Abstract
Objective:
To determine whether commencement of antibiotics within 3 postnatal days in preterm, very low birth weight infants (VLBW, ≤1500 g), is associated with the development of necrotizing enterocolitis (NEC).
Study design:
Pre-planned statistical analyses were done to study the association between early antibiotic treatment and later NEC development, using the NEOMUNE-NeoNutriNet cohort of VLBW infants from 13 neonatal intensive care units (NICUs) in five continents (n=2831). NEC incidence was compared between infants who received early antibiotics and those who did not, with statistical adjustments for NICU, gestational age, birth weight, sex, delivery mode, antenatal steroids, Apgar score, and type and initiation of enteral nutrition.
Results:
The incidence of NEC was 9.0% in the group of infants who did not receive early antibiotics (n=269) versus 3.9% in the remaining infants (n=2562). NEC incidence remained lower in the early-antibiotic group after stepwise statistical adjustments for NICU (OR 0.57; 95% CI, 0.35-0.94, P < .05) and other potential confounders (OR 0.25; CI, 0.12-0.47, P<0.0001).
Conclusions:
In this large international cohort of preterm VLBW infants, a minor proportion of infants did not receive antibiotics just after birth, yet these infants had a higher incidence of NEC. It is important to better understand the role of variables such as time, type, and length of antibiotic treatment on NEC incidence, immune development, gut colonization, and antibiotic resistance in the NICU.
Necrotizing enterocolitis (NEC), a severe intestinal inflammatory complication related to gut immaturity, occurs in 1-13% of very low birth weight (VLBW) infants.[1–3] Although early postnatal treatment with enteral antibiotics has been shown to reduce bacterial load in the gut and protect against NEC in animal models, and clinical studies,[4–6] this approach is not used to prevent NEC due to concerns of antimicrobial resistance and negative consequences from antibiotic therapy. Potentially, antibiotics administered intravenously can decrease microbial diversity, delay commensal colonization, and increase pathogenic bacteria.[7,8] Prolonged exposure to empirical intravenous antibiotics given in the first week of life has been associated with an increased risk of NEC, late-onset sepsis, and death in preterm infants in some studies.[9–14]
Due to risk of early onset sepsis, intravenous antibiotics are administered shortly after birth in the majority of VLBW infants, but more than 20% have very low risk of infection with questionable treatment indication.[14,15] The effect of such early antibiotic intervention on later NEC development, compared with no early treatment, is unclear. Data collected in the NeoNutriNet cohort from 13 neonatal intensive care units (NICUs) on five continents reported wide differences in enteral feeding practices and antibiotic use in VLBW infants.[1] Using data from this cohort, we investigated the within-hospital correlation between early use of antibiotics and NEC in VLBW infants. We hypothesized that initiation of antibiotics within three days of birth would increase the incidence of NEC.
METHODS
The NEOMUNE-NeoNutriNet cohort is a web-based database established on September 15, 2013 to collect data on in-hospital nutrition and clinical measures from VLBW infants. Each participating unit was requested to collect at least 100 infants, born consecutively between January 2011 and September 2014. A detailed description of the cohort has been published previously.[1] Inclusion criteria included infants born with a BW <1500 g and admitted to the participating NICUs within 24 h after birth. Exclusion criteria included major congenital abnormalities, metabolic disease, or died or were transferred to another hospital within 24 h of birth. Thirteen hospitals participated across Europe (3), Oceania (2), North America (1), Africa (1), and Asia (6). A total of 2947 infants were included from 13 participating NICUs, of which 2831 had data on both use of antibiotics and NEC outcomes and are included in the current study (Figure 1; available at www.jpeds.com). The incidence of NEC was defined as Bell stage ≥II, as described previously.[1,16] According to the timing of antibiotic use, these 2831 infants were divided into two groups: Early-antibiotic group if they received any antibiotic treatment within three days of birth or those that received antibiotic treatment beyond the third postnatal day, or never received any antibiotics. Antibiotics were administered intravenously in all cases. We compared NEC incidences between the early-antibiotic and no-early-antibiotic groups with adjustments for potential confounders.
Figure 1.
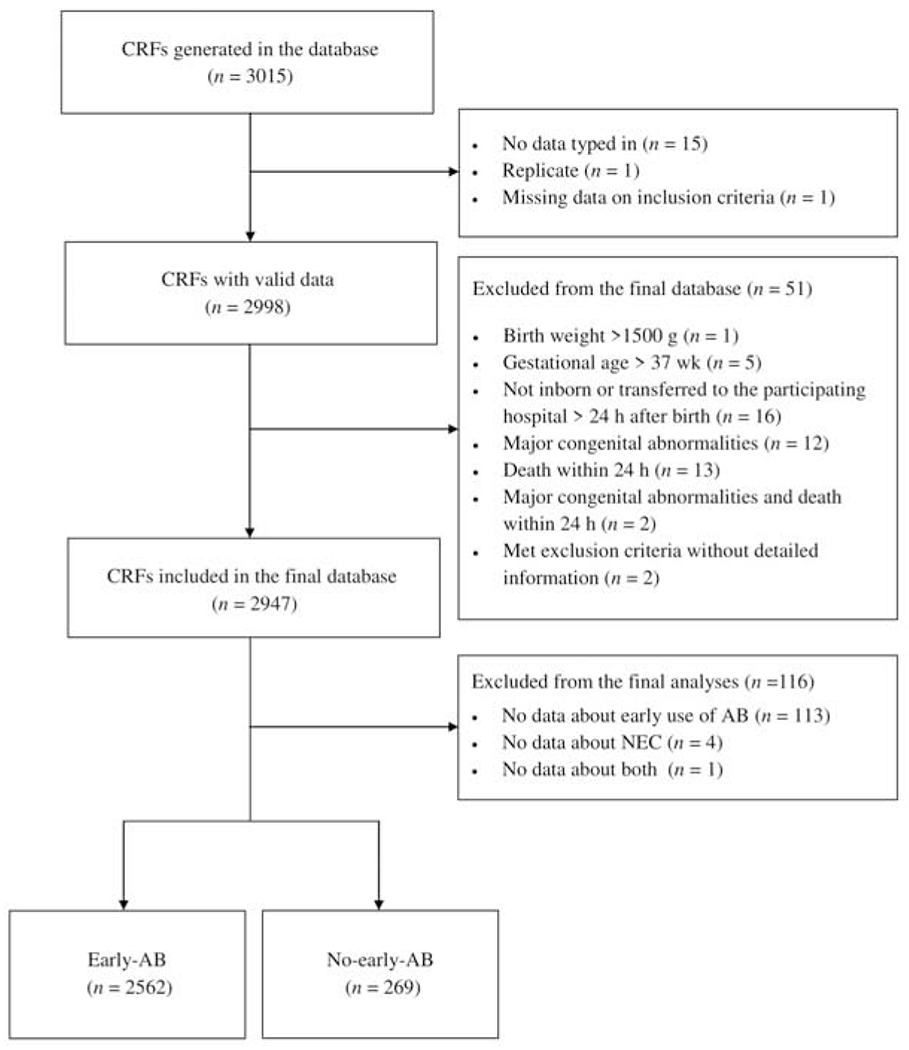
Flowchart for inclusion of study participants. AB, antibiotics; CRF, case report form
For explorative analyses, postmenstrual age (PMA) of NEC onset was compared between the early-antibiotic and no-early-antibiotic groups. NEC incidence was also assessed for correlation with the duration of early antibiotic treatment and whether the infants received prolonged antibiotic treatment (>3 days duration). In addition, to evaluate whether the association between NEC and early use of antibiotics differed in different geographical regions, we performed similar analyses within the five Guangdong ( South China) NICUs and the six Western NICUs, respectively (Table I).
TABLE 1.
Overview of antibiotic treatment at each participating NICU.
| Hospital | FOS | SWC | SNP | BWC | PWC | AUC | CHI | COP | AMS | NEW | PER | TAI | IBA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total included, n | 403 | 455 | 93 | 167 | 241 | 156 | 177 | 281 | 174 | 134 | 152 | 249 | 149 |
| Use of early antibiotics, n (%) | 394 (98) | 451 (99) | 91 (98) | 140 (84) | 228 (95) | 144 (92) | 172 (97) | 196 (70) | 131 (75) | 112 (84) | 138 (91) | 228 (92) | 137 (92) |
| Duration of early antibiotics, days, mean (SD) | 14 (9) | 10 (8) | 19 (13) | 10 (7) | 11 (6) | 6 (5) | 6 (4) | 6 (4) | 5 (2) | 3 (2) | 4 (2) | 5 (4) | 12 (7) |
| Types (class) of early antibiotics, n | UNK | ||||||||||||
| Penicillin | 370 | 449 | 5 | 112 | 224 | 144 | 129 | 131 | 111 | 137 | 227 | 129 | |
| Aminoglycoside | 4 | 9 | 1 | 0 | 1 | 140 | 192 | 131 | 111 | 137 | 200 | 135 | |
| Cephalosporin | 316 | 435 | 89 | 123 | 155 | 19 | 108 | 0 | 46 | 7 | 46 | 97 | |
| Carbapenem | 125 | 62 | 29 | 63 | 46 | 4 | 127 | 33 | 12 | 12 | 42 | 31 | |
| Macrolide | 77 | 102 | 7 | 57 | 0 | 0 | 3 | 0 | 1 | 0 | 2 | 3 | |
| Lincosamide | 1 | 2 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | |
| Glycopeptide | 30 | 21 | 7 | 36 | 16 | 10 | 69 | 47 | 45 | 46 | 35 | 11 | |
| Other | 8 | 10 | 0 | 5 | 4 | 19 | 49 | 13 | 30 | 5 | 58 | 42 |
AMS, Amsterdam University Medical Center, Vrije Universiteit, Emma Children’s Hospital, Netherlands; AUC, National Women’s Health, Auckland City Hospital, Auckland, New Zealand; BWC, Shenzhen Bao’an Maternal and Child Health Hospital, China; CHI, Rush University Children’s Hospital, Chicago, United States; COP, Dept of Neonatology, Rigshospitalet, Copenhagen, Denmark; FOS, Foshan Woman and Children’s Hospital, China; IBA, University College Hospital Ibadan, Nigeria; NEW, Newcastle Hospitals National Health Service Foundation Trust, United Kingdom; NICU, neonatal intensive care unit; PER, King Edward Memorial Hospital, Perth, Australia; PWC, Guangdong Women and Children Hospital, China; SNP, Shenzhen Nanshan People’s Hospital, China; SWC, Shenzhen Maternity& Child Health Care Hospital, China; TAI, Children’s Hospital of China Medical University, Taichung, Taiwan; UNK, unknown.
Guangdong NICUs: FOS, SWC, SNP, BWC, PWC and ‘Western’ NICUs: AUC,CHI,COP, AMS, NEW, PER
Statistical analyses
All statistical analyses were performed using the R software package (version 3.2.2). P values were two-sided and statistical significance was defined as P<0.05. Data were summarized using means and standard deviations (SD), numbers (n), and percentages as appropriate. For baseline comparisons between early-antibiotic and no-early-antibiotic groups, ANOVA was used for continuous outcomes (e.g. body weight, BW) and logistic regression was used for binary outcomes (e.g. small for gestational age, SGA) without any adjustment.
For our primary analysis, logistic regression was used to assess the difference in NEC incidence between early-antibiotic and no-early-antibiotic groups. With regard to adjustment for covariates and potential confounders, a predefined stepwise approach[17] was followed using logistic mixed-effects models. Model A was adjusted using NICU as the random effect. Model B was additionally adjusted for GA, BW, and sex. Model C further added delivery mode, use of antenatal steroids, and Apgar at 5 min. Model D added time when enteral nutrition was initiated, and type of enteral nutrition in the first week of life. To test whether SGA may influence the primary outcome, BW z-score and SGA status, respectively, was used in the model instead of BW in explorative analyses. BW z-score was calculated based on the Fenton 2013 growth chart[18] and SGA was defined as <10th percentile for weight at birth, as previously described[1]. Unadjusted ORs of each NICU were visualized in a forest plot and the heterogeneity across NICUs was examined by the Cochran Q test and quantified by the I2 index.
For further explorative analyses, a logistic mixed-effects model was used to assess associations between NEC incidence and early antibiotics duration, and the difference in NEC incidence among predefined subgroups, and a linear mixed-effects model was used to assess the difference in PMA at NEC onset between early-antibiotic and no-early-antibiotic groups and among predefined subgroups. Similarly, the stepwise approach with four models was used to adjust for covariates and potential confounders, as described above. Odds ratio (OR) and regression coefficient are reported for all models together with their 95% confidence intervals (CIs). To address possible bias from the loss of data of infants who died or were discharged or transferred early to step-down units or home before NEC development, we performed proportional hazards regression analysis for the primary outcome to test the robustness of our findings. Tukey post hoc analysis was used to adjust for multiple comparisons when required.
RESULTS
Across units, a majority of infants (2562/2831, ~90%) received antibiotics treatment within the first three days after birth. The prevalence of early antibiotics treatment ranged from 70% in COP to 99% in SWC and the mean duration of early antibiotics treatment varied from 3±2 days in NEW to 19±12 days in SNP (Table 1). The different agents used as early antibiotics are listed in Table 1. The predominant type of antibiotics used in the GD units was a combination of penicillin and cephalosporin, except for SNP using primarily cephalosporin. In the remaining units (non-GD), antibiotics was mostly a combination of penicillin and aminoglycoside. Relative to the early-antibiotic infants, the no-early-antibiotic group had higher BW and GA, lower birth z-score, more SGA infants, fewer males, more deliveries by caesarean delivery and received enteral nutrition earlier (all unadjusted P<0.05 and NICU-adjusted P<0.001, Table 2). Other demographic and nutritional characteristics did not differ between the two groups after adjustment for NICU (Table 2). Mortality adjusted by NICU tended to be higher in the early-antibiotic group (P=0.07).
TABLE 2.
Early use of antibiotics, demographic, nutritional, NEC and mortality data
| Early-antibiotics | No-early-antibiotics | P value1 | P value2 | |
|---|---|---|---|---|
| N | 2562 | 269 | ||
| Birth weight, g, mean (SD) | 1163 (240) | 1200 (246) | <0.05 | <0.0001 |
| Gestational age, wks, mean (SD) | 29.5 (2.4) | 31.0 (2.6) | <0.0001 | <0.0001 |
| Birth weight z-score | −0.38 (0.98) | −0.99 (1.08) | <0.0001 | <0.0001 |
| SGA, n (%) | 448 (18) | 99 (37) | <0.0001 | <0.0001 |
| Male, n (%) | 1366 (53) | 111 (41) | <0.001 | <0.001 |
| C-section, n (%) | 1538(60) | 217 (81) | <0.0001 | <0.0001 |
| Antenatal steroids, n (%) | 1500 (68) | 180 (74) | <0.05 | 0.25 |
| APGAR 5 <8, n (%) | 1504 (63) | 145(58) | 0.21 | 0.81 |
| Enteral nutrition start, n (%)3 | <0.0001 | <0.0001 | ||
| Slow | 479 (19) | 10 (4) | ||
| Medium | 763 (31) | 28 (11) | ||
| Quick | 1233 (50) | 226 (86) | ||
| Enteral nutrition type, n (%)4 | <0.0001 | 0.890 | ||
| IF | 1013 (45) | 54 (22) | ||
| HM | 1258 (55) | 193 (78) | ||
| Probiotics, n (%) | 675 (28) | 94 (38) | <0.01 | <0.01 |
| Initiation in week 1, n (%) | 362 (15) | 71 (29) | <0.0001 | 0.53 |
| Mortality, n (%) | 152 (5.9) | 15 (5.6) | 0.83 | 0.07 |
| NEC, n (%) | 99 (3.9) | 24 (9.0) | <0.001 | <0.05 |
Unadjusted P values.
Adjusted for NICU as a random effect.
Time when enteral nutrition was initiated was grouped into: Slow, never started or started later than postnatal day 4; Medium, started on postnatal day 3 or 4; Quick, started within postnatal day 2.
Type of enteral nutrition used in postnatal week 1. IF, exclusive IF or mixed feeding; HM: exclusive human milk feeding (MM and/or DM). HM, human milk; IF, infant formula; NEC, necrotizing enterocolitis; PMA, postmenstrual age; SD, standard deviation
In the no-early-antibiotic group 9.0% developed NEC compared with 3.9% in the remaining infants. As the primary outcome, unadjusted NEC incidence was lower in the early-antibiotic group (P<0.001, Table 2) and the respective ORs per NICU is shown in the forest plot (Figure 2). There was little statistical heterogeneity among NICUs (I2 = 20%, t2 = 0.23, P = 0.24). When adjusted for NICUs, NEC incidence remained lower in the early-antibiotic group with an odds ratio (OR) of 0.57 (P<0.05, model A, Figure 2). The significance level increased when other potential confounders were added to the models (ORs of 0.29, 0.27 and 0.25 for models B, C and D, respectively, all P<0.0001, Figure 2). The difference between the two groups remained robust when changing the covariates in the analysis from BW to z-score or SGA as a binary variable (data not shown). Furthermore, the primary outcome was analyzed in a sub-group of infants with GA < 28 weeks, which revealed even more pronounced differences between early-antibiotics (n=634) and no-early-antibiotics (n=39), with NEC incidences of 8.0% versus 20.5 %, respectively (P<0.05 for all models, ORs: 0.39-0.34). In the 123 NEC cases, the demographic, nutritional, and mortality characteristics differed in the same way as in all infants when comparing the two groups, except for BW (Table 3). Relative to early-antibiotic, no-early-antibiotic group had higher BW in all infants (Table 2), but similar in infants with NEC. When considering all-cause mortality and early discharge, the proportion of infants diagnosed with NEC was still lower in the early-antibiotic group compared with no-early-antibiotics infants after adjustment for potential confounders with a hazard ratio of 0.23 and 95% CI: 0.15-0.36 (P<0.0001).
Figure 2.
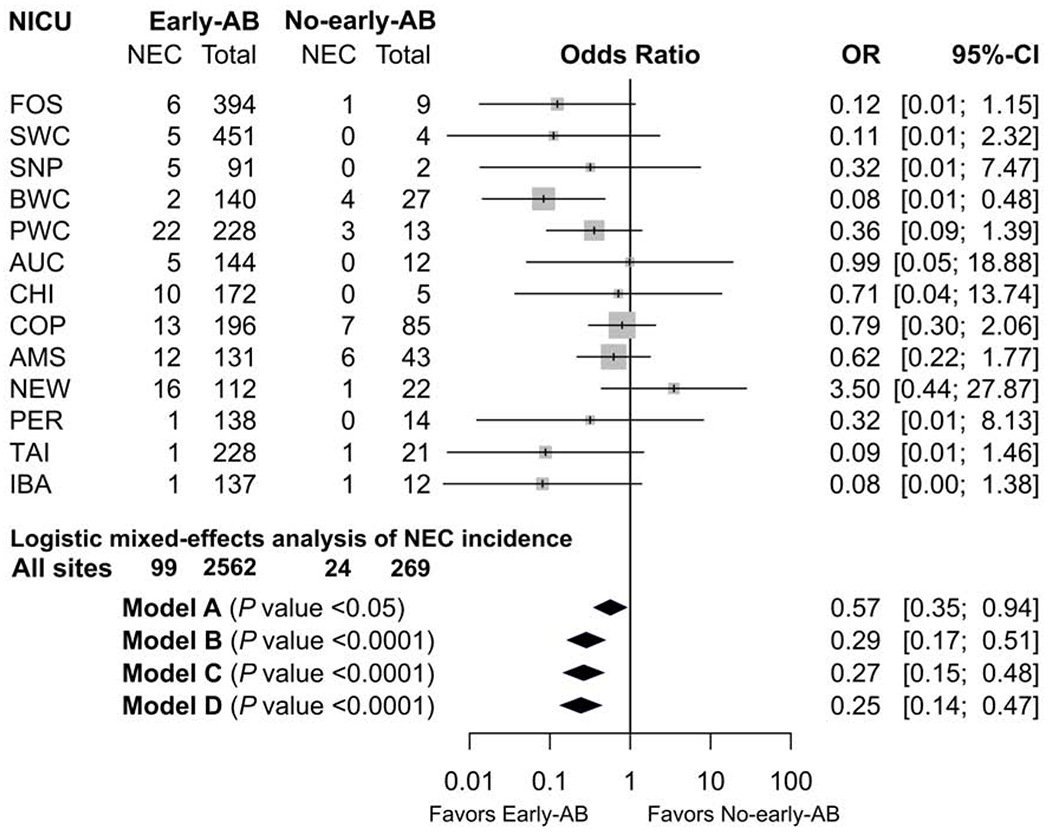
Forest plot of unadjusted OR of NEC incidence between early-antibiotics and no-early-antibiotics for each NICU. AB, antibiotics; CI, confidence interval; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; OR, odds ratio; Model A, NICU site as the random effect and no adjustment for other fixed-effects; model B, model A with adjustment for gestational age, birth weight, and sex as fixed-effects; model C, model B with additional adjustment for delivery mode, use of antenatal steroids, and Apgar score at 5 min; model D, model C with additional adjustment for initiation time of enteral nutrition and type of enteral nutrition in the first week of life
TABLE 3.
Demographic, nutritional, and mortality characteristics in NEC infants
| Early-antibiotics | No-early-antibiotics | P value1 | P value2 | |
|---|---|---|---|---|
| N | 99 | 24 | ||
| Birth weight, g, mean (SD) | 1012 (255) | 1026 (331) | 0.82 | 0.32 |
| Gestational age, wks, mean (SD) | 27.8 (2.5) | 29.5 (2.4) | <0.01 | <0.001 |
| Birth weight z-score | −0.10 (0.89) | −1.00 (0.89) | <0.0001 | <0.0001 |
| SGA, n (%) | 13 (13) | 9 (34) | <0.01 | <0.05 |
| Male, n (%) | 61(62) | 15 (63) | 0.94 | 0.73 |
| C-section, n (%) | 52 (53) | 21 (88) | <0.01 | <0.01 |
| Antenatal steroids, n (%) | 68 (72) | 17 (77) | 0.64 | 0.89 |
| APGAR 5 <8, n (%) | 52 (62) | 12 (52) | 0.40 | 0.39 |
| Enteral nutrition start, n (%)3 | <0.001 | <0.01 | ||
| Slow | 27 (29) | 0 (0) | ||
| Medium | 23(25) | 2 (8) | ||
| Quick | 43 (46) | 22 (92) | ||
| Enteral nutrition type, n (%)4 | 0.76 | 0.54 | ||
| IF | 20 (26) | 7 (29) | ||
| HM | 57 (74) | 17 (71) | ||
| Mortality, n (%) | 18 (18) | 7 (29) | 0.23 | 0.97 |
| Probiotics, n (%) | 22 (26) | 10 (43) | 0.10 | 0.53 |
| Probiotics prior to NEC onset, n (%) | 17 (20) | 8 (35) | 0.13 | 0.67 |
| Onset, postnatal day, mean (SD) | 18 (15) | 14 (7) | 0.78 | 0.62 |
| Onset, PMA in wks, mean (SD) | 30.2 (2.5) | 31.4 (2.4) | <0.05 | <0.05 |
| Bell stage, n (%) | 1.00 | 0.79 | ||
| II | 58 (67) | 16 (67) | ||
| III | 29 (33) | 8 (33) |
Unadjusted P values.
Adjusted for NICU as a random effect.
Time when EN was initiated was grouped into: Slow, never started or started later than postnatal day 4; Medium, started on postnatal day 3 or 4; Quick, started within postnatal day 2.
Type of enteral nutrition used in postnatal week 1. IF, exclusive IF or mixed feeding; HM: exclusive human milk feeding (MM and/or DM). HM, human milk; IF, infant formula; NEC, necrotizing enterocolitis; PMA, postmenstrual age; SD, standard deviation
Among the NEC infants, there was no significant difference in postnatal age at NEC onset between early-antibiotic and no-early-antibiotic groups (Table 3), whereas PMA at NEC onset was significantly greater in the no-early-antibiotic group before and after adjustment for NICUs (both P<0.05, Figure 2; OR: 0.96 for model A). When adjusted for other potential confounders including GA, the difference in PMA between early-antibiotic and no-early-antibiotic groups became non-significant in model B, C and D (P = 0.20-0.93, ORs: 1.00-1.02).
The differences in NEC outcomes were further investigated in additional subgroup analyses. Categorizing the infants by different geographic regions showed that the OR for NEC in the early-antibiotic compared with no-early-antibiotic groups was lower with stronger statistical significance in the five GD units than in the eight non-GD units (Table 4; available at www.jpeds.com). When only analyzing data from the five Western NICUs, the OR for NEC were attenuated, but remained significant after adjustment for potential confounders, such as BW and GA (P<0.05, for models B, C, or D, Table 4). The duration of early antibiotic treatment did not correlate with NEC incidence (ORs 0.99 for all models) for each day of early antibiotics duration increase. When grouping the infants into duration of early antibiotic treatment 1-3 days (n=559), or > 3 days (n=1991), NEC incidences were 4.3% and 3.7%, respectively, and did not differ between groups.
Table IV.
NEC incidence in the early antibiotic and no early antibiotic groups by geographic region
| 5 Guangdong NICUs |
8 non-Guangdong NICUs |
6 Western NICUs |
||||
|---|---|---|---|---|---|---|
| Models* | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value |
| A | 0.22 (0.09-0.51) | <.001 | 0.82 (0.45-1.49) | .49 | 0.96 (0.52-1.80) | .91 |
| B | 0.14 (0.05-0.35) | <000 | 0.34 (0.17-0.69) | <.01 | 0.39 (0.19-0.81) | <.05 |
| C | 0.13 (0.05-0.34) | <.000 | 0.32 (0.15-0.70) | <.01 | 0.38 (0.17-0.87) | <.05 |
| D | 0.11 (0.04-0.32) | <.000 | 0.33 (0.15-0.72) | <.01 | 0.39 (0.17-0.89) | <.05 |
Adjustment of models: model A. NICU as the random effect and no adjustment for other fixed effects, model B, model A with adjustments for gestational age, birth weight, and sex as fixed effects, model C. model B with additional adjustments for delivery model, use of antenatal steroids, and Apgar score at 5 min; model D, model C with additional adjustments for time of initiation of enteral nutrition and type of enteral nutrition in the first week of life.
DISCUSSION
Contrary to our hypothesis, NEC incidence was lower in infants who received early antibiotic treatment. This association was robust across NICUs, and was strengthened after adjusting for potential confounders, including enteral nutrition type and initiation time. The association was also unaffected by the use of probiotics, which varied widely among the different sites, although information on type of probiotics was not available. The association persisted when taking early death or discharge into account, and was robust to changing the covariates from BW to z-score or SGA as a binary variable, as well as subgroup analyses with only those less than 28 weeks GA. Furthermore, broad-spectrum antibiotic combinations are used as empiric treatment and the negative association between NEC rates and early antibiotic treatment did not appear to be restricted to specific types of antibiotics. In addition, the PMA of NEC onset was not affected by early antibiotic treatment when adjusted for gestational age and no association was found between duration of early antibiotic treatment and NEC incidence.
The major strengths of this study were a specified single a priori hypothesis before secondary analysis of the data from a large cohort of VLBW infants that were collected to study the relation between feeding and NEC and that the primary analysis addressed the within-hospital association. Although the incidence of NEC as well as mortality varied widely among the study NICUs, the range is comparable with previous reports of NEC incidence in very low birth weight infants.[19] However, the diagnosis of NEC is known to be variable,[20] and this study did not permit diagnosis of NEC after infant transfer to step-down units or sent home before fulfilling discharge criteria for socioeconomic reasons[1]. Nevertheless, the robust association across NICUs from several continents indicates potential general relevance, despite the widely differing guidelines for AB treatment.
Although the total cohort size for this study was large, an important limitation is that so few subjects were in the no-early-antibiotic group, and this group was characterized by significantly more mature (by approximately two gestational weeks) infants who were disproportionately SGA due to the ≤ 1500 g BW limit of the cohort. A critical question is if the adjustment for SGA in the statistical analysis was effective. We used three different sets of variables as covariates: GA and BW, GA and z-score, and GA and SGA status. These analyses showed very similar results. Furthermore, in the no-early antibiotic group, the proportion of SGA in infants diagnosed with NEC (34%) was similar to the overall proportion of SGA (37%), indicating that SGA was not a main reason for increased NEC in the no-early-antibiotic group. Also, when only including those with GA less than 28 weeks, this finding was still significant and even more pronounced. The proportion of caesarean deliveries was also higher in the no-early-antibiotic group. This likely reflected a higher incidence of physician-induced preterm birth where the absence of risk factors for infection makes it relevant to omit postnatal antibiotic treatment. Poor fetal growth associated with fetal Doppler ultrasound abnormalities may, however, increase the risk of NEC,[21,22] and thus the true effects of the prenatal causes of NEC may not be fully represented by SGA. Caesarean delivery can potentially lead to abnormal colonization in newborns[23] although several studies have found no increased risk of NEC after caesarean delivery.[24–27] Mode of delivery does not appear to be a confounding factor on the effect of early-antibiotics, as this would have been identified through our statistical analysis (Model D). Another limitation is that we did not include other potential confounding factors such as maternal status, use of surfactant, respiratory and circulatory challenges, and patent ductus arteriosus. Furthermore, potential confounding events that occurred between early-antibiotics and NEC onset were not recorded in the database. Related to this aspect, it is relevant to note that the two groups are defined by the very initial antibiotic treatment, whilst many subsequent diagnoses, complications and treatments prior to NEC onset are unclear. This includes antibiotics initiated after the first 3 postnatal days, which can comprise several regimen variations due to different reasons. In a subgroup of infants who had initiation of antibiotics beyond the third postnatal day, the NEC incidence was still lower in the early-antibiotic group (n=949) compared with the no-early-antibiotic group (n=137), with incidences of 7.9% versus 16.8%, respectively (P<0.01 for all models).
Two studies in preterm infants support the association between early antibiotics and lower NEC incidence. A case-control study with 56 NEC cases and 280 controls showed less NEC in infants that received antibiotic treatment within 24 h of birth.[21] A large cohort study in VLBW infants (n=14207) showed a small but significant reduction in the risk of NEC in infants treated with short duration early antibiotics (≤3 days), relative to infants not given such early antibiotic treatment[14]. In contrast, others have found increased NEC incidence with early antibiotic treatment, which was defined as initiation within the first week of life.[8] Early antibiotic treatment may delay the rapid colonization after birth in the immature preterm intestine.[28–30] A brief delay may provide time for intestinal postnatal adaptation of immune defense mechanisms, such as mucosal barrier function which undergoes significant maturation within the first few days after birth in preterm infants,[31] as also supported by studies in preterm pigs.[6,32]
Prolonged duration of antibiotic treatment, starting in the early neonatal period, has been linked to higher incidence of NEC as well as disruption of the intestinal microbial composition.[9–12,14] However, other studies[12,14,21] and the current study have not found this association. Nonetheless, compared with shorter duration, prolonged antibiotic therapy promotes development of antimicrobial resistance[33,34], which is a growing challenge and concern.[35] This is a large problem in NICUs, and infants who develop NEC often need long treatment courses of antibiotics.[35–37] Furthermore, the use of antibiotics in early life may have adverse consequences after the neonatal period.[38] Studies have found increased risk of obesity after antibiotic exposure in early life, in infants as well as in mouse studies.[35,39] Although antibiotics is a mainstay in treatment and prophylaxis of infections, excessive use of antibiotics should be avoided.[35,38,40]
In conclusion, in this large international cohort of VLBW infants, the 10% of infants who did not receive AB within three days of birth, showed higher NEC incidence. We do not yet suggest empirical antibiotics administration for every VLBW infant without antenatal risk factors or signs of neonatal infection, because there are other antibiotics-related neonatal morbidities,[14] potential long-term side effects, as well as the risks of antibiotic resistance.[35] Nonetheless, the no-early-antibiotic group in our study may represent an under-recognized group of infants with higher risk of NEC, despite their apparent lack of indication for AB treatment. A clinical trial, using early and short antibiotic treatment to prevent NEC, coupled with clinical signs of gut dysfunction (e.g. feeding intolerance, bacterial dysbiosis, fecal and blood biomarkers of immune dysfunction) could be considered. Furthermore, mechanistic animal studies on the optimal timing, dose, regimen, and type of antibiotic, incorporating detailed microbiological, immunological, and nutritional data, may help to clarify if, when, and how AB should be used for VLBW infants. A better understanding of antibiotics-related gut mechanisms could pave the way for more and better alternatives to the widespread antibiotic treatment of VLBW infants.
Table 5.
NEC incidence in early-antibiotic group compared with the no-early-antibiotic group in different geographic regions
| 5 GD NICUs |
8 non-GD NICUs |
6 western NICUs |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model1 | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value |
| A | 0.22 | 0.09-0.51 | <0.001 | 0.82 | 0.45-1.49 | 0.49 | 0.96 | 0.52-1.80 | 0.91 |
| B | 0.14 | 0.05-0.35 | <0.000 | 0.34 | 0.17-0.69 | <0.01 | 0.39 | 0.19-0.81 | <0.05 |
| C | 0.13 | 0.05-0.34 | <0.000 | 0.32 | 0.15-0.70 | <0.01 | 0.38 | 0.17-0.87 | <0.05 |
| D | 0.11 | 0.04-0.32 | <0.000 | 0.33 | 0.15-0.72 | <0.01 | 0.39 | 0.17-0.89 | <0.05 |
Adjustment of models: Model A, NICU as the random effect and no adjustment for other fixed-effect; model B, model A with adjustment for gestational age, birth weight, and gender as fixed-effects; model C, model B with additional adjustment for delivery model, use of antenatal steroids, and Apgar score at 5 min; model D, model C with additional adjustment for initiation time of enteral nutrition and type of enteral nutrition in the first week of life. GD, Guangdong province in China; NICU, neonatal intensive care units
Acknowledgements
We thank Dr Olukemi Tongo who helped with on-site data collection. We thank Christian Ritz for his kind advice in statistical analyses.
The study was part of the NEOMUNE project, sponsored by the Innovation Fund Denmark (12-132401 [to P.S.]). Data from Rush University Children’s Hospital were provided by support from the National Institute of Health (NR010009). H-C.L. was supported by the Taiwan China Medical University Hospital (DMR-107-183). J.M. and S.Y. were supported by Sanming Project of Medicine in Shenzhen (SZSM201612045) and Funding for the Construction of Key Medical Disciplines in Shenzhen (Affiliated Shenzhen Maternity & Child Healthcare Hospital, Southern Medical University). J.vG. is the director of the Dutch Human Milk Bank and is a member of the National Health Council. B.C. serves on scientific advisory boards for Nestlé Nutrition Institute and Danone/Nutricia. K.S. is the Director of the Human Milk Bank in Perth Australia and has received support from Medela and Nestlé Nutrition Institute. P.S. has received grant support from ARLA Foods, Medela, Danone/Nutricia, Biofiber-Damino, Mead Johnson Nutrition, and Nestlé Nutrition Institute. F.B. has received travel support for invited lectures from Abbot Nutrition and Nestlé Nutrition Institute and for participation in an expert working group from Danone/Nutricia. N.E. has received speakers’ honoraria from Nestlé Nutrition Institute and Danone/Nutricia, and his department has received research support from Prolacta Bioscience and Danone/Nutricia. The other authors declare no conflicts of interest.
Abbreviations:
- CI
confidence interval
- GA
gestational age
- GD
Guangdong
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- OR
odds ratio
- PMA
postmenstrual age
- SD
standard deviation
- SGA
small for gestational age
- VLBW
very low birth weight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].de Waard M, Li Y, Zhu Y, Ayede AI, Berrington J, Bloomfield FH, et al. Time to Full Enteral Feeding for Very Low-Birth-Weight Infants Varies Markedly Among Hospitals Worldwide But May Not Be Associated With Incidence of Necrotizing Enterocolitis: The NEOMUNE-NeoNutriNet Cohort Study. JPEN J Parenter Enteral Nutr 2019;43:658–67. 10.1002/jpen.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255–64. 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Juhl SM, Gregersen R, Lange T, Greisen G. Incidence and risk of necrotizing enterocolitis in Denmark from 1994-2014. PLoS One 2019;14:e0219268 10.1371/journal.pone.0219268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bury RG, Tudehope D. Enteral antibiotics for preventing necrotizing enterocolitis in low birthweight or preterm infants. Cochrane Database Syst Rev 2001. 10.1002/14651858.CD000405. [DOI] [PubMed] [Google Scholar]
- [5].Birck MM, Nguyen DN, Cilieborg MS, Kamal SS, Nielsen DS, Damborg P, et al. Enteral but not parenteral antibiotics enhance gut function and prevent necrotizing enterocolitis in formula-fed newborn preterm pigs. Am J Physiol Gastrointest Liver Physiol 2016;310:G323–33. 10.1152/ajpgi.00392.2015. [DOI] [PubMed] [Google Scholar]
- [6].Jensen ML, Thymann T, Cilieborg MS, Lykke M, Mølbak L, Jensen BB, et al. Antibiotics modulate intestinal immunity and prevent necrotizing enterocolitis in preterm neonatal piglets. Am J Physiol Gastrointest Liver Physiol 2014;306:G59–71. 10.1152/ajpgi.00213.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zwittink RD, Renes IB, van Lingen RA, van Zoeren-Grobben D, Konstanti P, Norbruis OF, et al. Association between duration of intravenous antibiotic administration and early-life microbiota development in late-preterm infants. Eur J Clin Microbiol Infect Dis 2018;37:475–83. 10.1007/s10096-018-3193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr 2014;165:23–9. 10.1016/j.jpeds.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr 2011;159:720–5. 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr 2011;159:392–7. 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged Duration of Initial Empirical Antibiotic Treatment Is Associated With Increased Rates of Necrotizing Enterocolitis and Death for Extremely Low Birth Weight Infants. Pediatrics 2009;123:58–66. 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Esaiassen E, Fjalstad JW, Juvet LK, van den Anker JN, Klingenberg C. Antibiotic exposure in neonates and early adverse outcomes: A systematic review and meta-analysis. J Antimicrob Chemother 2017;72:1858–70. 10.1093/jac/dkx088. [DOI] [PubMed] [Google Scholar]
- [13].Ting JY, Synnes A, Roberts A, Deshpandey A, Dow K, Yoon EW, et al. Association Between Antibiotic Use and Neonatal Mortality and Morbidities in Very Low-Birth-Weight Infants Without Culture-Proven Sepsis or Necrotizing Enterocolitis. JAMA Pediatr 2016;170:1181–7. 10.1001/jamapediatrics.2016.2132. [DOI] [PubMed] [Google Scholar]
- [14].Ting JY, Roberts A, Sherlock R, Ojah C, Cieslak Z, Dunn M, et al. Duration of Initial Empirical Antibiotic Therapy and Outcomes in Very Low Birth Weight Infants. Pediatrics 2019;143 10.1542/peds.2018-2286. [DOI] [PubMed] [Google Scholar]
- [15].Mukhopadhyay S, Puopolo KM. Clinical and Microbiologic Characteristics of Early-onset Sepsis Among Very Low Birth Weight Infants: Opportunities for Antibiotic Stewardship. Pediatr Infect Dis J 2017;36:477–81. 10.1097/INF.0000000000001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1–7. 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stock K, Schmid A, Griesmaier E, Gande N, Hochmayr C, Knoflach M, et al. The Impact of Being Born Preterm or Small for Gestational Age on Early Vascular Aging in Adolescents. J Pediatr 2018;201:49–54.e1. 10.1016/j.jpeds.2018.05.056. [DOI] [PubMed] [Google Scholar]
- [18].Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013; 13:59 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hein-Nielsen AL, Petersen SM, Greisen G. Unchanged incidence of necrotising enterocolitis in a tertiary neonatal department. Dan Med J 2015;62:1–5. [PubMed] [Google Scholar]
- [20].Juhl SM, Hansen ML, Fonnest G, Gormsen M, Lambaek ID, Greisen G. Poor validity of the routine diagnosis of necrotising enterocolitis in preterm infants at discharge. Acta Paediatr 2017;106:394–8. 10.1111/apa.13541. [DOI] [PubMed] [Google Scholar]
- [21].Berkhout DJC, Klaassen P, Niemarkt HJ, De Boode WP, Cossey V, Van Goudoever JB, et al. Risk Factors for Necrotizing Enterocolitis: A Prospective Multicenter Case-Control Study. Neonatology 2018;114:277–84. 10.1159/000489677. [DOI] [PubMed] [Google Scholar]
- [22].Ree IMC, Smits-Wintjens VEHJ, Rijntjes-Jacobs EGJ, Pelsma ICM, Steggerda SJ, Walther FJ, et al. Necrotizing enterocolitis in small-for-gestational-age neonates: a matched case-control study. Neonatology 2014;105:74–8. 10.1159/000356033. [DOI] [PubMed] [Google Scholar]
- [23].Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010;107:11971–5. 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Martin R, Makino H, Cetinyurek Yavuz A, Ben-Amor K, Roelofs M, Ishikawa E, et al. Early-Life Events, Including Mode of Delivery and Type of Feeding, Siblings and Gender, Shape the Developing Gut Microbiota. PLoS One 2016;11:e0158498 10.1371/journal.pone.0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Brumbaugh DE, Arruda J, Robbins K, Ir D, Santorico SA, Robertson CE, et al. Mode of Delivery Determines Neonatal Pharyngeal Bacterial Composition and Early Intestinal Colonization. J Pediatr Gastroenterol Nutr 2016;63:320–8. 10.1097/MPG.0000000000001124. [DOI] [PubMed] [Google Scholar]
- [26].Son M, Grobman WA, Miller ES. Is mode of delivery associated with the risk of necrotizing enterocolitis? Am J Obstet Gynecol 2016;215:389.e1-4. 10.1016/j.ajog.2016.04.058. [DOI] [PubMed] [Google Scholar]
- [27].Samuels N, van de Graaf RA, de Jonge RCJ, Reiss IKM, Vermeulen MJ. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr 2017;17:105 10.1186/s12887-017-0847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science 2016;352:539–44. 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Choi YS, Song IG. Fetal and preterm infant microbiomes: A new perspective of necrotizing enterocolitis. Korean J Pediatr 2017;60:307–11. 10.3345/kjp.2017.60.10.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gritz EC, Bhandari V. The human neonatal gut microbiome: a brief review. Front Pediatr 2015;3:17 10.3389/fped.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van Elburg RM, Fetter WPFF, Bunkers CM, Heymans HSAA. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch Dis Child Fetal Neonatal Ed 2003;88:F52–5. 10.1136/fn.88.1.f52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nguyen DN, Fuglsang E, Jiang P, Birck MM, Pan X, Kamal SBS, et al. Oral antibiotics increase blood neutrophil maturation and reduce bacteremia and necrotizing enterocolitis in the immediate postnatal period of preterm pigs. Innate Immun 2016;22:51–62. 10.1177/1753425915615195. [DOI] [PubMed] [Google Scholar]
- [33].Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 2000;162:505–11. 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- [34].Langford BJ, Morris AM. Is it time to stop counselling patients to “finish the course of antibiotics”? Can Pharm J 2017;150:349–50. 10.1177/1715163517735549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ramasethu J, Kawakita T. Antibiotic stewardship in perinatal and neonatal care. Semin Fetal Neonatal Med 2017;22:278–83. 10.1016/j.siny.2017.07.001. [DOI] [PubMed] [Google Scholar]
- [36].Morehead MS, Scarbrough C. Emergence of Global Antibiotic Resistance. Prim Care 2018;45:467–84. 10.1016/j.pop.2018.05.006. [DOI] [PubMed] [Google Scholar]
- [37].Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist 2018; 11:1645–58. 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science 2016;352:544–5. 10.1126/science.aad9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012;488:621–6. 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med 2016;8:39 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


