Abstract
Heart Failure (HF) incidence is increasing steadily worldwide, while prognosis remains poor. Though nutrition is a lifestyle factor implicated in prevention of HF, little is known about the effects of macro- and micronutrients as well as dietary patterns on the progression and treatment of HF. This is reflected in a lack of nutrition recommendations in all major HF scientific guidelines. In this state-of-the-art review, we examine and discuss the implications of evidence contained in existing randomized control trials as well as observational studies covering the topics of sodium restriction, dietary patterns and caloric restriction as well as supplementation of dietary fats and fatty acids, protein and amino acids and micronutrients in the setting of pre-existing HF. Finally, we explore future directions and discuss knowledge gaps regarding nutrition therapies for the treatment of HF.
Keywords: heart failure, sodium restriction, dietary patterns, fatty acids, caloric restriction, protein supplementation
Introduction
Heart Failure (HF) affects nearly 6.5 million adults in the United States and is steadily increasing in prevalence1. Prognosis for patients diagnosed with HF remains poor and there is a lack of dietary therapies which have demonstrated an improvement in clinical outcomes, reflected in a paucity of evidence-based nutrition recommendations from major HF guidelines2–6. Traditionally, nutrition management for HF has focused on sodium and fluid restriction, but in recent years, overarching dietary patterns, as well as specific micro- and macronutrients have garnered research interest7,8.
As HF is associated with risk factors such as chronic inflammation, coronary artery disease, hypertension, type 2 diabetes mellitus (T2DM), sarcopenia and obesity, it is useful to explore whether treating these comorbidities with nutrition interventions can aid in preventing as well as treating existing HF7. Additionally, as HF with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF) differ in their risk factors as well as response to pharmaceutical treatments9, they will be considered separately when reviewing the evidence surrounding nutrition interventions, though similarities may exist. Currently, there is a concerning lack of representation by patients with HFpEF in nutrition interventions in HF, which is problematic considering incidence of HFpEF is quickly exceeding HFrEF1,10. In this review, we appraise the current evidence surrounding dietary interventions in HFrEF and HFpEF in terms of caloric, micronutrients and macronutrients intake as well as specific dietary patterns. We finally present the ongoing clinical trials and future directions in the field.
Sodium Restriction
Sodium restriction, with or without concomitant fluid restriction, is perhaps the most widely used clinical nutrition therapy for patients with HF7. Despite recommendation across international guidelines, there is currently inadequate clinical evidence to suggest sodium restriction is beneficial in the setting of HF11. Furthermore, the level of suggested restriction in guidelines varies dramatically from <1500 milligrams (mg) to <3000 mg per day7. In patients with HF, increased renin-angiotensin-aldosterone system (RAAS) activation, along with increased vasopressin, can lead to worsening sodium and fluid retention (Figure 1)11. The mechanism of benefit for sodium restriction in HF is therefore theorized to be reduced fluid retention, which prevents worsening HF signs and symptoms (Figure 1)11. Notably, the opposing case against sodium restriction in HF argues that a low-sodium diet may actually increase RAAS activation, leading to worsening HF, although the evidence is limited11–13. An often overlooked factor is that patients with HF are at an increased risk of malnutrition. Sodium-restricted diets may inadvertently decrease intake of total calories and/or macro- and micronutrients, therefore worsening nutrition status8,14–17. In this regard, the method of patient education is important and may prevent the unintended reduction of other nutrients18.
Figure 1. Effects of sodium restriction in heart failure.
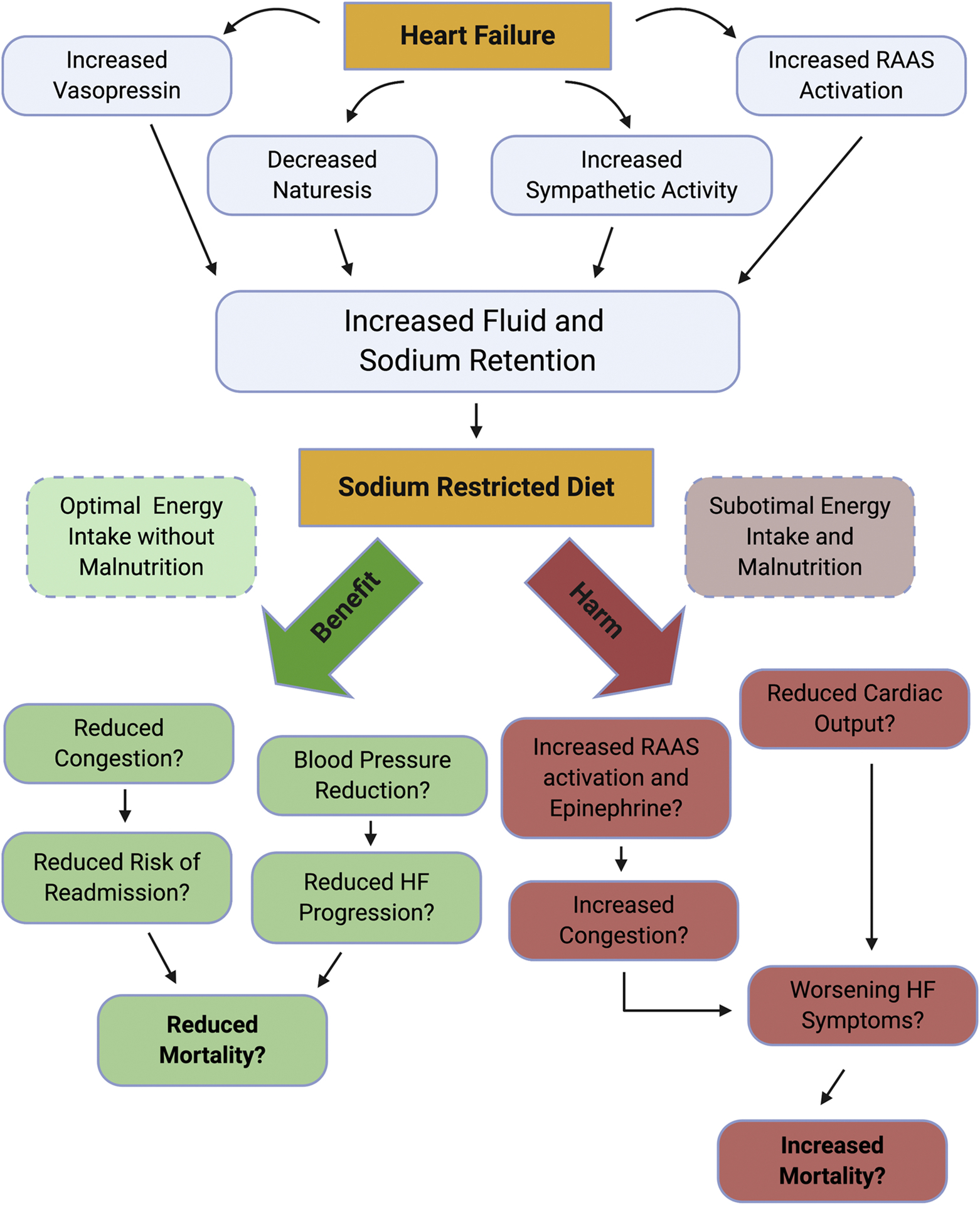
The figure illustrates the pathophysiology of sodium and fluid handling by the failing heart which results in fluid retention and worsening heart failure (HF) symptoms. As sodium restriction has not been proven to be beneficial nor harmful in randomized clinical trials, we show the potential mechanisms for benefits, which may result in lowered fluid retention and improved HF symptoms as well as the possible mechanisms of harm which paradoxically include increased fluid retention and worsening HF symptoms through compensatory mechanisms adapted from discussion in Khan et al11. RAAS: renin-angiotensin-aldosterone system.
Despite concerns, a few trials have demonstrated potential benefits of sodium restriction in HF (Table 1), with evidence to support the theory that restricting sodium may reduce HF signs and symptoms. In a 6-month outpatient trial, 65 subjects (left ventricular ejection fraction (LVEF) of 40±15.6 intervention, 42.3±15.5 control), were randomized to an intervention of sodium restriction 2000–2400 mg per day with fluid restriction of 1.5 liters (L) vs. control15. Dietitians educated the intervention group at baseline on sodium restriction and evaluated at 3 months with a 24-hour dietary recall and personalized suggestions while the control group received only generalized written instructions for HF self- care, including sodium restriction15. Sodium intake assessed by 3-day food records and 24-hour urinary sodium was collected at baseline and 6 months15. At follow up, subjects in the sodium restriction group demonstrated significant reductions in: 24-hour sodium excretion compared to the control group (−7.9 vs. 29.4%), extracellular water on bioelectrical impedance analysis (−1.1 vs. +1.4 liters (L)), self-reported fatigue (59.3 vs. 25.9%) and edema (37% vs. 7%) and a significant increase in questionnaire-assessed quality of life (QoL) versus the control group15. Notably, subjects in the intervention group demonstrated significantly reduced dietary energy intake (−16.8±24.7% sodium restriction, +10.7±30.6% control) along with reduction in sodium (−50.5±34.9% sodium restriction, 48.1±164.7% control) versus the control group15. Another trial also demonstrated improvements in HF symptoms, based on blinded physician assessment19. Based on the results of a 30 -patient pilot trial that showed no negative effects of sodium restriction on appetite, thirst or QoL20, 97 subjects (LVEF 34±11% intervention, 37±15% control) were randomized to 12 weeks of individualized counseling on sodium (2000–3000 mg) and fluid restriction (1.5 L) or control (standard nurse-led clinic education)19. Dietitians or nurses specially trained by dietitians administered the intervention education and followed up with patients every 2–3 weeks to enforce advice19. The primary endpoint was a composite outcome of clinical deterioration, improvement or unchanged status assessed by New York Heart Association (NYHA) class, hospitalization for HF, weight gain, edema on exam, subject-reported thirst and QoL19. If one category worsened, the patient status was classified as “deterioration”19. Significant improvements in the composite primary outcome was observed in the intervention group versus the control (51% sodium restriction,16% control)19.
Table 1.
Clinical studies of macronutrients, micronutrients and dietary patterns in the setting of Heart Failure (HF).
| Study | Trial Type | EF (%) | N | Dietary Intervention | Outcomes |
|---|---|---|---|---|---|
| Sodium Restriction | |||||
| Doukky et al (13) | Prospective, observational study | ≤40 | 902 | Outpatients enrolled in the HART trial, followed for 36 months Subjects divided in restricted (<2500 mg) and unrestricted (≥2500 mg) daily sodium intake based off serial food frequency questionnaires | In a multivariate adjusted, propensity matched analysis of 260 subjects, sodium restriction was associated with a significant increase in the rate of death or HF hospitalization (primary outcome). |
| Colín Ramírez et al. (15) | Randomized controlled trial | 40±15.6 Intervention 42.3±15.5 Control |
65 | 2,000–2,400 mg/day sodium vs. control for 6 months | Increase in physical activity and QoL assessed by a sum of KCCQ and MLHFQ scores and improvement in NYHA class in intervention group vs. control. |
| Philipson et al (19) | Multicenter, randomized controlled trial | 34±11 Intervention 37±15 Control |
97 | 2,000–3,000 mg/day sodium restriction vs. control for 12 weeks | The intervention group demonstrated signs of clinical improvement vs. control (composite primary outcome), driven mostly by improvement in NYHA class and edema. |
| Colín Ramírez et al. (21) | Open-label, randomized controlled pilot trial | 42.0 (25.050.5) | 38 | 1,500 mg/day (low) vs. 2,300 mg/day (moderate, control) sodium restriction for 6 months | BNP decreased from baseline in the low sodium group alone but there were no significant differences between groups; KCCQ clinical score increased in both groups with no difference between groups. |
| Paterna et al (22) | Randomized trial | <35 | 410 | 8 Groups of recently decompensated patients (30 days post-discharge): Groups A- D: 1 L/Day Fluid Restriction Groups E- H: 2 L/Day Fluid Restriction A + E.) 2760 mg NA/day with 250 mg Furosemide BID B + F.) 2670 mg Na/day with 125 mg Furosemide BID C+ G.) 1840 mg NA/day with 250 mg Furosemide BID D+ H.) 1840 mg NA/day with 125 mg Furosemide BID |
Group A demonstrated significantly lower readmission at 6 months as compared to all 7 other groups (primary outcome) |
| Paterna et al (23) | Randomized trial | <35 | 232 | 2,760 mg/day vs. 1,840 mg/day sodium in recently decompensated patients (30 days post-discharge) | 2,760 mg/day sodium group had lower readmissions at 180 days (primary outcome) and combined readmission and mortality at 90 days. |
| Song et al (25) | Prospective, observational study | 37±13 | 244 | Outpatients followed for a 12- month period, separated into groups consuming 2g, 2–3g and >3g of sodium per day based on a 4-day food diary at baseline | In subjects classified as NYHA class I/II, compared with subjects consuming 2–3 g sodium daily, those consuming <2 g sodium per day had a higher risk for hospitalization or death but those consuming greater than 3 g of sodium had a lower risk for hospitalization or death. In subjects classified as NYHA class III/IV, those who consumed >3 g sodium had a greater risk of hospitalization or death than those consuming 2–3 g, however there was no difference observed between those consuming <2 and 23 g sodium daily |
| Lennie et al (26) | Prospective, observational study | 34 ± 14 | 302 | Outpatients followed for 12 months, sodium intake based off a single 24-hour urine sample. | Subjects with NYHA class II/III HF consuming >3 g sodium at baseline had increased event free survival, while those in NYHA class III/IV consuming over >3 g had reduced event free survival. |
| Saleh et al (27) | Combined observational studies | 35 (13–79) | 107 | Outpatients with comorbid diabetes mellitus followed for a median of 337 days, sodium intake based off a single 24-hour urine sample | Event free survival was decreased in individuals with urinary sodium excretion over 3.8 g vs. those with sodium excretion below 3.8 g daily. |
| Aliti et al (31) | Randomized single-blind controlled trial | 26.0±8.7 | 75 | Inpatient, admitted for decompensated HF 800 mg/day sodium restriction vs. control until 7th day of hospitalization or discharge |
No differences in 3- day change in weight and clinical congestion score in intervention vs. control (primary outcome) and 30-day readmissions in intervention vs. control. |
| Machado d’Almeida et al (32) | Randomized Controlled Trial | 62 + 8 Intervention 60 + 7 Control |
53 | Inpatient, admitted for decompensated HF 800 mg/day sodium restriction with 800 ml/day fluid restriction vs. control until 7th day of hospitalization or discharge | The primary outcome of weight loss at 3 days did not differ between the two groups nor did clinical congestion score. |
| Dietary Patterns | |||||
| Levitan et al (39) | Prospective, observational study | Not Described | 3215 | Women enrolled in the WHI trial with a hospitalization for decompensated HF during the trial, followed for a median of 4.6 years. Adherence to MedDiet and DASH assessed by serial food frequency questionnaire |
Increasing adherence to the MedDiet was associated with a trend towards reduction in death from HF, while increasing adherence to the DASH diet was associated with a lower risk of death from HF |
| Miró et al (40) | Multicenter, prospective, observational study | 51±14 | 991 | Adherence to a MedDiet assessed by patient questionnaire in those seen in the emergency department for acute HF | After a mean follow up of 2.1±1.3 years, adherence to the Mediterranean diet was not associated a decrease in all-cause mortality (primary outcome) but was associated with a decrease in rehospitalizations for HF. |
| Rifai et al (41) | Randomized controlled trial | 41±13 Intervention 40±15 Control |
48 | 3 months of DASH diet intervention vs. control | Endothelial function was not better in the DASH group (primary outcome) but quality of life assessed by MLHFQ and 6MWT was improved vs. control. |
| Hummel et al (43)(44) | Open-label pilot trial | 69±6 | 13 | 21 days of home delivered DASH/sodium restricted diet (1,150 mg sodium/day) | DASH diet lowered clinic and 24-hour ambulatory blood pressure and BNP. Cardiac systolic and diastolic function, 6MWT and NYHA class were improved. |
| Hummel et al (45) | Multicenter, randomized single-blind controlled trial | 39±18 | 66 | Home delivered DASH/sodium restricted diet (1,500 mg sodium/day) vs. control for 30 days post-hospital discharge for HF exacerbation | No significant difference between groups in change of KCCQ summary score (primary outcomes). There was a trend towards lower HF re-hospitalization at 30 days in DASH vs. control. |
| Dietary Fat and Fatty Acids | |||||
| Carbone et al (56) | Cross sectional analysis | 60.4 (57.163.0) | 23 | Analysis of baseline 24-hour dietary recall in patients enrolled in a trial for anti-inflammatory therapy | Dietary UFA were positively associated with peak VO2, greater fat-free mass and more favorable diastolic function. |
| Carbone et al (57) | Single-arm pilot trial | 58±4 | 9 | In patients with comorbid obesity, 12 weeks of daily supplementation with 1 serving of food rich in UFA. Preferred recommendations for intake were extra-virgin olive oil (54 g), canola oil (54 g), lightly salted mixed tree nuts (walnuts, hazelnuts, almonds and pecans) or peanuts (28 g) | There was an increase in dietary UFA and plasma UFA (primary outcome). An increase in exercise time and oxygen pulse, as well as a trend towards an increase in peak VO2 was observed. |
| Mehra et al (58) | Randomized double-blind controlled trial | 18 ± 5 Intervention 16 ± 5 Control |
14 | Supplementation of N-3 PUFA 8g daily vs. placebo for 18 weeks | Trend towards reduction of TNF-α (primary outcome) and increase in body fat in intervention vs. placebo |
| Nodari et al (59) | Randomized, double-blind controlled trial | 36±7 Intervention 37±6 Placebo |
133 | N-3 PUFA 1g daily vs. placebo for 12 months | Increase in EF in N-3 PUFA vs. placebo (primary outcome), as well as improvement in diastolic function, decrease in inflammatory biomarkers and lower rate of HF admission. |
| Wu et al (60) | Randomized double-blind controlled trial | 28±1 | 31 | Supplementation of L-alanyl-L-glutamine (8 g/day) and N3 PUFA (6.5 g/day) vs. placebo for 3 months | No change in peak VO2, 6MWT, handgrip or leg/arm skeletal muscle function in intervention vs. placebo (primary outcomes). |
| GISSI-HF (61) | Multicenter randomized, double-blind controlled trial | 33.0±8.5 Intervention 33.2±8.5 Placebo |
6975 | N-3 PUFA 1g daily vs. placebo for a median follow up of 3.9 years | The N-3 PUFA group demonstrated lower all-cause mortality, as well as the combined outcome of lower all-cause mortality and hospital readmission (primary outcome). |
| Dietary Protein and Malnutrition | |||||
| Aquilini et al (65) | Randomized controlled Trial | 29.4 ± 17 Intervention 29.7 ± 9.2 Control |
44 | Outpatients with low muscle mass as assessed by arm muscle area below the 10th percentile for age and sex, randomized to consume 4 grams of essential amino acids twice daily vs. usual care control for 2 months | Subjects receiving the essential amino acids demonstrated a significantly greater improvement in CRF measured by peak VO2 on CPET and functional capacity measured by 6MWT than controls. |
| Lombardi et al (66) | Open-label, single armed study | 29.0% ± 7.9 | 13 | Outpatients, instructed to consume 4 g of essential amino acids twice daily for 3 months | No significant differences were observed in echocardiographic parameters or quality of life assessed by questionnaire, but peak VO2 and 6MWT increased at 3 months |
| Pineda-Juárez et al (67) | Randomized controlled trial | Not described | 66 | 3 months of resistance training plus branch-chain amino acid supplement (10 g/day) vs. resistance training alone | There were no effects of branch chain amino acids on muscle strength or peak VO2 |
| Deutz et al (69) | Randomized, double blind controlled trial | Not described | 652 | Older (>65 years) inpatients hospitalized for decompensated heart failure randomized to high calorie high protein oral supplement with HMB vs. placebo for 90 days | No significant difference in death or nonelective readmission postdischarge between groups, lower 90-day mortality in the intervention group vs. control (composite primary outcome) |
| Rozentryt et al (70) | Randomized, double-blind controlled pilot trial | 25±10 Intervention 24±5 Placebo |
29 | In patients with clinically significant weight loss, 6 weeks of a high calorie (600 kcal) high protein (20 g) oral nutrition supplement vs. placebo (12 kcal/day) | From baseline, intervention increased weight gain and overall MLHFQ score (primary outcomes), as well as 6MWT. Peak VO2 was not increased. |
| Bonilla-Palomas et al (71) | Randomized controlled trial | 48.5±17.3% | Inpatients hospitalized for decompensated HF with malnutrition established by Mini Nutrition Assessment Score randomized to 6 months of personalized nutrition counseling or usual care control, followed for 12 months | All-cause death or readmission due to HF was lower in the nutrition intervention group vs. control (composite primary outcome) | |
| Caloric Restriction | |||||
| Evangelista et al (80) | Randomized controlled trial | 26±7.3 | 14 | 12 weeks of a calorically restricted diet (500 to 800 kcal deficit), one group with high-protein (30% kcals), another group with standard protein (15% kcals) and a control diet without dietary changes | Greater weight loss, reduction in waist circumference, fat mass and increase in 6MWT and peak VO2 in the high-protein group vs. the other two groups |
| Kitzman et al (82) | Randomized controlled trial | 61±6 | 100 | 20 weeks of a 2×2 factorial study to assess the effects of exercise vs. diet 4 groups – exercise (supervised aerobic exercise 3× week), caloric restriction alone (400 kcal deficit), exercise and caloric restriction combined and control |
The independent effects of diet from baseline included increased peak VO2, but no effect on MLHF total score (primary outcomes). Exercise time, peak workload, 6MWT, reduction in weight, fat mass, inflammatory biomarkers and total KCCQ score also improved with diet. |
| Micronutrient Supplementation | |||||
| Witte et al (89) | Randomized, double-blind controlled trial | 26.1±6.7 | 32 | Daily multiple micronutrient supplement vs. placebo for 9 months | Increase in LVEF in the micronutrient group vs. placebo (primary outcomes), as well an increase in quality of life scores assessed by questionnaire. 6MWT, NYHA class and inflammatory biomarkers remained unchanged in both groups. |
| McKeag et al (90) | Randomized, double-blind, controlled trial | 38.3±11.4 Intervention 45.1±9.0 Control |
74 | Daily multiple micronutrient supplement vs. placebo for 12 months | No significant difference in EF between intervention and placebo (primary outcome) or in MLHFQ questionnaire score, 6MWT, NTproBNP and inflammatory biomarkers. |
| Beck-da-Silva et al (91) | Randomized, double-blind, controlled trial | 28 ± 7.8 | 23 | Outpatients with anemia randomized to: 1.) Iron Sucrose 200 mg intravenously, once a week, in 30 min infusions, for 5 weeks and placebo of oral presentation, three times a day 2.) Ferrous sulfate 200 mg, orally, three times a day, for 8 weeks and placebo of IV presentation once a week, for 5 weeks. 3.) Placebo of oral presentation, three times a day, for 8 weeks and placebo of IV presentation once a week, for 5 weeks. |
There was an increase in peak VO2 of 3.5 ml.kg.min−1 in the IV iron group and no improvement in the oral iron group |
| Lewis et al (92) | Randomized, double-blind controlled trial | 25 (20–34) | 225 | Outpatients with anemia randomized to 150 mg oral iron polysaccharide or placebo twice daily for 16 weeks | The primary outcome of peak VO2 did not differ between placebo and iron supplementation |
| Anker et al (93) | Combined Randomized Controlled Trials | 33.3 ±6.9 Intervention 34.5 ±7.1 Control |
844 | Combined analysis of 4 trials randomizing subjects to intravenous ferric carboxymaltose or placebo | Subjects on ferric carboxymaltose had lower rates of recurrent CV hospitalizations and CV mortality rate than those on placebo |
| Schoenenberger et al (95) | Randomized, double-blind controlled crossover trial | 29.5±2.5 | 9 | 28 days of treatments with 300 mg/day thiamine vs. placebo | Significant improvement in EF in thiamine group vs. placebo (primary outcome); trend towards improvement in 6MWT. |
| Shimon et al (96) | Randomized double-blind controlled trial | 28 ± 11 Intervention 26 ± 9 Placebo |
30 | 1 week of inpatient randomization to either IV thiamine 200 mg/day vs. placebo with 6 weeks of open label oral thiamine of 200 mg/day | In the patients completing the full 7 weeks of treatment, EF rose by 22%. |
| Witte et al (97) | Randomized double-blind controlled trial | 26.1±10.68 | 229 | 100 ug daily Vitamin D3 vs. placebo for 1 year | Vitamin D3 did not increase 6MWT vs. placebo (primary outcome), but did increase EF and improve remodeling. |
| Witham et al (98) | Randomized double-blind controlled trial | Not described | 105 | 100,000 IU Vitamin D2 vs. placebo at baseline and 10 weeks in older outpatients (≥70 years) | Vitamin D2 did not increase 6MWT or quality of life vs. placebo |
| Boxer et al (99) | Randomized double-blind controlled trial | 37.6±13.9 | 64 | 6 months of weekly 50,000 IU Vitamin D3 vs. placebo | There was no difference in changes in peak VO2 in the intervention group vs. control (primary outcome) nor in 6MWT. |
| Zittermann et al (100) | Randomized, double-blind controlled trial | 28 (23–34) Intervention 26 (24–35) Placebo |
892 | 4,000 IU Vitamin D3 daily vs. placebo for 3 years | Vitamin D3 did not reduce all-cause mortality vs. placebo (primary outcomes); there was an increased implantation of mechanical circulatory support in the Vitamin D3 group. |
| Ghatak et al (101) | Randomized, blind controlled trial | Not described | 12 | 400 mg oral Vitamin E vs. placebo once a day for 4 weeks | Vitamin E reduced oxidative stress indicated by greater reductions in malonyldialdehyde and superoxide anion levels in the intervention group vs. placebo |
| Keith et al (102) | Randomized double-blind controlled trial | 22.5 ± 5.5 | 56 | Outpatients randomized to 335.6 mg Vitamin E vs. placebo for 12 weeks | Vitamin E did not improve oxidative stress or quality of life vs. placebo |
| Shinke et al (104) | Randomized controlled trial | 39.0 | 19 | In outpatients presenting with HF post myocardial infarction, Single infusion of 2.0 grams Vitamin C followed by a 50 mg/ minute infusion for 10 minutes vs. control | Vitamin C enhances contractile response to p-adrenergic stimulation |
| Burkhard et al (105) | Randomized controlled trial | 22±2 Intervention 20 ± 3 Control |
15 | Single infusion of 25 mg/min Vitamin C over 10 minutes vs. control | Vitamin C improved flow-dependent dilation mediated by nitric oxide vs. control |
| Mortensen et al (108) | Randomized double-blind controlled trial | 31 ± 10 Intervention 31 ± 10 Placebo |
420 | CoQ10 100 mg TID or placebo for 106 weeks | CoQ10 reduced the primary outcome of major adverse cardiovascular events versus placebo |
6MWT, 6 Minute Walk Test; BID, twice a day; BNP, brain natriuretic peptide; CoQ10, Coenzyme Q10; DASH, Dietary Approaches to Stop Hypertension; EF, ejection fraction; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; kcals, kilocalories; MedDiet Score, Mediterranean Diet Score; MLHFQ, Minnesota Living with Heart Failure Questionnaire; N, number of participant; N-3 PUFA, N-3 Polyunsaturated Fatty Acids; Na, sodium; NYHA, New York Heart Association; peak VO2, peak oxygen consumption; QoL, quality of life; TID, three times daily; TNF-α, tumor necrosis factor alpha; UFA, Unsaturated Fatty Acids
Recently, a pilot trial enrolled 38 subjects with HF (LVEF 42%, 25.0–50.5) and randomized 1:1 to low sodium (1500 mg/day) or moderate sodium (2300 mg/day) for 6 months21. Subjects were provided baseline education and resources to aid in following the diet, called by a research dietitian on a monthly basis and 3 day food records were performed at baseline, 3 months and 6 month follow ups21. At 6 months, both groups significantly reduced their sodium intake with both groups consuming a mean sodium level below 1500 mg per day however there was no significant difference in the change between groups (low sodium group: 2137 (1304–3118) to 1398 (1090–2060) mg/day, moderate sodium group: 2678 (1797–3018) to 1461(1086–1765) mg/day)21. The low sodium group alone significantly improved their overall summary score measuring QoL by Kansas City Cardiomyopathy (KCCQ) questionnaire (59.6 (39.1–73.2) to 64.6 (50.3–86.1)) as well as significantly reducing their brain natriuretic peptide (BNP) levels (216 (25–670) to 71(39–222) picograms/mL), however, changes in these variables did not differ between groups21. In a post-hoc analysis, patients who achieved a sodium restriction below 1500 mg/day showed significant improvements in BNP and overall KCCQ score while those consuming sodium >1500 mg/day did not21. A multicenter dietitian-led follow-up trial (SODIUM-HF, NCT2012179) is ongoing, randomizing outpatients with HF to either <1500 mg sodium daily or usual care recommendations to simply reduce their dietary sodium with a primary composite outcome of all-cause mortality, cardiovascular (CV) hospitalizations and CV emergency department visits.
While potential benefits have been shown in a few trials, multiple studies have resulted in no benefit or even suggested negative effects resulting from sodium restriction (Figure 1). In a study enrolling subjects 30 days after discharge for an admission of acute decompensated HF (ADHF), 410 HFrEF patients with LVEF <35% were randomized to 8 groups in a 2×2×2 factorial design based on different sodium levels (1800 or 2800 mg), diuretic doses (125 vs. 250 mg furosemide twice daily) and fluid restrictions (1 vs 2 L)22. Subjects received meal plans prepared by dietitians which were standardized across the diets for other macronutrients, adherence to the diets was evaluated via food diaries22. At 180 days, individuals with normal sodium intake (2800 mg), 1 L fluid restriction and twice daily 250 mg furosemide dose, demonstrated a significantly decreased readmission rate versus all other groups22. A trial conducted by the same investigators with similar methodology, randomized 232 HFrEF LVEF <35% patients to either 1800 vs. 2300 mg of sodium, each with 1 L fluid restriction and demonstrated significant reductions in the primary outcome of readmissions at 180 days (7.6 vs 26.3%,) and the combined mortality and readmission (12.7 vs 39.5%) in the normal (2800 mg) versus low (1800 mg) sodium group23. Criticisms have been made regarding the results of these trials consisting of inclusion of only HFrEF patients, lack of readjustment of medication during the follow up period despite the high-dose diuretic regimen, the number of patients included who were not on guideline-recommended medical therapy and lastly, concerns of data replication in these studies has been raised24. Furthermore, these trials included other components such as fluid restriction and assigned diuretic dosing which may have altered outcomes versus sodium restriction alone22,23.
Other trials have suggested that perhaps the severity of HF influences whether sodium restriction of any level should be practiced (Table 1). A prospective, observational study compared outpatients with HF (LVEF 37±13) consuming either 2g, 2–3g and >3g of sodium per day over a 12-month follow-up period25. Sodium intake was based on a 4-day food diary at baseline25. Results differed among severity of HF defined by NYHA classes (21). In those with less severe HF, NYHA class I/II, subjects consuming <2 g sodium per day had a significantly higher risk for hospitalization or death (HR: 3.68, 95% CI 1.18–11.50), but subjects consuming greater than 3 g of sodium had a significantly lower risk for hospitalization or death (HR: 0.39, 95% CI 0.16–0.98) compared to subjects consuming 2–3 grams of sodium daily25. This relationship differed in subjects with more severe HF, NYHA class III/IV, in which subjects who consumed >3 g sodium had a significantly greater risk of hospitalization or death than those consuming 2–3 g (HR: 2.06, 95% CI 1.02–4.17), however, there was no difference observed between those consuming <2 and 2–3 grams sodium daily25. Although the authors discuss that intravascular depletion may occur due to compensatory mechanisms in those with NYHA class II–III HF leading to worsened outcomes, why outcomes may be improved in those consuming >3 g sodium is not discussed25. Likewise, the authors do not speculate on why differing outcomes are seen in those with NYHA class III/IV HF25. The results of this study echo an earlier prospective observational study from the same investigators that took a single 24-hour urine sample from 302 patients with HF (LVEF 34±14%) and followed outcomes for 12 months26. Subjects with NYHA class II/III HF consuming >3 g sodium at baseline had increased event free survival (HR: 0.44, 95% CI 0.20–0.97), while those in NYHA class III/IV consuming over >3 g had reduced event free survival (HR: 2.54, 95% CI 1.10–5.84)26.
Another recent prospective, observational study followed 107 patients with HF (LVEF 35%, 13–79) and diabetes mellitus, type undefined, for a median of 337 days27. Sodium consumption was again based off a single 24-hour urine sample27. This multivariate analysis was controlled for NYHA class and found event-free survival was significantly increased in individuals with urinary sodium excretion over 3.8 grams (HR: 2.8, 95% CI 1.3–5.7) versus those with sodium excretion below 3.8 grams daily27. Importantly, major issues are present with basing sodium consumption off a single 24-hour urinary collection, namely the reproducibility of a single sample - at least 3 measurements are needed for accurate sampling that reflects an individual’s sodium intake28 with very little evidence validating and standardizing such methods in individuals treated with diuretics (as in most HF patients)29. Another recent prospective observational study followed 902 NYHA class II/III, majority HFrEF (>75%) patients enrolled in the HF Adherence and Retention Trial (HART) for 36 months13. Sodium intake was averaged from food frequency questionnaires (FFQ) performed at years 1, 2 and 3 and patients were divided in restricted (<2500 mg) and unrestricted (≥2500 mg)daily sodium intake13. Notably, the authors assumed a baseline of consumption of 1,250 mg sodium to which they added all additional sodium intake quantified from the FFQ, which had not been validated for the measurement of sodium intake13,30. As patients weren’t randomized, they were propensity matched to a patient of the opposite group in a 1:1 ratio. In a multivariate adjusted, propensity matched analysis of 260 subjects, sodium restriction was associated with a significant increase in the primary outcome rate of death or HF hospitalization (HR: 1.72, 95% CI 1.12–2.65)13. This analysis also found a significantly increased risk of the primary outcome occurring with sodium restriction in NYHA class II patients (HR: 1.85, 95% CI 1.21 to 2.84) but not class III. The authors postulated that perhaps in less symptomatic NYHA class II patients, neurohormonal activation with sodium restriction outweighs the benefit of reduced fluid retention13. Furthermore, there was a significantly increased risk in patients not on angiotensin converting enzyme (ACE) inhibitors and Angiotensin II Receptor Blockers (ARB) therapy, while those prescribed these medications did not have significantly increased risk based on sodium restriction, suggesting that perhaps these medications nullify the compensatory neurohormonal effects of sodium restriction13.
Lastly, although inpatient sodium restriction is commonly practiced for ADHF admissions, only two trials have examined the effects of this strategy in HF inpatients (Table 1). Seventy five patients admitted for ADHF (EF 26±8.7%) were randomized to fluid (800 mL) and sodium restriction (800 mg) versus no restriction over a 3-day period31. Despite this tight restriction, 30-day readmission, transition from intravenous to oral diuretics, changes in weight and clinical congestion score did not differ significantly between the two groups31. Perceived thirst assessed by visual analog scale at the end of the 3-day period was significantly worse in the sodium and fluid restriction patients versus controls31. Another recent trial enrolled 53 inpatients with HFpEF (LVEF: 68±8% intervention, 60±7% control), and randomized them to a strict fluid restriction of 800 ml/day with sodium 800 mg/day versus a fluid and sodium unrestricted control group for 7 days or until hospital discharge32. The primary outcome of weight loss at 3 days did not differ significantly between the two groups nor did clinical congestion score32. Notably, the intervention group did display a significantly lower energy intake (1159.4 calories/day sodium restriction vs 1471.6 calories/day control).
Dietary Patterns
Although the traditional focus of nutrition therapies in HF has been on individual nutrients and specifically sodium, it is increasingly evident that overarching dietary pattern may have a pivotal role in preventing and treating HF33. The Mediterranean dietary pattern (MedDiet)34–40 and Dietary Approaches to Stop Hypertension (DASH) dietary pattern39,41–47 are the most frequently studied dietary patterns in regards to both preventing initial onset of HF as well as improving outcomes in those with preexisting HF33 (Table 1). These dietary patterns share many common characteristics - both emphasizing fruits, vegetables, whole grains and legumes while limiting saturated fatty acids (SFA)39 (Figure 2). While the DASH diet promotes a high potassium intake while limiting sodium, SFA and total fat48, the MedDiet emphasizes a greater intake of unsaturated fatty acids (UFA), which is composed of both monounsaturated (MUFA) and polyunsaturated fatty acids (PUFA) found in fatty fish, extra-virgin olive oil, canola oil and mixed nuts49.
Figure 2. Continuum of dietary pattern effects in heart failure.
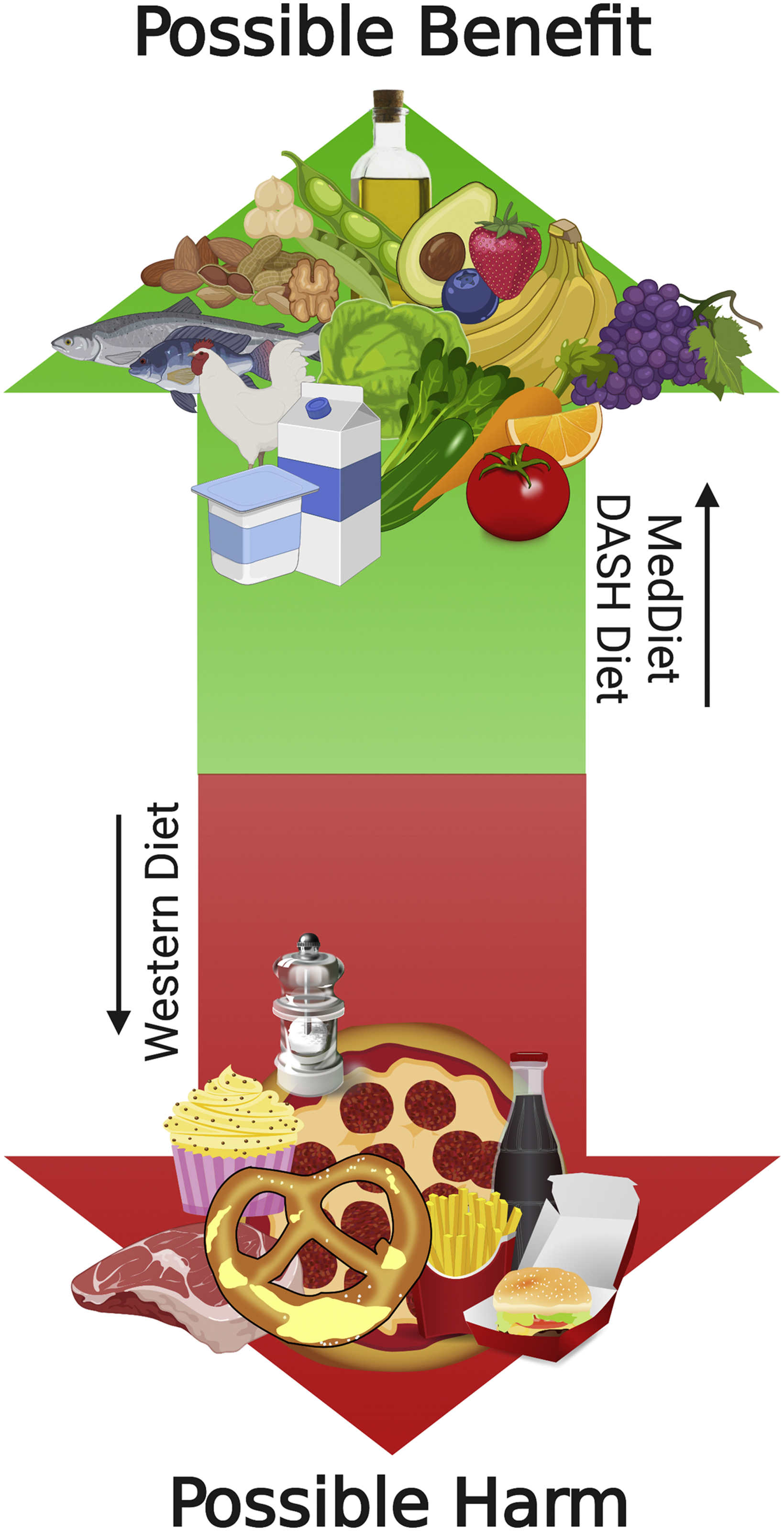
Existing literature from Hummel S et al.,(43–45) Rifai et al.(41), Miro et al.(40) and Carbone et al (56,57) demonstrates a possible benefit of the Dietary Approaches to Stop Hypertension (DASH) and Mediterranean dietary patterns and their components (i.e., unsaturated fatty acids) (MedDiet) on clinical outcomes in patients with HF. Preclinical and observational clinical data from Carbone et al (56,112) demonstrates possible worsening cardiac function from a Westernized dietary pattern, specifically a diet high in saturated fatty acids and sugars, though this requires confirmation in larger clinical studies.
The PREDIMED study (Prevención con Dieta Mediterránea) randomized subjects 1:1:1 to a MedDiet plus supplemental mixed nuts or extra-virgin olive oil versus a low-fat control group. Both supplemented groups had a lower incidence of the primary composite endpoint of myocardial infarction, stroke or CV death (extra-virgin olive oil, HR: 0.69, 95% CI 0.53–0.91; mixed nuts, HR: 0.72 (95% CI 0.54–0.95)50, but there was no significant difference in HF incidence after 4.8 years. The number of HF events was extremely low, likely resulting in the study being underpowered for these specific events35. However, in a subset of participants tested for HF biomarkers after 1 year of follow up, both MedDiet groups reduced their N-terminal pro-BNP (NT-proBNP) versus the low-fat control51.
Large observational trials have, however, demonstrated a reduction in HF incidence with adherence to a MedDiet. Two large prospective Swedish cohorts composed of over 30,000 men36 and women37, found that high compared to low adherence to the MedDiet resulted in a significant reduction in incidence of HF in both women (relative risk (RR): 0.79, 95% CI 0.68–0.93) and men (RR: 0.69, 95% CI 0.57–0.83) over a 10-year follow up. In a German cohort of over 24,000 subjects followed for 8 years, higher adherence to the MedDiet did not result in a significant decrease in HF incidence in a multivariate adjusted model (HR: 0.82, 95% CI 0.64–1.05) except when milk products were excluded from the MedDiet score (HR 0.75, 0.55–0.97)38.
Less is known about whether adherence to a MedDiet can improve clinical outcomes in patients with existing HF. In 3215 women with HF enrolled in the Women’s Health Initiative, a modified Block FFQ administered at baseline, year 1, 2 and 3 was used to evaluate adherence to the MedDiet or DASH diet39. The last FFQ before HF hospitalization was used to evaluate adherence to the dietary patterns39. After a median of 4.6 years follow up, adherence to the MedDiet was associated with a non-significant trend towards reduction in death from HF (highest quartile of adherence HR:0.85, 95% CI 0.70–1.02), while adherence to the DASH diet was associated with a significantly lower risk of death from HF (highest quartile of adherence HR: 0.84, 95% CI 0.70–1.00)39. In a prospective study of 991 patients admitted to Spanish emergency rooms with ADHF, adherence to the MedDiet was assessed with a 14-point score questionnaire with adherence defined as ≥9 points and nonadherence ≤8 points40. After a mean follow up of 2.1 years, no significant difference was observed between adherent and nonadherent patients on the primary outcome of mortality (HR: 0.86, 95% CI 0.73–1.02), but a significant reduction in rehospitalization from HF was observed in adherent patients (HR:0.74, 95% CI 0.61–0.90)40.
As aforementioned39, there has also been increasing interest in the role of the DASH diet to prevent and treat HF. In a large Swedish cohort of >35,000 Swedish women, a baseline FFQ was used to assess adherence to the DASH diet42. The women were followed for 7 years and demonstrated a significant linear reduction in development of HF in association with increasing DASH score, and women in the highest quartile of adherence to DASH demonstrated a significant lower risk of HF compared to the lowest quartile (HR: 0.63, 95% CI 0.48–0.81)42. This was also demonstrated in a large male Swedish cohort of nearly 39,000 participants, where a FFQ was also used to assess adherence to a DASH diet47. Participants were then followed for 9 years in which men in the top quartile of adherence to DASH demonstrated a significant reduction in risk of HF compared to the lowest quartile (HR: 0.78, 95% 0.65–0.95)47. Contrastingly, in a recent prospective cohort study of 4500 American older adults, DASH diet based on FFQ showed no significant effects on the development of HF over a 21.5 year follow-up period46. Notably, only two FFQs were performed over this long time period, one at baseline and again after 5 years46.
A few small trials have examined the effects of the DASH diet in patients with existing HF, with promising results41,43–45 (Table 1). A small trial of 13 patients with HFpEF (LVEF 69±6%) and hypertension consumed a DASH/sodium restricted (1,150 mg) diet which was provided to them in the form of all foods and beverages consumed for 21 days43,44. At follow-up, both clinic and ambulatory systolic (155±35 to 138±30 millimeters mercury (mmHg) clinical, 130±16 to 123±18 mmHg ambulatory) and diastolic blood pressure (79±15 to 72±8 mmHg clinical, 67±3 to 62±3 mmHg ambulatory) were significantly reduced, pulse wave velocity was significantly reduced (12.4± 3.0 to 11.0± 2.2 meters (m) per second), 6 minute walk test (6MWT) distance significantly increased (313± 86 to 337±91 m) and cardiac diastolic function (viscoelestance/relaxation −4.2±6.2 grams/second2, chamber stiffness −81±99 grams/second2) as well as ventricular-arterial coupling ratio (−0.2±0.3) significantly improved on echocardiogram43,44. In another small trial of 48 patients with HF (LVEF intervention 41±13%, LVEF control 40±15%), subjects were randomized to usual care control or DASH diet for 6 months41. The DASH diet group received baseline and then monthly sessions with a dietitian as well as weekly or biweekly phone calls to reinforce adherence to the diet41. Subjects in the usual care group received no instruction to change their diet41. At 3 months, subjects in the DASH group demonstrated significantly greater QoL assessed by Minnesota Living with Heart Failure (MLWHF) questionnaire (29±20 to 21±15 DASH, 38±4 to 39±22 control), a non-significant trend towards lower NT-ProBNP (102 ±97 to 94 ±97 DASH, 253 ± 472 to 314±510 control) and a significantly improved 6MWT distance (254±119 to 292±124 DASH, 202±77 to 197±81 control) versus controls41. Recently, the GOURMET-HF trial (Geriatric-Out-of-Hospital Randomized Meal Trial in Heart Failure) randomized 66 patients with HF to home delivered DASH/sodium restricted (1500 mg/day) meals or usual care control for 4 weeks post HF hospitalization45. There was no difference in the primary outcome of the KCCQ summary score (46±23 to 59±20 DASH, 43±19 to 53±24 control), but 30 day readmissions (11 vs. 27%) and days re-hospitalized during that time period (17 vs. 55) trended lower in patients on the DASH/sodium restricted meal plan45, supporting a larger study to further investigate the effects of this strategy.
Overall, both the MedDiet and DASH diet are rich in plant-based foods and low in processed foods and red meat (Figure 2)39. A plant-based dietary pattern has been found to be associated with a significantly lower risk of HF in a large nationally representative American cohort (HR: 0.59, 95% CI 0.41–0.86)52. Additionally, high intake of fruits and vegetables compared to low has been significantly associated with a lower risk of HF in a large Swedish female cohort (RR: 0.82, 95% CI 0.72 to 0.94), with the lowest rate of HF occurrence in women consuming over >5 servings of fruits and vegetables per day53. At this time, both the MedDiet and DASH diet are reasonable candidates for large RCT investigating their ability to decrease the risk of HF and improve secondary outcomes in both HFrEF and HFpEF.
Dietary Fatty Acids
The MedDiet is not only rich in plant-based foods, but also UFA found in fatty fish, mixed nuts and extra-virgin olive oil54. There has been a long misunderstanding by the public and clinical communities regarding the role of dietary fat in CV disease (CVD)54. While SFA does increase low-density lipoprotein cholesterol and may increase the risk of cardiometabolic diseases, UFA appear have the opposite effect- possibly exerting a protective effect against the development of obesity, T2DM and CVD54. Furthermore, low-fat diets do not lead to lower incidence or mortality from cardiometabolic disease and evidence does not warrant their use in prevention of these conditions54,55.
Small studies have shown that UFA supplementation through dietary sources may have a beneficial effect on patients with HF. A cross sectional analysis of 23 patients with HFpEF showed that consumption of dietary SFA, MUFA, PUFA, and UFA as total amount and percent of daily calories correlated with increased cardiorespiratory fitness (CRF) measured by peak VO2 on maximal CPET56. Upon multivariate analysis, UFA remained significantly associated with peak oxygen consumption (peak VO2) while SFA was no longer significant56. Greater intake of UFA was also associated with more effective diastolic function on echocardiogram and more favorable body composition exemplified by higher percentage of fat-free mass and greater fat free mass to fat mass ratio measured with BIA56. The UFA-Preserved pilot study, a recent dietitian-led pilot trial of 9 subjects with HFpEF, supplemented at least one serving of UFA-rich foods every day for 12 weeks57. Recommended food choices included at least 54 g extra-virgin olive oil or canola oil and 28 g unsalted or lightly salted mixed nuts, but subjects had further choices including fatty fish, avocado and seeds to suit personal or cultural preferences57. Importantly, the study did not provide recommendations on caloric intake and did not provide any upper limit for consumption for UFA-rich foods. After 12 weeks, subjects demonstrated significant increases in UFA through both percent of median daily calories reported on dietary recall (from 19.4 [16.1–22.5]% to 34.5 [28.2–56.1]%), without significant changes in caloric intake, and plasma fatty acids (from 1,319 [1,224–1,477] to 1,621 [1,261–2,110] μg/mL) as an objective operator-independent measure of adherence57. Moreover, exploratory analyses revealed significant improvements in exercise time (7.3%), oxygen pulse (+13.7%) and non-significant favorable trend towards improvement in peak VO2 (+11.6%), without significant changes in self-reported physical activity57. Of note, changes in plasma UFA were also positively associated with changes in peak VO2 (R=+0.71). Dietary supplementation of UFA may represent a strategy to improve CRF and clinical outcomes in HFpEF patients, but further trials with larger numbers of patients will be needed to confirm this57. Of note, the UFA-Preserved 2 (Unsaturated Fatty Acids to Improve Cardiorespiratory Fitness in Obesity and HFpEF) (NCT03966755) is currently ongoing, testing daily dietary supplementation of UFA versus standard of care control of sodium and SFA following the Dietary Guideline for Americans 2015–2020 in patients with HFpEF. Lastly, dietary supplementation of UFA has yet to be examined in subjects with HFrEF.
Although interest in overall UFA supplementation in patients with HF is relatively new, there has been sustained interest in the benefits of N-3 PUFA supplementation in patients with HF. A small double-blind trial randomized 14 advanced HFrEF (EF 18±5% N-3 PUFA, 16±5% control) patients to 8 g/day N-3 PUFA or placebo for 18 weeks to examine effects on systemic inflammation58. After 18 weeks, the N-3 PUFA patients demonstrated a trend towards reduction in inflammatory biomarker tumor necrosis factor alpha (TNF-α) versus placebo (−59% N-3 PUFA, +44% placebo, p=0.07)58. In a subset of 8 patients (4 placebo, 4 N-3 PUFA), patients taking N-3 PUFA showed a trend towards decrease in inflammatory biomarker interleukin 1 (−39%, p=0.09), while no change occurred in the placebo group58. Furthermore, a significant increase in body fat occurred in patients assigned to N-3 PUFA vs. control (+13% N-3 PUFA, −5% control), which demonstrated a statistically significant inverse association with the change in TNF-α (r= −0.6)58. Another randomized trial of N-3 PUFA supplementation in HFrEF patients (LVEF 37±6%), randomized 133 patients to a loading dose of 5 g daily of N-3 PUFA or placebo for the first month, which then dropped to 2 g for the remainder of the 12 months59. After 12 months, subjects supplementing with N-3 PUFA demonstrated significant improvements in CRF by peak VO2 on cardiopulmonary exercise test (CPET) (19.5±3.8 to 20.7±4.3 mL•kg−1•min−1 N-3 PUFA, 18.3±4.4 to 17.4±4.2 mL•kgLM−1•min−1 placebo), exercise time on CPET (10.4±1.9 to 11.2±2.1 minutes N-3 PUFA, 10.6 ± 2.1 to 10.0 ±2 .0 minutes placebo) and the primary endpoint of EF (36±7 to 39±6 % N-3 PUFA, 37±6 to 35±6% placebo) compared to placebo59. Importantly, hospitalizations for CV causes (14.9% vs. 39.4%,) and worsening HF (5.9% vs. 30.3) were also significantly lower for subjects in the N-3 PUFA group versus placebo59. In contrast, a randomized, double blind control trial of 6.5 g of PUFA daily with 8 g of the dipeptide L-alanyl-L-Glutamine demonstrated no improvements in echocardiography or peak VO2 after 3 months of supplementation in 31 patients with HFrEF (LVEF 28% ± 1)60. It is possible despite the high dosage (6.5 g N-3 PUFA daily) that the 3-month duration of the trial was too short to see an effect59–61. Lastly, in the randomized, double blind control trial, GISSI-HF (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico HF), over 7,000 subjects with HF (LVEF 33%,±8.5) were assigned to 1 g N-3 PUFA daily or placebo and followed for a median of 3.9 years61. There were modest but significant reductions in the co-primary endpoint of all-cause mortality (HR: 0.91, 95% CI 0.833–0.998) and combined all-cause death and admission for CV causes (HR: 0.92, 99% CI 0.849–0.999) in the N-3 PUFA group versus placebo61. Notably, over 91% of patients enrolled in this trial had HFrEF62, and outcome trials are needed to examine the effect of N-3 PUFA in clinical outcomes in HFpEF.
Overall, it appears that N-3 PUFA supplementation may have beneficial effects on clinical outcomes in HFrEF patients, however, trials have used differing doses and maximally effective dose is unclear, with greater doses being typically associated with more favorable results. Additionally, work so far has focused almost entirely on HFrEF patients and future trials will need to establish the effectiveness of N-3 PUFA supplementation in the HFpEF population.
Dietary Protein and Malnutrition
Lean mass (LM) abnormalities are common in HF and contribute to worse QoL, CRF and clinical outcomes63. In SICA-HF (Studies Investigating Comorbidities Aggravating Heart Failure), male patients were evaluated for sarcopenia, a loss of muscle strength and mass, and cachexia, an unintentional total weight and LM loss, and the impact of these conditions64. Both patients with cachexia and sarcopenia presented with a lower weight-adjusted peak VO2 versus those without these conditions, and coexisting sarcopenia and cachexia compounded the worsening of peak VO2, muscle strength and QoL64. With the known impact of LM abnormalities in HF, protein intake, an essential building block of lean tissue, has been evaluated as a therapeutic strategy that may help to increase muscle mass, strength and potentially result in improved outcomes.
As amino acids (AA) are the building blocks of proteins, several studies have examined supplementing combinations or single AA rather than whole protein mixtures. In a study of 44 patients with HF (LVEF 29.4±17% Intervention, 29.7±9.2% control) and low muscle mass as assessed by arm muscle area below the 10th percentile for age and sex, patients were randomized to receive 8 g essential AA per day or usual care control65. At two months, subjects in both groups demonstrated a significant increase in body weight, which was, however, significantly greater in the intervention group (+2.96±1.56 kg essential AA, 2.30±0.80 control). Patients in both groups also experienced a significant increase in arm muscle area, which was, however, not different between groups65. Subjects receiving the essential AA demonstrated a significantly greater improvement in CRF measured by peak VO2 on CPET (13.5±1.7 to 14.9±1.9 ml/kg/min−1 essential AA, 12.9±2.7 to 13.0±3.5 ml/kg/min−1 controls )and functional capacity measured by 6MWT distance (331±124 to 405±130 m essential AA, 298±142 to 310±155 m controls)65. In another small trial of 13 patients with HF (LVEF 29.0±7.9%), patients consumed supplements containing 8 grams of essential AA daily for 3 months66. After 3 months, no significant differences were observed in echocardiographic parameters or QoL assessed by questionnaire, but peak VO2 (14.8±3.9 to 16.8±5.1 mL•kg−1•min−1) and 6MWT distance (439.1±64.3 to 474.2±89.0 m) significantly increased at 3 months, suggesting peripheral improvements in the skeletal muscle rather than central cardiac improvements; however body composition and strength were not assessed66.
In contrast, other combinations of AA have not improved parameters of interest in patients with HF. A trial of 66 patients with HF (LVEF not available), randomized patients to 10 g branch chain AA daily with resistance training or resistance training alone67. After 3 months, peak VO2 and muscle strength increased significantly in both groups with no differences between groups67. Dietary recalls performed by research dietitians revealed no between group differences in other dietary factors67. It is possible that a greater dose of AA is needed to induce additional muscle protein synthesis in the setting of HF and exercise training. An ongoing double-blind randomized control trial is assigning 22 patients with HFrEF who are beginning cardiac rehabilitation, consisting of both aerobic and strength training, to receive an isocaloric supplement providing either 30 grams of whey protein isolate or 30 grams of carbohydrate daily for 12 weeks on a primary outcome of muscle mass and strength68.
As cachexia, or unintentional weight and LM loss, worsens functional capacity and outcomes of patients with HF63, other studies have combined protein or AA supplementation with additional calories in the form of high-calorie high-protein oral supplements to counteract this wasting process. One trial combined an oral nutrition supplement with beta-hydroxy-beta-methylbutyrate (HMB), a metabolite of the AA leucine, in 652 older (≥65 years) patients hospitalized for HF (LVEF not available) assessed as malnourished on the Subjective Global Assessment69. Patients were randomized to either the supplement (350 calories, 20 g protein, 44 g carbohydrate, 1.5 g calcium-HMB) or a placebo (48 calories, 12 g carbohydrate) twice daily for 90 days69. At 90 days, there was no significant difference in the composite primary endpoint of death or nonelective readmission post-discharge between groups, however, there was a significantly lower 90-day mortality in the intervention group versus control (4.8% vs 9.7%, respectively)69. In a similar trial, 29 patients with HFrEF (LVEF Intervention 25±10, placebo 24±5%) and cachexia, defined as edema-free weight loss of >7.5% over at least 6 months, were randomized to a high-calorie, high-protein supplement (600 calories, 20 g protein) divided into two daily doses for 6 weeks or a placebo (12 calories)70. A total of 23 subjects received the intervention supplement while only 6 received placebo70. In the intervention group alone there was a significant increase in body weight at 6 weeks (2.0±1.7 kg), which was maintained at 18 weeks (2.3±3.1 kg), most of which was deemed to be fat mass by dual energy X-ray absorptiometry70. QoL assessed by MLWHF questionnaire (47±5 to 37±6) and 6MWT distance (366±23 m to 410±24 m) also significantly improved at 6 weeks, but peak VO2 and LVEF did not change in the intervention group70.
Lastly, instead of direct supplementation of protein, amino acids and/or calories, the PICNIC trial (The Nutritional Intervention Program in Hospitalized Patients with Heart Failure who are Malnourished) randomized hospitalized patients with malnutrition, established by Mini Nutrition Assessment Score, and HF (LVEF 48.5±17.3%) to individualized nutrition counseling or usual care control71. The nutrition intervention was performed by a physician specialist aided by a nutritionist and focused on general recommendations for caloric and macronutrient intake, tailored to the patient’s individual needs and comorbidities71,72. At 12 months, the composite primary endpoint of all-cause death or readmission due to HF was significantly lower in the nutrition intervention group (HR: 0.45, 95% CI 0.19–0.62)71. Though the intervention only lasted 6 months, at 24 months the intervention continued to benefit patients who had undergone nutrition counseling with a significant reduction in all-cause death or readmission due to HF in the intervention group versus control (HR: 0.45, 95% CI 0.31–0.89)73.
Overall, amino acid and high-calorie high-protein supplements may improve outcomes69 as well as increase weight, LM and exercise and functional capacity65,66,70. Importantly, current interventions are small and vary in protein and amino acids supplemented. Moreover, interventions to date have often been performed in HFrEF or in a mixed group of patients with HFpEF/HFrEF leaving little understanding of how amino acid and protein interventions impact patients with HFpEF. Further work will need to focus on comparing differing amounts of proteins and amino acids, amino acids versus whole protein, amino acids or protein alone versus in combination with high calorie supplements and the effects of these interventions in HFpEF versus HFrEF.
Caloric Restriction
As previously mentioned, loss of total body weight and/or LM in HF presents a major concerns for worsening outcomes63. The obesity paradox holds that patients with class I–II obesity may have better survival than their normal and underweight peers in the setting of HF as well as other CVD, especially in those with reduced CRF74–76. Furthermore, weight loss is associated with increased mortality risk though this is likely the unintentional weight loss associated with cardiac cachexia rather than weight loss associated with intentional lifestyle change77. Despite this, it is important to note the impact of obesity on the risk of developing HF, especially HFpEF, as well as decreasing CRF in this population75,78. Rather than fat mass being protective, it is more likely that LM serves in a protective role75. Therefore sarcopenic obesity, a condition characterized by excess fat mass that impairs health (i.e., obesity) and reduced LM and related strength and functionality (i.e., sarcopenia), may be particularly detrimental for HF patients75,79.
Few trials examining intentional weight loss from caloric restriction have been performed to date in patients with HF. A feasibility trial in 14 outpatients with HF (LVEF 26± 7) examined caloric restriction in the setting of a high protein and standard protein diet versus a normal diet control for 12 weeks80. Both the standard and high protein diet included a 500–800 calorie deficit based on estimated energy requirements80. At 12 weeks, patients in the high protein diet demonstrated significant reductions in weight in kg (−9.9±2.0 high protein, −5.6±0.8 standard protein, +1.51±0.6 control), percent (%) fat mass (−2.5±1.9 high protein, −1.1±1.9 standard protein, −1.2±2.1 control) and significant improvements in 6MWT distance in m (+287.3±69.0 high protein, −12.3±69.0 standard protein, 138.4±77.1 control) and peak VO2 in mL•kg−1•min−1 (3.1±1.0 high protein, −0.3±1.0 standard protein, −0.3±1.1 control) compared to the other two diets80. Those on the high protein diet also significantly improved their lipid panels including lowering low density lipoprotein cholesterol in mg/deciliter (mg/dL) (−4.5±1.9 high protein, −0.2 ± 0.8 standard protein, 31.3±2.2 control) and increasing high density lipoprotein cholesterol in mg/dL (15.2±2.1 high protein, 0.2±1.8 standard protein, −0.3±2.1 control) versus the other two groups80. Notably, a larger follow-up trial was designed, which compared a high and standard protein diet in HF, but had poor attrition (63% completed primary outcome visit) and, to date, results have not been reported81.
The SECRET trial randomized 100 patients with HFpEF in a 2×2 factorial design to aerobic exercise training, caloric restriction, aerobic exercise training and caloric restriction or control for 20 weeks82. All meals were provided outside of breakfast, the prescribed deficit for the caloric restriction was 400 calories and the deficit for the caloric (+1.36 mL.kg.min−1, 95% CI 0.2–2.3) and exercise (+2.3 mL.kg.min−1, 95% CI 1.0–3.1) both resulted in a significant increase in the primary outcome of peak VO2, but not its co-primary outcome of MLWHF questionnaire score (i.e., QoL)82. Exercise Training and caloric restriction both decreased body weight, but while caloric restriction decreased LM and fat mass, exercise training decreased fat mass alone, confirming the protective role of exercise against LM loss during caloric restriction82. Notably, patients did not experience clinically significant changes in cardiac function in either group, indicating the improvements in peak VO2 mostly stemmed from peripheral changes82. Currently the SECRET-II is ongoing, testing caloric restriction and aerobic exercise training versus caloric restriction, aerobic and resistance exercise training in 84 patients with obesity and HFpEF (NCT02636439).
In the context of an overall healthy dietary pattern and absence of malnutrition, long-term caloric restriction has been linked to reduced mortality and even remarkably long lifespans83. In the multicenter Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy 2 (CALERIE-2) trial, young adult participants who were normal or overweight (BMI 22.0–28.0 kg/m2) were randomized to an ad-libitum normal diet or a 25% caloric restriction from their baseline energy needs, with no changes in normal physical activity, for two years84. Although the caloric restriction participants fell somewhat short of the prescribed 25% restriction in energy, they experienced sustained weight loss (−10.4 ± 0.4%), a reduction in inflammatory biomarkers C-Reactive Protein and TNF-a, a reduction in blood pressure, an increase in high density lipoprotein (HDL) cholesterol and improvements in glucose control at 24 months84. Few adverse events occurred and no significant self-reported changes in hunger, mood or cognitive function were observed84. Furthermore, weight loss was primarily composed of fat mass (69% of weight lost at 24 months) and although half of participants were normal weight, only one became clinically underweight (<18.5 kg/m2)84. A recent analysis of clinical trials, including SECRET, in older adults revealed that fat mass regain is common after participation in clinical trials involving caloric restriction are completed, suggesting that perhaps caloric restriction needs to be implemented in the form of a sustained lifestyle change rather than a temporary intervention. Perhaps future interventions in HF patients should be designed to evaluate the safety and feasibility of long-term caloric restriction85. Reduction in resting metabolic rate with caloric restriction was significantly lower (−71 ± 12 calories/day) from baseline in CALERIE-2, a factor that may contribute to weight gain should the participant stop adhering to caloric restriction84. Little is known about resting metabolic rate in patients with HF, especially HFpEF, and it is unclear how and if changes in resting metabolic rate could affect long term feasibility of caloric restriction in this population. Thus far, both completed trials evaluating caloric restriction80,82 in patients with HF have indicated promising improvements, including increases in peak VO2. However, larger trials in both HFrEF and HFpEF need to be completed with long term outcomes to demonstrate that caloric restriction resulting in sustained weight loss alone is safe and results in improved morbidity and mortality outcomes.
Micronutrients
Outside of the concern for excess dietary sodium, deficiency of dietary micronutrients, defined here as vitamins and minerals, has posed a greater concern for patients with HF86,87. A clear association of multiple micronutrient deficiencies on HF outcomes as well as outcome changes associated with multiple micronutrient supplementation has yet to be demonstrated. Deficiencies of multiple micronutrients have been associated with a greater risk of hospitalization and mortality in some analyses86,87, but not in others88. A double-blind pilot trial of 30 patients with HFrEF (LVEF≤35%) randomized patients to a multiple micronutrient supplement for 9 months demonstrated a significant increase in LVEF (+5.3±1.4%) and QoL measured by questionnaire in the intervention group versus controls89. In contrast, a more recent double-blind randomized control trial of 74 patients with HFrEF (LVEF≤45%) demonstrated no increase in LVEF or QoL measured by questionnaire in patients taking the supplement versus control over a period of 12 months90. Notably, these two trials differed somewhat in micronutrients supplemented as well as dosage amount and baseline LVEF89,90 and no patients with HFpEF were included.
Iron deficiency is perhaps the most well characterized nutrient deficiency comorbidity in HF and is associated with reduced CRF and QoL. Importantly, oral iron supplementation does not improve peak VO2 in patients with HFrEF91,92. Contrastingly, compared to placebo in randomized control trials, intravenous iron supplementation in patients with iron deficiency and HFrEF significantly reduces CV hospitalizations and mortality (HR: 0.59, 95% CI 0.40–0.88)93.
Thiamine constitutes another well characterized deficiency in patients with HF, possibly resulting from increased urinary excretion from diuretics and/or lessened oral intake, absorption and altered metabolism94. Two small, short term randomized control trials have demonstrated a significant increase in LVEF in patients with HFrEF with high dose thiamine supplementation (200–300 mg/day), but this has not been demonstrated in a larger cohort95,96. Currently, an ongoing feasibility study is establishing the possibility of a larger trial of thiamine supplementation in patients with HFrEF at McMaster University in Ontario, Canada (THIAMINE-HF, NCT03228030). Vitamin D deficiency is also common in patients with HF and is associated with poor outcomes, but oral supplementation failed to improve 6MWT distance in HFrEF97,98 or peak VO2 in a mixed cohort of HFrEF and HFpEF99. In a double-blind trial of 410 patients with HFrEF (LVEF 28% (23–34) Vitamin D, 27 % (24–35) placebo) subjects were randomized to receive 4000 international units (IU) Vitamin D or placebo for 3 years. After 3 years, the primary endpoint of all-cause mortality was not significantly different in the vitamin D vs. placebo (HR:1.09, 95% CI 0.69–1.71)100. Moreover, the placement rate for mechanical circulatory support was significantly higher in the Vitamin D supplemented patients than placebo, indicating possible harm (HR:1.96, 95% CI 1.04–3.66).
As oxidative stress has been postulated to be involved in the progression of HF101, several studies have studied the vitamin antioxidants C and E in HF101–105. Although vitamin E appeared to increase endogenous antioxidant and reduce plasma prooxidant activity in a group of very young acute HFrEF patients101, a subsequent randomized control trial showed no changes in oxidative stress over a 12 week period of oral supplementation102. Furthermore, in an open-label randomized control trial of 8415 patients, randomized post-myocardial infarction and presenting with left ventricular dysfunction, vitamin E supplementation resulted in a significant increased risk of developing HF (HR:1.50, 95% CI 1.03–2.20), possibly indicating the potential for harm in patients with HF103. Few studies have examined vitamin C supplementation in HF, but very small studies have indicated a possible increase in myocardial contractility and endothelial function104,105.
Coenzyme Q10 (CoQ10), also known as ubiquinone, plays an important role in the electron transport chain contributing to the creation of adenosine triphosphate (ATP) as well as improving endothelial function and serving as an antioxidant106. Lower levels of CoQ10 are associated with lower LVEF and higher NT-proBNP in patients with HF107 and adverse effects appear to be negligible, although more evidence is needed106. The Q-SYMBIO (Coenzyme Q10 as Adjunctive Treatment of Chronic Heart Failure: A Randomized, Double-blind, Multicentre Trial With Focus on Symptoms, Biomarker Status) multi-center trial randomized 420 patients with NYHA class III-IV HF, regardless of LVEF, in a double-blind format to CoQ10 100mg 3 times daily or placebo for 2 years108. While there were no differences in the short-term primary outcomes of change in 6MWT, NYHA class or NT-proBNP at 16 weeks, there was a 50% RR reduction in the long term primary outcome of major adverse cardiovascular events (MACE) (HR:0.50, 95% CI 0.32–0.80) at 106 weeks in patients randomized to CoQ10 versus placebo108. Furthermore, there was a reduction in CV mortality (HR 0.51, 95% CI 0.28–0.92), hospitalization for HF (HR 0.51, 95% CI 0.27–0.95) and all-cause mortality (HR 0.51, 95% CI 0.30–0.89) in patients assigned to CoQ10 versus placebo108. Despite these impressive results, the low annual mortality rate of 7% and relatively small number of patients enrolled prompt caution in interpreting the results of this trial106. Follow-up trials of CoQ10 in patients with HF, but with a greater sample size, are warranted.
Currently, intravenous iron supplementation in patients with both HFrEF and iron deficiency is the only vitamin or mineral micronutrient supplementation strategy shown to definitively improve outcomes in HF93, despite evidence of micronutrient deficiency being associated with poor outcomes in HF86,87. It is possible, however, that a nutrient dense dietary pattern providing a wealth of micronutrients along with dietary fiber, UFA and plant-based protein may be the supplementation strategy that most likely benefits patients with HF (Figure 2)39,40,43–45.
Future Directions
Currently, dietary therapies proven to improve outcomes in HF are lacking. Outside of vitamin and mineral micronutrients, other dietary supplementation strategies such as nitrates in beetroot juice109,110 have demonstrated promising results. Ongoing trials are continuing to evaluate sodium restriction in both outpatients with HFpEF or HFrEF (SODIUM-HF, NCT02012179) and in inpatients with HFrEF (SALT, NCT02689635). Although it is impossible to conduct a blinded trial with dietary patterns, both the DASH diet and MedDiet have demonstrated promising results on HF outcomes and should be tested in larger, randomized interventions39,40,43–45,111. Dietary supplementation strategies, such demonstrated with UFA57 and protein and amino acids65,66,69,70, may demonstrate sustainable therapies for future, larger interventions as patients are not necessarily asked to give up or change dietary habits. Future strategies will also need to focus on the emerging issues of obesity as well as sarcopenia in the HF population7.
Conclusion
There are few existing dietary strategies proven to improve clinical outcomes in HF (Table 1 and Figure 2). Healthy dietary patterns and supplementation strategies on top of regular dietary intake represent promising areas for continued work. Importantly, due to the growing population of patients with HFpEF and the lack of beneficial pharmacologic therapies in this population, there is an urgent need to test nonpharmacologic strategies, such as dietary interventions, to improve clinical outcomes.
Acknowledgements
The authors thank Rand Gabriel Buenaventura for his assistance with the graphic design of Figures 1 and 2 for the manuscript during his summer fellowship funded by the American Heart Association.
Disclosures:
Dr Hummel is supported by a grant from Veterans Affairs, CARA-009-16F9050. Dr Carbone is supported by a Career Development Award 19CDA34660318 from the American Heart Association and by the Clinical and Translational Science Awards Program UL1TR002649 from National Institute of Health. The remaining authors have nothing to disclose.
Abbreviations
- 6MWT
Six-Minute Walk Test
- ACE
Angiotensin-Converting Enzyme
- ATP
Adenosine Triphosphate
- ARB
Angiotensin II Receptor Blocker
- ADHF
Acute Decompensated Heart Failure
- AA
Amino Acids
- BNP
Brain Natriuretic Peptide
- CPET
Cardiopulmonary Exercise Test
- CRF
Cardiorespiratory Fitness
- CV
Cardiovascular
- CVD
Cardiovascular Disease
- DASH
Dietary Approaches to Stop Hypertension
- FFQ
Food Frequency Questionnaire
- HF
Heart Failure
- HFpEF
Heart Failure with Preserved Ejection Fraction
- HFrEF
Heart Failure with Reduced Ejection Fraction
- HMB
Beta-hydroxy-beta-methylbutyrate
- HR
Hazard Ratio
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- L
Liters
- LM
Lean Mass
- LVEF
Left Ventricular Ejection Fraction
- m
Meters
- MedDiet
Mediterranean Diet
- Mg
Milligrams
- mL
Milliliter
- mL•kg−1•min−1
Milliliters per kilograms per minute
- MLWHF
Minnesota Living with Heart Failure questionnaire
- mmHg
Millimeters mercury
- MUFA
Monounsaturated Fatty Acids
- NT-proBNP
N-terminal pro-Brain Natriuretic Peptide
- N-3 PUFA
N-3 Polyunsaturated Fatty Acids
- NYHA
New York Heart Association
- Peak VO2
Peak Oxygen Consumption
- PUFA
Polyunsaturated Fatty Acids
- QoL
Quality of life
- CoQ10
Coenzyme Q10
- RAAS
Renin Angiotensin Aldosterone System
- SFA
Saturated Fatty Acid
- T2DM
Type 2 Diabetes Mellitus
- TNF-α
Tumor Necrosis Factor Alpha
- UFA
Unsaturated Fatty Acids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Salim VS, Alvaro A, BE J, et al. Heart Disease and Stroke Statistics—2020 Update. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. [DOI] [PubMed] [Google Scholar]
- 3.America HFS of. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16(6):e1–e2.20610207 [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of Amer. Circulation. 2017;136(6):e137–e161. [DOI] [PubMed] [Google Scholar]
- 5.Coats AJS, Pieske B, Linde C, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(27):2129–2200. [DOI] [PubMed] [Google Scholar]
- 6.Kuehneman T, Gregory M, de Waal D, et al. Academy of Nutrition and Dietetics Evidence-Based Practice Guideline for the Management of Heart Failure in Adults. J Acad Nutr Diet. 2018;118(12):2331–2345. [DOI] [PubMed] [Google Scholar]
- 7.Vest AR, Chan M, Deswal A, et al. Nutrition, Obesity, and Cachexia in Patients With Heart Failure: A Consensus Statement from the Heart Failure Society of America Scientific Statements Committee. J Card Fail. 2019;25(5):380–400. [DOI] [PubMed] [Google Scholar]
- 8.Allen KE, Billingsley HE, Carbone S. Nutrition, Heart Failure, and Quality of Life. JACC Hear Fail. July 2020:1266. doi: 10.1016/j.jchf.2020.04.006 [DOI] [Google Scholar]
- 9.Jason R, Nicholas H, Anthony R. Why Don’t We Have Proven Treatments for HFpEF? Circ Res. 2017;120(8):1243–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10(4):401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan MS, Jones DW, Butler J. Salt, No Salt, or Less Salt for Patients With Heart Failure? Am J Med. 2020;133(1):32–38. [DOI] [PubMed] [Google Scholar]
- 12.Alvelos M, Ferreira A, Bettencourt P, et al. The effect of dietary sodium restriction on neurohumoral activity and renal dopaminergic response in patients with heart failure. Eur J Heart Fail. 2004;6(5):593–599. [DOI] [PubMed] [Google Scholar]
- 13.Doukky R, Avery E, Mangla A, et al. Impact of Dietary Sodium Restriction on Heart Failure Outcomes. JACC Hear Fail. 2016;4(1):24 LP – 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jefferson K, Ahmed M, Choleva M, et al. Effect of a Sodium-Restricted Diet on Intake of Other Nutrients in Heart Failure: Implications for Research and Clinical Practice. J Card Fail. 2015;21(12):959–962. [DOI] [PubMed] [Google Scholar]
- 15.Colín Ramírez E, Castillo Martínez L, Orea Tejeda A, Rebollar González V, Narváez David R, Asensio Lafuente E. Effects of a nutritional intervention on body composition, clinical status, and quality of life in patients with heart failure. Nutrition. 2004;20(10):890–895. [DOI] [PubMed] [Google Scholar]
- 16.Colin-Ramirez E, McAlister FA, Zheng Y, Sharma S, Ezekowitz JA. Changes in dietary intake and nutritional status associated with a significant reduction in sodium intake in patients with heart failure. A sub-analysis of the SODIUM-HF pilot study. Clin Nutr ESPEN. 2016;11:e26–e32. [DOI] [PubMed] [Google Scholar]
- 17.Bilgen F, Chen P, Poggi A, et al. Insufficient calorie intake worsens post-discharge quality of life and increases readmission burden in heart failure. JACC Hear Fail. 2020;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arcand JAL, Brazel S, Joliffe C, et al. Education by a dietitian in patients with heart failure results in improved adherence with a sodium-restricted diet: A randomized trial. Am Heart J. 2005;150(4):716.e1–716.e5. [DOI] [PubMed] [Google Scholar]
- 19.Philipson H, Ekman I, Forslund HB, Swedberg K, Schaufelberger M. Salt and fluid restriction is effective in patients with chronic heart failure. Eur J Heart Fail. 2013;15(11):1304–1310. [DOI] [PubMed] [Google Scholar]
- 20.Philipson H, Ekman I, Swedberg K, Schaufelberger M. A pilot study of salt and water restriction in patients with chronic heart failure. Scand Cardiovasc J. 2010;44(4):209–214. [DOI] [PubMed] [Google Scholar]
- 21.Colin-Ramirez E, McAlister FA, Zheng Y, Sharma S, Armstrong PW, Ezekowitz JA. The long-term effects of dietary sodium restriction on clinical outcomes in patients with heart failure. The SODIUM-HF (Study of Dietary Intervention Under 100 mmol in Heart Failure): A pilot study. Am Heart J. 2015;169(2):274–281.e1. [DOI] [PubMed] [Google Scholar]
- 22.Paterna S, Parrinello G, Cannizzaro S, et al. Medium Term Effects of Different Dosage of Diuretic, Sodium, and Fluid Administration on Neurohormonal and Clinical Outcome in Patients With Recently Compensated Heart Failure. Am J Cardiol. 2009;103(1):93–102. [DOI] [PubMed] [Google Scholar]
- 23.Paterna S, Gaspare P, Fasullo S, Sarullo FM, Di Pasquale P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci. 2008;114(3):221–230. [DOI] [PubMed] [Google Scholar]
- 24.Konerman MC, Hummel SL. Sodium restriction in heart failure: benefit or harm? Curr Treat Options Cardiovasc Med. 2014;16(2):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song EK, Moser DK, Dunbar SB, Pressler SJ, Lennie TA. Dietary sodium restriction below 2 g per day predicted shorter event-free survival in patients with mild heart failure. Eur J Cardiovasc Nurs. 2013;13(6):541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lennie TA, Song EK, Wu J-R, et al. Three Gram Sodium Intake is Associated With Longer Event-Free Survival Only in Patients With Advanced Heart Failure. J Card Fail. 2011;17(4):325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saleh ZT, Lennie TA, Alhurani AS, Almansour IM, Alduraidi H, Moser DK. High Dietary Sodium Intake is Associated with Shorter Event-Free Survival in Patients with Heart Failure and Comorbid Diabetes. Clin Nurs Res. November 2019:1054773819888743. [DOI] [PubMed] [Google Scholar]
- 28.Sun Q, Bertrand KA, Franke AA, Rosner B, Curhan GC, Willett WC. Reproducibility of urinary biomarkers in multiple 24-h urine samples. Am J Clin Nutr. 2016;105(1):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basuray A, Dolansky M, Josephson R, et al. Dietary sodium adherence is poor in chronic heart failure patients. J Card Fail. 2015;21(4):323–329. doi: 10.1016/j.cardfail.2014.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hummel SL, Konerman MC. Dietary Sodium Restriction in Heart Failure: A Recommendation Worth its Salt? JACC Heart Fail. 2016;4(1):36–38. doi: 10.1016/j.jchf.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 31.Aliti GB, Rabelo ER, Clausell N, Rohde LE, Biolo A, Beck-da-Silva L. Aggressive Fluid and Sodium Restriction in Acute Decompensated Heart Failure: A Randomized Clinical Trial. JAMA Intern Med. 2013;173(12):1058–1064. [DOI] [PubMed] [Google Scholar]
- 32.Machado d’Almeida KS, Rabelo-Silva ER, Souza GC, et al. Aggressive fluid and sodium restriction in decompensated heart failure with preserved ejection fraction: Results from a randomized clinical trial. Nutrition. 2018;54:111–117. [DOI] [PubMed] [Google Scholar]
- 33.Billingsley H, Rodriguez-Miguelez P, Del Buono MG, Abbate A, Lavie CJ, Carbone S. Lifestyle Interventions with a Focus on Nutritional Strategies to Increase Cardiorespiratory Fitness in Chronic Obstructive Pulmonary Disease, Heart Failure, Obesity, Sarcopenia, and Frailty. Nutrients. 2019;11(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chrysohoou C, Panagiotakos DB, Aggelopoulos P, et al. The Mediterranean diet contributes to the preservation of left ventricular systolic function and to the long-term favorable prognosis of patients who have had an acute coronary event. Am J Clin Nutr. 2010;92(1):47–54. [DOI] [PubMed] [Google Scholar]
- 35.Papadaki A, Martinez-Gonzalez MA, Alonso-Gomez A, et al. Mediterranean diet and risk of heart failure: results from the PREDIMED randomized controlled trial. Eur J Heart Fail. 2017;19(9):1179–1185. [DOI] [PubMed] [Google Scholar]
- 36.Tektonidis TG, Akesson A, Gigante B, Wolk A, Larsson SC. Adherence to a Mediterranean diet is associated with reduced risk of heart failure in men. Eur J Heart Fail. 2016;18(3):253–259. [DOI] [PubMed] [Google Scholar]
- 37.Tektonidis TG, Åkesson A, Gigante B, Wolk A, Larsson SC. A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: A population-based cohort study. Atherosclerosis. 2015;243(1):93–98. [DOI] [PubMed] [Google Scholar]
- 38.Wirth J, di Giuseppe R, Boeing H, Weikert C. A Mediterranean-style diet, its components and the risk of heart failure: a prospective population-based study in a non-Mediterranean country. Eur J Clin Nutr. 2016;70(9):1015–1021. [DOI] [PubMed] [Google Scholar]
- 39.Levitan EB, Lewis CE, Tinker LF, et al. Mediterranean and DASH Diet Scores and Mortality in Women With Heart Failure. Circ Hear Fail. 2013;6(6):1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miró Ò, Estruch R, Martín-Sánchez FJ, et al. Adherence to Mediterranean Diet and All-Cause Mortality After an Episode of Acute Heart Failure: Results of the MEDIT-AHF Study. JACC Hear Fail. 2018;6(1):52–62. [DOI] [PubMed] [Google Scholar]
- 41.Rifai L, Pisano C, Hayden J, Sulo S, Silver MA. Impact of the DASH diet on endothelial function, exercise capacity, and quality of life in patients with heart failure. Proc (Bayl Univ Med Cent). 2015;28(2):151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levitan EB, Wolk A, Mittleman MA. Consistency With the DASH Diet and Incidence of Heart Failure. Arch Intern Med. 2009;169(9):851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hummel SL, Seymour EM, Brook RD, et al. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail. 2013;6(6):1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hummel SL, Mitchell SE, Brook RD, et al. Low-Sodium Dietary Approaches to Stop Hypertension Diet Reduces Blood Pressure, Arterial Stiffness, and Oxidative Stress in Hypertensive Heart Failure With Preserved Ejection Fraction. Hypertension. 2012;60(5):1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hummel SL, Wahida K, Gillespie B, et al. Home-Delivered Meals Postdischarge From Heart Failure Hospitalization. Circ Hear Fail. 2018;11(8):e004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Gobbo LC, Kalantarian S, Imamura F, et al. Contribution of Major Lifestyle Risk Factors for Incident Heart Failure in Older Adults: The Cardiovascular Health Study. JACC Heart Fail. 2015;3(7):520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levitan EB, Wolk A, Mittleman MA. Relation of Consistency With the Dietary Approaches to Stop Hypertension Diet and Incidence of Heart Failure in Men Aged 45 to 79 Years. Am J Cardiol. 2009;104(10):1416–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rifai L, Silver MA. A Review of the DASH Diet as an Optimal Dietary Plan for Symptomatic Heart Failure. Prog Cardiovasc Dis. 2016;58(5):548–554. [DOI] [PubMed] [Google Scholar]
- 49.Martínez-González MA, Fuster V, Hu F, et al. Benefits of the Mediterranean diet beyond the Mediterranean Sea and beyond food patterns. BMC Med. 2016;14(1):157. doi: 10.1186/s12916-016-0714-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Estruch R, Ros E, Salas-Salvadó J, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018;378(25):e34. [DOI] [PubMed] [Google Scholar]
- 51.Montserrat F, Estruch R, Jordi S-S, et al. Effect of the Mediterranean diet on heart failure biomarkers: a randomized sample from the PREDIMED trial. Eur J Heart Fail. 2014;16(5):543–550. [DOI] [PubMed] [Google Scholar]
- 52.Lara KM, Levitan EB, Gutierrez OM, et al. Dietary Patterns and Incident Heart Failure in U.S. Adults Without Known Coronary Disease. J Am Coll Cardiol. 2019;73(16):2036–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rautiainen S, Levitan EB, Mittleman MA, Wolk A. Fruit and vegetable intake and rate of heart failure: a population-based prospective cohort of women. Eur J Heart Fail. 2015;17(1):20–26. [DOI] [PubMed] [Google Scholar]
- 54.Billingsley H, Carbone S, Lavie C. Dietary Fats and Chronic Noncommunicable Diseases. Nutrients. 2018;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):655–666. [DOI] [PubMed] [Google Scholar]
- 56.Carbone S, Canada JM, Buckley LF, et al. Dietary Fat, Sugar Consumption, and Cardiorespiratory Fitness in Patients With Heart Failure With Preserved Ejection Fraction. JACC Basic to Transl Sci. 2017;2(5):513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carbone S, Billingsley HE, Canada JM, et al. Unsaturated Fatty Acids to Improve Cardiorespiratory Fitness in Patients with Obesity and HFpEF- The UFA PRESERVED Pilot Study. JACC Basic to Transl Sci. 2019;4(4):563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehra MR, Lavie CJ, Ventura HO, Milani RV. Fish Oils Produce Anti-inflammatory Effects and Improve Body Weight in Severe Heart Failure. J Hear Lung Transplant. 2006;25(7):834–838. doi: 10.1016/j.healun.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 59.Nodari S, Triggiani M, Campia U, et al. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2011;57(7):870–879. [DOI] [PubMed] [Google Scholar]
- 60.Wu C, KT S, Ruiping J, et al. Supplementation of l-Alanyl-l-Glutamine and Fish Oil Improves Body Composition and Quality of Life in Patients With Chronic Heart Failure. Circ Hear Fail. 2015;8(6):1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.GISSI-HF investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–1230. [DOI] [PubMed] [Google Scholar]
- 62.Siscovick DS, Barringer TA, Fretts AM, et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease. Circulation. 2017;135(15):e867–e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carbone S, Billingsley HE, Rodriguez-Miguelez P, et al. Lean Mass Abnormalities in Heart Failure: The Role of Sarcopenia, Sarcopenic Obesity, and Cachexia. Curr Probl Cardiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emami A, Saitoh M, Valentova M, et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Eur J Heart Fail. 2018;20(11):1580–1587. [DOI] [PubMed] [Google Scholar]
- 65.Aquilani R, Opasich C, Gualco A, et al. Adequate energy-protein intake is not enough to improve nutritional and metabolic status in muscle-depleted patients with chronic heart failure. Eur J Heart Fail. 2008;10(11):1127–1135. [DOI] [PubMed] [Google Scholar]
- 66.Lombardi C, Carubelli V, Lazzarini V, et al. Effects of oral amino Acid supplements on functional capacity in patients with chronic heart failure. Clin Med Insights Cardiol. 2014;8:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pineda-Juárez JA, Sánchez-Ortiz NA, Castillo-Martínez L, et al. Changes in body composition in heart failure patients after a resistance exercise program and branched chain amino acid supplementation. Clin Nutr. 2016;35(1):41–47. [DOI] [PubMed] [Google Scholar]
- 68.Dos Santos EM, de Moraes R, Tibiriça EV, Huguenin GVB, Moreira ASB, De Lorenzo AR. Whey protein supplementation for the preservation of mass and muscular strength of patients with heart failure: study protocol for a randomized controlled trial. Trials. 2018;19(1):431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deutz NE, Matheson EM, Matarese LE, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin Nutr. 2016;35(1):18–26. [DOI] [PubMed] [Google Scholar]
- 70.Rozentryt P, von Haehling S, Lainscak M, et al. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double-blind pilot study. J Cachexia Sarcopenia Muscle. 2010;1(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonilla-Palomas JL, Gámez-López AL, Castillo-Domínguez JC, et al. Nutritional Intervention in Malnourished Hospitalized Patients with Heart Failure. Arch Med Res. 2016;47(7):535–540. [DOI] [PubMed] [Google Scholar]
- 72.Gámez-López AL, Bonilla-Palomas JL, Anguita-Sánchez M, et al. Rationale and design of PICNIC study: nutritional intervention program in hospitalized patients with heart failure who are malnourished. Rev Esp Cardiol (Engl Ed). 2014;67(4):277–282. [DOI] [PubMed] [Google Scholar]
- 73.Bonilla-Palomas JL, Gámez-López AL, Castillo-Domínguez JC, Moreno-Conde M, López-Ibáñez MC, Anguita-Sánchez M. Does nutritional intervention maintain its prognostic benefit in the long term for malnourished patients hospitalised for heart failure? Rev Clínica Española. 2018;218(2):58–60. [DOI] [PubMed] [Google Scholar]
- 74.Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ. Obesity paradox in cardiovascular disease: where do we stand? Vasc Health Risk Manag. 2019;15:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carbone S, Lavie CJ, Arena R. Obesity and Heart Failure: Focus on the Obesity Paradox. Mayo Clin Proc. 2017;92(2):266–279. [DOI] [PubMed] [Google Scholar]
- 76.Carbone S, Lavie CJ, Elagizi A, Arena R, Ventura HO. The Impact of Obesity in Heart Failure. Heart Fail Clin. 2020;16(1):71–80. [DOI] [PubMed] [Google Scholar]
- 77.Pocock SJ, McMurray JJV, Dobson J, et al. Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29(21):2641–2650. [DOI] [PubMed] [Google Scholar]
- 78.Carbone S, Canada JM, Buckley LF, et al. Obesity Contributes to Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2016;68(22):2487–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ventura HO, Carbone S, Lavie CJ. Muscling up to improve heart failure prognosis. Eur J Heart Fail. 2018;20(11):1588–1590. doi: 10.1002/ejhf.1314 [DOI] [PubMed] [Google Scholar]
- 80.Evangelista LS, Heber D, Li Z, Bowerman S, Hamilton MA, Fonarow GC. Reduced body weight and adiposity with a high-protein diet improves functional status, lipid profiles, glycemic control, and quality of life in patients with heart failure: a feasibility study. J Cardiovasc Nurs. 2009;24(3):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Motie M, Evangelista LS, Horwich T, et al. Pro-HEART - a randomized clinical trial to test the effectiveness of a high protein diet targeting obese individuals with heart failure: rationale, design and baseline characteristics. Contemp Clin Trials. 2013;36(2):371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kitzman DW, Brubaker P, Morgan T, et al. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Most J, Tosti V, Redman LM, Fontana L.Calorie restriction in humans: An update. Ageing Res Rev. 2017;39:36–45. doi: 10.1016/j.arr.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ravussin E, Redman LM, Rochon J, et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. Journals Gerontol Ser A. 2015;70(9):1097–1104. doi: 10.1093/gerona/glv057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Houston DK, Miller ME, Kitzman DW, et al. Long-Term Effects of Randomization to a Weight Loss Intervention in Older Adults: A Pilot Study. J Nutr Gerontol Geriatr. March 2019:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.LT A, Christina A, Kay RM, et al. Micronutrient Deficiency Independently Predicts Time to Event in Patients With Heart Failure. J Am Heart Assoc. 2018;7(17):e007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song EK, Kang S-M. Micronutrient Deficiency Independently Predicts Adverse Health Outcomes in Patients With Heart Failure. J Cardiovasc Nurs. 2017;32(1). [DOI] [PubMed] [Google Scholar]
- 88.Levitan EB, Shikany JM, Ahmed A, et al. Calcium, magnesium and potassium intake and mortality in women with heart failure: the Women’s Health Initiative. Br J Nutr. 2013;110(1):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Witte KKA, Nikitin NP, Parker AC, et al. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26(21):2238–2244. [DOI] [PubMed] [Google Scholar]
- 90.McKeag NA, McKinley MC, Harbinson MT, et al. The Effect of Multiple Micronutrient Supplementation on Left Ventricular Ejection Fraction in Patients With Chronic Stable Heart Failure: A Randomized, Placebo-Controlled Trial. JACC Hear Fail. 2014;2(3):308–317. [DOI] [PubMed] [Google Scholar]
- 91.Beck-da-Silva L, Piardi D, Soder S, et al. IRON-HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol. 2013;168(4):3439–3442. [DOI] [PubMed] [Google Scholar]
- 92.Lewis GD, Malhotra R, Hernandez AF, et al. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF Randomized Clinical Trial. JAMA. 2017;317(19):1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anker SD, Kirwan B-A, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail. 2018;20(1):125–133. [DOI] [PubMed] [Google Scholar]
- 94.Kattoor AJ, Goel A, Mehta JL. Thiamine Therapy for Heart Failure: a Promise or Fiction? Cardiovasc Drugs Ther. 2018;32(4):313–317. [DOI] [PubMed] [Google Scholar]
- 95.Schoenenberger AW, Schoenenberger-Berzins R, der Maur CA, Suter PM, Vergopoulos A, Erne P. Thiamine supplementation in symptomatic chronic heart failure: a randomized, double-blind, placebo-controlled, cross-over pilot study. Clin Res Cardiol. 2012;101(3):159–164. [DOI] [PubMed] [Google Scholar]
- 96.Shimon H, Almog S, Vered Z, et al. Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am J Med. 1995;98(5):485–490. [DOI] [PubMed] [Google Scholar]
- 97.Witte KK, Byrom R, Gierula J, et al. Effects of Vitamin D on Cardiac Function in Patients With Chronic HF: The VINDICATE Study. J Am Coll Cardiol. 2016;67(22):2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Witham MD, Crighton Linda J, Gillespie ND, Struthers AD, McMurdo MET. The Effects of Vitamin D Supplementation on Physical Function and Quality of Life in Older Patients With Heart Failure. Circ Hear Fail. 2010;3(2):195–201. [DOI] [PubMed] [Google Scholar]
- 99.Boxer RS, Kenny AM, Schmotzer BJ, Vest M, Fiutem JJ, Pina IL. A randomized controlled trial of high dose vitamin D3 in patients with heart failure. JACC Heart Fail. 2013;1(1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zittermann A, Ernst JB, Prokop S, et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J. 2017;38(29):2279–2286. [DOI] [PubMed] [Google Scholar]
- 101.Ghatak A, Brar MJ, Agarwal A, et al. Oxy free radical system in heart failure and therapeutic role of oral vitamin E. Int J Cardiol. 1996;57(2):119–127. [DOI] [PubMed] [Google Scholar]
- 102.Keith ME, Jeejeebhoy KN, Langer A, et al. A controlled clinical trial of vitamin E supplementation in patients with congestive heart failure. Am J Clin Nutr. 2001;73(2):219–224. [DOI] [PubMed] [Google Scholar]
- 103.Marchioli R, Levantesi G, Macchia A, et al. Vitamin E increases the risk of developing heart failure after myocardial infarction: results from the GISSI-Prevenzione trial. J Cardiovasc Med. 2006;7(5). [DOI] [PubMed] [Google Scholar]
- 104.Shinke T, Shite J, Takaoka H, et al. Vitamin C restores the contractile response to dobutamine and improves myocardial efficiency in patients with heart failure after anterior myocardial infarction. Am Heart J. 2007;154(4):645.e1–645.e8. [DOI] [PubMed] [Google Scholar]
- 105.Burkhard H, Naoshi A, Christoph K, Helmut D. Vitamin C Improves Endothelial Function of Conduit Arteries in Patients With Chronic Heart Failure. Circulation. 1998;97(4):363–368. [DOI] [PubMed] [Google Scholar]
- 106.Sharma A, Fonarow GC, Butler J, Ezekowitz JA, Felker GM. Coenzyme Q10 and Heart Failure: A State-of-the-Art Review. Circ Heart Fail. 2016;9(4):e002639. doi: 10.1161/CIRCHEARTFAILURE.115.002639 [DOI] [PubMed] [Google Scholar]
- 107.McMurray JJV, Dunselman P, Wedel H, et al. Coenzyme Q, Rosuvastatin, and Clinical Outcomes in Heart Failure. JACC. 2010;56(15):1196 LP – 1204. [DOI] [PubMed] [Google Scholar]
- 108.Mortensen SA, Rosenfeldt F, Kumar A, et al. The Effect of Coenzyme Q10 on Morbidity and Mortality in Chronic Heart Failure. JACC Hear Fail. 2014;2(6):641–649. [DOI] [PubMed] [Google Scholar]
- 109.Coggan AR, Broadstreet SR, Mahmood K, et al. Dietary Nitrate Increases VO2peak and Performance but Does Not Alter Ventilation or Efficiency in Patients With Heart Failure With Reduced Ejection Fraction. J Card Fail. 2018;24(2):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eggebeen J, Kim-Shapiro DB, Haykowsky M, et al. One Week of Daily Dosing With Beetroot Juice Improves Submaximal Endurance and Blood Pressure in Older Patients With Heart Failure and Preserved Ejection Fraction. JACC Hear Fail. 2016;4(6):428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tuttle KR, Shuler LA, Packard DP, et al. Comparison of Low-Fat Versus Mediterranean-Style Dietary Intervention After First Myocardial Infarction (from The Heart Institute of Spokane Diet Intervention and Evaluation Trial). Am J Cardiol. 2008;101(11):1523–1530. [DOI] [PubMed] [Google Scholar]
- 112.Carbone S, Mauro AG, Mezzaroma E, et al. A high-sugar and high-fat diet impairs cardiac systolic and diastolic function in mice. Int J Cardiol. 2015;198:66–69. doi: 10.1016/j.ijcard.2015.06.136 [DOI] [PubMed] [Google Scholar]


