe physical (e.g., urinary tract symptoms, gastritis, and liver and kidney dysfunction) (Cheung et al., 2011; Pal et al., 2013; Poon et al., 2010; Yiu-Cheung, 2012) and mental health problems (e.g., depression) (Fan et al., 2016; Tang et al., 2015).
Ketamine’s abuse potential has long been attributed to its indirect effects on increasing striatal dopamine release (Kokkinou et al., 2018). However, compared with psychostimulants such as cocaine and methamphetamine, ketamine only mildly stimulates striatal dopamine release (Kokkinou et al., 2018). In addition, dopamine receptor blockers failed to block the “high” effects of ketamine in healthy volunteers (Krystal et al., 1999), and the self-administration behavior in a rodent model (Carlezon and Wise, 1996). Given that fast and large amounts of dopamine release in the ventral striatum is necessary to reinforce the effects of substances with misuse potential (Volkow et al., 2017), how dopamine is involved in ketamine’s addictive effect is not yet clear (Kokkinou et al., 2018). Recent data also suggested that ketamine’s addictive effect might be related to its action on opioid receptors (Williams et al., 2018). Furthermore, ketamine users often co-use psychostimulants (e.g., cocaine, methamphetamine) (Liang et al., 2015; Tang et al., 2011), and ketamine may exacerbate the craving for psychostimulant use (Kegeles et al., 2000), similar to how tobacco use in cocaine users may augment the craving for their cocaine use (Brewer et al., 2013).
In contrast to ketamine, psychostimulants cause marked increases in dopamine release in the striatum. Individuals who chronically used psychostimulants showed larger striatal volumes (Andres et al., 2016; Chang et al., 2005a; Ersche et al., 2011; Fein and Fein, 2013; Grodin and Momenan, 2017; He et al., 2018; Jacobsen et al., 2001; Jernigan et al., 2005; Mackey et al., 2014). Similarly, rodents and nonhuman primates also showed enlarged striatal volumes after chronic methamphetamine administration (Groman et al., 2013; Thanos et al., 2016). These enlarged striatal structures are thought to be an adaptive response to dopamine depletion or the lack of dopamine D2 receptor (D2R) signal pathways, because larger striatal volumes were also seen following chronic D2R blockade with antipsychotic treatments (Fan et al., 2019; Hashimoto et al., 2018; Leung et al., 2011). In addition, glial activation and neuroinflammation might also contribute to enlarged striatal volumes found in stimulant users (Andres et al., 2016; Groman et al., 2013; Taylor et al., 2007; Thanos et al., 2016). However, larger striatal volumes were seen in non-user siblings of stimulant users as well, suggesting that this phenotype could also be a pre-morbid vulnerability for stimulant use disorder (Ersche et al., 2013). Whether ketamine users and those who co-use psychostimulants also show similarly enlarged striatal structures remains unclear.
In contrast to the numerous morphometry studies in stimulant users, only one study used voxel-based morphometry to evaluate for possible volume abnormalities in chronic ketamine users, but no abnormalities in the subcortical regions were reported (Liao et al., 2011). However, this study did not assess how recent regular psychostimulant co-use might impact the brain volumes in ketamine users. Several studies also find enlarged white matter volumes in psychostimulant users (Chang et al., 2005a; Mon et al., 2014; Thompson et al., 2004), and abnormal white matter integrity in chronic ketamine users (Liao et al 2010) compared with non-users. A few studies further evaluated cognition in relation to striatal volume alterations in stimulant users, but the findings were inconsistent. For instance, in current or abstinent methamphetamine users, enlarged striatal volumes did not correlate with the severity of cognitive impairment (Jernigan et al., 2005; Thayer et al., 2019), but did correlate with better verbal fluency, motor function or inhibitory function (Chang et al., 2005a; Jan et al., 2012). Similarly, nonhuman primates chronically treated with methamphetamine showed enlarged striatal structures and poorer inhibitory control (Groman et al., 2012). However, whether ketamine users with or without stimulant co-use show similar associations between striatal or white matter volumes and cognitive and behavioral function remains unknown. Lastly, ketamine is well known for its antidepressant effects (Yang et al., 2019). Several studies found that ketamine used as an antidepressant led to increased structural (Vasavada et al., 2016) and functional (Abdallah et al., 2017) connectivity, increased glucose metabolism (Nugent et al., 2014) and increased neural activity (Murrough et al., 2015) in the striatum. While depressive symptoms are prevalent among regular ketamine users (Fan et al., 2016; Tang et al., 2015), no study examined the relationship between striatum volumes and depressive symptoms in regular ketamine users. The goal of this study was to measure the striatal and white matter volumes and their associations with cognitive and depressive symptom measures in chronic ketamine (Primarily-K) users, with or without additional regular polysubstance use (PolyS), especially psychostimulants, compared with non-drug users. We hypothesized that due to the chronic indirect effects on dopamine release (Kokkinou et al., 2018), the striatal structures (caudate, putamen and globus pallidus) and white matter volumes in Primarily-Ketamine users would be enlarged, but even more in ketamine users who co-use polysubstances (K+PolyS) since they would have even greater dopamine release and stimulation. Based on prior studies that showed the enlarged striatal structures may reflect a compensatory response and facilitate maintenance of cognitive performance in stimulant users (Chang et al., 2005a), we also expected that ketamine users, especially K+PolyS, with larger striatal or white matter volumes would have better cognitive performance and fewer depressive symptoms.
2. Materials and methods
2.1. Participants
The participants were recruited during 2011– 2015 from several non-governmental organizations (NGOs) in Hong Kong that offered drug rehabilitation services. Ketamine users were referred by counselling centers, residential treatment centers, and youth outreach teams. Healthy non-drug (ND) user controls were recruited from community service centers. Both ketamine users and ND user controls who met the following inclusion criteria were enrolled: 1) age 18 – 40 years; 2) right-handed; 3) capable and willing to provide valid informed consent. In addition, ketamine users were enrolled if they 4) were attending a drug rehabilitation service at an NGO; 5) used ketamine at least 24 times and other illicit psychoactive substances less than 1x/week over 6 months (< 24 times), within the last 2 years for the “primarily” ketamine (Primarily-K) users,; and 6) used both ketamine at least 24 times, as well as other illicit psychoactive substances at least 24 times over 6 months within the last 2 years, for K+PolyS users. Since tobacco smoking is highly prevalent (70 −100%) amongst ketamine users in mainland China (Li et al., 2017; Liao et al., 2011) and Hong Kong (Lee et al., 2005), nicotine dependence and social alcohol drinking were permitted for both user groups. For any illegal substances that were abused by the participants, lifetime use was defined as any amounts ever used in their lifetime, and regular use was defined as use of the substance for more than once per week over a 6-month period. The participants in the healthy ND control group had no history of substance use (tobacco smoking and recreational alcohol consumption were allowed). Exclusion criteria for all participants were: 1) any neurological disorders including history of significant brain injury (e.g., traumatic brain injury with loss of consciousness, strokes, etc.), or any significant chronic medical disease (e.g., hepatic or renal dysfunction) that required regular medications; 2) reported current or history of major psychiatric disorders (e.g., major depression, schizophrenia or bipolar disorder) with the exception of substance use disorder; 3) unable to provide written consent; 4) any MRI contraindications; 5) pregnancy (by self-report); 6) any significant brain lesion(s) (the MRI scans were reviewed by a senior Radiologist). The study was approved by the Survey and Behavioral Research Ethics Committee of the Chinese University of Hong Kong. All participants provided written consent. Each participant was compensated with a shopping coupon worth HK$500 (approximately US$65) upon the completion of the study for their travel costs and missed work hours. All participants were native Cantonese speakers and the entire study protocol was administered using Cantonese language. The entire protocol was designed to be completed in two days, with cognitive and psychological assessments on the first day and MRI scans on the second day.
2.2. Assessments for psychopathological symptoms and cognitive function
A research assistant interviewed each participant at the NGO or at our research center and performed the psychological assessment. The entire assessment lasted 2 h and included smoking breaks.
The Severity of Dependence Scale (SDS) (Gossop et al., 1995), a 5-item self-report scale, was used to measure the degree of dependence for each substance in the previous month or the month before abstinence. Each item of the SDS was scored from 0 to 3, with higher scores indicating greater severity of dependence. The 21-item version of the Beck Depression Inventory (BDI) (Shek, 1990) was used to screen for depressive symptoms. The anxiety subscale of the Hospital Anxiety Depression Scale (HADSA) (Leung et al., 1993) was used to assess anxiety symptoms. The HADSA comprised 7 items, each graded from 0 to 3; higher scores indicate greater severity of symptoms. Addiction Severity Inventory-Lite Version (Cacciola et al., 2007; McLellan et al., 1980) was used to evaluate substance use patterns, including alcohol consumption quantitation. The Structured Clinical Interview (SCID) for DSM-IV Axis I Disorders was used to determine the criteria used in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM IV) (American Psychiatric Association, 1994) for the diagnosis of drug dependency.
The cognitive assessments provided measures for seven cognitive domains: Working Memory (Wechsler Adult Intelligence Scale [WAIS]-III Digit Span Forward and Backward, Arithmetic) (Wechsler et al., 2002), Verbal Memory (WMS-III Logic Memory delay recall, retention and recognition, WMS-III Word List delay recall and retention) (Hua et al., 2005; Wechsler, 1997a, 1997b), Visual memory (Rey-Osterrieth Complex Figure test [RCF] (Osterrieth, 1944; Taylor, 1959) delay recall and retention), Learning (WMS-III Logic Memory immediate recall, WMS-III Word List immediate recall and RCF immediate recall), Executive function (Modified Verbal Fluency Test (Chiu et al., 1997), Stroop interference (Stroop, 1935), Wisconsin Card Sorting Test (Heaton et al., 1993) and RCF copy (Flak et al., 2019; Shin et al., 2006)), Information Processing Speed (Stroop color reading, Digit Symbol Coding) and Language (Modified Boston Naming test (Cheung et al., 2000; Goodglass and Kaplan, 1983) (Table 2). For tasks that included immediate recall and delayed recall (Logic Memory, Word List, and RCF), retention (% delayed recall/copy or immediate recall) rather than delayed recall was used as the index of memory component.
Table 2.
Neuropsychological Performance of participants (Mean ± S.E.) and p-values from One-way ANCOVA
| ND (N=56) | Primarily-K Users (K, N=60) | K+PolyS Users (K+PS, N=64) | 1-way ANCOVA p-value |
post hoc p-value (adjusted) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Education | Group | ND v. K | ND v. K+PS | K v. K+PS | ||||
| Working memory | ||||||||||
| Digit span forward | 15.5 ± 0.2 | 15.5 ± 0.2 | 15.2 ± 0.2 | 0.191 | 0.990 | 0.597 | 0.321 | |||
| Digit span backward | 9.8 ± 0.5 | 8.9 ± 0.4 | 8.5 ± 0.4 | 0.069 | 0.459 | 0.047 | 0.217 | |||
| Arithmetic | 16.2 ± 0.6 | 13.5 ± 0.5 | 13.2 ± 0.4 | 0.748 | 0.013 | <0.001 | <0.001a | 0.002 | <0.001 | 0.624 |
| Verbal Memory | ||||||||||
| LM retentionc | 87.8 ± 3.7 | 71.2 ± 3.0 | 81.4 ± 2.8 | 0.075 | 0.293 | 0.493 | 0.003a | 0.007 | 0.621 | 0.022 |
| LM recognition | 25.2 ± 0.7 | 21.2 ± 0.5 | 22.2 ± 0.5 | 0.009 | 0.012 | 0.435 | <0.001a | <0.001 | 0.004 | 0.414 |
| WL retentionc | 86.4 ± 4.1 | 77 ± 3.7 | 84.2 ± 3.6 | 0.014 | 0.830 | 0.617 | 0.053 | |||
| Visual Memory | ||||||||||
| RCF retentionc | 1 ± 0 | 1.1 ± 0 | 1. ± 0 | 0.858 | 0.398 | 0.699 | 0.328 | |||
| Learning | ||||||||||
| LM immediate recall | 40.7 ± 2.4 | 27.2 ± 2.2 | 33.3 ± 2.1 | 0.019 | 0.154 | 0.068 | <0.001a | <0.001 | 0.002 | 0.210 |
| WL first recall | 5.8 ± 0.3 | 5.1 ± 0.2 | 5.3 ± 0.2 | 0.005 | 0.442 | 0.263 | 0.246 | |||
| RCF immediate recall | 25.3 ± 1.2 | 21.2 ± 1.0 | 19.0 ± 0.9 | 0.857 | 0.231 | 0.841 | 0.001a,b | 0.070 | 0.001 | 0.277 |
| Executive Function | ||||||||||
| Verbal Fluencyd | 47.9 ± 1.6 | 41.2 ± 1.5 | 46.8 ± 1.4 | 0.414 | 0.157 | 0.039 | 0.121 | |||
| Stroop interference | 8.2 ± 1.1 | 10.7 ± 0.9 | 10.7 ± 0.8 | 0.327 | 0.387 | 0.768 | 0.246 | |||
| WCST total errors | 20.5 ± 2.9 | 24.8 ± 2.4 | 25 ± 2.2 | 0.012 | 0.832 | 0.104 | 0.517 | |||
| RCF copy | 33.6 ± 0.5 | 33.9 ± 0.4 | 32.6 ± 0.4 | 0.757 | 0.635 | 0.049 | 0.016 | |||
| Speed of Information Processing | ||||||||||
| Stroop color reading | 13.4 ± 0.5 | 12.6 ± 0.4 | 12.4 ± 0.4 | 0.803 | 0.076 | 0.003 | 0.407 | |||
| Digit symbol coding | 89.2 ± 2.5 | 88.1 ± 2.1 | 85.0 ± 1.9 | 0.009 | 0.019 | 0.004 | 0.325 | |||
| Language | ||||||||||
| MBNT | 15.1 ± 0.2 | 14.5 ± 0.1 | 14.4 ± 0.1 | 0.363 | 0.052 | 0.708 | 0.056 | |||
Tukey’s Test of post hoc comparisons were performed only for groups that have p values remain significant after Holm-Bonferroni correction.
p-value for RCF immediate recall was 0.005 after covariated for RCF copy.
LM retention: LM delayed recall/LM immediate recall; WL retention: WL delayed recall/WL 5th immediate recall; RCF retention: RCF delay recall/RCF copy.
Sex-by-group interaction showed a trend for significance for Verbal Fluency, p=0.030 (not significant after Holms-Bonferroni correction).
Abbreviations: LM: Wechsler Adult Intelligence Test-III (WAIS-III) Logical Memory; RCF: Rey-Osterrieth Complex Figure test; WL: WMS-III Words Learning; WCST: Wisconsin Card Sorting Test; MBNT: Modified Boston Naming test.
2.3. Image acquisition
All participants were scanned on a 3T scanner (Philips Achieva 3.0T, X Series, Quasar Dual MRI System, Best, Netherlands) at the Prince of Wales Hospital in Hong Kong. Each participant was asked to refrain from illegal drug use for 4–7 days, and each provided a drug-free urine sample on the day of the scan. In addition, all participants had a negative alcohol Breathalyzer test. However, to avoid acute withdrawal effects from nicotine, tobacco smokers were allowed a smoke break before the scans. Structural images included a T1-weighted 3D Spoiled Gradient Echo sequence (TE/TR = 2.23/25ms, flip angle=30 degrees, pixel resolution: 0.89×0.89×1.5mm) and a T2-weighted transversal fluid attenuated inversion recovery (FLAIR) sequence (TE/TR = 125/11000ms, flip angle=120 degrees, inversion time=280ms, pixel resolution=0.65×0.87×5mm). Structural images were assessed by a certified radiologist (C.W.) for possible neuroanatomical abnormalities. One participant was found to have a small cerebellar cyst, but none of the scans were excluded due to neuroanatomical abnormality.
2.4. Image processing
Three striatal structures (caudate, putamen, and globus pallidus) were selected as volumes of interests (VOIs) and were automatically segmented using a published customized analysis procedure (Figure 1A) (Chen et al., 2016; Herskovits et al., 2015). The analysis procedure involved brain extraction (Smith, 2002), followed by normalization (Jenkinson et al., 2012), segmentation (Jenkinson et al., 2012), and parcellation of these volumes, which were overlaid on the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002). All results from the skull-stripping, tissue segmentation, and parcellation were visually inspected by the authors (HL, TE and LC). One scan had poor segmentation in the caudates and was removed from the final analyses. The nucleus accumbens and ventral striatum were included at the bottom portions of the neostriatal structures (caudate and putamen) in the Atlas used in this study (Figure 1A) (Rolls et al., 2020). The total grey matter and total white matter volumes, and intracranial volume (ICV) were also determined. Since prior studies did not find hemispheric differences in white matter abnormalities in stimulant users (Beard et al., 2019; Chang et al., 2005a; Mon et al., 2014; Schlaepfer et al., 2006; Thompson et al., 2004), to minimize the number of ROIs measured, these whole brain measures were not separated into left and right hemispheres. Volumes of selected regions were normalized by the estimated ICV (sum of the volumes of total grey matter, total white matter, and cerebrospinal fluid).
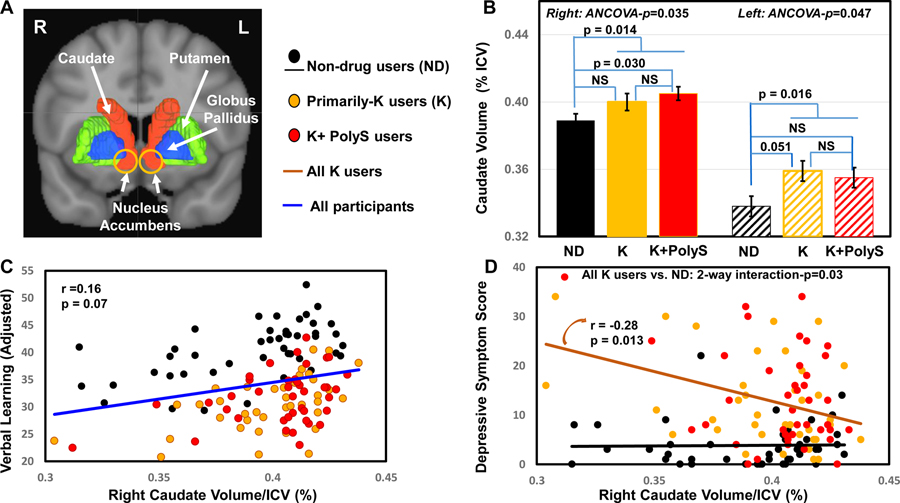
Figure 1. Caudate Volumes: Partial Correlations with Verbal Learning and Depressive Symptoms.
A) Volumes of interests (VOIs) segmented from the Automated Anatomical Labeling (AAL) atlas are shown; note that the nucleus accumbens is included in the caudate VOI (yellow circle). B) Right caudate volumes were different across three groups (1-way ANCOVA, p= 0.035). Ketamine polysubstance (K+PolyS) users had the largest right caudates across groups, adjusted for age and sex. There was a linear trend in the right caudates, with K+PolyS>Primarily-K>Non-drug users (p=0.011). The two ketamine user groups combined also had larger right caudate volume than ND group (p=0.014). Adjusted p-values were derived from Tukey’s post hoc tests. In the left caudates, group difference across the three groups was also significant (p=0.047); Primarily-K users showed a trend for larger left caudates than ND controls (P=0.051). The two ketamine groups combined also had larger left caudates than ND controls (p=0.016). C) Across all participants (blue line), larger right caudate volumes showed a trend for correlation with better verbal learning (r=0.16, p=0.07), adjusted for age, sex and education. D) Among all ketamine users (brown line), but not the control subjects, larger right caudate volumes predicted with lower depressive symptom scores (Interaction-p=0.030; in ketamine users r=−0.28, p=0.013), adjusted for age and sex. NS=non-significant.
2.5. Statistical analyses
Data were analyzed using R (version 3.5.2 https://www.R-project.org/)(R Core Team, 2019). Continuous variables were described and reported as mean±SE, median and range, and categorical variables as N (%). The demographic and clinical variables were compared across groups using analysis of variance (ANOVA), T-test, Chi-square, Kruskal-Wallis test or Mann-Whitney U, depending on the type and distribution of the variables. One-way analysis of covariance (ANCOVA) was used to assess group differences on the raw scores of cognitive performance and brain volumes; age, sex and education were included as covariates. A “trend test” was performed for volume measures that showed significant group difference to explore whether the group differences followed a linear trend (Wiens and Nilsson, 2017). However, education did not have an effect and was removed from the final models for brain volume comparisons. Sex-by-ketamine use interactions were also evaluated for cognitive and brain volume measures. The significance level was set at p<0.05. Corrections for multiple comparisons were performed using Holm-Bonferroni correction for the cognitive measures, but not for the brain VOIs measured, since we had a priori hypotheses that these selected brain regions would be larger bilaterally. Tukey’s Post hoc analyses were conducted for VOIs with group differences. Tukey’s Post hoc analyses for cognitive performance were limited to measures with significant group differences on the ANCOVA after Holm-Bonferroni correction. Exploratory regression analyses between 3 VOIs (% ICV) (left and right caudate and total white matter) and 5 cognitive measures (Arithmetic, LM retention, LM recognition, LM immediate recall and RCF immediate recall) that showed group differences were explored using the following linear regression model: cognitive performance as the dependent variable; group (ketamine or ND dummy code), volume and their 2-way interactions as independent variables; and age, sex and years of education as covariates. Additional exploratory correlations between ROI measures and ketamine use patterns, stimulants use patterns, SDS and BDI scores were also explored. Uncorrected p-values were presented for exploratory regression analyses.
3. Results
3.1. Participant characteristics
In total, 60 Primarily-K users, 64 K+PolyS users, and 56 ND user controls were enrolled in the study and completed the psychopathological and cognitive assessments. All participants were Chinese. Sex proportion and monthly incomes were similar across groups. However, both groups of ketamine users were slightly older (p=0.048), and had lower levels of education (p<0.001), than the ND controls (Table 1). Primarily-K users also had more depressive/anxiety symptoms than ND controls (higher BDI and HADSA scores, p-values<0.001). Across both ketamine user groups, a greater amount of lifetime ketamine use was associated with more depressive symptoms (r=0.27, p=0.008). The two ketamine user groups had similar ketamine use patterns and similar alcohol use levels, but the K+PolyS group tended to start ketamine use at younger ages (P=0.05). The majority of ketamine users (85 % in Primarily-K group, 90 % in the K+PolyS group) fulfilled the DSM-IV criteria for lifetime dependency for ketamine. After ketamine, the next most popular drugs used regularly within the past 2 years by K+PolyS users were cocaine and methamphetamine. The K+PolyS group used more cocaine (P<0.001) and marijuana (P=0.002) in the past 2 years than the Primarily-K users. None of the participants, except for one in the K+PolyS group, used opioids in the past two years. Six participants in the Primarily-K user group and 7 in the K+PolyS group reported sedative use in the past 2 years (p=0.89) and only 1 Primarily-K user and 2 K+PolyS users reported using cough medicine in the previous 2 years (Table 1). None of the ketamine users fulfilled the DSM-IV lifetime dependency criteria for other substances except for ketamine and tobacco smoking.
Table 1:
Participants’ Demographics and Clinical Characteristics (mean ± S.E. or median & range)
| Non-Users (N=56) | Primarily-K (N=60) | K + PolyS (N=64) | p-value | |
|---|---|---|---|---|
| Age (years) | 23.9 ± 0.6 | 26.0 ± 0.6 | 25.6 ± 0.6 | 0.048 a |
| Age range | (18 – 41) | (19 – 39) | (18 – 41) | |
| # Men (%) | 24 (42.1%) | 37 (61.7%) | 36 (56.3%) | 0.092b |
| Education (years) | 13.6 ± 0.3 | 9.2 ± 0.3 | 9.6 ± 0.2 | <0.001 a |
| Monthly Income (*1000HKD) | 8.9 ± 1.2 | 5.3 ± 0.9 | 5.4 ± 1.5 | 0.063a |
| BDI total score (0–63) | 3.8 ± 0.6 | 12.5 ± 1.3 | 14.7 ± 1.3 | <0.001 a |
| HADSA (0–21) | 2.8 ± 0.3 | 5.3 ± 0.5 | 6.9 ± 0.5 | <0.001 a |
| SDS scale score (0–15) | - | 8.3 ± 0.4 | 8.5 ± 0.4 | 0.666c |
| Lifetime alcohol user, n (%) | 34 (60.7%) | 44 (73.3%) | 58 (92.1%) | 0.148b |
| Days of alcohol use in past month | 0.9 ± 1.4 | 3.1 ± 6.8 | 3.3 ± 7.0 | 0.914d |
| Completed MRI scans, n (%) | 46 (80.7%) | 39 (65.0%) | 41 (64.1%) | 0.090b |
| Ketamine usage pattern | ||||
| Age of first use | - | 17.0 ± 0.4 | 16.0 ± 0.4 | 0.05c |
| Age of regular use | - | 19.0 ± 0.6 | 18.4 ± 0.4 | 0.139c |
| Duration of regular use (months) | - | 76.3 ± 5.5 | 77.7 ± 5.4 | 0.855c |
| Days of use in past 2 years (median, range) | - | 512.4 (97–730) | 650 (92–730) | 0.286d |
| Daily dosage (gram) | - | 3.2 (0.3–28) | 3 (0.2–28) | 0.879e |
| Lifetime usage (Log, gram) | - | 3.6 ± 0.1 | 3.7 ± 0.1 | 0.621c |
| Days since last use (median, range) | 63.0 (0 – 666) | 30.0 (0 – 486) | 0.075e | |
| DSMIV-dependence in past year | - | 50 (84.7%) | 57 (90.5%) | 0.615b |
| Cocaine Co-Use | ||||
| Lifetime use, n (%) | - | 45 (76.3%) | 55 (84.6%) | 0.240b |
| Lifetime regular use, n (%) | - | 17 (29.3%) | 45 (69.2%) | <0.001 b |
| Duration of regular use (months) | - | 12 (1–48) | 33 (1.5–105) | 0.007 e |
| # user (past 2 year) | 18 (30.5%) | 41 (64.1%) | <0.001 b | |
| Days of use in past 2 year | 21 (1–51) | 258 (5–728) | <0.001 d | |
| Months since last use (median, range) | - | 28 (0.5–133) | 3.5 (0–145.2) | <0.001 d |
| Methamphetamine Co-Use | ||||
| Lifetime use, n (%) | - | 27 (45.8%) | 31 (47.7%) | 0.830b |
| Lifetime regular user, n (%) | - | 10 (16.9%) | 19 (29.7%) | 0.096b |
| Duration of regular use (months) | - | 16 (1–144) | 12 (1.5–108) | 0.808e |
| # user (past 2 year) | 6 (10.2%) | 14 (21.9%) | 0.079b | |
| Days of use in past 2 year | 31 (0–62) | 97 (1–254) | 0.772d | |
| Months since last use (median, range) | 39 (0.8 – 212) | 14.1 (0–107.6) | 0.047 d | |
| Marijuana Co-Use | ||||
| Lifetime use, n (%) | - | 37 (62.7%) | 44 (67.7%) | 0.561b |
| Lifetime regular use, n (%) | - | 6 (10.2%) | 18 (27.7%) | 0.014 b |
| Duration of regular use (months) | - | 96 (3–136) | 12 (2–196) | 0.663e |
| # user (past 2 year) | 8 (13.8%) | 9 (14.3%) | 0.938b | |
| Days of use in past 2 year | 0 (0–4) | 96 (5–526) | 0.002 d | |
| Months since last use (median, range) | 63 (0.23 −276.3) | 53 (0.03–200.3) | 0.375d | |
| Hallucinogens | ||||
| Lifetime use, n (%) | - | 35 (61.4%) | 35 (53.8%) | 0.400b |
| Lifetime regular use, n (%) | - | 18 (31.6%) | 18 (27.7%) | 0.639b |
| Duration of regular use (months) | - | 24 (3–120) | 18 (1–72) | 0.677e |
| # Any use in past 2 years, n (%) | - | 0 | 0 | - |
| Months since last use (median, range) | - | 77 (25–179) | 98.6 (26–236.9) | 0.197e |
ANOVA;
χ2;
T test;
Kruskal-Wallis test;
Mann-Whitney U;
p-values in bold indicates < 0.05. Primarily-K use: use ketamine >1/week in any 6 months in past 2 years; use other drugs less than 1/w in any 6 months in past 2 years. Days of use in past 2 years: calculated only among users, one missing value from cough medicine user; Duration of regular use: calculated only among users; Regular use: ≥1/week x 6 months. BDI = The 21-item version of Beck Depression Inventory. HADSA = Hospital Anxiety Depression Scale (Anxiety). SDS=Severity of Dependence Scale
Among the participants who completed the neuropsychological assessments on Day 1 of the study, 39 Primarily-K, 41 K+PolyS users, and 46 ND controls also completed the brain MRI scans on Day 2. Retention rate was slightly higher in the ND group (80.7 %) than in the two ketamine user groups (64 %−65 %, p=0.09). Those who did not complete the study were more likely to be ketamine users compared with those who completed the study (80 % vs. 63 %, p=0.028). Compared to participants who completed the study (n=126), those who did not return for the MRI scans (n=55) had fewer years of education (p=0.023), shorter duration of ketamine use (p=0.015) and shorter duration of abstinence from cocaine use (p=0.018). Those who did not return to complete the MRI scans also performed worse on Arithmetic (p=0.036), LM recognition (p=0.044), LM and RCF immediate recall, RCF copy (p=0.035) and Verbal Fluency tasks (p=0.003), compared to participants who completed the study.
3.2. Neuropsychological performance
Compared to ND controls, ketamine users had poorer performance in several cognitive domains, including Working memory (Arithmetic), Verbal Memory (Logical Memory Retention and Recognition), and Learning (Logical Memory immediate recall and RCF immediate recall). In addition, the poorer performance on RCF immediate recall remained significant after the RCF copy score was included as a covariate. The ANCOVA-p-values for these group differences ranged from 0.003 to <0.001, adjusted for age, sex, and years of education (Table 2). These p-values remained significant after Holm-Bonferroni correction. However, the sex-by-group interactions were not significant for any of the measures, except for a trend for significance for the fluency scores (p=0.030; not significant after Holm-Bonferroni correction).
On post hoc analyses, both ketamine user groups performed poorer compared to ND controls on Working Memory-Arithmetic, (Primarily-K vs. ND: −20 %, p=0.002; K+PolyS vs. ND: −23 %, p<0.001) and Verbal Memory-Logical Memory Recognition (Primarily-K vs. ND: −19 %, p<0.001; K+ PolyS vs. ND: −14 %, p=0.004). In the Verbal Memory domain, Primarily-K users also had poorer Logical Memory Retention than ND (Primarily-K vs. ND: −23 %, p=0.007). In the Learning domain, compared to ND, both ketamine user groups also performed poorer in LM immediate recall (Primarily-K vs. ND: −49 %; p<0.001; K+PolyS vs. ND: −22 %, p=0.002), and the K+PolyS users had lower RCF immediate recall (K+PolyS vs. ND:−33 %, p=0.001). On post hoc comparisons between the two ketamine groups, Primarily-K performed poorer than K+PolyS users on Logic Memory retention (p=0.022) (Table 2). Sex-by-ketamine use effect was found only in the Verbal Fluency test (interaction-p=0.022), with the male Primarily-K users showing the lowest scores, which were lower than the male K+PolyS users (−16 %, p=0.006) and the female Primarily-K users (−13 %, p=0.058).
3.3. Brain morphometry and associations with cognition and drug Use
Comparisons of regional brain volumes were adjusted for age and sex, but not for education since it had no effects on the volume measurements (see Table 3). Consistent with prior studies (Wyciszkiewicz and Pawlak, 2014), the right caudates were larger than the left caudates across all participants. Ketamine users had larger right caudate volumes than ND controls (ANCOVA-p=0.035; Linear trend for K+PolyS > Primarily-K > ND -p=0.011; K+PolyS vs. ND, +4 %, p=0.030) (Figure 1B). Post hoc analyses showed that the right caudate volumes were not different between the two ketamine groups and between Primarily-K and ND groups (p=0.206). Group differences in the left caudate were also significant (ANCOVA-p=0.047) (Figure 1B). Post hoc analyses showed a trend for larger left caudates in the Primarily-K group compared to ND controls (p=0.051). In addition, for comparison with other studies that might evaluate ketamine users without separating the Primarily-K users from the K+PolyS users (Liao et al., 2011; Liao et al., 2010), and since the two ketamine groups were not significantly different, we combined the two ketamine groups and found that the combined ketamine user group showed larger caudates on both sides (right: +3.1%, p=0.014; left: +5.0%, p=0.016) than the ND group (Figure 1B).
Table 3.
Total Brain and Subcortical Volumes (% relative to ICV) Across Groups
| ROI/ICV (%) | Non-User (N=46) | Primarily-K (K, N=39) | K+PolyS (N=41) | 1-way ANCOVA p-value* | post hoc (corrected-p**) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Group | Linear trend | K vs. ND | K+PolyS vs. ND | K vs. K+PolyS | ||||
| Total Grey Matter | 38.7 ± 0.2 | 38.8 ± 0.2 | 38.5 ± 0.2 | <0.001 | <0.001 | 0.491 | NS | --- | --- | --- |
| Total White Matter | 42.8 ± 0.1 | 42.9 ± 0.2 | 43.3 ± 0.1 | 0.140 | 0.157 | 0.009 | 0.004 | 0.857 | 0.011 | 0.05 |
| L Caudate | 0.338 ± 0.006 | 0.359 ± 0.006 | 0.355 ± 0.006 | 0.014 | 0.026 | 0.047 | 0.061 | 0.051 | 0.146 | 0.863 |
| R Caudate | 0.389 ± 0.004 | 0.400± 0.004 | 0.405 ± 0.004 | 0.073 | 0.140 | 0.035 | 0.011 | 0.206 | 0.030 | 0.698 |
| L Putamen | 0.428 ± 0.002 | 0.431 ± 0.002 | 0.43 ± 0.002 | 0.054 | 0.048 | 0.716 | NS | --- | --- | --- |
| R Putamen | 0.453 ± 0.002 | 0.454 ± 0.002 | 0.454 ± 0.002 | 0.115 | 0.071 | 0.873 | NS | --- | --- | --- |
| L Globus Pallidus | 0.12 ± 0.0 1 | 0.12 ± 0.0 1 | 0.12 ± 0.001 | 0.515 | 0.170 | 0.972 | NS | --- | --- | --- |
| R Globus Pallidus | 0.117 ± 0.001 | 0.116 ± 0.001 | 0.115 ± 0.001 | 0.494 | 0.024 | 0.687 | NS | --- | --- | --- |
P-values are from 1-way ANCOVA covaried for Age and Sex. P-values < 0.05 are bolded.
Corrected-p values are derived from the “adjusted p-values” from the Tukey tests.
L=left; R=right
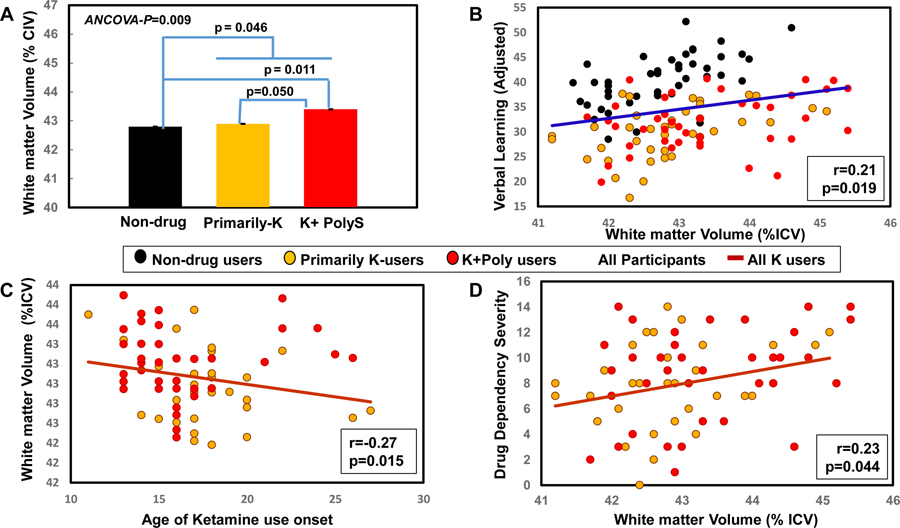
Total white matter volumes were significantly different amongst the 3 groups (ANCOVA-p=0.009). More specifically, K+PolyS users had larger total white matter volumes than Primarily-K users (+0.9 %, p=0.05) and ND controls (+1.2 %, p=0.011), resulting in a linear trend for K+PolyS > Primarily-K > ND (p= 0.004; Figure 2A). Again, for comparison with other studies, the two combined ketamine groups also had larger white matter volumes than the ND group (+0.7 %, p=0.046). No group differences were found in the other brain regions assessed, including the putamen, total grey matter and estimated intracranial volume. No sex-by-ketamine use interactions were observed on any of the basal ganglia sub-regions. In addition, since marijuana use might impact white matter and subcortical volumes (Orr et al., 2016), we added the days of marijuana use in the past 2 years as a covariate, the group differences in either caudate or total white matter volumes remained significant.
Figure 2. White matter volumes : Partial correlations with verbal learning and dependency severity.
A) The Total white matter volumes were significantly different across the three groups (ANCOVA-p=0.009). Ketamine polydrug (K+PolyS) users had larger white matter volumes than ND users (p=0.011) and Primarily-K users (p=0.050), covaried for age and sex, with a linear trend showing K+PolyS>Primarily-K>Non-drug users (p=0.004). Adjusted p-values were derived from Tukey’s post hoc tests. In addition, all ketamine users combined had larger white matter volumes than ND controls (p=0.046). B) Across all participants (blue line), larger white matter volumes predicted better verbal learning, after adjustments for age, sex and education (r=0.21, p=0.019). C) Across both ketamine user groups (brown line), earlier age of onset of ketamine use (years) predicted larger white matter volumes (r=−0.27, p=0.015). D) Across both ketamine user groups (brown line), larger white matter volumes predicted more severe drug dependency, after adjustments for age and sex (r=0.23, p=0.044).
We further explored the relationships between volume measurements and cognitive function, depressive symptoms, drug dependency scores and ketamine and cocaine use patterns. The two ketamine groups were combined in the regression analyses since the regression models were not significantly different between the two ketamine user groups. Across all participants, larger right caudate volumes did not predict better Verbal Learning, but the p-value approached significance (r=0.16, p=0.07) (Figure 1C). Across all ketamine users, but not in ND controls, those with larger right caudates also had fewer depressive symptoms (r=−0.28, p=0.013) (Interaction-p=0.03) (Figure 1D). Although more days of concurrent cocaine use did not predict larger right caudate volumes in K+PolyS users, the p-value also approached significance (r=0.36, p=0.058; data not shown). However, larger white matter volumes predicted better verbal learning across all participants (r=0.21, p=0.019) (Figure 2B), Earlier age of ketamine use (years) predicted larger white matter volumes (r=−0.27, p=0.015, Figure 2C), which in turn predicted higher SDS dependence score in all ketamine users (r=0.23, p=0.044) (Figure 2D).
4. Discussion
The major findings of the current study are: 1) Consistent with our hypothesis, Primarily-K users and K+PolyS users both had larger caudates in both hemispheres compared to ND controls, although the two groups were not different in their caudate volumes. 2) Both ketamine user groups also had larger whole brain white matter volumes, with the K+PolyS group showing even greater white matter hypertrophy than the ND controls. 3) Although larger caudates did not predict better verbal learning, larger white matter volume predicted better verbal learning, across all participants. 4) Larger caudates also predict lesser depressive symptoms in both K user groups. 5) Earlier onset of ketamine use predicted larger white matter volume, which in turn predicted greater drug dependency severity, across both ketamine user groups.
4.1. Basal ganglia and white matter volumes in the ketamine users
Consistent with our hypothesis, larger caudates were observed in both groups of ketamine users compared with ND controls. Although the caudate volumes between the two ketamine groups were not significantly different, a linear trend of K+PolyS > K > ND was found on the right side, and the right caudate volume in K+PolyS showed a correlational non-significant trend with total days of cocaine use. Our finding of larger caudates in ketamine users is likely due to a similar adaptive mechanism as the enlarged striatal structures observed in stimulant users (Andres et al., 2016; Chang et al., 2005a; Ersche et al., 2011; He et al., 2018; Jacobsen et al., 2001; Jernigan et al., 2005), since ketamine also leads to dopamine release. In addition, the extent of caudate enlargement (3 – 5%) in ketamine users was less than that found in methamphetamine users (9 – 10%) (Chang et al., 2005a), consistent with the relatively reduced dopamine release previously observed in ketamine users compared to that in methamphetamine users (Kokkinou et al., 2018). Evidence that supports the neuroadaptive mechanism of enlarged striatum found in chronic stimulant users also come from findings of longitudinal studies and intervention studies. For example, findings that enlarged striatal volumes recovered or normalized after prolonged abstinence from stimulant use (Andres et al., 2016; He et al., 2018), or the striatal volume enlargements observed following antipsychotic treatments blocking D2 receptors (Fan et al., 2019; Hashimoto et al., 2018; Leung et al., 2011). In addition, individuals with ADHD consistently showed smaller basal ganglia structures, including the caudates, than those in the controls (Chen et al., 2018; Hoogman et al., 2017), but individuals taking stimulant medications were associated with normalization of the striatal volumes (Hoogman et al., 2017; Nakao et al., 2011). The speculation that D2/D3 receptors blockade and down-regulation may lead to enlarged striatal volumes is further supported by a recent animal study that chronic administration of a D2 receptor blocker, haloperidol, increased striatal volumes in wild type mice but not in D2 receptor gene knockout mice (D2KO) (Guma et al., 2018). However, enlarged striata were also seen in non-user siblings of stimulant users, and healthy elderly with lower D2 availability (Roussotte et al., 2015); groups at risk for addictive behaviors. Therefore, the greater caudate volumes in our ketamine users might also be due to a combined effect of pre-morbid vulnerability and the consequence of ketamine or/and stimulant use disorder. Stimulants reportedly affect DAT and D2/3 receptor levels similarly in caudate, putamen and globus pallidus (Proebstl et al., 2019); however, our ketamine users showed larger volumes than ND controls only in the caudates in the current study. Previous studies in methamphetamine users also inconsistently found larger than normal putamen only (Chang et al., 2005a) or both caudate and putamen (Jernigan et al., 2005). Different study methods, study populations or the variable drug use patterns, might have led to inconsistent findings across studies (Berman et al., 2008; Roussotte et al., 2015). Lastly, variable imaging techniques across studies, including ours, might have led to variable signal intensities in the basal ganglia structures across studies, and resulting in less consistent segmentation of the putamen and GP volumes.
Our finding of larger white matter volume, especially in K+PolyS users, is similar to the white matter hypertrophy (Mon et al., 2014; Thompson et al., 2004) and larger posterior corpus callosum (Chang et al., 2005a) reported in methamphetamine or cocaine users. Larger striatal and white matter volumes in stimulant users might also be due to neuroinflammation, resulting from glial activation (Chang et al., 2005b; Sekine et al., 2008; Taylor et al., 2007). The even larger white matter volume in K+PolyS than the Primarily-K group indicates an additive effect of ketamine and stimulants on glial activation/neuroinflammation in the brain, since dopamine receptors are involved in modulating astroglia and microglial activity and neuroinflammation (Xia et al., 2019). For example, in the striatum of chronic methamphetamine users, larger volumes was associated with more restricted tissue water diffusion (Andres et al., 2016), suggestive of gliosis, while longer duration of methamphetamine use was associated with higher levels of the glial marker myo-inositol (Taylor et al., 2007). Increased glial cell density was also found in the enlarged striatum in saline treated D2KO mice and in the limbic cortical area in haloperidol treated wild types, indicating that blockade of the D2 receptor pathway may be involved in the gliosis process (Guma et al., 2018).
4.2. Dependency, depression and cognitive function of ketamine users
Although ketamine dependence was not an enrollment criterion for this study, 85 – 90% of the ketamine users in this study fulfilled the DSM-IV criteria for drug dependence and used ketamine daily, which is contrary to the statement that “ketamine dependence [is] relatively scarce” (Zanos et al., 2018). In contrast to the three case reports described in Zanos’s article, several more recent studies reported the harmful effects of ketamine in hundreds of regular ketamine users (Chen et al. 2014, Zhang et al. 2018, Li et al. 2019) and discussed the difficulty of their cessation from ketamine use (Wang et al. 2010). In particular, the psychological addiction, rather than the physical withdrawal symptoms, is the reason that cessation of ketamine use is difficult for the regular users (Chen et al., 2014; Wang et al., 2010). Our findings, together with these prior reports suggest that the incidence of ketamine dependence might be underestimated. The reinforcing effect of ketamine may result from its indirect effects on increasing both glutamatergic and dopaminergic release. Ketamine blocks NMDA receptors on the GABAergic interneurons, leading to disinhibition of glutamatergic neurons projecting to the midbrain dopamine neurons, and subsequently increasing dopamine neuron firing and release of dopamine in the striatum, nucleus accumbens and the frontal cortex, as seen in acute ketamine administration in rodent studies with medium to very large effect sizes (Kokkinou et al., 2018).
In the current study, ketamine users had more depressive symptoms than ND controls. This finding is consistent with previous studies that showed approximately 80 % of frequent ketamine users had moderate to severe depressive symptoms (Fan et al., 2016; Tang et al., 2015), especially pronounced when comparing current users with abstinent ketamine users (Tang et al., 2013). The high prevalence of depressive symptoms in ketamine users, and the anti-depressant effects of ketamine (Yang et al., 2019), suggest that ketamine misuse may reflect an attempt at “self-medication”. Our finding of larger right caudates in the ketmaine users with fewer depressive symptoms support prior neuroimaging studies that found striatal structural and functional changes in depressed patients who responded to ketamine treatment. Specifically, responders to ketamine treatment showed increased (normalized) brain activation within the right caudates to positive emotional faces (Murrough et al., 2015), increased global and caudate connectivity (Abdallah et al., 2017), and increased glucose metabolism in the right ventral striatum (Nugent et al., 2014). Although it remains unclear whether antidepressants treatment affects striatal volumes, non-significantly smaller caudate volumes were found in patients with major depressive disorder in the ENIGMA-MDD Workgroup (Schmaal et al., 2016). Furthermore, responders to electroconvulsive therapy for depression also showed enlarged striatal structures after the treatment (Wade et al., 2016), which is also in line with our finding. Taken together, our finding and prior studies suggested caudate might be involved in ketamine’s effects on emotional regulation.
Another clinical feature of the chronic ketamine users in this study was their significantly impaired learning and memory function compared to ND controls. Ketamine blocks NDMA receptors which in turn interrupts synaptic plasticity and leads to learning and memory deficits in users (Morgan et al., 2012). Furthermore, Primarily-K users performed poorly on learning and memory measures, and had even worse performance on Logical Memory retention, when compared with K+PolyS users. These findings suggest that chronic ketamine use may have profound neurotoxic effects. Similarly, another study of 565 ketamine users also reported impaired cognitive function in both ketamine users with and without poly-substance co-use (Zhang et al., 2018). In addition, frequent ketamine users showed greater verbal memory deficit compared to individuals with methamphetamine use disorder (Wang et al., 2018). Taken together, chronic ketamine use appears to have deleterious effects on cognition, particularly on memory and learning functions.
4.3. Correlations between caudate and white matter volume and psychological function
Although ketamine users had poorer cognitive performance than the non-user controls, similar to previous studies of stimulant users that found larger striatal volumes were associated with better cognition (Chang et al., 2005a; Jan et al., 2012), our results suggest larger caudates might be associated with better verbal learning and fewer depressive symptoms and that those with larger white matter volumes also had better cognition. In the current study of ketamine users with or without stimulant co-use, the enlarged striatal and white matter volumes might reflect a neuroadaptive response following repeated dopamine stimulation and dopamine modulated glial activation. Stimulant administration may lead to both microglial and astrocytic activation (Friend and Keefe, 2013; Sekine et al., 2008), and this may apply also to our Primarily-K users since the drug may also lead to dopamine release. While chronic microglia activation promotes neuroinflammation and mediates neurotoxic effects of stimulant exposure (Clark et al., 2013), astrocyte activation might have neuroprotective effects by providing trophic support, reconstructing the blood-brain barrier, and maintaining extracellular homeostasis (Friend and Keefe, 2013; Pekny et al., 2016). All of these findings further suggest that larger striatal and white matter volumes may be a compensatory reponse to chronic stimulant use (Chang et al., 2005b).
5. Limitations
This study has several limitations. First, we did not collect detailed information regarding tobacco smoking or e-cigarette use, which might be other contributing factors to larger subcortical volumes (Durazzo et al., 2012; Durazzo et al., 2017; Franklin et al., 2014; Hanlon et al., 2016; Li et al., 2015; Wetherill et al., 2015) and poorer cognitive performances (Durazzo et al., 2010; Liang et al., 2018; Vermeulen et al., 2018). Based on prior studies, tobacco smoking is highly prevalent among ketamine users in Asia. Previous reports found that 100% of the ketamine users were tobacco smokers in studies from mainland China (Li et al., 2017; Liao et al., 2011) and Hong Kong (Lee et al., 2005) and 72% or higher in studies of ketamine users from Taiwan (Chen et al., 2014; Li et al., 2017). Second, the MRI scans did not have isotropic voxels which led to poor contrast for grey white segmentation on axial and sagittal images, and also precluded our ability to assess cortical thickness with other automated techniques. Future studies with improved grey-white matter delineations are needed for more detailed cortical morphometric analyses. Third, the lifetime regular stimulant use in some Primarily-K users, although less prevalent than that in the K+PolyS group, also might have confounded our striatal or white matter volume measurements in this group. Fourth, since we chose our regions of interest based on a priori hypotheses, we did not perform corrections for multiple comparisons for the selected volume measurements, which could lead to type 2 errors. Future studies with a larger sample size and evaluating more brain regions should include corrections for multiple comparisons. Lastly, due to the cross-sectional study design, we were not able to determine the causation between larger caudate volume and ketamine or polysubstances use. Nevertheless, the strength of the current study is the detailed information on other substances used, which allowed us to compare Primarily-K users to K+PolyK users.
6. Conclusion
Ketamine users had larger caudate and total white matter volumes than non-drug users. Ketamine with stimulant co-use appears to have a greater effect than ketamine on white matter hypertrophy. However, across both ketamine user groups, those with larger caudates had lower depression scores while those with larger white matter volumes had better learning function, which suggest that the larger volumes might be a compensatory response to repeated ketamine and polysubstance use.
Highlights.
Ketamine users had larger caudates and white matter volumes compared to non-users.
Ketamine users who co-use other substances had even larger white matter volumes.
Ketamine users had poorer cognition and more depressive symptoms than non-users.
Larger caudates predicted fewer depressive symptoms.
Acknowledgements
This work was supported by the Department of Diagnostic Radiology and Nuclear Medicine at the University Of Maryland School Of Medicine, Baltimore, Maryland, USA. The Beat Drugs Fund, Narcotics Division, Security Bureau, the Government of the Hong Kong Special Administrative Region. We are grateful to our research participants and referrals from the following counselling centers: Caritas Wong Yiu Nam Center; Christian New Being Fellowship - Life Training Base; Drug Addicts Counselling and Rehabilitation Services (DACARS) - Enchi Lodge Hong Kong Christian Service - Jockey Club Lodge of the Rising Sun; Hong Kong Christian Service - The Barnabas Charitable Service Association Limited; Hong Kong Christian Service - Yuen Long District Youth Outreaching Social Work Team; Hong Kong Lutheran Social Service Cheer Lutheran Center; Operation Dawn Girl Center; The Evangelical Lutheran Church of Hong Kong-Ling Oi Tan Ka Wan Centre; The Evangelical Lutheran Church of Hong Kong, Enlighten Centre – Yuen Long; The Society for the Aid and Rehabilitation of Drug Abusers - Adult Female Rehabilitation Centre and Shek Kwu Chau Treatment & Rehabilitation Centre. We also thank Ms. Bridgette Pocta for language editing on the manuscript.
Role of funding source
This work was supported by The Beat Drugs Fund (BDF101020), Narcotics Division, Security Bureau, the Government of the Hong Kong Special Administrative Region, and by the National Institute on Drug Abuse, National Institute of Health.
Footnotes
Declarations of interest
None.
References
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, DeWilde KE, Wong E, Anticevic A, Tang CY, Iosifescu DV, Charney DS, Murrough JW, 2017. Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacology 42(6), 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 1994. Diagnostic and statistical manual of mental disorders. American Psychiatric Association, Whasington DC. [Google Scholar]
- Andres T, Ernst T, Oishi K, Greenstein D, Nakama H, Chang L, 2016. Brain Microstructure and Impulsivity Differ between Current and Past Methamphetamine Users. J Neuroimmune Pharmacol 11(3), 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard CL, Schmitz JM, Soder HE, Suchting R, Yoon JH, Hasan KM, Narayana PA, Moeller FG, Lane SD, 2019. Regional differences in white matter integrity in stimulant use disorders: A meta-analysis of diffusion tensor imaging studies. Drug Alcohol Depend 201, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED, 2008. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci 1141, 195–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer AJ 3rd, Mahoney JJ 3rd, Nerumalla CS, Newton TF, De La Garza R 2nd, 2013. The influence of smoking cigarettes on the high and desire for cocaine among active cocaine users. Pharmacol Biochem Behav 106, 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, McLellan AT, Lin YT, Lynch KG, 2007. Initial evidence for the reliability and validity of a “Lite” version of the Addiction Severity Index. Drug Alcohol Depend 87(2–3), 297–302. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr., Wise RA, 1996. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci 16(9), 3112–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T, 2005a. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry 57(9), 967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS, 2005b. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry 162(2), 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Nixon E, Herskovits E, 2016. Advanced Connectivity Analysis (ACA): a Large Scale Functional Connectivity Data Mining Environment. Neuroinformatics 14(2), 191–199. [DOI] [PubMed] [Google Scholar]
- Chen WY, Huang MC, Lin SK, 2014. Gender differences in subjective discontinuation symptoms associated with ketamine use. Subst Abuse Treat Prev Policy 9, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Sudre G, Sharp W, Donovan F, Chandrasekharappa SC, Hansen N, Elnitski L, Shaw P, 2018. Neuroanatomic, epigenetic and genetic differences in monozygotic twins discordant for attention deficit hyperactivity disorder. Mol Psychiatry 23(3), 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Chan AS, Law SC, Chan JH, Tse VK, 2000. Cognitive function of patients with nasopharyngeal carcinoma with and without temporal lobe radionecrosis. Arch Neurol 57(9), 1347–1352. [DOI] [PubMed] [Google Scholar]
- Cheung RY, Chan SS, Lee JH, Pang AW, Choy KW, Chung TK, 2011. Urinary symptoms and impaired quality of life in female ketamine users: persistence after cessation of use. Hong Kong Med J 17(4), 267–273. [PubMed] [Google Scholar]
- Chiu HF, Chan CK, Lam LC, Ng KO, Li SW, Wong M, Chan WF, 1997. The modified Fuld Verbal Fluency Test: a validation study in Hong Kong. J Gerontol B Psychol Sci Soc Sci 52(5), P247–250. [DOI] [PubMed] [Google Scholar]
- Clark KH, Wiley CA, Bradberry CW, 2013. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotox Res 23(2), 174–188. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Fryer SL, Rothlind JC, Vertinski M, Gazdzinski S, Mon A, Meyerhoff DJ, 2010. Measures of learning, memory and processing speed accurately predict smoking status in short-term abstinent treatment-seeking alcohol-dependent individuals. Alcohol Alcohol 45(6), 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Insel PS, Weiner MW, Alzheimer Disease Neuroimaging I, 2012. Greater regional brain atrophy rate in healthy elderly subjects with a history of cigarette smoking. Alzheimers Dement 8(6), 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Yoder KK, Murray DE, 2017. Cigarette smoking is associated with amplified age-related volume loss in subcortical brain regions. Drug Alcohol Depend 177, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET, 2011. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain 134(Pt 7), 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Smith DG, Bullmore ET, Robbins TW, 2013. Distinctive personality traits and neural correlates associated with stimulant drug use versus familial risk of stimulant dependence. Biol Psychiatry 74(2), 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Xiang H, Tan S, Yang F, Fan H, Guo H, Kochunov P, Wang Z, Hong LE, Tan Y, 2019. Subcortical structures and cognitive dysfunction in first episode schizophrenia. Psychiatry Res Neuroimaging 286, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan N, Xu K, Ning Y, Rosenheck R, Wang D, Ke X, Ding Y, Sun B, Zhou C, Deng X, Tang W, He H, 2016. Profiling the psychotic, depressive and anxiety symptoms in chronic ketamine users. Psychiatry Res 237, 311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Fein D, 2013. Subcortical volumes are reduced in short-term and long-term abstinent alcoholics but not those with a comorbid stimulant disorder. Neuroimage Clin 3, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak MM, Hol HR, Hernes SS, Chang L, Engvig A, Bjuland KJ, Pripp A, Madsen BO, Knapskog AB, Ulstein I, Lona T, Skranes J, Lohaugen GCC, 2019. Adaptive Computerized Working Memory Training in Patients With Mild Cognitive Impairment. A Randomized Double-Blind Active Controlled Trial. Front Psychol 10, 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wetherill RR, Jagannathan K, Johnson B, Mumma J, Hager N, Rao H, Childress AR, 2014. The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLoS One 9(8), e104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DM, Keefe KA, 2013. Glial reactivity in resistance to methamphetamine-induced neurotoxicity. J Neurochem 125(4), 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, 1983. The Assessment of Aphasia and Related Disorders., 2nd ed. Lea & Febiger, Philadelphia:. [Google Scholar]
- Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W, Strang J, 1995. The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 90(5), 607–614. [DOI] [PubMed] [Google Scholar]
- Grodin EN, Momenan R, 2017. Decreased subcortical volumes in alcohol dependent individuals: effect of polysubstance use disorder. Addict Biol 22(5), 1426–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, London ED, Jentsch JD, 2012. Dysregulation of D(2)-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci 32(17), 5843–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Morales AM, Lee B, London ED, Jentsch JD, 2013. Methamphetamine-induced increases in putamen gray matter associate with inhibitory control. Psychopharmacology (Berl) 229(3), 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guma E, Rocchetti J, Devenyi GA, Tanti A, Mathieu A, Lerch JP, Elgbeili G, Courcot B, Mechawar N, Chakravarty MM, Giros B, 2018. Regional brain volume changes following chronic antipsychotic administration are mediated by the dopamine D2 receptor. Neuroimage 176, 226–238. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Owens MM, Joseph JE, Zhu X, George MS, Brady KT, Hartwell KJ, 2016. Lower subcortical gray matter volume in both younger smokers and established smokers relative to non-smokers. Addict Biol 21(1), 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Ito YM, Okada N, Yamamori H, Yasuda Y, Fujimoto M, Kudo N, Takemura A, Son S, Narita H, Yamamoto M, Tha KK, Katsuki A, Ohi K, Yamashita F, Koike S, Takahashi T, Nemoto K, Fukunaga M, Onitsuka T, Watanabe Y, Yamasue H, Suzuki M, Kasai K, Kusumi I, Hashimoto R, Cocoro, 2018. The effect of duration of illness and antipsychotics on subcortical volumes in schizophrenia: Analysis of 778 subjects. Neuroimage Clin 17, 563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Huang X, Turel O, Schulte M, Huang D, Thames A, Bechara A, Hser YI, 2018. Presumed structural and functional neural recovery after long-term abstinence from cocaine in male military veterans. Prog Neuropsychopharmacol Biol Psychiatry 84(Pt A), 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R, Chelune G, Talley JL, Curtiss G, 1993. Wisconsin Card Sorting Test Manual: Revised and Expanded. Psychological Assessment Resources. Inc,, Odessa. [Google Scholar]
- Herskovits EH, Hong LE, Kochunov P, Sampath H, Chen R, 2015. Edge-Centered DTI Connectivity Analysis: Application to Schizophrenia. Neuroinformatics 13(4), 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, van Hulzen KJE, Medland SE, Shumskaya E, Jahanshad N, Zeeuw P, Szekely E, Sudre G, Wolfers T, Onnink AMH, Dammers JT, Mostert JC, Vives-Gilabert Y, Kohls G, Oberwelland E, Seitz J, Schulte-Ruther M, Ambrosino S, Doyle AE, Hovik MF, Dramsdahl M, Tamm L, van Erp TGM, Dale A, Schork A, Conzelmann A, Zierhut K, Baur R, McCarthy H, Yoncheva YN, Cubillo A, Chantiluke K, Mehta MA, Paloyelis Y, Hohmann S, Baumeister S, Bramati I, Mattos P, Tovar-Moll F, Douglas P, Banaschewski T, Brandeis D, Kuntsi J, Asherson P, Rubia K, Kelly C, Martino AD, Milham MP, Castellanos FX, Frodl T, Zentis M, Lesch KP, Reif A, Pauli P, Jernigan TL, Haavik J, Plessen KJ, Lundervold AJ, Hugdahl K, Seidman LJ, Biederman J, Rommelse N, Heslenfeld DJ, Hartman CA, Hoekstra PJ, Oosterlaan J, Polier GV, Konrad K, Vilarroya O, Ramos-Quiroga JA, Soliva JC, Durston S, Buitelaar JK, Faraone SV, Shaw P, Thompson PM, Franke B, 2017. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry 4(4), 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Liang D, Lan YC, Vicknasingam BK, Chakrabarti A, 2016. Drug Abuse, HIV, and HCV in Asian Countries. J Neuroimmune Pharmacol 11(3), 383–393. [DOI] [PubMed] [Google Scholar]
- Hua MS, Chang BS, Lin KN, Yang JM, Lu SR, Chen SY, 2005. Wechsler Memory Scale III (Chinese): manual., 3rd. ed. Chinese Behavior Science Corp, Taipei. [Google Scholar]
- Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH, 2001. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry 158(3), 486–489. [DOI] [PubMed] [Google Scholar]
- Jan RK, Lin JC, Miles SW, Kydd RR, Russell BR, 2012. Striatal volume increases in active methamphetamine-dependent individuals and correlation with cognitive performance. Brain Sci 2(4), 553–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, 2012. Fsl. Neuroimage 62(2), 782–790. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I, 2005. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry 162(8), 1461–1472. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Zea-Ponce Y, Rodenhiser-Hill J, Mann JJ, Van Heertum RL, Cooper TB, Carlsson A, Laruelle M, 2000. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry 48(7), 627–640. [DOI] [PubMed] [Google Scholar]
- Kokkinou M, Ashok AH, Howes OD, 2018. The effects of ketamine on dopaminergic function: meta-analysis and review of the implications for neuropsychiatric disorders. Mol Psychiatry 23(1), 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, Cassello K, Bowers MB Jr., Vegso S, Heninger GR, Charney DS, 1999. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology (Berl) 145(2), 193–204. [DOI] [PubMed] [Google Scholar]
- Lee AM, Chan RCK, Chen EYH, Tang SW, Chum KWC, 2005. A Study on the Cognitive Impairment and Other Harmful Effects Caused by Ketamine Abuse Narcotics Division, Security Bureau, Hong Kong. [Google Scholar]
- Leung CM, Ho S, Kan CS, Hung CH, Chen CN, 1993. Evaluation of the Chinese version of the Hospital Anxiety and Depression Scale. A cross-cultural perspective. Int J Psychosom 40(1–4), 29–34. [PubMed] [Google Scholar]
- Leung M, Cheung C, Yu K, Yip B, Sham P, Li Q, Chua S, McAlonan G, 2011. Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta-analyses with sample size weighting. Schizophr Bull 37(1), 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Zhang S, Hung CC, Chen CM, Duann JR, Lin CP, Lee TS, 2017. Depression in chronic ketamine users: Sex differences and neural bases. Psychiatry Res 269, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yuan K, Cai C, Feng D, Yin J, Bi Y, Shi S, Yu D, Jin C, von Deneen KM, Qin W, Tian J, 2015. Reduced frontal cortical thickness and increased caudate volume within fronto-striatal circuits in young adult smokers. Drug Alcohol Depend 151, 211–219. [DOI] [PubMed] [Google Scholar]
- Liang H, Chang L, Chen R, Oishi K, Ernst T, 2018. Independent and Combined Effects of Chronic HIV-Infection and Tobacco Smoking on Brain Microstructure. J Neuroimmune Pharmacol 13(4), 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang HJ, Tang KL, Chan F, Ungvari GS, Tang WK, 2015. Ketamine users have high rates of psychosis and/or depression. J Addict Nurs 26(1), 8–13. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Corlett PR, Wang X, Yang M, Chen H, Liu T, Chen X, Hao W, Fletcher PC, 2011. Reduced dorsal prefrontal gray matter after chronic ketamine use. Biol Psychiatry 69(1), 42–48. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X, Liu T, Chen X, Fletcher PC, Hao W, 2010. Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain 133(Pt 7), 2115–2122. [DOI] [PubMed] [Google Scholar]
- Mackey S, Stewart JL, Connolly CG, Tapert SF, Paulus MP, 2014. A voxel-based morphometry study of young occasional users of amphetamine-type stimulants and cocaine. Drug Alcohol Depend 135, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge J, Winstock A, Hunt N, Mitcheson L, 2007. 5-Year trends in use of hallucinogens and other adjunct drugs among UK dance drug users. Eur Addict Res 13(1), 57–64. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP, 1980. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis 168(1), 26–33. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Abe C, Gazdzinski S, Pennington D, Schmidt T, Meyerhoff DJ, 2014. Structural brain differences in alcohol-dependent individuals with and without comorbid substance dependence. Drug Alcohol Depend 144, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV, Independent Scientific Committee on, D., 2012. Ketamine use: a review. Addiction 107(1), 27–38. [DOI] [PubMed] [Google Scholar]
- Morley KI, Lynskey MT, Moran P, Borschmann R, Winstock AR, 2015. Polysubstance use, mental health and high-risk behaviours: Results from the 2012 Global Drug Survey. Drug Alcohol Rev 34(4), 427–437. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Collins KA, Fields J, DeWilde KE, Phillips ML, Mathew SJ, Wong E, Tang CY, Charney DS, Iosifescu DV, 2015. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry 5, e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D, 2011. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry 168(11), 1154–1163. [DOI] [PubMed] [Google Scholar]
- Narcotics Division SB, The Government of The Hong Kong Special Adminstrative Region, 2017. Central Registry of Drug Abuse Sixty-fifth Report, Mar 1, 2017. ed., p. 10.
- Nugent AC, Diazgranados N, Carlson PJ, Ibrahim L, Luckenbaugh DA, Brutsche N, Herscovitch P, Drevets WC, Zarate CA Jr., 2014. Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord 16(2), 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr JM, Paschall CJ, Banich MT, 2016. Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. Neuroimage Clin 12, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieth PA, 1944. Le test de copie d’une figure complex: Contribution a l’etude de la perception et de la memoire [The Complex Figure Test: Contribution to the study of perception and memory]. Archives of Psycholog 28, 1021–1034. [Google Scholar]
- Pal R, Balt S, Erowid E, Erowid F, Baggott MJ, Mendelson J, Galloway GP, 2013. Ketamine is associated with lower urinary tract signs and symptoms. Drug Alcohol Depend 132(1–2), 189–194. [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A, 2016. Astrocytes: a central element in neurological diseases. Acta Neuropathol 131(3), 323–345. [DOI] [PubMed] [Google Scholar]
- Poon TL, Wong KF, Chan MY, Fung KW, Chu SK, Man CW, Yiu MK, Leung SK, 2010. Upper gastrointestinal problems in inhalational ketamine abusers. J Dig Dis 11(2), 106–110. [DOI] [PubMed] [Google Scholar]
- Proebstl L, Kamp F, Manz K, Krause D, Adorjan K, Pogarell O, Koller G, Soyka M, Falkai P, Kambeitz J, 2019. Effects of stimulant drug use on the dopaminergic system: A systematic review and meta-analysis of in vivo neuroimaging studies. Eur Psychiatry 59, 15–24. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2019. R: A language fnad environment for statistical computing. In. Vinnea, Austrial: R Foundation for Statistical Computing. [Google Scholar]
- Rolls ET, Huang CC, Lin CP, Feng J, Joliot M, 2020. Automated anatomical labelling atlas 3. Neuroimage 206, 116189. [DOI] [PubMed] [Google Scholar]
- Roussotte FF, Jahanshad N, Hibar DP, Thompson PM, Alzheimer’s Disease Neuroimaging I, 2015. Altered regional brain volumes in elderly carriers of a risk variant for drug abuse in the dopamine D2 receptor gene (DRD2). Brain Imaging Behav 9(2), 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Lancaster E, Heidbreder R, Strain EC, Kosel M, Fisch HU, Pearlson GD, 2006. Decreased frontal white-matter volume in chronic substance abuse. Int J Neuropsychopharmacol 9(2), 147–153. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N, Loehrer E, Tiemeier H, Hofman A, Niessen WJ, Vernooij MW, Ikram MA, Wittfeld K, Grabe HJ, Block A, Hegenscheid K, Volzke H, Hoehn D, Czisch M, Lagopoulos J, Hatton SN, Hickie IB, Goya-Maldonado R, Kramer B, Gruber O, Couvy-Duchesne B, Renteria ME, Strike LT, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie NA, Wright MJ, Hall GB, MacQueen GM, Frey EM, Carballedo A, van Velzen LS, van Tol MJ, van der Wee NJ, Veer IM, Walter H, Schnell K, Schramm E, Normann C, Schoepf D, Konrad C, Zurowski B, Nickson T, McIntosh AM, Papmeyer M, Whalley HC, Sussmann JE, Godlewska BR, Cowen PJ, Fischer FH, Rose M, Penninx BW, Thompson PM, Hibar DP, 2016. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 21(6), 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AJ, Bourne A, Weatherburn P, Reid D, Marcus U, Hickson F, Network E, 2016. Illicit drug use among gay and bisexual men in 44 cities: Findings from the European MSM Internet Survey (EMIS). Int J Drug Policy 38, 4–12. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL, 2008. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci 28(22), 5756–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek DT, 1990. Reliability and factorial structure of the Chinese version of the Beck Depression Inventory. J Clin Psychol 46(1), 35–43. [DOI] [PubMed] [Google Scholar]
- Shin MS, Park SY, Park SR, Seol SH, Kwon JS, 2006. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat Protoc 1(2), 892–899. [DOI] [PubMed] [Google Scholar]
- Sinner B, Graf BM, 2008. Ketamine. Handb Exp Pharmacol(182), 313–333. [DOI] [PubMed] [Google Scholar]
- Smith SM, 2002. Fast robust automated brain extraction. Hum Brain Mapp 17(3), 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR, 1935. Studies of interference in serial verbal reactions. J Exp Psychol 18, 643. [Google Scholar]
- Tang A, Liang HJ, Ungvari GS, Tang WK, 2011. Referral patterns and clinical characteristics of subjects referred to substance abuse clinic of a regional hospital in Hong Kong. East Asian Arch Psychiatry 21(1), 22–27. [PubMed] [Google Scholar]
- Tang WK, Liang HJ, Lau CG, Tang A, Ungvari GS, 2013. Relationship between cognitive impairment and depressive symptoms in current ketamine users. J Stud Alcohol Drugs 74(3), 460–468. [DOI] [PubMed] [Google Scholar]
- Tang WK, Morgan CJ, Lau GC, Liang HJ, Tang A, Ungvari GS, 2015. Psychiatric morbidity in ketamine users attending counselling and youth outreach services. Subst Abus 36(1), 67–74. [DOI] [PubMed] [Google Scholar]
- Taylor EM, 1959. Psychological Appraisal of Children with Cerebral Defects, Fourth Printing. Harvard University Press, Cambrige, Massachusetts. [Google Scholar]
- Taylor MJ, Schweinsburg BC, Alhassoon OM, Gongvatana A, Brown GG, Young-Casey C, Letendre SL, Grant I, Group H, 2007. Effects of human immunodeficiency virus and methamphetamine on cerebral metabolites measured with magnetic resonance spectroscopy. J Neurovirol 13(2), 150–159. [DOI] [PubMed] [Google Scholar]
- Thames AD, Mahmood Z, Burggren AC, Karimian A, Kuhn TP, 2016. Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care 28(5), 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Kim R, Delis F, Ananth M, Chachati G, Rocco MJ, Masad I, Muniz JA, Grant SC, Gold MS, Cadet JL, Volkow ND, 2016. Chronic Methamphetamine Effects on Brain Structure and Function in Rats. PLoS One 11(6), e0155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer RE, YorkWilliams SL, Hutchison KE, Bryan AD, 2019. Preliminary results from a pilot study examining brain structure in older adult cannabis users and nonusers. Psychiatry Res Neuroimaging 285, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED, 2004. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci 24(26), 6028–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- Vasavada MM, Leaver AM, Espinoza RT, Joshi SH, Njau SN, Woods RP, Narr KL, 2016. Structural connectivity and response to ketamine therapy in major depression: A preliminary study. J Affect Disord 190, 836–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen JM, Schirmbeck F, Blankers M, van Tricht M, Bruggeman R, van den Brink W, de Haan L, Genetic R, Outcome of Psychosis, i., 2018. Association Between Smoking Behavior and Cognitive Functioning in Patients With Psychosis, Siblings, and Healthy Control Subjects: Results From a Prospective 6-Year Follow-Up Study. Am J Psychiatry 175(11), 1121–1128. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, Baler R, 2017. The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 18(12), 741–752. [DOI] [PubMed] [Google Scholar]
- Wade BS, Joshi SH, Njau S, Leaver AM, Vasavada M, Woods RP, Gutman BA, Thompson PM, Espinoza R, Narr KL, 2016. Effect of Electroconvulsive Therapy on Striatal Morphometry in Major Depressive Disorder. Neuropsychopharmacology 41(10), 2481–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LJ, Chen CK, Lin SK, Chen YC, Xu K, Huang MC, 2018. Cognitive profile of ketamine-dependent patients compared with methamphetamine-dependent patients and healthy controls. Psychopharmacology (Berl) 235(7), 2113–2121. [DOI] [PubMed] [Google Scholar]
- Wang YC, Chen SK, Lin CM, 2010. Breaking the drug addiction cycle is not easy in ketamine abusers. Int J Urol 17(5), 496; author reply 497. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1997a. WAIS-III Administration and Scoring Manual, 3rd ed. The Psychological Corporation, San Antonio:TX. [Google Scholar]
- Wechsler D, 1997b. Wechsler Memory Scale, 3rd ed. The Psychological Corporation, San Antonio: TX. [Google Scholar]
- Wechsler D, Chen YH, Chen XY, 2002. WAIS-III Chinese Version Technical Manual. Psychological Corporation, San Antonio, TX:. [Google Scholar]
- Wetherill RR, Jagannathan K, Hager N, Childress AR, Rao H, Franklin TR, 2015. Cannabis, Cigarettes, and Their Co-Occurring Use: Disentangling Differences in Gray Matter Volume. Int J Neuropsychopharmacol 18(10), pyv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens S, Nilsson ME, 2017. Performing Contrast Analysis in Factorial Designs: From NHST to Confidence Intervals and Beyond. Educ Psychol Meas 77(4), 690–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, Hawkins J, Birnbaum J, Lyons DM, Rodriguez CI, Schatzberg AF, 2018. Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. Am J Psychiatry 175(12), 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff K, Winstock AR, 2006. Ketamine : from medicine to misuse. CNS Drugs 20(3), 199–218. [DOI] [PubMed] [Google Scholar]
- Wyciszkiewicz A, Pawlak MA, 2014. Basal Ganglia Volumes: MR-Derived Reference Ranges and Lateralization Indices for Children and Young Adults. Neuroradiol J 27(5), 595–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia QP, Cheng ZY, He L, 2019. The modulatory role of dopamine receptors in brain neuroinflammation. Int Immunopharmacol 76, 105908. [DOI] [PubMed] [Google Scholar]
- Yang C, Yang J, Luo A, Hashimoto K, 2019. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry 9(1), 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu-Cheung C, 2012. Acute and chronic toxicity pattern in ketamine abusers in Hong Kong. J Med Toxicol 8(3), 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr., Gould TD, 2018. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev 70(3), 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Tang WK, Liang HJ, Ungvari GS, Lin SK, 2018. Other drug use does not impact cognitive impairments in chronic ketamine users. Drug Alcohol Depend 186, 1–8. [DOI] [PubMed] [Google Scholar]