Abstract
Background and Aim
Myosteatosis is a prognostic factor in cancer and liver cirrhosis. It can be determined noninvasively using computed tomography or, as shown recently, by magnetic resonance (MR) imaging. The primary aim was to analyze the reproducibility of skeletal muscle signal intensity on routine MR‐enterographies, as indicator of myosteatosis, in Crohn's disease (CD) and to explore the association between skeletal muscle signal intensity at diagnosis with time to intestinal resection.
Methods
CD patients undergoing MR‐enterography within 6 months from diagnosis and having a maximum of 5 years follow‐up were included. Skeletal muscle signal intensity was analyzed on T1‐weighted fat‐saturated post‐contrast images. Intra‐observer and inter‐observer reproducibilities were assessed by intra‐class correlation coefficient and Cohen's kappa. Intra‐observer and inter‐observer variabilities were determined by Pearson correlation coefficient and displayed by Bland–Altman plots. Time to intestinal resection was studied by Kaplan–Meier analysis.
Results
Median time between diagnosis and MR‐enterography was 5 weeks (inter‐quartile range 1–9) in 35 CD patients. Skeletal muscle signal intensity showed good intra‐class correlation and substantial agreement (for intra‐observer, intraclass correlation coefficient = 0.948, κ = 0.677; and inter‐observer reproducibility, intraclass correlation coefficient = 0.858, κ = 0.622). Resection free survival was shorter in the low skeletal muscle signal intensity group (P = 0.037).
Conclusion
Skeletal muscle signal intensity on routine MR‐enterographies is reproducible and was associated with unfavorable disease outcome, indicating potential clinical relevance.
Keywords: disease outcome, IBD, myosteatosis
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing–remitting disease of the gastrointestinal tract, with Crohn's disease (CD) and ulcerative colitis as main subtypes. Fatigue is a common symptom reported among IBD patients, not only during flares but also in periods of quiescent disease with 40% of IBD patients still reporting fatigue. 1 , 2 Because of fatigue, IBD patients are less physically active. 3 Immobility as well as chronic inflammation, malnutrition, and corticosteroid use, also present in IBD patients, have been described as risk factors for both loss of muscle mass and strength, that is, sarcopenia. 4
A cross‐sectional study reported that 12% of the CD patients have sarcopenia. 4 Muscle strength can be affected because of fatty infiltration of the muscle, that is, myosteatosis. 5 Myosteatosis is associated with poor survival in patients with cancer as well as liver cirrhosis. 6 , 7 , 8 , 9 In CD, myosteatosis is associated with a more complicated disease phenotype and in IBD patients undergoing a surgical resection with longer hospital stay and readmission within 30 days. 10 , 11 The effect of myosteatosis on disease outcome parameters over time in CD patients has not been extensively studied. Although CD patients are at risk to develop myosteatosis based on risk factors described in other populations like glucocorticoid treatment, immobility, and systemic inflammation response. 6 , 12 , 13
A non‐invasive quantitative assessment of muscles can be performed with computed tomography (CT) by measuring skeletal muscle radiation attenuation, a parameter related to muscle fat content, 14 on a single slice at the third lumbar vertebra (L3). 15 Recently, a study reported a new non‐invasive method for the assessment of myosteatosis by the determination of skeletal muscle signal intensity on magnetic resonance (MR) imaging in periampullary cancer. 16 The signal intensity of the dorsal muscle group was normalized against the signal intensity of the cerebrospinal fluid at a single slice at L3 on T2‐weighted images. 16 In this study, MR skeletal muscle signal intensity was found to correlate with CT‐derived muscle radiation attenuation in patients with periampullary cancer (r = −0.614, P < 0.001).
MR enterography is performed to determine disease extension, activity, and/or complications in routine clinical practice as part of the work‐up for CD patients, without radiation exposure. Predicting patients at risk for unfavorable outcome at diagnosis, or at recurrence after surgery, is increasingly important given the availability of novel drugs and treatment strategies.
The primary aim of the present study was to analyze the reproducibility for assessing skeletal muscle signal intensity in patients with CD, on MR‐enterographies performed as part of routine clinical care. Our secondary aim was to explore the association of skeletal muscle signal intensity at time of diagnosis with time to intestinal resection, as proxy for unfavorable outcome, in these patients.
Methods
Study population
The Inflammatory Bowel Disease South Limburg (IBDSL) cohort is a Dutch population‐based inception cohort that has been used to study IBD epidemiology and disease course since 1991. All IBD patients over 18 years of age at diagnosis were included and followed prospectively. 17 Data on demographics, phenotype (according to the Montreal classification 18 ), and surgery were retrieved from medical records, using standardized registration forms. Disease localization was determined by ileocolonoscopy and MR‐enterography. IBDSL cohort has been approved by the Ethics Committee of the Maastricht University Medical Centre+ (NL31636.068.10), is registered in ClinicalTrials.gov (NCT02130349), and meets the ethical standard of the revised Declaration of Helsinki. 19
Thirty‐five CD patients, participating in the IBDSL cohort with the availability of good quality fat‐saturated post‐contrast T1‐weighted MR‐enterography images within 6 months from diagnosis as part of the routine work‐up for CD, were used for the present study. The patients had a maximum clinical follow‐up period of 5 years.
Imaging
MR‐enterography scans (Philips Medical Systems Intera, 1.5 Tesla, Best, The Netherlands) with fat‐saturated post‐contrast T1‐weighted images were used. Slice thickness was either 4 or 5 mm. The injected contrast was gadolinium based, and the amount administered was weight based. Two researchers, one from the department of radiology and nuclear medicine and one from the gastroenterology department, evaluated the MR images. Both researchers were trained in radiologic anatomy and body composition analysis (TL and CS). All MR images were evaluated once by one observer (TL) and twice by the other observer (CS), the analyses were performed independently of each other. The observers were blinded for each other's results and the patient's outcome.
At first, each observer determined the L3 level because muscle volume at this level is representative for total body skeletal muscle mass. 20 , 21 The L3 slice with best visualization of both transverse processes was chosen. Subsequently, the muscle area was defined using sliceOmatic V5.0 software (Tomovision, Canada).
Only dorsal muscles were used for analysis because of quality issues of the ventral portion of the MR images, for example, to artifacts by movement of the abdominal wall or air in the intestine. The dorsal muscles were defined as the muscles dorsal to a virtual horizontal line through the most ventral tip of the L3 vertebra (Fig. 1).
Figure 1.
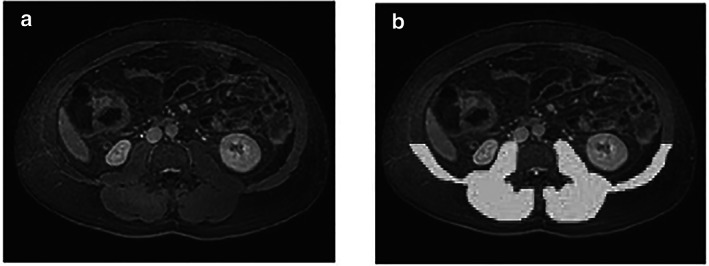
Skeletal muscle signal intensity on magnetic resonance (MR)‐enterography. MR‐enterography images showing the region of interest of dorsal muscles (white) and cerebrospinal fluid (white) at the third lumbar vertebra (L3); (a) and (b) show, respectively, the original and marked MR images.
Magnetic resonance skeletal muscle signal intensity
The area and signal intensity of the dorsal muscles were determined (Fig. 1). The mean signal intensity of the dorsal muscles was normalized against the mean signal intensity of the cerebrospinal fluid. A standardized square of 9 pixels with the lowest signal intensity inside the cervical canal was marked, and the mean signal intensity of this square was used to estimate the cerebrospinal fluid signal intensity. The normalization of the skeletal muscle signal intensity against cerebrospinal fluid was performed because signal intensity in MR imaging is scaled per acquired sequence. 16
MRs were derived following a standardized MR‐enterography protocol for fat suppression post‐contrast settings on T1‐weighted images. 22 This means that fatty tissue on T1‐weighted MR‐enterography images has a low signal intensity.
Statistical analysis
Baseline characteristics are presented as medians with corresponding inter‐quartile ranges (IQRs) for numerical variables and as number of patients with corresponding percentages for categorical ones. For comparison between groups, Mann–Whitney U tests and Fisher's exact tests were used for, respectively, numerical and categorical variables.
For the determination of low MR skeletal muscle signal intensity, pointing to muscle fat content, sex‐specific cut‐off points were set at the lowest 20th percentile. The intra‐reproducibility and inter‐reproducibility were assessed by determination of the intra‐class correlation coefficient (ICC) (two‐way mixed and absolute agreement) and Cohen's kappa (κ). In addition, the intra‐observer and inter‐observer variabilities of the skeletal muscle signal intensity were assessed using the Pearson correlation coefficient (r) and displayed by Bland–Altman plots. For the inter‐observer analyses, the first measurements of observer one (CS) were used.
An additional analysis was performed to determine whether region of interest (ROI) also showed agreement. On MR, there are several slices at L3. When the identical slice at L3 in the same patient was chosen by both observers, the overlap (in %) between ROI was calculated. Therefore, all overlapping colored pixels of the dorsal muscles were divided by the total number of pixels colored by both observers (Fig. 2). To determine the corresponding ROI of the cerebrospinal fluid, all overlapping colored pixels were divided by the total number of pixels colored by both observers.
Figure 2.

Region of interest. White region: pixels colored by both observers. Gray region: pixels colored by one of the two observers.
To explore the relevance of skeletal muscle signal intensity in relation to disease outcome in CD, the association between skeletal muscle signal intensity and time to intestinal resection was assessed by a Kaplan–Meier analysis. The average value of the two observers on skeletal muscle signal intensity was used for the analysis. Furthermore, the association between time to intestinal resection and disease behavior, localization, age at diagnosis, all according to the Montreal classification, 18 and increased CRP (≥ 10 mg/L) was analyzed.
Statistical analysis was performed using IBM SPSS statistics for Windows, Version 25 (IBM, Armonk, NY). A two‐sided P value ≤ 0.05 was considered significant. For the ROI agreement determination, MATLAB Release 2018a (MathWorks, Inc., Natick, MA) was used.
Results
Patient cohort
For this study, T1 fat‐saturated post‐contrast MR images at time of diagnosis of 35 CD patients were included. Baseline characteristics are presented in Table 1. Of the 35 patients, 42.9% were men, median age at diagnosis is 37 years (IQR 26–43), and median time between diagnosis and MR is 5 weeks (IQR 1–9). For all patients, 5‐year follow‐up data were available except for two, of which one was lost to follow‐up for personal reasons. These two had a follow‐up between 2.8 and 4.5 years. During the 5‐year follow‐up, the disease behavior of three patients changed from non‐stricturing non‐penetrating into stricturing, and the disease localization of one other patient from ileal into ileocolonic.
Table 1.
Baseline characteristics
| CD patients (n = 35) | High SMSI (n = 29) | Low SMSI (n = 6) | P value | |
|---|---|---|---|---|
| Male, n (%) | 15 (42.9%) | 13 (44.8%) | 2 (33.3%) | 0.680 |
| Montreal at diagnosis † | ||||
| Age | ||||
| A2, 17–40 years, n (%) | 20 (57.1%) | 17 (58.6%) | 3 (50.0%) | 1.000 |
| A3, > 40 years, n (%) | 15 (42.9%) | 12 (41.4%) | 3 (50.0%) | 1.000 |
| Disease location | ||||
| L1, ileal, n (%) | 15 (42.9%) | 13 (44.8%) | 2 (33.3%) | 0.680 |
| L2, colonic, n (%) | 11 (31.4%) | 9 (31.0%) | 2 (33.3%) | 1.000 |
| L3, ileocolonic, n (%) | 9 (25.7%) | 7 (24.1%) | 2 (33.3%) | 0.635 |
| L4, upper GI only, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Disease behavior | ||||
| B1, non‐stricturing non‐penetrating, n (%) | 23 (65.7%) | 20 (69.0%) | 3 (50%) | 0.391 |
| B2, stricturing, n (%) | 10 (28.6%) | 8 (27.6%) | 2 (33.3%) | 1.000 |
| B3, penetrating, n (%) | 2 (5.7%) | 1 (3.4%) | 1 (16.7%) | 0.318 |
|
Perianal disease at diagnosis, n (%) |
4 (11.4%) | 4 (13.8%) | 0 (0.0%) | 1.000 |
| Upper GI location at diagnosis, n (%) | 1 (2.9%) | 1 (3.4%) | 0 (0.0%) | 1.000 |
| Time between diagnosis and MR‐enterography in weeks (median, IQR) | 5.0 (1.0–9.0) | 5.0 (1.0–8.5) | 5.0 (2.0–16.0) | 0.404 |
| Skeletal muscle signal intensity (median, IQR) | 2.7 (2.4–3.1) | 2.7 (2.6–3.2) | 2.1 (1.6–2.3) |
CD, Crohn's disease; GI, gastrointestinal; IQR, inter‐quartile range; MR, magnetic resonance; SMSI, skeletal muscle signal intensity; n, number of patients.
classification according to Montreal Classification. 18 .
Reproducibility of image analysis
For the intra‐observer reproducibility, the ICC was 0.948 (95% confidence interval [CI] 0.899–0.973) and intra‐observer agreement κ = 0.677 (95% CI 0.328–1). The intra‐observer variability for skeletal muscle signal intensity was r = 0.947 (Fig. 3a,b, including Bland–Altman plot).
Figure 3.
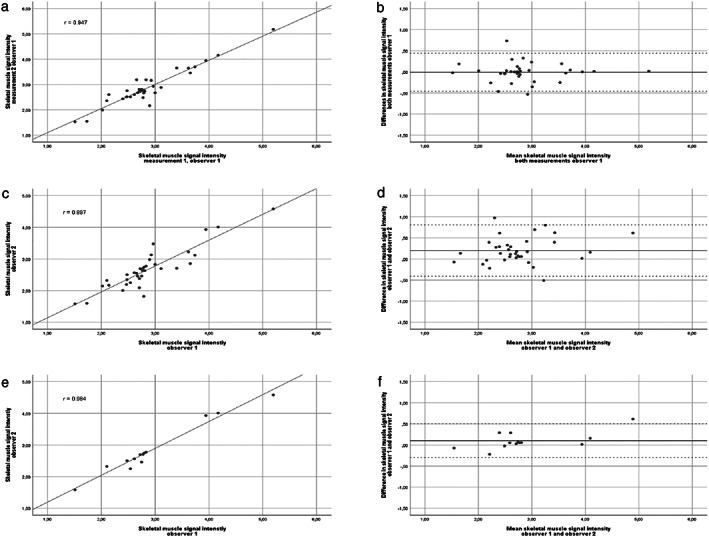
Intra‐observer and inter‐observer variability skeletal muscle signal intensity. Panels (a), (c), and (e): Pearson correlation coefficient of skeletal muscle signal intensity for, respectively, both measurements by one observer, r = 0.947, y = 0.941x + 0.156; both observers r = 0.897, y = 0.985x + 0.239; and similar slices at L3 (n = 13) of both observers r = 0.984, y = 1.141x − 0.300. Panels (b), (d), and (f): Bland–Altman plot for difference and mean of skeletal muscle signal intensity for both measurements by one observer (b), mean difference displayed by solid black line (−0.01), with limits of agreement by the dashed lines (−0.46 and 0.44); by both observers (d), mean difference displayed by the solid black line (0.20), with limits of agreement by the dashed lines (−0.41 and 0.80), and of the similar slices at L3 (n = 13) (f) by both observers, mean difference displayed by the solid black line (0.10), with limits of agreement by the dashed lines (−0.30 and 0.50).
For the inter‐observer reproducibility, the ICC was 0.858 (95% CI 0.627–0.938) and inter‐observer agreement κ = 0.622 (95% CI 0.338–0.906) and an inter‐observer variability of r = 0.897 (Fig. 3c,d, including Bland–Altman plot).
In 13 patients, the same slice at L3 was chosen by both observers. For these 13 MR images, the ICC was 0.969 (95% CI 0.881–0.985) and inter‐observer agreement was κ = 0.755 (95% CI 0.308–1), with r = 0.984 for the intra‐observer variability (Fig. 3e,f, including Bland–Altman plot).
The percentage of the overlapping ROI of the dorsal muscles was between 81.9% and 92.1% (Fig. 2). The overlap between the ROIs of the cerebrospinal fluid was overlapping for 100% in three patients, 50% in nine patients, and 20% in one patient.
Resection free survival
In total, 6 of the 35 patients were classified as having a low skeletal muscle signal intensity, with sex‐specific cut‐off points below 2.27 and 2.42 for male (n = 2) and female (n = 4) patients, respectively. There were no significant differences in baseline characteristics between the low versus high skeletal muscle signal intensity groups. One of the four patients with change in disease phenotype had a low skeletal muscle signal intensity.
In the Kaplan–Meier analysis, patients with a low skeletal muscle signal intensity had a shorter intestinal resection free period (P = 0.037) compared with a high skeletal muscle signal intensity (Fig. 4). The median intestinal resection free survival time in the low skeletal muscle signal intensity was 4.3 years (IQR 1.7–5.0) and in the high skeletal muscle signal intensity 5.0 years (IQR 5.0–5.0; range 0.5–5 years).
Figure 4.
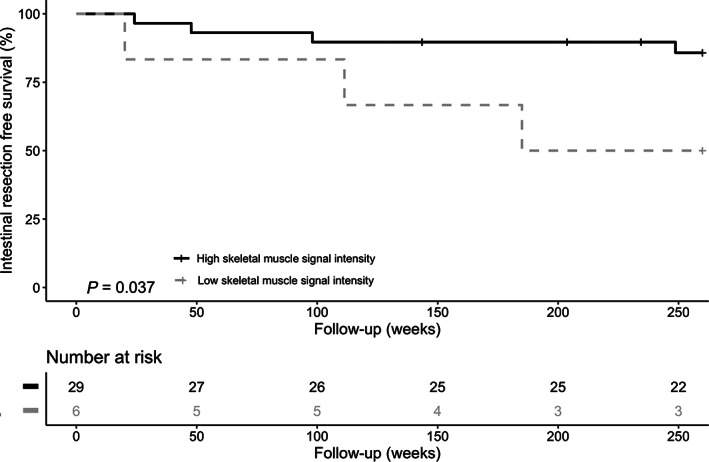
Kaplan–Meier analysis on intestinal resection free survival. Kaplan–Meier analysis on intestinal resection free survival time and high versus low skeletal muscle signal intensity.
When only the patients with a minimum follow‐up of 4 years were taken into account (n = 34), similar results on a shorter intestinal resection free period in patients with a low skeletal muscle signal intensity were found (P = 0.011).
In the study population, patients with non‐stricturing non‐penetrating disease phenotype (n = 23) had a longer intestinal resection free period (P = 0.014) compared with those with stricturing or penetrating (n = 12) disease phenotype. Furthermore, patients who were younger at diagnosis (age 17–40 years; n = 20) had a longer intestinal resection free period compared with a higher age at diagnosis (age > 40 years; n = 15), although not significant (P = 0.082). No significant differences were seen on intestinal resection free period and disease localization at diagnosis (colonic vs ileal or ileocolonic) or increased CRP.
Discussion
The present study shows that the assessment of skeletal muscle signal intensity on routine MR‐enterographies is a reproducible method. Furthermore, as explorative analyses, we found this to have potential clinical relevance as it was associated with unfavorable disease outcome in CD.
To our knowledge, the used method on skeletal muscle signal intensity, as a parameter for myosteatosis, was described only once in patients with periampullary cancer. 16 The use of MR‐enterographies to interpret muscle fat content is of special interest because MR‐enterographies are used in the routine clinical practice in CD patients to assess disease extension and complications. In contrast to CT, MR‐enterographies do not expose (often young) patients to (repeated) radiation exposure. We found a good reproducibility based on an excellent intra‐observer ICC and good inter‐observer ICC as well as a substantial intra‐observer and inter‐observer agreement. 23 , 24 The inter‐observer variability was good, although selecting a different slice at L3 for the analyses may have influenced the inter‐observer variability. The Bland–Altman plot indicates small systemic difference between the two observers (Fig. 3d). This is probably because of selecting a different level at L3 because the mean difference is smaller when the results of only the similar slices are displayed (Fig. 3f). The inter‐observer variability was similar to the study of van Dijk et al. 16 when the skeletal muscle signal intensity was determined for the subjects of which the same slice on MR was chosen by both observers, with an excellent ICC. 23 The results of the present study indicate that the outcome parameter (low vs high skeletal muscle signal intensity) is not affected by the substantial inter‐observer agreement when both observers determined the L3 slice independently. 24 Because the outcome parameter is not affected by the level of L3 slice, it can be considered to use the described technique in both research and eventual clinical settings.
MRs that are conducted following an enterography protocol, as is common practice in CD, do not include plane T2‐weighted images. Therefore, the T1‐weighted fat‐saturated post‐contrast MR images from the standard MR‐enterography protocol were used for the analysis in the present study. 22 The administered amount of the gadolinium‐based contrast was weight dependent and because skeletal muscle signal intensity of back muscles is relative stable during different contrast phases, the post‐contrast phase of the T1‐weighted images will not influence our results on skeletal muscle signal intensity. 25 The border of cerebrospinal fluid is harder to visualize on T1‐weighted images compared to T2‐weigthed images; therefore, the cerebrospinal fluid was demarked by a standardized square of nine pixels. To normalize the skeletal muscle signal intensity, the cerebrospinal fluid signal intensity was chosen because it is available at L3, the signal intensity is similar to water, and the signal intensity of cerebrospinal fluid is assumed to be very similar and stable between different individuals. 16
Predicting an unfavorable outcome at diagnosis or a high risk for recurrence after surgery is of importance to select the appropriate treatment algorithm (e.g. accelerated step‐up or top‐down therapy, biological treatment to prevent post‐operative recurrence) in CD. The currently available biomarkers do not sufficiently predict disease outcome. 26 Further insights in factors affecting disease outcome are therefore warranted. In cancer and liver cirrhosis patients, myosteatosis is found to be a prognostic factor. 6 , 7 , 8 , 9 The results of our explorative analyses showed a significantly shorter intestinal resection free period in the low skeletal muscle signal intensity group, pointing to increased muscle fat content.
In this study population, patients with a severe disease behavior at diagnosis also had a shorter intestinal resection free period. This finding is in line with the results of risk factors for surgery in a population‐based CD cohort. 27 However, we could not confirm an effect of disease localization and age at diagnosis on intestinal resection free survival, 28 which might be because of the small numbers of patients.
The results of myosteatosis on resection free survival illustrate the potential clinical relevance. This needs to be confirmed in future, larger studies and compared or combined with other predictor parameters, such as disease behavior.
In the current study, we used intestinal resection as proxy for unfavorable disease outcome, which could reliably be retrieved from the retrospective data set. In the future, it is relevant to add other disease outcome parameters, such as flares, change of disease phenotype, hospitalizations, and post‐operative complications and outcomes. These parameters are of interest because CT muscle attenuation radiation, as a parameter related to muscle fat content, has previously been reported to negatively affect duration of hospitalization and readmission rate after intestinal resection in IBD patients 11 and to be associated with a complicated disease phenotype in CD. 10 Because of the small number of patients in the present methodological study, we could not confirm the latter results.
The strength of the present study is the combination of the assessment of reproducibility of skeletal muscle signal intensity and the possibility to link the data to the long‐term follow‐up data of the IBDSL cohort. The association found between low skeletal muscle signal intensity and time to surgical intervention points to the potential of skeletal muscle signal intensity for future research as well as its clinical relevance. It should be noted that the number of patients for this exploratory part of the study was rather small. Because of the fat‐saturated post‐contrast T1 images, it was not possible to reliably determine the visceral fat mass, which is a limitation of the present technique. In future studies with larger patient groups, it would be interesting to also measure other parameters of body composition like visceral and subcutaneous fat because these parameters are also reported to correlate with adverse outcomes and complicated disease phenotype in CD on CT. 10 , 29 There are several limitations of the present study. First, although MR‐enterographies in routine clinical care are performed using standardized scanning protocols, the included MR‐enterographies for the present study were conducted (in part) by three different MR scanners, all being Philips Intera. Possible relative small differences in scan setting over time (slice thickness 4 or 5 mm, duration in scanning time of the fat‐saturated post‐contrast T1‐weighted transverse slice) may have influenced the measurements. The skeletal muscle signal intensity was normalized against the signal intensity of the cerebrospinal fluid to make comparison between patients and different scanner settings possible. Second, as described previously, we had to make some adjustments to the assessment protocol as was described by van Dijk et al. 16 because the standardized MR‐enterography protocol does not contain plane T2‐weighted images. Furthermore, for skeletal muscle signal intensity, sex‐specific cut‐off points have not yet been determined. The reported prevalence in CD of sarcopenia, comprising muscle mass and muscle strength, ranges between 12% and 41.6%. 4 , 30 In the present study, the sex‐specific cut‐off, to identify patients with low skeletal muscle signal intensity, was set at the lowest 20% for this study.
In conclusion, we found that assessment of skeletal muscle signal intensity on routine MR‐enterographies at diagnosis can be used with substantial to good reproducibility in CD patients and has the potential to predict unfavorable disease outcome. This relatively fast and simple image analysis is of relevance because MR‐enterography is available in routine clinical practice in CD patients and does not contain radiation exposure.
Acknowledgments
We would like to thank Alex Roth for his support with MATLAB.
Spooren, C. E. G. M. , Lodewick, T. M. , Beelen, E. M. J. , van Dijk, D. P. J. , Bours, M. J. L. , Haans, J. J. , Masclee, A. A. M. , Pierik, M. J. , Bakers, F. C. H. , and Jonkers, D. M. A. E. (2020) The reproducibility of skeletal muscle signal intensity on routine magnetic resonance imaging in Crohn's disease. Journal of Gastroenterology and Hepatology, 35: 1902–1908. 10.1111/jgh.15068.
Declaration of conflict of interest: CS, MP, and DJ report a grant from European commission outside the submitted work. Part of the work of DJ is financed by Grant Top Knowledge Institute (Well on Wheat), the Carbokinietics program as part of the NWO‐CCC Partnership program and H2020 Nr. 848228/DISCOvERIE. The other contributing authors have no conflicts of interest to declare in connection with this paper.
References
- 1. Romberg‐Camps MJ, Bol Y, Dagnelie PC et al Fatigue and health‐related quality of life in inflammatory bowel disease: results from a population‐based study in the Netherlands: the IBD‐South Limburg cohort. Inflamm. Bowel Dis. 2010; 16: 2137–2147. [DOI] [PubMed] [Google Scholar]
- 2. Minderhoud IM, Oldenburg B, van Dam PS, van Berge Henegouwen GP. High prevalence of fatigue in quiescent inflammatory bowel disease is not related to adrenocortical insufficiency. Am. J. Gastroenterol. 2003; 98: 1088–1093. [DOI] [PubMed] [Google Scholar]
- 3. Tew GA, Jones K, Mikocka‐Walus A. Physical activity habits, limitations, and predictors in people with inflammatory bowel disease: a large cross‐sectional online survey. Inflamm. Bowel Dis. 2016; 22: 2933–2942. [DOI] [PubMed] [Google Scholar]
- 4. Bryant RV, Ooi S, Schultz CG et al Low muscle mass and sarcopenia: common and predictive of osteopenia in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015; 41: 895–906. [DOI] [PubMed] [Google Scholar]
- 5. Correa‐de‐Araujo R, Harris‐Love MO, Miljkovic I, Fragala MS, Anthony BW, Manini TM. The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging‐related muscle dysfunctions: a symposium report. Front. Physiol. 2017; 8: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rollins KE, Tewari N, Ackner A et al The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin. Nutr. 2016; 35: 1103–1109. [DOI] [PubMed] [Google Scholar]
- 7. Okumura S, Kaido T, Hamaguchi Y et al Impact of skeletal muscle mass, muscle quality, and visceral adiposity on outcomes following resection of intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2016; 24: 1037–1045. [DOI] [PubMed] [Google Scholar]
- 8. van Dijk DP, Bakens MJ, Coolsen MM et al Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J. Cachexia. Sarcopenia Muscle 2016; 8: 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montano‐Loza AJ, Angulo P, Meza‐Junco J et al Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J. Cachexia. Sarcopenia Muscle 2016; 7: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cravo ML, Velho S, Torres J et al Lower skeletal muscle attenuation and high visceral fat index are associated with complicated disease in patients with Crohn's disease: an exploratory study. Clin. Nut. ESPEN. 2017; 21: 79–85. [DOI] [PubMed] [Google Scholar]
- 11. O'Brien S, Kavanagh RG, Carey BW, Maher MM, O'Connor OJ, Andrews EJ. The impact of sarcopenia and myosteatosis on postoperative outcomes in patients with inflammatory bowel disease. Eur. Radiol. Exp. 2018; 2: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamrick MW, McGee‐Lawrence ME, Frechette DM. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front Endocrinol. (Lausanne) 2016; 7: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malietzis G, Johns N, Al‐Hassi HO et al Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann. Surg. 2016; 263: 320–325. [DOI] [PubMed] [Google Scholar]
- 14. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J. Appl. Physiol. (1985) 2000; 89: 104–110. [DOI] [PubMed] [Google Scholar]
- 15. Aubrey J, Esfandiari N, Baracos VE et al Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf.) 2014; 210: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Dijk DPJ, Bakers FCH, Sanduleanu S et al Myosteatosis predicts survival after surgery for periampullary cancer: a novel method using MRI. HPB (Oxford) 2018; 20: 715–720. [DOI] [PubMed] [Google Scholar]
- 17. van den Heuvel TR, Jonkers DM, Jeuring SF et al Cohort profile: the Inflammatory Bowel Disease South Limburg Cohort (IBDSL). Int. J. Epidemiol. 2017; 46: e7. [DOI] [PubMed] [Google Scholar]
- 18. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Medical A . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 20. Schweitzer L, Geisler C, Pourhassan M et al What is the best reference site for a single MRI slice to assess whole‐body skeletal muscle and adipose tissue volumes in healthy adults? Am. J. Clin. Nutr. 2015; 102: 58–65. [DOI] [PubMed] [Google Scholar]
- 21. Shen W, Punyanitya M, Wang Z et al Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J. Appl. Physiol. (1985) 2004; 97: 2333–2338. [DOI] [PubMed] [Google Scholar]
- 22. Fidler JL, Guimaraes L, Einstein DM. MR imaging of the small bowel. Radiographics 2009; 29: 1811–1825. [DOI] [PubMed] [Google Scholar]
- 23. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016; 15: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174. [PubMed] [Google Scholar]
- 25. Becker‐Weidman DJ, Hope TA, Doshi PH, Patel A, Mitchell DG. Transient washout of hepatic hemangiomas: potential pitfall mimicking malignancy. Radiol. Case Rep. 2016; 11: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barnes EL, Burakoff R. New biomarkers for diagnosing inflammatory bowel disease and assessing treatment outcomes. Inflamm. Bowel Dis. 2016; 22: 2956–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ronnblom A, Holmstrom T, Karlbom U, Tanghoj H, Thorn M, Sjoberg D. Clinical course of Crohn's disease during the first 5 years. Results from a population‐based cohort in Sweden (ICURE) diagnosed 2005‐2009. Scand. J. Gastroenterol. 2017; 52: 81–86. [DOI] [PubMed] [Google Scholar]
- 28. Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn's disease. Ann. Surg. 2000; 231: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thiberge C, Charpentier C, Gillibert A et al Lower Subcutaneous or visceral adiposity assessed by abdominal computed tomography could predict adverse outcome in patients with Crohn's disease. J. Crohns Colitis 2018; 12: 1429–1437. [DOI] [PubMed] [Google Scholar]
- 30. Ryan E, McNicholas D, Creavin B, Kelly ME, Walsh T, Beddy D. Sarcopenia and inflammatory bowel disease: a systematic review. Inflamm. Bowel Dis. 2018; 25: 67–73. [DOI] [PubMed] [Google Scholar]


