Highlights
-
•
Non-human animal research shows stress alters amygdala–medial prefrontal cortex (mPFC) connectivity.
-
•
It is unclear how prenatal stress may alter human infant connectivity.
-
•
Prenatal stress was associated with decreased amygdala–mPFC functional connectivity.
-
•
Prenatal stress was associated with increased amygdala–mPFC structural connectivity.
-
•
This work provides insight into how stress contributes to neurodevelopmental risk.
Keywords: Prenatal stress, Infant neuroimaging, Amygdala, Medial prefrontal cortex, Resting-state, Diffusion weighted imaging
Abstract
Stressful experiences are linked to neurodevelopment. There is growing interest in the role of stress in the connectivity between the amygdala and medial prefrontal cortex (mPFC), a circuit that subserves automatic emotion regulation. However, the specific timing and mechanisms that underlie the association between stress and amygdala–mPFC connectivity are unclear. Many factors, including variations in fetal exposure to maternal stress, appear to affect early developing brain circuitry. However, few studies have examined the associations of stress and amygdala–mPFC connectivity in early life, when the brain is most plastic and sensitive to environmental influence. In this longitudinal pilot study, we characterized the association between prenatal stress and amygdala–mPFC connectivity in young infants (approximately age 5 weeks). A final sample of 33 women who provided data on preconception and prenatal stress during their pregnancy returned with their offspring for a magnetic resonance imaging scan session, which enabled us to characterize amygdala–mPFC structural and functional connectivity as a function of prenatal stress. Increased prenatal stress was associated with decreased functional connectivity and increased structural connectivity between the amygdala and mPFC. These results provide insight into the influence of prenatal maternal stress on the early development of this critical regulatory circuitry.
1. Introduction
Brain development is preprogrammed to unfold according to a set of genetic instructions. Yet, environmental factors play a crucial role in this progression, prompting or delaying the initiation of neurodevelopmental processes and changing neural structure and connectivity. Knowledge of the impact of the environment on brain development has grown immensely in recent decades as we have gained a greater understanding of the opportunities and vulnerabilities associated with periods of increased sensitivity to the environment. The brain develops rapidly in early life; thus, the environment during gestation merits close attention. Maternal stress during pregnancy has been linked to a host of outcomes in offspring (e.g., altered development and later psychiatric symptoms; Nazzari et al., 2020; Van Den Bergh et al., 2005), suggesting that variations in the intrauterine environment can contribute to risk for negative outcomes (Scheinost et al., 2017). Of particular focus is the amygdala–medial prefrontal cortex (mPFC) circuit, which is thought to be important for early learning about the environment, and serves as a foundational structure in affective development (Tottenham and Gabard-Durnam, 2017). Rodent research finds that early life stress exposure is associated with increased mRNA expression of corticotropin-releasing hormone in the amygdala (Hatalski et al., 1998), along with structural and functional alterations in amygdala circuitry (Molet et al., 2014), suggesting a possible pathway from stress exposure to changes in amygdala connectivity (Buss et al., 2012a; Gee et al., 2013b; VanTieghem and Tottenham, 2018). There is a gap to fill by characterizing the early consequences of prenatal stress on this circuit in order to inform our understanding of long-term developmental trajectories of emotion regulation.
The hypothesis that stress “accelerates” the development of amygdala–mPFC circuitry is based in part on rodent research (Callaghan and Richardson, 2011; Moriceau and Sullivan, 2006). In humans, there is some evidence that amygdala–mPFC connectivity is affected by stress experienced during childhood, yet as noted by a recent review (Colich et al., 2019), approximately half of these studies indicate more positive amygdala–mPFC connectivity, whereas the other half indicate less positive connectivity in those children exposed to early adversity. In adults, alterations of this circuitry is linked to anxiety (e.g., Casey and Lee, 2015) and emotion regulation (e.g., Banks et al., 2007; Ochsner and Gross, 2005). Most consider this circuit to serve a “top-down” process where the mPFC regulates the amygdala. However, earlier in development, “bottom-up” processes may work to establish this later regulatory relationship, as it has been theorized that amygdala–mPFC connections are bidirectionally shaped (Tottenham and Gabard-Durnam, 2017), which is supported by work in rodents (Quirk et al., 2006; Sotres-Bayon and Quirk, 2010). Obtaining a greater understanding of the potential impact of stress on amygdala–mPFC circuitry—and implications for later emotional functioning—requires information about development, including knowledge about typical development of this circuitry in early infancy.
Previous magnetic resonance imaging (MRI) work examining brain connectivity in infants provide promise for the usefulness of this tool. While these studies have typically focused on maternal depression, rather than stress, as a predictor of infant brain connectivity (Posner et al., 2016; Qiu et al., 2015; Rifkin-Graboi et al., 2013), each finds support that prenatal experiences may be reflected in infant brain outcomes. These studies have examined functional and structural connectivity primarily through resting-state functional MRI (rsfMRI) and diffusion-weighted imaging (DWI) techniques. rsfMRI examines the temporal association between hemodynamic signals of given brain regions to index co-activation—positive correlations indicate that two regions are typically engaged at the same time and are therefore “functionally” connected. DWI provides the ability to examine anatomical connections between brain regions by indexing the diffusion of water across the brain—more restricted diffusion is interpreted as tighter structural connections, such as more tightly bundled myelinated axons. Previous work demonstrates that structural connectivity between these two regions emerges as early as 13 weeks gestation (Vasung et al., 2010). Thus, it is likely that variations in the intrauterine environment could exert effects on this developing circuit beginning in early pregnancy. Endocrine and inflammatory mediators in the gestational environment may influence fetal brain circuitry either directly—higher levels of circulating cortisol can pass through the fetal blood-brain barrier and act on key signaling pathways and structures in the fetal brain (Lenniger et al., 2020; Noorlander et al., 2006)—or indirectly, by changing the amount or activity of critical neurotrophic factors, hormones, or neurotransmitters (Buss et al., 2012b). Functional connectivity between the amygdala and PFC has also been identified in neonates within four days of birth (Rogers et al., 2017). Thus, using a multi-modal approach to examine the connectivity between the amygdala and mPFC in early infancy is likely to provide important insight into this circuit’s development.
In this pilot study of newborn infants, we prospectively examine maternal stress assessed in pregnancy with functional and structural connectivity of the amygdala–mPFC circuit. Examining individual differences in early brain development may be important for understanding differences in the developmental trajectories of this circuitry and contribute to knowledge about whether stress may accelerate or delay the development of typical amygdala–mPFC circuitry. Although it is unclear whether findings from studies assessing stress and brain circuitry in older children will generalize to early infancy, we expect to observe positive resting-state functional connectivity (rsFC) between these regions. Further, given the possibility that exposure to stress in gestation may affect development in this circuitry, we hypothesize that stress will be associated with rsFC, although it is not clear whether acceleration would manifest as greater or lesser rsFC between these regions. In terms of structural connectivity, we expect to detect white matter tracts between these regions, and that among those infants exposed to greater prenatal stress, there will be greater evidence of structural connectivity between the amygdala and mPFC. Thus, our aims are to examine the state of connectivity in this sample of young infants and to examine the association between maternal stress in pregnancy and infant connectivity. Importantly, there have been calls to consider the effect of preconception adversity when examining the potential association between prenatal stress and offspring outcomes (Scheinost et al., 2017), in part because prenatal stress may be a marker of cumulative stress rather than capture the isolated influence of stress specifically during gestation. As a result, as a third aim, we plan to include maternal preconception stress as a covariate in secondary analyses to allow for the independent examination of prenatal stress.
2. Materials and methods
2.1. Participants
Participants were recruited from the greater Nashville area from obstetrics clinics and through digital and print advertisements to take part in a study of infant brain and behavioral development. All study procedures were approved by the Vanderbilt University Institutional Review Board, and all participants provided informed consent prior to participating, including for their infant to participate following birth. Sixty-one pregnant women completed an initial session during their pregnancy involving questionnaires and interviews related to lifetime and prenatal stress. Thirty-nine of these women returned for a second visit when their infants were approximately five weeks old to complete an infant MRI scan session. Thirty-three mother–infant dyads (maternal age = 21.19–38.29 years, M ± SD = 29.54 ± 4.92 years; infant age = 3.57–6.86 weeks, M ± SD = 4.77 ± 0.88 weeks, 14 male) completed both prenatal stress assessments and provided usable MRI data for neuroimaging analyses. Six infants did not provide usable scan data. See Table 1 for detailed sample characteristics.
Table 1.
Sample characteristics.
| Included in Neural Analysis (N = 33) |
Excluded from Neural Analysis (N = 6) |
t or χ2 | |
|---|---|---|---|
| Infant Age at Scan Mean (SD) months | 4.81 (0.93) |
5.05 (1.28) |
−0.66 |
| Infant Race Number (percent) | 3.15 | ||
| White/Caucasian American | 25 (76 %) |
5 (83 %) |
|
| Asian American | 0 (0 %) |
0 (0 %) |
|
| Black/African American | 6 (18 %) |
1 (17 %) |
|
| Native Hawaiian/Pacific Islander | 0 (0 %) |
0 (0 %) |
|
| American Indian or Alaska Native | 1 (3 %) |
0 (0 %) |
|
| Other/Biracial | 1 (3 %) |
0 (0 %) |
|
| Infant Ethnicity Number (percent) | 0.32 | ||
| Hispanic or Latinx | 3 (9 %) |
1 (17 %) |
|
| Infant Sex N (percent) | 0.17 | ||
| Male | 14 (42 %) |
2 (33 %) |
|
| Mother Age Mean ± SD years | 29.55 ± 4.92 | 29.09 ± 5.76 | 0.21 |
| Maternal Race Number (percent) | 0.38 | ||
| White/Caucasian American | 26 (79 %) |
5 (83 %) |
|
| Asian American | 0 (0 %) |
0 (0 %) |
|
| Black/African American | 5 (15 %) |
1 (17 %) |
|
| Native Hawaiian/Pacific Islander | 0 (0 %) |
0 (0 %) |
|
| American Indian or Alaska Native | 1 (3 %) |
0 (0 %) |
|
| Other/Biracial | 1 (3 %) |
0 (0 %) |
|
| Maternal Ethnicity Number (percent) | |||
| Hispanic or Latinx | 3 (9 %) |
1 (17 %) |
0.32 |
| Maternal Education Number (percent) | 3.63 | ||
| High School Diploma/GED | 1 (3 %) |
1 (17 %) |
|
| Some College | 5 (15 %) |
0 (0 %) |
|
| Associate's Degree | 3 (9 %) |
1 (17 %) |
|
| Trade/Technical School | 0 (0 %) |
0 (0 %) |
|
| Bachelor's Degree | 13 (39 %) |
3 (50 %) |
|
| Graduate Degree | 11 (33 %) |
1 (17 %) |
|
| Annual household income Number (percent) | 3.52 | ||
| Less than $5,000 | 0 (0 %) |
0 (0 %) |
|
| $5,001-15,000 | 1 (3 %) |
0 (0 %) |
|
| $15,001-30,000 | 5 (15 %) |
0 (0 %) |
|
| $30,001-60,000 | 6 (18 %) |
1 (17 %) |
|
| $60,001-90,000 | 5 (15 %) |
1 (17 %) |
|
| $90,001-150,000 | 9 (27 %) |
3 (50 %) |
|
| More than $150,000 | 7 (21 %) |
0 (0 %) |
|
| Did not provide | 0 (0 %) |
1 (17 %) |
Note. M (SD) or %.
*p < .05; **p < .01; ***p < .001.
2.2. Study procedure
Interested participants were first screened over the phone to assess study eligibility for an initial session during pregnancy. Eligible participants were 18 years or older, currently pregnant with a singleton pregnancy, fluent in English, a U.S. Citizen or permanent resident, and reported no plans to move out of the greater Nashville area in the following year. Eligible participants were invited to an initial laboratory visit when they were between 16 and 32 weeks gestation to complete questionnaires and interviews.
After their due date, participants were screened a second time to assess their infant’s eligibility to undergo MRI scanning. At this stage, exclusion criteria included severe complications during birth, infant head trauma, infant premature birth (prior to 36 weeks gestation) and any infant MRI contraindication (e.g., metal implant). Eligible infants underwent MRI scanning during natural sleep when they were between four to six weeks of age, adjusted for due date (M ± SD age at scan = 4.77 ± 0.88 weeks; 14 male). Study data were collected and managed using REDCap electronic data capture tools hosted at Vanderbilt University (Harris et al., 2019). Participants were compensated at the end of each visit.
Data collection is ongoing. Of the 61 mother-infant dyads who have completed the prenatal visits, 17 mothers did not continue in the study and 5 infants did not meet eligibility criteria for the MRI visit (e.g., premature birth, medical complications). See Supplemental Table 1 for sample characteristics of the 17 participants who did not continue in the study. Thus, 39 infants underwent MRI scanning at their newborn visit. Thirty-three infants provided at least one usable scan (i.e., rsfMRI or DWI); of these, 32 infants (M ± SD age at scan = 4.79 ± 0.88 weeks; 14 male) provided usable rsfMRI and 24 infants (M ± SD age at scan = 4.71 ± 0.79 weeks; 11 male) provided usable multi-shell DWI data. Five infants did not provide MRI data for either of these sequences and data from one infant was removed due to excessive motion.
2.3. Prenatal stress
At their prenatal visit, participants completed the Crisis in Family Systems–Revised (CRISYS; Berry et al., 2001), a self-report measure assessing 72 possible stressors from the preceding six months. The CRISYS demonstrates good internal reliability in this sample (Cronbach’s α = .86). Items assess a range of stressful experiences from daily hassles to significant stressors, and response options are binary (1 = yes, 0 = no) for all items; thus, all items are weighted equally, and total scores represent the total number of stressors endorsed by each participant. Scores for our sample ranged from 0-18.
2.4. Preconception stress
At their prenatal visit, women also participated in interviews about their lifetime exposure to stress and trauma using a modified version of the Life Stressor Checklist–Revised (Wolfe et al., 1996). We added the follow-up question: “Did this happen during your pregnancy?” in order to isolate stressors that occurred prior to conception. The number of types of events endorsed were summed to quantify preconception stress.
2.5. MRI imaging acquisition
Newborn infants were imaged using the “swaddle and soothe” method during natural sleep in the evening as previously described (Camacho et al., 2019). Mothers arrived in the evening (sessions typically started between 6:30 and 7:30PM). The infant was undressed, changed into a disposable diaper, and swaddled in a muslin cloth before being placed into a MedVac immobilizer designed for infants. Mothers were then encouraged to feed their baby and begin their bedtime routine. Once the infant had been soundly sleeping for 10 min, earplugs were gently placed in the baby’s ears, secured with skin-safe medical tape, and covered with Natus MiniMuffs for additional hearing protection. The infant was then transferred to the scanning bed. If the infant remained soundly asleep for five more minutes, the baby was moved into the scanner and acquisition was initiated. A researcher remained with the infant at all times, including during MRI acquisition, in order to alert the scan operator when the infant woke and to soothe the baby back to sleep as needed. If the infant woke, acquisition was paused until the baby was soundly asleep again. This process of soothing and scanning was repeated until either all data were collected or their parent decided to end the session.
MR images were acquired at the VUIIS Center for Human Imaging using a Philips Ingenia Elition 3.0 T X equipped with a 32-channel head coil (funded by S10OD021771 01). High resolution T2-weighted anatomical images were collected using a 3D volume isotropic turbo spin echo (VISTA) sequence (0.8 mm x 0.8 mm x 0.8 mm voxel, 180 sagittal slices, 276 × 276 acquisition matrix, flip angle = 90 degrees, TR = 2500 ms, TE = 310 ms). Resting state functional MRI were obtained using a simultaneous multi-slice echo planar imaging sequence (2.0 mm x 1.9 mm x 1.9 mm voxel, 54 axial slices, 96 × 96 acquisition matrix, flip angle = 60 degrees, TR = 1410 ms, TE = 30 ms, 3 simultaneous slices, 270 contiguous volumes). The six-minute-and-20-second sequence was repeated if the infant remained asleep after the first acquisition and again at the end of the full MR protocol resulting in 12−18 min of data from each infant. Diffusion weighted images were acquired using a simultaneous multi-slice multi-shell diffusion weighted echo planar imaging sequence (1.79 mm x 1.79 mm x 2.0 mm voxel, 48 axial slices, 100 × 100 acquisition matrix, flip angle = 77 degrees, TR = 3400 ms, TE = 125.9 ms, 3 simultaneous slices, number of unweighted B-value 0 volumes = 11, number of weighted B-value 700 volumes = 30, number of weighted B-value 2000 volumes = 64). The volumes obtained at the three diffusion weightings were collected in a roughly interleaved order with un-weighted volumes (B = 0) occurring after every 10 or so weighted volumes (B = 700 and B = 2000). For each of the simultaneous multi-slice images, a shorter acquisition of each was collected in the opposite phase encoding direction. These short acquisitions were combined with the first volumes of each of the full acquisitions in order to correct for field distortions (see MRI Processing). All MR images were visually inspected for artifacts prior to processing.
2.6. MRI processing
MR image processing was conducted in Python version 3.7 using the NiPype framework (Gorgolewski et al., 2011) leveraging tools from FSL version 5.0.11(https://fsl.fmrib.ox.ac.uk), FreeSurfer version 6.0 (https://surfer.nmr.mgh.harvard.edu), SPM version 12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12), AFNI version 18.1.01 (https://afni.nimh.nih.gov), and ANTs (http://stnava.github.io/ANTs). Diffusion data were further processed using DSI Studio (http://dsi-studio.labsolver.org). All templates and scripts used in this analysis are publicly available at https://github.com/vanderbiltsealab/connectivity_analysis.
2.6.1. T2-weighted anatomical MRI
First, T2-weighted images (T2w) were corrected for nonuniform intensities (Tustison et al., 2010); then, the brain was extracted using FSL’s brain extraction tool (BET; Jenkinson et al., 2005), down-sampled to a 2 mm x 2 mm x 2 mm voxel size, and registered to an age-specific T2-weighted anatomical template (Jenkinson et al., 2002).
2.6.2. Resting state fMRI
rsfMRI data were corrected for field distortions by taking the first five volumes of the rsfMRI sequence combined with the five volumes collected in the opposite phase encoding direction to characterize the off-resonance field distortions using FSL’s TOPUP tool (Andersson et al., 2003). This correction was then applied to the rsfMRI sequence and visually inspected for accurate distortion removal. Next, data were slice-time corrected and rigidly aligned to the middle volume in the acquisition using FSL’s MCFLIRT (Jenkinson et al., 2002), which produced frame-by-frame measurements of translation and rotation in each direction that were used in later de-noising (see rsfMRI Noise Characterization and Removal). Next, rsfMRI data were co-registered to the down-sampled T2w (see T2-weighted anatomical MRI), and the transform from the T2w to template registration was applied to the rsfMRI, warping it into the template space using FSL’s FLIRT (Jenkinson et al., 2002). Registrations were visually inspected for accuracy and modified as needed to correct alignment.
Measurement noise from motion and global signals are both known to dramatically influence BOLD signal and connectivity measurements (Fox, 2017; Power et al., 2012; Satterthwaite et al., 2012; Van Dijk et al., 2012). Thus, we took careful steps to denoise rsfMRI data (see rsfMRI Noise Characterization and Removal). Frames with motion greater than 0.25 mm of framewise displacement were replaced with temporally interpolated values before bandpass filtering to retain signal fluctuations between 0.008 and 0.09 Hz (Hallquist et al., 2013). After bandpass filtering, interpolated frames were removed. Data were then concatenated within subjects before final connectivity analyses were performed. Each infant contributed between 6.9 and 15.5 min (M ± SD = 11.5 ± 1.7 min) of low-motion data for connectivity analysis.
2.6.3. rsfMRI noise characterization and removal
Global signals: Global signals were characterized on both the subject-specific and context-specific levels. Physiological noise is detectable in white matter and cerebral spinal fluid (CSF), thus signal in these regions are often used as subject-specific proxies for denoising gray matter voxels (Behzadi et al., 2007; Birn, 2012). An additional consideration for infant imaging is session-specific variation in the magnetic environment that is a byproduct of having an additional person in the MRI room (Howell et al., 2019). Thus, to characterize this noise, BOLD signal from outside the brain (“session noise”) and from white matter and cerebral spinal fluid within the brain (“physiological noise”) were each isolated by masking out the brain and gray matter respectively. Each noise volume was then smoothed with a 4 x 4 x 4mm gaussian kernel, filling the removed brain areas with neighboring noise signal. For the session noise volume, the kernel base was extended to 22 mm to avoid zero values in the inner voxels of the brain (instead, these were near-zero values). To characterize noise associated with the general procedural context (e.g., the scanner sequence and the scanning experience), runs of rsfMRI were temporally averaged across all participants, producing voxel-level estimates of procedural noise. Procedural noise captures effects of pulse sounds or other aspects of the scanning environment that may produce erroneous correlations. Thus, removing this signal may enhance detection of individual differences in connectivity. These steps produced three voxel- and time-specific regressors for each participant: session, physiological, and procedural.
Motion: Derivatives were calculated from the six motion parameters (translation and rotation in each of the three directions) produced from rsfMRI rigid-realignment. Nonlinear influences of each of these six motion parameters on BOLD signal were characterized by creating a Volterra series from the motion derivatives (Friston et al., 2000). Specifically, each motion parameter was lagged four times, capturing a “memory effect” of motion on the signal up to 5.64 s later. In total, this procedure generated 30 motion regressors: 6 original parameters and 24 lagged derivatives.
Final denoising for each rsfMRI run ultimately included three voxel-specific nuisance regressors (session, physiological, and procedural) and thirty motion regressors (six original motion parameters and their derivatives lagged 4 times). These regressors were entered into a general linear model (GLM) and the residuals were used in subsequent processing steps.
2.6.4. Multi-shell diffusion-weighted MRI
DWI sequences were first corrected for off-resonance distortions estimated from the first volume (B = 0) of the DWI and from a volume collected in the opposite phase encoding direction using FSL’s TOPUP tool (Andersson et al., 2003). This correction was next applied to the DWI sequence. Using DSI Studio’s preprocessing interface, each directional volume was visually inspected for artifact, and volumes contaminated by motion or distortions were removed. Eddy current and motion correction was applied with b-table rotation to the remaining volumes (Andersson and Sotiropoulos, 2016). Between 100–107 volumes were retained for each infant after quality control (M ± SD = 105.04 ± 2.01 volumes). Fiber orientation was estimated in subject space using a generalized q-sampling procedure (Yeh et al., 2010), which takes a model-free approach to fiber tracking, avoiding assumptions about the underlying diffusion structure. Specifically, the orientation distribution for diffusion at each voxel is estimated and used to determine fiber orientation, allowing for improved modeling of crossing fibers (Yeh et al., 2011), and has been shown to be reliable to histology (Gangolli et al., 2017). Fibers were identified using deterministic fiber tracking from 100,000 random seeds throughout the brain. Fifty percent of the previous directional information was used to predict the subsequent step (step size 1 mm) and only fibers that were 10−200 mm in length were retained. An angular threshold of 75 degrees was used. Due to relatively low axonal myelination in newborn infants (Brody et al., 1987), a generous low threshold of 0.01 quantitative anisotropy (QA) was used for each fiber in order to be able to model infants with lower prefrontal myelination. QA is an index of the anisotropic spins that diffuse along the fiber, is more robust to partial volume effects than fractional anisotropy (Yeh et al., 2013), and was used as an index of axonal myelination.
2.7. Functional and structural connectivity estimation
2.7.1. Functional connectivity
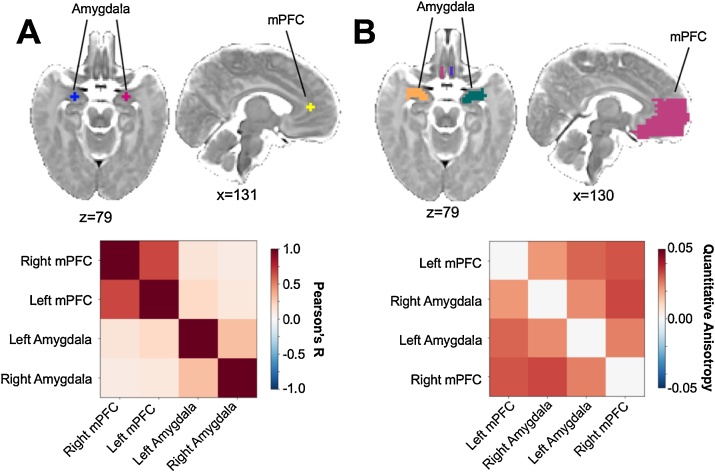
Regions of interest (ROIs) were created based on the anatomical landmarks visible on the neonate template brain. Spheres (6 mm diameter) were centered on the amygdala and the medial prefrontal cortex (corresponding to a small region in the center of Brodmann area 32) separately for each hemisphere. Specific coordinates for the sphere centers are listed in template space in Table 2 and visualized in Fig. 1a. Pearson’s correlations were calculated for each participant between each seed. The average functional connectivity matrix for the sample is included in Fig. 1a.
Table 2.
Coordinates for the rsfMRI connectivity analyses.
| Extent | Coordinates |
|||
|---|---|---|---|---|
| Region | mm3 | X | Y | Z |
| Left Amygdala | 56 | 55 | 67 | 39 |
| Right Amygdala | 56 | 72 | 67 | 39 |
| Left Medial Prefrontal Cortex | 56 | 60 | 81 | 50 |
| Right Medial Prefrontal Cortex | 56 | 66 | 81 | 50 |
Fig. 1.
Group level mean connectivity across regions of interest. A. Mean functional connectivity (Pearson’s R; bottom) between the amygdala and the medial prefrontal cortex (mPFC; top). B. Mean structural connectivity characterized as quantitative anisotropy (QA; bottom) for the same regions extended to the white matter and full structural region (top).
2.7.2. Structural connectivity
Given the relatively low levels of myelin in neonates (Deoni et al., 2011), a large medial PFC ROI for each hemisphere was manually created to encapsulate the main medial PFC gyrus, overlapping with the mPFC ROI investigated in the functional connectivity analysis. Amygdala ROIs were extended into the neighboring white matter and manually modified to cover the entire anatomical amygdala structure. Connectivity was operationalized as the average QA of all fibers connecting two regions. ROIs and the average structural connectivity matrix for the sample are visualized in Fig. 1b.
2.8. Prenatal stress and newborn neural connectivity analysis
A mixed modeling approach was used to account for the nested structure of the data. Four measures were included for each infant for both functional and structural connectivity (R amygdala–R mPFC, R amygdala–L mPFC, L amygdala–R mPFC, and L amygdala–L mPFC). Not all participants had complete data for all four outcomes, and mixed modeling allows for unbalanced data and accounts for the variance within participants. As such, participants were included if any of the four metrics of interest were provided (with more structural metrics missing [21 of 96 possible metrics from 24 included scans] than functional connectivity metrics [0 of 128 possible metrics from 32 included scans]). For the analyses without stress, the intercept is presented without adjusting for covariates. For the full models examining stress, amygdala and mPFC hemisphere were included as fixed predictors, along with infant corrected age, sex, gestational age at the prenatal stress assessment, and number of prenatal stressors endorsed. For exploratory analyses examining the specificity of stress, number of preconception stressful events endorsed was included as an additional fixed predictor. All variables were mean centered with the exception of dummy-coded variables which were coded as 0 and 1, with 1 assigned as the label. Last, given the small sample size, we emphasize the importance of effect size and the 95 % confidence interval for these effects.
3. Results
3.1. Resting-state functional connectivity between the amygdala and medial prefrontal cortex
The average functional connectivity between the seeds of the amygdala and mPFC was significantly positive (B = 0.13 [0.08, 0.17], t(127) = 5.67, p < .001), indicating that, on average, our participants were showing positive connectivity between these regions. For analyses using the same larger seed region for the mPFC please see the supplement.
3.2. Structural connectivity between the amygdala and medial prefrontal cortex
The average structural connectivity between the amygdala and entire mPFC was significantly positive (B = 0.03 [0.03, 0.04], t(74) = 32.12, p < .001), indicating that, on average, our participants were showing detectable structural connectivity between these regions.
3.3. Prenatal stress and newborn amygdala–medial prefrontal cortex connectivity
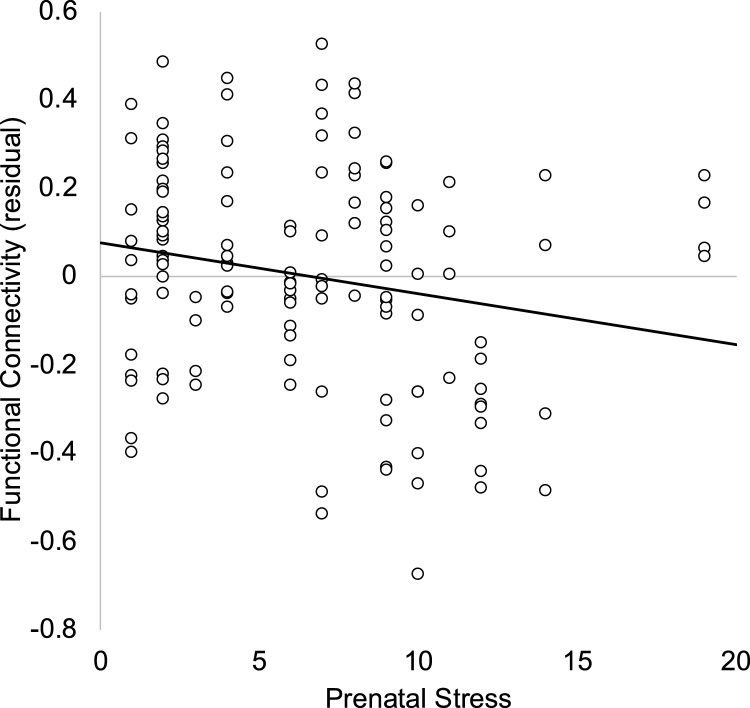
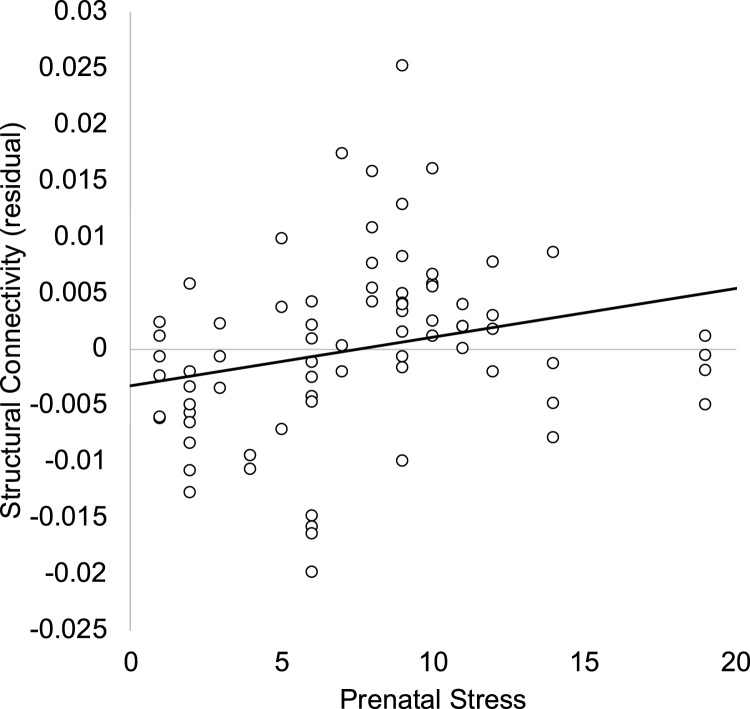
In a full model including covariates (noted above; see Table 3), prenatal stress was associated with less positive rsfMRI connectivity between the amygdala and mPFC (see Fig. 2). For structural connectivity, within the full model (see Table 4), prenatal stress was associated with greater QA in the fiber tracts linking the amygdala and mPFC (see Fig. 3).
Table 3.
Fixed effects from the mixed model examining resting-state functional connectivity between the amygdala and medial prefrontal cortex.
| Parameter | B | 95 % CI | β | 95 % CI | df | t | p |
|---|---|---|---|---|---|---|---|
| Intercept | 0.15 | 0.07, 0.24 | 121 | 3.55 | .001 | ||
| Right amygdala | −0.07 | −0.16, 0.02 | −0.14 | −0.31, 0.03 | 121 | 1.59 | .114 |
| Right prefrontal cortex | −0.05 | −0.14, 0.04 | −0.09 | −0.26, 0.08 | 121 | 1.10 | .274 |
| Corrected age | −0.05 | −0.11, 0.003 | −0.19 | −.40, 0.01 | 121 | −1.89 | .061 |
| Sex (1=male) | 0.07 | −0.02, 0.16 | 0.14 | −0.03, 0.32 | 121 | 1.62 | .108 |
| Gestational age at stress assessment (weeks) | 0.01 | −0.001, 0.01 | 0.19 | 0.03, 0.41 | 121 | 1.71 | .090 |
| Prenatal stress | −0.01 | −0.02, -0.002 | −0.28 | −0.50, -0.05 | 121 | −2.41 | .018 |
Fig. 2.
Maternal stress in pregnancy is associated with less positive functional coupling between amygdala and medial prefrontal cortex. Residual after accounting for laterality, infant corrected age, sex, and gestational age at the prenatal stress assessment. Note. Given connectivity is calculated between each amygdala and mPFC hemisphere, participants can contribute up to 4 data points to the analyses and scatterplot.
Table 4.
Fixed effects from the mixed model examining structural connectivity between the amygdala and medial prefrontal cortex.
| Parameter | B | 95 % CI | β | 95 % CI | df | t | p |
|---|---|---|---|---|---|---|---|
| Intercept | 0.03 | 0.03, 0.03 | 68 | 16.65 | <.001 | ||
| Right amygdala | 0.001 | −0.003, 0.01 | 0.07 | −0.13, 0.27 | 68 | −0.67 | .508 |
| Right prefrontal cortex | 0.002 | −0.002, 0.01 | 0.11 | −0.09, 0.31 | 68 | −1.10 | .277 |
| Corrected age | −0.01 | −0.01, -0.003 | −0.53 | −0.79, -0.27 | 68 | −4.06 | <.001 |
| Sex (1=male) | 0.002 | −0.002, 0.01 | .10 | −0.10, 0.29 | 68 | 0.96 | .341 |
| Gestational age at stress assessment (weeks) | −0.0001 | −0.0004, 0.0002 | −.09 | −0.35, 0.16 | 68 | −0.77 | .446 |
| Prenatal stress | 0.0005 | 0.0001, 0.001 | .28 | 0.03, 0.52 | 68 | 2.27 | .026 |
Fig. 3.
Maternal stress in pregnancy is associated with greater quantitative anisotropy (QA) in fiber tracts linking the amygdala and medial prefrontal cortex. Residual after accounting for laterality, infant corrected age, sex, and gestational age at the prenatal stress assessment. Note. Given connectivity is calculated between each amygdala and mPFC hemisphere, participants can contribute up to 4 data points to the analyses and scatterplot.
3.4. Specificity of prenatal stress
Given that preconception stress may be associated with variations in the intrauterine environment, and that preconception and prenatal stress may be associated, we examined whether the above associations held when the number of preconception (i.e., whole life until conception of this child) events were included in the model. In the full sample, there was a significant association between preconception and prenatal stress (r(59) = .33 [.08, .54], p = .011). Importantly, even after accounting for preconception stress (B=−0.01 [−0.02, 0.01], β=−0.10 [−0.29, 0.09], t(120)= −1.01, p = .315), prenatal stress was significantly associated with less positive functional coupling (B=−0.01 [−0.02, −0.002], β=−0.26 [−0.49, −0.03], t(120)= −2.28, p = .024). In addition, the association between prenatal stress also remained highly similar (B=0.001 [0.0001, 0.001], β=0.29 [0.07, 0.52], t(67) = 2.60, p = .011) after including the number of types of preconception stressful life events in the model, which was also significantly associated with amygdala–mPFC structural connections (B=0.001 [0.0005, 0.002], β=0.34 [0.16, 0.53], t(67) = 3.69, p < .001).
4. Discussion
In a small sample of young infants (i.e., approximately five weeks old) who underwent MRI, we characterized amygdala–mPFC connectivity both functionally, using rsfMRI, and structurally, using DWI, to obtain measures of the structural integrity of white matter. We found evidence of positive rsFC, on average, between these two brain regions as well as the presence of white matter tracts at this age. Further, we examined these measures of functional and structural connectivity as a function of maternal stress during pregnancy. We found evidence that greater prenatal stress was associated with less positive rsFC and greater structural integrity between the amygdala and mPFC. These findings held even when covarying for preconception stress, in support of the hypothesis that it is specifically stress during pregnancy that may be associated with these patterns, rather than cumulative stress in the mother’s lifespan.
The results from this study build on prior research indicating that experiences may influence emotion regulation circuitry begin early in life, including during gestation and perhaps prior to significant caregiving experiences. As for potential mechanisms linking prenatal stress exposure and downstream consequences to the fetus, other investigations suggest that maternal cortisol and inflammation may be important (Graham et al., 2018, 2019). Importantly, in pregnancy, both cortisol and inflammation have been linked to experiences of stress (Entringer et al., 2015). Graham and colleagues (2018) did not specifically examine amygdala–mPFC connectivity, but found that maternal cortisol predicted less positive connectivity between the right amygdala and dorsal lateral PFC in newborn girls. In the same sample, maternal inflammation levels (i.e., IL-6) were associated with newborn amygdala connectivity (Graham et al., 2018). It has been suggested that fetal programming of brain connectivity in utero occurs primarily through endocrine and inflammatory mediators that pass through the placenta and fetal blood-brain barrier and precipitate developmental cascades via direct signaling or by modifying the availability and activity of critical neural growth factors and hormones (Buss et al., 2012b). For example, there is evidence that unusually high concentrations of glucocorticoids modify the trajectories of brain development both by binding directly to regions dense in glucocorticoid receptors (including the amygdala and PFC) and initiating local changes (Welberg et al., 2001), as well as by modulating the activity of proteins that facilitate neuronal differentiation and determine cell fates (Kumamaru et al., 2008; Lussier et al., 2009; Shimizu et al., 2010). However, it is unclear whether the influences of these potential mechanisms are “by design” (i.e., adaptive processes that will benefit the developing fetus postnatally) or are byproducts of other biological tradeoffs that negatively affect fetal development. It has been theorized that biological signals during gestation may cue the fetus about the postnatal environment, thus shaping developmental processes in an adaptive way that was formerly primarily considered to be detrimental (Barker, 2004). More recently, this process has been reconceptualized as potentially adaptive in specializing for success in that future environment (Barker, 1990, 2007; Blair and Raver, 2012; Del Giudice et al., 2011; Wadhwa et al., 2010). As such, it is possible that stress-related brain changes may well be disadvantageous in some contexts, but may also be beneficial in others (for more on this perspective, see Frankenhuis and de Weerth, 2013). The importance of the prenatal environment for brain development is further underscored by work in older children that finds that prenatal environments predict brain structure and function in children (Sarkar et al., 2014).
We chose specifically to explore seed-to-seed connectivity between the amygdala and mPFC to directly examine this circuit; however, past research examining whole-brain connectivity of the amygdala during natural sleep helps to provide context for this decision. For instance, in a whole-brain approach to examining amygdala functional connectivity, Qiu et al. (2015) found more positive amygdala–ACC coactivation in analyses of infants whose mothers had more symptoms of depression during pregnancy. Posner et al. (2016) used a similar multimodal approach to study amygdala–PFC circuitry in infants with and without exposure to maternal depression during pregnancy, finding less positive functional connectivity between the amygdala and dorsal PFC, as well as decreased structural connectivity between right amygdala and right ventral PFC (Posner et al., 2016). Scheinost et al. (2017) examined amygdala rsFC in both preterm and full-term newborns, finding decreased connectivity in preterm infants and for those full-term infants exposed to prenatal stress. Though the mPFC was not specifically examined, their finding that the amygdala showed decreased connectivity with other PFC sub-regions was consistent with our results. Turesky et al. (2019) found a positive association between amygdala–precuneus rsFC and family conflict across a small sample of low- and high-income two-month-old Bangladeshi infants. In another sample of infants assessed shortly after birth, higher levels of maternal anxiety assessed during the second trimester of pregnancy predicted lower fractional anisotropy—an index of structural integrity—in a number of brain regions, including the right uncinate fasciculus (Rifkin-Graboi et al., 2015). Based on this limited work, increased prenatal stress is associated with decreased amygdala–PFC functional connectivity in infancy and has qualitatively different patterns of association relative to other types of caregiving stress such as exposure to maternal depression. The structural basis of this functional association may differ by region of the mPFC, and this decrease in functional coupling may be associated with increased coupling of other emotion processing regions. Further work is needed to replicate our findings and to test this theory.
This study examined the association between prenatal stress and amygdala–mPFC connectivity at a single time point, but may inform our understanding of the baseline for developmental trajectories following stress. Previous work has documented that amygdala–mPFC functional connectivity may change differentially for those with and without early adversity exposure (Gee et al., 2013a; Thijssen et al., 2017). Yet, it is worth noting that a recent meta-analysis and review found support for the hypothesis that early adversity accelerates biological aging, but found no consistent evidence for altered amygdala–mPFC connectivity (Colich et al., 2019). Several studies supported potential stress-related acceleration (Colich et al., 2017; Gee et al., 2013a; Keding and Herringa, 2016) and several others that indicated potential delayed development of this circuitry (Cisler et al., 2013; Marusak et al., 2015; Silvers et al., 2017). Non-human animal research permits more stringent controls than does human research and allows for causal conclusions regarding specific forms of early adversity or stress and developing brain connectivity. Two recent studies that examined connectivity in rodent models following severe maternal deprivation and maltreatment, respectively, indicate that these adverse early experiences were associated with delayed or dampened maturation of corticolimbic circuitry across development (Honeycutt et al., 2020; Yan et al., 2017).
Our results indicated that prenatal stress was associated with less coactivation between the amygdala and mPFC, but greater structural connections. It is not clear how this pattern of less functional but stronger structural connectivity maps onto predictions regarding whether stress accelerates or delays maturation of this circuitry. On the one hand, reduced functional coupling and greater structural integrity in white matter tracts connecting these regions may resemble relatively mature circuitry. Yet, in this developmental period, rsFC between these regions may not yet be serving the regulatory function it serves later. Weaker functional coupling of the amygdala and mPFC has been postulated to be a marker of less effective communication (Park et al., 2018), though it is unclear when in development we might expect to see regulatory control of the amygdala by the mPFC. Connectivity is expected to change across development (Gabard-Durnam et al., 2018), making it difficult to compare these findings to older children and adults. Despite some longitudinal work on typical developmental changes in amygdala–mPFC connectivity (e.g., Gabard-Durnam et al., 2018; Gee et al., 2013b; Kujawa et al., 2016; Silvers et al., 2017; Wu et al., 2016), we are not yet confident in the typical developmental trajectory of this circuitry or when top-down regulation of the amygdala by the mPFC may be expected to come online. Connectivity between the amygdala and mPFC is often described as bidirectional, although most research has been conducted in adults. Examining the connectivity from a developmental perspective indicates that given the difference in maturation rates of these two brain regions, where the amygdala develops earlier in life, it is likely that initial connections are driven from the amygdala in a "bottom-up" fashion rather than following a "top down" signal from the mPFC (Tottenham and Gabard-Durnam, 2017). Furthermore, our results provide new evidence for the role of the prenatal environment in accelerating changes in the structure and function of the amygdala–mPFC circuitry. This finding introduces the possibility of adding an even earlier timeframe to a currently-held theoretical model which argues that the postnatal caregiving environment is responsible for accelerated changes in the amygdala–mPFC circuity (Callaghan and Tottenham, 2016).
There are challenges in recruitment and collection of infant imaging data to consider. First, there are methodological constraints to imaging infants that result in shorter acquisitions and increased measurement noise. For example, due to the time and physical constraints involved in scanning non-sedated infants, many past studies has examined less than 10 min of censored data (Gao et al., 2015, 2013; Graham et al., 2018; Qiu et al., 2015; Salzwedel et al., 2019; Smyser et al., 2010; Sylvester et al., 2018; Thomas et al., 2019; Toulmin et al., 2015). Longer acquisitions have been shown to dramatically improve functional connectivity estimates, with a dramatic improvement in reliability when using more than ten minutes of data (Birn et al., 2013; Gordon et al., 2017; Laumann et al., 2015). Relatedly, many studies failed to report adequate control for motion and physiological noise, both of which are known to dramatically influence functional connectivity measures (Birn, 2012; Power et al., 2012; Satterthwaite et al., 2012; Van Dijk et al., 2012). Our longer duration (mean 11.5 min of low motion rsfMRI data), with careful motion and noise correction, are notable strengths of this investigation. Second, there are dramatic changes in neurobiology across the first year of life—including the growth of foundational brain structure (Deoni et al., 2011; Knickmeyer et al., 2008), expansion of neurovasculature (Harb et al., 2013; Norman and Oʼkusky, 1986), and likely, the entrainment of the neuronal-hemodynamic response that gives rise to the BOLD signal (Benasich et al., 2008; Mateo et al., 2017; Takano and Ogawa, 1998)—which make comparisons of non-overlapping age groups difficult. Thus, there is a critical need for more work in this domain in order to gain a full understanding of how stress influences the developing infant brain and if our findings differ due to differences in neurobiological development or due to methodological differences.
Several limitations should be noted. The small sample size of this pilot study limits the confidence in these associations. Nevertheless, this work contributes to a growing understanding of connectivity of the amygdala to the mPFC across early development. Further, because of the limited sample size, we were underpowered to examine sex differences. Infants were scanned during natural sleep, and there is much unknown about sleeping brain activity as a function of sleep stage and in relation to waking brain activity (Graham et al., 2015). It is likely that, given the types of stressors assessed on the CRISYS, many of the items endorsed would be ongoing in nature. Our measure of prenatal stress was captured mid-pregnancy, and given the 6 month retrospective assessment includes some of the preconception period for approximately half of participants (mean of 0.72 months across the sample). This measure precludes us from identifying specific timing during gestation or capturing potential changes in stress across pregnancy. Other studies examining more extreme stressors (e.g., an ice storm; King and Laplante, 2005) and deaths of close family members (Hansen et al., 2000) have a greater ability to inform whether specific periods of gestation may be more or less associated with amygdala-mPFC circuitry. Relatedly, our assessment of preconception stress covered participants' whole life, and it is important to acknowledge there are only moderate associations between prospectively assessed and retrospectively reported life events (Baldwin et al., 2019). Lastly, we generalized the structural analysis to the entire mPFC due to methodological constraints, as young infants have relatively low prefrontal myelination, requiring that we consider a larger region in our fiber tracking. This makes it somewhat more difficult to draw direct comparisons to the rsFC between the amygdala and a more restricted mPFC seed. Of course, function is not necessarily dependent on the most proximal distal connections (such as when neuronal firing propagates through local cortical connections before propagating distally).
An open question is the degree to which connectivity patterns established during gestation can be further modified postnatally, either through additional stressors (i.e., a second hit) or through protective experiences and nurturing caregiving environments. Notably, the variation in stress is likely due to experiences during gestation, prior to the opportunity for explicit caregiver regulation. The prolonged development of the brain throughout a long childhood period may provide further opportunities for these associations to be tuned (Tottenham, 2014). Longitudinal studies that scan infants multiple times throughout their childhood will better enable investigations of risk and protective factors in further shaping this circuitry.
5. Conclusions
Experiences of stress during gestation may be influencing the connectivity between the amygdala and mPFC, circuitry important for emotion regulation in later development. Establishing the role of prenatal stress on brain development informs our understanding of early functional and structural amygdala–mPFC connectivity. It also points to pregnancy as an important target when considering prevention efforts for children at high risk for stress exposure, particularly given that these results held when covarying for women’s preconception stress. Future investigations examining longitudinal changes in brain development will inform whether and how subsequent environmental experiences change the trajectory of this circuitry across development along with corresponding changes in emotion regulation.
Funding
This work was supported by the Vanderbilt Kennedy Center; Vanderbilt Institute for Clinical and Translational Research [VR53419]; the Jacobs Foundation, Zürich, Switzerland [Early Career Research Fellowship 2017-1261-05 to Kathryn L. Humphreys]; and the National Science Foundation Graduate Research Fellowship to M. Catalina Camacho.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We are grateful to our study participants for their time and trust in our research team. We wish to acknowledge the following individuals who aided in the collection of this data: Emilia Cardenas, Regan Carell, Kaitlyn Couch, Nelson Eiselstein, Sean Gallagher, Addison Glover, Paige Hamilton, Sarah Lempres, Mia Letterie, Hannah Piersiak, Sara Schunck, Saikat Sengupta, and Seth Smith. We are also grateful to Sanjana Ravi and Sydney Takemoto for their feedback on this manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100877.
Contributor Information
Kathryn L. Humphreys, Email: k.humphreys@vanderbilt.edu.
M.C. Camacho, Email: camachoc@wustl.edu.
Marissa C. Roth, Email: marissa.c.roth@vanderbilt.edu.
Elizabeth C. Estes, Email: elizabeth.c.estes@vanderbilt.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Skare S., Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20:870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Baldwin J.R., Reuben A., Newbury J.B., Danese A. Agreement between prospective and retrospective measures of childhood maltreatment: a systematic review and meta-analysis. JAMA Psych. 2019;76:584–593. doi: 10.1001/jamapsychiatry.2019.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. Amygdala–frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. 1990. The Fetal and Infant Origins of Adult Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J.P. Developmental origins of adult health and disease. J. Epidemiol. Community Health. 2004;58 doi: 10.1136/jech.58.2.114. 114 LP – 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich A.A., Gou Z., Choudhury N., Harris K.D. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behav. Brain Res. 2008;195:215–222. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C., Quinn K., Shalowitz M., Wolf R. Validation of the crisis in family systems–revised, a contemporary measure of life stressors. Psychol. Rep. 2001;88:713–724. doi: 10.2466/pr0.2001.88.3.713. [DOI] [PubMed] [Google Scholar]
- Birn R.M. The role of physiological noise in resting-state functional connectivity. Neuroimage. 2012;62:864–870. doi: 10.1016/j.neuroimage.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Birn R.M., Molloy E.K., Patriat R., Parker T., Meier T.B., Kirk G.R., Nair V.A., Meyerand M.E., Prabhakaran V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., Raver C.C. Child development in the context of adversity;Experiential canalization of brain and behavior. Am. Psychol. 2012;67:309–318. doi: 10.1037/a0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody B.A., Kinney H.C., Kloman A.S., Gilles F.H., Brody B.A., Kloman A.S., Gilles F.H. Sequence of central nervous system myelination in human Infancy: an autopsy study of myelination. J. Neuropathol. Exp. Neurol. 1987;46:283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- Buss C., Davis E.P., Shahbaba B., Pruessner J.C., Head K., Sandman C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E1312. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C., Entringer S., Wadhwa P.D. Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology. Sci. Signal. 2012;5:1–8. doi: 10.1126/scisignal.2003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav. Neurosci. 2011;125:20–28. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. The stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016 doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho M.C., King L.S., Ojha A., Garcia C.M., Sisk L.M., Cichocki A.C., Humphreys K.L., Gotlib I.H. Cerebral blood flow in 5‐ to 8‐month‐olds: Regional tissue maturity is associated with infant affect. Dev. Sci. 2019 doi: 10.1111/desc.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Lee F.S. Optimizing treatments for anxiety by age and genetics. Ann. N. Y. Acad. Sci. 2015;1345:16–24. doi: 10.1111/nyas.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., James G.A., Tripathi S., Mletzko T., Heim C., Hu X.P., Mayberg H.S., Nemeroff C.B., Kilts C.D. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol. Med. 2013;43:507–518. doi: 10.1017/S0033291712001390. [DOI] [PubMed] [Google Scholar]
- Colich N.L., Williams E.S., Ho T.C., King L.S., Humphreys K.L., Price A.N., Ordaz S.J., Gotlib I.H. The association between early life stress and prefrontal cortex activation during implicit emotion regulation is moderated by sex in early adolescence. Dev. Psychopathol. 2017;29:1851–1864. doi: 10.1017/S0954579417001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich N.L., Rosen M.L., Williams E.S., McLaughlin K.A. Biological aging in childhood and adolescence following experiences of threat and deprivation: a systematic review and meta-analysis. bioRxiv. 2019 doi: 10.1101/642405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M., Ellis B.J., Shirtcliff E.A. The adaptive calibration model of stress responsivity. Neurosci. Biobehav. Rev. 2011 doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S.C.L., Mercure E., Blasi A., Gasston D., Thomson A., Johnson M., Williams S.C.R., Murphy D.G.M. Mapping infant brain myelination with magnetic resonance imaging. J. Neurosci. 2011;31:784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S., Buss C., Wadhwa P.D. Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 Curt Richter Award paper. Psychoneuroendocrinology. 2015;62:366–375. doi: 10.1016/j.psyneuen.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–173. doi: 10.1016/J.NEUROIMAGE.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhuis W.E., de Weerth C. Does early-life exposure to stress shape or impair cognition? Curr. Dir. Psychol. Sci. 2013;22:407–412. doi: 10.1177/0963721413484324. [DOI] [Google Scholar]
- Friston K.J., Mechelli A., Turner R., Price C.J. Nonlinear responses in fMRI: the balloon model, Volterra kernels, and other hemodynamics. Neuroimage. 2000;12:466–477. doi: 10.1006/nimg.2000.0630. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., O’Muircheartaigh J., Dirks H., Dean D.C., Tottenham N., Deoni S. Human amygdala functional network development: a cross-sectional study from 3 months to 5 years of age. Dev. Cogn. Neurosci. 2018;34:63–74. doi: 10.1016/j.dcn.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangolli M., Holleran L., Hee Kim J., Stein T.D., Alvarez V., McKee A.C., Brody D.L. Quantitative validation of a nonlinear histology-MRI coregistration method using generalized Q-sampling imaging in complex human cortical white matter. Neuroimage. 2017;153:152–167. doi: 10.1016/j.neuroimage.2017.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Gilmore J.H., Shen D., Smith J.K., Zhu H., Lin W. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb. Cortex. 2013;23:594–603. doi: 10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Alcauter S., Elton A., Hernandez-Castillo C.R., Smith J.K., Ramirez J., Lin W. Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb. Cortex. 2015;25:2919–2928. doi: 10.1093/cercor/bhu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Hare T.A., Bookheimer S.Y., Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Hare T.A., Bookheimer S.Y., Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.M., Laumann T.O., Gilmore A.W., Newbold D.J., Greene D.J., Berg J.J., Ortega M., Hoyt-Drazen C., Gratton C., Sun H., Hampton J.M., Coalson R.S., Nguyen A.L., McDermott K.B., Shimony J.S., Snyder A.Z., Schlaggar B.L., Petersen S.E., Nelson S.M., Dosenbach N.U. Precision functional mapping of individual human brains. Neuron. 2017;95 doi: 10.1016/j.neuron.2017.07.011. 791-807.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K., Burns C.D., Madison C., Clark D., Halchenko Y.O., Waskom M.L., Ghosh S.S. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinform. 2011;5:13. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Pfeifer J.H., Fisher P.A., Lin W., Gao W., Fair D.A. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev. Cogn. Neurosci. 2015 doi: 10.1016/j.dcn.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Rasmussen J.M., Rudolph M.D., Heim C.M., Gilmore J.H., Styner M., Potkin S.G., Entringer S., Wadhwa P.D., Fair D.A., Buss C. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol. Psychiatry. 2018;83:109–119. doi: 10.1016/j.biopsych.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Rasmussen J.M., Entringer S., Ben Ward E., Rudolph M.D., Gilmore J.H., Styner M., Wadhwa P.D., Fair D.A., Buss C. Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol. Psychiatry. 2019;85:172–181. doi: 10.1016/j.biopsych.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M.N., Hwang K., Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D., Lou H.C., Olsen J. Serious life events and congenital malformations: a national study with complete follow-up. Lancet. 2000;356:875–880. doi: 10.1016/S0140-6736(00)02676-3. [DOI] [PubMed] [Google Scholar]
- Harb R., Whiteus C., Freitas C., Grutzendler J. In vivo Iimaging of cerebral microvascular plasticity from birth to death. J. Cereb. Blood Flow Metab. 2013;33:146–156. doi: 10.1038/jcbfm.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., Duda S.N. The REDCap consortium: Building an international community of software partners. J. Biomed. Inform. 2019 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatalski C.G., Guirguis C., Baram T.Z. 1998. Corticotropin Releasing Factor mRNA Expression in the Hypothalamic Paraventricular Nucleus and the Central Nucleus of the Amygdala Is Modulated by Repeated Acute Stress in the Immature Rat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt J.A., Demaestri C., Peterzell S., Silveri M.M., Cai X., Kulkarni P., Cunningham M.G., Ferris C.F., Brenhouse H.C. Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity. Elife. 2020;9 doi: 10.7554/eLife.52651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.R., Styner M.A., Gao W., Yap P.T., Wang L., Baluyot K., Yacoub E., Chen G., Potts T., Salzwedel A., Li G., Gilmore J.H., Piven J., Smith J.K., Shen D., Ugurbil K., Zhu H., Lin W., Elison J.T. The UNC/UMN Baby Connectome Project (BCP): an overview of the study design and protocol development. Neuroimage. 2019 doi: 10.1016/j.neuroimage.2018.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain Images. Neuroimage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Pechaud M., Smith S. BET2-MR-based estimation of brain, skull and scalp surfaces. Eleventh Annual Meeting of the Organization for Human Brain Mapping. 2005 [Google Scholar]
- Keding T.J., Herringa R.J. Paradoxical prefrontal-amygdala recruitment to angry and happy expressions in pediatric posttraumatic stress disorder. Neuropsychopharmacology. 2016;41:2903–2912. doi: 10.1038/npp.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S., Laplante D.P. The effects of prenatal maternal stress on children’s cognitive development: project Ice Storm. Stress. 2005;8:35–45. doi: 10.1080/10253890500108391. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K., Hamer R.M., Lin W., Gerig G., Gilmore J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A., Wu M., Klumpp H., Pine D.S., Swain J.E., Fitzgerald K.D., Monk C.S., Phan K.L. Altered development of amygdala-anterior cingulate cortex connectivity in anxious youth and young adults. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1:345–352. doi: 10.1016/j.bpsc.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamaru E., Numakawa T., Adachi N., Yagasaki Y., Izumi A., Niyaz M., Kudo M., Kunugi H. Glucocorticoid prevents brain-derived neurotrophic factor-mediated maturation of synaptic function in developing hippocampal neurons through reduction in the activity of mitogen-activated protein kinase. Mol. Endocrinol. 2008;22:546–558. doi: 10.1210/me.2007-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann T.O., Gordon E.M., Adeyemo B., Snyder A.Z., Joo S.J., Chen M.-Y., Gilmore A.W., McDermott K.B., Nelson S.M., Dosenbach N.U.F., Schlaggar B.L., Mumford J.A., Poldrack R.A., Petersen S.E. Functional system and areal organization of a highly sampled individual human brain. Neuron. 2015;87:657–670. doi: 10.1016/j.neuron.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenniger C., Espinoza-Heredia C., Trentacosta C., Thomason M.E. Associations between prenatal maternal cortisol levels and the developing human brain. Biol. Psychiatry. 2020;87:S126. doi: 10.1016/j.biopsych.2020.02.340. [DOI] [Google Scholar]
- Lussier A.L., Caruncho H.J., Kalynchuk L.E. Repeated exposure to corticosterone, but not restraint, decreases the number of reelin-positive cells in the adult rat hippocampus. Neurosci. Lett. 2009;460:170–174. doi: 10.1016/j.neulet.2009.05.050. [DOI] [PubMed] [Google Scholar]
- Marusak H.A., Martin K.R., Etkin A., Thomason M.E. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology. 2015;40:1250–1258. doi: 10.1038/npp.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo C., Knutsen P.M., Tsai P.S., Shih A.Y., Kleinfeld D. Entrainment of arteriole vasomotor fluctuations by neural activity is a basis of blood-oxygenation-level-dependent “resting-state” connectivity. Neuron. 2017;96 doi: 10.1016/j.neuron.2017.10.012. 936-948.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet J., Maras P.M., Avishai-Eliner S., Baram T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014;56:1675–1688. doi: 10.1002/dev.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R.M. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzari S., Fearon P., Rice F., Ciceri F., Molteni M., Frigerio A. Neuroendocrine and immune markers of maternal stress during pregnancy and infant cognitive development. Dev. Psychobiol. 2020:1–11. doi: 10.1002/dev.21967. [DOI] [PubMed] [Google Scholar]
- Noorlander C.W., De Graan P.N.E., Middeldorp J., Van Beers J.J.B.C., Visser G.H.A. Ontogeny of hippocampal corticosteroid receptors: effects of antenatal glucocorticoids in human and mouse. J. Comp. Neurol. 2006;499:924–932. doi: 10.1002/cne.21162. [DOI] [PubMed] [Google Scholar]
- Norman M.G., Oʼkusky J.R. The growth and development of microvasculature in human cerebral cortex. J. Neuropathol. Exp. Neurol. 1986;45:222. doi: 10.1097/00005072-198605000-00003. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005 doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Park A.T., Leonard J.A., Saxler P.K., Cyr A.B., Gabrieli J.D.E., Mackey A.P. Amygdala-medial prefrontal cortex connectivity relates to stress and mental health in early childhood. Soc. Cogn. Affect. Neurosci. 2018;13:430–439. doi: 10.1093/scan/nsy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Cha J., Roy A.K., Peterson B.S., Bansal R., Gustafsson H.C., Raffanello E., Gingrich J., Monk C. Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl. Psychiatry. 2016;6:4–11. doi: 10.1038/tp.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A., Anh T.T., Li Y., Chen H., Rifkin-Graboi A., Broekman B.F.P., Kwek K., Saw S.-M., Chong Y.-S., Gluckman P.D., Fortier M.V., Meaney M.J. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl. Psychiatry. 2015;5:e508. doi: 10.1038/tp.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G.J., Garcia R., González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol. Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Bai J., Chen H., Hameed W.B.R., Sim L.W., Tint M.T., Leutscher-Broekman B., Chong Y.S., Gluckman P.D., Fortier M.V., Meaney M.J., Qiu A. Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol. Psychiatry. 2013;74:837–844. doi: 10.1016/j.biopsych.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Meaney M.J., Chen H., Bai J., Hameed W.B., Tint M.T., Broekman B.F.P., Chong Y.-S., Gluckman P.D., Fortier M.V., Qiu A. Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54 doi: 10.1016/j.jaac.2015.01.013. 313-321.e2. [DOI] [PubMed] [Google Scholar]
- Rogers C.E., Sylvester C.M., Mintz C., Kenley J.K., Shimony J.S., Barch D.M., Smyser C.D. Neonatal amygdala functional connectivity at rest in healthy and preterm infants and early internalizing symptoms. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56:157–166. doi: 10.1016/j.jaac.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel A.P., Stephens R.L., Goldman B.D., Lin W., Gilmore J.H., Gao W. Development of amygdala functional connectivity during infancy and its relationship with 4-year behavioral outcomes. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019;4:62–71. doi: 10.1016/j.bpsc.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Craig M.C., Dell’Acqua F., O’Connor T.G., Catani M., Deeley Q., Glover V., Murphy D.G.M. Prenatal stress and limbic-prefrontal white matter microstructure in children aged 6-9 years: a preliminary diffusion tensor imaging study. World J. Biol. Psychiatry. 2014;15:346–352. doi: 10.3109/15622975.2014.903336. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H., Gur R.C., Gur R.E. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D., Sinha R., Cross S.N., Kwon S.H., Sze G., Constable R.T., Ment L.R. Does prenatal stress alter the developing connectome? Pediatr. Res. 2017 doi: 10.1038/pr.2016.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H., Arima H., Ozawa Y., Watanabe M., Banno R., Sugimura Y., Ozaki N., Nagasaki H., Oiso Y. Glucocorticoids increase NPY gene expression in the arcuate nucleus by inhibiting mTOR signaling in rat hypothalamic organotypic cultures. Peptides. 2010;31:145–149. doi: 10.1016/j.peptides.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Silvers J.A., Insel C., Powers A., Franz P., Helion C., Martin R.E., Weber J., Mischel W., Casey B.J., Ochsner K.N. VlPFC-vmPFC-amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cereb. Cortex. 2017;27:3502–3514. doi: 10.1093/cercor/bhw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser C.D., Inder T.E., Shimony J.S., Hill J.E., Degnan A.J., Snyder A.Z., Neil J.J. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F., Quirk G.J. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C.M., Smyser C.D., Smyser T., Kenley J., Ackerman J.J., Shimony J.S., Petersen S.E., Rogers C.E. Cortical functional connectivity evident after birth and behavioral inhibition at age 2. Am. J. Psychiatry. 2018;175:180–187. doi: 10.1176/appi.ajp.2017.17010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Ogawa T. Characterization of developmental changes in EEG-gamma band activity during childhood using the autoregressive model. Pediatr. Int. 1998;40:446–452. doi: 10.1111/j.1442-200X.1998.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Thijssen S., Muetzel R.L., Bakermans-Kranenburg M.J., Jaddoe V.W.V., Tiemeier H., Verhulst F.C., White T., Van Ijzendoorn M.H. Insensitive parenting may accelerate the development of the amygdala-medial prefrontal cortex circuit. Dev. Psychopathol. 2017;29:505–518. doi: 10.1017/S0954579417000141. [DOI] [PubMed] [Google Scholar]
- Thomas E., Buss C., Rasmussen J.M., Entringer S., Ramirez J.S.B., Marr M., Rudolph M.D., Gilmore J.H., Styner M., Wadhwa P.D., Fair D.A., Graham A.M. Newborn amygdala connectivity and early emerging fear. Dev. Cogn. Neurosci. 2019;37 doi: 10.1016/j.dcn.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. The importance of early experiences for neuro-affective development. Curr. Top. Behav. Neurosci. 2014;16:109–129. doi: 10.1007/7854_2013_254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Gabard-Durnam L.J. The developing amygdala: a student of the world and a teacher of the cortex. Curr. Opin. Psychol. 2017 doi: 10.1016/j.copsyc.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmin H., Beckmann C.F., O’Muircheartaigh J., Ball G., Nongena P., Makropoulos A., Ederies A., Counsell S.J., Kennea N., Arichi T., Tusor N., Rutherford M.A., Azzopardi D., Gonzalez-Cinca N., Hajnal J.V., Edwards A.D. Specialization and integration of functional thalamocortical connectivity in the human infant. Proc. Natl. Acad. Sci. 2015;112:6485–6490. doi: 10.1073/pnas.1422638112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesky T.K., Jensen S.K.G., Yu X., Kumar S., Wang Y., Sliva D.D., Gagoski B., Sanfilippo J., Zöllei L., Boyd E., Haque R., Hafiz Kakon S., Islam N., Petri W.A., Nelson C.A., Gaab N. The relationship between biological and psychosocial risk factors and resting-state functional connectivity in 2-month-old Bangladeshi infants: a feasibility and pilot study. Dev. Sci. 2019;22 doi: 10.1111/desc.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison N.J., Avants B.B., Cook P.A., Zheng Y., Egan A., Yushkevich P.A., Gee J.C. N4ITK: improved N3 Bias correction. IEEE Transl. Med. Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bergh B.R.H., Mulder E.J.H., Mennes M., Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci. Biobehav. Rev. 2005;29:237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem M.R., Tottenham N. Current Topics in Behavioral Neurosciences. Springer Verlag; 2018. Neurobiological programming of early life stress: functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology; pp. 117–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L., Huang H., Jovanov-Milošević N., Pletikos M., Mori S., Kostović I. Development of axonal pathways in the human fetal fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J. Anat. 2010;217:400–417. doi: 10.1111/j.1469-7580.2010.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa P.D., Buss C., Entringer S., Swanson J.M. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2010;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg L.A.M., Seckl J.R., Holmes M.C. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/S0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Wolfe J., Kimerling R., Brown P.J., Chrestman K.R., Levin K. Psychometric review of the life stressor checklist-revised. Meas. Stress. trauma, Adapt. 1996:198–201. [Google Scholar]
- Wu M., Kujawa A., Lu L.H., Fitzgerald D.A., Klumpp H., Fitzgerald K.D., Monk C.S., Phan K.L. Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Hum. Brain Mapp. 2016;37:1684–1695. doi: 10.1002/hbm.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Rincón-Cortés M., Raineki C., Sarro E., Colcombe S., Guilfoyle D.N., Yang Z., Gerum S., Biswal B.B., Milham M.P., Sullivan R.M., Castellanos F.X. Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2016.276. e1005–e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F.-C., Wedeen V.J., Tseng W.-Y.I. Generalized q-sampling imaging. IEEE Trans. Med. Imaging. 2010;29:1626–1635. doi: 10.1109/TMI.2010.2045126. [DOI] [PubMed] [Google Scholar]
- Yeh F.-C., Wedeen V.J., Tseng W.-Y.I. Estimation of fiber orientation and spin density distribution by diffusion deconvolution. Neuroimage. 2011;55:1054–1062. doi: 10.1016/j.neuroimage.2010.11.087. [DOI] [PubMed] [Google Scholar]
- Yeh F.-C., Verstynen T.D., Wang Y., Fernández-Miranda J.C., Tseng W.-Y.I. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.