Abstract
The symptom burden of HIV-associated neurocognitive disorder (HAND) is high among older individuals, and treatment options are limited. Mindfulness-based stress reduction (MBSR) has potential to improve neurocognitive performance, psychosocial wellbeing, and quality of life, but empirical studies in this growing vulnerable population are lacking. In this trial, participants (N=180) age 55 and older who are living with HIV infection, are on combination antiretroviral therapy with suppressed viral loads, and yet continue to experience behavioral and cognitive symptoms of HAND, are randomized to MBSR or to a waitlist control arm that receives MBSR following a 16-week period of standard care. Primary outcomes (attention, executive function, stress, anxiety, depression, everyday functioning, quality of life) and potential mediators (affect, mindfulness) and moderators (social support, loneliness) are assessed at baseline and weeks 8, 16, and 48 in both groups, with an additional assessment at week 24 (post-MBSR) in the crossover control group. Assessments include self-report and objective measures (e.g., neuropsychological assessment, neurological exam, clinical labs). In addition, a subset of participants (n=30 per group) are randomly selected to undergo fMRI to evaluate changes in functional connectivity networks and their relationship to changes in neuropsychological outcomes. Forthcoming findings from this randomized controlled trial have the potential to contribute to a growing public health need as the number of older adults with HAND is expected to rise.
Keywords: HIV, HIV-associated neurocognitive disorder, mindfulness, cognition, older adults, stress
Introduction
More than 327,000 people living with HIV (PLWH) in the United States are over age 55, and the proportion of older PLWH continues to expand.1–4 HIV-associated neurocognitive disorder (HAND) can persist despite viral suppression with antiretroviral therapy (ART), and no proven treatment options exist for these patients.5–13 The symptom burden of HAND is particularly high among older patients.14–21 Older PLWH have high rates of medical comorbidities and polypharmacy; even when these are managed well, they can contribute to cognitive impairment.22,23 For older PLWH, HAND in the setting of undetectable plasma viral loads is best conceived as a geriatric syndrome – i.e., a clinical condition driven by multiple disease processes and occurring with greater frequency in old age. Despite inevitable growth of this population, data to inform treatment are limited.7
Although evidence-based interventions for HAND do not exist, Mindfulness-Based Stress Reduction (MBSR) is a viable option. MBSR aims to improve the capacity for mindfulness, which facilitates disengagement from thoughts and emotions that can negatively influence mental health.24 There are no published reports of MBSR being tested specifically for HAND, but mindfulness in other samples is associated with outcomes that are particularly relevant for older adults with HAND. For example, in studies with younger PLWH, other chronic illness groups, and uninfected older adults, MBSR is associated with improvements in quality of life, psychological symptoms (e.g., decreased stress, depression, anxiety; increased positive emotions), and medical outcomes (e.g., improved immune function, decreased pain).25–36 Moreover, mindfulness trials in non-HAND samples, including patients with mild cognitive impairment (MCI), have revealed improved neuropsychological testing performance in attention, working memory and executive control domains, while resting state fMRI (rs-fMRI) has shown improved functional connectivity in the default mode network (DMN) and salience network (SAL).30,37–49 This suggests that MBSR may improve brain function in networks that support attention and emotion regulation and are affected by HIV.38,47–51
Potential moderators of MBSR include social support and loneliness, which are tightly linked to outcomes in geriatric medicine. For example, the quality and quantity of one’s social support is associated with cardiovascular, neuroendocrine, and immune function,52,53 and to older adults’ cognitive function.54 Loneliness in older adults increases risk for Alzheimer’s disease, and predicts poorer quality of life, cognitive and functional decline, and death.55–57 Participants with lower levels of social support or higher levels of loneliness may benefit most from a group-based intervention such as MBSR, but these hypotheses must be carefully tested, as the nature of social support in PLWH substantially differs from that of HIV-uninfected populations.58–60
We are conducting the first known test of the efficacy of MBSR to improve HAND-associated symptoms (poor attention, executive function, stress, anxiety, depressive symptoms), functioning, and quality of life in older PLWH. We additionally are investigating factors that mediate and moderate the effects of MBSR. In a subset of participants, we also are determining whether resting state functional connectivity (rs-fc) improves in response to MBSR and correlates with neuropsychological performance.
Study Design and Methods
Overview
This randomized controlled trial (RCT) evaluates the efficacy of MBSR to reduce symptom burden in patients aged 55 and older who are living with HIV infection, are on combination antiretroviral therapy (cART) with suppressed viral loads, and yet continue to experience behavioral and cognitive symptoms of HIV-associated neurocognitive disorder (HAND). Participants (N=180) are randomized to 8 weeks of Mindfulness-Based Stress Reduction (MBSR arm) or standard of care operationalized as a waitlist control (CO arm) (Figure 1). Participants in the MBSR arm receive a booster call between completion of the intervention and the follow-up assessment, while those in the CO arm are only contacted for scheduling purposes. After evaluating sustained MBSR effects at 16 weeks, the CO arm is offered MBSR to boost retention and to increase power for evaluation of the secondary aim. All participants are followed for 48 weeks.
Figure 1.
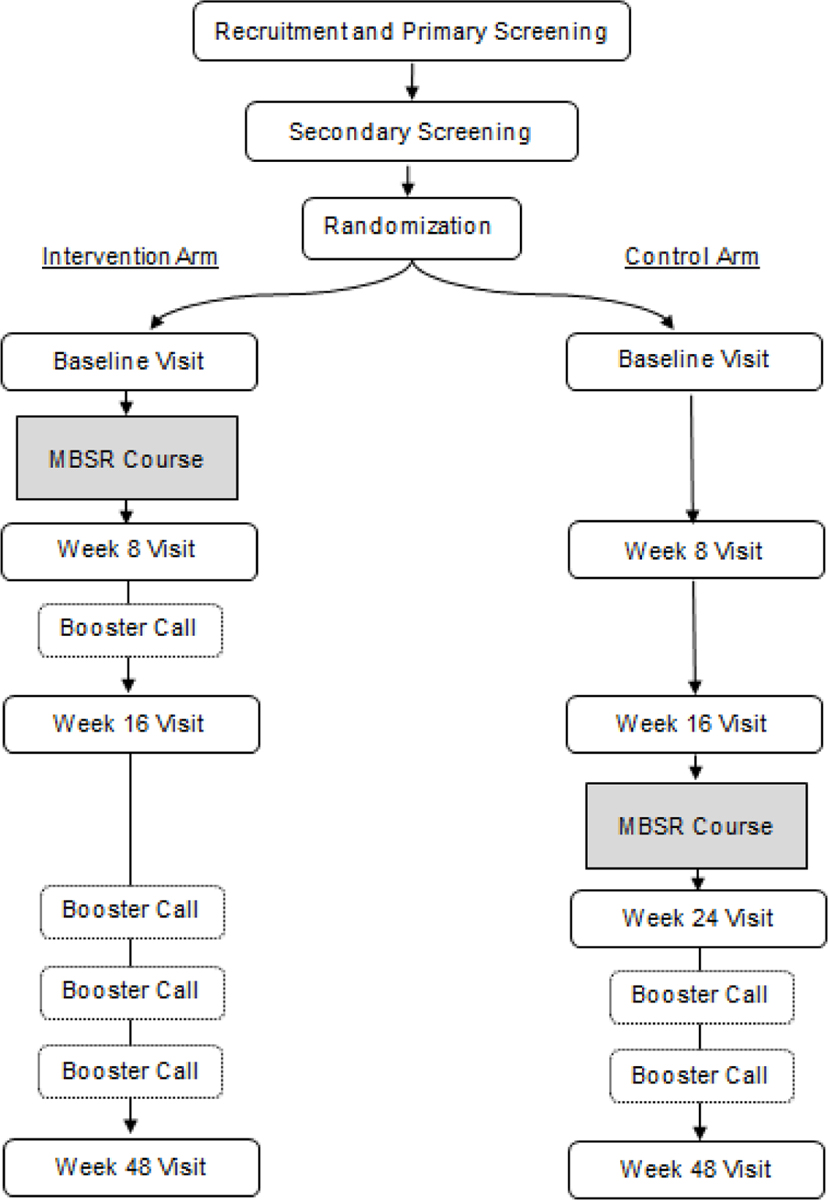
Study Flow
Aims of the trial are: (1) to test the efficacy of MBSR to improve cognitive (attention, executive function) and psychological symptoms (stress, anxiety, depression), everyday function, and quality of life among PLWH age 55 and over who have undetectable plasma HIV RNA, HAND, and no targeted treatment options; (2) to perform rs-fc analyses in a subset of participants to determine if MBSR affects the salience (SAL) and default mode networks (DMN), and to examine whether changes in rs-fc within these networks correlates with improved attention and executive functioning on neuropsychological tests; and (3) to investigate whether the observed effects of MBSR are moderated by the quality of social support and loneliness and mediated by change in positive and negative affect and mindfulness. All procedures were approved by the Institutional Review Board at the University of California San Francisco (UCSF) and registered with ClinicalTrials.gov (NCT02936401).
Participants
Eligibility for enrollment is determined through a primary screening call and a secondary screening visit. To be eligible for enrollment, participants must: 1) be 55 years of age or older (lowered from the initial eligibility criterion of age ≥60, to facilitate enrollment of N=180); 2) be HIV-positive, on cART for at least 12 months with an undetectable plasma viral load (<100 copies/mL) for at least the previous six months; and 3) endorse mild-moderate cognitive symptoms and meet criteria for HAND based on neuropsychological testing performance informed by clinical acumen. Participants are excluded if they: 1) demonstrate a detectable plasma viral load (≥100 copies/mL) at the in-person screening visit; 2) endorse use of cocaine or methamphetamine in the previous six months; 3) currently practice or have extensive experience with mindfulness; 4) fail to attend screening visits after two attempts and despite support offered; 5) have a treatable condition that may impact cognition (e.g., neurosyphilis, thyroid disorder, B12 deficiency, cancer requiring chemotherapy) or other major confounding cognitive factors (e.g., recent head injury or stroke, cognitive impairment caused by alcohol or substance use, active brain infection other than HIV); 6) are unable to complete cognitive testing in English; 7) are unable to provide informed consent or assent with a legal surrogate to sign consent; or 8) have an unstable psychiatric condition (e.g., active psychosis, suicidal or homicidal ideation) making it difficult to complete the intervention.
Recruitment
To achieve enrollment of N=180 participants, we recruited through a variety of methods that include outreach to hospitals, health clinics, community centers, and faith-based organizations and through advertising in publications, in newsletters, and various community settings (e.g., public transit, local businesses) in the San Francisco Bay Area. Informational letters are also mailed through UCSF’s Recruitment Letter Service directly to medical center patients who may fit criteria. When interested individuals contact study staff, they are given a detailed description of the study purpose, screening procedures, and the intervention, and are asked to complete a phone interview (primary screen). After giving verbal consent, participants are queried about basic eligibility criteria, such as age and treatment status. Participants also complete the Patient’s Assessment of Own Functioning Inventory (PAOFI) to quantify symptoms.61 Those who meet basic criteria (i.e., age ≥55, stable cART with undetectable viral load) and endorse at least one cognitive symptom on the PAOFI as “almost always,” “very often,” or “fairly often” are invited for secondary screening. Based on our prior research evaluating HAND22 and conducting trials of MBSR in PLWH,28 we estimated that 400 phone screenings would be necessary to enroll N=180.
Consent, Screening and Randomization
Participants who pass the primary screen complete a secondary screen including cognitive testing in person on the UCSF campus. On the day of the visit and prior to evaluation, all participants receive detailed information regarding the assessments to be completed at the screening and RCT study visits, and provide informed consent. During this visit, participants complete a full cognitive62 and functional evaluation, assessment of geriatric syndromes, and a neurological exam. A full list of clinical labs and instruments used during this secondary screening visit is included in Table 1.
Table 1.
Secondary Screening Instruments
| Cognitive Testing |
|---|
| • Learning and Memory: California Verbal Learning Task-II (CVLT-II)-63 sum of trials 1–5, immediate free recall, and long-delay free recall; Benson Figure Recall64 |
| • Language: Boston Naming Test,65 Category Fluency,66 Lexical Fluency,66 Wide Range Achievement Test-4,66 Sentence Comprehension66 |
| • Attention/Working Memory: CVLT-II trial 1,63 Digits Forward,67 Digits Backward,67 1-back,66 2-back66 |
| • Executive Function: Trails B,67 Design Fluency,68 Stroop Interference66 |
| • Psychomotor Speed: Trails A,67 Stroop Color Naming66 |
| • Visuospatial: Benson Figure Copy,64 Visual Object and Space Perception,66 Comprehensive Affect Testing System Face Matching Test69 |
| • Motor/Manual Dexterity: Grooved pegboard,70 Finger tapping71 |
| • Mood/Affect: Geriatric Depression Scale (30 item)72 |
|
Functional Testing |
| • Neuropsychological Assessment Battery – Daily Living Module |
|
Neurological Exam |
| • Eye movements, strength, sensation, coordination & extrapyramidal findings |
| • Targeted cognitive/behavioral review of symptoms, including date of first symptom to estimate duration of HAND |
| • Medical history including HIV variables (e.g., cART history & duration of cART and viral suppression, adherence, CD4 nadir), comorbidities |
| • Current medications and supplements |
| • Family history of neurological disorders |
| • Mood and psychiatric illnesses |
| • Drug and alcohol history |
|
Geriatric Syndromes Assessment |
| • Whispered voice test |
| • Snellen eye chart |
| • Physical activity query |
| • Grip strength |
| • Short performance physical battery |
| • Timed gait |
| • Katz & Lawton Activities of Daily Living (ADL)73 and Instrumental Activities of Daily Living (IADL)74 scales |
|
Clinical Labs |
| • Lymphocyte subset panel |
| • Plasma HIV RNA |
| • Complete blood count |
| • Hepatitis C antibody |
| • Alanine aminotransferase and Aspartate aminotransferase |
| • Creatinine |
| • Hemoglobin A1c |
| • Rapid plasma reagin |
| • Thyroid stimulating hormone |
| • Vitamin B12 |
Data from each secondary screening visit is reviewed at consensus conference, attended by a neurologist and neuropsychologist who utilize clinical acumen to determine the participant’s cognitive diagnosis, guided by the 2007 Frascati HAND criteria.75 Individuals are eligible for randomization if they meet criteria for HAND, specifically mild neurocognitive disorder (MND),75 as evidenced by performance at least 1.0 SD below the mean for demographically adjusted norms in at least two cognitive domains and mild interference in daily functioning as measured by ADL/IADL assessments at in-person screening (Table 1). Randomization occurs on a rolling basis using a blinded scheme, with MBSR sessions starting every 16 weeks. Participants who have indications for non-HIV processes that could affect cognition are referred to their primary care physician to consider further clinical screening to rule out non-HAND causes of cognitive impairment. Individuals with treatable conditions can be re-screened 6 months after receiving appropriate therapy. Participants with severe cognitive deficits consistent with dementia are not randomized unless the study team agrees that the deficits are mild enough to withstand rigors of MBSR.
Intervention
The intervention follows the standard MBSR protocol consisting of eight weekly classes (2.5 hours per class), one silent retreat (8 hours, week 6), and home practice assignments (45 minutes per day of formal mindfulness practice, plus 15 minutes per day of informal practice, 6 days per week).76 MBSR courses take place at the UCSF Osher Center for Integrative Medicine and are led by experienced MBSR instructors. Instructors follow a manualized course outline, completing a checklist after each course to assure fidelity. To facilitate adherence, learning and home practice, all participants receive a workbook containing the class schedule and assignments, guided audio, and a copy of Full Catastrophe Living.76 Instructors record participants’ class attendance/absences. The MBSR program uses a group format with 10–15 individuals in each course, all of whom are enrolled in the study.
Intervention content adhered to the standard MBSR protocol and was not tailored to include HIV- or HAND-specific topics. Formal meditation methods taught in MBSR include: body scan meditation; gentle yoga postures practiced with mindful awareness of the body; sitting meditation with mindfulness of breath, body, thoughts, and emotions, and intentional awareness; and walking meditation. Informal mindfulness meditation practices taught in MBSR include: awareness of pleasant and unpleasant events; and deliberate awareness of routine activities such as eating, walking, and interpersonal communications. Didactics on stress physiology and reactivity addressing the effects of perception, appraisal, and attitude on health habits, behavior and interpersonal communication are included.
At weeks 12, 24, 32, and 40, study staff conducts booster calls with participants who complete the course. These calls are estimated to last approximately 5 minutes and are intended to encourage ongoing mindfulness practice and enhance study retention. Self-reported adherence to formal and informal mindfulness practice is also collected during these calls.
Control Condition
Individuals randomized to the CO arm receive standard care between baseline and week 16, and during this period have no contact with study staff outside of study visits, other than that needed for scheduling. Upon completion of the week 16 evaluation, participants in the CO arm are offered the standard MBSR course. Study staff conducts booster calls at weeks 32 and 40 with CO arm participants who complete the MBSR course. The length and content of these calls is the same as those done in the MBSR arm.
Participant Compensation
Participants are paid $50 for the completion of the secondary screening visit, $25 each for baseline, week 8, week 16 and week 48 visits and $30 for each MRI scan. Participants are also reimbursed for parking at study visits. Participants are paid $15 at each MBSR session to offset the cost of transportation and parking and are paid $250 for successful completion of the MBSR course and the post-intervention visit.
Measures
An overview of the schedule of assessments is provided in Table 2. Primary outcome measures and assessment of mediators and moderators are captured at baseline, week 8, week 16, and week 48. Basic clinical labs (CD4 count and plasma HIV RNA) are run at these same time points. In addition to screening all participants with MRI at baseline, at least 30 cases/arm are randomly selected to undergo MRI at weeks 8 and 16 to evaluate the imaging aims of the trial. Participants in the CO arm who complete the MBSR course between weeks 16 and 24 are also evaluated for primary outcome measures and assessment of mediators and moderators at week 24.
Table 2.
Schedule of Assessments
| Primary Screening | Secondary Screening | Baseline | Week 8 | Week 16 | Week 24c | Week 48 |
|---|---|---|---|---|---|---|
| Assessment of basic eligibility criteria Symptom quantification |
Cognitive testing Functional testing Neurological exam Geriatric syndromes assessment Clinical labs |
Primary outcome measures Mediators & moderators Clinical labsa MRIb |
Primary outcome measures Mediators & moderators Clinical labsa MRIb |
Primary outcome measures Mediators & moderators Clinical labsa MRIb |
Primary outcome measures Mediators & moderators |
Primary outcome measures Mediators & moderators Neurological exam Clinical labsa |
Plasma HIV RNA and lymphocyte subset panel only
n=30 participants per arm
Control participants only
Detailed descriptions of measures can be found in Table 3. Assessments are completed one-on-one by trained staff using computer assistance for self-reported measures. In addition to the standardized assessments indicated in Table 3, participants complete a brief mixed methods evaluation of the MBSR program at their post-intervention assessment (week 8 for the intervention group, week 24 for the control group). This evaluation assesses participants’ rating of the program overall and of the instructor (1 very poor to 5 excellent) and whether they would recommend the program to others (1 definitely not to 5 definitely). Two open-ended items ask what participants like most/least and what they found most/least helpful in the MBSR program.
Table 3.
Study Measures
| Primary Outcome Measures |
|---|
| • Attention & Executive Function: Continuous Performance Task,77 Symbol-Digit modalities test,78 WAIS Letter Number Sequencing79 |
| • Stress, Anxiety, Depression: Perceived Stress Scale,80 State-Trait Anxiety Inventory,81 Geriatric Depression Scale82 |
| • Everyday function: Katz & Lawton ADL73 and IADL74 scales |
| • Quality of Life: World Health Organization Quality of Life – HIV Scale83 |
| • Neuropsychiatric: Buss-Durkee Irritability subscale,84 Center for Neurologic Study – Lability Scale,85 Affective Intensity Measure86 |
|
Assessment of Mediators and Moderators |
| • Social Networks: Norbeck Social Support Scale,87 Medical Outcome Study Social Support Survey88 |
| • Loneliness: Revised UCLA Loneliness Scale89 |
| • Affect: Modified Differential Emotions Scale90 |
| • Mindfulness: Five Facet Mindfulness Questionnaire,91 Mindfulness Attention Awareness Scale92 |
Imaging
Participants undergo whole brain MRI on a Siemens 3 Tesla Prisma Fit Scanner with a 64-channel coil to collect 3D-T1 Magnetization Prepared Rapid Acquisition Gradient-Echo (MPRAGE) sequences (1.0x1.0x1.0mm3) and rs-fc scan at resting state with eyes open. We will calculate functional connectivity strength within networks from 2 six-minute blood oxygen level dependent (BOLD) rs-fc MRI scans wherein the participant is asked to rest quietly in the scanner with eyes open and without falling asleep. T-1 isometric structural scans are used for co-registration.50,93,94 The BOLD time-series will be extracted from a total of 36 previously defined seeds constituting the DMN and SAL networks as well as the dorsal attention (DAN), executive control (CON), and sensorimotor (SMN) networks.93 Relationships between each of the 36 seeds will be calculated by constructing 36x36 cross-correlation matrices and Fisher z-transforming the resulting correlation values.50 DMN and SAL data will be summarized as composite scores representing mean intra-network functional connectivity. A whole-brain analysis will then be performed on the entire matrix of pairwise relationships to explore treatment effects in other functional brain systems.
Planned Analyses
Descriptive analysis, including graphical displays, frequency tables, measures of central tendency and variability, will be used to describe primary outcomes, and important demographic, clinical, and neuropsychological characteristics across and within the two study arms.95 Medical variables (e.g., duration of HIV infection, cART history) that could influence neurocognitive outcomes through differential penetration of the blood-brain barrier96 will be examined in bivariate correlational analyses to identify statistically significant covariates. Proportions will be compared between groups using the Fisher’s exact test.97 Continuous variables, including the primary outcomes, will be examined using Wilcoxon tests.98
Reasons for any missing data will be examined. Individuals with missing data on key characteristics will be compared to those with complete data to assess for potential bias due to nonrandom missing data.99 If the mechanism generating missing data is deemed informative, we will perform sensitivity analysis.99 We will also capture information on participants who fail the screen due to missing two appointments and describe these as they inform the external validity of our findings in relation to PLWH over age 55 in the United States.
To examine the statistical significance of the impact of MBSR on the primary outcomes (Table 3) and on intra-network functional connectivity, we will conduct the following analyses:
In the primary analysis of the randomized intervention effect, we will employ two-sample Wilcoxon rank sum tests to compare the two treatment arms with regard to change in outcomes between weeks 0 and 8 and between weeks 0 and 16. We will first perform an intent-to-treat analysis of all randomized cases, followed by a complete case analysis of those who completed week 16. No adjustment for multiple comparisons is made as the endpoint of week 0 to 16 change is studied to assess the durability of effects.
We will employ the Wilcoxon signed rank test to test for change in the primary outcomes before and after MBSR within each arm. This analysis will be conducted both on the immediate MBSR arm only (considering change from week 0 to both weeks 8 and 16) and in the pooled data from both arms, where pre- and post-MBSR change in the control arm is measured before and after MBSR. We will also conduct a repeated measures ANOVA to investigate the impact of MBSR intervention on all time points, treating the MBSR exposure as a time varying-intervention and parameterized with a time-varying indicator variable (which is always 1 in the immediate MBSR arm, and changes from 0 to 1 after week 15 in the control arm). We will include in this model an interaction of this intervention indicator with time to investigate whether its impact varies over time (e.g., attenuates after completion of MBSR).
Analyses of moderation will investigate the effect of baseline covariates such as loneliness and social support and of interaction of these covariates with the randomized intervention assignment on the primary outcomes. Medical covariates, such as HAND severity, will also be examined as potential moderators, allowing us to identify whether MBSR in non-research settings should be targeted to patients with a particular clinical presentation. Multiple least-squares regression will be used for this purpose; both the outcomes measuring change from 0 to 8 weeks and 0 to 16 weeks will be considered. Secondary analysis of effect modification will investigate the effect of MBSR on change in outcomes within participants pooled across the treatment arms as noted above. For these analyses, the “baseline” covariates will be measured at week 16 in the control arm—immediately prior to the initiation of MBSR. We will also conduct a repeated measures ANOVA on the pooled data with time-varying treatment indicator for mindfulness and include as covariates measures of moderators at the initiation of MBSR (week 0 for MBSR arm and week 16 for control arm) and the interaction of these moderators with treatment. To protect against over fitting, 10-fold cross-validation procedures will be used for all procedures. Adjustment for multiple comparisons will not be undertaken, as the goal is to examine consistency of findings across all analyses, recognizing that the randomized comparisons are most interpretable but have the least number of participants. The advantage of the pooled analyses is increase in sample size, but these secondary analyses are seen as an aid to interpretation of the primary analyses rather than as primary analyses in themselves. The Sobel test will be used to test whether the change in positive and negative affect and mindfulness measured at weeks 8 and 16 is a significant mediator of study outcomes—change from week 0 to weeks 8 and 16. Regression analyses required for these computations will be based on ordinary least squares regression.
Discussion
MBSR has potential to decrease symptom burden and improve quality of life among older adults with HAND. To test this hypothesis, this study employs a randomized controlled trial of MBSR to target neuropsychological symptoms (attention, executive function, stress, anxiety, depression), functioning, and quality of life among adults aged 55 and older who have HAND and have maximized treatment options. In addition to primary outcomes measured by self-report and neuropsychological evaluation, we will conduct functional connectivity analyses of rs-fc in a subset of participants to demonstrate increased strength of brain networks corresponding to improved cognition. In the full sample, we also will examine psychosocial factors to determine if they mediate (affect, mindfulness) or moderate (social support, loneliness) our main findings.
Together this work employs geriatric, neuroscience and complementary medicine disciplines to reduce symptom burden in aging HIV-infected patients. Research is beginning to demonstrate benefits of MBSR in PLWH 28,31 but has not specifically targeted those with HAND. Given the lack of disease-targeted approaches to decreasing the burden of HAND among older PLWH,6,8,9,12,100 efficacious strategies for managing neuropsychological symptoms are critically needed in this population. Existing evidence regarding the effectiveness of MBSR for improving cognition is mixed, with one systematic review finding no evidence for improvements in attention and only preliminary support for improvements in other elements of cognition such as working memory.101 However, most of the MBSR trials included in that review were conducted with healthy samples,101 while very few studies have included participants who demonstrate cognitive impairment at baseline.102 The current study of older adults with HAND therefore has potential to contribute not only to clinical recommendations for this population, but also to the science of neurocognition in response to MBSR.
Strengths of this study include the use of both self-reported (e.g., symptoms) and objective measures (i.e., neuropsychological examination, fMRI). This is particularly relevant in this clinical sample, as compared to prior use of objective neuropsychological assessment of MBSR outcomes, which has been conducted largely in healthy samples.101 Additional strengths of this study, compared to the methods of previous MBSR trials,31,101 include: use of trained MBSR instructors, methods for monitoring treatment fidelity, and exclusion of participants who are already experienced with mindfulness and therefore may demonstrate ceiling effects. Although these rigorous research procedures increase the internal validity of this study, results may not generalize to other populations, such as PLWH without access to a major academic healthcare center or willingness to participate in complex research protocols and those with subclinical HIV-related neurologic dysfunction. If this trial supports efficacy of MBSR for older adults with HAND, additional studies will be warranted to examine the effectiveness and implementation in community-based settings and with subclinical neurocognitive changes.
Despite these strengths, a minor limitation to our approach to allowing controls to eventually undergo MBSR is that there will be no controls at 48 weeks to perform cross-group comparisons. Instead of maintaining participants in a prolonged control arm, we will longitudinally model individual change over the course of the study to determine the trajectory of change (positive or negative) after each time point and compare individuals to their visit immediately before MBSR initiation. The use of monetary compensation to enhance completion of the course may not match real life settings, thus impacting the scalability of this intervention. Nonetheless, future research will need to determine whether any changes in response to MBSR among older adults with HAND are maintained over long-term follow-up. Additional trials that enroll an HIV-negative cohort may also be useful to distinguish effects of MBSR on general aging vs. HAND-specific processes.
MBSR has been applied to patients with HIV, elders, and patients with MCI in published work,30,34,49,103 but to our knowledge, this is the first randomized controlled trial of MBSR for older adults with HAND. To account for the possibility of increased attrition in this group, we have over-estimated failure to complete the intervention. This ensures that, even if rates of retention and adherence are low, we will be fully powered to examine primary outcomes. As the number of older adults with HAND rises, this study has the potential to contribute to a growing public health need.
Funding:
This work is supported by the National Institutes of Health [R01 NR015223]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ANOVA
Analysis of Variance
- BOLD
Blood Oxygen Level Dependent
- cART
Combination Antiretroviral Therapy
- CON
Executive Control Network
- DAN
Dorsal Attention Network
- DMN
Default Mode Network
- fMRI
functional Magnetic Resonance Imaging
- HAND
HIV-associated neurocognitive disorder
- HIV
Human Immunodeficiency Virus
- MBSR
Mindfulness Based Stress Reduction
- MCI
Mild Cognitive Impairment
- MND
Mild Neurocognitive Disorder
- MPRAGE
Magnetization Prepared Rapid Acquisition Gradient-Echo MRI Magnetic Resonance Imaging
- PAOF
Patient’s Assessment of Own Functioning
- PLWH
People living with HIV
- RCT
Randomized Controlled Trial
- rs-fMRI
resting state fMRI
- rs-fc
resting state functional connectivity
- SAL
Salience Network
- SMN
Sensorimotor Network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Estimated HIV Incidence and Prevalence in the United States, 2010–2016. CDC; 2019. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-24-1.pdf [Google Scholar]
- 2.Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA 2013;309(13):1397–1405. doi: 10.1001/jama.2013.2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep 2010;7(2):69–76. doi: 10.1007/s11904-010-0041-9 [DOI] [PubMed] [Google Scholar]
- 4.Mills EJ, Bärnighausen T, Negin J. HIV and aging--preparing for the challenges ahead. N Engl J Med 2012;366(14):1270–1273. doi: 10.1056/NEJMp1113643 [DOI] [PubMed] [Google Scholar]
- 5.Carroll A, Brew B. HIV-associated neurocognitive disorders: recent advances in pathogenesis, biomarkers, and treatment. F1000Research 2017;6. doi: 10.12688/f1000research.10651.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev 2009;19(2):169–185. doi: 10.1007/s11065-009-9092-3 [DOI] [PubMed] [Google Scholar]
- 7.Eggers C, Arendt G, Hahn K, et al. HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. J Neurol 2017;264(8):1715–1727. doi: 10.1007/s00415-017-8503-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS Lond Engl 2007;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27 [DOI] [PubMed] [Google Scholar]
- 9.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol 2002;8(2):136–142. doi: 10.1080/13550280290049615 [DOI] [PubMed] [Google Scholar]
- 10.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 1999 2007;45(2):174–182. doi: 10.1097/QAI.0b013e318042e1ee [DOI] [PubMed] [Google Scholar]
- 11.Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology 2004;63(5):822–827. doi: 10.1212/01.wnl.0000134665.58343.8d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vance DE, Fazeli PL, Grant JS, Slater LZ, Raper JL. The role of neuroplasticity and cognitive reserve in aging with HIV: recommendations for cognitive protection and rehabilitation. J Neurosci Nurs J Am Assoc Neurosci Nurses 2013;45(5):306–316. doi: 10.1097/JNN.0b013e31829d8b29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bougea A, Spantideas N, Galanis P, Gkekas G, Thomaides T. Optimal treatment of HIV-associated neurocognitive disorders: myths and reality. A critical review. Ther Adv Infect Dis 2019;6:2049936119838228. doi: 10.1177/2049936119838228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghidei L, Simone MJ, Salow MJ, et al. Aging, antiretrovirals, and adherence: a meta analysis of adherence among older HIV-infected individuals. Drugs Aging 2013;30(10):809–819. doi: 10.1007/s40266-013-0107-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodkin K, Wilkie FL, Concha M, et al. Aging and neuro-AIDS conditions and the changing spectrum of HIV-1-associated morbidity and mortality. J Clin Epidemiol 2001;54 Suppl 1:S35–43. doi: 10.1016/s0895-4356(01)00445-0 [DOI] [PubMed] [Google Scholar]
- 16.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 1999 2012;60 Suppl 1:S1–18. doi: 10.1097/QAI.0b013e31825a3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu SM, Chow DC, Valcour V, et al. The Impact of Depressive Symptoms on Neuropsychological Performance Tests in HIV-Infected Individuals: A Study of the Hawaii Aging with HIV Cohort. World J AIDS 2011;1(4):139–145. doi: 10.4236/wja.2011.14020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS Lond Engl 2004;18 Suppl 1:S79–86. doi: 10.1097/00002030-200401001-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valcour V, Watters MR, Williams AE, Sacktor N, McMurtray A, Shikuma C. Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. J Neurovirol 2008;14(5):362–367. doi: 10.1080/13550280802216494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watters MR, Poff PW, Shiramizu BT, et al. Symptomatic distal sensory polyneuropathy in HIV after age 50. Neurology. 2004;62(8):1378–1383. doi: 10.1212/01.wnl.0000120622.91018.ea [DOI] [PubMed] [Google Scholar]
- 21.Woods SP, Dawson MS, Weber E, Grant I, HIV Neurobehavioral Research Center (HNRC) Group. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. J Clin Exp Neuropsychol 2010;32(4):398–407. doi: 10.1080/13803390903130737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greene M, Covinsky KE, Valcour V, et al. Geriatric Syndromes in Older HIV-Infected Adults. J Acquir Immune Defic Syndr 2015;69(2):161–167. doi: 10.1097/QAI.0000000000000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greene M, Steinman MA, McNicholl IR, Valcour V. Polypharmacy, drug-drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J Am Geriatr Soc 2014;62(3):447–453. doi: 10.1111/jgs.12695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown KW, Ryan RM, Creswell JD. Mindfulness: Theoretical Foundations and Evidence for its Salutary Effects. Psychol Inq 2007;18(4):211–237. doi: 10.1080/10478400701598298 [DOI] [Google Scholar]
- 25.Abbott RA, Whear R, Rodgers LR, et al. Effectiveness of mindfulness-based stress reduction and mindfulness based cognitive therapy in vascular disease: A systematic review and meta-analysis of randomised controlled trials. J Psychosom Res 2014;76(5):341–351. doi: 10.1016/j.jpsychores.2014.02.012 [DOI] [PubMed] [Google Scholar]
- 26.Anheyer D, Haller H, Barth J, Lauche R, Dobos G, Cramer H. Mindfulness-Based Stress Reduction for Treating Low Back Pain: A Systematic Review and Meta-analysis. Ann Intern Med 2017;166(11):799–807. doi: 10.7326/M16-1997 [DOI] [PubMed] [Google Scholar]
- 27.Garland EL, Hanley AW, Goldin PR, Gross JJ. Testing the mindfulness-to-meaning theory: Evidence for mindful positive emotion regulation from a reanalysis of longitudinal data. PloS One 2017;12(12):e0187727. doi: 10.1371/journal.pone.0187727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hecht FM, Moskowitz JT, Moran P, et al. A Randomized, Controlled Trial of Mindfulness-Based Stress Reduction in HIV Infection. Brain Behav Immun Published online 2018. doi: 10.1016/j.bbi.2018.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoury B, Sharma M, Rush SE, Fournier C. Mindfulness-based stress reduction for healthy individuals: A meta-analysis. J Psychosom Res 2015;78(6):519–528. doi: 10.1016/j.jpsychores.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 30.Moynihan JA, Chapman BP, Klorman R, et al. Mindfulness-Based Stress Reduction for Older Adults: Effects on Executive Function, Frontal Alpha Asymmetry and Immune Function. Neuropsychobiology. 2013;68(1):34–43. doi: 10.1159/000350949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riley KE, Kalichman S. Mindfulness-based stress reduction for people living with HIV/AIDS: preliminary review of intervention trial methodologies and findings. Health Psychol Rev 2015;9(2):224–243. doi: 10.1080/17437199.2014.895928 [DOI] [PubMed] [Google Scholar]
- 32.Rouleau CR, Garland SN, Carlson LE. The impact of mindfulness-based interventions on symptom burden, positive psychological outcomes, and biomarkers in cancer patients. Cancer Manag Res 2015;7:121–131. doi: 10.2147/CMAR.S64165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott-Sheldon LAJ, Balletto BL, Donahue ML, et al. Mindfulness-Based Interventions for Adults Living with HIV/AIDS: A Systematic Review and Meta-analysis. AIDS Behav Published online July 27, 2018:1–16. doi: 10.1007/s10461-018-2236-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gayner B, Esplen MJ, DeRoche P, et al. A randomized controlled trial of mindfulness-based stress reduction to manage affective symptoms and improve quality of life in gay men living with HIV. J Behav Med 2012;35(3):272–285. doi: 10.1007/s10865-011-9350-8 [DOI] [PubMed] [Google Scholar]
- 35.Haller H, Winkler MM, Klose P, Dobos G, Kümmel S, Cramer H. Mindfulness-based interventions for women with breast cancer: an updated systematic review and meta-analysis. Acta Oncol Stockh Swed 2017;56(12):1665–1676. doi: 10.1080/0284186X.2017.1342862 [DOI] [PubMed] [Google Scholar]
- 36.Wells RE, Kerr C, Dossett ML, et al. Can Adults with Mild Cognitive Impairment Build Cognitive Reserve and Learn Mindfulness Meditation? Qualitative Theme Analyses from a Small Pilot Study. J Alzheimers Dis 2019;70(3):825–842. doi: 10.3233/JAD-190191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azulay J, Smart CM, Mott T, Cicerone KD. A pilot study examining the effect of mindfulness-based stress reduction on symptoms of chronic mild traumatic brain injury/postconcussive syndrome. J Head Trauma Rehabil 2013;28(4):323–331. doi: 10.1097/HTR.0b013e318250ebda [DOI] [PubMed] [Google Scholar]
- 38.Farb NAS, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Soc Cogn Affect Neurosci 2013;8(1):15–26. doi: 10.1093/scan/nss066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farb NAS, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci 2007;2(4):313–322. doi: 10.1093/scan/nsm030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gotink RA, Meijboom R, Vernooij MW, Smits M, Hunink MGM. 8-week Mindfulness Based Stress Reduction induces brain changes similar to traditional long-term meditation practice - A systematic review. Brain Cogn 2016;108:32–41. doi: 10.1016/j.bandc.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 41.Hölzel BK, Ott U, Gard T, et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc Cogn Affect Neurosci 2008;3(1):55–61. doi: 10.1093/scan/nsm038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jensen CG, Vangkilde S, Frokjaer V, Hasselbalch SG. Mindfulness training affects attention--or is it attentional effort? J Exp Psychol Gen 2012;141(1):106–123. doi: 10.1037/a0024931 [DOI] [PubMed] [Google Scholar]
- 43.Jha AP, Denkova E, Zanesco AP, Witkin JE, Rooks J, Rogers SL. Does mindfulness training help working memory ‘work’ better? Curr Opin Psychol 2019;28:273–278. doi: 10.1016/j.copsyc.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 44.Johansson B, Bjuhr H, Rönnbäck L. Mindfulness-based stress reduction (MBSR) improves long-term mental fatigue after stroke or traumatic brain injury. Brain Inj 2012;26(13–14):1621–1628. doi: 10.3109/02699052.2012.700082 [DOI] [PubMed] [Google Scholar]
- 45.Singleton O, Hölzel BK, Vangel M, Brach N, Carmody J, Lazar SW. Change in Brainstem Gray Matter Concentration Following a Mindfulness-Based Intervention is Correlated with Improvement in Psychological Well-Being. Front Hum Neurosci 2014;8:33. doi: 10.3389/fnhum.2014.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness Meditation Training and Executive Control Network Resting State Functional Connectivity: A Randomized Controlled Trial. Psychosom Med 2017;79(6):674–683. doi: 10.1097/PSY.0000000000000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang JH, Jung WH, Kang D-H, et al. Increased default mode network connectivity associated with meditation. Neurosci Lett 2011;487(3):358–362. doi: 10.1016/j.neulet.2010.10.056 [DOI] [PubMed] [Google Scholar]
- 48.Kilpatrick LA, Suyenobu BY, Smith SR, et al. Impact of Mindfulness-Based Stress Reduction training on intrinsic brain connectivity. NeuroImage 2011;56(1):290–298. doi: 10.1016/j.neuroimage.2011.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wells RE, Yeh GY, Kerr CE, et al. Meditation’s impact on default mode network and hippocampus in mild cognitive impairment: a pilot study. Neurosci Lett 2013;556:15–19. doi: 10.1016/j.neulet.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80(13):1186–1193. doi: 10.1212/WNL.0b013e318288792b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guha A, Wang L, Tanenbaum A, et al. Intrinsic network connectivity abnormalities in HIV-infected individuals over age 60. J Neurovirol 2016;22(1):80–87. doi: 10.1007/s13365-015-0370-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uchino BN. Social support and health: A review of physiological processes potentially underlying links to disease outcomes. J Behav Med 2006;29(4):377–387. doi: 10.1007/s10865-006-9056-5 [DOI] [PubMed] [Google Scholar]
- 53.Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull 1996;119(3):488–531. doi: 10.1037/0033-2909.119.3.488 [DOI] [PubMed] [Google Scholar]
- 54.Kelly ME, Duff H, Kelly S, et al. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: a systematic review. Syst Rev 2017;6(1):259. doi: 10.1186/s13643-017-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amieva H, Stoykova R, Matharan F, Helmer C, Antonucci TC, Dartigues J-F. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med 2010;72(9):905–911. doi: 10.1097/PSY.0b013e3181f5e121 [DOI] [PubMed] [Google Scholar]
- 56.Ekwall AK, Sivberg B, Hallberg IR. Loneliness as a predictor of quality of life among older caregivers. J Adv Nurs 2005;49(1):23–32. doi: 10.1111/j.1365-2648.2004.03260.x [DOI] [PubMed] [Google Scholar]
- 57.Perissinotto CM, Stijacic Cenzer I, Covinsky KE. Loneliness in older persons: a predictor of functional decline and death. Arch Intern Med 2012;172(14):1078–1083. doi: 10.1001/archinternmed.2012.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green G Social support and HIV. AIDS Care. 1993;5(1):87–104. doi: 10.1080/09540129308258587 [DOI] [PubMed] [Google Scholar]
- 59.Knowlton A, Hua W, Latkin C. Social support among HIV positive injection drug users: implications to integrated intervention for HIV positives. AIDS Behav 2004;8(4):357–363. doi: 10.1007/s10461-004-7320-7 [DOI] [PubMed] [Google Scholar]
- 60.Qiao S, Li X, Stanton B. Social Support and HIV-related Risk Behaviors: A Systematic Review of the Global Literature. AIDS Behav 2014;18(2):419–441. doi: 10.1007/s10461-013-0561-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chelune GJ, Lehman RAW. Neuropsychological and personality correlates of patients’ complaints of disability In: Goldstein G, Tarter RE, eds. Advances in Clinical Neuropsychology. Plenum Press; 1986:95–118. [Google Scholar]
- 62.Milanini B, Catella S, Perkovich B, et al. Psychiatric symptom burden in older people living with HIV with and without cognitive impairment: the UCSF HIV over 60 cohort study. AIDS Care. 2017;29(9):1178–1185. doi: 10.1080/09540121.2017.1281877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Second Edition. Adult Version. Manual Psychological Corporation; 2000. [Google Scholar]
- 64.Possin KL, Laluz VR, Alcantar OZ, Miller BL, Kramer JH. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer’s disease and behavioral variant frontotemporal dementia. Neuropsychologia. 2011;49(1):43–48. doi: 10.1016/j.neuropsychologia.2010.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mack WJ, Freed DM, Williams BW, Henderson VW. Boston Naming Test: shortened versions for use in Alzheimer’s disease. J Gerontol 1992;47(3):P154–158. doi: 10.1093/geronj/47.3.p154 [DOI] [PubMed] [Google Scholar]
- 66.UCSF Memory & Aging Center Normative Data.
- 67.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS). The Psychological Corporation; 2001. [Google Scholar]
- 69.Froming KB, Levy M, Schaffer SG, Ekman P. The Comprehensive Affect Testing System. Psychology Software, Inc.; 2006. [Google Scholar]
- 70.Matthews C, Klove H. Instruction Manual for the Adult Neuropsychology Test Battery. University of Wisconsin Medical School; 1964. [Google Scholar]
- 71.Heaton RK, Miller SW, Taylor MJ, Grant-Isibor I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Published online January 1, 2004. Accessed February 14, 2020 https://www.scienceopen.com/document?vid=6df6d828-eaad-4a57-8405-063033bebbd8
- 72.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 73.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged: The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963;185(12):914–919. doi: 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 74.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Nurs Res 1970;19(3):278. [PubMed] [Google Scholar]
- 75.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kabat-Zinn J Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. Delta trade pbk. reissue.. Delta Trade Paperbacks; 2005. [Google Scholar]
- 77.Rosvold HE, Mirsky AF, Sarason I, Bransome ED Jr., Beck LH. A continuous performance test of brain damage. J Consult Psychol 1956;20(5):343–350. doi: 10.1037/h0043220 [DOI] [PubMed] [Google Scholar]
- 78.Smith A Symbol Digit Modalities Test. Published online 1973.
- 79.Wechsler D Wechsler adult intelligence scale - Fourth Edition (WAIS-IV). Published online 2008.
- 80.Cohen S Perceived stress in a probability sample of the United States In: The Social Psychology of Health. The Claremont Symposium on Applied Social Psychology. Sage Publications, Inc; 1988:31–67. [Google Scholar]
- 81.Spielberger CD. State-Trait Anxiety Inventory for Adults. Published online 1983. doi: 10.1037/t06496-000 [DOI] [Google Scholar]
- 82.Brink TL, Yesavage JA, Lum O, Heersema PH, Adey M, Rose TL. Screening Tests for Geriatric Depression. Clin Gerontol 1982;1(1):37–43. doi: 10.1300/J018v01n01_06 [DOI] [PubMed] [Google Scholar]
- 83.WHOQOL-HIV Group. Preliminary development of the World Health Organsiation’s Quality of Life HIV instrument (WHOQOL-HIV): analysis of the pilot version. Soc Sci Med 2003;57(7):1259–1275. doi: 10.1016/S0277-9536(02)00506-3 [DOI] [PubMed] [Google Scholar]
- 84.Buss AH, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol 1957;21(4):343–349. doi: 10.1037/h0046900 [DOI] [PubMed] [Google Scholar]
- 85.Moore SR, Gresham LS, Bromberg MB, Kasarkis EJ, Smith RA. A self report measure of affective lability. J Neurol Neurosurg Psychiatry 1997;63(1):89–93. doi: 10.1136/jnnp.63.1.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Larsen RJ, Diener E. A multitrait-multimethod examination of affect structure: hedonic level and emotional intensity. Personal Individ Differ 1985;6(5):631–636. doi: 10.1016/0191-8869(85)90013-3 [DOI] [Google Scholar]
- 87.Norbeck JS, Lindsey AM, Carrieri VL. The development of an instrument to measure social support. Nurs Res 1981;30(5):264–269. doi: 10.1097/00006199-198109000-00003 [DOI] [PubMed] [Google Scholar]
- 88.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1982. 1991;32(6):705–714. [DOI] [PubMed] [Google Scholar]
- 89.Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66(1):20–40. doi: 10.1207/s15327752jpa6601_2 [DOI] [PubMed] [Google Scholar]
- 90.Fredrickson BL. Positive Emotions Broaden and Build In: Devine P, Plant A, eds. Advances in Experimental Social Psychology. Vol 47 Academic Press; 2013:1–53. doi: 10.1016/B978-0-12-407236-7.00001-2 [DOI] [Google Scholar]
- 91.Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using Self-Report Assessment Methods to Explore Facets of Mindfulness. Assessment. 2006;13(1):27–45. doi: 10.1177/1073191105283504 [DOI] [PubMed] [Google Scholar]
- 92.Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol 2003;84(4):822–848. doi: 10.1037/0022-3514.84.4.822 [DOI] [PubMed] [Google Scholar]
- 93.Brier MR, Thomas JB, Snyder AZ, et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. J Neurosci Off J Soc Neurosci 2012;32(26):8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shulman GL, Pope DLW, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci Off J Soc Neurosci 2010;30(10):3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tukey JW. Some thoughts on clinical trials, especially problems of multiplicity. Science. 1977;198(4318):679–684. doi: 10.1126/science.333584 [DOI] [PubMed] [Google Scholar]
- 96.Caniglia EC, Cain LE, Justice A, et al. Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology. 2014;83(2):134–141. doi: 10.1212/WNL.0000000000000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Agresti A An Introduction to Categorical Data Analysis. 2nd ed. Wiley Inter-Science; 2007. [Google Scholar]
- 98.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Second edition. Springer; 2015. [Google Scholar]
- 99.Little RJA, Rubin DB. Statistical Analysis with Missing Data. John Wiley & Sons; 2019. [Google Scholar]
- 100.Cysique LA, Maruff P, Brew BJ. Variable benefit in neuropsychological function in HIV-infected HAART-treated patients. Neurology. 2006;66(9):1447–1450. doi: 10.1212/01.wnl.0000210477.63851.d3 [DOI] [PubMed] [Google Scholar]
- 101.Lao S-A, Kissane D, Meadows G. Cognitive effects of MBSR/MBCT: A systematic review of neuropsychological outcomes. Conscious Cogn 2016;45:109–123. doi: 10.1016/j.concog.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 102.Vago DR, Gupta RS, Lazar SW. Measuring cognitive outcomes in mindfulness-based intervention research: a reflection on confounding factors and methodological limitations. Curr Opin Psychol 2019;28:143–150. doi: 10.1016/j.copsyc.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gallegos AM, Hoerger M, Talbot NL, et al. Toward identifying the effects of the specific components of Mindfulness-Based Stress Reduction on biologic and emotional outcomes among older adults. J Altern Complement Med N Y N 2013;19(10):787–792. doi: 10.1089/acm.2012.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]


