Abstract
Study Objectives
Obstructive sleep apnea (OSA) is characterized by recurrent partial to complete upper airway obstructions during sleep, leading to repetitive arousals and oxygen desaturations. Although many OSA biomarkers have been reported individually, only a small subset have been validated through both cross-sectional and intervention studies. We sought to profile serum protein biomarkers in OSA in unbiased high throughput assay.
Methods
A highly multiplexed aptamer array (SomaScan) was used to profile 1300 proteins in serum samples from 713 individuals in the Stanford Sleep Cohort, a patient-based registry. Outcome measures derived from overnight polysomnography included Obstructive Apnea Hypopnea Index (OAHI), Central Apnea Index (CAI), 2% Oxygen Desaturation index, mean and minimum oxygen saturation indices during sleep. Additionally, a separate intervention-based cohort of 16 individuals was used to assess proteomic profiles pre- and post-intervention with positive airway pressure.
Results
OAHI was associated with 65 proteins, predominantly pathways of complement, coagulation, cytokine signaling, and hemostasis which were upregulated. CAI was associated with two proteins including Roundabout homolog 3 (ROBO3), a protein involved in bilateral synchronization of the pre-Bötzinger complex and cystatin F. Analysis of pre- and post intervention samples revealed IGFBP-3 protein to be increased while LEAP1 (Hepicidin) to be decreased with intervention. An OAHI machine learning classifier (OAHI >=15 vs OAHI<15) trained on SomaScan protein measures alone performed robustly, achieving 76% accuracy in a validation dataset.
Conclusions
Multiplex protein assays offer diagnostic potential and provide new insights into the biological basis of sleep disordered breathing.
Keywords: apnea, proteomics, polysomnography, serum, sleep-disordered breathing, biomarkers, oxygen saturation
Statement of Significance.
Sleep apnea is a prevalent sleep disorder caused by recurrent collapse of upper airway leading to oxygen desaturation and arousals, with consequences for increased daytime sleepiness, impaired performance, and cardiovascular morbidity. Although, overnight polysomnography (PSG) is the gold standard in diagnosis of sleep apnea, it is costly, cumbersome, and limited in availability. Here we implemented blood serum-based proteomic assays in 713 individuals to find protein biomarkers of apnea correlating these measures with gold-standard PSG. Obstructive sleep apnea was associated with 65 proteins, predominantly modulating complement and coagulation pathways, while central apnea was associated with ROBO3 and cystatin F proteins. Our study identifies proteomic signatures and associated biological pathways in sleep apnea.
Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder whose prevalence increases with obesity. It occurs more frequently in men, although the gender ratio equalizes following menopause in women [1]. OSA is characterized by recurrent complete or partial upper airway obstruction during sleep resulting in oxygen desaturation and repetitive arousals. In OSA, sleep fragmentation results in excessive daytime sleepiness [2–4] and recurrent hypoxemia resulting in a predisposal to cardiometabolic disorders and increased cardiovascular risk [2, 5–10].
The gold-standard diagnostic test for OSA is attended overnight polysomnography (PSG) in a sleep laboratory [11]. OSA severity is typically evaluated based on the average number of apneas and hypopneas per hour of sleep or the apnea–hypopnea index (AHI). This reliance on AHI is problematic because the number varies greatly depending on the hypopnea definition. The American Academy of Sleep Medicine (AASM) criteria define hypopnea as at least 10 s of reduced (30%) upper airway respiratory flow resulting in either a 3% oxygen desaturation or/and an electroencephalogram arousal [12], while the “Medicare” definition of hypopnea (generally used for older patients) is 10 s of reduced airflow with at least 4% oxygen desaturation. Based on AASM criterion, Peppard et al. [13] estimated that 26% of adults between 30 and 70 years have an AHI greater than 5 and 10% have an AHI greater than 15. Other recent studies have reported the prevalence of moderate-to-severe sleep apnea (AHI ≥15) at 23.4% and 49.7% in women and men, respectively [14]. Some studies emphasize the 4% desaturation criterion for scoring hypopneas to focus on the increased cardiovascular risk due to hypoxemia because this definition tends to exclude events leading to arousals. Other studies use AASM’s definition with a cut point of AHI at least 15, because cardiovascular risk is elevated at this level. The Medicare definition of hypopnea tends to miss patients with early disease where events are short and primarily associated with arousals (i.e. patients who are young, female, and lean) [2, 15]. Although the cardiovascular effects of OSA without hypoxemia are not established, these patients experience issues with daytime alertness [2]. Furthermore, in a recent population-based study, those with high arousal-based indices were found to transition to events with hypoxemia with aging and/or increasing weight [2], suggesting mild disease cannot be ignored.
Alternatives to overnight PSG measures of AHI are Home Sleep Testing (HST), devices that are increasingly employed for OSA diagnosis. HST devices have limitations. They generally measure respiratory effort and oxygen saturation without electroencephalography, so cannot capture arousals and sleep fragmentation associated with OSA. AHI is generally underestimated because there is variation in both the number and type of sensors depending on the device. Additionally, sensors can shift during these unattended studies, resulting in unreliable measurements.
In summary, the prevalence of OSA is high (particularly with the obesity pandemic) and accurately diagnosing OSA is challenging and costly. Qualified sleep laboratories are not universally available resulting in delayed diagnosis. HST has become more acceptable but misses arousals and sleep fragmentation. Therefore, there is a need to develop more efficient and cost-effective approaches to OSA diagnosis. An ideal biomarker should correlate with severity of disease and also indicate treatment response. The biomarker should help differentiate cases with recurrent hypoxemia versus with arousal/sleep fragmentation only and help differentiate between obstructive and central apnea. Identifying key biomarkers of OSA will also facilitate our understanding of its pathophysiology and complications. Although numerous efforts to profile biomarkers in OSA have been reported, none have been consistently reproduced nor do they meet the criteria for routine diagnostic use. Some studies have used high-throughput gene expression assays in moderate-to-severe OSA with reports of increased expression of endothelial junction, proapoptotic [16] and inflammatory gene signatures [17]. Studies have profiled microRNAs [18] and found that myocardial ischemia and heart failure associated microRNAs to be elevated in OSA [19]. Other studies used more conventional single- or low-throughput multiprotein measurements either by ELISA or Luminex to profile differences in OSA versus controls using plasma serum or CSF. Notably, reports showed associations with elevated Tau [20–23], amyloid beta [24, 25] in CSF, elevated blood IL-6 cytokine levels [22, 26–37], CRP [26, 32, 34], increased insulin [37], and elevated monocyte to high-density lipoprotein (HDL) cholesterol ratio [38]. Other groups have performed high-throughput Luminex-based proteomic assays with a focus on characterizing the cognitive impairment in OSA by profiling 254 serum proteins. These authors found a prominent insulin-related protein signature [39]. Notably, several others have utilized mass spectrometry. Characterization of the red blood cell proteome in OSA patients found associations with proteins involved in catalytic oxidoreductase and response to stress [40], while dysregulation of lipids was found by other groups [41].
Developing suitable biomarkers has been hampered by the inability to measure multiple biomarkers in the same patient cohort. Recent technological advances have enabled time-efficient, cost-effective measurement of multiple circulating biomarkers. In this study, we used the SomaScan array to profile 1,300 proteins to identify novel sleep apnea biomarkers and to develop multivariate constructs to predict sleep apnea phenotypes based on proteomic profiles.
Methods
The Stanford sleep cohort
The Stanford sleep cohort includes 1,070 participants aged 18–91 years enrolled at the Stanford Sleep Clinic starting in 1999, from which a subset of 713 individuals were used as part of this current study (Table 1) [42, 43]. Approximately 8.5–10.0 mL of blood was drawn from each participant (typically fasting) the morning after the initial diagnostic overnight PSG using one glass red-top serum Vacutainer tube and allowed to clot for a minimum of 30 min, the serum was then aliquoted and stored at −80°C until assay. Laboratory PSG studies for cohort participants were scored using the alternate AASM hypopnea definition for AHI and standard criteria for the central apnea index (CAI) [12]. The lowest oxygen saturation values were also available. Approximately 49.2% of participants had an obstructive apnea hypopnea index (OAHI, with hypopneas defined with arousal or 3% desaturation) above or equal to 15/h. The first inclusion criteria were participants who had both PSG and SomaScan proteomics (n = 772 participants), from here participants who did not have continuous positive airway pressure (CPAP) treatment nor non-missing demographic variables, for example, age, gender, body mass index (BMI), and date of PSG were included in the analysis (n = 713). No prior sample size calculations were performed owing to the design of the study which was exploratory with an aim to generate unbiased hypotheses.
Table 1.
Summary of Variables Classified by Apnea Status in the Study Cohort (n = 713)
| Variables | Moderate/severe (n = 351) (Range or N) | Control/mild apnea (n = 362) (Range or N) | p | Statistic | 95% CI |
|---|---|---|---|---|---|
| PSG variables | |||||
| Sleep stage s1 % | 14.34 (0–66.6) | 9.62 (0–49.46) | 1.3e−10 | 6.53 | 3.3 ± 6.13 |
| Sleep stage s2 % | 62.13 (19.52–91.94) | 63.27 (14.92–98.2) | 0.21180 | −1.25 | −2.94 ± 0.65 |
| Sleep stage s3 % | 4.61 (0–25.26) | 6.73 (0–36.88) | 1.4e−06 | −4.87 | −2.98 ± −1.27 |
| Sleep stage s4 % | 2.56 (0–28.85) | 3.7 (0–44.63) | 0.00994 | −2.59 | −2 ± −0.27 |
| REM ratio % | 16.37 (0–37.13) | 16.67 (0–36.28) | 0.55591 | −0.59 | −1.32 ± 0.71 |
| Sleep efficiency % | 77.77 (27.34–97.38) | 78.16 (21.91–98.1) | 0.68846 | −0.4 | −2.33 ± 1.54 |
| 2% Oxygen desaturation events | 162.89 (5–651) | 61.68 (0–459) | <2e−16 | 15.56 | 88.43 ± 113.99 |
| 3% Oxygen desaturation events | 76.9 (0–432) | 21.13 (0–239) | <2e−16 | 13.32 | 47.54 ± 64.01 |
| Mean SaO2 % | 95.39 (16.2–99.4) | 96.3 (0–100.2) | 0.03624 | −2.1 | −1.75 ± −0.06 |
| Low SaO2 % | 87.53 (32–98.3) | 92.3 (76.7–98.9) | <2e−16 | −11.59 | −5.58 ± −3.96 |
| Baseline SaO2 % awake | 97.4 (92.38–100) | 97.94 (92.68–100.08) | 9.2e−06 | −4.47 | −0.77 ± −0.3 |
| Demographic variables | |||||
| Age (years) | 48.87 (18.9–90.5) | 42.49 (13–77.9) | 2.0e−10 | 6.45 | 4.44 ± 8.32 |
| BMI | 28.44 (9.77–73.52) | 25.89 (15.08–78.66) | 1.3e−07 | 5.34 | 1.61 ± 3.49 |
| Height (m) | 1.74 (1.01–2.27) | 1.72 (1.22–1.98) | 0.02436 | 2.26 | 0 ± 0.03 |
| Weight (kg) | 85.79 (39.7–170.1) | 76.61 (43.5–227.32) | 4.0e−10 | 6.35 | 6.34 ± 12.02 |
| Gender, male % | 67.5% (237) | 52.4% (184) | 6.7e−06 | 0.5 | 0.36 ± 0.68 |
| Systolic BP (mm/Hg) | 129.22 (90–181) | 124.6 (84–184) | 0.00049 | 3.5 | 2.03 ± 7.2 |
| Diastolic BP (mm/Hg) | 80.67 (40–112) | 77.89 (33–116) | 0.00164 | 3.16 | 1.05 ± 4.5 |
| Comorbidities | |||||
| Hypertension | 42.7% (150) | 30.5% (107) | 0.00032 | 0.56 | 0.41 ± 0.78 |
| Depression | 15.1% (53) | 19.7% (69) | 0.16526 | 1.32 | 0.88 ± 2 |
| Asthma | 3.4% (12) | 4.8% (17) | 0.45045 | 1.39 | 0.62 ± 3.25 |
| Thyroid disorders | 5.7% (20) | 2.6% (9) | 0.03637 | 0.42 | 0.17 ± 0.99 |
| Type 2 diabetes | 1.4% (5) | 1.4% (5) | 1.00000 | 0.97 | 0.22 ± 4.25 |
| Gastroesophageal reflux disease | 6% (21) | 4.3% (15) | 0.30604 | 0.68 | 0.32 ± 1.41 |
| Hypercholesterolemia | 26.2% (92) | 16.8% (59) | 0.00132 | 0.55 | 0.37 ± 0.8 |
| Blood variables | |||||
| Glucose (mg/dL) | 91.32 (46–174) | 88.34 (4–205) | 0.01594 | 2.42 | 0.56 ± 5.4 |
| Triglycerides (mg/dL) | 141.35 (25–741) | 121.85 (28–508) | 0.00223 | 3.07 | 7.03 ± 31.97 |
| Low density lipoproteins (mg/dL) | 129.69 (52–251) | 123.09 (19–331) | 0.01370 | 2.47 | 1.36 ± 11.85 |
| High density lipoproteins (mg/dL) | 47.85 (6–106) | 52.46 (23–100) | 2.5e−05 | −4.24 | −6.74 ± −2.47 |
| Total cholesterol (mg/dL) | 197.47 (105–345) | 193.01 (87–339) | 0.13147 | 1.51 | −1.34 ± 10.26 |
| Very low density lipoproteins (mg/dL) | 8.17 (2–66) | 7.7 (2–32) | 0.75662 | 0.31 | −2.54 ± 3.49 |
Fisher’s exact test for categorical variables or student’s t-test was performed for continuous variables. 95% CI is the difference in means between the apnea and control.
OSA prevalence (moderate to severe ≥15 events/h) was 49.2% (n = 351/713) and average age in the moderate-to-severe apnea group was 48.8 years and predominantly male (67.5%; p = 6.7e–06) with significantly increased BMI (mean BMI 28.4 vs 25.8; p = 1.3e−07). The cohort was not followed up for any neurodegenerative disorders nor was there any data on comorbidities such as stroke, dementia, mild cognitive impairment, and congestive heart failure (likely low prevalence), but hypertension (42.7% vs 30.5%) and hypercholesteremia (26.2% vs 16.8%) was predominant in the moderate-to-severe apnea group (p < 0.001). Notably, blood HDL levels were significantly decreased in the moderate-to-severe apnea group (mean HDL 47.8 vs 52.46; p p = 2.5e–05), while other variables were unremarkable. Table 1 describes other clinical characteristics including summary PSG data stratified by moderate-to-severe apnea versus mild/control apnea.
Positive airway pressure intervention cohort
Plasma samples from 16 participants in a study at Washington University in Saint Louis, described in detail elsewhere [44], were used for protein measures. These 16 individuals had mild OSA (AHI of ≥5 and <15/h) or moderate‐to‐severe OSA (AHI ≥15/h) and gave a preintervention blood sample. The participants received positive airway pressure (PAP) treatment. Participants adherent to PAP, defined as usage at least 4 h on at least 70% of 30 preceding nights recorded by the PAP machine, provided a postintervention blood sample. The 16 individuals were randomly selected to assay proteomics from a bigger cohort described in detail elsewhere [44].
Protein measures
The relative expression levels of 1,300 serum proteins were assayed with SomaScan, a highly multiplexed aptamer approach (see Supplementary Table S1 for a complete list of proteins assayed) as previously detailed elsewhere [45–48]. Several studies have performed evaluation of the SomaScan platform by characterizing protein quantitative trait loci (pQTL) and then comparing them to a Luminex-based platform [49, 50] with good concordance. SomaScan (SomaLogic Inc., Boulder CO) was designed to have extended dynamic range from fM to μM, with both extracellular and intracellular proteins (including soluble domains of membrane proteins) being included with predominantly proteins in the secretome being targeted. Serum (150 μL of each sample) was used for protein measurement assay. More detailed information on the procedure can be found at the manufacturer’s website (http://somalogic.com/wp-content/uploads/2017/06/SSM-002-Technical-White-Paper_010916_LSM1.pdf). Data quality control (QC) was performed by SomaScan (described in detail in http://somalogic.com/wp-content/uploads/2017/06/SSM-071-Rev-0-Technical-Note-SOMAscan-Data-Standardization.pdf) at both sample and protein levels. Sample-level QC involved using hybridization controls during hybridization to adjust for systematic variability, further median of the signal over all the protein dilution sets (0.005%, 1%, and 40%) was used to adjust for within-run variability. These hybridization and median scale factors were used to normalize data across samples in a run and acceptance criteria were for these values to be in the range of 0.5–1.8. Protein-level QC involved using same replicate calibrator serum sample, and median values from the calibrator sample signal are used to calculate a scale factor to correct for between-run variability.
PSG data
About 713 participants had PSG data available in European Data Format. The following parameters were extracted: baseline wake oxygen saturation and oxygen desaturation index (ODI) as described in the study of Koch et al. [2], detailed description of the subsample can be found in the studies of Andlauer et al. [51] and Moore et al. [52]. All indices were parsed from scored event files using custom python scripts. Parameters of interest chosen wake baseline oxygen saturation (SaO2), ODI2% and ODI3%, minimum oxygen saturation, AHI, OAHI, and CAI after performing cross-correlation with other PSG variables (data not shown).
SomaScan data analysis
SomaScan data were received in ADAT format and were parsed with custom scripts in python into a tidy format [53]. SomaLogic performed both inter- and intra-assay normalization as described previously [46]. The 1,300 proteins were measured in three dilutions (0.005%, 1%, and 40%) to capture the dynamic range (see Supplementary Table S1 for details). Principal component plots were computed to gauge underlying data structure and no deviations or outliers from expected structure were found by us or other researchers who used the platform [54]. Boxplots of RFU (relative fluorescence units) protein measures binned by individuals were further visually inspected, and there were no individuals with consistently high interquartile range greater than 75 relative to other individuals. Log-normalized RFU protein measures were analyzed for associations in a linear model with empirical Bayes moderation using the Limma library in R [55]. The model design included covariates such as Age, Gender, BMI, BMI2, Age × Gender × BMI, and years from blood draw to assay.
In the intervention cohort, the SomaScan protein measures were analyzed in a paired approach, comparing preintervention to postintervention profiles within each individual. Significance analysis of microarrays [56] was used in paired mode to find proteins differentially expressed utilizing a permutation procedure to estimate null statistical distribution, local false discovery rate (FDR) was estimated as described by Storey et al. [57], and corresponding q values computed.
Pathway analysis
All 5% FDR significant protein sets were analyzed for biological pathway enrichment using the module Toppfun of the Toppgene suite [58]. The toppgene suite determines the similarity between candidate and known proteins/genes with the exception that this is modeled as network structure. This entails modeling proteins/genes as nodes in a graph while interactions between proteins/genes are modeled as edges or connections between the nodes. The goal of this network-based prioritization is to identify nodes (proteins/genes) that are relevant to biological processes or diseases. Candidate proteins/genes are scored based on their network distance to the known protein/genes.
OAHI classifier
Protein measures were used to train a lasso model with L1 regularization [59] to predict OAHI outcome (OAHI ≥15 or OAHI <15). The hyperparameters of the models were tuned via a 10-fold cross-validation approach: A 75% train–25% test split was adopted, SomaScan data on 549 individuals was used for model training, while the remaining data on 164 individuals was used for validation (total sample size 713 individuals). Three models were trained to classify OAHI. Model 1 incorporated demographic information (age, gender, and BMI) in addition to the protein measures, model 2 incorporated only protein measures, and model 3 incorporated only demographic information. All used same test-train split and same lasso-based approach. The R glmnet library [60] was used to train A L1 regularized lasso models [59] and class-balanced test-train splits and metrics were computed using caret library [61]. Receiver operating curves (ROCs) were constructed using R libraries plotROC and ggplot2 and statistical differences between the different model area under the curves (AUCs) were calculated using pROC package [62].
Results
Differential expression of proteins associated with OAHI
SomaScan protein measures were modeled as linear functions of OAHI adjusted for demographic variables (age, gender, and BMI) in 713 individuals. Sixty-five proteins (34 upregulated and 31 downregulated) were identified as differentially expressed at 5% FDR (Table 2). Among the top differentially expressed proteins (DEPs) (FDR p-value <0.005) were tPA (tissue-type plasminogen activator), laminin, aminoacylase-1, growth hormone receptor, and IL-18 Ra proteins which were upregulated, that is, positively correlated with OAHI index, while IGFBP-1 (insulin-like growth factor-binding protein 1), carbonic anhydrase I, and UNC5H4 (Netrin receptor UNC5D) were downregulated and negatively correlated with OAHI (see Supplementary Table S2 for full list of associations). Robust adjustment for BMI as described in Methods was performed as it is a major risk factor for elevated OAHI [1]. After adjustment, we did not find any evidence of residual effects of BMI on the differentially associated proteins as indicated by absence of BMI-associated proteins, for instance, leptin (data not shown).
Table 2.
Top differentially expressed proteins (n = 65) associated with Apnea index (OAHI) post 5% FDR adjustment.
| Protein Variables | t | p | LogFC | B (log odds) | Adjusted p |
|---|---|---|---|---|---|
| Laminin | 4.851424 | 1.51E−06 | 0.003258 | 2.075003 | 0.000980002 |
| tPA | 4.893682 | 1.23E−06 | 0.003815 | 2.274444 | 0.000980002 |
| IGFBP-1 | −4.58588 | 5.35e−06 | −0.00972 | 0.858886 | 0.002314424 |
| Aminoacylase-1 | 4.22325 | 2.73e−05 | 0.006367 | −0.69741 | 0.006852415 |
| Carbonic anhydrase I | −4.26599 | 2.26e−05 | −0.01055 | −0.5203 | 0.006852415 |
| Growth hormone receptor | 4.18514 | 3.21e−05 | 0.003941 | −0.85391 | 0.006852415 |
| IL-18 Ra | 4.152204 | 3.7e−05 | 0.0019 | −0.98808 | 0.006852415 |
| LG3BP | 4.079381 | 5.03e−05 | 0.004581 | −1.28114 | 0.008160917 |
| UNC5H4 | −4.02498 | 6.32e−05 | −0.00321 | −1.49685 | 0.00910507 |
| TFPI | 3.97311 | 7.83e−05 | 0.002999 | −1.69992 | 0.010152077 |
| Factor H | 3.89366 | 0.000108 | 0.001877 | −2.0061 | 0.012500848 |
| NRX1B | −3.87705 | 0.000116 | −0.00343 | −2.06937 | 0.012500848 |
| MBD4 | −3.83584 | 0.000136 | −0.00243 | −2.22522 | 0.013609224 |
| Coagulation Factor IXab | 3.799048 | 0.000158 | 0.002919 | −2.36299 | 0.014262141 |
| DHH | −3.75605 | 0.000187 | −0.00364 | −2.52238 | 0.014262141 |
| Factor I | 3.784641 | 0.000167 | 0.001665 | −2.41659 | 0.014262141 |
| PDE11 | 3.768198 | 0.000178 | 0.002108 | −2.47753 | 0.014262141 |
| Integrin a1b1 | 3.739266 | 0.0002 | 0.003899 | −2.58415 | 0.014382903 |
| Coagulation Factor IX | 3.697546 | 0.000235 | 0.002423 | −2.73649 | 0.015106209 |
| Endothelin-converting enzyme 1 | 3.670881 | 0.00026 | 0.00183 | −2.83299 | 0.015106209 |
| GDF2 | −3.67736 | 0.000254 | −0.00423 | −2.8096 | 0.015106209 |
| LYVE1 | −3.70172 | 0.000231 | −0.00312 | −2.72133 | 0.015106209 |
| S100A4 | −3.65204 | 0.00028 | −0.00527 | −2.90078 | 0.015106209 |
| suPAR | 3.662345 | 0.000269 | 0.001941 | −2.86375 | 0.015106209 |
| SAP | 3.601961 | 0.000338 | 0.002407 | −3.07932 | 0.017541381 |
| CD70 | 3.55832 | 0.000398 | 0.003407 | −3.23296 | 0.018453038 |
| IFN-lambda 1 | −3.57117 | 0.00038 | −0.0023 | −3.18792 | 0.018453038 |
| MCP-4 | −3.56662 | 0.000386 | −0.00346 | −3.20389 | 0.018453038 |
| PLPP | −3.52351 | 0.000453 | −0.00455 | −3.35423 | 0.020282104 |
| Calcineurin | 3.455373 | 0.000583 | 0.002789 | −3.58825 | 0.023488989 |
| Coagulation Factor Xa | 3.444959 | 0.000605 | 0.002159 | −3.62363 | 0.023488989 |
| NACA | −3.45357 | 0.000586 | −0.00528 | −3.5944 | 0.023488989 |
| NOTC2 | 3.440176 | 0.000616 | 0.004877 | −3.63984 | 0.023488989 |
| Peroxiredoxin-6 | −3.47393 | 0.000544 | −0.00683 | −3.52497 | 0.023488989 |
| a-Synuclein | −3.42896 | 0.000641 | −0.00688 | −3.67778 | 0.023765541 |
| C5b, 6 Complex | 3.404628 | 0.0007 | 0.001938 | −3.75967 | 0.024456845 |
| CD39 | 3.398232 | 0.000717 | 0.001537 | −3.7811 | 0.024456845 |
| IL-13 Ra1 | 3.412849 | 0.00068 | 0.001627 | −3.73207 | 0.024456845 |
| CK2-A1:B | −3.3751 | 0.000779 | −0.00558 | −3.85828 | 0.025890244 |
| IFN10 | 3.366182 | 0.000804 | 0.004 | −3.88791 | 0.026060153 |
| EPB41 | −3.34035 | 0.000881 | −0.00779 | −3.97328 | 0.027206702 |
| Myokinase, human | −3.3412 | 0.000878 | −0.00762 | −3.97049 | 0.027206702 |
| NADPH-P450 Oxidoreductase | 3.325114 | 0.00093 | 0.004275 | −4.02334 | 0.028045661 |
| PAFAH beta subunit | −3.3062 | 0.000994 | −0.00353 | −4.08515 | 0.029295837 |
| Heparin cofactor II | 3.288655 | 0.001057 | 0.002256 | −4.14221 | 0.030462655 |
t is the empirical Bayes moderated t-statistic; B is th empirical Bayes log odds of differential expression.
We further stratified our analysis based on the severity index of OAHI and performed several comparisons outlined below. (1) Proteomic profiles of individuals (n = 351) with moderate-to-severe apnea (OAHI ≥15) were compared to individuals (n = 362) with mild to no apnea (OAHI <15), we found nine proteins to be associated with moderate-to-severe apnea (OAHI ≥15) at 5% FDR (Supplementary Table S3). Interestingly, Factor I and calcineurin proteins were upregulated while SOST (sclerostin), NRX1B (neurexin-1b), GDF-2 (growth differentiation factor 2), lymphotactin, carbonic anhydrase I, CRTAM (cytotoxic and regulatory T cell molecule), and SuPAR (soluble urokinase-type plasminogen activator receptor) to be downregulated. (2) Proteomic profiles of individuals (n = 159) with severe apnea (OAHI ≥30) were compared to control (OAHI <5) individuals (n = 162), at 5% FDR threshold we found carbonic anhydrase I and Factor I proteins to be associated with moderate-to-severe apnea (Supplementary Table S4). We also ran association analyses and compared the proteomic profiles in individuals (n = 176) with moderate apnea (OAHI ≥15 and OAHI <30) to control individuals (OAHI <5, n = 162), at 5% FDR we did not find any proteins to be associated (Supplementary Table S5). Similarly, comparing proteomic profiles in individuals with mild apnea versus control individuals, no DEPs were observed at 5% FDR threshold (Supplementary Table S6).
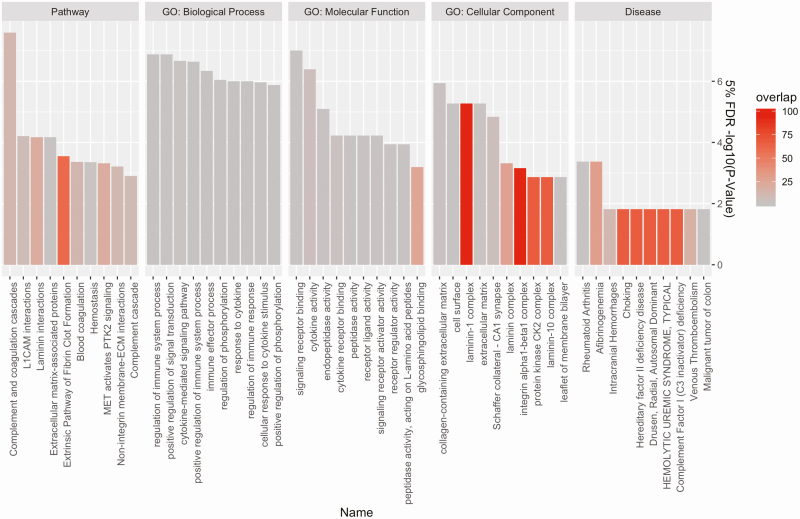
Pathway analysis and gene ontology (GO) biological process analyses done in Toppgene [58] webserver (FDR p-value <0.05) of DEPs in OAHI (modeled as a continuous variable) revealed overrepresentation of DEPs in several pathways (Figure 1)—Complement and coagulation cascades, L1CAM and Laminin interactions, extracellular matrix and extracellular matrix-associated proteins, Extrinsic Pathway of Fibrin Clot Formation and Hemostasis—in general agreement with previous reports of coagulation being associated with apnea events [63, 64]. GO biological processes analysis (FDR p-value <0.05) revealed the involvement of regulation of immune system process, positive regulation of signal transduction and cytokine-mediated signaling pathway, and others (see Supplementary Table S7 for full list). Of additional interest, we also exclusively used either upregulated and downregulated proteins associated with OAHI for overrepresentation in pathways or biological processes, at 5% FDR in agreement with overall analyses, upregulated pathways were complement and coagulation cascades and laminin interactions (Supplementary Table S8), while downregulated pathways at 5% FDR were protein kinase CK2 complex, positive regulation of signal transduction, and organophospate catabolic processes among others (Supplementary Table S9).
Figure 1.
Pathway analysis and GO biological process analyses done in Toppgene webserver of 65 differentially expressed proteins (FDR p-value <0.05) in moderate-to-severe apnea. The y-axis is the negative logarithm 10 of the adjusted p-value of the overrepresented pathways, while the x-axis are the pathways and processes binned by categories. GO: Biological Process: the pathways and larger processes to which that gene product’s activity contributes. GO: Molecular Function: the molecular activities of individual gene products. GO: Cellular Component: where the gene products are active. The color gradient indicates the % shared overlap between the candidate apnea proteins and known pathway/processes-related proteins. GO, gene ontology; L1CAM, L1 cell adhesion molecule; MET, MET proto-oncogene alias hepatocyte growth factor receptor; PTK2, protein tyrosine kinase 2; ECM, extracellular matrix; CA1, the first region in the hippocampal circuit; CK2, casein kinase 2.
ROBO3 protein and Cystatin-F are increased in central apneas
CAI was fit as linear function of protein measures, this analysis revealed ROBO3 (Roundabout homolog 3) and CYTF (Cystatin-F) to be strongly upregulated in central apneas (FDR p-value <5e−4, Table 3 and Supplementary Table S10).
Table 3.
Differentially Expressed Proteins Associated With Central Apnea Index (CAI), 2% Oxygen Desaturation Events, Low Oxygen Saturation Levels (Low SaO2), and Mean Oxygen Saturation Levels (Mean SaO2)
| Protein variables | Variable | t | p | LogFC | B (log odds) | Adjusted p |
|---|---|---|---|---|---|---|
| CYTF | Central apnea | 4.965793 | 8.61e−07 | 0.071963 | 4.894893 | 0.000558 |
| ROBO3 | Central apnea | 6.93464 | 9.29e−12 | 0.052575 | 15.97869 | 1.2E−08 |
| Laminin | 2% Oxygen desaturation events | 4.258327 | 2.38e−05 | 0.004826 | −0.03975 | 0.023049 |
| UNC5H4 | 2% Oxygen desaturation events | −4.11609 | 4.38e−05 | −0.00554 | −0.6197 | 0.023049 |
| tPA | 2% Oxygen desaturation events | 4.069173 | 5.33e−05 | 0.005345 | −0.80692 | 0.023049 |
| UNC5H4 | Low SaO2 | 4.941248 | 9.72e−07 | 1.222975 | 4.817064 | 0.001261 |
| NovH | Mean SaO2 | −4.44758 | 1.01e−05 | −2.50109 | 3.027134 | 0.013093 |
| UNC5H4 | Mean SaO2 | 4.267287 | 2.25e−05 | 3.88582 | 2.340392 | 0.014594 |
All associations were adjusted at 5% FDR. t is the empirical Bayes moderated t-statistic; B is the empirical Bayes log odds of differential expression.
Proteins associated with low oxygen saturation indices
ODI2% was fit as a function of protein measures adjusted for demographic variables, and three proteins were found to be differentially expressed (FDR p-value <0.05). Laminin and tPA were upregulated while UNC5H4 was downregulated (Table 3 and Supplementary Table S11). ODI3% was not associated with any proteins at 5% FDR (data not shown). Mean SaO2 levels during sleep was significantly associated (FDR p-value <0.05) with downregulated NovH (Protein NOV homolog) and upregulated UNC5H4 (Table 3 and Supplementary Table S12). Minimum SaO2 level during sleep was associated only with increased expression of UNC5H4 and trend to decreased tPA protein expression (Table 3 and Supplementary Table S13).
Proteins associated with PAP intervention
Paired analyses of pre- and postintervention samples from the intervention cohort who were treated with PAP showed that IGFBP-3 and BMP-1 (bone morphogenetic protein 1) were increased, while LEAP-1 (hepicidin) was decreased, postintervention (5% FDR, see Supplementary Table S14 for list of proteins).
Moderate-to-severe apnea can be predicted by SomaScan protein panel
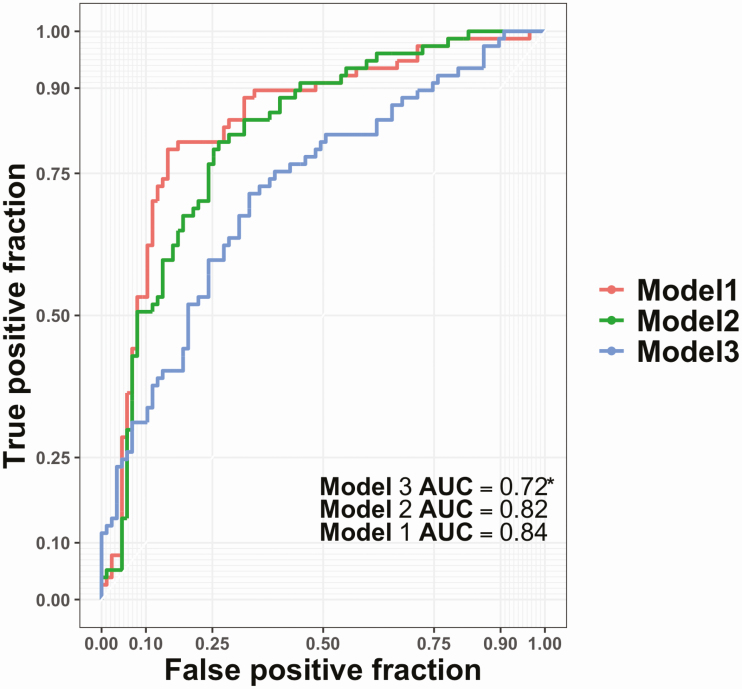
Since we found a relatively large number of proteins (n = 65) differentially expressed and significantly associated with OAHI when modeled as continuous variable and additionally among all the stratified analyses (see above) the largest number of proteins was associated with moderate-to-severe apnea versus mild/control apnea, we capitalized on these associations to train a machine learning classifier on SomaScan protein measures in a test set (n = 549) to predict moderate-to-severe apnea (OAHI ≥15 was treated as case while OAHI <15 was treated as control or mild apnea) in an validation set (n = 164). Model 1 incorporating demographic variables and protein measures achieved 77.5% accuracy in classifying OAHI, model 2 (only SomaScan measures) achieved 76.1% accuracy, and model 3 (only demographic variables) achieved a lower accuracy of 68.3% (see Figure 2 for ROC and AUCs and Table 4 for classifier metrics and Supplementary Table S15 for confusion matrix). The sensitivity for detecting OAHI at least 15 status was 73.8% for model 1, 72.1% for model 2, and 65.1% for model 3. The specificity for identifying control status was 81.2% for model 1, 80.8% for model 2, and 71.6% for model 3. The corresponding negative predictive values (NPVs) were 74.7%, 72.4%, and 66.7% for models 1, 2, and 3, respectively.
Figure 2.
The receiver operating curve (ROC) of an OAHI classifier to classify severe-to-moderate apnea (≥15/h). The training set included 549 individuals and untouched test set included 164 individuals. Three models were trained: model 1 incorporating demographic variables and SomaScan protein measures, model 2 trained only SomaScan protein measures, and model 3 that was trained only demographic variables (age, gender, and BMI). The y-axis represents the true positive fraction while the x-axis represents the false positive fraction. *Model 3 AUC was statistically significant when compared to model 1 and model 2 AUC (p < 0.05).
Table 4.
Performance Metrics of a Machine Learning OAHI Classifiers (OAHI ≥15), the Model Was Trained on 549 Individuals and Validated in 164 Individuals (total individuals = 713)
| Model name | F1 score | Accuracy | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|---|---|
| Model 1 includes SomaScan proteins, age, gender, and BMI | 0.77 | 0.775 | 0.738 | 0.812 | 0.805 | 0.747 | 0.84 |
| Model 2 includes only SomaScan proteins | 0.761 | 0.764 | 0.721 | 0.808 | 0.805 | 0.724 | 0.815 |
| Model 3 includes only age, gender, and BMI | 0.675 | 0.683 | 0.651 | 0.716 | 0.701 | 0.667 | 0.72 |
PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve.
Proteins associated with age of serum samples
The serum samples assayed in this study were collected and processed with a mean delay of 11.6 years (blood draw to assay). Although above results were carefully adjusted for sample age, we observed 535 proteins to have significant (FDR p-value <0.05) differential expression (464 proteins downregulated and 71 upregulated) when age of samples was fit as a function of the protein matrix. The full list of proteins associated with age of samples is given in Supplementary Table S16.
Discussion
To our knowledge, this is the first study to look at highly multiplexed protein biomarkers (1,300) at once in association with sleep apnea and oxygen desaturation indices utilizing an aptamer-based approach (SomaScan assay) in the Stanford sleep cohort of 713 individuals, a patient-based registry. The overall prevalence of OAHI in our cohort was 49.2% predominantly biased in men (67.5 %), while the overall CAI prevalence was relatively lower at 1.3%. We analyzed differential expression patterns in cross-sectional Stanford sleep cohort of 713 individuals as a function of OAHI that revealed 65 proteins to be dysregulated. On the other hand, CAI was associated with only two proteins including ROBO3, a protein involved in bilateral synchronization of the pre-Bötzinger complex and cystatin F. Further analysis of pre- and post-CPAP intervention in longitudinal cohort of 16 individuals was less revealing with three proteins that were differentially associated with active CPAP treatment: IGFBP3 and BMP-1 increased while LEAP1 (hepicidin) decreased with intervention.
We found significant changes with OAHI in 65 proteins, notably, tPA, laminin, growth hormone receptor, IL-18 Ra, aminoacylase-1 (involved in oxidative stress), and LG3BP (adhesion molecule) were increased with apnea while IGFBP-1, carbonic anhydrase I, and UNC5H4 were inversely associated with moderate-to-severe apnea and observed to be decreased. Pathway analysis (FDR p-value <0.05) of DEPs in OAHI revealed overrepresentation of DEPs in complement and coagulation cascades, L1CAM and Laminin interactions, extracellular matrix and extracellular matrix-associated proteins, and extrinsic pathway of fibrin clot formation and hemostasis. These changes show that OAHI is associated with disturbances of multiple pathways, growth factors, cell adhesion factors, enzymes, notably with previous reports of increased coagulation including tPA being associated with apnea events [65, 66], which may play a role in the increased risk of stroke in these patients [67].
tPA is a serine protease found on endothelial cells that line blood vessels and functions to catalyze the conversion of plasminogen to plasmin. Plasminogen is the pro-enzyme of plasmin and is implicated in fibrin degradation in blood vessels [68]. tPA while being circadian dependent [69] has been linked to host of disorders. For instance, tPA increase has been associated with acute myocardial infarction [70, 71] and coronary artery disease [70] and while deficiencies in tpA production have been linked to deep vein thrombosis [72]. Interestingly, previous studies have found an accumulation of fibrin in patients with sleep apnea [73], while other investigators have found increased plasminogen activator inhibitor-1 protein levels in patients with sleep apnea [74]. In agreement our data indicate increased tPA levels in patients with high OAHI indices.
Interestingly, growth hormone receptor and IGFBP-1 proteins were associated with moderate-to-severe apnea in our study, these associations could be attributed to disruptions in growth hormone pathway in which both IGFBP-1 and IGFBP-3 (associated with CPAP treatment in our study) are important conduits [75–77]. It is established that growth hormone is secreted predominantly during slow-wave sleep [78, 79], while OSA is associated with disruptions in slow-wave sleep [80–82]. Furthermore, decreased IGFBP-1 levels have been recognized as important predictors to development of glucose tolerance/diabetes [83–85] and increased cardiovascular risk. Our findings are consistent with these observations and establish dysregulation of insulin and growth hormone pathway associated proteins in apnea. We should however note that the incidence of diabetes was low (<5%) in our cohort, although this could be attributed to missing data. Other data on IGFBP-1 are sparse in the context of apnea, with one study suggesting no association with apnea nor CPAP treatment [86]. Additional studies are warranted to understand the role of these proteins in apnea. Taken together, recurrent episodes of hypoxia and sleep disruption dispose to a state of hypercoagulability and fibrin dysregulation, which in turn may promote the incidence of coronary events in patients with sleep apnea.
The strong positive association of OAHI with IL18RA (IL18 receptor alpha, called IL18R1, 2q12) is particularly interesting considering the interleukin 18 receptor IL18RAP associated protein (IL18 beta chain) is one of two genome-wide significant findings reported for minimum oxyhemoglobin saturation (rs78136548) during sleep [87], a variable correlated with OAHI. IL18R1 and IL18RAP are located close to each other and rs78136548 and linked markers are an eQTL for both transcripts in various tissues, and for the nearby gene SLC9A4, a proton gene pump [87]. As the rs78136548 region linked with minimum oxygen desaturation in the Cade et al. study [87] regulates both subunits of the ILR18, it is plausible that the ILR18 is a causal pathway for sleep apnea. Problematically, however, ILR18RAP was also measured in this study and did not vary with OSA (data not shown). Notably, low oxygen saturation in this study was not significantly associated with IL18RA. Finally, Suhre et al. [50], using the same technology as this study, found significant cis pQTL but not trans QTL for these loci in blood. Additional studies of the IL18 pathway in OSA are thus warranted to follow-up on these findings.
Proteomic signatures associated with CPAP treatment were much less pronounced, we found a cluster of three proteins that were associated with CPAP treatment. Among the three proteins, notably IGFBP-3 protein level was positively correlated with CPAP treatment, this finding is in line with other investigators who also found increased IGFBP-3 protein levels post-CPAP adherence using an ELISA assay [88]. BMP-1 protein was found to be increased in response to CPAP treatment, incidentally it is also associated with moderate-to-severe apnea in our cross-sectional cohort (Table 2), while it is recognized as a major player in tissue remodeling and repair [89], its role in relation to apnea remains to be elucidated. Strikingly, LEAP-1 (hepicidin) was decreased in response to CPAP treatment in the current study, hepicidin is recognized as a liver-derived protein that regulates iron homeostasis and also plays a key role as an antimicrobial agent preferentially inhibiting growth of fungal pathogens. This finding is in agreement with other reports of increased hepcidin in severe apnea [90], although we could not find any association with moderate-to-severe apnea in our cross-sectional cohort.
Central apneas were associated with two proteins, ROBO3 and CYTF. ROBO3 is a critical protein for proper neural migration and axon guidance. At least 19 different mutations in the ROBO3 gene have been identified in people with horizontal gaze palsy with progressive scoliosis. This gene is required for hindbrain axon midline crossing [91]. Interestingly, ROBO3 has been shown to be a critical component of pre-Bötzinger complex (pre-BötC) responsible for pace inspiration. Inactivation of ROBO3 results in left–right de-synchronization of the pre-BötC oscillator, with asymmetric independence of left–right breathing activities and diaphragm contractions in Robo3 null mice, although rhythm generation is unaffected [92]. Although the role of ROBO3 post-development is unknown, it may be that increased ROBO3 levels in central apnea is a compensatory mechanism in response to repeated central apnea events that aim at strengthening the central pacemaker. Previous studies of ROBO3 have identified eQTLs for expression in various tissues, but not in blood using the aptamer technology [50]. Further investigations of the role of ROBO3 in the regulation of central sleep apnea are warranted. CYTF on the other hand is a cysteine protease inhibitor and is selectively enriched in immune cell subsets [93], and CYTF has been reported to have a strong affinity to cathepsin L that is implicated in normal lysomal mediated protein turnover. Interestingly, CYTF has been found to promote neovascularization after ischemia via cathepsin L [94]. It is unclear what role increased CYTF plays in central apneas but it is possible that CYTF promotes tissue repair, further studies are needed to understand this mechanism. Central apnea incidence was 1.9% in our cohort, this is a limitation that larger cohorts will need to address.
Finding a robust blood biomarker for sleep apnea could be very useful in clinical practice. In this work, we used a regularized lasso model to classify moderate-to-severe apnea (OAHI ≥15/h) on 549 participants with validation in 164 additional participants. We found that training a machine learning classifier using only protein measures could achieve 76.1% accuracy (72.1% sensitivity) in classification of moderate-to-severe apnea (OAHI ≥15/h), while a classifier also incorporating demographic variables (age, gender, and BMI) performed with higher accuracy of 77.5%. In comparison, the STOP-Bang questionnaire [95] had higher sensitivity at 94% to detect moderate-to-severe OSA (AHI ≥15/h), but a specificity of 34% and an NPV of 75%. The Berlin questionnaire had 54% sensitivity, 97% specificity to classify moderate-to-severe OSA (AHI ≥15/h) [96]. Our classifier (model 2 using only protein measures) had 80.8% specificity with an NPV of 72.4%. This suggests that although there is modest and comparatively similar performance of the protein measures in classifying moderate-to-severe OSA (AHI ≥15/h) with questionnaire-based methods, larger studies are warranted.
While the aptamer-based approach holds considerable promise in exploratory analysis of sleep disorders and can generate hypotheses that can be studied in greater detail, we note several limitations to our study. First, our serum samples were old, with mean blood draw to assay time being 11.6 years. Sample storage time (the samples were kept at −80ºC) correlated with many measures, and while we controlled for sample age in our analyses, ideally this type of analysis should be conducted on recent samples. Second, all samples were derived at a single sleep center. Replicating findings with samples from other cohorts would be needed to show generalization. Third, the protein expression patterns from the cross-sectional cohort (Stanford sleep cohort) may not be directly comparable to the longitudinal CPAP interventional cohort, this can be attributed to the fact that while Stanford sleep cohort was assayed using serum samples the CPAP cohort used plasma samples. Fourth, we did not have data on comorbidities such as stroke, dementia, mild cognitive impairment, and congestive heart failure in our cohort, these conditions could present as altered protein expression profiles. Finally, the study used an earlier SomaScan panel of proteins that included only 1,300 proteins, while a more recent panel includes 5,500 proteins.
To summarize, this large proteomic analysis of sleep-disordered breathing identified differential protein expression patterns associated with obstructive respiratory events, oxygen desaturations, central apneas, and PAP treatment for OSA. Multiplex protein assays offer diagnostic potential and provide new insights into the biological basis of sleep-disordered breathing.
Supplementary Material
Acknowledgments
We are thankful to the individuals who consented to be studied as part of the Stanford sleep cohort, further we thank Jing Zhang at the Stanford Center for sleep science and medicine for sample biobanking. We thank the anonymous reviewers whose inputs have improved the manuscript.
Funding
The Stanford Sleep cohort was supported by unrestricted funds of Stanford Center for Sleep Sciences and Medicine and NIH 5R01HL071515. Longitudinal CPAP cohort study was supported by National Institutes of Health awards K23-NS089922, UL1RR024992 Sub-Award KL2-TR000450, and the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. SomaScan assays for the entire cohort were supported by Biomedical Research Program at Weill Cornell Medicine Qatar funded by Qatar Foundation.
Disclosure Statement
Nonfinancial disclosures: All authors report no disclosures, we certify that the submission is not under review at any other publication. The corresponding author, EM, takes full responsibility for the data, the analyses and interpretation, and the conduct of the research. He has full access to all of the data, and he has the right to publish any and all data separate and apart from any sponsor.
Financial disclosures: EM occasionally consults and has received contracts from Jazz Pharmaceuticals, is and has been a Principal Investigator on clinical trials using sodium oxybate and Solriamfetol, Jazz Pharmaceutical products, for the treatment of Type 1 Narcolepsy; none of these have any scientific relationships to the current study. There exist no financial or other relationships that might lead to a perceived conflict of interest.
Conflict of interest statement. None declared.
References
- 1. Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 2. Koch H, et al. Breathing disturbances without hypoxia are associated with objective sleepiness in sleep apnea. Sleep. 2017;40(11). doi: 10.1093/sleep/zsx152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colt HG, et al. Hypoxemia vs sleep fragmentation as cause of excessive daytime sleepiness in obstructive sleep apnea. Chest. 1991;100:1542–1548. [DOI] [PubMed] [Google Scholar]
- 4. Roehrs T, et al. Predictors of objective level of daytime sleepiness in patients with sleep-related breathing disorders. Chest. 1989;95:1202–1206. [DOI] [PubMed] [Google Scholar]
- 5. Kim JS, et al. Association of novel measures of sleep disturbances with blood pressure: the Multi-Ethnic Study of Atherosclerosis. Thorax. 2020;75:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shell B, et al. Neural control of blood pressure in chronic intermittent hypoxia. Curr Hypertens Rep. 2016;18:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gottlieb DJ, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peppard PE. Is obstructive sleep apnea a risk factor for hypertension?—differences between the Wisconsin Sleep Cohort and the Sleep Heart Health Study. J Clin Sleep Med. 2009;5:404–405. [PMC free article] [PubMed] [Google Scholar]
- 9. Punjabi NM, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yaggi HK, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. [DOI] [PubMed] [Google Scholar]
- 11. Kapur VK, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berry RB, et al. AASM scoring manual updates for 2017 (version 2.4). J Clin Sleep Med. 2017;13:665–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peppard PE, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heinzer R, et al. Prevalence of sleep apnoea syndrome in the middle to old age general population. Lancet Respir Med. 2016;4:e5–e6. [DOI] [PubMed] [Google Scholar]
- 15. Anttalainen U, et al. Is ‘MILD’ sleep-disordered breathing in women really mild? Acta Obstet Gynecol Scand. 2010;89:605–611. [DOI] [PubMed] [Google Scholar]
- 16. Chen YC, et al. Genome-wide gene expression array identifies novel genes related to disease severity and excessive daytime sleepiness in patients with obstructive sleep apnea. PLoS One. 2017;12:e0176575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dumaine JE, et al. Acute sleep fragmentation induces tissue-specific changes in cytokine gene expression and increases serum corticosterone concentration. Am J Physiol Regul Integr Comp Physiol. 2015;308:R1062–R1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khurana S, et al. Canvassing the aetiology, prognosis and molecular signatures of obstructive sleep apnoea. Biomarkers. 2019;24:1–16. [DOI] [PubMed] [Google Scholar]
- 19. Freitas LS, et al. Severe obstructive sleep apnea is associated with circulating microRNAs related to heart failure, myocardial ischemia, and cancer proliferation. Sleep Breath. (2020):1–10. [DOI] [PubMed] [Google Scholar]
- 20. Bubu OM, et al. Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes. Sleep. 2019;42(6). doi: 10.1093/sleep/zsz048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baril AA, et al. ; Canadian Sleep and Circadian Network. Biomarkers of dementia in obstructive sleep apnea. Sleep Med Rev. 2018;42:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Motamedi V, et al. Elevated tau and interleukin-6 concentrations in adults with obstructive sleep apnea. Sleep Med. 2018;43:71–76. [DOI] [PubMed] [Google Scholar]
- 23. Liguori C, et al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep. 2017;40(5). doi: 10.1093/sleep/zsx011 [DOI] [PubMed] [Google Scholar]
- 24. Ju YE, et al. Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann Neurol. 2016;80:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bu XL, et al. Serum amyloid-beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep. 2015;5:13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fitzgibbons CM, et al. Physical activity in overlap syndrome of COPD and obstructive sleep apnea: relationship with markers of systemic inflammation. J Clin Sleep Med. 2019;15:973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Z, et al. Association study of genetic variations of inflammatory biomarkers with susceptibility and severity of obstructive sleep apnea. Mol Genet Genomic Med. 2019;7:e801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bozic J, et al. Adropin and inflammation biomarker levels in male patients with obstructive sleep apnea: a link with glucose metabolism and sleep parameters. J Clin Sleep Med. 2018;14:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shalitin S, et al. Hepcidin, soluble transferrin receptor and IL-6 levels in obese children and adolescents with and without type 2 diabetes mellitus/impaired glucose tolerance and their association with obstructive sleep apnea. J Endocrinol Invest. 2018;41:969–975. [DOI] [PubMed] [Google Scholar]
- 30. Lu D, et al. Pulmonary surfactant-associated proteins and inflammatory factors in obstructive sleep apnea. Sleep Breath. 2018;22:99–107. [DOI] [PubMed] [Google Scholar]
- 31. Li Y, et al. Objective, but not subjective, sleepiness is associated with inflammation in sleep apnea. Sleep. 2017;40(2). doi: 10.1093/sleep/zsw033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang YS, et al. Inflammatory cytokines in pediatric obstructive sleep apnea. Medicine (Baltimore). 2016;95:e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vicente E, et al. Upper airway and systemic inflammation in obstructive sleep apnoea. Eur Respir J. 2016;48:1108–1117. [DOI] [PubMed] [Google Scholar]
- 34. Fleming WE, et al. Blood biomarkers of endocrine, immune, inflammatory, and metabolic systems in obstructive sleep apnea. Clin Biochem. 2016;49:854–861. [DOI] [PubMed] [Google Scholar]
- 35. Arnardottir ES, et al. Effects of obesity on the association between long-term sleep apnea treatment and changes in interleukin-6 levels: the Icelandic Sleep Apnea Cohort. J Sleep Res. 2015;24:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee RN, et al. Disordered breathing during sleep and exercise in idiopathic pulmonary fibrosis and the role of biomarkers. QJM. 2015;108:315–323. [DOI] [PubMed] [Google Scholar]
- 37. Maeder MT, et al. Comprehensive biomarker profiling in patients with obstructive sleep apnea. Clin Biochem. 2015;48:340–346. [DOI] [PubMed] [Google Scholar]
- 38. Inonu Koseoglu H, et al. Monocyte count/HDL cholesterol ratio and cardiovascular disease in patients with obstructive sleep apnea syndrome: a multicenter study. Clin Appl Thromb Hemost. 2018;24:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lal C, et al. Proteomic biomarkers of cognitive impairment in obstructive sleep apnea syndrome. Sleep Breath. 2019;23:251–257. [DOI] [PubMed] [Google Scholar]
- 40. Feliciano A, et al. Evening and morning alterations in obstructive sleep apnea red blood cell proteome. Data Brief. 2017;11:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silva LOE, et al. Metabolic profile in patients with mild obstructive sleep apnea. Metab Syndr Relat Disord. 2018;16:6–12. [DOI] [PubMed] [Google Scholar]
- 42. Stephansen JB, et al. Neural network analysis of sleep stages enables efficient diagnosis of narcolepsy. Nat Commun. 2018;9:5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andlauer O, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol. 2013;70:891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ju YS, et al. Obstructive sleep apnea treatment, slow wave activity, and amyloid-β. Ann Neurol. 2019;85:291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lourdusamy A, et al. ; AddNeuroMed Consortium; Alzheimer’s Disease Neuroimaging Initiative. Identification of cis-regulatory variation influencing protein abundance levels in human plasma. Hum Mol Genet. 2012;21:3719–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gold L, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One. 2010;5:e15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hathout Y, et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2015;112:7153–7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kraemer S, et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: a SOMAmer-based, streamlined multiplex proteomic assay. PLoS One. 2011;6:e26332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sun BB, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suhre K, et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat Commun. 2017;8:14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Andlauer O, et al. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep. 2012;35:1247–155F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moore H 4th, et al. Design and validation of a periodic leg movement detector. PLoS One. 2014;9:e114565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wickham H. Tidy data J Stat Softw 2014;59(10):1–23.26917999 [Google Scholar]
- 54. Kim CH, et al. Stability and reproducibility of proteomic profiles measured with an aptamer-based platform. Sci Rep. 2018;8:8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smyth GK. Limma: linear models for microarray data. In: Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York, NY: Springer; 2005: 397–420. [Google Scholar]
- 56. Tusher VG, et al. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Storey JD, et al. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen J, et al. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37:W305–W311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Park MY, et al. L1‐regularization path algorithm for generalized linear models. J R Stat Soc Ser B. 2007;69:659–677. [Google Scholar]
- 60. Friedman J, et al. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 61. Kuhn M. Building predictive models in R using the caret package. J Stat Softw. 2008;28:1–26.27774042 [Google Scholar]
- 62. Robin X, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hong SN, et al. Association between hypercoagulability and severe obstructive sleep apnea. JAMA Otolaryngol Head Neck Surg. 2017;143:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. von Känel R, et al. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest. 2003;124:1956–1967. [DOI] [PubMed] [Google Scholar]
- 65. Martin RA, et al. The effect of acute aerobic exercise on hemostasis in obstructive sleep apnea. Sleep Breath. 2017;21:623–629. [DOI] [PubMed] [Google Scholar]
- 66. Steffanina A, et al. The Plasminogen system and transforming growth factor-β in subjects with obstructive sleep apnea syndrome: effects of CPAP treatment. Respir Care. 2015;60:1643–1651. [DOI] [PubMed] [Google Scholar]
- 67. Redline S, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Litvinov RI, et al. What is the biological and clinical relevance of fibrin? Semin Thromb Hemost. 2016;42:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Andreotti F, et al. Circadian variation of fibrinolytic activity in blood. Chronobiol Int. 1991;8:336–351. [DOI] [PubMed] [Google Scholar]
- 70. Wiman B, et al. Plasma levels of tissue plasminogen activator/plasminogen activator inhibitor-1 complex and von Willebrand factor are significant risk markers for recurrent myocardial infarction in the Stockholm Heart Epidemiology Program (SHEEP) study. Arterioscler Thromb Vasc Biol. 2000;20:2019–2023. [DOI] [PubMed] [Google Scholar]
- 71. Bom JGvd, et al. Tissue plasminogen activator and risk of myocardial infarction. Circulation. 1997;95:2623–2627. [DOI] [PubMed] [Google Scholar]
- 72. Juhan-Vague I, et al. Deficient t-PA release and elevated PA inhibitor levels in patients with spontaneous or recurrent deep venous thrombosis. Thromb Haemost. 1987;57: 67–72. [PubMed] [Google Scholar]
- 73. Nobili L, et al. Morning increase of whole blood viscosity in obstructive sleep apnea syndrome. Clin Hemorheol Microcirc. 2000;22:21–27. [PubMed] [Google Scholar]
- 74. Ishikawa J, et al. Increased low-grade inflammation and plasminogen-activator inhibitor-1 level in nondippers with sleep apnea syndrome. J Hypertens. 2008;26:1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Carter-Su C, et al. Growth hormone signaling pathways. Growth Horm IGF Res. 2016;28:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Firth SM, et al. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. [DOI] [PubMed] [Google Scholar]
- 77. Kaplan SA, et al. The somatomedin hypothesis 2007: 50 years later. J Clin Endocrinol Metab. 2007;92:4529–4535. [DOI] [PubMed] [Google Scholar]
- 78. Sassin JF, et al. Human growth hormone release: relation to slow-wave sleep and sleep-walking cycles. Science. 1969;165:513–515. [DOI] [PubMed] [Google Scholar]
- 79. Van Cauter E, et al. A quantitative estimation of growth hormone secretion in normal man: reproducibility and relation to sleep and time of day. J Clin Endocrinol Metab. 1992;74:1441–1450. [DOI] [PubMed] [Google Scholar]
- 80. Heinzer R, et al. Slow-wave activity in sleep apnea patients before and after continuous positive airway pressure treatment: contribution to daytime sleepiness. Chest. 2001;119:1807–1813. [DOI] [PubMed] [Google Scholar]
- 81. Ratnavadivel R, et al. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5:519–524. [PMC free article] [PubMed] [Google Scholar]
- 82. Ren R, et al. Interaction between slow wave sleep and obstructive sleep apnea in prevalent hypertension. Hypertension. 2020;75:516–523. [DOI] [PubMed] [Google Scholar]
- 83. Lewitt MS, et al. Insulin-like growth factor-binding protein-1 in the prediction and development of type 2 diabetes in middle-aged Swedish men. Diabetologia. 2008;51:1135–1145. [DOI] [PubMed] [Google Scholar]
- 84. Sandhu MS, et al. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet. 2002;359:1740–1745. [DOI] [PubMed] [Google Scholar]
- 85. Haywood NJ, et al. Insulin-like growth factor binding protein 1 could improve glucose regulation and insulin sensitivity through its RGD domain. Diabetes. 2017;66:287–299. [DOI] [PubMed] [Google Scholar]
- 86. Shah N, et al. Sleep and insulin-like growth factors in the Cardiovascular Health Study. J Clin Sleep Med. 2013;9:1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cade BE, et al. Associations of variants in the hexokinase 1 and interleukin 18 receptor regions with oxyhemoglobin saturation during sleep. PLoS Genet. 2019;15:e1007739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Archontogeorgis K, et al. Serum levels of vascular endothelial growth factor and insulin-like growth factor binding protein-3 in obstructive sleep apnea patients: effect of continuous positive airway pressure treatment. Open Cardiovasc Med J. 2015;9:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chakraborty S, et al. Role of BMP1/tolloid like proteases in bone morphogenesis and tissue remodeling. In: Chakraborti S, Dhalla NS, eds. Proteases in Physiology and Pathology. Singapore: Springer Singapore; 2017: 77–88. [Google Scholar]
- 90. Liu Y, et al. Association of serum hepcidin levels with the presence and severity of obstructive sleep apnea syndrome. Med Sci Monit. 2015;21:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jen JC, et al. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304:1509–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bouvier J, et al. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13:1066–1074. [DOI] [PubMed] [Google Scholar]
- 93. Hamilton G, et al. Cystatin F is a cathepsin C-directed protease inhibitor regulated by proteolysis. EMBO J. 2008;27:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Urbich C, et al. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med. 2005;11:206–213. [DOI] [PubMed] [Google Scholar]
- 95. Nagappa M, et al. Validation of the STOP-bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One. 2015;10:e0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Netzer NC, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.