Summary
Clinical scenario
A 65‐year‐old man presented with a 12‐h history of deteriorating rash. Two weeks previously he had completed a course of neoadjuvant chemotherapy for ductal carcinoma of the breast. On examination there were bullae, widespread atypical targetoid lesions and 15% epidermal detachment. There was no mucosal involvement on presentation, but subsequently it did evolve. Skin biopsy showed subepidermal blistering with epidermal necrosis. This confirmed our clinical diagnosis of overlap Stevens–Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). On transfer to intensive care he was anxious and fearful.
Management question
What are the psychological impacts of SJS/TEN on this man's life?
Background
SJS and TEN have devastating outcomes for those affected.
Objectives
To conduct a Critically Appraised Topic to (i) analyse existing research related to the psychological impact of SJS and TEN and (ii) apply the results to the clinical scenario.
Methods
Seven electronic databases were searched for publications focusing on the psychological impact of SJS/TEN on adults over 18 years of age.
Results
Six studies met the inclusion criteria. Healthcare practitioners’ (HCPs’) lack of information around the disorder was highlighted. Patients experienced undue stress and fear. Some patients had symptoms aligned to post‐traumatic stress disorder (PTSD), anxiety and depression.
Discussion and recommendation
The evidence suggests that SJS and TEN impact psychologically on patients’ lives. Education of HCPs, to address their lack of awareness and information on SJS/TEN, should facilitate their capacity to provide information and support to patients, thereby reducing patient anxiety. On discharge, a follow‐up appointment with relevant HCPs to reduce the possibility of PTSD occurring should be considered.
Short abstract
What's already known about this topic?
Many long‐term sequelae have been identified in patients with Stevens–Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), with cutaneous and ocular problems being among the most common.
A search of the existing literature has identified a lack of research relating to the psychological impact on patients.
What does this study add?
SJS and TEN have long‐lasting psychosocial implications for the lives of those affected and their significant others.
Healthcare practitioners’ lack of information around the disorder, particularly in terms of its diagnosis, causes stress and anxiety for patients.
Following discharge from the hospital, individuals expressed fear of taking medication and in attending their doctor, frequently leading them to engage in avoidance behaviours.
Patients were distressed, with symptoms aligned to post‐traumatic stress disorder.
Plain language summary available online
Clinical scenario
A 65‐year‐old man presented as being acutely unwell with a 12‐h history of deteriorating rash. In the preceding 2 weeks, he had completed a course of neoadjuvant chemotherapy for locally advanced invasive human epidermal growth factor receptor 2‐positive ductal carcinoma of the left breast. His medications included his chemotherapy agents (docetaxel, carboplatin and trastuzumab) and intermittent dexamethasone and domperidone. On examination, there were bullae, widespread atypical targetoid lesions and 15% epidermal detachment. There was no mucosal involvement on presentation but subsequently it did evolve. Skin biopsy showed subepidermal blistering with epidermal necrosis. This confirmed our clinical diagnosis of overlap Stevens–Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). His Score of Toxic Epidermal Necrosis (SCORTEN) was 4, with a projected mortality risk of 58%. On transfer to the intensive care unit he was anxious and fearful.
What are the psychological impacts of SJS/TEN on this man's life?
Background
SJS and TEN are severe immune‐mediated mucocutaneous reactions, usually occurring as a result of medications.1 Many medications have been identified as causing the conditions, some of which include allopurinol,2, 3, 4 sulfonamide antibiotics,3 anticonvulsant agents,4 oxicam, nonsteroidal anti‐inflammatory drugs,3 chlormezanone,4 corticosteriods4 and nevirapine.3 Some ‘over‐the‐counter’ medications, such as acetaminophen, metamizole and ibuprofen, have also been linked to the conditions.2, 5, 6 However, Roujeau et al.7 highlight that the link between medications such as acetaminophen, ibuprofen and secretolytics in causing SJS and TEN may be a result of protopathic bias. These conditions can be linked to infections such as HIV,8 herpes virus9 and mycoplasma pneumonia.10 A rare genetic predisposition (the genotype HLA‐B*1502), which occurs only in people of Chinese and Southeast Asian descent, is linked to causing the conditions, where the genotype reacts with an environmental trigger, such as the absorption of carbamazepine.11
SJS and TEN are characterized in the acute phase by a febrile illness followed by cutaneous erythema with blister formation, and skin and mucous membrane necrosis and detachment.12 The individual becomes critically ill within a short period of time and is treated as a medical emergency. The U.K. guidelines and French national care protocols for the management of SJS and TEN have noted that, if patients are not referred to a specialist area such as an intensive care unit (ICU) or burns centre, the mortality outcome for the patient may be affected.1, 13 SJS and TEN are on the same disease continuum.14 A severity‐of‐illness score, SCORTEN, which is calculated on days 1 and 3, is a measure for determining the seriousness of the conditions.15 In SJS, a body surface area of < 10% is affected, in the case of TEN ≥ 30% of the body surface is affected, while 10–30% involvement is referred to as SJS/TEN overlap.16 Sekula et al.17 reported mortality rates of 23% and 34% at 6 weeks and 1 year, respectively.
The literature indicates that these conditions are rare in the general population, with a rate of 1–2 per million per year.18, 19 A U.K. study of SJS/TEN has highlighted an incidence rate of 5·76 cases of SJS/TEN per million person‐years between 1995 and 2013.20 The study noted that the incidence rate has been higher in patients aged < 10 years or ≥ 80 years. An increased susceptibility to SJS in patients aged > 80 years may be partly explained by polypharmacy within this age cohort.21 Difficulties in differentiating between erythema multiforme majus and SJS in people in these age groups may account for the higher incidence rates.22, 23
Notwithstanding the rarity of the conditions outlined above, they have devastating outcomes for those affected. Many long‐term sequelae have been identified, with cutaneous and ocular problems being among the most common;24 the respiratory and gastrointestinal tracts may also be affected.25 At the onset, the focus of care is on preservation and maintenance of life. While the British Association of Dermatologists’ guidelines provide comprehensive details on the medical care and management of adult patients with SJS and TEN, the authors have recommended further research into what constitutes optimal supportive care.1 According to Nogueira et al.,26 the conditions have significant psychological effects on the patients, causing them distress and anxiety. The French national diagnosis and care protocol for SJS and TEN refers to the importance of managing pain and psychological distress.13 However, a search of the existing literature identified a dearth of research relating to the psychological impact of SJS and TEN on patients. According to Lee et al.,25 the long‐term psychiatric and psychosocial sequelae of SJS and TEN remain unclear.
The objective of this Critically Appraised Topic is (i) to critically appraise existing research related to the psychological impact of SJS and TEN and (i) to apply the results to the patient scenario.
Methods
A Critically Appraised Topic was completed based on the four main steps outlined by Callander et al.27
Formation of a focused question
The original question arose from clinical practice. Using the population, exposure, outcome/theme format we devised a review question.28, 29 We considered empirical evidence, which focused on (population) adults over 18 years of age presenting with SJS or TEN; (exposure) adverse reaction diagnosed as SJS or TEN; and (outcome) the psychological impact such as emotional upset or trauma, post‐traumatic stress, anxiety, and other impacts on individuals’ wellbeing and self‐concept (Table S1; see Supporting Information).
Search for the best available evidence
Following the PRISMA guidelines,30 a systematic search was conducted sourcing and synthesizing empirical evidence that used qualitative and/or quantitative research.29 The protocol for this review was registered with the International Prospective Register of Systematic Reviews (CRD42018111369).31
This Critically Appraised Topic used the integrative review methodological process outlined by Whittemore and Knafl.29 This method facilitated the concurrent synthesis of qualitative and quantitative empirical evidence, which was necessary to answer our clinical question. Qualitative and quantitative empirical studies were included, and also unpublished research theses and dissertations.
We searched seven electronic international databases for publications and identified additional records through other sources, dating from January 2008 to December 2018 (Fig. 1). The search strategy was developed through consensus across the review team. Two information specialists (librarians; I.D. and L.D.) were on the review team and led the search process. We carried out a preliminary search on MEDLINE to determine the sensitivity and accuracy of the selected search terms. The search terms included synonyms and medical subject headings describing SJS and TEN and their psychological impact. We exported all records to EndNote and removed duplicates and studies outside of the date range.
Figure 1.
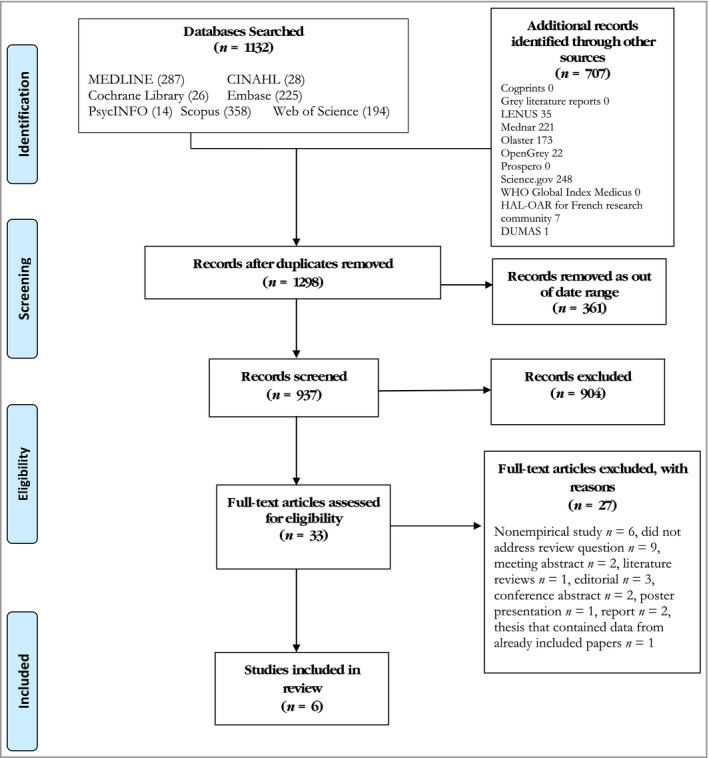
PRISMA flow diagram.30
Adhering to the inclusion and exclusion criteria, five authors (P.O'R., C.K., P.M., I.D., S.R.) screened the titles and abstracts (n = 937), with four working in pairs and one reviewer (C.K.) acting as arbitrator, if required. Review meetings were held to discuss outcomes and to agree on final studies for the next stage of the process, namely full‐text review (n = 33). Two reviewers screened the full texts of the articles and a meeting was held to agree on the rationale for exclusion of studies. Six studies that met the review inclusion criteria were included (Table 1). Two reviewers (P.O'R. and S.R.) were involved in the data extraction of the final included studies.
Table 1.
Characteristics of the included studies
| Study | Country | Aim/purpose | Type | Study participants | Key message | CASP |
|---|---|---|---|---|---|---|
| Butt 201134 | U.K. | To explore the experiences and beliefs of patients who had SJS/TEN and how these influenced their attitudes towards medicines and ADRs | Retrospective qualitative study | 14 participants (8 female, 6 male). Age range 21–82 years | Life‐threatening ADRs such as SJS and TEN may continue to affect patients’ lives long after the event, having an impact on their current lives physically and psychologically. Patients can lose trust in healthcare professionals and in medicines in general | 19/20 |
| Butt 201235 | U.K. | To illuminate patient experiences of SJS and TEN by analysing unsolicited internet narratives or postings of those who have had personal experience of drug‐induced SJS and TEN | Qualitative study | 208 internet descriptions (128 female, 68 male, 12 unknown). 139 posts from those with direct experience of SJS/TEN and 69 by relatives | Patients and relatives with experience of SJS or TEN posted on support group websites to share their experiences, provide support to other people affected and obtain advice from others with similar experiences. Findings indicted that patients and their relatives had many concerns about the ADR, often long after the event | 20/20 |
| Raspaud 201436 | France | To describe the sequelae of SJS and TEN including impact on quality of life | Quantitative descriptive study; doctoral thesis | 21 participants (18 female, 3 male) | SJS and TEN can cause long‐term sequelae including cutaneous (70%), psychological (60%) and ocular (45%) complications. Psychological sequelae are an important part of SJS and TEN complications and may be underestimated | 16/24 |
| Hefez 201837 | France | To assess the prevalence of PTSD and its risk factors in a population of adults with SJS/TEN who had benefited from early psychological evaluation | Monocentric prospective study | 31 patients (19 female, 12 male) | Despite frequent prescription of hydroxyzine at the acute phase, almost one‐quarter (23%) of patients with SJS/TEN had PTSD at 6 months. A systematic psychiatric evaluation should be offered regularly for at least 1 year after the acute phase of the disease | 24/24 |
| Dodiuk‐Gad 201638 | Canada | To explore the long‐term psychological complications and HRQOL of survivors of SJS/TEN | Quantitative study | 17 participants (11 female, 6 male) | Survivors of SJS/TEN had major long‐term, overlooked and treatable psychological complications and decreased HRQOL following a mean 51·6 ± 74·7 months (median 9, range 1–228) after SJS/TEN. Psychological support during hospitalization, prior to discharge and throughout follow‐up should be offered to all patients | 20/24 |
| Zitha 201539 | South Africa | To investigate prospectively the presence of anxiety and depression in patients with severe cutaneous ADRs, and assess their quality of life at two time intervals, using validated scoring systems | Quantitative Study | 48 participants (35 female, 13 male). 34 participants were diagnosed with SJS, TEN or SJS/TEN overlap | There is a high prevalence of anxiety and depression among patients with SJS/TEN and the disease negatively affects quality of life. At 6 weeks after diagnosis 13 of 34 patients with SJS/TEN had both anxiety and depression, while at 6 months 11 of 26 had comorbid anxiety and depression. At 6 months the quality of life of 14 of 26 patients was moderately to extremely affected | 20/24 |
ADR, adverse drug reaction; CASP, Critical Appraisal Skills Programme;32 HRQOL, health‐related quality of life; PTSD, post‐traumatic stress disorder; SJS, Stevens–Johnson syndrome; TEN, toxic epidermal necrolysis.
Critical appraisal of the evidence
The six studies were critically appraised and screened using the Critical Appraisal Skills Programme (CASP).32 Two of the studies were qualitative and four were quantitative, two of which included a doctoral thesis and a master's thesis. The CASP Qualitative Checklist and CASP Cohort Study Checklist were used as appropriate. Each paper was assigned a score: 0–20 for qualitative papers and 0–24 for the cohort study papers (Table 1), whereby the answers were scored as ‘yes’ = 2, ‘cannot tell’ = 1 and ‘no’ = 0. Of the six studies, the CASP scores were as follows: 16 (n = 1), 19 (n = 1), 20 (n = 3) and 24 (n = 1).
Interpreting and applying the results
Data extraction was performed on the findings and results sections of the included studies. The original studies were reread and compared with the data extract files for verification purposes and to identify any extraction errors. The data were then analysed inductively using the steps outlined by Whittemore and Knafl,29 namely data reduction, categorization, data comparison and conclusion drawing (thematic development). To strengthen the data analysis process, the data were interpreted using Braun and Clarke33 thematic framework analysis. Key words and statements were highlighted and formed initial codes, which were both descriptive and interpretive.33 To establish a broader level of meaning, codes were clustered together to form potential themes, thus ensuring that they were relevant in answering the review question.33 Cross‐comparisons were made across themes to ensure consistency and to rule out any duplication.33 Six reviewers were involved in the data analysis stage (P.O'R., C.K., P.M., I.D., B.W., S.R.).
Results
At the first stage of screening, 33 full‐text articles were assessed for eligibility and 27 full‐text studies were excluded with reasons (Fig. 1). Six studies were included in the Critically Appraised Topic. Two of the included studies were qualitative34, 35 and four were quantitative (Table 1).36, 37, 38, 39 Two of the studies were from the U.K.,34, 35 two were from France,36, 37 one was from Canada38 and one was from South Africa.39 Following analysis, two overarching themes were identified, namely (i) the impact of diagnosis now and for the future and (ii) living with the psychosocial impact. For each of these, two further subthemes were developed (Table 2). Quotations from the qualitative papers are presented in Table 2.
Table 2.
| Theme 1 Impact of diagnosis for now and the future |
| Subtheme 1 Healthcare practitioners not knowing – the distress on patients |
| ‘Well, I'd never heard of it, and when the doctors themselves didn't know anything about it, it was all a bit scary’ (Patient 1)34 |
| ‘I definitely feel that the medical profession is not aware enough of Stevens–Johnson’ (internet description 195)35 |
| ‘I was amazed at the lack of knowledge on the part of the medical professionals. I clearly knew more about this disorder than anyone else I dealt with’ (internet description 67)35 |
| ‘…the doctor diagnosed me with things like chicken pox, measles and flea bites’ (internet description 136)35 |
| ‘…my mom looked frantically through a book of medical problems and came to Steven–Johnsons syndrome. It fit the description perfectly but when she asked the doctor if it could be that he said no, it's too rare’ (internet description 136)35 |
| ‘I'm totally clueless about SJS though. Am I now a carrier? I'm aware of the fact it was due to an allergic reaction to the drugs prescribed to me. I think I was extremely lucky as it only affected my mouth and not the rest of my body. Could anyone update me on what happens now with regards to SJS and me being a carrier? I'm aware I shouldn't use that medicine ever again’ (internet description 216)35 |
| ‘Well I felt bitter that I should not have been given cefalexin, but it was on my notes it said I'm allergic to penicillin…and there is a train of thought that cefalexin is closely related to penicillin, and she [the GP] shouldn't have given me that knowing my history, all my notes say no penicillin. I feel she [the GP] should have looked it up on the internet, she's got the means, she should have inquired rather than handing out willy‐nilly’ (patient 4)34 |
| ‘But the only thing now is, it's made me so scared of taking pills. I won't go to the doctors if I can help it now…um, you know if you got infections or anything like that, I won't go, and if I had to go, was forced to go, he gives me tablets, I ask him. I must be the worst person, the worst nightmare they've had! [smiles]. I ask him, then I ask the chemist [laughs], then I think, I'm not taking them! Just in case, you know? It's frightening’ (patient 1)34 |
| ‘I am still quite confused by this syndrome. Will it stay in her system forever? Since it is a syndrome, does it always come back and never go away? I don't know anymore and I am scared for my daughter, please help’ (internet description 71)35 |
| Subtheme 2 Minimizing the risks |
| ‘I stopped taking any medication unnecessarily, like paracetamol, penicillin, Nurofen, and Lockets [medicated lozenges], because they're like medicated inside aren't they…and, so I stopped taking all that kind of stuff…and I get really bad migraines as well, that will actually make me throw up, but I still don't take Nurofen…because of the chance’ (patient 7)34 |
| ‘I think it's just made me aware of everything really…um, if er, if new sweets have come on [to the market] or anything…from different foods, you think, knowing that it's stupid! But it does…you think about it!’ (patient 1)34 |
| ‘…in the meantime, we live one day at a time, suspicious of all meds, suspicious of all foods, and even suspicious of the air that James breathes…why, why, why???’ (internet description 92)35 |
| Theme 2 Living with the psychosocial impact |
| Subtheme 1 Living with the distress |
| ‘Yes…being depressed, yes, because as I said I get flashbacks, your memory goes but you remember certain things like when I'm having a shower or taking my top off or look in the mirror it all comes back again’ (patient 13)34 |
| ‘I will never forget. I feel traumatized and sometimes I feel very afraid that this might happen again’ (internet description 34)35 |
| ‘Yes because I'm scarred in my mind as well as scarred on my body…I have flashbacks to my illness…the doctors were great and the hospital was great…but what let me down was the aftercare because OK, I got home and had to go back for checkups, but I said what about my scars? And the doctor was great he said you're a big strong lad, you'll be able to cope, but really I don't’ (patient 13)34 |
| Subtheme 2 Impact on self and others |
| ‘My parents told me the doctors expected side‐effects to be along the lines of blindness, deafness or sterility. Thus far I can see and hear just fine, but I'm a 19‐year‐old virgin, and I still live with this fear in the back of my mind that I might not be able to have kids when I'm ready to’ (internet description 25)35 |
| ‘I gave birth to our son September 13, 2002 and am looking for information on heredity and drugs known to cause SJS, as everything I've read says it is genetic and blood relations have a greater chance of developing SJS. I cannot imagine anyone having to go through that, and I need to protect my son’ (internet description 70)35 |
| ‘…and the day when I'll be released from the hospital finally came…after one and a half months. What I looked like that time made people a little disgusted and scared. I still have scars, I didn't have nails, and only a little hair were left. I had no friends in elementary school’ (internet description 124)35 |
| ‘My friends left me alone. They did not want anything to do with me. There was this one word, which was following me every moment. That word was “disabled and handicapped” (internet description 1)35 |
| ‘So now, 13 years later, I am finally married to a very understanding wife but to be honest, our sex life is not what it could or should be simply because I cannot enjoy sex or achieve orgasm – lack of sensitivity on my part. You know, if my arms, feet, toes, fingers or legs would have blistered and ruptured, I could have dealt with that. I really feel that it has left some long‐term effects that I will never overcome’ (internet description 79)35 |
| ‘The fever comes and goes. I asked her [the doctor] to check my genital area as no other doctor has. She ran a full battery of tests and reviewed the lab results from my ER visit the night before. The blisters are now covering my lips and mouth, down my throat, in my ears, nose and vagina. She tested me for every known STD. After enduring an extremely painful vaginal exam, she told me she wanted to treat me for herpes. As you can imagine, this has caused great stress on my marriage’ (internet description 133)35 |
| ‘I could see the look of disgust on the face of my aunt and my wife and the visitors for what was happening to me. Everyone, who saw me, could not believe the way I looked. Even my kids, when they visited me, could not recognize me as if I had turned into a monster’ (internet description 73)35 |
SJS, Steven–Johnson syndrome; GP, general practitioner; ER, emergency room; STD, sexually transmitted disease.
Theme 1: impact of diagnosis for now and the future
The lack of information from healthcare practitioners (HCPs) regarding SJS and TEN appeared to have caused stress and anger in those affected. Engaging in avoidance behaviour has been identified as one of the ways in which individuals appear to cope with the incumbent stress from SJS or TEN.
Subtheme 1: healthcare practitioners not knowing – the distress on patients
Patients described feeling distressed when they initially had symptoms of SJS or TEN and presented to their doctor or hospital. They often did not know what to expect in the ‘here and now’ and into the future, due in part to failures in diagnosing the condition. They eventually found out that they had this devastating disease and, while it would have a significant impact on their lives, the degree of information that they received from HCPs was minimal, leading to feelings of anger, confusion and a lack of trust in HCPs.34, 35 They also expressed anger and regret that they were not listened to by the doctor, especially when they were providing the doctors with information that might have helped in the prevention of SJS or TEN and/or in its diagnosis.34, 35
Subtheme 2: minimizing the risks
Patients learned to deal with the stress and distress of being diagnosed with SJS or TEN in different ways. In the case of the observed data, coping with the condition frequently led to individuals not only avoiding medications that might have given rise to SJS or TEN, but also being suspicious of all medications. Consequently, the impact of the diagnosis led to long‐term avoidance behaviour.34, 35 Similarly, a study by Raspaud36 highlighted that 11 out of 15 patients indicated that they had a fear of taking medications. In the study of Dodiuk‐Gad et al.,38 with 17 participants, the questionnaire ‘Impact of Events Scale‐Revised’40 was used to establish evidence of post‐traumatic stress disorder (PTSD). One of the factors within the scale was avoidance, and 47% of participants (n = 8) were above the cutoff score. One patient expressed that they had a fear not only of medication but also of attending the doctor.34
Theme 2: living with the psychosocial impact
Individuals who experience SJS or TEN refer to it as being a traumatic event in their lives. The evidence suggests that the conditions have long‐lasting impact on the lives of those affected and their significant others.
Subtheme 1: living with the distress
Patients who experienced SJS or TEN used language aligned to that of experiencing a traumatic event. As with research from those who have experienced PTSD, patients articulated that they sometimes experienced depression and flashbacks, constantly living with the fear that it might happen again.34, 35
They were physically stripped of their skin, which was an external manifestation of what was happening to them emotionally. One man described how he was still trying to cope with the trauma of the illness. He described being scarred in his mind as well as in his body.34 Trauma was also a key finding of the papers that quantitatively assessed the impact of SJS or TEN. In the study of Dodiuk‐Gad et al.,38 71% of patients (n = 12) had scores indicating clinically significant psychological distress; 65% of participants (n = 11) had symptoms of post‐traumatic stress. In addition, 29% of participants (n = 5) had total scores consistent with ‘clinical signs of possible PTSD’.38 While many patients experienced psychological distress, anxiety or depression, only a small minority were assessed by a mental health professional during the period following diagnosis. Similarly, Raspaud36 reported that following discharge with an initial diagnosis of TEN, 12 patients from a sample of 15 presented with anxiety, depression, irritability, insomnia and nightmares. Zitha39 found that of 26 patients diagnosed with SJS, TEN or SJS/TEN overlap (16, 7 and 3, respectively), 11 had anxiety and 13 were depressed at 6 months following the initial diagnosis. Using the Hospital Anxiety Depression Scale (HADS),41 42% of patients (n = 11) presented with a comorbidity of anxiety and depression, with a median HADS score of 15·3 (interquartile range 0·75–19·25). The study by Hefez et al.,37 with 31 participants, found that 23% of patients (n = 7) with SJS or TEN showed PTSD at 6 months.
Subtheme 2: impact on self and others
The diagnosis of SJS or TEN affected not only all aspects of the patient's life but also their relationships with significant others. Living with SJS or TEN caused them to fear for the future, particularly in relation to protecting their children from the condition. As highlighted in theme 1, patients were frequently left with many unanswered questions by HCPs; this posed many challenges. The future could feel uncertain and unsafe; difficulty in coping with this uncertainty was particularly challenging.35
A sense of loss and sadness was evident in some patients’ descriptions, with one man reporting how the illness had a long‐term impact on his relationship with his wife due to him not being able to enjoy sex.35 Similarly, the misdiagnosis of SJS by a doctor led to stress and stigma, whereby a patient described being tested for every known sexually transmitted disease, which affected her personal relationship.35
There is evidence from the data that SJS and TEN affected the individual's self‐image and how others viewed them.35 They were ‘stripped bare’ of their self‐image and of their friends. One patient described the look of disgust on the face of those who visited him and described himself as a ‘monster’.35
SJS and TEN affected all aspects of quality of life. Using the Dermatology Life Quality Index,42 Zitha39 reported that at 6 months following the initial diagnosis, the quality of life of 53% of patients (n = 14) diagnosed with SJS, TEN or SJS/TEN overlap was moderately to extremely affected. Raspaud36 referred to the medical file of one patient where it was noted that there was a repercussion on the patient's socioprofessional and personal life, a restriction on their daily activity and an aesthetic discomfort. Dodiuk‐Gad et al.38 found ‘major long‐term, overlooked and treatable psychological complications and decreased [health‐related quality of life] among survivors following a mean 51·6 ± 4·7 months (median 9, range 1–228) after SJS/TEN’. They also found that only 29% of participants (n = 5) were employed following diagnosis. This was a common theme, where Raspaud36 also found that one‐third of participants (n = 7) were in receipt of an allowance for adults with disabilities or disability compensation benefit. Patients highlighted that they had lost their job or had difficulty in getting one because of the condition.35
Discussion
Empirical literature exploring SJS and TEN is focused predominantly on the concomitant care, management and physical sequelae related to the conditions. There is a paucity of studies focused solely on the psychological consequences and on the impact of the conditions on patients’ lives. Our Critically Appraised Topic has identified six studies exploring the psychological impact of SJS and TEN and has revealed limited evidence. This lack of research may be attributed to the rarity of the disorder.
The available evidence suggests that SJS and TEN have a psychological impact that might have lasting implications on the quality of life of those affected and their significant others. Some patients were left feeling distressed, with symptoms aligned to PTSD, anxiety and depression. Patients revealed that they were confused and unaware of how it was going to affect their lives from the time of diagnosis onwards, which could also negatively impact their relationships with significant others. Patient accounts identified a lack of knowledge on the part of the HCPs around the disorder. Consequently, patients were poorly informed. Following discharge from hospital, individuals expressed fear around taking medication and in attending their doctor and, subsequently, engaged in avoidance behaviour.
Many experts in the area have highlighted the importance of managing, researching and preventing the psychological sequelae in patients with SJS and TEN.13, 16, 25, 43 Although some evidence exists, more clarity and research are required regarding the impacts on individuals’ lives.25 Eckert et al.,44 in a meeting abstract, have documented the importance of HCPs putting strategies (not identified) in place to prevent possible psychological sequelae such as PTSD in patients with SJS and TEN.
Patients with SJS or TEN are considered a similar cohort to patients with burns in terms of experiencing a traumatic event. Identifying how the psychological care is managed in patients with burns may be beneficial in the development of psychological support strategies for patients with SJS and TEN. Evidence has highlighted that there is a very close link between PTSD and the use of avoidance coping behaviours in patients with burns and traumatized patients, which can affect recovery from the traumatic event.45, 46, 47 Renneberg et al.48 have proposed the implementation of routine screening of psychological distress and the ability to cope in patients with burns. In addition, hypnosis, distraction techniques and relaxation therapy are some supportive interventions used with such patients in managing pain and psychological concerns;49, 50 this may be relevant to patients with SJS and TEN.
While the rarity of SJS and TEN poses research challenges,14 particularly in terms of participants, it is an opportunity to commence research dialogue on a global front, so as to address such anomalies. This approach has been adopted by the International Rare Diseases Research Consortium.51
Clinical message
We conclude that there is limited evidence detailing how best to minimize the psychological impact of SJS and TEN on patients. More research is required to address how best to incorporate psychological care into the patient's supportive care plan.
Our patient was cared for in the ICU by a multidisciplinary team, encompassing the expertise of the dermatological team and the ICU specialists. This provided reassurance to both the patient and their family. In light of the findings from our Critically Appraised Topic we took the following actions, which should be considered in reducing the psychological impact on patients presenting with SJS or TEN.
Patients have a right to information regarding their condition, and the provision of it should allay some potential stresses. It is crucial to have meaningful patient involvement, from the onset, so as to ensure that their experiences inform decision‐making and care pathways.
HCPs need to be aware of SJS and TEN to assist in early patient diagnosis. Lack of awareness and information from HCPs has caused stress and anxiety for patients. Using education and training to increase HCP awareness and knowledge concerning SJS and TEN should facilitate their capacity to provide information, support or referral opportunities to patients and family members, thereby reducing anxiety while increasing satisfaction with care.
The patient's care environment needs to be as stress free and person centred as possible.
On discharge, a follow‐up appointment with the relevant HCP, such as a psychologist, psychotherapist and/or social worker, should be considered so as to reduce the possibility of PTSD occurring.
Supporting information
Table S1 MEDLINE search strategy.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.
Funding sources This research was funded by the Health Research Institute, University of Limerick, Ireland.
Conflicts of interest None to declare.
*Plain language summary available online
References
- 1. Creamer D, Walsh SA, Dziewulski P et al U.K. guidelines for the management of Stevens–Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br J Dermatol 2016; 174:1194–227. [DOI] [PubMed] [Google Scholar]
- 2. Rodríguez‐Martín S, Martín‐Merino E, Lerma V et al Incidence of Stevens‐Johnson syndrome/toxic epidermal necrolysis among new users of different individual drugs in a European population: a case–population study. Eur J Clin Pharmacol 2019; 75:237–46. [DOI] [PubMed] [Google Scholar]
- 3. Mockenhaupt M, Viboud C, Dunant A et al Stevens‐Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR‐study. J Invest Dermatol 2008; 128:35–44. [DOI] [PubMed] [Google Scholar]
- 4. Roujeau JC, Kelly JP, Naldi L et al Medication use and the risk of Stevens‐Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 1995; 333:1600–8. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen KD, Tran TN, Nguyen MLT et al Drug‐induced Stevens‐Johnson syndrome and toxic epidermal necrolysis in Vietnamese spontaneous adverse drug reaction database: a subgroup approach to disproportionality analysis. J Clin Pharm Ther 2019; 44:69–77. [DOI] [PubMed] [Google Scholar]
- 6. Biswal S, Sahoo SS. Paracetamol induced Stevens‐Johnson syndrome–toxic epidermal necrolysis overlap syndrome. Int J Dermatol 2014; 53:1042–4. [DOI] [PubMed] [Google Scholar]
- 7. Roujeau JC, Dunant A, Mockenhaupt M. Epidermal necrolysis, ocular complications, and ‘cold medicines’. J Allergy Clin Immunol 2018; 6:703–4. [DOI] [PubMed] [Google Scholar]
- 8. Barvaliya M, Sanmukhani J, Patel T et al Drug‐induced Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS‐TEN overlap: a multicentric retrospective study. J Postgrad Med 2011; 57:115. [DOI] [PubMed] [Google Scholar]
- 9. Detjen PF, Patterson R, Noskin GA et al Herpes simplex virus associated with recurrent Stevens‐Johnson syndrome: a management strategy. Arch Intern Med 1992; 152:1513–16. [PubMed] [Google Scholar]
- 10. Schalock PC, Dinulos JG. Mycoplasma pneumoniae‐induced Stevens‐Johnson syndrome without skin lesions: fact or fiction? J Am Acad Dermatol 2005; 52:312–15. [DOI] [PubMed] [Google Scholar]
- 11. Foster MW, Sharp RR. Beyond race: towards a whole‐genome perspective on human populations and genetic variation. Nat Rev Genet 2004; 5:790. [DOI] [PubMed] [Google Scholar]
- 12. Bastuji‐Garin S, Rzany B, Stern RS et al Clinical classification of cases of toxic epidermal necrolysis, Stevens‐Johnson syndrome, and erythema multiforme. JAMA Dermatol 1993; 129:92–6. [PubMed] [Google Scholar]
- 13. Ingen‐Housz‐Oro S, Duong TA, Bensaid B et al Epidermal necrolysis French national diagnosis and care protocol (PNDS; Protocole National de Diagnostic et de Soins). Orphanet J Rare Dis 2018; 13:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohanim S, Palioura S, Saeed HN et al Stevens‐Johnson syndrome/toxic epidermal necrolysis – a comprehensive review and guide to therapy. I. Systemic disease. Ocul Surf 2016; 14:2–19. [DOI] [PubMed] [Google Scholar]
- 15. Fouchard N, Bertocchi M, Roujeau J‐C et al SCORTEN: a severity‐of‐illness score for toxic epidermal necrolysis. J Invest Dermatol 2000; 115:149–53. [DOI] [PubMed] [Google Scholar]
- 16. Wolkenstein P, Wilson YT. Toxic epidermal necrolysis: the past, the guidelines and challenges for the future. J Plast Reconstr Aesthet Surg 2016; 69:733–5. [DOI] [PubMed] [Google Scholar]
- 17. Sekula P, Dunant A, Mockenhaupt M et al Comprehensive survival analysis of a cohort of patients with Stevens‐Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol 2013; 133:1197–204. [DOI] [PubMed] [Google Scholar]
- 18. Roujeau JC, Guillaume J‐C, Fabre J‐P et al Toxic epidermal necrolysis (Lyell syndrome): incidence and drug etiology in France, 1981–1985. Arch Dermatol 1990; 126:37–42. [DOI] [PubMed] [Google Scholar]
- 19. Rzany B, Mockenhaupt M, Baur S et al Epidemiology of erythema exsudativum multiforme majus, Stevens‐Johnson syndrome, and toxic epidermal necrolysis in Germany (1990–1992): structure and results of a population‐based registry. J Clin Epidemiol 1996; 49:769–73. [DOI] [PubMed] [Google Scholar]
- 20. Frey N, Jossi J, Bodmer M et al The epidemiology of Stevens‐Johnson syndrome and toxic epidermal necrolysis in the U.K. J Invest Dermatol 2017; 137:1240–7. [DOI] [PubMed] [Google Scholar]
- 21. Mannesse CK, Derkx FH, de Ridder MA et al Contribution of adverse drug reactions to hospital admission of older patients. Age Ageing 2000; 29:35–9. [DOI] [PubMed] [Google Scholar]
- 22. Ziemer M, Wiesend CL, Vetter R et al Cutaneous adverse reactions to valdecoxib distinct from Stevens‐Johnson syndrome and toxic epidermal necrolysis. JAMA Dermatol 2007; 143:711–16. [DOI] [PubMed] [Google Scholar]
- 23. Liss Y, Mockenhaupt M. Erythema exsudativum multiforme majus versus Stevens–Johnson syndrome: differences in clinical pattern and etiology. Meeting on Cutaneous Adverse Drug Reactions and ESDR, Paris, France, 7–9 September 2006. J Invest Dermatol 2006; 126:106. [Google Scholar]
- 24. Yang CW, Cho YT, Chen KL et al Long‐term sequelae of Stevens‐Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol 2016; 96:525–9. [DOI] [PubMed] [Google Scholar]
- 25. Lee HY, Walsh SA, Creamer D. Long‐term complications of Stevens‐Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow‐up. Br J Dermatol 2017; 177:924–35. [DOI] [PubMed] [Google Scholar]
- 26. Nogueira R, Franca M, Lobato MG et al [Quality of life of patients with Stevens–Johnson syndrome]. Arq Bras Oftalmol 2003; 66:67–70 (in Portuguese). [Google Scholar]
- 27. Callander J, Anstey AV, Ingram JR et al How to write a Critically Appraised Topic: evidence to underpin routine clinical practice. Br J Dermatol 2017; 177:1007–13. [DOI] [PubMed] [Google Scholar]
- 28. Khan KS, Kunz R, Kleijnen J et al Five steps to conducting a systematic review. J R Soc Med 2003; 96:118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs 2005; 52:546–53. [DOI] [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J et al Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Reilly P, Kennedy C, Coffey A et al The psychological impact of Stevens Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) on an adult's well‐being: protocol for an integrative literature review. PROSPERO 2018 CRD42018111369. Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018111369 (last accessed 4 December 2019).
- 32. Critical Appraisal Skills Programme . CASP checklists. Available at: https://casp-uk.net/casp-tools-checklists (last accessed 4 December 2019).
- 33. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006; 3:77–101. [Google Scholar]
- 34. Butt TF, Cox AR, Lewis H et al Patient experiences of serious adverse drug reactions and their attitudes to medicines: a qualitative study of survivors of Stevens‐Johnson syndrome and toxic epidermal necrolysis in the UK. Drug Saf 2011; 34:319–28. [DOI] [PubMed] [Google Scholar]
- 35. Butt TF, Cox AR, Oyebode JR et al Internet accounts of serious adverse drug reactions: a study of experiences of Stevens‐Johnson syndrome and toxic epidermal necrolysis. Drug Saf 2012; 35:1159–70. [DOI] [PubMed] [Google Scholar]
- 36. Raspaud B. La nécrolyse épidermique toxique: le suivi des séquelles sur une série de cas au CHU de Bordeaux. [Toxic epidermal necrolysis: the follow‐up of sequelae of a case series at Bordeaux University Hospital]. Sciences Pharmaceutiques. Available at: https://dumas.ccsd.cnrs.fr/dumas-01105303/document (last accessed 4 December 2019).
- 37. Hefez L, Zaghbib K, Sbidian E et al Post‐traumatic stress disorder in Stevens‐Johnson syndrome and toxic epidermal necrolysis: prevalence and risk factors. A prospective study of 31 patients. Br J Dermatol 2019; 180:1206–13. [DOI] [PubMed] [Google Scholar]
- 38. Dodiuk‐Gad RP, Olteanu C, Feinstein A et al Major psychological complications and decreased health‐related quality of life among survivors of Stevens‐Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol 2016; 175:422–4. [DOI] [PubMed] [Google Scholar]
- 39. Zitha E. Prevalence of anxiety and depression in a predominantly HIV‐infected population with severe cutaneous adverse drug reactions (Master of Science dissertation, University of Cape Town). Available at: https://open.uct.ac.za/handle/11427/21378 (last accessed 4 December 2019).
- 40. Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale – Revised. Behav Res Ther 2003; 41:1489–96. [DOI] [PubMed] [Google Scholar]
- 41. Poole NA, Morgan JF. Validity and reliability of the Hospital Anxiety and Depression Scale in a hypertrophic cardiomyopathy clinic: the HADS in a cardiomyopathy population. Gen Hosp Psychiatry 2006; 28:55–8. [DOI] [PubMed] [Google Scholar]
- 42. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19:210–16. [DOI] [PubMed] [Google Scholar]
- 43. Walsh S, Lew T, Lee HY. Psychological sequelae of toxic epidermal necrolysis: further insights. Br J Dermatol 2016; 175:241. [DOI] [PubMed] [Google Scholar]
- 44. Eckert A, Cavigli A, Lauth M et al Posttraumatic stress disorder in survivors of Stevens‐Johnson syndrome and toxic epidermal necrolysis. J Am Acad Dermatol 2018; 79 (Suppl. 1):AB225. [Google Scholar]
- 45. Pineles SL, Mostoufi SM, Ready CB et al Trauma reactivity, avoidant coping, and PTSD symptoms: a moderating relationship? J Abnorm Psychol 2011; 120:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Andrews RM, Browne AL, Drummond PD et al The impact of personality and coping on the development of depressive symptoms in adult burns survivors. Burns 2010; 36:29–37. [DOI] [PubMed] [Google Scholar]
- 47. Lawrence JW, Fauerbach JA. Personality, coping, chronic stress, social support and PTSD symptoms among adult burn survivors: a path analysis. J Burn Care Rehabil 2003; 24:63–72. [DOI] [PubMed] [Google Scholar]
- 48. Renneberg B, Ripper S, Schulze J et al Quality of life and predictors of long‐term outcome after severe burn injury. J Behav Med 2014; 37:967–76. [DOI] [PubMed] [Google Scholar]
- 49. Wiechman SA, Patterson DR. Psychosocial aspects of burn injuries. BMJ 2004; 329:391–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wiechman Askay S, Patterson DR, Sharar SR et al Pain management in patients with burn injuries. Int Rev Psychiatry 2009; 21:522–30. [DOI] [PubMed] [Google Scholar]
- 51. Austin CP, Cutillo CM, Lau LP et al Future of rare diseases research 2017–2027: an IRDiRC perspective. Clin Transl Sci 2018; 11:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 MEDLINE search strategy.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.


