INTRODUCTION
Among the several challenges faced by the growing elderly population with increasing longevity in India, postmenopausal osteoporosis (PMO) is emerging as one of the major public health issues. Osteoporosis is an asymptomatic or “silent” disease and generally presents as a fragility fracture. Typical osteoporotic fractures are those of the hip, spine, and wrist. Global data indicate that 20% of women with hip fracture die within 1 year of the fracture and 50% of them never regain their functional independence.[1] Vertebral fractures can also have significant mortality and are associated with increased long-term morbidity.[2] The World Health Organization (WHO) has identified osteoporosis as an important noncommunicable disease. Osteoporotic fractures impose great financial, medical, and social burden on society. These guidelines are intended to be used as a resource document by the healthcare providers involved in postmenopausal women's health at all levels of healthcare with specific reference to India. Although framed for India, it is hoped that these guidelines will be useful for menopause practitioners across the globe.
This is one of the endeavors of the Indian Menopause Society to work toward the slogan, “Fit @ Forty Strong @ Sixty, and Independent @ Eighty”.
BASIC CONCEPTS
Definitions
1. Osteoporosis: The WHO defines osteoporosis as “a systemic skeletal disease characterized by low bone mass (measured as bone mineral density [BMD]) and micro-architectural deterioration of the bone tissue with a consequent increase in bone fragility and susceptibility to fractures, involving the wrist, spine, hip, pelvis, ribs, or humerus”[3]
2. Fragility fracture, the end point of inadequate skeletomuscular health, has been defined by the WHO as “a fracture caused by injury, which would be insufficient to fracture normal bone: the result of reduced compressive and/or torsional strength of bone.” Clinically, a fragility fracture can be defined as one which occurs as a result of minimal trauma, such as a fall from a standing height or less, or no identifiable trauma[4]
3. The most common sites of fragility fracture are the hip, spine, and forearm. The other sites are pelvis, proximal femur, proximal humerus, proximal tibia, and fractures involving three ribs simultaneously[5]
4. Sarcopenia is a progressive and generalized skeletal muscle disorder that is associated with increased likelihood of adverse outcomes, including falls, fractures, physical disability, and mortality. Severe sarcopenia is confirmed by the presence of low muscle quantity or quality and/or low physical performance. Sarcopenia is diagnosed in the presence of low muscle quantity or quality. Low muscle strength indicates the probability of sarcopenia[6]
5. Frailty: Physical frailty is defined as “a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual's vulnerability for developing increased dependency and/or death.”[7]
CRITERIA FOR DIAGNOSIS OF OSTEOPOROSIS
6. The diagnosis of an osteoporosis is by the presence of fragility fracture (clinical or radiological) and/or by BMD (T-score below or equal to −2.5) in a postmenopausal woman [Table 1]
Table 1.
Diagnosis of osteoporosis
| Grading | Score |
|---|---|
| Normal | T-score above (i.e., better than) −1.0 |
| Osteopenia or low bone mass | T-score between −1.0 and −2.5 |
| Osteoporosis | T-score below (i.e., worse than) or equal to −2.5 |
| Severe osteoporosis | T-score below −2.5 with fragility fracture |
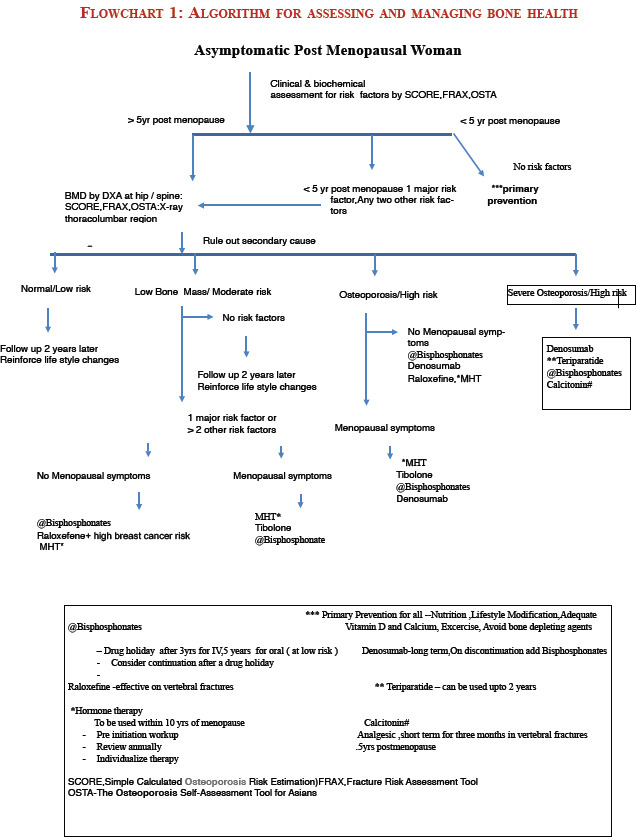
7. The “gold standard” method of BMD testing is by dual X-ray absorptiometry (DXA). Its value is expressed in standard deviation (SD) units from the population mean in young adults (T-score) or from the mean in an age-matched population (Z-score)
8. The reference range recommended by the International Osteoporosis Foundation, International Society of Clinical Densitometry (ISCD), WHO, and National Osteoporosis Foundation (NOF) for calculating the T-score in the postmenopausal women is the National Health and Nutrition Examination Survey III reference database in Caucasian women aged 20–29 years (Grade C)[8,9,10,11]
9. The ISCD diagnostic criteria for osteoporosis in postmenopausal women and in men aged 50 and older is if the T-score of the lumbar spine, total hip, or femoral neck is −2.5 or less. In certain circumstances, the 33% of radius (also called 1/3 radius) may be utilized[12]
10. The Z-score describes the number of SDs by which the BMD in an individual differs from the mean value expected for age and sex. It is mostly used in children, adolescents, and premenopausal women. A Z-score below −2 is regarded as abnormal and should be referred to as “low for age.” A low Z-score in a postmenopausal woman indicates the need to evaluate for secondary osteoporosis.[12]
TYPES OF OSTEOPOROSIS
11. Osteoporosis is classified as primary (includes type I and type II) and secondary
-
Primary osteoporosis is seen in the postmenopausal women in whom there is no specific pathogenetic mechanism other than age
- Type I or PMO affects mainly trabecular bone occurring in the early part of the menopause transition. There is an accelerated bone loss at the rate of 1%–2% per year (range 1%–5% yearly) due to declining estrogen levels and is seen in the first 5–7 years after menopause[13]
- Type II or senile osteoporosis is age related, and bone loss occurs at a rate of 1% per year in both sexes and affects the cortical and trabecular bone.
Secondary osteoporosis is due to specific causes.[14]
12. Osteoporosis and osteomalacia: Bone is a dynamic tissue with a continuous remodeling, leading to the formation of new bone and resorption of old bone. A mismatch of this process forms the basis for osteoporosis, while defective mineralization of the newly formed osteoid is called osteomalacia.[15]
EPIDEMIOLOGY
13. There is a wide prevalence of low dietary calcium in Indians of all age groups, with majority of postmenopausal women consuming <400 mg/day. This extends to all other age groups (infancy, adulthood, postmenopausal women, pregnancy, and lactation)[16,17,18,19,20]
14. Studies on bone mineral health from different parts of India indicate a wide prevalence of Vitamin D deficiency in all age groups, including infancy, adulthood, postmenopausal women, pregnancy, and lactation[21,22,23,24,25,26,27,28,29,30,31]
PEAK BONE MASS
15. Peak bone mass (PBM) is the highest level of bone mass achieved as a result of normal growth and is important as it determines resistance or susceptibility to osteoporosis and fractures. PBM is the result of the interaction of the various factors: genetic, hormonal, racial, nutritional, lifestyle, and physical exercise.[32,33,34,35,36] Environmental factors modulate the expression of the genetic potential to achieve PBM[37,38]
16. Age, sex, and genetic predisposition are important nonmodifiable risk factors for osteoporosis
17. Although PBM is achieved by 25–30 years, 40%–50% of bone mass is achieved by the age of 18 years. At skeletal maturity, women have 10%–15% lower bone mass than men. Asian Indians have a significant lower PBM than Caucasians.[39,40,41]
SCREENING AND DIAGNOSIS
18. Osteoporosis is asymptomatic unless a fracture occurs. Fracture risk is defined by BMD (both primary and secondary causes) and clinical risk factors. For treatment purpose, combining BMD with clinical risk factors provides a better estimate of fracture risk. We simply should not treat T-scores but must take a patient's full clinical status into account when we make therapeutic decisions
19. Early diagnosis in the asymptomatic period is essential, and timely management of osteoporosis will prevent the associated morbidity and mortality. Osteoporotic fracture risk screening of large-scale whole population groups is not likely to be cost-effective, so more selective approaches, i.e., targeted screening for disease detection, are advocated. In the absence of a validated population screening tool for PMO in India, a case-finding strategy utilizing clinical risk factors with the addition of DXA as needed is suggested (Grade C)
20. Asymptomatic women: Opportunistic screening for women above 40 years is suggested [Flowchart 1 Refer Appendix 1]
21. Risk assessment factors for fractures are derived by history and clinical examination. It is important to distinguish between those risk factors that lead to reduced bone mass from those that predispose to osteoporotic fractures with a BMD not in the osteoporotic range. Risk assessment tools such as The Osteoporosis Self-Assessment Tool (OSTA)8788] for Asians and Simple Calculated Risk Estimation Score (SCORE)89 are simple and cost-effective to screen women at risk for osteoporotic fracture
22. The WHO Fracture Risk Assessment Tool (FRAX) is country specific, and an online tool is available for India (http: www.shef.ac.uk/FRAX). FRAX is used to identify patients in the osteopenia group most likely to benefit from treatment. It predicts the 10-year absolute risk for a fracture in an individual, and the cost-effective analysis determines the interventional threshold above which the treatment is cost-effective. FRAX is country specific, and until more Indian data are available on the prevalence of osteoporotic fractures and mortality rates, it may not serve the true purpose for the usage of FRAX in the Indian context (Grade C)
23. Major risk factors defined by the WHO are (Grade A):
Advancing age is a single most significant risk factor
Low body mass index (BMI)
Prior history of a fracture
Parental history of hip fracture
Smoking
Alcohol
Use of glucocorticoid
Rheumatoid arthritis.
24. Environmental factors include nutrition (calcium intake using the quick dietary calculator, protein), physical activity and sunlight exposure, and risk of falling, which are the important modifiable risk factors
25. Secondary osteoporosis: Case finding for secondary osteoporosis is practiced in high-risk disease subgroups, such as chronic glucocorticoid users and patients with rheumatoid arthritis, collagen vascular disease or inflammatory bowel disease, hypogonadism, thyroid dysfunction, type 2 diabetes, and use of aromatase inhibitors in breast cancer survivors (Grade A)
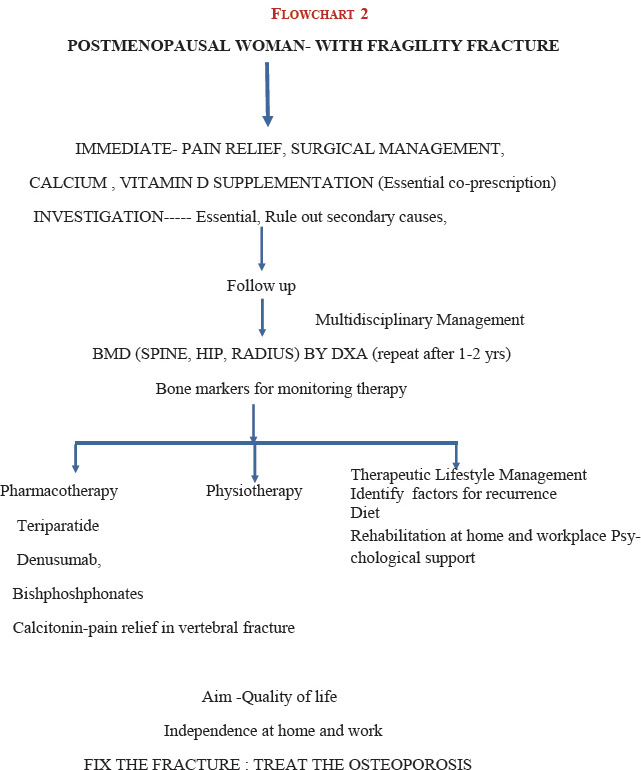
26. Symptomatic women [Flowchart 2 Refer Appendix 2]
27. Women presenting with fragility fracture, complain of severe pain, which is sudden in onset with minimal trauma, or chronic pain localized to the mid-back may radiate to the abdomen. Generalized bone pain indicates osteomalacia or metastasis. A multifactorial fall assessment is recommended. In Vitamin D deficiency, proximal muscle is affected more than the distal, so activities such as using a squatting toilet, climbing stairs, and getting out of low chair can be particularly difficult. Tenderness on the pretibial and sternum can be elicited
28. Physical examination should include recording the height and weight annually, check for balance and gait, and ask the women to get up from chair without using their arms (get up and go test). The occiput to wall distance in standing position is ideally zero, inability to touch the occiput to wall, while standing implies a thoracic fracture. Inability to insinuate the four fingers of the hand between the lower rib cage and anterior superior iliac crest implies a lumbar fracture. Kyphosis and Dowager's hump are seen in the late stage of osteoporosis (Grade A)
29. Laboratory tests: It is essential to rule out secondary causes.
-
Essential (Grade A)
- Complete blood picture, ESR
- Random blood sugar
- Serum calcium
- Preferably fasting serum phosphorus
- Serum creatinine
- Serum albumin
- Alkaline phosphatase
- Serum TSH 25 hydroxy vitamin D
- X-ray of the thoracolumbar spine (lateral view)
- PTH (based on clinical judgment).
Dual X-ray absorptiometry
30. It is suggested to conduct central DXA of the spine and hip in all women 5 years beyond the natural age of menopause and in women less than 5 years since menopause with one high clinical risk or more than two clinical risk factors. This suggestion is based on the following: (Grade C)
Early age of natural menopause, i.e., 46.7 years in an Indian women[42,43]
Life expectancy of an Indian woman is 70.3 years (WHO Statistics 2018)
Early age of presentation of fracture. Accelerated bone loss in the immediate 5 years of menopause[44,45,46]
Stratification by age shows that the prevalence of low bone mass is more than 40% from the age of 40 years and increases to more than 80% by the age of 65 years.[47,48,49,50,51,52,53,54,55]
31. Indications for DXA (Grade B)
All postmenopausal women more than 5 years of menopause
Postmenopausal women less than 5 years of menopause with risk factors
Women in menopause transition with secondary causes
Radiological evidence of osteopenia and the presence of vertebral compression fracture
Women with fragility fractures by radiology or DXA
Ideally, before initiating pharmacotherapy for osteoporosis
Emerging indications are to measure total body fat and lean tissue mass.
32. The lowest BMD score obtained from all the sites is used for diagnosis (Grade A).
33. Screen postmenopausal women for secondary osteoporosis if history or examination shows systemic disease or low Z-scores on DXA (Grade A).
34. To monitor therapy, the interval to the next DXA should depend on the calculated individual risk and would mostly be scheduled between 1 and 5 years later.
35. Peripheral DXA (X-ray based) may be used as a mass screening tool because of its high negative predictive value (Grade C).
RADIOGRAPHY: QUANTITATIVE ULTRASOUND
36. X-ray abnormality is a feature of advanced bone disease. We recommend X-rays in all the diagnostic protocols for osteoporosis (Grade A). In areas of endemic fluorosis, it is not advisable to follow ultrasound-based bone densitometry for diagnosis of osteoporosis.[56]
BONE TURNOVER MARKERS
37. Bone turnover markers (BTMs) are not a part of the routine tests to be used for clinical diagnosis. They may be used as an initial evaluation. They help identify the fast losers (Grade A). BTMs are also useful to assess compliance and efficacy of therapy and preferably follow the broad guidelines given below (Grade B):
-
Type of markers
- Bone resorption: Serum C-terminal telopeptide (CTX)
-
Bone formation: Serum procollagen type 1 N-terminal propeptide (PINP), bone-specific alkaline phosphatase.Use one marker of bone resorption and one marker of bone formation. More specifically, use markers for bone resorption when on antiresorptives and use bone formation markers when on anabolic agents
Monitoring: Baseline, and for resorption markers at three or six months, for formation markers at six months after treatment has been initiated
Timing of sample: Morning (before 9 am) after an overnight fast for CTX and anytime for PINP
Try to use the same laboratory services and same assay or method for monitoring intervals of measurement and compare the difference with the least significance change in terms of percentages or absolute values.
MANAGEMENT
Therapeutic lifestyle management
38. Therapeutic lifestyle management is an essential part in the management of osteoporosis. This includes a balanced diet, adequate physical activity and exposure to sunlight, and avoidance of bone-depleting agents such as tobacco, alcohol, caffeine and excessive salt. It is advisable to limit the intake of sodium to 5 g/day (1 tsp), decrease caffeine intake (<3 cups/day), limit alcohol, and avoid use of tobacco (Grade B). The quantity of protein intake should be 0.8–1 g/kg body weight
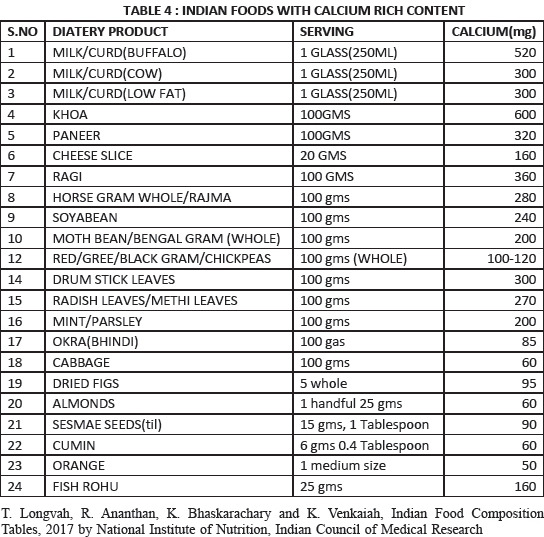
39. The recommended dietary allowance (RDA) of calcium intake for an adult Indian women is given in Table 2.[57] Assess the total calcium intake from dietary sources by using the NOF tool depicted in Table 3.[58] If needed, supplements are used to correct the deficient balance. The intake should exceed >800 mg/day (Grade B)
Table 2.
Recommended dietary allowance of calcium
| Group | Calcium (mg) |
|---|---|
| Adult women | 600 |
| Pregnancy | 1200 |
| Lactation | 1200 |
| Postmenopausal women | 800 |
Table 3.
Quick dietary calcium assessment chart
| Source | Calcium (mg)* | Number of servings | Total calcium (mg) |
|---|---|---|---|
| Diary source | 300-525/1 glass 300/1 katori curds | x | |
| Non diary | 200-300 | x |
Total intake of calcium in mg. *Approximate estimates. Calculate the total daily dietary intake by entering the sources and the number of servings from diary and nondiary sources before supplementation
Encourage dietary intake [Refer Appendix 3]; supplements are added to correct the deficient balance. The risk of cardiovascular events and calculi are not observed with the recommended doses of calcium
Limit 500 mg calcium at one time from food and/or supplements. Spread calcium sources throughout the day
Dietary calcium restriction is no longer recommended for patients with hypercalciuria
The 2016 NOF guideline on the safety and benefit of calcium supplementation stated that calcium intake below the UL (2,000-2,500 mg/d) is not associated with CVD risk in generally healthy adults
The data on supplemental calcium intake over and above the RDA is currently controversial. In case where calcium supplementation is medically necessary, patients should be encouraged to take their calcium supplements with a meal and should be monitored for hypercalciuria
Absorption of calcium is decreased when taken with foods rich in oxalic acid, phytates and tannins (spinach, fibres, Iron, zinc, spinach, tea, alcohol), vitamin D deficiency, estrogen deficiency, ageing, decreased gastric acid production, and malabsorptive disorders. Thyroid medications, corticosteroids, tetracyclines, and anticonvulsants and calcium should be taken separately
24-h urine calcium is the best method of evaluating adequacy of calcium intake and absorption.
Vitamin D
40. Vitamin D deficiency can be considered as a National Nutritional Deficiency pandemic. In the background of widespread Vitamin D deficiency in all age groups, it is prudent to adopt the US Endocrine Society 2011 RDA[59,60] [Table 4]
Table 4.
US Endocrine Society 2011 recommended dietary allowance 69
| Life stage group | RDA (IU) | Upper limit |
|---|---|---|
| Adults (18 years and above) | 1500-2000 | 10,000 |
| Pregnancy and lactation | 1500-2000 | 10,000 |
| Children and adults at risk* | 2-3 times the normal requirement for their age |
*Obesity, HIV infection, on glucocorticoids, anticonvulsant, antifungal and antiviral therapy. A desirable range is between 30 and 60 ng/mL, although levels up to 100 ng/mL are unlikely to result in Vitamin D toxicity. Except in granuloma disorders, wherein it is advisable to maintain the serum levels of 25(OH) D up to >30 ng/mL. RDA: Recommended dietary allowance
It is preferable to get Vitamin D through sunlight by exposing 15%–30% of body surface area (face, neck, and both arms and forearms) without sunscreen for at least 30 min between 10 am and 3 pm, depending on the season, latitude, altitude, pollution, and skin pigmentation. This is equivalent to consuming 340–490 IU of Vitamin D every day based on the reports that 100 IU of Vitamin D intake will raise serum 25(OH)D by 1 ng/ml
Dietary sources are limited; hence, the Government of India has permitted fortification of food which would enable population at large to enhance an intake of RDA of Vitamin D by 30%–50% (200–300 IU). This would be of value if one assumes that the consumption of milk/milk products is 700 ml per day and of oil is 30 ml/day. However, implementing intake from the natural sources has practical limitations. Hence, it is recommended to use Vitamin D as supplements (Grade A)
-
Recommendations for the management of Vitamin D deficiency and maintenance are given below (Grade B):[61,62,63,64,65,66,67,68]
- Cholecalciferol (Vitamin D3) is available in the form of oral tablets (conventional morcellized or nanoemulsion formulations), granules, and oral spray. Dosages of 1000, 2000, and 60,000 IU are available
- Intramuscular (IM) injections of Vitamin D3 are available in doses of 300,000 and 600,000 IU per ampoule. Injections of cholecalciferol are cost-effective and may be recommended in cases of malabsorption and also to increase compliance. The disadvantages are painful and erratic blood levels.
Cholecalciferol is the preferred therapy for correction of deficiency and maintenance.
Management of deficiency: Cholecalciferol (Vitamin D3) 60000 IU/orally once a week for 8 weeks preferably with milk is given. One IM injection of 600,000 IU is given to correct the deficiency (not to be repeated before three months and may be given after confirmation of persistent low levels of Vitamin D). This is followed by maintenance therapy
Maintenance therapy: Cholecalciferol 60,000 IU once a month in summer or twice a month in winter is preferred. Vitamin D supplements of 2000 IU/day or injection of cholecalciferol 300,000 IU IM twice a year or 600,000 IU IM once a year is given
Cholecalciferol, 1000 IU daily, will raise blood levels, on average, by approximately 10 ng/ml
Upper acceptable limit: The dose for treatment should not exceed 4000 IU/day and hypercalcemia has been reported when the dose exceeds 10,000 IU/day
Vitamin D derivatives: Calcitriol, the active form of Vitamin D, is reserved only for patients with chronic renal and hepatic disease. Alfacalcidol is a synthetic analog of the active Vitamin D metabolite calcitriol (1,25-dihydroxyvitamin-D3), and it is metabolized to calcitriol by its 25-hydroxylation in the liver. It is less potent than calcitriol. The use of Vitamin D derivatives necessitates monitoring of serum and possibly urine calcium. There is the risk of hypercalcemia and hypercalciuria. Adverse effects of prolonged hypercalcemia include impairment of renal function and nephrocalcinosis
In postmenopausal women, the intake of Vitamin D should be in addition to sunlight exposure. Vitamin D supplementation (≥500–2000 IU/day) was favorable in the reduction of hip fracture and any nonvertebral fracture in persons aged 65 years of age or older
41. Vitamin K: For women of postmenopausal age, 180–350 μg/day of Vitamin K2–7 may need to be supplemented along with the recommended intake of calcium, magnesium, Vitamin D, and a balanced diet. Current RDA of Vitamin K2–7 WHO/FAO of 65–80 μg/day is too low and needs to be raised up to at least 100 μg/day throughout life, with larger doses when needed. Both bone and cardiovascular health of women with osteoporosis would benefit from Vitamin K2–7 intake[69] (Grade C)
42. Exposure to complex nutrients and food constituents interact to affect bone mass; hence, it is the individual clinician to decide on supplementing Vitamin A, Vitamin B12, and phytoestrogens (Grade B).
PHYSICAL ACTIVITY/EXERCISE
43. Adequate physical activity is needed to maintain bone health. Appropriate resistance, weight-bearing aerobics, and core-stabilizing exercisers are needed to maintain bone health (Grade A). Balance exercises are necessary to prevent falls. Brisk walking 4–5 times a week for 30 min is part of maintaining health but on its own would not be sufficient for bone health[70]
44. Patients with severe osteoporosis should avoid engaging in motions, such as forward flexion exercises, using heavy weights, or even performing side-bending exercises, because pushing, pulling, lifting, and bending exert compressive forces on the spine that may lead to fracture (Grade A)[71]
45. Prevention of falls: Patients should receive a multifactorial risk assessment and intervention because it is the most consistently effective strategy to prevent falls (Grade A).[72,73,74]
PHARMACOTHERAPY
46. It is good to understand the term prevention and treatment in the context of osteoporosis
The term prevention is used to denote the prevention of bone loss in the postmenopausal women with low bone mass (T-score between −1 and −2.5) and increased fracture risk
Treatment is defined as reduction in fracture risk in the postmenopausal women with osteoporosis.
47. Indications for pharmacotherapy[75,76,77,78,79,80,81,82]
Fragility fractures (clinical, height loss of >4 cm, kyphosis or morphometric by X-rays, or VFA by DXA) (Grade A)
BMD T-scores ≤−2.5 at the femoral neck or spine or wrist by DXA (Grade A)
Women with low bone mass by DXA with one major or two other minor risk factors (or) eligible by OSTA for Asians, FRAX, and SCORE (Grade A)
In the absence of BMD measurements by DXA, intervention is individualized, based on the clinical risk assessment fracture risk tools such as the SCORE, OSTA, and FRAX (interventional threshold –10-year risk score ≥3% for hip fracture and ≥20% for major osteoporotic fracture), the cost–benefit analysis, and risk–benefit outcome (Grade B).
48. The choice of medication depends on drug-related (risk–benefit), patient profile (age, years since menopause, symptoms, and comorbidities), and environment-related factors (economics and social). Patients should be educated in PMO and its treatment and empowered to take part in shared decision-making to improve adherence. They should be calcium and Vitamin D replete
49. Patients should be monitored initially, every 3–6 months for 2–3 contacts, and then annually for clinical assessment. Assess for side effects and compliance. We suggest that markers of bone resorption and formation may be tested at baseline and after 3–6 months of therapy in certain situations and research settings (Grade C)
50. We suggest that DXA should be performed every 2 years on the same machine to monitor osteoporosis therapy (Grade B)
Measurement error must be considered when interpreting serial BMD assessments to determine whether the change is real and not simply random fluctuation or artifact. Follow the ISCD guidelines for conducting and interpreting DXA
Each center should determine its precision error to estimate the least significant change (i.e., the change in BMD required to have 95% confidence that the change is real). This is a standard of care and has to be meticulously followed by the DXA centers
Most osteoporosis therapies do not cause increase in BMD, and the anitifracture effect of treatment is only partly explained by the relatively small changes in DXA
Stable BMD is consistent with successful treatment.
51. Nonresponders to PMO therapy may be due to poor adherence, poor calcium/Vitamin D health, untreated secondary osteoporosis, concomitant therapy with skeletotropic drugs, inappropriate choice of drugs, or wrong choice of monitoring strategies (Grade C)
52. Duration of therapy has to be individualized depending on the patient's profile, drug used, and response to therapy
53. There is no specific recommendation on combination therapies and sequential therapies these should be planned as per the individual patient's need. Although teriparatide and denosumab combination has been documented with the highest BMD outcomes till date, some guidelines recommend sequential therapies for maintaining BMD gains and long-term protection against fracture. Drug holidays are planned in patients on bisphosphonates, depending on the categorization of risk for fracture
54. There are no-head-to-head trials of the various drugs comparing their effects on fracture rates. The details of drug therapy are given in Appendix 4.54
55. Hormone therapy, alendronate, and risedronate may be considered as initial options for most early postmenopausal women with low or moderate fracture risk. In women who are intolerant of oral bisphosphonates or in whom they are contraindicated, intravenous bisphosphonates or denosumab should be considered (Grade A recommendation)
56. Women with breast cancer risk and with osteoporosis of the spine may be benefitted with raloxifene
57. In older postmenopausal women, injectable agents such as denosumab, zoledronic acid, or teriparatide can be considered as initial therapy for those who have the highest fracture risk, older women who have had multiple vertebral fractures or hip fractures, or those who have very low T-scores, those who have upper gastrointestinal problems, and those might not tolerate or absorb oral medication and patient preference
58. Bisphosphonates are recommended as the first-line drugs for treating postmenopausal women, with proven efficacy in the prevention of vertebral and nonvertebral fractures, including hip fractures (Grade A)
MENOPAUSAL HORMONE THERAPY
59. Estrogen progesterone therapy/estrogen therapy (EPT/ET) may be used for low bone mass and treatment of osteoporosis in the early post- menopause with vasomotor symptomatic unless there is a contraindication. (Grade A)[83,84,85]
60. Pre-menopausal hormone therapy (MHT) workup and an annual follow-up are essential when prescribing MHT. The dose and duration of MHT should be individualized, and a risk–benefit assessment was carried out annually. A full gynecological assessment is mandatory before starting MHT and at regular intervals thereafter. Self-breast examination is advised monthly and clinical breast examination at least annually. A mammogram where available should be carried out 1–3 yearly, if the initial mammogram is normal (Grade C)
61. All preparations including low-dose, nonoral routes of estrogen are effective in preserving bone mass. In women with hypertriglyceridemia, obesity, glucose intolerance, history of deep vein thrombosis, and tobacco users, nonoral route should be preferred (Grade B)
62. MHT should not be started solely for bone protection after 10 years of menopause. Extended use of MHT in women with reduced bone mass is an option after considering the risk–benefit analysis compared to the other available therapies for osteoporosis (Grade B)
63. MHT is indicated as primary therapy to prevent bone loss in women with premature menopause and secondary amenorrhea (Grade C)
64. Progestogens should be added to estrogen therapy in women with uterus (Grade A)
65. If MHT is given to women below the age of 60 or within 10 years of menopause, the risks are rare. Table 7 elaborates the risks and benefits in terms that can be used during counseling for easy and understandable communication [Table 5]
Table 7.
Based on WHI: number of less events on ET and EPT versus placebo per 10,000 women per year of HT use between the age group of 50 years and 59 years
| Disease | Number of less events with ET |
|---|---|
| Heart Disease | 12 |
| Breast Cancer | 8 |
| Disease | Number of less events with E / EPT |
| Overall Mortality | 10 |
| Fractures | 5 |
| Colorectal cancer | 6 |
Table 5.
Council for International Organisations of Medical Sciences definitions are as follows which can be easily communicated to the lay person
| Term | n | Colloquial |
|---|---|---|
| Very common | 1/1 to 1/10 | A person in family |
| Common | 1/10 to 1/100 | A person in street |
| Uncommon | 1/100 to 1/1000 | A person in village |
| Rare | 1/1000 to 1/10000 | A person in a small town |
| Very rare | Less than 1/10000 | A person in a large town |
Harms: Based on WHI, the number of excess events on MHT versus placebo per 10,000 women per year of MHT. Use between the age group of 50 and 59 years (Grade A) [Table 6][83]
Benefits of hormone therapy are presented in Table 7 (Grade A).
Table 6.
Based on WHI, Number of excess events on HT versus placebo per 10,000 women per year of HT use between the age group of 50-59 years
| Disease | Estrogen | WHO/CIOMS definition of risk | Estrogen + progesterone | WHO/CIOMS definition of risk |
|---|---|---|---|---|
| Venous thromboembolism | 4 | Rare | 11 | Rare |
| Stroke | 1 | Rare | 4 | Rare |
| Breast cancer | 5 | Rare | ||
| Cardiovascular disease | 5 | Rare |
66. Tibolone may be preferable to MHT in symptomatic menopausal women with mammographically dense breast tissue (Grade A). It can be used as an add-back therapy with GnRH analogs for vasomotor symptoms and to maintain BMD (Grade B). Tibolone should be used with caution in women over 60 years and should not be used in those who have strong risk factors for stroke, in breast cancer survivors (Grade A)
67. Selective estrogen receptor modulators (SERMs, e.g., raloxifene at 60 mg daily) has been shown to be beneficial in reducing new vertebral fracture risk by 69% in the postmenopausal women with osteoporosis and 47% in the postmenopausal women with osteopenia over 3 years simultaneous reduction by 76% in the risk of invasive breast cancer (Grade A)
68. Raloxifene can be used as therapy for the prevention and treatment of osteoporosis, especially for women with an increased risk of breast cancer. It has shown to reduce the risk of invasive breast cancer by 76% (Grade A)
69. Raloxifene and estrogen are associated with a similar increased risk of venous thromboembolism (VTE) (Grade A). However, no cases of VTE were reported among young healthy postmenopausal Asian women while on therapy
70. Bazedoxifene (BZA) is an SERM that has been purposely synthesized to specifically improve skeletal and lipid parameters, while benefiting or having no effect on hot flushes. Conjugated estrogens/bazedoxifene (CE/BZA) is the first FDA-approved medication that combines conjugated estrogens with an estrogen agonist/antagonist, BZA, and is an option for vasomotor symptoms as well as for the prevention of osteoporosis. The combination of CE/BZA has been labeled the tissue-selective estrogen complex, yet to be launched in India.
TERIPARATIDE
71. Teriparatide is reserved for treating women at high risk for fracture, including those with very low BMD and with a previous vertebral fracture. 20 mcg/day subcutaneously (SC) is given for 18 months. Serum calcium and serum uric acid are monitored at 1, 6, and 12 months
72. A recommendation can be made for the treatment with antiresorptive therapy (bisphosphonates) following discontinuation of teriparatide (Grade A)
73. Adverse effects are headache, hypercalcemia, hypercalciuria, renal adverse effects, nausea, rhinitis, and arthralgia. It is contraindicated in hypocalcemia and hypersensitivity.
CALCITONIN
74. Calcitonin is approved for PMO treatment but not for prevention. It helps in relieving pain in vertebral fractures in short-term period only.
DENOSUMAB
75. It is a monoclonal antibody approved recently in India, specifically targets RANKL, and is approved for postmenopausal women with osteoporosis at high risk of fracture
76. It increases both trabecular and cortical bone strength; reduces vertebral, nonvertebral, and hip fracture risk; and increases BMD more than bisphosphonates, thereby providing benefits over 10 years therapy without any drug holiday
77. 60 mg is given SC once in 6 months which has good patient convenience, well tolerated even in patients with creatinine clearance <30 ml/min where bisphosphonates and teriparatide are contraindicated
78. Denosumab is cost-effective. When the antiresorptive drugs are discontinued, there is rebound bone resorption over variable time frames, leading to the risk of multiple vertebral fractures, which is also seen with denosumab discontinuation. Thus, Swiss association guidelines have mandated the sequential administration of alendronate or zoledronic acid for 2 years; starting it at 6 months from the last dose of denosumab. Follow-on therapy of alendronate or zoledronic acid helps maintain the continuous BMD gained while on denosumab and prevents the increased risk of multiple vertebral fractures on discontinuation of denosumab.
SURGICAL MANAGEMENT
79. Vertebral fractures
Vertebral compression fractures are common but are often silent consequences of osteoporosis
All vertebral compression fractures without neurological deficit should be treated conservatively for 3 weeks as majority get better during this period
In a recent Cochrane review, the role of percutaneous vertebroplasty and kyphoplasty in the management of those vertebral compression fractures that do not respond to nonoperative treatment has being questioned[86]
Occult hip fractures are not uncommon. In intracapsular fractures, internal fixation could be considered, if the fracture can be reduced anatomically (Grade B)
Hemiarthroplasty should be considered to eliminate thigh pain secondary to loosening and is ideal for elderly patients with limited life expectancy (Grade A)
Total hip replacement should be considered when internal fixation is inappropriate or contraindicated in physiologically younger patients for improved quality of life (Grade B).
80. All patients who suffer from fracture should be subjected to BMD after surgery where possible and appropriate treatment for osteoporosis initiated (Grade A)
81. Postfracture fixation – patient-specific osteoporosis-related medical management is used to avoid subsequent fractures (Grade A)
82. Postoperatively, start appropriate pharmacological therapy for osteoporosis. Drugs such as teriparatide which facilitates osteoblastic bone formation can be started (Grade A). Antiresorptives such as denosumab, when started before or after 6 weeks of postfracture, did not affect fracture healing as it is fracture neutral and does not accumulate at the fracture rims; denosumab can also be given along with teriparatide; bisphosphonates are started 4–6 weeks later (Grade B). All need to be calcium and Vitamin D replete
83. Anabolic steroids may be used in very old frail women with sarcopenia for 6 months.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
APPENDIX 1
APPENDIX 2
APPENDIX 3
APPENDIX 4
Appendix 4.
DRUG CHART
| Drug | Dosage | Route | Position in therapy | Vertebral* | Hip* | Non- vertebral* | Precautions |
|---|---|---|---|---|---|---|---|
| Alendronate | 5/10 mg daily | Oral | 1st line | Yes, 50% | Yes, 51-56% | Yes, 49% | Hypocalcemia, Vitamin D status, should not be used in patients with eGFR below 30 ml/min, pregnancy, lactation, pediatric, ONJ, AFF |
| 35/70 mg weekly | |||||||
| 150 mg monthly | |||||||
| Risedronate | 5 mg daily | Oral | 1st line | Yes, 41-49% | Yes, 30% | Yes, 36% | Hypocalcemia, Vitamin D status, should not be used in patients with eGFR below 30 ml/min, pregnancy, lactation, pediatric, ONJ, AFF |
| 35 mg weekly | |||||||
| 150 mg monthly | |||||||
| Zoledronate | 5 mg | IV | 1st line | Yes, 70% | Yes, 41% | Yes, 25% | Hypocalcemia, Vitamin D status, should not be used in patients with eGFR below 30 ml/min, pregnancy, lactation, pediatric, ONJ, AFF |
| Teriparatide | 20 mcg | SC | For severe osteoporosis | Yes, 65% | Insufficient data | Yes, 53% | Hypocalcemia, Vitamin D status, Hypersensitivity, local tissue damage, pregnancy, lactation, pediatric, |
| Denosumab | 60 mg | SC | 1st line | Yes, 68% | Yes, 40% | Yes, 20% | Hypocalcemia, Vitamin D status, pregnancy, lactation, pediatric, |
| MHT | Various regimes | Various regimes | 1st line with menopausal symptoms (<10 years menopause) | Yes, 30-70% | Yes, 40% | Yes, 27% | Blood clots, Cancer (such as breast, uterine, or endometrial), Heart or liver disease, Heart attack, Known or suspected pregnancy, Stroke |
| Raloxifene | 60 mg | Oral | At risk of breast cancer, without Vasomotor symptoms, <10 years menopause | Yes, 40% | No | No | With a low risk of DVT and for whom bisphosphonates or denosumab are not appropriate, or with a high risk of breast cancer |
| Tibolone | 2.5 mg | Oral | 1st line <10 years menopause | Yes, 50% | Yes, 26% | Yes, 26% | To stop tibolone a few weeks before any operation to reduce the risk of a blood clot, drug interaction with warfarin |
| Calcitonin | 200 IU | Nasal spray | 2nd line | Yes, 21% | No | No | Serious hypersensitivity reactions, including fatal anaphylaxis, reported; consider skin testing prior to treatment |
| Drug | Advantages | Disadvantages | Contraindications | Adverse effects | |||
| Alendronate | Most commonly used drug | Inconvenient administration - Stay upright for 30 min on intake, drink lots of water, no food before taking the drug, drug holiday may be needed after 3-5 years | Hypocalcemia, Hypersensitivity, Compromised renal function, Upper GI disease - Abnormalities of the esophagus which delay esophageal emptying such as stricture of achalasia, patients at increased risk of aspiration | Dyspepsia, esophagitis abdominal pain, musculoskeletal pain | |||
| Risedronate | Inconvenient administration - Stay upright for 30 min on intake, drink lots of water, no food before taking the drug, drug holiday may be needed after 3-5 years anaphylaxis, including fatal events | Hypocalcemia, hypersensitivity, compromised renal function, Upper GI disease - Abnormalities of the esophagus which delay esophageal emptying such as stricture of achalasia, patients at increased risk of aspiration | Rash, abdominal pain, dyspepsia, diarrhea, arthralgia | ||||
| Zoledronate | 1st line drug, | Hypocalcemia, hypersensitivity, compromised renal function | Acute reaction (flu such as symptoms, fever, myalgia) may occur within 3 days of infusion, hypotension, fatigue, eye inflammation, more nausea, vomiting, abdominal pain | ||||
| Teriparatide | Potent bone forming activity, Large increase in spine BMD over 2 years | Reserved line drug, 2 years usage, daily injections required, | Hypocalcemia, hypersensitivity | Headache, hypercalcemia (high-quality); hypercalciuria, renal adverse effects, nausea, rhinitis, arthralgia | |||
| Denosumab | 1st line drug, Rise of BMD reported over 10 years at spine, hip and nonvertebral sites, can be used in patients in eGFR 15-30 ml/min | Loss of effect and drop in BMD after discontinuation (should be continued on bisphosphonates) | Hypocalcemia, Hypersensitivity | Dermatitis, rash, mild bone/muscle pain, UTIs | |||
| MHT | Less musculoskeletal symptoms of aches and pains and possibly sarcopenia (or muscle wasting) | breast cancer VTE, stroke, potentiation of preexisting breast cancer, increased risk of gall stones, depression, headache, premenstrual syndrome, breast tenderness, skin irritation, weight gain, menstrual bleeding | Active endometrial and gynecological hormone- dependent cancers Active breast cancer, Undiagnosed, abnormal vaginal bleeding Moderate and high risk for breast cancer Established CVD and at severe increased risk of CVD, Previous personal or family history of venous thromboembolism Systematic lupus erythematous, Diabetes with end organ disease Severe active liver disease with impaired or abnormal liver function x Previous personal or family history of venous thromboembolism x Known or suspected pregnancy | Bloating, Breast swelling or tenderness, Headaches, Mood changes, Nausea, Vaginal bleeding | |||
| Raloxifene | benefit of a reduced incidence of invasive estrogen receptor-positive breast cancer both during treatment and for at least 5 years after completion | Daily oral administration | Pregnancy, lactation, Active history of thromboembolic disorders | Venous thromboembolism, stroke | |||
| Tibolone | Increases BMD, decreases cholesterol and triglycerides similar to conventional MHT | Reduction of HDL levels and its high cost | Pregnancy and lactation, breast cancer, estrogen-dependent malignant tumors (e.g., endometrial cancer) Undiagnosed genital bleeding, Untreated endometrial hyperplasia, venous thromboembolism (deep venous thrombosis pulmonary embolism, thrombophilic disorders, arterial thromboembolic disease (e.g., angina, myocardial infarction, stroke or TIA), Acute liver disease, or a history of liver disease, Hypersensitivity to the active substance(s), Porphyria | May increase stroke rates in women over 60 years of age, Weight gain, Unscheduled bleeding | |||
| Calcitonin | Ease of administration | Circulating antibodies to calcitonin-salmon may develop, and may cause loss of response to treatment | Hypersensitivity to calcitonin-salmon | Rhinitis, epistaxis, and allergic reactions | |||
*% reduction in fracture in individual pivotal studies only and not in head-head studies. eGFR: Estimated glomerular filtration rate, HDL: High-density lipoprotein, BMD: Bone mineral density, DVT: Deep vein thrombosis, GI: Gastrointestinal, MHT: Menopause hormone therapy, TIA: Transient ischaemic attack, ONJ: Osteonecrosis of the jaw
REFERENCES
- 1.Cooper C, Campion G, Melton LJ., 3rd Hip fractures in the elderly: A world-wide projection. Osteoporos Int. 1992;2:285–9. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 2.Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: A prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215–20. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 3.Consensus development conference: Prophylaxis and treatment of osteoporosis. Am J Med. 1991;90:107–10. doi: 10.1016/0002-9343(91)90512-v. [DOI] [PubMed] [Google Scholar]
- 4.Brown JP, Josse RG. Scientific Advisory Council of the Osteoporosis Society of Canada. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada [published correction appears in CMAJ. 2003 Feb 18;168(4):400] [published correction appears in CMAJ. 2003 Mar 18;168(6):676.] [published correction appears in CMAJ. 2003 Mar 4;168(5):544] CMAJ. 2002;167(10 Suppl):S1–S34. [PMC free article] [PubMed] [Google Scholar]
- 5.Rose, Steven H, Melton L, Joseph III, Morrey, Bernard F, Ilstrup, Duane M, Riggs B. Lawrence Epidemiologic Features of Humeral Fractures. Clinical Orthopaedics and Related Research. 1982;168:24–30. [PubMed] [Google Scholar]
- 6.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R. Frailty consensus: A call to action. J Am Med Dir Assoc. 2013;14:392–7. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Assessment of Fracture Risk and its Implication to Screening for Postmenopausal Osteoporosis: Technical Report Series 843. Geneva: WHO; 1994. [PubMed] [Google Scholar]
- 9.NIH consensus development panel on osteoporosis prevention, diagnosis, and therapy, March 7-29, 2000: Highlights of the conference. South Med J. 2001;94:569–73. [PubMed] [Google Scholar]
- 10.Kanis JA. UK: University of Shiffield; 2007. Assessment of Osteoporosis at the Primary Health Care Level 2008 World Health Organization Scientific Group. WHO Collaboration Centre for Metabolic Bone Diseases. [Google Scholar]
- 11.Dawson-Hughes B National Osteoporosis Foundation Guide Committee. A revised clinician's guide to the prevention and treatment of osteoporosis. J Clin Endocrinol Metab. 2008;93:2463–5. doi: 10.1210/jc.2008-0926. [DOI] [PubMed] [Google Scholar]
- 12.Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone. 2008;43:1115–21. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 13.Dobbs MB, Buckwalter J, Saltzman C. Osteoporosis: The increasing role of the orthopaedist. Iowa Orthop J. 1999;19:43–52. [PMC free article] [PubMed] [Google Scholar]
- 14.Parfitt AM. The coupling of bone formation to bone resorption: A critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res. 1982;4:1–6. doi: 10.1016/0221-8747(82)90002-9. [DOI] [PubMed] [Google Scholar]
- 15.Morgan B. Osteomalacia and osteoporosis. Postgrad Med J. 1968;44:621–5. doi: 10.1136/pgmj.44.514.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shatrugna V, Kulkarni B, Kumar PA, Rani KU, Balakrishna N. Bone status of Indian women from a low-income group and its relationship to the nutritional status. Osteoporos Int. 2005;16:1827–35. doi: 10.1007/s00198-005-1933-1. [DOI] [PubMed] [Google Scholar]
- 17.Raj JP, Venkatachalam S, Shekoba M, Norris JJ, Amaravati RS. Dietary calcium intake and physical activity levels among people living in Karnataka, India – An observational hospital-based study. J Family Med Prim Care. 2018;7:1411–6. doi: 10.4103/jfmpc.jfmpc_153_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raj JP, Oommen AM, Paul TV. Dietary calcium intake and physical activity levels among urban South Indian postmenopausal women. J Family Med Prim Care. 2015;4:461–4. doi: 10.4103/2249-4863.161355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D, Srinivasarao PV, Sarma KV, et al. High prevalence of low dietary calcium, high phytate consumption, and Vitamin D deficiency in healthy south Indians. Am J Clin Nutr. 2007;85:1062–7. doi: 10.1093/ajcn/85.4.1062. [DOI] [PubMed] [Google Scholar]
- 20.Harinarayan CV, Ramalakshmi T, Venkataprasad U. High prevalence of low dietary calcium and low Vitamin D status in healthy South Indians. Asia Pac J Clin Nutr. 2004;13:359–64. [PubMed] [Google Scholar]
- 21.Harinarayan CV, Sachan A, Reddy PA, Satish KM, Prasad UV, Srivani P. Vitamin D status and bone mineral density in women of reproductive and postmenopausal age groups: A cross-sectional study from South India. J Assoc Physicians India. 2011;59:698–704. [PubMed] [Google Scholar]
- 22.Marwaha RK, Tandon N, Reddy DR, Aggarwal R, Singh R, Sawhney RC, et al. Vitamin D and bone mineral density status of healthy schoolchildren in Northern India. Am J Clin Nutr. 2005;82:477–82. doi: 10.1093/ajcn.82.2.477. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal N, Faridi MM, Aggarwal A, Singh O. Vitamin D Status of term exclusively breastfed infants and their mothers from India. Acta Paediatr. 2010;99:1671–4. doi: 10.1111/j.1651-2227.2010.01912.x. [DOI] [PubMed] [Google Scholar]
- 24.Marwaha RK, Tandon N, Garg MK, Kanwar R, Narang A, Sastry A, et al. Vitamin D status in healthy Indians aged 50 years and above. J Assoc Physicians India. 2011;59:706–9. [PubMed] [Google Scholar]
- 25.International Osteoporosis Foundation. The Asian Audit: Epidemiology, Costs and Burden of Osteoporosis in Asia 2009. International Osteoporosis Foundation. 2009. [Last accessed 2019 Jun 10]. Available from: http://www.iofbonehealth.org .
- 26.Sachan A, Gupta R, Das V, Aggarwal A, Awasthi PK, Bhatia V. High prevalence of Vitamin D deficiency among pregnant women and their newborns in Northern India. Am J Clin Nutr. 2005;81:1060–4. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- 27.Arya V, Bhambri R, Godbole MM, Mithal A. Vitamin D status and its relationship with bone mineral density in healthy Asian Indians? Osteoporos Int. 2004;15:56–61. doi: 10.1007/s00198-003-1491-3. doi:10.1007/s00198-003-1491-32. [DOI] [PubMed] [Google Scholar]
- 28.Aparna P, Muthathal S, Nongkynrih B, Gupta SK. Vitamin D deficiency in India. J Family Med Prim Care. 2018;7:324–30. doi: 10.4103/jfmpc.jfmpc_78_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beloyartseva M, Mithal A, Kaur P, Manash PB. Widespread vitamin D deficiency among Indian health care professionals? Arch Osteoporos. 2012;7:187–192. doi: 10.1007/s11657-012-0096-x. doi:10.1007/s11657-012-0096-x. [DOI] [PubMed] [Google Scholar]
- 30.Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N, Kochupillai N. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472–5. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 31.Zargar AH, Ahmad S, Masoodi SR, Wani AI, Bashir MI, Laway BA, Shah ZA. Vitamin D status in apparently healthy adults in Kashmir Valley of Indian subcontinent. Postgrad Med J. 2007;83:713–6. doi: 10.1136/pgmj.2007.059113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: The Hertfordshire cohort study. Pediatr Res. 2005;57:582–6. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- 33.Cooper C, Cawley M, Bhalla A, Egger P, Ring F, Morton L, et al. Childhood growth, physical activity, and peak bone mass in women. J Bone Miner Res. 1995;10:940–7. doi: 10.1002/jbmr.5650100615. [DOI] [PubMed] [Google Scholar]
- 34.Cooper C, Fall C, Egger P, Hobbs R, Eastell R, Barker D. Growth in infancy and bone mass in later life. Ann Rheum Dis. 1997;56:17–21. doi: 10.1136/ard.56.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayer AA, Cooper C. Fetal programming of body composition and musculoskeletal development. Early Hum Dev. 2005;81:735–44. doi: 10.1016/j.earlhumdev.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Cooper C, Eriksson JG, Forsén T, Osmond C, Tuomilehto J, Barker DJ. Maternal height, childhood growth and risk of hip fracture in later life: A longitudinal study. Osteoporos Int. 2001;12:623–9. doi: 10.1007/s001980170061. [DOI] [PubMed] [Google Scholar]
- 37.Marwaha RK, Tandon N, Shivaprasad C, Kanwar R, Mani K, Aggarwal R, et al. Peak bone mineral density of physically active healthy Indian men with adequate nutrition and no known current constraints to bone mineralization. J Clin Densitom. 2009;12:314–21. doi: 10.1016/j.jocd.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Marwaha RK, Puri S, Tandon N, Dhir S, Agarwal N, Bhadra K, Aini SN. Effect of sports training & Nutrition on Bone mineral density in young Indian healthy females. Indian J Med Res. 2011;134:307–313. [PMC free article] [PubMed] [Google Scholar]
- 39.CMIR Taskforce Study. Population based reference standards of peak bone mineral density of Indian males and female. An ICMR Multicentre Task Force Study. New Delhi: Indian Council of Medical Research. 2010 [Google Scholar]
- 40.Shivane VK, Sarathi V, Lila AR. Peak bone mineral density and its determinants in an Asian Indian population. J Clin Densitom. 2012;15:152–8. doi: 10.1016/j.jocd.2011.12.007. doi:10.1016/j.jocd.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Ganpule A, Yajnik CS, Fall CHD, Rao S, Fisher DJ, Kanade A, et al. Bone Mass in Indian Children—Relationships to Maternal Nutritional Status and Diet during Pregnancy: the Pune Maternal Nutrition Study. The Journal of Clinical Endocrinology & Metabolism. 2006;91:2994–3001. doi: 10.1210/jc.2005-2431. [DOI] [PubMed] [Google Scholar]
- 42.Singh M. Early age of natural menopause in India, a biological marker for early preventive health programs. Climacteric. 2012;15:581–6. doi: 10.3109/13697137.2011.643514. [DOI] [PubMed] [Google Scholar]
- 43.Ahuja M. Age of menopause and determinants of menopause age: A PAN India survey by IMS. J Midlife Health. 2016;7:126–31. doi: 10.4103/0976-7800.191012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sankaran B. Clinical studies: Incidence of fracture neck of femur and intertrochanteric fractures in three Delhi hospitals. In: Sankaran B, editor. Osteoporosis. New Delhi: South East Asia Regional Office, World Health Organization; 2000. pp. 9–18. [Google Scholar]
- 45.Jha RM, Mithal A, Malhotra N, Brown EM. Pilot case-control investigation of risk factors for hip fractures in the urban Indian population. BMC Musculoskelet Disord. 2010;11:49. doi: 10.1186/1471-2474-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marwaha RK, Tandon N, Gupta Y, Bhadra K, Narang A, Mani K, et al. The prevalence of and risk factors for radiographic vertebral fractures in older Indian women and men: Delhi Vertebral Osteoporosis Study (DeVOS) Arch Osteoporos. 2012;7:201–7. doi: 10.1007/s11657-012-0098-8. [DOI] [PubMed] [Google Scholar]
- 47.Meeta M. Evaluation of Risk Factors for Dexa Referral in Indian Women Poster Presented at International Menopause Meeting, Spain. 2008 [Google Scholar]
- 48.Meeta M. Data from Vomed diagnostic on prevalence of osteoporosis. Osteoporosis Alert. 2002 [Google Scholar]
- 49.Aggrawal N, Bathla S, Juneja S. Measurement of bone mineral density by dexa scan in postmenopausal women. Obs Gynae Today. 2004;9:768–71. [Google Scholar]
- 50.Meeta M. Two Hundred and Six Reasons to be Informed about Osteoporosis. Poster at the Annual Indian Menopause Society meeting at Chandigarh. 2006 [Google Scholar]
- 51.Unni J, Garg R, Pawar R. Bone mineral density in women above 40 years. J Midlife Health. 2010;1:19–22. doi: 10.4103/0976-7800.66989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savardekar LS, Shah RS, Iddya U, Balaiah D, Parihar A, Jaknkaria B. Bone density in normal Indian women: Assessment by USG and DEXA. Obst Abd Gynae Today. 2004;9:772–6. [Google Scholar]
- 53.Nangia S, Arya V, Gujral Ratni B, Mithal A. Lucknow: Presented at 27th Annual Meeting of The Endocrine Society of India; Spinal bone mineral density in normal Indian females. 1997 [Google Scholar]
- 54.Singh M, Magon N, Singh T. Major and minor discordance in the diagnosis of postmenopausal osteoporosis among Indian women using hip and spine dual-energy X-ray absorptiometry. J Midlife Health. 2012;3:76–80. doi: 10.4103/0976-7800.104457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reddy PG, Mithal A, Rao DS. Bone mineral density in healthy Asian Indian women: Development of a reference database and implications for diagnosis of osteoporosis in Indian women living in the United States [abstract] J Bone Miner Res. 2002;17(Suppl 1):SA270. [Google Scholar]
- 56.Harinarayan CV, Ramalakshmi T, Prasad UV, Kumar EGTV, Srinivasa Rao PVLN. Ultrasound bone mineral density of os calcis - its relationship with bone mineral markers and 25(OH) vitamin D in endemic fluorotic and non-fluorotic villages. J Clin Sci Res. 2012;1:157–62. [Google Scholar]
- 57.National Institute of Nutrition, ICMR, Dietary Guidelines for Indians: A Manual. Annex 3. 2011 [Google Scholar]
- 58.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–81. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline [published correction appears in J Clin Endocrinol Metab.2011 Dec;96(12):3908]? J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. doi:10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 60.Moyer VA. U.S. Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;158:691–6. doi: 10.7326/0003-4819-158-9-201305070-00603. [DOI] [PubMed] [Google Scholar]
- 61.Harinarayan CV, Appicatlaa L, Nalini BA, Joshi S, et al. (2012) Efficacy and Safety of Cholecalciferol Supplementation in Vitamin D Deficient Subjects Based on Endocrine Society Clinical Practice Guidelines? Endocrinol Metabol Syndrome. 2012;S4:004. doi:10.4172/2161-1017.S4-004. [Google Scholar]
- 62.Goswami R, Gupta N, Ray D, Singh N, Tomar N. Pattern of 25-hydroxy vitamin D response at short (2 month) and long (1 year) interval after 8 weeks of oral supplementation with cholecalciferol in Asian Indians with chronic hypovitaminosis D. Br J Nutr. 2008;100:526–9. doi: 10.1017/S0007114508921711. [DOI] [PubMed] [Google Scholar]
- 63.Harinarayan CV, Joshi SR. Vitamin D status in India--its implications and remedial measures. J Assoc Physicians India. 2009;57:40–48. [PubMed] [Google Scholar]
- 64.Marwaha RK, Garg MK, Sethuraman G, Gupta N, Mithal A, Dang N, et al. Impact of three different daily doses of Vitamin D3 supplementation in healthy schoolchildren and adolescents from North India: A single-blind prospective randomised clinical trial. Br J Nutr. 2019;121:538–48. doi: 10.1017/S0007114518003690. [DOI] [PubMed] [Google Scholar]
- 65.Marwaha RK, Dabas A. Interventions for prevention and control of epidemic of Vitamin D deficiency. Indian J Pediatr. 2019;86:532–7. doi: 10.1007/s12098-019-02857-z. [DOI] [PubMed] [Google Scholar]
- 66.Marwaha RK, Dev T, Mittal A. A randomised controlled trial comparing the efficacy of micellised and fat-soluble Vitamin D3 supplementation in healthy adults. Br J Nutr. 2019;121:859–65. doi: 10.1017/S0007114518003215. [DOI] [PubMed] [Google Scholar]
- 67.Nandgaye, Krishnakumar M. Relative oral bioavailability of three formulations of vitamin D3: An open-label, three-treatment study. [Last accessed on 2020 Jul 27];International Journal of Basic & Clinical Pharmacology, [S.l.] 2018 8:138–142. doi: http://dx.doi.org/10.18203/2319-2003.ijbcp20185172. Available at: https://www.ijbcp.com/index.php/ijbcp/article/view/2996 . [Google Scholar]
- 68.Gupta N, Farooqui KJ, Batra CM, Marwaha RK, Mithal A. Effect of oral versus intramuscular Vitamin D replacement in apparently healthy adults with Vitamin D deficiency. Indian J Endocrinol Metab. 2017;21:131–6. doi: 10.4103/2230-8210.196007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emaus N, Gjesdal CG, Almås B, Christensen M, Grimsgaard AS, Berntsen GK, et al. Vitamin K2 supplementation does not influence bone loss in early menopausal women: A randomised double-blind placebo-controlled trial. Osteoporos Int. 2010;21:1731–40. doi: 10.1007/s00198-009-1126-4. [DOI] [PubMed] [Google Scholar]
- 70.Granacher U, Gollhofer A, Hortobágyi T, Kressig RW, Muehlbauer T. The importance of trunk muscle strength for balance, functional performance and fall prevention in seniors: A systematic review. Sports Med. 2013;43:627–41. doi: 10.1007/s40279-013-0041-1. [DOI] [PubMed] [Google Scholar]
- 71.Sinaki M. Exercise for patients with osteoporosis: Management of vertebral compression fractures and trunk strengthening for fall prevention. PM R. 2012;4:882–8. doi: 10.1016/j.pmrj.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG, Close JC. Effective exercise for the prevention of falls: A systematic review and meta-analysis. J Am Geriatr Son. 2008;56:2234–43. doi: 10.1111/j.1532-5415.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 73.Choi M, Hector M. Effectiveness of intervention programs in preventing falls: A systematic review of recent 10 years and meta- analysis. J Am Med Dir Assoc. 2012;13:188.13–e21. doi: 10.1016/j.jamda.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 74.Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;12:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanis JA, Harvey NC, McCloskey E, Bruyère O, Veronese N, Lorentzon M, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporosis Int. 2020;31:1–12. doi: 10.1007/s00198-019-05176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J Clin Endocrinol Metab. 2020;105:dgaa048. doi: 10.1210/clinem/dgaa048. doi:10.1210/clinem/dgaa048. [DOI] [PubMed] [Google Scholar]
- 77.Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohammed K, Benkhadra K. Efficacy of Pharmacological Therapies for the Prevention of Fractures in Postmenopausal Women: A Network Meta-Analysis. The Journal of Clinical Endocrinology & Metabolism. 2019;104:1623–1630. doi: 10.1210/jc.2019-00192. [DOI] [PubMed] [Google Scholar]
- 78.Barrionuevo P, Gionfriddo MR, Castaneda-Guarderas A, Zeballos-Palacios C, Bora P, Mohammed K, et al. Women's values and preferences regarding osteoporosis treatments: A systematic review. J Clin Endocrinol Metab. 2019;104:1631–6. doi: 10.1210/jc.2019-00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12:43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Camacho Pm, Petak Sm, Binkley N. American association of clinical endocrinologists and american college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(Suppl 4):1–42. doi: 10.4158/EP161435.GL. Doi:10.4158/Ep161435.Gl. [DOI] [PubMed] [Google Scholar]
- 81.Meier C, Uebelhart B, Aubry-Rozier B, Birkhäuser M, Bischoff-Ferrari HA, Frey D, et al. Osteoporosis drug treatment: duration and management after discontinuation. A position statement from the SVGO/ASCO. Swiss Med Wkly. 2017;147:w14484. doi: 10.4414/smw.2017.14484. Published 2017. doi:10.4414/smw.2017.14484. [DOI] [PubMed] [Google Scholar]
- 82.Meeta M, Harinarayan CV, Marwah R, Sahay R, Kalra S, Babhulkar S. Clinical practice guidelines on postmenopausal osteoporosis: An executive summary and recommendations. J Midlife Health. 2013;4:107–26. doi: 10.4103/0976-7800.115293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calman KC, Royston G. Personal paper: Risk language and dialects. Br Med J. 1997;315:939–42. doi: 10.1136/bmj.315.7113.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.National Institute for Health and Care Excellence, Menopause, Clinical Guideline; June. 2015 [Google Scholar]
- 85.Meeta M, Digumarti L, Agarwal N, Vaze N, Shah R, Malik S. Clinical practice guidelines on postmenopausal osteoporosis: An executive summary and recommendations. J Midlife Health. 2013;4:107–26. doi: 10.4103/0976-7800.115290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buchbinder R, Johnston RV, Rischin KJ, Homik J, Jones C, Golmohammadi K, Kallmes DF. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database of Systematic Reviews. 2018;(Issue 11) doi: 10.1002/14651858.CD006349.pub4. Art. N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koh LK, Sedrine WB, Torralba TP, Kung A, Fujiwara S, Chan SP. A simple tool to identify Asian women at increased risk of osteoporosis. Osteoporos Int. 2001;12:699–705. doi: 10.1007/s001980170070. [DOI] [PubMed] [Google Scholar]
- 88.Latt TS, Aye TT, Ko K, Myint T, Hlaing NN, Thaung M, Chit TT. Myanmar Clinical Practice Guideline for Osteoporosis. Journal of the Asian Federation of Endocrine Societies. 27:151–5. DOI: 10.15605/jafes.027.02.03. [Google Scholar]
- 89.Lydick E, Cook K, Turpin J, Melton M, Stine R, Byrnes C. Development and validation of a simple questionnaire to facilitate identification of women likely to have low bone density. Am J Manag Care. 1998;4:37–48. [PubMed] [Google Scholar]


