Abstract
Recent outbreaks of Zika virus (ZIKV) have been associated with birth defects, including microcephaly and neurological impairment. However, the mechanisms which confer potential susceptibility to ZIKV during pregnancy remain unclear. We hypothesized that poor outcomes from ZIKV infection during pregnancy are due in part to pregnancy-induced alteration of innate immune cell frequencies and cytokine expression. To examine the impact of pregnancy on innate immune responses, we inoculated immunocompetent pregnant and non-pregnant female C57BL/6 mice with 5×105 FFU of ZIKV intravaginally. Innate immune cell frequencies and cytokine expression were measured by flow cytometry at day 3 post infection. Compared to non-pregnant mice, pregnant mice exhibited higher frequencies of uterine macrophages (CD68+) and CD11c+ CD103+ and CD11c+ CD11b+ dendritic cells. Additionally, ZIKV-infected pregnant mice had lower frequencies of CD45+ IL-12+ and CD11b+ IL-12+ cells in the uterus and spleen. Next, we measured the frequencies of antigen-experienced CD4 (CD4+ CD11a+ CD49d+) and CD8 (CD8lo CD11ahi) T cells at day 10 post-infection to determine the impact of pregnancy-associated changes in innate cellular IL-12 responses on the adaptive immune response. We found that pregnant mice had lower frequencies of uterine antigen-experienced CD4 T cells and ZIKV-infected pregnant mice had lower frequencies of uterine antigen-experienced CD8 T cells compared to ZIKV-infected non-pregnant mice. These data show that pregnancy results in altered innate and adaptive immune responses to ZIKV infection in the reproductive tract of mice and that pregnancy-associated immune modulation may play an important role in the severity of acute ZIKV infection.
Introduction
Zika virus (ZIKV) is a neurotropic flavivirus originally isolated from a febrile rhesus macaque in the Zika Forest of Uganda (1). Although ZIKV was first identified in humans in 1952, only sporadic infections occurred in humans until 2007, when the first major outbreak was reported on Yap Island (2). Since its emergence, infections have been reported in Africa, Asia, the Pacific Islands, and the Americas. ZIKV is transmitted to humans predominantly through bites from Aedes mosquitos, but infections after sexual contact and blood transfusions have also been reported (3, 4). The majority of infected individuals are asymptomatic, with some infections causing mild symptoms such as fever, rash, conjunctivitis, muscle and joint pain, malaise, and headache (5). However, ZIKV infections in French Polynesia in 2013 and 2014 were linked to an increase in Guillain-Barré syndrome in adults (6). In 2015, a ZIKV outbreak in Brazil was associated with a marked increase in microcephaly in infants born to acutely infected mothers (7–9). Other reports also show that ZIKV infection in fetuses may cause a spectrum of disease from severe microcephaly to more subtle brain and developmental abnormalities together referred to as congenital ZIKV syndrome (8, 10–12). Multiple studies have provided additional evidence of vertical transmission of ZIKV from infected pregnant mothers to the fetus (9, 13). Vertical transmission of ZIKV often occurs following periods of prolonged maternal viremia, and this is supported by data from both human studies and nonhuman primate models of congenital ZIKV infection (14, 15).
The mechanisms underlying the increased severity of ZIKV infection during pregnancy remain understudied. During pregnancy, women are at increased risk for infection and increased severity of infection with ZIKV and several other pathogens, including listeria, cytomegalovirus (CMV), herpes simplex virus (HSV), influenza virus, and HIV (16–21). In general, successful pregnancy relies on tolerance of the maternal immune system towards the semi-allogeneic fetus, which is often referred to as immunotolerance. This results in changes at multiple levels of the maternal immune system. For example, human natural killer (NK) cells lose their cytotoxic abilities and instead take on a supportive role during pregnancy (22). Additionally, the decidua contains an abundance of regulatory T cells (Tregs) during early pregnancy, which maintain tolerance, prevent inflammation, and promote implantation of the embryo (23–25). Many of these changes are linked to the induction of pregnancy hormones. Several studies have suggested that human chorionic gonadotropin (HCG) plays a role in the recruitment of Tregs to the maternal-fetal interface and promotes the generation of tolerogenic dendritic cells (DCs) (26, 27). These pregnancy-induced immune changes impact susceptibility to several pathogens. For example, changes in the immune response during pregnancy result in progesterone-dependent increased susceptibility to HSV2 in the genital tract of mice, resulting in lower HSV2-specific IgG and IgA responses in the genital tract following infection (28). Additionally, influenza infection in pregnant ferrets results in decreased total CD8+ T-cells and decreased H1N1-specific B-cell responses compared to non-pregnant ferrets (29).
While different stages of pregnancy clearly modulate adaptive immune responses, less is known about pregnancy-induced innate immune responses during viral infection. In a pregnant mouse model of influenza infection, Cox-2, PGE2, and PGF2α were increased, resulting in remodeling of the placental architecture, preterm labor, impaired fetal growth, and increased fetal and maternal mortality and morbidity (18). In another study of late stage pregnancy, viral infection of the placenta triggered an inflammatory response, including the secretion of IL-1, IL-6, IL-8, and TNFα, and fetal abnormalities in the absence of direct fetal infection (30, 31). Additionally, a study of ZIKV-infected mothers reported the presence of interferon gamma-inducible protein-10 (IP-10), IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), vascular endothelial growth factor (VEGF), and granulocyte-colony stimulating factor (G-CSF) in the amniotic fluid of mothers whose infants were born with microcephaly (32). Another study found that IP-10, CCL5, IL-9, interferon gamma (IFNγ), IL-7, IL-5, and IL-1ra were upregulated in the plasma of acute ZIKV-infected individuals compared to healthy donors (33). In the recovery phase, IL-12p70 and basic fibroblast growth factor (FGF) were found to be upregulated (33). However, it is unclear how the immune response to ZIKV is impacted by pregnancy, especially in early pregnancy.
Few studies have examined the effect of pregnancy on innate immune responses in the reproductive tract at the early stages of pregnancy just after embryo implantation. Previous studies have shown that ZIKV infections in the first trimester of pregnancy confer a greater risk of microcephaly compared to second and third trimester infections (34). Since the severity of ZIKV congenital disease increases with infection during early stages of pregnancy, we examined pregnancy-associated changes in the innate immune response during early pregnancy. In order to evaluate the innate immune cellular response in the reproductive tract, we utilized an immune competent murine model of ZIKV intravaginal inoculation which has been described previously (35). Following intravaginal inoculation of ZIKV at embryonic day 4.5 (E4.5), we found that pregnant mice exhibited increased frequencies of CD11c+ CD103+ and CD11c+ CD11b+ DCs in the uterus and a higher frequency of uterine macrophages (CD68+) compared to ZIKV-inoculated non-pregnant mice at day 3 post-infection (E7.5). We next found that ZIKV-infected pregnant mice exhibited significantly lower frequencies of CD45+ IL-12+ cells and CD11b+ IL-12+ in the uterus and spleen. We next determined the impact of pregnancy-induced decreases of IL-12 expression on the adaptive immune system by measuring the frequencies of total CD4 and CD8 T cells and antigen-experienced CD4 (CD4+ CD11a+ CD49d+) and CD8 (CD8lo CD11ahi) T cells at day 10 post-infection. While ZIKV-infected mice had higher frequencies of uterine CD8+ T cells compared to mock-infected mice, ZIKV-infected pregnant mice had lower frequencies of antigen-experienced CD8+ T cells compared to infected non-pregnant mice. Additionally, pregnant mice had lower frequencies of antigen-experienced CD4+ T cells. Taken together, these results suggest that pregnancy alters the local innate and adaptive immune responses to ZIKV infection, which may decrease immune control of acute viral infection.
Materials and Methods
Ethics Statement
All animal research was approved by the University of Colorado and Denver VAMC local Institutional Animal Care and Use Committees (approval number 1098v3). All laws and regulations regarding animal care and euthanasia were followed according to guidelines from the PHS/NIH/OLAW policy, Animal Care Policy (USDA), and the AVMA guidelines on euthanasia.
Virus propagation and cell culture
Vero cells (ATCC, Manassas, VA) and C6/36 cells (ATCC) were cultured at 37°C and 5% CO2 in complete Minimal Essential Media (MEM) supplemented with 10% fetal bovine serum (FBS, HyClone, Thermo Fisher Scientific, Waltham, MA). ZIKV strain PRVABC59 (GenBank: KU501215) was provided by the Centers for Disease Control (CDC, Atlanta, GA). ZIKV stocks were propagated in Vero cells at passage 4 and C6/36 cells at passage 1, and cell culture supernatants were harvested at 6 days post infection. Virus stocks were titrated in Vero cells using a focus forming assay (FFA) and were aliquoted and stored at −80°C.
Mice
Six-week-old C57BL/6J (stock no. 000664) male and female mice were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were housed in a pathogen-free animal facility at the University of Colorado Anschutz Medical Campus (Aurora, CO) and maintained on a 12:12 light/dark cycle at 21–24°C. Eight-week-old female mice were mated with male mice ranging from 8–30-weeks-old. While mating, each mating pair was housed separately. Otherwise, 5 female mice were housed together. 10–20 female mice were used per experiment.
Hormone Treatment
To increase the likelihood of pregnancy in the mice, female mice were treated with exogenous gonadotropins to increase ovulation (36). Two days before mating, female mice were injected intraperitoneally with 2.5 iu of Pregnant Mare Serum Gonadotropin (bioWORLD, Dublin, OH). 48 hours later, they were intraperitoneally injected with 2.5 iu human chorionic gonadotropin (HCG, Sigma Aldrich, St. Louis, MO) and immediately mated with male mice overnight (16 hours). The males and females were separated the following morning (E0.5).
Zika virus infection
On day E4.5, eight-week-old pregnant and non-pregnant female mice were randomly assigned to either the mock or ZIKV infection groups. The mice were anaesthetized with isoflurane (McKesson Corporation, Irving, TX) and infected intravaginally with 5×105 FFU of PRVABC59 ZIKV in 15 μL of HBSS (Gibco, Thermo Fisher). Mock-infected mice received 15 μL of HBSS intravaginally. Spleen and uterine tissues were harvested for flow cytometry at 3 days post infection (E7.5) for analysis of innate immune cells (macrophages, dendritic cells) or 10 days post infection (E14.5) for analysis of adaptive immune cells (T cells).
Vaginal lavages
Vaginal lavages were performed 48 hours post infection. Mice were anaesthetized with isoflurane, and 50 μL of sterile phosphate buffered saline (PBS, Corning, Corning, NY) was inserted into the urogenital tract using a micropipette. The liquid was expelled slowly into the urogenital tract and then drawn back up and mixed with 200 μL of sterile PBS supplemented with 1% FBS. The samples were vortexed for 30 seconds and then aliquoted and stored at −80°C.
RNA extraction and qPCR
ZIKV RNA was isolated from the vaginal lavage samples using the E.Z.N.A. Viral RNA Kit (Omega Bio-tek, Norcross, GA) according to the manufacturer’s instructions. The primer and probe set Zika1087/1108FAM/1163c (IDT, Coralville, Iowa) was used to detect viral RNA. Real-time qPCR was performed using the Luna Universal Probe qPCR Master Mix (New England Biolabs, Ipswich, MA) with amplification on the Biorad CFX96 Real Time PCR Detection system, both per the manufacturer’s instructions. The sensitivity of this assay was evaluated by testing known dilutions of an RNA transcript copy of the ZIKV P1 plasmid. Concentration of viral RNA (copies/microliter) was calculated using the standard curve generated by the CFX96 instrument.
Tissue processing
Spleen and uterine tissues were dissected and placed in Eppendorf or conical tubes containing R10 media (RPMI with L-glutamine (Corning) + 10% FBS + 1% Penicillin/Streptomycin (Corning) + 1% HEPES (Gibco) + 1% Sodium Pyruvate (Gibco) + 1% MEM Non-essential amino acids (MEM-NEAA, Gibco)) on ice before being processed. Spleen tissues were processed into single-cell suspensions by mechanical dissociation; tissues were crushed through a 70 μm cell strainer (CELLTREAT, Pepperell, MA) using disposable plastic pestles (CELLTREAT). Red blood cells (RBC) were removed by incubating the cell suspensions in 5 mL 1X RBC Lysis Buffer (eBioscience, Thermo Fisher) for 5 minutes at room temperature. The cells were then washed in 30 mL of R10 media, vortexed, and centrifuged at 500 rcf.
Before processing, the placenta, decidua, and fetuses were carefully removed from the uterus. The uterine tissues were then enzymatically digested using Liberase TL (Roche, Basel, Switzerland) at a final concentration of 160 μg/mL in HBSS (Gibco, Thermo Fisher). First, each tissue was suspended in 500 μL of cold liberase + HBSS in a 1.5 mL Eppendorf tube and mechanically dissociated using small surgical scissors. Next, another 500 μL of Liberase + HBSS was added to each tissue, and the samples were incubated at 37°C for 35 minutes with occasional vortexing. Samples were kept on ice in between steps. After incubation at 37°C, the dissociated tissues were filtered through 100 μm cell strainers (CELLTREAT).
After preparation of single-cell suspensions, the samples were centrifuged at 500 rcf for 5 minutes, counted using Trypan blue (Corning), and resuspended at a concentration of 1×106 cells/mL in R10 media. The cells were then aliquoted into FACS tubes (0.25–1×106 cells/tube) with strainer caps (BD Biosciences, San Jose, CA).
Flow cytometry
The following antibodies were used for extracellular flow cytometry: anti-mouse CD45 BV650 (clone 30-F11, Biolegend, San Diego, CA), anti-mouse/human CD11b APC-Cy7 (clone M1/70, Biolegend), anti-mouse CD11c PE-eFluor 610 (clone N418, eBioscience), anti-mouse I-A/I-E FITC (clone M5/114.15.2, Biolegend), anti-mouse CD103 BV711 (clone 2E7, Biolegend), anti-mouse Ly6C BV785 (clone HK1.4, Biolegend), anti-mouse CD24 PerCP-eFluor 710 (clone M1/69, eBioscience), anti-mouse CD86 PE-Cy7 (clone GL-1, Tonbo Biosciences, San Diego, CA), anti-mouse F4/80 BUV395 (clone T45–2342, BD Biosciences), anti-mouse CD8a BV711 (clone 53–6.7, Biolegend), anti-mouse CD4 PE-Cy5.5 (clone RM4–5, eBioscience), anti-mouse CD25 BUV395 (clone PC61, BD Biosciences), anti-mouse CD69 FITC (clone H1.2F3, Tonbo Biosciences), anti-mouse CD11a PE-Cy7 (clone M17/4, Biolegend), anti-mouse CD49d PE Dazzle 594 (clone R1–2, Biolegend), and anti-mouse NK1.1 APC (clone PK136, BD Biosciences). The following antibodies were used for intracellular flow cytometry: anti-mouse CD68 PE-Cy7 (clone FA-11, Biolegend), anti-mouse CD3 BUV395 (clone 145–2C11, BD Biosciences), anti-mouse CD3 BV785 (clone 17A2, Biolegend), anti-mouse IL-12 (p40/p70) PE (clone C15.6, BD Biosciences), anti-mouse IL-6 APC (clone MP5–20F3, BD Biosciences), anti-mouse IL-10 APC (clone JES5–16E3, Biolegend), anti-mouse FoxP3 BV421 (clone MF-14, Biolegend), anti-mouse CD3 BV785 (clone 17A2, Biolegend), anti-mouse interferon gamma PE (clone XMG1.2, Biolegend), and anti-mouse TNF-alpha APC-Cy7 (clone MP6-XT22, Biolegend). Ghost Violet 510 dye (Tonbo Biosciences) was used to assess viability.
Single cell suspensions were washed in PBS, centrifuged at 500 rcf for 5 minutes, and briefly vortexed. Next, 10 μL of viability dye (0.1 μL dye + 10 uL FACS buffer (1% FBS in PBS) per sample) was added to each sample, vortexed, and incubated at room temperature for 10 minutes. Next, 50 μL of extracellular antibodies prepared in FACS buffer were added to each sample, vortexed, and incubated at 4°C for 25 minutes. 210 μL of Cytofix/Cytoperm solution (BD Biosciences) per sample was then added to permeabilize the cells, followed by vortexing and incubation for 20 minutes at 4°C. When staining for FoxP3, permeabilization was instead performed using the FoxP3/transcription factor fixation/permeabilization kit (eBioscience) per the manufacturer’s instructions. The cells were then washed with 1 mL of Flow Cytometry Perm Buffer (Tonbo Biosciences) or FoxP3 permeabilization buffer (eBioscience, for FoxP3 staining) twice, centrifuged at 700 rcf for 5 minutes, and vortexed. Next, 50 μL of intracellular antibodies in perm buffer or FoxP3 buffer were added to each sample, vortexed, and incubated at 4°C of 45 minutes. The samples were then washed once more in Perm or FoxP3 buffer, centrifuged at 700 rcf for 5 minutes, vortexed, and finally fixed in 1% paraformaldehyde (Thermo Fisher).
The data was acquired on a LSRII flow cytometer (BD) using voltages standardized according to previously published methods (37). FlowJo software (FlowJo, LLC, Ashland, Oregon) was used to analyze the data. Cells were first gated to eliminate doublets and debris (FSC-H vs FSC-A), followed by elimination of dead cells (selection of cells negative for viability dye), and selection of CD45+ cells. CD− and CD3+ cells were then gated for further analysis of innate immune cells (macrophages, monocytes, and dendritic cells) and T cells, respectively. Since unequal numbers of cells were obtained for different tissues and between different experimental groups, the flow cytometry data are presented as frequencies (percentages).
Statistics
All statistical analysis was performed in Prism software (GraphPad, San Diego, CA), versions 7 and 8. One-way ANOVA and Tukey’s multiple comparison tests (indicated with * in the figures) were used to compare cell frequencies between pregnant and non-pregnant mock and ZIKV-infected mice when the data were parametric, and the Kruskal-Wallis test with Dunn’s multiple comparison test was used for non-parametric data (indicated with + in the figures). T tests were used when only two groups were compared. While all the treatment groups were compared to one another, only the statistically significant comparisons between the mock non-pregnant and mock pregnant, ZIKV non-pregnant and ZIKV pregnant, and mock pregnant and ZIKV pregnant groups are shown in the figures. Due to high variability in the data, the ROUT method was used to identify outliers in the data presented in Figures 6 and 7 using a Q value of 1%. All the data are presented as mean ± standard deviation. P<0.05 was considered statistically significant. All data shown represent 2–3 experiments of 10–20 mice each (n=38–40 mice total for each parameter measured).
Fig 6. Frequencies of uterine and splenic CD4+ and CD8+ T cells during pregnancy and ZIKV-infection.
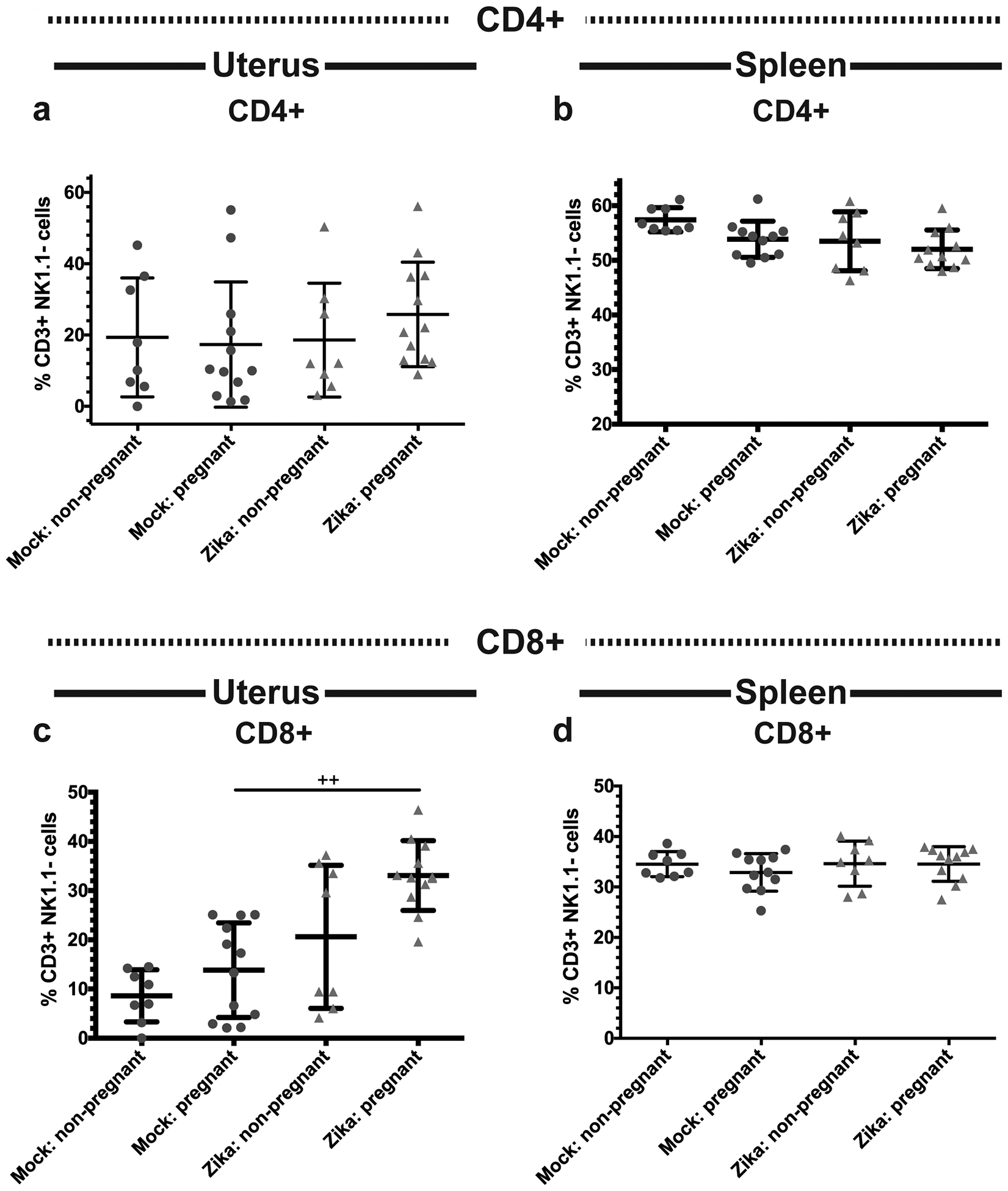
Flow cytometry was performed on the uterine (a,c) and splenic (b,d) immune cells of ZIKV-infected (red triangles) and mock-infected (blue circles) pregnant and non-pregnant mice at day 10 post infection. Frequencies of CD4+ (a-b) and CD8+ (c-d) T cells were measured. *p<0.05, one-way ANOVA and Tukey’s multiple comparisons test; ++p<0.01, Kruskal-Wallis test and Dunn’s multiple comparisons test. N=40 (3 independent experiments of 10 or 20 mice, N=8 mock non-pregnant, N=12 mock pregnant, N=8 ZIKV non-pregnant, N=12 ZIKV pregnant). Error bars represent the mean ± standard deviation.
Fig 7. Pregnancy alters the frequencies of antigen-experienced T cells.
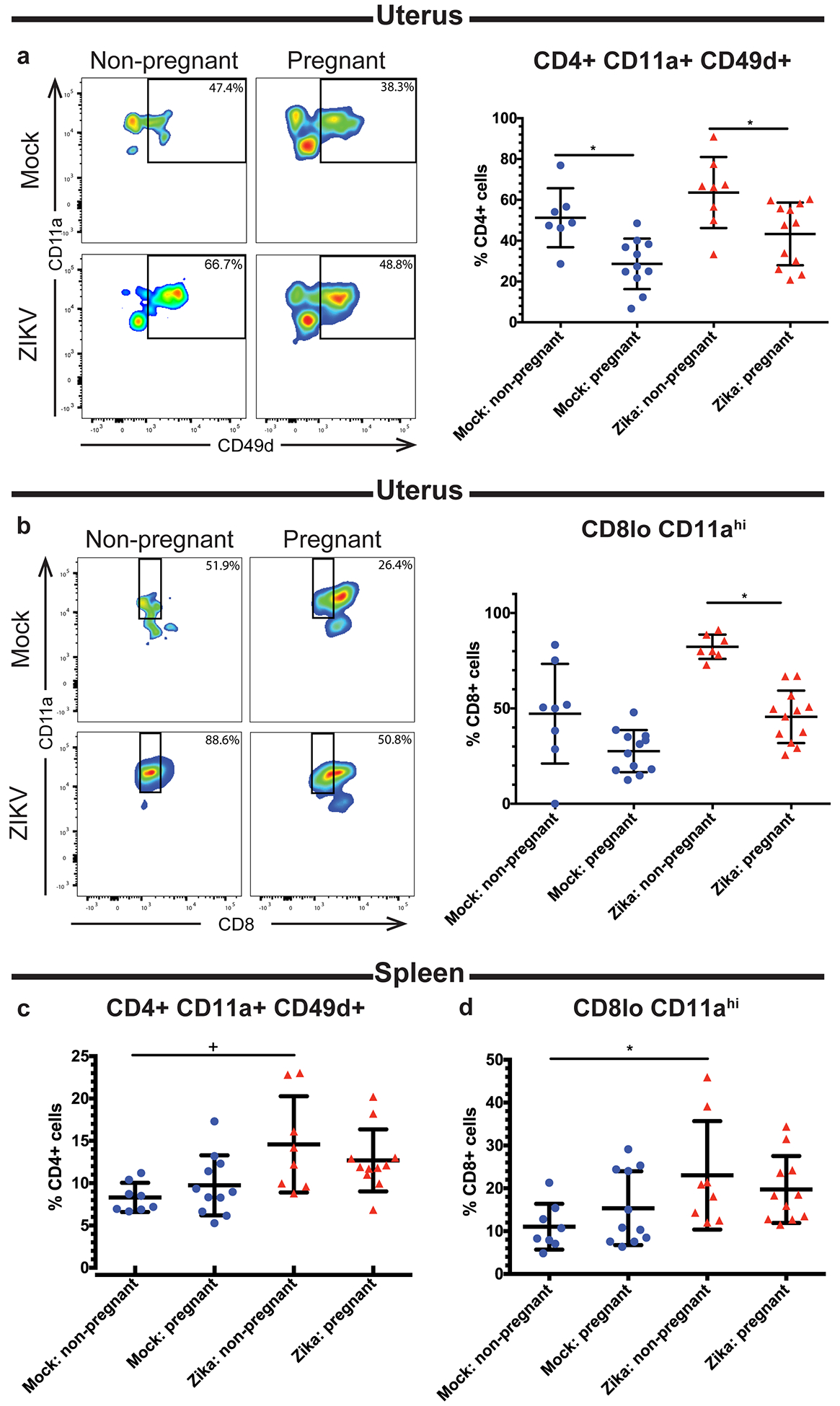
Flow cytometry was performed on the uterine (a-b) and splenic (c-d) immune cells of ZIKV-infected (red triangles) and mock-infected (blue circles) pregnant and non-pregnant mice at day 10 post infection. Frequencies of CD4+ CD11a+ CD49d+ (a,c) and CD8lo CD11ahi (b,d) T cells were measured. Representative flow plots of CD4+ CD11a+ CD49d+ and CD8lo CD11ahi cells are shown in (a) and (b), respectively. *p<0.05 one-way ANOVA and Tukey’s multiple comparisons test; +p<0.05 Kruskal-Wallis with Dunn’s multiple comparisons test. N=40 (3 independent experiments of 10 or 20 mice, N=8 mock non-pregnant, N=12 mock pregnant, N=8 ZIKV non-pregnant, N=12 ZIKV pregnant). Error bars represent the mean ± standard deviation.
Results
Intravaginal ZIKV infection in C57BL/6 mice
To generate a mouse model of ZIKV infection during pregnancy, 8-week-old, female C57BL/6 mice were injected with 2.5 international units (iu) pregnant mare serum gonadotropin (PMSG) by intraperitoneal (ip) inoculation, ip injected with 2.5 iu human chorionic gonadotropin (HCG) 48 hours later, and mated with male mice overnight (16 hours). The males and females were separated the following morning (E0.5). At E4.5, the female mice were inoculated with 5×105 FFU of ZIKV (PRVABC59) or mock inoculum. Intravaginal washes and tissue harvests for analysis of virus and immune parameters, respectively, were performed at specific time points post-infection (Fig. 1a). Pregnancy rates ranged from 30–80% with this approach, allowing for prospective cohort analysis of both pregnant and non-pregnant mice that were treated at the same time with the same hormones prior to ZIKV infection. At 48 hours post-infection, ZIKV PCR of vaginal wash fluid revealed evidence of ZIKV RNA in the genital tract of both pregnant and non-pregnant mice (Fig. 1b). ZIKV RNA levels peaked at day 2 post-infection, and the levels of ZIKV RNA did not differ significantly between the pregnant and non-pregnant mice (p=0.2601). Additionally, the mice were weighed every other day beginning at the date of infection (day 0, E4.5). The weights of the mice in each experimental group did not differ significantly until day 8 (E12.5), where the mock and ZIKV-infected pregnant groups weighed significantly more than the mock non-pregnant group (p<0.05, Fig. 1c). The ZIKV non-pregnant group did not differ significantly in weight from the mock non-pregnant group. Lastly, we dissected and counted the numbers of whole fetuses present in each mouse in the mock and ZIKV-infected pregnant groups and found no significant differences (Fig. 1d).
Fig 1. Intravaginal ZIKV infection in C57BL/6 mice.
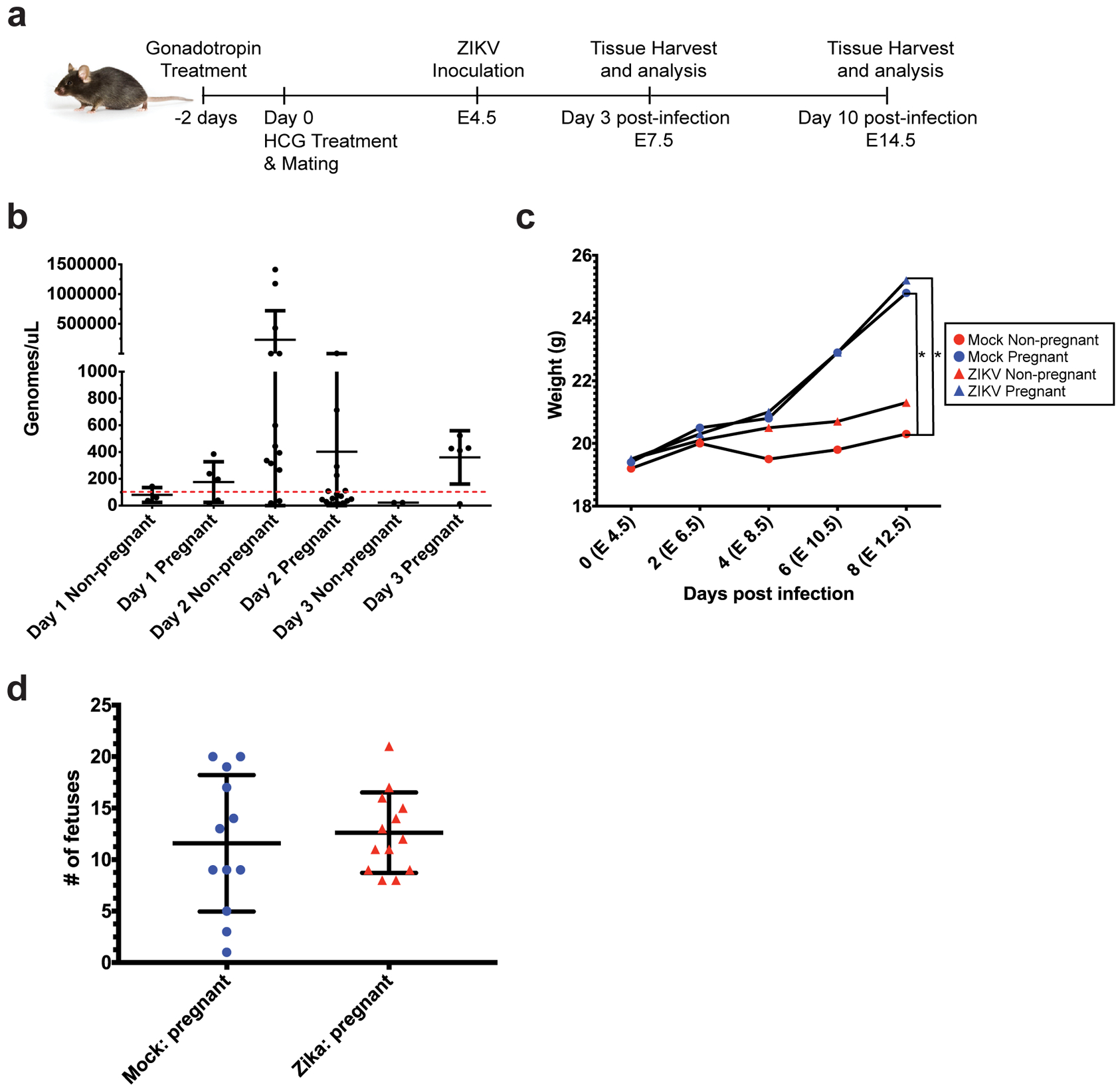
a) Experimental methodology: female C57BL/6 mice were treated with gonadotropins, mated, and then infected intravaginally with ZIKV at embryonic day 4.5 (E4.5). Spleen and uterine tissues were harvested at day 3 post infection (E7.5) or day 10 post-infection (E14.5). b) Vaginal lavages were performed at 24, 48, and 72 hours post-infection, and ZIKV RNA was detected by qPCR. N=80 (4 independent experiments of 20 mice each, only samples with a Cq <36 are shown (N=42 total, N=7 Day 1, N=28 Day 2, N=7 Day 3)) c) Mice in each experimental group were weighed every other day from E4.5 (day of infection) to E12.5 (day 8 post-infection). Average weights for each group are shown. N=39 (2 independent experiments with 10 or 19 mice, N=8 mock non-pregnant, N=12 mock pregnant, N=7 ZIKV non-pregnant, N=12 ZIKV pregnant). *p<0.05 Two-way ANOVA with Tukey’s multiple comparisons. d) Whole fetuses from pregnant ZIKV-infected (red triangles) and mock-infected (blue circles) mice were dissected and counted. Each symbol (triangle or circle) represents one mouse. N=25 mice (12 mock pregnant, 13 ZIKV pregnant, taken from 2 independent experiments). Error bars represent the mean ± standard deviation in all the graphs.
Pregnancy-induced changes in the innate cellular response during ZIKV infection
Next, we analyzed the innate cellular immune responses in the pregnant and non-pregnant mice at 3 days post-infection, immediately following peak ZIKV infection of the reproductive tract. Following mock and ZIKV intravaginal inoculation, non-pregnant and pregnant mice were euthanized at day 3 post-infection (E7.5), and spleen and uterine tissue were analyzed by flow cytometry. We measured the frequencies of cells expressing several markers of innate immune cells, including CD68 (macrophages), CD11b (expressed on monocytes, macrophages, and DCs), CD11c (expressed on DCs, monocytes, macrophages, and granulocytes), and Ly6C (expressed on macrophages, monocytes, and neutrophils). Following infection, both ZIKV-infected and mock-infected pregnant mice exhibited increased frequencies of uterine CD45+ CD68+ macrophages compared to their non-pregnant counterparts (Fig. 2a; p= 0.0055 mock non-pregnant vs. mock pregnant, p= 0.0004, ZIKV non-pregnant vs. ZIKV pregnant). Furthermore, there was no difference in this population between the mock-infected pregnant and ZIKV-infected pregnant groups (p=0.9381). These results suggest that the increase in CD45+ CD68+ cells in the uterus was due to pregnancy rather than infection. In the splenic tissue, ZIKV infection during pregnancy resulted in a significantly increased frequency of CD45+ CD68+ macrophages compared to ZIKV-infected non-pregnant mice (Fig. 2b, p= 0.0095). However, there was no significant difference in the frequency of CD45+ CD68+ macrophages between the mock-inoculated pregnant and non-pregnant mice (p=0.5849) or the mock-inoculated pregnant and ZIKV-infected pregnant mice (p=0.6762), suggesting that both pregnancy and ZIKV infection were necessary to increase splenic CD68+ cell frequencies. In both the spleen and uterus, mock and ZIKV-infected pregnant and non-pregnant mice exhibited similar frequencies of CD45+ CD11b+ cells (Fig. 2c; p= 0.7906 spleen; p= 0.057, uterus). ZIKV-infected pregnant mice exhibited increased frequencies of CD45+ CD11c cells in the spleen compared to ZIKV-infected non-pregnant mice (Fig. 2d, p= 0.0109), but no significant differences were seen when the pregnant and non-pregnant mock-infected mice were compared (p=0.7237). No significant differences in CD45+ CD11c+ cells were seen in the uterus (p= 0.7392). Additionally, ZIKV infection during pregnancy resulted in a significantly decreased frequency of CD45+ Ly6C+ cells in the spleen compared to ZIKV-infected non-pregnant mice (Fig. S1a, p= 0.042). In comparison, mock-inoculated pregnant and non-pregnant mice exhibited no significant changes in the frequency of splenic CD45+ Ly6C+ cells (p= 0.4801). In mice, Ly6C expression in CD11b+ monocytes distinguishes pro-inflammatory monocytes from anti-inflammatory patrolling monocytes which participate in tissue repair, with the pro-inflammatory group having high expression of Ly6C (Ly6C hi) and the anti-inflammatory group having low expression of Ly6C (Ly6C lo). All groups had similar frequencies of splenic CD11b+ Ly6C hi (Fig. S1b, p= 0.5446 mock non-pregnant vs. mock pregnant, p=0.5933 ZIKV non-pregnant vs. ZIKV pregnant, p=0.9148 mock pregnant vs. ZIKV pregnant) and CD11b+ Ly6C lo cells (Fig. S1c, p= 0.2948 mock non-pregnant vs. mock pregnant, p=0.0806 ZIKV non-pregnant vs. ZIKV pregnant, p=0.8819, mock pregnant vs. ZIKV pregnant). These data show that Ly6C expression on CD11b+ monocytes is significantly decreased in the spleens of pregnant mice during acute viral infection but not in the absence of viral infection. However, there were no differences in CD45+ Ly6C+ (p= 0.1022), CD11b+ Ly6C hi (p= 0.1915), or CD11b+ Ly6C lo (p= 0.0794) cells in the uterus between groups. These data suggest that the combined effects of pregnancy and acute ZIKV infection, but not pregnancy or infection alone, alter the frequencies of splenic CD11c+ and total CD11b+ Ly6C+ cells without altering these cell populations in the uterus.
Fig 2. Changes to the immune response in the uterus and spleen during pregnancy and ZIKV infection.
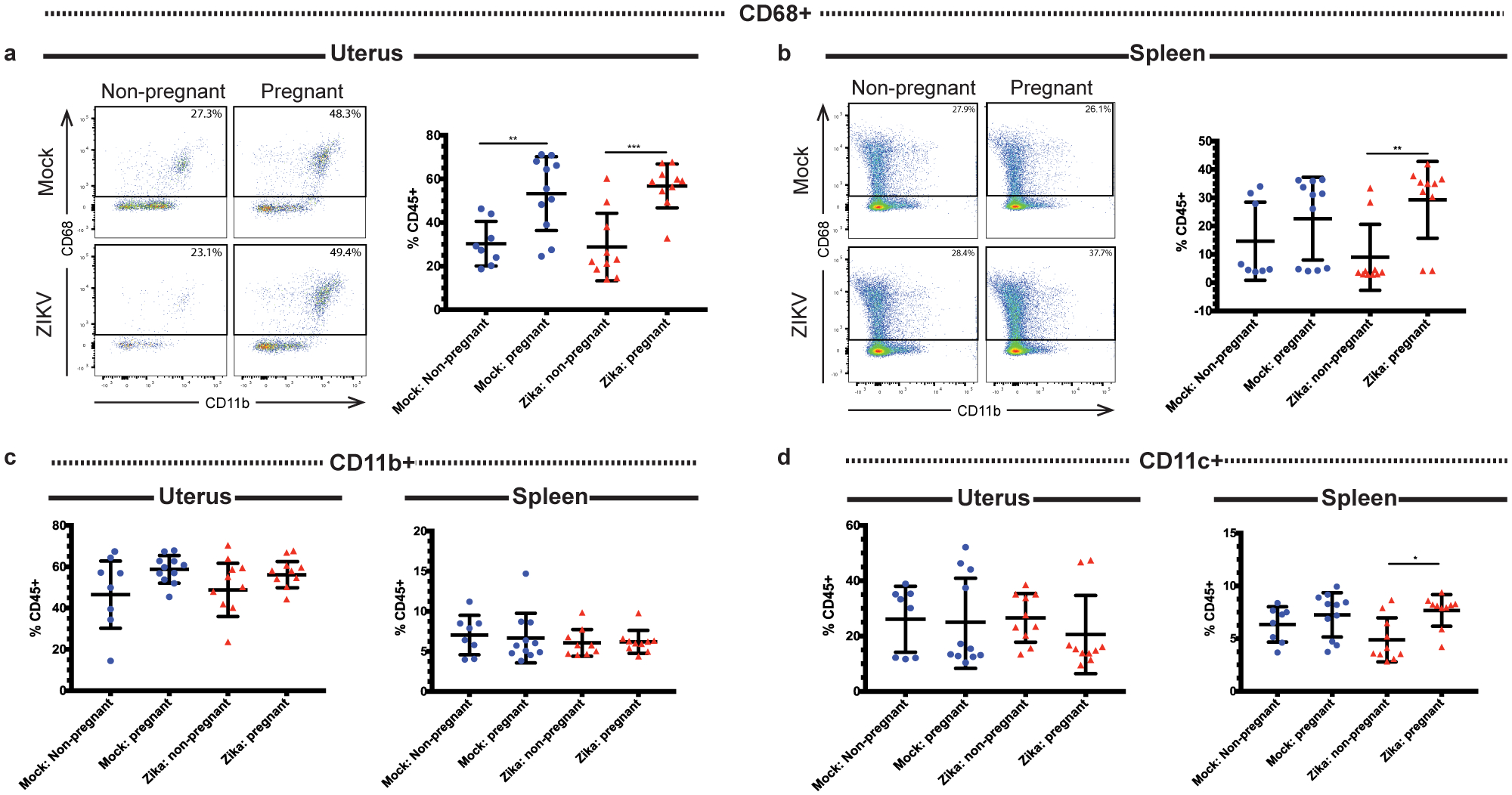
Flow cytometry was performed on immune cells from the uteruses and spleens of ZIKV-infected (red triangles) and mock-infected (blue circles) pregnant and non-pregnant mice at day 3 post infection, and the frequencies of CD68+ (a-b), CD11b+ (c), and CD11c+ (d) cells were measured. Representative pseudocolor plots of CD68 gating are shown for the (a) (uterus) and (b) (spleen). *p<0.05, **p <0.01, ***p<0.001; one-way ANOVA and Tukey’s multiple comparisons test. N=39 (2 independent experiments of 19–20 mice, N=8 mock non-pregnant, N=11 mock pregnant, N=10 ZIKV non-pregnant, N=10 ZIKV pregnant). Error bars represent the mean ± standard deviation.
Pregnant mice have higher frequencies of CD11b+ CD11c+ and CD11c+ CD103+ DCs
Since infiltrating macrophages did not express pregnancy-associated changes in activation in the uterine tissue following acute viral infection, we evaluated dendritic cells (DCs) for evidence of pregnancy-induced changes in activation during acute viral infection. DCs are important antigen presenting cells which coordinate the innate immune response and support the development of adaptive immune responses. Several lines of evidence suggest that uterine dendritic cells take on a tolerogenic phenotype during pregnancy (38, 39). Tolerogenic DCs are potent secretors of anti-inflammatory mediators such as IL-10 and weak producers of pro-inflammatory cytokines including IL-12 and TNFα (40, 41). Two types of tolerogenic DCs are present in the murine uterus: those positive for CD103 (CD11c+ CD103+) and those double-positive for CD11c and CD11b (CD11b+ CD11c+) (42). Despite what is known about these cells during pregnancy, little is known about DC activation during acute viral infection of the reproductive tract. Therefore, we evaluated uterine and splenic tissue for changes in tolerogenic DC populations after ZIKV infection. At 3 days post-infection, ZIKV-inoculated pregnant mice exhibited significantly increased frequencies of uterine CD45+ CD11b+ CD11c+ cells compared to non-pregnant mice (Fig. 3a, p=0.0004). A similar trend was apparent when the mock-inoculated pregnant and non-pregnant mice were compared, although the difference was not statistically significant (p=0.0638). Additionally, no significant difference in the frequency of this population was observed between the mock pregnant and ZIKV-infected pregnant mice (p=0.5769). These results indicate that pregnancy, rather than ZIKV infection, was the likely cause of the increase in CD11b+ CD11c+ DCs in the uterine tissue. Within the CD45+ CD11b+ CD11c+ population, ZIKV-inoculated pregnant mice also exhibited a significant increase in MHCII+ cells compared to ZIKV-inoculated non-pregnant mice (p=0.0081) while no significant difference was seen between the mock non-pregnant and mock pregnant mice (p=0.2159) or the mock pregnant and ZIKV-infected pregnant mice (p=0.7160) (Fig. 3b). Additionally, ZIKV-infected pregnant mice had a significantly greater frequency of splenic CD11b+ CD11c+ cells compared to mock-inoculated pregnant mice (Fig. 3c; p=0.015 mock pregnant vs. ZIKV pregnant) but no significant differences in the MHCII+ subset were found between groups (Fig. 3d, p=0.6382). Despite the change in MHCII, both the mock and ZIKV-inoculated pregnant mice exhibited markedly decreased frequencies of CD11b+ CD11c+ MHCII+ CD86+ cells in the uterus compared to their non-pregnant counterparts (Fig. 3e; p=0.0170 mock non-pregnant vs. mock pregnant, p=0.0007 ZIKV non-pregnant vs. ZIKV pregnant, p=0.6539 mock pregnant vs. ZIKV pregnant), indicating a pregnancy-induced decrease in CD11b+ CD11c+ DC activation. While frequencies of CD11b+ CD11c+ MHCII+ cells increased in the uterus with pregnancy, we found that the frequency of IL-10 expression in the uterus decreased during pregnancy, although not significantly during ZIKV infection (Fig. 3f, p= 0.0311 mock non-pregnant vs. pregnant, p=0.306 ZIKV non-pregnant vs. pregnant, p >0.9999 mock pregnant vs. ZIKV pregnant). CD86 expression in the splenic CD11b+ CD11c+ population did not differ between groups (Fig. 3g, p=0.2215), and no differences in splenic CD11b+ CD11c+ IL-10+ cells were seen between groups (Fig. 3h, p=0.4512). These data show that pregnancy induces an increase in uterine CD11b+ CD11c+ DCs during ZIKV infection while decreasing the frequency of CD86+ cells in this subset. Moreover, IL-10 expression is significantly decreased during this early stage of pregnancy in mock infected animals, but IL-10 is not significantly suppressed in pregnant mice during ZIKV infection. These data show that during pregnancy, ZIKV infection induces increased expression of tolerogenic signals (decreased CD86 and less suppression of IL-10) on CD11b+ CD11c cells.
Fig 3. Pregnant mice have higher frequencies of CD11b+ CD11c+ dendritic cells in the uterus.
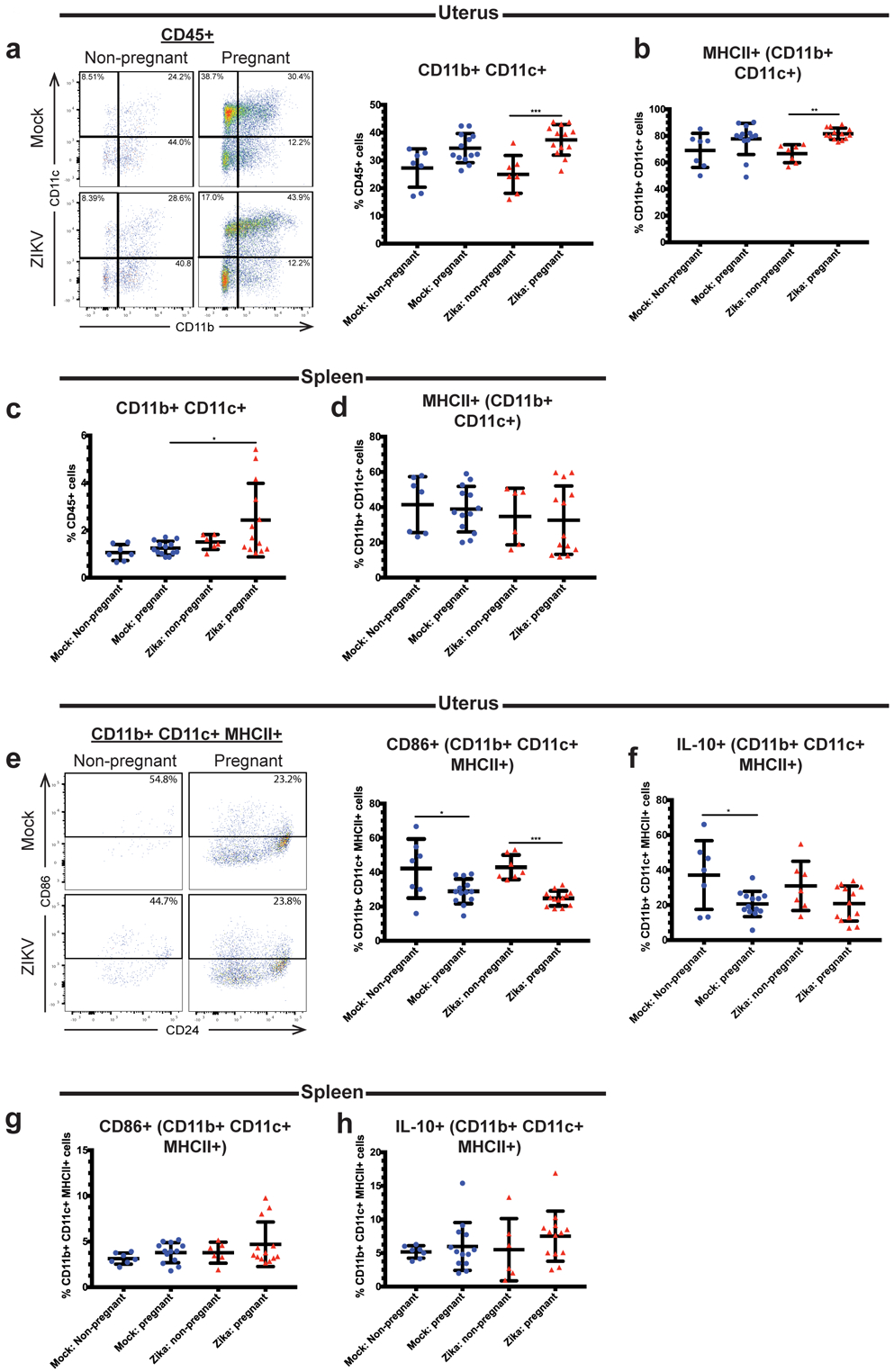
Flow cytometry was performed on the uterine (a-b, e-f) and splenic (c-d, g-h) immune cells of ZIKV-infected (red triangles) and mock-infected (blue circles) pregnant and non-pregnant mice at day 3 post infection. Frequencies of CD11b+ CD11c+ cells (a,c), CD11b+ CD11c+ MHCII+ cells (b,d) CD86+ CD11b+ CD11c+ MHCII+ cells (e,g), and IL-10+ CD11b+ CD11c+ MHCII+ (f,h) cells were measured. Representative flow cytometry plots are also shown in (a) (CD11b+ CD11c+ gating) and (e) (CD86+ gating). *p<0.05, **p<0.01, ***p<0.001; one-way ANOVA and Tukey’s multiple comparisons test. N=40 (2 independent experiments of 20 mice, N=7 mock non-pregnant, N=13 mock pregnant, N=7 ZIKV non-pregnant, N=13 ZIKV pregnant). Error bars represent the mean ± standard deviation.
Similar to CD11b+ CD11c+ cells, we found that ZIKV-inoculated pregnant mice exhibited a significant increase in frequency of uterine CD11c+ CD103+ cells compared to ZIKV-infected non-pregnant mice (Fig. 4a, p= 0.0004 ZIKV non-pregnant vs. ZIKV pregnant). Similarly, uterine CD11c+ CD103+ cells were also significantly increased in the mock-inoculated pregnant mice compared to the mock-inoculated non-pregnant mice (p=0.0476), although the difference was less than what was seen between the ZIKV-infected groups. No significant difference in CD11c+ CD103+ cells was found between the mock pregnant and ZIKV-infected pregnant groups (p=0.9248). Within the CD11c+ CD103+ population, there was no significant change in the CD86+ (p=0.2347) or IL-10+ (p=0.7101) subsets between treatment groups (Fig. 4b–c). In the spleen, there were no significant differences in the frequencies of CD11c+ CD103+ (p=0.3143), CD11c+ CD103+ CD86+ (p=0.895), or CD11c+ CD103+ IL-10+ (p=0.2012) cells between groups (Fig. 4d–f). These data show that local factors in the uterus likely mediate the maintenance of tolerogenic dendritic cell phenotypes during pregnancy regardless of viral infection.
Fig 4. Pregnant mice have higher frequencies of CD11c+ CD103+ dendritic cells in the uterus.
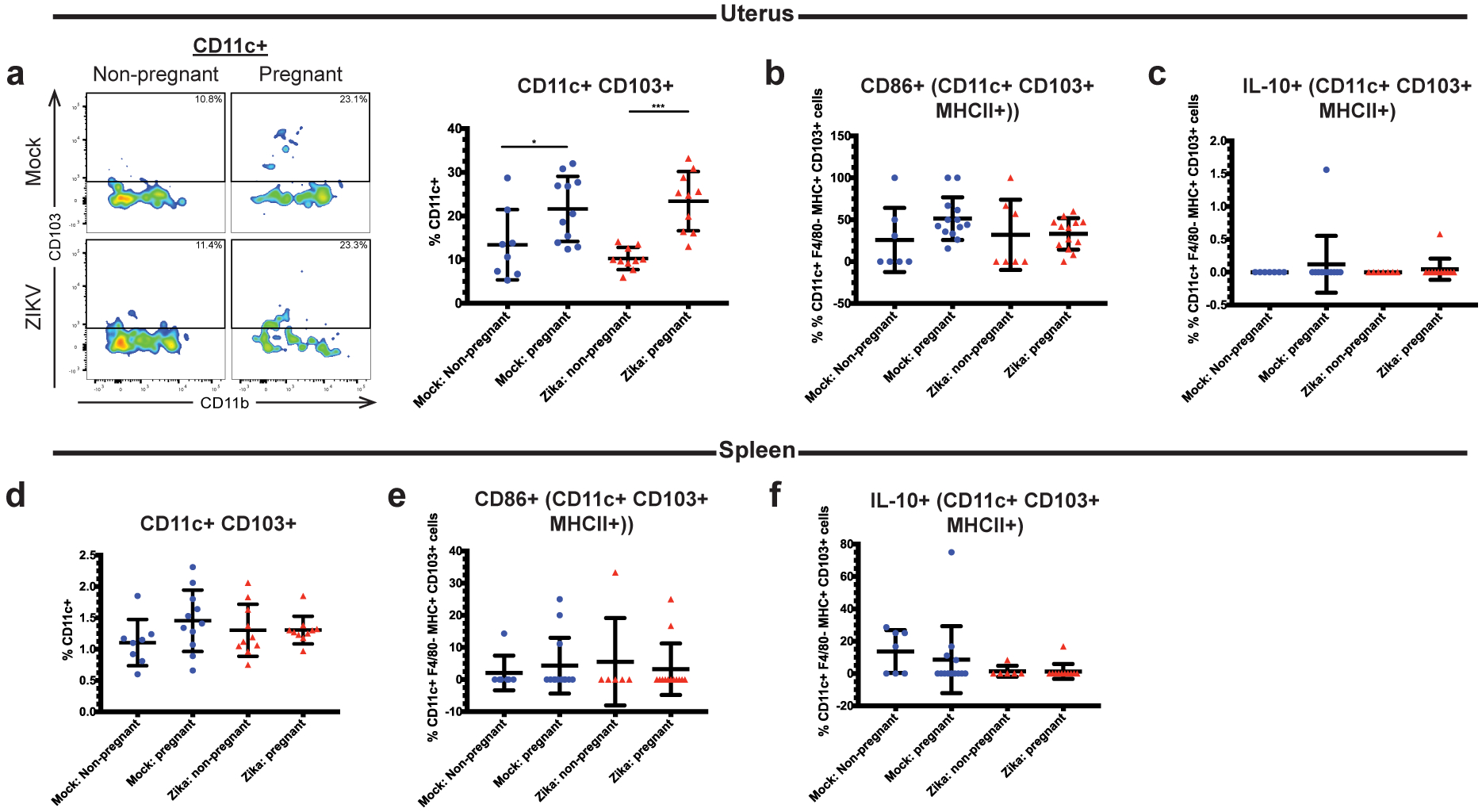
Flow cytometry was performed on the uterine (a-c) and splenic (d-f) immune cells of ZIKV-infected (red triangles) and mock-infected (blue circles) pregnant and non-pregnant mice at day 3 post infection. Frequencies of CD11c+ CD103+ cells (a,d), CD11c+ CD103+ CD86+ cells (b,e), and CD11c+ CD103+ IL-10+ (c,f) cells were measured. A representative flow cytometry plot (CD11c+ CD103+ gating) is shown in (a). *p<0.05, ***p<0.001; one-way ANOVA and Tukey’s multiple comparisons test. N=39 (2 independent experiments of 20 mice, N=8 mock non-pregnant, N=11 mock pregnant, N=10 ZIKV non-pregnant, N=10 ZIKV pregnant). Error bars represent the mean ± standard deviation.
Pregnant mice exhibit decreased IL-12 responses to ZIKV infection in the uterus
Next, we evaluated the expression of pro-inflammatory cytokines in the uterine innate immune cells. IL-12 promotes the differentiation of T cells into Th1 cells and activates NK cells, and it is upregulated during certain viral infections (43, 44). Additionally, multiple studies have shown that IL-12 levels are increased in the blood and endometrial tissue of in women with recurrent pregnancy loss, suggesting that IL-12 may be detrimental during pregnancy (45, 46). In uterine tissue at day 3 post-infection, we found that the frequency of CD45+ cells producing IL-12 was decreased in ZIKV-infected pregnant mice compared to ZIKV-infected non-pregnant mice (Fig. 5a, p=0.0081). This difference was not seen when non-pregnant and pregnant mock-infected mice were compared (p=0.9535). We also found a significant decrease in splenic CD45+ IL-12+ cells in pregnant ZIKV-infected mice compared to non-pregnant ZIKV-infected mice (Fig. 5b, p=0.0323). Next, we further analyzed IL-12 expression in several immune cell subtypes. We found that uterine IL-12+ CD11b+ (Fig. 5c, p=0.007), IL-12+ CD68+ (Fig. 5d, p=0.0018), and IL-12+ Ly6C+ (Fig. 5f, p=0.004) cells were significantly decreased in ZIKV-infected pregnant mice compared to ZIKV-infected non-pregnant mice. Uterine CD45+ CD103+ cells exhibited a trend towards decreased IL-12 expression during pregnancy in both mock and ZIKV-infected mice when compared to non-pregnant mice, but the differences were not statistically significant (p= 0.0912 mock non-pregnant vs. mock pregnant, p=0.1647 ZIKV non-pregnant vs. ZIKV pregnant) (Fig. 5e). There was no significant difference in CD11c+ IL-12+ cells between groups (Fig. 5g, p=0.1943). In the spleen, we found that frequencies of IL-12+ cells were significantly decreased within the CD11b+ (Fig. 5c, p=0.0093) population in ZIKV-infected pregnant mice compared to ZIKV-infected non-pregnant mice. Similar to the uterus, inhibition of IL-12 expression appeared to be specific to monocytes and macrophages, as CD11c+ (Fig. 5g, p=0.2629) and CD103+ (Fig. 5e, p=0.9808) cells did not exhibit significant changes in IL-12 expression between treatment groups. The frequency of Ly6C+ IL-12+ cells trended toward a decrease in the ZIKV-infected pregnant mice compared to the other groups, but this change did not reach significance when the groups were compared to each other (Fig. 5f, p=0.0478). These data show that pregnancy in ZIKV-infected mice results in decreases in IL-12-expressing cells, chiefly IL-12+ monocytes and macrophages. Pregnancy alone did not result in alterations of any of the IL-12+ cell frequencies measured, as no significant differences were seen when the mock non-pregnant and mock pregnant mice were compared. Taken together, these data suggest that IL-12 production during ZIKV infection is suppressed in pregnant mice.
Fig 5. Pregnant mice have lessened IL-12 responses during Zika virus infection.
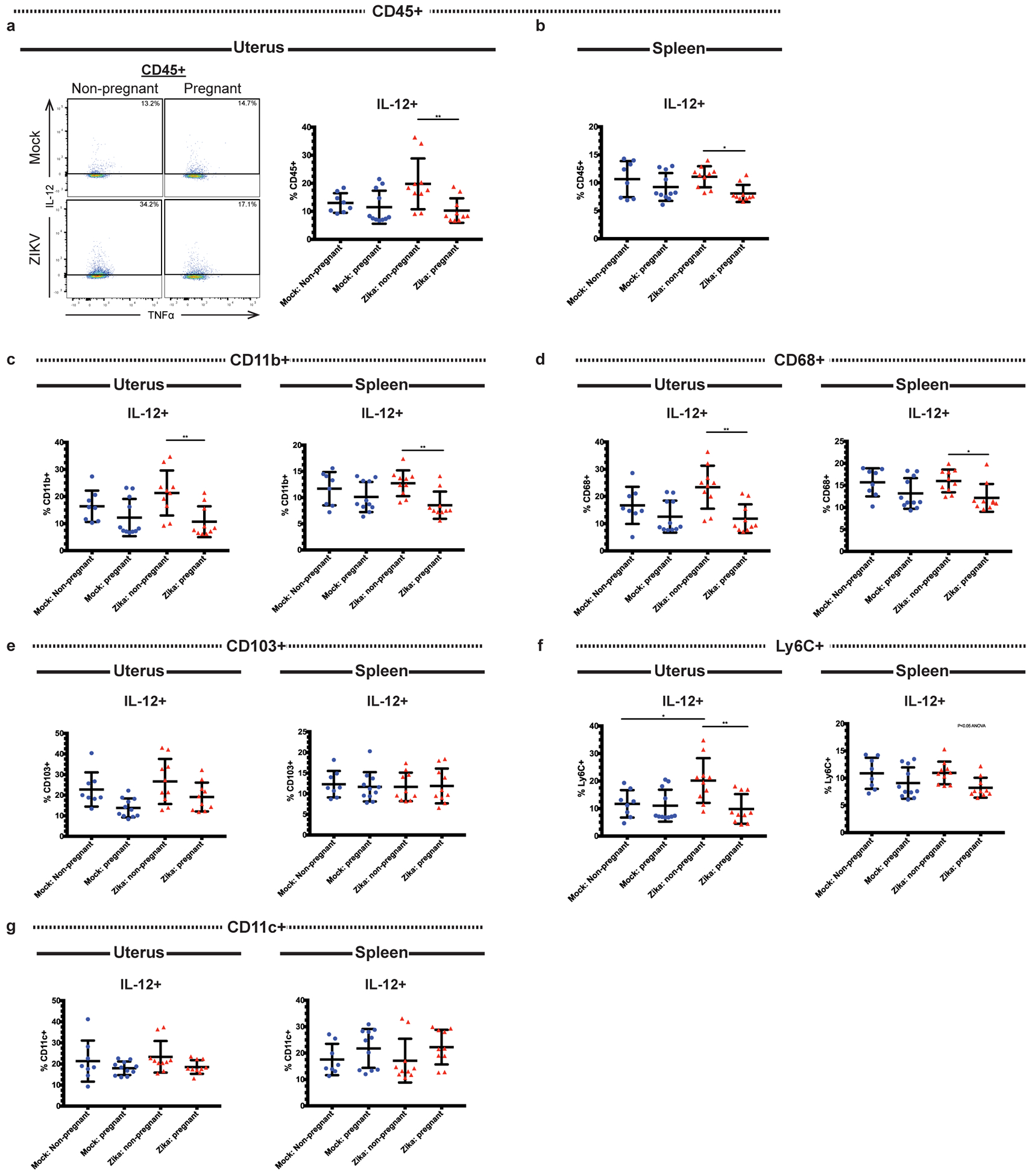
Flow cytometry was performed on the uterine and splenic immune cells of ZIKV-infected (red triangles) and mock-infected (blue circles) pregnant and non-pregnant mice at day 3 post infection. Frequencies of IL-12-expressing cells were measured within the CD45+ (a-b), CD11b+ (c), CD68+ (d), CD103+ (e), Ly6C+ (f), and CD11c+ (g) populations. Representative flow cytometry plots depicting IL-12+ gating are shown in (a). *p<0.05, **p<0.01; one-way ANOVA and Tukey’s multiple comparisons test. N=39 (2 independent experiments of 20 mice, N=8 mock non-pregnant, N=11 mock pregnant, N=10 ZIKV non-pregnant, N=10 ZIKV pregnant). Error bars represent the mean ± standard deviation.
Additionally, we measured the frequencies of IL-6+ cells within each of these populations, as it has been reported that IL-6 is upregulated during ZIKV infection (47). We found no significant differences in the frequencies of uterine IL-6+ CD45+ (p=0.3776 uterus), IL-6+ CD68+ (p=0.2023 uterus), IL-6+ CD11b+ (p=0.2719 uterus), IL-6+ CD11c+ (p=0.5416 uterus), IL-6+ Ly6C+ (p=0.3119 uterus), or IL-6+ CD103+ (p=0.0569 uterus) cells between groups (Fig. S2). Similar to the results seen in the uterus, IL-6 expression did not differ significantly in any of the splenic cell subsets tested (p=0.0645 CD45+ IL-6+, p=0.2721 CD68+ IL-6+, p=0.5910 CD11b+ IL-6+, p=0.1166 CD11c+ IL-6+, p=0.8750 Ly6C+ IL-6+, p=0.2587 CD103+ IL-6+).
Pregnancy alters the frequencies of antigen-experienced CD4 and CD8 T cells
Since pregnant mice had increased frequencies of DCs associated with immunotolerance and decreased frequencies of cells expressing pro-inflammatory IL-12, we next examined the impact of immunotolerance on the adaptive immune response to ZIKV infection during pregnancy. First, we measured the total frequencies of uterine and splenic CD4+ and CD8+ T cells. We found no differences in total CD4+ T cell frequencies in the uterus (Fig. 6a, p= 0.9929 mock non-pregnant vs. mock pregnant, p=0.7665 ZIKV non-pregnant vs. ZIKV pregnant, p=0.5840 mock pregnant vs. ZIKV pregnant) or spleen (Fig. 6b, p=0.1872 mock non-pregnant vs. pregnant, p=0.8311 ZIKV non-pregnant vs. ZIKV pregnant, p=0.6621 mock pregnant vs. ZIKV pregnant) between groups. However, ZIKV-infected pregnant mice had significantly more uterine CD8+ T cells compared to their mock-inoculated counterparts (Fig. 6c, p <0.01) while no significant differences were seen within infection groups (p >0.05 mock non-pregnant vs. mock pregnant, p >0.05 ZIKV non-pregnant vs. ZIKV pregnant). No differences were seen in CD8+ T cell frequencies in the spleen (Fig. 6d, p= 0.5201). These results indicate that CD8+ T cells are recruited to the uterus during ZIKV infection in pregnant mice.
Previous studies have established that antigen-specific CD4 and CD8 T cell responses after viral infection can be measured using surrogate markers of activation. For example, CD4 T cells become CD11a+ CD49+ after antigen encounter during various infections and maintain this phenotype into the memory phase(48–50). Similarly, CD8 T cells downregulate CD8 and upregulate CD11a (CD8lo CD11ahi) after antigen encounter during viral infections, including ZIKV(51, 52). These phenotypes are strictly dependent upon antigen stimulation rather than inflammatory cytokine expression, making this a useful tool for tracking antigen-specific T cell responses post infection. When we measured the frequencies of CD4+ CD11a+ CD49d+ and CD8lo CD11ahi cells at day 10 post-ZIKV infection, we found that mock and ZIKV-infected pregnant mice had similar frequencies of uterine CD4+ CD11a+ CD49d+ cells (p=0.1034), and both pregnant groups had fewer CD4+ CD11a+ CD49d+ cells compared to their non-pregnant counterparts (Fig. 7a, p= 0.0171 mock non-pregnant vs pregnant, p= 0.0252 ZIKV non-pregnant vs. pregnant). Similarly, we found that ZIKV-infected pregnant mice had lower frequencies of CD8lo CD11ahi cells in the uterus compared to ZIKV-infected non-pregnant mice (p <0.05). A similar trend was seen when the mock non-pregnant and mock pregnant mice were compared, but the difference was not statistically significant (p >0.05). Additionally, no significant difference was seen between the mock pregnant and ZIKV-infected pregnant groups (p >0.05) (Fig. 7b). These results indicate that pregnancy results in a decrease in antigen-experienced T cells in the uterus. In the spleen, ZIKV-infected non-pregnant mice had significantly higher frequencies of both CD4+ CD11a+ CD49d+ (Fig. 7c, p<0.05) and CD8lo CD11ahi (Fig. 7d, p<0.05) cells compared to mock-infected non-pregnant mice, but no other significant differences were found. This suggests that the frequencies of antigen-experienced T cells in the spleen were increased after infection but not impacted by pregnancy. Additionally, we measured the frequencies of activated CD4 T cells (CD4+ CD69+ and CD4+ CD25+) and found no significant difference in CD69+ (Fig. 8a–b, p= 0.7439 uterus, p= 0.7056 spleen) or CD25+ (Fig. 8c–d, p= 0.3393 uterus, p= 0.3456 spleen) CD4+ cell frequencies in either the uterus or spleen. Since Tregs play an important role in maintaining immunotolerance during pregnancy, we also measured the frequencies of uterine (Fig. 8e) and splenic (Fig. 8f) CD4+ Tregs (CD4+ CD25+ FoxP3+). Surprisingly, we found no significant differences in Treg frequencies between any of the experimental groups (p=0.9827 uterus, p=0.095 spleen). Taken together, these results suggest that pregnancy-associated immunotolerance may impact the local adaptive immune response to viral infection in the uterus.
Fig 8. Frequencies of activated T cells and Tregs in ZIKV-infected pregnant and non-pregnant mice.
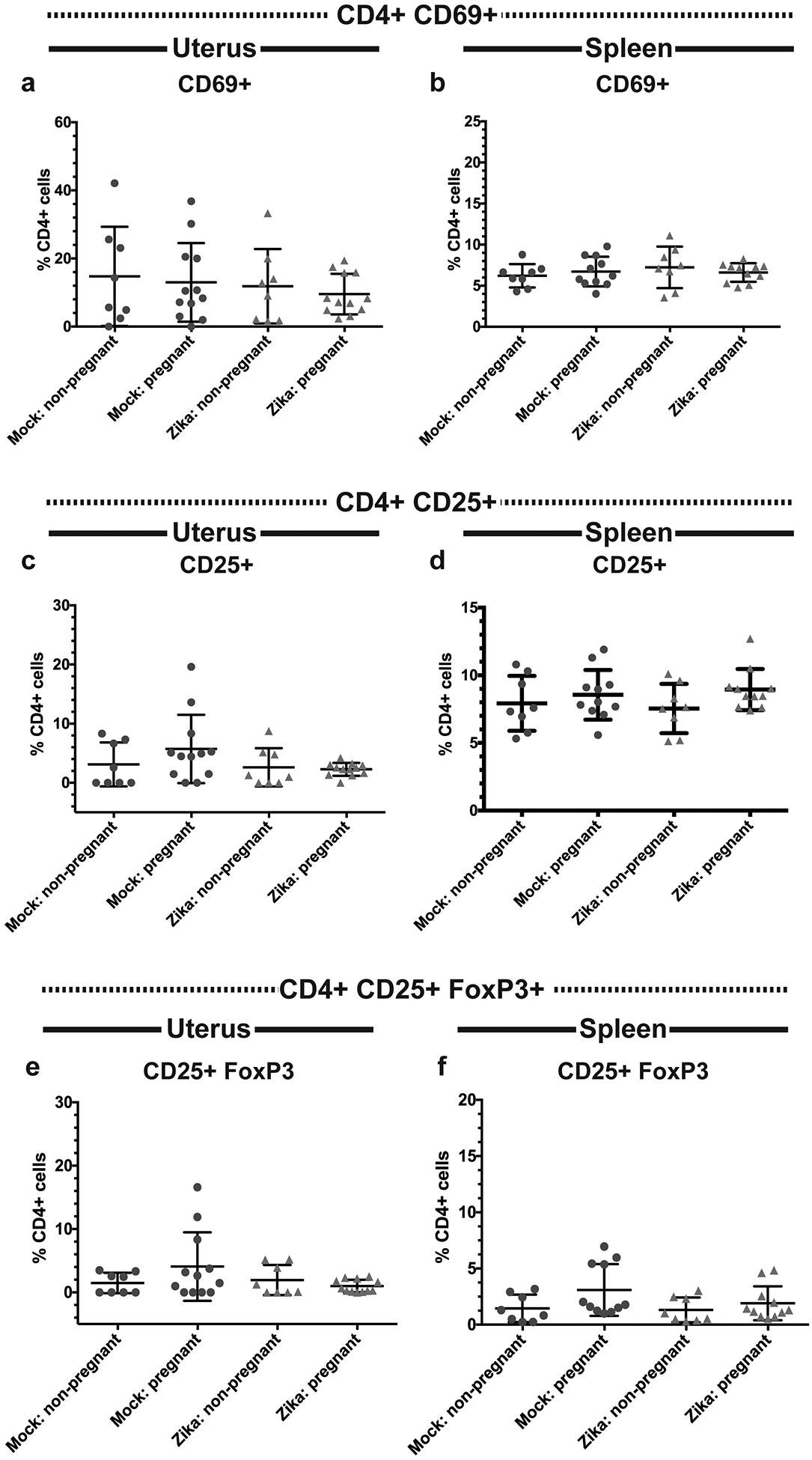
Flow cytometry was performed on the uterine (a,c,e) and splenic (b,d,f) immune cells of ZIKV-infected (red triangles) and mock-infected (blue circles) pregnant and non-pregnant mice. Frequencies of CD4+ CD69+ (a-b), CD4+ CD25+ (c-d) CD4 T cells, and CD4+ CD25+ FoxP3+ Tregs (e-f) were measured. N=40 (3 independent experiments of 10 or 20 mice, N=8 mock non-pregnant, N=12 mock pregnant, N=8 ZIKV non-pregnant, N=12 ZIKV pregnant). Error bars represent the mean ± standard deviation.
Discussion
In this study, we evaluated acute innate immune cellular responses in the reproductive tract of immune competent mice to Zika virus infection during early pregnancy and their impact on the downstream adaptive immune response. The data show that the early stages of pregnancy result in inhibition of innate cellular activation and maintenance of tolerogenic immune cell changes despite acute viral infection in the reproductive tract. We show that pregnant mice exhibited decreased CD45+Ly6C+ cells in the spleen following acute viral infection. We also found that pregnancy induces expression of tolerogenic DCs (CD45+ CD11b+CD11c+) that express increased MHCII with ZIKV infection during pregnancy while also decreasing expression of the activation marker CD86. Moreover, IL-10 expression in CD11b+ CD11c+ DCs was significantly decreased during this early stage of pregnancy (E7.5) in mock infected animals, potentially representing the importance of some inflammatory responses in supporting early pregnancy. However, during acute viral infection, IL-10 was not significantly suppressed in pregnant mice during ZIKV infection. These data imply that ZIKV infection induces increased expression of tolerogenic signals (decreased CD86 and less suppression of IL-10) on CD11b+ CD11c cells during pregnancy. It is surprising that IL-10 was decreased during pregnancy in our mouse model, since previous studies have shown that IL-10 increases during early pregnancy in mice and peaks at E12 (53, 54). It is possible that cell types other than DCs are responsible for increased IL-10 production during pregnancy, including non-immune cells. Additionally, since we only measured IL-10 production by flow cytometry at a single timepoint, our data do not capture the possibility that more IL-10 was produced but then secreted from the DCs at a timepoint prior to our analysis. Overall, these findings indicate that pregnancy inhibits virus-induced acute inflammation during early implantation, likely as a mechanism to protect the developing fetus.
We also found that some of the inhibitory cell phenotypes were specific to the uterine tissue. For example, during acute ZIKV infection, non-pregnant mice significantly suppress immunotolerant CD11c+ CD103+ cells to support acute inflammation during infection. However, pregnant mice exhibit a significant increase in CD11c+ CD103+ cells in the uterus. These changes were not seen in the spleen, implying that the expression of CD11c+ CD103+ cells in the uterus is regionally regulated to support the developing pregnancy. In contrast, some of the inhibitory cell phenotypes were found in both the uterus and the spleen. We found that CD45+ IL-12 responses to acute viral infection were significantly decreased in both the uterus and spleen of pregnant ZIKV-infected mice compared to ZIKV-infected non-pregnant mice. In the uterus, decreased IL-12 production was largely due to CD11b+, CD68+, and Ly6C+ cells, and in the spleen, decreased IL-12 production was largely due to CD11b+ cells. These data show that both systemic and regional responses during pregnancy modulate the innate immune cellular response to acute viral infection.
Macrophages are involved in remodeling of the spiral arteries during early pregnancy, a process which is crucial in establishing blood flow to the placenta (55, 56). Macrophages are broadly classified into two subtypes: classically activated M1 macrophages, which are considered pro-inflammatory, and alternatively activated M2 macrophages, which have anti-inflammatory properties and are involved in tissue repair (57–59). We found that pregnant mice exhibited higher frequencies of uterine CD68+ macrophages with lower expression of IL-12 upon ZIKV infection. Previous studies have shown that several M2 markers, including CD206, CCL18, CD163, IL-10, and mannose receptor c type (MRC)-1 are expressed on decidual macrophages (60–62). Human placental macrophages, or Hofbauer cells, are targets of ZIKV and promote dissemination of the virus. Infected Hofbauer cells produce pro-inflammatory cytokines, including MCP-1, IL-6, IP-10, and type I interferons (63). It is unclear how pregnancy-induced immunotolerance would impact responses of Hofbauer cells, and future studies should examine the interaction between maternal and placental immune regulation during acute viral infection.
CD11c+ DCs are crucial for early placentation and regulate tissue remodeling and angiogenesis (64). While the inflammatory activity of decidual DCs is important to support early implantation, they are altered by the local environment, resulting in loss of migration of uterine DCs to the lymph nodes (65). However, the specific changes in activation and cytokine production in DCs during acute infection have not yet been characterized. Tolerogenic DCs, including the CD11b+ CD11c+ and CD11c+ CD103+ populations, promote immunotolerance toward the fetus during pregnancy and secrete anti-inflammatory mediators including IL-10 (38–41). We found that both subtypes were upregulated in pregnant mice, regardless of infection. Interestingly, we found that there were fewer CD11b+ CD11c+ IL-10+ cells in the uteruses of mock-inoculated pregnant mice than mock-inoculated non-pregnant mice. However, ZIKV-infected pregnant mice did not have decreased IL-10+ or CD11c+ CD103+ cells. These results suggest that pregnancy signals maintain immunotolerant signaling and cell types despite acute viral infection in these tissues. Additionally, pregnant mice exhibited fewer CD86+ CD11b+ CD11c+ cells in the uterus, which is indicative of less mature, unactivated DCs. Taken together, these results indicate that pregnancy-associated tolerogenic DCs are not significantly suppressed by ZIKV infection.
DCs serve as a bridge between innate and adaptive immunity, recognizing antigen and priming T and B cell responses. Through a combination of TCR stimulation, co-stimulation, and cytokine secretion, DCs drive the polarization of naïve CD4+ T helper cells into effector T cells(66). Therefore, DCs are critical regulators of the downstream immune response. We found that pregnant ZIKV-infected mice had lower frequencies of antigen-experienced CD4+ CD11a+ CD49+ and CD8lo CD11ahi T cells compared to their non-pregnant counterparts. Together with the increased frequencies of tolerogenic DCs seen in the pregnant mice, this suggests that immunotolerance during pregnancy may prevent proficient activation of the anti-viral T cell response. Tolerogenic DCs support immunotolerance through several mechanisms, including: 1) possessing an immature phenotype characterized by low expression of co-stimulatory molecules and MHC; 2) secretion of immunosuppressive cytokines, such as TGF-β or IL-10; and 3) increased expression of inhibitory receptors, such as PDL-1 or CTLA-4(67). Although the ZIKV-infected mice had greater frequencies of CD11b+ CD11c+ MHCII+ cells compared to non-pregnant mice, they had significantly lower frequencies of CD11b+ CD11c+ CD86+ cells, which indicates that these DCs have a more immature phenotype. Furthermore, we saw a decrease in IL-10+ cells within this population in the ZIKV-infected pregnant mice. These results suggest that lack of co-stimulation through CD86 during pregnancy, rather than production of IL-10, may prevent proper activation of T cells during ZIKV infection. However, we did not find any difference in the frequencies of activated T cells as measured by CD69 and CD25. A similar lack of significant increase in activation markers, including CD69, CD11a, CD43, was noted in another study of wild-type C57BL/6 mice infected with ZIKV(68), suggesting that upregulation of CD69 and other activation markers may not be a feature of ZIKV infection in wild-type C57BL/6 mice. The aforementioned study also did not find any difference in FoxP3+ CD4 T cells in the spleen after infection, which is consistent with our results. Additionally, we did not find any significant difference in Tregs in the pregnant mice, suggesting that the impacts of immunotolerance on the T cell responses in this model are not due to an expansion of Tregs.
Co-stimulation through CD86 has been shown to play an important role in the immune response to several viral infections, as anti-viral CD8+ T cell responses are significantly impaired in mice when CD86/CD28 co-stimulation is disrupted genetically or with blocking reagents (69–72). The interaction between CD28 and CD86 or CD80 promotes T cell activation, proliferation, and cytokine production (73). Therefore, it is possible that the reduced frequencies of antigen-experienced CD4 and CD8 T cells in the uteruses of pregnant mice are a direct result of the lack of CD86 on immunotolerant DCs during pregnancy. However, further studies are needed to determine the exact effects of CD86 downregulation in the uterus during ZIKV infection.
In the presence of IL-12 and IFNγ, which are often secreted in response to viral infections, naïve T cells differentiate into T helper 1 (Th1) cells(43, 74–76). Conversely, IL-4 promotes the development of T helper 2 (Th2) cells, and IL-23, IL-6, and TGF-β promote the development of T helper 17 (Th17 cells)(77–80). A previous study of ZIKV infection in wild-type C7BL/6 mice found that antigen-experienced CD4+ CD11a+ CD49d+ cells secreted typical Th1 cytokines when re-stimulated with PMA and ionomycin 7 days post-infection, including IFNγ, IL-2, and TNFα and expressed the Th1 transcription factor T-bet but did not produce substantial amounts of Th2 (IL-5) or Th17 (IL-17) cytokines. This suggests that T cells are primarily polarized toward a Th1 phenotype after ZIKV infection. The same study also demonstrated a robust CD8+ T cell response to ZIKV infection, with antigen-experienced CD8 cells producing IFNγ and TNFα. When we evaluated IFNγ and TNFα expression in our mice, we detected very little, if any, expression of either cytokine (data not shown). It is likely that any cytokines produced by these mice were secreted in vivo, and therefore we were not able to detect them by flow cytometry without re-stimulation. Further studies are needed to determine how pregnancy impacts T cell polarization after ZIKV infection.
Our data also show that uterine monocytes, macrophages, and DCs are deficient in IL-12 production upon challenge with ZIKV during pregnancy. See supplementary Table 1 for summary of results. Since IL-12 is an important activator of NK cell responses(81), suppression of IL-12-induced activation of uterine NK cells is likely important to prevent NK activation and increased risk to the pregnancy. Our data show that virus-induced production of IL-12 by CD45+ cells is significantly reduced in pregnant mice compared to non-pregnant mice. This may be an important mechanism by which the localized immune response in the decidua is modulated to protect the pregnancy while still mounting an immune response to viral infection that is efficacious but not deleterious to the developing fetus. These findings should be evaluated as a potential biomarker of pregnancy loss during acute infection, as these markers may provide prognostic value for pregnancy loss and complications. As mentioned above, IL-12 also drives polarization of naïve T cells to Th1 cells, which promotes an anti-viral T cell response. Future studies are needed to clarify the role of IL-12 during ZIKV infection in the reproductive tract and to determine how IL-12 deficiency impacts T cell polarization and resolution of ZIKV infection.
The findings of this study may be broadly applicable to other acute infections during pregnancy, and further studies are needed to evaluate pregnancy-induced immune modulation during acute infection and vaccination. Additionally, our data show that activation of important antigen presenting cells are modulated during pregnancy. These findings have important implications for vaccine studies during pregnancy as well, since dendritic cells and other antigen presenting cells are vital for development of the adaptive immune response that defines vaccine outcomes. In conclusion, our results show that pregnancy-induced changes to the immune response impacts the acute innate cellular response to ZIKV infection and inhibits important features of the acute anti-viral immune response.
Supplementary Material
Key Points.
Pregnancy results in recruitment of tolerogenic dendritic cells to the uterus.
Pregnancy results in lessened IL-12 responses to Zika virus infection.
Pregnant mice have fewer antigen-experienced T cells after Zika infection.
References
- 1.Dick GW, Kitchen SF, and Haddow AJ. 1952. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 46: 509–520. [DOI] [PubMed] [Google Scholar]
- 2.Kindhauser MK, Allen T, Frank V, Santhana RS, and Dye C. 2016. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ 94: 675–686C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musso D, Roche C, Robin E, Nhan T, Teissier A, and Cao-Lormeau VM. 2015. Potential sexual transmission of Zika virus. Emerg Infect Dis 21: 359–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barjas-Castro ML, Angerami RN, Cunha MS, Suzuki A, Nogueira JS, Rocco IM, Maeda AY, Vasami FG, Katz G, Boin IF, Stucchi RS, Resende MR, Esposito DL, de Souza RP, da Fonseca BA, and Addas-Carvalho M. 2016. Probable transfusion-transmitted Zika virus in Brazil. Transfusion 56: 1684–1688. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer E, Leshem E, Lustig Y, Gottesman G, and Schwartz E. 2016. The Clinical Spectrum of Zika Virus in Returning Travelers. The American journal of medicine 129: 1126–1130. [DOI] [PubMed] [Google Scholar]
- 6.Heukelbach J, and Werneck GL. 2016. Surveillance of Zika virus infection and microcephaly in Brazil. Lancet 388: 846–847. [DOI] [PubMed] [Google Scholar]
- 7.Campos GS, Bandeira AC, and Sardi SI. 2015. Zika Virus Outbreak, Bahia, Brazil. Emerg Infect Dis 21: 1885–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martines RB, Bhatnagar J, Keating MK, Silva-Flannery L, Muehlenbachs A, Gary J, Goldsmith C, Hale G, Ritter J, Rollin D, Shieh WJ, Luz KG, Ramos AM, Davi HP, Kleber de Oliveria W, Lanciotti R, Lambert A, and Zaki S. 2016. Notes from the Field: Evidence of Zika Virus Infection in Brain and Placental Tissues from Two Congenitally Infected Newborns and Two Fetal Losses - Brazil, 2015. MMWR Morb Mortal Wkly Rep 65: 159–160. [DOI] [PubMed] [Google Scholar]
- 9.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, and Avsic Zupanc T. 2016. Zika Virus Associated with Microcephaly. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- 10.de Paula Freitas B, de Oliveira Dias JR, Prazeres J, Sacramento GA, Ko AI, Maia M, and Belfort R Jr. 2016. Ocular Findings in Infants With Microcephaly Associated With Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, Doriqui MJ, Neri JI, Neto JM, Wanderley HY, Cernach M, El-Husny AS, Pone MV, Serao CL, Sanseverino MT, and Brazilian F Medical Genetics Society-Zika Embryopathy Task. 2016. Possible Association Between Zika Virus Infection and Microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep 65: 59–62. [DOI] [PubMed] [Google Scholar]
- 12.Waggoner JJ, and Pinsky BA. 2016. Zika Virus: Diagnostics for an Emerging Pandemic Threat. J Clin Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, Timofeev J, Rodriguez FJ, Levanov L, Razak J, Iyengar P, Hennenfent A, Kennedy R, Lanciotti R, du Plessis A, and Vapalahti O. 2016. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, Aliota MT, Weiler AM, Barry GL, Weisgrau KL, Vosler LJ, Mohns MS, Breitbach ME, Stewart LM, Rasheed MN, Newman CM, Graham ME, Wieben OE, Turski PA, Johnson KM, Post J, Hayes JM, Schultz-Darken N, Schotzko ML, Eudailey JA, Permar SR, Rakasz EG, Mohr EL, Capuano S 3rd, Tarantal AF, Osorio JE, O’Connor SL, Friedrich TC, O’Connor DH, and Golos TG. 2017. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog 13: e1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meaney-Delman D, Oduyebo T, Polen KN, White JL, Bingham AM, Slavinski SA, Heberlein-Larson L, St George K, Rakeman JL, Hills S, Olson CK, Adamski A, Culver Barlow L, Lee EH, Likos AM, Munoz JL, Petersen EE, Dufort EM, Dean AB, Cortese MM, Santiago GA, Bhatnagar J, Powers AM, Zaki S, Petersen LR, Jamieson DJ, Honein MA, and Group USZPRPVW. 2016. Prolonged Detection of Zika Virus RNA in Pregnant Women. Obstet Gynecol 128: 724–730. [DOI] [PubMed] [Google Scholar]
- 16.Aronoff DM, Correa H, Rogers LM, Arav-Boger R, and Alcendor DJ. 2017. Placental pericytes and cytomegalovirus infectivity: Implications for HCMV placental pathology and congenital disease. Am J Reprod Immunol 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baud D, and Greub G. 2011. Intracellular bacteria and adverse pregnancy outcomes. Clin Microbiol Infect 17: 1312–1322. [DOI] [PubMed] [Google Scholar]
- 18.Littauer EQ, Esser ES, Antao OQ, Vassilieva EV, Compans RW, and Skountzou I. 2017. H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. PLoS Pathog 13: e1006757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louie JK, Acosta M, Jamieson DJ, Honein MA, and California Pandemic Working G. 2010. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med 362: 27–35. [DOI] [PubMed] [Google Scholar]
- 20.Palacios R, Senise J, Vaz M, Diaz R, and Castelo A. 2009. Short-term antiretroviral therapy to prevent mother-to-child transmission is safe and results in a sustained increase in CD4 T-cell counts in HIV-1-infected mothers. HIV Med 10: 157–162. [DOI] [PubMed] [Google Scholar]
- 21.Beckham JD, Pastula DM, Massey A, and Tyler KL. 2016. Zika Virus as an Emerging Global Pathogen: Neurological Complications of Zika Virus. JAMA Neurol 73: 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma S 2014. Natural killer cells and regulatory T cells in early pregnancy loss. Int J Dev Biol 58: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, and Saito S. 2004. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 10: 347–353. [DOI] [PubMed] [Google Scholar]
- 24.Quinn KH, and Parast MM. 2013. Decidual regulatory T cells in placental pathology and pregnancy complications. Am J Reprod Immunol 69: 533–538. [DOI] [PubMed] [Google Scholar]
- 25.Teles A, Schumacher A, Kuhnle MC, Linzke N, Thuere C, Reichardt P, Tadokoro CE, Hammerling GJ, and Zenclussen AC. 2013. Control of uterine microenvironment by foxp3(+) cells facilitates embryo implantation. Front Immunol 4: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schumacher A, Brachwitz N, Sohr S, Engeland K, Langwisch S, Dolaptchieva M, Alexander T, Taran A, Malfertheiner SF, Costa SD, Zimmermann G, Nitschke C, Volk HD, Alexander H, Gunzer M, and Zenclussen AC. 2009. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol 182: 5488–5497. [DOI] [PubMed] [Google Scholar]
- 27.Wan H, Versnel MA, Leijten LM, van Helden-Meeuwsen CG, Fekkes D, Leenen PJ, Khan NA, Benner R, and Kiekens RC. 2008. Chorionic gonadotropin induces dendritic cells to express a tolerogenic phenotype. J Leukoc Biol 83: 894–901. [DOI] [PubMed] [Google Scholar]
- 28.Kaushic C, Ashkar AA, Reid LA, and Rosenthal KL. 2003. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol 77: 4558–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon SW, Wong SS, Zhu H, Chen R, Li L, Zhang Y, Guan Y, and Webby RJ. 2018. Dysregulated T-Helper Type 1 (Th1):Th2 Cytokine Profile and Poor Immune Response in Pregnant Ferrets Infected With 2009 Pandemic Influenza A(H1N1) Virus. J Infect Dis 217: 438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, Kim JS, Edwin SS, Gomez R, and Draghici S. 2010. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol 63: 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salaun B, Romero P, and Lebecque S. 2007. Toll-like receptors’ two-edged sword: when immunity meets apoptosis. Eur J Immunol 37: 3311–3318. [DOI] [PubMed] [Google Scholar]
- 32.Ornelas AM, Pezzuto P, Silveira PP, Melo FO, Ferreira TA, Oliveira-Szejnfeld PS, Leal JI, Amorim MM, Hamilton S, Rawlinson WD, Cardoso CC, Nixon DF, Tanuri A, Melo AS, and Aguiar RS. 2017. Immune activation in amniotic fluid from Zika virus-associated microcephaly. Ann Neurol 81: 152–156. [DOI] [PubMed] [Google Scholar]
- 33.Barros JBS, da Silva PAN, Koga RCR, Gonzalez-Dias P, Carmo Filho JR, Nagib PRA, Coelho V, Nakaya HI, Fonseca SG, and Pfrimer IAH. 2018. Acute Zika Virus Infection in an Endemic Area Shows Modest Proinflammatory Systemic Immunoactivation and Cytokine-Symptom Associations. Front Immunol 9: 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, Fontanet A, and Mallet HP. 2016. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 387: 2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, and Iwasaki A. 2016. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell 166: 1247–1256 e1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fowler RE, and Edwards RG. 1957. Induction of superovulation and pregnancy in mature mice by gonadotrophins. J Endocrinol 15: 374–384. [DOI] [PubMed] [Google Scholar]
- 37.Perfetto SP, Ambrozak D, Nguyen R, Chattopadhyay PK, and Roederer M. 2012. Quality assurance for polychromatic flow cytometry using a suite of calibration beads. Nat Protoc 7: 2067–2079. [DOI] [PubMed] [Google Scholar]
- 38.Blois SM, Kammerer U, Alba Soto C, Tometten MC, Shaikly V, Barrientos G, Jurd R, Rukavina D, Thomson AW, Klapp BF, Fernandez N, and Arck PC. 2007. Dendritic cells: key to fetal tolerance? Biol Reprod 77: 590–598. [DOI] [PubMed] [Google Scholar]
- 39.Tagliani E, and Erlebacher A. 2011. Dendritic cell function at the maternal-fetal interface. Expert Rev Clin Immunol 7: 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salamone G, Fraccaroli L, Gori S, Grasso E, Paparini D, Geffner J, Perez Leiros C, and Ramhorst R. 2012. Trophoblast cells induce a tolerogenic profile in dendritic cells. Hum Reprod 27: 2598–2606. [DOI] [PubMed] [Google Scholar]
- 41.Blois SM, Alba Soto CD, Tometten M, Klapp BF, Margni RA, and Arck PC. 2004. Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod 70: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 42.Collins MK, Tay CS, and Erlebacher A. 2009. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest 119: 2062–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komastu T, Ireland DD, and Reiss CS. 1998. IL-12 and viral infections. Cytokine Growth Factor Rev 9: 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orange JS, and Biron CA. 1996. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol 156: 1138–1142. [PubMed] [Google Scholar]
- 45.Wilson R, McInnes I, Leung B, McKillop JH, and Walker JJ. 1997. Altered interleukin 12 and nitric oxide levels in recurrent miscarriage. Eur J Obstet Gynecol Reprod Biol 75: 211–214. [DOI] [PubMed] [Google Scholar]
- 46.Comba C, Bastu E, Dural O, Yasa C, Keskin G, Ozsurmeli M, Buyru F, and Serdaroglu H. 2015. Role of inflammatory mediators in patients with recurrent pregnancy loss. Fertil Steril 104: 1467–1474 e1461. [DOI] [PubMed] [Google Scholar]
- 47.Kotzky K, Allen JE, Robinson LR, Satterfield-Nash A, Bertolli J, Smith C, Ornelas Pereira I, Faria ESSAC, and Peacock G. 2019. Depressive Symptoms and Care Demands Among Primary Caregivers of Young Children with Evidence of Congenital Zika Virus Infection in Brazil. J Dev Behav Pediatr 40: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDermott DS, and Varga SM. 2011. Quantifying antigen-specific CD4 T cells during a viral infection: CD4 T cell responses are larger than we think. J Immunol 187: 5568–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimura D, Miyakoda M, Kimura K, Honma K, Hara H, Yoshida H, and Yui K. 2016. Interleukin-27-Producing CD4(+) T Cells Regulate Protective Immunity during Malaria Parasite Infection. Immunity 44: 672–682. [DOI] [PubMed] [Google Scholar]
- 50.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, and Harty JT. 2011. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rai D, Pham NL, Harty JT, and Badovinac VP. 2009. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol 183: 7672–7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pardy RD, Rajah MM, Condotta SA, Taylor NG, Sagan SM, and Richer MJ. 2017. Analysis of the T Cell Response to Zika Virus and Identification of a Novel CD8+ T Cell Epitope in Immunocompetent Mice. PLoS Pathog 13: e1006184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng SB, and Sharma S. 2015. Interleukin-10: a pleiotropic regulator in pregnancy. Am J Reprod Immunol 73: 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, and Wegmann TG. 1993. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol 151: 4562–4573. [PubMed] [Google Scholar]
- 55.Smith SD, Dunk CE, Aplin JD, Harris LK, and Jones RL. 2009. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol 174: 1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faas MM, Spaans F, and De Vos P. 2014. Monocytes and macrophages in pregnancy and pre-eclampsia. Front Immunol 5: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nathan CF, Murray HW, Wiebe ME, and Rubin BY. 1983. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 158: 670–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stein M, Keshav S, Harris N, and Gordon S. 1992. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 176: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mills CD, Kincaid K, Alt JM, Heilman MJ, and Hill AM. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164: 6166–6173.10843666 [Google Scholar]
- 60.Gustafsson C, Mjosberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, and Ernerudh J. 2008. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One 3: e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laskarin G, Cupurdija K, Tokmadzic VS, Dorcic D, Dupor J, Juretic K, Strbo N, Crncic TB, Marchezi F, Allavena P, Mantovani A, Randic L, and Rukavina D. 2005. The presence of functional mannose receptor on macrophages at the maternal-fetal interface. Hum Reprod 20: 1057–1066. [DOI] [PubMed] [Google Scholar]
- 62.Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, and Ernerudh J. 2011. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol 187: 3671–3682. [DOI] [PubMed] [Google Scholar]
- 63.Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, Schinazi RF, Chakraborty R, and Suthar MS. 2016. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe 20: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, and Jung S. 2008. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest 118: 3954–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morelli AE, and Thomson AW. 2007. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol 7: 610–621. [DOI] [PubMed] [Google Scholar]
- 66.Kapsenberg ML 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 3: 984–993. [DOI] [PubMed] [Google Scholar]
- 67.Domogalla MP, Rostan PV, Raker VK, and Steinbrink K. 2017. Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity. Front Immunol 8: 1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winkler CW, Myers LM, Woods TA, Messer RJ, Carmody AB, McNally KL, Scott DP, Hasenkrug KJ, Best SM, and Peterson KE. 2017. Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes. J Immunol 198: 3526–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fuse S, Obar JJ, Bellfy S, Leung EK, Zhang W, and Usherwood EJ. 2006. CD80 and CD86 control antiviral CD8+ T-cell function and immune surveillance of murine gammaherpesvirus 68. J Virol 80: 9159–9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edelmann KH, and Wilson CB. 2001. Role of CD28/CD80–86 and CD40/CD154 costimulatory interactions in host defense to primary herpes simplex virus infection. J Virol 75: 612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bertram EM, Lau P, and Watts TH. 2002. Temporal segregation of 4–1BB versus CD28-mediated costimulation: 4–1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol 168: 3777–3785. [DOI] [PubMed] [Google Scholar]
- 72.Thebeau LG, and Morrison LA. 2003. Mechanism of reduced T-cell effector functions and class-switched antibody responses to herpes simplex virus type 2 in the absence of B7 costimulation. J Virol 77: 2426–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lenschow DJ, Walunas TL, and Bluestone JA. 1996. CD28/B7 system of T cell costimulation. Annu Rev Immunol 14: 233–258. [DOI] [PubMed] [Google Scholar]
- 74.Luckheeram RV, Zhou R, Verma AD, and Xia B. 2012. CD4(+)T cells: differentiation and functions. Clin Dev Immunol 2012: 925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maloy KJ, Burkhart C, Junt TM, Odermatt B, Oxenius A, Piali L, Zinkernagel RM, and Hengartner H. 2000. CD4(+) T cell subsets during virus infection. Protective capacity depends on effector cytokine secretion and on migratory capability. J Exp Med 191: 2159–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, and Murphy KM. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260: 547–549. [DOI] [PubMed] [Google Scholar]
- 77.Martinez-Sanchez ME, Huerta L, Alvarez-Buylla ER, and Villarreal Lujan C. 2018. Role of Cytokine Combinations on CD4+ T Cell Differentiation, Partial Polarization, and Plasticity: Continuous Network Modeling Approach. Front Physiol 9: 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, and Paul WE. 2008. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Immunol 181: 2943–2951. [PubMed] [Google Scholar]
- 79.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, and Stockinger B. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24: 179–189. [DOI] [PubMed] [Google Scholar]
- 80.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, and Littman DR. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8: 967–974. [DOI] [PubMed] [Google Scholar]
- 81.Askenase MH, Han SJ, Byrd AL, Morais da Fonseca D, Bouladoux N, Wilhelm C, Konkel JE, Hand TW, Lacerda-Queiroz N, Su XZ, Trinchieri G, Grainger JR, and Belkaid Y. 2015. Bone-Marrow-Resident NK Cells Prime Monocytes for Regulatory Function during Infection. Immunity 42: 1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


