Abstract
Salinity is one of the most vicious environmental constraints that hamper agricultural production. Experiments were done to explore the significant role of sole and synergistic supplementation of kinetin (100 µM KN) and putrescine (100 µM PUT) on Luffa acutangula in NaCl (100 mM) treatment. The harmful effects of salinity on growth were manifested by decreased seedling length, biomass, and pigment contents. We studied the effect of KN, and PUT in preventing salt (NaCl) induced physiological disorders and oxidative damages in 20-day-old Luffa acutangula seedlings. The individual application of KN and PUT increased growth and biochemical parameters, whereas combined KN + PUT treatment showed significant enhancement in growth, photosynthetic pigment content, and osmolyte accumulation in salt-affected plants. Application of KN and PUT also prevented hydrogen peroxide and superoxide production as confirmed by inhibition in electrolyte leakage and lipid peroxidation. Kinetin and PUT application upregulated the antioxidant defense system by enhancing antioxidant enzymes and non-enzymatic contents. Luffa seedlings treated with NaCl + KN + PUT showed 79, 26, 74, and 73% rise in superoxide dismutase, catalase, ascorbate peroxidase, and glutathione reductase enzymes, respectively, in comparison to NaCl-stressed Luffa acutangula. Findings revealed that synergistic utilization of KN and PUT modulate growth and biochemical processes in seedlings efficaciously in comparison to the individual application under salt stress, and it may be due to a regulatory crosstalk mechanism.
Keywords: Antioxidants, Phytohormones, Polyamine, Priming, ROS, Osmoprotection, Salinity
Introduction
Soil salinity is a serious problem that adversely influence crop production and sustainable development all over the world. Salinity, because of salt accretion in cultivable soil, is a major threat to agricultural activities (Negrao et al. 2017; D’Amelia et al. 2018; Liu et al. 2020). Application of fertilizers and excessive irrigation have enhanced salinity in crop fields out of which twenty percent of total global irrigated area is adversely impacted by NaCl stress, and more than half of fertile land is anticipated to be salinized by 2050 (Fischer et al. 2012; Shokat and Grosskinsky 2019). Salinity exerts a detrimental effect on the development of plants as the presence of excessive Na+ in soil alters the texture of the soil, checks soil porosity and aeration, reduces microbes and availability of nutrients on the earth surface, and negatively affects water uptake by plants (Vimal et al. 2017; Hmaeid et al. 2019). Soil containing salts gives rise to osmotic shock due to water deficiency, nutrient imbalance, ion toxicity, and oxidative stress, which may cause malfunctioning of metabolic pathways and finally, death of plant cells (Wu et al. 2013). Salinization increases reactive oxygen species (ROS) production, which degrades biomolecules and initiates lipid peroxidation and changes in nucleic acid (Yang et al. 2019). ROS induced oxidative stress hinders redox homeostasis which indirectly cause loss of photosynthetic efficiency, mineral absorption capacity, level of phytohormones, and gene expression in plants (Hasanuzzaman et al. 2013; Fallah et al. 2017; Ma et al. 2018). However, to combat the adverse impact of salt stress, plants develop complex antioxidant defense machinery composed of enzymatic and non-enzymatic components like ascorbate (AsA), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), etc., which keep ROS under control and contribute to stress endurance mechanism (Hasanuzzaman et al. 2019b).
Phytohormone priming has been reported as a potent method for growth enhancement in plants in adverse environmental constraints (Sytar et al. 2019). The growth hormones of plants take part in the modulation of key metabolic pathways in the state of adverse conditions as they crosstalk with other biomolecules to evoke protection mechanisms (Ahmad et al. 2016). Kinetin (KN) promotes growth of different crop plants exposed to salinity (Wu et al. 2012), heavy metals (Singh and Prasad 2014), and waterlogging (Younis et al. 2003). Kinetin has been reported to regulate cell division, morphogenesis, seed germination, nutrients absorption, and delay senescence in plants (Ahanger et al. 2020). Polyamines show significant functions against abiotic stresses, and because of the presence of cations, they preserve different biomolecules containing negative charges (Lopez-Gomez et al. 2017; Hasanuzzaman et al. 2019a). Polyamines have been found to linked with salt stress tolerance mechanisms as they help in scavenging of ROS and affect antioxidant activity at molecular and gene expression levels (Sang et al. 2016). The exogenous supplementation of polyamines protects cell membrane integrity, maintains ion balance in cells, minimizes inhibition of morphological parameters induced by NaCl stress, and restricts ROS generation by controlling the defense mechanism of plants under natural constraints (Lopez-Gomez et al. 2017). Putrescine (PUT; C4H12N2), a ubiquitous low-molecular-weight polyamine, contains two amino groups (Baniasadi et al. 2018). It is a central product of the polyamine biosynthetic pathway and acts as a precursor of spermidine (Spd) and spermine (Spm; Xu et al. 2009). Putrescine inhibits accumulation of Na+ and Cl− ions in cells of Atropa belladonna under salt stress (Ali 2000) and also reduces superoxide levels in plant cells in order to check oxidative stress (Ali et al. 2020). PUT increased the growth and viability of Oryza sativa leaves under salt stress (Lutts et al. 1996). This may be due to the inhibition of ethylene biosynthesis as PUT checks NaCl‐induced conversion of aminocyclopropane-1-carboxylic acid to ethylene.
Luffa acutangula L. belongs to the Cucurbitaceae family and is also widely used in traditional medicinal system to treat various ailments. It exhibits several therapeutic properties and is used to cure dysentery, headache, jaundice, diabetes, fungal infections, and leprosy. However, during the initial growth phase, this plant is sensitive to salinity. Remediation of saline soil is a challenging task, but the application of phytohormones and polyamines can be an environmentally safe and sustainable approach for crop production under salinity. The function of KN and PUT in diminishing the adverse impact of abiotic stresses has been well documented, but the synergistic application by which they alleviate salinity-induced changes in Luffa plants has not been studied yet. It was hypothesized that the interaction between KN and PUT might trigger an effective defense mechanism against NaCl stress as observed by Ahanger et al. (2018, 2019). The present study may open new avenues by kinetin and putrescine synergistic application for the regulation of the salt tolerance mechanism in crop plants, specifically those cultivated in saline areas, and to increase the crop production via low-cost additives such as KN and PUT. Hence, the present investigation was executed to explore individual and synergistic effects of KN and PUT on L. acutangula grown under salt stress as a combined application can enhance salt tolerance capacity by modulating physiological, biochemical, and antioxidant enzyme responses.
Materials and methods
Cultivation of Luffa acutangula plants and treatment
Sponge gourd (Luffa acutangula cv. Pusa Sneha) seeds were sterilized with 10% NaOCl for 7 min, then proper cleaning was done with autoclaved distilled water. Kinetin (6-furfurylaminopurine, KN; molecular weight: 215.21 g mol−1) and putrescine (PUT; molecular weight: 161.07 g mol−1) were procured from Merck. Seeds of Luffa were immersed in distilled water and KN solution (100 μM) for 7 h. The sowing of seeds was done in plastic pots contained disinfected sand with a full-strength Hoagland solution. In a growth chamber, pots with germinated seedlings were placed under photo-synthetically active radiation of 150 μmol photons m−2 s−1 in 16:8 h day and night duration at 26 ± 2 °C with 90% moisture and sprayed with full-strength Hoagland solution after two days. After a week, two seedlings were kept in each pot. Application of modified Hoagland solution with NaCl (100 mM) was induced, and a mixture of 100 μM PUT with tween-20 (spreading agent) was applied on the foliage and the seedlings which were sprayed with distilled water considered as untreated. Total of eight groups were made: (1). Control (full-strength Hoagland solution with distilled water) (2). 100 mM NaCl (3). 100 µM KN (4). 100 µM PUT (5). 100 µM KN + 100 µM PUT (6). 100 mM NaCl + 100 µM KN (7). 100 mM NaCl + 100 µM PUT (8). 100 mM NaCl + 100 µM KN + 100 µM PUT. Twenty day old seedlings, after 10 days of KN and PUT supplementation, and 100 mM NaCl treatment, respectively were used for the assessment of physiological processes.
Determination of growth parameters and relative water content
Growth parameters were examined in terms of seedling length and biomass. In relative water content assessment, the same size round shaped Luffa leaves were collected from control and treated samples and fresh weight was evaluated. After 12 h floating in distilled water at 25 ± 2 °C under dark conditions, turgid weight was analyzed for dry biomass measurement, and leaves were put at 80 °C in an electric oven for 2 days. RWC was analyzed with the formula: RWC (%) = Fw − Dw/Tw − Dw × 100.
Determination of pigment content
Luffa acutangula leaves (100 mg) were ground with 80% acetone and optical density of the extracted chlorophyll and carotenoid pigments were measured at 663, 646, and 470 nm by UV–visible spectrophotometer. Chlorophyll a, b, total chl and carotenoids were measured following the procedure of Lichtenthaler (1987).
Estimation of sugar, proline, and glycine betaine
Hedge and Hofreiter (1962) method was applied for the analysis of sugars. Leaves (0.1 g) were ground with 95% ethanol (5 ml), and after centrifugation, anthrone reagent (4 ml) was added in the supernatant (1 ml), and the mixture was kept for heating for 15 min. At 620 nm, absorbance was noted, and the sugar amount was analyzed by standard curve prepared from glucose.
The proline amount was calculated by Bates et al. (1973). Luffa leaves were treated with sulphosalicylic acid (3%), and acid ninhydrin and acetic acid were mixed in aliquot and boiled at 100 °C for 1 h. The absorbance was assessed at 520 nm after the addition of toluene (4 ml).
The content of glycine betaine was measured by the procedure proposed by Grieve and Grattan (1983). The periodide crystals were observed due to reaction with KI–I2 reagent at low pH, absorbance was noted at 365 nm, and concentration was measured by standard curve.
Estimation of protein and nitrate reductase activity
The fresh Luffa leaves (10 mg) were grounded with 1 N NaOH at 100 °C for 5 min. After 10 min of adding alkaline copper reagent (5 ml) in the test tubes, Folin–Ciocalteau reagent (0.5 ml) was mixed in the sample. After 30 min, absorbance was taken at 650 nm, and protein amount was recorded by Bovine Serum Albumin standard curve (Lowry et al. 1951).
Nitrate reductase enzyme activity was measured following the protocol of Jaworski (1971). Luffa leaves (250 mg) were treated with the medium (4.5 ml) containing 100 mM phosphate buffer, 3% potassium nitrate and 5% propanol and the aliquot (0.4 ml) was treated with 3% sulphanilamide (0.3 ml) in 3 N HCl and 0.02% N-1-NEDD (0.3 ml). At 540 nm, absorbance was calculated and nitrate reductase activity was analyzed by sodium nitrite standard curve.
Estimation of electrolyte leakage, hydrogen peroxide, superoxide
Electrolyte leakage was estimated by the method of Dionisio-Sese and Tobita (1998).
Electrolyte leakage (%) = (EC1 − EC0)/(EC2 − EC0) × 100.
Hydrogen peroxide was determined by the method of Velikova et al. (2000). At 390 nm, absorbance was taken and concentration of hydrogen peroxide was measured by the standard curve.
Superoxide concentration was measured by fusing fresh leaves with potassium phosphate buffer (65 mM, pH 7.8) and centrifugation for 10 min at 5,000 × g. The hydroxylamine hydrochloride (10 mM) was added to the supernatant and sulphanilamide and naphthylamine were also added after 20 min. Absorbance was noted at 530 nm and concentration was assessed by standard curve prepared from sodium nitrite (Yang et al. 2011).
Lipid peroxidation and lipoxygenase activity
Malondialdehyde content was determined to identify oxidative damage of lipids in Luffa leaves (Heath and Packer, 1968). Two hundred milligrams luffa leaves were mashed in trichloroacetic acid (0.1%) and centrifuged at 10,000 g for 10 min. Filtrate (1 ml) was added in 0.5% TBA (4 ml) and kept at 95 °C temperature for 30 min and after proper cooling, centrifugation was done. The optical density of filtrate was calculated at 532 nm and a correction was made by reducing non-specific absorbance at 600 nm. MDA concentration was determined by applying 155 mM–1 cm–1extinction coefficient.
Doderer et al. (1992) method was applied for calculation of lipoxygenase activity. Absorbance was taken at 234 nm and the calculation was done with an extinction coefficient of 25 mM−1 cm−1.
Assay of antioxidant enzymes
The enzyme extracts were produced by fusing leaves of Luffa (500 mg) with 0.1 M sodium phosphate buffer with polyvinyl pyrrolidone. The mixture was centrifuged at 14,000g at 4 °C for 30 min in a cooling centrifuge and filtrate was taken for analysis of various enzymatic components which are given below:
Superoxide dismutase was measured by nitroblue tetrazolium photochemical assay with the method of Beyer and Fridovich (1987). The enzyme (0.4 ml) was mixed with 20 mM methionine, 0.15 mM EDTA, 0.12 mM NBT, 13 μM riboflavin and 0.05 M sodium carbonate. Test tubes containing samples were kept under fluorescent lamps for 30 min and the same set which was unilluminated considered as blank. The purple-colored formazan shows NBT reduction under light, and it was calculated at 560 nm and analyzed with those samples which were without enzyme. One unit of enzyme activity is elucidated as the content of the enzyme needed to show 50% suppression in NBT reduction.
The activity of CAT was analyzed by separation of hydrogen peroxide at 240 nm for 1 min by applying an extinction coefficient of 39.4 mM–1 cm–1 (Cakmak and Marschner, 1992). Enzyme extract (0.2 ml) was mixed with 50 mM potassium phosphate buffer, 1 mM EDTA, and 10 mM H2O2. The one unit of CAT activity is known as 1 nmol H2O2 dissociated min−1.
Ascorbate peroxidase was determined by following Nakano and Asada (1981). Two millilitres of reaction mixture was composed of 25 mM potassium phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.25 mM ascorbate, enzyme extract (0.2 ml) and hydrogen peroxide (1 mM). Absorbance was calculated at 290 nm with an extinction coefficient of 2.8 mM−1 cm–1.
Foster and Hess (1980) method was used for the analysis of GR. The reaction mixture (3 ml) was composed of potassium phosphate buffer (100 mM), EDTA (1 mM), NADPH (50 µM), oxidized glutathione (100 µM) and enzyme (100 µl) and change in absorbance was calculated at 340 nm.
Determination of ascorbate and reduced glutathione
The procedure of Mukherjee and Choudhuri (1983) was applied for the calculation of ascorbate content. The fresh leaves were crushed with 6%TCA and dinitrophenylhydrazine (2%) and thiourea (10%) were added. The samples were kept in a water bath for 15 min, then after proper cooling 80% H2SO4 (5 ml) was added, and absorbance was measured at 530 nm, and a standard curve of ascorbate with known concentration.
The reduced glutathione content was analyzed by Ellman (1959). Luffa leaves (100 mg) were macerated with phosphate buffer and filtrate (500 µl) was mixed with 5,5-dithiobis(2-nitrobenzoic acid). Absorbance was measured at 412 nm and GSH content was analyzed by its standard curve.
Statistical analysis
Treatments were organized as a randomized block design with three replicates. The data were calculated using ANOVA and SPSS software and mean was calculated by using Duncan’s multiple range test (DMRT) at P < 0.05.
Results
Growth parameters and relative water content
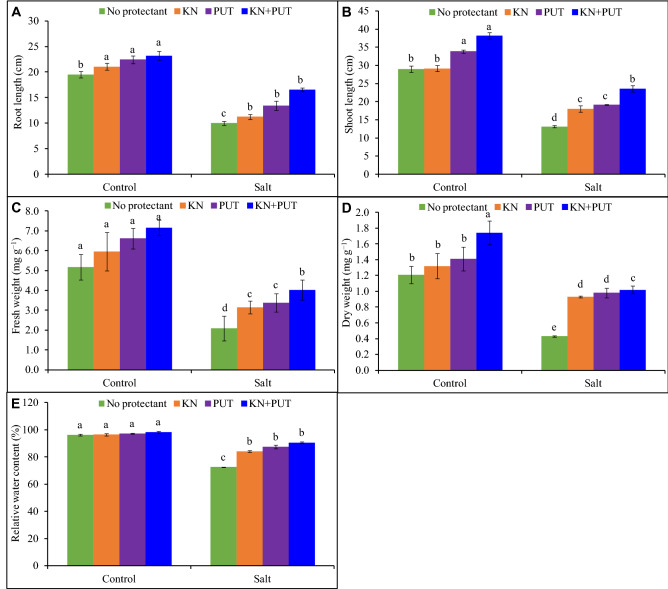
Luffa acutangula seedlings under the influence of NaCl stress, showed an obvious decrease in root and shoot length and weight in comparison to control. Under salt stress (100 mM), 49, 55, 60, and 64% decrease in lengths of root and shoot and fresh and dry weight, respectively, were observed over control (Fig. 1). Supplementation of KN and PUT reflected enhancement in seedling length and biomass and synergistic treatment of KN + PUT showed 32 and 38% rise in length of shoots and fresh weight in comparison to control. As compared to salt treated Luffa seedlings, NaCl + KN + PUT treatment reflected 66 and 137% stimulation in the length of roots and dry weight. Relative water status showed 25% reduction in NaCl treated seedlings, but RWC was elevated to 33, 34, and 36% in KN, PUT, and KN + PUT treated seedlings in comparison to salt treated seedlings alone. The kinetin priming and PUT spray improved relative water content in control along with salt stress. Relative turgidity was elevated by 25% in Luffa leaves treated with NaCl + KN + PUT in comparison to NaCl stressed seedlings (Fig. 1).
Fig. 1.
Effect of salt stress on seedling length, biomass, and relative water content of Luffa acutangula L. with or without kinetin and putrescine. Data are mean ± standard error of three replicates. In a bar, different letter(s) indicate a significant mean difference at P < 0.05 according to Duncan’s multiple range test (DMRT)
Pigment content
Pigments are indispensable component for the development of plants. NaCl stress adversely affected chl contents (a, b and total) and carotenoids. Seedlings either primed with kinetin or foliar sprayed with PUT showed an increase in chl and carotenoids contents. Supplementation of KN and PUT individually enhanced total chl by 5 and 14% and carotenoids content by 19 and 34%, respectively (Table 1). The combined application of KN + PUT remarkedly stimulated chl (a + b) and carotenoids by 25 and 42%, respectively, as compared to control. Thus, KN priming or PUT use inhibited reduction in pigment content with maximum improvement in salt affected Luffa seedlings exposed to KN + PUT.
Table 1.
Effect of salt stress on pigment contents of Luffa acutangula L. seedlings with or without kinetin and putrescine. Data are mean ± standard error of three replications. Values in a column with different letter(s) show significant mean difference at P < 0.05 as per DMRT
| Treatment | Chl a (mg g−1 FW) | Chl b (mg g−1 FW) | Chl (a + b) (mg g−1 FW) | Carotenoids (mg g−1 FW) |
|---|---|---|---|---|
| Control | 2.98 ± 0.14b | 0.96 ± 0.01b | 3.94 ± 0.64b | 0.62 ± 0.08b |
| NaCl | 1.31 ± 0.12c | 0.54 ± 0.07c | 1.85 ± 0.19d | 0.39 ± 0.03d |
| KN | 3.10 ± 0.07a | 1.04 ± 0.10b | 4.14 ± 0.11a | 0.74 ± 0.08b |
| PUT | 3.24 ± 0.11a | 1.26 ± 0.10a | 4.50 ± 0.19a | 0.83 ± 0.03a |
| KN + PUT | 3.56 ± 0.13a | 1.38 ± 0.06a | 4.94 ± 0.13a | 0.88 ± 0.03a |
| NaCl + KN | 1.87 ± 0.11c | 0.66 ± 0.01c | 2.53 ± 0.24c | 0.45 ± 0.09c |
| NaCl + PUT | 2.15 ± 0.04b | 0.75 ± 0.09c | 2.9 ± 0.19c | 0.49 ± 0.03c |
| NaCl + KN + PUT | 2.43 ± 0.19b | 0.89 ± 0.07c | 3.32 ± 0.33b | 0.54 ± 0.11c |
Oxidative stress indicators
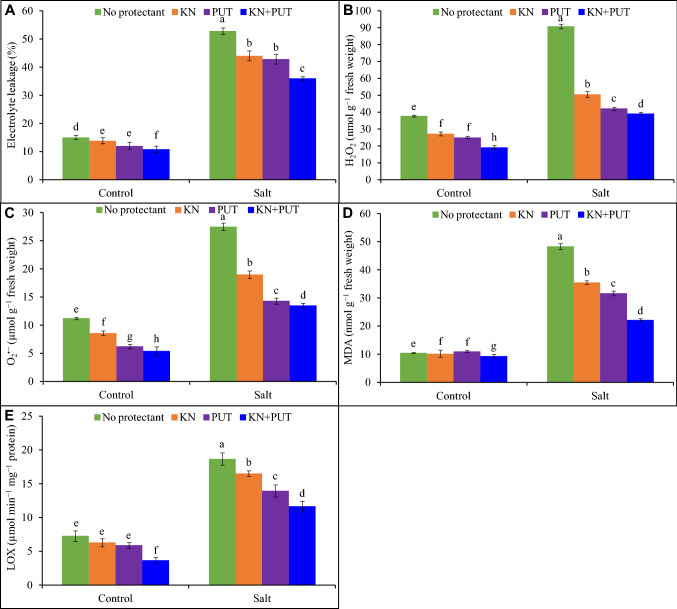
Salt stress increased H2O2, superoxide, electrolyte leakage, MDA, and LOX activity by 140, 145, 251, 358, and 157%, respectively, in seedlings in comparison to control. Kinetin and PUT application inhibited electrolyte leakage by 74 and 77%, respectively, over NaCl stress. Synergistic application of KN + PUT markedly decreased H2O2 and O2·− production and checked oxidative stress due to the lipid peroxidation and electrolyte leakage (Fig. 2).
Fig. 2.
Electrolyte leakage, hydrogen peroxide, superoxide, MDA content and lipoxygenase activity in Luffa acutangula seedlings grown under salt stress with and without kinetin and putrescine. In a bar, different letter(s) indicate a significant mean difference at P < 0.05 according to DMRT
Salinity exhibited notable stimulation in MDA content 358% and lipoxygenase activity 157% in Luffa leaves as compared to control. The exposure to KN or PUT reflected a reduction in LOX activity with 80% decline in KN + PUT administration over NaCl treatment in Luffa leaves (Fig. 2).
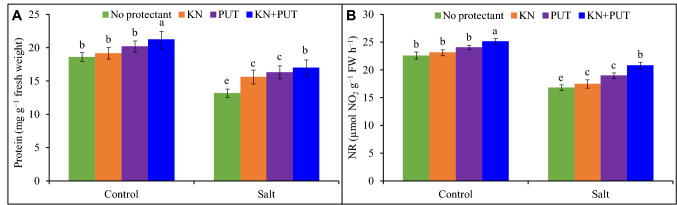
Protein content and nitrate reductase activity
Protein amount and NR activity were significantly affected by salt exposure. In protein content, 29% reduction was found in Luffa leaves with NaCl stress in comparison to control while it was stimulated to 3 and 8% with KN and PUT supplementation (Fig. 3). The 2 and 6% stimulation in NR activity was achieved in KN, and PUT treated seedlings in comparison to control. The 11% rise was recorded in NR activity in combined treatment, i.e., KN + PUT.
Fig. 3.
Protein and nitrate reductase enzyme activity in Luffa acutangula leaves grown under salt stress with and without kinetin and putrescine. Data are mean ± standard error of three replicates. In a bar, different letter(s) indicate a significant mean difference at P < 0.05 according to DMRT
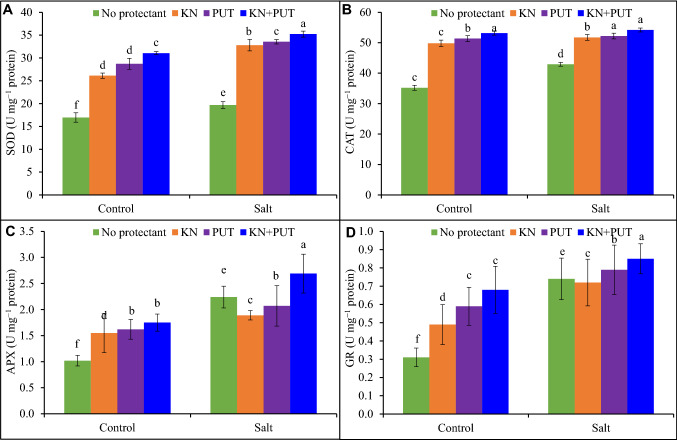
Activities of antioxidant enzymes
Enzymatic antioxidants showed different responses in Luffa acutangula during treatment with KN or PUT sole or in combination. Treatment of seedlings with KN or PUT individually and synergistically revealed enhancement in antioxidant activity over control and NaCl stress (Fig. 4). Salt stress promoted the activities of antioxidant enzymes as compared to untreated leaves, but stimulation was recorded when KN and PUT were applied to Luffa acutangula under NaCl stress. Seedlings exposed to NaCl + KN + PUT reflected augmentation of 79, 26, 74, and 73% in SOD, CAT, APX, and GR, respectively over salt-stressed leaves.
Fig. 4.
Activities of SOD, CAT, APX and GR in Luffa acutangula leaves grown under salt stress with and without kinetin and putrescine. Data are mean ± standard error of three replicates. In a bar, different letter(s) indicate a significant mean difference at P < 0.05 according to DMRT
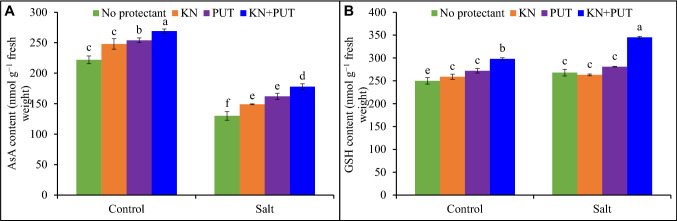
The synergistic impact of KN and PUT was more prominent in comparison to sole treatment. Salt stress inhibited AsA accumulation and enhanced GSH content, both KN and PUT increased AsA and GSH in control and salt-stressed Luffa seedlings. The KN + PUT application improved 21% AsA and 19% GSH over control seedlings (Fig. 5).
Fig. 5.
Ascorbate and reduced glutathione contents in Luffa acutangula leaves grown under salt stress with and without kinetin and putrescine. Data are mean ± standard error of three replicates. In a bar, different letter(s) indicate a significant mean difference at P < 0.05 according to DMRT
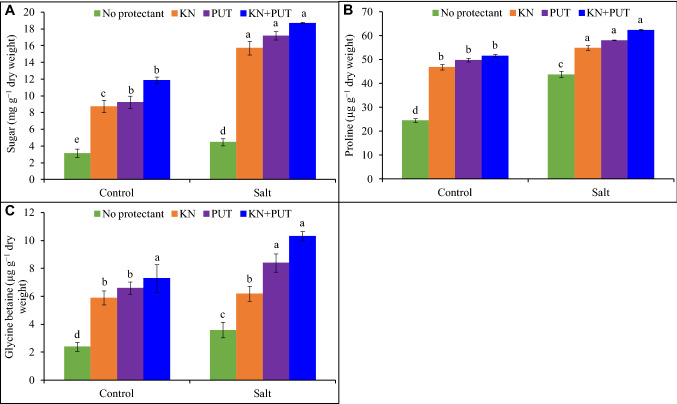
Osmolytes content
Kinetin and PUT supplementation alone or, in combination, enhanced the levels of osmolytes (Fig. 6). The treatment of NaCl to Luffa acutangula increased contents up to 42, 79, and 50% of sugar, proline, and glycine betaine in comparison to control. Enhancement in osmoprotectants concentration was recorded by KN and PUT application with a significant rise 495, 155, and 330% was reported in Luffa seedlings treated with NaCl + KN + PUT in comparison to control (Fig. 6).
Fig. 6.
Effect of salt stress on sugar, proline, and glycine betaine contents of Luffa acutangula L. seedlings with or without kinetin and putrescine. In a bar, different letter(s) indicate a significant mean difference at P < 0.05 according to DMRT
Discussion
High salt concentration in soil restricts seed germination, water, and nutrients uptake, reduces hydraulic conductivity, pigment synthesis, and other metabolic processes required for plant development (Gong et al. 2018). The inhibition in seedling length, as well as the reduction in biomass of Luffa acutangula because of NaCl stress was observed (Manai et al. 2014; Martinez et al. 2018). Salt stress impelled growth restriction might be due to inhibition in the elongation of plant cells (Queiros et al. 2011). Imbalance in the homeostasis of nutrients was another aspect of NaCl-induced damage in Luffa seedlings. Zhao et al. (2007) stated that alteration in the root plasma membrane by ion channels disruption and removal of nutrients by excessive Na+ influx was responsible for decreased nutrient uptake and imbalance in metabolic processes under NaCl stress. In this study, salinity induced decrease in growth was mitigated by KN priming or PUT or by their combined application (Ali et al. 2020). Our findings of the mitigation of salt-induced reduction in seedling length and biomass by kinetin are similar, as recorded by Kaya et al. (2010). Hamayun et al. (2015) observed kinetin increased resistance in soybean against salt stress by its interaction with growth hormones. Polyamines have been proved to be efficient to increase salt resistance by modifying membrane permeability, ion transport mechanism, ion channels, root and shoot growth (Lutts et al. 1996), Na+ intrusion and extrusion (Pottosin et al. 2012), photosynthesis (Shu et al. 2012), increase in nitrate reductase enzyme activity (Yadav et al. 2018) and antioxidative responses (Ikbal et al. 2014).
Relative water content is applied to check water content and osmotic adjustment by the plants under different environmental conditions (Ravikumar et al. 2014). More relative water content sustains the structure of proteins and improves photoassimilates mobilization. Gurmani et al. (2018) found that seeds of wheat primed with kinetin increased water status.
Salt enhances chlorophyllase activity and decreases the stability of the pigment–protein complex, whereas ROS produced due to NaCl stress degrades pigments (Shu et al. 2012; Martinez et al. 2018). Salt stress decreased pigment content might be due to decreased ribulose 1,5 bisphosphate carboxylase/oxygenase activity, disorganization of chloroplast structure or thylakoid membrane and loss of membrane proteins in the chloroplast (Gengmao et al. 2015).
Kinetin increases protochlorophyllide-synthesizing enzyme concentration (Demetriou, 2008). Significant stimulation in chl synthesis and number of chloroplasts have been reported in leaves of cowpea and maize by KN application (Tounekti et al. 2011; Acidri et al. 2020). Enhanced growth of Luffa seedlings with KN and PUT treatment was associated with its positive impact on photosynthesis. Ahanger et al. (2018) observed an increase in chl synthesis due to the foliar application of KN. Behera et al. (2002) reported that kinetin triggered carotenoids synthesis and protected photosynthetic apparatus from ROS by upregulating pigment synthesizing enzymes. Chattopadhayay et al. (2002) revealed that polyamine application on rice plants inhibited chl loss and leaf senescence. Shu et al. (2013) observed that PUT increased polyamine levels in chloroplast and managed negative impacts of salinity on photosynthesis. The interaction of polyamines with PSII proteins stabilized protein structure and enhanced chloroplast function in cucumber under NaCl stress. Kinetin and PUT supplementation promoted photoprotection, which influenced other attributes such as maintenance of water potential, antioxidant system, mineral assimilation, and metabolite accumulation (Oliveira et al. 2019). The role of KN and PUT might be oxidative stress reduction, maintenance of pH in the cells, protection of thylakoid membranes and pigment-protein complexes and maintenance of pigment synthesis and catabolic processes.
Osmolytes act as signaling molecules that reduce unfavorable effects of NaCl stress on biochemical pathways by preserving enzyme activities, photosynthetic oxygen-evolving complex and control redox balance and energy status during salt-induced stress (Khan et al. 2014; Ahanger and Agarwal 2017). Gharsallah et al. (2016) and Ahanger and Agarwal (2017) reported an increase in osmolyte concentration under NaCl stress. Plants with a higher amount of osmolytes show better stress withstanding ability and fast free radicals elimination (Slama et al. 2015; Ahanger et al. 2019). High relative water content improves photoassimilate mobility and stabilizes protein structure. More osmolytes accumulation exhibited maintenance of water potential in tissues which leads to lowering of water potential to facilitate continuous uptake of water (Ahanger et al. 2020). Application of KN and PUT significantly enhanced sugar, proline, and glycine betaine contents in NaCl-treated seedlings by inhibiting the efflux of K+ by improving ROS scavenging and preventing photoinhibition (Cuin and Shabala 2007).
The high electrolyte leakage indicates damage in the membrane as noted in Luffa plants during treatment with salt stress (Hussain et al. 2017). The rise in MDA level because of salinity indicates oxidative stress owing to excessive reactive oxygen species production in Luffa seedlings but KN and PUT application significantly decreased the lipid peroxidation.
Seedlings treated with NaCl showed H2O2 production which was responsible for the peroxidation of lipids, however KN and PUT treatment significantly mitigated salt incited lipid peroxidation by decreasing the concentration of H2O2 (Ahanger et al. 2018). Kinetin and PUT protect the photosynthetic electron transport chain by modulation in NADP+ and NADPH contents and check electrons movement to molecular oxygen and restricts O2·− production as observed in our results (Wu et al. 2012). Under NaCl stress, LOX enzyme activity increased, but a decline in LOX activity was found in plants exposed to KN and PUT (Sofo et al. 2004). The reduction in LOX activity was an indication of enhanced proteins and lipids stability in KN and PUT treated seedlings and improved oxidative stress tolerance capacity.
The decrease in protein content in seedlings with NaCl treatment was due to the decrease in protein synthesis or increased protein degradation (Hussain et al. 2017). Nitrogen metabolism is related to carbon metabolism. In the form of nitrate, nitrogen is absorbed by the plants which promotes development. Nitrate reductase, a substrate-incited enzyme, converts nitrate to nitrite. Significant reduction in NR activity in leaves under NaCl stress may be due to a reduced rate of carbon fixation or reduced nitrate uptake by roots (Kleinhofs and Warner 1990). The notable escalation was reported by the supplementation of KN and PUT may be due to improved photosynthetic ability and NO3− uptake by roots (Yadav et al. 2018).
Salt stress causes a reduction of osmosis, ion cytotoxicity, and overproduction of free radicals which causes oxidative stress and hampers biochemical processes in plants (Kamran et al. 2020). The enzymatic and non-enzymatic components are an integral components of plant defense mechanism and can scavenge ROS in order to stabilize membrane structure and prevent membrane lipid peroxidation. SOD converts O2·− to H2O2, whereas CAT removes the H2O2 by converting H2O2 to H2O. APX scavenges H2O2 in cytosol and chloroplast and prevents its entry into different cells to avoid injury. The supplementation of KN and PUT either individually or synergistically prevented salt-induced changes by triggering of antioxidant enzymes (Ahanger et al. 2018). Ascorbate and GSH are vital constituents of AsA-GSH radical scavenging cycle. This cycle contains APX and GR enzymes which play a significant role in AsA and GSH production. Ascorbate is water-soluble, low molecular weight antioxidant, and helps in removal of H2O2. The maintenance of AsA and GSH by upregulation of APX and GR activities results in inflow of electrons to oxygen in the chloroplast, thereby preventing superoxide radical production (Noctor and Foyer 1998). Polyamines function in the regulation of the AsA-GSH cycle and antioxidants might contribute to stress resistance (Nahar et al. 2016). Supplementation of spermidine to salinized nutrient solution stimulated SOD and CAT activities and mitigating NaCl-incited membrane injury and pigment synthesis reduction and polyamine content was enhanced (Duan et al. 2008). The increased Luffa seedling resistance against salt stress was correlated with enhanced antioxidant activity. Wu et al. (2012) and Siddiqui et al. (2015) stated that kinetin incited the upregulation of antioxidants. Acidri et al. (2020) observed that KN-treated coffee plants upregulated the non-enzymatic antioxidants and it was associated with enhanced free radicals scavenging capacity. Kinetin and PUT interact with other molecules and provide coordinated action towards stress resistance (Hasanuzzaman et al. 2019a). Ahanger et al. (2018) observed that kinetin treated tomato plants exhibited an increase in osmolyte content, regulation in AsA-GSH cycle with a robust antioxidant system. Nahar et al. (2016) observed that polyamine supplementation to salt-stressed mung bean increased GSH and AsA contents and antioxidant enzymes activities and inhibited oxidative stress. Hence, synergistic KN and PUT application induced up-regulation of osmolytes and antioxidant components which strengthens endurance strategies in Luffa acutangula against salt stress.
Conclusion
Salt stress markedly reduced the morphological parameters of Luffa acutangula seedlings. It reduced the contents of pigment and protein and nitrate reductase enzymes. It stimulated the accumulation of osmolytes and caused electrolyte leakage and lipid peroxidation. Significant enhancement in seedling length and biomass has been observed with KN, PUT, and their combined treatment, i.e. KN + PUT. Application of KN and PUT alleviated unfavorable effects of salt stress on morphological and biochemical components such as pigment and protein contents and NR activity of Luffa acutangula by triggering the up-regulation of osmolytes and antioxidants. The increase in antioxidant enzymes due to kinetin and putrescine supplementation exhibited a defensive role against salt stress. Thus, KN and PUT ameliorate the unfavorable impact of salt stress by strengthening the defense system of Luffa seedlings. Kinetin and PUT mediated growth promotion in Luffa seedlings under NaCl stress may be due to the development of a detoxifying system against ROS by incorporation between various signaling constituents. The present study shows probable crosstalk between KN and PUT in preventing NaCl stress. Further investigations are required to unravel the molecular mechanism in relation to KN and PUT application for plant stress resistance.
Acknowledgements
Dr. Kapoor is grateful to Amity Institute of Biotechnology, Amity University Uttar Pradesh, for providing the laboratory facilities to carry out the work.
Author contributions
RTK and MH designed and planned the experiment. RTK conducted the experiment. MH analyzed the data. RTK and MH prepared the manuscript draft. MH edited the manuscript. Both authors approved the final version of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acidri R, Sawai Y, Sugimoto Y, Handa T, Sasagawa D, Masunaga T, Sadahiro Yamamoto S, Nishihara E. Exogenous kinetin promotes the nonenzymatic antioxidant system and photosynthetic activity of coffee (Coffea arabica L.) plants under cold stress conditions. Plants. 2020;9(2):281. doi: 10.3390/plants9020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahanger MA, Agarwal RM. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol Biochem. 2017;115:449–460. doi: 10.1016/j.plaphy.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Ahanger MA, Alyemeni MN, Wijaya L, Alamri SA, Alam P, Ashraf M. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE. 2018;13:e0202175. doi: 10.1371/journal.pone.0202175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahanger MA, Qin C, Maodong Q, Dong XX, Ahmad P, AbdAllah EF, Zhang L. Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol Biochem. 2019;144:1–13. doi: 10.1016/j.plaphy.2019.09.021. [DOI] [PubMed] [Google Scholar]
- Ahanger MA, Mirb RA, Alyemenic MN, Ahmad P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol Biochem. 2020;147:31–42. doi: 10.1016/j.plaphy.2019.12.007. [DOI] [PubMed] [Google Scholar]
- Ahmad P, Abdel Latef AA, Hashem A, AbdAllah EF, Gucel S, Tran LSP. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci. 2016;7:347. doi: 10.3389/fpls.2016.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali RM. Role of putrescine in salt tolerance of Atropa belladonna plant. Plant Sci. 2000;152:173–179. [Google Scholar]
- Ali R, Hassan S, Shah D, Sajjad N, Bhat EA. Role of polyamines in mitigating abiotic stress. In: Roychoudhury A, Tripathi DK, editors. Protective chemical agents in the amelioration of plant abiotic stress: biochemical and molecular perspectives. New York: Wiley; 2020. pp. 291–305. [Google Scholar]
- Baniasadi F, Saari VR, Moud AAM. Physiological and growth responses of Calendula ocinalis L. plants to the interaction effects of polyamines and salt stress. Sci Hortic. 2018;234:312–317. [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Beyer WF, Fridovich JL. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Behera RK, Mishra PC, Choudhury NK. High irradiance and water stress induce alterations in pigment composition and chloroplast activities of primary wheat leaves. J Plant Physiol. 2002;159:967–973. [Google Scholar]
- Cakmak I, Marschner H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves. Plant Physiol. 1992;98:1222–1227. doi: 10.1104/pp.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhayay MK, Tiwari BS, Chattopadhyay G, Bose A, Sengupta DN, Ghosh B. Protective role of exogenous polyamines on salinity-stressed rice (Oryza sativa) plants. Physiol Plant. 2002;116:192–199. doi: 10.1034/j.1399-3054.2002.1160208.x. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Shabala S. Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ. 2007;30:875–885. doi: 10.1111/j.1365-3040.2007.01674.x. [DOI] [PubMed] [Google Scholar]
- D’Amelia L, Dell’Aversana E, Woodrow P, Ciarmiello LF, Carillo P (2018) Metabolomics for crop improvement against salinity stress. In: Kumar V, Wani SH, Suprasanna P,Tran LS (eds) Salinity Responses and Tolerance in Plants, vol 2. Springer, Cham. pp. 267–287.
- Demetriou LF. Effect of kinetin on the state of the protochlorophyll pigment in the inner membrane system of etioplasts. Biochem (Moscow) Suppl A Membr Cell Biol. 2008;2:237–242. [Google Scholar]
- Dionisio-Sese ML, Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998;135:1–9. [Google Scholar]
- Doderer A, Kokkelink I, van der Veen S, Valk B, Schram A, Douma A. Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim Biophys Acta. 1992;112:97–104. doi: 10.1016/0167-4838(92)90429-h. [DOI] [PubMed] [Google Scholar]
- Duan J, Li J, Guo S, Kang Y. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol. 2008;165:1620–1635. doi: 10.1016/j.jplph.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fallah F, Nokhasi F, Ghaheri M, Kahrizi D, Beheshti Ale Agha A, Ghorbani T, Kazemi E, Ansarypour Z. Effect of salinity on gene expression, morphological and biochemical characteristics of Stevia rebaudiana Bertoni under in vitro conditions. Cell Mol Biol. 2017;63(7):102–106. doi: 10.14715/cmb/2017.63.7.17. [DOI] [PubMed] [Google Scholar]
- Fischer G, Hizsnyik E, Prieler S, Wiberg D (2012) Scarcity and abundance of land resources: competing uses and the shrinking land resource base. In: Proceedings of the worlds within reach: from science to policy-IIASA 40th Anniversary Conference, Laxenburg, Austria, 24–26 October 2012.
- Foster JG, Hess JL. Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 1980;66:482–487. doi: 10.1104/pp.66.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengmao Z, Shihui L, Xing S, Yizhou W, Zipan, C (2015) The role of silicon in physiology of the medicinal plant (Lonicera japonica L.) under salt stress. Sci Rep 5:12696. [DOI] [PMC free article] [PubMed]
- Gharsallah C, Fakhfakh H, Grubb D, Gorsane F (2016) Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 8, lw055. [DOI] [PMC free article] [PubMed]
- Gong DH, Wang GZ, Si WT, Zhou Y, Liu Z, Jia J. Effects of salt stress on photosynthetic pigments and activity of Ribulose-1,5-bisphosphate Carboxylase/Oxygenase in Kalidium foliatum. Russ J Plant Physiol. 2018;65:98–103. [Google Scholar]
- Grieve CM, Grattan SR. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983;70:303–307. [Google Scholar]
- Gurmani AR, Khan SU, Ali A, Rubab T, Schwinghamer T, Jilani G, Farid A, Zhang J. Salicylic acid and kinetin mediated stimulation of salt tolerance in cucumber (Cucumis sativus L.) genotypes varying in salinity tolerance. Hortic Environ Biotechnol. 2018;59:461–471. [Google Scholar]
- Hamayun M, Hussain A, Khan SA, Irshad M, Khan AL, Waqas M, Shahzad R, Iqbal A, Ullah N, Rehman G, Kim HY, Lee IJ. Kinetin modulates physiohormonal attributes and isoflavone contents of Soybean grown under salinity stress. Front Plant Sci. 2015;6:377. doi: 10.3389/fpls.2015.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci. 2013;14:9643–9684. doi: 10.3390/ijms14059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Alhaithloul HAS, Parvin K, Bhuyan MHMB, Tanveer M, Mohsin SM, Nahar K, Soliman MH, Al Mahmud J, Fujita M. Polyamine action under metal/metalloid stress: regulation of biosynthesis, metabolism and molecular interactions. Int J Mol Sci. 2019;20:3215. doi: 10.3390/ijms20133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M, Bhuyan MHM, Anee TI, Parvin K, Nahar K, Mahmud JA, Fujita M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants. 2019;8:384. doi: 10.3390/antiox8090384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedge JE, Hofreiter BT. Estimation of carbohydrate. In: Whistler RL, Be Miller JN, editors. Methods in carbohydrate chemistry. New York: Academic Press; 1962. pp. 17–22. [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hmaeid N, Wali M, Mahmoud OM, Pueyo JJ, Ghnaya T, Abdelly C. Efficient rhizobacteria promote growth and alleviate NaCl-induced stress in the plant species Sulla carnosa. Appl Soil Ecol. 2019;133:104–113. [Google Scholar]
- Hussain I, Singh NB, Singh A, Singh H, Singh SC, Yadav V. Exogenous application of phytosynthesized nanoceria to alleviate ferulic acid stress in Solanum lycopersicum. Sci Hortic. 2017;214:158–164. [Google Scholar]
- Ikbal FE, Hernández JA, Barba-Espín G, Koussa T, Aziz A, Faize M. Enhanced salt-induced antioxidative responses involve a contribution of polyamine biosynthesis in grapevine plants. J Plant Physiol. 2014;171:779–788. doi: 10.1016/j.jplph.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Jaworski E. Nitrate reductase assay in intact plant tissue. Biochem Biophys Res. 1971;43:1274–1279. doi: 10.1016/s0006-291x(71)80010-4. [DOI] [PubMed] [Google Scholar]
- Kamran M, Parveen A, Ahmar S, Malik Z, Hussain S, Chattha MS, Saleem MH, Adil M, Heidari P, Chen JT. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int J Mol Sci. 2020;21(148):1–27. doi: 10.3390/ijms21010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya C, Tuna AL, Okant AM. Effect of foliar applied kinetin and indole acetic acid on maize plants grown under saline conditions. Turk J Agric For. 2010;34:529–538. [Google Scholar]
- Khan MIR, Asgher M, Khan NA. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycine betaine and ethylene in mungbean (Vigna radiata L.) Plant Physiol Biochem. 2014;80:67–74. doi: 10.1016/j.plaphy.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Kleinhofs A, Warner RL (1990) Advances in nitrate assimilation: the biochemistry of plants, vol 16. Academic Press, New York
- Lopez-Gomez M, Hidalgo-Castellanos J, Munoz-Sanchez JR, Marin-Pena AJ, Lluch C, Herrera-Cervera JA. Polyamines contribute to salinity tolerance in the symbiosis Medicago truncatula-Sinorhizobium meliloti by preventing oxidative damage. Plant Physiol Biochem. 2017;116:9–17. doi: 10.1016/j.plaphy.2017.04.024. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophyll and carotenoids: pigments of photosynthetic bio-membranes. In: Packer L, Douce R, editors. Methods in Enzymology. San Diego: Academic Press; 1987. pp. 350–382. [Google Scholar]
- Liu B, Peng X, Han L, Hou L, Li B. Effects of exogenous spermidine on root metabolism of cucumber seedlings under salt stress by GC-MS. Agronomy. 2020;10:459. [Google Scholar]
- Lowry OH, Rosebrough NS, Farrand AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:263–275. [PubMed] [Google Scholar]
- Lutts S, Kinet JM, Bouharmont J. Ethylene production in relation to salinity by leaves of rice (Oryza sativa L.) tolerance and exogenous putrescine application. Plant Sci. 1996;116:15–25. [Google Scholar]
- Ma NL, Che Lah WA, Abd Kadir N, Mustaqim M, Rahmat Z, Ahmad A, Lam SD, Ismail MR. Susceptibility and tolerance of rice crop to salt threat: physiological and metabolic inspections. PLoS ONE. 2018;13(2):e0192732. doi: 10.1371/journal.pone.0192732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manai J, Gouia H, Corpas FJ. Redox and nitric oxide homeostasis are affected in tomato (Solanum lycopersicum) roots under salinity-induced oxidative stress. J Plant Physiol. 2014;171:1028–1035. doi: 10.1016/j.jplph.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Martinez V, Nieves-Cordones M, Lopez-Delacalle M, Rodenas R, Mestre TC, Garcia-Sanchez F, Rubio F, Nortes PA, Mittler R, Rivero RM. Tolerance to stress combination in tomato plants: new insights in the protective role of melatonin. Molecules. 2018;23:535. doi: 10.3390/molecules23030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant. 1983;58:166–170. [Google Scholar]
- Nahar K, Hasanuzzaman M, Rahman A, Alam AA, Mahmud JA, Suzuki T, Fujita M. Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front Plant. 2016;7:1104. doi: 10.3389/fpls.2016.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach-chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Negrao S, Schmockel SM, Tester M. Evaluating physiological responses of plants to salinity stress. Ann Bot. 2017;119(1):1–11. doi: 10.1093/aob/mcw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Oliveira VP, Lima MDR, Silva BRS, Batista BL, Lobato AKS. Brassinosteroids confer tolerance to salt stress in Eucalyptus urophylla plants enhancing homeostasis, antioxidant metabolism and leaf anatomy. J Plant Growth Regul. 2019;38(2):557–573. [Google Scholar]
- Pottosin I, Velarde-Buendía AM, Zepeda-Jazo I, Dobrovinskaya O, Shabala S. Synergism between polyamines and ROS in the induction of Ca2C and KC fluxes in roots. Plant Signal Behav. 2012;7:1084–1087. doi: 10.4161/psb.21185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiros F, Rodrigues JA, Almeida JM, Almeida DP, Fidalgo F. Differential responses of the antioxidant defence system and ultrastructure in a salt-adapted potato cell line. Plant Physiol Biochem. 2011;49:1410–1419. doi: 10.1016/j.plaphy.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Ravikumar G, Manimaran P, Voleti SR, Subrahmanyam D, Sundaram RM, Bansal KC, Viraktamath BC, Balachandran SM. Stress inducible expression of AtDREB1A transcription factor greatly improves drought stress tolerance in transgenic indica rice. Transgenic Res. 2014;23:421–439. doi: 10.1007/s11248-013-9776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang T, Shan X, Li B, Shu S, Sun J, Guo S. Comparative proteomic analysis reveals the positive effect of exogenous spermidine on photosynthesis and salinity tolerance in cucumber seedlings. Plant Cell Rep. 2016;35:1769–1782. doi: 10.1007/s00299-016-1995-x. [DOI] [PubMed] [Google Scholar]
- Shokat S, Grosskinsky DK. Tackling salinity in sustainable agriculture-what developing countries may learn from approaches of the developed world. Sustainability. 2019;11:4558. [Google Scholar]
- Shu S, Yuan LY, Guo SR, Sun J, Liu CJ. Effects of exogenous spermidine on photosynthesis, xanthophyll cycle and endogenous polyamines in cucumber seedlings exposed to salinity. Afr J Biotechnol. 2012;11:6064–6074. [Google Scholar]
- Shu S, Yuan LY, Guo SR, Sun J, Yuan YH. Effects of exogenous Spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplastsin Cucumis sativus L. under salt stress. Plant Physiol Biochem. 2013;63:209–216. doi: 10.1016/j.plaphy.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Siddiqui MW, Singh JP, Nayyer MA, Barman K, Ahmad MS, Kumar V. 6-Benzylaminopurine affects lipid peroxidation and membrane permeability and thereby preserves curd quality and antioxidants during storage of cauliflower. Acta Physiol Plant. 2015;37:96. [Google Scholar]
- Singh S, Prasad SM. Growth, photosynthesis and oxidative responses of Solanum melongena L. seedlings to cadmium stress: mechanism of toxicity amelioration by kinetin. Sci Horticul. 2014;176:1–10. [Google Scholar]
- Slama I, Abdelly C, Bouchereau A, Flowers T, Savoure A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot. 2015;115:433–447. doi: 10.1093/aob/mcu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofo A, Dichio B, Xiloyannis C, Masia A. Lipoxygenase activity and proline accumulation in leaves and roots of olive trees in response to drought stress. Physiol Plant. 2004;121:58–65. doi: 10.1111/j.0031-9317.2004.00294.x. [DOI] [PubMed] [Google Scholar]
- Sytar O, Kumari P, Yadav S, Brestic M, Rastogi A. Phytohormone priming: regulator for heavy metal stress in plants. J Plant Growth Regul. 2019;38:739–752. [Google Scholar]
- Tounekti T, Hernández I, Müller M, Khemira H, Munné-Bosch S. Kinetin applications alleviate salt stress and improve the antioxidant composition of leaf extracts in Salvia officinalis. Plant Physiol Biochem. 2011;49:1165–1176. doi: 10.1016/j.plaphy.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain treated bean plants. Plant Sci. 2000;151:59–66. [Google Scholar]
- Vimal SR, Singh JS, Arora NK, Singh S. Soil-plant-microbe interactions in stressed agriculture management: a review. Pedosphere. 2017;27:177–192. [Google Scholar]
- Wu X, Zhu Z, Li X, Zha D. Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters and antioxidative system in seedlings of eggplant (Solanum melongena L.) under salinity stress. Acta Physiol Plant. 2012;34:2105–2114. [Google Scholar]
- Wu D, Cai S, Chen M, Ye L, Chen Z, Zhang H, Zhang G. Tissue metabolic responses to salt stress in wild and cultivated barley. PLoS ONE. 2013;8:e55431. doi: 10.1371/journal.pone.0055431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wu X, Zhang H. Impact of D-Arg on drought resistance and endogenous polyamines in mycorrhizal Pinus massoniana. J Nanjing For Univ. 2009;33:019–023. [Google Scholar]
- Yadav V, Singh NB, Singh H, Singh A, Hussain I. Putrescine affects tomato growth and response of antioxidant defense system due to exposure to cinnamic acid. Int J Veg Sci. 2018;25:259–277. [Google Scholar]
- Yang H, Wu F, Cheng J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011;127:1237–1242. doi: 10.1016/j.foodchem.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wang C, Xue Y, Liu X, Chen S, Song C, Yang Y, Guo Y. Calcium-activated 14-3-3 proteins as a molecular switch in salt stress tolerance. Nat Commun. 2019;10(1):1199. doi: 10.1038/s41467-019-09181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis ME, El-Shahaby OA, Nemat Alla MN, El-Bastawisy ZM. Kinetin alleviates the influence of waterlogging and salinity on growth and affects the production of plant growth regulators in Vigna sinensis and Zea mays. Agronomie. 2003;23:277–285. [Google Scholar]
- Zhao F, Song CP, He J, Zhu H. Polyamines improve KC/NaC homeostasis in barley seedlings by regulating root ion channel activities. Plant Physiol. 2007;145:1061–1072. doi: 10.1104/pp.107.105882. [DOI] [PMC free article] [PubMed] [Google Scholar]